Abstract
When animals walk overground, mechanical stimuli activate various receptors located in muscles, joints and skin. Afferents from these mechanoreceptors project to neuronal networks controlling locomotion in the spinal cord and brain. The dynamic interactions between the control systems at different levels of the neuraxis ensures that locomotion adjusts to its environment and meets task demands. In this review, we describe and discuss the essential contribution of somatosensory feedback to locomotion. We start with a discussion of how biomechanical properties of the body affect somatosensory feedback. We follow with the different types of mechanoreceptors and somatosensory afferents and their activity during locomotion. We then describe central projections to locomotor networks and the modulation of somatosensory feedback during locomotion and its mechanisms. We then discuss experimental approaches and animal models used to investigate the control of locomotion by somatosensory feedback before providing an overview of the different functional roles of somatosensory feedback for locomotion. Lastly, we briefly describe the role of somatosensory feedback in the recovery of locomotion after neurological injury. We highlight the fact that somatosensory feedback is an essential component of a highly integrated system for locomotor control.
Introduction
During terrestrial locomotion, neurons that respond to mechanical stimuli, with specialized receptors (mechanoreceptors) located in muscles, tendons, joints and/or skin, inform the central nervous system (CNS) of the body segments’ relative position and motion, the forces that muscles generate and exert on bones, as well as characteristics of the terrain. From the mechanoreceptors, afferents send action potentials to the spinal cord and/or brainstem where they contact different neuronal targets. Inputs from peripheral mechanoreceptors, collectively termed somatosensory feedback, are then transmitted, processed and integrated at different levels of the CNS where they influence the control of locomotion directly or indirectly. We can broadly divide somatosensory feedback into proprioceptive and tactile. Proprioception, a term first introduced by Charles Sherrington (760) and taken from the Latin word proprius, meaning property, refers to perception of one’s own body and movements through information generated inside the body. Proprioception is mainly provided by muscle receptors, muscle spindles and Golgi tendon organs (GTOs), but also by some cutaneous receptors. Tactile information, sensed by receptors in the skin, is concerned with sensory stimuli originating outside the body, such as the physical characteristics of the environment. In this review, we will not consider stimuli associated with the visual, olfactory and vestibular systems that also play a role in locomotor control. Signals from proprioceptive and tactile afferents evoke coordinated motor patterns, such as reflexes and automatic postural responses, which rapidly modify the locomotor pattern in response to perturbations or unexpected changes in the environment. They also change ongoing motor patterns in response to internal demands (e.g., anticipatory motor actions). Without somatosensory feedback, locomotion is not functional, as shown in people who have lost the senses of touch and proprioception following viral infections or because of genetic mutations (147; 160; 476). In these rare cases, most people do not recover the capacity to stand and walk and if they do, they must train intensely for months to years to adopt control strategies that require planning each step and relying heavily on vision. Other mammals, such as mice, rats and cats, recover a much higher degree of functionality following the loss of somatosensory feedback. The reason for this is unclear but likely relates to the fact that humans stand upright on two legs and require a more precise postural control, which is mediated in part by somatosensory feedback informing supraspinal centers.
In this review, we describe and discuss the functional roles of somatosensory feedback in the control of terrestrial locomotion in mammals, mainly in mice, cats and humans. The control of locomotion is often described as tri-partite: the spinal locomotor central pattern generator (CPG) produces the basic motor pattern, which is continuously adjusted by somatosensory feedback and by descending commands from the brainstem and other brain structures [reviewed in (339; 455; 625; 718). Figure 1 shows some conceptual models of the neural control of locomotion and the dynamic interactions between the different control mechanisms. Although the spinal CPG generates the basic pattern of muscle activations for locomotion, stimulation of somatosensory afferents was instrumental in validating Brown’s original hypothesis for a spinal locomotor center (106). Indeed, Jankowska, Lundberg and colleagues showed alternation between flexor and extensor nerve/motoneuron discharges that followed electrical stimulation of high threshold muscle and cutaneous afferents in acute spinal-transected decerebrate cats curarized and treated with l-3,4-dihydroxyphenylalanine (L-DOPA) and nialamide (Fig. 1A) (440; 441). Grillner and Zangger (1979) then showed a centrally generated locomotor-like rhythm, termed fictive locomotion because of the absence of movement, in acute spinal-transected decerebrate curarized cats with dorsal roots sectioned caudal to the spinal transection and treated with L-DOPA and nialamide (344). With stimulation of somatosensory afferents, either by stimulating cut dorsal roots or the dorsal columns, a locomotor-like pattern emerged, consisting of flexor-extensor alternation on one side and left-right alternation between homologous muscle nerves. Grillner developed a model of CPG organization where distinct functional units, or unit burst generators (UBGs), activate synergistic muscles acting at individual joints. Each UBG can generate its own rhythm and different UBGs can be coupled or uncoupled depending on task demands (Fig. 1B) (337). Since then, experimental studies have confirmed the existence of the spinal locomotor CPG and the important interactions between somatosensory feedback and signals from supraspinal structures. This has led to more elaborate conceptual models of locomotor control, such as the two-level CPG, originally proposed to explain the activity of bifunctional muscles (661), which separates rhythm generation and pattern formation (Fig. 1C). It is now accepted that each limb is controlled by a distinct CPG with various pathways coordinating their activities (Fig. 1D) (275). In this review, we highlight the fact that somatosensory feedback is an essential component of a highly integrated system for locomotor control. Table 1 lists the abbreviations used throughout the review.
Figure 1. Neural control of locomotion.
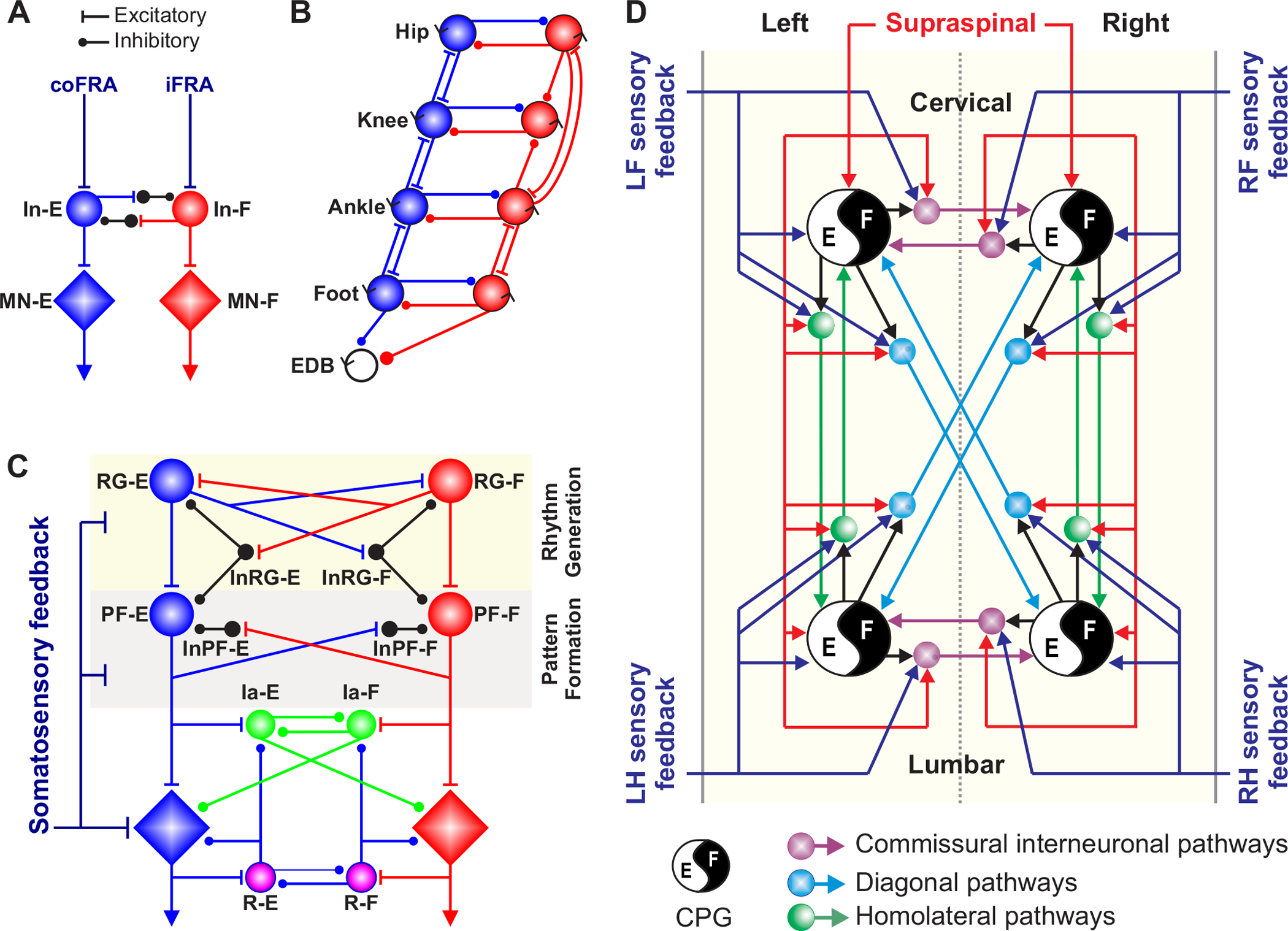
A) The half-center model is composed of populations of last-order interneurons that control extensor (In-E) and flexor (In-F) motoneurons [reproduced and modified from (440)]. MN-E and MN-F represent extensor and flexor motoneurons, respectively. The In-E and In-F populations receive excitatory inputs from contralateral (coFRA) and ipsilateral (iFRA) flexor reflex afferents, respectively, and these interneuron populations mutually inhibit each other. FRAs have been used to characterize a collection of high-threshold afferents from muscle, joint and cutaneous receptors involved in generating ipsilateral limb flexion (and crossed extension). However, stimulating FRAs produce excitatory and inhibitory responses in both ipsilateral flexors and extensors as well as muscles of the other limbs and the term can lead to confusion. B) The unit burst generator model originally proposed by Grillner (1981) [reproduced and modified from (343)]. EDB, extensor digitorum brevis. C) The two-layer central pattern generator model separating rhythm generation (RG) and pattern formation (PF) [adapted from (729; 730)]. Last-order extensor (PF-E) and flexor (PF-F) populations of interneurons at the PF level control extensor (MN-E) and flexor (MN-F) motoneuron pools, respectively. PF-E and PF-F mutually inhibit each other via inhibitory interneurons (InPF-E and InPF-F). The pattern formation level receives inputs from extensor (RG-E) and flexor (RG-F) populations of interneurons located at the rhythm generation level. The RG-E and RG-F populations can mutually excite or inhibit each other. Somatosensory feedback projects to neurons at the RG and PF levels as well as motoneurons. Motoneurons also receive inputs from Ia inhibitory interneurons (Ia-E and Ia-F) and motoneuron collaterals project to Renshaw cells (R-E and R-F). D) Schematic representation of the neural control of interlimb coordination [reproduced from (275)]. A distinct spinal locomotor CPG controls each limb. Commissural interneurons ensure left-right coordination at cervical and lumbar levels. Descending and ascending propriospinal pathways, with homolateral and diagonal projections, coordinate cervical and lumbar CPGs. Propriospinal pathways can consist of neurons with long or short axonal projections. Supraspinal inputs and somatosensory feedback from the limbs access spinal CPGs via commissural and propriospinal pathways. Arrows represent excitatory or inhibitory influences. E, extensor; F, flexor; LF, left forelimb; LH, left hindlimb; RF, right forelimb; RH, right hindlimb.
Table 1.
Abbreviations used throughout review.
| Table of abbreviations | |
|---|---|
| AAV | Adeno associated virus |
| CNS | Central nervous system |
| CoM | Center of mass |
| CoP | Center of pressure |
| CPG | Central pattern generator |
| CS | Conditioned stimulus |
| DOF | Degrees of freedom |
| DRG | Dorsal root ganglia |
| DSCT | Dorsal spinocerebellar tract |
| DTA | Diphtheria toxin light chain A |
| DTR | Diphtheria toxin receptor |
| DTX | Diphtheria toxin |
| E1, E2, E3 | First, second and third extension phases |
| EDL | Extensor digitorum longus |
| Egr3 | Early growth response 3 |
| EMG | Electromyography |
| ENG | Electroneurography |
| EPSP | Excitatory post-synaptic potential |
| F | Flexion phase |
| GABA | Gamma-Aminobutyric acid |
| GTO | Golgi Tendon Organ |
| HTMR | High-threshold mechanoreceptor |
| IPSP | Inhibitory post-synaptic potential |
| KO | Knock out |
| L-DOPA | l-3,4-dihydroxyphenylalanine |
| LMTR | Low-threshold mechanoreceptor |
| PAD | Primary afferent depolarization |
| pmRF | Pontomedullary reticular formation |
| PNI | Peripheral nerve injury |
| Pv | Parvalbumin |
| RA1 | Rapidly-adapting type 1 |
| RA2 | Rapidly-adapting type 2 |
| SA1 | Slowly-adapting type 1 |
| SA2 | Slowly-adapting type 2 |
| SCI | Spinal cord injury |
| SCT | Spinocervical tract |
| SP | Superficial peroneal |
| SR | Superficial radial |
| TA | Tibialis anterior |
| UBG | Unit burst generator |
| US | Unconditioned stimulus |
| VGLUT1 and 2 | Vesicular glutamate transporter type 1 and 2 |
| VN | Vestibular nucleus |
| VSCT | Ventral spinocerebellar tract |
Locomotor preparations
Before starting our discussion of various topics related to somatosensory feedback during locomotion, it is important to describe the locomotor preparations used experimentally. Real locomotion, which can be constrained or unconstrained (e.g. animal held in stereotaxic frame), refers to stepping movements done over ground or on a treadmill. Real locomotion is studied in intact animals, including healthy people, and following different types of naturally occurring or experimentally-induced pathological conditions or lesions. We refer to real locomotion in animals with a complete spinal transection, or spinal animals, as spinal locomotion. The spinal preparation has contributed extensively to our understanding of how somatosensory feedback interacts with spinal locomotor circuits. Another type of locomotor preparation in experimental studies is decerebrate or decorticate animals, where a portion of the cerebral cortex and brain is removed [reviewed in (850)]. Because of the loss of sentience, the decerebrate preparation allows the study of real locomotion with more invasive procedures than what would be possible in an awake behaving animal or in an acute preparation performed with anesthesia that inhibits the excitability of neural networks (774). In decerebrate/decorticate preparations, stepping movements generally occur on a treadmill and the limbs are relatively free to move, with the head and/or trunk of the animals immobilized in a stereotaxic frame. Treadmill locomotion in decerebrate animals is generally less smooth than locomotion in animals stepping freely. Decerebrate locomotion can occur spontaneously or by electrically stimulating regions of the brainstem or cerebellum, depending on the transection level of the decerebration, and/or by electrically stimulating the spinal cord (427; 587; 588; 731; 763; 850).
Another important preparation is that of fictive locomotion, where motor outputs are recorded from peripheral nerves or ventral roots (ENG, electroneurography) following systemic administration of a curare-like drug, which blocks transmission at neuromuscular junctions throughout the body, or in isolated/partially-isolated spinal cord preparations. The term ‘fictive’ refers to the absence of movement, even though motor outputs from the spinal cord remain and are recorded. The term ‘fictitious’ locomotion, first introduced in curarized cats (662), was later modified to fictive locomotion (254). Fictive locomotion is extremely useful to isolate the contribution of specific sensory inputs as phasic somatosensory feedback is absent due to the lack of movement. In cats, fictive locomotion is recorded following decerebration and as described above, it occurs spontaneously or is evoked by electrical brainstem, cerebellar or spinal cord stimulation. Fictive locomotion can be elicited in spinal cats, although it requires pharmacology and/or electrical stimulation of the spinal cord or dorsal roots (281; 344; 648). In isolated spinal cord preparations of neonatal rats or mice, fictive locomotion requires pharmacology and is facilitated by electrical stimulation of the spinal cord and/or dorsal roots (133; 134; 458; 462). The various techniques and approaches described below have been used during real or fictive locomotion to answer different scientific questions pertaining to the control of locomotion by somatosensory feedback. When interpreting results, it is vital to consider the preparation and its limitations, the type of locomotion and the species.
The biomechanics of terrestrial locomotion in relation to somatosensory feedback
Before describing mechanoreceptors, somatosensory afferents and their role in locomotor control, we provide an overview of the biomechanics of terrestrial locomotion to gain an appreciation of their influence on somatosensory feedback. Quadrupedal and bipedal terrestrial mammals have evolved to move over a range of distances with various speeds and forms of locomotion that depend on their evolutionary history, habitat and behavioral goals. It is important to consider that the relative role of somatosensory feedback in controlling locomotion likely differs across species. For instance, humans have unique postural requirements and somatosensory information might be more important for postural control compared to quadrupeds. Moreover, large mammals with greater mass and a high center of gravity likely make use of somatosensory feedback differently than minuscule mammals, such as mice. When discussing the role of somatosensory feedback during locomotion, it is important to consider the biomechanical characteristics of the animal and its movements within its environment. Despite great differences in body size, morphological characteristics, and behavioral demands, terrestrial mammals have developed common modes of locomotion, known as gaits (295; 328; 374). Animals select specific gaits to optimize their success of achieving behavioral goals and to minimize mechanical and metabolic energy expenditure (15; 132).
Kinematics of locomotion
Locomotor gaits
We normally define modes of locomotion, or gaits, by sequences of stance and swing phases of each limb and by the relative time of phase onsets and offsets between limbs. Using two parameters, the duty cycle (the ratio of stance duration to cycle duration of a limb) and the phase difference between the homolateral hindlimb and forelimb footfalls, Hildebrand classified locomotor gaits of various mammalian species (374) (Fig. 2). We call gaits identified in Figure 2 symmetric. In symmetric quadrupedal gaits, as opposed to asymmetric, the duty cycle of the two limbs at the shoulder (forelimbs) and pelvic (hindlimbs) girdles, or pair of legs in bipeds, is the same, with a relative phase difference of 50% of the cycle period. In this review, we focus on the most common walking and running gaits, distinguished by the duty cycle. In walking and running gaits, duty cycles are greater or smaller than 50%, respectively. In other words, at least one leg contacts the ground at all times during walking, while a portion of time with no ground contact occurs during running (also called an aerial phase). An exception to this rule is the singlefoot running gaits where the duty cycle of each limb is below 50% and there is always a support phase throughout the cycle (Fig. 2, singlefoot running gaits). Both walking and running gaits have a wide range of phase differences between homolateral limbs, from zero (synchronous motion of the fore- and hindlimbs, as in pacing) to an out-of-phase motion (50% difference, as in trotting). Animals with relatively long legs compared to trunk length, such as camels, alpacas, giraffes, and some breeds of dogs, use gaits approaching pacing to avoid collisions between the fore- and hindlimbs (66; 177; 373; 667). Cats often adopt a pacing-like gait on a treadmill as opposed to the more common walking with a lateral sequence of foot falls or trotting gait during overground locomotion (82). Although humans are bipeds, they use a trot-like gait with in-phase motion of the diagonal limbs (e.g. right leg with left arm).
Figure 2. Examples of mammalian gaits.
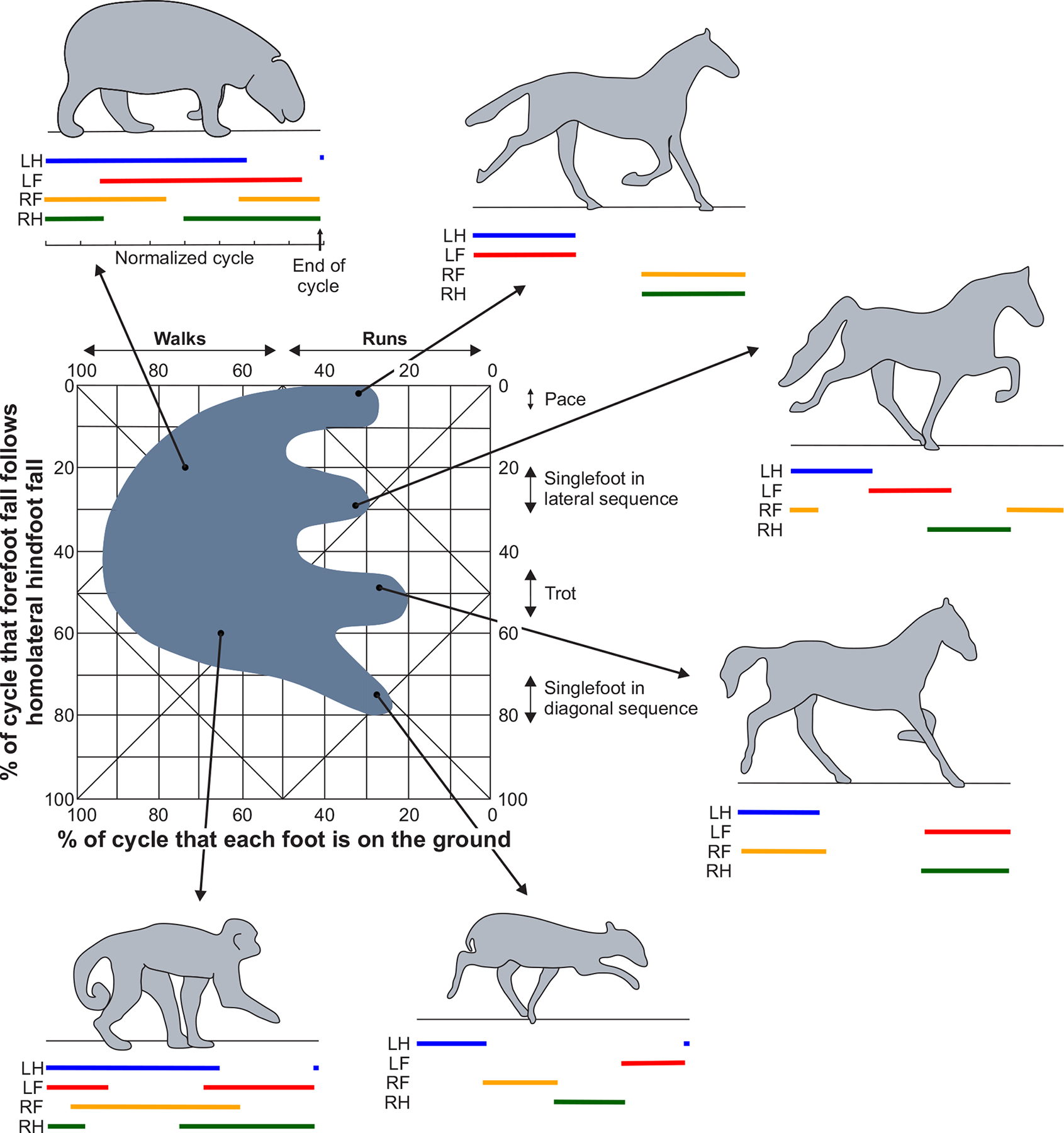
Examples of quadrupedal symmetric gaits classified based on the duty cycle (the horizontal axis in the graph) and the phase difference between the homolateral hindlimb and forelimb footfalls (vertical axis). Examples of symmetric gaits include walking with a lateral sequence of foot falls of the pygmy hippopotamus with a lateral limb support sequence (top left); three running gaits of the horse, including a pace with in-phase 2-beat movements of the homolateral limbs (top right), trot with in-phase 2-beat movements of the contralateral fore- and hindlimbs (middle bottom) and a 4-beat lateral sequence gait with a single foot on the ground and the other limbs in swing (middle top); a 4-beat diagonal sequence gait with a single foot on the ground and the other limbs in swing in the duiker, an African antelope (bottom right); and walking with a diagonal sequence of foot falls in the monkey. Modified from (374).
The major factor affecting gait selection for a given species is locomotor speed. Animals normally use walking gaits at slow speeds and prefer running gaits (e.g., trotting and galloping) to move faster in order to minimize metabolic and mechanical energy expenditure (15; 398; 536). Quadrupedal gaits, such as walk, trot and gallop, have their own optimal locomotion speed, where oxygen consumption per travelled distance is about the same for all three gaits (398). The energy expenditure per travelled distance is higher at gait transition speeds. Gait transition speeds between walking, trotting and galloping increase with animal size across and within species (336; 367). For example, mice change from walking to running (trotting) gaits at speeds of 0.10–0.15 m/s (490), cats at about 0.9 m/s (316) and humans around 2.0 m/s (399; 811). Different terrestrial quadrupedal and bipedal mammals appear to locomote in a dynamically similar fashion despite large differences in animal size. Dynamically similar motions are those that can be made equivalent by multiplying motion length-related characteristics by a length constant, the time-related characteristics by a time constant, and all forces by a force constant (15). For dynamically similar locomotion, the Froude number (normalized locomotion speed) should be the same at the maximal speed of a given gait and at transition speeds between different gaits. The Froude number Fr is defined as (16):
| (1) |
In this equation, v is locomotion speed, m is body mass, g is gravitational acceleration and l is leg length. The nominator and denominator in the first ratio in the equation represents the centripetal and gravitational force, respectively. The Froude number Fr = 1 corresponds to the maximum walking speed. As motion of the body’s center of mass (CoM) during human walking can be accurately described by motion of an inverted pendulum, or as a sequence of arcs with radius l (16; 132; 789), walking is possible only if the centripetal force mv2/l does not exceed the gravitational force mg so the CoM continues to travel along the arc, i.e. (see eq. 1). Otherwise, the foot loses contact with the ground (transition to running). According to the above condition, humans with leg lengths of ~0.9 m have their maximal walking speed () around 3 m/s, whereas cats with leg lengths of 0.25 m cannot walk faster than 1.6 m/s. With increasing locomotor speed, animals prefer to transition to another gait at a much slower speed than the maximum possible speed of the previous gait. Despite large difference in size, mammals, from mice to elephants, prefer to transition from walking to running gaits at approximately the same normalized locomotion speed Fr = 0.4–0.5 (14; 336). There is a slight difference in the normalized gait transition speed between cursorial animals (those that stand and run on almost straight legs), such as cats, horses and humans, compared to non-cursorial animals, normally small mammals, such as mice and rats that stand and run on strongly bent legs. Small rodents have slightly higher normalized gait transitions speeds (15). Thus, quadrupedal and bipedal mammals generally use a dynamically similar locomotion.
Factors triggering gait transitions are still not fully established. One kinematic variable that appears to trigger the walk-to-run transition in humans is ankle flexion angular velocity because it is abruptly reduced at this transition (399). Adding additional mass to the foot (524) or fatiguing ankle flexors (750) reduces the walk-to-run transition speed in humans, supporting the potential role of ankle kinematics and effort of ankle flexors as factors triggering the walk-to-run transition. The ankle flexion angular velocity and the corresponding effort of ankle flexors cannot explain the run-to-walk transition with decreasing locomotor speeds because both increase at this transition. Other studies have suggested that high values of hip angular displacements and velocities and corresponding hip muscle actions and effort trigger the walk-to-run transition (28; 579; 675; 682). Therefore, it appears that walking kinematics, characterized by a more extended leg in swing, causing larger leg inertia, compared to running, requires greater swing-related flexor muscle activity and effort with increasing locomotor speeds. Transitioning to running decreases the mean leg length and leg moment of inertia during swing and thus mechanical demands on leg muscles (682; 794). Total leg muscle activity during walking and running at different speeds in humans supports the idea that muscle effort required to swing the leg triggers the walk-to-run transition, whereas increased activity of leg extensors in the stance phase of running with decreasing speeds triggers the run-to-walk transition (423; 682; 794). Proprioceptive feedback could play the key role in signaling kinematic factors triggering gait transitions.
Other kinematic variables
Stride and step lengths, cycle and phase durations, as well as stride frequency depend on locomotor speed and animal size. The relationships between these kinematic variables and speed have generally similar trends among different animals with a wide range of sizes, from fruit flies (801), mice (156), cats (316; 350), goats (307) to humans (609). Specifically, cycle and stance durations both decrease with increasing speed, while swing duration does not change substantially. Stride frequency increases linearly with speed of walking and trotting in mice, rats, dogs and horses with the slope of the frequency-speed relationship decreasing with increasing animal size. After transitioning to gallop, the stride frequency becomes constant and speed increases by increasing stride length (Heglund et al., 1974).
As discussed (Fig. 2), we can characterize locomotor gaits using limb support phases. In quadrupedal animals, we can divide the locomotor cycle into a total of 8 support phases, with swing and support phases for each limb (Fig. 3), or four phases for bipedal animals. For example, during quadrupedal locomotion in cats at slow to moderate treadmill speeds (0.3 – 0.7 m/s), 2 to 4 limbs are in contact with the ground at all times while at faster speeds (0.8 – 1.0 m/s) 4-limb support phases disappear (279).
Figure 3. Support phases during walking in the cat and related static and dynamic stability measures.
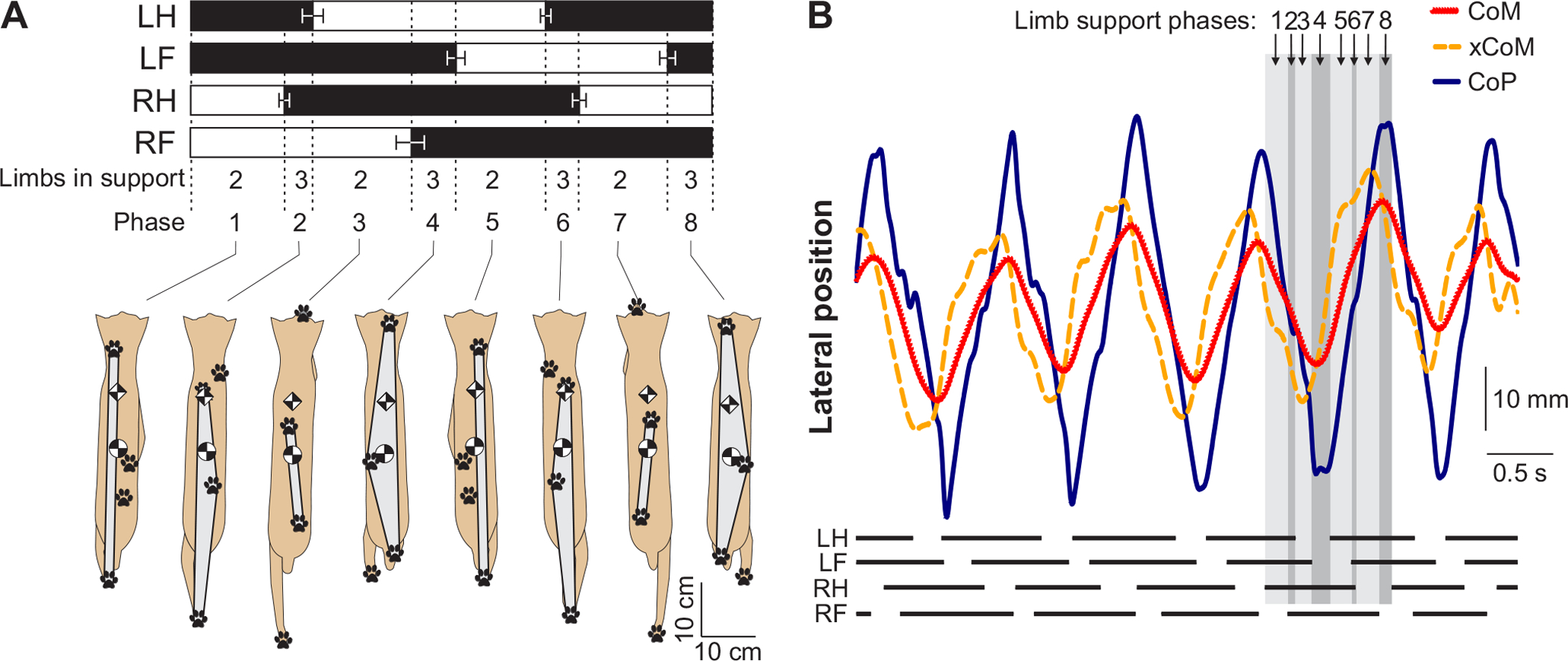
A) Limb support phases (top), the corresponding cat body configurations with the base of support (in gray), paw prints, center of mass (circles), and extrapolated center of mass (diamonds) shown for each limb support phase during cat overground walking; reproduced with permission from (251). B) Support phases during treadmill walking for the left hindlimb (LH), left forelimb (LF), right hindlimb (RH) and right forelimb (RF) shown by horizontal lines at bottom. The traces correspond to the position of the center of mass (CoM), extrapolated center of mass (xCoM) and the center of pressure (CoP) in the left-right direction as a function of time. Vertical shaded rectangles correspond to the 8 support phases indicated in B; reproduced with permission from (636).
The combination of support limbs determines configuration of the support area for the body (Fig. 3A). For example, the body is statically stable in phases with 3 limbs on the ground (phases 4 and 8) or in diagonal 2-limb support (phases 3 and 7), with the body’s CoM lying within the area of support. During 2-limb homolateral support (phases 1 and 5), the CoM may be statically unstable in the medial-lateral direction. On the other hand, cats appear dynamically unstable in the forward direction in the diagonal 2-limb support phase of overground walking (phases 3 and 7), and dynamically unstable in the medial-lateral direction during 2-limb homolateral support of treadmill locomotion (Fig. 3B). Dynamic stability recovers with placement of the other forepaw on the ground and onset of the next 3-limb support phase. Dynamic stability is defined by the margin of dynamic stability, which is the difference between the edge of the support area (center of pressure, CoP) and the extrapolated CoM (xCoM, CoM plus additional displacement that depends on CoM speed) (379). The limb support pattern is sensitive to stability demands. In unstable environments, quadrupeds and bipeds increase step width, the area of support and the duration of 2-limb (in humans) and 4-limb (in quadrupeds) support phases while decreasing the duration of diagonal 2-limb support (in quadrupeds) and CoM height (135; 294; 555; 743; 855).
Philippson provided one of the first quantifications of hindlimb joint kinematics (669) based on instantaneous images of dog locomotion made by himself and by Marey (535). Philippson divided the locomotor cycle into 4 phases based on joint angle peaks in the swing and stance phases. The flexion phase (F) starts from maximum hip and ankle extension at swing onset and ends when ankle dorsiflexion and knee flexion reach their maximum in swing. The early extension phase (E1) starts at maximal ankle and knee flexion and lasts until full extension at the ankle and knee and maximal hip flexion at stance onset. In the next extension phase (E2), the ankle and knee joints flex, or yield, because of loading of the limb in early stance. The last extension phase (E3) starts from maximal ankle and knee flexion until the end of stance.
Changes in limb joint angles and limb segment elevation angles with respect to the vertical during quadrupedal walking, trotting and galloping (130; 260; 316; 743), as well as during human walking and running (422; 631), are stereotyped and similar across species. Joint angles during locomotion determine changes in muscle-tendon length, defined as the distance between the muscle-tendon origin and insertion. We can calculate this length for limb muscles using geometric models of the limb (83; 316; 331; 649) to provide (together with electromyography) indirect information about the type of muscle-tendon unit action (isometric, concentric or eccentric) and potential muscle length-related sensory feedback. An isometric contraction is when the muscle develops tension without changing length. Concentric and eccentric contractions refer to the muscle developing tension while shortening and lengthening, respectively. As discussed later on, muscle fascicle length changes may differ from changes in the muscle-tendon unit, especially in distal muscles with a long tendon and aponeurosis and short muscle fibers. For example, fascicles of the active gastrocnemius muscle can shorten or remain at a constant length while the entire muscle-tendon unit elongates during the ankle yield in the stance phase of locomotion because of its long tendon with aponeurosis. Thus, conclusions about whether muscle fibers are stretching or shortening during specific phases of movement made based on changes in joint angles or muscle-tendon unit lengths may be inaccurate (172; 380; 418).
During the F phase, ankle and knee extensor muscle-tendon units stretch, although no stretch-related muscle activity (EMG, electromyography) is normally present (317; 649). In the E1 phase, muscle-tendon units of ankle and knee flexors stretch and the hamstring muscles show EMG activity that increases with speed (202; 609; 678; 682; 784). The magnitude of ankle and knee yield in E2 and joint extensions in E3 increases with locomotor speed, paralleled by an increase in extensor EMG activity (202; 306; 316; 317; 609). In downslope locomotion, hindlimb extensor muscle-tendon units and muscle fibers stretch more during stance than in level walking, while they shorten during upslope walking. The EMG activity of extensors is highest in upslope quadrupedal locomotion, followed by level and downslope walking (127; 329; 331; 464; 522; 544; 562).
We define elevation angle of a leg segment as the acute angle between the long axis of the segment and the vertical. Describing locomotor kinematics using limb segment elevation angles has revealed a kinematic synergy called the planar covariation of elevation angles. In this synergy, a three-dimensional trajectory of the elevation angles of the foot, shank, and thigh for the leg/hindlimb or the carpals, forearm, and upper arm for the forelimb, is situated in a plane. The variance of limb elevation angles in the cycle is accurately described by two principal components that correlate with limb orientation and limb length (81; 198; 422). The planar covariation of elevation angles occurs in various human locomotor behaviors, such as walking, crouched walking, running and hopping, in terrestrial and aquatic locomotion in dogs (130), in running birds (612) and in macaques walking bipedally (611). Thus, it appears that the locomotor control system reduces the dimensionality of limb control from three to two degrees of freedom (DOF). Neural, rather than kinematic, constraints seem to produce this reduction (63; 369; 424; 492).
To reduce the variability (increase precision) of important kinematic variables, such as foot position at stance onset, by covariation of other variables, such as segment elevation angles or joint angles, the CNS appears to take advantage of the abundance of degrees of freedom in the musculoskeletal system during locomotion (483; 592; 744). This kinematic synergy allows for precise placement of the foot during locomotion, presumably improving balance control (465; 470). The CNS also controls stereotypic CoM paths in the horizontal plane when changing walking direction by adjusting walking speed to the path curvature and maximizing path smoothness (368; 668). These CoM kinematic synergies resemble those of skilled hand movements (262; 474).
