Abstract
Spatial and temporal changes in sedimentary nucleic acid concentrations in an abyssal locality of the northeastern Atlantic Ocean were investigated in relation to fluxes of nucleic acids produced in the photic layer. Sediment trap material, collected between 1996 and 1998 at depths of 1,000, 3,000, and 4,700 m, and sediment samples were analyzed for DNA and RNA content. Nucleic acid concentrations in the sediments were very high and displayed significant temporal changes, whereas mesoscale variability was low. DNA and RNA concentrations generally displayed opposite temporal patterns, which are likely to be dependent on the nature and characteristics of DNA and RNA molecules. Nucleic acid fluxes were high and displayed clear seasonal changes apparently coupled with seasonal pulses of primary production. However, while median values of DNA fluxes were relatively similar in all sediment traps, median values of RNA fluxes almost doubled from the 1,000- to the 4,700-m depth, suggesting differences in the metabolic activity of microbes associated with sinking particles. Significant relationships between DNA concentrations in the sediments and DNA fluxes and between RNA concentrations and RNA fluxes, indicating the presence of a clear pelagic-benthic coupling of particulate nucleic acids, were observed. The benthic system investigated was not steady state since we estimated that, from September 1996 to October 1998, nucleic acid concentration in the sediments decreased by about 165 mg of DNA m−2. Vertical profiles revealed a significant decrease in DNA concentration with depth in the sediments, reaching an asymptotic value of about 5 μg g−1. This DNA fraction constitutes a pool of potentially refractory DNA (accounting for 16 to 40% of the total DNA pool) that might be buried in the sediments.
Phytodetritus sinking to the sea floor contains large amounts of living (e.g., microbial [28]) and detrital (15, 27) DNA, and the latter fraction accounts for the large majority of DNA pools in marine sediments (6). In all marine environments, nucleic acids are present in low concentrations in comparison to other organic macromolecules, but nonetheless, detrital nucleic acids represent an important N and P trophic source (21). This might apply, to an even larger extent, to the deep-sea environments, where organic N, P, and/or nucleotides may limit secondary production (5, 6).
The available information on sedimentary nucleic acid concentrations demonstrated the presence of large amounts of DNA (about 0.5 g of DNA m−2 cm−1) also in deep-sea environments such as in the highly oligotrophic eastern Mediterranean Sea (5). Such DNA pools (converted to carbon equivalents) were 1.3 times higher than the total benthic biomass (5). Nucleic acid accumulation in the eastern Mediterranean Sea could be a basin scale anomaly due to an uncoupling between input and consumption (4) or a worldwide phenomenon in deep-sea sediments.
In general terms, the quantitative relevance of the nucleic acid concentrations in the sediments is the result of a complex budget, including (i) fluxes from the photic layer, (ii) inputs from resuspension and/or lateral advection, (iii) biomass accumulation and turnover, (iv) production of detrital DNA, (v) rate of DNA uptake by heterotrophs, (vi) burial of the detrital DNA, and (vii) export due to resuspension. On the other hand, information on these processes is still too limited to fully understand pathways, dynamics, and ecological significance of nucleic acids in marine sediments.
In the present study, we investigated DNA and RNA dynamics in abyssal sediments of the northeastern Atlantic Ocean in order to (i) gather information on pelagic-benthic coupling of nucleic acids and (ii) provide quantitative estimates of DNA distribution and accumulation in deep-sea sediments.
(This research was conducted by A. Dell’Anno in partial fulfillment of the requirements for a Ph.D. from the University of Messina, Messina, Italy, 1996 to 1999.)
MATERIALS AND METHODS
Study area and sampling.
Sediment sampling and trap deployments were carried out in the Porcupine Abyssal Plain (PAP; northeastern Atlantic Ocean, at a 4,800-m depth at 48°50.2′N, 16°29.9′W). This area is characterized by high sedimentation rates (18) and a strong seasonality (23).
Undisturbed sediment samples were collected with a multicorer (Maxicorer; inside diameter, 9.0 cm; depth penetration, >20 cm) in September 1996; March, July, and October 1997; and March and October 1998. During each cruise, 4 to 10 cores were taken from four to seven different deployments. Upon recovery, all cores were vertically divided into nine layers of 0 to 5, 5 to 10, 10 to 20, 20 to 30, 30 to 40, 40 to 50, 50 to 60, 60 to 100, and 100 to 150 mm, and frozen at −20°C until analysis. For bacterial analyses, three to five replicate subsamples (0.63 cm3) were collected in September 96 with sterile cutoff syringes from the same cores utilized for the nucleic acid analyses. The subsamples were fixed with filtered (0.2-μm pore size) and sterilized seawater containing 2% buffered formalin and stored at 4°C until later analysis (within 20 days).
