Abstract
Despite the availability of over 30 antiseizure medications (ASMs), there is no “one size fits it all,” so there is a continuing search for novel ASMs. There are divergent data demonstrating that modulation of distinct serotonin (5‐hydroxytryptamine, 5‐HT) receptors subtypes could be beneficial in the treatment of epilepsy and its comorbidities, whereas only a few ASM, such as fenfluramine (FA), act via 5‐HT. There are 14 different 5‐HT receptor subtypes, and most epilepsy studies focus on one or a few of these subtypes, using different animal models and different ligands. We reviewed the available evidence of each 5‐HT receptor subtype using MEDLINE up to July 2021. Our search included medical subject heading (MeSH) and free terms of each “5‐HT subtype” separately and its relation to “epilepsy or seizures.” Most research underlines the antiseizure activity of 5‐HT1A,1D,2A,2C,3 agonism and 5‐HT6 antagonism. Consistently, FA, which has recently been approved for the treatment of seizures in Dravet syndrome, is an agonist of 5‐HT1D,2A,2C receptors. Even though each study focused on a distinct seizure/epilepsy type and generalization of different findings could lead to false interpretations, we believe that the available preclinical and clinical studies emphasize the role of serotonergic modulation, especially stimulation, as a promising avenue in epilepsy treatment.
Keywords: 5‐HT, antiseizure medication, epilepsy treatment, fenfluramine, SUDEP
Key points.
Over 30 antiseizure medications (ASMs) are available, yet one‐third of epilepsy patients do not achieve appropriate seizure control.
Hence, there is an unmet need for ASMs with innovative mechanisms of action, such as serotonergic (5‐HT) modulation.
Until now only one ASM acts via 5‐HT, that is, fenfluramine (FA), which likely is a 5‐HT1D,2A,2C agonist and approved for Dravet syndrome.
Interestingly, numerous studies show that 5‐HT1A,1D,2A,2C,3 agonists and 5‐HT6 antagonists are promising candidates for epilepsy treatment.
Although generalizing these studies could lead to false interpretations, 5‐HT modulation withholds a novel avenue in epilepsy treatment.
1. INTRODUCTION
Epilepsy is a prevalent neurological disease, affecting up to 70 million people worldwide. The ultimate goal for patients with epilepsy (PWE) is to achieve complete seizure control without drug‐induced adverse events and preserve the quality of life. The mainstay of epilepsy treatment is controlling seizures by antiseizure medications (ASMs; previously referred to as anti‐epileptic drugs, AEDs) that can act through different pathways, that is, an increase of neuronal inhibition and/or a decrease of neuronal excitation. 1
The first‐generation ASMs mainly act by blocking sodium channels or stimulating the neurotransmission by γ‐aminobutyric acid (GABA), 2 while second‐ and third‐generation ASMs have distinct molecular targets. 3 Moreover, the pharmacokinetic profile of the newer ASMs has improved, which led to more predictable dose–response effects, fewer side effects, and fewer/no drug–drug interactions. 4 Nonetheless, more than 30% of the PWE seizures cannot be controlled with the currently available ASMs. 5
In general, ASM discovery and development are encouraged for orphan diseases (ie affecting <5/10 000 people in the general population), such as Dravet syndrome (DS). 6 , 7 This strategy has led to the discovery of fenfluramine (FA), a serotonergic drug, that successfully reduces seizures in DS patients. 8 This serotonergic drug is also under evaluation for other severe epilepsy syndromes, such as Lennox–Gastaut and Sunflower syndrome. 9 , 10 , 11 Since preliminary data with this serotonergic drug are promising, it is expected that serotonergic modulation is a promising target to stop (drug‐resistant) seizures. Even though FA is believed to affect non‐serotonergic pathways as well, such as sigma‐1 (σ1) receptors, 12 , 13 , 14 the exact anti‐epileptic mechanisms are still elusive and it seems likely that serotonin is involved based on preclinical data. 13 , 15 , 16 , 17
With this in mind, other studies have underlined that serotonergic receptors seem to be an interesting target for future ASMs. 2 , 18 , 19 In addition, ample preclinical and clinical evidence is available to suggest the importance of serotonergic neurotransmission in epilepsy, depression, headache, and sudden unexplained death in epilepsy patients (SUDEP). 18 , 20 , 21 , 22 , 23 , 24 , 25 , 26 , 27 , 28 , 29 Most compelling evidence of the 14 different serotonin receptors and its role in epilepsy have been reviewed by Gharedaghi and colleagues in 2014. 18
Therefore, we aimed to update this review with the available research of the last 7 years and provide a comprehensive overview of the modulation of each serotonin receptor in the pathology/treatment of epilepsy.
2. MATERIALS AND METHODS
We reviewed the existing literature by means of MEDLINE (using PubMed) up to July 2021, following the PRISMA guidelines (Table S1). The following search with medical subject heading (MeSH) and free terms was used: (((((((((((((5‐HT1A receptor) OR (5‐HT1A receptor[MeSH Terms])) AND ((((epilepsy) OR (epilepsy[MeSH Terms])) OR (seizures)) OR (seizures[MeSH Terms]))) OR (((5‐HT1B receptor[MeSH Terms]) OR (5‐HT1B receptor)) AND ((((epilepsy) OR (epilepsy[MeSH Terms])) OR (seizures)) OR (seizures[MeSH Terms])))) OR (((5‐HT1D receptor) OR (5‐HT1D receptor[MeSH Terms])) AND ((((epilepsy) OR (epilepsy[MeSH Terms])) OR (seizures)) OR (seizures[MeSH Terms])))) OR (((5‐HT1E receptor) OR (5‐HT1E receptor[MeSH Terms])) AND ((((epilepsy) OR (epilepsy[MeSH Terms])) OR (seizures)) OR (seizures[MeSH Terms])))) OR (((5‐HT1F receptor) OR (5‐HT1F receptor[MeSH Terms])) AND ((((epilepsy) OR (epilepsy[MeSH Terms])) OR (seizures)) OR (seizures[MeSH Terms])))) OR (((5‐HT2A receptor) OR (5‐HT2A receptor[MeSH Terms])) AND ((((epilepsy) OR (epilepsy[MeSH Terms])) OR (seizures)) OR (seizures[MeSH Terms])))) OR (((5‐HT2B receptor) OR (5‐HT2B receptor[MeSH Terms])) AND ((((epilepsy) OR (epilepsy[MeSH Terms])) OR (seizures)) OR (seizures[MeSH Terms])))) OR (((5‐HT3 receptor) OR (5‐HT3 receptor[MeSH Terms])) AND ((((epilepsy) OR (epilepsy[MeSH Terms])) OR (seizures)) OR (seizures[MeSH Terms])))) OR (((5‐HT4 receptor) OR (5‐HT4 receptor[MeSH Terms])) AND ((((epilepsy) OR (epilepsy[MeSH Terms])) OR (seizures)) OR (seizures[MeSH Terms])))) OR (((5‐HT5 receptor) OR (5‐HT5 receptor[MeSH Terms])) AND ((((epilepsy) OR (epilepsy[MeSH Terms])) OR (seizures)) OR (seizures[MeSH Terms])))) OR (((5‐HT6 receptor) OR (5‐HT6 receptor[MeSH Terms])) AND ((((epilepsy) OR (epilepsy[MeSH Terms])) OR (seizures)) OR (seizures[MeSH Terms])))) OR (((5‐HT7 receptor) OR (5‐HT7 receptor[MeSH Terms])) AND ((((epilepsy) OR (epilepsy[MeSH Terms])) OR (seizures)) OR (seizures[MeSH Terms]))).
A review of the full text of the obtained articles was performed to exclude articles: (1) without the notion of the role of the 5‐hydroxytryptamine (5‐HT) receptor subtype in epilepsy and/or seizures and (2) studies that consisted of insufficient data to evaluate one or more 5‐HT receptor subtypes in epilepsy and/or seizures. Outcomes of interest were no, proconvulsive or anticonvulsive effects of modulating a distinct 5‐HT receptor subtype. In addition, other neurological features by stimulating or blocking the 5‐HT receptor subtypes were documented.
Our search has led to 229 publications during the last 20 years, of which 93 elaborated on the effects of distinct 5‐HT ligands and epilepsy/seizure treatment. Due to subsequent analyses of these publications' references, 81 other valuable articles were identified. Finally, this led to the inclusion of 174 articles about 5‐HT and seven articles about epilepsy and ASM (n = 181 total) (Figure S1: Citation flowchart of search strategy).”
3. RESULTS
3.1. 5‐HT receptors and epilepsy
Our search has led to 229 publications during the last twenty years, of which 93 elaborated on the effects of distinct 5‐HT ligands and epilepsy/seizure treatment. Due to subsequent analyses of these publications' references, 61 other valuable articles were identified. Finally, this led to the inclusion of 154 articles about 5‐HT and seven articles about epilepsy and ASM (n = 161 total).
Current research highlights the potential of modulating serotonergic transmission and targeting distinct serotonin (5‐HT) receptors in the treatment of epilepsy. 20 , 30 Consistently, 5‐HT is involved in different types of epilepsy, both in a preclinical and clinical setting. 31 This monoaminergic neurotransmitter, 5‐HT, affects numerous processes in the human body. During the late 1940s, 5‐HT was discovered in the blood causing vasoconstriction of blood vessels. 32 Soon thereafter, its presence was confirmed also in blood vessel walls, blood platelets, enterochromaffin cells, the lungs, and the heart. Even though the majority of 5‐HT is present in the gastrointestinal tract (90%, enterochromaffin cells), it is a key player in maintaining normal brain physiology. Hence, it is not surprising that defects in serotonergic transmission have been related to numerous neurological diseases, such as epilepsy and depression. 20 , 33 , 34 , 35
5‐HT‐related research has exploded since the discovery in 1940‐1950 resulting in successful approaches to characterize the different 5‐HT receptor subtypes. Already by 1980; 5‐HT1‐like, 5‐HT2, and 5‐HT3 were characterized. However, the classification has changed in the following years due to better insights into molecular biology and secondary pathways. For example, 5‐HT1C receptors are now referred to as 5‐HT2C sine they have 78% sequence homology with 5‐HT2 receptors. This receptor subtype is coupled to a phosphoinositol (PI) pathway, like the other two 5‐HT2 receptors (5‐HT2A and 5‐HT2B). Nowadays, receptor classification is based on similarities regarding structural (nucleotide and amino acid components), transduction (secondary pathways), and ligand‐binding profiles (drug‐related). Overall, 14 5‐HT receptor subtypes have been identified and are currently categorized into seven families (5‐HT1‐5‐HT7). 36 All 5‐HT receptor subtypes are G‐protein coupled receptors (GPCR), except the 5‐HT3 subtype. This latter receptor is a ligand‐gated sodium‐potassium channel, which causes depolarization of the cell membrane, that is, an excitatory effect. The other receptors are GPCR, that is, seven‐transmembrane receptors that activate intracellular second messenger cascades. Members of the 5‐HT1 family (5‐HT1A,1B,1D,1E,1F) and 5‐HT5 decrease adenylyl cyclase (AC) and subsequently cyclic adenosine monophosphate (cAMP) in the cell, causing inhibitory effects. The 5‐HT2 family (5‐HT2A,2B,2C) increases intracellular concentrations of inositol triphosphate (IP3) and diacylglycerol (DAG) by phospholipase C (PLC) activation, inducing excitation. The following subtype receptors increase AC: 5‐HT4, 5‐HT6, and 5‐HT7, and thus causing excitation as well. 36 There is evidence that targeting different 5‐HT receptors and/or affecting 5‐HT metabolism and transport could be efficacious in the treatment of epilepsy and its comorbidities (eg, depression). 20 , 21 , 31 , 33 , 37 , 38 In 2014, Gharedaghi and colleagues showed that stimulating 5‐HT1, 5‐HT2, or 5‐HT3 receptor subtypes produce anticonvulsive effects. Regarding inhibition of the 5‐HT4‐, 5‐HT6‐, or 5‐HT7 receptors, the debate is still ongoing and more data are needed regarding the pro‐ or anticonvulsive effects of the 5‐HT5 receptor. 18 , 22 , 31 In addition, various studies have used different compounds that are not always highly selective, different animal models, administration routes, and doses. 18 , 39 Moreover, controversial and sometimes contradictory findings were published. Thus, all these data should be interpreted with caution and require further research to determine which specific 5‐HT ligand(s) could be effective in treating a certain form of epilepsy/epilepsy syndrome or other (neurological) diseases (Table 1).
