Abstract
Background
To observe different roles of direct bilirubin (Dbil) on portopulmonary hypertension (POPH) and idiopathic pulmonary arterial hypertension (IPAH).
Methods
Thirty incident patients with POPH and 180 with IPAH (matched by the WHO functional classification in a 1 : 6 ratio) between March 2010 and December 2020 were included. The receiver operating curve and Kaplan–Meier method were applied to estimate the ability to distinguish between the two and survival, respectively. Univariate and forward multiple stepwise regression analyses were performed to access the relationship between pulmonary vascular resistance (PVR) and clinical indices.
Results
Compared to IPAH, the POPH group had better hemodynamics including PVR (7.08 ± 3.95 vs. 14.89 ± 7.11, P < 0.001) and higher total bilirubin (Tbil) and Dbil. Tbil and Dbil had a negative correlation with PVR in the POPH group (r = −0.394, P=0.031; r = −0.364, P=0.048, respectively) but positive correlation in the IPAH group (r = 0.218, P=0.003; r = 0.178, P=0.018, respectively). Increased neutrophil counts (r = 0.394, P=0.031) and elevated NT-proBNP (r = 0.433, P < 0.001) would help predict the elevation of PVR in POPH and IPAH groups independent of Dbil, respectively. Dbil could distinguish POPH from IPAH (AUC = 0.799, P=0.009), and the ability was elevated when taking aspartate aminotransferase together (AUC = 0.835, P < 0.001). The overall survival was better in POPH than in IPAH (7 dead cases of POPH and 96 of IPAH, P=0.002). Survival was better in POPH than in IPAH in the group of Dbil ≥7 μmol/L (P=0.001) but showed no significant difference between POPH and IPAH in the group of Dbil <7 μmol/L (P=0.192).
Conclusions
The POPH group had a better hemodynamic profile than IPAH. Dbil was associated oppositely with the elevation of PVR in POPH and IPAH. Patients with POPH had better survival than those with IPAH in the total cohort and in the group of Dbil ≥7 μmol/L, but limited dead cases of POPH should be noted.
1. Introduction
Portopulmonary hypertension (POPH) is a life-threatening disease with damage to both pulmonary circulation and portal circulation with or without liver diseases, defined as Group 1 pulmonary hypertension (PH) and a severe complication of portal hypertension [1–5]. According to epidemiology, POPH could explain 5–15% of pulmonary arterial hypertension (PAH) causing a specific associated PAH form [6, 7]. Hemodynamic studies showed that 2–6% of patients with portal hypertension had significantly obvious pulmonary hypertension [8]. To date, pieces of literature regarding POPH stay a few, but POPH has still not been well recognized. In spite of many puzzles of POPH, POPH was generally thought to share some similar pathobiological mechanisms to other forms of PAH [2, 9].
Bilirubin, including total bilirubin and direct bilirubin, is one indicator of liver function abnormality and had an association with PAH. During the 1990s, higher expression levels of total bilirubin were found to be a risk factor for early postoperative mortality in 31 patients with primary PH and 31 patients with Eisenmenger syndrome who all underwent heart-lung transplantation [10]. Meanwhile, Takeda et al. [11] and colleagues conducted the research regarding bilirubin and mortality in 18 patients with idiopathic PAH (IPAH) and 19 with connective tissue disease-associated PAH (CTD-PAH) and found hyperbilirubinemia and total bilirubin concentration to be risk predictors of death independently of WHO functional classification and brain natriuretic peptide (BNP), respectively. Our previous study investigated 404 patients with IPAH at enrollment. The results suggested that the expression level of direct serum bilirubin was much higher in nonsurvivors than in survivors, and the baseline expression level of direct serum bilirubin could predict severity and outcomes of IPAH [12].
POPH, as a disease related to liver abnormality, probably exists in abnormal bilirubin. However, the comparisons of bilirubin between POPH and IPAH stay unclear. Thus, our objective was to make comparisons between IPAH and POPH.
2. Materials and Methods
2.1. Ethics and Population
This study was approved by the Ethics Committee of the Shanghai Pulmonary Hospital with the approval number k16-293 and according to the Declaration of Helsinki. Written consent was obtained from each patient.
Patients with portal hypertension were diagnosed at other centers and would come to our center for PH confirmation. The acute reports regarding hepatic venous portal pressure gradient (HVPG) values were not obtained. All patients were firstly screened by echocardiography (systolic pulmonary artery pressure ≥40 mmHg) and then underwent right heart catheterization (RHC) to confirm PAH at our center. There were 34 cases with POPH confirmed at our center from March 2010 to December 2020. Of those, 2 cases with Budd-Chiari syndrome and 2 cases with schistosomiasis were mechanically different from the other 30 cases (pure sinusoidal portal hypertension) and were excluded from the study. To include a total of 30 patients with POPH in this retrospective study, patients with IPAH were matched by WHO functional classification (WHO FC) in a 1 : 6 ratio to generate a typical landscape of IPAH and increase the test power. The flow diagram is shown in Figure 1. The diagnosis of IPAH and POPH was made in accordance with the standard guideline as to the following. The criteria for IPAH included (i) mean pulmonary arterial pressure (mPAP) ≥25 mmHg, mean pulmonary arterial wedge pressure (mPAWP) ≤15 mmHg, and pulmonary vascular resistance (PVR) ≥3 wood units measured at rest through RHC; (ii) exclusion of pulmonary hypertension due to left heart disease or lung diseases or chronic thromboembolism pulmonary hypertension; and (iii) exclusion of other forms of PAH via special tests containing hematology, biochemistry, immunology, serology, ultrasound, etc. The diagnosis of POPH was according to (i) mPAP ≥ 25 mmHg, mPAWP < 15 mmHg, and PVR ≥ 3 wood units measured at rest through RHC and (ii) portal hypertension (ascites, splenomegaly, and varicose veins).
Figure 1.
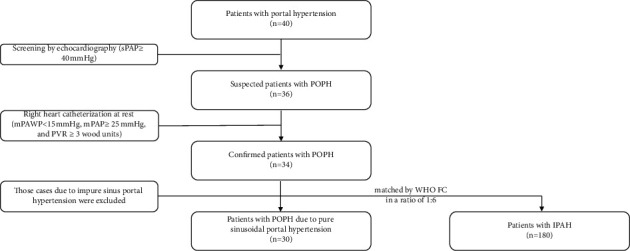
The flowchart of POPH confirmation, the inclusion and the exclusion. POPH, portopulmonary hypertension; IPAH, idiopathic pulmonary arterial hypertension. sPAP, systolic pulmonary arterial pressure; mPAP, mean pulmonary arterial pressure; mPAWP, mean pulmonary artery wedge pressure; PVR, pulmonary vascular resistance; WHO FC, World Health Organization functional classification.
