Abstract
Objective
This study investigates the presence of cerebrovascular injuries in a large sample of civilian penetrating brain injury (PBI) patients, determining the prevalence, radiographic characteristics, and impact on short-term outcome.
Methods
We retrospectively reviewed patients with PBI admitted to our institution over a two-year period. Computed tomography head scans, computer tomography angiograms and venograms of the intracranial vessels were evaluated to determine the wound trajectory, intracranial injury characteristics, and presence of arterial (AI) and venous sinus (VSI) injuries. Demographics, clinical presentation, and treatment were also reviewed. Discharge disposition was used as surrogate of short-term outcome.
Results
Seventy-two patients were included in the study. The mechanism of injury was gunshot wounds in 71 patients and stab wound in one. Forty-one of the 72 patients (60%) had at least one vascular injury. Twenty-six out of 72 patients suffered an AI (36%), mostly pseudoaneurysms and occlusions, involving the anterior and middle cerebral arteries. Of the 72 patients included, 45 had dedicated computed tomography venograms, and of those 22 had VSI (49%), mainly manifesting as superior sagittal sinus occlusion. In a multivariable regression model Intraventricular hemorrhage at presentation was associated with AI (OR=9.9, p=0.004). The same was not true for VSI.
Conclusion
Acute traumatic cerebrovascular injury is a prevalent complication in civilian PBI, frequently involving both the arterial and venous sinus systems. Although some radiographic features might be associated with presence of vascular injury, assessment of the intracranial vasculature in the acute phase of all PBI is essential for early diagnosis. Treatment of vascular injury remains variable depending on local practice.
Keywords: Traumatic cerebrovascular injury, Penetrating brain injury, cerebrovascular injury, penetrating brain injury
Background
Traumatic cerebrovascular injury is a common potential complication of penetrating brain injury (PBI)1,2. The current guidelines are almost two decades old and are based only on eight retrospective studies from the 1990s.3 The studies’ findings are vague and their recommendations are broad suggesting PBI providers maintain “an index of suspicion for the presence of vascular injury […]” and “when these are detected, therapeutic measures […] are indicated”3.
The identification of arterial or venous injury is important. Often times those injuries may lead to secondary brain injury which worsens the primary insult and complicates care. However, the main body of published literature about PBI comes from the military setting; specifically, the Iraqi conflict, Lebanese conflict, and Afghanistan war1–8. Arguably, the military context differs from the civilian setting in that it more frequently involves blast injuries as well as high velocity penetrating injuries. Those studies mainly focus on development of delayed arterial traumatic intracranial aneurysms (TICAs), reporting an incidence as high as 34%5. Risk factors for development of TICAs include presence of intraparenchymal hemorrhage, subarachnoid hemorrhage (SAH), shrapnel and bone fragments, and absence of an exit wound1,4. The largest military case series to date (n=64)5 reports a natural history of arterial rupture between 4 and 32 days post injury, with some untreated TICAs subsequently healing and resolving spontaneously.
Guidelines for diagnosis of cerebrovascular injuries in PBI are antiquated, and mostly predate the era of high-quality modern CT angiography. The current guidelines and much of the subsequent literature use digital subtraction angiography (DSA) as the preferred modality of investigation, mostly given the limitations introduced by artifact from retained bone and shrapnel.3 Reported criteria for performing screening DSA in military trauma patients8 include (1) penetrating head injury of any kind, (2) a known surgically treated TICA, (3) nonpenetrating head injury associated with blast and a presenting Glasgow Coma Scale (GCS) score less than 8, (4) transcranial Doppler ultrasound evidence of vasospasm, and (5) spontaneous decrease in the partial pressure of brain-tissue oxygen or cerebral blood flow in an otherwise stable patient.
In the civilian population, where blast injuries are rare, and the mechanism of injury continues to predominantly involve lower velocity ballistics, the studies on the topic are scant and the majority of the literature is in the form of case reports and small case series.9–20 The reported prevalence of vascular injury in civilian PBI ranges between 3%−50%.21,22 The largest series to date of arterial injury in the civilian population (n=55)23,24 identified several CT findings associated with the presence of vascular injury. These include frontobasal and temporal entry sites (20/55), injury in the proximity of the circle of Willis (COW) (10/55), presence of SAH (20/55), and presence of intraventricular hemorrhage (9/55). A wound trajectory in the proximity of COW was the best predictor of arterial injury (OR 6.8). CTA was found to be an effective screening tool in this population, with 100% sensitivity and 100% specificity in identifying TICAs involving the first-order branches of intracranial arteries (n=7), decreasing to 77.3% sensitivity and 90.3% specificity for vascular injury involving more distal branches (n=22)25. However, this research mainly focused on TICAs, leaving other forms of vascular injury largely uninvestigated. Data on VSI is even more scarce with no large cohorts describing the frequency or management of VSI in PBI.
Given the limitations and modicum of literature on cerebrovascular injuries in civilian PBI, we systematically investigated both arterial and venous sinus injury in a large sample of PBI patients at our Level I Trauma center with a relatively high proportion of penetrating injuries (40% of all trauma activations). Here we report the prevalence, associated radiographic characteristics, management strategies, and impact on length of stay and short-term clinical outcome of these injuries.
Methods
Study Population
We performed a retrospective analysis of patients with penetrating brain injury admitted to our institution between the years of 2018 and 2020. Our institution maintained level I trauma accreditation during the entire duration of the study. The Institutional Review Board at the University of Chicago approved the protocol (IRB-19-0220). Inclusion criteria were: 1) admission diagnosis of penetrating brain injury; 2) age ≥ 16 years; 3) surviving Trauma Bay resuscitation; and 4) performance of head CTA or CTV during the hospital stay. Exclusion criteria were: 1) absence of dural penetration, 2) artifact obscuring the first-order branches of intracranial vessels on CTA. Demographic data, imaging, treatment, length of stay (LOS), and outcome surrogates defined as discharge dispositions, and Glasgow outcome scale extended (GOSE) were reviewed. Arterial Cranio-vascular injury was defined as an injury evident on CTA. Venous Sinus Cranio-vascular injury was defined as an injury evident on CTV. LOS was defined as the number of midnights spent in the hospital. Due to challenges with long-term follow up in our population, discharge disposition and GOSE at discharge were used as surrogates of clinical outcome. Also, clinical outcome was dichotomized into favorable outcome (discharge to home or an acute inpatient rehabilitation facility), versus unfavorable outcome (discharge to LTACH, hospice, or in-hospital death).
