Abstract
Background
Group B Streptococcus (GBS) remains a leading cause of infant morbidity and mortality. A candidate vaccine targets 6 GBS serotypes, offering a potential alternative to intrapartum antibiotic prophylaxis to reduce disease burden. However, our understanding of the contributions of specific capsule types to GBS colonization and disease remains limited.
Methods
Using allelic exchange, we generated isogenic GBS strains differing only in the serotype-determining region in 2 genetic backgrounds, including the hypervirulent clonal complex (CC) 17. Using a murine model of vaginal cocolonization, we evaluated the roles of the presence of capsule and of expression of specific capsular types in GBS vaginal colonization fitness independent of other genetic factors.
Results
Encapsulated wild-type strains COH1 (CC17, serotype III) and A909 (non-CC17, serotype Ia) outcompeted isogenic acapsular mutants in murine vaginal cocolonization. COH1 wild type outcompeted A909. Notably, expression of type Ia capsule conferred an advantage over type III capsule in both genetic backgrounds.
Conclusions
Specific capsule types may provide an advantage in GBS vaginal colonization in vivo. However, success of certain GBS lineages, including CC17, likely involves both capsule and noncapsule genetic elements. Capsule switching in GBS, a potential outcome of conjugate vaccine programs, may alter colonization fitness or pathogenesis.
Keywords: Streptococcus agalactiae, capsular polysaccharide, vaginal colonization
Using isogenic group B Streptococcusstrains in a murine model of asymptomatic vaginal colonization, we found a role for capsular serotype in colonization fitness, as well as a contribution of noncapsule genetic features.
Streptococcus agalactiae (group B Streptococcus [GBS]) remains a leading cause of neonatal disease [1–3]. The use of intrapartum antibiotic prophylaxis (IAP) has reduced the burden of early-onset GBS disease in newborns [4, 5]. However, rates of maternal GBS colonization and late-onset GBS disease remain nearly unchanged [5–8]. Furthermore, GBS disease burden remains high in regions where screening and IAP may not be widely available or feasible, particularly in sub-Saharan African nations [2, 7].
Maternal GBS vaccination may prove more feasible and effective than IAP in preventing GBS disease worldwide. The polysaccharide capsule is an important GBS virulence factor [9, 10]. Placental transfer of anticapsular maternal antibodies has been demonstrated to protect against early-onset GBS disease [11–13]. GBS6, a hexavalent capsular polysaccharide conjugate vaccine targeting serotypes Ia, Ib, II, III, IV, and V is well tolerated and immunogenic in healthy nonpregnant adults and has the potential to provide protection against most GBS isolates that cause invasive disease [8, 14–17]. However, it is possible that the implementation of a hexavalent polysaccharide vaccine may lead to serotype replacement with nonvaccine type strains, as was observed following pneumococcal vaccination in the United States [18, 19]. GBS capsular switching, including within the CC17 hypervirulent clone, has been documented [20–23].
Individual GBS serotypes vary in frequency of isolation from colonization or disease states [2, 3, 8, 16, 24]. Serotype III strains from clonal complex (CC) 17 are particularly common in late-onset GBS disease [8, 16, 25]. However, because serotype is nonrandomly associated with GBS genetic backgrounds as assessed by sequence type (ST) or CC assignment, it has been extremely challenging to determine whether there are independent effects of specific GBS serotypes on colonization or disease. Here, we used techniques to modify the GBS chromosome to generate otherwise isogenic bacterial strains differing only in capsular serotype. Employing these strains in a murine model of vaginal cocolonization probed the specific roles of type Ia and III capsule types in GBS fitness at the vaginal mucosal surface.
METHODS
Bacterial Strains and Growth Conditions
GBS wild-type (WT) strains A909 (serotype Ia, ST7; American Type Culture Collection [ATCC] BAA-1138) and COH1 (serotype III, CC17; ATCC BAA-1176) were used. HY106, a capsule-deficient mutant of COH1, was the kind gift of Dr Kelly Doran. Strain HY106 has a deletion of a portion of the cps locus that abolishes capsule synthesis [26]. HY106, WT strains, and their derivatives were grown at 37°C under stationary conditions in tryptic soy broth (Fisher). Chemically competent Escherichia coli DH5α (New England Biolabs) was stored and transformed according to the manufacturer’s instructions. E. coli growth was in LB medium at 37°C unless transformed with a temperature-sensitive plasmid, in which case the growth temperature was 28°C.
Murine Vaginal Colonization Model
All experimental procedures were reviewed and approved by the New York University Langone Institutional Animal Care and Use Committee. Female C57BL6/J mice, 6–8 weeks old, were purchased from Jackson Laboratories. Vaginal colonization was performed as previously described [27]. Animals were subcutaneously injected with 10 μg of water-soluble 17β-estradiol (Sigma-Aldrich) at 48 and 24 hours before colonization to synchronize the estrous cycle.
For the monocolonization model with WT A909 (Ia) or COH1 (III), bacterial cultures were grown overnight to stationary phase. Following overnight growth, the optical densities of the 2 strains were normalized to one another, and cultures were then centrifuged and resuspended in a 1:1 mixture of phosphate-buffered saline (PBS) and sterile 10% gelatin for a final concentration of 109 colony-forming units (CFU)/mL. Mice were anesthetized with 3%–5% isoflurane (Baxter), and 50 μL GBS suspension (total inoculum 5 × 107 CFU) was administered intravaginally using a sterile pipette. Mice were housed in separate cages for the remainder of the experiment. Vaginal swab specimens were collected using a sterile swab that was vigorously shaken into 300 μL of PBS. Serial dilutions were plated on CHROMagar (CHROMagar) for enumeration of CFU/mL.
For the cocolonization model, bacterial cultures were grown and centrifuged as described above. Cultures were resuspended in PBS, and a 1:1 mixture was made of the 2 resuspended strains to be competed. This mixture was then mixed 1:1 with sterile 10% gelatin. Mice were colonized, housed, and swabbed as described above. In addition to plating serial dilutions, dilutions were spread on CHROMagar plates with glass beads for determination of serotype by colony immunoblot.