The above kinematic synergies could be a consequence of a dimensionality reduction in muscle control by concurrently activating groups of muscles, or muscle synergies, instead of controlling individual muscles (74; 75; 149; 175; 198; 813; 880). Muscle synergy control has been revealed by computational methods of dimensionality reduction of EMG activity patterns during locomotion using principal component analysis (421; 617), nonnegative matrix factorization (148; 818) and by cluster analysis of EMG burst onsets and offsets (191; 471; 538). Studies have accurately reconstructed locomotor EMG activity patterns of up to 40 muscles with 3–6 muscle synergies (or muscle groups) and their time-varying activation patterns. These muscle synergies are consistent across quadrupedal and bipedal animals, including mice, rats, guineafowls, cats, monkeys and humans (19; 149; 196; 198; 359; 464; 739; 880).
During locomotion, at least one flexor synergy, involving major leg flexors, controls leg elevation during the swing phase. At least two extensor synergies, active during early and late stance, engage two groups of leg extensors that control leg yield and extension. The muscle composition of extensor synergies depends on the species. Additional muscle synergies involve two-joint hip and knee muscles (e.g., hamstring and rectus femoris) active during the stance-to-swing and swing-to-stance transitions. During these transition periods, there is a combination of hip flexion/knee extension and hip extension/knee flexion resultant joint moments (651; 683). Activation of the two-joint hamstrings and rectus femoris during phase transitions is advantageous because of their mechanical advantage (680; 681). The EMG activity of the hamstrings and rectus femoris strongly depends on motion-related somatosensory feedback (224; 538; 660) and appears responsible for moving the leg in the forward and backward directions (188; 359).
The robust kinematic and muscle synergies observed during terrestrial locomotion in different mammalian species may indicate common solutions to locomotor control problems developed by the nervous system in the course of evolution, individual development and learning. One potential advantage of these solutions is the reduction in the number of controlled variables. Somatosensory feedback must be involved in refining these solutions during motor learning and in their modifications in response to external and internal perturbations.
Kinetics of locomotion
Kinetic variables, such as ground reaction forces and resultant joint moments, are directly responsible for CoM and joint kinematics, respectively. Three-dimensional ground reaction forces during locomotion are well documented in quadrupedal and bipedal animals of different sizes (see Fig. 4). The normal component of the ground reaction force (vertical component in level locomotion) typically has two peaks during walking that correspond to the deceleration (E2) and acceleration (E3) phases, while in running there is typically one peak at the transition between the E2 and E3 phases (14).
Figure 4. Ground reaction forces of different quadrupedal species and humans during locomotion.
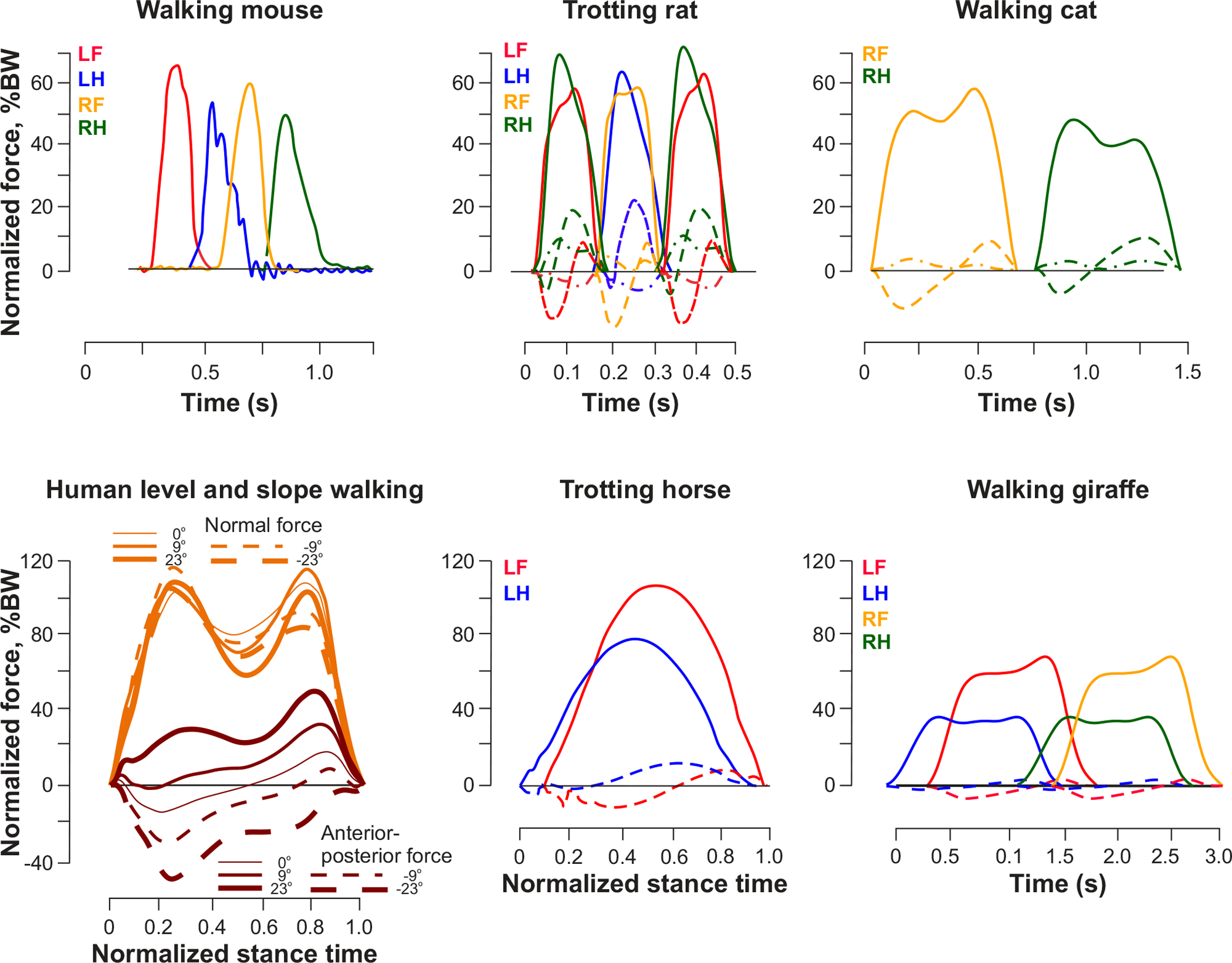
In quadrupeds of different sizes from a mouse to giraffe, vertical ground reaction force (GRF) applied to the forelimbs is typically greater than the GRF applied to hindlimbs. The forelimbs generate larger breaking force impulses than accelerating impulses in the anterior-posterior direction. The accelerating force impulses of the hindlimbs are larger than breaking ones. In all panels except for human walking, solid lines are vertical forces, dashed lines are anterior-posterior forces, and dashed lines with dots are medio-lateral forces (medio-lateral ground reaction applied to the foot is directed toward the body, i.e. the foot applies force on the ground in the opposite outward direction). From top left to bottom right: GRFs of walking mouse (modified from (156), GRFs of trotting rat (modified from (591), GRFs of walking cat (251), normal and anterior-posterior forces during level, upslope and downslope walking in humans (modified from (487)), GRFs of trotting horse (modified from (157) and GRFs of walking giraffe (modified from (66). BW, bodyweight; LF, left forelimb; LH, left hindlimb; RF, right forelimb; RH, right hindlimb.
The vertical ground reaction force applied to the forelimbs of quadrupedal animals during quiet standing or level locomotion is about 20% higher than the vertical force applied to the hindlimbs (~50% in giraffes) as the body’s CoM is slightly shifted rostrally because of the weight of the head and neck. We see this shift in all studied quadrupeds, including mice (156), rats (31), cats (251; 484), goats (634), horses (215) and giraffes (66). The positive tangential ground reaction force impulse, measured as the area under the force-time curve, during level locomotion is greater for the hindlimbs compared to the forelimbs, while the negative force impulse is greater for the forelimbs, indicating that the hindlimbs accelerate the body forward and the forelimbs decelerate the body (251; 634). During upslope walking, ground reaction forces applied to the hindlimbs are higher than on the forelimbs, while the situation reverses during downslope walking (31; 215; 329).
Resultant joint moments reflect the summed moments of force produced by all structures around the joint, including moments produced by active and passive forces of agonists and antagonists, as well as ligaments (862; 884). The contribution of passive structures to joint moments in normal movements is relatively low (less than ~15%) but becomes substantial at extreme joint positions (365; 712). During locomotion in predictable environments, such as on a treadmill or walkway in the laboratory, co-activation of antagonist muscles is low and there is a close correspondence between patterns of joint moments and EMG activity (27; 421; 649; 832). Thus, resultant joint moments are convenient variables to quantify muscle action at the joints during in vivo locomotion. The magnitude and direction of joint moments depend on locomotor phase, speed, slope of the ground and the type of gait. Extension joint moments reach their maximum during the stance phase in all types of locomotion compared to the extension or flexion moments during the swing phase (421; 561; 675; 687; 861).
The resultant joint moments during stance have the greatest contribution to joint kinematics. During the swing phase, joint kinematics are substantially influenced by motion-dependent interaction moments and body segment gravitational force in addition to the resultant joint moment of force produced by the muscles (Zatsiorsky, 2002). For example, during the swing phase of walking and running of relatively large animals, such as cats and humans, knee extension occurs through passive interaction moments produced without knee extensor activity (296; 682; 863). In contrast, smaller animals, like mice and rats, require knee extensor activity during swing (13; 739). This is because animal size and limb segment inertia contribute to the magnitude of the interaction and gravitational moments at the joint. In small animals, the interaction moments are negligible (391). The inertia of limbs in multi-segment extremities during the swing phase of locomotion or in reaching causes unpredictable motion-dependent perturbations and requires constant somatosensory corrections (75). Disruptions of somatosensory feedback due to illness or experimental interventions make coordinated movements in relatively large animals, such as cats and humans, difficult (166; 733). On the other hand, mice demonstrate robust coordinated overground locomotion without functional muscle spindles (10).
Mechanosensitive receptors, afferents, and their activity during locomotion
During locomotion, muscles shorten and stretch, joints move, the skin stretches and hairs deflect. Most mechanoreceptors and their afferents will activate during normal locomotion and their relative contribution will change dynamically with the phase of the step cycle, context and task demands. As discussed in the following sections, the somatosensory system has about a dozen different types of low-threshold mechanoreceptors (LTMRs) that respond to different stimuli and signal different properties, often with overlapping functions.
Muscle receptors and afferents
Muscle spindles
Muscle spindles are sensory end organs that respond to changes in the rate (dynamic component) and magnitude (static component) of muscle stretch [reviewed in (54; 55; 58; 245; 402; 547–549; 691; 699; 810)]. The human muscular system contains over 44,000 muscle spindles (55). Muscle spindles are composed of intrafusal fibers with contractile polar regions and a non-contractile central or equatorial region organized in parallel with extrafusal muscle fibers. Intrafusal fibers include dynamic nuclear bag 1 fibers, sensitive to both the rate of stretch and change in length, as well as static nuclear bag 2 fibers and static nuclear chain fibers that are mainly sensitive to absolute length changes (57). The central region of muscle spindles is innervated by generally one group Ia afferent (also referred to as primary spindle afferents), which wraps around all three types of nuclear fibers, and one or more group II afferents (also referred to as secondary spindle afferents) that wrap around static bag 2 and chain fibers. The polar contractile regions of intrafusal fibers receive motor innervation from β and γ motoneurons, often referred to as fusimotor drive (54; 55; 245; 534; 547; 549). The β motoneurons in vertebrates innervate both intrafusal and extrafusal fibers (246), whereas γ motoneurons, found in mammals exclusively, only innervate intrafusal fibers. Thus, γ motoneurons cannot directly affect the contractile force of muscle, while β motoneurons can. The presence of γ motoneurons in mammals indicates that the sensitivity of muscle spindles can be regulated independently of extrafusal fiber activity, which is controlled by α-motoneurons. The γ-motoneurons can be static or dynamic (545), and based on experimental data and simulations, it was proposed that static and dynamic sensitivities are independently regulated (695). Static γ-motoneurons innervate static nuclear bag 2 fibers and nuclear chain fibers while dynamic γ-motoneurons innervate dynamic nuclear bag 1 fibers (53; 55; 62). Because of this organization, primary spindle afferents show high dynamic sensitivity, whereas secondary spindle afferents mainly respond to static muscle length changes.
Most of our knowledge on fusimotor drive during locomotion comes from inferences based on spindle afferent recordings in decorticate/decerebrate or intact cats stepping on a treadmill (658; 659; 695; 755). However, some studies have recorded and identified γ motoneuron activity from hindlimb muscle nerves during fictive locomotion in decerebrate curarized cats, showing phasic or tonic discharge (76; 77; 245; 594; 595; 806). Thus, although γ motoneuron receive numerous converging inputs from different types of somatosensory afferents (341; 596; 597), their phasic modulation can be generated centrally. It had been proposed that α and γ motoneurons receive common synaptic drive during movement, referred to as α-γ co-activation (76; 325; 779; 825). While this may hold true for certain motor behaviors and for certain fusimotor neurons, in dynamic conditions, such as locomotion, studies have shown that only a portion of the pattern of fusimotor drive can be explained by α-γ co-activation (503; 691; 693; 695). Prochazka et al. (1985) introduced the concept of ‘fusimotor set’, where the pattern of dynamic and/or static fusimotor drive adjusts according to task demands. In this control scheme, the pattern of dynamic fusimotor drive during undemanding conditions, such as locomotion of a cat on a flat surface, is low while static drive is high. When the task becomes more challenging and/or unpredictable, such as ladder walking, dynamic fusimotor drive increases.
How do spindle afferents discharge during locomotion and what potential information do they provide? A few groups have succeeded in obtaining stable recordings from various types of somatosensory afferents from DRGs or dorsal roots during treadmill or over ground locomotion, first in decorticate/decerebrate cats (658; 659; 755) and then in freely behaving intact cats (503–506; 697; 845; 846). Primary spindle afferents can discharge at peak rates greater than 200 Hz during treadmill locomotion in intact cats, with a normal range of 50–100 Hz and an ensemble mean of ~80 Hz (506; 692). Discharge rates can be much higher (e.g. 500 Hz) when cats land from a fall and ankle extensors are rapidly stretched or when the animal receives an unexpected load by applying pressure to their back during stance (698) or perform fast paw-shaking (694). Primary spindle afferents discharge at rest and generally display phasic patterns during stepping, but discharge rate is highly sensitive to the animal’s state of arousal (697).
The discharge of some spindle afferents is clearly linked to mechanical events of the step cycle whereas in other afferents it is less clear. For example, primary spindle afferents from ankle extensors display peak activity when muscles are passively stretched in the F phase (697; 698). These afferents show reduced discharge during E1 followed by a burst of activity during early stance, when the muscle-tendon unit is lengthening, although muscle fascicles might undergo shortening or isometric contractions (172; 380; 418; 522). Variable discharge is then observed for the remainder of stance when these muscles contract. Although maintained primary spindle afferent firing while ankle extensors shorten is consistent with high fusimotor drive, afferent discharge did not correspond to EMG activity, consistent with weak α-γ co-activation. Studies have shown similar patterns of activity in primary spindle afferents of ankle extensors during decerebrate locomotion in cats, albeit with much higher activity during stance (755; 756).
The hamstring muscles include semitendinosus, semimembranosus and biceps femoris that flex the knee and extend the hip. Hamstring muscles have different compartments with preferential actions at the knee and hip (137; 250). For example, the anterior biceps femoris is a one-joint hip extensor whereas the posterior biceps femoris is more active with knee flexion. Thus, spindle afferents from hamstring muscles can signal changes in muscle lengths that occur at the knee and/or hip joints. A few studies have recorded from hamstring primary spindle afferents in intact cats during overground locomotion (697; 698). High discharge rates were found during the F and E1 phases, when the hamstrings stretch passively, with maintained discharge during stance when these muscles shorten, consistent with fusimotor drive (698).
Primary spindle afferents of vastii muscles, pure knee extensors, displayed strong activity during early to mid-stance with lesser activity during the F phase of swing when the muscles were passively stretched, and weak or absent activity in E1 and late stance when muscles shortened passively (506). Spindle afferents from the sartorius muscle, a mechanically complex muscle with compartments that flex the hip and extend (anterior sartorius) or flex (medial sartorius) the knee (381; 679), showed more variable patterns (506). The most consistent pattern consisted of peak activity during swing and stance when the muscle was rapidly shortening and lengthening, respectively. The sartorius primary spindle afferents were silent in late stance and early swing, when the muscle was at maximal length. Other primary spindle afferents from sartorius did not correlate with anatomical or kinematic changes. Interestingly, Ia afferents from the rectus femoris, another muscle that extends the knee and flexes the hip, only fired during stance, similar to the vastii muscles (500).
We know less about secondary spindle afferents firing during locomotion, because of their smaller size. However, from the few recordings available, it appears that group II afferent firing from different hindlimb muscles correlates well with changes in muscle-tendon length during overground or treadmill locomotion in intact cats (692). Secondary spindle afferent firing never falls silent, consistent with strong static fusimotor drive, and depending on the muscle, there is some evidence of an α-γ linkage, particularly for triceps surae muscles, although it appears weak or absent for other muscles, such as the hamstrings (692).
Figure 5 (left and middle column) shows examples of activity of spindle Ia and II afferents from different muscle groups recorded in freely walking cats. So what information do spindle afferents from various leg muscles provide during locomotion? Simulation studies based on spindle afferent recordings during locomotion in intact cats have shown that ensemble firing rates from a few (≤ 10) primary afferents accurately estimate muscle lengths, hindlimb joint positions and/or velocities (692; 693; 845). Depending on the muscle, including fusimotor drive in the simulation improves the estimation (692; 693). Therefore, as different muscles shorten and lengthen at specific times during the step cycle, spindle afferents, under fusimotor control, provide the CNS with continuous detailed information on dynamic and static muscle fascicle lengths and thus, the position and velocity of the limb and individual joints. As discussed below, cutaneous and joint afferents complement spindle afferent information.
Figure 5. Schematic representation of ensemble activity of muscle afferents.
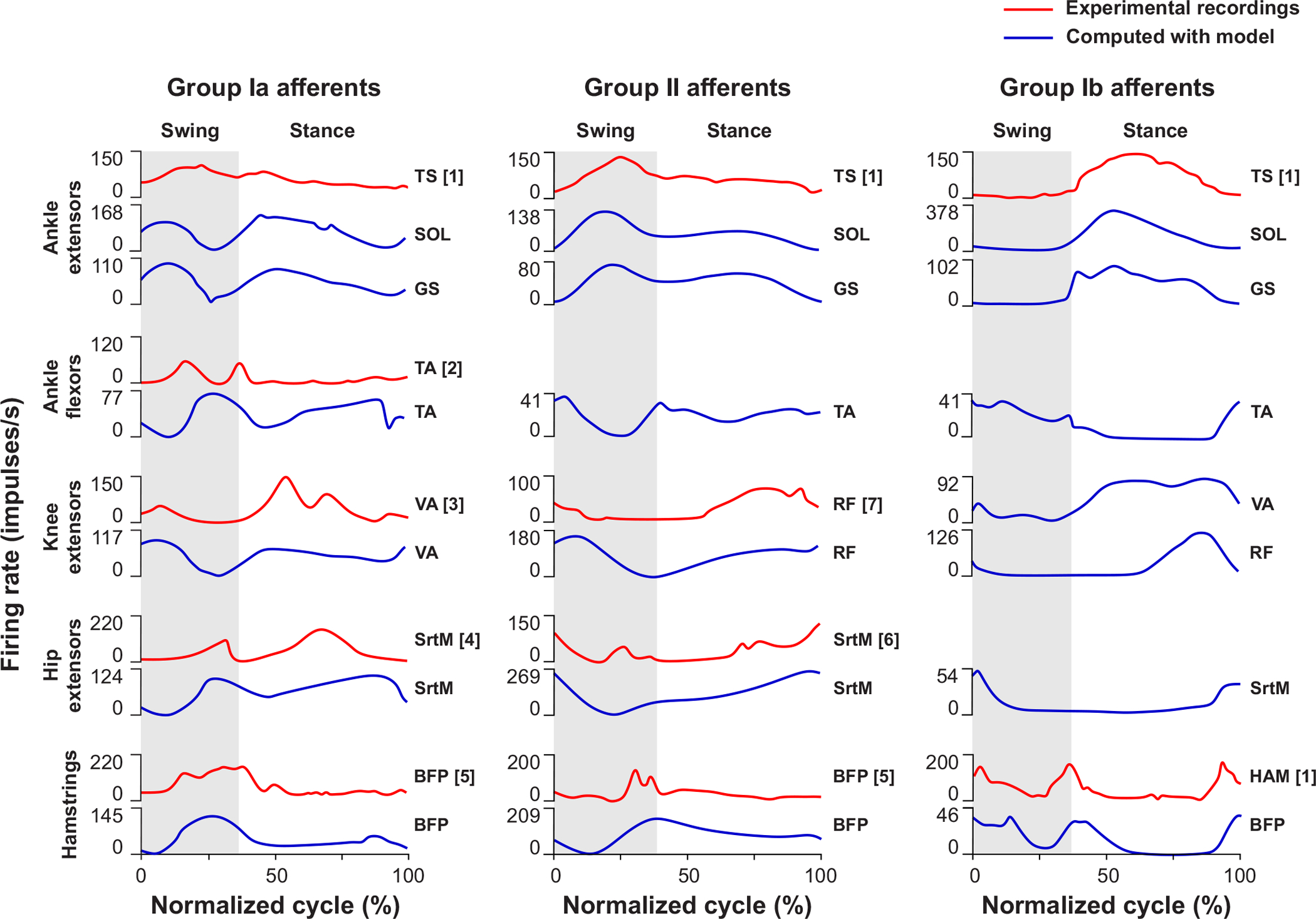
The figure shows afferent activity recorded in freely walking cats (red lines) and computed using a neuromechanical model relating afferent activity to instantaneous muscle fascicle length and velocity and tendon force (blue lines). Length-related spindle primary (Ia) and secondary (II) afferents from ankle (tibialis anterior) and knee (biceps femoris posterior) flexors demonstrate increased activity at the swing-to-stance transition, while spindle secondary afferents from hip flexors (rectus femoris and medial sartorius) show increased activity at the stance-to-swing transition. These patterns of activity are consistent with muscle fascicle length changes. Length-related activity of spindle afferents and force-related activity of GTO afferents of ankle (triceps surae, soleus, gastrocnemius) and knee (vasti and rectus femoris) extensors is high during the stance phase and corresponds to high EMG activity of these muscles in stance. High activity of GTO afferents of the knee flexor–hip extensor hamstrings at phase transitions also corresponds to EMG activity pattern of this muscle. BFP, posterior biceps femoris; GS, gastrocnemius; HAM, hamstrings; RF, rectus femoris; SOL, soleus; SrtM, medial sartorius TA, tibialis anterior; TS, triceps surae; [1] Prochazka, Gorassini, 1998; [2] and [5], Loeb, Duysens, 1979; [3], [4] and [6], Loeb et al., 1985; [7] Loeb, 1981. Computed afferent activity taken from (686).
Golgi tendon organs
Golgi tendon organs (GTOs) are encapsulated corpuscles located at muscle-tendon junctions innervated by a large myelinated Ib afferent whose endings are entwined between tendon fibers from 10–20 motor units (i.e. the α-motoneuron and all muscle fibers it innervates) [reviewed in (58; 431; 604; 691)]. They respond to small changes in active and passive muscle tension (332; 395; 795) and are found in nearly all mammalian limb muscles. GTOs are plentiful in muscles of the face, neck, and tail in cats (310; 431; 710) but they seem to be only present in limb muscles of the mouse (870). However, one recent study in mice reported GTO afferents from an unspecified axial muscle (615). Differences between mouse studies could be due to experimental approaches. Although we can only speculate, maybe GTOs developed in axial muscles of larger mammals in more plentiful numbers to help stabilize posture during movement. Overall, however, GTOs are less numerous than muscle spindles (58).
In physiological conditions, GTO afferent firing provides an accurate indicator of total muscle force (171; 692). For example, during isometric contractions, the discharge rate of GTO afferents parallels muscle force, with frequent step-like increases in discharge rate, particularly with slower increases in force (171). It is also clear that GTOs are sensitive to the rate of change in active force (i.e. its dynamic component). During locomotion in intact cats, GTO afferents show peak activity when parent muscles contract (36; 500; 692; 697). For instance, GTO afferents from ankle extensors, such as the lateral gastrocnemius and flexor digitorum longus, showed peak activity in stance (E2 and E3) during overground locomotion in intact cats when these muscles generate active force (36; 697). At peak EMG activity/muscle force, ankle extensor GTO afferents often discharged in excess of 100 Hz, whereas they became silent when the muscles were inactive. Figure 5 (right column) shows examples of activity of group Ib afferents from different muscle groups recorded in freely walking cats.
Cutaneous receptors and afferents
The glabrous and hairy skin of mammals contains no less than 7 main types of mechanoreceptors that respond to low-threshold stimuli, signaling different functional aspects of innocuous touch [reviewed in (3; 187; 449; 489)] that may contribute to locomotion in several ways. In glabrous skin, LTMRs include Meissner corpuscles (RA1, rapidly adapting Type 1), Pacinian corpuscles (RA2, rapidly adapting type 2), Merkel discs (SA1, slowly adapting Type 1) and Ruffini endings (SA2, slowly adapting type 2). The hairy skin has three main types of mechanoreceptors, Guard hairs (or monotrich, 1–2% of hairs), Awl-Auchene hairs (~25% of hairs) and Zigzag hairs (or D-Hair, Down, >70% of hairs) (3; 104; 113; 114; 489; 493). Merkel discs and Pacinian corpuscles are also found in hairy skin. Tactile afferents from glabrous skin and hair LTMRs are of the Aβ (thickly myelinated), Aδ (thinly myelinated) or C (unmyelinated) fiber types, based on their conduction velocities, and can adapt slowly, maintaining discharge during sustained stimulation, or rapidly, discharging briefly at stimulus onset and offset.
Due to the shape and properties of their end organs, which determines their rate of adaptation, as well as their depth in the skin, the different LTMR afferents respond preferentially to specific stimuli, such as indentation, stretch, motion and/or vibration (3; 489). The size of their receptive fields dictates their spatial acuity. The RA1 receptors in glabrous (Merkel cells) and hairy (Guard/Awl-Auchene hair cells) skin are sensitive to skin or hair movements and low frequency vibration (1–10 Hz). In humans, they are found in high densities in the glabrous skin of the hand and the soles of the feet (3). RA2 receptors (Pacinian corpuscles) in glabrous skin have large receptive fields and are extremely sensitive to high frequency (80–300 Hz) low amplitude vibration in the nanometer range (3; 432; 520). SA1 receptors have small receptive fields and high spatial resolution to indentation, particularly with motion across the skin. The SA2 receptors have larger receptive fields than SA1s and although they respond less to indentation, they are much more sensitive to the rate and magnitude of skin stretch. Just like muscle spindles, they make an important contribution to proprioception (699). Rapidly adapting receptors respond more strongly when objects move across the skin. The SA1, SA2, RA1 and RA2 receptors send their information to the spinal cord via Aβ afferents. Rapidly adapting Aδ afferents from Awl-Auchene and Zigzag hair cells have relatively large receptive fields and are highly sensitive to dynamic hair deflections (3; 489). The C afferent LTMRs (C-LTMRs) from Awl-Auchene and Zigzag hair cells have an intermediate adaptation rate and are sensitive to hair deflections, particularly slow moving stimuli. They are associated with the pleasant nature of touch (614).
It is likely that information from cutaneous mechanoreceptors combine to provide a precise representation of the shape, pressure and motion of objects contacting the skin. Specifically, those from the footpads can provide precise information of the load on the limb. Abraira and Guinty (2013) proposed that arrays of cutaneous mechanoreceptors and their afferents are organized into ‘sensory units’ that convey specific tactile features. As discussed later on, cutaneous feedback ascends to the brain and converge with inputs from different sources to provide richer representations of the tactile world. In the fore- and hindpaws of animals or the human foot, the dynamic sensitivity of cutaneous mechanoreceptors makes them perfectly suited to provide detailed information about the terrain. Afferents from cutaneous LTMRs discharge when their receptive fields are contacted, as shown from DRG recordings in intact cats during treadmill locomotion (500; 502). Loeb and colleagues also reported that afferents from cutaneous mechanoreceptors were larger, more easily identifiable and found in greater number compared to muscle afferents when sampling from lumbar and sacral DRG (500). Cutaneous receptors sensitive to stretch, such as SA2 and hair cells, were activated by skin motion during locomotion.
Joint receptors and afferents
The most understudied group of receptors and afferents in relation to their contribution to locomotor control are those that supply the joints and their role in proprioception in general is contentious (445; 691; 699). Joint receptors include Ruffini endings, Pacinian corpuscles, GTO-like receptors and free nerve endings located in the joint capsule, ligaments and/or menisci [reviewed in (445; 447; 453; 529)]. Joint Ruffini endings are slowly adapting LTMRs that signal static and dynamic joint position (445). Pacinian corpuscles are rapidly adapting LTMRs signaling mechanical stress and vibration (445). The GTO-like receptors, mostly found in ligaments, adapt slowly and have high thresholds, being mostly silent without movement (445). Historically, joint receptors were considered to detect the limits of joint movements and to serve a protective function (32; 96; 273; 445; 780). Studies have reported that some joint afferents respond throughout the entire range of motion (445; 453). However, other studies have instead insisted that these afferents came from nearby muscles and that joint afferents do not provide information related to the entire range of motion (155; 333; 563).
Stimulation of high threshold joint afferents, like those of muscles and skin, evokes flexion reflexes of the limb (383). Inputs from joint afferents interact with other reflex pathways, such as those from group Ib muscle afferents (517). Although inputs from low-threshold joint afferents have weak effects on α-motoneurons, they have a powerful influence on the γ-motoneurons of various hindlimb muscles, eliciting EPSPs and IPSPs, as demonstrated in cats (341; 445–447; 837). Thus, the role of joint afferents in locomotor control might be in shaping fusimotor drive to muscles and regulating the sensitivity of muscle spindles.
Loeb and colleagues recorded the activity of knee joint afferents during locomotion in the intact cat (500; 502). They found that these afferents discharged infrequently during locomotion and responded preferentially to axial rotations of the knee joint. Some units belonging to the posterior articular nerve showed most of their activity during stance despite greater knee joint angle changes during swing (500). In the same study, increasing hindlimb loading by placing the forelimbs on an elevated stationary platform also increased the activity of knee joint afferents.
In summary, afferents from mechanoreceptors in muscles, skin and joints discharge during locomotion and can contribute to its control in different yet complementary ways. Table 2 summarizes the main afferent types, their end organs, their preferential stimulus and their potential main sensory functions during locomotion.
Table 2.
Types of mechanoreceptors and their main potential sensory function for locomotion.
| Afferent type | End organ | Preferential stimulus | Main sensory function |
|---|---|---|---|
| Muscle: | |||
| Group Ia afferents | Muscle spindles | Dynamic muscle stretch | Limb/segment velocity and position |
| Group II afferents | Muscle spindles | Static muscle stretch | Limb/segment position |
| Group Ib afferents | Golgi tendon organs | Muscle tension | Contractile muscle force |
| Skin: | |||
| SA1 (Aβ afferents) | Merkel cells | Skin indentation | Maintained contact/terrain characteristics |
| SA2 (Aβ afferents) | Ruffini endings | Skin stretch/tension | Forces applied distally |
| RA1 (Aβ afferents) | Meissner corpuscles | Movement across skin | Motion of foot on terrain and its characteristics |
| Foot contact and liftoff | |||
| RA2 (Aβ afferents) | Pacinian corpuscles | Vibration | Light contact to body |
| Aδ-LTMR (Aδ afferents) | Longitudinal lanceolate ending | Hair cell deflection | None for locomotion (affective touch) |
| C-LTMR (C afferents) | Longitudinal lanceolate ending | Hair cell deflection | Flexion reflex-crossed extension |
| HTMR (Aβ, Aδ and C afferents) | Free nerve endings | Noxious mechanical | |
| Joint: | Limb/segment position | ||
| Ruffini-like endings | Joint position | Ground reaction forces | |
| Pacinian-like corpuscles | Mechanical stress/vibration | Limiting angular excursions | |
| Golgi tendon organ-like receptors | Joint position/extremes | Limiting angular excursions | |
| Free nerve endings | Joint position/extremes |
Developmental origin of somatosensory afferents
The early process of the developmental process giving rise to different types of somatosensory afferent neurons in the dorsal root ganglia (DRG) is delamination of the neural crest cells from the dorsal neural tube at around embryonic days 8 to 10 (E8 to E10) in the mouse (753). These neural crest cells, once delaminated, migrate ventrolaterally where some become glial cells while others become DRG or autonomic ganglia neurons (706). Neurogenesis of somatosensory neurons in DRG occurs in two separate waves, initiated by the expression of the transcription factor Neurogenin 2 (Ngn2) for large caliber neurons and Neurogenin 1 for smaller caliber neurons (521). Somatosensory neurons expressing the receptors for Brain-derived neurotrophic factor (BDNF) and neurotrophin 3 (NT3), as well as the Tropomyosin receptor kinases B and C (TrkB and TrkC), become large caliber neurons in the early wave of neurogenesis, eventually becoming proprioceptive afferent neurons (713). Somatosensory neurons expressing the receptor for nerve growth factor and Tropomyosin receptor kinase A (TrkA) become smaller caliber neurons in the later wave of neurogenesis that eventually give rise to smaller non-proprioceptive afferents (539; 713).
The early wave embryonic somatosensory neurons project their axons to peripheral targets in the skin or muscles. Once the axons reach the skin or muscles, target-derived trophic factors determine the combination of genes subsequently expressed in somatosensory neurons (12; 142; 415). The interaction between the sensory afferent and its target determines the maturation of the peripheral ending and the central projection through the expression and activation of specific transcription factors (142; 415). In the mouse, somatosensory neurons reach target muscles around E11 to E14 and diverge into group Ib afferents, giving rise to the endings in GTOs, and group Ia and II afferents that innervate muscle spindles. This divergence is mainly associated with the continuing expression of the Pou4f3 transcription factor selectively in Group Ib afferents at later developmental stages (615). The laminar organization of the spinal cord and the spatial organization of motoneuron pools is important for establishing distinctive projection patterns and selective synaptic connections of somatosensory afferents with target neurons in the spinal cord (40; 443). Findings regarding central projections of different somatosensory afferent types have mainly focused on their terminations within the spinal grey matter and monosynaptic connections of spindle afferents with motoneurons.
Somatosensory afferents innervating the skin can be divided into i) LTMRs that include Aβ-, Aδ- and C-LTMRs and ii) high-threshold mechanoreceptors (HTMRs), such as C-HTMRs that are maximally sensitive to noxious stimuli (3). Using mouse genetics, it was shown that afferents from LTMRs project into the dorsal horn and terminate in laminae in an organized manner (493). Afferents from C-LTMRs terminate into lamina II, Aδ-LTMRs into lamina III and Aβ-LTMRs into laminae IV and V. The LTMR subclasses express differential genes. The C-LTMRs express the enzyme tyrosine hydroxylase, which catalyzes the production of L-DOPA from tyrosine. The Aδ-LTMRs express the tyrosine receptor kinase B, a receptor for BDNF and neurotophin 4. Finally, the Aβ-LTMRs express the neuropeptide Y receptor-2, with the exception of SA-LTMRs. The somatosensory afferent neurons, their gene expression patterns, and their projection patterns into the dorsal horn of the spinal cord is now well described, providing new avenues to understand their functional roles.
Significant insights into the development of central projections within the spinal cord have been made by studying the monosynaptic connection between group Ia afferent fibers and motoneurons of homonymous (projections from and to the same muscle) and synergist (muscles with similar mechanical actions) muscles. Electrophysiological studies in the cat showed that group Ia afferents form strong excitatory synaptic connections with motoneurons innervating homonymous muscles that project to the same muscle from which the afferent arises and weaker excitatory connections with synergist motoneurons that project to other muscles with similar functions (226). Motoneurons innervating individual muscles have a specific location in the ventral horn of the spinal cord (443; 715; 716; 830; 876). The specific identity of motoneuron pools are determined by the expression of a set of homeobox (Hox) genes (182). The selective elimination of FoxP1, a transcription factor acting as a Hox-accessory factor, in motoneurons changes the pattern of motoneuron connectivity and motoneurons choose muscles randomly (181). Despite the change in motoneuron connectivity and localization, central axons of group Ia afferents terminate in the same place as they would have if motoneuron pools had maintained their normal positions (799). The approaching angle of afferent axons relative to the orientation of motoneuron dendrites determines contact formation (52). Smaller approaching angles correlate with the formation of higher synaptic densities. In addition to spatial parameters, activity-dependent mechanisms refine the connectivity of group Ia afferents with synergist motoneurons (570). Functional synaptic connections with the motoneurons and Group Ia afferents are established around E17 (earliest measured time point), but the maturation continues at least within the first postnatal week in mice (142; 472; 565). Thus, afferent endings have genetically-determined coordinates within the spinal cord and experience-dependent mechanisms shape and refine connections for appropriate functional outcomes.
Somatosensory afferent projections to and within the central nervous system
Textbooks understandably simplify somatosensory afferents as they enter the spinal cord and make synaptic connections. The reality is complex even for senior sensorimotor neuroscientists. For example, the monosynaptic reflex pathway, considered the simplest sensorimotor pathway, generally refers to the direct synaptic connections of group Ia afferents onto homonymous (or agonist) α-motoneurons in the ventral horn of the spinal cord. However, group Ia afferents also make monosynaptic connections with synergist motoneurons, various spinal interneurons that project locally and across several segments, as well as neurons that ascend to supraspinal structures (Fig. 6) (419; 433; 435; 437; 721; 835). Other somatosensory afferents make similar widespread projections. Within the spinal cord, spinal interneurons, some with dendritic trees that span large areas, receive afferent inputs from different sources and make synaptic contacts with various excitatory and inhibitory neurons that project ipsilaterally, contralaterally or bilaterally (Jankowska, 2015). Spinal interneurons that project directly to motoneurons (pre-motor or last-order) of different muscles overlap extensively (434; 819). Additionally, α-motoneurons have extremely large dendritic trees (533) and receive converging inputs from multiple excitatory and inhibitory pre-motor interneurons that receive inputs from various somatosensory afferents. In other words, a single somatosensory afferent affects the excitability of neural targets throughout the CNS, directly or indirectly, and neuronal targets receive converging inputs from many afferent types. Because of the highly integrated nature of somatosensory feedback with other control systems, its impact on movement control is not surprising. Below, we briefly review some key concepts and principles of somatosensory afferent projections derived from anatomical and physiological studies.