Trap material was collected by using three sediment traps (PARFLUX; surface, 0.5 m2) mounted on the same mooring line and placed at 1,000, 3,000 and about 4,700-m depths (the deepest trap was about 100 m above the bottom) and armed with 13 collecting funnels. Sediment traps were deployed during four sampling periods covering September 1996 to September 1998. Each 6-month deployment was programmed for open and closed periods of 7 and 28 days, respectively (generally longer in winter and shorter in spring-summer). Sediment trap samples were fixed in situ with buffered and prefiltered formalin in order to minimize bacterial activity (9). Trap samples were processed by the method of Heussner et al. (10). The sediment trap material was split into eight fractions and stored in the dark until analysis.
Nucleic acid analysis in the sediments.
Before analysis, large macroscopic organisms (macrofauna; i.e., organisms larger than 0.5 mm) were removed from the sediment. Nucleic acid extraction and determination from sediment samples were carried out by the spectrophotometric method described by Dell’Anno et al. (6). Briefly, 1 g of wet sediment (three replicates) was treated with 3.0 ml of 0.5 N perchloric acid, stirred for 3 min, and sonicated three times for 1 min (with intervals of 30 s). Nucleic acids were hydrolyzed at 75°C for 30 min with continuous stirring. After centrifugation (3,000 × g, 10 min), the absorbance of the total nucleic acid content (TNA) in the supernatant was measured at 260 nm. DNA absorbance was determined with a diphenylamine (2% in acetic acid) light-activated reaction (40 W, 12 h) at 598 nm and converted into concentration by using standard solutions of DNA type I from calf thymus. DNA concentration was then expressed as equivalents of absorbance at 260 nm in order to calculate (by difference) the absorbance (ABS) due to RNA: ABSRNA = ABSTNA − ABSDNA. RNA absorbance (260 nm) was then converted into concentration by using standard solutions of RNA type III from baker’s yeast. The interference in TNA determination due to organic and inorganic compounds was tested on sediment subsamples, previously treated at 100°C (2 h) and in a muffle furnace (550°C, 4 h), respectively. Data were normalized to sediment dry weight after desiccation (60°C, constant weight).
Nucleic acid analysis in sediment trap material.
Subsamples (5 to 10 ml) of sediment trap material were filtered, in duplicate, under vacuum (<100 mm Hg) onto GS-type membrane filters (0.22-μm pore size). Particulate nucleic acid extraction was carried out by the procedure described by Bailiff and Karl (1). The filters were extracted in 100% acetone for 1 h at −20°C and centrifuged, and the supernatants were discarded. The pellets were newly resuspended in cold acetone (100%, −20°C) and extracted for 30 min at −20°C until the filters were completely dissolved (usually two or three separate rinses were required). The samples were then washed once with 90% acetone (4°C), once with 10% trichloroacetic acid (TCA (4°C) and twice with 95% ethanol (4°C), and the resulting pellet was resuspended in NH4OH. In order to determine DNA and RNA from the same subsample, we combined spectrofluorometric DNA determination (using diaminobenzoic acid [1]) and spectrophotometric analyses for TNA (by absorbance at 260 nm [7]). Particulate DNA concentrations in trap samples were calculated by using a calibration curve of calf thymus DNA and then expressed as equivalents of absorbance at 260 nm in order to calculate (by difference) the absorbance due to RNA as explained above.
Bacterial analyses.
For bacterial analyses, subsamples were sonicated three times (Sonifier Branson 2200; 60 W for 1 min) (3), diluted 100 times, stained with acridine orange (0.01%, final concentration), and filtered on black Nuclepore 0.2-μm-pore-size filters. The filters were analyzed under epifluorescence microscopy (3). Bacterial DNA and bacterial RNA contribution to the total DNA and RNA pool were calculated by assuming a conversion factor of 3.3 fg of DNA cell−1 (26) and 4.2 fg of RNA cell−1 (8). These conversion factors were selected as estimated for cells of the same average size encountered in this study. Data were normalized to sediment dry weight after desiccation (60°C, constant weight).
RESULTS
Nucleic acid concentrations in the sediments.
