Abstract
A previous study reported that a 400-mg dose of medroxyprogesterone acetate (MPA) reduced male reindeer aggression and blocked development of secondary sexual characteristics but did not completely impair fertility. Here we have repeated that protocol in two separate trials. In 2017, tissues and blood samples, collected from MPA and control (CTL) reindeer bulls, euthanized at 30 and 60 d post-treatment were used to evaluate testes histology and morphometrics, cfos activity in the brain and androgen levels. While testes weight tended to decline from August to September in both groups, indices of spermatogenesis remained high. By September, indices of spermatogenesis were declining in both groups with sperm density lower (P = 0.05) in MPA compared to CTL bulls. Aug CTL bulls had the highest concentrations of androstenedione (A4) (P = 0.009) and testosterone (T) (P = 0.08), whereas these androgens were baseline in Aug MPA bulls. By September, A4 and T levels in CTL bulls declined to levels measured in MPA bulls. Cfos activity had a greater number (P = 0.02) of cfos positive neurons in the central amygdala in MPA compared to CTL bulls, suggesting a heightened fear response among the MPA bulls. In the second trial (2019), MPA-treated bulls, with (E, n = 4) and without (IE, n = 4) breeding experience, were blood sampled at key points from July through September when they were put in individual harems with estrous-synchronized cows. Concentrations of T were greatest (P < 0.001) among E bulls prior to MPA treatment but 1 mo after treatment, both T and A4 were baseline in all eight reindeer. Semen collected by electroejaculation at 60 d post-MPA treatment revealed only minor differences in sperm abnormalities between E and IE bulls using both fresh and frozen/thawed semen. Only three bulls (2 E and 1 IE) sired offspring. Breeding success was not related to previous breeding experience, body weight, or bull age. The failure of some MPA bulls to breed appears to be a behavioral, not a physiological, limitation. Limited application of MPA is clearly a useful tool for managing rut-aggression in non-breeding reindeer. However, the possibility that semen could be collected from MPA-treated bulls using restraint and mild sedation rather than general anesthesia should be investigated. This could improve the quality of semen collection while enhancing the safety of both handlers and animals.
Keywords: androgens, cfos brain activity, medroxyprogesterone acetate, reindeer, spermatogenesis, sperm characteristics
A single dose of MPA given to reindeer bulls at the beginning of rut suppresses rut-related changes with no apparent effects on spermatogenesis in the short-term (30 d) though fertility may be impacted by 60 d. While some MPA bulls successfully breed, an increase in cfos activity in the central amygdala of MPA-treated bulls may be inhibiting breeding through an enhanced fear response.
Introduction
Reindeer (Rangifer tarandus) are an important resource (meat, fur, and antlers) throughout the circumpolar north and are herded on traditional pastures today (Nieminen, 2018). More recently in North America, reindeer farming behind fences for agrotourism, meat, and antlers has become increasingly popular (https://reindeerowners.com/). Husbandry practices developed for intensive management provide an important resource that can inform reindeer conservation efforts as climate change (Tryland et al., 2018) and politics (Tyler et al., 2021) alter traditional practices and ranges.
Farmed reindeer are generally docile and easy to handle except for rutting bulls. Reindeer in rut will direct their aggression towards herd mates, people, and infrastructure. Hypophagia, a natural feature of rutting bulls (Nagy et al., 2021), can last ~2–3 mo and, in farmed situations, affects all male reindeer including younger, subordinate bulls (Barboza et al., 2004). In extreme cases, hypophagia-induced emaciation can be difficult to reverse (Blake et al., 2007).
Medroxyprogesterone acetate (MPA), a synthetic progestin, has been widely used to control male aggression in a variety of mammals: humans (Emory et al., 1995), primates (Zumpe et al., 1991), horses (Squires et al., 1997), dogs (Hart, 1981), and, most commonly, in zoos and captive wildlife settings (Patton et al., 2005). Anecdotal information from reindeer production settings suggests that MPA administered on a short-term basis (2-4 mo) will reduce aggression in non-breeding male reindeer or may be given to males following breeding to reduce rutting aggression (Blake et al., 2007).
In a recent study, a single dose (400 mg) of MPA produced a marked reduction in rut-related aggression that lasted for approximately 2 mo in a mixed-age group of reindeer males (Rowell et al., 2019). Although testosterone (T) levels were not measured, the treatment eliminated rut-related aggressive behavior, rut-induced hypophagia, and interfered with the final cleaning of velvet antlers, all T dependent events (Stokkan et al., 1980; Bubenik et al., 1997; Barboza et al., 2004). In the study by Rowell et al. (2019), the single MPA dose was effective in all individuals regardless of age or weight. However, even though the MPA-treated bulls behaved like castrates, fertility was not abolished by this treatment as indicated by similar sperm characteristics and breeding success of an MPA-treated bull 45 d post-treatment. Alternatively, an MPA-treated bull the following year showed no interest in estrous females and did not produce offspring even though the breeding soundness exam (BSE) did not indicate impaired fertility (Rowell et al., 2019). This bull had no previous breeding experience, which may have affected his response to estrous females in harem.
Using MPA to reduce rut-related behaviors among non-breeding bulls and to reverse hypophagia in bulls at the end of the breeding season is a useful tool for managing reindeer males. However, the unexpected finding that some MPA-treated bulls successfully bred females while others showed no interest in breeding, raises questions about previous breeding experience, the mechanism of MPA actions, and the role of androgens in rutting reindeer.
In 2017, the objective was to evaluate plasma androgen concentrations and tissues from testes and brains collected from MPA-treated and CTL reindeer bulls harvested ~30 and 60 d post-treatment. Specific goals were to describe and identify treatment similarities and/or differences in testes morphometric measures, sperm density counts, systemic levels of T and androstenedione (A4), and to identify cfos activity in different regions of the brain.
In 2019, the objective was to evaluate breeding success, systemic T and A4 concentrations, and semen characteristics in MPA-treated bulls with and without prior breeding experience.
Materials and Methods
Animals and experimental design
Reindeer were housed at the Robert G. White Large Animal Research Station (LARS), University of Alaska Fairbanks (UAF). All reindeer protocols were approved by the UAF Institutional Animal Care and Use Committee, Protocol #1081766 (Objective 1) and #1448250 (Objective 2).
Objective 1 (2017) testes measurements, cfos activity in the brain, and plasma androgen concentrations from MPA and CTL reindeer bulls
Four of eight reindeer bulls (MPA) between 2 and 5 yr of age were administered MPA (400 mg im; Rood and Riddle Veterinary Pharmacy, Lexington, KY) on July 28, approximately 1 wk before antler cleaning and 2 wk before anticipated onset of overt, rut-related aggression. Overt stress associated with injections stems primarily from handling and restraint, so the remaining four bulls were similarly handled (restrained in the hydraulic chute and weighed) on July 28 but remained as untreated controls (CTL). On August 28–29 (31–32 d post-treatment), MPA (n = 2) and CTL bulls (n = 2) were anesthetized using intramuscular injection of 0.6 mg/kg xylazine (Xylazine Injection 100 mg/mL, Covetrus North America, Dublin, OH) and 8 mg/kg ketamine (Ketathesia 100 mg/mL Covetrus North America, Dublin, OH). After induction, blood samples were collected followed by an intravenous injection of 80 IU of sodium heparin to improve the brain perfusion by reducing blood clotting. Anesthetized animals were then transferred to a necropsy facility where body weights were recorded. Following euthanasia, intravenous injection of 60–80 mg/kg pentobarbital (Euthasol 390mg/ml, Virbac AH, Inc, Fort Worth, TX), the head was immediately removed for brain perfusion. Tissue collection and standard post-mortem examination followed.
