Abstract
Connexins (Cxs) constitute a large family of transmembrane proteins that form gap junction channels, which enable the direct transfer of small signaling molecules from cell to cell. In blood vessels, Cx channels allow the endothelial cells (ECs) to respond to external and internal cues as a whole and, thus, contribute to the maintenance of vascular homeostasis. While the role of Cxs has been extensively studied in large arteries, a growing body of evidence suggests that they also play a role in the formation of microvascular networks. Since the formation of new blood vessels requires the coordinated response of ECs to external stimuli, endothelial Cxs may play an important role there. Recent studies in developmental and pathologic models reveal that EC Cxs regulate physiological and pathological angiogenesis through canonical and noncanonical functions, making these proteins potential therapeutic targets for the development of new strategies aimed at a better control of angiogenesis.
In multicellular organisms, cells constantly exchange chemical and electrical signals with each other to coordinate their functions. At the level of the organ, this intercellular communication plays a central role in maintaining tissue homeostasis and biological functions.
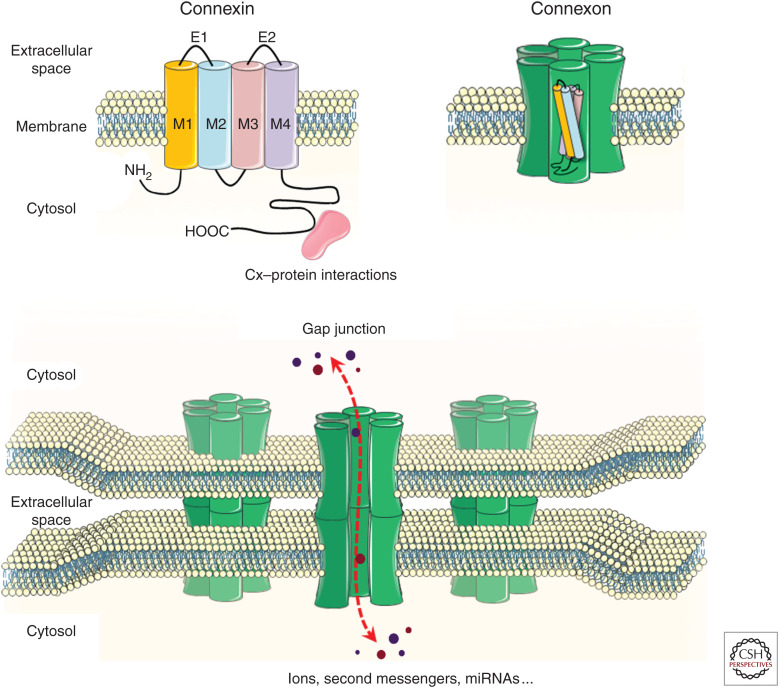
One way of cell-to-cell communication relies on the existence of specialized regions of the plasma membrane forming intercellular channels called gap junctions (GJs). GJs enable the passage of ions, metabolites, nucleotides, and second messengers up to about 1 kDa between the cytosols of adjacent cells (Fig. 1). This direct intercellular communication allows cells to assess the functional state of their neighbors, and to coordinately adapt their own function, thus playing a critical role in the regulation of many biological processes.
Figure 1.
Organization of connexins into gap-junction channels. The diagram shows the assembly of connexins into a connexon, and the intercellular joining of two connexons to form an intercellular channel. Clusters of intercellular channels form gap junctions. Each intercellular channel provides an axial pore for the bidirectional exchange of molecules (dashed red arrow) between the cytosols of apposed cells.
Connexins (Cxs) are the building blocks of these intercellular channels, and are encoded by a family of highly conserved genes (Yeager et al. 1998). Twenty different Cx genes have been identified in the mouse genome and 21 in the human genome (Willecke et al. 2002; Sohl and Willecke 2004). All Cx isoforms share the same organization with four-transmembrane domains, one cytoplasmic loop, two extracellular loops, and both the carboxy and the amino terminals in the cytoplasm (Fig. 1). The carboxy-terminal tail varies in length and sequence between the different isoforms, thus conferring to Cxs functional domains for posttranslational modifications and protein–protein interactions that modulate the permeability properties of the channels (Bruzzone et al. 1996).
Once synthesized, Cxs assemble into hexameric structures termed connexons, which are inserted in the plasma membrane. A Cx channel is formed when one connexon interacts with another connexon of an adjacent cell. In most tissues, multiple Cx isoforms can be expressed by a single-cell type, to form homo- (formed by one type of Cx) as well as heteromeric connexons (formed by different Cxs). In addition, connexons of one cell can join a companion connexon or a connexon of another type expressed by a neighboring cell, establishing homotypic and heterotypic GJ channels between cells expressing different Cxs (Fig. 1). This diversity in Cx isoforms and connexon combinations allows for a large diversity in the permeability properties of the GJ channels, and raises the question of what are the Cx-specific functional roles.
Beside their role in the formation of GJs, some Cxs also exert channel-independent functions, contributing to the regulation of cytoskeleton organization (Chen et al. 2015), proliferation (Gellhaus et al. 2010), and gene expression (Martins-Marques et al. 2015). These functions rely on the ability of Cxs to bind and regulate a wide range of proteins, usually at their carboxy-terminal cytoplasmic tails (Leithe et al. 2018). Thus, Cxs can potentially influence intercellular as well as intracellular signaling by regulating GJs and acting as scaffolding proteins, respectively (Fig. 1).
Given that Cxs are expressed in almost every cell type of the human body, and are involved in multiple cell functions, their dysfunction often results in disease (Delmar et al. 2018). In blood vessels, the presence of various types of GJs between endothelial cells (ECs) and smooth muscle cells (SMCs) provides a rapid mean for the intercellular communications that are required for coordinated changes in vessel diameter (Segal 2015). Accordingly, alterations in EC and SMC Cx expression and function have been associated with vascular dysfunction (Molica et al. 2018; Pohl 2020). While initially most studies of vascular Cxs have focused on large elastic arteries, due to their easy accessibility, increasing evidence now supports a critical role of Cxs in the formation and maintenance of the microvasculature. The aim of this paper is to review this more recent knowledge, with a particular emphasis on the experimental studies that have revealed unique roles of Cx-dependent signaling during physiological and tumoral angiogenesis, as well as in blood vessel morphogenesis.
ROLE OF ENDOTHELIAL Cxs IN VASCULAR FUNCTIONS
Four Cxs (Cx45, Cx43, Cx40, and Cx37) have been detected in the wall of many different vessels, in a pattern that differs between ECs and SMCs, and varies with the type of vascular compartment.