Figure 6. Projections from single group Ia afferent to neuronal targets of spinal cord.
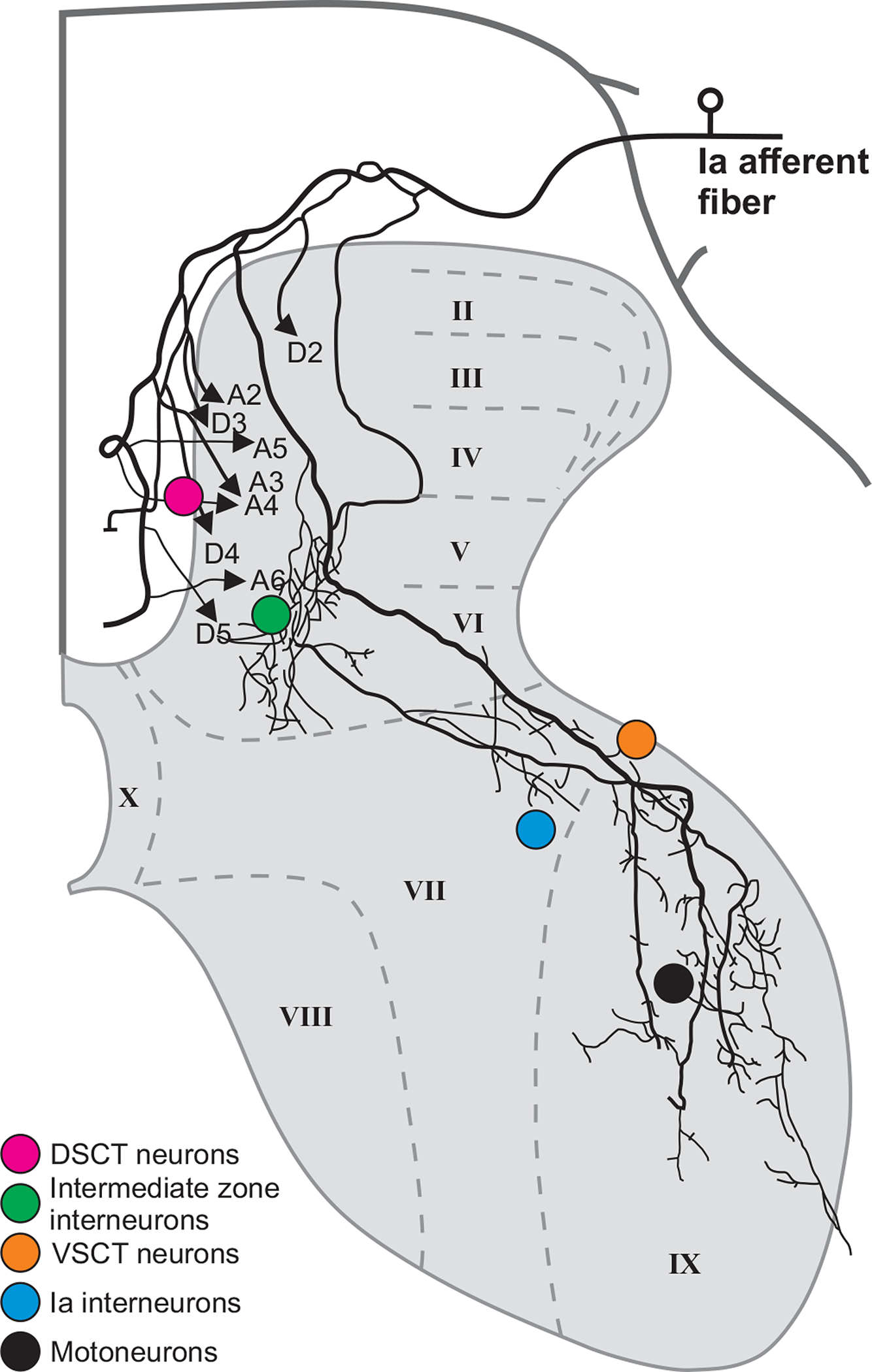
The figure shows the trajectory of axon collaterals of a muscle spindle primary afferent (group Ia) from the medial gastrocnemius intra-axonally labelled with horseradish peroxidase. Colored circles indicate the location of five populations of target cells contacted by terminal branches of the group Ia afferent. Approximate locations of spinal cord laminae are shown (from Roman numerals II to X). Adapted and reproduced with permission from (435) using material from (419). DSCT, dorsal spinocerebellar tract; VSCT, ventral spinocerebellar tract).
Mechanoreceptive afferents terminate in spinal cord laminae
Somatosensory neurons have their cell bodies in DRG and are pseudo-unipolar, with an axon extending to a peripheral end organ and an afferent branch terminating centrally in the spinal cord where it releases the excitatory neurotransmitter glutamate. Somatosensory afferents express the vesicular glutamate transporter VGLUT1 and can be differentiated from central excitatory neurons that express VGLUT2 (25; 814; 831). The spinal cord grey matter is often divided into anatomical subdivisions, or laminae, based on the types of neurons and terminal projections observed histologically in the cat (708). Upon entering the spinal cord, large myelinated LTMR afferents bifurcate rostrally and caudally and branch extensively across a few or several spinal segments. There is extensive overlap in synaptic terminations from different types of somatosensory afferents, as these afferents have numerous collateral branches.
Group Ia and II afferents from muscle spindles project mainly to laminae V-VII in the intermediate zone and laminae IX of the ventral horn (Fig. 7) (103; 238; 419; 844). These areas contain neurons of the ventral (VSCT) and dorsal (DSCT) spinocerebellar tracts, propriospinal neurons, pre-motor or last-order interneurons, Ia inhibitory interneurons and/or agonist/synergist motoneurons, as shown primarily in cats but also rats (239; 435; 437; 439; 835). Group II afferents also make monosynaptic contacts with dorsal horn interneurons in laminae IV, a region containing another population of DSCT cells that do not receive group I inputs (437; 771; 835). In the cat, group II afferents also make monosynaptic contacts with a distinct functional population of contralaterally projecting last-order interneurons in lamina VIII (436; 437), a population of commissural interneurons not observed in the rat (835).
Figure 7. Muscle afferent projections in the rat spinal cord.
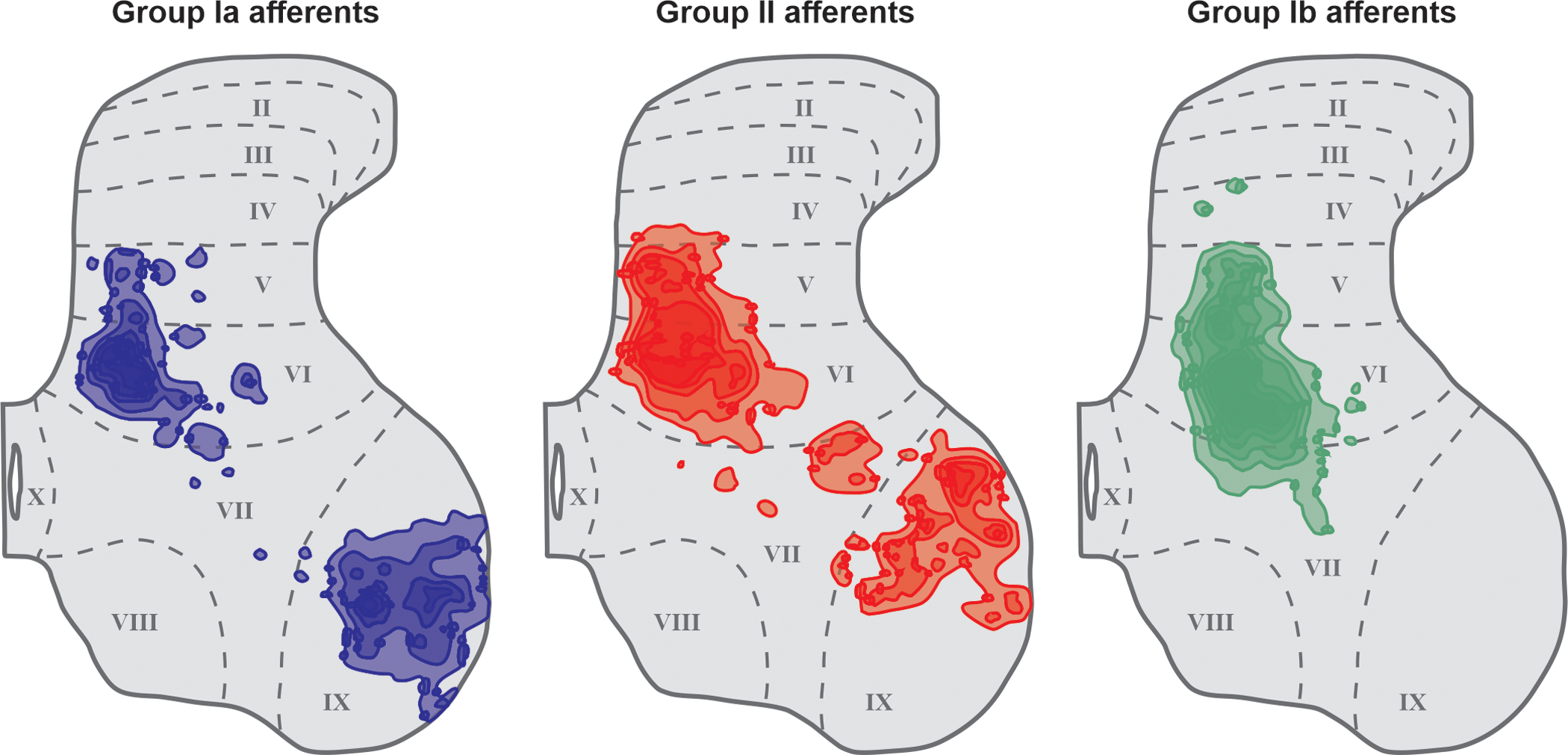
Figure shows contour maps of varicosities from 3 afferents of each class (Group Ia, II and Ib). Contour maps created by calculating the density of varicosities and outlining areas above a certain threshold. Approximate locations of spinal cord laminae are shown. Modified from (835).
In the cat spinal cord, upon entering the dorsal horn, group Ib afferent fibers bifurcate, sending projections rostrally and caudally over several segments, with ascending fibers traveling in the dorsal column (431). Collaterals run ventrally in the dorsal horn and terminate mainly in laminae V-VII. Extensive branching is found in Clarke’s column in the lumbar enlargement, which gives rise to a portion of the DSCT. Group Ib afferents also project to neurons of the VSCT in lamina VII (771). Similar projection patterns of group Ib afferents were observed in the rat spinal cord, with the majority of varicosities found in lamina VI (Fig. 7) (835).
In mammals, all LTMR cutaneous afferents send axonal projections to the dorsal horn of the spinal cord, with terminals primarily in laminae III-IV, where the cell bodies of the postsynaptic dorsal column neurons and spinocervical tract (SCT) neurons are located (3; 101; 102; 489; 493; 664). These projections have a somatotopic organization, with distal to proximal skin sites sending inputs along a mediolateral gradient. Upon entering the spinal cord, the Aβ fibers send projections rostrally and caudally, with collaterals terminating throughout the superficial and intermediate laminae. The smaller caliber Aδ and C afferents do not bifurcate, they terminate a few segments rostral in the dorsal horn (493). Most tactile afferents synapse on interneurons that project locally within the spinal cord, while a smaller proportion send long ascending projections directly or indirectly to the brain (3).
As discussed above, projections from different afferent types overlap extensively within the spinal grey matter, mainly in laminae of the intermediate zone (V-VII). While some intermediate zone spinal interneurons receive selective inputs from one afferent type (e.g. group I or group II afferents), the majority receive converging inputs from different types, as demonstrated from electrophysiological studies in the cat [reviewed in (235; 433; 437)]. Indeed, cutaneous and joint afferents project directly (monosynaptic) or indirectly (di- or oligosynaptic) to intermediate zone spinal interneurons receiving group I and II afferent inputs (237; 361; 518; 711). The majority of these intermediate zone interneurons project directly to α-motoneurons (437). The pattern of convergence on individual intermediate zone interneurons appears, at present, to be random, with no clear defining patterns of inputs and outputs (434; 435). Jankowska (2008, 2015) argued that the role of the spinal circuitry is to increase or decrease specific aspects of somatosensory inputs to meet task demands.
Somatosensory information ascends directly or indirectly to multiple supraspinal structures
Somatosensory information from peripheral afferents ascends through spinal pathways to reach different supraspinal structures. The main targets are the brainstem, the cerebellum, the thalamus and the cerebral cortex that, in turn, influence sensorimotor structures and pathways involved in locomotion. Neurons in these supraspinal structures generally discharge during locomotion and lesions lead to locomotor deficits.
Brainstem
The brainstem is an important structure for the control of locomotion and posture. In the 1960s, a Russian group identified a region within the midbrain, which they termed the mesencephalic locomotor region (MLR), that when electrically stimulated initiated quadrupedal locomotion on a treadmill in decerebrate cats and modulated speed (763). Neurons within brainstem nuclei, such as those of the lateral vestibular nucleus, giving rise to the vestibulospinal tract, the reticular formation, giving rise to the reticulospinal tract, and the red nucleus, giving rise to the rubrospinal tract, are rhythmically active during locomotion in the cat (207; 543; 618; 619; 622; 623; 654). These brainstem pathways project to spinal circuits that affect muscle activity during locomotion (205; 208; 624; 656; 709).
Several brainstem nuclei receive somatosensory afferent inputs directly or indirectly. For instance, somatosensory information from the limbs and trunk carried in the dorsal columns (DC) terminate in DC nuclei of the brainstem, the cuneate and gracile nuclei, either directly or indirectly [reviewed in (3; 510)]. Tactile and proprioceptive afferents send long ascending projections directly to DC nuclei neurons in the brainstem, termed the direct DC pathway. In this direct pathway, afferents travel in the ipsilateral gracile and cuneate fasciculi in the DC and synapse on their respective brainstem nuclei. However, the bulk of information from somatosensory afferents is transmitted indirectly to the DC nuclei, with afferents first synapsing on postsynaptic dorsal column projection neurons in laminae III-IV of the spinal cord that then ascend in the ipsilateral gracile and cuneate fasciculi (3; 101; 510). Ultimately, somatosensory information from the direct and indirect DC pathways converge on brainstem nuclei neurons. Gracile nuclei neurons, with cell bodies in the medial-dorsal medulla, mainly receive somatosensory inputs from the hindlimbs and trunk. The cuneate nuclei, located lateral to the gracile nuclei in the brainstem, receive somatosensory inputs from the forelimbs and trunk. The DC nuclei send projections back to the spinal cord, locally to other brainstem neurons, to the cerebellum and to subcortical and cortical structures of the brain and are a main sensorimotor integration and processing center (72; 510; 714).
Studies in anesthetized or decerebrate cats have shown that neurons of the lateral vestibular nucleus (VN), also called Deiter’s nucleus, respond to somatosensory inputs from muscle and cutaneous afferents of the fore- and hindlimbs via direct spinal pathways (676; 858; 859), or indirectly via cerebellar projections (17; 18). The direct spinal pathways appear to be mainly from cutaneous, muscle group II and higher threshold afferents, with weak or absent muscle group I inputs (859). Mossy or climbing fiber collaterals also contribute to the activation of lateral VN neurons. In turn, lateral VN neurons project to all levels of the spinal cord. Wilson et al. (1966) proposed that ascending somatosensory inputs to Deiter’s nucleus provide a general facilitation of its neurons, which also received converging and more organized inputs from other sources, such as the cerebellum. Interestingly, a small proportion of cells (10–15%) that receive somatosensory information from the fore- and hindlimbs, project to the lumbosacral and cervico-thoracic cord, respectively, suggesting a role in coordinating the four limbs (17). Studies have also shown that VN neurons discharge in response to active or passive limb movements in decerebrate or conscious cats (47; 556). McCall et al. (2016) showed that about two thirds of recorded VN neurons responded to passive hindlimb movements in the conscious cat. Of these, about half responded to parasagittal plane movements in any direction while about 40% showed directional specificity. Thus, the phasic discharge of VN neurons during locomotion in the conscious cat could partly be due to incoming somatosensory inputs (543).
Another region of the brainstem receiving somatosensory inputs from trunk, forelimb and hindlimb afferents that plays an important role in controlling posture and locomotion is the medial pontomedullary reticular formation (pmRF) (206; 207; 230; 576; 663; 677; 751; 766; 773). In conscious or decerebrate cats, cells in the pmRF respond to active and/or passive trunk and limb movements, as well as electrical stimuli to peripheral nerves or natural stimulation of the skin (230; 576; 773). Somatosensory inputs from the limbs and trunk reach the pmRF through direct spinal pathways, termed spinoreticular neurons, and via the cerebellar nuclei, such as the fastigial nucleus (230; 550). Spinoreticular neurons have their cell bodies primarily in laminae VII and VIII of the lumbosacral cord and project to the ipsilateral or contralateral pmRF, or bilaterally (258; 550; 847). Siegel and Tomaszewski (1983) found that pmRF cells responded with greater proportion to trunk movements followed by forelimb and hindlimb movements, with limb receptive fields located proximally, with few located on the distal limbs. However, most pmRF cells have large and complex receptive fields, responding from low to high threshold stimuli applied to the head, neck, trunk and all four limbs, consistent with broad convergence of somatosensory inputs onto these cells (206; 550; 773). During locomotion, Drew et al. (1996) stimulated cutaneous nerves in all four limbs and recorded responses in medullary reticulospinal neurons in intact cats. They observed low-threshold (mainly Aβ afferents) short-latency excitatory responses that were modulated by phase, peaking mostly during swing. The authors suggested that cutaneous inputs from the limbs modify the activity of reticulospinal neurons in response to external perturbations.
Cerebellum
The cerebellum is a main target of somatosensory information from peripheral afferents receiving mossy fiber inputs from the brainstem and spinal cord and climbing fiber inputs from the inferior olive. Somatosensory information from the fore- and hindlimbs reaches the cerebellum via direct and indirect spinal pathways [reviewed in (84; 91; 298; 420; 444; 515; 531; 628; 792; 828)]. Hindlimb afferents project to DSCT and VSCT neurons that ascend directly to the cerebellum where they terminate as mossy fiber inputs. The DSCT ascends in the ipsilateral dorsolateral funiculus while VSCT travels in the contralateral ventral funiculus. The VSCT then crosses back so that both spinocerebellar pathways mainly transmit somatosensory information from the ipsilateral side of the body. VSCT neurons also receive sensory inputs from the contralateral hindlimb (45).
In the thoracolumbar spinal cord, cell bodies of the DSCT are located in laminae V, VI and VIII. The DSCT neurons can be grouped into different populations, according to their location (e.g. dorsal horn, Clarke’s column and lamina VIII) (771). DSCT neurons receive mono-, di- and/or polysynaptic inputs from all types of LTMR afferents (84; 91). However, the population in Clarke’s column receives denser projections from group I afferents while the one in the dorsal horn receives inputs mainly from group II and cutaneous afferents (231; 238; 239; 757; 771). Cell bodies of the VSCT, located in laminae VII and VIII, are also distributed across lumbar segments with greater numbers at L3–L6 (84; 792). Although VSCT neurons also receive excitatory inputs from LTMR afferents, to a lesser degree than DSCT neurons, they receive more dense projections, both excitatory and inhibitory (mainly glycinergic pre-motor interneurons), from spinal interneurons and descending pathways (228; 253; 352; 757; 771; 792).
Forelimb muscle, joint and cutaneous afferents send projections to the cerebellum through two main pathways, the cuneocerebellar tract (CCT) and the rostral spinocerebellar tract (RSCT) (84; 159; 163; 164; 510; 542; 627; 628; 630; 665; 666). The CCT, with features resembling the DSCT, originates in the external cuneate and main cuneate nuclei and projects ipsilaterally to the cerebellum through the inferior cerebellar peduncle, terminating as mossy fiber inputs. The CCT carries information from all types of LTMR afferents, including monosynaptic inputs from group I/II muscle afferents and disynaptic inputs from cutaneous afferents (384). The RSCT, with cell bodies originating in the cervical enlargement, responds to group I (mainly group Ib) and higher threshold afferents from the forelimbs (630). It enters the cerebellum via the ipsilateral superior and inferior cerebellar peduncles, terminating as mossy fiber inputs (542).
What information is encoded by spinocerebellar neurons receiving somatosensory inputs, particularly as it relates to locomotor control? The rhythmic activity of DSCT neurons has traditionally been considered to be driven by phasic somatosensory inputs, as it disappeared after deafferentation in mesencephalic cats during MLR-evoked treadmill locomotion, while that of VSCT neurons persisted (45; 46). However, it was later shown that both DSCT (70% of cells) and VSCT (100% of cells) neurons in the lumbar cord were rhythmically active during MLR-evoked fictive locomotion in decerebrate curarized cats, indicating that their rhythmic activity does not require phasic somatosensory inputs (253). Thus, both DSCT and VSCT neurons receive rhythmic drive from the spinal locomotor CPG [see also (650)]. Fedirchuk et al. (2013) also showed that DSCT neurons active during the extension phase responded to group I afferent inputs from both flexor and extensor muscles. The convergence of flexor and extensor group I inputs was also shown at rest in anesthetized cats (466). It is important to note that due to convergence, DSCT neurons do not receive site-specific (muscle or skin) information and more complex movements, such as locomotion, activate most of the population (91; 459; 626). Thus, the DSCT is thought to provide detailed information about whole limb kinematics, as neuronal activity more accurately reflects measures of multi-joint movements, as opposed to single-joint movements (91). The apparent representation of whole limb kinematics by DSCT population activity can be largely explained by musculoskeletal geometry and convergence of muscle length inputs (150). It was proposed that VSCT and RSCT neurons, which receive greater amounts of inputs from spinal interneurons and descending pathways, play a role in conveying efference copies of motor commands (21; 159; 515). However, as DSCT and VSCT neurons both receive input from somatosensory afferents and spinal pre-motor interneurons, including those of the spinal locomotor CPG, they probably contribute jointly to providing the cerebellum information about the intention and execution of locomotor movements. Simple spike activity in Purkinje cells, generated from mossy fiber inputs, generally increases during locomotion and rhythmic discharges, time-locked to the phase of stepping have been observed in the cat (41; 620).
Inferior olive
The inferior olive is a major target of somatosensory inputs from the spinal cord and brainstem nuclei and evokes complex spikes in a few cerebellar Purkinje neurons through climbing fibers (37; 88; 299; 302–304; 727; 728). The role of the inferior olive is not entirely clear and has been proposed to provide information to the cerebellum regarding movement errors (37) or to associate a sensory signal with a somatosensory event (304).
Lesioning the inferior olive with intraperitoneal injections of 3-acetylpyridine in the rat (498) or kainic acid injections directly in the inferior olive in cats (394) produces an ataxic gait and increased limb flexion during swing, with some deficits worsening over time. The progressive ataxia is due to the loss of inhibitory control of cerebellar nuclei, as lesions to the paravermal cortex, a main target of climbing fiber inputs, produce similar deficits (136). Somatosensory inputs from the limbs and trunk to the inferior olive are somatotopically organized, reaching different regions of the inferior olive and cerebellum via spino-olivocerebellar (SOC) pathways [see Fig 1. Of (37)]. One SOC pathway, ascending in the lateral funiculus and terminating in the C2 cerebellar zone, transmits converging somatosensory information from the four limbs in the cat (37; 43; 481). Another SOC pathway, ascending in the dorsal funiculus and terminating in C1 and C3 cerebellar zones, transmits somatosensory information from the ipsilateral limbs in the cat (37; 244; 629).
Some studies showed no change in complex spike activity in Purkinje cells during locomotion in the cat (29; 42; 620) while others showed phase-dependent changes (460; 823). Perhaps the discrepancies are because climbing fiber activity is strongly modulated with state (e.g. decerebrate vs intact preparations) and task (e.g. rest versus locomotion). In intact cats and rats, responses in SOC pathways evoked by stimulating forelimb cutaneous afferents are generally decreased from rest to locomotion (37; 39; 786). During locomotion, climbing fiber responses evoked by stimulating the superficial radial nerve were shown to be phase-dependent, with the modulation depending on the target cerebellar zone (37; 38). For example, responses in the C1 region were largest around footfall while those in C2 peaked in late stance (495). Interestingly, the phase-dependent modulation in cerebellar responses do not follow the modulation of cutaneous reflexes in forelimb muscles, consistent with different central gating mechanisms.
Thalamus
Pathways from the spinal cord and DC nuclei that receive afferent inputs from the limbs and trunk project to the contralateral ventral posterior (VP) nucleus of the thalamus (187; 270; 452; 513; 514; 532; 554). In mammals, the VP is somatotopically organized, with the forelimb areas more medial than the hindlimbs, and can be divided into subregions that receive different sensory modalities with varying receptive field sizes (270; 452). For example, in the rat, the rostral ventroposterior lateral (VPL) nucleus receives mainly proprioceptive information with large cutaneous receptive fields on distal limb segments, the middle portion of the VPL is mostly cutaneous with small receptive fields, while the caudal VPL mainly receives cutaneous inputs with large receptive fields (270).
In the rat, responses in VPL, with receptive fields on the contralateral forepaw, display short- (4–10 ms) and longer-latency (10–25 ms) responses when electrically stimulating the skin of the contralateral forepaw (769). In this study, about half of VPL cells responded strongly around paw contact and short- and longer-latency evoked responses were differentially modulated with phase. Lesion studies have shown that short-latency responses are mediated by a relay in the cuneate nucleus while longer-latency responses are unaffected by such lesions (138). Cells in the somatosensory thalamus also exhibit task-dependent modulation from rest to locomotion, with higher discharge rates observed during locomotion in the rat VPL (768; 769). In contrast, responses in the VPL nucleus evoked by stimulating the skin of the contralateral forepaw were reduced from rest to locomotion. Thus, even though the discharge rate of VPL neurons increases, their responsiveness to cutaneous inputs decreases, indicating that increased neuronal discharge is mediated by inputs from other sources. Similarly, activity of neurons in ventrolateral thalamus during locomotion in intact cats cannot be fully explained by somatosensory inputs and are likely modulated in part by inputs from supraspinal sources, including the cerebellum and cerebral cortex (69).
Cerebral cortex
Thalamocortical projections relaying proprioceptive and tactile information diverge and converge on several areas of the cerebral cortex, such as the primary somatosensory cortex (S1), located in Brodmann’s areas 3b, 3a, 1 and 2 of the anterior parietal cortex, the secondary somatosensory cortex (S2), the parietal ventral area and the posterior parietal cortex (areas 5 and 7) (187; 428; 510). Because of the crossed projection from DC nuclei to the VP thalamic nucleus, the cerebral cortex receives somatosensory information from the contralateral limbs. Neurons in areas 3b and 1 mainly respond to cutaneous inputs while those in 3a mainly receive muscle proprioceptive inputs. Neurons in area 2, S2 and the parietal ventral area respond to both cutaneous and proprioceptive inputs (187; 412; 461). Extensive connections between the different somatosensory and motor cortical areas exist.
The discharge rate of neurons within S1 changes during locomotion, with most neurons having a preferred phase (140; 252; 261; 770). The activity of S1 neurons during locomotion also depends on behavioral context and task demands (139; 140). Favorov et al. (2015) showed that neurons in S1, with receptive fields in the contralateral forelimb, showed a clear phase-dependent modulation during locomotion in the cat. Although S1 neurons were highly heterogeneous in terms of response properties, overall, neurons with proximal receptive fields in the upper arm/shoulder had greater peak activity during mid-swing while neurons with more distal receptive fields displayed peak activity at the swing-to-stance transition. Interestingly, cells with receptive fields on the plantar surface of the forepaw started discharging vigorously before paw contact, consistent with anticipation of the paw making contact. This was also shown in rats (140). In other words, these S1 neurons predicted the sensory event, instead of responding to it. Fiztsimmons et al. (2009) showed that S1 neurons of the hindlimb area showed peak activity during the swing phase, particularly at the stance-to-swing transition, in rhesus macaques trained to step bipedally. Ensemble recordings from S1 neurons predicted leg kinematics and EMGs and accuracy increased with sample size. S1 recordings also predicted future motor actions.
Summary
In summary, limb and trunk afferents have dense projections within the spinal cord, diverging and converging on neuronal targets. Somatosensory afferents ascend to several supraspinal structures via multiple direct and indirect pathways, providing critical information regarding the state of the locomotor system and how the body interacts with the environment. A general feature of somatosensory processing is that neurons from the spinal cord to the cerebral cortex are broadly tuned for direction and position (91). The spinal cord circuitry encodes whole limb kinematics and precise knowledge of the position and motion of the limbs are then integrated at successively higher (or more rostral) stages of processing, such as cervical propriospinal neurons, the brainstem, the cerebellum, the thalamus and the cerebral cortex. In other words, the CNS makes use of converging inputs from muscle, joint and cutaneous afferents at spinal levels and at higher levels of processing. The spinal cord receives the most detailed information and integration at successive levels simplifies the information to make appropriate decisions about the movement and the physical environment.
Modulation and gating of somatosensory feedback during locomotion
Somatosensory feedback modulates central neuronal networks and is in turn modulated by these same neuronal circuits. The modulation of somatosensory feedback during locomotion is best exemplified by reflex modulation. A reflex is an involuntary response to a somatosensory stimulus. It is important to note that they are not stereotyped responses. Reflex responses can be reorganized in a task-dependent manner (task-dependent modulation) or change according to phase (phase-dependent modulation). Reflex responses can also display long-term or persistent modifications when exposed to prolonged training or environmental stimuli (reflex conditioning). Task- and phase-dependent reflex modulation can be considered short-term modulation, as they transiently respond to task demands, whereas reflex conditioning can be considered a long-term modulation, with changes in reflex responses occurring gradually and persisting over time. In this section, we briefly discuss the three types of reflex modulation before describing the neuronal mechanisms potentially involved.
Task-dependent modulation
The fact that reflexes are not stereotyped responses and are modulated by behavioral status has been acknowledged since around the mid-20th century (689). For instance, there is modulation during motor preparation, as the strength of reflex responses changes shortly before the movement starts (347). Reflexes are then modulated from a resting state or a standing position to a locomotor state, as shown in cats and humans (122; 221; 319; 644). However, the modulation of stretch or H-reflexes from standing to walking might be due to differences in background EMG activity, as studies showed similar stretch or H-reflex amplitudes in the two tasks at matched EMG activity backgrounds in cats and humans (582; 777). The classic example of task-dependent modulation is the reversal of Ib inhibition at rest to excitation during locomotion in hindlimb extensors of acute or chronic spinal decerebrate cats during treadmill locomotion (644) and in decerebrate curarized cats during fictive locomotion (319). This task-dependent reflex reversal is thought to reinforce extensor activity during stance, as discussed later on.
Another example of task-dependent modulation is found with an increase in speed or a change in gait. Studies have shown modulation of the soleus H-reflex from walking to running in humans, with one study showing a reduction (123) while another showed an increase (775). The main difference between the two studies concerns methodology, with the latter correcting for movement of the recording and stimulating electrodes, which can profoundly impact surface recordings (278). Cutaneous reflexes from the foot are also modulated with increasing speed in intact and spinal cats (406; 407) and during human locomotion (222).
A change in the direction of stepping also alters reflex modulation. For instance, stimulating the foot dorsum during the swing phase of forward locomotion in cats and humans produces the stumbling corrective reaction, which initially involves activation of knee flexors, as well as ankle and hip extensors to move the leg away from and over the stimulus or obstacle (110; 265; 742). During backward locomotion, however, similar muscle activations would not produce the desired effect. As such, when the plantar surface of the foot makes contact with an object during the swing phase of backward locomotion, hip and ankle flexors are activated while knee flexor activity is suppressed to move the leg forward and up to step over the obstacle (110). Using electrical stimuli, studies showed different patterns of reflex modulation during forward and backward locomotion (110; 220).
Split-belt locomotion, which simulates features of stepping along a circular path, also modulates cutaneous reflexes when compared to tied-belt locomotion (406). In this study, split-belt locomotion significantly reduced reflex modulation (from minimum to maximum responses across the locomotor cycle) in all ipsilateral and contralateral muscles of intact and spinal cats compared with tied-belt locomotion at matched speeds, independently of which limb was stepping on the slow or fast belt. The authors proposed that asymmetric sensory feedback from the left and right legs altered the state of the spinal network, thereby reducing cutaneous reflexes to prevent somatosensory inputs from destabilizing the pattern. Therefore, it is clear from these studies that task, or behavioral context, modulates reflexes to meet functional demands.
Task-dependent modulation of responses to muscle and cutaneous afferent inputs has also been shown during fictive locomotion in curarized decerebrate cats (186; 282). For instance, Degtyarenko et al. (1998) showed a reduction in cutaneous reflexes when going from a fictive locomotor rhythm to a scratch one. Frigon and Gossard (2010) showed that stimulating plantaris muscle group I afferents reset the fictive locomotor rhythm from flexion to extension but had no effect on the scratch rhythm. Similarly, tonic dorsiflexion of the ankle prolonged extensor bursts and cycle duration during fictive locomotion (Fig. 8A) but had no effect on the scratch cycle (Fig. 8B), although it did slightly prolong extensor bursts. However, another study showed that tonic electrical stimulation of ankle extensor group I afferents increased the frequency of the scratch rhythm, with a transient increase in extensor burst duration and a larger continuous decrease in flexor burst duration (655). Moreover, electrical stimulation could reset the scratch rhythm from flexion to extension, although the resetting was less abrupt compared to fictive locomotion. The different findings of these two studies can be explained by differences in stimulation parameters and the level of decerebration (pre-collicular versus post-collicular). It is likely that the locomotor and scratch networks have common and specialized mechanisms to modulate somatosensory inputs.
Figure 8. Task-dependent modulation of somatosensory feedback.
Effects of manually dorsiflexing the ankle (~5°) during spontaneous A) fictive locomotion and B) fictive scratching in a decerebrate curarized cat. Figure shows activity from extensor and flexor nerves. EDL, extensor digitorum longus; MG, medial gastrocnemius; SmAB, semimembranosus-anterior biceps; TA, tibialis anterior. Modified from (282).
Phase-dependent modulation
It is well known that reflexes are modulated with phase during locomotion. For example, the effect of cutaneous afferent stimulation on muscle activity during locomotion shows a reversal from swing to stance (218; 761). In spontaneously stepping thalamic cats, stimulating cutaneous afferents from the plantar surface of the foot increased the ongoing extensor muscle activity during stance whereas the same stimulus during swing prolonged flexor activity (218). Several studies in cats (intact, decerebrate and spinal), rodents and humans have confirmed the modulation of cutaneous reflexes in limb muscles across the locomotor cycle, including reflex reversals from excitatory to inhibitory responses (2; 51; 217; 268; 269; 285; 408; 552; 616; 672; 829; 887). The classic functional example is the stumbling corrective reaction with mechanical or electrical stimulation of the foot dorsum during swing, which lifts the limb away and up over the obstacle (265). The same stimulation during stance does not lift the limb, instead flexor and extensor muscles are co-activated to increase limb stiffness, a response termed the stumbling preventive correction. We describe these responses and the neuronal circuits involved in more detail later on.
Phase-dependency can be controlled at a spinal level, as several studies have shown phase-dependent modulation of cutaneous reflexes in spinal cats during treadmill or fictive locomotion (268; 269; 285; 286; 406; 407; 473). Figure 9 shows phase-dependent modulation of cutaneous reflexes evoked by stimulating the superficial peroneal (SP) nerve during treadmill locomotion at 0.4 m/s in the ipsilateral semitendinosus, vastus lateralis and lateral gastrocnemius muscles. In the semitendinosus, short- and longer-latency excitatory responses, or P1 and P2, respectively, are independently modulated across the locomotor cycle (Fig. 9A). In ipsilateral extensors, such as vastus lateralis, short-latency inhibitory responses, or N1, are frequent during the muscle’s period of activity (Fig. 9B). It should be noted that some differences exist between reflex responses in the intact and spinal cat, such as the reduction of P2 responses and the appearance of short-latency excitatory responses in ankle extensors during stance, as opposed to short-latency inhibition in intact cats (Fig. 9C) (285). The appearance of these responses reflects functional changes in spinal neuronal circuits, the loss of supraspinal inputs and their interactions with somatosensory feedback after spinal transection.
Figure 9. Phase-dependent modulation of cutaneous reflexes.