TABLE 1.
Serotonin (5‐HT) receptor modulation and their preclinical/clinical potential
| Improvement of (neurological) diseases and/or health features by | |||||
|---|---|---|---|---|---|
| 5‐HT receptor | Stimulation | Inhibition | Main reference(s) | ||
| Preclinical | Clinical | Preclinical | Clinical | ||
| 1A | Aggression, anxiety, craving, depression, epilepsy, impulsivity, sleep | TLE (?), anxiety, depression | Absence epilepsy, cognition | Depression | 43, 172 |
| 1B | Aggression, locomotor activity, sleep | — | — | — | 58, 172 |
| 1D | Depression, epilepsy | Migraine | — | — | 59, 172 |
| 1E | — | — | — | — | 64 |
| 1F | Migraine | — | — | — | 68 |
| 2A | Appetite, the absence epilepsy thermoregulation, sleep, SUDEP | Cognition | — | Psychosis, sleep | 70 |
| 2B | — | — | Cardiotoxicity, schizophrenia, drug addiction | Pulmonary hypertension | 94 |
| 2C | Appetite, (absence) epilepsy | Appetite, epilepsy | — | — | 83 |
| 3 | Epilepsy, SUDEP | — | Anxiety, cognition, depression, migraine, pain | Nausea, vomiting, psychosis | 173 |
| 4 | Cognition, depression epilepsy, SUDEP | Constipation, IBS, reflux | Anxiety, epilepsy | 117 | |
| 5 | Cognition | — | — | — | 120 |
| 6 | Depression | — | Cognition, depression, epilepsy | TLE (?) | 121 |
| 7 | Behavior, cognition, epilepsy, mood | — | Cognition, depression, epilepsy | — | 128 |
(?) indicates uncertain effects of pharmacological modulation of this receptor subtype. See text for all the references of these (pre)clinical studies.
Abbreviations: IBS, irritable bowel syndrome; OCD, obsessive‐compulsive behavior; sudden unexplained death in epilepsy patients (SUDEP); TLE, temporal lobe epilepsy.
3.1.1. 5‐HT1A receptors
5‐HT1A receptors are the most widely studied receptors in the 5‐HT research. Structurally, they differ significantly from the other 5‐HT receptors and show similarities to adrenergic receptors that potentially explain the high affinity of several adrenergic agents (eg, propranolol) to 5‐HT1A receptors.
Agonists of the 5‐HT1A receptor carry potential anxiolytic, antidepressant, anti‐epileptic, cognition‐enhancing, and neuroprotective effects. 40 , 41 , 42 , 43 Currently, several 5‐HT1A agonists are used in the clinic for the treatment of anxiety and depression, such as tandospirone and buspirone. 44 Moreover, several researchers suggest the involvement in addiction, alcoholism, behavior, impulsivity, and in the different phases of sleep. 36 , 45
Regarding epilepsy, some evidence shows that inhibition of the 5‐HT1A receptor is anticonvulsive in animal models of the absence epilepsy. For example, several 5‐HT1A antagonists reduced spike‐wave discharges in Wistar Albino Glaxo/Rijswijk (WAG/Rij) rats and in Groggy (GRY) rats, two validated genetic strains of the absence epilepsy. 31
In contrast, pharmacological 5‐HT1A stimulation could be involved in the anti‐epileptic mechanism of several known and novel compounds, such as 8‐OH‐DPAT, 46 pyrrolidin‐2‐one derivatives, 47 cannabidiol (CBD), 48 , 49 and curcumin. 50 Also, endogenous substances, such as hormones, can interact with 5‐HT1A receptors as suggested by the cross‐talk between estrogenic and serotonergic (5‐HT1A and 5‐HT3) pathways. 51 Finally, it could increase the seizure threshold in other seizure types as reviewed by Gharedaghi and colleagues. 18 In addition, 5‐HT1A activation can be involved in non‐pharmacological therapies, such as the preclinically used low‐frequency stimulation (LFS) and nervus vagus stimulation (VNS) in the clinical setting. LFS showed inhibitory activity against seizures in amygdala‐kindled rats and was counteracted by a selective 5‐HT1A antagonist. 52 VNS can enhance tonic forebrain activation of postsynaptically located 5‐HT1A receptors, 53 although its role in epilepsy has not been clarified. 5‐HT1A receptors can play a role in status epilepticus,, 54 in epileptogenesis, 55 and in patients with temporal lobe epilepsy (TLE) having decreased 5‐HT1A receptor availability. 31 , 56 5‐HT1A gene polymorphisms can also contribute to the psychiatric comorbidities in TLE patients, indicating a potential role of this receptor subtype in TLE. 57
Thus, current data suggest a beneficial role of 5‐HT1A stimulation in most preclinical epilepsy models (except the absence epilepsy) and patients with TLE. Moreover, anxiety‐reducing effects have been reported in a patient with Angelman syndrome (AS) by buspirone. 42
3.1.2. 5‐HT1B and 5‐HT1D receptors
Most of the 5‐HT1B receptors are located postsynaptically, although some of them are presynaptically where they are involved in the 5‐HT release. Similarities to 5‐HT1D receptors for both location and structure have impeded examining the functional role of 5‐HT1B receptors and showing overlap with 5‐HT1D receptors. Recent studies with newer 5‐HT1B ligands, showing high selectivity to 5‐HT1B receptors compared to 5‐HT1D receptors, suggest a role in behavior, locomotor activity, and sleep regulation. 58 Consistently, 5‐HT1B receptor KO mice show aggressive behavior and locomotor impairments. Of interest, these KO mice did not show an epileptic phenotype and several 5‐HT1B ligands did not affect seizure activity in animal models. Overall, no straightforward data demonstrate an anticonvulsant role of 5‐HT1B agonists in epilepsy. 36
5‐HT1D receptors show a wide distribution throughout the CNS and preclinical research suggests a role in anxiety, depression, and brain disorders (like migraine and Huntington's disease). 36 , 59 Whereas 5‐HT1D agonists are potentially antidepressants more research is needed to determine whether agonists or antagonists could be efficacious in other brain disorders. 36
Only a few studies are in favor of 5‐HT1B/1D agonism for potential epilepsy treatment. Several studies using the drug‐resistant DS zebrafish model showed that 5‐HT1D agonists significantly reduced seizures. 13 , 60 , 61 Interestingly, triptans that are already on the market for the treatment of migraine, showed locomotor reducing activity in two zebrafish models of chemically induced seizures 62 and a chemically induced seizure mouse model (pentylenetetrazol, PTZ). 61 Last but not least, one of the 5‐HT1D agonists used in the zebrafish model and another triptan (zolmitriptan) were also effective in a mice model of DS and even significantly improved survival of these mice. 63 The aforementioned data underline the possibility of ameliorating drug‐resistant seizures and increasing survival in DS by 5‐HT1D agonism. Overall, further research is needed to investigate the potential role of 5‐HT1B/1D agonism in the treatment of epilepsy and potential some of its comorbidities such as migraine.
3.1.3. 5‐HT1E and 5‐HT1F receptors
As members of the 5‐HT1 family, these receptors are negatively coupled to AC, although the coupling to AC can be achieved by distinct pathways for the 5‐HT1E receptor, determined by the density and cellular environment of the receptors. Structurally, the 5‐HT1F receptor is most closely related to the 5‐HT1E receptor with nearly 60% amino acid homology. In addition, there are several similar pharmacological characteristics. 36 , 64 Thus, one could expect comparable physiological effects and clinical significance for these two receptor subtypes. Consistently, 5‐HT1E and 5‐HT1F agonists have been suggested in the treatment of memory impairments, 65 , 66 though only 5‐HT1F agonists are under clinical evaluation for treating migraine. 67 , 68 , 69
5‐HT1E receptors are highly present in the olfactory bulb glomeruli (Table S2), the molecular layer of the dentate gyrus (DG), and the adventitial layer of cerebral arteries. These receptors have a more dominant expression in neurons, compared to glia cells. Agonists of 5‐HT1E receptors inhibit AC activity in the DG, thereby modulating hippocampal activity, which makes these agonists a potential drug for the treatment of TLE since hyperactivity in the hippocampus has been linked to TLE. 66
5‐HT1F receptors show a similar expression profile as 5‐HT1E receptors. Stimulation of this receptor subtype is assumed to inhibit impulses of the trigeminal nerves, hyperpolarizing nerve terminals. Therefore, 5‐HT1E agonists, the “Ditans,” are currently being investigated for migraine treatment. 68 , 69 Nonetheless, we cannot confirm that this receptor subtype would modulate seizures based on the current knowledge.
3.1.4. 5‐HT2 receptors
A lot has changed since the initial identification of 5‐HT2 receptors. The primarily CNS‐located 5‐HT2 receptors were later renamed to 5‐HT2A receptors, together with the discovery of the 5‐HT2B receptors that are predominantly distributed in the peripheral system. In addition, the 5‐HT1C receptors were called 5‐HT2C receptors due to similarities in structure and secondary pathways, for example, PLC activation. 36 In the past decades, numerous selective agents have been developed that are able to discriminate between these three subpopulations, resultantly making it possible to examine their distinct clinical significance. 70 In vitro data show that augmented serotonin increases cortical excitation through activation of 5‐HT2 and 5‐HT3 receptors, 71 suggesting that antagonism of these receptors would induce anti‐epileptic effects. Clinical data underline the potential of 5‐HT2 and 5‐HT3 antagonism, by mirtazapine, in the treatment of sleep disturbances in patients with AS. 72 As delineated below, subtypes of the 5‐HT2 receptors (5‐HT2A, ‐2B, and ‐2C) can play a crucial role in current and future epilepsy treatment.