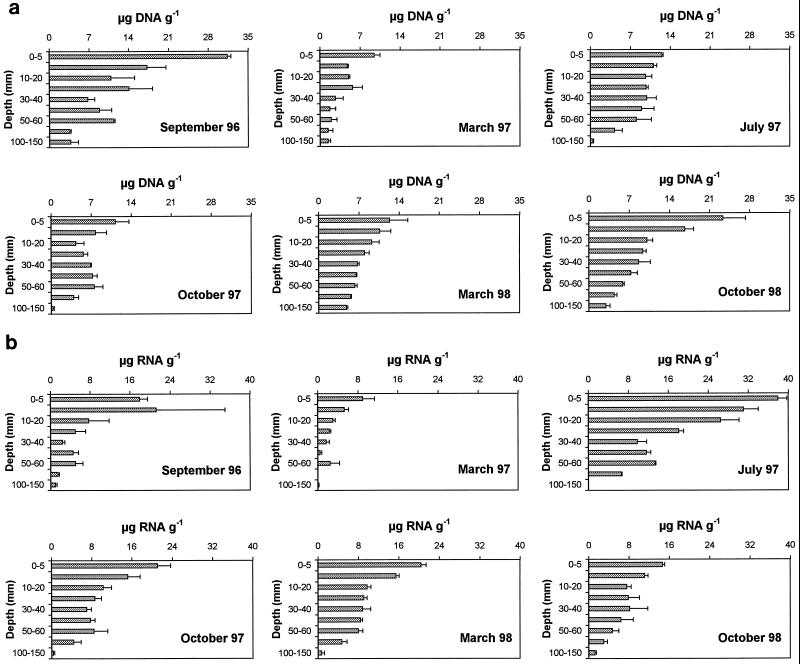
Temporal changes in DNA and RNA concentrations in the top 5 mm of the sediment and values integrated in the whole sediment core (0 to 150 mm) are reported in Fig. 1. In the top 5 mm of the sediment, DNA concentrations were characterized by significant temporal changes (analysis of variance [ANOVA], F = 9.84, P < 0.001), with the highest values in September 1996 (31.3 ± 0.6 μg g−1 [mean ± standard error]) and the lowest in March 1997 (9.5 ± 1.0 μg g−1). Similar temporal patterns were observed for DNA concentrations integrated down to a 150-mm depth, which ranged from 2.5 ± 0.9 to 7.3 ± 2.7 μg g−1 in March 1997 and September 1996, respectively. By contrast, RNA concentrations generally showed an opposite temporal pattern, with the highest values in July 1997 (37.9 ± 1.7 and 9.4 ± 3.9 μg g−1 in the 0- to 5-mm depth and in the integrated 0- to 150-mm depth, respectively) and the lowest in March 1997 (9.0 ± 2.3 and 1.2 ± 0.9 μg g−1 in the 0- to 5-mm depth and in the integrated 0- to 150-mm depth, respectively). DNA and RNA spatial variability on mesoscale (here defined as coefficient of variation calculated from data obtained from sediment cores of different deployments [CV]) was low (on average, about 10% for both parameters) (Table 1).
FIG. 1.
Nucleic acid concentrations in the top 0 to 5 mm of sediment and in the whole sediment core (integrated 0 to 150 mm) in the PAP. (a) Temporal changes of DNA concentrations; (b) temporal changes of RNA concentrations. Standard errors are shown by error bars. Data are expressed as micrograms per gram of sediment dry weight.
TABLE 1.
Mesoscale variability of nucleic acid concentrationsa
| Sampling period | Depth (mm) | DNA or RNA concn (μg g−1) of:
|
DNA or RNA concn (μg g−1) of both deployments
|
CV | ||||
|---|---|---|---|---|---|---|---|---|
| Deployment 1
|
Deployment 2
|
|||||||
| Mean | SD | Mean | SD | Mean | SE | |||
| DNA concn | ||||||||
| September 1996 | 0–5 | 30.5 | 15.9 | 32.2 | 9.4 | 31.3 | 0.6 | 1.9 |
| March 1997 | 0–5 | 10.9 | 4.6 | 8.1 | 2.8 | 9.5 | 1.0 | 10.3 |
| July 1997 | 0–5 | 12.8 | 3.7 | 12.2 | 0.7 | 12.5 | 0.2 | 1.6 |
| October 1997 | 0–5 | 8.0 | 2.0 | 14.5 | 3.5 | 11.3 | 2.3 | 20.8 |
| March 1998 | 0–5 | 16.8 | 6.9 | 7.9 | 1.5 | 12.4 | 3.2 | 25.6 |
| October 1998 | 0–5 | 17.9 | 8.6 | 28.8 | 0.8 | 23.4 | 3.9 | 16.8 |
| RNA concn | ||||||||
| September 1996 | 0–5 | 20.1 | 1.5 | 15.8 | 4.8 | 17.9 | 1.6 | 8.7 |
| March 1997 | 0–5 | 5.8 | 1.6 | 12.3 | 3.4 | 9.0 | 2.3 | 25.8 |
| July 1997 | 0–5 | 40.4 | 11.3 | 35.5 | 11.5 | 37.9 | 1.7 | 4.6 |
| October 1997 | 0–5 | 24.7 | 21.0 | 17.5 | 0.2 | 21.1 | 2.6 | 12.1 |
| March 1998 | 0–5 | 21.8 | 2.1 | 19.1 | 9.0 | 20.4 | 1.0 | 4.8 |
| October 1998 | 0–5 | 15.2 | 7.2 | 14.1 | 10.3 | 14.7 | 0.4 | 2.9 |
Reported are DNA and RNA concentrations from two different deployments, the means of DNA and RNA concentrations (as the average concentrations of nucleic acids from the two different deployments), standard deviations, standard errors, and the coefficient of variation of DNA and RNA concentrations. All data are relative to all sampling periods and to the top 0 to 5 mm of sediment.