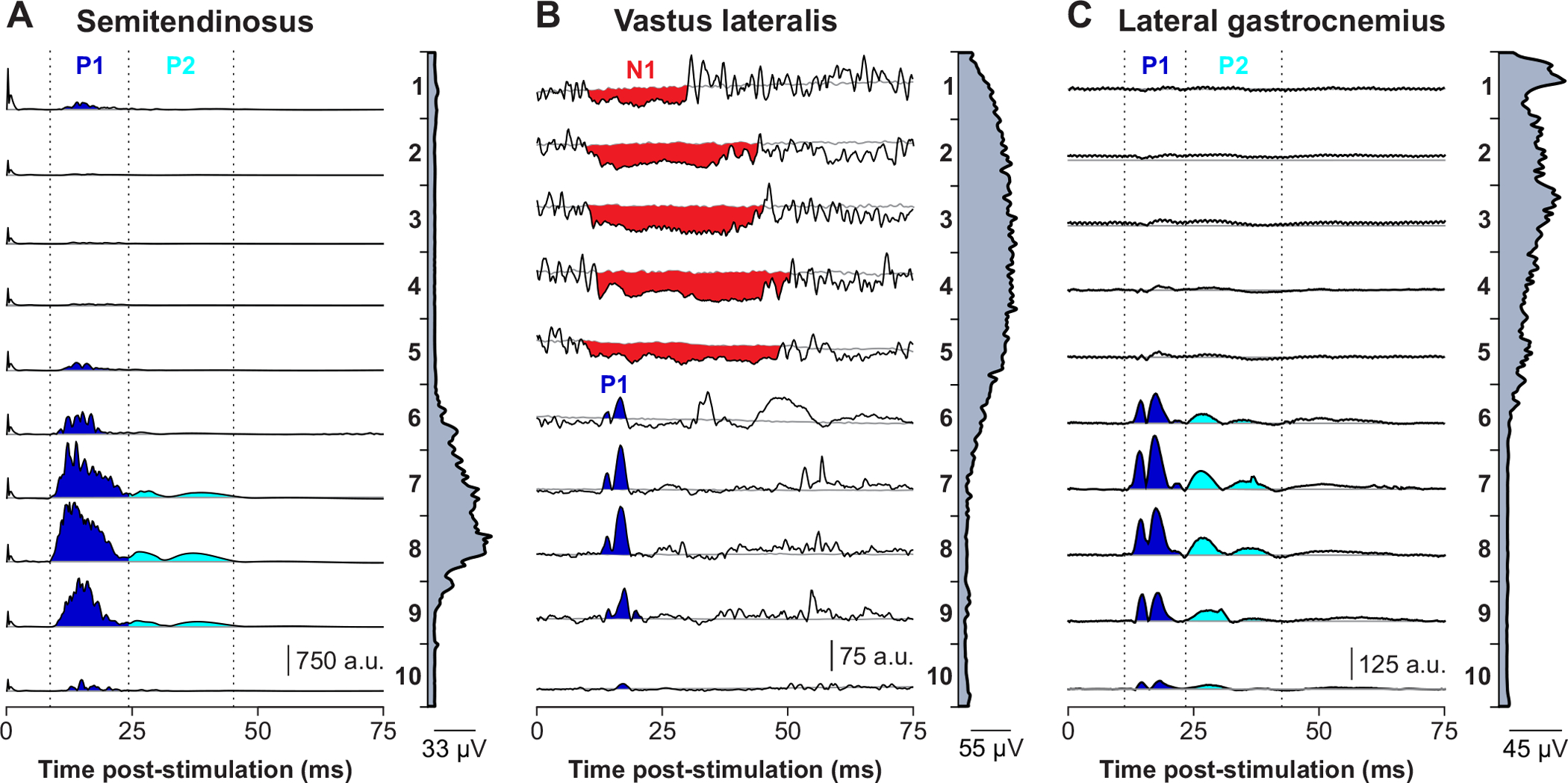
Phase-and speed-dependent modulation of cutaneous reflexes evoked by electrically stimulating the superficial peroneal nerve (single 0.2 ms pulse at 1.2 times the motor threshold) in A) semitendinosus, B) vastus lateralis and C) lateral gastrocnemius at a treadmill speed of 0.4 m/s in a spinal cat. Cutaneous reflexes are separated into 10 bins. Rectified EMG waveforms obtained with stimulation are separated into 10 bins (average of 5–17 cycles per bin). The red lines show the background level of EMG in each bin (average of ~90 control cycles). The EMG waveform shown vertically on the right of each panel is the rectified activity of the muscle across the normalized cycle. Modified from (407).
Stretch or H-reflexes are also modulated with phase during animal and human locomotion. In humans and cats, stretch and H-reflexes in triceps surae muscles peak in amplitude during the stance phase and are suppressed or reduced during swing (11; 122; 123; 211; 582; 775; 777).
Studies have also shown phase-dependent modulation of reflex responses, EPSPs and IPSPs, evoked by stimulating group I/II muscle or cutaneous afferents, during fictive locomotion in curarized decerebrate cats, with intact or transected spinal cords (33; 318; 473; 560; 703–705; 745; 759). The modulation of responses in spinal cats during fictive locomotion is consistent with a role of the spinal locomotor CPG in reflex modulation. Yamaguchi and colleagues have also identified reflex modulation in the forelimb during fictive locomotion in curarized decerebrate cats (378; 752; 877). Figure 10 shows phase dependent modulation of post-synaptic potentials in a hip extensor motoneuron evoked by stimulating the plantaris and sartorius nerves at group I strength (1.8–2.0 T) during an episode of spontaneous fictive locomotion in a curarized decerebrate cat. As can be seen, stimulating the sartorius nerve evokes an IPSP followed by an EPSP during flexion whereas only an IPSP is observed during extension. Stimulation of the plantaris nerve evokes EPSPs during both flexion and extension with a larger amplitude in flexion.
Figure 10. Phase-dependent modulation of post-synaptic potentials evoked by stimulating extensor and flexor muscle afferents at group I strength during fictive locomotion.
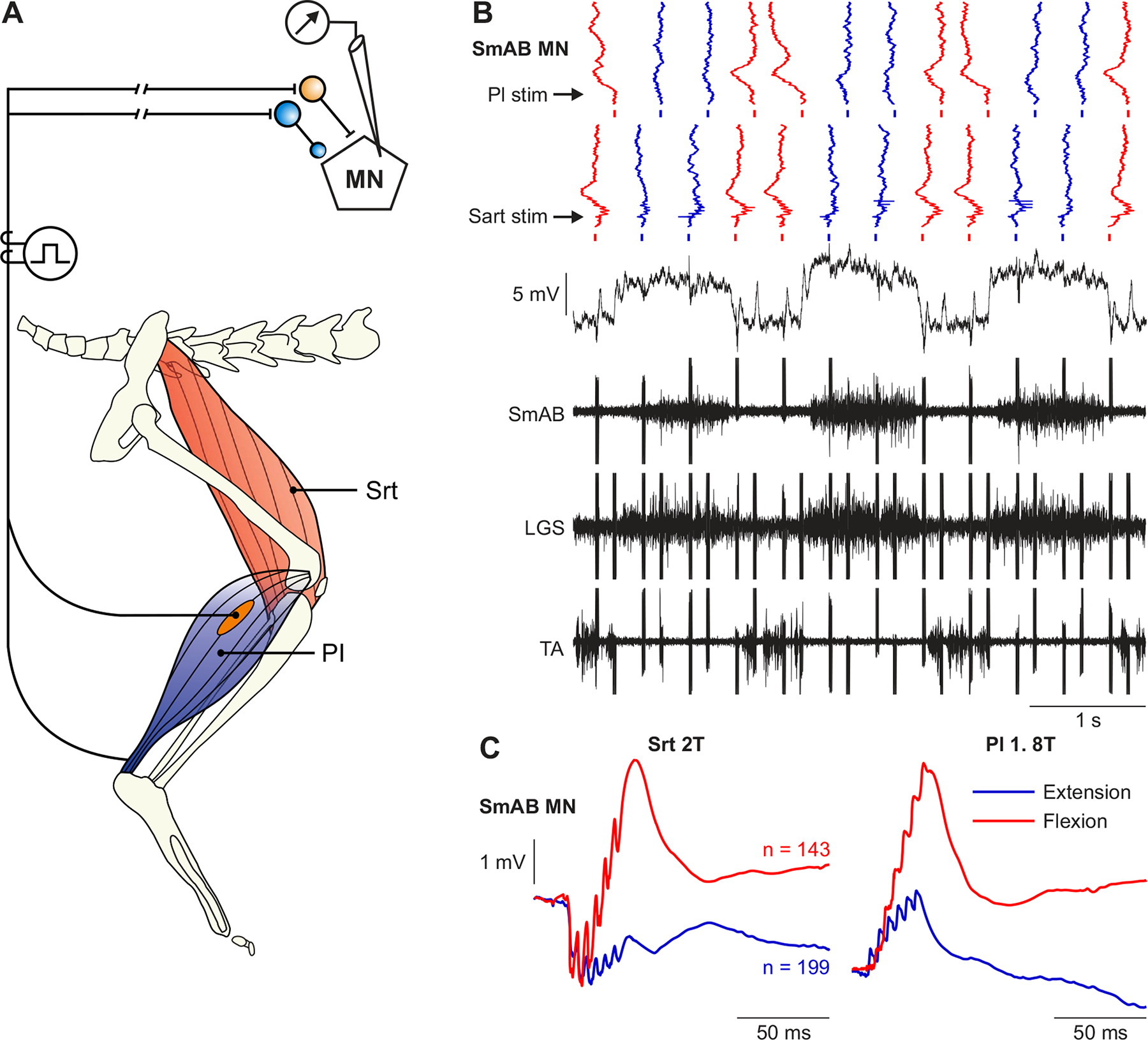
A) Nerves to plantaris (Pl) and sartorius (Srt) muscles were stimulated at group I strength during spontaneous fictive locomotion in a decerebrate curarized cats. Intracellular recordings from antidromically-identified motoneurons were made with glass micropipettes filled with QX-314. The type (i.e. inhibitory or excitatory) of last-order interneuron activated within a given reflex pathway can be inferred by the sign of the post-synaptic potential recorded in the motoneuron. B) During a fictive locomotor episode, the 2 nerves were stimulated with high frequency and short trains (6 pulses at 200 Hz) in alternation, with an interval of 150–250 ms between nerve stimulations. From top to bottom: Evoked responses tilted 90 degrees evoked by Srt and Pl nerve stimulation given in alternation; intracellular membrane potential oscillations in the SmAB motoneuron showing the locomotor-drive potential and superimposed evoked responses; ENGs from extensors, semimembranosus-anterior biceps (SmAB) and lateral gastrocnemius-soleus (LGS) and from a flexor, tibialis anterior (TA). The locomotor cycle was divided into extension and flexion phases according to the extensor ENG onset and offsets. C) Post-synaptic potentials evoked by stimulating Srt and Pl nerves were divided and averaged into extension and flexion phases. Data were recorded in Jean-Pierre Gossard’s lab at the Université de Montréal.
Reflex conditioning
Experiments in various mammalian species, including humans, have shown modification of spinal reflex pathways in response to prolonged stimulation, exposure to repeated experiences and training, which parallel modifications in the locomotor pattern (326; 865). For example, professional ballet dancers have small or absent stretch and H-reflexes in ankle plantarflexors compared to age-matched trained athletes and untrained individuals (312; 607). Nielsen et al. (1993) attributed smaller H-reflexes to the frequent use of co-contractions of flexors and extensors to maintain balance during ballet postures. A study later showed 30 min of training consisting of co-activating ankle flexors and extensors reduced soleus H-reflexes (652). Thus, co-contractions performed by professional ballet dancers over several years potentially lead to permanent changes in stretch and H-reflex pathways. Interestingly, professional ballet dancers also adopt a distinctive walking pattern after years of rigorous training, indicating that prolonged training affects other sensorimotor circuits in parallel (865).
The work by Jonathan Wolpaw and colleagues on operant conditioning in rodents, monkeys and humans clearly established that spinal reflexes undergo gradual and persistent increases (up-conditioning) or decreases (down-conditioning) in amplitude in response to a reward (129; 144; 807; 866). The up- or down-conditioning persists after spinal transection, indicating changes within spinal circuits. Operant conditioning requires neuronal plasticity, which is distributed throughout the brain and spinal cord, involving the corticospinal tract, inferior olive and cerebellum, as well as changes in motoneuron properties, synaptic inputs and spinal interneurons (864; 865; 867). Moreover, operant conditioning of spinal reflexes can be used to alter the locomotor pattern in the intact state and following an incomplete spinal cord injury, as shown in rats and humans (145; 808; 809). For instance, up-conditioning of the soleus H-reflex in rats increased the EMG burst amplitude of the soleus during locomotion, without affecting other spatiotemporal variables (146). Following a unilateral mid-thoracic lateral hemisection on the right side, Chen et al. (2006) observed an asymmetry in the phasing of soleus bursts in the left and right hindlimbs during locomotion in rats. Up-conditioning of the right soleus H-reflex increased the EMG burst in this muscle and restored symmetry in the phasing. Interestingly, in humans, down-conditioning of the soleus H-reflex improves locomotion, by restoring left-right symmetry, increasing gait speed, and improving balance, possibly by reducing spasticity and its gait impairing effects (808).
While successful reward-based operant conditioning of spinal reflexes requires signals from the brain, spinal reflexes can also be conditioned in spinal-transected animals. For example, studies have shown classical conditioning of hindlimb flexion reflexes in spinal cats, or tail flick responses in spinal rats, by pairing conditioned (CS) and unconditioned (US) stimuli (212–214; 327; 414; 637). In these studies, the CS (e.g. saphenous nerve stimulation) elicits weak flexion reflexes while the US (e.g. SP nerve stimulation) evokes strong flexion reflexes. In the conditioning period, CS and US are paired, producing large flexion reflexes. After this conditioning, CS presented alone generate flexion reflexes that remain large, consistent with retention of the conditioned response in spinal circuits. Over time, flexion reflexes evoked by CS-alone return to pre-conditioning values, a phenomenon termed extinction. In other words, somatosensory inputs can leave a memory trace within the spinal cord and alter its function without inputs from the brain.
Therefore, it is clear that the spinal cord and the neuronal circuits that integrate, modulate and transmit somatosensory inputs to motoneurons are plastic, with the potential to modify spinal reflexes and motor behaviors, including locomotion, before and after spinal cord injury.
Gating/modulatory mechanisms
In the three previous sections, we described reflex modulation during locomotion in the short- and long-term. What potential mechanisms modulate somatosensory feedback during locomotion as a function of task and phase and in the long-term with prolonged training or conditioning? As discussed below, the modulation of spinal reflexes can occur at several sites and through various mechanisms.
Gating starts in the periphery
First, we need to consider that mechanoreceptors located in the periphery have modulatory properties that ultimately determine if afferents reach firing threshold and transmit action potentials to the spinal cord. We refer to this as peripheral gating, as opposed to central gating, which occurs within the CNS. In the late 1960s, it was discovered that cat spindle afferent endings have ‘synaptic-like vesicles’, suggesting a purely peripheral modulation of proprioceptive afferents (5). It was later discovered that these synaptic-like vesicles undergo activity-dependent recycling and contain glutamate (56) as well as the vesicular glutamate transporter 1 (VGLUT1) protein (871), suggesting that glutamate release alters the sensitivity of spindle afferents in the periphery (80). Indeed, glutamate released by spindle afferent endings increases the firing rate of the afferents themselves to a given stretch, or in other words, spindle afferent possess a positive feedback loop (79). This positive feedback is countered by the activation of Ca2+ and K+(Ca) ion channels (79). The functional role of this peripheral modulation of spindle afferents remains unclear for locomotion and movement in general. As discussed earlier, the sensitivity of spindle afferents is also regulated by γ- and β-motoneurons, which are controlled by central mechanisms and are rhythmically active during locomotion (245). Thus, spindle afferents, before releasing neurotransmitters at their synaptic terminal in the spinal cord, undergo complex modulation in the periphery.
Gating by presynaptic inhibition
Somatosensory afferents terminate in various laminae of the spinal cord, where they release neurotransmitters, primarily glutamate. The presynaptic release of neurotransmitters can be decreased or inhibited via GABAergic interneurons that make axo-axonic contacts with sensory afferents, a process termed presynaptic inhibition, first demonstrated in the cat spinal cord by Eccles (227; 229). These GABAergic interneurons form highly specific synapses on sensory terminals and express GAD2 (also known as GAD65), one of two enzymes that synthesize GABA (78; 259; 401). Because the reversal potential of chloride is more depolarized than the resting potential in primary afferents, the activation of GABAA receptors produces a net efflux of Cl- ions that depolarizes the membrane, a phenomenon termed primary afferent depolarization (PAD) (232; 718; 725). The backward propagating, or antidromic, potential is thought to collide with the incoming, or orthodromic, input from the periphery, thus inhibiting or shunting the sensory response.
Presynaptic inhibition of somatosensory afferent terminals, inferred by measuring PAD, is modulated during locomotion with task and phase [reviewed in (718)]. For instance, antidromic spike numbers in cut dorsal rootlets increase from rest to fictive locomotion but are reduced during fictive scratch in curarized decerebrate cats (169). The increase in PAD pathway transmission from rest to locomotion is consistent with the generalized decrease in somatosensory transmission observed during fictive locomotion (315; 657). Studies in curarized decerebrate cats during fictive locomotion have also shown phase-dependent modulation of PAD in individual muscle (322; 567) and cutaneous (320; 321) afferents. Recordings of antidromic discharges in dorsal roots were also made during real locomotion in cats and rats, with some units discharging tonically and others rhythmically (68; 210; 673)
Pathways transmitting PAD are modulated by different sources during locomotor activity, including from muscle and cutaneous afferents (318; 323; 558; 567; 568), the spinal locomotor CPG (718) and supraspinal inputs (723; 724; 726; 778). Sirois et al. (2013) showed that cutaneous and muscle afferents converge on common PAD interneurons, whereas reticulospinal inputs use PAD pathways different from those of somatosensory afferents. Additionally, different collaterals from the same afferent can be controlled by different PAD interneurons, as shown in anesthetized cats (508). The release of monoamines, such as 5-HT, NA and dopamine in the spinal cord also modulates presynaptic inhibition at the afferent terminal (297). These complex interactions modulate presynaptic inhibition and hence the influence of somatosensory feedback on central locomotor networks. Figure 11 schematically illustrates the modulation of PAD pathways by synaptic inputs from group I afferents and a CPG neuron that converge on the same GABAergic interneuron and from a reticulospinal neuron that affects PAD through an independent pathway.
Figure 11. Presynaptic inhibition through primary afferent depolarization.
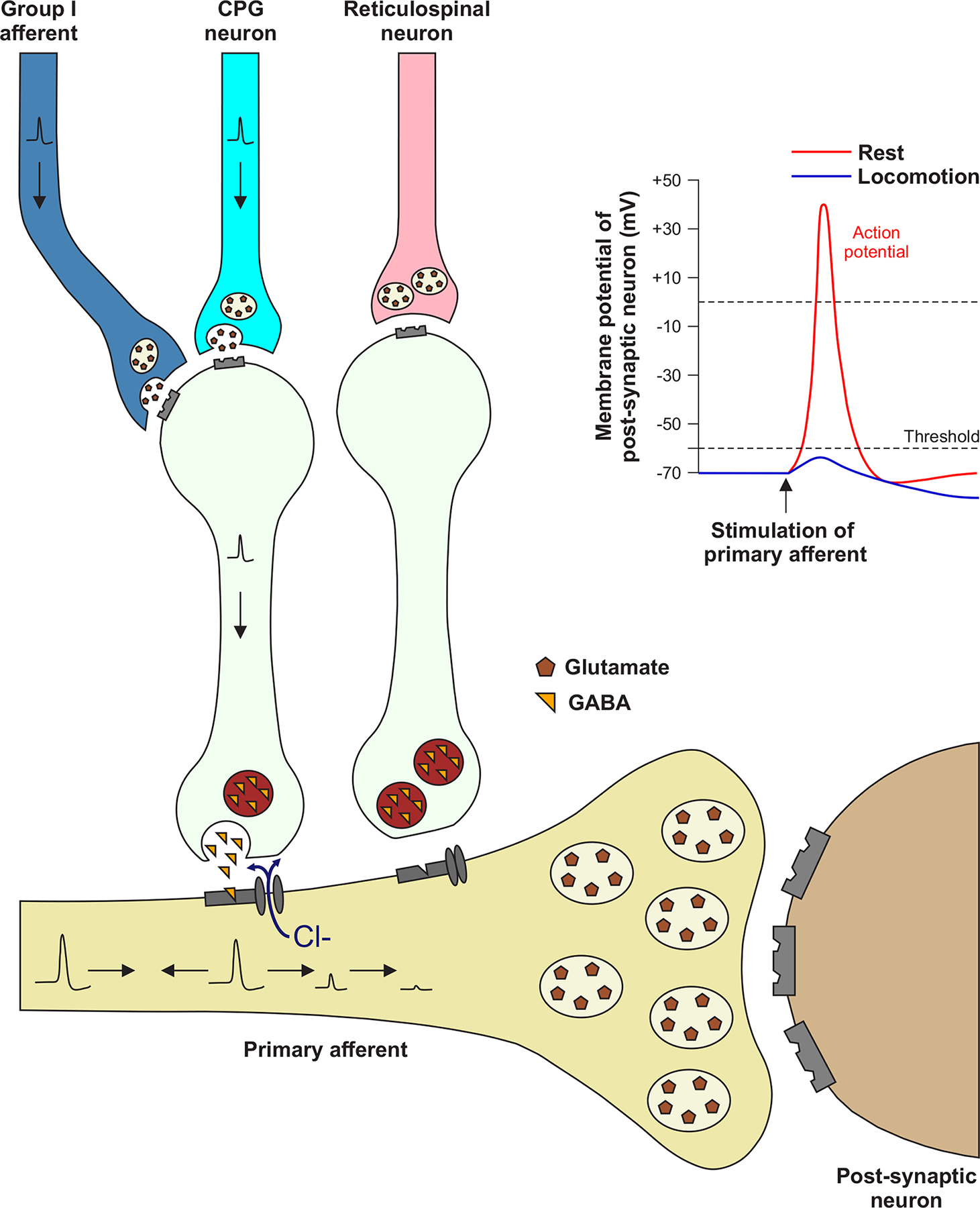
The figure shows synaptic contact between a primary afferent and a post-synaptic neuron. Two GABAergic interneurons make axo-axonic contacts on the primary afferent. One GABAergic interneuron receives converging glutamatergic inputs from a group I afferent and from a CPG neuron while the other GABAergic interneuron receives input from a reticulospinal neuron. During locomotion, the group I afferent and CPG neuron depolarize their target GABAergic interneuron, leading to the release of GABA. GABA binds to GABAA ionotropic receptors on the primary afferent and opens Cl- channels. Cl- exits the primary afferent leading to a depolarization (PAD, primary afferent depolarization) and a electrotonic potential that travels in both directions. The antidromic PAD collides with the action potential coming from the periphery, reducing the orthodromic response and preventing release of glutamate at the primary afferent terminal. The electrotonic potential travelling in the orthodromic direction weakens in magnitude the further it travels and as it reaches the terminal, it is too weak to affect transmitter release.
The modulation of presynaptic inhibition has functional consequences. Recently, a study showed that disrupting interneurons that control the presynaptic inhibition of somatosensory afferents impaired forelimb reaching movements in mice (259). Genetic ablation of GAD2-expressing interneurons in the cervical/upper thoracic spinal cord severely reduced reaching accuracy, with erratic forepaw trajectories and oscillations. However, no changes were observed during skilled locomotion on a horizontal ladder, indicating a task-dependent role of presynaptic inhibition. Thus, the functional role of presynaptic inhibition during locomotion remains unclear and will need to be tested in a range of locomotor behaviors, including with sensory perturbations, and with genetic manipulation of presynaptic inhibition in the hindlimbs as well.
Gating by selecting and modulating spinal neurons
The activity of spinal neurons determines if somatosensory inputs influence motor behaviors. Spinal neuronal activity is regulated by the spinal locomotor CPG, somatosensory feedback, supraspinal inputs and the release of neuromodulators, such as monoamines, that activate intrinsic membrane properties. Aside from monosynaptic connections between spindle afferents and spinal motoneurons, all other spinal reflex pathways involve at least one interposed interneuron. Thus, changing the excitability of spinal motoneurons, and in most cases spinal interneurons, directly affects spinal reflexes. During locomotion, some spinal neurons generate rhythmic excitatory and inhibitory patterns of activity (e.g. those of the CPG) while others receive these rhythmic excitatory and inhibitory drives (107; 450; 758). If an excitatory somatosensory input reaches a neuron while it is hyperpolarized, the probability of it reaching firing threshold is low. On the other hand, if the target neuron is near firing threshold or currently discharging, excitatory somatosensory inputs will increase the likelihood that it will discharge or increase its firing rate. Studies in decerebrate cats and neonatal rats have shown that populations of spinal interneurons discharge rhythmically during locomotion but their peak phase of activity occurs at different times in the cycle (119; 599; 621; 877). If one population is active only during flexion while another is active during extension or at phase transitions, then somatosensory inputs reaching these populations can only affect the locomotor pattern at these times.
As described earlier, somatosensory feedback projects to neurons at different spinal segments and either directly or indirectly to various supraspinal structures. Gating of somatosensory feedback occurs at all levels and these structures in turn project to spinal neurons that receive and integrate cutaneous, muscle and joint afferent inputs. Brainstem pathways not only provide the excitatory glutamatergic drive necessary for the voluntary activation of the spinal locomotor CPG, they also release neuromodulators, such as serotonin or noradrenaline, which affect transmission in reflex pathways. For example, the activation of lamina VIII commissural interneurons by group II afferents is facilitated by serotonin but reduced by noradrenaline in the cat spinal cord (351).
Figure 12 illustrates some of the mechanisms and interactions modulating inputs from an ankle extensor group Ib afferent. As it enters the spinal cord, the Ib afferent makes synaptic contacts with neurons of the DSCT, the spinal locomotor CPG as well as inhibitory and excitatory last-order interneurons that project to ankle extensor motoneurons. The Ib afferent also ascends and terminates in brainstem nuclei that transmit this information to the cerebellum, thalamus and cerebral cortex. At rest, the disynaptic inhibitory pathway is open and the excitatory pathway is inhibited. However, during locomotion, the spinal CPG inhibits the inhibitory pathway and releases the excitatory pathway from inhibition (disinhibition). At the same time, various supraspinal structures interact dynamically with each other and with spinal circuits, such as the spinal CPG and local reflex circuits. These dynamic interactions ensure that group Ib inputs can reinforce extensor activity during stance.
Figure 12. Modulation of somatosensory feedback and its interactions with central locomotor networks.
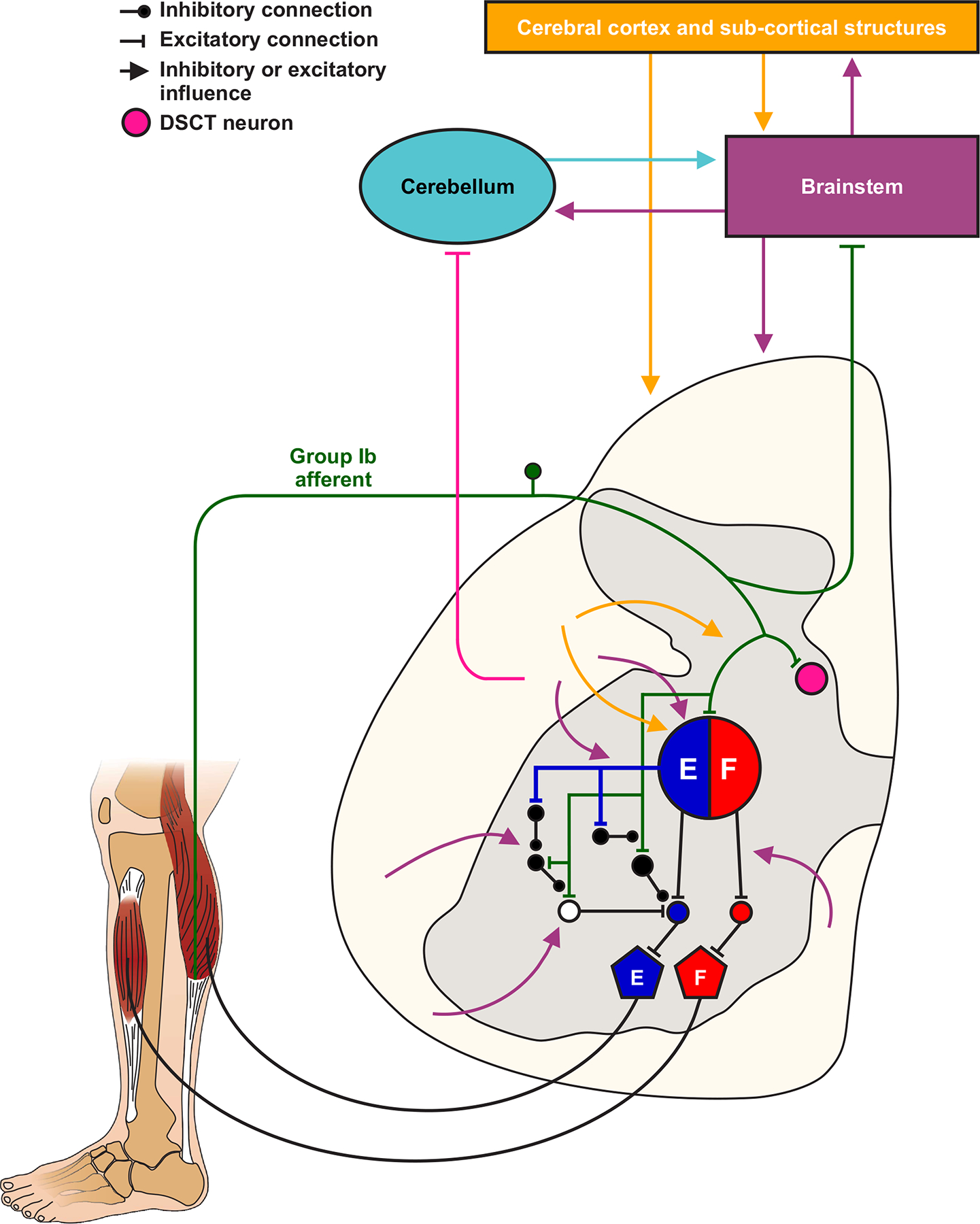
The figure shows potential mechanisms and interactions modulating inputs from a group Ib afferent from an ankle extensor. Upon entering the spinal cord, the Ib afferent makes synaptic contacts with neurons of the dorsal spinocerebellar tract (DSCT), the spinal locomotor CPG, represented as extensor and flexor half-centers, as well as inhibitory and excitatory last-order interneurons that project to ankle extensor motoneurons. The Ib afferent also ascends to brainstem nuclei that transmit the information to the cerebellum, thalamus and cerebral cortex. At rest, the disynaptic inhibitory pathway is open and the excitatory pathway is inhibited. During locomotion, the spinal CPG inhibits the inhibitory pathway and releases the excitatory pathway from inhibition through disinhibition. At the same time, various supraspinal structures interact dynamically with each other and with spinal circuits, such as the spinal CPG and local reflex circuits.
Long-term changes in reflex pathways observed with conditioning or training can occur by changing the synaptic strength of CNS neurons involved in integrating and transmitting somatosensory feedback or in their modulation. This can occur throughout the CNS, starting within the spinal cord, which can support both long-term potentiation and depression (737; 738). Training also modifies pathways mediating synaptic inhibition. For example, successful down-conditioning of the soleus H-reflex in rats involves an increase in the number, size and density of GABAergic terminals on soleus motoneurons (842). It also increases the number of GABAergic interneurons in the ventral horn (843). In contrast, successful up-conditioning of the soleus H-reflex does not change the number of GABAergic terminals with slight increases in terminal axonal diameter and soma coverage (674). Instead, up-conditioning seems to involve changes in F-terminals (inhibitory synapses) and C-boutons, which are large cholinergic synapses on motoneurons (256). These studies indicate that increasing or decreasing the gain of a reflex does not necessarily involve the same plasticity mechanisms within the spinal cord and that a complex interplay is at work.
Experimental approaches to investigate the role of somatosensory feedback during locomotion
In the previous sections, we provided an overview of somatosensory receptors and afferents, their activity during locomotion and the potential mechanisms involved in modulating somatosensory inputs. In the next half of the review, we will discuss the functional roles of somatosensory feedback during locomotion. Before that, we describe the approaches and animal models to study the functional roles of somatosensory feedback during movement and discuss their advantages and limitations.
Mechanical stimulation
Arguably, the simplest approach to study the role of somatosensory feedback during locomotion is to activate the mechanoreceptors that transmit tactile and proprioceptive information. We can do this by applying contact or pressure to a body part, stretching a muscle, inducing or stopping joint movement with different levels of resistance, increasing or decreasing load and by applying vibration. The main advantage of mechanical stimulation in experimental studies is that the stimulus is natural, closely mimicking real world situations. However, there are limitations. Mechanical stimulation activates different types of mechanoreceptors and afferents to varying degrees depending on the nature of the stimulus and the force with which it is applied, making it difficult to precisely control. In this regard, animal preparations are particularly useful because mechanical stimulation can be combined with other procedures to isolate and identify the type of receptors and afferents activated.
Vibration is an interesting approach to investigate the role of somatosensory feedback because different mechanoreceptors preferentially respond to certain stimulus parameters, such as frequency and amplitude. For example, in the skin, afferents from SA1 (Merkel discs), RA1 (Meissner’s corpuscles) and RA2 (Pacinian corpuscles) mechanoreceptors have their peak sensitivity around 5 Hz, 40 Hz and 250 Hz, respectively (187; 271; 272; 593; 732; 804). Afferent fibers from primary muscle spindles are extremely sensitive to vibration whereas those from secondary spindles are generally insensitive, as are those from Golgi tendon organs at rest (105). The main disadvantage is that vibration activates different types of mechanoreceptors concurrently, as they respond to overlapping frequency and amplitude ranges. The effects of vibration also depend on muscle contraction. In a resting muscle, vibration mainly increases the discharge of primary spindle afferents but when the muscle is contracting, afferents from Golgi tendon organs also discharge (105; 699). Thus, using vibration to investigate the role of specific types of somatosensory feedback, particularly in dynamic conditions where muscle length and force changes, has limitations.
Electrical stimulation
Electrically stimulating dorsal roots and cutaneous nerves evokes a pure sensory volley while stimulating a muscle nerve activates both sensory and motor axons. However, because of the larger axonal diameter of group Ia afferents, which are recruited first by external electrical currents, it is possible to obtain a sensory-evoked response, the H-reflex, before the appearance of the motor-response, the M-wave (580; 885). The H-reflex is often described as monosynaptic group Ia afferent activation of the motoneuron pool but several types of afferents, with oligo- or polysynaptic connections, contribute to the response (115; 116). The idea of a purely ‘monosynaptic’ reflex response between Ia afferents and agonist motoneurons is likely inaccurate, as group Ia afferents diverge upon entering the spinal cord, making synaptic contacts with multiple neuronal targets and many types of afferents converge on spinal interneurons that project to motoneurons (433; 435).
The main advantage of electrical stimulation is that the stimulus is highly reproducible and parameters, such as pulse duration, frequency and intensity can be accurately controlled. This facilitates the assessment of task- and phase-dependent modulation of reflex responses. In animal studies, chronic implantations of electrodes, placed around nerves using cuffs and sown into muscles, allow for stable stimulations and recordings across several days or months. This can be used to assess reflex changes before and over time after neurological injury in the same animal (277; 284; 285). The precise timing of electrical stimulation also allows determining response latency and inferring the number of synapses in the pathway, particularly in animal studies with intracellular recordings.
There are disadvantages to electrical nerve stimulation. First, afferents are recruited from the largest to the smallest. For example, the effects of smaller axonal diameter group II afferents are only observed after recruiting larger group I afferents. Second, with electrical stimulation, multiple sensory axons are activated synchronously, which is not how afferents are normally activated during locomotion. Third, in humans, electrodes are generally placed on the surface of the skin to stimulate nerves and record from muscles. In dynamic conditions, such as locomotion, movement of the nerve and muscle underneath the electrodes changes the population of afferents activated and muscle fibers recorded (278; 775). This is not a major concern for assessing burst durations or response timing, but it is a challenge when assessing amplitude changes in different phases and tasks, and even more so across days. As such, locomotor studies in humans using surface electrodes need to be carefully designed and results interpreted with caution.
Surgical lesions and repair
Another simple approach to study the role of somatosensory feedback is to remove it surgically, by sectioning dorsal roots before they enter the spinal cord or cutaneous nerves in the periphery. Sectioning dorsal roots, or deafferentation, removes somatosensory feedback at specific spinal segments (106; 308; 309; 345; 849). Although deafferentation does not disrupt the motor innervation, which exits the spinal cord via ventral roots, it lacks specificity, as it removes all types of afferents, often from several points of origin.
Although sectioning a cutaneous nerve, or denervation, is more specific, here again, all types of skin afferents within the nerve are disrupted. Thus, the relative contribution from different types of cutaneous receptors or afferents cannot be studied separately. In contrast to cutaneous denervations, sectioning muscle nerves is not amenable to specifically study the role of somatosensory feedback because it also removes the motor component, producing an immediate motor impairment. To circumvent this issue, investigators have used muscle self-reinnervation where the muscle nerve is cut and immediately reattached (161; 311). Studies have shown that the output from spinal motoneurons recovers while some aspects of muscle afferent inputs do not, inferred by the loss of the stretch reflex (167; 168; 519). Self-reinnervation studies have shown that the partially recovered afferent inputs (161; 348; 569) are blocked centrally by retraction of glutamatergic synapses (VGLUT1) on α-motoneurons in the ventral horn (23; 24; 112). In other words, the sensory volley enters the spinal cord but it does not reach the motoneuron pools.
An important limitation of deafferentation, denervation or self-reinnervation studies is that compensatory changes occur rapidly and in the long-term, thus confounding or underestimating the role of lesioned afferents. Animals might make locomotor adjustments (e.g. shift their weight or make other postural adjustments, co-activate antagonists, etc.) to palliate the sensorimotor deficits and central neuronal circuits can be rapidly reorganized due to the removal of inhibitory and excitatory connections.
Studies have also applied or injected anesthetic agents on the skin or around a nerve to block somatosensory afferent activity during locomotion [e.g. (334; 636; 841)]. The main advantage of this approach is that it can block somatosensory feedback and it is reversible. However, the completeness of the block is difficult to assess, and specific afferent types cannot be targeted.