In essence, compelling preclinical and clinical evidence indicates that 5‐HT2A/2C stimulation leads to antiseizure activity and could ameliorate epilepsy‐related comorbidities, for example, depression and SUDEP. In clear contrast, only one zebrafish research group suggested the anticonvulsive role of 5‐HT2B stimulation. 73 In addition, the prominent expression of this receptor subtype in the heart 74 and related cardiotoxic effects 75 , 76 , 77 , 78 underscore that the 5‐HT2B subtype is not an interesting target for epilepsy treatment. 21 , 79
3.1.5. 5‐HT2A receptors and 5‐HT2C receptors
These receptors are present in the brain and the highest densities are found in the neocortex. As described for the 5‐HT1E receptor, two pathways can be activated depending on the location and cellular environment of the 5‐HT2A receptor. 36 These distinct pathways can explain the hallucinogenic properties of some 5‐HT2A agonists (mainly activating arachidonic acid pathways, eg, LSD) and the absence of hallucinations by other 5‐HT2A agonists (mainly affecting phosphoinositide signaling, eg, lisuride). 80 , 81 Structurally, the 5‐HT2A receptor is very closely related to the 5‐HT2C receptor with almost 80% homology in the transmembrane (TM) portions, possibly explaining some binding overlap of ligands for both receptor subtypes. As a consequence, many clinical effects can involve both 5‐HT2A and 5‐HT2C receptors; such as appetite control, thermoregulation, locomotor activity, and sleep. In addition, several researchers suggested these receptors to be promising targets for antidepressants, antipsychotics, and definitely antiepileptic drugs. 33 , 36 , 78 , 82 , 83 , 84 A review by Guiard and Di Giovanni thoroughly describes the controversial role of 5‐HT2A receptors in epilepsy wherein proconvulsant properties are likely to be attributed to the use of high doses of 5‐HT2A ligands and/or off‐target effects by modulating other receptors. Of interest, FA is not only increasing 5‐HT in the synaptic cleft (indirect) but also directly targeting 5‐HT2A (and 5‐HT2C) receptors. 76
Regarding the absence epilepsy, stimulation of the 5‐HT2A and/or 5‐HT2C receptor could be beneficial since it inhibits the rhythmic thalamic burst firings which are likely to be the electrical burst origin of the absence epilepsy. Consistently, several 5‐HT2A agonists show promise in treating atypical the absence seizures and 5‐HT2A antagonists increase the severity of seizures 21 , 85 and diminished the anti‐epileptic effect of FA in several preclinical models. 15 , 16
Furthermore, the 5‐HT2A receptor regulates mood 33 and modulates CO2‐induced arousal and stimulation of this receptor subtype can rescue animals from SUDEP. 29 , 86 , 87 These findings underline that 5‐HT2A agonists can decrease epilepsy and ameliorate its comorbidities such as depression and SUDEP.
The 5‐HT2C receptor is likely to be involved in the epileptiform activity as well since 5‐HT2C KO mice display an epileptic phenotype and 5‐HT2C antagonists worsen the seizure phenotype 88 , 89 and can counteract the anti‐epileptiform activity of FA. 13 Additionally, 5‐HT2C agonists are anticonvulsive in models of atypical absences, 21 acute seizure models, 83 , 84 drug‐resistant seizures in the zebrafish model of DS, 13 , 28 and have been used in human studies to treat drug‐resistant epilepsy. 90 , 91 Nonetheless, there are few studies that demonstrate no antiseizure effects by 5‐HT2A and/or 5‐HT2C agonism. 18 Moreover, overstimulation of these receptors can be proconvulsive 92 and 5‐HT2A antagonism showed antiseizure effects in one rodent epilepsy model. 93 In addition, 5‐HT2A antagonism had a positive effect on short‐term memory, which possibly expands the role of modulating this receptor subtype in other diseases, beyond epilepsy. 18
3.1.6. 5‐HT2B receptors
Even as 5‐HT2B receptors exhibit almost 70% homology to the other two 5‐HT2 receptor subtypes, their expression profile is nearly absent in the brain and appears to be mainly involved in vasoconstrictive effects in the vascular and cardiac system. 74 , 75 , 77 Hence, it is not surprising that only scanty data are available regarding the role of 5‐HT2B receptors in neurological disorders.
Recent rodent data indicate that 5‐HT2B antagonists hold promise for treating schizophrenia and drug addiction, due to the interaction of 5‐HT2B and dopamine. 94 Additionally, three studies suggest the role of 5‐HT2B in epilepsy treatment. First, in a PTZ‐kindling rat model of chronic epilepsy increased immunoreactivity of the 5‐HT2B receptor was found in the cortex and medulla, while it was decreased in the hippocampus. However, further behavioral/functional studies are necessary to elucidate the meaning of this immunoreactivity alteration. 95 Second, the novel ASM, CBD, reduced seizures in pilocarpine‐induced SE in rats that was attributed to both CB1 and 5‐HT2B receptors, 96 although 5‐HT2B receptors are probably not involved in CBD's mechanism of action as shown by Dos Santos et al 48 and Pelz et al 97 Third, Baraban et al showed that 5‐HT2B agonists can reduce seizures in the zebrafish DS model. Nonetheless, numerous other researchers did not observe any seizure reduction by 5‐HT2B modulation in different animal models of epilepsy. 18 , 29 , 63 , 84 , 89 , 90 , 98 , 99 , 100 , 101 , 102 , 103 , 104 , 105 , 106 For example, Sourbron et al did not demonstrate any beneficial effect of several 5‐HT2B agonists in the aforementioned zebrafish DS model 13 , 28 and 5‐HT2B antagonism was not able to counteract the anti‐epileptiform activities of the serotonergic drug, FA, in this DS model. 13 Therefore, FA is unlikely to be anti‐epileptic through 5‐HT2B agonism. Nevertheless, the N‐dealkylated metabolite of FA, norfenfluramine (NORFA), displays higher affinity and activity at the 5‐HT2B and 5‐HT2C receptors. This activation of 5‐HT2B receptors is associated with cardiac valve hypertrophy, and the drug‐induced valvulopathy has resulted in the withdrawal of FA from the market in the 1990s. 107 Even though drug‐induced valvulopathy could lead to pulmonary hypertension (PH), clinical trials with lower dose FA monitor cardiac side effects and until now its safety has been guaranteed. 108 In contrast, 5‐HT2B antagonists are a potential novel therapeutic target for treating PH, for example, terguride, a potent 5‐HT2B antagonist, is being investigated as a PH treatment. 109
3.1.7. 5‐HT3 receptors
5‐HT3 receptors are expressed in the peripheral nervous system but also in the CNS. From a clinical perspective, 5‐HT3 antagonists have shown efficacy in treating nausea and vomiting, if induced by chemotherapy or radiation but not if it is triggered by motion sickness or apomorphine. 36 Moreover, clinical data demonstrate its use in migraine treatment and preclinical studies showed a potential of 5‐HT3 ligands in anxiety, cognition, depression, dementia, memory enhancement, and psychosis. The effect on seizures is in ongoing debate and some authors suggest that this receptor is not involved. Most preclinical data are in favor of 5‐HT3 stimulation to suppress seizures 18 , 110 ; for example, 5‐HT3 antagonists increase the frequency of hippocampal theta bursts, which is related to generalized tonic‐clonic seizures and eliminated the anticonvulsive properties of 5‐HT3 agonists. 111 , 112 In addition, 5‐HT3 agonism blocked seizure‐induced respiratory arrest in a mouse model of SUDEP 100 and alleviated acute seizure activity 103 and in PTZ‐kindling in mice. 113 Even as the 5‐HT3 receptor is an excitatory receptor it is mainly located on inhibitory interneurons (in cortex and hippocampus) leading to hyperpolarisation and thus less excitation in the brain, comparable to the 5‐HT2A/2C receptors. 21 Nevertheless, it can cause NO production by activation of neuronal nitrite oxide synthase, which is potentially proconvulsive in several seizure models. 114 This could explain that the 5‐HT3 antagonism decreases seizures in PTZ‐kindled mice 102 and that ondansetron, a 5‐HT3 antagonist is anticonvulsant in the MES test. 18 , 22 Lamotrigine, an ASM already available on the market, acts on sodium and calcium channels but also inhibits 5‐HT3‐activated currents. 115 Until now, it is unknown if this latter pathway is involved in its anti‐epileptic activity.
Altogether, most data attribute an anticonvulsive role to 5‐HT3 stimulation, especially in models for generalized seizures, for example, acute PTZ and PTZ kindling murine models. 31 , 103 Recent data even show that the anticonvulsant effect of various SSRIs involves 5‐HT3 stimulation. 113 In conclusion, 5‐HT3 agonists could be interesting in ASM development although side effects can be anticipated due to stimulation of the chemoreceptor trigger zone that can cause bradycardia, nausea, and vomiting. 31
3.1.8. 5‐HT4 receptors
5‐HT4 receptors have an extended tissue distribution and play a role in the slow excitatory responses to 5‐HT in neurons. Structurally, there is some overlap between 5‐HT4 and 5‐HT3 ligands. Currently, research is focused on both central and peripheral effects of 5‐HT4 ligands in diseases, such as addiction, anxiety, cognition, irritable bowel syndrome, and gastroesophageal reflux. 36 , 116 , 117 Available data are very limited in the epilepsy field, although the majority is in favor of 5‐HT4 antagonists as potential anticonvulsant treatment. These compounds nullified epileptiform spikes, induced by 5‐HT4 agonists, and reduced forelimb clonus in amygdala‐kindled rats. 18 In contrast, 5‐HT4 stimulation increases GABA inhibitory currents in the hippocampal dentate gyrus of guinea pigs, 118 5‐HT4 KO mice experience more aggravated PTZ‐induced seizures, compared to WT mice, 119 and recent data show that FA prevented SUDEP and reduced seizures by 5‐HT4 receptor stimulation, although 5‐HT2 and 5‐HT7 receptors could be involved as well. 16 In conclusion, data are contradictory and future preclinical studies should elaborate on the exact role of 5‐HT4 receptors in different seizure models.
3.1.9. 5‐HT5 receptors
5‐HT5 receptors are structurally unrelated to other 5‐HT receptor subtypes, however, they share some pharmacological properties with 5‐HT1D receptors. Not much is known about the clinical significance but based on their localization (cortex, astrocytes); suggestions have been made regarding anxiety, brain development, cognition, depression, feeding, and locomotor activity. 120 It is currently not known if these receptors are involved in epileptogenesis and/or epilepsy. 18 , 31
3.1.10. 5‐HT6 receptors
The clinical significance of the 5‐HT6 receptors subtype is currently unknown but it appears to be involved in several neuropsychiatric processes (depression, psychosis, and obsessive‐compulsive behavior) and recent evidence underlined a procognitive role of both 5‐HT6 agonists and antagonists. 121 Even though data regarding the role of this receptor subtype in epilepsy are very limited, the beneficial effects of highly selective 5‐HT6 antagonists are relatively more robust in animal models of seizures 122 , 123 , 124 and mossy fiber sprouting, 125 in contrast to 5‐HT6 agonists. Spontaneous seizures in the post‐SE pilocarpine rat model were reduced after treatment with a highly selective 5‐HT6 antagonist. In addition, 5‐HT6 receptor expression was upregulated in the hippocampus and neocortex of these post‐SE rats. 31 , 105 , 126 In line with these findings, clinical data of patients with drug‐resistant TLE show an upregulation of this receptor as well. 105 Thus, current data favor a proconvulsive role of the 5‐HT6 receptor.
