Abstract
Background
Antibiotics, such as inhaled tobramycin, are used to eradicate new-onset Pseudomonas aeruginosa (PA) infections in patients with cystic fibrosis (CF) but frequently fail due to reasons poorly understood. We hypothesized that PA isolates’ resistance to neutrophil antibacterial functions was associated with failed eradication in patients harboring those strains.
Methods
We analyzed all PA isolates from a cohort of 39 CF children with new-onset PA infections undergoing tobramycin eradication therapy, where 30 patients had eradicated and 9 patients had persistent infection. We characterized several bacterial phenotypes and measured the isolates’ susceptibility to neutrophil antibacterial functions using in vitro assays of phagocytosis and intracellular bacterial killing.
Results
PA isolates from persistent infections were more resistant to neutrophil functions, with lower phagocytosis and intracellular bacterial killing compared to those from eradicated infections. In multivariable analyses, in vitro neutrophil responses were positively associated with twitching motility, and negatively with mucoidy. In vitro neutrophil phagocytosis was a predictor of persistent infection following tobramycin even after adjustment for clinical risk factors.
Conclusions
PA isolates from new-onset CF infection show strain-specific susceptibility to neutrophil antibacterial functions, and infection with PA isolates resistant to neutrophil phagocytosis is an independent risk factor for failed tobramycin eradication.
Keywords: cystic fibrosis, Pseudomonas aeruginosa, antibiotic eradication therapy, neutrophil phagocytosis
Pseudomonas aeruginosa (PA) isolates from new-onset cystic fibrosis (CF) infection show strain-specific susceptibility to neutrophil antibacterial functions, and infection with PA isolates resistant to neutrophil phagocytosis is an independent risk factor for failed tobramycin eradication therapy in children with CF.
Cystic fibrosis (CF) is a multisystem genetic disorder caused by mutations in the gene encoding the cystic fibrosis transmembrane conductance regulator (CFTR). CFTR dysfunction leads to impaired mucociliary clearance and airway mucus plugging, which favor bacterial colonization and chronic infection [1]. Chronic infections with Pseudomonas aeruginosa (PA), the predominant opportunistic respiratory pathogen in CF, are associated with lung function decline and worse clinical outcomes [2]. To avoid progression to chronic infection, new-onset PA infections are routinely treated with antibiotics, most commonly inhaled tobramycin in North America [3, 4]. Unfortunately, even with early interventions, antibiotic eradication therapy fails and PA infections persist in 28%–40% of patients [3–7].
Several studies to date in different pediatric CF cohorts—namely, in the US Early Pseudomonas Infection Control (EPIC) study [8, 9], the Netherlands and Denmark [10], Australia [11], and our study cohort at the Hospital for Sick Children in Canada [12]—have examined clinical and microbiological parameters to identify predictors of persistent infection following eradication therapy. Although these studies varied in design and sampling method for the recovery of PA, none found significant differences in the clinical characteristics (age, age at diagnosis, sex, CFTR genotypes, eradication treatments, and lung function) between patients with successful eradication and those with persistent infection.
Interestingly, Mayer-Hamblett et al [9] (EPIC study) and Vidya et al [12] (Hospital for Sick Children cohort study) also investigated phenotypic characteristics of the infecting PA isolates for their association with failed eradication therapy. Bacterial phenotypes commonly associated with chronic infections, namely mucoidy and lack of twitching motility, as well as wrinkly colonies surface and irregular edges, showed association with eradication failure [9, 12]. Such bacterial phenotypes may impair the efficacy of antimicrobials [13, 14], but also evade host defense mechanisms that are important for bacterial clearance [15, 16]. Neutrophils are innate immune cells that carry out phagocytic and bacterial killing functions critical to PA eradication and enhance the efficacy of antibacterial therapy [17, 18]. Multiple bacterial factors, such as surface molecules [19], motility appendages [20, 21], and overproduction of exopolysaccharides (EPSs; alginate, Psl and Pel) [22–24], alter neutrophil-mediated antibacterial clearance. Bacterial characteristics of PA isolates at the time of new-onset infection may thus be determinants of neutrophil-PA interactions and be associated with outcomes of eradication therapy in CF patients.
Although many studies have investigated host-PA interactions in experimental in vitro and animal models of infection, few have linked these findings to clinical outcomes [10, 25–28]. In this study, we characterized all PA isolates collected from new-onset infections of children with CF from the Hospital for Sick Children cohort study [12] using in vitro assays for neutrophil phagocytosis and intracellular bacterial killing. We sought to identify the bacterial phenotypes that contributed most to in vitro resistance to neutrophil antibacterial functions, and to determine whether such resistance was associated with failed eradication therapy in CF patients.
MATERIALS AND METHODS
Cohort Design
Bacterial isolates and clinical data from a prospective cohort of CF children followed at the Hospital for Sick Children (Toronto, Canada) were used for this study [12]. Patients included in this study were (1) 5–18 years old, (2) able to produce sputum, and (3) diagnosed with new-onset PA infection during the period 2011–2014, and all were treated with inhaled tobramycin twice per day for 28 days. All patients from this cohort were included except for those with PA isolates with tobramycin minimum inhibitory concentration (MIC) >2 μg/mL to exclude tobramycin resistance as a cause of eradication failure and because the in vitro neutrophil assays can only be performed on aminoglycoside-susceptible isolates. New-onset PA infection was defined as a PA-positive sputum culture following at least 3 PA-negative sputum cultures in the previous 12 months. A sputum culture (posttreatment) was collected 1 week after the end of tobramycin therapy to determine the outcome of eradication therapy, based on previous definitions [3, 5]. The infection was considered “persistent” if the posttreatment sputum culture was positive for PA, and “eradicated” if the posttreatment culture was negative [29]. This study was approved by the research ethics board (REB) at the Hospital for Sick Children.