Imaging Study and Interpretation
CTA and CTV images were acquired using helical scanners with 0.6 mm by 40 mm collimation with 4.5 and 19.0 second delays after bolus triggering for CTA and CTV, respectively. Multiplanar reformatted images and maximum intensity projection (MIP) images were also obtained. All studies were performed using our institutional cerebral CTA and CTV protocols.
Images were initially evaluated by the board-certified attending neuroradiologist on service at the time of acquisition. A second board-certified attending neuroradiologist (D.G.) who was blind to the initial findings, reviewed the images. To describe the presumed site of entry, the cranium was divided into three regions: frontobasal, which included the frontal bone and orbitofacial region; temporal which included the pterion, temporal bone, and sphenoid bone; and parieto-occipital, which included the parietal bone and occipital bone. Presumed site of exit, when applicable, was divided into the same region of the site of entry. For each vascular study we evaluated the presence or absence of vascular injury, the arterial and/or venous sinus involvement, the vessels involved, and the type of injury. In our institution, brain CTA and CTV is routinely obtained for all PBI patients who survive initial resuscitation. The goal is to perform those studies within the first 24–48 hours. However, exceptions partially related to patients’ hemodynamic stability may prevent the accomplishment of that goal. The studies included were all done within this time frame.
Statistical Analysis
Descriptive statistics were presented as means with standard deviations or medians with interquartile ranges (as appropriate) for continuous variables and as percentages for categorical variables. In univariate analyses, categorical variables were compared by chi-square or Fisher test, as appropriate, and continuous variables were compared by Mann-Whitney U tests or t-tests as appropriate. Given the sample size, statistical significance was set at p <0.05.
Results
Basic characteristics of the population
Within the duration of the study, 128 patients presented with a penetrating brain injury. Thirty-six patients died during initial resuscitation. Of the ninety-two remaining patients, twenty patients did not have CTA or CTV as they expired within the first 72 hours and vascular imaging was not obtained due to hemodynamic instability and/or perceived medical futility. The remaining seventy-two patients were analyzed (supplemental figure 1). Median age of our cohort was 26.5(16) years, and 60 patients (83%) were male. The predominant race was black (92%). The intent was assault in 61 patients (85%). The mechanism of injury was gunshot wounds in seventy-one patients and stab wound in one patient. 60 patients had isolated PBI (83%) while 12 patients suffered from polytrauma. Median GCS (IQR) at presentation was 7 (11). Pupillary response was bilaterally reactive in 40 patients (55.6%), one fixed in 9 (12.5%), and bilateral fixed in 23 (31.9%). Sixteen patients (22%) were discharged home, twenty-one (29%) to acute rehabilitation facility, eight (11%) to long term acute care hospital (LTACH), and twenty-seven (38%) died in the hospital. Overall, a favorable outcome was observed in 38 patients (51%).
Vascular Injury
We define an occlusion as a vessel blockage evidenced by lack of contrast filling due to a clot, transection as a traumatic rupture of the vessel wall, intimal injury as disruption of the inner wall of the vessel, dissection as extension of intravascular blood into the vessel wall via a disruption to intima or vasa vasorum and finally we define impingement as extrinsic compression of a vessel. Of the 72 patients included in this study, all had CTAs and 47(65%) had CTVs performed. Forty-one patients (57%) were found to have at least one vascular injury. Fourteen patients (19%) were found to have multiple concomitant vascular injuries. Thirty-seven arterial injuries were identified in 26 patients (Table 1). The type of arterial injuries was as follows: 11 TICAs, 7 occlusions, 8 transections, 6 dissections, and 4 intimal injuries. The arterial injuries were predominantly located in the anterior circulation. The ACA, MCA and ICA were equally affected with 9 injuries in each. The internal carotid artery injury was intradural in 6 cases and extra-dural in 3. The posterior circulation involved primarily the vertebral artery in 7 cases, followed by posterior cerebral artery in 2, and posterior communicating artery in 1. (Table 1, supplemental table 1)
Table 1.
Summary characteristics of all arterial injuries
| Pseudoaneurysm | Occlusion | Transection | Intimal Injury | Dissection | CCF | Total | |
|---|---|---|---|---|---|---|---|
|
| |||||||
| ACA | 4 | 1 | 2 | 1 | 1 | 0 | 9 |
| MCA | 4 | 1 | 3 | 0 | 1 | 0 | 9 |
| ICA | 3 | 0 | 3 | 1 | 1 | 1 | 9 |
| VA | 0 | 2 | 0 | 2 | 3 | 0 | 7 |
| PCA | 0 | 2 | 0 | 0 | 0 | 0 | 2 |
| PCOM | 0 | 1 | 0 | 0 | 0 | 0 | 1 |
|
| |||||||
| Total | 11 | 7 | 8 | 4 | 6 | 1 | 37 |
ACA = anterior cerebral artery; MCA = middle cerebral artery; ICA = internal carotid artery; VA = vertebral artery; PCA = posterior cerebral artery; PCOM = posterior communicating artery; CCF = carotid-cavernous fistula
Out of 47 patients who got a CTV, 22 patients (47%) were found to have at least one venous sinus injury. A total of twenty-three venous sinus injuries were detected as one patient had concomitant injury of the transverse and sigmoid sinuses. The predominant injury type was an occlusion which was observed in 15 patients, followed by impingement in 6, and finally transection in 2 cases. Sixteen VSI involved the superior sagittal sinus, followed by the transverse sinus in 4 and sigmoid sinus in 3 (Table 2, supplemental table 2)
Table 2.