3.1.11. 5‐HT7 receptors
Due to the wide distribution of 5‐HT7 receptors in the CNS, it is not surprising that it can be involved in several neurological processes and pathophysiology. Structurally, there is less than 50% TM sequence homology between this and the other 5‐HT receptor subtypes. This receptor affects cognitive processes, mood, circadian rhythm, and the relaxation of coronary arteries. Consequently, 5‐HT7 ligands could be effective in treating memory impairments, behavioral dysfunction, sleep disorders of circadian nature, and coronary heart disease. 127 , 128 Regarding epilepsy, most researchers are in favor of a proconvulsive role of the 5‐HT7 receptor. For example, numerous 5‐HT7 antagonists were proven to be anticonvulsant in different animal models of seizures like the pilocarpine rat model of TLE, WAG/Rij rats, and the DBA/2 J mice model of the absence epilepsy. 18 , 129 This latter finding could be attributed to the fact that the thalamus, considered to be the origin of electrical discharges in the absence epilepsy is enriched with 5‐HT7 binding sites. However, 5‐HT7 agonism decreased seizures in mice picrotoxin‐induced seizure 130 and partially rescued the brain anomalies and epileptic phenotype in Cdkl5 KO mice. 131 In addition, 5‐HT7 KO mice have decreased seizure thresholds for electrical and chemical‐induced seizures. 132
Overall, several experimental data are in favor of a proconvulsive role of the 5‐HT7 receptor, in line with patient data with drug‐resistant TLE that have an upregulated expression of 5‐HT7 receptors in the neocortex, 106 although the exact role is not uniform. 133
3.2. 5‐HT system and epilepsy, comorbidities and mortality
Involvement of the 5‐HT system in epilepsy, comorbidities, and mortality has been suggested by several researchers 20 , 21 , 22 , 23 , 26 , 27 , 30 , 38 , 134 (Figure 1) and until now FA is potentially the only ASM modulating the 5‐HT system (Figure 2). The importance of shared risk factors and especially genetics appear to be key players. 134 Some even hypothesize that the cause could directly be related to 5‐HT system impairments. For instance, a patient with a de novo mutation in the sodium voltage‐gated channel alpha subunit (SCN2A) had drug‐resistant epilepsy that responded to treatment with the 5‐HT precursor, 5‐ hydroxytryptophan (5‐HTP). 135 In addition, decreased hippocampal 5‐HT levels were observed in patients with TLE. 136 Preclinical evidence is even more prominent showing, for example, that 5‐HTP was anticonvulsant in drosophila with an SCN1A mutation. 137 In addition, a zebrafish model of DS (scn1a mutation) demonstrated a lower 5‐HT brain content that could be related to the scn1a mutation. 60
FIGURE 1.
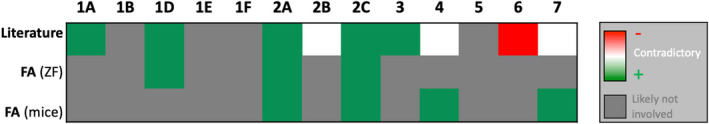
Fourteen serotonin (5‐HT) receptor subtypes. Stimulation (+) of several receptor subtypes (5‐HT1A,1D,2A,2C,3) and inhibition (−) of the 5‐HT6 subtype have been implicated in antiseizure activity (row literature). For the 5‐HT2B,4,7 subtypes data are contradictory, depicted in white. 5‐HT1D,2A,2C subtypes are likely involved in the mechanism of fenfluramine (zebrafish (ZF) data). 5‐HT2A,2C,4,7 subtypes are likely involved in the mechanism of fenfluramine (mice data). See text for the references of these preclinical studies
FIGURE 2.
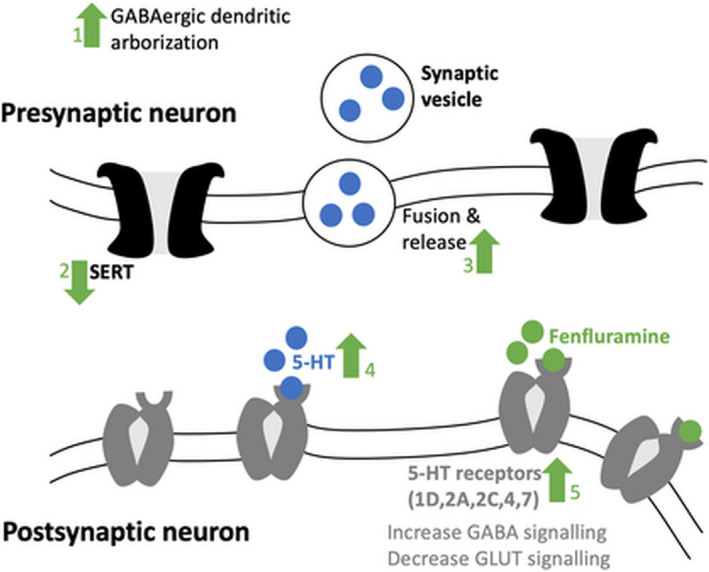
Serotonergic mechanisms of action of fenfluramine: (1) increase of GABAergic dendritic arborization via 5‐HT and GABAergic activity 168 ; (2) decrease of serotonin reuptake by inhibition of SERT 169 ; (3) increase of fusion and release of synaptic vesicles 170 ; (4) the two previous modulatory lead to an increase of 5‐HT in the synaptic cleft and thereby stimulation of 5‐HT receptor subtypes; and (5) fenfluramine directly stimulates at least five serotonin (5‐HT) receptor subtypes (5‐HT1D,2A,2C,4,7) (zebrafish and mice data), 13 , 171 thereby increasing gamma‐aminobutyric acid inhibitory input and decreasing glutaminergic excitatory output. Regarding the sigma receptor modulation, we refer to Martin et al 2020. 12 5‐HT = serotonin; GABA = gamma aminobutyric acid; GLUT = glutamine; SERT = serotonin transporter
For numerous epilepsy‐associated problems, the 5‐HT system seems to be involved and reduced 5‐HT transmission seems to negatively impact several epilepsy comorbidities, such as motor functions, 138 behavior, 139 depression, migraine, and cognitive impairments. 140 Regarding depression, a plethora of evidence underlines the antidepressant activity of 5‐HT and effective depression therapy with SSRIs. 38 Interestingly, a relation between epilepsy and depression was linked to 5‐HT1A 141 , 142 and 5‐HT2A 33 receptors.
With regards to migraine, experimental and patient evidence exists regarding the efficacy of agonists for the 5‐HT1B, 5‐HT1D, and 5‐HT1F receptors. More recent yet limited data demonstrate anti‐migraine activities for 5‐HT2B and 5‐HT7 antagonists in animal models of migraine. 143 The link between 5‐HT and cognition has been extensively studied and 5‐HT1A, 5‐HT1B, 5‐HT2A, 5‐HT2C, 5‐HT3, 5‐HT4, 5‐HT6, and 5‐HT7 ligands show preclinical and clinical efficacy in treating cognitive defects. Nevertheless, these data are limited and focus on the older population and patients with AD and schizophrenia. Moreover, controversial findings impede making a general conclusion toward the potential of certain 5‐HT agonists or antagonists in treating cognitive defects. 144
SUDEP applies to death in PWE that is not related to known causes like injury and drowning. Studies imply that the overall risk for SUDEP is greater than 0.1% in the general epilepsy population although estimates vary significantly. 145 Moreover, SUDEP is one of the most severe consequences for patients with drug‐resistant epilepsy and SUDEP appears to be the major cause of death in patients with drug‐resistant DS. 146 , 147 Currently, the exact mechanisms SUDEP are unknown and the majority of research points out that SUDEP can be the result of respiratory dysfunction that is immediately followed by a seizure. 148 , 149 Several experimental studies suggest the importance of 5‐HT in SUDEP. For example, 5‐HT2C KO mice have spontaneous seizures and earlier mortality due to respiratory arrest, compared to WT mice. 89 In addition Lmx1b KO, mice lacking the development of serotonergic neurons, have a relatively higher seizure threshold and higher chance to die from respiratory failure that was prevented by stimulating 5‐HT2A receptors. 29 Moreover, human studies have shown that many PWE can have hypoxia after a seizure, 150 which can be reduced by taking SSRIs, increasing 5‐HT in the synaptic cleft. 151 The overall importance of 5‐HT in SUDEP can be related to the 5‐HT‐dependent regulation of breathing and sleep arousal to keep normal blood CO2 and pH values. 152 Recently, Cross and colleagues showed that the all‐cause and SUDEP mortality rates during FA treatment of patients with DS significantly decreased (1.7/1000 person‐years), compared to literature reports (9.3‐15.8/1000 person‐years). 153
3.3. 5‐HT system and cardiovascular side effects
Serotonergic drugs that directly or indirectly lead to 5‐HT2B receptor activation are indeed associated with cardiac valve hypertrophy. Stimulation of 5‐HT2B receptors, which are GPCR (Table S2), leads to activation of PLC that subsequently activates protein kinase C (PKC). PKC mobilizes intracellular calcium and DAG. Via other, yet to be explored, pathways this GPCR can also induce Src phosphorylation and activation of extracellular regulated kinases (ERK1/2). Moreover, phosphorylated Src modulates the transforming growth factor β (TGF‐β) receptor, enhancing the 5‐HT2B‐stimulated mitogenesis that involves the phosphorylation of retinoblastoma protein (Rb‐P). Moreover, the PKC and ERK1/2 similarly modulate Rb‐P leading to excessive mitogenesis, thereby causing overgrowth valvulopathy and valvular dysfunction. Hence, drugs that stimulate the 5‐HT2B receptor could induce cardiac valvulopathies. 76 , 154 , 155
Fenfluramine was initially used as an anti‐obesity drug but was withdrawn from the market due to drug‐induced valvulopathy that was related to abuse, use of other amphetamine‐like drugs and/or high doses. 75 Even though several preclinical data show that 5‐HT2B stimulation is not mandatory for a seizure reduction by FA, FA can increase 5‐HT and thereby indirectly stimulate 5‐HT2B receptors. Fortunately, much lower dosages are used in the clinic and clinical trials nowadays 156 , 157 and after more than 3 years of treatment with low‐dose FA, no cardiotoxic events have been observed. 158 These data, together with the durability and magnitude of FA's reduction of drug‐resistant seizures, 159 strongly suggest that significant benefits of FA could outweigh potential cardiac risks. Regarding other, possibly serotonergic side effects, clinical trials mainly report decreased appetite (potential role of 5‐HT2C) and somnolence (5‐HT?). 160 , 161 , 162
4. CONCLUSION
Antiseizure medication development has been focusing on neurotransmitters and ion channels involved in excitatory and inhibitory neurotransmission. 163 In the last decade, research implies that the complex variety of pathways involved in epilepsy, such as serotonergic (5‐HT) transmission, are neglected by this simplistic view. Nonetheless, the exact role of each serotonin (5‐HT) receptor subtype remains elusive, in part due to contradictory findings. 18 , 31
Our review underlines that most evidence is in favor of 5‐HT1A,1D,2A,2C,3 agonism and 5‐HT6 antagonism to treat epilepsy. Even though the role of the other receptor subtypes is unclear, one should be cautious to generalize these findings and discrepancies might arise due to a number of factors, for example, the difference in animal seizure models and the difference of compounds and their doses. 18 , 164
Interestingly, serotonergic ASMs are currently under development for rare, severe epilepsy syndromes and one of them, FA, showed promising results by numerous clinical studies. 9 , 11 , 108 , 165 , 166 , 167 Even as FA likely displays (part of its) anti‐epileptic activity via sigma1 (σ1) receptors, 12 , 14 other zebrafish and mice studies have shown that FA‐induced 5‐HT1D,2A,2C agonism plays a crucial role in its antiseizure activity 13 , 15 and even 5‐HT4,7 agonism has been suggested by one study. 17 Serotonergic agonism of 5‐HT2A,2C,4 receptors can also ameliorate epilepsy‐related mortality (SUDEP). 16 , 87
In conclusion, the available research strongly suggests that serotonergic modulation, especially stimulation, should be a novel avenue for future ASMs to treat epilepsy and its comorbidities.
CONFLICT OF INTEREST
LL received grants, and is a consultant and/or speaker for Zogenix; LivaNova, UCB, Shire, Eisai, Novartis, Takeda/Ovid, NEL, Epihunter. LL has a patent for ZX008 (fenfluramine) for the treatment of Dravet syndrome and infantile epilepsies assigned to his institution and licensed to Zogenix. The remaining author (JS) has no conflict of interest to disclose. We confirm that we have read the Journal's position on issues involved in ethical publication and affirm that this report is consistent with those guidelines.