ECs, which cover the internal surface of blood vessels, express mainly Cx37 and Cx40 (Fig. 2; Hill et al. 2002; Simon and McWhorter 2002; Rummery and Hill 2004; Alonso et al. 2010b), allowing the endothelium to respond as a coordinated assembly to external and internal cues. Cx43 is specifically induced in ECs during remodeling (Gabriels and Paul 1998; Kwak et al. 2005).
Figure 2.
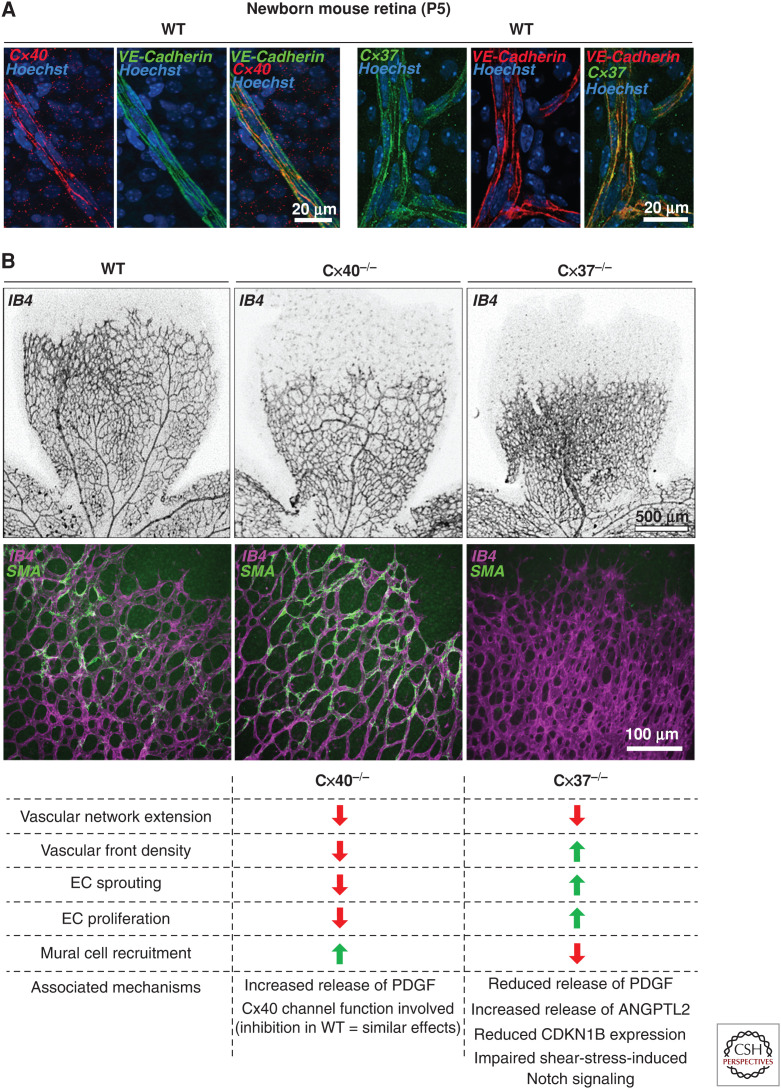
Contribution of endothelial Cx40 and Cx37 to developmental retinal angiogenesis. (A) Immunostaining of whole-mounted retinas from P5 wild-type (WT) mice shows the dual labeling of endothelial cells (ECs) for Cx40 (red) and the EC marker vascular endothelial (VE)-cadherin (green) (left panels), and for Cx37 (green) and VE-cadherin (red) (right panels). (B) Labeling of retina whole-mounts for the EC marker IB4 (black) reveals reduced extension of the vascular network in P5 Cx40−/− and Cx37−/− mice, compared to WT. Strikingly, the density of angiogenic vessels was reduced in the retinas of Cx40−/− mice, but increased in those of Cx37−/− animals (upper panels). Immunostaining shows increased coverage of neovessels identified by IB4 (purple) with α-smooth muscle actin (αSMA, green)-positive cells in the retina of a Cx40−/− mouse. In contrast, Cx37−/− animals display a reduced coverage of retinal neovessels by αSMA-positive mural cells (middle panels). The lower panel summarizes the main angiogenesis defects of the developing retinas of Cx40−/− and Cx37−/− mice.
Cx43 and Cx45 are the prominent isoforms observed in the SMCs (Kruger et al. 2000; Rummery et al. 2002). However, Cx37 has also been detected in this cell type under pathological conditions (Rummery et al. 2002; Simon and McWhorter 2002; Alonso et al. 2010b).
In arteries, GJs integrate the function of SMCs by coordinating changes in both membrane potential and intracellular calcium (Ca2+) concentrations between adjacent cells (Christ et al. 1996; Segal 2015). Within the endothelium, Cxs contribute to the control of agonist-induced vasodilatory responses by regulating the spreading of Ca2+ transients between adjacent ECs (Boittin et al. 2013; Segal 2015), as well as by controlling endothelial nitric oxide (NO) production through direct interactions with the endothelial NO synthase (Alonso et al. 2010a; Pfenniger et al. 2010; Meens et al. 2015). In arterioles, in which a single layer of SMCs surrounds the monolayered endothelium, myoendothelial GJs, established through discontinuities of the endothelial basement membrane, permit bidirectional electrical and chemical communications between ECs and SMCs, ensuring that both cell types display equivalent membrane potential (Emerson and Segal 2000; de Wit et al. 2008). These heterocellular communications also contribute to the control of blood flow and vasomotor responses along large distances, by either the spreading of cytosolic Ca2+ (Dora 2001) or the transmission of hyperpolarization and NO from the endothelium to the smooth muscle (Sandow et al. 2002; Figueroa et al. 2013).
The observations that a number of polymorphisms of EC Cx genes are associated with several human vascular diseases (Boerma et al. 1999; Firouzi et al. 2006; Zoidl and Dermietzel 2010; Schmidt et al. 2015), and that the targeted deletion of selected Cx genes induce vascular phenotypes in mice (de Wit et al. 2000, 2003; Simon and McWhorter 2002; Haefliger et al. 2004, 2006), suggest that EC Cxs are also implicated in other physiological and pathological functions of vessels. In particular, Cx40−/− mice are hypertensive due to aberrant renin angiotensin system activity (Krattinger et al. 2007; Wagner et al. 2007; Le Gal et al. 2014), and display an impaired endothelial-dependent dilation of arteries (Alonso et al. 2010a; Jobs et al. 2012; Meens et al. 2015). In contrast, Cx37−/− animals show defective formation of lymphatic and venous valves (Kanady and Simon 2011; Munger et al. 2013), and develop atherosclerotic lesions (Wong et al. 2006). Intriguingly, the expression of EC Cx40 and Cx37 decreases during diabetes (Hou et al. 2008), inflammation (Simon et al. 2004; Rignault et al. 2007), and in the early stages of atherosclerosis (Kwak et al. 2002), while their expression increases during hypertension (Alonso et al. 2010b). These observations suggest that Cx expression is somehow linked to alterations in the structure and/or function of vessels. The further finding that Cx40 and Cx37 channels contribute to control the production and diffusion of EC-derived NO (Alonso et al. 2010a; Pfenniger et al. 2010; Figueroa et al. 2013) provides a first mechanistic clue to our understanding of how Cx signaling contributes to EC function and blood vessel homeostasis.