Vertical distributions of DNA and RNA concentrations in the sediment core are illustrated in Fig. 2. DNA concentrations were characterized by a significant decrease with increasing depth in the sediment (ANOVA, F = 11.8, P < 0.001), with the highest values in the top 0 to 5 mm of sediment (on average for the entire data set, 16.7 ± 3.2 μg g−1) and the lowest values in the deepest sediment layers (100- to 150-mm section; on average, 2.3 ± 0.7 μg g−1). Significant differences in RNA concentrations were observed among sediment layers (range, 0.6 ± 0.2 to 20.2 ± 3.6 μg g−1 in the 100- to 150- and 0- to 5-mm depths of the sediment, respectively; ANOVA, F = 12.1, P < 0.001) (Fig. 2b). A detailed analysis of the vertical patterns of DNA during different sampling periods revealed different shapes. Two example cases that summarize extreme conditions are reported in Fig 3.
FIG. 2.
Vertical distribution of nucleic acids in the sediments. (a) DNA distribution; (b) RNA distribution. Standard errors are shown by error bars. Data are relative to all sampling periods and to all depths in the sediment.
FIG. 3.
DNA vertical distribution in two different sampling periods (September 1996 and March 1998).
Nucleic acid fluxes.
DNA and RNA concentrations determined on trap subsamples (5 to 10 ml) were converted to nucleic acid fluxes as follows. (i) DNA and RNA concentrations from subsamples relative to the nucleic acid concentrations present in the total volume of the trap cups were calculated. (ii) Total DNA and RNA concentrations were then multiplied by 2 to be reported to the surface area of 1 m2 (since the surface area of the collecting trap was 0.5 m2). (iii) This value was divided by the number of days occurring at each deployment (i.e., the interval between open and closed trap for each sampling period). DNA and RNA fluxes were then expressed as micrograms per square meter per day.
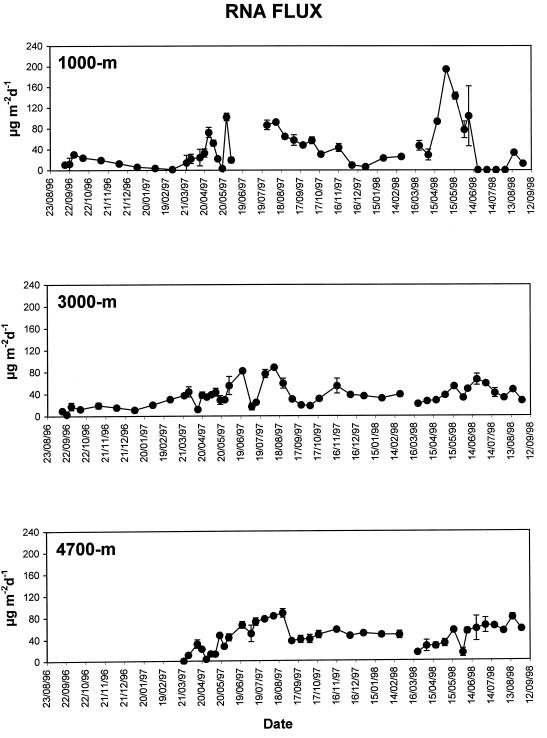
DNA flux in the PAP area was characterized by evident seasonal changes (Fig. 4). In the trap at a depth of 1,000 m, both the highest (172 μg m−2 day−1 in April 1997) and the lowest (1.2 μg m−2 day−1 in December 1997) DNA fluxes were observed. On average for the entire sampling period, DNA fluxes were rather similar at all trap depths (ANOVA, F = 3.05, P = 0.27 [not significant]), ranging from 30.4 ± 3.2 to 36.8 ± 5.6 μg m−2 day−1 (at 4,700- and 1,000-m depths, respectively). Median values of DNA fluxes for the entire sampling period were 24.3, 25.3, and 27.3 μg m−2 day−1, respectively, at 1,000-, 3,000-, and 4,700-m depths. RNA fluxes showed wide seasonal changes, with the highest values in May 1998 (194.9 μg m−2 day−1) and the lowest in July 1998 (<0.1 μg m−2 day−1, at 1,000-m depth) (Fig. 5). On average for the entire sampling period, RNA fluxes ranged from 35.7 ± 2.7 to 45.1 ± 3.5 μg m−2 day−1 (at 3,000- and 4,700-m depths, respectively) and did not change significantly between traps (ANOVA, F = 3.05, P = 0.65 [not significant]). Median values of RNA fluxes for the entire sampling period were 25.0, 33.0, and 47.3 μg m−2 day−1, respectively, at 1,000-, 3,000-, and 4,700-m depths.