PA Clinical Isolates
All PA clinical isolates were recovered from patients’ sputum collected prior to the initiation of inhaled tobramycin as previously described [12]. In brief, sputum samples were homogenized with sputolysin and stored at –80°C. PA isolates were recovered from frozen sputum, included multiple colony morphotypes when present, and were confirmed by matrix-assisted laser desorption/ionization–time of flight mass spectrometry for species identification.
Phenotypic Characterization of PA Isolates
Phenotypic assays for twitching and swimming motility, biofilm formation by crystal violet (CV) assay, and mucoidy status were done as previously reported [12]. In brief, mucoidy was determined by colony morphology following growth on yeast extract mannitol (YEM) agar for 24–48 hours. Swimming motility was determined by inoculating a single colony into 0.3% Luria-Bertani (LB) agar and measuring the diameter (mm) of the zone of bacterial growth after overnight incubation at 37°C. Twitching motility was determined by inoculating a single colony into 1% thin LB agar plate and measuring the diameter (mm) of the twitching zone following staining with 0.1% CV. Biofilm formation was measured by inoculating polystyrene 96-well plates with 100 µL of overnight bacteria cultures diluted 1:100 in LB medium. After static incubation overnight at 37°C, the adherent biofilm biomass was stained with 0.1% CV, resolubilized with 95% ethanol, and measured at optical density (OD) 600 nm. The Congo red binding assay was used to assess EPS-mediated bacterial aggregation in liquid culture [30]. One hundred microliters of overnight PA cultures was diluted into 4 mL Vogel-Bonner minimal medium (VBMM) containing 40 µg/mL Congo red (Sigma catalog number C6767) and incubated for 18 hours at 37°C with shaking at 250 rpm. Bacterial cells were then spun down and the absorbance of the supernatant was measured at OD at 490 nm (OD490nm). The Congo red binding was calculated as (OD490nm [VBMM + Congo red control] – OD490nm [sample]).
In Vitro Neutrophil Phagocytosis and Intracellular Bacterial Killing Assays
A gentamicin protection assay was used to measure the phagocytic uptake of PA isolates by human neutrophil–like cells derived from immortalized HL-60 cells. In brief, HL-60 cells were cultured in Iscove’s modified Dulbecco’s medium (Wisent) and differentiated with 1.3% dimethyl sulfoxide and 2.3 µM all-trans retinoic acid for 3 days to generate neutrophil-like cells (dHL-60s) with >95% viability. Bacteria were grown in 5 mL LB medium overnight (18 hours) at 37°C with shaking at 250 rpm, then spun down, washed twice with Hanks’ balanced salt solution without calcium, magnesium, and phenol red (Wisent), and diluted to 107 colony-forming units (CFU)/mL. Two hundred fifty microliters of the diluted bacterial suspension was opsonized with 20% human serum (Millipore Sigma) and coincubated with 2.5 × 105 dHL-60s at a multiplicity of infection of 10 for 30 minutes at 37°C. Following this incubation, 100 µg/mL gentamicin was added for another 30 minutes (T = 60 minutes) for the phagocytosis assay or 90 minutes (T = 120 minutes) for the intracellular bacterial killing assay. Cells were then washed twice, lysed with 0.1% Triton, and plated on LB agar for enumeration of viable intracellular bacteria by counting CFUs after overnight incubation at 37°C. The phagocytosis index was calculated as the number of internalized bacteria at T = 60 divided by total bacteria at T = 0. The intracellular bacterial killing was calculated as the number of internalized bacteria at T = 60 minus T = 120, then divided by total bacteria at T = 0. All experiments were done with at least 4 replicates in at least 2 independent experiments.
Statistical Analyses
Comparisons were performed using the Mann–Whitney nonparametric test or χ 2 test as indicated. Univariable Spearman correlation was first used to measure the association between each bacterial phenotype and in vitro neutrophil responses (phagocytosis or intracellular bacterial killing), and those with a P value ≤ .2 were included in a subsequent multivariable model. A random-effects model was used to determine the association between different bacterial phenotypes and in vitro neutrophil responses while accounting for clustering due to occurrence of multiple isolates per patient. A logistic regression model was used to determine the association between in vitro neutrophil responses and a persistent infection outcome. For the adjusted multivariable logistic regression model, clinical parameters or bacterial phenotypes were considered based on prior literature or selection using the Spearman correlation above, and only 1 additional variable was tested in the model at a time given the power of our limited sample size. Statistical comparisons and regression analyses were performed using SPSS version 26 software (IBM SPSS, Chicago, Illinois). A P value of ≤.05 was considered significant.
RESULTS
Characteristics of Patients With Eradicated or Persistent PA Infections
From a total of 43 eligible patients, 39 patients were analyzed in this study, while 4 patients harboring 6 isolates with tobramycin MIC >2 were excluded. The study thus included 30 patients with eradicated infections and 9 patients with persistent infections (ie, failed tobramycin eradication therapy), resulting in 52 eradicated PA isolates and 19 persistent PA isolates. Several patients were infected with >1 morphologically distinct PA isolate (morphotypes), with a median of 2 (range, 1–3) morphotypes in patients with persistent infections, and a median of 1 (range, 1–4) morphotype in patients with eradicated infections (Supplementary Figure 1). Consistent with our previous report [12], demographic and baseline clinical characteristics were similar between patients who failed eradication therapy and those who succeeded (Table 1).
Table 1.