Many recent studies have now identified that EC Cxs also play significant roles in the microcirculation. Specifically, a growing body of evidence points to a crucial role for these proteins in the development, growth, and remodeling of microvascular networks, under both physiological and pathological conditions. In the following sections, we shall review these studies, with a special focus on endothelial Cxs, given the prominent role of ECs in the formation and maintenance of microvessels.
ROLE OF ENDOTHELIAL Cxs DURING DEVELOPMENTAL ANGIOGENESIS
The finding that mice lacking both Cx40 and Cx37 die perinatally due to hemorrhages and vascular dysmorphia (Simon and McWhorter 2002), provided the first clue that EC Cxs likely participate in the formation of blood vessels, at least in the skin, the testis, gastrointestinal tissues and lungs. Interestingly, EC–cell contacts, tight junction protein expression and vascular permeability were unaffected in the Cx40 and Cx37 double deficient vessels outside of the hemorrhagic areas, suggesting that EC Cxs are not required for the establishment of EC contacts, but are likely involved in the maintenance of microvascular integrity. The authors further suggested an overlapping role for Cx37 and Cx40 during vascular development, inasmuch as the expression of some Cx37 in Cx40−/− Cx37+/− animals was sufficient to prevent the embryonic lethality and the vascular defects observed in Cx40−/− Cx37−/− mice (Simon and McWhorter 2002).
More recent studies have established that the loss of either Cx40 or Cx37 is sufficient to affect the formation of microvascular networks, inasmuch as the selective knockdown of either one of these two Cx isoforms in ECs decreased the number of capillary branches formed in in vitro angiogenesis assays (Gärtner et al. 2012). Further studies have extended these findings to in vivo physiological angiogenesis. Thus, in the newborn mouse retina model (Stahl et al. 2010), the loss of either Cx37 or Cx40 results in a decreased extension of the growing microvascular network (Fig. 2; Haefliger et al. 2017; Hamard et al. 2020). However, the defects in vascular development and maturation differed between the loss of Cx40 and of Cx37 (Fig. 2; Fang et al. 2017; Haefliger et al. 2017; Hamard et al. 2020).
Thus, the loss of Cx40 reduced EC proliferation, sprouting, and capillary density at the angiogenic front, while enhancing the mural cell coverage of the neovessels (Fig. 2; Haefliger et al. 2017). These findings correlated with an increased secretion of PDGF-BB by the ECs lacking Cx40, in line with the established role of GJ-dependent cell-to-cell communication in multiple secretory systems (Bosco et al. 2011). Strikingly, the selective reestablishment of Cx40 in ECs was sufficient to correct the angiogenesis defects observed in the retinas of Cx40−/− mice, whereas the inhibition of Cx40 channels in wild-type animals, after the intravitreal injections of peptides that functionally target Cx40, closely mimicked the effects of Cx40 deficiency during retinal angiogenesis (Haefliger et al. 2017).
The developing vascular network of perinatal retina observed after loss of Cx37 markedly differs from that observed in Cx40−/− mice (Fig. 2). Thus, loss of Cx37 increased the vascular density at the angiogenic front, due to an increased proliferation and sprouting of ECs (Hamard et al. 2020), in line with the previously reported suppressive growth function of Cx37 (Burt et al. 2008). In the developing retina, this role affects the maturation and remodeling of the primitive vascular plexus, initiated by blood flow–induced shear stress, which activates a NOTCH-Cx37-p27 cascade, leading to the down-regulation of the EC cycle and to arteriovenous specification (Fang et al. 2017). Loss of Cx37 also reduced the mural cell recruitment to newly formed vessels (Hamard et al. 2020). This defect in neovessel maturation was associated with reduced release of PDGF-BB and increased secretion of angiopoietin-2 by Cx37-deficient ECs, in contrast with the increased secretion of PDGF-BB observed in ECs lacking Cx40. Altogether, the data indicate that despite their coexpression in EC GJs, Cx40 and Cx37 play distinct, yet complementary roles during physiological angiogenesis, differentially controlling EC growth and neovessel maturation and specification (Fig. 2). However, further studies are needed to elucidate whether the vascular defects associated with EC Cx deficiency during development affect visual acuity or interfere with retinal vascular diseases, such as ischemic retinopathy and age-related macular degeneration, at adulthood.
ROLE OF ENDOTHELIAL Cxs DURING PATHOLOGICAL ANGIOGENESIS
Recent studies reported that both Cx37 and Cx40 influence the pathogenesis of hereditary hemorrhagic telangiectasia (HHT), a genetic vascular disorder characterized by anastomoses between arterioles and venules (arteriovenous malformation [AVM]), and by focal dilations of the vessels, which compromise their functionality and integrity. The disease is caused by different mutations in the genes encoding components of the BMP9/10-ALK1-Smad1/5/9 signaling pathway (Robert et al. 2020). In a mouse model of HHT with disrupted SMAD1/5 signaling, AVM-like vascular malformations selectively develop in regions lacking Cx37 (Peacock et al. 2020), supporting the view that this Cx, whose expression is controlled by shear stress (Pfenniger et al. 2012), is key for a proper response of EC to increased blood flow (Peacock et al. 2020). Cx40 may also be associated with the pathogenesis of HHT, inasmuch as its expression is reduced in EC within the skin of HHT2 patients (Gkatzis et al. 2016). Furthermore, AVM-like vascular malformations are frequently observed in ALK1/Cx40 double heterozygous mice, which show increased production of reactive oxygen species (Gkatzis et al. 2016). Taken together, these data suggest that low expression of either Cx37 or Cx40 predisposes to vascular HHT lesions, and that human polymorphisms in EC Cx genes may influence the progression of the disease.
In addition to their roles in developmental angiogenesis and morphogenesis, different studies have also identified a role for EC Cxs during blood vessel formation in adult tissues. For example, deletion of Cx40, as well as the targeting of this protein by peptides designed to bind to its extracellular domains, decreases the angiogenic potential of ECs, both ex vivo in the aortic ring-sprouting assay and in vivo in subcutaneously implanted Matrigel plugs (Alonso et al. 2016).