FIG. 4.
Seasonal and vertical changes in DNA fluxes at 1,000-, 3,000-, and 4,700-m depths. Standard deviations are reported. d, day.
FIG. 5.
Seasonal and vertical changes in RNA fluxes at 1,000-, 3,000-, and 4,700-m depths. Standard deviations are reported. d, day.
The two deepest traps displayed rather similar temporal patterns for both DNA and RNA fluxes. Since there are no significant quantitative differences between nucleic acids fluxes at the 3,000- and 4,700-m depths, in this study we compared nucleic acid concentrations in the sediments with fluxes of nucleic acid from trap at the 3,000-m depth as this trap displayed the more complete data set.
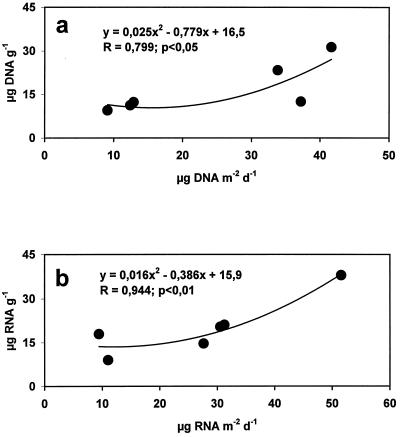
In order to identify a pelagic-benthic coupling in nucleic acid concentrations, sediment trap fluxes, integrated to 1 month before sediment sampling, were compared to sedimentary DNA and RNA concentrations. Significant relationships between DNA concentrations in the sediments and DNA fluxes (Fig. 6a) and between RNA concentrations and RNA fluxes (Fig. 6b) were observed.
FIG. 6.
Relationships between nucleic acid fluxes at 3,000-m depth and nucleic acids in the sediments in the six sampling periods. Relationships between DNA concentrations and DNA fluxes (a) and relationships between RNA concentrations and RNA fluxes (b) are shown. d, day.
Benthic bacteria.
Bacterial density in the sediments of the PAP displayed highest values in the top 0 to 5 mm of sediment [(3.2 ± 0.8) × 108 cells g−1] and lowest values in the 100- to 150-mm section [(0.6 ± 0.1) × 108 cells g−1] (Table 2). Bacteria decreased significantly, up to fivefold, from the upper 5-mm section to the 100- to 150-mm section. Bacterial DNA from intact cells (as determined by epifluorescence microscopy) accounted on average for 4% of the total sedimentary DNA pools, showing limited variations along the sediment core (range, 1.8 to 6.8% in the 50- to 60- and the 60- to 100-mm sections, respectively) (Table 2). By contrast, bacterial RNA accounted on average for about 12% of the total RNA pool, and its importance increased irregularly with increasing depth in the sediment core (Table 2).
TABLE 2.
Vertical distribution of bacterial density, bacterial DNA and RNA concentrations, and their relative contributions to the nucleic acid pools in sediments collected in September 1996
| Depth (mm) | Bacterial density (cells [108 g−1]) (SE) | Concn (μg g−1)a
|
Relative contribution (%)b
|
||
|---|---|---|---|---|---|
| B-DNA | B-RNA | B-DNA/DNA | B-RNA/RNA | ||
| 0–5 | 3.2 (0.8) | 1.1 | 1.4 | 3.4 | 7.6 |
| 5–10 | 3.1 (0.2) | 1.0 | 1.3 | 5.9 | 6.1 |
| 10–20 | 1.7 (0.3) | 0.5 | 0.7 | 5.0 | 9.0 |
| 20–30 | 1.1 (0.0) | 0.4 | 0.5 | 2.7 | 9.5 |
| 30–40 | 0.9 (0.2) | 0.3 | 0.4 | 4.4 | 15.6 |
| 40–50 | 0.8 (0.1) | 0.3 | 0.3 | 2.8 | 7.0 |
| 50–60 | 0.6 (0.1) | 0.2 | 0.3 | 1.8 | 5.2 |
| 60–100 | 0.7 (0.0) | 0.2 | 0.3 | 6.8 | 20.9 |
| 100–150 | 0.6 (0.1) | 0.2 | 0.2 | 5.2 | 24.5 |
B-DNA, bacterial DNA; B-RNA, bacterial RNA.
Relative contributions of bacterial DNA to the DNA pool (B-DNA/DNA) and of bacterial RNA to the RNA pool (B-RNA/RNA) are given.