Baseline Characteristics of Study Patients by Persistent or Eradicated Status
| Characteristic | Persistent (n = 9) | Eradicated (n = 30) | P Value |
|---|---|---|---|
| Age, y, median (range) | 10 (6.3–17.1) | 11.3 (6.5–17.5) | .52 |
| Female sex, No. (%) | 4 (44) | 18 (60) | .41 |
| Age at diagnosis, No. (%) | |||
| <2 y | 8 (89) | 21 (70) | .08 |
| >2 y | 1 (11) | 9 (30) | |
| FEV1 % predicted, median (range) | 85.8 (55.4–120.5) | 88.8 (39.4–126.8) | .52 |
| Genotypes, No. (%) | |||
| Homozygous ΔF508 | 4 (44) | 16 (53) | .83 |
| Heterozygous ΔF508 | 3 (33) | 10 (33) | |
| Other | 2 (22) | 4 (13) | |
| Pancreatic insufficiency, No. (%) | 8 (89) | 29 (97) | .28 |
| CFRD, No. (%) | 1 (11) | 2 (6.7) | .91 |
| BMI z score, median (range) | –0.55 (–1.63 to 1.21) | –0.07 (–1.44 to 2.44) | .51 |
Data were previously published in Vidya et al [12] and modified accordingly to the current study population.
Abbreviations: BMI, body mass index; CFRD, cystic fibrosis–related diabetes mellitus; FEV1, forced expiratory volume in 1 second.
PA isolates from patients with persistent infections are more resistant to neutrophil phagocytosis and intracellular bacterial killing compared to those from patients with eradicated infections
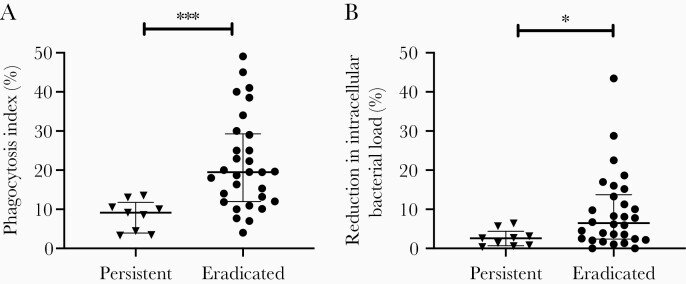
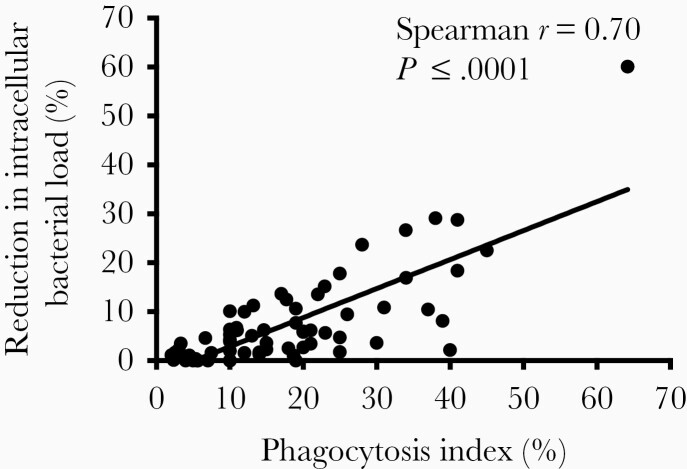
We compared the phagocytic uptake and intracellular bacterial killing by dHL-60s of PA isolates from the eradicated group to those from the persistent group. Since some patients were infected with >1 PA isolate, we analyzed our data by combining the neutrophil assay data for all morphotypes in each patient. As shown in Figure 1, the average phagocytic index was >2-fold lower (9.1% vs 19.5%; P = .0003) in patients with persistent infections compared to those with eradicated infections. We measured the reduction in neutrophil intracellular bacterial load as a measure of intracellular bacterial killing and found it to be 2.5-fold lower (2.6% vs 6.7%; P = .018) in the persistent group compared to the eradicated group. Notably, similar results were obtained when we used the maximal value (Supplementary Figure 2A and 2B) or the median (Supplementary Figure 2C and 2D) of combined neutrophil data from each patient. We also observed similar findings when all PA isolates were analyzed independently (Supplementary Figure 3) and noted that the phagocytic index was significantly correlated with intracellular bacterial killing (r = 0.70, P < .0001; Figure 2). These results thus indicated that patients who fail eradication therapy harbor PA isolates that are more resistant to neutrophil antibacterial functions than patients with successful eradication.
Figure 1.
Pseudomona s aeruginosa isolates from patients with persistent infections exhibit lower neutrophil antibacterial functions compared to those from patients with eradicated infection. Neutrophil phagocytosis (A) and intracellular bacterial killing (B) of the persistent (n = 9 patients) group and the eradicated (n = 30 patients) group. The data were analyzed per patient by averaging the neutrophil results of all P. aeruginosa isolates from each patient. Results are shown as median and interquartile range. Statistical comparisons were performed using Mann–Whitney test (*P < .05, ***P < .001).
Figure 2.
Neutrophil phagocytosis is significantly associated with intracellular bacterial killing. Association was calculated by Spearman correlation coefficient.
Loss of Twitching Motility and Mucoidy Are Associated With Impaired Neutrophil Antibacterial Functions
We first characterized the flagellum-mediated swimming motility, type IV pilus–mediated twitching motility, mucoidy (alginate overproduction), biofilm formation, and Congo red binding (Psl and Pel EPS-mediated bacterial aggregation) in all PA isolates (Table 2). In initial univariable analyses, neutrophil phagocytosis was only significantly correlated with twitching motility (r = 0.43, P < .001) and not any other bacterial phenotype (Table 3). Neutrophil intracellular bacterial killing was significantly associated with twitching motility (r = 0.43, P < .001), mucoidy (r = 0.26, P = .03), and Congo red binding (r = 0.32, P < .01).
Table 2.