Supporting information
Supplementary Material
Sourbron J, Lagae L. Serotonin receptors in epilepsy: Novel treatment targets? Epilepsia Open. 2022;7:231–246. 10.1002/epi4.12580
REFERENCES
- 1. Beghi E. The epidemiology of epilepsy. Neuroepidemiology. 2020;54(2):185–91. [DOI] [PubMed] [Google Scholar]
- 2. Löscher W, Klitgaard H, Twyman RE, Schmidt D. New avenues for anti‐epileptic drug discovery and development. Nat Rev Drug Discov. 2013;12(10):757–76. [DOI] [PubMed] [Google Scholar]
- 3. Sills GJ, Rogawski MA. Mechanisms of action of currently used antiseizure drugs. Neuropharmacology. 2020;168:107966. 10.1016/j.neuropharm.2020.107966. Epub. [DOI] [PubMed] [Google Scholar]
- 4. Rogawski MA, Löscher W. The neurobiology of antiepileptic drugs. Nat Rev Neurosci. 2004;5(7):553–64. 10.1038/nrn1430 [DOI] [PubMed] [Google Scholar]
- 5. Kwan P, Arzimanoglou A, Berg AT, Brodie MJ, Allen Hauser W, Mathern G, et al. Definition of drug resistant epilepsy: consensus proposal by the ad hoc Task Force of the ILAE Commission on Therapeutic Strategies. Epilepsia. 2010;51(6):1069–77. [DOI] [PubMed] [Google Scholar]
- 6. Meekings KN, Williams CSM, Arrowsmith JE. Orphan drug development: an economically viable strategy for biopharma R&D. Drug Discov Today. 2012;17(13–14):660–4. [DOI] [PubMed] [Google Scholar]
- 7. Binder DK, Boison D, Eid T, Frankel WN, Mingorance A, Smith BN, et al. Epilepsy benchmarks area II: prevent epilepsy and its progression. Epilepsy Curr. 2020;20(1_suppl):14S–22. 10.1177/1535759719895274 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Schoonjans A‐S, Ceulemans B. An old drug for a new indication: repurposing fenfluramine from an anorexigen to an antiepileptic drug. Clin Pharmacol Ther. 2019;106(5):929–32. [DOI] [PubMed] [Google Scholar]
- 9. Lagae L, Schoonjans A‐S, Gammaitoni AR, Galer BS, Ceulemans B. A pilot, open‐label study of the effectiveness and tolerability of low‐dose ZX008 (fenfluramine HCl) in Lennox‐Gastaut syndrome. Epilepsia. 2018;59(10):1881–8. 10.1111/epi.14540 [DOI] [PubMed] [Google Scholar]
- 10. Gogou M, Cross JH. Fenfluramine as antiseizure medication for epilepsy. Dev Med Child Neurol. 2021;63(8):899–907. [DOI] [PubMed] [Google Scholar]
- 11. Geenen KR, Doshi SP, Patel S, Sourbron J, Falk A, Morgan A, et al. Fenfluramine for seizures associated with Sunflower syndrome. Dev Med Child Neurol. 2021;63(12):1427–32. [DOI] [PubMed] [Google Scholar]
- 12. Martin P, de Witte PAM, Maurice T, Gammaitoni A, Farfel G, Galer B. Fenfluramine acts as a positive modulator of sigma‐1 receptors. Epilepsy Behav. 2020;105:106989. [DOI] [PubMed] [Google Scholar]
- 13. Sourbron J, Smolders I, de Witte P, Lagae L. Pharmacological analysis of the anti‐epileptic mechanisms of fenfluramine in scn1a mutant zebrafish. Front Pharmacol. 2017;8:191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Martin P, Reeder T, Sourbron J, de Witte PAM, Gammaitoni AR, Galer BS. An emerging role for sigma‐1 receptors in the treatment of developmental and epileptic encephalopathies. Int J Mol Sci. 2021;22(16):8416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Rodríguez‐Muñoz M, Sánchez‐Blázquez P, Garzón J. Fenfluramine diminishes NMDA receptor‐mediated seizures via its mixed activity at serotonin 5HT2A and type 1 sigma receptors. Oncotarget. 2018;9(34):23373–89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Faingold C, Tupal S. The action of fenfluramineto prevent seizure‐induced death in the DBA/1 mouse SUDEP model is selectively blocked by an antagonist or enhanced by an agonist for the serotonin 5‐HT4 receptor [Internet]. 2019. Available from https://www.zogenix.com/wp‐content/uploads/2019/12/04.‐FINAL‐52353‐Tupal‐Faingold‐AES‐poster‐2019‐11‐27.pdf
- 17. Parthena M, Boyd B, Gail F, Boyd B, Galer B. An examination of the mechanism of action of fenfluramine in Dravet syndrome: A look beyond serotonin [Internet]. 2016. Available from http://www.zogenix.com/c/newsroom/publications‐presentations.php
- 18. Gharedaghi MH, Seyedabadi M, Ghia J‐E, Dehpour AR, Rahimian R. The role of different serotonin receptor subtypes in seizure susceptibility. Exp Brain Res. 2014;232(2):347–67. [DOI] [PubMed] [Google Scholar]
- 19. Löscher W. Fit for purpose application of currently existing animal models in the discovery of novel epilepsy therapies. Epilepsy Res. 2016;126:157–84. [DOI] [PubMed] [Google Scholar]
- 20. Deidda G, Crunelli V, Di Giovanni G. 5‐HT/GABA interaction in epilepsy. Prog Brain Res. 2021;259:265–86. [DOI] [PubMed] [Google Scholar]
- 21. Venzi M, David F, Bellet J, Cavaccini A, Bombardi C, Crunelli V, et al. Role for serotonin2A (5‐HT2A) and 2C (5‐HT2C) receptors in experimental absence seizures. Neuropharmacology. 2016;108:292–304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Bagdy G, Kecskemeti V, Riba P, Jakus R. Serotonin and epilepsy. J Neurochem. 2007;100(4):857–73. [DOI] [PubMed] [Google Scholar]
- 23. De Deurwaerdere P, Di Giovanni G. 5‐HT interaction with other neurotransmitters: an overview. Prog Brain Res. 2021;259:1–5. [DOI] [PubMed] [Google Scholar]
- 24. Lei S. Serotonergic modulation of neural activities in the entorhinal cortex. Int J Physiol Pathophysiol Pharmacol. 2012;4(4):201–10. [PMC free article] [PubMed] [Google Scholar]
- 25. Chugani DC. Serotonin in autism and pediatric epilepsies. Ment Retard Dev Disabil Res Rev. 2004;10(2):112–6. [DOI] [PubMed] [Google Scholar]
- 26. Theodore WH. Does serotonin play a role in epilepsy? Epilepsy Curr. 2003;3(5):173–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Crunelli V, Carmignoto G, Steinhauser C. Novel astrocyte targets: new avenues for the therapeutic treatment of epilepsy. Neuroscientist. 2015;21(1):62–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Sourbron J, Schneider H, Kecskés A, Liu Y, Buening EM, Lagae L, et al. Serotonergic modulation as effective treatment for Dravet syndrome in a zebrafish mutant model. ACS Chem Neurosci. 2016;7(5):588–98. [DOI] [PubMed] [Google Scholar]
- 29. Buchanan GF, Murray NM, Hajek MA, Richerson GB. Serotonin neurones have anti‐convulsant effects and reduce seizure‐induced mortality. J Physiol. 2014;592(Pt 19):4395–410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Gilliam FG, Hecimovic H, Gentry MS. Serotonergic therapy in epilepsy. Curr Opin Neurol. 2021;34(2):206–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Svob Strac D, Pivac N, Smolders IJ, Fogel WA, De Deurwaerdere P, Di Giovanni G. Monoaminergic mechanisms in epilepsy may offer innovative therapeutic opportunity for monoaminergic multi‐target drugs. Front Neurosci. 2016;10:1–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Rapport M, Green A, Page I. Serum vasoconstrictor, serotonin; isolation and characterization. J Biol Chem. 1948;176(3):1243–51. [PubMed] [Google Scholar]
- 33. Guiard BP, Di GG. Central serotonin‐2A (5‐HT2A) receptor dysfunction in depression and epilepsy: the missing link? Front Pharmacol. 2015;6:46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Di Giovanni G. Serotonin in the pathophysiology and treatment of CNS disorders. Exp Brain Res. 2013;230(4):371–3. [DOI] [PubMed] [Google Scholar]
- 35. Zarcone D, Corbetta S. Shared mechanisms of epilepsy, migraine and affective disorders. Neurol Sci. 2017;38(S1):73–6. 10.1007/s10072-017-2902-0 [DOI] [PubMed] [Google Scholar]
- 36. Glennon RA, Dukat MM. Serotonin receptors and drugs affecting serotonergic neurotransmission. In: Foye's textbook of medical chemistry. Baltimore: Williams and Wilkins Inc, 2002; p. 366–96. Available from http://downloads.lww.com/wolterskluwer_vitalstream_com/sample‐content/9781609133450_Lemke/samples/Chapter_11.pdf [Google Scholar]
- 37. Quesseveur G, Gardier AM, Guiard BP. The monoaminergic tripartite synapse: a putative target for currently available antidepressant drugs. Curr Drug Targets. 2013;14(11):1277–94. [DOI] [PubMed] [Google Scholar]
- 38. Bombardi C, Grandis A, Pivac N, Sagud M, Lucas G, Chagraoui A,, et al. Serotonin modulation of hippocampal functions: From anatomy to neurotherapeutics. Elsevier; 2021. Available from https://www.sciencedirect.com/science/article/pii/S0079612321000315 [DOI] [PubMed] [Google Scholar]
- 39. Panczyk K, Golda S, Waszkielewicz A, Zelaszczyk D, Gunia‐Krzyzak A, Marona H. Serotonergic system and its role in epilepsy and neuropathic pain treatment: a review based on receptor ligands. Curr Pharm Des. 2015;21(13):1723–40. [DOI] [PubMed] [Google Scholar]
- 40. Lopez‐Meraz M‐L, Gonzalez‐Trujano M‐E, Neri‐Bazan L, Hong E, Rocha LL. 5‐HT1A receptor agonists modify epileptic seizures in three experimental models in rats. Neuropharmacology. 2005;49(3):367–75. [DOI] [PubMed] [Google Scholar]
- 41. Zweckberger K, Simunovic F, Kiening KL, Unterberg AW, Sakowitz OW. Anticonvulsive effects of the dopamine agonist lisuride maleate after experimental traumatic brain injury. Neurosci Lett. 2010;470:150–4. [DOI] [PubMed] [Google Scholar]
- 42. Balaj K, Nowinski L, Walsh B, Mullett J, Palumbo ML, Thibert RL, et al. Buspirone for the treatment of anxiety‐related symptoms in Angelman syndrome: a case series. Psychiatr Genet. 2019;29(2):51–6. [DOI] [PubMed] [Google Scholar]
- 43. Albert PR, Vahid‐Ansari F. The 5‐HT1A receptor: signaling to behavior. Biochimie. 2019;161:34–45. [DOI] [PubMed] [Google Scholar]
- 44. Wang L, Zhang Y, Du X, Ding T, Gong W, Liu F. Review of antidepressants in clinic and active ingredients of traditional Chinese medicine targeting 5‐HT1A receptors. Biomed Pharmacother. 2019;120:109408. [DOI] [PubMed] [Google Scholar]
- 45. Hannon J, Hoyer D. Molecular biology of 5‐HT receptors. Behav Brain Res. 2008;195(1):198–213. [DOI] [PubMed] [Google Scholar]
- 46. Orban G, Pierucci M, Benigno A, Pessia M, Galati S, Valentino M, et al. High dose of 8‐OH‐DPAT decreases maximal dentate gyrus activation and facilitates granular cell plasticity in vivo. Exp Brain Res. 2013;230(4):441–51. [DOI] [PubMed] [Google Scholar]