2.2. Demographics, Hemodynamics, Clinical Variables, and Outcome Collection
Demographics, World Health Organization functional classification (WHO FC), target therapy, liver function, etiological originals of portal hypertension, and other related data were collected. The MELD (model of end-stage liver disease) equation was applied to calculate the score for severity as 9.57 × ln (creatinine, mg/dL) + 3.78 × ln (total bilirubin, mg/dL) + 11.20 × ln (international normalized ratio) + 6.43. The minimal values were forced to 1.0 for calculation purposes [13]. Baseline hemodynamics were measured via RHC at rest in all patients. The patients were followed up until 23 December 2020 through telephone interviews and outpatient clinic visits. All-cause mortality was the observational endpoint. During the period of follow-up, no one was lost in this study.
2.3. Data Analysis and Statistics
Continuous variables with normal distribution were expressed as mean ± SD, and the independent Student's t-test was used for the comparisons. Continuous variables with nonnormal distribution were expressed as median (first and third interquartile), and the Mann-Whitney U test was applied for the comparisons. Categorical variables were expressed as the number of patients and relative frequencies (n, %), and the Chi-square test was used for the comparisons. The receiver operating characteristic (ROC) curve was employed to assess the ability of distinguishing POPH from IPAH. The predictive ability in the models should be compared with integrated discrimination improvement (IDI) [14] and net reclassification improvement (NRI) [15]. Correlations were generated by Pearson or Spearman correlation analyses. Univariate and forward multiple stepwise regression analysis was performed to assess the relationship of PVR and clinical indices, which were adjusted by gases of blood, metabolic comorbidities, Child-Pugh score, and concomitant medications.
Overall survival time was defined from the date of diagnostic RHC to death. Surviving patients were censored on the date of the last clinical contact. The Kaplan–Meier method was used to estimate the proportion of patients surviving at each time point. Survival curves were compared with the log-rank test. All comparisons were employed with a two-sided test through SPSS (Statistical Package for Social Science, Chicago, IL) version 22.0, and a P value less than 0.05 was considered significant. All figures were concluded via GraphPad Prism (San Diego, CA, USA) version 7.0 software.
3. Results
3.1. Demographic and Clinical Indices at Baseline
A total of 30 POPH (8 men) and 180 IPAH (57 men) patients were included in this study. The mean time of follow-up was 70.23 ± 41.74 months. Data are illustrated in Table 1. Patients with POPH were older than patients with IPAH (49.5 ± 13.1 vs. 39.1 ± 14.9 years old, P < 0.001). In patients with POPH, the most common etiology of liver disease was hepatitis B virus infection (19 cases, 63.4%) and the second one was cryptogenic disease (7 cases, 23.3%). The Child-Pugh class of POPH was mainly distributed in class A (13 cases, 43.3%) and class B (14 cases, 46.7%). D-dimer (399 (218, 1673) vs. 149 (106, 202) ng/ml, P < 0.001) and PCO2 (33.96 ± 11.68 vs. 28.43 ± 5.07 mmHg, P=0.019) were significantly higher, while NT-proBNP (324 (81, 893) vs. 739 (262, 1904) pg/ml, P=0.004) was significantly lower in the POPH group rather than in IPAH group. Compared to the IPAH group, patients in the POPH group were more likely to receive the treatment of phosphodiesterase 5 inhibitors (19 cases (63.2%) vs. 77 cases (42.7%), P=0.036).
Table 1.
Baseline demographics and clinical indices of portopulmonary hypertension and idiopathic pulmonary arterial hypertension.
| Parameters | POPH (n = 30) | IPAH (n = 180) | P value |
|---|---|---|---|
| Demographics | |||
| Age at diagnosis (year) | 49.5 ± 13.1 | 39.1 ± 14.9 | <0.001 |
| Female : male | 22 : 8 | 123 : 57 | 0.854 |
| Height (cm) | 162.7 ± 7.6 | 161.9 ± 6.9 | 0.584 |
| Weight (kg) | 63.0 ± 12.9 | 58.5 ± 9.9 | 0.032 |
| Body surface area (m2) | 1.70 ± 0.29 | 1.62 ± 0.18 | 0.046 |
| Onset of symptoms (mo) | 23 (10–37) | 19 (9–44) | 0.856 |
| WHO FC (I : II : III : IV) | 1 : 12 : 14 : 3 | 2 : 61 : 107 : 10 | 0.571 |
| Etiology of liver disease, n (%) | |||
| Cryptogenic | 7 (23.3%) | NA | |
| Hepatitis B | 19 (63.4%) | NA | |
| Hepatitis C | 1 (3.3%) | NA | |
| Alcohol | 1 (3.3%) | NA | |
| PBC | 2 (6.7%) | NA | |
| Child-Pugh class, n (%) | |||
| A | 13 (43.3%) | NA | |
| B | 14 (46.7%) | NA | |
| C | 3 (10.0%) | NA | |
| Hemodynamics | |||
| mRAP (mmHg) | 4.74 ± 3.54 | 8.48 ± 5.49 | <0.001 |
| sPAP (mmHg) | 80.46 ± 21.71 | 99.25 ± 24.81 | <0.001 |