Comparison of Bacterial Phenotypes in Persistent Versus Eradicated Pseudomonas aeruginosa Isolates
| PA Phenotypes | Persistent PA (n = 19) | Eradicated PA (n = 52) | P Value |
|---|---|---|---|
| Biofilm production (OD595nm), median (IQR) | 0.21 (0.15−0.24) | 0.21 (0.10−0.32) | .97 |
| Twitching, mm, median (IQR) | 18.0 (3.7−20.8) | 26.9 (19.3−36.7) | <.01 |
| Swimming, mm, median (IQR) | 11.2 (0−17) | 13.9 (10.1−16.0) | .33 |
| Mucoidy, No. (%) | 12 (63) | 18 (35) | .03 |
| Congo red binding (OD495nm), median (IQR) | −0.25 (−0.32 to −0.21) | −0.23 (−0.31 to −0.15) | .14 |
Statistical comparisons were performed using Mann–Whitney or χ 2 test.
Abbreviations: IQR, interquartile range; OD, optical density; PA, Pseudomonas aeruginosa.
Table 3.
Correlation Between Bacterial Phenotypes and In Vitro Neutrophil Antibacterial Responses in Univariable Analysis
| PA Phenotypes | Phagocytosis | Intracellular Bacterial Killing | ||
|---|---|---|---|---|
| Coefficient | P Value | Coefficient | P Value | |
| Twitching | 0.43 | <.001 | 0.43 | <.001 |
| Swimming | 0.07 | .55 | 0.05 | .69 |
| Mucoidy | –0.19 | .09 | –0.26 | .03 |
| Congo red binding | 0.18 | .13 | 0.32 | <.01 |
The association between each bacterial phenotype and neutrophil phagocytosis or intracellular bacterial killing was calculated using the Spearman correlation coefficient.
Abbreviation: PA, Pseudomonas aeruginosa.
Next, since some patients have multiple morphologically distinct PA isolates, which could lead to clustering due to repeated measures, we calculated the intraclass correlation (ICC) for neutrophil phagocytosis and bacterial killing in our dataset. With an ICC value of 0.5, our data showed moderate clustering of neutrophil measurements within each patient; that is, neutrophil responses elicited by PA isolates from the same patient are more similar to each other than those between different patients. To account for this data clustering, we used a random-effects model to determine the relationship between neutrophil phagocytosis or intracellular bacterial killing, and the 3 bacterial phenotypes identified in univariate analyses with a P ≤ .2, namely twitching, mucoidy, and Congo red binding (Table 4). We found that twitching motility (r = 0.27, P = .02) and mucoidy (r = –5.60, P = .04) were significantly associated with neutrophil phagocytosis, that is, a 1-mm increase in twitching was associated with a 0.27% increase in phagocytosis, and the presence of mucoidy was associated with an average 5.6% reduction in phagocytosis. However, Congo red binding showed no association with phagocytosis. Twitching motility (r = 0.20, P = .03) and mucoidy (r = –4.53, P = .03) were also significantly associated with intracellular bacterial killing (Table 4).
Table 4.
Association Between Bacterial Phenotypes and In Vitro Neutrophil Antibacterial Responses in Multivariable Analysis
| PA Phenotype | Phagocytosis | Intracellular Bacterial Killing | ||||
|---|---|---|---|---|---|---|
| Coefficienta | (95% CI) | P Value | Coefficienta | (95% CI) | P Value | |
| Twitching | 0.27 | (.04–.51) | .02 | 0.20 | (.02–.38) | .03 |
| Mucoidy | –5.60 | (–11.04 to –.14) | .04 | –4.53 | (–8.63 to –.42) | .03 |
| Congo red binding | –13.94 | (–49.81 to 21.11) | .44 | –8.06 | (–35.31 to 19.17) | .56 |
Abbreviations: CI, confidence interval; PA, Pseudomonas aeruginosa.
aThe regression coefficient was calculated using a random-effects model fitted on all isolates (n = 19 persistent and n = 52 eradicated isolates).
Multivariate Analysis to Predict Persistent Infection Following Inhaled Tobramycin Therapy
We used a logistic regression model to assess whether the resistance of PA isolates to neutrophil phagocytosis is an independent predictor of failed eradication therapy after adjusting for other covariables. In patients infected with >1 PA morphotype, we used the mean of neutrophil results as done for Figure 1. We observed a trend toward association between in vitro intracellular bacterial killing and persistent infection, but this did not reach statistical significance. However, in vitro neutrophil phagocytosis was a significant predictor of persistent infection (odds ratio, 0.76 [95% confidence interval, .62–.94]; P = .01), indicating that every percentage increase in neutrophil phagocytosis was associated with a 24% reduction in the odds of a persistent infection outcome (Table 5). In addition, we further determined that in vitro neutrophil phagocytosis remained a significant predictor of failed eradication therapy even after adjustment for other clinical parameters (age, sex, age at diagnosis, forced expiratory volume in 1 second, body mass index, CFTR genotypes, pancreatic insufficiency, cystic fibrosis–related diabetes mellitus) and bacterial phenotypes (twitching, mucoidy) (Table 5).
Table 5.