DISCUSSION
Nucleic acid concentrations in the PAP sediments.
Previous studies have shown that nucleic acid concentrations in the sediments might vary according to changes in environmental conditions and that higher sedimentary nucleic acid values are generally observed in highly productive systems (5). Sedimentary DNA concentrations reported in this study were high (ranging from 9.5 to 31.3 μg g−1 in the top 0 to 5 mm of sediment) when compared to literature data (summarized previously [5]), indicating that this abyssal system shares trophic conditions (i.e., organic matter accumulation) typical of continental shelf environments. Also, RNA concentrations in the PAP area were extremely high and comparable to those found in highly productive systems (such as upwelling area and locations characterized by the presence of a Posidonia bed [3]) and about four times higher than those reported in highly oligotrophic bathyal sediments of the Cretan Sea (5).
DNA and RNA concentrations in PAP sediments showed significant temporal changes, whereas spatial variability of both DNA and RNA concentrations on mesoscale was low. Therefore, it is likely that the observed temporal patterns cannot be explained with spatial heterogeneity of DNA and RNA pools.
DNA and RNA concentrations generally displayed opposite temporal patterns that are likely to be dependent on the nature and characteristics of the DNA and RNA molecules. In fact, DNA is subjected to lower turnover (13) and longer degradation rates than RNA (20). In this regard, RNA concentrations in the PAP area showed similar temporal patterns reported for bacterial secondary production (measured as [3H]leucine uptake rates), determined synoptically in the same sediments (24). These results suggest that RNA concentrations reflect changes in bacterial metabolic activity, which in turn is induced by pulses of organic matter sedimentation (29).
Previous studies carried out on deep-sea sediments of the eastern Mediterranean Sea clearly demonstrated that DNA pools were largely unaccounted for by DNA associated with living biomass (detrital DNA represented about 90% of the total DNA pool [5]). Accordingly, in PAP sediments, bacterial DNA accounted for a quasinegligible fraction of the total DNA pool (on average, 4%). Since bacteria in the PAP sediments display limited temporal changes (22), the concentrations and temporal changes of DNA pools in the investigated abyssal sediments are apparently independent of bacterial dynamics. As far as RNA concentrations are concerned, reports of previous studies of the oligotrophic eastern Mediterranean Sea indicated that bacteria alone accounted for about 26% of the total RNA pool (5), whereas in the PAP sediments, bacterial RNA, with the same conversion factor, accounted for about 12%. This is not surprising since (i) bacteria contribute to a larger fraction of the total benthic biomass in oligotrophic than in eutrophic systems (3, 4) and (ii) detrital nucleic acids might represent a more important organic source to bacteria in food-limited than in food-rich environments (5). Data reported here are in agreement with these hypotheses, indicating that the microbial loop (reported here in terms of bacterial capability to recycle detrital organic compounds otherwise lost to the benthic food webs) might play a much more important role in nucleic acid dynamics in oligotrophic deep-sea sediments than in more productive systems, such as the PAP area.
Nucleic acid fluxes and pelagic-benthic coupling in nucleic acid concentrations.
Previous studies have shown that large amounts of DNA and RNA may be supplied to the benthos from particle sedimentation (1, 11, 12, 28). Temporal changes in DNA concentrations in deep-sea sediments have been hypothesized to be related to DNA inputs from the photic layer (5), but a direct pelagic-benthic coupling between nucleic acid fluxes and nucleic acid concentrations in the sediments has never been demonstrated. In the PAP area, nucleic acid fluxes were high and displayed clear seasonal fluctuations apparently coupled with seasonal pulses of primary production (23). However, while median values of DNA fluxes were relatively constant in all sediment traps, median values of RNA fluxes almost doubled from 1,000- to 4,700-m depths, suggesting an increased microbial metabolic activity associated with particles during sinking. These results might suggest that RNA fluxes are subjected, more than DNA, to changes related to microbial growth and colonization of the settling particles.
The relationships between nucleic acid concentrations in the sediments and nucleic acid vertical fluxes indicate the presence of a clear pelagic-benthic coupling. Such relationships are not spurious, as DNA and RNA concentrations followed opposite temporal patterns and provide, to our knowledge, the first direct evidence on the relationships between changes of sedimentary nucleic acid concentrations and nucleic acid inputs from the photic layer.