Colony Immunoblotting
Following growth at 37°C, colonies were counted to identify plates with 20–200 colonies for subsequent immunoblotting. Plates were briefly overlaid with nitrocellulose membranes (Amersham) to allow adherence of GBS material to the membrane. Membranes were blocked in 3% bovine serum albumin (BSA) in PBS (blocking solution) for 1 hour. Blots were then transferred to type Ia or type III Streptococcus group B type antisera (Statens Serum Institut) and incubated for 30 minutes with gentle shaking. Type Ia antisera was diluted 1:2000 in blocking solution, and type III antisera was diluted 1:5000 in blocking solution. Blots were then washed 3 times in PBS. Blots were incubated in horseradish peroxidase (HRP)-conjugated goat anti-rabbit secondary antibody (Pierce) diluted 1:1000 in blocking solution for 2 hours. Blots were washed 3 times in PBS and stained using a 3,3\'-diaminobenzidine tetrahydrochloride substrate kit (Abcam). Colonies positive for each capsule type were counted. In experiments comparing WT to acapsular mutants, nonreacting GBS colonies representing the acapsular mutants were also counted. Competitive index (CFU strain 1 recovered/CFU strain 1 inoculated)/(CFU strain 2 recovered/CFU strain 2 inoculated) was calculated and log-transformed for calculation of geometric mean and 95% confidence interval for each condition and time point.
Generation of Capsule Switch, Capsule Deficient, and Revertant Strains
Prior work using plasmid overexpression suggested that cpsH expression in trans was sufficient to mediate expression of either type Ia or type III capsule in a heterologous strain background with an intact native cps locus [28]. Comparison of the cps loci between GBS types Ia and III demonstrate a region of difference consisting of the 3ʹ end of cpsG and the full open reading frame of cpsH [29]. We generated capsule switch strains by constructing a cassette consisting of the heterologous region of difference flanked by 500bp of homology to the target strain on each side. Each cassette was cloned into the temperature-sensitive plasmid pHY304 by Gibson assembly and transformed into electrocompetent E. coli DH5α. The insert was verified by polymerase chain reaction (PCR) and sequencing. Allelic exchange was performed essentially as described [30]. Briefly, the plasmid was transformed into electrocompetent WT A909 (Ia) or COH1 (III). Temperature-based selection was used to isolate single-cross and double-cross plasmid insertion and excision mutants, which were confirmed by PCR. A909 capsule switch mutants expressed type III capsule and COH1 capsule switch mutants expressed type Ia capsule, while revertant strains, which occur based on the direction of plasmid excision at the final step of the allelic exchange procedure, expressed their native capsule type. Capsule types of WT, capsule switch, and revertant strains were confirmed by latex agglutination with type Ia and type III sera (Immulex Strep-B kit; Statens Serum Institut) and by sequencing of the capsule locus.
CpsE catalyzes an essential step in the synthesis of the GBS polysaccharide repeating unit, and therefore inactivation of the cpsE gene leads to inactivation of capsule synthesis [31]. To generate capsule-deficient A909ΔcpsE, we amplified 500-bp homology arms to construct a cassette for in-frame deletion of the full open reading frame of cpsE. The cassette was cloned into pMBsacB, a temperature-sensitive and sucrose-counterselectable mutagenesis shuttle vector [30] using Gibson assembly and transformed into electrocompetent E. coli DH5α. The insert was verified by PCR and sequencing. Allelic exchange was performed essentially as described [30]. A909ΔcpsE was verified by PCR and loss of latex agglutination with type Ia sera. Revertant strains continued to express type Ia capsule.
Enzyme-Linked Lectin Assay
An enzyme-linked lectin assay (ELLA) was used to assess the amount of capsule present in each strain. All GBS capsules possess a terminal α-2,3 sialic acid. Sialylation has been shown to be important for the full synthesis of the GBS capsule [32], and sialic acid has therefore been used as a means to recognize capsule expression [33]. A previously described protocol [33] was adapted for whole bacteria [34]. Maackia amurensis I lectin (MAL-I; Vector Labs), which recognizes sialic acid as Neu5Acα-2,3-Galβ-1,4-GlcNAc [35], was used for recognition of sialic acid on GBS type Ia and type III capsule.
GBS strains were grown in 10mL of tryptic soy broth under stationary conditions at 37°C to an optical density at 600nm of 0.6. Cultures were then centrifuged and resuspended in 10mL of coating buffer (0.087 M NaHCO3 and 0.015 M Na2CO3 in sterile water, pH 9.5). Prepared suspensions were diluted 1:10 in coating buffer and 100 μL was distributed in the wells of an enzyme-linked immunosorbent assay (ELISA) plate. This plate was incubated overnight at 37°C to allow the wells to dry.
The following day, samples were fixed with 50 μL of 100% methanol and allowed to dry for 40 minutes at 37°C. Wells were then washed with 200 μL of PBS for 15 minutes at room temperature. Samples were blocked with 200 μL/well of 1% BSA in PBS for 4 hours, covered, at 37°C. Following blocking, 50 μL of 10 μg/mL biotinylated MAL-I in PBS with 1% BSA and 0.05% Tween (PBSAT) was distributed in each well, and the plate was incubated at room temperature for 1 hour. Wells were then washed 3 times with 200 μL PBSAT for 5 minutes. Streptavidin-HRP (Pierce) was diluted 1:20000 in PBSAT and 50 μL was distributed in each well. The plate was incubated at 37°C for 15 minutes. Wells were washed 3 times with PBSAT. 100 μL/well of TMB-ELISA substrate solution (Pierce) was added and the plate was incubated for 10 minutes at room temperature, protected from the light. The reaction was stopped with the addition of 50 μL/well of 1N H2SO4. Finally, the absorbance was read at 450nm in a plate reader.
Statistical Analyses
Persistence of vaginal monocolonization between WT A909 (Ia) and WT COH1 (III) was compared using the Log-rank (Mantel-Cox) test, and CFUs recovered from the vagina at each day were compared by 2-way analysis of variance with Sidak multiple comparison test. ELLA absorbance outcomes between strains were compared using Kruskal-Wallis test (Prism, GraphPad Software).