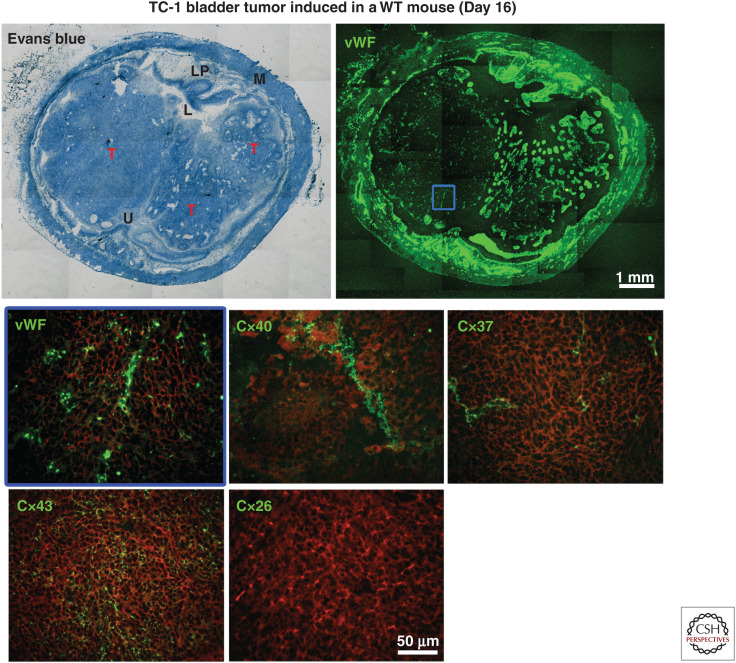
There is also a large body of circumstantial evidence associating altered GJs, Cx expression, and/or subcellular localization to the abnormal growth of many cancer cells (Aasen et al. 2016). Accordingly, reinduction of Cx expression and GJ communication between tumor cells may be sufficient to reduce their growth, indicating a tumor suppressor role of at least some Cx isoforms (Asencio-Barría et al. 2019). In light of the presence of Cx40 and Cx37 in the vessels of animal models of cancer (Fig. 3) and human tumors (Alonso et al. 2016), and the well-established influence of increased angiogenesis in tumor progression, several studies have questioned whether EC Cxs could also play this role and participate in tumor progression (McLachlan et al. 2006; Alonso et al. 2016; Thuringer et al. 2016a,b; Okamoto et al. 2019). Direct experimental testing of this hypothesis has revealed that, in mice, the loss of Cx40, as well as the systemic targeting of Cx40 channels by mimetic peptides, reduces angiogenesis, resulting in an inhibition of tumor growth, which significantly extended the survival of the tumor-bearing mice (Alonso et al. 2016). These effects were associated with enhanced perfusion of the tumoral vessels, which also featured increased mural cell recruitment, suggesting an improvement of the vessel structure and function. In turn, these changes improved the effectiveness of an experimental, systemic chemotherapy, which resulted in a prolonged survival of the Cx40-null mice carrying the tumors. If these observations in mice were translatable to the clinic, a pharmacological inhibition of Cx40 could be an obvious candidate strategy for the development of innovative cancer therapies. However, given that Cx40 also plays important functions in the cardiac conduction system and in renin-secreting cells of the kidney by regulating cardiac contractions and blood pressure, respectively (Desplantez et al. 2007; Bosco et al. 2011), the tissue selectivity of the purported treatments should be addressed. Even though no serious off target secondary effects have been reported in mice exposed to the Cx40 mimetic peptides, additional investigations should evaluate local delivery systems to make the strategy available in the human clinic. Comparable experiments are addressing whether Cx37 could also be a suitable target to inhibit tumoral angiogenesis and growth (K Sathiyanadan, F Alonso, S Domingos-Pereira et al., unpubl.). Preliminary observations suggest that the loss of Cx37 signaling also reduces tumor angiogenesis and growth. However, the mechanisms involving Cx37 probably differ from those involving Cx40, as the structure and function of tumor vessels did not appear altered in Cx37-deficient animals. These data also suggest that the two Cxs act together in promoting tumor growth, given that the systemic targeting of Cx40 channels in Cx37−/− mice resulted in a more pronounced antitumor effect compared to untreated Cx37−/− and wild-type treated animals (K Sathiyanadan, F Alonso, S Domingos-Pereira et al., unpubl.).
Figure 3.
Detection of Cx40 and Cx37 within the vasculature of TC-1 tumors. Representative tumors (Ts) grown 16 days after the instillation into the mouse bladder of TC-1 cells. (M) Muscle, (LP) lamina propria, (L) lumen, (U) urothelium (upper left panel). Immunostaining for the endothelial cell marker Von Willebrand factor (vWF) reveals the microvessels of bladder and tumors (upper right panel). Higher magnification of the tumor area delimited by the blue square is shown. Immunostaining for different connexin isoforms (green), performed on consecutive sections, reveals Cx40 and Cx37 in the tumor vessels. The different distribution of Cx43 indicates that this connexin is expressed by tumor cells. No Cx26 was observed in the tumors (Cx26 is known to be expressed between normal bladder urothelial cells). The red background is produced by Evans blue counterstain when activated with green light (lower panels).
In certain systems, EC Cxs may mediate a direct cross talk between ECs and tumor cells. Such heterocellular communication may influence tumor progression by enabling the transfer of regulatory microRNAs with proangiogenic or antitumoral effects (Thuringer et al. 2016a,b). At first glance, this effect may not seem consistent with the observations reported above, in which an antitumoral effect was found after loss of EC Cxs. Understanding this apparent discrepancy awaits future studies comparing the different types of cancer, and takes into account the likely quantitative and functional differences of Cx channels in different models.
In addition to its role in tumor growth, heterocellular communication between ECs and tumor cells is also implicated in tumor cell invasion and metastasis (El-Sabban et al. 2002; Bazarbachi et al. 2004). As a consequence, new combinatorial therapies in preclinical studies targeting tumor vascularization and GJ communication have shown promising results in preventing tumor cell extravasation and metastasis (Zibara et al. 2015).