Assuming that measured DNA fluxes represent the input of DNA to the benthos, we tentatively estimated the amount of DNA utilized by benthic heterotrophs. In about 2 years (from September 1996 to September 1998), 24 mg of DNA m−2 reached the sediments through particulate fluxes. In the absence of heterotrophic uptake, this input of DNA would accumulate in the sediments, but, conversely, DNA concentrations in the 0- to 150-mm sediment layer decreased (from September 1996 to October 1998) to about 141 mg of DNA m−2. Given the low contribution of living biomass to the total sedimentary DNA pool, DNA decrease could be dependent upon a progressive utilization of detrital DNA by benthic heterotrophs. Since total organic carbon flux was low when compared to measurements in situ of CO2 and dissolved organic carbon release by benthic activities (14), the utilization of labile compounds (such as detrital DNA) would represent an important trophic source to sustain benthic metabolic requirements. The total amount of DNA potentially removed from the system during the study period was equivalent to 165 mg of DNA m−2. This result suggests that detrital DNA is utilized by deep-sea benthic organisms, but the pathway of utilization (as nucleotides or N and P source) and turnover rates of sedimentary DNA pools are as yet unknown.
Vertical distribution of nucleic acid concentrations in the PAP sediments.
Vertical profiles of DNA and RNA concentrations revealed a significant decrease with depth in the sediments. This is obvious and expected since most benthic parameters follow similar patterns and there are no reasons for enhanced nucleic acid synthesis in deeper sediment layers. Since living biomass accounted for an almost negligible fraction of the total sedimentary DNA pool, vertical distribution of DNA is likely to be dependent on three main factors: (i) quantity of the DNA reaching the seafloor, (ii) sediment mixing, and (iii) DNA utilization rates along the sediment core. In all sampling periods, DNA concentrations in deeper sediment layers seem to reach an asymptotic value of about 5 μg g−1. Mayer (17), describing vertical distribution of organic carbon in marine sediments, assumed this asymptotic value to represent the refractory fraction throughout the sediment core. Since extracellular DNA bound to mineral particles and to highly refractory organic compounds (i.e., humic acids) is resistant to DNase degradation (2, 16, 25), such a DNA fraction recalcitrant to enzymatic degradation (i.e., refractory DNA) might persist in the sediments (19).
Refractory DNA in the top sediment layer would account, depending upon the different seasons and ecological conditions, for 16 to 40% of the total DNA pool (in September 1996 and March 1998, respectively). Available data are too limited to draw general conclusions, but the different contributions of the refractory DNA fraction in the two periods are likely to be related to the increasing time lag between DNA input in the sediments and sediment collection and/or different utilization rates of detrital DNA in different periods. Further studies are needed to fully understand the bioavailability and pathways of detrital DNA utilization in marine sediments.
ACKNOWLEDGMENTS
This research has been undertaken in the framework of the BENGAL programme. We acknowledge the support from 60% of University of Bari and the European Commission’s Marine Science and Technology Program (MAST III) under contract MAS3-CT-950018.
Two anonymous referees contributed to improve the quality of the manuscript.
REFERENCES
- 1.Bailiff D M, Karl D M. Dissolved and particulate DNA dynamics during a spring bloom in the Antarctic Peninsula region, 1986–87. Deep-Sea Res. 1991;38:1077–1095. [Google Scholar]
- 2.Blum S A E, Lorenz M G, Wackernagel W. Mechanisms of retarded DNA degradation and prokaryotic origin of DNases in non-sterile soil. Syst Appl Microbiol. 1997;20:513–521. [Google Scholar]
- 3.Danovaro R, Della Croce N, Fabiano M. Labile organic matter and microbial biomasses in deep-sea sediments (E-Mediterranean) Deep-Sea Res. 1993;40:953–965. [Google Scholar]
- 4.Danovaro R, Dell’Anno A, Della Croce N. Lack or evidence for a pelagic-benthic coupling in the Eastern Mediterranean Sea? Mediterr Target Project Newsl. 1997;5:14–15. [Google Scholar]
- 5.Danovaro R, Dell’Anno A, Pusceddu A, Fabiano M. Nucleic acid concentrations (DNA, RNA) in the continental and deep-sea sediments of the eastern Mediterranean: relationships with seasonally varying organic inputs and bacterial dynamics. Deep-Sea Res. 1999;46:1077–1094. [Google Scholar]