Investigations in pathological states
The importance of somatosensory feedback in the control of movement cannot be overstated and is exemplified by several diseases or medical conditions that affect the senses of touch and proprioception to varying degrees by destroying peripheral afferents while leaving motor innervations mostly or completely intact. These sensory neuropathies include diabetes mellitus (255), Charco-Marie-Tooth disease with its most prevalent type IA form (600), certain viral infections (160; 476) and some genetic mutations (147). Depending on the pathology and its severity, proprioception and touch can be abolished while leaving the sensations of pain and temperature generally intact. Pathologies produce varying reductions in proprioception and touch because they often destroy peripheral afferents of different sizes. For instance, Charco-Marie-Tooth disease type IA mainly affects large caliber afferents while diabetic sensory neuropathies also destroy smaller afferents (741). Sensory neuropathies generally proceed from distal to proximal limb segments (494). As such, tactile sensation from the foot sole and proprioception from muscles controlling the ankle joint are affected first and to a greater degree. People with sensory neuropathies tend to walk more slowly, with a wider step width, shorter step length and longer double support phases compared to healthy controls (600). Indeed, step width is an often-used outcome measure in animal models of neurotrauma and disease. Thus, when working with clinical populations, a detailed neurophysiological and functional evaluation is needed on an individual basis to assess remaining sensations and sensorimotor functions.
In very rare cases, humans can be completely deprived of tactile and proprioceptive information, as seen in the 1998 documentary ‘The Man Who Lost His Body’ by the British Broadcasting Corporation. In these individuals, where a viral infection triggers an auto-immune response that permanently destroys large sensory myelinated afferents from the limbs and trunk, abolishing proprioception and light touch, movement is severely impaired and the ability to stand and walk is initially lost (160; 476). Rare instances of recovery require consciously planning every movement and months of intense rehabilitation to restore even the most basic movements, such as sitting up from a supine position. The automaticity and fluidity of movement are permanently lost, highlighting the essential role that somatosensory feedback normally contributes for initiating, coordinating and controlling movements.
Human clinical populations are important to better understand human motor control. Another advantage is that humans can perform various motor tasks that animals cannot and provide verbal feedback of what they are perceiving. However, there are several limitations in addition to those with human experimentation in general. Pathologies are rarely selective in terms of the peripheral afferents destroyed and motor innervations are often impaired to some degree, causing muscle weakness. Some damage may also occur to the central nervous system, impairing vision, vestibular and other sensorimotor functions (671; 827). Because clinical populations are highly heterogeneous in terms of damaged systems, age, pathological severity and level of functionality, results are often difficult to interpret and generalize. As with animal models, humans can adopt various strategies to compensate for sensorimotor loss and neuroplastic changes can occur in the short- and long-term, obscuring the role of somatosensory feedback.
Extracellular recordings
Electrodes placed on or around DRG or fibers have been used to record the electrical activity of somatosensory afferents during locomotion, first in decerebrate cats (658; 659; 756) and then in freely behaving intact cats (500; 502; 503; 697; 698; 846). In intact cats, the approach consists of exposing the DRG or dorsal roots at a specific spinal segment and implanting a floating electrode array that can sample from a few units (502; 697; 846). Recording somatosensory afferents provides a window into their behavior during locomotion and how their discharge characteristics inform the CNS of ongoing movements and external perturbations. The main disadvantage of these experiments is that they are technically challenging in terms of obtaining stable recordings and unambiguously identifying the type and origin of the afferents. The number of afferents recorded per animal has generally been low, often only 1–3 afferents per cat, with a bias for afferents of larger size. However, more recent studies have considerably improved the yield per cat, with > 20 afferents recorded simultaneously (353; 845).
Intracellular recordings
To study synaptic potentials evoked by somatosensory afferents in a locomotor state, intracellular recordings can be made in neurons of the brain and spinal cord during fictive locomotion [e.g. (185; 186; 518; 703; 705; 748)]. In these experiments, nerves can be electrically stimulated, muscle-tendon units can be stretched, vibrated or palpated and joints can be manipulated to investigate how somatosensory feedback interacts with locomotor networks. Recording inhibitory (IPSPs) and excitatory (EPSPs) post-synaptic potentials in motoneurons in response to electrical nerve stimulation helps determine response latency and hence the number of synapses involved between the primary afferent terminal and the motoneuron (433). Although we can investigate the effects of specific somatosensory inputs during fictive locomotion, these are generally exaggerated compared to real locomotion because of the absence of phasic somatosensory feedback and other interactions that normally occur within spinal sensorimotor circuits during real locomotion. Achieving stable intracellular recordings during fictive locomotion is also technically challenging and the number of recorded neurons per animal can be low due to many experimental factors.
Chemical and pharmacological compounds
Somatosensory afferents can be destroyed or modulated by naturally occurring or synthetic chemical compounds. For example, pyridoxine, or Vitamin B6, at high doses selectively and permanently destroys afferents of large size, including group I afferents and the larger size group II muscle and Aβ cutaneous afferents, in various mammals, including humans (392; 469; 647; 791; 873). Cats treated with high levels of pyridoxine, are unable to stand or walk for a few days after intoxication, although they progressively recover these functions, albeit not completely, over a period of several weeks (647). Functional recovery is not due to regeneration of peripheral afferents, as a second pyridoxine intoxication has no additional effect (647). The main disadvantage of pyridoxine is that it is not selective to a specific type of afferent, as it destroys most afferents over a certain size and partially smaller afferents.
Other chemical or pharmacological compounds have been shown to preferentially affect transmission in specific sensory afferent pathways. For example, noradrenergic and serotonergic agonists depress group II afferent transmission, albeit at different sites within the spinal cord, while leaving group I afferent transmission largely unaffected in anesthetized cats (98). However, there are limitations to using pharmacological compounds to investigate sensory afferent transmission. First, drugs have central effects, particularly monoamines, altering the excitability of spinal interneurons and motoneurons, as well as the state of the spinal network. Second, studies in animal models showing selective depression in somatosensory pathways were often performed in anesthetized and/or curarized decerebrate preparations, which might not accurately reflect neural transmission in awake freely behaving animals. Third, there are important inter-species differences in how chemical and pharmacological compounds affect neural transmission and network function. For instance, noradrenergic agonists facilitate spinal locomotion in cats, but have a depressive effect in mice and rats, where, instead, serotonergic agonists facilitate spinal locomotion (719). Thus, results using pharmacological compounds, based on experiments in other animals, should be interpreted with caution.
Genetics
Proprioceptive
We can selectively remove proprioceptive feedback from muscles using mouse genetics (Fig. 13). For example, normal development of muscle spindles requires the zinc finger transcription factor Early growth response 3 (Egr3) and in the absence of its expression, as in Egr3 knock out (Egr3-KO) mice, muscle spindles degenerate postnatally (Fig. 13A) (143; 817). Studies have used Egr3-KO mice to investigate the role of proprioceptive feedback from muscle spindles during locomotion (10; 803). Alternatively, proprioceptive afferents from muscle spindles and GTOs can be destroyed embryonically by the selective expression of the diphtheria toxin light chain A (DTA) (Fig. 13B) (838). To do this, a mouse line was used bearing the gene that encodes the DTA under the control of the transcription factor Isl2 with an upstream stop sequence flanked by loxp sites, making the DTA expression dependent on the presence of cre recombinase (Isl2::DTA mouse) (881). The Isl2::DTA mice is crossed with another mouse line, the Pv::cre mice (377), which expresses the cre recombinase by controlling the expression of parvalbumin (Pv), a calcium binding protein selectively expressed in proprioceptive DRG neurons. In the offspring of this cross (Pv::cre;Isl2::DTA mouse), all proprioceptive afferents are selectively destroyed, as Isl2 is expressed in all DRG neurons and Pv in all proprioceptive neurons (838). Consequently, the Pv::cre;Isl2::DTA mouse is an animal model where all proprioceptive afferents from muscle spindles and GTOs are destroyed during embryonic development. One limitation of the Pv::cre;Isl2::DTA mouse is that the CNS circuitry might reorganize due to the embryonic loss of proprioceptive afferents (819). Therefore, any measured phenotype might be due to this reorganization in addition to the removal of proprioceptive afferents.
Figure 13. Proprioceptive feedback and mouse genetics.
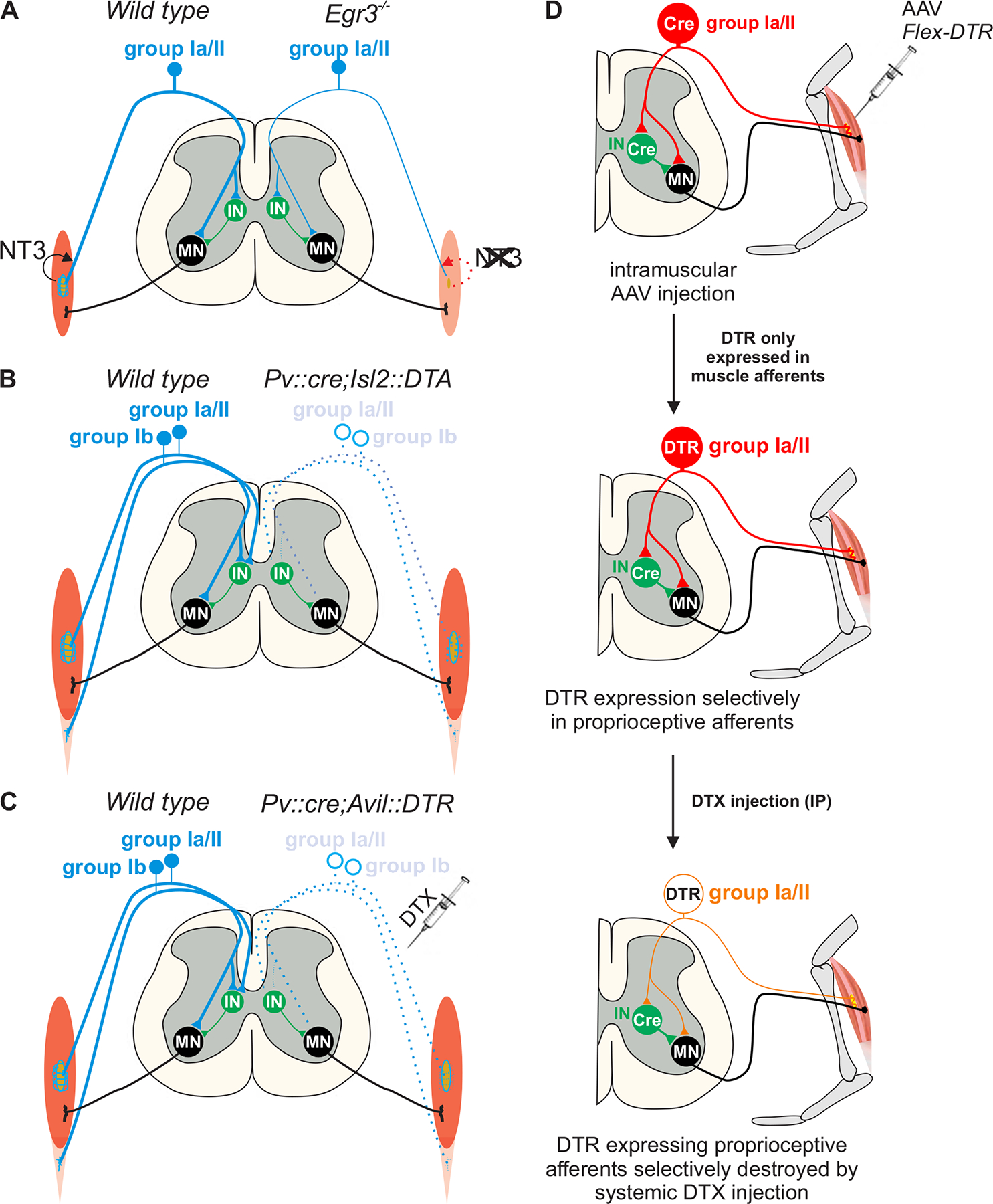
A) Selective removal of muscle spindles in Egr3-KO mice occurs through loss of neurotrophin 3 (NT3) expression in the muscle spindles resulting in their degeneration postnatally. B) Proprioceptive afferent neurons are selectively removed by making them selectively express the highly toxic DTA using the calcium binding protein Parvalbumin (Pv) and the transcription factor Isl2, both genes collectively expressed in proprioceptive afferents only. C) Alternatively, proprioceptive afferent neurons can be made susceptible to the diphtheria toxin (DTX) by making them express the gene that encodes the diphtheria toxin receptor (DTR), normally not expressed in mice. In these mice, systemic injection of DTX results in acute removal of the proprioceptors. D) The gene that encodes the DTR in a cre-dependent manner can be postnatally delivered via adeno associated virus (AAV) injections into selected muscles. Later, as in C, proprioceptive afferents only from the AAV injected muscles can be destroyed by systemic injection of DTX.
An alternative method to remove proprioceptive feedback uses the fact that the wild type mouse does not express the diphtheria toxin receptor (DTR) and, as such, is not susceptible to the diphtheria toxin (DTX). Therefore, the Pv::cre mouse was crossed with another mouse line that carries the gene encoding the DTR, in a cre-dependent manner, under the control of the DRG neuron-specific actin binding protein advillin (Avil::DTR mouse) (790). This makes proprioceptive afferents selectively susceptible to DTX (Fig. 13C) (802). In the offspring of this cross, the Pv::cre;Avil::DTR mouse, proprioceptive neurons develop normally but can be acutely destroyed at an adult age by administering DTX. Alternatively, the gene that encodes the DTR in a cre-dependent manner can be postnatally delivered via adeno associated virus (AAV) injections into selected muscles (Fig. 13D) (553). When AAVs are injected into a selected muscle of a Pv::cre mouse, they infect motoneurons and proprioceptive neurons that innervate this muscle. Because only proprioceptive neurons express Pv, the DTR is expressed only in these and not in motoneurons. With this method, Pv expressing premotor interneurons are not infected as AAVs do not cross synapses (22). With systemic administration of DTX (e.g. in the drinking water), only proprioceptive afferents from the muscle injected with the AAV are affected.
Cutaneous
Tactile sensation from the skin can also be genetically removed or silenced. Within the last decade, some of the genes selectively expressed in subsets of cutaneous afferents have been described (300). Earlier descriptions of the molecular signature of some cutaneous afferents were the Transient receptor potential channels in thermosensitive afferents (131) and the Mas 1-related G protein-coupled receptor expression in itch sensitive afferents (497). Subsequent differentiation was achieved by identifying Neurofilament 200, which is expressed in myelinated A-fibers (486), whereas Isolectin B4, Substance P and Calcitonin gene-related peptide are expressed in C-fibers (405). More recent single cell RNA sequencing techniques allowed for a higher resolution classification of cutaneous afferent neurons (824; 890). However, how these molecularly defined subclasses relate to different cutaneous neurons remains unclear.
The molecular signature of the Merkel cell complex, responsible for signaling fine touch, consisting of the epithelial Merkel cells and SA1 afferents, is better known (868). The mechanosensitive ion channel Piezo2 is the main transducer in the Merkel cell complex (707; 869). In addition, the epithelial Merkel cells express the epithelial Keratin protein Krt-14 (821) and the transcription factor Atoh1 (71), which led to the creation of the Krt14::cre mouse (183) and the Atoh1 cre-conditional KO (Atoh1-cKO) mouse lines (70; 772). Mouse lines have been created that either selectively lack Merkel cells, such as Krt-14::cre;Atoh1-cKO mice (590), or where Merkel cells are insensitive to mechanical stimulation, as in the Krt::14;Piezo2fl/fl mouse line (869). Although behavioral experiments were performed to understand the role of Merkel cells in light touch sensation (707), their role in locomotion has not been specifically explored. There are also mouse lines with selective ablation of spinal interneuron populations that transmit cutaneous information (4; 92; 111; 632). We expect that these and new mutant mouse lines, making use of optogenetics and/or chemogenetics to manipulate somatosensory afferents, combined with behavioral and physiological experiments will provide new insights into the control of locomotor networks by cutaneous afferents.
Mathematical and computational models
A powerful way to investigate how motion-related somatosensory feedback interacts with central circuits is to perform neuromechanical modeling and simulations. In these models, we first compute locomotor movement-related mechanical variables (muscle fascicle length/velocity, muscle force and external loads applied to the skin of body segments) that are the inputs to somatosensory mechanoreceptors. Subsequently, using models of mechanoreceptors, we calculate the firing rates of somatosensory afferents. The computed motion-related feedback in combination with models of the musculoskeletal system and locomotor neural networks allows for the systematic investigation of the role of somatosensory feedback in controlling locomotion, which is often incomplete from experimental studies alone.
Forward dynamics models
Differential equations of motion describe the cause-effect relationships between forces and moments and the resulting motion. These equations can be solved in both directions if we know the forces/moments or motion. If motion (time-dependent positions of body segments) is known, e.g. using motion capture, we can calculate velocities and accelerations of body segments by numerical differentiation and compute forces and moments from the accelerations and measured inertial properties of body segments as well as external ground reaction forces. We call finding forces and moments from recorded motion an inverse dynamics analysis. If muscle forces and/or joint moments are recorded or estimated from muscle activity, accelerations of body segments derived from equations of motion are integrated to obtain motion, such as velocity and displacement of body segments. We call this process forward dynamics analysis [reviewed in (832; 862; 884)]. Forward dynamics models are especially useful to study locomotor control by somatosensory feedback because of closed-loop neuromechanical simulations, where we can manipulate the properties and organization of somatosensory pathways to analyze changes in locomotor activity (90; 242; 243; 356; 366; 426; 613; 640).
Constructing a typical neuromechanical model consists of several steps. It involves: modeling the muscle excitation-activation dynamics using EMG or neural activity as input; modeling the muscle-tendon interaction dynamics that describe the development of muscle contractile force as a function of activation, muscle fascicle length/velocity and tendon properties; transforming muscle forces to joint moments; deriving and integrating equations of limb dynamics; and modeling motion-dependent somatosensory outputs from mechanoreceptors. Lastly, it involves modeling neural rhythm/pattern generating locomotor circuits that use somatosensory feedback as inputs and produce motoneuronal activity as outputs. Here, we briefly review some of these steps relevant to modeling somatosensory feedback.
The muscle excitation-activation dynamics describe the process of muscle membrane depolarization and the release of calcium (Ca2+) from (and subsequent reuptake by) the sarcoplasmic reticulum into (from) the cytoplasm and the formation of connections between myosin and actin myofilaments via cross-bridges developing force. In a simplified form, we can describe this process by a first-order differential equation that relates the neural input (muscle/motoneuronal excitation) to the rate of muscle activation (concentration of Ca2+ in the cytoplasm and muscle force development) (366; 883).
We can describe the process of force development in the muscle-tendon unit by muscle contraction dynamics or dynamics of muscle-tendon interactions. This process involves interactions between actin and myosin myofilaments via cross-bridge cycling attachments, pulling on the actin and detaching. In cross-bridge cycling, actin filaments slide with respect to myosin, shortening muscle sarcomeres, and thus muscle fascicles. This stretches the elastic aponeurosis and tendon attached to muscle fascicles in series and develops force in the muscle-tendon unit. Models of different complexity have described various aspects of contraction dynamics, from molecular mechanisms and biochemical kinetics (190; 364; 411), to phenomenological models, so-called Hill-type models, which describe the empirical input (activation, muscle/tendon length and velocity) and output (tendon force) relationships (366; 736; 820; 840; 883). Researchers often use phenomenological muscle models in neuromechanical simulations because they are simpler and sufficiently accurate for predicting muscle forces within physiological ranges during locomotion.
A typical Hill-type model consists of three elements: a contractile element, a parallel elastic element and a series elastic element (Fig. 14A). Models of the muscle contractile element describe the force-length-velocity relationships of the muscle fibers. The overlap between actin and myosin myofilaments determines the isometric force-length relationship (Fig. 14B) and demonstrates the maximal force production at the muscle’s mid length (314).
Figure 14. Hill-type muscle model and its properties.
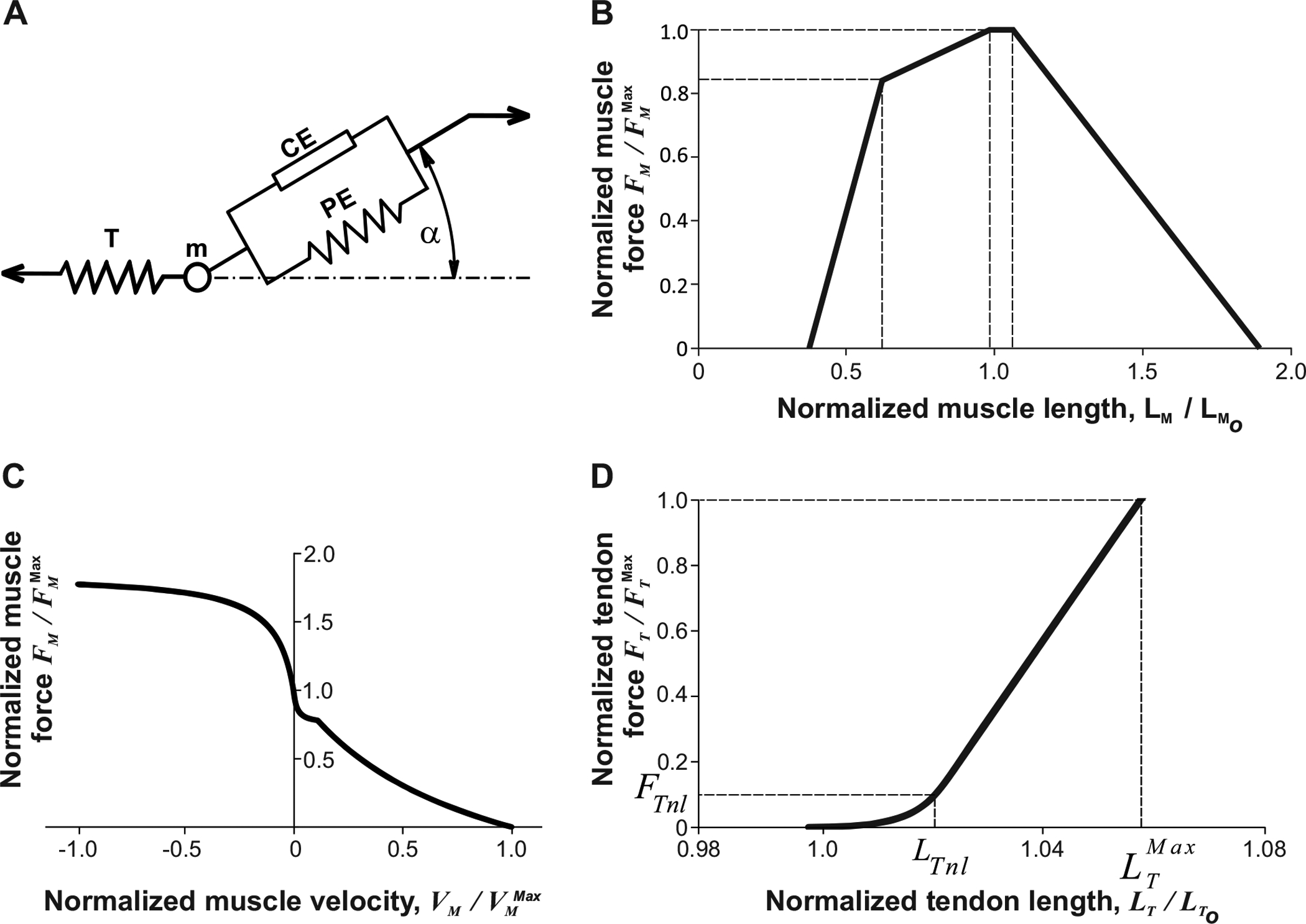
A) Three-element Hill-type model. CE, contractile element; PE, parallel elastic element; T, tendon; m, mass of muscle fascicles. B) Normalized force-length relationship of the contractile element fully activated and producing force isometrically, i.e. without length change. Force is normalized to the maximum muscle force developed by the fully activated muscle at its optimal length. Length is normalized to the optimal muscle fascicle length. C) Normalized force-velocity relationship of the contractile element. Velocity is normalized to the maximum shortening velocity. Positive velocity corresponds to shortening. D) Normalized tendon force-length relationship. Parameters FTnl, LTnl and are empirical constants; LTo is the resting (slack) tendon length. Adapted from (686).
The force-velocity relationship (Fig. 14C) is an empirical relationship demonstrating that a muscle’s force production decreases with shortening velocity (positive velocity values) and increases with muscle stretch (257; 375). The parallel elastic element represents passive tissues surrounding the contractile element. We describe its force-length properties by a passive force-length relationship similar to that of the tendon. The series elastic element represents passive elastic properties of the internal tendon (aponeurosis) and the external tendon. We describe its force-length relationship as shown in Fig. 14D.
The physiological and mechanical properties of the muscle-tendon unit have important implications for the output of mechanoreceptors. For example, muscle spindles are embedded inside the muscle belly parallel to extrafusal fibers and spindle length changes reflect length changes in extrafusal fibers (546). During postural sway and locomotion, length changes in the muscle belly and the series elastic element can decouple, especially in distal muscle-tendon units that have relatively long tendons and short muscle fascicles. As such, there may be situations where the muscle-tendon unit is lengthening during locomotion, as in the yield phase of stance, while muscle fascicles and spindles are shortening or maintaining a nearly constant length (172; 247; 380; 509).
The next step in developing a neuromechanical model to investigate somatosensory control is the transformation of tendon forces to joint moments, the main contributors to limb dynamics. For this transformation, we multiply muscle forces by corresponding muscle moment arms, defined as the shortest distance between the joint center and the line of muscle action (204; 633). We can measure moment arms directly from X-ray or MRI images of the tendon path or by the method of tendon elongation because the muscle-tendon unit moment arm equals the derivative of the muscle-tendon length with respect to the joint angle (26; 882). We can also estimate moment arms using geometric models of muscle-tendon paths with respect to joint centers (83; 141; 316; 649). After determining the moment arms of muscles with respect to the axis of joint rotation, we calculate the corresponding joint moment as the sum of the products of the corresponding tendon forces and moment arms.
We can then derive equations of limb dynamics. These consist of standard equations of motion relating accelerations of body segments with their inertial properties (mass and moments of inertia) and forces/moments applied to body segments. Forces/moments include joint moments produced by muscles (see above), external forces/moments (e.g., ground reaction), and those that depend on body segment motion, such as Coriolis and centrifugal forces. Integrating these equations with those corresponding to muscle excitation-activation and contraction dynamics, starting from a given state of the system, generates, in each integration step, new velocities/displacements of body segments, velocity/length of muscle fascicles/tendons, as well as tendon and external forces applied to body segments as a function of time. In each integration step, we can calculate motion-related somatosensory afferent signals from the instantaneous muscle fascicle/tendon lengths and velocities, tendon forces as well as external forces applied to body segments. In turn, we can use these sensory signals as inputs to the equations describing dynamics of the neuronal locomotor control system to compute its output (i.e. motoneuronal activity), muscle forces and locomotor motion.
As evident from the above description, neuromechanical modeling is complex and time consuming, requiring expertise in various disciplines, including musculoskeletal physiology and mechanics, neurophysiology, computational neuroscience and biomechanics, as well as others. However, having a comprehensive neuromechanical model that accurately reproduces locomotor behaviors allows for testing/generating new hypotheses and obtaining new insights into the control of locomotion by somatosensory feedback not possible otherwise (49; 90; 343; 356; 537; 874). Open-source or commercial software packages have considerably simplified the process of neuromechanical modeling. For example, the open-source software OpenSim (189) allows users to develop complex biomechanical models of musculoskeletal systems and to conduct computer simulations of locomotion and other movements. The open-source software Neuron (376) allows developing models of neurons and neural networks. The open-source software AnimatLab (158) combines biomechanical and neuronal packages to develop comprehensive neuromechanical models and simulations.
Modeling outputs from mechanoreceptors
Any neuromechanical model includes a description of the transformation of mechanical input variables into a model of mechanoreceptor outputs (i.e. the firing rate of corresponding afferents). We can model this transformation by: 1) modeling a receptor’s input-output relationship, 2) a detailed modeling of the anatomical structure and function of mechanoreceptors and 3) by developing and investigating physical robotic receptor systems or hybrid living receptor-computer model systems.
The first approach, describing a receptor’s input-output relationship, involves in situ or in vivo recordings of mechanical variables, such as tendon forces, muscle fascicle length/velocity and pressure applied to the skin, with simultaneous recordings of firing rates of identified afferents from dorsal root filaments or DRG (502; 697; 835; 846). We can then fit a mathematical transfer function or regression equation to describe input-output characteristics of mechanoreceptors. These relationships have been obtained for the main somatosensory afferents in the cat (Table 3). Despite the relative simplicity of this approach, the available input-output models are relatively accurate. The accuracy of afferent activity prediction is of course limited to the range of mechanical variables tested experimentally. Most in vivo recordings were made during normal gaits, where firing rates do not normally exceed 250 Hz (500; 503; 505; 692; 693; 696; 698; 846). It is not clear if the maximal firing rates (range 400–700 Hz) of group Ia afferents recorded from cat triceps surae and biceps femoris posterior-semitendinosus muscles during paw shake (694) could be accurately predicted by the models in Table 3, although see (690). Within the physiological range of locomotor movements, predicted firing rates correlate highly with the recorded afferent activity and root mean square errors are small. This is especially true for spindle afferents of proximal muscles, such as the hamstrings (692; 693) that have relatively long muscle fascicle lengths and short tendons and thus little decoupling between their length changes. Better performance of input-output models for GTOs compared to muscle spindles could be because GTOs behave as a nearly linear, time-invariant system within the physiological ranges of inputs and are not affected by modulation of gain (receptor sensitivity, i.e. the ratio of the receptor’s response to its input signal) (395). The input-output characteristics of muscle spindles are more complex because their response patterns depend on various non-linear properties of the intrafusal fibers, separate gain control of static and dynamic components and levels of γ-motoneuron activation (87; 97; 173; 363; 605; 787).
Table 3.
Input-output relationships of somatosensory mechanoreceptors in the cat
| Afferent type | Equation | Experiment | References |
|---|---|---|---|
| Spindle group Ia | RIa = 65 * v0.5 + 200 * d + kEMG + R0, where RIa, firing rate, Hz; v and d, muscle-tendon unit (MTU) elongation velocity, MTU rest length/s and MTU elongation normalized to rest length; k, percent of maximum EMG recruitment; EMG, rectified, averaged and normalized EMG; R0, the mean rate. | In vivo recordings from multiple hindlimb muscles | (690; 692; 693) |
| Spindle group II | RII = 13.5 * d + 20 * EMG + R0, where RIa, firing rate, Hz; d, MTU elongation, mm; EMG and R0 are the same as above. | In vivo recordings from multiple hindlimb muscles | (690; 692; 693) |
| Golgi tendon group Ib | RIb = k * F * (s + 0.15)(s + 1.5)(s + 16)/ (s + 0.2)(s + 2)(s + 37), where RIb, firing rate, Hz; s is the Laplace variable. | In situ, soleus and medial gastrocnemius | (396) |
| Cutaneous | , Rc, firing rate, Hz; k1 and k12, empirical constants; Fy and , vertical ground reaction force in N and its time derivative in N/s. | In vivo recordings from paw pad afferents | (686; 846) |
Modeling the anatomical structure and function of mechanoreceptors
To improve the accuracy of firing rate predictions of complex mechanoreceptors, researchers have developed sophisticated mechanistic models that describe the structure and function. For example, one muscle spindle model incorporates two types of nuclear bag intrafusal fibers and nuclear chain intrafusal fibers as a Hill-type muscle model, representing the polar zones, in series with the elastic equatorial sensory zone (573). The firing rate of group Ia and II afferents is determined by stretch velocity and length of the sensory zone, which in turn depends on the fusimotor activation of the polar zones and their dynamic interactions with the equatorial zones. For other models, see (363; 496; 530; 722; 740). A recent muscle spindle model described the history-dependent mechanical behavior of intrafusal fibers using a computational cross-bridge cycling model (86). A population of Hodgkin-Huxley-style neurons defined the transformation of the graded receptor potential into spindle afferent action potentials. The model predicted and explained many experimental features of primary and secondary spindle afferent responses to muscle length changes. These include the non-linear dependence of afferent firing rates on stretch velocity (165; 173), the history-dependence of afferent firing rates on muscle mechanical states (605; 700; 701) and the partial occlusion of combined effects of static and dynamic fusimotor stimulation (59).
One study detailed a model of the GTO that captures important anatomical and functional features (574). The model includes innervated and non-innervated collagen fibers and a sensory region modeled as viscoelastic material with specific stress-strain characteristics. The interactions between these structures in response to the activation of the motor units attached to the receptor determines the amount of stretch of the sensory region and the firing rate of Ib afferents. The model captures GTO responses to developing muscle force, including static and dynamic sensitivities of slow, fast fatigue resistant and fast fatiguing muscle fibers, as well as self- and cross-adaptation of responses to prior activation of the same or different motor units.
Although detailed comprehensive models of muscle receptors provide accurate receptor output and account for major properties of corresponding afferent responses, these models require an estimation of multiple parameters, which is often difficult or impractical. Depending on study goals, researchers can select a model or sets of models of appropriate complexity to model different aspects of somatosensory function and their role in locomotion.
Physical robotic receptor systems
Physical models of mechanoreceptors and somatosensory motor control may offer important advantages over computational models. The computational approach deals with mathematical abstractions and simplifications that might not accurately represent the modeled system and its interactions with the real environment. Designing robotic systems that mimic the basic structure and functions of the animal’s body while reproducing basic motor behaviors in the physical world gives researchers important insight about the demands for the control system and the necessary somatosensory feedback (413; 463; 467; 788; 800). Mechatronic robotic hands with haptic capabilities have been used to understand the interactions between active exploration of the external environment and somatosensory feedback (507). Such systems have found utility in prosthetic hands and feet capable of sensing physical contact and evoking the corresponding tactile perceptions in the user via electrical stimulation of residual cutaneous nerves (151; 541). Another advantage of physical robotic models is that they can generate sensorimotor control of large-scale neuromechanical systems in real time using special-purpose hardware based on large-scale integrated-circuit technology (754). In one implementation of such a robotic system, researchers emulated the control of robotic and cadaveric fingers using over 2000 sensory, motor and cortical neurons (430).
Hybrid receptor-robot-computer model systems
Limb prostheses with neural interfaces mentioned above represent hybrid systems used to gain new insight into the role of somatosensory feedback in movement control. Having a living system in the sensory feedback control loop allows for rigorous testing of somatosensory control hypotheses by systematic manipulations of gains of receptors and sensory pathways or parameters of external perturbations. Having an intact or reduced animal preparation in the feedback loop allows for investigating neuronal mechanisms of sensorimotor integration. Interfacing the lamprey with a robotic system via recorded activities of the contralateral reticulospinal pathways receiving input from vestibular afferents revealed that lamprey postural stabilization in the roll plane results from the subtraction of signals from the left and right reticulospinal pathways (891). The role of proprioceptive feedback in controlling leg movements and in the reversal of length-dependent reflexes from resistive to assistive between quiescent and locomotor states was investigated by connecting an in-vitro preparation of the crayfish thoracic nerve cord to a detailed computational model of the animal’s neuromechanical system (49; 152). They found that proprioceptive feedback increases the frequency of rhythmic locomotor activity by nearly three times. Such hybrid systems are powerful research tools to test and understand detailed neuromechanics of the somatosensory control of movement.
Model systems
Cat
The cat model has a long tradition for investigating the neural and biomechanical control of locomotion (276; 337; 718). Many of the basic principles of the neural control of locomotion have been derived from studies in the cat model [recently reviewed in (276)]. These include: 1) the basic locomotor pattern is generated by a spinal network, now commonly called the spinal locomotor CPG (106; 344; 441). 2) A region within the brainstem, the MLR, initiates locomotion and regulates speed (731; 763). 3) Spinal reflexes shape locomotor outputs in response to external perturbations in a task- and phase-dependent manner (265; 269; 319; 644; 761). 4) Somatosensory feedback has direct access to the spinal locomotor network (162; 646; 747). The first intracellular recordings from mammalian neurons were also done in the cat and physiological knowledge of its sensorimotor circuitry is more detailed than in any other mammalian species (100; 117; 433; 438; 516; 557; 796; 797). Locomotor preparations, such as treadmill locomotion after decerebration (763), fictive locomotion (254; 344; 662) and chronic EMG recordings and nerve stimulation during unrestrained behaviors (217; 248) were pioneered in the cat. It should be emphasized that recordings were made, for the first time, in the cat model of the activity of non-identified and identified spinal neurons (Ia inhibitory interneurons, γ-motoneurons, Renshaw cells, motoneurons), as well as neurons of ascending (e.g. spinocerebellar and spino-reticular tracts) and descending (vestibulospinal, reticulospinal, rubrospinal, corticospinal) during real locomotion (625). Thus, the cat model provided not only the basic organization of the locomotor system in mammals but also an analysis of the principles governing its operation.
The cat model offers several advantages to investigate the role of somatosensory feedback during locomotion. Because of its size, we can record or stimulate several muscles and nerves in the same animal during locomotion (191; 408; 471). Due to the cat’s robust nature, we can perform chronic recordings and stimulations over several months before and after different types of lesions (277; 284; 285). The cat was domesticated at least four thousand years ago (400) and as a result, we can train it to perform a variety of locomotor tasks with positive reinforcement, such as food rewards and affection. Investigating the control of locomotion in large animals is important from a translational perspective because the biomechanical requirements to generate movement and its neural control depend on body size. As discussed below, several findings first observed in the cat model were then demonstrated in humans. The main disadvantage of the cat model is that newer molecular genetic techniques are not yet available, although these will be important to develop in the cat or other relatively large mammals for preclinical studies (575).