In September, the remaining four bulls (two MPA and two CTL) were randomly assigned to one of four harems consisting of either five or six estrous-synchronized females. They remained in harem for 1 wk (September 6–13). Ultrasound for pregnancy was conducted at approximately 8 wk post-harem. On September 25–26, the remaining four bulls were similarly euthanized (59–60 d post-treatment) and the same sampling protocol followed.
Estrus synchronization and breeding protocol
Estrus synchronization in these reindeer is a well-established, routinely applied practice at the onset of the breeding season (Blake et al., 2007). Progesterone-releasing vaginal insert (EAZI-BREED CIDR Sheep Insert; Zoetis Animal Health, Troy Hills, NJ) was placed in breeding females for 1 wk. At CIDR removal, Prostaglandin F2α (Lutalyse, 15 mg; Zoetis Animal Health, Troy Hills, NJ) was administered by intramuscular injection and the females placed in harem with a bull for 1 wk. After harem break up, females remained separated from all males until calving.
Tissue collection and analyses
Body weights and blood sampling
Body weights and jugular blood samples were collected prior to euthanasia, serum harvested, and stored frozen until analyzed.
Testes
Following excision and removal of the epididymis, testes were weighed and measured (length × width) with digital calipers. Each testis was bisected longitudinally, and samples (approximately 2 cm3) were collected from the top, middle and bottom regions of the testis. Tissues were fixed in Bouin’s solution overnight, rinsed in 70% ethanol, dehydrated, and embedded in paraffin following standard histological procedures (Abedal-Majed et al., 2022). Each paraffin block was serially sectioned (20 slides). Sections (6µ) were stained with hematoxylin and eosin.
Calculation of sperm density
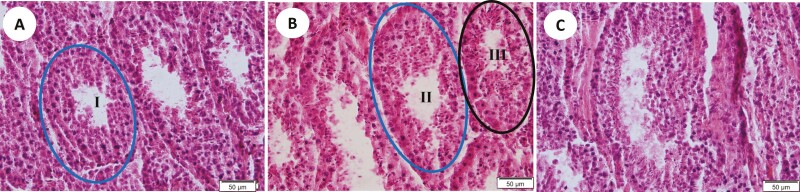
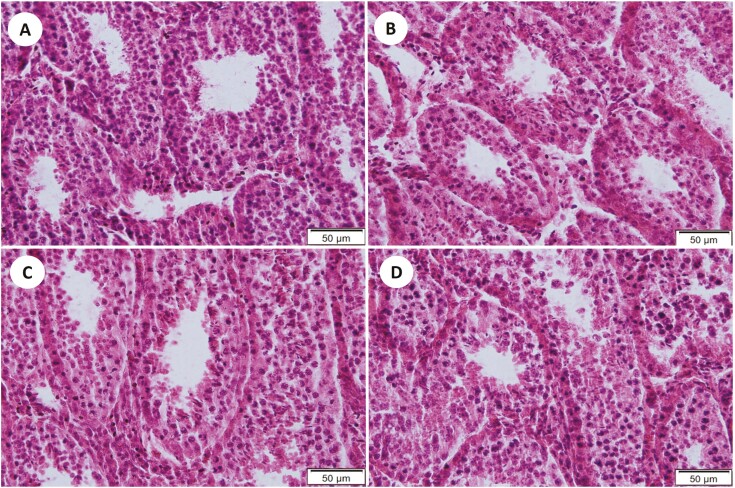
On each slide, three separate areas of the seminiferous tubule were analyzed by comparing them to representative, standardized images with sperm density ranked 0, 1, 2, and 3 (Table 1; Figure 1A–C). The proportion of seminiferous tubules containing developing sperm was calculated by dividing those containing developing sperm by total number of seminiferous tubules in each unit area (400×). A similar calculation was made for tubules containing mature sperm (MS). Sperm density per unit area was derived by determining the density of sperm per tubule in each unit area (400×) and averaging per unit area and bull. Overall, nine unit-areas were counted per bull with three unit-areas obtained in serial sections at slides 5, 10, and 15. Since three separate tissue samples of the testis were obtained per bull, the analyzed slides at 5, 10, and 15 were randomized as to which sample was quantified. Each slide was evaluated by three independent technicians. Totals were averaged for each sample within technician and overall.
Table 1.
Rank scale for sperm density counts
| Rank | Description | Photomicrograph |
|---|---|---|
| 0 | No developing spermatids/sperm | Fig 1A |
| 1 | Few developing spermatids/sperm | Fig 1B |
| 2 | Several developing spermatids/sperm | Fig 1B |
| 3 | Many spermatids/sperm concentrated around the lumen | Fig 1C |
Within each seminiferous tubule, the number of developing spermatids or mature sperm was estimated and the seminiferous tubule ranked according to the following scale.
Figure 1.
Examples of seminiferous tubules from reindeer testes representing various stages of spermatogenesis as defined in Table 1. (A) The seminiferous tubule circled I is an example of a tubule ranked 0; (B) the seminiferous tubule labeled II is an example of a tubule ranked 1; the seminiferous tubule labeled III is an example of a tubule ranked 3; (C) the seminiferous tubule in the middle is an example of a tubule with mature sperm (6µ H&E).
Hormone assays
Androstenedione was measured using an ImmuChem Double Antibody Androstenedione RIA kit from MP Biomedicals (Solon, OH). Serum samples were extracted and treated according to manufacturer’s instructions. Samples were analyzed in multiple assays with intra- and inter- coefficient of variation ranging between 3%–8% and 10%–14%, respectively (Summers et al., 2014). Testosterone was measured using an ImmuChem Double Antibody Testosterone RIA kit from MP Biomedicals (Solon, OH). Serum samples were treated according to manufacturer’s instructions and run in multiple assays with reported intra- and inter-coefficient of variation ranging between 9%–11% and 12%, respectively (Desaulniers et al., 2015). Assay sensitivity for both assays was 10 pg/mL. To validate reindeer plasma, charcoal-stripped reindeer plasma was spiked with high standards for A4 and T then serially diluted 1:2 to fall within the range of the standard curve. The reindeer samples exhibited parallelism with similarly treated bovine samples (previously validated for these assays). Recoveries were 90% of expected with CV’s all below 12%.
Brain tissue
Immediately following euthanasia, heads were removed and perfused via the carotid artery with one liter of 0.9% saline containing 75,000 units of heparin followed by 5 liters of 4% paraformaldehyde in 0.1 M Sorensen’s phosphate buffer. When perfusion was complete, the skull was opened using a Stryker saw and the brain removed following a previously described necropsy procedure (Miller and Zachary, 2017). Olfactory bulbs and brains were removed from the cranium and the intact brain was postfixed in a 4% paraformaldehyde solution and stored in 70% ethanol.