| mPAP (mmHg) | 46.54 ± 11.95 | 60.58 ± 15.03 | <0.001 |
| mPAWP (mmHg) | 8.23 ± 3.59 | 8.44 ± 3.16 | 0.755 |
| TPG (mmHg) | 38.24 ± 11.21 | 52.38 ± 14.77 | <0.001 |
| Cardiac index (L/min/m2) | 4.02 ± 1.59 | 2.47 ± 0.81 | <0.001 |
| PVR (wood unit) | 7.08 ± 3.95 | 14.89 ± 7.11 | <0.001 |
| SVR (wood unit) | 15.78 ± 7.37 | 22.01 ± 8.23 | 0.001 |
| SvO2 (%) | 65.50 ± 9.73 | 60.97 ± 10.00 | 0.004 |
| Target therapy, n (%) | |||
| ERAs | 3 (10.1%) | 29 (16.1%) | 0.389 |
| PDE-5is | 19 (63.2%) | 77 (42.7%) | 0.036 |
| Prostacyclin analogues | 1 (3.3%) | 14 (7.8%) | 0.382 |
| Combined therapies | 3 (10.1%) | 39 (21.7%) | 0.139 |
| No specific treatment | 4 (13.3%) | 21 (11.7%) | 0.794 |
| Laboratory variables | |||
| Uric acid (μmol/L) | 366.57 ± 156.03 | 404.13 ± 125.95 | 0.149 |
| BUN (mmol/L) | 5.20 ± 1.81 | 5.51 ± 1.96 | 0.415 |
| Creatinine (μmol/L) | 61.57 ± 21.00 | 67.23 ± 17.43 | 0.114 |
| D-dimer (ng/ml) | 399 (218, 1673) | 149 (106, 202) | <0.001 |
| International normalized ratio | 1.31 ± 0.32 | 1.22 ± 0.43 | 0.315 |
| NT-proBNP (pg/ml) | 324 (81, 893) | 739 (262, 1904) | 0.004 |
| BNP (pg/ml) | 120 (80, 402) | 221 (55, 427) | 0.727 |
| Arterial blood gas | |||
| PH | 7.45 ± 0.03 | 7.45 ± 0.03 | 0.522 |
| PO2 (mmHg) | 72.16 ± 13.72 | 72.13 ± 18.26 | 0.994 |
| PCO2 (mmHg) | 33.96 ± 11.68 | 28.43 ± 5.07 | 0.019 |
| SO2 (%) | 93.35 ± 5.13 | 93.00 ± 5.62 | 0.780 |
| Blood cell counts (109/L) | |||
| WBC | 4.18 ± 1.78 | 6.68 ± 2.09 | <0.001 |
| Neutrophils | 2.26 ± 1.40 | 3.88 ± 1.79 | <0.001 |
| RBC | 4.24 ± 0.85 | 4.92 ± 0.63 | <0.001 |
| PLT | 114.73 ± 85.67 | 178.92 ± 72.54 | <0.001 |
| MELD scores | 11 (8–13) | NA |
POPH, portopulmonary hypertension; IPAH, idiopathic pulmonary arterial hypertension; WHO FC, World Health Organization functional classification; PBC, primary biliary cirrhosis; sPAP, systolic pulmonary artery pressure; mRAP, mean right atrial pressure; mPAP, mean pulmonary artery pressure; mPAWP, mean pulmonary artery wedge pressure; PVR, pulmonary vascular resistance; TPG, transpulmonary gradient; SVR, systemic vascular resistance; SvO2, mixed venous oxygen saturation; ERAs, endothelin receptor antagonists; PDE-5is, phosphodiesterase 5 inhibitors; BUN, blood urea nitrogen; NT-proBNP, N-terminal pro brain natriuretic peptide; BNP, brain natriuretic peptide; PH, power of hydrogen; PO2, partial pressure of oxygen; PCO2, partial pressure of carbon dioxide; SO2, oxygen content of arterial blood; WBC, white blood cell; RBC, red blood cell; PLT, platelet; MELD score, model for end-stage liver disease scores; NA, not applicable. Hemodynamics were measured via right heart catheterization.
In the aspect of arterial blood gas, patients with POPH had higher PCO2 (33.96 ± 11.68 vs. 28.43 ± 5.07 mmHg, P=0.019) than patients with IPAH. With respect to blood cell counts, the POPH group had lower white blood cell counts (4.18 ± 1.78 vs. 6.68 ± 2.09 109/L, P < 0.001), neutrophil counts (2.26 ± 1.40 vs. 3.88 ± 1.79 109/L, P < 0.001), red blood cell counts (2.26 ± 1.40 vs. 3.88 ± 1.79 109/L, P < 0.001), and platelet counts (114.73 ± 85.67 vs. 178.92 ± 72.54 109/L, P < 0.001) than the IPAH group.
Hemodynamic variables were quite different between POPH and IPAH. Compared to patients with IPAH, patients with POPH had lower systolic pulmonary artery pressure (sPAP, 80.46 ± 21.71 vs. 99.25 ± 24.81 mmHg, P < 0.001), mean right atrial pressure (mRAP, 4.74 ± 3.54 vs. 8.48 ± 5.49 mmHg, P < 0.001), mean pulmonary artery pressure (mPAP, 46.54 ± 11.95 vs. 60.58 ± 15.03 mmHg, P < 0.001), pulmonary vascular resistance (PVR, 7.01 ± 4.07 vs. 14.82 ± 7.00 wood unit, P < 0.001), transpulmonary gradient (TPG, 38.24 ± 11.21 vs. 52.38 ± 14.77 mmHg, P < 0.001), and systemic vascular resistance (SVR, 15.78 ± 7.37 vs. 22.01 ± 8.23 wood unit, P=0.001) but higher cardiac index (CI, 4.02 ± 1.59 vs. 2.47 ± 0.81 L/min/m2, P < 0.001) and mixed venous oxygen saturation (SvO2, 65.50 ± 9.73 vs. 60.97 ± 10.00%, P=0.004). Of these differential variables, the gap of PVR between the POPH group and the IPAH group exhibited a two-fold change.
3.2. Variables of the Liver Function Test
Patients with POPH showed significantly higher expression of total bilirubin (25.0 (17.0, 44.3) vs. 19.0 (14.0, 28.3) μmol/L, P=0.013), direct bilirubin (10.5 (6.0, 17.0) vs. 6.0 (4.0, 10.5) μmol/L, P=0.013), and aspartate aminotransferase (AST, 37.5 (30.0, 44.0) vs. 25.0 (20.0, 30.0) IU/L, P < 0.001) and lower expression of albumin (32.88 ± 4.27 vs. 38.41 ± 5.14 g/L, P < 0.001) than patients with IPAH. In addition, alanine aminotransferase (ALT, 26.5 (17.8, 38.0) vs. 24.0 (19.0, 32.0) IU/L, P=0.566) showed no difference between the POPH group and IPAH group. These data are generated in Table 2.
Table 2.