Mouse
In combination with the molecular genetic techniques described above, applicable in vitro and in vivo physiological methods have made the mouse an important animal model for locomotor research. In the in vitro approach, the spinal cord is dissected out of a mouse and kept alive in a dish by superfusing it with an oxygenated Ringer’s solution (456). The advantage of this method is that the activity of individual neurons can be recorded during fictive locomotor-like activity initiated by a mixture of serotonergic or glutamatergic agonists (108; 456). Several methods can be combined in the in vitro mouse preparation, such as extracellular recordings from ventral roots (454), neuronal activity imaging using activity- or calcium-dependent dyes (610), electrical stimulation of dorsal roots or peripheral nerves (111) and activation of interneurons using optogenetic tools (457). However, there are limitations to using in vitro preparations. The spinal cord is typically taken from a neonatal mouse, not older than one week, because as the size of the spinal cord increases, so does an anoxic region in its center, making it difficult to maintain viable for experiments (856). In neonates, the spinal cord is still maturing and does not fully represent the spinal cord of an adult. To overcome this, slices of adult spinal cords can be maintained alive in a dish for physiological experiments (583). However, as the spinal locomotor circuitry spans multiple spinal segments, slice preparations do not capture its complexity, with preparations often limited to less than one spinal segment (583). Additionally, important elements of the nervous system, such as somatosensory feedback and supraspinal inputs, are drastically reduced in in vitro preparations. Despite these limitations, the in vitro approach combined with mouse genetics has been instrumental in advancing our understanding of sensorimotor spinal circuits.
In vivo approaches, using the mouse to understand the role of somatosensory feedback in locomotion, has also garnered increasing interest. The first step was the use of chronically implantable electrodes to record EMG during unrestrained behaviors (9; 488; 643). In addition to EMG recordings, miniature cuff electrodes have been implanted to stimulate peripheral nerves to activate somatosensory afferents and characterize reflexes during free behavior (8; 128; 475). Finally, new advances in implantable optical fibers have allowed the application of optogenetics to activate or silence specific brain areas during free behavior (120; 124; 451; 826). Combining these methods with mouse genetics, behavioral analyses and computational modeling (179; 180; 552; 739) has been a powerful approach to understand the role of somatosensory feedback during locomotion. The main limitation of the in vivo approach is that obtaining cellular level information of the spinal circuitry is not as feasible as with the in vitro approach. Mice also perform rapid movements with flexed limbs and their neuromechanical control might not generalize to larger cursorial mammals.
Human
In comparison to most terrestrial mammals, humans are a strange beast. They evolved their locomotor behavior to use two straight legs in an upright position, which when compared to quadrupeds, is a highly unstable position. The obvious advantage is that it frees the arms to perform other actions and the pendular movement of the straight legs requires minimal energy expenditure. However, it requires a more precise control of posture to avoid falling. Locomotor research in human subjects is critical to understand how we walk and to facilitate locomotor recovery in various movement disorders. Movement kinematics and kinetics are easily measured in humans, as is the EMG activity of several muscles using surface electrodes. To study the role of somatosensory feedback, mechanical perturbations are applied or nerves are electrically stimulated with surface electrodes in healthy human subjects and in people with various pathological conditions. Humans can readily perform a variety of locomotor tasks that other animals cannot. Many studies, mainly inspired by experiments and results in cats, have shown the importance of somatosensory feedback in human locomotion (223; 404; 638; 639; 886; 889). Experiments in human infants are also useful because, as supraspinal pathways are not fully formed, the control of locomotion is principally accomplished by spinal circuits interacting with somatosensory feedback (878). A main limitation of human research is that invasive techniques, such as lesions or direct neuronal recordings, are not available, which limits the neurophysiological knowledge obtainable. Moreover, only nerves located superficially, just under the skin, can be stimulated. Because EMG and nerve stimulations are performed with surface electrodes, movement of the skin relative to the underlying structures can easily induce errors in recorded signals, particularly in amplitude.
Functional roles of somatosensory feedback during locomotion
As discussed in the next few sections, somatosensory feedback contributes in multiple ways to the control of locomotion in mammals.
Somatosensory feedback contributes to postural control during quiet standing and locomotion
During locomotion, animals must maintain balance, or equilibrium, and the orientation of their body segments in relation to each other and the environment. This is critical in animals with long straight limbs and a relatively high center of gravity, like cats and particularly humans that walk bipedally in an upright posture. The goal of this section is not to provide a comprehensive discussion of postural control but an overview of the specific role played by somatosensory feedback in controlling posture.
As discussed earlier, the loss of touch and proprioceptive feedback leads to an inability to stand upright in humans (85; 476), indicating an essential role of somatosensory information in controlling posture, at least in humans. In a deafferented subject without proprioceptive and tactile information, where recovery was achieved through several months of intense rehabilitation, despite a relatively stable gait, the individual walked with a wider base of support and at slower speeds than healthy controls (476). The deafferented subject tilted his shoulders and head forward to see his legs during walking. He also locked the knee joint by co-activating knee extensors and flexors during stance, thus reducing the number of DOF to control. It should be noted that walking without visual feedback was not possible.
To maintain equilibrium, animals must provide adequate anti-gravity muscle tone and maintain the body’s CoM within the base of support in the horizontal plane (293). Although properties of the mechanical system play an important role in maintaining postural balance, active neural control is also required. What is the role of somatosensory feedback in this neural control? Maintaining anti-gravity tone (i.e. in extensor muscles) does not require supraspinal signals, as spinal cats with a transection at thoracic levels recover full weight bearing hindlimb standing and locomotion (61; 94; 293; 360; 511; 512; 527; 785). This indicates that interactions between sensory feedback from the limbs and spinal circuits are sufficient for anti-gravity muscle tone. Spinal cats can also adjust to different imposed distances between the fore- and hindlimbs, or anteroposterior distances, during quiet standing (293). Spinal cats, however, shift more weight to the forelimbs for support. In the same study, the alignment of the trunk axis, which depends on shoulder and hip height, and hindlimb axis was similar in intact and spinal cats, although hindlimb geometry slightly differed. Spinal cats maintained hip height with greater extension at the knee to compensate for reduced angles at the ankle and metatarsophalangeal joints. Fung and Macpherson (1999) concluded that the spinal cord has the rudimentary circuitry for determining postural orientation of the trunk and hindlimbs. This neural control is undoubtedly informed by somatosensory feedback, most likely length feedback from muscle spindles.
Another role of somatosensory feedback in controlling posture is to rapidly inform the CNS of a perturbation. When balance is unexpectedly perturbed, the nervous system generates automatic postural responses (APRs), consisting of stereotyped patterns of EMG activity in several muscles tuned to the direction and velocity (or acceleration) of the disturbance, as shown in cats (417; 525–527; 791; 812) and humans (393; 416; 528). These APRs increase limb stiffness and decelerate the CoM to restore its position and maintain balance. Studies in intact cats, using unexpected horizontal translations of the support surface in 12–16 directions, showed that APRs occur at latencies of 40–80 ms in several fore- and hindlimb muscles (417; 527; 791). In humans, APRs occur at latencies of 80–120 ms following the disturbance (601). Muscle activations in APRs occur in specific combinations, or muscle synergies, as shown in intact cats (813; 816) and healthy humans (154). In cats, a set of 4–5 hindlimb muscle synergies account for APRs during standing, with each synergy corresponding to a specific endpoint force vector (813; 816). This indicates that individual synergies produce force in a specific direction.
In spinal cats that had recovered the ability to stand unassisted, balance was severely impaired in response to unexpected disturbances due to the disruption of APRs and postural muscle synergies (153; 527). Indeed, with sudden disturbances, only a portion of extensor muscles activated, mainly those that were active prior to the perturbation, while APRs in flexors were abolished (153; 527). In extensors displaying APRs, EMG activity was delayed, more variable, smaller in amplitude and briefer than in the intact state. However, some directional tuning remained, indicating that the spinal cord retains some ability to interpret the direction of the perturbation signaled by somatosensory feedback, even though properly responding to unexpected perturbations was largely lost and functionally inappropriate. Muscle synergies of APRs in spinal cats do not correspond to force production, in contrast to intact cats (153). Responses in spinal cats most likely assist in maintaining weight support and not balance. In other words, spinal circuits interacting with somatosensory feedback are not sufficient to maintain posture during perturbed stance and certainly not in dynamic conditions, such as locomotion.
To determine the specific contribution of somatosensory feedback in generating APRs, Stapley et al. (2002) used pyridoxine (vitamin B6) intoxication in intact cats, which destroyed large caliber (above 7 um) group I muscle afferents and large group II and cutaneous afferents. In their experiments, cats stood quietly on four force plates embedded in a movable platform that was horizontally displaced in 12 directions (5.5 cm displacement at 15 cm/s) (Fig. 15A). Seven days after pyridoxine intoxication, cats recovered the ability to stand quietly on the platform but horizontal displacements led to falls or compensatory stepping responses in ~40% of trials, which were not observed before pyridoxine. The onset of APRs in all 7 hindlimb muscles studied was significantly delayed after pyridoxine intoxication, going from 40–65 ms in control trials to 91–222 ms with pyridoxine (Fig. 15B). The timing and amplitude of muscle activity following perturbations were also more variable after pyridoxine. After pyridoxine, perturbations led to larger CoM displacements that took longer to reach peak values (Fig. 15C). These results indicate that somatosensory feedback from large tactile and proprioceptive afferents are essential for the proper timing and amplitude of postural responses to unexpected perturbations. Similar delays of APRs in response to horizontal translations of the support surface were found in humans with diabetic peripheral neuropathy, which mainly affects distal limb segments (416). Inglis et al. (1994) also showed more variable APRs and reduced scaling with translations of different velocities and amplitudes in subjects with neuropathies compared to healthy controls.
Figure 15. Pyridoxine intoxications reveals that large diameter somatosensory afferents contribute to postural control.
A) Schematic of the experimental set-up. Filled circles represent kinematic markers. The antero-posterior (AP) stance distance is the horizontal distance between the wrist and ankle joints. Forelimb, hindlimb, and trunk axis indicated by dashed lines. Reproduced and modified from (293). B) Rectified averaged EMG activity of the gluteus medius and biceps femoris medial head before (control) and 7 days after pyridoxine intoxication, which destroys large diameter somatosensory afferents, in one cat with translation of the support surface at 240°. The dashed vertical line indicates the onset of platform acceleration. Under each trace, the arrows indicate response onset in control trials and at day 7. C) Amplitude maximum initial displacement of the CoM and time of maximum displastement in relation to platform translation at 240°. Error bars indicate SE. **Significantly different from control values (p < 0.001, one-way ANOVA). B and C reproduced and modified from (791). Copyright 2002 Society for Neuroscience.
In another study, Ting and Macpherson (2004) investigated the type of somatosensory input that encoded the direction of the perturbation and tuned APRs. They performed unexpected rotations or horizontal translations in 16 directions in intact cats. Interestingly, rotations and translations evoked similar APRs for a given degree of perturbation, particularly in extensors, consistent with a common neural strategy, despite different initial passive changes in kinematics (limb axis and joint angles) and kinetics (vertical and horizontal ground reaction forces). They found that the only consistent initial feature generated by rotations and translations was the change in the angle of the ground reaction force vector, which was in the same direction under the four limbs. The ground reaction force angle is the ratio of shear (horizontal) and loading (vertical) forces. What sensory signals encode these features? Ting and Macpherson (2004) proposed that cutaneous afferents from mechanoreceptors of the paw pads detect the change in ground reaction force angle. Indeed, removing cutaneous inputs from the paw pads in intact dogs delayed postural responses to horizontal support displacements (589). In another study, removing cutaneous inputs from the hindpaws of decerebrate cats substantially reduced the magnitude of muscle responses to fast horizontal displacements of the support surface in 16 directions (387). However, the tuning of muscle responses was largely preserved, indicating that proprioceptive feedback also signals horizontal support translations, as shown in other studies (386; 388).
How do these results during quiet or perturbed standing apply to locomotion? During locomotion, muscle activation patterns in response to perturbations are also organized into postural synergies (154). Chvatal and Ting (2012) showed 6–8 muscle synergies during unperturbed and perturbed (horizontal translations in four directions) locomotion in humans. During perturbed walking in early stance, the largest reactive responses in the ipsilateral leg depended on the muscle composition of the synergy and the perturbation direction. During anterior displacements, muscle synergies comprising hip flexors, ankle flexors and knee extensors showed their highest activation levels, whereas the muscle synergy dominated by trunk and ankle extensors activated with posterior displacement.
To investigate the role of cutaneous feedback for balance during locomotion, Bolton and Misiaszek (2009) performed lateral translations of the support surface while cats stepped on a walkway before and after bilaterally sectioning the 5 cutaneous nerves that supply the hindpaw. During undisturbed locomotion, the effects of cutaneous denervation were subtle. Cats stepped with a slightly crouched posture, with slightly more rostral and caudal positions of the hindpaws at contact and liftoff, respectively, along with longer hindlimb double support periods. Intact cats made a corrective step following medial or lateral displacements of the support surface at stance onset of one hindlimb (diagonal forelimb is also in stance and displaced) by changing the trajectory of the contralateral hindlimb (89; 581). After cutaneous denervation of the hindlimbs, larger deviations from the original path were observed and all four limbs now participated in step corrections (89). The cutaneous denervation reduced the EMG activity in the medial gastrocnemius and gluteus medius of the perturbed stance leg, although response onset and pattern remained unaffected. The authors proposed that cutaneous inputs normally scale the magnitude of APRs, which are themselves triggered by other somatosensory cues, such as proprioceptive feedback. As noted, the diagonal forelimb, which was not denervated, was also displaced. It is possible that cutaneous information from the forelimbs was sufficient to instruct the CNS to trigger APRs in the four limbs. In another study, cats with unilaterally anesthetized fore- and hindpaws shifted their body position and weight towards the anesthetized side during split-belt locomotion (636). This change in locomotor strategy resembles the increase in grip force of human subjects following anesthesia of the fingers (584; 848). When holding an object with their fingertips anesthetized, blindfolded subjects increase grip force, possibly to recruit additional mechanoreceptors located in deeper layers of the skin or in muscles to improve sensation and feedback.
To summarize, somatosensory feedback plays an essential role in controlling posture during locomotion. It is required for the proper timing and scaling of APRs and postural synergies. Although somatosensory feedback interacting with spinal circuits can produce anti-gravity muscle tone, APRs and postural synergies are not functional after spinal transection, consistent with an essential supraspinal contribution in postural control. Both proprioceptive and tactile feedback likely participate in APRs, with relative contributions depending on task demands and contextual cues.
Somatosensory feedback is required for skilled locomotion and proper paw/foot placement
Walking or stepping on a flat surface for prolonged periods is a rarity for animals, including humans, in their daily routine. The terrain is often irregular, changes in direction must be performed at varying speeds and obstacles in the environment must be negotiated. Many mammals must also accomplish highly skilled locomotor tasks, such as climbing trees, stepping and jumping on branches or the top of narrow support surfaces, such as fences or cables. Following the loss of proprioceptive and/or tactile feedback, quadrupedal mammals, such as mice, rats and cats, recover a high degree of proficiency during simple locomotor tasks, such as overground or treadmill locomotion on a level surface. However, during skilled locomotor tasks, movement errors become pronounced and frequent, if the task is accomplished at all. For example, following the selective and permanent destruction of large size somatosensory afferents with pyridoxine intoxication, cats recover the ability to stand and walk after a few months (647). However, maximal locomotor speed is reduced and cats lose the ability to perform difficult tasks, such as stepping along a narrow beam or on elevated pegs (647).
In another study, the five cutaneous nerves of the hindpaws of cats were sectioned and locomotion was tested during simple and more difficult tasks (93). As originally shown by Sherrington (761), removing cutaneous inputs had little effect on level treadmill or overground locomotion. However, cutaneous denervation of the hindpaws impaired stepping on an incline, with greater yield at the ankle at foot contact, reduced knee joint movement and increased hip flexion, which recovered towards pre-denervation values within about 3 weeks. Impaired incline locomotion likely reflects a role of cutaneous inputs in scaling the amplitude of muscle activity (89). The most striking change after cutaneous denervation observed by Bouyer and Rossignol (2003a) occurred during horizontal ladder walking. Initially, cats refused to step on the ladder and, although they eventually recovered this ability, they did so with a different strategy. Instead of contacting the rungs with their digits, they made contact with the mid-foot and gripped the rungs of the ladder by curling the toes while stepping more slowly (Fig. 16A). In a similar vein, when Egr3-KO mice that lack functional muscle spindle feedback step on a horizontal ladder, the paws frequently miss or slip off the rungs (Fig. 16B) (10; 803). Increased missteps during a horizontal ladder task were also observed with a proprioceptive sensory neuropathy induced chemically by Oxaliplatin in rats (397; 836).
Figure 16. Somatosensory feedback is required for proper paw placement during skilled locomotion.
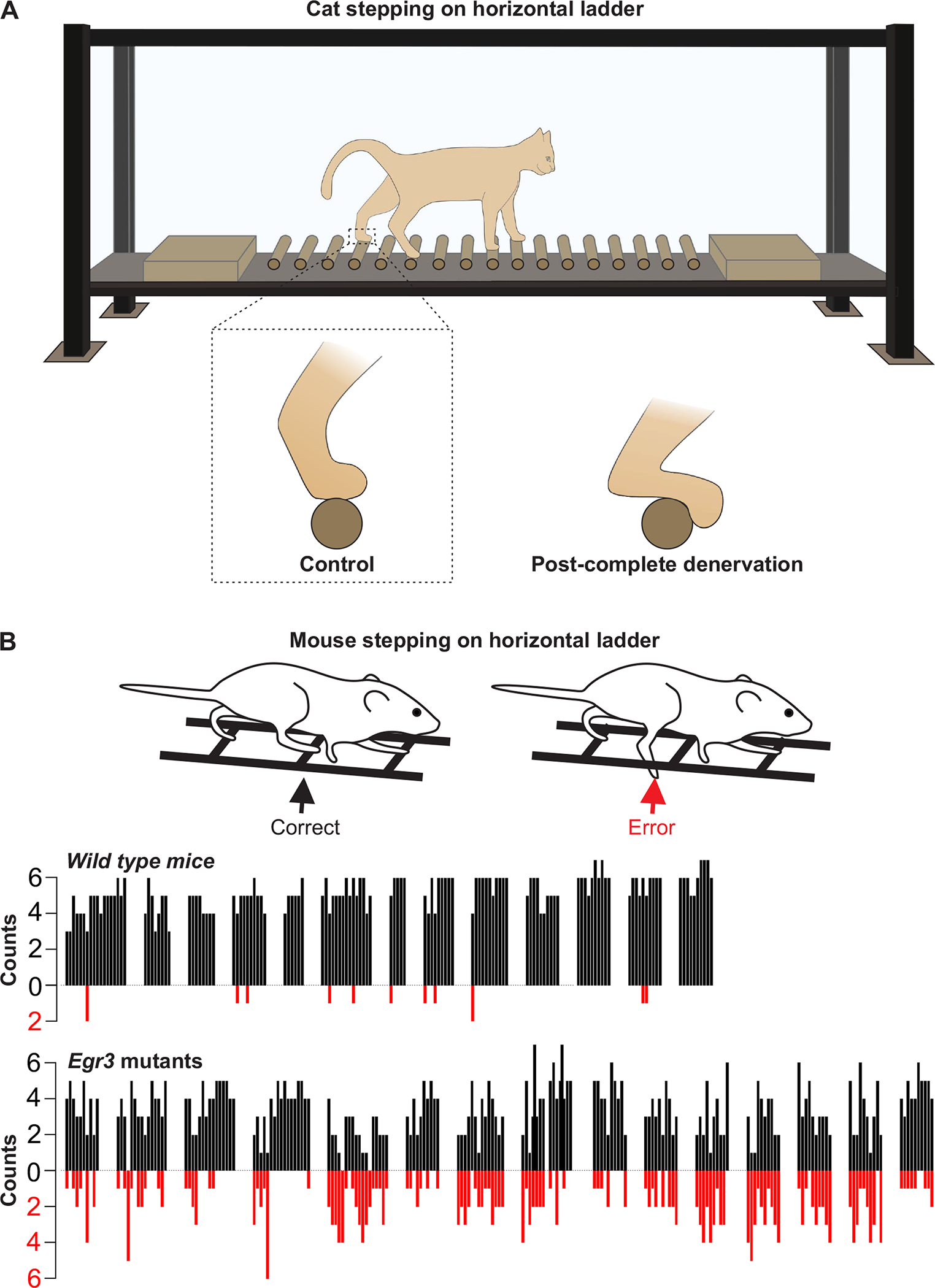
A) Cat stepping on a horizontal ladder before (control) and after a complete cutaneous denervation of the hindpaws. Experimental results from (93). B) Egr3 mutant mice make more errors during walking on a horizontal ladder, determined as more frequent foot droppings between rungs than in wild types. Each bar indicates the number of steps that landed on a rung (black bars) or missed the rung (red bars) counted during one run. Each set of bar represents a run from different mouse (n = 13 for wild type and n = 15 for Egr3 mutants). Reproduced and modified from (10).
Mayer et al. (2018) investigated muscle activity patterns in mice with muscle spindles selectively removed unilaterally from knee or ankle extensors by using gene delivery through an AAV and genetic manipulations. They showed that following acute and selective muscle spindle removal, mice can locomote at comparable speeds as wild types. However, the speed-dependent modulation of ankle extensor activity disappeared following removal of spindle feedback from ankle extensors but not knee extensors. Their findings echoed previous findings showing the powerful activation of extensors throughout the hindlimb from ankle extensor group I afferents, but not knee extensor group I afferents during MLR-evoked fictive locomotion in curarized decerebrate cats (346; 557). Interestingly, Egr3-KO mice that lack functional muscle spindle feedback can step overground or on a treadmill, with EMG patterns resembling those of wild type mice (Fig. 17A), but they cannot swim (10; 803). The pattern of EMG activity during swimming attempts in Egr3-KO mice is strikingly different from swimming wild-type mice (Fig. 17B). Swimming is a task that does not engage load receptors and depends highly on proprioceptive feedback. Altogether, these findings show that mice with reduced or absent muscle spindle feedback can step overground, albeit with impairments in the fine regulation of leg muscle activity and movements. However, during skilled locomotion, impairments become more pronounced and movement errors appear.
Figure 17. The loss of muscle spindle feedback in mice impairs swimming but not treadmill locomotion.
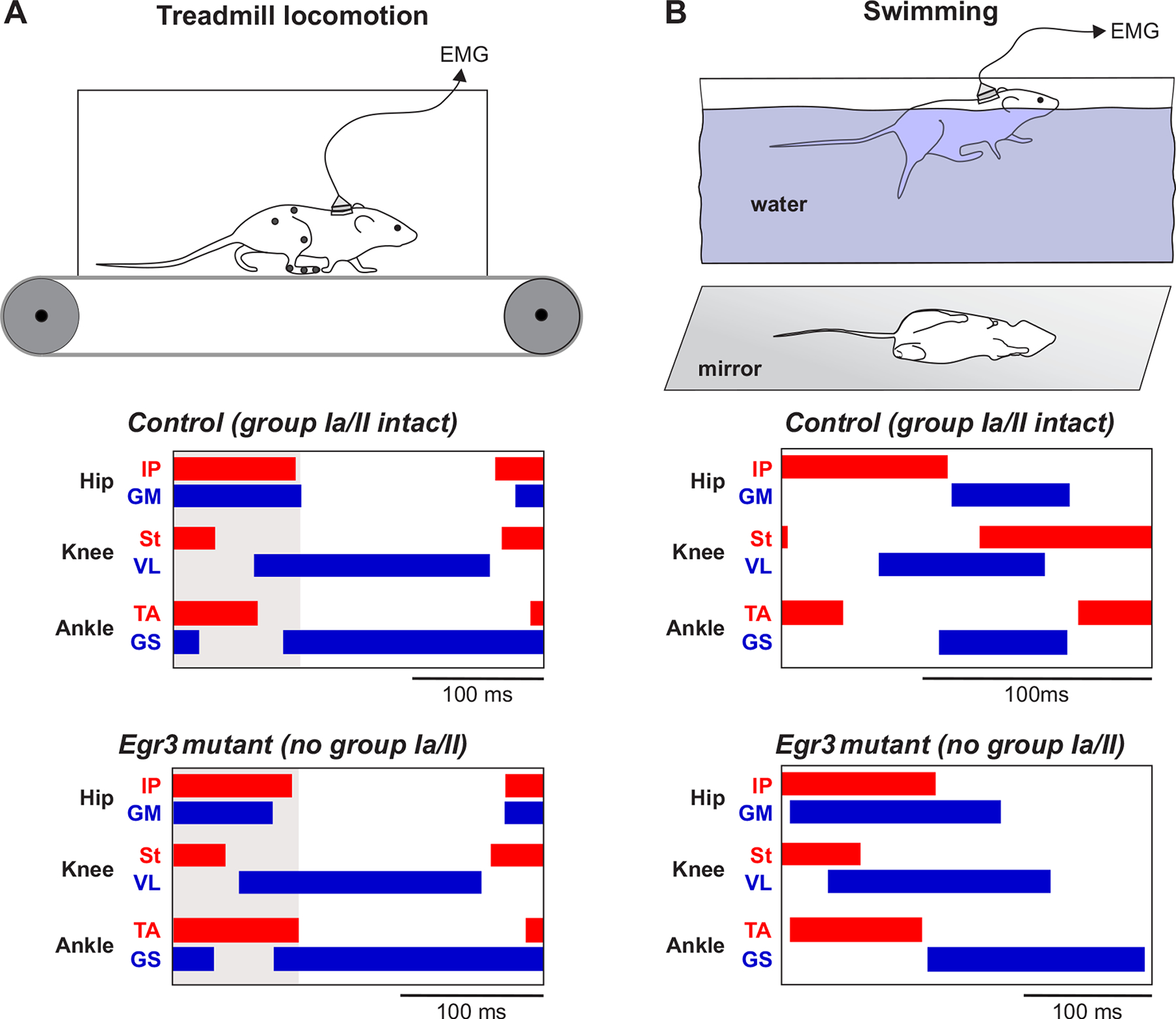
Locomotor pattern gradually degrades with removal of proprioceptive feedback. A) Chronic EMG recordings were made during treadmill locomotion and swimming in wild-type and Egr3 mutants that lack functional muscle spindle feedback. B) Bar diagram illustrating the activity of flexor (red) and extensor (blue) muscles during treadmill walking and swimming in wild-type (n = 16 for walking and n = 14 for swimming) and Egr3 mutant (n = 15 for walking and swimming) mice. Each horizontal bar is the average EMG activity in a normalized locomotor cycle (between successive swing or iliopsoas burst onsets for walking and swimming, respectively). GM, gluteus maximum; IP, iliopsoas; St, semitendinosus; TA, tibialis anterior; VL, vastus lateralis. Reproduced and modified from (10).
Lesions to the somatosensory cerebral cortex also impairs skilled locomotion. In one study, focal lesions to the forepaw area of the primary somatosensory cortex in rats impaired locomotion on a rotating beam, mainly by disrupting coordination between rostral and caudal parts of the body (872). Lesioned rats recovered rotating beam locomotion within 2–3 weeks, consistent with compensatory strategies involving interactions between somatosensory feedback and subcortical mechanisms. Another study recorded responses in the motor cortex of cats to low threshold electrical stimulation of forelimb nerves (superficial radial and ulnar nerves) at rest (quiet sitting) and during ladder walking (540). Overall, cortical responses to forelimb nerve stimulation were shorter in duration and smaller in amplitude during ladder walking with notable phase-dependent modulation. This might reflect a tighter regulation of somatosensory responses for skilled locomotion. They also found that most neurons responding to forelimb inputs had greater responsiveness around the time the forepaw made contact with the ladder rung, consistent with a role in paw placement and in controlling early stance.
Therefore, these results highlight the role of somatosensory feedback in properly placing the paws at contact and in controlling dynamic balance, features of locomotion essential for skilled locomotion. In mammals that need to perform skilled locomotion to avoid predators or capture prey, the loss of somatosensory feedback means certain death.
Somatosensory feedback regulates phase durations and transitions
The locomotor cycle can be broadly divided into two phases, a stance and a swing phase, where extensor and flexor muscles, respectively, are mostly active. Although the spinal locomotor CPG sets the basic rhythm and controls phase durations and transitions, somatosensory inputs and supraspinal signals modulate this control, advancing or delaying phase transitions. The effect of somatosensory feedback on phase durations and transitions and its interactions with the spinal locomotor CPG has been extensively described, primarily stemming from studies in decerebrate cats during fictive or treadmill locomotion (274; 403; 559; 641; 642; 646; 718). Below, we describe how somatosensory inputs prolong phases or reset the rhythm, change cycle/phase variations and entrain the rhythm. It is important to note that the effects of somatosensory inputs on the locomotor cycle depend on the preparation (e.g. different types of fictive locomotion, treadmill or overground locomotion), the state of the spinal cord (e.g. intact versus spinal), decerebrate versus conscious locomotion, and the method used to evoke the locomotor rhythm (e.g. spontaneously occurring, electrically- or pharmacologically-evoked).
Stance-to-swing transition
Classic experiments in the cat during treadmill locomotion demonstrated that stretch inputs from hip muscles, load-related inputs from limb extensors and cutaneous afferents from the foot regulate the stance-to-swing transition (216; 219; 342; 851). Duysens and Pearson (1980) showed that loading ankle extensors of one leg prevented its swing onset while the other three limbs continued to step. The force on the leg had to decrease below a certain threshold to initiate swing onset. Whelan et al. (1995) confirmed this by electrically stimulating hindlimb extensor nerves at group I strength at high frequency (> 100 Hz) during treadmill locomotion in decerebrate cats, prolonging stance and resetting the rhythm to extension with stimulation during early swing. Group I inputs from knee and ankle extensors synergize in prolonging stance and for phase resetting. Keir Pearson and colleagues suggested that a condition to initiate swing is the unloading of extensors. Functionally, this means that weight support will be maintained if the leg is loaded, thus reducing the risk of a fall. Cutaneous afferents from the foot also signal contact or pressure to the skin. Low-threshold stimulation of the sural and distal tibial nerves, which innervate the lateral edge and plantar surface of the foot (73), prolongs the stance phase during treadmill locomotion in decerebrate cats (216).
The other proposal to facilitate the stance-to-swing transition is that the hip must extend to an angle of at least 95° for swing onset, as first shown in the spinal cat during treadmill locomotion (342). Hiebert et al. (1996) confirmed that stretching hip and ankle flexors advanced flexor bursts and swing onset in decerebrate cats stepping on a treadmill, consistent with a role of hip flexor group Ia/II afferents in regulating the stance-to-swing transition. The position of the contralateral leg is also important for the stance-to-swing transition. During slow walking or trot, a limb cannot transition from stance to swing if the other limb is not bearing weight, indicating that somatosensory inputs from the contralateral leg regulates the ipsilateral stance-to-swing transition. Also, during split-belt locomotion with large differences in speed between the slow and fast sides, the slow hindlimb can transition from stance to swing with the paw rostral to the hip (i.e. with a hip angle less than 90°), as shown in spinal cats (280). In this scenario, sensory cues other than those related to hip position become more important for the stance-to-swing transition. Thus, the regulation of the stance-to-swing transition relies on multiple somatosensory signals weighted according to task demands.
Swing-to-stance transition
The swing-to-stance transition is a critical part of the step cycle because the foot must be properly placed on the ground to accept weight transfer and ensure balance. In the intact and spinal cat, the relative position of the foot at contact remains invariant with increasing speed (178; 280; 350). Thus, sensory signals from the limbs must inform the spinal locomotor CPG at the end of swing to bring the foot down. Indeed, studies in decerebrate cats during treadmill locomotion showed that assisting hip flexion during swing shortened the hip flexor burst and advanced extensor burst onset (478; 564). Additionally, by comparing hindlimb extensor onset in various locomotor tasks, McVea et al. (2005) showed that extensor burst onset was closely associated with hip angle, as opposed to the other joints, consistent with a role of sensory signals from the hip in regulating the swing-to-stance transition. They attributed this to group I and II feedback from hip muscles. The authors also acknowledged that other afferents likely contribute to the swing-to-stance transition. In the cat forelimb, somatosensory feedback from shoulder muscle afferents appears to play a similar role as hip muscle afferents. Shoulder protraction in mid- to late flexion shortens the flexor burst and advances extensor burst onset, as shown in decerebrate curarized cats during fictive locomotion (735).
Types of somatosensory afferents involved
To identify the somatosensory afferents involved in regulating phase durations and transitions, fictive locomotor studies in decerebrate cats have been used. Stimulating group I afferents from hindlimb extensors during the flexion phase terminates the flexor burst and resets the rhythm to extension (Fig. 18A), whereas the same stimulation during extension prolongs the extensor burst (Fig. 18B) (162; 282; 288; 346; 747). Both group Ia and Ib afferents appear equally effective in prolonging extension while resetting from flexion to extension requires activation of group Ib afferents (346). In addition, group I afferents from distal extensors, such as those from the ankle, are more effective than those from more proximal hip or knee extensors. Prolongation of ipsilateral extension is paired with increased duration of contralateral flexor bursts, likely via interactions between left and right spinal CPGs. During MLR-evoke fictive locomotion in decerebrate curarized cats, group I extensor stimulation can increase the electroneurographic (ENG) amplitude of some extensors without affecting it in others while the ENG amplitude of contralateral flexors is generally unaffected. Stimulation of some extensor nerves, such as the quadriceps, prolongs extension and increases ENG activity in some extensors while producing inhibitory responses in others, such as ankle extensors (346). This is consistent with some excitatory or inhibitory pathways accessing both the rhythm generation and pattern formation levels of the spinal locomotor CPG with others only accessing one level. Moreover, some group I stimuli disrupt the timing of the rhythm while others affect amplitude but not the rhythm, again indicating access to different CPG levels. Increasing stimulation intensity to activate group II strength does not change the group I effect. Studies have identified disynaptic pathways transmitting EPSPs to extensor motoneurons via intercalated interneurons and polysynaptic pathways that interact with the rhythm generator to mediate group I enhancement of extension during fictive locomotion in the cat (33; 34; 197; 319; 560). Similar findings have been reported during treadmill locomotion in decerebrate or intact cats as well as during human locomotion, although the effects on phase durations and transitions are smaller than during fictive locomotion because of the presence of phasic somatosensory inputs from various sources (852). Functionally, this means that stretching and/or loading of extensors throughout the legs activates group I afferents to enhance extensor activity, either to reinforce stance or to terminate swing to initiate a new support phase.
Figure 18. Effect of stimulating muscle afferents during spontaneous fictive locomotion in decerebrate curarized cats.
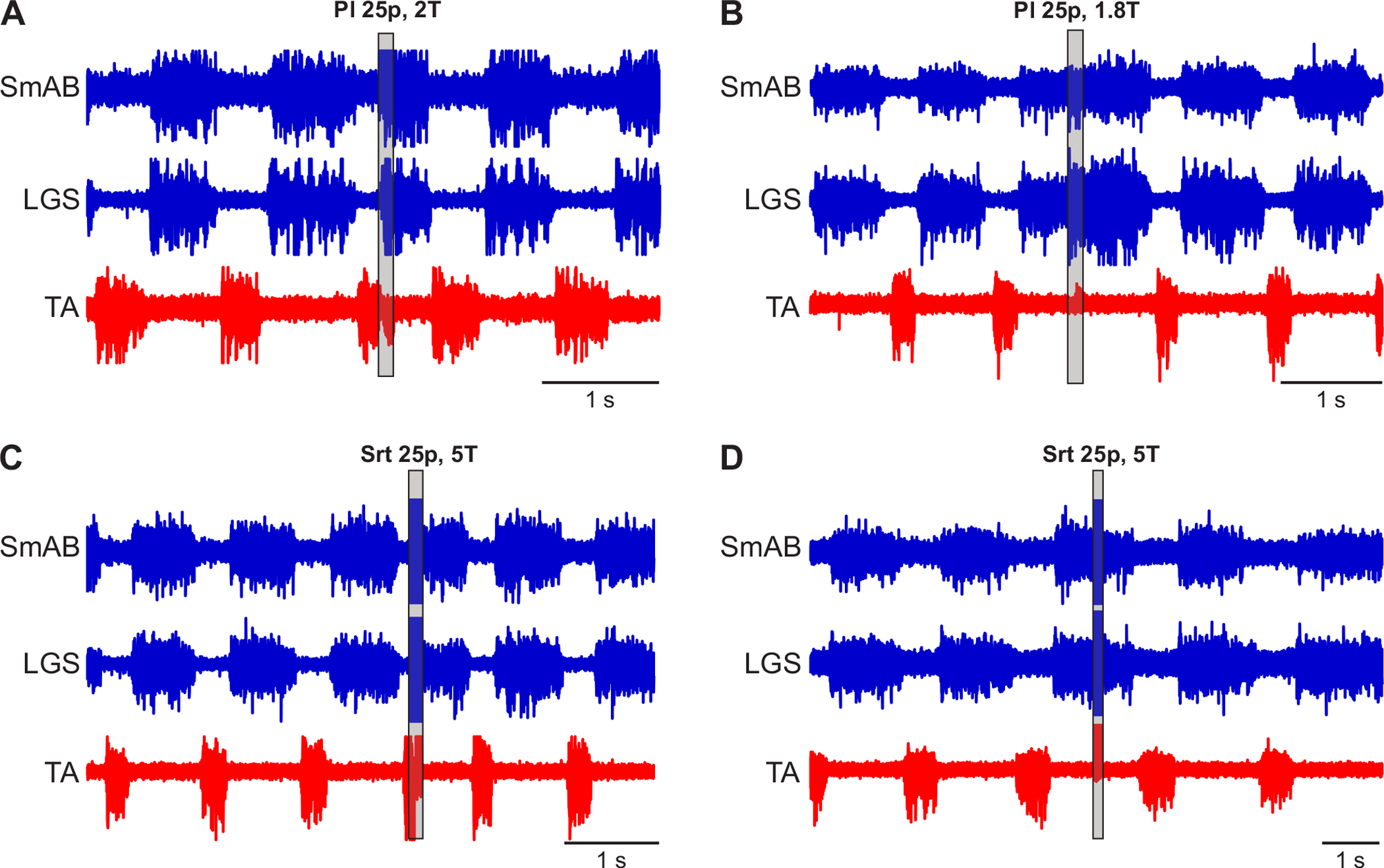
The figure shows the effect of stimulating the plantaris (Pl) nerve at group I strength and the sartorius (Srt) nerve at group II strength on the raw ENG bursts of activity during spontaneous fictive locomotion. Stimulation of the Pl nerve during A) mid-flexion reset the rhythm to extension while stimulation during B) late extension prolongs the extensor burst. Stimulation of the Srt nerve during C) early flexion resets the rhythm to extension while stimulation during D) mid- to late extension has no visible effect. LGS, lateral gastrocnemius-soleus; SmAB, semimembranosus-anterior biceps; T, threshold; TA, tibialis anterior. Reproduced and modified from (288).