RESULTS
COH1 Outcompetes A909 in a Mouse Model of Vaginal Cocolonization
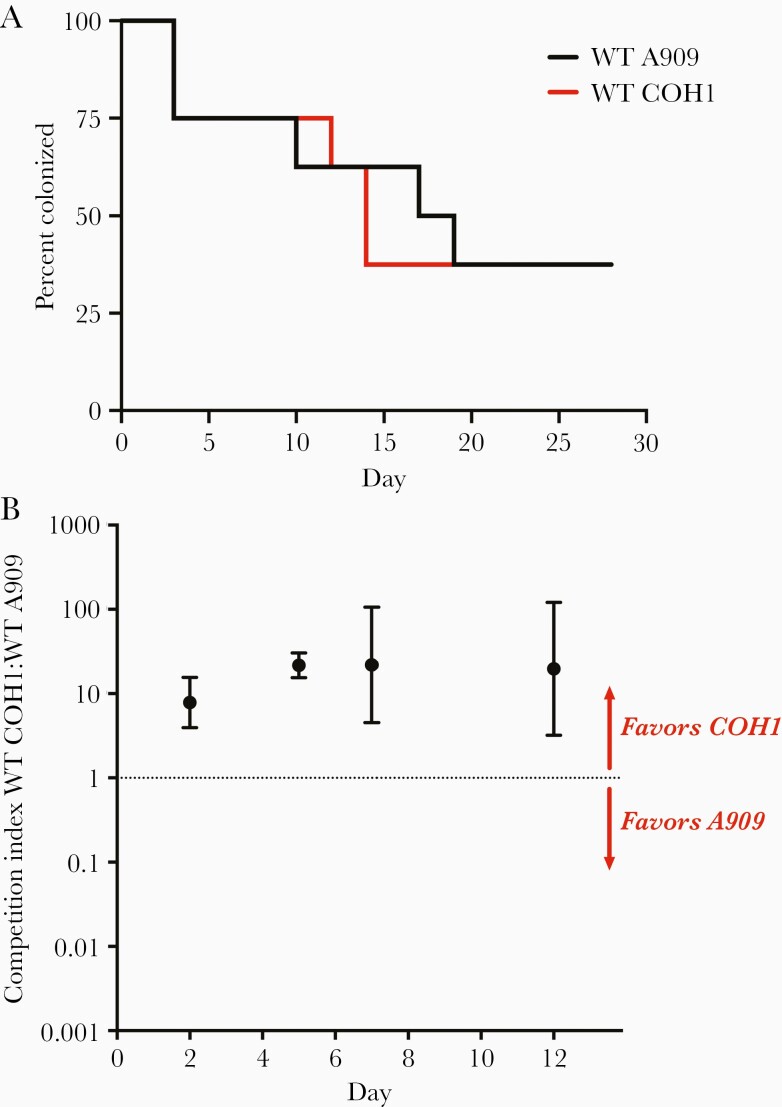
We vaginally colonized mice with WT A909 (Ia) or WT COH1 (III) and monitored colonization persistence and bacterial burden with vaginal swabbing (monocolonization model). Both A909 and COH1 were able to establish persistent vaginal colonization under conditions of monocolonization (Figure 1A). There was no significant difference in duration of colonization (Figure 1A) or in recovered CFU between WT A909 (Ia) and COH1 (III) strains (Supplementary Figure 1).
Figure 1.
COH1 outcompetes A909 in a mouse model of vaginal cocolonization. A, Adult nonpregnant C57/BL6J mice were vaginally colonized with wild-type (WT) COH1 or A909 (n=8 for each strain). Kaplan-Meier curve represents colonization persistence, as determined by vaginal swabs every 2–3 days (P=not significant, Log-rank test). B, Adult nonpregnant C57/BL6J mice were simultaneously vaginally colonized with a 1:1 mixture of WT A909 + WT COH1 (n=5). Samples collected by vaginal swabs at 4 time points postcolonization were spread on Chromagar plates. Immunoblot with type Ia and type III primary antibody was used to differentiate between A909 and COH1. Data points represent geometric mean competition indices and error bars represent 95% confidence intervals.
In clinical settings, multiple GBS serotypes are frequently found to cocolonize the vagina [36–38]. We therefore used a competition model to more carefully assess differences in establishment and persistence of GBS vaginal colonization by simultaneously colonizing the vagina with 2 GBS strains. Geometric mean competitive index was greater than 7 by 2 days postcolonization, and exceeded 20 by 5 days postcolonization, suggesting a competitive advantage for WT COH1 (III, CC17) over WT A909 (Ia, ST7) strain in vaginal colonization fitness (Figure 1B).
Presence of Type Ia or Type III Capsule Confers an Advantage Over Capsule-Deficient Strains
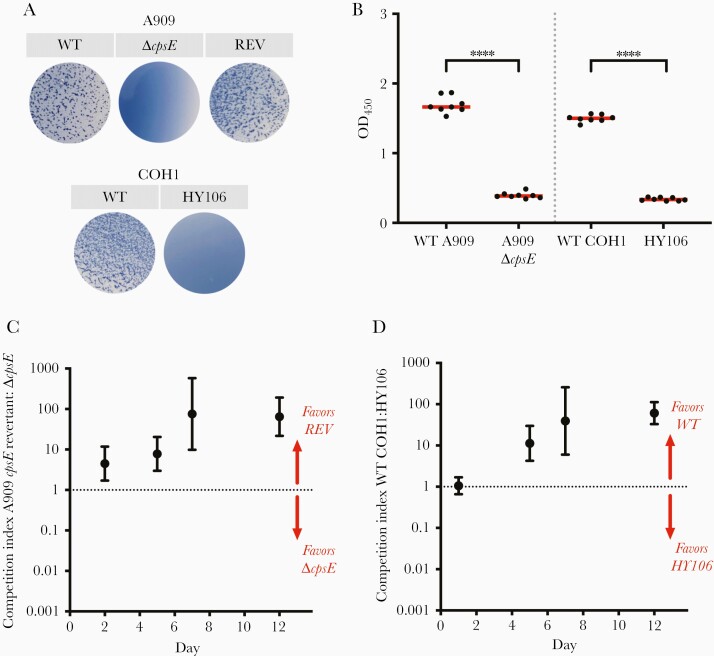
The GBS capsule is an important virulence factor. We therefore sought to assess the role of capsule presence versus absence as well as potential effects of specific capsule types in vaginal colonization fitness. We created a capsule-deficient A909 strain, A909ΔcpsE, and utilized a capsule-deficient mutant of COH1, HY106. We confirmed the abolition of type Ia or type III capsule production in these mutant strains by latex agglutination with type Ia sera for A909 strains and type III sera for COH1 strains (Figure 2A). We then performed an ELLA, which confirmed that these capsule-deficient mutants did not produce any detectable level of capsule, in contrast to their WT counterparts (Figure 2B).