Summary characteristics of all venous sinus injuries
| Occlusion | Impingement | Transection | Total | |
|---|---|---|---|---|
|
| ||||
| SSS | 11 | 4 | 1 | 16 |
| Transverse | 2 | 1 | 1 | 4 |
| Sigmoid | 2 | 1 | 0 | 3 |
|
| ||||
| Total | 15 | 6 | 2 | 23 |
SSS = superior sagittal sinus
Clinical and radiographic features of patients with vascular injury
Forty-one patients with vascular injury (VI) and 31 patients without vascular injury (nVI) are compared (Table 3). Baseline demographics were not significantly different between the two groups. Lower GCS is more likely associated with VI (OR=0.85,95% C.I=0.74–0.99, p=0.026), Imaging characteristics including site of entry are not significantly different across the two group. As most of the injuries were penetrating but not perforating, the exit wound was not frequent enough to allow for meaningful statistics. Also, whether an injury was penetrating or perforating was not significantly associated with VI.
Table 3.
Comparison of clinical and radiographic variables between vascular injury and non-vascular injury groups (in patients where dedicated vascular imaging was completed)
| Clinical Variables | Vascular Injury (N=41) | Non-Vascular Injury (N=31) | P Value |
|---|---|---|---|
| Median age (IQR), yrs | 24 (15) | 27(19) | 0.406 |
| Male (%) | 34 (83) | 26 (84) | 0.915 |
| Median GCS (IQR) | 5 (7) | 11 (10) | *0.023 |
| CT Variables | |||
| Presumed Entry Site | |||
| Frontobasal (%) | 22 (54) | 13 (42) | 0.324 |
| Facial (%) | 4 (10) | 0(0) | 0.074 |
| Orbital (%) | 5 (12) | 4 (13) | 0.928 |
| Frontal (%) | 13 (32) | 9 (29) | 0.807 |
| Temporal (%) | 5 (12) | 8 (26) | 0.137 |
| Parieto-occipital (%) | 14 (34) | 10 (32) | 0.866 |
| Parietal (%) | 9 (22) | 4 (13) | 0.323 |
| Occipital (%) | 5 (12) | 6 (19) | 0.403 |
| Injury | |||
| Bihemispheric involvement (%) | 18 (44) | 9 (29) | 0.197 |
| Frontal lobe (%) | 26 (63) | 19 (61) | 0.854 |
| Parietal lobe (%) | 21 (51) | 12 (39) | 0.291 |
| Temporal lobe (%) | 13 (32) | 11 (35) | 0.736 |
| Occipital lobe (%) | 6 (15) | 7 (23) | 0.385 |
| Thalamus (%) | 9 (22) | 6 (19) | 0.788 |
| Posterior fossa (%) | 3 (7) | 4 (13) | 0.428 |
| Brainstem (%) | 3 (7) | 3 (!0) | 0.72 |
| IVH (%) | 15 (37) | 6 (19) | 0.111 |
| SAH (%) | 32 (78) | 28 (90) | 0.166 |
| Intracerebral bullet fragments (%) | 27 (66) | 20 (64) | 0.906 |
| Intracerebral bone fragments (%) | 33 (80) | 22 (71) | 0.346 |
| Outcome Variables | |||
| Median Length of Stay (IQR), d | 5 (14) | 7 (13) | 0.363 |
| Median GOSE at discharge (IQR) | 2 (3) | 5 (4) | *0.003 |
| Favorable Outcome (%) | 16 (39) | 21 (68) | *0.016 |
| Mortality (%) | 20 (49) | 7 (23) | *0.023 |
GCS = Glasgow Coma Scale; IVH = intraventricular hemorrhage; SAH = subarachnoid hemorrhage; GOSE = extended Glasgow Outcome Scale
Upon separately considering variables associated with arterial injury (AI) and variables associated with venous sinus injury (VSI) (Supplementary Table 1 and 2), intraventricular hemorrhage at presentation was found in 54% of patients with AI compared to 15% in patients without. In a multivariate logistic regression model accounting for significantly different imaging variables(p<0.05), IVH remains significantly associated (OR=9.9,95% CI=2.1–47.6,p=0.004) No significant differences in radiographic characteristics were found in VSI subgroup analysis.
Treatment of vascular injuries
Given that no established guidelines for the management of vascular complications in civilian PBI exist, the treatment of arterial and venous sinus injuries were individualized based on a multidisciplinary approach involving the neurointensivist, neurosurgeon, and neurointerventionalist. Management included observation, medical treatment with antithrombotics, open surgical intervention, and endovascular therapy. Specifically, three pseudoaneurysms were treated with endovascular embolization (one had an associated injury of the internal carotid artery that was also sacrificed), one pseudoaneurysm was treated surgically, one carotid-cavernous fistula was treated with flow-diverting stent, two arterial dissections were treated with antiplatelet therapy, and two venous sinus thrombosis were treated with anticoagulants. The majority of vascular injuries were observed (32/41) due to refractory intracranial hypertension, hemodynamic instability that prohibited interventions, or contraindication to start antithrombotic therapy. Of the patients that did receive surgical or endovascular treatment, patient 11 received endovascular embolization of pseudoaneurysm and sacrifice of left internal carotid artery. He was discharged to an acute rehabilitation facility with GOSE at discharge of 4. Patient 16 had surgical treatment of a left M4-MCA pseudoaneurysm. The patient was discharged to an LTACH with GOSE at discharge of 3. Patient 18 had endovascular embolization of a pseudoaneurysm in the right M2-MCA. He was discharged home with GOSE at discharge of 6. Patient 26 had embolization of a pseudoaneurysm in the left M1-MCA. The patient died in the hospital. Finally patient 32 had carotid-cavernous fistula that was treated with endovascular flow diverting stent. The patient was discharged to an LTACH with GOSE at discharge of 3. Individual description of each patient characteristics, treatment, and outcome is presented in table 4.
Table 4.