EC Cxs influence tissue recovery following ischemic events (Yu et al. 2019). Mice with endothelial-specific deletion of Cx40 featured an increased infarct size after myocardial ischemia reperfusion injury (Morel et al. 2014). Moreover, Cx40, in association with VEGFR2, was identified as an early marker of the endocardial-to-endothelial switch that occurs after myocardial infarction in mice, to promote angiogenesis and arteriogenesis during cardiac repair (Miquerol et al. 2015). In a mouse model of hindlimb ischemia, Cx40−/− animals showed reduced limb perfusion and survival due to compromised arteriogenesis (Fang et al. 2012). In contrast, Cx37−/− mice featured a faster recovery of ischemic lesions, involving an enhanced number of collateral vessels as well as an increased collateral remodeling and angiogenesis (Fang et al. 2011). Together, these results demonstrate the crucial role of both vascular EC Cxs in postischemic responses, with Cx40 required for postischemic tissue survival and collateral remodeling, and Cx37 exerting a growth suppressive effect. One should expect that the proangiogenic activity of Cx40 and the growth inhibitory function of Cx37 would be critical for the fine tuning of blood vessel formation, which is needed to avoid uncontrolled angiogenesis and poor neovessel function. Different cell types, expressing high levels of Cx40, are now available for a direct experimental testing of innovative approaches to control pathological angiogenesis, as well as ischemia-reperfusion injuries (Joo et al. 2015; Vilà-Gonzàlez et al. 2019).
CONCLUSIONS
Studies performed during the last 20 years have unraveled a role for EC Cxs in the formation of blood vessels. These studies demonstrate that altered EC Cx expression or function may contribute to microvascular malformations, and possibly to angiogenesis-related diseases. Specifically, Cx40 displays proangiogenic functions by promoting vessel growth and limiting mural cell recruitment, whereas Cx37 mediates growth-suppressive effects and is required for proper arteriovenous differentiation. Thus, these two Cxs, by regulating different, yet complementary mechanisms, contribute to regulate the fine balance between the stimulatory and inhibitory signals required for physiological angiogenesis. They further suggest that the two Cxs may regulate pathological angiogenesis, notably in the context of tumor growth. Obviously, further studies are required to interrogate our ideas of the molecular mechanisms whereby EC Cxs regulate blood vessel morphogenesis, as well as to determine the usefulness and safety of Cx-targeted approaches in the development of novel strategies to control abnormal angiogenesis in cancer and ischemia reperfusion injury.
ACKNOWLEDGMENTS
This work was supported by a grant from the Fondation Lefoulon-Delalande (to F.A.), and grants from the SNF (31003A-175452/1) and the Swiss Cancer League (KFS-3796-02-2016) (to J.-A.H.).
Footnotes
Editors: Diane R. Bielenberg and Patricia A. D'Amore
Additional Perspectives on Angiogenesis: Biology and Pathology available at www.perspectivesinmedicine.org
REFERENCES
- Aasen T, Mesnil M, Naus CC, Lampe PD, Laird DW. 2016. Gap junctions and cancer: communicating for 50 years. Nat Rev Cancer 16: 775–788. 10.1038/nrc.2016.105 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alonso F, Boittin FX, Bény JL, Haefliger JA. 2010a. Loss of connexin40 is associated with decreased endothelium-dependent relaxations and eNOS levels in the mouse aorta. Am J Physiol Heart Circ Physiol 299: H1365–H1373. 10.1152/ajpheart.00029.2010 [DOI] [PubMed] [Google Scholar]
- Alonso F, Krattinger N, Mazzolai L, Simon A, Waeber G, Meda P, Haefliger JA. 2010b. An angiotensin II- and NF-κB-dependent mechanism increases connexin 43 in murine arteries targeted by renin-dependent hypertension. Cardiovasc Res 87: 166–176. 10.1093/cvr/cvq031 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alonso F, Domingos-Pereira S, Le Gal L, Derré L, Meda P, Jichlinski P, Nardelli-Haefliger D, Haefliger JA. 2016. Targeting endothelial connexin40 inhibits tumor growth by reducing angiogenesis and improving vessel perfusion. Oncotarget 7: 14015–14028. 10.18632/oncotarget.7370 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Asencio-Barría C, Defamie N, Sáez JC, Mesnil M, Godoy AS. 2019. Direct intercellular communications and cancer: a snapshot of the biological roles of connexins in prostate cancer. Cancers (Basel) 11: 1370. 10.3390/cancers11091370 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bazarbachi A, Abou Merhi R, Gessain A, Talhouk R, El-Khoury H, Nasr R, Gout O, Sulahian R, Homaidan F, de The H, et al. 2004. Human T-cell lymphotropic virus type I-infected cells extravasate through the endothelial barrier by a local angiogenesis-like mechanism. Cancer Res 64: 2039–2046. 10.1158/0008-5472.CAN-03-2390 [DOI] [PubMed] [Google Scholar]
- Boerma M, Forsberg L, Van Zeijl L, Morgenstern R, De Faire U, Lemne C, Erlinge D, Thulin T, Hong Y, Cotgreave IA. 1999. A genetic polymorphism in connexin 37 as a prognostic marker for atherosclerotic plaque development. J Inter Med 246: 211–218. 10.1046/j.1365-2796.1999.00564.x [DOI] [PubMed] [Google Scholar]
- Boittin FX, Alonso F, Le Gal L, Allagnat F, Bény JL, Haefliger JA. 2013. Connexins and M3 muscarinic receptors contribute to heterogeneous Ca2+ signaling in mouse aortic endothelium. Cell Physiol Biochem 31: 166–178. 10.1159/000343358 [DOI] [PubMed] [Google Scholar]
- Bosco D, Haefliger JA, Meda P. 2011. Connexins: key mediators of endocrine function. Physiol Rev 91: 1393–1445. 10.1152/physrev.00027.2010 [DOI] [PubMed] [Google Scholar]
- Bruzzone R, White TW, Goodenough DA. 1996. The cellular internet: on-line with connexins. Bioessays 18: 709–718. 10.1002/bies.950180906 [DOI] [PubMed] [Google Scholar]