Figure 2.
Presence of type Ia or type III capsule confers an advantage over capsule-deficient strains. A, Latex agglutination was used to confirm production of capsule. Type Ia antisera was used for wild-type (WT) A909, A909 ΔcpsE, and revertant (REV) strains. Type III antisera was used for WT COH1 and HY106. B, Enzyme-linked lectin assay using Maackia amurensis (MAL-I) lectin was used to estimate capsule amount in WT and capsule-deficient A909 and COH1 strains. Data points represent technical replicates (n=8 for each strain), and median optical density (OD) was determined. Data shown are representative of 3 experiments. (∗∗∗∗ P<.0001, Kruskal-Wallis test). C and D, Adult nonpregnant C57/BL6J mice were vaginally colonized with a 1:1 mixture of a capsule-producing and capsule-deficient strain (C, n=8; D, n=6). Samples collected by vaginal swabs at 4 time points postcolonization were spread on Chromagar plates. Immunoblot with type Ia (C) or type III (D) primary antibody was used to differentiate between encapsulated and capsule-deficient strains. Data points represent geometric mean competition indices and error bars represent 95% confidence intervals.
In the murine vaginal colonization model, we compared the relative fitness of capsule-producing and capsule-deficient strains of the same strain background (ie, encapsulated A909 vs A909ΔcpsE and WT COH1 vs HY106). In the setting of cocolonization with the encapsulated revertant A909 versus A909ΔcpsE, the geometric mean competitive index exceeded 4 at 2 days postcolonization and exceeded 75 by 7 days postcolonization, suggesting a robust competitive advantage of encapsulated A909 over the capsule-deficient A909ΔcpsE (Figure 2C). Likewise, encapsulated COH1 outcompeted the acapsular HY106 mutant, with a mean competitive index exceeding 10 by 5 days postcolonization and 30 by 7 days postcolonization (Figure 2D). Encapsulated A909 and COH1 strains therefore efficiently outcompeted isogenic acapsular strains, suggesting that GBS capsule likely provides an advantage in the maintenance of vaginal colonization.
Characterization of Capsule Switch and Revertant Strains
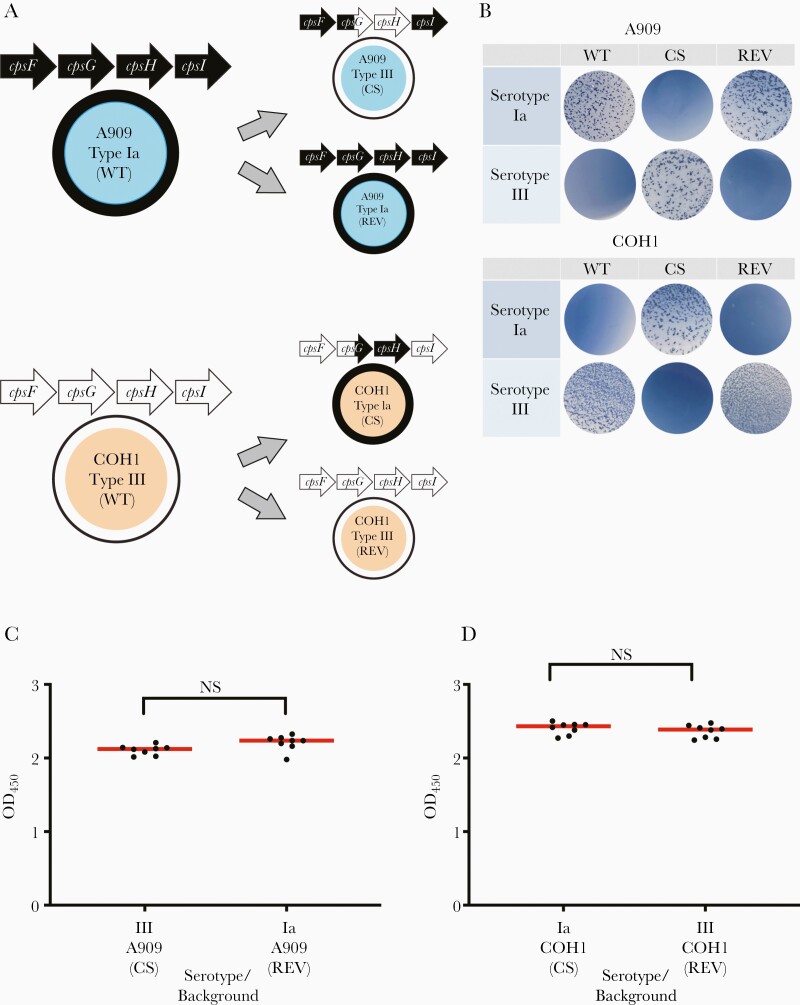
Capsule switch strains, specifically, A909 expressing type III capsule and COH1 expressing type Ia capsule, were created by allelic exchange of a fragment including the 3ʹ end of cpsG and the full open reading frame of cpsH (Figure 3A). Latex agglutination confirmed capsular switching of mutants, and revertant strains were identified as strains that had undergone plasmid insertion but reverted to their native capsule type based on the direction of plasmid excision (Figure 3B). Revertant strains were used for comparison to control for alterations in phenotype that could have been introduced by the allelic exchange procedure.
Figure 3.
A909 and COH1 capsule switch strains produce similar amounts of capsule. A, pMBsacB mutagenesis was used for allelic exchange of cpsG and cpsH of the cps locus from a type III strain to a type Ia strain and vice versa. Capsule switch (CS) strains, A909 type III and COH1 type Ia, differ from the wild type (WT) in only these sections of the cps locus, but produce a different capsule type. Revertant strains (REV), A909 type Ia and COH1 type III, have undergone pMBsacB mutagenesis, but revert back to the native cpsG and cpsH, therefore producing their native capsule type. B, Latex agglutination with type Ia and type III antisera was used to confirm capsular switching in A909 and COH1 mutant strains. C and D, Enzyme-linked lectin assay using Maackia amurensis (MAL-I) lectin was used to estimate capsule amount in CS and REV A909 and COH1 strains. Data points represent technical replicates (n=8 for each strain) and median optical density (OD) was determined. The x-axis label indicates the expressed serotype (either Ia or III), the strain background (either A909 or COH1), and either CS or REV to indicate the specific strain type. Data shown are representative of multiple individual experiments (P=not significant [NS], Kruskal-Wallis test).