Summary characteristics of all patients with vascular injury
| Patient | Age | Sex | Mechanism | GCS | Presumed site of entry | Vessel(s) injured | Injury type | Time to vascular imaging (days) | Treatment | Outcome |
|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 16 | M | GSW | 15 | Frontobasal | SSS | Occlusion | 0 | Medical | Favorable |
| 2 | 34 | M | GSW | 3T | Parieto-occipital | 1. L ICA (intradural) 2. R ICA (intradural) |
1. Transection 2. Transection |
0 | Observation | Unfavorable |
| 3 | 17 | M | GSW | 7T | Frontobasal | SSS | Occlusion | 0 | Observation | Unfavorable |
| 4 | 21 | M | GSW | 15 | Parieto-occipital | SSS | Occlusion | 0 | Observation | Favorable |
| 5 | 46 | M | GSW | 10 | Frontobasal | L VA | Dissection | 0 | Medical | Favorable |
| 6 | 20 | M | GSW | 3T | Frontobasal | 1. R ACA 2. L VA |
1. Intimal injury 2. Intimal injury |
0 | Observation | Unfavorable |
| 7 | 17 | M | GSW | 3T | Frontobasal | 1. L VA 1. L Sigmoid Sinus |
1. Occlusion 2. Occlusion |
0 | Observation | Unfavorable |
| 8 | 24 | M | GSW | 4T | Parieto-occipital | 1. R ACA 2. L ACA 3. SSS |
1. Transection 2. Transection 3. Occlusion |
0 | Observation | Unfavorable |
| 9 | 45 | M | GSW | 4T | Frontobasal | L MCA | Transection | 0 | Observation | Unfavorable |
| 10 | 20 | M | GSW | 11 | Frontobasal | SSS | Impingement | 0 | Observation | Unfavorable |
| 11 | 19 | M | GSW | 8T | Temporal | 1. L ICA (skull base) 2. L ICA (skull base) 3. L Transverse Sinus 4. L Sigmoid Sinus |
1. Dissection 2. Pseudoaneurysm 3. Occlusion 4. Occlusion |
0 | Endovascular | Favorable |
| 12 | 20 | M | GSW | 4T | Frontobasal | 1. L ACA 2. R ACA |
1. Pseudoaneurysm 2. Pseudoaneurysm |
0 | Observation | Unfavorable |
| 13 | 24 | M | GSW | 5T | Parieto-occipital | L VA | Occlusion | 0 | Observation | Unfavorable |
| 14 | 31 | F | GSW | 15 | Temporal | 1. L Transverse Sinus 2. L Sigmoid Sinus |
1. Impingement 2. Impingement |
0 | Observation | Unfavorable |
| 15 | 33 | F | GSW | 3T | Temporal | 1. L ACA 2. R ACA |
1. Pseudoaneurysm 2. Pseudoaneurysm |
0 | Observation | Unfavorable |
| 16 | 18 | M | GSW | 5T | Parieto-occipital | L MCA | Pseudoaneurysm | 0 | Surgical | Unfavorable |
| 17 | 32 | M | GSW | 4T | Parieto-occipital | R ACA | Occlusion | 0 | Observation | Unfavorable |
| 18 | 40 | M | GSW | 15 | Frontobasal | R MCA | Pseudoaneurysm | 0 | Endovascular | Favorable |
| 19 | 35 | M | GSW | 3T | Frontobasal | SSS | Occlusion | 2 | Observation | Unfavorable |
| 20 | 29 | M | GSW | 5T | Frontobasal | 1. R MCA 2. L ACA |
1. Pseudoaneurysm 2. Occlusion |
1 | Observation | Unfavorable |
| 21 | 23 | M | GSW | 6T | Frontobasal | 1. R MCA 2. SSS |
1. Occlusion 2. Transection |
0 | Observation | Favorable |
| 22 | 51 | M | GSW | 10 | Frontobasal | L VA | Dissection | 7 | Medical | Favorable |
| 23 | 29 | M | GSW | 3T | Frontobasal | R ICA (skull base) | Intimal injury | 0 | Observation | Unfavorable |
| 24 | 30 | M | GSW | 3T | Frontobasal | 1. L MCA 2. L PCA 3. L PCOM |
1. Transection 2. Occlusion 3. Occlusion |
0 | Observation | Unfavorable |
| 25 | 37 | M | GSW | 14 | Frontobasal | SSS | Occlusion | 1 | Observation | Favorable |
| 26 | 44 | M | Stab wound | 3T | Frontobasal | L MCA | Pseudoaneurysm | 1 | Endovascular | Unfavorable |
| 27 | 48 | M | GSW | 15 | Frontobasal | SSS | Impingement | 2 | Observation | Favorable |
| 28 | 30 | M | GSW | 4T | Parieto-occipital | L VA | Intimal injury | 0 | Observation | Unfavorable |
| 29 | 22 | M | GSW | 15 | Parieto-occipital | SSS | Occlusion | 0 | Observation | Favorable |
| 30 | 30 | F | GSW | 4T | Parieto-occipital | R VA | Dissection | 0 | Observation | Unfavorable |
| 31 | 22 | M | GSW | 10T | Parieto-occipital | L Transverse Sinus | Transection | 0 | Observation | Unfavorable |
| 32 | 23 | F | GSW | 7T | Frontobasal | R ICA (skull base) | CCF | 1 | Endovascular | Unfavorable |
| 33 | 18 | F | GSW | 3T | Parieto-occipital | 1. R MCA 2. R PCA |
1. Transection 2. Occlusion |
0 | Observation | Unfavorable |
| 34 | 48 | F | GSW | 9T | Frontobasal | SSS | Impingement | 0 | Observation | Favorable |
| 35 | 44 | M | GSW | 3T | Temporal | 1. R ICA 2. L ICA 3. SSS |
1. Pseudoaneurysm 2. Pseudoaneurysm 3. Occlusion |
0 | Observation | Unfavorable |
| 36 | 19 | M | GSW | 6T | Frontobasal | 1. L MCA 2. L ACA 3. L Transverse Sinus |
1. Dissection 2. Dissection 3. Occlusion |
0 | Observation | Favorable |
| 37 | 20 | M | GSW | 4T | Parieto-occipital | SSS | Occlusion | 0 | Observation | Unfavorable |
| 38 | 21 | M | GSW | 4T | Temporal | R ICA | Transection | 0 | Observation | Unfavorable |
| 39 | 21 | M | GSW | 15 | Parieto-occipital | SSS | Impingement | 0 | Observation | Favorable |
| 40 | 21 | F | GSW | 15 | Parieto-occipital | SSS | Occlusion | 0 | Medical | Favorable |
| 41 | 42 | M | GSW | 3T | Frontobasal | SSS | Occlusion | 0 | Observation | Unfavorable |
GSW = gunshot wound; SSS = superior sagittal sinus; ICA = internal carotid artery; VA = vertebral artery; ACA = anterior cerebral artery; MCA = middle cerebral artery; PCA = posterior cerebral artery; PCOM = posterior communicating artery; CCF = carotid-cavernous fistula
Outcome of vascular injury
The median hospital LOS of patients was not significant between those with and without a vascular injury (Table 3). Mortality was more likely in patients with vascular injury. Presence of vascular injury was more associated with unfavorable outcome (OR=0.3, CI=0.11–0.8, p=0.02). Median GOSE was higher at discharge in patients without vascular injury (Median GOSE of 2 in VI vs 5 in nVI, p=0.003). However, when accounting for GCS on presentation, the presence of vascular injury is not statistically relevant in explaining unfavorable outcome.