Logistic Regression Model Suggest That Impaired In Vitro Neutrophil Phagocytosis of Pseudomonas aeruginosa Isolates Is Associated With Failed Eradication Therapy in Patients With Cystic Fibrosis
| Unadjusted Model | |||||||
|---|---|---|---|---|---|---|---|
| OR | (95% CI) | P Value | |||||
| In vitro neutrophil phagocytosis | 0.76 | (.62–.94) | .01 | ||||
| In vitro neutrophil intracellular bacterial killing | 0.76 | (.66–1.01) | .06 | ||||
| Adjusted Modela | |||||||
| OR | (95% CI) | P Value | Variablea | OR | (95% CI) | P Value | |
| In vitro neutrophil phagocytosis | 0.76 | (.62–.94) | .01 | Age | 0.91 | (.67–1.25) | .57 |
| 0.76 | (.62–.94) | .01 | Female sex | 1.27 | (.19–8.58) | .81 | |
| 0.76 | (.60–.95) | .02 | Age at diagnosis >2 y | 0.85 | (.06–12.43) | .91 | |
| 0.73 | (.57–.95) | .02 | Heterozygous | 0.76 | (.06–9.05) | .83 | |
| Other | 2.01 | (.13–33.8) | .61 | ||||
| 0.77 | (.62–.95) | .02 | BMI | 0.82 | (.54–1.23) | .33 | |
| 0.71 | (.56–.92) | <.01 | Pancreatic insufficiency | 0.02 | (.00–3.23) | .14 | |
| 0.76 | (.62–.94) | .01 | CFRD | 0.67 | (.01–36.72) | .84 | |
| 0.76 | (.61–.94) | .01 | FEV1 %, predicted | 1 | (.96–1.05) | .94 | |
| 0.77 | (.63–.95) | .01 | Mucoidy | 0.39 | (.05–2.74) | .34 | |
| 0.79 | (.63–.90) | .04 | Twitching | 0.96 | (.87–1.06) | .38 | |
Abbreviations: BMI, body mass index; CFRD, cystic fibrosis–related diabetes mellitus; CI, confidence interval; FEV1, forced expiratory volume in 1 second; OR, odds ratio.
aIn the adjusted model, each additional clinical parameter or bacterial phenotype was included 1 at a time. P = .05, with OR evaluated using a 2-sided .05 level of significance.
DISCUSSION
Although several studies have previously reported on the phenotypic characteristics of PA isolates in patients undergoing eradication therapy [9–12, 31], our study is the first to examine the association between bacterial–neutrophil interactions (ie, phagocytosis and intracellular killing) and outcomes of PA antimicrobial eradication therapy in patients with CF. Our study showed that PA isolates from patients who failed tobramycin eradication therapy were more resistant to neutrophil phagocytosis and intracellular bacterial killing than those from patients with successful eradication. Furthermore, loss of twitching motility and mucoidy were associated with resistance to neutrophil antibacterial functions.
Among the few studies that have characterized bacterial phenotypes in patients undergoing eradication therapy, Douglas et al also identified a high proportion of mucoidy among isolates from new-onset infections (18.2%), and 3 of 6 isolates from patients who failed eradication were mucoid [11]. Interestingly, Mayer-Hamblett et al tested 22 bacterial phenotypes and reported that wrinkly colony surface and irregular colony edges morphologies were associated with eradication failure in the 194 participants from the EPIC trial; they concluded that eradication failure was associated with PA phenotypes typical of chronic infection [9] and host adaptation [32, 33]. Whether the occurrence of such “chronic phenotypes” reflect a preexisting infection [34], acquisition of shared strains, or patient-to-patient transmission [35] remains unknown. A recent study of our cohort by Stapleton et al reported that 41% of CF patients with new-onset PA infection shared strains with other patients based on whole genome sequencing of their PA isolates, and patient-to-patient transmission was potentially involved in a third of patients with shared strains [36], a proportion similar to results reported by Marvig et al [37].
Numerous PA phenotypes modulate host–pathogen interactions important to bacterial clearance, but few studies have examined whether such interactions are associated with infection outcomes in patients with CF. Tramper-Stranders et al found no differences in bacterial motility, protease, or pyocyanin production between persistent and eradicated isolates, but did observe that persistent isolates caused greater cytotoxicity in IB3-1CF bronchial cells compared to the eradicated isolates [10]. In a study that examined bronchoalveolar lavage fluid (BALF) at the time of new-onset PA infection in a cohort of 26 children with CF, Douglas et al reported a trend toward higher neutrophil counts, neutrophil elastase, and IL-1ß in the BALF of patients who failed eradication therapy [11].
Neutrophils are the primary phagocytic cells recruited to eradicate PA in the lung [16, 17], yet appear ineffective at eliminating PA in chronic stages of infections [38]. In addition to the inflammatory milieu of the CF lung, neutrophil functions are also modulated by the CF-adapted PA phenotypes commonly encountered in chronic infections. Several studies have examined how phagocytic responses differ upon infection with CF-adapted clinical isolates. Mahenthiralingam et al originally observed that chronic infection PA isolates commonly lacked swimming and twitching motility, were mucoidy, and were resistant to nonopsonic phagocytosis by murine macrophages, compared to their clonally related isolates recovered from new-onset PA infection [39]. Two more recent studies compared neutrophil extracellular trap (NET) formation in response to pairs of clonally related PA isolates from early and chronic CF infections, and found that CF adapted isolates elicited significantly less NET formation [40, 41]. However, none of these studies investigated whether the neutrophil responses to early-infection PA isolates were predictive of subsequent clinical outcomes.
Neutrophil phagocytosis and intracellular killing of bacteria are complex processes that involve interactions with numerous bacterial surface molecules and motility appendages [42]. For example, complement deposition, opsonization, and reactive oxygen species–mediated killing are hindered by the overexpression of EPSs such as alginate in mucoid isolates, and Psl and Pel in wrinkly colony isolates [24, 43, 44]. Furthermore, pili act as ligands for nonopsonic phagocytosis [45], and nonmotile mucoid PA are resistant to nonopsonic phagocytosis [22]. The association of loss of twitching motility and mucoidy with impaired neutrophil-mediated bacterial clearance in our PA clinical isolates is thus mechanistically plausible and could increase the risk of persistent infection in CF patients. Future studies with larger collections and more comprehensive phenotyping of PA isolates may identify other bacterial characteristics associated with impaired neutrophil antibacterial functions.