Fixed brains were dissected using surface landmarks. The amygdala was dissected on the rostral/caudal plane at the level of the optic nerve. The preoptic area of the hypothalamus was identified anterior of the optic chiasm, ventral to the anterior commissure, and lateral to the third ventricle (Mirto et al., 2017). The ventral tegmental area (VTA) was dissected from the midbrain tegmentum at the midline fixed laterally by the cerebral peduncles and the red nucleus (Kramer et al., 2017). Fixed brain tissue was cryoprotected in a 20% sucrose fixative solution at 4 °C and then rapidly frozen on dry ice and stored at −80 °C until sectioned.
Blocked tissues were embedded in Tissue-Tek O. C. T. compound (VWR International, LLC, Radnor, PA). Using a Reichert HistoStat Cryostat microtome, samples where sliced anterior to posterior at 30 μm for a total of 24 slices per animal. Tissue slices were placed in cryoprotectant (50% 0.05M sodium phosphate buffer, 30% ethylene glycol, 20% glycerol) and stored at −20 °C.
Neuronal activity was evaluated by measuring fos positive cells in brain tissues. Every third tissue slice for a total of 8 slices per animal were stained using rabbit polyclonal antibody (FRP, Ab-2, PC38, Calbiochem, San Diego, CA; diluted 1:20,000) in free-floating sections (Mirto et al., 2017). Negative controls were obtained in the absence of primary antibody. Stained sections were visualized on an Olympus Vannox microscope (10×) equipped with a digital camera. The preoptic area, amygdala nuclei (central, cortical and medial), and VTA were photographed. Images were quantified using Image J software (USA National Institute of Health) for fos positive cells. Stained cells on the border of images were excluded from quantification. Cells with positive staining were counted in each region and averaged for statistical analysis.
Objective 2 (2019): the role of breeding experience in MPA-treated bulls
Breeding trial
Eight reindeer bulls (2- and 4-yr-old) were used in this trial. Four of the bulls successfully bred females the previous fall and were classed as experienced breeders (E). The remaining four bulls (matched for age) were kept isolated with no breeding opportunity and were considered inexperienced (IE). All eight bulls were administered MPA (400 mg i.m.; Rood and Riddle Veterinary Pharmacy, Lexington, KY) on July 24, approximately 1 wk before antler cleaning and 2 wk before anticipated onset of rut-related aggression. For logistic reasons, harems were staggered temporally. Four harems (n = 4 estrous-synchronized females each) were set up September 4–12 and the remaining 4 harems (n = 3 or 4 estrous-synchronized females each) were set up September 12–19. Bulls were randomly assigned to harems, ensuring that each harem replicate included 2 E and 2 IE bulls. Jugular blood samples were collected on July 16, July 24 (MPA injection), August 21 when bulls are normally in full rut, immediately pre-harem and again post-harem. Additional untreated bulls (n = 2; 2- and 4-yr-old) were blood sampled on September 25, with the MPA bulls. These two bulls did not go into harem. All samples were analyzed for T and A4 as described above. Ultrasound for pregnancy was conducted at approximately 8 wk post-harem. A single pregnancy within a harem was considered indicative of a successful breeding bull.
Semen collection and analyses
Semen was collected by electroejaculation from all eight bulls in the breeding trial, September 23–26. The bulls were physically restrained in a hydraulic chute and given an intramuscular injection of ketamine and xylazine (as detailed above). Immediately following injection, they were released into an adjacent pen for anesthetic induction, approximately 5–10 min. All reindeer were monitored (temperature, pulse and respiration, as well as oxygen saturation) and administered supplemental oxygen at a rate of 1.5–2 L/min via an intranasal tube. Once immobilized, each bull was suspended upright in a sling (Munk’s Livestock Sling Mfg., Anacortes, WA 98221). Semen was collected using an electroejaculator as described previously (Rowell et al., 2019). Semen was evaluated immediately following collection using a modified BSE (Rowell et al., 2019). Remaining semen was extended in Triladyl extender (Minitube USA, Inc, Verona, WI 53593) at a concentration of approximately 1 × 108 and frozen in 0.5 mL straws as described previously (Rowell et al., 2019). Flow cytometric analyses of sperm were conducted on frozen/thawed sperm to evaluate viability, acrosome integrity, mitochondrial energy potential, and oxidative stress as described previously (Geary et al., 2021).
Statistics
Statistical analysis of hormone data was conducted using the Proc Mixed Procedure in SAS (version 9.3). For physical testes parameters (i.e., testis length), the Proc Mixed Procedures in SAS were used to determine significance. The test was first conducted with an interaction between MPA treatment and collection date included in the model and if no significance was found, then the interaction was removed from the model. GLM procedures of Minitab (Minitab Inc., State College, PA) were used to determine significant differences in brain tissues. GLM procedures (SigmaPlot version 14.5, Systat Software Inc, San Jose, CA) were used to determine differences between E and IE bull body weight. A 2-factor (sampling date and breeding experience) RMANOVA was used to analyze hormone concentrations in 2019. Semen characteristics were analyzed using the mixed model in SAS (Version 9.4) including bull and prior breeding experience as independent variables. A separate analysis of semen characteristics to determine effects on bulls siring offspring used the mixed model with bull, breeding experience, and sired offspring as independent variables. In all cases, P ≤ 0.05 was considered significant.
Results
Objective 1 (2017) reindeer
Two CTL bulls and one MPA bull successfully bred in September 2017. In harems of six females, the two CTL bulls sired four calves each while the MPA bull (a reindeer that had previous breeding experience) sired three calves. The second MPA bull had no prior breeding experience, exhibited no sexual interest in the females, and sired no offspring.