Liver function test of portopulmonary hypertension and idiopathic pulmonary arterial hypertension at baseline.
| Parameters | POPH (n = 30) | IPAH (n = 180) | P value |
|---|---|---|---|
| Total bilirubin (μmol/L) | 25.0 (17.0, 44.3) | 19.0 (14.0, 28.3) | 0.013 |
| Direct bilirubin (μmol/L) | 10.5 (6.0, 17.0) | 6.0 (4.0, 10.5) | <0.001 |
| Albumin (g/L) | 32.88 ± 4.27 | 38.41 ± 5.14 | <0.001 |
| ALT (IU/L) | 26.5 (17.8, 38.0) | 24.0 (19.0, 32.0) | 0.566 |
| AST (IU/L) | 37.5 (30.0, 44.0) | 25.0 (20.0, 30.0) | <0.001 |
POPH, portopulmonary hypertension; IPAH, idiopathic pulmonary arterial hypertension; ALT, alanine aminotransferase; AST, aspartate aminotransferase.
3.3. Correlation between PVR and Differential Laboratory Variables and Liver Function Test in POPH and IPAH
Table 3 illustrates the correlations between PVR and the clinical variables and indices in POPH and IPAH. (PVR, total bilirubin, direct bilirubin, AST, D-dimer, blood cell counts, NT-proBNP, and PCO2 were regarded as continuous variables. Target therapies and MELD scores were deemed as categorical variables.) The analysis found that total bilirubin and direct bilirubin presented a negative correlation with PVR in the POPH group (r = −0.394, P=0.031; r = −0.364, P=0.048, respectively), while they showed a positive correlation with PVR in the IPAH group (r = 0.218, P=0.003; r = 0.178, P=0.018, respectively). NT-proBNP was positively related to PVR in the IPAH group (r = 0.438, P < 0.001), and neutrophil counts had a positive correlation with PVR in the POPH group (r = 0.394, P=0.031).
Table 3.
Correlation between PVR and laboratory variables, liver function test, and therapies in POPH and IPAH.
| POPH | IPAH | |||
|---|---|---|---|---|
| r | P | r | P | |
| Liver function test | ||||
| Total bilirubin | −0.394 | 0.031 | 0.218 | 0.003 |
| Direct bilirubin | −0.364 | 0.048 | 0.178 | 0.018 |
| AST | −0.136 | 0.474 | 0.139 | 0.066 |
| D-dimer | 0.009 | 0.964 | 0.016 | 0.913 |
| Blood cell counts | ||||
| WBC | 0.275 | 0.142 | 0.179 | 0.117 |
| Neutrophils | 0.394 | 0.031 | 0.107 | 0.167 |
| NT-proBNP | 0.203 | 0.299 | 0.438 | <0.001 |
| PCO2 | 0.014 | 0.940 | 0.004 | 0.976 |
| Target therapies | −0.076 | 0.690 | 0.133 | 0.076 |
| MELD scores (per unit) | −0.243 | 0.195 | — | — |
Data were conducted via Pearson or Spearman correlation analyses. PVR, total bilirubin, direct bilirubin, AST, D-dimer, blood cell counts, NT-proBNP, and PCO2 were regarded as continuous variables. Target therapies and MELD scores were deemed as categorical variables. PVR, pulmonary vascular resistance; POPH, portopulmonary hypertension; IPAH, idiopathic pulmonary arterial hypertension; AST, aspartate aminotransferase; WBC, white blood cell; NT-proBNP, N-terminal pro brain natriuretic peptide; PCO2, partial pressure of carbon dioxide; MELD scores, model for end-stage liver disease scores; —, not applicable.
3.4. Independent Determinants to Predict PVR Elevation in POPH and IPAH
Taking those potentially correlated variables (the significance is shown in Table 3) into account, we applied a multiple stepwise regression analysis to determine the strength of the prediction of the elevation of the PVR (as a continuous variable) after adjusting for gases of blood (continuous variable), metabolic comorbidities (yes or not, categorical variable, including hypertension, diabetes, obesity, and dyslipidaemia), Child-Pugh score (categorical variable), and concomitant medications (categorical variable). In the POPH group, elevated neutrophil counts as an independent predictor purported rising PVR accounting for 15.6% change (standardized β = 0.394, P=0.030). NT-proBNP had the sole role in positively predicting PVR elevation in the IPAH group and made 18.7% variation clear (standardized β = 0.433, P < 0.001). This information is illustrated in Table 4.
Table 4.
Independent determinants of PVR elevation from differential laboratory variables in POPH and IPAH.
| Independent predictors | R 2 | Standardized β | 95% confidence interval | P value | |
|---|---|---|---|---|---|
| POPH | Neutrophils | 0.156 | 0.394 | 0.109, 0.118 | 0.030 |
| IPAH | NT-proBNP | 0.187 | 0.433 | 0.001, 0.002 | <0.001 |
Data were conducted via linear regression analyses. Models were adjusted by gases of blood (continuous variable), metabolic comorbidities (yes or not, categorical variable, including hypertension, diabetes, obesity, and dyslipidaemia), Child-Pugh score (categorical variable), and concomitant medications (categorical variable). PVR, total bilirubin, direct bilirubin, blood cell counts, and NT-proBNP were regarded as continuous variables. PVR, pulmonary vascular resistance; POPH, portopulmonary hypertension; IPAH, idiopathic pulmonary arterial hypertension; NT-proBNP, N-terminal pro brain natriuretic peptide.
3.5. Ability of Direct Bilirubin to Identify POPH from Total Patients
ROC analysis was performed to assess the ability of direct bilirubin, AST, and their combination to identify POPH from total patients (Figure 2). We found the significant strength of direct bilirubin in distinguishing POPH from total patients with AUC = 0.799 and P=0.009. Meanwhile, another abnormal liver function index of AST also showed the significant ability to identify POPH from IPAH, of which the AUC was 0.701 (P < 0.001). When combining the two variables together, the ability to identify POPH from IPAH had been improved and the AUC was elevated to 0.835 (P < 0.001). We also calculated the value of IDI and NRI to evaluate the elevated ability of the combined model relative to the direct bilirubin model, and the results were following: absolute IDI = 0.064, relative IDI = 0.074, and NRI = 0.030.
Figure 2.
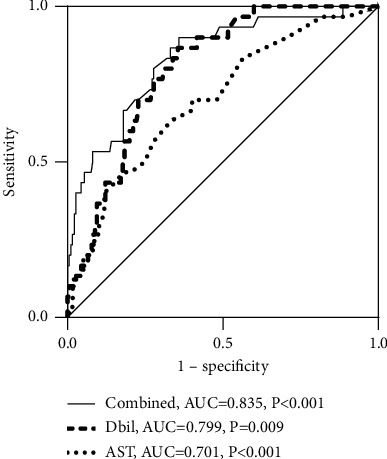
The ability of direct bilirubin (Dbil), aspartate aminotransferase (AST), and their combination to distinguish POPH from the total cohort. POPH, portopulmonary hypertension; IPAH, idiopathic pulmonary arterial hypertension; AUC, area under the curve.