In a recent study of a subset of our cohort, Beaudoin et al observed higher levels of the exopolysaccharide Psl in biofilm-grown persistent PA isolates [31]. The wrinkly colony morphology described by Mayer-Hamblett et al to be associated with eradication failure is also typically caused by the overproduction of exopolysaccharides Psl and Pel [9, 13]. Since the overproduction of Psl and/or Pel reduces complement deposition, confers resistance to neutrophil antibacterial functions [24], and promotes bacterial persistence in mouse infection models [46], these observations raised the possibility that high Psl and/or Pel expression may be associated with eradication failure through their effects on neutrophil functions. However, using the Congo red aggregation assay [30], we found no differences between persistent and eradicated isolates, nor any association with differential neutrophil responses.
Our study has several limitations. Chronic PA infections of the CF lung are genetically and phenotypically highly diverse, and occur in the context of polymicrobial communities. Multiple PA sublineages often coexist [47, 48], and Stapleton et al reported that mixed strains were found in 16% of new-onset PA infections [36]. Since we only tested morphologically distinct PA clones recovered from each single sputum sample, we may have overlooked some of the phenotypic diversity. However, when multiple PA morphotypes were present, we analyzed all neutrophil assay results, using either the maximal, the median, or the mean value for each patient and found similar results, suggesting that the in vitro neutrophil phenotypes were robust measures. Additionally, the status of persistent infection was defined based on a positive sputum culture after the completion of tobramycin treatment, without confirmation by whole genome sequencing to exclude the possibility of a new PA infection. We chose to test neutrophil responses using the HL-60 cell lines to obtain robust and reproducible measurements of neutrophilic functions in response to a large number of PA isolates. However, we recognize that our results have not been validated in primary human neutrophils, and our experimental system does not account for acquired or intrinsic neutrophil defects associated with the CF lung milieu or CFTR defect [49, 50]. Finally, the multivariable analyses were limited by the small sample size, particularly for patients with persistent infections. Our study sample size only had sufficient power for 1 additional variable to be included in the logistic regression analyses, leading us to examine the effect of different clinical parameters and bacterial phenotypes in a sequential manner.
Our study suggests that decreased neutrophil phagocytosis of PA is an independent predictor of failed tobramycin eradication. Whether in vitro neutrophil assays could be used in a clinical setting remains unknown, and further studies are required to address the potential polyclonal nature of new-onset PA infections and to validate our results in an independent cohort. Nonetheless, our results provide biological insights into why eradication therapy might fail in CF patients. Although the mechanisms underlying the failure of tobramycin eradication are likely multifactorial, our study supports the notion that strain-specific PA–neutrophil interactions are important determinants of the outcome of inhaled tobramycin eradication treatment. These results thus highlight the possibility that novel nonantibiotic therapies that target PA–neutrophil interactions and enhance neutrophil-mediated antibacterial functions should be considered to improve the outcome of PA eradication in CF.
Supplementary Data
Supplementary materials are available at The Journal of Infectious Diseases online. Consisting of data provided by the authors to benefit the reader, the posted materials are not copyedited and are the sole responsibility of the authors, so questions or comments should be addressed to the corresponding author.
Notes
Acknowledgments. We thank Dr Brent Berwin and his team for technical support, and Dr Daniel Wozniak for providing the Pseudomonas aeruginosa strains used as controls in our assays.
Financial support. This work was supported by Cystic Fibrosis Canada (grant number 559985 to D. N.); and the Cystic Fibrosis Foundation (grant number WATERS17G0 to V. W.). K. K. was supported by a scholarship from Cystic Fibrosis Canada and the Meakins Christie Studentship. D. N. was supported by a Fonds de Recherche en Santé du Quebec (FRQS) salary award.
Potential conflicts of interest. V. W. has served as consultant for AstraZeneca anti-Psl monoclonal antibody trials. All other authors report no potential conflicts of interest.
All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
Presented in part: 2018 Neutrophil International Symposium, Quebec City, Canada, 2–6 June 2018; and 2019 North American Cystic Fibrosis Conference, Nashville, Tennessee, 31 October–2 November 2019.
Contributor Information
Kelly Kwong, Department of Microbiology and Immunology, McGill University, Montreal, Canada; Meakins Christie Laboratories, Research Institute of the McGill University Health Centre, Montreal, Canada.
Andrea Benedetti, Department of Epidemiology, Biostatistics and Occupational Health, McGill University, Montreal, Canada; Centre for Health Outcome Research, Research Institute of the McGill University Health Centre, Montreal, Canada.
Yvonne Yau, Department of Laboratory Medicine and Pathobiology, University of Toronto, Toronto, Canada; Division of Microbiology, Department of Pediatric Laboratory Medicine, The Hospital for Sick Children, Toronto, Canada.
Valerie Waters, Department of Laboratory Medicine and Pathobiology, University of Toronto, Toronto, Canada; Division of Infectious Diseases, Department of Pediatrics, The Hospital for Sick Children, University of Toronto, Toronto, Canada.
Dao Nguyen, Department of Microbiology and Immunology, McGill University, Montreal, Canada; Meakins Christie Laboratories, Research Institute of the McGill University Health Centre, Montreal, Canada; Department of Medicine, McGill University, Montreal, Canada.