- 47. Sapa J, Zygmunt M, Kulig K, Malawska B, Dudek M, Filipek B, et al. Evaluation of anticonvulsant activity of novel pyrrolidin‐2‐one derivatives. Pharmacol Rep. 2014;66(4):708–11. [DOI] [PubMed] [Google Scholar]
- 48. Dos Santos RG, Hallak JEC, Crippa JAS. Neuropharmacological effects of the main phytocannabinoids: a narrative review. Adv Exp Med Biol. 2021;1264:29–45. [DOI] [PubMed] [Google Scholar]
- 49. Martínez‐Aguirre C, Carmona‐Cruz F, Velasco AL, Velasco F, Aguado‐Carrillo G, Cuéllar‐Herrera M, et al. Cannabidiol acts at 5‐HT(1A) receptors in the human brain: relevance for treating temporal lobe epilepsy. Front Behav Neurosci. 2020;14:611278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Arbabi Jahan A, Rad A, Ghanbarabadi M, Amin B, Mohammad‐Zadeh M. The role of serotonin and its receptors on the anticonvulsant effect of curcumin in pentylenetetrazol‐induced seizures. Life Sci. 2018;211:252–60. [DOI] [PubMed] [Google Scholar]
- 51. Pottoo FH, Javed MN, Barkat MA, Alam MS, Nowshehri JA, Alshayban DM, et al. Estrogen and Serotonin: complexity of interactions and implications for epileptic seizures and epileptogenesis. Curr Neuropharmacol. 2019;17(3):214–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Gharib A, Komaki A, Manoochehri Khoshinani H, Saidijam M, Barkley V, Sarihi A, et al. Intrahippocampal 5‐HT(1A) receptor antagonist inhibits the improving effect of low‐frequency stimulation on memory impairment in kindled rats. Brain Res Bull. 2019;148:109–17. [DOI] [PubMed] [Google Scholar]
- 53. Manta S, Dong J, Debonnel G, Blier P. Enhancement of the function of rat serotonin and norepinephrine neurons by sustained vagus nerve stimulation. J Psychiatry Neurosci. 2009;34(4):272–80. [PMC free article] [PubMed] [Google Scholar]
- 54. Yang Y, Guo Y, Kuang Y, Wang S, Jiang Y, Ding Y, et al. Serotonin 1A receptor inhibits the status epilepticus induced by lithium‐pilocarpine in rats. Neurosci Bull. 2014;30(3):401–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Hatini PG, Commons KG. Serotonin abnormalities in Dravet syndrome mice before and after the age of seizure onset. Brain Res. 2019;1724:146399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Fonseca NC, Joaquim HPG, Talib LL, Vincentiis S, Gattaz WF, Valente KD. 5‐hydroxytryptamine1A receptor density in the hippocampus of patients with temporal lobe epilepsy is associated with disease duration. Eur J Neurol. 2017;24(4):602–8. [DOI] [PubMed] [Google Scholar]
- 57. Pernhorst K, van Loo KMJ, von Lehe M, Priebe L, Cichon S, Herms S, et al. Rs6295 promoter variants of the serotonin type 1A receptor are differentially activated by c‐Jun in vitro and correlate to transcript levels in human epileptic brain tissue. Brain Res. 2013;1499:136–44. [DOI] [PubMed] [Google Scholar]
- 58. Tiger M, Varnäs K, Okubo Y, Lundberg J. The 5‐HT(1B) receptor – a potential target for antidepressant treatment. Psychopharmacology. 2018;235(5):1317–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Villalón CM, VanDenBrink AM. The role of 5‐hydroxytryptamine in the pathophysiology of migraine and its relevance to the design of novel treatments. Mini Rev Med Chem. 2017;17(11):928–38. [DOI] [PubMed] [Google Scholar]
- 60. Sourbron J, Schneider H, Kecskes A, Liu Y, Buening EM, Lagae L, et al. Serotonergic modulation as effective treatment for Dravet syndrome in a zebrafish mutant model. ACS Chem Neurosci. 2016;7(5):588–98. [DOI] [PubMed] [Google Scholar]
- 61. Gooshe M, Ghasemi K, Rohani MM, Tafakhori A, Amiri S, Aghamollaii V, et al. Biphasic effect of sumatriptan on PTZ‐induced seizures in mice: modulation by 5‐HT1B/D receptors and NOS/NO pathway. Eur J Pharmacol. 2018;824:140–7. [DOI] [PubMed] [Google Scholar]
- 62. Sourbron J, Partoens M, Scheldeman C, Zhang Y, Lagae L, de Witte P. Drug repurposing for Dravet syndrome in scn1Lab(‐/‐) mutant zebrafish. Epilepsia. 2019;60(2):e8–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Hatini PG, Commons KG. A 5‐HT1D‐receptor agonist protects Dravet syndrome mice from seizure and early death. Eur J Neurosci. 2020;52(10):4370–4. 10.1111/ejn.14776 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Barnes NM, Sharp T. A review of central 5‐HT receptors and their function. Neuropharmacology. 1999;38(8):1083–152. [DOI] [PubMed] [Google Scholar]
- 65. Meneses A. Chapter 6–5‐HT1E/1F receptor. In: Meneses A, editor. The role of 5‐HT systems on memory and dysfunctional memory. San Diego: Academic Press; 2014. p. 27–8. Available from: http://www.sciencedirect.com/science/article/pii/B9780128008362000064 [Google Scholar]
- 66. Klein MT, Teitler M. Distribution of 5‐ht(1E) receptors in the mammalian brain and cerebral vasculature: an immunohistochemical and pharmacological study. Br J Pharmacol. 2012;166(4):1290–302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Ramadan NM, Skljarevski V, Phebus LA, Johnson KW. 5‐HT1F receptor agonists in acute migraine treatment: a hypothesis. Cephalalgia. 2003;23(8):776–85. [DOI] [PubMed] [Google Scholar]
- 68. Vila‐Pueyo M. Targeted 5‐HT(1F) therapies for migraine. Neurother J Am Soc Exp Neurother. 2018;15(2):291–303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Capi M, De Angelis V, De Bernardini D, De Luca O, Cipolla F, Lionetto L, et al. CGRP receptor antagonists and 5‐HT1F receptor agonist in the treatment of migraine. J Clin Med. 2021;10(7):1429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Maroteaux L, Ayme‐Dietrich E, Aubertin‐Kirch G, Banas S, Quentin E, Lawson R, et al. New therapeutic opportunities for 5‐HT2 receptor ligands. Pharmacol Ther. 2017;170:14–36. [DOI] [PubMed] [Google Scholar]
- 71. Puzerey PA, Decker MJ, Galan RF. Elevated serotonergic signaling amplifies synaptic noise and facilitates the emergence of epileptiform network oscillations. J Neurophysiol. 2014;112(10):2357–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Hanzlik E, Klinger SA, Carson R, Duis J. Mirtazapine for sleep disturbances in Angelman syndrome: a retrospective chart review of 8 pediatric cases. J Clin Sleep Med. 2020;16(4):591–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Griffin AL, Jaishankar P, Grandjean J‐M, Olson SH, Renslo AR, Baraban SC. Zebrafish studies identify serotonin receptors mediating antiepileptic activity in Dravet syndrome. Brain Commun. 2019;1(1):fcz008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. Launay J‐M, Herve P, Peoc'h K, Tournois C, Callebert J, Nebigil CG, et al. Function of the serotonin 5‐hydroxytryptamine 2B receptor in pulmonary hypertension. Nat Med. 2002;8(10):1129–35. [DOI] [PubMed] [Google Scholar]
- 75. Elangbam CS. Drug‐induced valvulopathy: an update. Toxicol Pathol. 2010;38(6):837–48. [DOI] [PubMed] [Google Scholar]
- 76. Rothman RB, Baumann MH. Serotonergic drugs and valvular heart disease. Expert Opin Drug Saf. 2009;8(3):317–29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77. Rothman RB, Baumann MH, Savage JE, Rauser L, McBride A, Hufeisen SJ, et al. Evidence for possible involvement of 5‐HT(2B) receptors in the cardiac valvulopathy associated with fenfluramine and other serotonergic medications. Circulation. 2000;102(23):2836–41. [DOI] [PubMed] [Google Scholar]
- 78. Isaac M. Serotonergic 5‐HT2C receptors as a potential therapeutic target for the design antiepileptic drugs. Curr Top Med Chem. 2005;5(1):59–67. [DOI] [PubMed] [Google Scholar]
- 79. Guiard BP, Di Giovanni G, Di GG. Central serotonin‐2A (5‐HT2A) receptor dysfunction in depression and epilepsy: the missing link? Front Pharmacol. 2015;6:46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80. McLean TH, Parrish JC, Braden MR, Marona‐Lewicka D, Gallardo‐Godoy A, Nichols DE, et al. 1‐Aminomethylbenzocycloalkanes: conformationally restricted hallucinogenic phenethylamine analogues as functionally selective 5‐HT2A receptor agonists. J Med Chem. 2006;49(19):5794–803. [DOI] [PubMed] [Google Scholar]
- 81. Karaki S, Becamel C, Murat S, Mannoury la Cour C, Millan MJ, Prézeau L, et al. Quantitative phosphoproteomics unravels biased phosphorylation of serotonin 2A receptor at Ser280 by hallucinogenic versus nonhallucinogenic agonists. Mol Cell Proteomics. 2014;13:1273–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82. Pehrson AL, Jeyarajah T, Sanchez C. Regional distribution of serotonergic receptors: a systems neuroscience perspective on the downstream effects of the multimodal‐acting antidepressant vortioxetine on excitatory and inhibitory neurotransmission. CNS Spectr. 2016;21(2):162–83. [DOI] [PubMed] [Google Scholar]
- 83. Cheng J, Kozikowski AP. We need 2C but not 2B: developing serotonin 2C (5‐HT2C) receptor agonists for the treatment of CNS disorders. ChemMedChem. 2015;10(12):1963–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84. Silenieks LB, Carroll NK, Van Niekerk A, Van Niekerk E, Taylor C, Upton N, et al. Evaluation of selective 5‐HT2C agonists in acute seizure models. ACS Chem Neurosci. 2019;10(7):3284–95. [DOI] [PubMed] [Google Scholar]
- 85. Watanabe K, Ashby CRJ, Katsumori H, Minabe Y. The effect of the acute administration of various selective 5‐HT receptor antagonists on focal hippocampal seizures in freely‐moving rats. Eur J Pharmacol. 2000;398(2):239–46. [DOI] [PubMed] [Google Scholar]
- 86. Buchanan GF, Smith HR, MacAskill A, Richerson GB. 5‐HT2A receptor activation is necessary for CO2‐induced arousal. J Neurophysiol. 2015;114(1):233–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87. Petrucci AN, Joyal KG, Purnell BS, Buchanan GF. Serotonin and sudden unexpected death in epilepsy. Exp Neurol. 2020;325:113145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88. Ishiura S. Serotonin receptor knockout mice. Nihon Shinkei Seishin Yakurigaku Zasshi. 1999;19(5):257–60. [PubMed] [Google Scholar]