3.6. Survival Assessment between POPH and IPAH
There were a total of 103 dead cases (7 POPH) during the follow-up. Of those dead cases, 72 patients with IPAH (75.0%) and 2 patients with POPH (28.6%) died of right heart failure. With respect to liver disease, no one died of liver failure in IPAH, while 5 patients with POPH (71.4%) died of liver failure. The 1st-year, 3rd-year, 5th-year, and 10th-year overall survival rates were found to be 67.2%, 53.2%, 41.0%, and 35.2% in the IPAH group and 89.3%, 81.5%, 81.5%, and 71.3% in the POPH group, respectively (log-rank = 0.002, Figure 3(a)). With the division of direct bilirubin (both IPAH and POPH), we found that there was no significantly different survival between POPH and IPAH in the group of direct bilirubin <7 μmol/L (log-rank = 0.192, Figure 3(b)), but it showed better survival in POPH than in IPAH in the group of direct bilirubin ≥7 μmol/L (log-rank = 0.001, Figure 3(c)).
Figure 3.
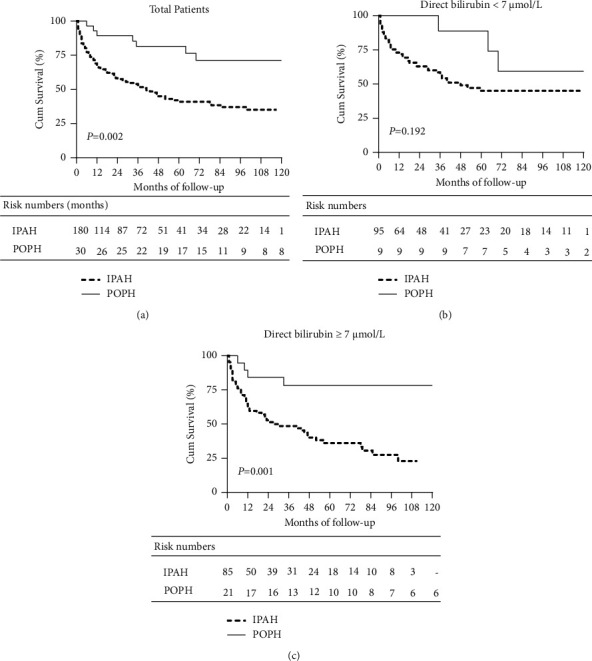
Comparison of estimated survival between IPAH and POPH in total patients (a). When divided by the upper limit of the normal range of direct bilirubin (both IPAH and POPH), the different survival situations between POPH and IPAH patients with direct bilirubin <7 μmol/L (b) and patients with direct bilirubin ≥7 μmol/L (c). POPH, portopulmonary hypertension; IPAH, idiopathic pulmonary arterial hypertension.
4. Discussion
To date, there have still been limited types of literature talking about the differences in bilirubin between IPAH and POPH. Our study demonstrated some interesting findings as follows: (I) when matched by WHO FC, patients with POPH had better hemodynamics and survival than patients with IPAH; (II) patients with POPH had worse liver function than patients with IPAH, which showed that the medians (first and third interquartile) of direct bilirubin were 10.5 (6.0, 17.0) and 6.0 (4.0, 10.5) μmol/L, respectively; (III) total bilirubin and direct bilirubin associated with PVR positively in the IPAH group but negatively in the POPH group; (IV) elevated neutrophil counts and elevated NT-proBNP were independent predictors of PVR increase in POPH and IPAH, respectively; (V) direct bilirubin with AST could better help identify POPH from IPAH than separate direct bilirubin or AST.
No matter from the REHAP registry [16], the REVEAL registry [17], or the data of the National Research Project on Intractable Disease in Japan [18], POPH subjects were considered with better hemodynamics, which was similar to our data. However, the western population [13, 14] showed a worse prognosis in POPH than in IPAH or heritable PAH (HPAH) when referring to the survival situation. In contrast, patients with POPH from the Japanese population showed no significant difference in survival compared to IPAH ones [18]. Our study showed better survival in POPH rather than in IPAH when the populations were limited into a similar WHO functional classification. Although a recent study from China [19] had shown a trend similar to the western population, the limited cases of 10 PHT-post-splenectomy-PH and 20 IPAH were not that easy to generate the typical landscape of IPAH. Our study contained 30 cases with POPH and 180 cases with IPAH, which could help recognize the difference between POPH and a typical landscape of IPAH. It is easy to consider that race might be an indicator of different survival among countries and regions. However, a study from the REVEAL registry, including a total of 3046 patients in which 100 were deemed as Asian or Pacific Islander, demonstrated that the race was not significantly associated with survival after age under 60 years adjustment [20]. Meanwhile, DuBrock's study [21] found that patients with POPH had lower socioeconomic status than patients with IPAH, in which lower education level would associate with more emergency department visits in American patients. Different survival among countries and regions requires more clinical investigations concerning the development of economy and medication, diets, mental situation, the spectrum of primary diseases, etc. Another difference in our study from the western population was the etiology of liver disease. Our data showed that the most etiology was hepatitis B virus infection of POPH rather than alcohol. Actually, the burden of liver diseases differed a lot from countries and regions [22]. In the western population, alcohol was the major cause of liver diseases, including POPH, while hepatitis B virus infection was higher than alcohol factor in East Asia [22, 23]. Meanwhile, our study was with a small sample size, which means that the small dead cases of POPH limited the survival comparison between POPH and IPAH. A new national study is expected in the future.