- Burt JM, Nelson TK, Simon AM, Fang JS. 2008. Connexin 37 profoundly slows cell cycle progression in rat insulinoma cells. Am J Physiol Cell Physiol 295: C1103–C1112. 10.1152/ajpcell.299.2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen CH, Mayo JN, Gourdie RG, Johnstone SR, Isakson BE, Bearden SE. 2015. The connexin 43/ZO-1 complex regulates cerebral endothelial F-actin architecture and migration. Am J Physiol Cell Physiol 309: C600–C607. 10.1152/ajpcell.00155.2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Christ GJ, Spray DC, el-Sabban M, Moore LK, Brink PR. 1996. Gap junctions in vascular tissues. evaluating the role of intercellular communication in the modulation of vasomotor tone. Circ Res 79: 631–646. 10.1161/01.RES.79.4.631 [DOI] [PubMed] [Google Scholar]
- Delmar M, Laird DW, Naus CC, Nielsen MS, Verselis VK, White TW. 2018. Connexins and disease. Cold Spring Harb Perspect Biol 10: a029348. 10.1101/cshperspect.a029348 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Desplantez T, Dupont E, Severs NJ, Weingart R. 2007. Gap junction channels and cardiac impulse propagation. J Membr Biol 218: 13–28. 10.1007/s00232-007-9046-8 [DOI] [PubMed] [Google Scholar]
- de Wit C, Roos F, Bolz SS, Kirchhoff S, Krüger O, Willecke K, Pohl U. 2000. Impaired conduction of vasodilation along arterioles in connexin40-deficient mice. Circ Res 86: 649–655. 10.1161/01.RES.86.6.649 [DOI] [PubMed] [Google Scholar]
- de Wit C, Roos F, Bolz SS, Pohl U. 2003. Lack of vascular connexin 40 is associated with hypertension and irregular arteriolar vasomotion. Physiol Genomics 13: 169–177. 10.1152/physiolgenomics.00169.2002 [DOI] [PubMed] [Google Scholar]
- de Wit C, Boettcher M, Schmidt VJ. 2008. Signaling across myoendothelial gap junctions—fact or fiction? Cell Commun Adhes 15: 231–245. 10.1080/15419060802440260 [DOI] [PubMed] [Google Scholar]
- Dora KA. 2001. Cell-cell communication in the vessel wall. Vasc Med 6: 43–50. 10.1177/1358836X0100600108 [DOI] [PubMed] [Google Scholar]
- El-Sabban ME, Merhi RA, Haidar HA, Arnulf B, Khoury H, Basbous J, Nijmeh J, de Thé H, Hermine O, Bazarbachi A. 2002. Human T-cell lymphotropic virus type 1-transformed cells induce angiogenesis and establish functional gap junctions with endothelial cells. Blood 99: 3383–3389. 10.1182/blood.V99.9.3383 [DOI] [PubMed] [Google Scholar]
- Emerson GG, Segal SS. 2000. Electrical coupling between endothelial cells and smooth muscle cells in hamster feed arteries: role in vasomotor control. Circ Res 87: 474–479. 10.1161/01.RES.87.6.474 [DOI] [PubMed] [Google Scholar]
- Fang JS, Angelov SN, Simon AM, Burt JM. 2011. Cx37 deletion enhances vascular growth and facilitates ischemic limb recovery. Am J Physiol Heart Circ Physiol 301: H1872–H1881. 10.1152/ajpheart.00683.2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fang JS, Angelov SN, Simon AM, Burt JM. 2012. Cx40 is required for, and cx37 limits, postischemic hindlimb perfusion, survival and recovery. J Vasc Res 49: 2–12. 10.1159/000329616 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fang JS, Coon BG, Gillis N, Chen Z, Qiu J, Chittenden TW, Burt JM, Schwartz MA, Hirschi KK. 2017. Shear-induced Notch-Cx37-p27 axis arrests endothelial cell cycle to enable arterial specification. Nat Commun 8: 2149. 10.1038/s41467-017-01742-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Figueroa XF, Lillo MA, Gaete PS, Riquelme MA, Sáez JC. 2013. Diffusion of nitric oxide across cell membranes of the vascular wall requires specific connexin-based channels. Neuropharmacology 75: 471–478. 10.1016/j.neuropharm.2013.02.022 [DOI] [PubMed] [Google Scholar]
- Firouzi M, Kok B, Spiering W, Busjahn A, Bezzina CR, Ruijter JM, Koeleman BP, Schipper M, Groenewegen WA, Jongsma HJ, et al. 2006. Polymorphisms in human connexin40 gene promoter are associated with increased risk of hypertension in men. J Hypertens 24: 325–330. 10.1097/01.hjh.0000200512.40818.47 [DOI] [PubMed] [Google Scholar]
- Gabriels JE, Paul DL. 1998. Connexin43 is highly localized to sites of disturbed flow in rat aortic endothelium but connexin37 and connexin40 are more uniformly distributed. Circ Res 83: 636–643. 10.1161/01.RES.83.6.636 [DOI] [PubMed] [Google Scholar]
- Gärtner C, Ziegelhöffer B, Kostelka M, Stepan H, Mohr FW, Dhein S. 2012. Knock-down of endothelial connexins impairs angiogenesis. Pharmacol Res 65: 347–357. 10.1016/j.phrs.2011.11.012 [DOI] [PubMed] [Google Scholar]
- Gellhaus A, Wotzlaw C, Otto T, Fandrey J, Winterhager E. 2010. More insights into the CCN3/Connexin43 interaction complex and its role for signaling. J Cell Biochem 110: 129–140. [DOI] [PubMed] [Google Scholar]
- Gkatzis K, Thalgott J, Dos-Santos-Luis D, Martin S, Lamandé N, Carette MF, Disch F, Snijder RJ, Westermann CJ, Mager JJ, et al. 2016. Interaction between ALK1 signaling and Connexin40 in the development of arteriovenous malformations. Arterioscler Thromb Vasc Biol 36: 707–717. 10.1161/ATVBAHA.115.306719 [DOI] [PubMed] [Google Scholar]
- Haefliger JA, Nicod P, Meda P. 2004. Contribution of connexins to the function of the vascular wall. Cardiovasc Res 62: 345–356. 10.1016/j.cardiores.2003.11.015 [DOI] [PubMed] [Google Scholar]
- Haefliger JA, Krattinger N, Martin D, Pedrazzini T, Capponi A, Doring B, Plum A, Charollais A, Willecke K, Meda P. 2006. Connexin43-dependent mechanism modulates renin secretion and hypertension. J Clin Invest 116: 405–413. 10.1172/JCI23327 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haefliger JA, Allagnat F, Hamard L, Le Gal L, Meda P, Nardelli-Haefliger D, Génot E, Alonso F. 2017. Targeting Cx40 (Connexin40) expression or function reduces angiogenesis in the developing mouse retina. Arterioscler Thromb Vasc Biol 37: 2136–2146. 10.1161/ATVBAHA.117.310072 [DOI] [PubMed] [Google Scholar]