- 89. Brennan TJ, Seeley WW, Kilgard M, Schreiner CE, Tecott LH. Sound‐induced seizures in serotonin 5‐HT2c receptor mutant mice. Nat Genet. 1997;16(4):387–90. [DOI] [PubMed] [Google Scholar]
- 90. Di Giovanni G, De Deurwaerdère P. New therapeutic opportunities for 5‐HT2C receptor ligands in neuropsychiatric disorders. Pharmacol Ther. 2016;157:125–62. [DOI] [PubMed] [Google Scholar]
- 91. Tolete P, Knupp K, Karlovich M, DeCarlo E, Bluvstein J, Conway E, et al. Lorcaserin therapy for severe epilepsy of childhood onset. Neurology. 2018;91(18):837–9. 10.1212/WNL.0000000000006432 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92. Halberstadt AL. Pharmacology and toxicology of N‐Benzylphenethylamine (“NBOMe”) hallucinogens. Curr Top Behav Neurosci. 2017;32:283–311. [DOI] [PubMed] [Google Scholar]
- 93. Collins SA, Huff C, Chiaia N, Gudelsky GA, Yamamoto BK. 3,4‐methylenedioxymethamphetamine increases excitability in the dentate gyrus: role of 5HT2A receptor‐induced PGE2 signaling. J Neurochem. 2016;136(5):1074–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94. Devroye C, Cathala A, Piazza PV, Spampinato U. The central serotonin(2B) receptor as a new pharmacological target for the treatment of dopamine‐related neuropsychiatric disorders: rationale and current status of research. Pharmacol Ther. 2018;181:143–55. [DOI] [PubMed] [Google Scholar]
- 95. Akyuz E, Doganyigit Z, Paudel YN, Koklu B, Kaymak E, Villa C, et al. Immunoreactivity of muscarinic acetylcholine M2 and serotonin 5‐HT2B receptors, norepinephrine transporter and kir channels in a model of epilepsy. Life. 2021;11(4):276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96. Colangeli R, Di Maio R, Pierucci M, Deidda G, Casarrubea M, Di Giovanni G. Synergistic action of CB(1) and 5‐HT(2B) receptors in preventing pilocarpine‐induced status epilepticus in rats. Neurobiol Dis. 2019;125:135–45. [DOI] [PubMed] [Google Scholar]
- 97. Pelz MC, Schoolcraft KD, Larson C, Spring MG, López HH. Assessing the role of serotonergic receptors in cannabidiol's anticonvulsant efficacy. Epilepsy Behav. 2017;73:111–8. [DOI] [PubMed] [Google Scholar]
- 98. Tupal S, Faingold CL. Fenfluramine, a serotonin‐releasing drug, prevents seizure‐induced respiratory arrest and is anticonvulsant in the DBA/1 mouse model of SUDEP. Epilepsia. 2019;60(3):485–94. [DOI] [PubMed] [Google Scholar]
- 99. Hawkins NA, Anderson LL, Gertler TS, Laux L, George AL, Kearney JA. Screening of conventional anticonvulsants in a genetic mouse model of epilepsy. Ann Clin Transl Neurol. 2017;4(5):326–39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100. Faingold CL, Randall M, Zeng C, Peng S, Long X, Feng HJ. Serotonergic agents act on 5‐HT(3) receptors in the brain to block seizure‐induced respiratory arrest in the DBA/1 mouse model of SUDEP. Epilepsy Behav. 2016;64(Pt A):166–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101. Saigal N, Bajwa AK, Faheem SS, Coleman RA, Pandey SK, Constantinescu CC, et al. Evaluation of serotonin 5‐HT(1A) receptors in rodent models using [18F]mefway PET. Synapse. 2013;67(9):596–608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102. Mishra A, Goel RK. Chronic 5‐HT(3) receptor antagonism ameliorates seizures and associated memory deficit in pentylenetetrazole‐kindled mice. Neuroscience. 2016;339:319–28. [DOI] [PubMed] [Google Scholar]
- 103. Li B, Wang L, Sun Z, Zhou Y, Shao D, Zhao J, et al. The anticonvulsant effects of SR 57227 on pentylenetetrazole‐induced seizure in mice. PLoS One. 2014;9(4):e93158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104. Yang Z, Liu X, Yin Y, Sun S, Deng X. Involvement of 5‐HT(7) receptors in the pathogenesis of temporal lobe epilepsy. Eur J Pharmacol. 2012;685(1–3):52–8. [DOI] [PubMed] [Google Scholar]
- 105. Wang L, Lv Y, Deng W, Peng X, Xiao Z, Xi Z, et al. 5‐HT6 receptor recruitment of mTOR modulates seizure activity in epilepsy. Mol Neurobiol. 2015;51(3):1292–9. [DOI] [PubMed] [Google Scholar]
- 106. Yang Z, Liu X, Yin Y, Sun S, Deng X. Involvement of 5‐HT₇ receptors in the pathogenesis of temporal lobe epilepsy. Eur J Pharmacol. 2012;685(1–3):52–8. [DOI] [PubMed] [Google Scholar]
- 107. Rothman RB, Baumann MH, Savage JE, Rauser L, McBride A, Hufeisen SJ, et al. Evidence for possible involvement of 5‐HT(2B) receptors in the cardiac valvulopathy associated with fenfluramine and other serotonergic medications. Circulation. 2000;102(23):2836–41. [DOI] [PubMed] [Google Scholar]
- 108. Schoonjans A‐S, Ceulemans B. A critical evaluation of fenfluramine hydrochloride for the treatment of Dravet syndrome. Expert Rev Neurother. 2021;26:1–14. 10.1080/14737175.2021.1877540 [DOI] [PubMed] [Google Scholar]
- 109. Dumitrascu R, Kulcke C, Konigshoff M, Kouri F, Yang X, Morrell N, et al. Terguride ameliorates monocrotaline‐induced pulmonary hypertension in rats. Eur Respir J. 2011;37(5):1104–18. [DOI] [PubMed] [Google Scholar]
- 110. Zhao H, Lin Y, Chen S, Li X, Huo H. 5‐HT3 receptors: a potential therapeutic target for epilepsy. Curr Neuropharmacol. 2018;16(1):29–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111. Amiri Gheshlaghi S, Mohammad Jafari R, Algazo M, Rahimi N, Alshaib H, Dehpour AR. Genistein modulation of seizure: involvement of estrogen and serotonin receptors. J Nat Med. 2017;71(3):537–44. [DOI] [PubMed] [Google Scholar]
- 112. Payandemehr B, Bahremand A, Rahimian R, Ziai P, Amouzegar A, Sharifzadeh M, et al. 5‐HT(3) receptor mediates the dose‐dependent effects of citalopram on pentylenetetrazole‐induced clonic seizure in mice: involvement of nitric oxide. Epilepsy Res. 2012;101(3):217–27. [DOI] [PubMed] [Google Scholar]
- 113. Alhaj MW, Zaitone SA, Moustafa YM. Fluvoxamine alleviates seizure activity and downregulates hippocampal GAP‐43 expression in pentylenetetrazole‐kindled mice: role of 5‐HT3 receptors. Behav Pharmacol. 2015;26(4):369–82. 10.1097/FBP.0000000000000127 [DOI] [PubMed] [Google Scholar]
- 114. Kwan C, Bédard D, Frouni I, Gaudette F, Beaudry F, Hamadjida A, et al. Pharmacokinetic profile of the selective 5‐HT(3) receptor antagonist ondansetron in the rat: an original study and a minireview of the behavioural pharmacological literature in the rat. Can J Physiol Pharmacol. 2020;98(7):431–40. [DOI] [PubMed] [Google Scholar]
- 115. Kim KJ, Jeun SH, Sung K‐W. Lamotrigine, an antiepileptic drug, inhibits 5‐HT(3) receptor currents in NCB‐20 neuroblastoma cells. Korean J Physiol Pharmacol. 2017;21(2):169–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116. Vidal R, Castro E, Pilar‐Cuéllar F, Pascual‐Brazo J, Díaz A, Rojo ML, et al. Serotonin 5‐HT4 receptors: a new strategy for developing fast acting antidepressants? Curr Pharm Des. 2014;20(23):3751–62. [DOI] [PubMed] [Google Scholar]
- 117. Rebholz H, Friedman E, Castello J. Alterations of expression of the serotonin 5‐HT4 receptor in brain disorders. Int J Mol Sci. 2018;19(11):3581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118. Bijak M, Misgeld U. Effects of serotonin through serotonin1A and serotonin4 receptors on inhibition in the guinea‐pig dentate gyrus in vitro. Neuroscience. 1997;78(4):1017–26. [DOI] [PubMed] [Google Scholar]
- 119. Compan V, Zhou M, Grailhe R, Gazzara RA, Martin R, Gingrich J, et al. Attenuated response to stress and novelty and hypersensitivity to seizures in 5‐HT4 receptor knock‐out mice. J Neurosci. 2004;24(2):412–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120. Nelson DL. 5‐HT5 receptors. Curr Drug Targets CNS Neurol Disord. 2004;3(1):53–8. [DOI] [PubMed] [Google Scholar]
- 121. Karila D, Freret T, Bouet V, Boulouard M, Dallemagne P, Rochais C, et al. Therapeutic potential of 5‐HT6 receptor agonists. J Med Chem. 2015;58(20):7901–12. [DOI] [PubMed] [Google Scholar]
- 122. Routledge C, Bromidge SM, Moss SF, Price GW, Hirst W, Newman H, et al. Characterization of SB‐271046: a potent, selective and orally active 5‐HT(6) receptor antagonist. Br J Pharmacol. 2000;130(7):1606–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123. Stean TO, Hirst WD, Thomas DR, Price GW, Rogers D, Riley G, et al. Pharmacological profile of SB‐357134: a potent, selective, brain penetrant, and orally active 5‐HT(6) receptor antagonist. Pharmacol Biochem Behav. 2002;71(4):645–54. [DOI] [PubMed] [Google Scholar]
- 124. Hirst WD, Stean TO, Rogers DC, Sunter D, Pugh P, Moss SF, et al. SB‐399885 is a potent, selective 5‐HT6 receptor antagonist with cognitive enhancing properties in aged rat water maze and novel object recognition models. Eur J Pharmacol. 2006;553(1–3):109–19. [DOI] [PubMed] [Google Scholar]
- 125. Lin W, Huang W, Chen S, Lin M, Huang Q, Huang H. The role of 5‐HTR6 in mossy fiber sprouting: activating Fyn and p‐ERK1/2 in pilocarpine‐induced chronic epileptic rats. Cell Physiol Biochem. 2017;42(1):231–41. [DOI] [PubMed] [Google Scholar]
- 126. Liu C, Wen Y, Huang H, Lin W, Huang M, Lin R, et al. Over‐expression of 5‐HT6 receptor and activated Jab‐1/p‐c‐Jun play important roles in pilocarpine‐induced seizures and learning‐memory impairment. J Mol Neurosci. 2019;67(3):388–99. [DOI] [PubMed] [Google Scholar]
- 127. Glennon RA, Dukat M. Serotonin receptors and drugs affecting serotonergic neurotransmission. In: Foye's Principles of Medicinal Chemistry [internet]. 2012:366–96. Available from: http://downloads.lww.com/wolterskluwer_vitalstream_com/sample‐content/9781609133450_Lemke/samples/Chapter_11.pdf [Google Scholar]
- 128. Blattner KM, Canney DJ, Pippin DA, Blass BE. Pharmacology and therapeutic potential of the 5‐HT(7) receptor. ACS Chem Neurosci. 2019;10(1):89–119. [DOI] [PubMed] [Google Scholar]
- 129. Thirumaran S‐L, Lepailleur A, Rochais C. Structure‐activity relationships of serotonin 5‐HT(7) receptors ligands: a review. Eur J Med Chem. 2019;183:111705. [DOI] [PubMed] [Google Scholar]