We chose PVR to conduct the correlation analysis not only because of the two-time fold change between POPH and IPAH but also because PVR could predict mortality and graft failure in transplantation patients with POPH [24]. Although total bilirubin and direct bilirubin were positively correlated with PVR, the independent predictor of PVR elevation was NT-proBNP in the IPAH group. Bilirubin has been considered to positively correlate with the hemodynamic profile in patients with heart failure (HF) [25]. And then, total bilirubin was among the most highly significant predictors of mortality in a large cohort of chronic HF patients in a clinical trial [26]. As aware, right HF was the leading cause of death of IPAH [27, 28], and NT-proBNP is one of the most valuable biomarkers for diagnosing HF. Furthermore, a prior study has demonstrated a positive correlation between serum bilirubin concentration and lognormal concentration of BNP [11]. Therefore, it is not difficult to understand the positive correlation between bilirubin and the independent predicted value of NT-proBNP to the elevation of PVR in the IPAH group. However, there was no significant association between NT-proBNP and PVR in the POPH group, and it indicated that HF might not be the main cause of death in POPH patients when the most likely cause of death in POPH patients was liver disease. MELD scores could present the severity of liver disease, and it was deemed as a predictor of mortality in POPH [29]. However, we found that MELD scores had no significant correlation with PVR in POPH when the previous study found that MELD scores correlated poorly with PVR (r = −0.01) [30]. The differences might come from the small sample size in our study and the measured means of echocardiography in the later study.
The negative correlation between PVR and direct bilirubin was a decent finding in the POPH group. Horsfall [31] and colleagues conducted an extensive, statin-treated cohort (without liver disease or cardiovascular disease) research and found that low expression levels of serum bilirubin were associated with an increased risk of cardiovascular disease, suggesting a beneficial effect of elevated bilirubin levels. Meanwhile, a cohort containing 504,206 adults from a UK primary care research database showed the negative association between serum bilirubin and incidence of respiratory disease (chronic obstructive pulmonary disease and lung cancer) and all-cause mortality [32]. In the general population of Korea, the association between serum bilirubin and cardiovascular disease exhibited a trend similar to the before two [33]. In fact, bilirubin was thought to have antioxidant and anti-inflammatory effects over the past decades [34, 35], in which a bilirubin-biliverdin cycling mechanism could help explain the biological effects [36]. In in vitro experiments, Mazzone et al. [37] found that Human Umbilical Vein Endothelial Cells (HUVECs) prevented the adhesion induced by TNFα after being treated with different serum unconjugated bilirubin concentrations, in which the expression of E-selectin VCAM-1 and ICAM-1 was reduced.
Meanwhile, the neutrophil counts were the independent predictor of PVR elevation in POPH after taking total bilirubin, direct bilirubin, and neutrophil counts into the linear regression analysis. In fact, bilirubin had some association with neutrophils in some pulmonary diseases. Biliverdin, which can be interconverted with bilirubin, improved pulmonary inflammation induced by hemorrhagic shock and resuscitation (HSR). The authors used biliverdin treatment before HSR in rats and found markedly decreased neutrophil infiltration in the lung sections (neutrophils were stained by the naphthol AS-D chloroacetate method) compared to the sham group [38]. In the mouse model of chronic obstructive pulmonary disease induced from cigarette smoke exposure (CSE), the liver growth factor (LGF), an albumin-bilirubin complex, exhibited the antifibrotic, antioxidant, and antihypertensive actions at extrahepatic sites. After CSE mice were treated with LGF, the circulating T lymphocytes were significantly decreased and neutrophils from peripheral blood tended to be reduced compared to the CSE group [39]. Nevertheless, whether the bilirubin would influence the pathological and physiological changes of POPH and IPAH is still unknown, and more studies exploring the different mechanisms between POPH and IPAH should be conducted.
5. Conclusions
When matched by WHO FC, POPH patients had a distinct demographic, clinical, and hemodynamic profile compared to IPAH patients. Total bilirubin and direct bilirubin had a negative correlation with PVR in POPH but positive in IPAH. Patients with POPH had better survival than IPAH patients in the group of total patients and the group of patients with direct bilirubin ≥7 μmol/L, but the limited dead cases of POPH should be noted.
Acknowledgments
All the work in this study was completed at the Division of Cardio-Pulmonary Circulation, Shanghai Pulmonary Hospital Affiliated to Tongji University School of Medicine, Shanghai, China. The authors would thank all colleagues for the supportive work and all the participants for their devotion to scientific improvement. This study was funded by the Youth Program of the National Natural Science Foundation of China (81900050 and 82000059), the Project of Science and Technology Commission Shanghai Municipality (201409004100), and the Natural Science Foundation of Shanghai (15ZR1431500).
Contributor Information
Jinming Liu, Email: jinmingliu@tongji.edu.cn.
Sugang Gong, Email: gongsugang@tongji.edu.cn.
Data Availability
Data used to support the findings of this study are available from the corresponding author upon reasonable request.
Additional Points
Limitations. First, the small sample size of POPH patients resulted from a single tertiary center with a referral difference. Patients who developed obvious cardiopulmonary symptoms and signs would come to our center for PH confirmation. However, most patients would turn to some general hospitals for treatment of primary liver disease. Second, because bilirubin is dynamically changing during disease development, data measured at baseline might not be the best indicators and long-term monitoring of bilirubin in POPH should be studied further.
Disclosure
Yuan Li and Hongling Qiu are the equal contributors.
Conflicts of Interest
The authors declare that there are no conflicts of interest.
Authors' Contributions
Conceptualization was performed by Jinming Liu and Sugang Gong; Data curation was carried out by Qinhua Zhao, Jing He, Rong Jiang, Wenhui Wu, Cijun Luo, Huiting Li, and Lan Wang; Formal analysis was conducted by Yuan Li, Hongling Qiu, Qinhua Zhao, Jing He, Rong Jiang, Wenhui Wu, Cijun Luo, Huiting Li, and Lan Wang; Funding acquisition was performed by Qinhua Zhao, Lan Wang, and Sugang Gong; Investigation was done by Yuan Li and Hongling Qiu; Methodology was developed by Jinming Liu; Supervision was carried out by Jinming Liu and Sugang Gong; Visualization was conducted by Yuan Li and Hongling Qiu; the original draft was written by Yuan Li; review and editing were carried out by Hongling Qiu, Qinhua Zhao, Jing He, Rong Jiang, Wenhui Wu, Cijun Luo, Huiting Li, Lan Wang, Jinming Liu, and Sugang Gong.
Supplementary Materials
We have collected related data from echocardiography because patients would like to accept a noninvasive test (echocardiography) rather than an invasive test (right heart catheterization) and found that there was no significant change between the baseline and last follow-up time in POPH. The escaped time between the baseline and the last follow-up time in POPH was 65.1 ± 17.3 months. Those data are summarized in Supplement Figure 1. Supplement Figure 1: Comparisons of indices measured by means of echocardiography between the time of baseline and the last follow-up in POPH, including sPAP (a), TAPSE (b), and LVEF (c). sPAP, systolic pulmonary artery pressure; TAPSE, tricuspid annular plane systolic excursion; LVEF, left ventricular ejection fractions.