The effect of stimulating flexor muscle afferents on phase durations and transitions during fictive or real locomotion is more variable compared to extensor group I afferents. During MLR-evoked or spontaneously occurring fictive locomotion, electrical stimulation of flexor muscle afferents at group II strength during flexion generally resets the rhythm to extension (Fig. 18C), while stimulation during extension produces no effect or a prolongation of extension (Fig. 18D) (288; 653; 793). However, when tested in the same preparation, flexor group II afferents from synergist muscles, such as tibialis anterior (TA) and extensor digitorum longus (EDL), both ankle flexors, could reset the rhythm from flexion to extension (TA stim) or prolong flexion (EDL stim) (793). Prolongation of extension at group II strength is mainly observed by stimulating hip flexors, such as sartorius and rectus femoris, which also extend the knee, although stimulating hip flexor afferents at group II strength can also enhance ongoing flexion (793). An extension enhancement from group II flexor reflex afferents is not observed during treadmill locomotion in decerebrate cats (371). Stimulation of flexor afferents at group I strength in flexion during fictive or treadmill locomotion in decerebrate cats generally increases flexor activity and prolongs the ongoing phase while stimulation during extension resets the rhythm to flexion (371; 478; 479; 653; 793). Thus, group I and II flexor muscle afferents appear to have competing effects on phase durations and transitions during locomotion, at least during fictive locomotion, which likely explain the variable results of stimulation at group II strength, where group I afferents are also maximally activated. Oligosynaptic excitatory pathways appear to mediate flexion enhancement by group I flexor afferents (186; 703). Functionally, these results point to a facilitation of swing by low threshold flexor muscle afferents.
Low-threshold electrical stimulation of cutaneous afferents from the paw pads (distal tibial nerve) during MLR-evoked fictive locomotion in decerebrate cats or L-DOPA-induced fictive locomotion in high spinal cats produces similar effects as group I ankle extensor afferents, with resetting of the rhythm from flexion to extension and prolongation of the on-going extension phase (216; 346; 747). On the other hand, cutaneous afferents from the foot dorsum (superficial peroneal nerve) can prolong extension during extension and enhance flexion during flexion (346), although resetting from extension to flexion has also been reported in high spinal cats treated with L-DOPA and nialamide (747). Mechanical stimulation of the skin overlying the lumbar region also effectively resets the locomotor rhythm to flexion in rabbits and cats (290; 571; 833; 834). Tonic lumbar skin stimulation stops locomotion, abolishes weight support and maintains the hindlimbs in hyperflexion. Although high-threshold Aδ fibers from the lumbar skin have been implicated in this phenomenon (834), light pressure is sufficient to inhibit locomotion and weight support, consistent with a strong contribution from Aβ fibers (409)
Electrical stimulation of high threshold afferents that include joint, cutaneous and group II and III muscle afferents can reset the rhythm from extension to flexion or prolong on-going flexion or produce opposite effects, with resetting from flexion to extension and prolongation of extension (747). The effect depends on the nerve being stimulated, with high threshold flexor muscle afferents generally enhancing flexion while those from extensor muscles, particularly those from distal muscles, generally promoting extension, albeit with a shift to flexion enhancement at higher stimulation intensity, particularly in proximal hip and knee extensors.
Cycle and phase variations
During real locomotion in mammals, including humans, cycle duration varies with the duration of the stance phase while swing phase duration remains relatively invariant with increasing speed [reviewed in (274; 324; 337)]. During fictive locomotion occurring spontaneously in decerebrate cats or in pharmacologically-evoked fictive locomotion in acute or chronic spinal-transected decerebrate cats (281; 282; 338), cycle duration varies with the extension phase, also termed extensor-dominated, similar to what occurs during real locomotion in animals and humans (44; 174; 316; 340; 350). In contrast, with electrical stimulation of the MLR, the proportion of the flexion phase increases and the cycle varies more with flexion duration, also termed flexor-dominated (281; 875). Indeed, when MLR stimulation was performed during spontaneously occurring fictive locomotion, the rhythm changed from extensor- to flexor-dominated (281). This indicates that supraspinal inputs can change the control of phase variations by the spinal locomotor CPG. Somatosensory inputs also alter phase variations. For example, a slight tonic dorsiflexion of the ankle, which stretches ankle extensors, strengthens extensor dominance during spontaneous fictive locomotion in decerebrate cats (282). The same study also showed that during fictive scratch, which is a flexor-dominated rhythm, ankle dorsiflexion changed the rhythm from flexor- to extensor-dominated. Thus, phase variations are extremely sensitive to somatosensory inputs.
Entrainment
Another way to demonstrate the regulation of phase durations and transitions by somatosensory inputs is by entraining the rhythm by changing treadmill speed in spinal cats or during fictive locomotion with electrical or mechanical stimulation at varying frequencies, as shown in decerebrate cats (30; 162; 468; 644). Entrainment refers to the consistent timing of a phase onset to an event or stimulation over a range of frequencies, such as the onset of extensor bursts timed to sinusoidal stretches of extensor muscles. When the hindlimbs of spinal cats are placed on a treadmill, they match belt speed, changing their step frequency (178; 184; 191; 266; 280; 283). Moreover, when the hindlimbs of spinal cats are placed on a split-belt treadmill, each limb will adjust to the speed of its respective belt (267; 280; 283; 482). A recent study showed that spinal cats produced hindlimb locomotion and adjusted to speed in the backward direction, including backward split-belt locomotion (359). To perform these adjustments, somatosensory feedback from the limbs interacts with the spinal locomotor network.
Evidently, during treadmill locomotion in the spinal preparation, many different types of somatosensory afferents can entrain the rhythm for speed adjustments in the forward or backward directions. However, strong candidates are stretch-related inputs from hip muscles and load or stretch-activated inputs from extensor muscles. For instance, Kriellaars et al. (1994) showed that sinusoidal hip flexion and extension movements entrained the rhythm during MLR-evoked fictive locomotion in decerebrate cats. Extensor and flexor bursts were timed to hip flexion (stretching of hip extensors) and extension (stretching of hip flexors), respectively, over a range of frequencies. They observed entrainment with small hip movements and while progressive denervation of hip muscles weakened entrainment, it remained present until all hip muscles were denervated. Denervating the joint capsule did not affect entrainment when hip muscle afferents were present, excluding a role of joint afferents. Variations in the cycle matched variations in extensor burst durations, indicating that phasic stretch-related low threshold afferent inputs (group I and II) from hip extensor muscles controlled extensor burst durations and entrained the rhythm. In another study in acute spinal-transected decerebrate cats treated with L-DOPA and nialamide, stretch of ankle extensors entrained the fictive locomotor rhythm (162). Extensor burst onset was timed to stretching and loading of ankle extensors. They argued that group Ib afferent inputs entrained the rhythm. Cutaneous inputs can also entrain locomotor-like rhythms, as shown in spinal cats with trains of electrical stimuli to the SP or distal tibial nerves (571; 572).
To summarize, various types of somatosensory afferents compete to enhance flexion or extension during locomotion and hence the transitions between phases. Generally, extensor muscle afferents and cutaneous afferents from the plantar surface promote extensor activity while those from flexor muscle afferents and cutaneous afferents from the foot dorsum promote flexor activity. The spinal locomotor CPG uses redundant sources of somatosensory information from multiple muscles and skin regions to regulate the rhythm and its phase durations and transitions, changing the weighting of each according to task demands. As discussed earlier, this requires gating or modulation of these inputs.
Somatosensory feedback regulates the magnitude of muscle activity
Somatosensory feedback can change the magnitude of muscle activity, to reinforce or weaken muscle contractions to precisely control force and meet task demands. In animal models and humans, the magnitude of muscle activity in limb flexors and extensors is modulated with locomotor speed (407; 410; 425; 553; 670; 784), in different gaits (126; 350; 784), when stepping on an incline or decline (127; 329; 372; 464; 783; 815) and differentially in the slow and fast limbs during split-belt locomotion (195; 280; 289) or when walking on a circular path (170). All these modulations occur in spinal cats, indicating that somatosensory feedback interacting with spinal locomotor circuits plays an important part in regulating muscle activity.
Proprioceptive feedback from extensor muscles
Load- and stretch-sensitive receptors in extensor muscles become activated at stance onset as these muscles contract while being lengthened (although fascicles of some distal muscles might shorten), thus activating group Ib, Ia and II afferents. As stance progresses, extensor muscles shorten to propel the body forward. Studies have shown that somatosensory feedback makes a major contribution to extensor EMG amplitude during stance. For example, when a hindlimb of a decerebrate cat stepped in a hole on the treadmill, the EMG amplitude of knee and ankle extensors decreased by about 30% (Fig. 19A) (370). Increasing the force at the ankle (hence force feedback) during foot-in-hole trials restored EMG amplitude to normal values (Fig. 19B). Partial deafferentation by sectioning dorsal root at L4–L6 also decreased EMG amplitude of knee extensors by about 50% without affecting ankle extensor activity. A more extensive deafferentation that included L7–S2 roots decreased ankle extensor amplitude by more than 50%.
Figure 19. Proprioceptive feedback from extensors muscles contributes to the magnitude of extensor activity.
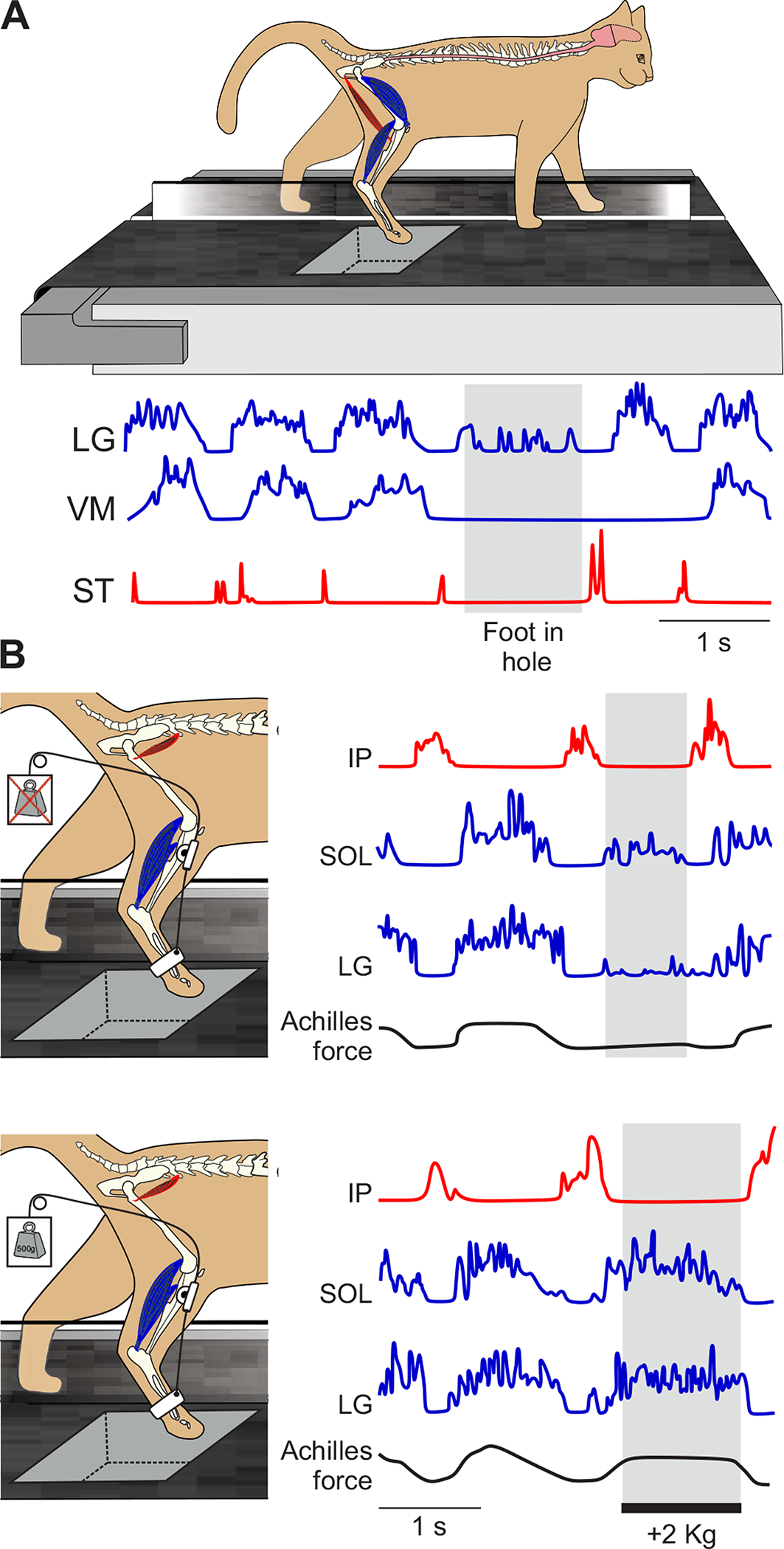
A) When the hindlimb of a decerebrate cat steps in a hole during treadmill locomotion, the EMG activity in ankle (LG, lateral gastrocnemius) and knee extensor (VM, vastus medialis) muscles reduced. The shaded area indicates the time the foot entered the hole. B) Loading ankle extensor muscles during foot-in-hole trials restored normal levels of EMG activity in ankle extensor muscles. The shaded area indicates the time the foot entered the hole without (top) and with (bottom) load applied to the Achilles tendon. Reproduced and modified from (370).
During spontaneous fictive locomotion in decerebrate curarized cats, a slight tonic dorsiflexion of the ankle considerably increases the amplitude of extensor muscles throughout the hindlimb (282). Increasing the level of dorsiflexion or the force applied to ankle extensors abolishes the locomotor-like rhythm, maintaining it in extension (219). Brief trains of electrical stimulation to ankle extensor nerves during extension also increases ipsilateral extensor EMG amplitude during spontaneous or drug-induced fictive locomotion in spinal-intact and spinal cats, respectively (see Fig. 18B) (162; 288). This effect is mainly attributed to group Ib afferents.
As stated earlier, stimulating ankle extensor group Ib afferents at rest evokes inhibition in extensor muscles, or a negative feedback (225). However, during locomotion, group Ib afferents activate an alternative pathway that elicits excitation in extensor muscles during the extension phase or stance (319; 644), although some inhibitory effects have also been reported (717). Studies have shown that this positive feedback reinforces the magnitude of extensor activity during the stance phase in cats (199–201; 370) and humans (6; 335; 608). This positive feedback could play an important role when increasing speed by increasing extensor activity to stabilize the leg at contact and for propulsion at push-off. In healthy and spinal cord-injured humans, reducing bodyweight with a harness reduces leg EMG amplitude, particularly in antigravity muscles, during walking (357). This modulation was more closely associated with peak load, as opposed to muscle-tendon length or stretch velocity, consistent with a role of force feedback. Intracellular recordings during MLR-evoked or drug-induced fictive locomotion in decerebrate curarized spinal-intact or spinal cats showed that stimulating ankle extensor afferents at group I strength evoked disynaptic EPSPs in hip, knee and ankle extensors during the extension phase, but not during flexion (33; 560). Hip and knee extensor afferents also evoked group I disynaptic EPSPs in homonymous motoneurons. Disynaptic EPSPs were attributed to both group Ia and Ib afferents.
Although the evidence points to a relatively more important role of force feedback from limb extensors in regulating EMG activity, studies also report that stretch-sensitive feedback from muscle spindles plays a role. In humans, sudden stretching or shortening of ankle extensor muscle during stance modulates extensor muscle activity, an influence attributed mainly to group II afferents (194; 776; 879), although some have attributed this to load feedback (6). In cats, stretching of the ankle extensors muscle-tendon units normally occurs when the foot contacts the ground in early stance (E2 phase). This stretching of ankle extensor muscles coincided with activation of spindle afferents and could contribute to the overall activity of extensor muscles (696). More recently, using genetically modified mice in combination with gene delivery, Mayer et al. (2018) selectively removed muscle spindle feedback from ankle extensor muscles, which abolished the speed-dependent modulation of ankle extensor activity. However, removing spindle feedback from ankle extensors did not significantly affect the speed-dependent modulation of EMG amplitude in other hindlimb extensors.
Proprioceptive feedback from flexor muscles
Proprioceptive feedback from flexor muscles also regulates the magnitude of muscle activity during locomotion, as shown in various cat preparations. For example, Hiebert et al. (1996) stretched or vibrated hip and ankle flexors, or electrically stimulated their afferents, during spontaneous treadmill locomotion in decerebrate cats. They found that stretching flexor muscles or electrically stimulating their nerves during stance weakened the amplitude of extensors and advanced swing onset. For proprioceptive feedback from TA, the reduction in extensor amplitude required stimulation at group II strength while for EDL they observed a clear effect at group I strength. Vibration of the EDL confirmed the involvement of group Ia afferents. In contrast, the same stimulations during swing did not noticeably change the EMG amplitude of flexors. Other studies from Keir Pearson’s group showed that proprioceptive feedback from the hip flexor sartorius plays a key role in reinforcing hip flexor activity during swing (478; 479). Blocking hip flexion during swing normally increased hip flexor activity by 20–50%. Detaching the iliopsoas from its insertion did not change the 20–50% increase in hip flexor activity of the iliopsoas and both compartments of the sartorius (anterior and medial) when blocking hip flexion during swing. However, after detaching the sartorius muscles distally or by blocking their nerve conduction, the percent increase in the iliopsoas when blocking hip flexion during swing virtually disappeared. In another study, Lam and Pearson (2002) showed that stimulating sartorius muscle afferents at group I strength increased EMG amplitude in the iliopsoas muscle during swing while stimulation at group II strength produced inhibition in iliopsoas and TA.
During MLR-evoked fictive locomotion in decerebrate curarized cats, stimulating flexor muscle afferents at group I or II strengths or stretching/vibrating flexor muscles can increase or decrease the magnitude of activity in hindlimb nerves, and elicit EPSPs or IPSPs, depending on the phase of the cycle (186; 703; 793). For instance, Quevedo et al. (2000) found that the largest group I disynaptic EPSPs in ankle flexor motoneurons occurred with homonymous stimulation and peaked in flexion. Although smaller, EPSPs also occurred during the extension phase. They also observed large EPSPs with stimulation of afferents from synergist ankle flexor muscles and from bifunctional muscles, such as sartorius and semitendinosus. They attributed the effects to both group Ia and Ib afferents. Flexor muscle afferents also elicit disynaptic IPSPs in antagonist muscles and other extensors, with IPSPs generally peaking in flexion (186; 703). Thus, proprioceptive feedback from flexor muscles is distributed to several hindlimb motor pools and could fine tune muscle activity during locomotion, particularly during the swing phase.
Tactile feedback
In the human hand, cutaneous afferents play a key role in adjusting grip strength while holding or manipulating objects (448; 449; 848). They do this by scaling and tuning the magnitude of muscle activity. The paws of animals and the human foot likely accomplish a similar function, to maintain appropriate contact forces with the ground. Cutaneous afferent stimulation provides short-latency excitatory and inhibitory synaptic inputs to motoneurons innervating trunk and limb muscles (118; 349; 857). During locomotion in cats, electrically stimulating cutaneous nerves of the fore- or hindpaw evokes short-latency (~10 ms) and longer-latency (~25 ms) excitatory or inhibitory responses in limb muscles. Studies have described these responses as P1 and P2 for short- and longer-latency positive/excitatory responses, respectively, or as N1 and N2 for short- and longer-latency negative/inhibitory responses (shown in Fig. 9) (2; 209; 217; 501). In humans, P1 and P2 responses have onsets around 50 ms and 70–80 ms, respectively (51; 220). Stimulating cutaneous nerves during stance generally elicits N1 responses followed by P2 or P3 (> 35 ms) responses in extensor muscles and P1 and P2 responses in flexors of the stimulated fore- or hindlimb. During swing, P1 and P2 responses in flexors peak in amplitude with weak or absent responses in extensors. At higher stimulation intensity or with longer trains, cutaneous inputs alter limb trajectory. Figure 20 shows examples with stimulation of the superficial radial (SR) and SP nerves during mid-stance and mid-swing of the stimulated limb. During stance, nerve stimulations can co-activate extensors and flexors (Fig. 20A) to ensure stable support (Fig. 20B). However, during swing, the same stimulations flex and elevate the limb by changing muscle activity, particularly by increasing flexor activity.
Figure 20. Cutaneous inputs regulate muscle activity and alter limb trajectory in a phase-dependent manner.
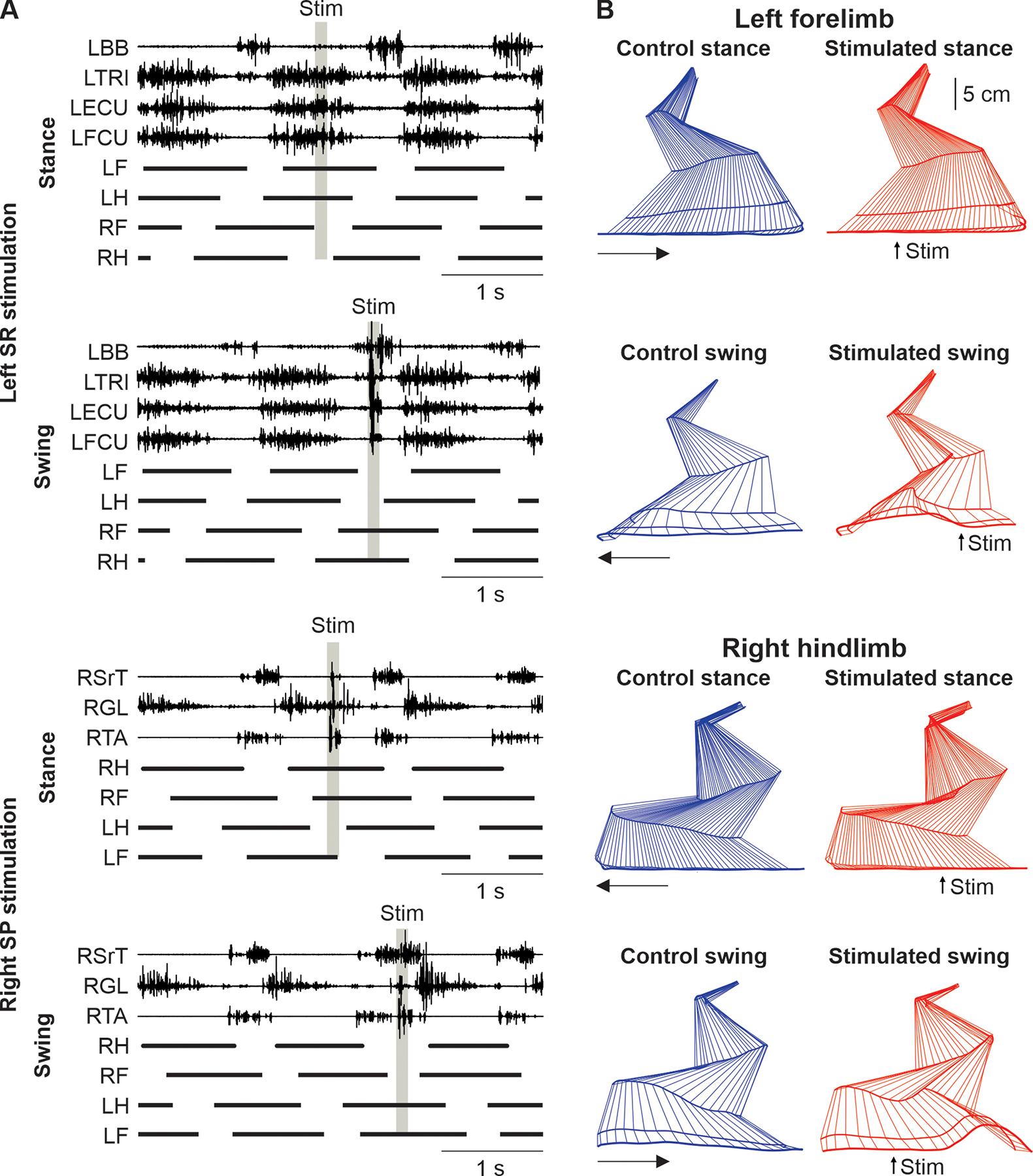
A) The effects of stimulating the superficial radial (SR) and superficial peroneal (SP) nerves on the EMG activity of selected muscles and the phases of the four limbs during treadmill locomotion at 0.4 m/s in an intact cat. The SR and SP nerves were stimulated during mid-stance and mid-swing of the homonymous limb. The shaded area indicates the period of stimulation (25 pulses of 0.2 ms duration at 200 Hz and at 1.2 times the motor threshold). B) Kinematic reconstruction of the forelimb (top panels) and hindlimb (bottom panels) without (control) and with stimulation during stance and swing. Note that in the top panels the left SR was stimulated while in the bottom panels the right SP was stimulated. Unpublished data from Frigon lab. BB, biceps brachii; ECU, extensor carpi ulnaris; FCU, flexor carpi ulnaris; GL, gastrocnemius lateralis; L, left; F, forelimb; H, hindlimb; R, right; Srt, anterior sartorius; ST, stance; TA, tibialis anterior; TRI, triceps brachii.
Cutaneous inputs from other body regions also exert powerful influences on locomotion. As discussed in the previous section, stimulating the lumbar skin stops locomotion and abolishes hindlimb weight support in rabbits and cats (409; 833). The weakening of extensor activity with lumbar skin stimulation could help rapidly lower the animal’s hindquarters following contact to avoid injury or capture. In contrast, stimulating the area under the tail, the perineal region (vulva, scrotum and inguinal fold), increases locomotor activity. This facilitation of locomotion has been shown in a variety of spinal mammals, including mice (488), rats (20) and cats (360; 572; 718). In spinal cats, perineal stimulation can turn a weak locomotor pattern into a robust one by increasing the activity of flexors and extensors throughout the hindlimbs. The function of this sensorimotor pathway and its interactions with the spinal locomotor CPG remain unclear but it could serve an important survival function by facilitating escape from predators.
Somatosensory feedback regulates muscle and inter-joint coordination
Despite the great variety of body shapes and sizes of terrestrial mammals and the terrains over which they move, they have evolved common musculoskeletal design features and solutions to motor control challenges [for reviews see (15; 241; 822). A common design feature is motor redundancy of the musculoskeletal system, which has a large number of kinematic DOF (i.e., total number of rotation directions in all joints), with over 240 in humans, and a large number of muscles and compartments exceeding the number of DOF by almost 3 times. Each muscle or compartment has the ability to produce a moment of force with respect to approximately 4 DOF on average (e.g., about the flexion-extension and adduction-abduction axes in one joint and flexion-extension and pronation-supination axes in an adjacent joint), indicating that most muscles control multiple DOF and span two or more joints (688). Motor redundancy offers great flexibility in choosing motor strategies for locomotion, including choice of gaits, trajectories of the CoM, limb segments and joint angles, as well as muscle activation patterns. It also offers resiliency against injuries and external perturbations. On the other hand, motor redundancy and non-linear properties of the neuromuscular system make it difficult, if not impossible, to perform accurate coordinated movements without constant sensory corrections (75). The reasons for this include: 1) motion-dependent interaction moments arising in multi-segmented extremities that perturb the ongoing movement (382; 702; 734); 2) the continually changing capacity of a muscle to produce force at the same activation level because of non-linear muscle force-length-velocity properties (330; 684; 840) and tendon elasticity (380; 418; 522); 3) the number of recruited motor units and their firing patterns (313; 381); 4) noise in the neural control system (67); and 5) unexpected external perturbations.
Muscle proprioceptive feedback is well suited for quick, functionally appropriate responses to postural and movement perturbations and for efficient locomotion (121; 225; 226; 233; 234; 291; 390; 602; 606; 680; 681; 860). Length-dependent monosynaptic excitatory pathways support activation of muscle synergists through similar muscle stretch and α-γ motoneuronal drive. These muscles normally act together as a group during locomotion (359; 421; 471; 538) and in response to postural perturbations (385; 527; 813), forming a basis for muscle locomotor and postural flexor and extensor synergies. The length-dependent links among synergistic groups are not always symmetric. For example, excitation is normally stronger from medial to lateral synergists than in the opposite direction, which likely reflects differences in mechanical actions of synergistic groups with respect to different DOF (602) and because the limbs exert forces on the ground in the outward direction in relation to the body’s long axis during standing and locomotion (251; 292).
Circuits within the spinal cord coordinate muscles crossing single and multiple joints. For example, length-dependent afferents from synergists acting at a joint give rise to disynaptic pathways via Ia-inhibitory interneurons to strict anatomical antagonists (233; 499). The Ia-inhibitory interneurons mediate inhibition of antagonists evoked by stretch of the synergists and by receiving a parallel central drive to motoneurons of the synergies (254; 301). Monosynaptic length-dependent pathways also link one-joint extensors across neighboring hindlimb joints, ankle, knee and hip (226; 233; 234; 854), with functionally similar links in the forelimb (121; 291). These cross-joint links appear to support the coordinated actions of muscle groups against gravity during standing and the stance phase of locomotion (249; 471; 526; 538). Some cross-joint links from group Ia afferents exhibit a proximal to distal excitation gradient (226; 234; 291; 854).
Force-dependent disynaptic and trisynaptic pathways from GTOs have more distributed actions across motoneurons of hindlimb muscles (225; 431; 604). Apart from autogenic inhibitory actions, these links provide inhibition to extensor motoneurons from synergists of the same joints and extensors of neighboring joints (225; 854). Within a group, inhibitory actions appear stronger from two-joint muscles compared to their one-joint synergists, such as from gastrocnemius to soleus (176; 603) or from rectus femoris to vastii (854). Force-dependent pathways also provide excitation from extensor to flexor motoneurons of antagonists at the same joint, such as from triceps surae, plantaris and flexor digitorum longus to pretibial flexors or at neighboring joints, such as from quadriceps to pretibial flexors (225).
Length- and force-dependent pathways were suggested to have important functional implications for regulating limb stiffness and stability, resisting postural perturbations and performing efficient locomotion (233; 291; 604). Because of the complexity of musculoskeletal design features and spinal reflex pathways, a full understanding of their significance for muscle and inter-joint coordination is still missing. One attempt explained some features of muscle coordination using a relatively simple musculoskeletal human leg model, assuming that people minimize fatigue and sense of effort when performing skilled locomotor behaviors and postural tasks (680; 681). Computed muscle forces that minimize muscle fatigue for given mechanical demands (the required joint moments) of different motor tasks, such as walking, cycling, exerting external forces in different directions and arm postural tasks, show surprisingly similar patterns to corresponding recorded forces and EMG. For example, synergists show simultaneous force production. One-joint muscles demonstrate strict reciprocal action at a given joint. Two-joint muscles produce the greatest force when they can contribute to desired moments at the two joints spanned by the muscle. For example, the computed force of the two-joint gastrocnemius, an ankle extensor and knee flexor, is greater than the soleus force, a one-joint ankle extensor, when the movement requires ankle extension and knee flexion moments. If the magnitude of the knee flexor moment continuously increases and the ankle extension moment does not change, the soleus force continues to decrease and reaches zero. At this point, the one-joint antagonist tibialis anterior starts to produce increasing force.
Features of muscle coordination predicted by minimizing muscle fatigue have been consistently observed during locomotion, postural corrective responses, paw shakes, and other automatic and reflex responses. This coordination does not seem to depend on the type of muscle action (i.e. isometric, concentric or eccentric) because it has been observed during isometric tasks (109; 429), upslope and downslope locomotion (127; 783), fast paw shake responses (566; 782) and relatively slow load lifting (566; 685). Removing monosynaptic length-dependent input to motoneurons from gastrocnemius and soleus in the cat by muscle self-reinnervation did not change the synergistic activation of the triceps surae muscles during level, upslope and downslope walking (329; 635) or the selective inhibition of soleus and the enhanced EMG activity of the gastrocnemii during paw shake (566). On the other hand, removal of the local stretch reflex from gastrocnemii and soleus by self-reinnervation produced task-dependent changes in inter-joint coordination in cats (1). In this study, after self-reinnervation of the triceps surae muscles, which abolishes autogenic length feedback (519), inter-joint coordination was affected (greater ankle yield) during the stance phase of downslope overground locomotion, whereas level and incline stepping were unaffected. During downslope walking, ankle extensors undergo greater stretching compared to level and incline walking (522) and the animals appeared unable to compensate for the loss of autogenic length feedback to correct inter-joint coordination. At the same time, the greater ankle yield and increased knee angle could be a compensatory mechanism to increase length feedback from spared ankle extensors and to increase the passive ankle extensor moment (523; 687).
Cutaneous feedback also contributes to coordination. As stated, an important functional response mediated by cutaneous feedback that requires rapid control of inter-joint coordination occurs when the foot contacts an obstacle during the swing phase, when the foot is off the ground. Quevedo and colleagues described the sequential activation of hindlimb motoneurons with stimulation of the SP nerve during the flexor phase of MLR-evoked fictive locomotion in decerebrate curarized cats (704; 705). The stimulation train initially excites motoneurons of knee flexors/hip extensors (posterior biceps and semitendinosus) followed by a brief ankle extensor excitation (lateral gastrocnemius) and inhibition of ankle flexors (TA). The activation of these motoneurons, with central latencies of ~2 ms occurs through di- or tri-synaptic pathways. Following a delay, ankle flexor and hip flexor (sartorius, psoas) motoneurons are excited, sometimes following an initial inhibition. Figure 21 illustrates the spinal circuitry involved in the sequential activation of motor pools innervating muscles crossing different joints by SP nerve afferents during the flexion phase.
Figure 21. Cutaneous inputs regulate inter-joint coordination during locomotion.
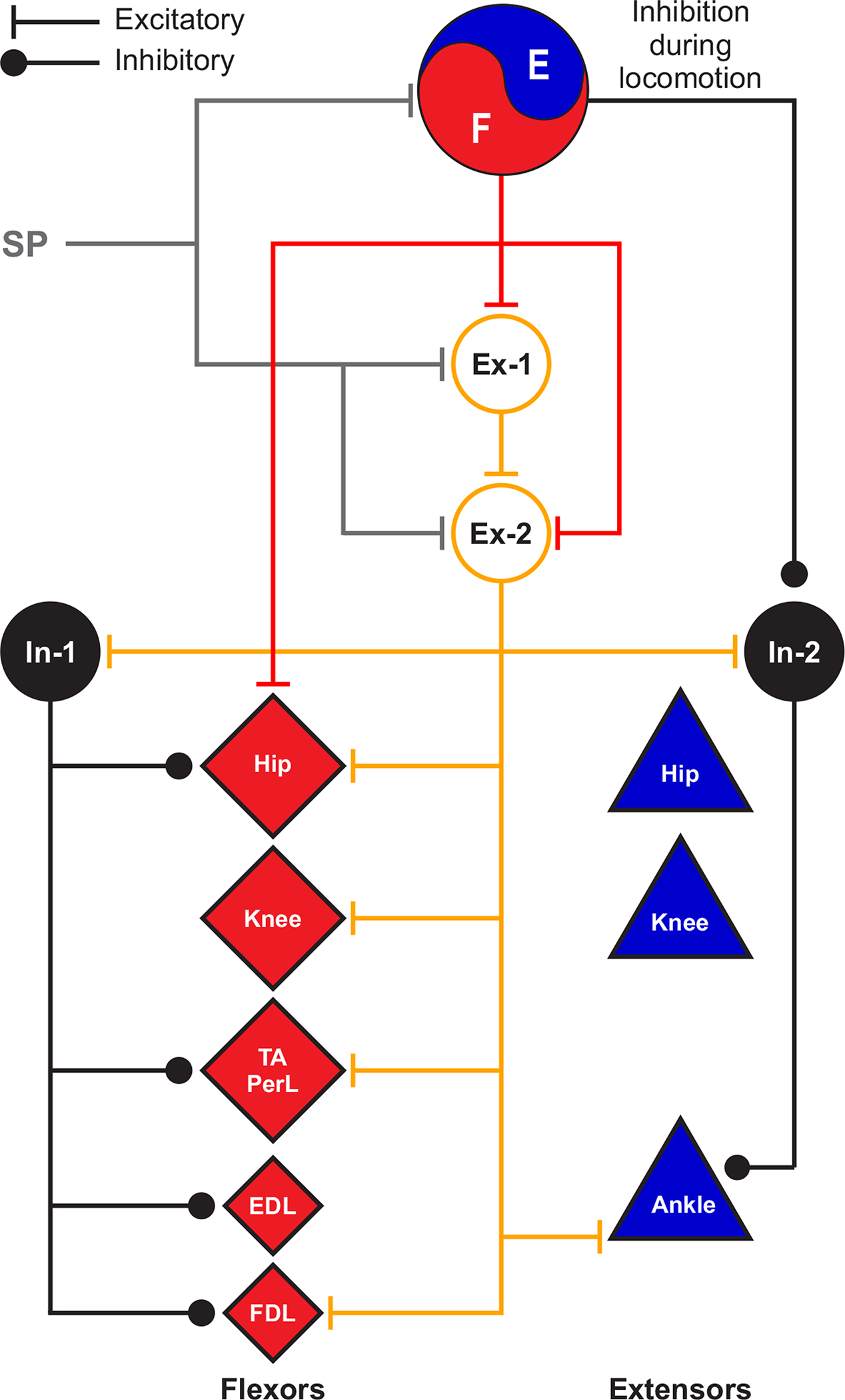
The figure shows short-latency pathways from cutaneous afferents of the superficial peroneal (SP) nerve to different hindlimb motoneurons during the flexion phase of fictive locomotion. The central pattern generator (CPG) is shown with mutually inhibiting extensor (E) and flexor (F) parts. The CPG can phasically modulate interneurons mediating di- and trisynaptic excitation of hindlimb motoneurons from SP afferents. The inhibitory pathway to ankle extensor motoneurons observed at rest (last-order inhibitory interneuron In-2) is inhibited by the spinal locomotor CPG. Reproduced and modified from (705). Ex-1 and Ex-2, excitatory interneurons 1 and 2. In-1 and In-2, inhibitory interneurons 1 and 2.