- 130. Pericić D, Svob SD. The role of 5‐HT(7) receptors in the control of seizures. Brain Res. 2007;1141:48–55. 10.1016/j.brainres.2007.01.019 [DOI] [PubMed] [Google Scholar]
- 131. Vigli D, Rusconi L, Valenti D, La Montanara P, Cosentino L, Lacivita E, et al. Rescue of prepulse inhibition deficit and brain mitochondrial dysfunction by pharmacological stimulation of the central serotonin receptor 7 in a mouse model of CDKL5 Deficiency Disorder. Neuropharmacology. 2019;144:104–14. [DOI] [PubMed] [Google Scholar]
- 132. Witkin JM, Baez M, Yu J, Barton ME, Shannon HE. Constitutive deletion of the serotonin‐7 (5‐HT(7)) receptor decreases electrical and chemical seizure thresholds. Epilepsy Res. 2007;75(1):39–45. [DOI] [PubMed] [Google Scholar]
- 133. Nikiforuk A. Targeting the serotonin 5‐HT7 receptor in the search for treatments for CNS disorders: rationale and progress to date. CNS Drugs. 2015;29(4):265–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134. Keezer MR, Sisodiya SM, Sander JW. Comorbidities of epilepsy: current concepts and future perspectives. Lancet Neurol. 2016;15(1):106–15. 10.1016/S1474-4422(15)00225-2 [DOI] [PubMed] [Google Scholar]
- 135. Horvath GA, Demos M, Shyr C, Matthews A, Zhang L, Race S, et al. Secondary neurotransmitter deficiencies in epilepsy caused by voltage‐gated sodium channelopathies: a potential treatment target? Mol Genet Metab. 2016;117(1):42–8. [DOI] [PubMed] [Google Scholar]
- 136. da Fonseca NC, Joaquim HPGG, Talib LL, de Vincentiis S, Gattaz WF, Valente KD. Hippocampal serotonin depletion is related to the presence of generalized tonic‐clonic seizures, but not to psychiatric disorders in patients with temporal lobe epilepsy. Epilepsy Res. 2015;111:18–25. [DOI] [PubMed] [Google Scholar]
- 137. Schutte RJ, Schutte SS, Algara J, Barragan EV, Gilligan J, Staber C, et al. Knock‐in model of Dravet syndrome reveals a constitutive and conditional reduction in sodium current. J Neurophysiol. 2014;112(4):903–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138. Miguelez C, Morera‐Herreras T, Torrecilla M, Ruiz‐Ortega JA, Ugedo L. Interaction between the 5‐HT system and the basal ganglia: functional implication and therapeutic perspective in Parkinson's disease. Front Neural Circuits. 2014;8:21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139. Numan M. Neurobiology of social behavior: Toward an understanding of the prosocial and antisocial brain. Elsevier Science; 2014. Available from: https://books.google.be/books?id=FDrLAwAAQBAJ [Google Scholar]
- 140. Srinivas H, Shah U. Comorbidities of epilepsy. Neurol India. 2017;65(7):18–24. [DOI] [PubMed] [Google Scholar]
- 141. Yohn CN, Gergues MM, Samuels BA. The role of 5‐HT receptors in depression. Mol Brain. 2017;10(1):28. 10.1186/s13041-017-0306-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142. Hasler G, Bonwetsch R, Giovacchini G, Toczek MT, Bagic A, Luckenbaugh DA, et al. 5‐HT(1A) receptor binding in temporal lobe epilepsy patients with and without major depression. Biol Psychiatry. 2007;62:1258–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143. Barbanti P, Aurilia C, Egeo G, Fofi L, Palmirotta R. Serotonin receptor targeted therapy for migraine treatment: an overview of drugs in phase I and II clinical development. Expert Opin Investig Drugs. 2017;26(3):269–77. [DOI] [PubMed] [Google Scholar]
- 144. Svob Strac D, Pivac N, Muck‐Seler D. The serotonergic system and cognitive function. Transl Neurosci. 2016;7(1):35–49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145. Thurman DJ, Hesdorffer DC, French JA. Sudden unexpected death in epilepsy: assessing the public health burden. Epilepsia. 2014;55(10):1479–85. [DOI] [PubMed] [Google Scholar]
- 146. Shmuely S, Sisodiya SM, Gunning WB, Sander JW, Thijs RD. Mortality in Dravet syndrome: a review. Epilepsy Behav. 2016;64(Pt A):69–74. [DOI] [PubMed] [Google Scholar]
- 147. Al‐Baradie RS. Dravet syndrome, what is new? Neurosciences. 2013;18(1):11–7. [PubMed] [Google Scholar]
- 148. Massey CA, Sowers LP, Dlouhy BJ, Richerson GB. Mechanisms of sudden unexpected death in epilepsy: the pathway to prevention. Nat Rev Neurol. 2014;10(5):271–82. 10.1038/nrneurol.2014.64 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149. Ryvlin P, Nashef L, Tomson T. Prevention of sudden unexpected death in epilepsy: a realistic goal? Epilepsia. 2013;54(Suppl 2):23–8. [DOI] [PubMed] [Google Scholar]
- 150. Bateman LM, Li C‐S, Seyal M. Ictal hypoxemia in localization‐related epilepsy: analysis of incidence, severity and risk factors. Brain. 2008;131(Pt 12):3239–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151. Bateman LM, Li C‐S, Lin T‐C, Seyal M. Serotonin reuptake inhibitors are associated with reduced severity of ictal hypoxemia in medically refractory partial epilepsy. Epilepsia. 2010;51(10):2211–4. [DOI] [PubMed] [Google Scholar]
- 152. Richerson GB. Serotonergic neurons as carbon dioxide sensors that maintain pH homeostasis. Nat Rev Neurosci. 2004;5(6):449–61. [DOI] [PubMed] [Google Scholar]
- 153. Cross JH, Galer BS, Gil‐Nagel A, Devinsky O, Ceulemans B, Lagae L, et al. Impact of fenfluramine on the expected SUDEP mortality rates in patients with Dravet syndrome. Seizure. 2021;93:154–9. [DOI] [PubMed] [Google Scholar]
- 154. Hutcheson JD, Setola V, Roth BL, Merryman WD. Serotonin receptors and heart valve disease–it was meant 2B. Pharmacol Ther. 2011;132(2):146–57. 10.1016/j.pharmthera.2011.03.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155. Roth BL. Drugs and valvular heart disease. N Engl J Med. 2007;356(1):6–9. [DOI] [PubMed] [Google Scholar]
- 156. Schoonjans A‐S, Marchau F, Paelinck BP, Lagae L, Gammaitoni A, Pringsheim M, et al. Cardiovascular safety of low‐dose fenfluramine in Dravet syndrome: a review of its benefit‐risk profile in a new patient population. Curr Med Res Opin. 2017;33(10):1773–81. [DOI] [PubMed] [Google Scholar]
- 157. Balagura G, Cacciatore M, Grasso EA, Striano P, Verrotti A. Fenfluramine for the treatment of Dravet syndrome and Lennox‐Gastaut syndrome. CNS Drugs. 2020;34(10):1001–7. [DOI] [PubMed] [Google Scholar]
- 158. Lai WW, Galer BS, Wong PC, Farfel G, Pringsheim M, Keane MG, et al. Cardiovascular safety of fenfluramine in the treatment of Dravet syndrome: analysis of an ongoing long‐term open‐label safety extension study. Epilepsia. 2020;61(11):2386–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159. Lagae L. Dravet syndrome. Curr Opin Neurol. 2021;34(2):213–8. [DOI] [PubMed] [Google Scholar]
- 160. Strzelczyk A, Pringsheim M, Mayer T, Polster T, Klotz KA, Muhle H, et al. Efficacy, tolerability, and retention of fenfluramine for the treatment of seizures in patients with Dravet syndrome: compassionate use program in Germany. Epilepsia. 2021;62(10):2518–27. [DOI] [PubMed] [Google Scholar]
- 161. Devi N, Madaan P, Asrar MM, Sahu JK, Bansal D. Comparative short‐term efficacy and safety of add‐on anti‐seizure medications in Dravet syndrome: an indirect treatment comparison. Seizure. 2021;91:316–24. [DOI] [PubMed] [Google Scholar]
- 162. Specchio N, Pietrafusa N, Doccini V, Trivisano M, Darra F, Ragona F, et al. Efficacy and safety of fenfluramine hydrochloride for the treatment of seizures in Dravet syndrome: a real‐world study. Epilepsia. 2020;61(11):2405–14. [DOI] [PubMed] [Google Scholar]
- 163. Bialer M, Johannessen SI, Koepp MJ, Levy RH, Perucca E, Tomson T, et al. Progress report on new antiepileptic drugs: a summary of the Fourteenth Eilat Conference on New Antiepileptic Drugs and Devices (EILAT XIV). II. Drugs in more advanced clinical development. Epilepsia. 2018;59(10):1842–66. 10.1111/epi.14555 [DOI] [PubMed] [Google Scholar]
- 164. Ramage A. Problems of drug selectivity and dose – pharmacology. J Physiol. 2005;569:711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165. Schoonjans A‐S, Lagae L, Ceulemans B. Low‐dose fenfluramine in the treatment of neurologic disorders: experience in Dravet syndrome. Ther Adv Neurol Disord. 2015;8(6):328–38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166. Cross JH, Caraballo RH, Nabbout R, Vigevano F, Guerrini R, Lagae L. Dravet syndrome: treatment options and management of prolonged seizures. Epilepsia. 2019;60(Suppl 3):S39–48. [DOI] [PubMed] [Google Scholar]
- 167. Bialer M, Cross H, Hedrich UBS, Lagae L, Lerche H, Loddenkemper T. Novel treatment approaches and pediatric research networks in status epilepticus. Epilepsy Behav. 2019;101:106564. [DOI] [PubMed] [Google Scholar]
- 168. Tiraboschi E, Martina S, van der Ent W, Grzyb K, Gawel K, Cordero‐Maldonado ML, et al. New insights into the early mechanisms of epileptogenesis in a zebrafish model of Dravet syndrome. Epilepsia. 2020;61(3):549–60. [DOI] [PubMed] [Google Scholar]
- 169. Rothman RB, Zolkowska D, Baumann MH. Serotonin (5‐HT) transporter ligands affect plasma 5‐HT in rats. Ann N Y Acad Sci. 2008;1139:268–84. [DOI] [PubMed] [Google Scholar]
- 170. Gobbi M, Frittoli E, Mennini T, Garattini S. Releasing activities of d‐fenfluramine and fluoxetine on rat hippocampal synaptosomes preloaded with [3H]serotonin. Naunyn Schmiedebergs Arch Pharmacol. 1992;345(1):1–6. [DOI] [PubMed] [Google Scholar]
- 171. Tupal S, Faingold CL. Serotonin 5‐HT4 receptors play a critical role in the action of fenfluramine to block seizure‐induced sudden death in a mouse model of SUDEP. Epilepsy Res. 2021;177:106777. [DOI] [PubMed] [Google Scholar]
- 172. De Deurwaerdère P, Bharatiya R, Chagraoui A, Di Giovanni G. Constitutive activity of 5‐HT receptors: factual analysis. Neuropharmacology. 2020;168:107967. [DOI] [PubMed] [Google Scholar]
- 173. Fakhfouri G, Rahimian R, Dyhrfjeld‐Johnsen J, Zirak MR, Beaulieu JM, et al. 5‐HT3 receptor antagonists in neurologic and neuropsychiatric disorders: the iceberg still lies beneath the surface. Witkin JM, editor. Pharmacol Rev. 2019;71(3):383–412. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Material