References
- 1.Simonneau G., Robbins I. M., Beghetti M., et al. Updated clinical classification of pulmonary hypertension. Journal of the American College of Cardiology . 2009;54(1):S43–S54. doi: 10.1016/j.jacc.2009.04.012. [DOI] [PubMed] [Google Scholar]
- 2.Navarro-Vergara D. I., Roldan-Valadez E., Cueto-Robledo G., Jurado-Hernandez M. Y. Portopulmonary hypertension: prevalence, clinical and hemodynamic features. Current Problems in Cardiology . 2021;46(3) doi: 10.1016/j.cpcardiol.2020.100747.100747 [DOI] [PubMed] [Google Scholar]
- 3.Thomas C., Glinskii V., De Jesus Perez V., Sahay S. Portopulmonary hypertension: from bench to bedside. Frontiers in Medicine . 2020;7 doi: 10.3389/fmed.2020.569413.569413 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Machicao V. I., Balakrishnan M., Fallon M. B. Pulmonary complications in chronic liver disease. Hepatology . 2014;59(4):1627–1637. doi: 10.1002/hep.26745. [DOI] [PubMed] [Google Scholar]
- 5.Rodriguez-Roisin R., Krowka M. J., Herve P., Fallon M. B., ERS Task Force Pulmonary-Hepatic Vascular Disorders (PHD) Scientific Committee Pulmonary-hepatic vascular disorders (PHD) European Respiratory Journal . 2004;24(5):861–880. doi: 10.1183/09031936.04.00010904. [DOI] [PubMed] [Google Scholar]
- 6.Le Pavec J., Souza R., Herve P., et al. Portopulmonary hypertension: survival and prognostic factors. American Journal of Respiratory and Critical Care Medicine . 2008;178(6):637–643. doi: 10.1164/rccm.200804-613oc. [DOI] [PubMed] [Google Scholar]
- 7.Sithamparanathan S., Nair A., Thirugnanasothy L., et al. Survival in portopulmonary hypertension: outcomes of the United Kingdom national pulmonary arterial hypertension registry. The Journal of Heart and Lung Transplantation . 2017;36(7):770–779. doi: 10.1016/j.healun.2016.12.014. [DOI] [PubMed] [Google Scholar]
- 8.Colle I., Moreau R., Godinho E., et al. Diagnosis of portopulmonary hypertension in candidates for liver transplantation: a prospective study. Hepatology . 2003;37(2):401–409. doi: 10.1053/jhep.2003.50060. [DOI] [PubMed] [Google Scholar]
- 9.Simonneau G., Montani D., Celermajer D. S., et al. Haemodynamic definitions and updated clinical classification of pulmonary hypertension. European Respiratory Journal . 2019;53(1) doi: 10.1183/13993003.01913-2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kramer M. R., Marshall S. E., Tiroke A., Lewiston N. J., Starnes V. A., Theodore J. Clinical significance of hyperbilirubinemia in patients with pulmonary hypertension undergoing heart-lung transplantation. The Journal of Heart and Lung Transplantation: The Official Publication of the International Society for Heart Transplantation . 1991;10(2):317–321. [PubMed] [Google Scholar]
- 11.Takeda Y., Takeda Y., Tomimoto S., Tani T., Narita H., Kimura G. Bilirubin as a prognostic marker in patients with pulmonary arterial hypertension. BMC Pulmonary Medicine . 2010;10(1):p. 22. doi: 10.1186/1471-2466-10-22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Xu X.-Q., Lv Z.-C., Liu Q.-Q., et al. Direct bilirubin: a new risk factor of adverse outcome in idiopathic pulmonary arterial hypertension. International Journal of Cardiology . 2017;228:895–899. doi: 10.1016/j.ijcard.2016.11.036. [DOI] [PubMed] [Google Scholar]
- 13.Schlegel A., Linecker M., Kron P., et al. Risk assessment in high- and low-MELD liver transplantation. American Journal of Transplantation . 2017;17(4):1050–1063. doi: 10.1111/ajt.14065. [DOI] [PubMed] [Google Scholar]
- 14.Kerr K. F., Mcclelland R. L., Brown E. R., Lumley T. Evaluating the incremental value of new biomarkers with integrated discrimination improvement. American Journal of Epidemiology . 2011;174(3):364–374. doi: 10.1093/aje/kwr086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Pencina M. J., D’Agostino R. B., D’Agostino R. B., Jr., Vasan R. S. Evaluating the added predictive ability of a new marker: from area under the ROC curve to reclassification and beyond. Statistics in Medicine . 2008;27(2):157–172. doi: 10.1002/sim.2929. [DOI] [PubMed] [Google Scholar]
- 16.Lazaro Salvador M., Quezada Loaiza C. A., Rodríguez Padial L., et al. Portopulmonary hypertension: prognosis and management in the current treatment era—results from the REHAP registry. Internal Medicine Journal . 2021;51(3):355–365. doi: 10.1111/imj.14751. [DOI] [PubMed] [Google Scholar]
- 17.Krowka M. J., Miller D. P., Barst R. J., et al. Portopulmonary hypertension: a report from the US-based REVEAL registry. Chest . 2012;141(4):906–915. doi: 10.1378/chest.11-0160. [DOI] [PubMed] [Google Scholar]
- 18.Takahashi Y., Yamamoto K., Sakao S., et al. The clinical characteristics, treatment, and survival of portopulmonary hypertension in Japan. BMC Pulmonary Medicine . 2021;21(1):p. 89. doi: 10.1186/s12890-021-01452-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Huang L., Li W., Yang T., et al. Association between splenectomy and portal hypertension in the development of pulmonary hypertension. Pulmonary Circulation . 2020;10(1) doi: 10.1177/2045894019895426.2045894019895426 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Medrek S., Sahay S., Zhao C., Selej M., Frost A. Impact of race on survival in pulmonary arterial hypertension: results from the REVEAL registry. The Journal of Heart and Lung Transplantation . 2020;39(4):321–330. doi: 10.1016/j.healun.2019.11.024. [DOI] [PubMed] [Google Scholar]
- 21.Dubrock H. M., Burger C. D., Bartolome S. D., et al. Health disparities and treatment approaches in portopulmonary hypertension and idiopathic pulmonary arterial hypertension: an analysis of the pulmonary hypertension association registry. Pulmonary Circulation . 2021;11(3) doi: 10.1177/20458940211020913.20458940211020913 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Asrani S. K., Devarbhavi H., Eaton J., Kamath P. S. Burden of liver diseases in the world. Journal of Hepatology . 2019;70(1):151–171. doi: 10.1016/j.jhep.2018.09.014. [DOI] [PubMed] [Google Scholar]