Body weight and testes weight, length, and diameter
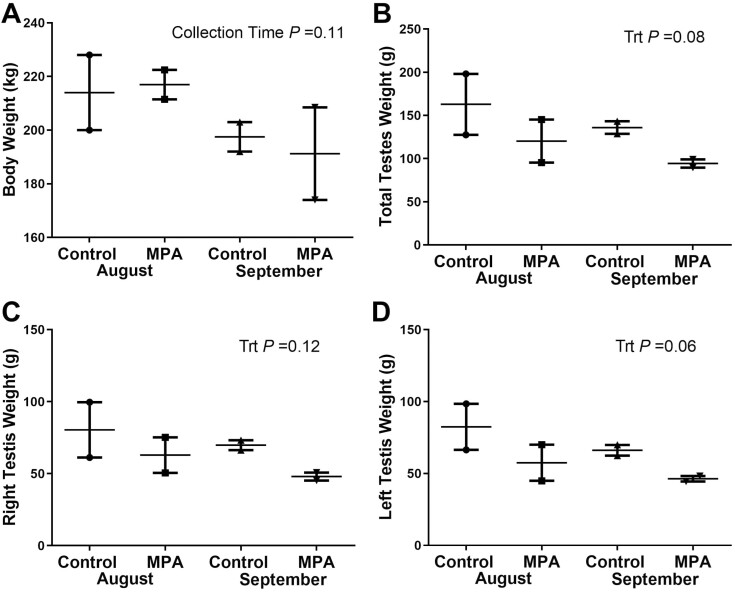
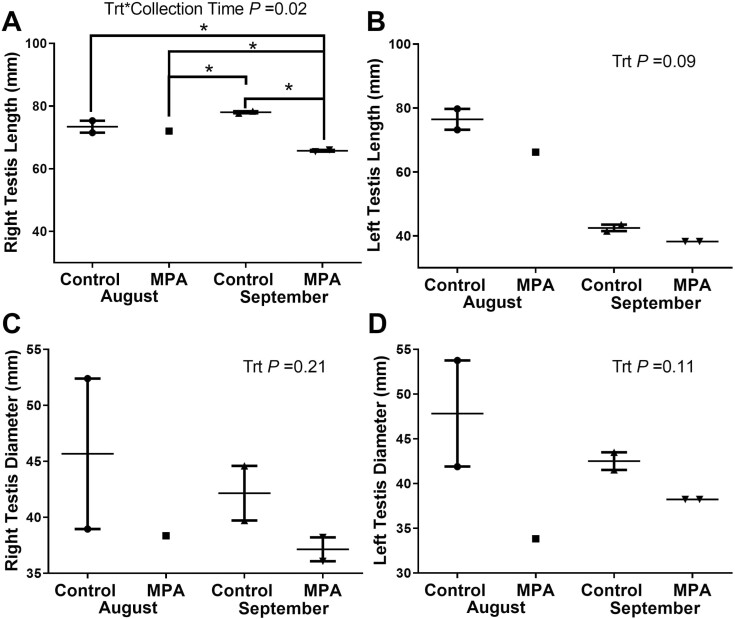
Reindeer body weight did not differ (P = 0.11) between MPA and CTL reindeer in either August or September. Combined testes weight was higher in CTL bulls (P = 0.08) than MPA bulls at both sampling dates, but no treatment differences were identified in left, right, or combined testis weight (Figure 2A–D). Testis length differed with treatment between left and right testes only in September where right testes length in MPA bulls was shorter than Sept CTL bulls (P = 0.05), and both CTL (P = 0.02) and MPA (P = 0.05) bulls in August (Figure 3A and B). Greater variation was observed in testes diameter with Aug CTL bulls having the greatest diameter in both left and right testes. Testes diameter generally decreased in September in both CTL and MPA bulls (Figure 3C and D).
Figure 2.
Body weight and testes weights collected from reindeer bulls. Reindeer bulls either treated with medroxyprogesterone acetate (MPA) or non-treated controls (CTL) were euthanized on August 28 (2 CTL and 2 MPA bulls) or September 25 (2 CTL and 2 MPA bulls). (A) Body weight. (B) Total combined testes weight. (C) Right testis weight. (D) Left testis weight.
Figure 3.
Length and diameter of testes from reindeer bulls. Reindeer bulls either treated with medroxyprogesterone acetate (MPA) or non-treated controls (CTL) were euthanized on August 28 (2 CTL and 2 MPA bulls) or September 25 (2 CTL and 2 MPA bulls). (A) Right testis length. (B) Left testis length. (C) Right testis diameter. (D) Left testis diameter.
Measures of spermatogenesis
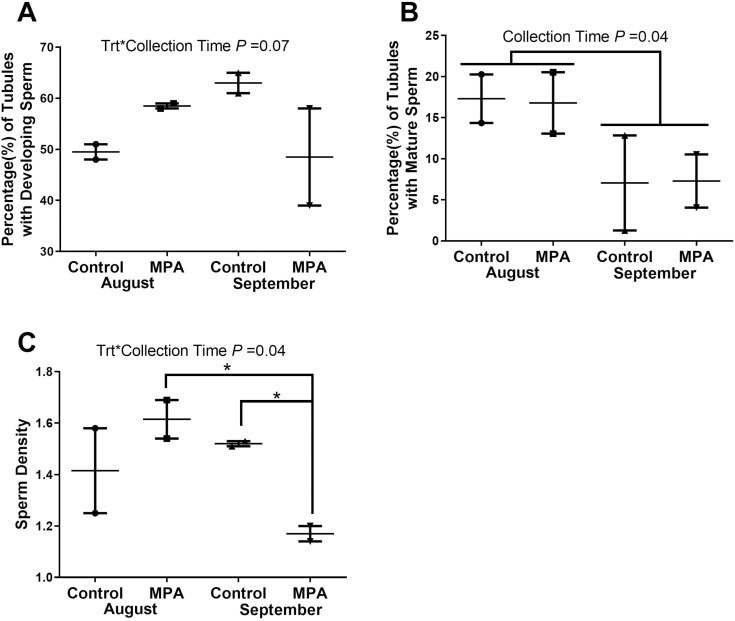
In August, there were no differences (P ≥ 0.10) between CTL and MPA bulls in the percentage of seminiferous tubules with developing spermatids, percentage of tubules with mature sperm, or sperm density. By September, developing spermatids remained high in CTL bulls but showed greater variation in MPA bulls (Figure 4A). The percentage of tubules with mature sperm did not differ with treatment in either August or September although this metric was lower (P = 0.04) for both groups in September (Figure 4B). Sperm density followed a similar pattern with high sperm density among the MPA reindeer in August vs. much lower values (P = 0.03) for MPA bulls in September (Figures 4C and 5A–D).
Figure 4.
Measures of spermatogenesis in testes of reindeer bulls. Reindeer bulls treated with medroxyprogesterone acetate (MPA) or non-treated controls (CTL) were euthanized on August 28 (2 CTL and 2 MPA bulls) or September 25 (2 CTL and 2 MPA bulls). (A) Percent of seminiferous tubules with developing spermatids. (B) Percent of tubules with mature sperm. (C) Average sperm per tubule = Sperm Density.
Figure 5.
Representative images of seminiferous tubules in reindeer testes. Reindeer bulls either treated with medroxyprogesterone acetate (MPA) or non-treated controls (CTL) were euthanized on August 28 (2 CTL and 2 MPA bulls) or September 25 (2 CTL and 2 MPA bulls). (A) CTL-August. (B) MPA-Aug. (C) CTL-Sept. (D) MPA-Sept (6µ; H&E).
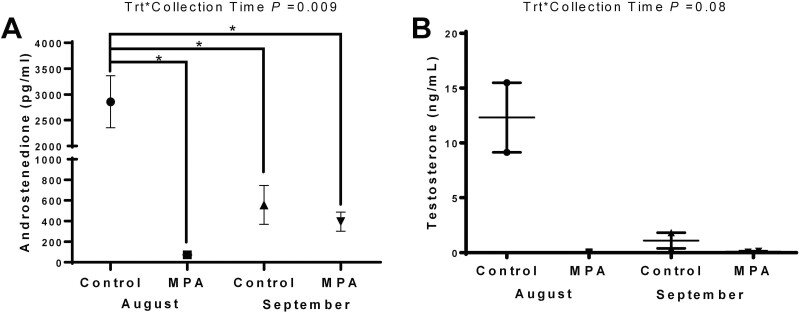
Testosterone and androstenedione concentrations
August CTL bulls exhibited the greatest concentrations of A4 (P = 0.009) and T (P = 0.08) between treatments and collection times. Among August MPA bulls, both A4 and T were baseline. In September, A4 concentrations in CTL bulls had declined (P = 0.004) from August levels and were similar to levels in Sept MPA bulls. Testosterone followed this pattern with elevated levels in Aug CTL bulls declining to MPA levels in September (Figure 6A and B).
Figure 6.