References
- 1. Ratjen F, Bell SC, Rowe SM, Goss CH, Quittner AL, Bush A. Cystic fibrosis. Nat Rev Dis Primers 2015; 1:15010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Gibson RL, Burns JL, Ramsey BW. Pathophysiology and management of pulmonary infections in cystic fibrosis. Am J Respir Crit Care Med 2003; 168:918–51. [DOI] [PubMed] [Google Scholar]
- 3. Treggiari MM, Retsch-Bogart G, Mayer-Hamblett N, et al. ; Early Pseudomonas Infection Control (EPIC) Investigators . Comparative efficacy and safety of 4 randomized regimens to treat early Pseudomonas aeruginosa infection in children with cystic fibrosis. Arch Pediatr Adolesc Med 2011; 165:847–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Stanojevic S, Waters V, Mathew JL, Taylor L, Ratjen F. Effectiveness of inhaled tobramycin in eradicating Pseudomonas aeruginosa in children with cystic fibrosis. J Cyst Fibros 2014; 13:172–8. [DOI] [PubMed] [Google Scholar]
- 5. Ratjen F, Munck A, Kho P, Angyalosi G; ELITE Study Group . Treatment of early Pseudomonas aeruginosa infection in patients with cystic fibrosis: the ELITE trial. Thorax 2010; 65:286–91. [DOI] [PubMed] [Google Scholar]
- 6. Schelstraete P, Haerynck F, Van daele S, Deseyne S, De Baets F. Eradication therapy for Pseudomonas aeruginosa colonization episodes in cystic fibrosis patients not chronically colonized by P. aeruginosa. J Cyst Fibros 2013; 12:1–8. [DOI] [PubMed] [Google Scholar]
- 7. Hewer SCL, Smyth AR, Brown M, et al. ; TORPEDO-CF Study Group . Intravenous versus oral antibiotics for eradication of Pseudomonas aeruginosa in cystic fibrosis (TORPEDO-CF): a randomised controlled trial. Lancet Respir Med 2020; 8:975–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Mayer-Hamblett N, Kronmal RA, Gibson RL, et al. ; EPIC Investigators . Initial Pseudomonas aeruginosa treatment failure is associated with exacerbations in cystic fibrosis. Pediatr Pulmonol 2012; 47:125–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Mayer-Hamblett N, Ramsey BW, Kulasekara HD, et al. . Pseudomonas aeruginosa phenotypes associated with eradication failure in children with cystic fibrosis. Clin Infect Dis 2014; 59:624–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Tramper-Stranders GA, van der Ent CK, Molin S, et al. . Initial Pseudomonas aeruginosa infection in patients with cystic fibrosis: characteristics of eradicated and persistent isolates. Clin Microbiol Infect 2012; 18:567–74. [DOI] [PubMed] [Google Scholar]
- 11. Douglas TA, Brennan S, Gard S, et al. . Acquisition and eradication of P. aeruginosa in young children with cystic fibrosis. Eur Respir J 2009; 33:305–11. [DOI] [PubMed] [Google Scholar]
- 12. Vidya P, Smith L, Beaudoin T, et al. . Chronic infection phenotypes of Pseudomonas aeruginosa are associated with failure of eradication in children with cystic fibrosis. Eur J Clin Microbiol Infect Dis 2015; 35:67–74. [DOI] [PubMed] [Google Scholar]
- 13. Starkey M, Hickman JH, Ma L, et al. . Pseudomonas aeruginosa rugose small-colony variants have adaptations that likely promote persistence in the cystic fibrosis lung. J Bacteriol 2009; 191:3492–503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Goltermann L, Tolker-Nielsen T. Importance of the exopolysaccharide matrix in antimicrobial tolerance of Pseudomonas aeruginosa aggregates. Antimicrob Agents Chemother 2017; 61:e02696-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Yonker LM, Cigana C, Hurley BP, Bragonzi A. Host-pathogen interplay in the respiratory environment of cystic fibrosis. J Cyst Fibros 2015; 14:431–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Lavoie EG, Wangdi T, Kazmierczak BI. Innate immune responses to Pseudomonas aeruginosa infection. Microbes Infect 2011; 13:1133–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Kolaczkowska E, Kubes P. Neutrophil recruitment and function in health and inflammation. Nat Rev Immunol 2013; 13:159–75. [DOI] [PubMed] [Google Scholar]
- 18. Ray VA, Hill PJ, Stover CK, et al. . Anti-Psl targeting of Pseudomonas aeruginosa biofilms for neutrophil-mediated disruption. Sci Rep 2017; 7:16065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. SenGupta S, Hittle LE, Ernst RK, Uriarte SM, Mitchell TC. A Pseudomonas aeruginosa hepta-acylated lipid A variant associated with cystic fibrosis selectively activates human neutrophils. J Leukoc Biol 2016; 100:1047–59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Speert DP, Eftekhar F, Puterman ML. Nonopsonic phagocytosis of strains of Pseudomonas aeruginosa from cystic fibrosis patients. Infect Immun 1984; 43:1006–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Floyd M, Winn M, Cullen C, et al. . Swimming motility mediates the formation of neutrophil extracellular traps induced by flagellated Pseudomonas aeruginosa. PLoS Pathog 2016; 12:e1005987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Cabral DA, Loh BA, Speert DP. Mucoid Pseudomonas aeruginosa resists nonopsonic phagocytosis by human neutrophils and macrophages. Pediatr Res 1987; 22:429–31. [DOI] [PubMed] [Google Scholar]