ELLA was used to compare the amount of capsule produced by capsule switch and revertant strains of the same genetic background. The amount of capsule produced by capsule switch and revertant strains did not differ in either the A909 or COH1 background (Figure 3C and 3D).
Strains Producing Type Ia Capsule Outcompete Strains Producing Type III Capsule Independent of Genetic Background
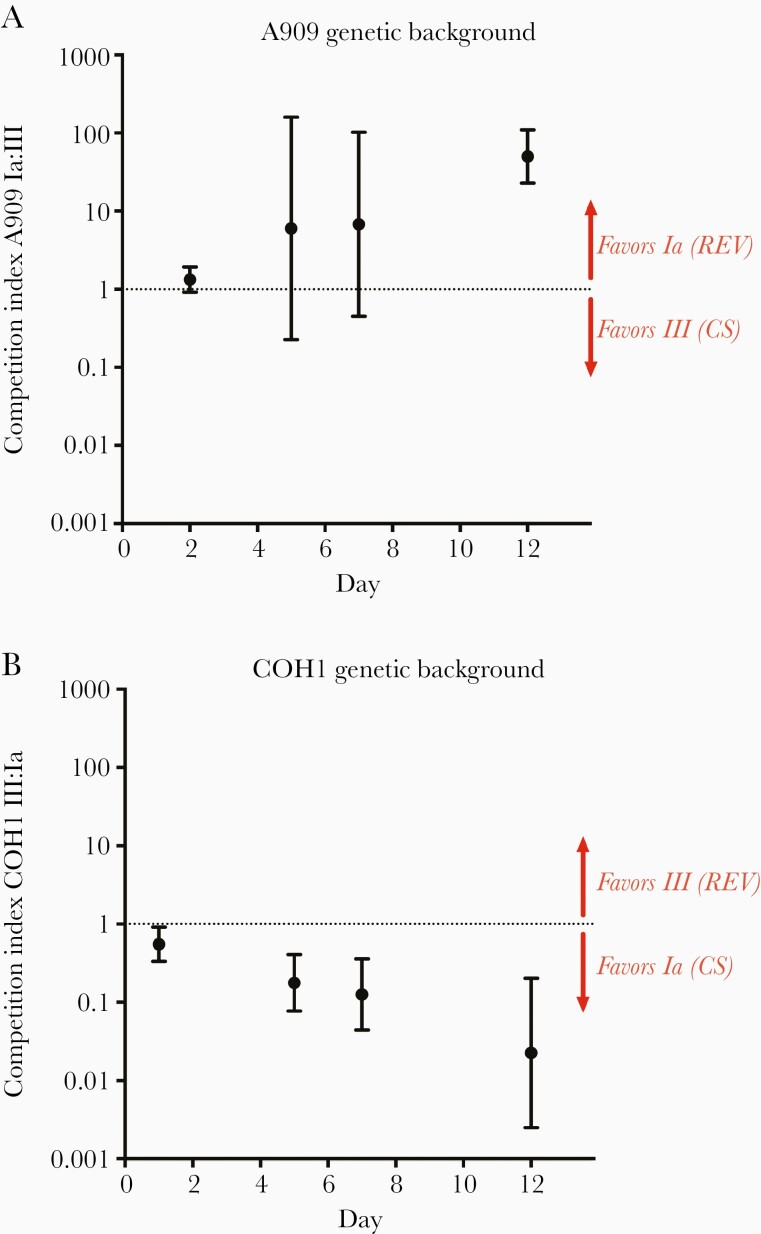
Following confirmation that matched capsule switch and revertant strains produce similar amounts of capsule, we competed these strain pairs in the murine vaginal cocolonization model. The A909 revertant strain expressing native type Ia capsule outcompeted the capsule-switch A909 strain expressing type III capsule, with geometric mean competitive index reaching approximately 50 by 12 days postcolonization (Figure 4A). Point estimates of competitive index at days 5 and 7 indicated an approximately 6-fold advantage for A909 expressing capsule type Ia, but the 95% confidence intervals crossed 1 at those time points. Notably, the COH1 capsule switch strain expressing type Ia capsule outcompeted the revertant strain expressing its native type III capsule. Geometric mean competitive index was approximately 0.18 (>5-fold difference) by 5 days postcolonization, and reached approximately 0.03 (>40-fold difference) by 12 days postcolonization, indicating a strong competitive advantage of the COH1 capsule switch expressing type Ia capsule over COH1 expressing its native type III capsule (Figure 4B). Taken together, these data suggest that the production of type Ia capsule may confer an advantage over type III capsule in vaginal colonization, even in a heterologous genetic background.
Figure 4.
Strains producing type Ia capsule outcompete strains producing type III capsule independent of genetic background. A and B, Adult nonpregnant C57/BL6J mice were vaginally colonized with a 1:1 mixture of a capsule switch (CS) and revertant (REV) strains, as in Figure 1 (A, n=8; B, n=6). Data points represent geometric mean competition indices and error bars represent 95% confidence intervals. The graph title indicates the strain genetic background (either A909 or COH1), and the right-side labels indicates the expressed serotype (either Ia or III) and either CS or REV to indicate the specific strain type.
DISCUSSION
GBS vaginal colonization plays a critical role in pathogenesis. Certain GBS capsular serotypes are more commonly isolated from vaginal colonization or invasive disease conditions [3, 16, 24], but it remains unclear whether capsular serotype itself drives differences in colonization success among GBS strains, particularly because capsular serotype and strain genetic background are nonrandomly associated. Prior work has suggested that GBS serotype may affect the magnitude of host inflammatory responses as well as colonization success [39, 40]. However, these studies have generally used clinical GBS isolates and thus cannot control rigorously for the effect of capsule versus noncapsule genetic features.
Rates of maternal GBS colonization have remained unchanged since the 1970s [5], and therefore understanding of the contribution of GBS capsule to vaginal colonization may help us understand the impact of the introduction of a GBS hexavalent vaccine. Furthermore, humoral immunity has been shown to enhance GBS clearance from the vaginal tract [41], suggesting that GBS vaccination strategies targeting vaginal colonization may aid in the control of neonatal GBS disease. Vaccination with a pneumococcal conjugate vaccine led to serotype replacement, or an increase in nonvaccine type strains among both asymptomatic carriers and strains causing invasive disease, likely due to both unmasking of nonvaccine type strains and recombination events leading to capsular switching and vaccine escape [18, 19]. Serotype replacement following the implementation of a conjugate vaccine program poses a threat to the success of vaccination in reducing GBS disease burden, and therefore continued monitoring of both vaginal colonization and disease burden will be important.