- 6.Dell’Anno A, Fabiano M, Duineveld G C A, Kok A, Danovaro R. Nucleic acid (DNA, RNA) quantification and RNA/DNA ratio determination in marine sediments: comparison of spectrophotometric, fluorometric and high-performance liquid chromatography methods and estimation of detrital DNA. Appl Environ Microbiol. 1998;64:3238–3245. doi: 10.1128/aem.64.9.3238-3245.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Fabiano M, Povero P, Danovaro R. Particulate organic matter composition in Terra Nova Bay (Ross Sea, Antarctica) during summer 1990. Antarct Science. 1996;8:7–13. [Google Scholar]
- 8.Fuhrman J A, Azam F. Thymidine incorporation as a measure of heterotrophic bacterioplankton production in marine surface waters: evaluation and field results. Mar Biol. 1982;66:109–120. [Google Scholar]
- 9.Gardner W D, Hinga K R, Marra J. Observation on the degradation of biogenic material in the deep ocean with implication on accuracy of sediment trap fluxes. J Mar Res. 1983;41:195–214. [Google Scholar]
- 10.Heussner S, Ratti C, Carbenne J. The PPS 3 time-series sediment trap and the sample processing technique used during the ECOMARGE experiment. Continental Shelf Res. 1990;10:943–958. [Google Scholar]
- 11.Karl D M, Knauer G A. Vertical distribution, transport, and exchange of carbon in the northeast Pacific Ocean: evidence for multiple zones of biological activity. Deep-Sea Res. 1984;31:221–243. [Google Scholar]
- 12.Karl D M, Knauer G A. Detritus-microbe interactions in the marine pelagic environment: selected results from the vertex experiment. Bull Mar Sci. 1984;35:550–565. [Google Scholar]
- 13.Karl D M, Novitsky J A. Dynamics of microbial growth in surface layers of a coastal marine sediment ecosystem. Mar Ecol Prog Ser. 1988;50:169–176. [Google Scholar]
- 14.Lampitt R S. BENGAL Workshop Gif Sur Yvette (France) 26–28 October. 1998. Personal communication. [Google Scholar]
- 15.Lochte K, Turley C M. Bacteria and cyanobacteria associated with phytodetrituts in the deep-sea. Nature. 1988;333:67–69. [Google Scholar]
- 16.Lorenz M G, Wackernagel W. Adsorption of DNA to sand and variable degradation rates of adsorbed DNA. Appl Environ Microbiol. 1987;53:2948–2952. doi: 10.1128/aem.53.12.2948-2952.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Mayer L M. The nature and determination of living sedimentary organic matter as food source for deposit-feeders. In: Lopez G, Tagon G, Levinton J, editors. Ecology of marine deposit-feeders, lecture notes on coastal and estuarine studies. New York, N.Y: Springer-Verlag; 1989. pp. 98–113. [Google Scholar]
- 18.Newton P R, Lampitt R S, Jickells T D, King P, Boutle C. Temporal and spatial variability of biogenic particle fluxes during the JGOFS NE Atlantic Process studies at 47°N 20°W. Deep-Sea Res. 1994;41:1617–1642. [Google Scholar]
- 19.Nielsen K M, Bones A M, Smalla K, van Elsas J D. Horizontal gene transfer from transgenic plants to terrestrial bacteria—a rare event. FEMS Microbiol Rev. 1998;22:79–103. doi: 10.1111/j.1574-6976.1998.tb00362.x. [DOI] [PubMed] [Google Scholar]
- 20.Novitsky J A. Degradation of dead microbial biomass in a marine sediment. Appl Environ Microbiol. 1986;52:504–509. doi: 10.1128/aem.52.3.504-509.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Paul J H, Jeffrey W H, DeFlaun M F. Dynamics of extracellular DNA in the marine environment. Appl Environ Microbiol. 1987;53:170–179. doi: 10.1128/aem.53.1.170-179.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Patching J W. BENGAL Workshop Gif Sur Yvette (France) 26–28 October. 1998. Personal communication. [Google Scholar]
- 23.Rice A L, Thurston M H, Bett B J. The IOSDL DEEPSEAS programme: introduction and photographic evidence for the presence and absence of a seasonal input of phytodetritus at contrasting abyssal sites in the northeastern Atlantic. Deep-Sea Res. 1994;41:1305–1320. [Google Scholar]
- 24.Rice A L. Annual Report BENGAL. Southampton, England: Southampton Oceanography Centre; 1998. High resolution temporal and spatial study of the benthic biology and geochemistry of a north-eastern atlantic abyssal locality; pp. 1–130. [Google Scholar]
- 25.Romanowski G, Lorenz M G, Wackernagel W. Adsorption of plasmid DNA to mineral surfaces and protection against DNase I. Appl Environ Microbiol. 1991;57:1057–1061. doi: 10.1128/aem.57.4.1057-1061.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Simon M, Azam F. Protein content and protein synthesis rates of planktonic bacteria. Mar Ecol Prog Ser. 1989;51:201–213. [Google Scholar]
- 27.Turley C M, Lochte K. Microbial response to the input of fresh detritus to the deep-sea bed. Paleogeog Paleoclim Paleoecol (Global and Planetary Change Section) 1990;89:3–23. [Google Scholar]
- 28.Turley C M, Mackie P J. Bacterial and cyanobacterial flux to the deep NE Atlantic on sedimenting particles. Deep-Sea Res. 1995;42:1453–1474. [Google Scholar]
- 29.Van Duyl F C, Kop A J. Bacterial production in North Sea sediments: clues to seasonal and spatial variations. Mar Biol. 1994;120:323–337. [Google Scholar]