Plasma androgen concentrations in reindeer bulls. Reindeer bulls either treated with medroxyprogesterone acetate (MPA) or non-treated controls (CTL) were euthanized on August 28 (2 CTL and 2 MPA bulls) or September 25 (2 CTL and 2 MPA bulls). Blood samples were collected just prior to euthanasia from CTL bulls (n = 2) and MPA bulls (n = 2) at each sampling date. (A) androstenedione. (B) Testosterone.
Brain (cfos) activity
Cfos activity increased (P = 0.05) from August to September in the preoptic area of the hypothalamus in both the CTL and MPA groups, but differences due to MPA treatment were not noted (P = 0.50). In the central amygdala, MPA bulls had a greater number (P = 0.02) of cfos positive neurons than CTL bulls. Differences due to treatment or collection period were not noted (P ≥ 0.30) in the medial and cortical amygdala. Differences in VTA cfos activity attributed to MPA treatment or season were not detected (P ≥0.20).
Objective 2 (2019) breeding experience and breeding success
Body weight did not change (P = 0.98) from the start of the study to onset of harem formation and did not differ (P = 0.10) between E and IE bulls in July or September. Both groups lost weight post-harem. Three of eight MPA bulls sired a single live calf each, two E bulls and one IE bull.
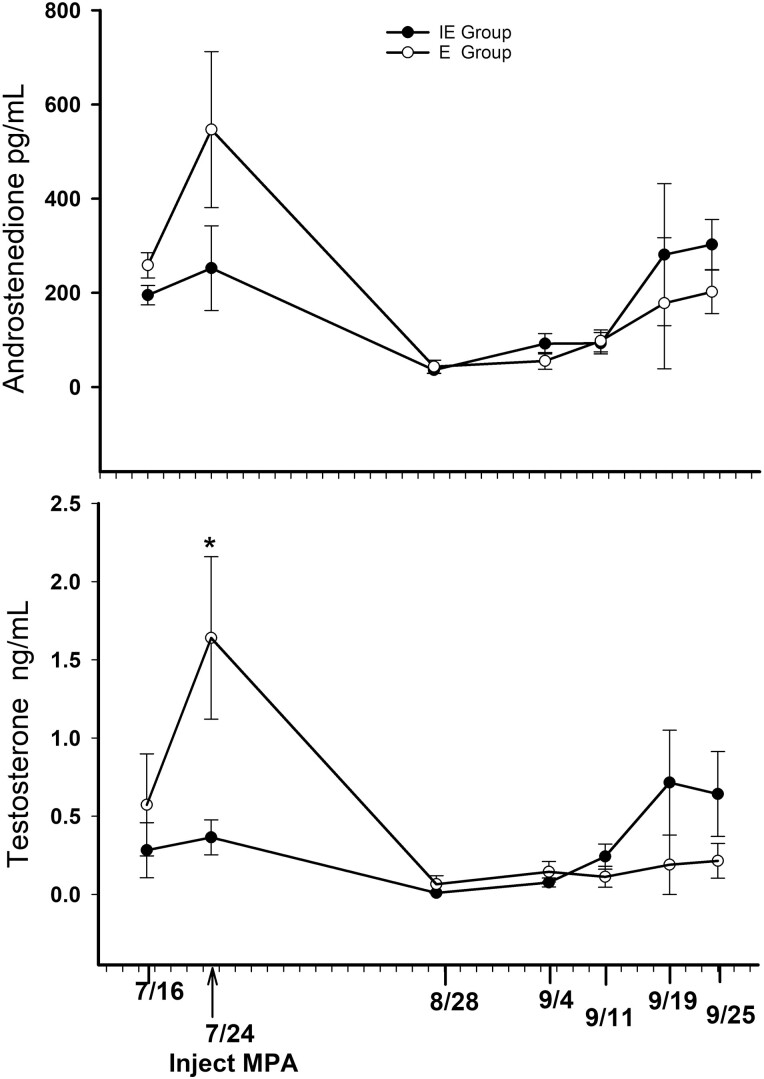
Testosterone and androstenedione
Concentrations of T and A4 (Figure 7A and B) follow a similar pattern over the 2-mo period. Concentrations of A4 did not differ (P = 0.30) between the E and IE groups on any sampling date. However, T concentrations in the E group on July 24 were greater (P = 0.001) than T concentrations in the IE group and greater (P < 0.05) than T concentrations in the E group after MPA treatment. By Aug 21, T and A4 concentrations were baseline or non-detectable in all individuals, increasing slowly and variably throughout September. The two untreated bulls that did not go into harem, had mean concentrations of 5.5 ng/mL T and 6,018 pg/mL A4, at sample collection on September 25.
Figure 7.
Plasma androstenedione and testosterone in reindeer bulls treated with medroxyprogesterone acetate (MPA) before the onset of rut. Eight bulls were divided into 2 groups: bulls (n = 4) with previous breeding experience (E) and bulls (n = 4) with no breeding experience (IE). The * indicates significance (P = 0.001). Testosterone levels in E bulls were greater than levels in IE bulls at all sampling dates and differed from E bulls at all post-MPA sampling dates.
Semen characteristics
Semen collected by electroejaculation revealed no differences (P > 0.10) in any of the semen measures in the modified BSE (Table 2). Flow cytometry measures of frozen/thawed semen from the same ejaculates revealed greater (P = 0.02) percentage of sperm with polarized mitochondria (energy potential) and fewer sperm with head defects (P = 0.04) in the ejaculates of E bulls, but differences were few (Table 2).
Table 2.
Semen characteristics (means ± sem) of ejaculate collected approximately 10 d after harem breakup, from reindeer bulls that had prior breeding experience (E) or no prior breeding experience (IE)
| E Bulls | IE Bulls | SEM | P | |
|---|---|---|---|---|
| BSE measures1 | ||||
| Ejaculate volume, mL | 1.3 | 1.7 | 0.2 | 0.22 |
| Sperm strength of motility rating2 | 2.3 | 2.3 | 0.4 | 1.00 |
| Progressive motility, % | 55.0 | 26.3 | 11.8 | 0.13 |
| Ejaculate concentration × 106 | 845.8 | 495.8 | 222.2 | 0.31 |
| Sperm in ejaculate × 106 | 1198.8 | 710.6 | 393.9 | 0.41 |
| Flow cytometry measures3 | ||||
| Live sperm, % | 42.7 | 31.3 | 5.1 | 0.17 |
| Live sperm with intact acrosome, % | 41.0 | 29.6 | 5.1 | 0.17 |
| Sperm with polarized mitochondria, % | 42.5 | 20.9 | 4.6 | 0.02 |
| Live sperm with strong antioxidant capacity, % | 13.3 | 6.4 | 2.3 | 0.08 |
| Live sperm 3 h post-thaw, % | 32.7 | 28.1 | 2.9 | 0.31 |
| Morphology measures4 | ||||
| Normal sperm, % | 57.3 | 24 | 7.4 | 0.22 |
| Knobbed acrosome, % | 1.5 | 2.8 | 1.2 | 0.47 |
| Head defects, % | 7.3 | 26.3 | 5.2 | 0.04 |
| Detached heads, % | 13.8 | 19.8 | 7.0 | 0.57 |
| Distal midpiece reflex, % | 3.80 | 5.8 | 1.3 | 0.31 |
| DAG-like Defect, % | 3.50 | 5.30 | 0.6 | 0.10 |
| Bowed midpiece, % | 1.50 | 1.00 | 0.9 | 0.70 |
| Proximal droplets, % | 5.3 | 10.5 | 3.6 | 0.34 |
| Distal droplets, % | 0.3 | 0.3 | 0.3 | 1.00 |
| Coiled principal piece, % | 0 | 1.3 | 0.2 | 0.36 |
| Bent principal piece, % | 7 | 4.3 | 3.0 | 0.55 |
Bulls were all treated with medroxyprogesterone acetate (400 mg) 60 d before semen collection.