Here, we demonstrated that the presence of type Ia or type III capsule confers a fitness advantage in GBS vaginal colonization over acapsular strains of the same genetic background. This finding establishes a role for common GBS capsule types at a critical stage of host-pathogen interaction. The interruption of the expression of type III capsule has been shown to result in a mutant that is avirulent in a neonatal rat model of sepsis [9]. Furthermore, the type III capsule has been shown to prevent C3 deposition on the bacteria, which blocks phagocytosis of GBS [42]. A recent study showed a role for GBS capsule in ascending infection during pregnancy using a murine model [43]. Capsular sialic acid has been shown to contribute to the resistance of serotype III GBS to opsonophagocytosis [44]. GBS sialic acid mimics host sialoglycans, leading to attenuated host immune responses, such as neutrophil responses and platelet activation, by engaging inhibitory Siglecs [10, 45, 46]. The specific mechanisms by which capsule enhances GBS colonization fitness at the vaginal mucosal surface remain unclear.
We created isogenic capsule switch and revertant strains to assess the specific role of type Ia and type III capsule in GBS vaginal colonization. In the A909 background, the native type Ia capsule-expressing strain outcompeted the capsule switch (type III) strain. Notably, the COH1 capsule switch strain (expressing type Ia capsule) outcompeted the revertant expressing native capsule type III, suggesting that type Ia capsule confers an advantage over type III capsule in vaginal cocolonization, independent of strain background. This finding was surprising, given that strains expressing type III capsule are more commonly isolated in vaginal colonization as well as early-onset and late-onset GBS disease than type Ia and that type III strains make up the majority of the hypervirulent CC17 strains [24, 47]. Furthermore, we observed that WT COH1, which expresses its native type III capsule, outcompeted WT A909, expressing type Ia capsule, consistent with the hypothesis that both capsule-dependent and capsule-independent factors contribute to colonization fitness. CC17 strains are highly associated with late-onset GBS disease, possibly due to the expression of a CC17-specific hypervirulent GBS adhesin, HvgA [48]. Other CC17-specific factors, including the serine-rich repeat adhesin Srr2, have also been implicated in colonization and pathogenesis [49]. The overall advantage of COH1 (III) over A909 (Ia), despite the fact that A909 expresses a type Ia capsule, indicates that these adhesins or other noncapsule features likely play an important but nonexclusive role in vaginal colonization fitness, consistent with prior findings [50]. Taken together, these findings also highlight the importance of using carefully constructed strains to assess the independent contribution of bacterial factors, including capsular serotype, to the outcome of host-pathogen interactions.
We note that there are technical limitations that may limit applicability of our findings. Importantly, we only tested 2 GBS strain backgrounds, A909 (Ia) and COH1 (III). At this point, we are unable to generalize beyond these strains, and construction of capsule switch and revertant strains in other backgrounds, as well as with other capsule types, will be necessary to fully understand the specific impact of capsule type on colonization and disease. The lectin-based technique to detect capsule amounts is only semiquantitative, and we are unable to precisely quantify capsule amounts produced by each strain. For that reason, the quantity of capsule produced by each strain may be a contributing factor in the outcomes of our experiments. In addition, we relied on a murine model of vaginal colonization for our findings. One advantage of this approach is that it occurs in complex tissues in the presence of an immune system. However, there are important differences between murine and human anatomy, as well as in host-pathogen interactions. In addition, the cellular composition of the murine vaginal mucosal surface may change in response to estrogen, which we use to synchronize estrus cycles. Ascending infection of the uterus was not explored in these experiments. We recognize the limitations of the murine model and caution against direct application of the findings reported here to considerations of human epidemiology.
GBS6, a candidate hexavalent capsular polysaccharide conjugate vaccine, targets the most prevalent GBS serotypes—Ia, Ib, II, III, IV, and V. Capsule switching has been documented in the CC17 hypervirulent clone from a type III to type IV capsule [20–23], and switching events involving other capsule types and genetic backgrounds are likely, particularly if vaccination becomes widespread. Our results suggest that capsule switch events may alter vaginal colonization fitness, in addition to their potential for mediating vaccine escape. Surveillance for such events and further studies to understand the role of GBS specific capsule types in colonization and disease are warranted.
Supplementary Data
Supplementary materials are available at The Journal of Infectious Diseases online. Supplementary materials consist of data provided by the author that are published to benefit the reader. The posted materials are not copyedited. The contents of all supplementary data are the sole responsibility of the authors. Questions or messages regarding errors should be addressed to the author.
Notes
Acknowledgment. We thank Dr Kelly Doran for the kind gift of GBS strain HY106.
Disclaimer . The content is solely the responsibility of the authors and does not necessarily represent the official views of the funding bodies.
Financial support. This work was supported by the National Institutes of Health (grant numbers R56 AI136499, R01 AI155476, and R01 AI143290 to A. J. R.; R21 AI147511 to A. J. R. and T. A. H.; and K08 AI132555 to T. A. H.).
Potential conflicts of interest. A. J. R. has acted as a consultant for Pfizer and on a compassionate use advisory board for CompAC (NYU/Janssen). T. A. H. has received consulting fees from Techspert.io. All other authors report no potential conflicts. All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
Contributor Information
Allison N Dammann, Department of Pediatrics, New York University Grossman School of Medicine, New York, New York, USA.
Anna B Chamby, Department of Pediatrics, New York University Grossman School of Medicine, New York, New York, USA.
Francisco J Gonzalez, Department of Pediatrics, New York University Grossman School of Medicine, New York, New York, USA.
Molly E Sharp, Department of Microbiology, New York University Grossman School of Medicine, New York, New York, USA.
Karina Flores, Department of Microbiology, New York University Grossman School of Medicine, New York, New York, USA.
Ifrah Shahi, Department of Microbiology, New York University Grossman School of Medicine, New York, New York, USA.
Sophia Dongas, Department of Pediatrics, New York University Grossman School of Medicine, New York, New York, USA.