Discussion
Our study represents a large series of patients with cerebrovascular injury in the acute setting of civilian PBI. In our dataset, the frequency of vascular injury, including both the arterial and venous sinus systems, was found to be 57%, higher than other civilian PBI series.21,22 In part, this may be due to our more inclusive definition of vascular injuries that is not restricted to only TICAs. Analogous to the previously reported literature, arterial injury was observed in 26/72 (36%) of our cohort. A total of 37 arterial injuries were described (some patients have more than one injury), the most prevalent of which was TICA (30%) and equal involvement of all major vessels in the anterior circulation (72 % of all injured vessels), with an equal split amongst ACA, MCA and ICA (Table 1).
With regards to venous sinus injury, the only report in civilian PBI comes from Jinkins et al.21 who described a single patient with left transverse venous sinus and internal jugular vein injury secondary to GSW to head. We describe a series of 47 patients that received CTVs and identify a total of 23 VSI (49%). The most common venous structure injured was the superior sagittal sinus (70% of the time) and the most common type of injury was occlusion (65% of the time). This represents the largest series of venous sinus injury in civilian PBI to date.
Given the high prevalence of vascular complications after penetrating brain injury, it is essential to obtain appropriate diagnostic imaging. Angiography, the gold standard in the diagnosis of vascular injury, may not always be feasible in the acute phase given the high prevalence of hemodynamic compromise and critical intracranial hypertension in this patient population. CTAs and CTVs represent a safe and rapid assessment of the intracranial vasculature and might be a useful alternative tool in early detection of vascular injury.
We found an association between the presence of intraventricular hemorrhage and arterial injury where the presence of IVH was associated with almost 10 times higher chance of having an intracranial arterial injury. Therefore, PBI patients presenting with IVH might be at higher risk of having an arterial complication and warrant special consideration. However, given the high prevalence of vascular injury even in patients with different imaging characteristics at presentation (i.e. other entry sites and no IVH), our practice favors early cerebrovascular imaging in all patients presenting with PBI.
There are no established practice guidelines for the treatment of vascular complications in PBI26. The current recommendations, now almost two decades old, suggest surgical or endovascular treatment of TICAs and arteriovenous fistulas, when identified. However, in the acute clinical setting, treatment of the above conditions is typically deferred pending clinical stability and control of intracranial hypertension. Current guidelines do not provide any recommendations pertaining to other (non-TICA) arterial or venous sinus injuries. Therefore, timing of treatment and choice of therapy has to be specifically tailored to each patient, balancing risks and benefits of potential interventions. For example, a venous sinus occlusion that would otherwise be treated with anticoagulation is challenging in PBI patients due to the concomitant high risk of developing or worsening intracranial hemorrhage. The same concern applies in patients with arterial dissection with regards to initiation of anticoagulation or antiplatelet therapy.
The current literature does not provide strong evidence about how vascular complications impact the outcome in civilian PBI. In our study, we used discharge dispositions as surrogates for short-term outcome. While the presence of vascular injury does appear to be associated with worse outcome, other predictors such as GCS on presentation remain the predominant variable associated with worse clinical outcome.
This study has several limitations. First, the study was a retrospective chart review. This introduces a potential selection bias, as 20 of the 92 patients with PBI who survived past presentation did not undergo cerebrovascular imaging; this may bias the outcome as individuals who expired in the first 48 hours may have sustained undetected vascular injury. Of those 20 patients, 18 had passed away within the first 48 hours and 2 did not undergo vascular imaging for unclear reasons. Second, the data was collected at a single level I trauma center, therefore the patient population may not be generalizable to other centers. Third, clinical and radiographic follow up was not available for many patients, limiting our assessment of long-term outcome and progression of the vascular injuries. Fourth, our reported treatments of vascular complications reflect the clinical practice of local neurointensivists, neurosurgeons, and neurointerventionalists, thus it may differ from current practice at other medical institutions. Finally, GOSE used as one of the outcome surrogates was determined at discharge, that is earlier than its intended use. Long term follow-up was not available at the time of writing this manuscript.
Conclusion
Traumatic cerebrovascular injury is a prevalent complication in civilians with PBI. Both arterial and venous sinus systems appear to be frequently involved with TICA being the most common arterial injury and occlusion of SSS the most common venous injury. IVH on presentation is significantly associated with higher odds of arterial injury, however assessment of the intracranial vasculature in the acute phase of all PBI is essential for early diagnosis. Selection of treatment of vascular injury remains limited to local clinical practice. Overall, vascular injury appears to be associated with more critical presentation, specifically lower GCS. Consequently, those patients tend to suffer worse overall outcome. Prospective studies regarding acute management, natural history, and long-term prognosis of vascular injury in the civilians with PBI are needed in order to improve the care of these patients.
Supplementary Material
Figure 1.