Breeding soundness evaluations were conducted on semen immediately after collection.
Sperm motility strength was measured on a scale of 0 to 5, where 0 indicates no sperm show straight-line movement and 5 indicates most sperm show very rapid and vigorous, straight-line movement and cross the 40× microscope field of view in less than 1 s.
Flow cytometry measures were conducted on frozen-thawed semen.
Morphology measures are based on descriptions in Barth and Oko (1989).
Discussion
The two experiments conducted over different breeding seasons (2017 and 2019) reinforce and expand on previously reported results (Rowell et al., 2019). This provides further evidence that male reindeer treated with MPA, using the same dose (400 mg) and timing of application, do not develop rut-related behaviors or rut-specific physical changes (e.g., neck muscle development, cleaning of antler velvet, and a rut associated odor in the urine). Common to all these studies is small sample size, in most cases 4 bulls per group. However, the basic protocol has now been repeated in three separate trials (including Rowell et al., 2019), in different years with different animals and metrics. The repeatability of the primary results supports the conclusions.
In 2017, blood sampling was limited to a single sample at the time of reindeer collection; however, the high concentration of A4 and T in Aug CTL bulls was expected and is consistent with previous work done at LARS (Bubenik et al., 1997; Barboza et al., 2004). The T and A4 concentrations in Sept CTL bulls were lower than expected and possibly a function of breeding activity two weeks prior to sample collection. In 2019, the T and A4 levels in July came from samples collected before and at the time of MPA administration. As such, they offer an untreated comparison to the baseline values obtained 1 mo later from all treated individuals. Androgen levels fluctuated within and between individuals in September, but in general remained low, especially when compared with the androgen levels in the two untreated bulls at the end of September. From these results, it does appear that, at least initially, MPA is blocking the expression of secondary sexual characteristics through suppression of systemic androgens.
While there was a trend for MPA bulls to have smaller testes, the small sample sizes, and high variability in testes measurements (weight, length, and diameter) make it hard to draw definitive conclusions. Older bulls come into rut earlier and have higher T concentrations during the rut (Stokkan et al., 1980; Bubenik et al., 1997). Leader-Williams (1979) documented seasonal and age-related increases in reindeer testes (and epididymal) weight as well as seminiferous tubular tissue area. Both these parameters increased earlier in the season and reached greater dimensions in older males. The variability in testes morphometrics in the present study reflects not only small sample size but also the 2 yr age difference between bulls in each sampling group.
There is scant information on the annual cycle of spermatogenesis in reindeer and what is available has been summarized in Nagy et al. (2021). In general, reindeer, like many temperate region cervids, undergo dramatic testicular involution during the winter. While minimum testes weight does not vary with age, maximum testes weight (during the breeding season) increases with age. In mature bulls, testis weight increases 4-fold (or greater) with seasonal recrudescence: 10–15 g in winter to ≥ 50 g during the rut (Meschaks and Nordkvist, 1962; Leader-Williams, 1979). Even given the variability in testes weights in this study, both CTL and MPA bulls at both collection points were within the testes weight ranges for rutting bulls reported by these authors.
The transition from maximum testes size at the end of the breeding season to involuted testes is rapid (approximately one month) and marked by the increased appearance of aberrant sperm, pyknotic nuclei, and cellular debris (Meschaks and Nordkvist, 1962; Leader-Williams, 1979). Despite smaller testes in the MPA group, all the measures of spermatogenesis (presence of developing spermatids/mature sperm and sperm density) remained high in August in both CTL and MPA bulls. Nor was there evidence of cellular debris, pyknotic nuclei, or cellular degeneration in either group at either sampling time. In 2017, only sperm density declined in Sept MPA reindeer, consistent with the finding of a lower sperm concentration in semen collected from MPA bulls in the original study (Rowell et al., 2019).
From January to March, reindeer testes are small and held close to the body wall. The tubular epithelium is greatly reduced, consisting primarily of a single cell layer epithelium with infrequent spermatogonia or spermatocytes (Meschaks and Nordkvist, 1962; Leader-Williams, 1979; Nagy et al., 2021). At LARS, simultaneous increases in LH and FSH in April are likely indicators that testis recrudescence has begun (Bubenik et al., 1997). Leader-Williams (1979) documented the first appearance of spermatids 1 mo before rut, with full spermatogenesis by the onset of rut in mature bulls. If cellular regeneration is beginning in April, spermatogenesis must be initiated in late May/early June, assuming 50–60 d for full spermatogenesis by early August. It is fair to assume that when these bulls were treated with MPA at the end of July, spermatogenesis was well underway. At 1 mo post-treatment, there was no apparent histological evidence in MPA bulls that spermatogenesis had been negatively affected, despite the complete suppression of circulating androgens in these reindeer. Although the late July administration of MPA reduced circulating androgens, it is possible that androgen binding hormone activity within the Sertoli cells at that time was sufficient to ensure that T levels within the testes were adequate to allow spermatogenesis to proceed.
The September data are more difficult to interpret. In addition to bull age, the lower androgen values and testes morphometrics may be further confounded by breeding activity 2 wk prior to the sample collection. Among the MPA bulls, there is behavioral evidence that MPA is wearing off in September with aggressive behavior reappearing in early October (Rowell et. al., 2019). Waning MPA may also be contributing to the fluctuating androgen levels seen in September 2019, suggesting a potential “rescue” of spermatogenesis. It is also approaching the end of the rutting season for CTL bulls. In the absence of estrous females, bull aggression normally tapers off in October (unpublished observations).
Regardless, 2 wk post-harem and almost 60 d post-treatment, semen quality determined on fresh semen and by flow cytometry on frozen-thawed semen was similar to previously reported values in both MPA and CTL reindeer (Rowell et al., 2019). Although the small sample size in both studies precludes evaluating individual differences in freezability, the viability of the semen post-thaw is similar between the two studies and similar to data presented in a recent, comprehensive review of assisted reproductive technologies in reindeer (Lindeberg et al., 2021).
In the original study and again in 2017, the only MPA bulls that successfully bred females had breeding experience. Yet in 2019, neither breeding experience nor body weight was a factor in breeding success and neither androgen levels (post-MPA treatment) nor semen characteristics varied with breeding experience. The failure of some MPA bulls to breed appears to be a behavioral, not a physiological, limitation.