- 23.Devarbhavi H., Asrani S. K., Kamath P. S. Reply to: “alcohol-associated liver disease, not hepatitis B, is the major cause of cirrhosis in Asia”. Journal of Hepatology . 2019;70(5):p. 1033. doi: 10.1016/j.jhep.2019.01.023. [DOI] [PubMed] [Google Scholar]
- 24.Jose A., Shah S. A., Anwar N., Jones C., Sherman K., Elwing J. Pulmonary vascular resistance predicts mortality and graft failure in transplantation patients with portopulmonary hypertension. Liver Transplantation . 2021;27:1811–1823. doi: 10.1002/lt.26091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Van Deursen V. M., Damman K., Hillege H. L., van Beek A. P., van Veldhuisen D. J., Voors A. A. Abnormal liver function in relation to hemodynamic profile in heart failure patients. Journal of Cardiac Failure . 2010;16(1):84–90. doi: 10.1016/j.cardfail.2009.08.002. [DOI] [PubMed] [Google Scholar]
- 26.Allen L. A., Felker G. M., Pocock S., et al. Liver function abnormalities and outcome in patients with chronic heart failure: data from the candesartan in heart failure: assessment of reduction in mortality and morbidity (CHARM) program. European Journal of Heart Failure . 2009;11(2):170–177. doi: 10.1093/eurjhf/hfn031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Zhang R., Dai L.-Z., Xie W.-P., et al. Survival of Chinese patients with pulmonary arterial hypertension in the modern treatment era. Chest . 2011;140(2):301–309. doi: 10.1378/chest.10-2327. [DOI] [PubMed] [Google Scholar]
- 28.Humbert M., Sitbon O., Yaici A., et al. Survival in incident and prevalent cohorts of patients with pulmonary arterial hypertension. European Respiratory Journal . 2010;36(3):549–555. doi: 10.1183/09031936.00057010. [DOI] [PubMed] [Google Scholar]
- 29.Savale L., Guimas M., Ebstein N., et al. Portopulmonary hypertension in the current era of pulmonary hypertension management. Journal of Hepatology . 2020;73(1):130–139. doi: 10.1016/j.jhep.2020.02.021. [DOI] [PubMed] [Google Scholar]
- 30.Krowka M. J., Swanson K. L., Frantz R. P., McGoon M. D., Wiesner R. H. Portopulmonary hypertension: results from a 10-year screening algorithm. Hepatology . 2006;44(6):1502–1510. doi: 10.1002/hep.21431. [DOI] [PubMed] [Google Scholar]
- 31.Horsfall L. J., Nazareth I., Petersen I. Cardiovascular events as a function of serum bilirubin levels in a large, statin-treated cohort. Circulation . 2012;126(22):2556–2564. doi: 10.1161/circulationaha.112.114066. [DOI] [PubMed] [Google Scholar]
- 32.Horsfall L. J., Rait G., Walters K., et al. Serum bilirubin and risk of respiratory disease and death. JAMA . 2011;305(7):691–697. doi: 10.1001/jama.2011.124. [DOI] [PubMed] [Google Scholar]
- 33.Suh S., Cho Y. R., Park M. K., Kim D. K., Cho N. H., Lee M.-K. Relationship between serum bilirubin levels and cardiovascular disease. PLoS One . 2018;13(2) doi: 10.1371/journal.pone.0193041.e0193041 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Sedlak T. W., Saleh M., Higginson D. S., Paul B. D., Juluri K. R., Snyder S. H. Bilirubin and glutathione have complementary antioxidant and cytoprotective roles. Proceedings of the National Academy of Sciences . 2009;106(13):5171–5176. doi: 10.1073/pnas.0813132106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Stocker R., Yamamoto Y., Mcdonagh A. F., Glazer A. N., Ames B. N. Bilirubin is an antioxidant of possible physiological importance. Science . 1987;235(4792):1043–1046. doi: 10.1126/science.3029864. [DOI] [PubMed] [Google Scholar]
- 36.McDonagh A. F. The biliverdin-bilirubin antioxidant cycle of cellular protection: missing a wheel? Free Radical Biology and Medicine . 2010;49(5):814–820. doi: 10.1016/j.freeradbiomed.2010.06.001. [DOI] [PubMed] [Google Scholar]
- 37.Mazzone G. L., Rigato I., Ostrow J. D., et al. Bilirubin inhibits the TNFα-related induction of three endothelial adhesion molecules. Biochemical and Biophysical Research Communications . 2009;386(2):338–344. doi: 10.1016/j.bbrc.2009.06.029. [DOI] [PubMed] [Google Scholar]
- 38.Kosaka J., Morimatsu H., Takahashi T., et al. Effects of biliverdin administration on acute lung injury induced by hemorrhagic shock and resuscitation in rats. PLoS One . 2013;8(5) doi: 10.1371/journal.pone.0063606.e63606 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Pérez-Rial S., Del Puerto-Nevado L., Girón-Martínez Á., et al. Liver growth factor treatment reverses emphysema previously established in a cigarette smoke exposure mouse model. American Journal of Physiology-Lung Cellular and Molecular Physiology . 2014;307(9):L718–L726. doi: 10.1152/ajplung.00293.2013. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
We have collected related data from echocardiography because patients would like to accept a noninvasive test (echocardiography) rather than an invasive test (right heart catheterization) and found that there was no significant change between the baseline and last follow-up time in POPH. The escaped time between the baseline and the last follow-up time in POPH was 65.1 ± 17.3 months. Those data are summarized in Supplement Figure 1. Supplement Figure 1: Comparisons of indices measured by means of echocardiography between the time of baseline and the last follow-up in POPH, including sPAP (a), TAPSE (b), and LVEF (c). sPAP, systolic pulmonary artery pressure; TAPSE, tricuspid annular plane systolic excursion; LVEF, left ventricular ejection fractions.
Data Availability Statement
Data used to support the findings of this study are available from the corresponding author upon reasonable request.