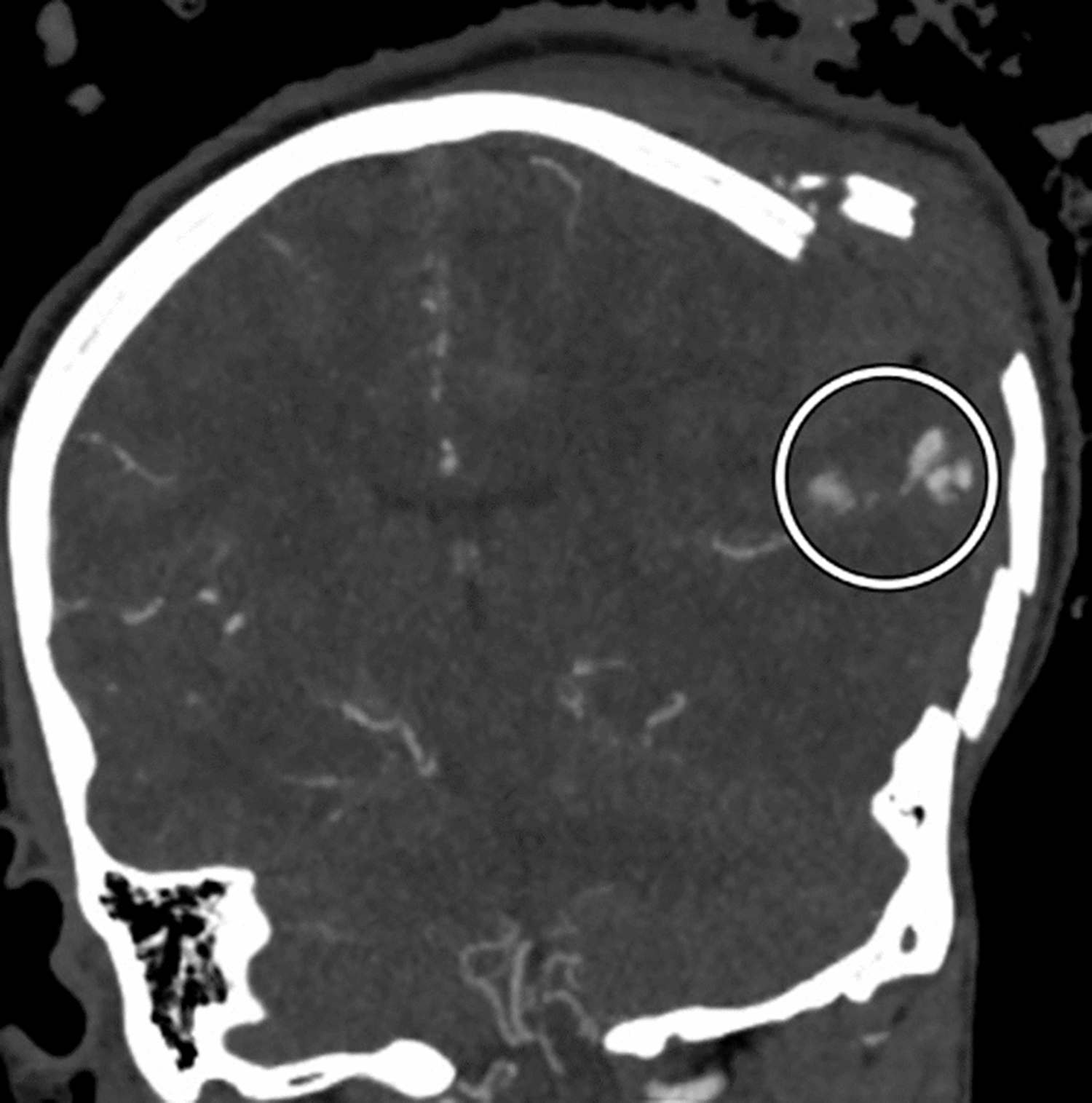
Coronal view of a comminuted left calvarial fracture with brain herniation and multiple areas of contrast extravasation from the left middle cerebral artery (encircled).
Figure 2.
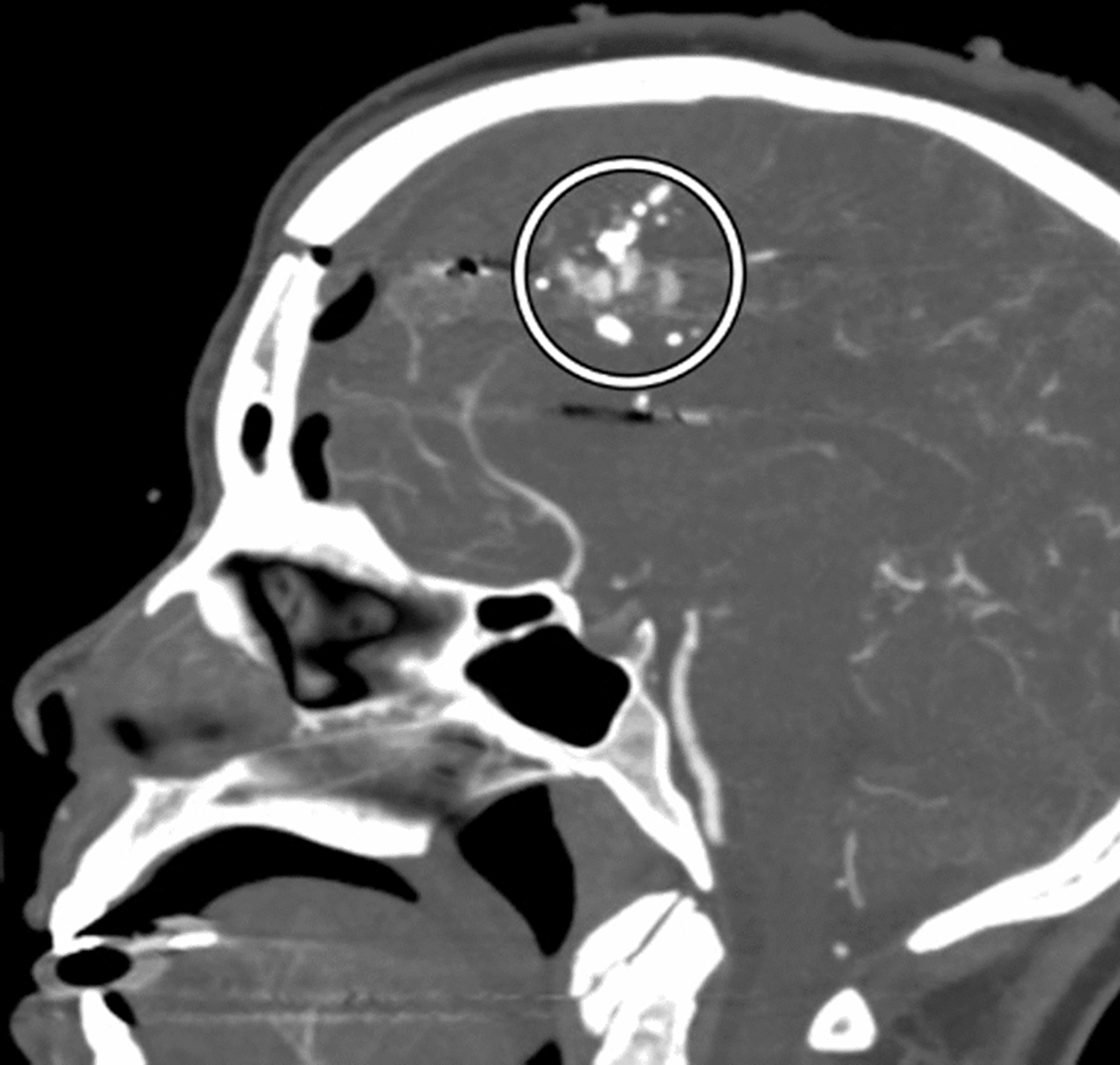
Sagittal CTA MIP image demonstrates a frontal calvarial fracture, pneumocephalus, and multiple areas of contrast extravasation from an anterior cerebral artery (encircled) adjacent to bullet fragments.