Increased fos activity in the hypothalamus, evident in both MPA and CTL bulls in September, is likely reflective of increased sexual cues in the environment, a phenomenon documented in sexually active rams exposed to urine from estrous vs. ovariectomized ewes (Mirto et al., 2017). However, the greater expression of fos activity in the central amygdala of MPA bulls was not expected since low-sexually performing rams have decreased fos activity in this region (Mirto et al., 2017). The central amygdala is responsible for initiating a state of arousal towards non-specific stimuli in the surrounding environment, and notably for a fear response (Campeau et al., 1991). The increased fos activity may be reflective of an increased fear response (King et al., 2005). The VTA contains dopamine neurons that are responsive to sexual stimuli. Sexually active rams exposed to putative sexual cues had greater fos activity in the VTA than low performing rams (Kramer et al., 2017), suggesting a greater biological reward (Bromberg-Martin et al., 2010). Since treatment differences in fos activity in the VTA were not noted, this suggests an equally responsive reward pathway for MPA bulls.
All these studies have used the same MPA dose and timing of administration. We expect that a 400 mg dose of MPA administered in late June/early July would potentially interfere with early development of spermatogenesis and impair subsequent fertility. If MPA treatment is administered earlier in spring (April/May) recrudescence of spermatogenesis might be blocked altogether. From this work, we cannot predict what effect a higher dose, or multiple doses might have on behavior, spermatogenesis, and antler development and/or antler casting. Once spermatogenesis has been initiated, androgens present in the testes appear adequate for the maintenance of spermatogenesis, at least for the first 30 d. The decreased semen quality (lower sperm density and lower sperm concentration) at 60 d may be reflecting the impact of T suppression on earlier cellular events critical to spermatogenesis (e.g., recruitment of spermatogonia, meiosis; Smith and Walker, 2014).
As a short-term tool to help producers manage rut-related aggression in non-breeding reindeer bulls, this dose of MPA and time of application is effective. To avoid problems with persistent velvet antler as well as the probability of bulls dropping their antlers prematurely, it is preferable for the administration of MPA to occur immediately after velvet cleaning is finished.
Collecting semen by electroejaculation under anesthesia is a commonly practiced technique. However, it does result in variable levels of sample contamination and volume. For a more complete discussion of the semen collection technique used here, see Rowell et al. (2019). The possibility that viable semen could be collected from MPA treated bulls, within the first 30 d post-treatment, using restraint and mild sedation rather than general anesthesia should be investigated. This could improve the quality of semen collection while enhancing the safety of both handlers and animals.
Acknowledgments
This research was supported by the Alaska Agricultural and Forestry Experiment Station (UAF) through USDA NIFA funding, specifically, Hatch Regular Research funding AK 17-03, accession 1009864, and Hatch Multistate funding 21-07, accession 1025520, as collaborative research in the Western Region Multistate Project W3112 Reproductive Performance in Domestic Ruminants. Hormonal analyses and testicular morphology in Objective 1 were supported by USDA multistate/hatch grants NEB26-202/W3112, Accession 1011127, NEB-26-252/W4112 (ASC). Undergraduate Creative and Research Experience (UCARE) grant by University of Nebraska-Lincoln for funding for (CS). Research on semen characteristics was supported in part by the USDA Agricultural Research Service. We would like to acknowledge the contributions of Alexandria Otto (undergraduate student, School of Natural Resources UNL) for helping with histology and counting sperm for the manuscript, Scott Kurz UNL Physiology laboratory manager for assistance with training students and androgen assays. We acknowledge the Robert G White Large Animal Research Station (UAF) for logistic support. Special thanks to Sarah Barcalow and the staff at LARS (UAF) for their care and expertise in handling reindeer and the Animal Resources Center (UAF) for assistance with necropsies and tissue collections. Mention of trade names or commercial products in this publication is solely for the purpose of providing specific information and does not imply recommendation or endorsement by the USDA. USDA is an equal opportunity provider and employer.
Glossary
Abbreviations
- A4
androstenedione
- BSE
breeding soundness exam
- CTL
control
- E
breeding experienced bulls
- IE
breeding inexperienced bulls
- LARS
Robert G. White Large Animal Research Station
- MPA
medroxyprogesterone acetate
- MS
mature sperm
- T
testosterone
- VTA
ventral tegmental area
Contributor Information
Janice E Rowell, Institute of Agriculture, Natural Resources and Extension, University of Alaska Fairbanks, Fairbanks, AK 99775, USA.
John E Blake, Animal Resources Center, University of Alaska Fairbanks, Fairbanks, AK 99775, USA.
Kathleen M Roth, School of Veterinary and Biomedical Science, University of Nebraska-Lincoln, Lincoln, NE 68583, USA.
Courtney M Sutton, Department of Animal Science, University of Nebraska-Lincoln, Lincoln, NE 68583, USA.
Colleen C Sachse, Department of Animal Science, University of Nebraska-Lincoln, Lincoln, NE 68583, USA.
Andrea S Cupp, Department of Animal Science, University of Nebraska-Lincoln, Lincoln, NE 68583, USA.
Thomas W Geary, Agricultural Research Service, USDA, Fort Keogh, Miles City, MT 59301, USA.
Abigail L Zezeski, Agricultural Research Service, USDA, Fort Keogh, Miles City, MT 59301, USA.
Brenda M Alexander, Department of Animal Science, University of Wyoming, Laramie, WY 82071, USA.
Robert L Ziegler, Department of Animal Science, University of Wyoming, Laramie, WY 82071, USA.
Milan P Shipka, Institute of Agriculture, Natural Resources and Extension, University of Alaska Fairbanks, Fairbanks, AK 99775, USA.
Conflict of Interest Statement
The authors declare no real or perceived conflicts of interest.