- Hamard L, Santoro T, Allagnat F, Meda P, Nardelli-Haefliger D, Alonso F, Haefliger JA. 2020. Targeting connexin37 alters angiogenesis and arteriovenous differentiation in the developing mouse retina. FASEB J 34: 8234–8249. 10.1096/fj.202000257R [DOI] [PubMed] [Google Scholar]
- Hill CE, Rummery N, Hickey H, Sandow SL. 2002. Heterogeneity in the distribution of vascular gap junctions and connexins: implications for function. Clin Exp Pharmacol Physiol 29: 620–625. 10.1046/j.1440-1681.2002.03699.x [DOI] [PubMed] [Google Scholar]
- Hou CJ, Tsai CH, Su CH, Wu YJ, Chen SJ, Chiu JJ, Shiao MS, Yeh HI. 2008. Diabetes reduces aortic endothelial gap junctions in ApoE-deficient mice: simvastatin exacerbates the reduction. J Histochem Cytochem 56: 745–752. 10.1369/jhc.2008.950816 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jobs A, Schmidt K, Schmidt VJ, Lübkemeier I, van Veen TA, Kurtz A, Willecke K, de Wit C. 2012. Defective Cx40 maintains Cx37 expression but intact Cx40 is crucial for conducted dilations irrespective of hypertension. Hypertension 60: 1422–1429. 10.1161/HYPERTENSIONAHA.112.201194 [DOI] [PubMed] [Google Scholar]
- Joo HJ, Song S, Seo HR, Shin JH, Choi SC, Park JH, Yu CW, Hong SJ, Lim DS. 2015. Human endothelial colony forming cells from adult peripheral blood have enhanced sprouting angiogenic potential through up-regulating VEGFR2 signaling. Int J Cardiol 197: 33–43. 10.1016/j.ijcard.2015.06.013 [DOI] [PubMed] [Google Scholar]
- Kanady JD, Simon AM. 2011. Lymphatic communication: connexin junction, what's your function? Lymphology 44: 95–102. [PMC free article] [PubMed] [Google Scholar]
- Krattinger N, Capponi A, Mazzolai L, Aubert JF, Caille D, Nicod P, Waeber G, Meda P, Haefliger JA. 2007. Connexin40 regulates renin production and blood pressure. Kidney Int 72: 814–822. 10.1038/sj.ki.5002423 [DOI] [PubMed] [Google Scholar]
- Kruger O, Plum A, Kim JS, Winterhager E, Maxeiner S, Hallas G, Kirchhoff S, Traub O, Lamers WH, Willecke K. 2000. Defective vascular development in connexin 45-deficient mice. Development 127: 4179–4193. 10.1242/dev.127.19.4179 [DOI] [PubMed] [Google Scholar]
- Kwak BR, Mulhaupt F, Veillard N, Gros DB, Mach F. 2002. Altered pattern of vascular connexin expression in atherosclerotic plaques. Arterioscler Thromb Vasc Biol 22: 225–230. 10.1161/hq0102.104125 [DOI] [PubMed] [Google Scholar]
- Kwak BR, Silacci P, Stergiopulos N, Hayoz D, Meda P. 2005. Shear stress and cyclic circumferential stretch, but not pressure, alter connexin43 expression in endothelial cells. Cell Commun Adhes 12: 261–270. 10.1080/15419060500514119 [DOI] [PubMed] [Google Scholar]
- Le Gal L, Alonso F, Wagner C, Germain S, Nardelli Haefliger D, Meda P, Haefliger JA. 2014. Restoration of connexin 40 (Cx40) in Renin-producing cells reduces the hypertension of Cx40 null mice. Hypertension 63: 1198–1204. 10.1161/HYPERTENSIONAHA.113.02976 [DOI] [PubMed] [Google Scholar]
- Leithe E, Mesnil M, Aasen T. 2018. The connexin 43 C-terminus: a tail of many tales. Biochim Biophys Acta Biomembr 1860: 48–64. 10.1016/j.bbamem.2017.05.008 [DOI] [PubMed] [Google Scholar]
- Martins-Marques T, Anjo SI, Pereira P, Manadas B, Girão H. 2015. Interacting network of the gap junction (GJ) protein connexin43 (Cx43) is modulated by ischemia and reperfusion in the heart. Mol Cell Proteomics 14: 3040–3055. 10.1074/mcp.M115.052894 [DOI] [PMC free article] [PubMed] [Google Scholar]
- McLachlan E, Shao Q, Wang HL, Langlois S, Laird DW. 2006. Connexins act as tumor suppressors in three-dimensional mammary cell organoids by regulating differentiation and angiogenesis. Cancer Res 66: 9886–9894. 10.1158/0008-5472.CAN-05-4302 [DOI] [PubMed] [Google Scholar]
- Meens MJ, Alonso F, Le Gal L, Kwak BR, Haefliger JA. 2015. Endothelial connexin37 and connexin40 participate in basal but not agonist-induced NO release. Cell Commun Signal 13: 34. 10.1186/s12964-015-0110-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miquerol L, Thireau J, Bideaux P, Sturny R, Richard S, Kelly RG. 2015. Endothelial plasticity drives arterial remodeling within the endocardium after myocardial infarction. Circ Res 116: 1765–1771. 10.1161/CIRCRESAHA.116.306476 [DOI] [PubMed] [Google Scholar]
- Molica F, Figueroa XF, Kwak BR, Isakson BE, Gibbins JM. 2018. Connexins and pannexins in vascular function and disease. Int J Mol Sci 19: 1663. 10.3390/ijms19061663 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morel S, Braunersreuther V, Chanson M, Bouis D, Rochemont V, Foglia B, Pelli G, Sutter E, Pinsky DJ, Mach F, et al. 2014. Endothelial Cx40 limits myocardial ischaemia/reperfusion injury in mice. Cardiovasc Res 102: 329–337. 10.1093/cvr/cvu063 [DOI] [PubMed] [Google Scholar]
- Munger SJ, Kanady JD, Simon AM. 2013. Absence of venous valves in mice lacking Connexin37. Dev Biol 373: 338–348. 10.1016/j.ydbio.2012.10.032 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Okamoto T, Usuda H, Tanaka T, Wada K, Shimaoka M. 2019. The functional implications of endothelial gap junctions and cellular mechanics in vascular angiogenesis. Cancers (Basel) 11: 237. 10.3390/cancers11020237 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peacock HM, Tabibian A, Criem N, Caolo V, Hamard L, Deryckere A, Haefliger JA, Kwak BR, Zwijsen A, Jones EAV. 2020. Impaired SMAD1/5 mechanotransduction and Cx37 (connexin37) expression enable pathological vessel enlargement and shunting. Arterioscler Thromb Vasc Biol 40: e87–e104. 10.1161/ATVBAHA.119.313122 [DOI] [PubMed] [Google Scholar]