Figure 3.
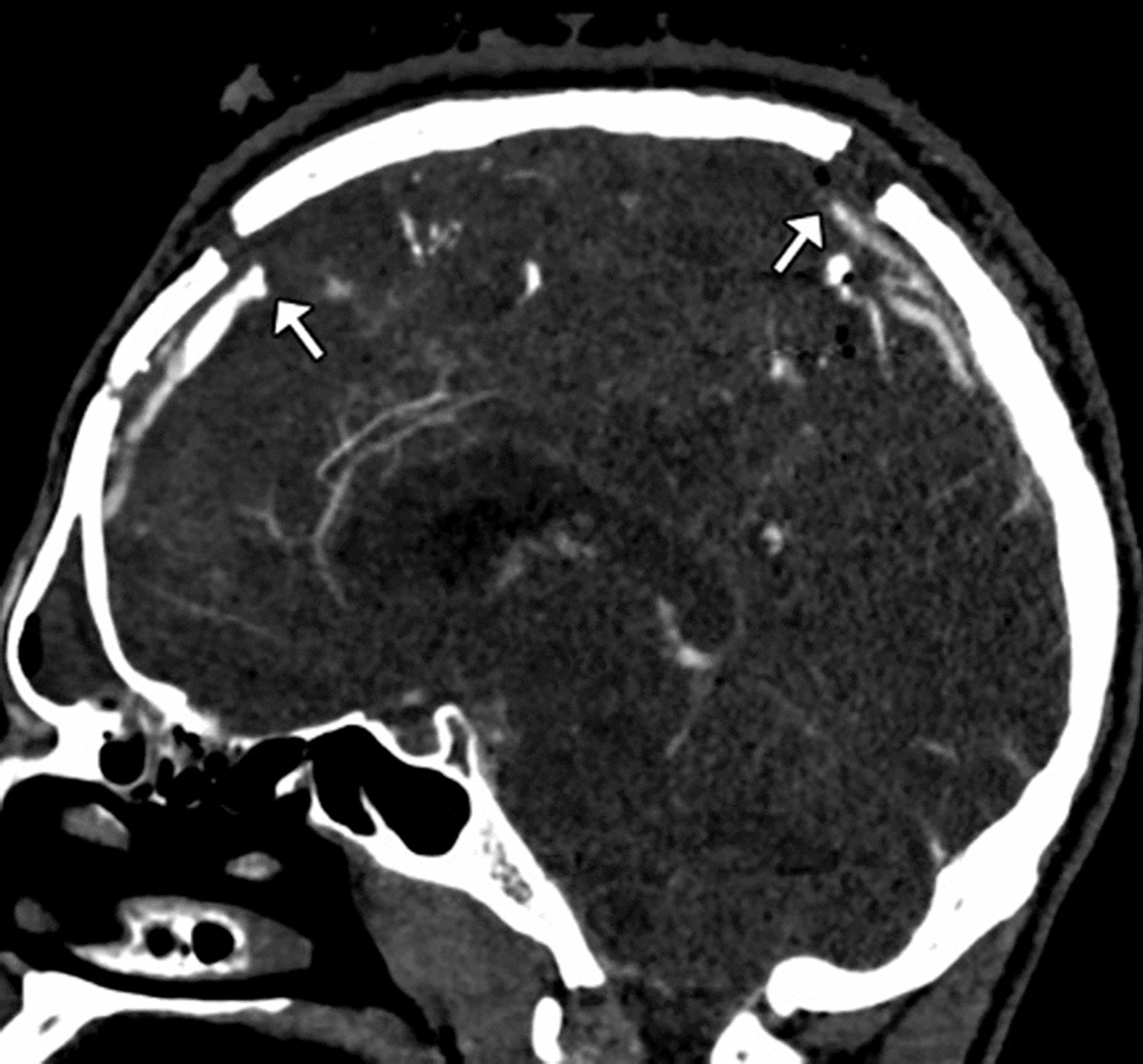
Sagittal CTV image demonstrates a displaced calvarial fracture with abrupt cut-offs (arrows) of a segment of the superior sagittal sinus.
Acknowledgments
We wish to acknowledge the support of the Department of Neurology, the Department of Radiology, Section of Neuroradiology, and the Department of Surgery, Sections of Neurosurgery and Trauma at the University of Chicago.
Funding
No sponsorship or funding was available for this study. The corresponding author (AM) had full access to all the data in the study and had final responsibility for the decision to submit for publication.
Footnotes
Disclosures
None of the authors has any conflicts of interest or financial disclosures to declare.
Publisher's Disclaimer: This Author Accepted Manuscript is a PDF file of an unedited peer-reviewed manuscript that has been accepted for publication but has not been copyedited or corrected. The official version of record that is published in the journal is kept up to date and so may therefore differ from this version.