Thomas A Hooven, Department of Pediatrics, University of Pittsburgh School of Medicine, Pittsburgh, Pennsylvania, USA; Richard King Mellon Institute for Pediatric Research, University of Pittsburgh Medical Center, Children’s Hospital of Pittsburgh, Pittsburgh, Pennsylvania, USA.
Adam J Ratner, Department of Pediatrics, New York University Grossman School of Medicine, New York, New York, USA; Department of Microbiology, New York University Grossman School of Medicine, New York, New York, USA.
References
- 1. Stoll BJ, Hansen NI, Sánchez PJ, et al. ; Eunice Kennedy Shriver National Institute of Child Health and Human Development Neonatal Research Network. . Early onset neonatal sepsis: the burden of group B streptococcal and E. coli disease continues. Pediatrics 2011; 127:817–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Madrid L, Seale AC, Kohli-Lynch M, et al. ; Infant GBS Disease Investigator Group. . Infant group B streptococcal disease incidence and serotypes worldwide: systematic review and meta-analyses. Clin Infect Dis 2017; 65:160–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Russell NJ, Seale AC, O’Driscoll M, et al. ; GBS Maternal Colonization Investigator Group. . Maternal colonization with group B Streptococcus and serotype distribution worldwide: systematic review and meta-analyses. Clin Infect Dis 2017; 65:100–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Schrag SJ, Zywicki S, Farley MM, et al. Group B streptococcal disease in the era of intrapartum antibiotic prophylaxis. N Engl J Med 2000; 342:15–20. [DOI] [PubMed] [Google Scholar]
- 5. Verani JR, McGee L, Schrag SJ; Division of Bacterial Diseases, National Center for Immunization and Respiratory Diseases, Centers for Disease Control and Prevention (CDC). . Prevention of perinatal group B streptococcal disease—revised guidelines from CDC, 2010. MMWR Recomm Rep 2010; 59:1–36. [PubMed] [Google Scholar]
- 6. Schrag SJ, Verani JR.. Intrapartum antibiotic prophylaxis for the prevention of perinatal group B streptococcal disease: experience in the United States and implications for a potential group B streptococcal vaccine. Vaccine 2013; 31(Suppl 4):D20–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Puopolo KM, Lynfield R, Cummings JJ.. Management of infants at risk for group B streptococcal disease. Pediatrics 2019; 144:e20191881. [DOI] [PubMed] [Google Scholar]
- 8. Nanduri SA, Petit S, Smelser C, et al. Epidemiology of invasive early-onset and late-onset group B streptococcal disease in the United States, 2006 to 2015: multistate laboratory and population-based surveillance. JAMA Pediatr 2019; 173:224–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Rubens CE, Wessels MR, Heggen LM, Kasper DL.. Transposon mutagenesis of type III group B Streptococcus: correlation of capsule expression with virulence. Proc Natl Acad Sci U S A 1987; 84:7208–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Carlin AF, Uchiyama S, Chang YC, Lewis AL, Nizet V, Varki A.. Molecular mimicry of host sialylated glycans allows a bacterial pathogen to engage neutrophil Siglec-9 and dampen the innate immune response. Blood 2009; 113:3333–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Baker CJ, Kasper DL.. Correlation of maternal antibody deficiency with susceptibility to neonatal group B streptococcal infection. N Engl J Med 1976; 294:753–6. [DOI] [PubMed] [Google Scholar]
- 12. Dangor Z, Kwatra G, Izu A, Lala SG, Madhi SA.. Review on the association of Group B Streptococcus capsular antibody and protection against invasive disease in infants. Expert Rev Vaccines 2015; 14:135–49. [DOI] [PubMed] [Google Scholar]
- 13. Le Doare K, Kampmann B, Vekemans J, et al. Serocorrelates of protection against infant group B Streptococcus disease. Lancet Infect Dis 2019; 19:e162–71. [DOI] [PubMed] [Google Scholar]
- 14. Absalon J, Segall N, Block SL, et al. Safety and immunogenicity of a novel hexavalent group B Streptococcus conjugate vaccine in healthy, non-pregnant adults: a phase 1/2, randomised, placebo-controlled, observer-blinded, dose-escalation trial. Lancet Infect Dis 2021; 21:263–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Buurman ET, Timofeyeva Y, Gu J, et al. A novel hexavalent capsular polysaccharide conjugate vaccine (GBS6) for the prevention of neonatal group B streptococcal infections by maternal immunization. J Infect Dis 2019; 220:105–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Bianchi-Jassir F, Paul P, To KN, et al. Systematic review of Group B streptococcal capsular types, sequence types and surface proteins as potential vaccine candidates. Vaccine 2020; 38:6682–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. McGee L, Chochua S, Li Z, et al. Multistate, population-based distributions of candidate vaccine targets, clonal complexes, and resistance features of invasive group B streptococci within the United States, 2015–2017. Clin Infect Dis 2021; 72:1004–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Brueggemann AB, Pai R, Crook DW, Beall B.. Vaccine escape recombinants emerge after pneumococcal vaccination in the United States. PLoS Pathog 2007; 3:e168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Weinberger DM, Malley R, Lipsitch M.. Serotype replacement in disease after pneumococcal vaccination. Lancet 2011; 378:1962–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Luan SL, Granlund M, Sellin M, Lagergård T, Spratt BG, Norgren M.. Multilocus sequence typing of Swedish invasive group B Streptococcus isolates indicates a neonatally associated genetic lineage and capsule switching. J Clin Microbiol 2005; 43:3727–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Bellais S, Six A, Fouet A, et al. Capsular switching in group B Streptococcus CC17 hypervirulent clone: a future challenge for polysaccharide vaccine development. J Infect Dis 2012; 206:1745–52. [DOI] [PubMed] [Google Scholar]
- 22. Seale AC, Koech AC, Sheppard AE, et al. Maternal colonization with Streptococcus agalactiae and associated stillbirth and neonatal disease in coastal Kenya. Nat Microbiol 2016; 1:16067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Lopes E, Fernandes T, Machado MP, et al. Increasing macrolide resistance among Streptococcus agalactiae causing invasive disease in non-pregnant adults was driven by a single capsular-transformed lineage, Portugal, 2009 to 2015. Euro Surveill 2018; 23:1700473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Fabbrini M, Rigat F, Rinaudo CD, et al. The protective value of maternal group B Streptococcus antibodies: quantitative and functional analysis of naturally acquired responses to capsular polysaccharides and pilus proteins in European maternal sera. Clin Infect Dis 2016; 63:746–53. [DOI] [PubMed] [Google Scholar]