Literature Cited
- Abedal-Majed, M. A., Springman S. A., Sutton C. M., Snider A. P., Bell B. E., Hart M., Kurz S. G., . et al. 2022. VEGFA165 can rescue excess steroid secretion, inflammatory markers, and follicle arrest in the ovarian cortex of high A4 cows. Biol. Reprod. 106:118–131. doi: 10.1093/biolre/ioab201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barboza, P.S., Hartbauer D.W., Hauer W.E., and Blake J.E.. . 2004. Polygynous mating impairs body condition and homeostasis in male reindeer (Rangifer tarandus tarandus). J. Comp. Physiol.[B] 174: 309–17. doi: 10.1007/s00360-004-0416-6. [DOI] [PubMed] [Google Scholar]
- Barth, A.D. and Oko R.. . 1989. Abnormal morphology of bovine spermatozoa. Ames (IA):Iowa State University Press. http://www.worldcat.org/oclc/925232491 [Google Scholar]
- Blake, J.E., Rowell J.E., and Shipka M.P.. . 2007. Reindeer reproductive management. In: Youngquist R.S. and Threlfall WR. editors, Current therapy in large animal theriogenology, 2nd ed., 970–74. St Louis (MI): Saunders, Elsevier. doi: 10.1016/B978-072169323-1.50134-3. [DOI] [Google Scholar]
- Bromberg-Martin, E. S., Matsumoto M., and Hikosaka O.. . 2010. Dopamine in motivational control: Rewarding, aversive, and alerting. Neuron 68:815–834. doi: 10.1016/J.NEURON.2010.11.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bubenik, G.A., Schams D., White R.G., Rowell J.E., Blake J.E., and Bartos L.. . 1997. Seasonal levels of reproductive hormones and their relationship to the antler cycle of male and female reindeer (Rangifer Tarandus). Comp. Biochem. Physiol. [A] 116B (2): 269–77. doi: 10.1016/s0305-0491(97)00183-1. [DOI] [PubMed] [Google Scholar]
- Campeau, S., Hayward M. D., Hope B. T., Rosen J. B., Nestler E. J., and Davis M.. . 1991. Induction of the c-fos proto-oncogene in rat amygdala during unconditioned and conditioned fear. Brain Res. 565:349–352. doi: 10.1016/0006-8993(91)91669-R. [DOI] [PubMed] [Google Scholar]
- Desaulniers, A. T., Cederberg R. A., Mills G. A., Ford J. J., Lents C. A., and White B. R.. . 2015. LH-Independent testosterone secretion is mediated by the interaction between gnrh2 and its receptor within porcine testes 1. Biol. Reprod. 93:45–46. doi: 10.1095/biolreprod.115.128082. [DOI] [PubMed] [Google Scholar]
- Emory, L. E., Cole C. M., and Meyer W. J.. . 1995. Use of depo-provera to control sexual aggression in persons with traumatic brain injury. J. Head Trauma Rehab. 10:47–58. doi: 10.1097/00001199-199506000-00005. [DOI] [Google Scholar]
- Geary, T., Waterman R., Van Emon M., Ratzburg C., Lake S., Eik B., Armstrong D., Zezeski A., and Heldt J.. . 2021. Effect of supplemental trace minerals on standard and novel measures of bull fertility. Theriogenology 172:307–314. doi: 10.1016/j.theriogenology.2021.07.006. [DOI] [PubMed] [Google Scholar]
- Hart, B. 1981. Progestin therapy for aggressive behavior in male dogs. J. Am. Vet. Med. 178:1070–1071. PMID: 7196903 [PubMed] [Google Scholar]
- King, J. A., de Oliveira W. L., and Patel N.. . 2005. Deficits in testosterone facilitate enhanced fear response. Psychoneuroendocrinology 30:333–340. doi: 10.1016/J.PSYNEUEN.2004.09.005. [DOI] [PubMed] [Google Scholar]
- Kramer, A.C., Mirto A.J., Austin K.J., Roselli C.E., and Alexander B.M.. . 2017. Tyrosine hydroxylase in the ventral tegmental area of rams with high or low libido—a role for dopamine. Anim. Reprod. Sci. 187 (December): 152–58. doi: 10.1016/J.ANIREPROSCI.2017.10.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leader-Williams, N. 1979. Age-related changes in the testicular and antler cycles of reindeer, Rangifer Tarandus. J. Reprod. Fertil. 57:117–126. doi: 10.1530/jrf.0.0570117. [DOI] [PubMed] [Google Scholar]
- Lindeberg, H., Nikitkina E., Nagy S. Z., Musidray A., Shiryaev G., Kumpula J., and Holand O.. . 2021. Potential applications of assisted reproductive technologies (ART) in reindeer (Rangifer tarandus). An. Reprod. Sci. 235:1–10. doi: 10.1016/j.anireprosci.2021.106890. [DOI] [PubMed] [Google Scholar]
- Meschaks, P., and Nordkvist M.. . 1962. On the sexual cycle in the reindeer male. Acta Vet. Scand. 3:151–162. doi: 10.1186/BF03547136. [DOI] [Google Scholar]
- Miller A., and Zachary J.. . 2017. Nervous system. In: Zachery J.F., editor. The pathologic basis of veterinary disease. St Louis:Elsevier, 6th ed, p. 805–908. ISBN 978-0-3233-5775-3. [Google Scholar]
- Mirto, A.J., Austin K.J., Uthlaut V.A., Roselli C.E., and Alexander B.M.. . 2017. Fos expression in the olfactory pathway of high- and low-sexually performing rams exposed to urine from estrous or ovariectomized ewes. Applied Anim. Behav. Sci. 186: 22–28. doi: 10.1016/J.APPLANIM.2015.09.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nagy, S.Z., Lindeberg H., Nikitkina E., Krutikova A., Smith E., Kumpula J., and Holand Ø.. . 2021. Reproduction of male reindeer (Rangifer Tarandus). Anim. Reprod. Sci. 227:106722. doi: 10.1016/j.anireprosci.2021.106722. [DOI] [PubMed] [Google Scholar]
- Nieminen, M. 2018. Reindeer and man: from hunting to domestication. In: Tryland M. and Kutz S. editors, Reindeer and caribou: health and disease. Boca Raton (FL):CRC Press. pp. 13–23. doi: 10.7557/2.38.1.4619. [DOI] [Google Scholar]
- Patton M, Jochle W., Penfold L.. . 2005. Contraception in ungulates. In: Asa, C.S. and Porton I.J., editors, Wildlife contraception: issues, methods, and applications. Baltimore (MD):The John Hopkins University Press. ISBN-10:0801883040. [Google Scholar]
- Rowell, J., Geary T., Blake J., Zezeski A. L., and Shipka M.. . 2019. The effects of short-term medroxyprogesterone acetate on rut related behaviors, semen characteristics and fertility in farmed reindeer bulls. Theriogenology 140:201–209. doi: 10.1016/j.theriogenology.2019.08.029. [DOI] [PubMed] [Google Scholar]
- Smith, L.B., and Walker W.H.. . 2014. The regulation of spermatogenesis by androgens. Seminars in Cell & Developmental Biology 30: 2–13. doi: 10.1016/j.semcdb.2014.02.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Squires, E., Badzinsky S., Amann R., McCue P., and Nett T.. . 1997. Effects of altrenogest on total scrotal width, seminal characteristics, concentrations of lh and testosterone and sexual behavior of stallions. Theriogenology 48:313–328. doi: 10.1016/S0093-691X(97)84078-2. [DOI] [PubMed] [Google Scholar]
- Stokkan, K-A., Hove K., and Carr W. R.. . 1980. Plasma concentrations of testosterone and luteinizing hormone in rutting reindeer bulls (Rangifer tarandrus). Can. J. Zool. 58:2081–2083. doi: 10.1139/z80-285 [DOI] [Google Scholar]
- Summers, A. F., Pohlmeier W. E., Sargent K. M., Cole B. D., Vinton R. J., Kurz S. G., McFee R. M., Cushman R. A., Cupp A. S., and Wood J. R.. . 2014. Altered theca and cumulus oocyte complex gene expression, follicular arrest and reduced fertility in cows with dominant follicle follicular fluid androgen excess. PLoS One 9:e110683. doi: 10.1371/journal.pone.0110683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tryland, M., Ravolainen V., and Pedersen Å.Ø.. . 2018. Climate change: potential impacts on pasture resources, health and diseases of reindeer and caribou. In: Tryland M. and Kutz S. J. editors. Reindeer and caribou: health and disease. Boca Raton (FL):CRC Press. p. 493–514. doi: 10.1201/9780429489617. [DOI] [Google Scholar]
- Tyler, N.J.C., Hanssen-Bauer I., Førland E.J., and Nellemann C.. . 2021. The shrinking resource base of pastoralism: Saami reindeer husbandry in a climate of change. Front. Sustain. Food Syst. 4:1–45. doi: 10.3389/fsufs.2020.585685. [DOI] [Google Scholar]
- Zumpe, D., Bonsall R., Kutner M., and Michael R.. . 1991. Medroxyprogesterone acetate, aggression, and sexual behavior in male cynomolgus monkeys (Macaca fascicularis). Horm. Behav. 25:394–409. doi: 10.1016/0018-506x(91)90010-f. [DOI] [PubMed] [Google Scholar]