References
- 1.Aarabi B Management of traumatic aneurysms caused by high velocity missile head wounds. Neurosurg Clin N Am. 1995;6:775–797 [PubMed] [Google Scholar]
- 2.Aarabi B Traumatic aneurysms of brain due to high velocity missile head wounds. Neurosurgery. 1988;22:1056–1063, [DOI] [PubMed] [Google Scholar]
- 3.Aarabi B, Alden T, Chestnut R. Vascular complications of penetrating brain injury. J Trauma. 2001;51(2 Suppl):S26–S28 [PubMed] [Google Scholar]
- 4.Amirjamshidi A, Rahmat H, Abbassioun K. Traumatic aneurysms and arteriovenous fistulas of intracranial vessels associated with penetrating head injuries occurring during war: principles and pitfalls in diagnosis and management. A survey of 31 cases and review of the literature. J Neurosurg. 1996;84:769–780 [DOI] [PubMed] [Google Scholar]
- 5.Bell RS, Vo AH, Roberts R, Wanebo J, Armonda RA. Wartime traumatic aneurysms: acute presentation, diagnosis, and multimodal treatment of 64 craniocervical arterial injuries. Neurosurgery. 2010;66:66–79 [DOI] [PubMed] [Google Scholar]
- 6.Haddad FS, Haddad GF, Taha J. Traumatic intracranial aneurysms caused by missiles: their presentation and manage- ment. Neurosurgery. 1991;28:1–7 [DOI] [PubMed] [Google Scholar]
- 7.Achram M, Rizk G, Haddad FS. Angiographic aspects of traumatic intracranial aneurysms following war injuries. Br J Radiol. 1980;53:1144–1149 [DOI] [PubMed] [Google Scholar]
- 8.Bank WO, Nelson PB, Drayer BP, Wilkins RH, Rosenbaum AE. Traumatic aneurysm of the basilar artery. AJR Am J Roentgenol. 1978;130:975–977. [DOI] [PubMed] [Google Scholar]
- 9.Hachemi M, Jourdan C, Di Roio C, et al. Delayed rupture of traumatic aneurysm after civilian craniocerebral gunshot injury in children. Childs Nerv Syst. 2007;23:283–287. [DOI] [PubMed] [Google Scholar]
- 10.Hemphill JC 3rd, Gress DR, Halbach VV. Endovascular therapy of traumatic injuries of the intracranial cerebral arteries. Crit Care Clin. 1999;15:811–829. [DOI] [PubMed] [Google Scholar]
- 11.Horowitz MB, Kopitnik TA, Landreneau F, et al. Multidisciplinary approach to traumatic intracranial aneurysms secondary to shotgun and handgun wounds. Surg Neurol. 1999;51:31–42. [DOI] [PubMed] [Google Scholar]
- 12.Chedid MK, Vender JR, Harrison SJ, McDonnell DE. Delayed appearance of a traumatic intracranial aneurysm. Case report and review of the literature. J Neurosurg. 2001;94:637–641. [DOI] [PubMed] [Google Scholar]
- 13.Shallat RF, Taekman MS, Nagle RC. Delayed complications of craniocerebral trauma: case report. Neurosurgery. 1981;8:569–573. [DOI] [PubMed] [Google Scholar]
- 14.Benoit BG, Wortzman G. Traumatic cerebral aneurysms: clinical features and natural history. J Neurol Neurosurg Psychiatry 1973;36:127–138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kieck CF, DeVillers JC. Vascular lesions due to transcranial stab wounds. J Neurosurg 1984;60:42–46. [DOI] [PubMed] [Google Scholar]
- 16.Benzel EC, Day WT, Kesterson L, et al. Civilian craniocerebral gunshot wounds. Neurosurgery. 1991;29:67–72. [DOI] [PubMed] [Google Scholar]
- 17.Holmes B, Harbaugh RE. Traumatic intracranial aneurysms: a contemporary review. J Trauma. 1993;35:855–860. [PubMed] [Google Scholar]
- 18.Larson PS, Reisner A, Morassutti DJ, Abdulhadi B, Harpring JE. Traumatic intracranial aneurysms. Neurosurg Focus. 2000;8:1–6. [DOI] [PubMed] [Google Scholar]
- 19.Parkinson D, West M. Traumatic intracranial aneurysms. JNeurosurg.1980;52:11–20. [DOI] [PubMed] [Google Scholar]
- 20.Jakobsson KE, Carlsson C, Elfverson J, Von Essen C. Traumatic aneurysms of cerebral arteries. A study of five cases. Acta Neurochir (Wien). 1984;71:91–98. [DOI] [PubMed] [Google Scholar]
- 21.Jinkins JR, Dadsetan MR, Sener RN, Desai S, Williams RG: Value of acute-phase angiography in the detection of vascular injuries caused by gunshot wounds to the head: analysis of 12 cases. AJR Am J Roentgenol. 1992;159:365–368. [DOI] [PubMed] [Google Scholar]
- 22.Levy ML, Rezai A, Masri LS, et al. The significance of subarachnoid hemorrhage after penetrating craniocerebral injury: correlations with angiography and outcome in a civilian population. Neurosurgery. 1993;32:532–540. [DOI] [PubMed] [Google Scholar]
- 23.Bodanapally UK, Saksobhavivat N, Shanmuganathan K, Aarabi B, Roy AK. Arterial injuries after penetrating brain injury in civilians: Risk factors on admission head computed tomography. Journal of neurosurgery. 2015;122:219–226. [DOI] [PubMed] [Google Scholar]
- 24.Bodanapally UK, Krejza J, Saksobhavivat N, et al. “Predicting arterial injuries after penetrating brain trauma based on scoring signs from emergency CT studies.” The neuroradiology journal. 2014;27:138–145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Bodanapally UK, Shanmuganathan K, Boscak AR, et al. Vascular complications of penetrating brain injury: comparison of helical CT angiography and conventional angiography. Journal of neurosurgery. 2014;121:1275–1283. [DOI] [PubMed] [Google Scholar]
- 26.Loggini A, Vasenina VI, Mansour A, et al. Management of civilians with penetrating brain injury: A systematic review. J Crit Care. 2020;56:159–166. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