- 23. Gunn JS, Bakaletz LO, Wozniak DJ. What’s on the outside matters: the role of the extracellular polymeric substance of gram-negative biofilms in evading host immunity and as a target for therapeutic intervention. J Biol Chem 2016; 291:12538–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Mishra M, Byrd MS, Sergeant S, et al. . Pseudomonas aeruginosa Psl polysaccharide reduces neutrophil phagocytosis and the oxidative response by limiting complement-mediated opsonization. Cell Microbiol 2012; 14:95–106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Skopelja S, Hamilton BJ, Jones JD, et al. . The role for neutrophil extracellular traps in cystic fibrosis autoimmunity. JCI Insight 2016; 1:e88912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Borkar DS, Fleiszig SM, Leong C, et al. . Association between cytotoxic and invasive Pseudomonas aeruginosa and clinical outcomes in bacterial keratitis. JAMA Ophthalmol 2013; 131:147–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Flitter BA, Hvorecny KL, Ono E, et al. . Pseudomonas aeruginosa sabotages the generation of host proresolving lipid mediators. Proc Natl Acad Sci U S A 2017; 114:136–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Quigley KJ, Reynolds CJ, Goudet A, et al. . Chronic infection by mucoid Pseudomonas aeruginosa associated with dysregulation in T-cell immunity to outer membrane porin F. Am J Respir Crit Care Med 2015; 191:1250–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Blanchard AC, Horton E, Stanojevic S, Taylor L, Waters V, Ratjen F. Effectiveness of a stepwise Pseudomonas aeruginosa eradication protocol in children with cystic fibrosis. J Cyst Fibros 2017; 16:395–400. [DOI] [PubMed] [Google Scholar]
- 30. Jones CJ, Wozniak DJ. Congo red stain identifies matrix overproduction and is an indirect measurement for c-di-GMP in many species of bacteria. Methods Mol Biol 2017; 1657:147–56. [DOI] [PubMed] [Google Scholar]
- 31. Beaudoin T, Yau YCW, Stapleton PJ, et al. . Staphylococcus aureus interaction with Pseudomonas aeruginosa biofilm enhances tobramycin resistance. NPJ Biofilms Microbiomes 2017; 3:25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Smith EE, Buckley DG, Wu Z, et al. . Genetic adaptation by Pseudomonas aeruginosa to the airways of cystic fibrosis patients. Proc Natl Acad Sci U S A 2006; 103:8487–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Hogardt M, Heesemann J. Adaptation of Pseudomonas aeruginosa during persistence in the cystic fibrosis lung. Int J Med Microbiol 2010; 300:557–62. [DOI] [PubMed] [Google Scholar]
- 34. Blanchard AC, Rooney AM, Yau Y, et al. . Early detection using qPCR of Pseudomonas aeruginosa infection in children with cystic fibrosis undergoing eradication treatment. J Cyst Fibros 2018; 17:723–8. [DOI] [PubMed] [Google Scholar]
- 35. Kosorok MR, Jalaluddin M, Farrell PM, et al. . Comprehensive analysis of risk factors for acquisition of Pseudomonas aeruginosa in young children with cystic fibrosis. Pediatr Pulmonol 1998; 26:81–8. [DOI] [PubMed] [Google Scholar]
- 36. Stapleton PJ, Izydorcyzk C, Clark S, et al. . Pseudomonas aeruginosa strain sharing in early infection among children with cystic fibrosis [manuscript published online ahead of print 16 June 2020]. Clin Infect Dis 2020. doi:10.1093/cid/ciaa788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Marvig RL, Sommer LM, Molin S, Johansen HK. Convergent evolution and adaptation of Pseudomonas aeruginosa within patients with cystic fibrosis. Nat Genet 2015; 47:57–64. [DOI] [PubMed] [Google Scholar]
- 38. Hartl D, Gaggar A, Bruscia E, et al. . Innate immunity in cystic fibrosis lung disease. J Cyst Fibros 2012; 11:363–82. [DOI] [PubMed] [Google Scholar]
- 39. Mahenthiralingam E, Campbell ME, Speert DP. Nonmotility and phagocytic resistance of Pseudomonas aeruginosa isolates from chronically colonized patients with cystic fibrosis. Infect Immun 1994; 62:596–605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Young RL, Malcolm KC, Kret JE, et al. . Neutrophil extracellular trap (NET)-mediated killing of Pseudomonas aeruginosa: evidence of acquired resistance within the CF airway, independent of CFTR. PLoS One 2011; 6:e23637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Yoo DG, Floyd M, Winn M, Moskowitz SM, Rada B. NET formation induced by Pseudomonas aeruginosa cystic fibrosis isolates measured as release of myeloperoxidase-DNA and neutrophil elastase-DNA complexes. Immunol Lett 2014; 160:186–94. [DOI] [PubMed] [Google Scholar]
- 42. Lovewell RR, Patankar YR, Berwin B. Mechanisms of phagocytosis and host clearance of Pseudomonas aeruginosa. Am J Physiol Lung Cell Mol Physiol 2014; 306:L591–603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Learn DB, Brestel EP, Seetharama S. Hypochlorite scavenging by Pseudomonas aeruginosa alginate. Infect Immun 1987; 55:1813–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Baltimore RS, Shedd DG. The role of complement in the opsonization of mucoid and non-mucoid strains of Pseudomonas aeruginosa. Pediatr Res 1983; 17:952–8. [DOI] [PubMed] [Google Scholar]
- 45. Kelly NM, Kluftinger JL, Pasloske BL, Paranchych W, Hancock RE. Pseudomonas aeruginosa pili as ligands for nonopsonic phagocytosis by fibronectin-stimulated macrophages. Infect Immun 1989; 57:3841–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Pestrak MJ, Chaney SB, Eggleston HC, et al. . Pseudomonas aeruginosa rugose small-colony variants evade host clearance, are hyper-inflammatory, and persist in multiple host environments. PLoS Pathogens 2018; 14:1–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Williams D, Evans B, Haldenby S, et al. . Divergent, coexisting Pseudomonas aeruginosa lineages in chronic cystic fibrosis lung infections. Am J Respir Crit Care Med 2015; 19:775–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Markussen T, Marvig RL, Gómez-Lozano M, et al. . Environmental heterogeneity drives within-host diversification and evolution of Pseudomonas aeruginosa. mBio 2014; 5:e01592–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Painter RG, Valentine VG, Lanson NA Jr, et al. . CFTR expression in human neutrophils and the phagolysosomal chlorination defect in cystic fibrosis. Biochemistry 2006; 45:10260–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Ratner D, Mueller C. Immune responses in cystic fibrosis: are they intrinsically defective? Am J Respir Cell Mol Biol 2012; 46:715–22. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.