- Pfenniger A, Derouette JP, Verma V, Lin X, Foglia B, Coombs W, Roth I, Satta N, Dunoyer-Geindre S, Sorgen P, et al. 2010. Gap junction protein Cx37 interacts with endothelial nitric oxide synthase in endothelial cells. Arterioscler Thromb Vasc Biol 30: 827–834. 10.1161/ATVBAHA.109.200816 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pfenniger A, Wong C, Sutter E, Cuhlmann S, Dunoyer-Geindre S, Mach F, Horrevoets AJ, Evans PC, Krams R, Kwak BR. 2012. Shear stress modulates the expression of the atheroprotective protein Cx37 in endothelial cells. J Mol Cell Cardiol 53: 299–309. 10.1016/j.yjmcc.2012.05.011 [DOI] [PubMed] [Google Scholar]
- Pohl U. 2020. Connexins: key players in the control of vascular plasticity and function. Physiol Rev 100: 525–572. 10.1152/physrev.00010.2019 [DOI] [PubMed] [Google Scholar]
- Rignault S, Haefliger JA, Waeber B, Liaudet L, Feihl F. 2007. Acute inflammation decreases the expression of connexin 40 in mouse lung. Shock 28: 78–85. 10.1097/shk.0b013e3180310bd1 [DOI] [PubMed] [Google Scholar]
- Robert F, Desroches-Castan A, Bailly S, Dupuis-Girod S, Feige JJ. 2020. Future treatments for hereditary hemorrhagic telangiectasia. Orphanet J Rare Dis 15: 4. 10.1186/s13023-019-1281-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rummery NM, Hill CE. 2004. Vascular gap junctions and implications for hypertension. Clin Exp Pharmacol Physiol 31: 659–667. 10.1111/j.1440-1681.2004.04071.x [DOI] [PubMed] [Google Scholar]
- Rummery NM, Hickey H, McGurk G, Hill CE. 2002. Connexin37 is the major connexin expressed in the media of caudal artery. Arterioscler Thromb Vasc Biol 22: 1427–1432. 10.1161/01.ATV.0000028814.45706.E5 [DOI] [PubMed] [Google Scholar]
- Sandow SL, Tare M, Coleman HA, Hill CE, Parkington HC. 2002. Involvement of myoendothelial gap junctions in the actions of endothelium-derived hyperpolarizing factor. Circ Res 90: 1108–1113. 10.1161/01.RES.0000019756.88731.83 [DOI] [PubMed] [Google Scholar]
- Schmidt K, Kaiser FJ, Erdmann J, Wit C. 2015. Two polymorphisms in the Cx40 promoter are associated with hypertension and left ventricular hypertrophy preferentially in men. Clin Exp Hypertens 37: 580–586. 10.3109/10641963.2015.1026043 [DOI] [PubMed] [Google Scholar]
- Segal SS. 2015. Integration and modulation of intercellular signaling underlying blood flow control. J Vasc Res 52: 136–157. 10.1159/000439112 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simon AM, McWhorter AR. 2002. Vascular abnormalities in mice lacking the endothelial gap junction proteins connexin37 and connexin40. Dev Biol 251: 206–220. 10.1006/dbio.2002.0826 [DOI] [PubMed] [Google Scholar]
- Simon AM, McWhorter AR, Chen H, Jackson CL, Ouellette Y. 2004. Decreased intercellular communication and connexin expression in mouse aortic endothelium during lipopolysaccharide-induced inflammation. J Vasc Res 41: 323–333. 10.1159/000079614 [DOI] [PubMed] [Google Scholar]
- Sohl G, Willecke K. 2004. Gap junctions and the connexin protein family. Cardiovasc Res 62: 228–232. 10.1016/j.cardiores.2003.11.013 [DOI] [PubMed] [Google Scholar]
- Stahl A, Connor KM, Sapieha P, Chen J, Dennison RJ, Krah NM, Seaward MR, Willett KL, Aderman CM, Guerin KI, et al. 2010. The mouse retina as an angiogenesis model. Invest Ophthal Vis Sci 51: 2813–2826. 10.1167/iovs.10-5176 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thuringer D, Boucher J, Jego G, Pernet N, Cronier L, Hammann A, Solary E, Garrido C. 2016a. Transfer of functional microRNAs between glioblastoma and microvascular endothelial cells through gap junctions. Oncotarget 7: 73925–73934. 10.18632/oncotarget.12136 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thuringer D, Jego G, Berthenet K, Hammann A, Solary E, Garrido C. 2016b. Gap junction-mediated transfer of miR-145-5p from microvascular endothelial cells to colon cancer cells inhibits angiogenesis. Oncotarget 7: 28160–28168. 10.18632/oncotarget.8583 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vilà-Gonzàlez M, Kelaini S, Magee C, Caines R, Campbell D, Eleftheriadou M, Cochrane A, Drehmer D, Tsifaki M, O'Neill K, et al. 2019. Enhanced function of induced pluripotent stem cell-derived endothelial cells through ESM1 signaling. Stem Cells 37: 226–239. 10.1002/stem.2936 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wagner C, de Wit C, Kurtz L, Grünberger C, Kurtz A, Schweda F. 2007. Connexin40 is essential for the pressure control of renin synthesis and secretion. Circ Res 100: 556–563. 10.1161/01.RES.0000258856.19922.45 [DOI] [PubMed] [Google Scholar]
- Willecke K, Eiberger J, Degen J, Eckardt D, Romualdi A, Güldenagel M, Deutsch U, Söhl G. 2002. Structural and functional diversity of connexin genes in the mouse and human genome. Biol Chem 383: 725–737. 10.1515/BC.2002.076 [DOI] [PubMed] [Google Scholar]
- Wong CW, Christen T, Roth I, Chadjichristos CE, Derouette JP, Foglia BF, Chanson M, Goodenough DA, Kwak BR. 2006. Connexin37 protects against atherosclerosis by regulating monocyte adhesion. Nat Med 12: 950–954. 10.1038/nm1441 [DOI] [PubMed] [Google Scholar]
- Yeager M, Unger VM, Falk MM. 1998. Synthesis, assembly and structure of gap junction intercellular channels. Curr Opin Struct Biol 8: 517–524. 10.1016/S0959-440X(98)80131-0 [DOI] [PubMed] [Google Scholar]
- Yu H, Kalogeris T, Korthuis RJ. 2019. Reactive species-induced microvascular dysfunction in ischemia/reperfusion. Free Radic Biol Med 135: 182–197. 10.1016/j.freeradbiomed.2019.02.031 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zibara K, Awada Z, Dib L, El-Saghir J, Al-Ghadban S, Ibrik A, El-Zein N, El-Sabban M. 2015. Anti-angiogenesis therapy and gap junction inhibition reduce MDA-MB-231 breast cancer cell invasion and metastasis in vitro and in vivo. Sci Rep 5: 12598. 10.1038/srep12598 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zoidl G, Dermietzel R. 2010. Gap junctions in inherited human disease. Pflugers Arch 460: 451–466. 10.1007/s00424-010-0789-1 [DOI] [PubMed] [Google Scholar]