- 25. Manning SD, Springman AC, Lehotzky E, Lewis MA, Whittam TS, Davies HD.. Multilocus sequence types associated with neonatal group B streptococcal sepsis and meningitis in Canada. J Clin Microbiol 2009; 47:1143–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Yim HH, Nittayarin A, Rubens CE.. Analysis of the capsule synthesis locus, a virulence factor in group B streptococci. Adv Exp Med Biol 1997; 418:995–7. [DOI] [PubMed] [Google Scholar]
- 27. Randis TM, Gelber SE, Hooven TA, et al. Group B Streptococcus β-hemolysin/cytolysin breaches maternal-fetal barriers to cause preterm birth and intrauterine fetal demise in vivo. J Infect Dis 2014; 210:265–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Chaffin DO, Beres SB, Yim HH, Rubens CE.. The serotype of type Ia and III group B streptococci is determined by the polymerase gene within the polycistronic capsule operon. J Bacteriol 2000; 182:4466–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Cieslewicz MJ, Chaffin D, Glusman G, et al. Structural and genetic diversity of group B Streptococcus capsular polysaccharides. Infect Immun 2005; 73:3096–103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Hooven TA, Bonakdar M, Chamby AB, Ratner AJ.. A counterselectable sucrose sensitivity marker permits efficient and flexible mutagenesis in Streptococcus agalactiae. Appl Environ Microbiol 2019; 85:e03009-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Cieslewicz MJ, Kasper DL, Wang Y, Wessels MR.. Functional analysis in type Ia group B Streptococcus of a cluster of genes involved in extracellular polysaccharide production by diverse species of streptococci. J Biol Chem 2001; 276:139–46. [DOI] [PubMed] [Google Scholar]
- 32. Chaffin DO, Mentele LM, Rubens CE.. Sialylation of group B streptococcal capsular polysaccharide is mediated by cpsK and is required for optimal capsule polymerization and expression. J Bacteriol 2005; 187:4615–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Alhhazmi A, Pandey A, Tyrrell GJ.. Identification of group B Streptococcus capsule type by use of a dual phenotypic/genotypic assay. J Clin Microbiol 2017; 55:2637–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Roy D, Takamatsu D, Okura M, et al. Capsular sialyltransferase specificity mediates different phenotypes in Streptococcus suis and group B Streptococcus. Front Microbiol 2018; 9:545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Geisler C, Jarvis DL.. Effective glycoanalysis with Maackia amurensis lectins requires a clear understanding of their binding specificities. Glycobiology 2011; 21:988–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Hoogkamp-Korstanje JA, Gerards LJ, Cats BP.. Maternal carriage and neonatal acquisition of group B streptococci. J Infect Dis 1982; 145:800–3. [DOI] [PubMed] [Google Scholar]
- 37. Ferrieri P, Hillier SL, Krohn MA, Moore D, Paoletti LC, Flores AE.. Characterization of vaginal & rectal colonization with multiple serotypes of group B streptococci using multiple colony picks. Indian J Med Res 2004; 119(Suppl):208–12. [PubMed] [Google Scholar]
- 38. Khatami A, Randis TM, Tavares L, Gegick M, Suzman E, Ratner AJ.. Vaginal co-colonization with multiple group B Streptococcus serotypes. Vaccine 2019; 37:409–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Flaherty RA, Aronoff DM, Gaddy JA, Petroff MG, Manning SD.. Distinct group B Streptococcus sequence and capsule types differentially impact macrophage stress and inflammatory signaling responses. Infect Immun 2021; 89:e00647-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Sweeney EL, Gardiner S, Tickner J, Trim L, Beagley KW, Carey AJ.. Group B Streptococcus serotypes Ia and V induce differential vaginal immune responses that may contribute to long term colonization of the female reproductive tract. Am J Reprod Immunol 2020; 83:e13199. [DOI] [PubMed] [Google Scholar]
- 41. Baker JA, Lewis EL, Byland LM, Bonakdar M, Randis TM, Ratner AJ.. Mucosal vaccination promotes clearance of Streptococcus agalactiae vaginal colonization. Vaccine 2017; 35:1273–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Marques MB, Kasper DL, Pangburn MK, Wessels MR.. Prevention of C3 deposition by capsular polysaccharide is a virulence mechanism of type III group B streptococci. Infect Immun 1992; 60:3986–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Noble K, Lu J, Guevara MA, et al. Group B Streptococcus cpsE is required for serotype V capsule production and aids in biofilm formation and ascending infection of the reproductive tract during pregnancy. ACS Infect Dis 2021; 7:2686–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Edwards MS, Kasper DL, Jennings HJ, Baker CJ, Nicholson-Weller A.. Capsular sialic acid prevents activation of the alternative complement pathway by type III, group B streptococci. J Immunol 1982; 128:1278–83. [PubMed] [Google Scholar]
- 45. Chang YC, Olson J, Beasley FC, et al. Group B Streptococcus engages an inhibitory Siglec through sialic acid mimicry to blunt innate immune and inflammatory responses in vivo. PLoS Pathog 2014; 10:e1003846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Uchiyama S, Sun J, Fukahori K, et al. Dual actions of group B Streptococcus capsular sialic acid provide resistance to platelet-mediated antimicrobial killing. Proc Natl Acad Sci U S A 2019; 116:7465–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Musser JM, Mattingly SJ, Quentin R, Goudeau A, Selander RK.. Identification of a high-virulence clone of type III Streptococcus agalactiae (group B Streptococcus) causing invasive neonatal disease. Proc Natl Acad Sci U S A 1989; 86:4731–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Tazi A, Disson O, Bellais S, et al. The surface protein HvgA mediates group B Streptococcus hypervirulence and meningeal tropism in neonates. J Exp Med 2010; 207:2313–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Six A, Bellais S, Bouaboud A, et al. Srr2, a multifaceted adhesin expressed by ST-17 hypervirulent Group B Streptococcus involved in binding to both fibrinogen and plasminogen. Mol Microbiol 2015; 97:1209–22. [DOI] [PubMed] [Google Scholar]
- 50. Wang NY, Patras KA, Seo HS, et al. Group B streptococcal serine-rich repeat proteins promote interaction with fibrinogen and vaginal colonization. J Infect Dis 2014; 210:982–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.