When the same cutaneous stimulation is delivered during ipsilateral stance in intact or spinal cats, increased extensor activity is observed throughout the limb to assist in weight support followed by greater flexor activity in the subsequent swing phase (110; 265; 269). This is termed the stumbling preventive reaction. Quevedo and colleagues also investigated hindlimb motoneuron activation during the extensor phase of MLR-evoked fictive locomotion in decerebrate curarized cats (704; 705). During the extensor phase, SP nerve stimulation evoked short-latency increases in the activity of hip, knee and ankle extensors as well as toe plantarflexors. The following flexion phase showed greater activity in hip, knee and ankle flexor motoneurons.
The stumbling corrective reaction and cutaneous reflexes from the foot are also modulated by locomotor direction, as shown by comparing responses during forward and backward locomotion in intact cats (110) and humans (220). Mechanical stimulation of the plantar surface of the paw during the swing phase of backward locomotion in intact cats elicits a response, albeit less frequently than in the forward direction, that moves the limb away from the contact that consists of hip and ankle flexion and reduced knee flexion (110). After this initial period, knee and ankle flexors raise the limb over the obstacle followed by hip and knee extension to complete the swing phase for weight acceptance. The phase- and task-dependent modulation of the stumbling corrective (or preventive) reaction highlights its functional relevance for maintaining balance during locomotion.
To summarize, proprioceptive and cutaneous feedback play critical roles in muscle and inter-joint coordination by promoting synergistic muscle activity, reciprocal inhibition of one joint antagonists, and by selective activation and inhibition of two-joint and one-joint muscles, respectively. Such muscle coordination promotes efficient fatigue-resistant movements, regulation of limb stiffness and postural stability during locomotion.
Somatosensory feedback regulates interlimb coordination
An effective locomotion in quadrupedal mammals and bipedal humans requires proper coordination between the limbs [recently reviewed by (275; 886)]. As described in previous sections, trunk and limb afferents project to spinal and supraspinal structures that then project to motor circuits controlling the four limbs. In the spinal cord, two main types of neurons coordinate the CPGs controlling the limbs, commissural interneurons and propriospinal neurons, and both types are strongly activated by somatosensory inputs.
It has long been known that stimulating cutaneous afferents in one leg evokes short-latency responses in the contralateral leg (761). This is often referred to as the crossed extensor reflex. As stated, stimulating cutaneous nerves or the skin of the foot/hindpaw during the swing phase evokes short-latency reflex responses in ipsilateral leg muscles but also in flexor and extensor muscles of the contralateral homologous limb, as part of the stumbling corrective reaction (depending on the nerve/foot region stimulated). Crossed responses are observed in intact and spinal cats (217; 269; 285; 287; 406–408), mice (475) and humans (193; 222; 798) and are thought to stabilize the support limb by increasing limb stiffness. Crossed responses are also observed between the forelimbs during locomotion with cutaneous nerve stimulation or limb perturbations (408; 720; 735). Crossed responses are mediated by commissural interneurons that have their cell bodies on one side of the cord, mainly in lamina VIII, and axonal projections to the contralateral side where they contact excitatory and inhibitory interneurons as well as motoneurons via a few to multiple collateral branches [recently reviewed in (551)]. Studies in cats and small rodents have shown that various types of proprioceptive and tactile inputs activate commissural interneurons (7; 48; 50; 60; 236; 240; 436) and assist in left-right coordination (551; 762; 805).
The stumbling corrective reaction also involves responses in muscles of all four limbs concurrently, as shown in intact cats (408) and humans (354). Thus, the corrective reaction is a whole-body response that involves pathways coordinating all four limbs, and likely trunk muscles. Indeed, cutaneous and muscle afferents activate propriospinal neurons that project between cervical and lumbosacral levels, thus coordinating the activity of cervico-thoracic and lumbosacral CPGs. Propriospinal pathways can be more or less direct with long axonal projections across several spinal segments (e.g. from cervical to lumbar levels or vice versa) or by involving a series of propriospinal relay neurons that project over short distances (263; 275; 477). Schomburg and colleagues stimulated muscle and cutaneous nerves in the forelimbs of decerebrate curarized cats with a high cervical spinal transection and recorded evoked responses in hindlimb motoneurons (746; 748; 749). At rest and during pharmacologically-evoked fictive locomotion, forelimb afferent inputs activated long descending propriospinal pathways that project to homolateral and diagonal hindlimb motor pools. Hindlimb muscle and cutaneous afferents also project to propriospinal neurons with long ascending projections to homolateral and diagonal forelimb motor pools, as shown in high spinal cats (577). During locomotion in cats and humans, cutaneous reflexes between the arms/forelimb and legs/hindlimbs are modulated by phase (192; 354; 355; 408; 480; 578; 888). Figure 22 shows interlimb reflexes during locomotion in an intact cat with electrical stimulation of the SP nerve. As can be seen, stimulating low threshold cutaneous afferents of the SP nerve evokes short- (7–10 ms) and longer-latency (18–25 ms) excitatory and/or inhibitory responses in all four limbs concurrently. Reflex responses are modulated by phase, generally peaking when the muscle is active. These interlimb reflex pathways are thought to play an important role in coordinating the upper and lower limbs during locomotion, particularly with unexpected perturbations.
Figure 22. Interlimb reflexes coordinate the four limbs during locomotion.
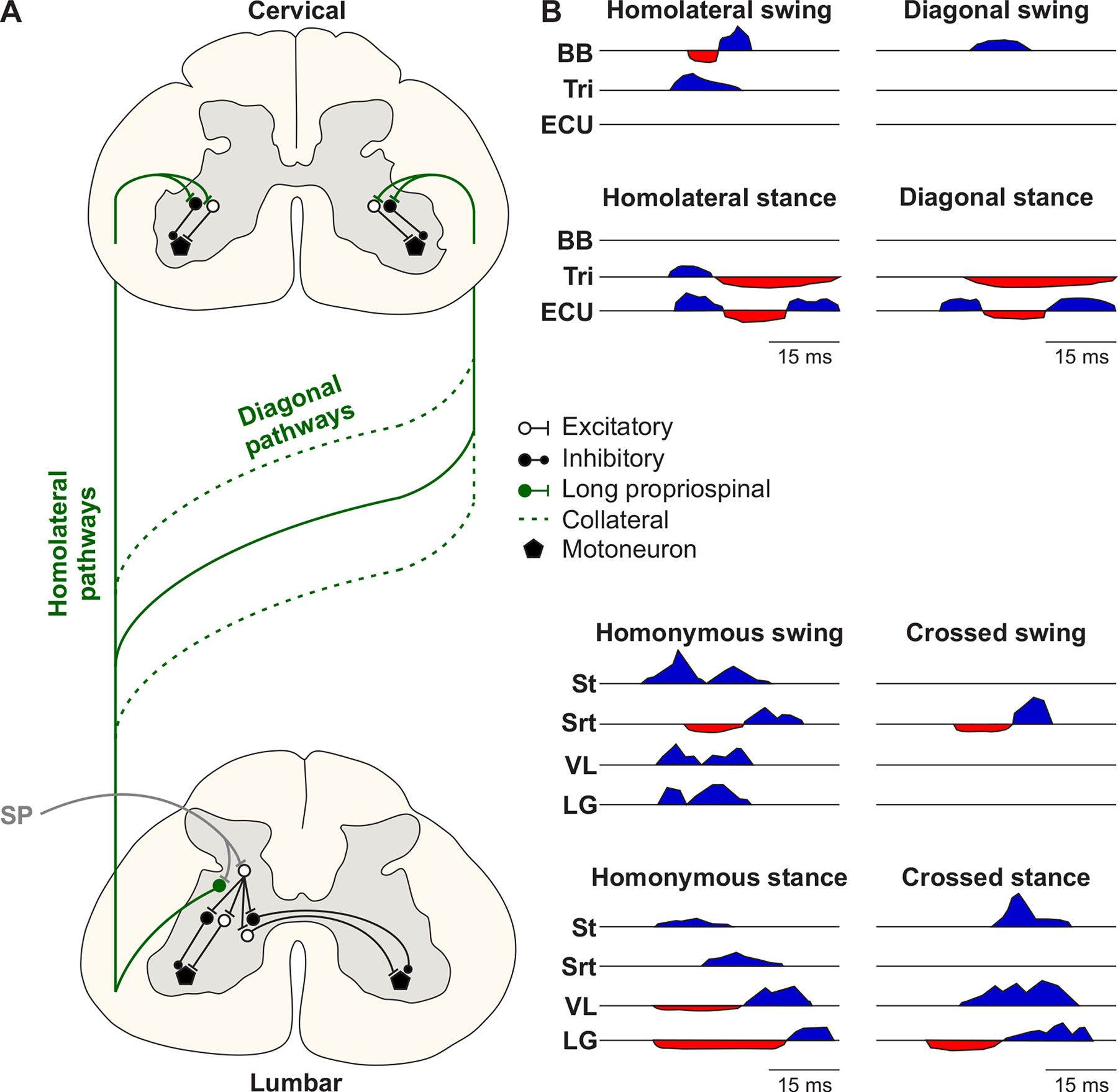
Schematic of reflex pathways and main responses during stance and swing evoked by SP nerve stimulation. A) Upon entering the spinal cord, primary afferents from the SP nerve contact 1) interneurons that project within the hemisegment (homonymous responses), 2) commissural interneurons that project contralaterally (crossed responses), and 3) ascending propriospinal neurons with their main axonal projections terminating ipsilaterally (homolateral responses) or on the other side (diagonal responses). Diagonal pathways cross at various segments along the length of the spinal cord and include collaterals from homolateral pathways that also project contralaterally. B) Panels show the main pattern of forelimb and hindlimb responses evoked with SP nerve stimulation when the different limbs are in mid-swing or mid-stance. Responses shaded in dark blue represent excitatory responses while those in red represent inhibitory responses. Responses are aligned to the start of the stimulation. Adapted from (408).
Several supraspinal structures that receive direct or indirect somatosensory information project to spinal motor circuits controlling the four limbs during locomotion. These include reticulospinal (205; 208; 656; 766), vestibulospinal (543; 544) and rubrospinal (389; 442) neurons in the brainstem. The brainstem reticular formation and the cerebellum also contribute to longer-latency reflex responses evoked in limb muscles following peripheral nerve stimulations, termed spino-bulbo-spinal reflexes (764; 765; 767). For example, in Figure 22, longer-latency responses could be mediated by pathways traversing the brainstem. In spinal cats, the longer-latency responses in hindimb muscles are generally diminished or abolished, depending on the muscle, consistent with a supraspinal contribution (406; 407; 473)
Other brain regions that control interlimb coordination are found throughout the sensorimotor cerebral cortex, which receives somatosensory information indirectly from the thalamus and from intra-cortical connections. The sensorimotor cerebral cortex projects to the spinal cord via the corticospinal tract and through relays in the brainstem (491). During locomotion in cats and humans, the corticospinal tract can influence muscle activity in the four limbs (64; 65; 99; 709). Other supraspinal structures that receive somatosensory inputs, such as the cerebellum and thalamus, affect interlimb coordination indirectly by modifying the output of the sensorimotor cerebral cortex and descending brainstem pathways.
The role of somatosensory feedback in functional recovery following injury
Somatosensory feedback plays an important, and even essential, role in the recovery of meaningful movement following neurological injury or in disease. This topic, in itself, could cover several reviews. Here, we will briefly describe the role of proprioceptive and tactile feedback in the recovery of locomotion following spinal cord injury (SCI) and peripheral nerve injury (PNI). To understand the role played by somatosensory feedback in motor recovery after SCI or PNI, it is important to underscore that the widespread loss of touch and proprioceptive information, with a viral infection for example, initially produces motor paralysis, despite intact CNS motor pathways and spinal motoneurons (476). Although people afflicted with such a disease will recover some movements, the capacity to stand and walk upright is generally permanently lost.
Spinal cord injury
Depending on the severity of the lesion, SCI partially or completely disrupts descending motor pathways but also ascending pathways that carry somatosensory information up to various CNS neurons and structures, either directly or indirectly. Thus, SCI not only disrupts the ability of descending motor pathways to control spinal sensorimotor circuits but also the control of supraspinal locomotor centers by somatosensory feedback. Not surprisingly, SCI leads to a host of sensorimotor deficits, such as impaired walking and balance and unwanted changes that can impede movements, such as hyperreflexia and muscle spasms.
In humans with an anatomically complete SCI, the ability to stand and walk does not recover. Some recovery is however possible with appropriate stimulation in humans with incomplete SCI (35; 305; 839). In contrast, in mammalian models, such as mice, rats, cats and dogs, an involuntary hindlimb locomotion can recover following a complete spinal transection (20; 61; 360; 362; 488; 511; 781). Even more remarkable, spinal mammals recover the ability to modulate speed, to step on a split-belt treadmill and to perform backward locomotion, as demonstrated primarily in spinal cats (191; 267; 280; 283; 358; 360). This is because the spinal locomotor CPG located at lumbar levels can still interact with somatosensory feedback from the limbs and trunk that enters the spinal cord caudal to the lesion. In spinal mammals, somatosensory feedback must initiate hindlimb locomotion and tune it for task demands, as signals from the brain cannot access the lumbar locomotor CPG.
How do proprioceptive and tactile inputs contribute to the recovery of locomotion and its control after SCI? In one study, one hindlimb was partially deafferented by sectioning the DRG from L3 to S1 after air stepping had recovered spontaneously in spinal cats (308). Initially, the ipsilesional hindlimb was flaccid, with reduced bilateral rhythmic hindlimb activity. While rhythmic activity spontaneously recovered in the non-deafferented hindlimb within a few weeks, the deafferented hindlimb took 3–4 months, with an erratic rhythm that required pinching of the tail or perineal region. Moreover, the coordination between the hindlimbs was unstable with poor alternation. When placed on a treadmill, the non-deafferented hindlimb had weight support, proper digitigrade paw placement at contact and followed treadmill speed. In contrast, the deafferented hindlimb made contact with the paw dorsum and bilateral coordination was impaired. Interestingly, when micturition occurred, bilateral rhythmic activity was facilitated. This is something we often observe in our spinal cats stepping on a treadmill (unpublished observations from the Frigon lab) and is likely the result of a general increase in spinal neuronal excitability that facilitates various sensorimotor circuits simultaneously.
While deafferentation highlights the role of somatosensory feedback in the recovery of rhythmic activity and in coordinating the hindlimbs, it does not identify the types of afferents that contribute. To address this, Bouyer and Rossignol (2003a,b) performed cutaneous denervations of the hindpaws in cats before or after spinal transection. In intact cats, completely denervating cutaneous inputs from the hindpaws does not noticeably affect treadmill locomotion (93). However, if the spinal cord is then transected, cats do not recover proper digitigrade paw placement at contact and weight support is severely impaired throughout stance (94). In the same study, increasing spinal neuronal excitability with clonidine, an α-2 noradrenergic agonist that facilitates spinal locomotion in cats, improved the locomotor pattern but did not restore proper paw placement during stance. Interestingly, sparing one of the five cutaneous nerves of the hindpaws is sufficient to allow proper recovery after spinal transection. If a complete cutaneous denervation is performed after the animal has recovered hindlimb locomotion after spinal transection, proper paw placement is lost, with reduced weight support. These results indicate that some cutaneous feedback is necessary for proper paw placement and weight support in the spinal cat. It also shows that spinal cats cannot compensate for the complete loss of cutaneous feedback, in contrast to intact cats.
More recently, genetic tools in mice have been used to determine the role of somatosensory feedback in locomotor recovery after SCI (802; 803). One study used Egr3 mutant mice, which lack functional muscle spindle feedback (817), to investigate locomotor recovery after SCI (803). Intact Egr3 mutant mice, although they display some ataxia, perform treadmill locomotion at slow walking speeds but have difficulty at higher speeds (> 0.4 m/s). After a lateral spinal hemisection at T10, although wild type mice recovered hindlimb kinematics to pre-lesion levels within a few weeks, the ipsilesional hindlimb of Egr3 mutant mice showed persistent dragging throughout the cycle. Another study from the same group used conditional and intersectional genetic approaches in mice to remove proprioceptive feedback before and after a T10 lateral spinal hemisection (802). As in their previous study, they showed that the ipsilesional hindlimb dragged during locomotion after incomplete SCI in mice lacking proprioceptive feedback. Selectively removing proprioceptive feedback entering cervical levels had no effect on hindlimb locomotor recovery, whereas removing it from lumbar levels produced severe impairments. This indicates that local proprioceptive feedback is critical for proper limb function after SCI. Local proprioceptive feedback is also necessary to maintain recovery, as its ablation 7 weeks after recovery reinstates ipsilesional hindlimb deficits. In other words, spared or reorganized descending motor pathways cannot compensate for the loss of local proprioceptive feedback and its interactions with spinal CPGs after incomplete SCI to generate meaningful limb movements.
Studies using electrical epidural stimulation of the spinal cord in rats, cats and humans with SCI have underscored that the activation of somatosensory afferents is the main contributor to the recovery of locomotion or its expression (125; 264; 485; 598). Using a computational model based on experimental data, Capogrosso et al. (2013) determined that epidural stimulation of the spinal cord recruited somatosensory afferents, which are located more dorsally, without directly activating spinal interneurons and motoneurons. In other words, it is the somatosensory afferents activated by epidural stimulation that recruit spinal sensorimotor circuits to generate standing and locomotion. Both tactile (203) and proprioceptive (264; 585; 586) inputs appear important for the full expression of locomotion with electrical epidural stimulation.
At present, the modality, afferent type and location of somatosensory information most important for locomotor recovery after SCI remain to be identified. As discussed above, the loss of tactile or proprioceptive afferents leads to a general decrease in spinal excitability, as fewer excitatory inputs enter the spinal cord, and the loss of either modality impairs paw placement and limb movements. This suggests that after SCI all available somatosensory inputs must participate to provide a sufficient level of spinal neuronal excitability so that locomotion can be effectively generated.
Peripheral nerve injury
Whereas SCI disrupts the transmission of somatosensory feedback to various central targets, PNI directly removes it in the periphery. In the case of mixed nerves, it also removes the motor component. To study recovery mechanisms after PNI, studies have used muscle or cutaneous denervations.
After partial denervation of ankle extensor muscles in cats, there is an increase in ankle flexion at the beginning of stance, or yield, which recovers within 1–2 weeks (284; 329; 645; 647). The recovery is due to changes in the EMG activity of several hindlimb muscles, particularly remaining synergists, reflecting a reorganization of spinal sensorimotor circuits. This functional recovery does not require descending inputs from the brain because the return of ankle yield to pre-denervation values is observed in spinal cats (95; 286). However, in cats treated with high doses of pyridoxine, the increase in ankle yield following partial denervation of ankle extensors is magnified and recovers slightly or not at all over time, consistent with a recovery mechanism mediated by somatosensory feedback (647). Indeed, studies have observed an increase in the effectiveness of proprioceptive feedback from remaining synergists after ankle extensor muscle denervation (853) and a reorganization of cutaneous reflex pathways from the hindpaws (284; 286).
Intact and spinal cats also recover hindlimb locomotion following cutaneous denervations of the hindpaws (89; 93; 94). However, as stated above, spinal cats require at least a minimum of cutaneous feedback for weight bearing and proper paw placement. If the cutaneous denervation is made progressively, spinal cats recover to pre-denervation values with each successive nerve section until the last cutaneous nerve is cut. This indicates that spared cutaneous inputs compensate for the loss of cutaneous feedback. This can be done by the expansion of receptive fields and/or through a reorganization of spinal sensorimotor circuits. Taken together, these results demonstrate an important role of remaining somatosensory inputs in functional recovery after PNI.
Lastly, while we only briefly discussed the role of somatosensory feedback in functional recovery after SCI and PNI, the same principles apply to other neurological movement disorders, such as stroke or neurodegenerative diseases (e.g. Parkinson’s disease, amyotrophic lateral sclerosis and multiple sclerosis). In these disorders, researchers and clinicians need to consider how affected neural structures normally contribute to the integration, processing and relaying of somatosensory inputs to motor circuits. Not surprisingly, restoring proprioceptive and tactile inputs or incorporating them into neuroprostheses is a very active and important area of research to restore motor functions after injury or in disease.
Conclusion
In this review, we described and discussed the contributions of somatosensory feedback to mammalian locomotion. We covered how biomechanical properties of the body and its interactions with the environment directly influence somatosensory feedback. It is important to note that without somatosensory feedback locomotion is not functional, particularly in humans who cannot stand or walk following the loss of proprioceptive and tactile inputs. The greater impairment in walking function in humans compared to quadrupeds, such as mice and cats, likely relates to their unique postural requirements and an important supraspinal contribution. Indeed, somatosensory feedback not only projects to spinal circuits involved in simple reflexes and locomotor pattern generation, it interacts with supraspinal centers that project back to the spinal cord to control posture and fine-tune locomotion. Several mechanisms regulate the inflow of somatosensory information at various levels of the neuraxis so that it remains functionally relevant according to phase, task and in response to training. Somatosensory feedback contributes in many ways to locomotor control, by regulating posture, ensuring proper paw placement during skilled tasks and coordinating muscle activations within and between limbs. Because somatosensory feedback is an essential component of a highly integrated system for locomotor control, it plays a vital role in the recovery of locomotion after neurological injury.
Studies in the cat model have been instrumental in establishing the functional roles of somatosensory feedback during locomotion and the types of afferents involved. These results have since been translated in many human and rodent studies, showing that different mammals share common neural strategies. Mouse genetics have started elucidating the effects of selectively removing certain types of somatosensory feedback from the whole organism or from selected muscles in health and disease. However, despite a large body of knowledge on the control of locomotion by somatosensory feedback, there are many unanswered questions. First, because we do not specifically know the neurons that form the spinal locomotor CPG, we do not know how somatosensory feedback interacts with them. Advances in mouse genetics combined with electrophysiology and computational models should provide some answers. Second, somatosensory feedback from multiple sources interacts with neuronal targets at different levels of the CNS during locomotion. How these complex interactions produce a smooth and efficient gait is largely unknown. Third, current genetic and molecular approaches are restricted to a few models, such as the mouse and zebrafish. We will need to develop these approaches in other mammalian models, particularly larger animals, to determine if cellular and molecular mechanisms are conserved across species. This is especially important if we want to use these approaches for therapies in humans. Lastly, because somatosensory feedback is so important for the control of posture and locomotion, it will continue to be an active area of research for decades to come.
Didactic Synopsis:
Major teaching points:
1) During locomotion, mechanical stimuli within and outside the body activate various types of mechanoreceptors located in muscles, joints and skin that inform the central nervous system of the body segments’ relative position and motion, the forces that muscles generate and exert on bones, as well as characteristics of the terrain.
2) Without somatosensory feedback, locomotion is not functional, and humans cannot stand or walk.
3) Several biomechanical properties of the musculoskeletal system affect the activity of somatosensory afferents during locomotion.
4) Somatosensory feedback projects and interacts with neuronal targets and networks within the spinal cord and brain that directly or indirectly control locomotion.
5) Several mechanisms regulate the inflow of somatosensory feedback during locomotion from the spinal cord to higher levels of the central nervous system.
6) Somatosensory feedback is required during locomotion for postural control and skilled tasks.
7) Somatosensory feedback regulates locomotor phase durations and transitions by interacting with spinal circuits that change the duration and magnitude of muscle activity.
8) Somatosensory feedback is critical in rapidly responding to external perturbations by coordinating muscles within and between limbs during locomotion.
9) Somatosensory feedback plays an essential role in the recovery of locomotion after spinal cord injury and peripheral nerve injury.
Acknowledgments
We thank Jonathan Harnie for help with some of the figures. We also thank Tatiana Deliagina, Dimitri Ryczko, Julia Kaltschmidt and E. Paul Zehr for helpful comments on a draft of this manuscript. Some of the work presented here was funded by grants from the Canadian Institutes of Health Research (PJT-156296 to A.F. and PJT-162357 to T.A.), the Natural Sciences and Engineering Research Council of Canada (RGPIN-2016-03790 to A.F. and RGPIN-2015-03871 to T.A.), the National Institutes of Health (R01 NS110550 to A.F. and B.I.P., R01 NS119268 and R01 NS115900 to T.A. and R01 NS100928 to B.I.P.) and by the National Science Foundation (2024414 to B.I.P.). Alain Frigon is a Fonds de Recherche-Santé Quebec Senior Research Scholar.
References
- 1.Abelew TA, Miller MD, Cope TC and Nichols TR. Local loss of proprioception results in disruption of interjoint coordination during locomotion in the cat. J Neurophysiol 84: 2709–2714, 2000. [DOI] [PubMed] [Google Scholar]
- 2.Abraham LD, Marks WB and Loeb GE. The distal hindlimb musculature of the cat. Cutaneous reflexes during locomotion. Exp Brain Res 58: 594–603, 1985. [DOI] [PubMed] [Google Scholar]
- 3.Abraira VE and Ginty DD. The sensory neurons of touch. Neuron 79: 618–639, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Abraira VE, Kuehn ED, Chirila AM, Springel MW, Toliver AA, Zimmerman AL, Orefice LL, Boyle KA, Bai L, Song BJ, Bashista KA, O’Neill TG, Zhuo J, Tsan C, Hoynoski J, Rutlin M, Kus L, Niederkofler V, Watanabe M, Dymecki SM, Nelson SB, Heintz N, Hughes DI and Ginty DD. The Cellular and Synaptic Architecture of the Mechanosensory Dorsal Horn. Cell 168: 295–310, 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Adal MN. The fine structure of the sensory region of cat muscle spindles. J Ultrastruct Res 26: 332–353, 1969. [DOI] [PubMed] [Google Scholar]
- 6.af Klint R, Mazzaro N, Nielsen JB, Sinkjaer T and Grey MJ. Load rather than length sensitive feedback contributes to soleus muscle activity during human treadmill walking. J Neurophysiol 103: 2747–2756, 2010. [DOI] [PubMed] [Google Scholar]
- 7.Aggelopoulos NC and Edgley SA. Segmental localisation of the relays mediating crossed inhibition of hindlimb motoneurones from group II afferents in the anaesthetized cat spinal cord. Neurosci Lett 185: 60–64, 1995. [DOI] [PubMed] [Google Scholar]
- 8.Akay T. Long-term measurement of muscle denervation and locomotor behavior in individual wild-type and ALS model mice. J Neurophysiol 111: 694–703, 2014. [DOI] [PubMed] [Google Scholar]
- 9.Akay T, Acharya HJ, Fouad K and Pearson KG. Behavioral and electromyographic characterization of mice lacking EphA4 receptors. J Neurophysiol 96: 642–651, 2006. [DOI] [PubMed] [Google Scholar]
- 10.Akay T, Tourtellotte WG, Arber S and Jessell TM. Degradation of mouse locomotor pattern in the absence of proprioceptive sensory feedback. Proc Natl Acad Sci U S A 111: 16877–16882, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Akazawa K, Aldridge JW, Steeves JD and Stein RB. Modulation of stretch reflexes during locomotion in the mesencephalic cat. J Physiol 329: 553–567, 1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Albers KM and Davis BM. The skin as a neurotrophic organ. Neuroscientist 13: 371–382, 2007. [DOI] [PubMed] [Google Scholar]
- 13.Alessandro C, Barroso FO, Prashara A, Tentler DP, Yeh HY and Tresch MC. Coordination amongst quadriceps muscles suggests neural regulation of internal joint stresses, not simplification of task performance. Proc Natl Acad Sci U S A 117: 8135–8142, 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Alexander RM. The gaits of bipedal and quadrupedal animals. The international journal of robotics research 3: 49–59, 1984. [Google Scholar]
- 15.Alexander RM. Optimization and gaits in the locomotion of vertebrates. Physiol Rev 69: 1199–1227, 1989. [DOI] [PubMed] [Google Scholar]
- 16.Alexander RM. Simple models of human locomotion. Journal of Theoretical Medicine 1: 129–135, 1997. [Google Scholar]
- 17.Allen GI, Sabah NH and Toyama K. Synaptic actions of peripheral nerve impulses upon Deiters neurones via the climbing fibre afferents. J Physiol 226: 311–333, 1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Allen GI, Sabah NH and Toyama K. Synaptic actions of peripheral nerve impulses upon Deiters neurones via the mossy fibre afferents. J Physiol 226: 335–351, 1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Allen JL, Kautz SA and Neptune RR. The influence of merged muscle excitation modules on post-stroke hemiparetic walking performance. Clin Biomech (Bristol, Avon) 28: 697–704, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Alluin O, Delivet-Mongrain H and Rossignol S. Inducing hindlimb locomotor recovery in adult rat after complete thoracic spinal cord section using repeated treadmill training with perineal stimulation only. J Neurophysiol 114: 1931–1946, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Alstermark B and Ekerot CF. The lateral reticular nucleus; integration of descending and ascending systems regulating voluntary forelimb movements. Front Comput Neurosci 9: 102, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Alvarez FJ, Jonas PC, Sapir T, Hartley R, Berrocal MC, Geiman EJ, Todd AJ and Goulding M. Postnatal phenotype and localization of spinal cord V1 derived interneurons. J Comp Neurol 493: 177–192, 2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Alvarez FJ, Rotterman TM, Akhter ET, Lane AR, English AW and Cope TC. Synaptic Plasticity on Motoneurons After Axotomy: A Necessary Change in Paradigm. Front Mol Neurosci 13: 68, 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Alvarez FJ, Titus-Mitchell HE, Bullinger KL, Kraszpulski M, Nardelli P and Cope TC. Permanent central synaptic disconnection of proprioceptors after nerve injury and regeneration. I. Loss of VGLUT1/IA synapses on motoneurons. J Neurophysiol 106: 2450–2470, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Alvarez FJ, Villalba RM, Zerda R and Schneider SP. Vesicular glutamate transporters in the spinal cord, with special reference to sensory primary afferent synapses. J Comp Neurol 472: 257–280, 2004. [DOI] [PubMed] [Google Scholar]
- 26.An KN, Takahashi K, Harrigan TP and Chao EY. Determination of muscle orientations and moment arms. J Biomech Eng 106: 280–282, 1984. [DOI] [PubMed] [Google Scholar]
- 27.Anderson FC and Pandy MG. Static and dynamic optimization solutions for gait are practically equivalent. J Biomech 34: 153–161, 2001. [DOI] [PubMed] [Google Scholar]
- 28.Andersson EA, Nilsson J and Thorstensson A. Intramuscular EMG from the hip flexor muscles during human locomotion. Acta Physiol Scand 161: 361–370, 1997. [DOI] [PubMed] [Google Scholar]
- 29.Andersson G and Armstrong DM. Complex spikes in Purkinje cells in the lateral vermis (b zone) of the cat cerebellum during locomotion. J Physiol 385: 107–134, 1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Andersson O and Grillner S. Peripheral control of the cat’s step cycle. II. Entrainment of the central pattern generators for locomotion by sinusoidal hip movements during “fictive locomotion.”. Acta Physiol Scand 118: 229–239, 1983. [DOI] [PubMed] [Google Scholar]
- 31.Andrada E, Mampel J, Schmidt A, Fischer MS, Karguth A and Witte H. From biomechanics of rats’ inclined locomotion to a climbing robot. International Journal of Design & Nature and Ecodynamics 8: 192–212, 2013. [Google Scholar]
- 32.ANDREW BL. The sensory innervation of the medial ligament of the knee joint. J Physiol 123: 241–250, 1954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Angel MJ, Guertin P, Jimenez I and McCrea DA. Group I extensor afferents evoke disynaptic EPSPs in cat hindlimb extensor motorneurones during fictive locomotion. J Physiol 494 (Pt 3): 851–861, 1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Angel MJ, Jankowska E and McCrea DA. Candidate interneurones mediating group I disynaptic EPSPs in extensor motoneurones during fictive locomotion in the cat. J Physiol 563: 597–610, 2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Angeli CA, Boakye M, Morton RA, Vogt J, Benton K, Chen Y, Ferreira CK and Harkema SJ. Recovery of Over-Ground Walking after Chronic Motor Complete Spinal Cord Injury. N Engl J Med 379: 1244–1250, 2018. [DOI] [PubMed] [Google Scholar]
- 36.Appenteng K and Prochazka A. Tendon organ firing during active muscle lengthening in awake, normally behaving cats. J Physiol 353: 81–92, 1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Apps R. Movement-related gating of climbing fibre input to cerebellar cortical zones. Prog Neurobiol 57: 537–562, 1999. [DOI] [PubMed] [Google Scholar]
- 38.Apps R and Lee S. Gating of transmission in climbing fibre paths to cerebellar cortical C1 and C3 zones in the rostral paramedian lobule during locomotion in the cat. J Physiol 516 (Pt 3): 875–883, 1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Apps R, Lidierth M and Armstrong DM. Locomotion-related variations in excitability of spino-olivocerebellar paths to cat cerebellar cortical c2 zone. J Physiol 424: 487–512, 1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Arber S. Motor circuits in action: specification, connectivity, and function. Neuron 74: 975–989, 2012. [DOI] [PubMed] [Google Scholar]
- 41.Armstrong DM and Edgley SA. Discharges of Purkinje cells in the paravermal part of the cerebellar anterior lobe during locomotion in the cat. J Physiol 352: 403–424, 1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Armstrong DM, Edgley SA and Lidierth M. Complex spikes in Purkinje cells of the paravermal part of the anterior lobe of the cat cerebellum during locomotion. J Physiol 400: 405–414, 1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Armstrong DM, Harvey RJ and Schild RF. Spino-olivocerebellar pathways to the posterior lobe of the cat cerebellum. Exp Brain Res 18: 1–18, 1973. [DOI] [PubMed] [Google Scholar]
- 44.Arshavskii I, Kots I, Orlovskii GN, Rodionov IM and Shik ML. [Study of biomechanics of running dogs]. Biofizika 10: 665–671, 1965. [PubMed] [Google Scholar]
- 45.Arshavsky YI, Berkinblit MB, Fukson OI, Gelfand IM and Orlovsky GN. Origin of modulation in neurones of the ventral spinocerebellar tract during locomotion. Brain Res 43: 276–279, 1972. [DOI] [PubMed] [Google Scholar]
- 46.Arshavsky YI, Berkinblit MB, Fukson OI, Gelfand IM and Orlovsky GN. Recordings of neurones of the dorsal spinocerebellar tract during evoked locomotion. Brain Res 43: 272–275, 1972. [DOI] [PubMed] [Google Scholar]
- 47.Arshian MS, Hobson CE, Catanzaro MF, Miller DJ, Puterbaugh SR, Cotter LA, Yates BJ and McCall AA. Vestibular nucleus neurons respond to hindlimb movement in the decerebrate cat. J Neurophysiol 111: 2423–2432, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Arya T, Bajwa S and Edgley SA. Crossed reflex actions from group II muscle afferents in the lumbar spinal cord of the anaesthetized cat. J Physiol 444: 117–131, 1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Bacque-Cazenave J, Chung B, Cofer DW, Cattaert D and Edwards DH. The effect of sensory feedback on crayfish posture and locomotion: II. Neuromechanical simulation of closing the loop. J Neurophysiol 113: 1772–1783, 2015. [DOI] [PubMed] [Google Scholar]
- 50.Bajwa S, Edgley SA and Harrison PJ. Crossed actions on group II-activated interneurones in the midlumbar segments of the cat spinal cord. J Physiol 455: 205–217, 1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Baken BC, Dietz V and Duysens J. Phase-dependent modulation of short latency cutaneous reflexes during walking in man. Brain Res 1031: 268–275, 2005. [DOI] [PubMed] [Google Scholar]
- 52.Balaskas N, Abbott LF, Jessell TM and Ng D. Positional Strategies for Connection Specificity and Synaptic Organization in Spinal Sensory-Motor Circuits. Neuron 102: 1143–1156, 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Banks RW. A histological study of the motor innervation of the cat’s muscle spindle. J Anat 133: 571–591, 1981. [PMC free article] [PubMed] [Google Scholar]
- 54.Banks RW. The motor innervation of mammalian muscle spindles. Prog Neurobiol 43: 323–362, 1994. [DOI] [PubMed] [Google Scholar]
- 55.Banks RW. The innervation of the muscle spindle: a personal history. J Anat 227: 115–135, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Banks RW, Bewick GS, Reid B and Richardson C. Evidence for activity-dependent modulation of sensory-terminal excitability in spindles by glutamate release from synaptic-like vesicles. Adv Exp Med Biol 508: 13–18, 2002. [DOI] [PubMed] [Google Scholar]
- 57.Banks RW, Harker DW and Stacey MJ. A study of mammalian intrafusal muscle fibres using a combined histochemical and ultrastructural technique. J Anat 123: 783–796, 1977. [PMC free article] [PubMed] [Google Scholar]
- 58.Banks RW, Hulliger M, Saed HH and Stacey MJ. A comparative analysis of the encapsulated end-organs of mammalian skeletal muscles and of their sensory nerve endings. J Anat 214: 859–887, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Banks RW, Hulliger M, Scheepstra KA and Otten E. Pacemaker activity in a sensory ending with multiple encoding sites: the cat muscle spindle primary ending. J Physiol 498 (Pt 1): 177–199, 1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Bannatyne BA, Edgley SA, Hammar I, Jankowska E and Maxwell DJ. Differential projections of excitatory and inhibitory dorsal horn interneurons relaying information from group II muscle afferents in the cat spinal cord. J Neurosci 26: 2871–2880, 2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Barbeau H and Rossignol S. Recovery of locomotion after chronic spinalization in the adult cat. Brain Res 412: 84–95, 1987. [DOI] [PubMed] [Google Scholar]
- 62.Barker D, Emonet-Denand F, Laporte Y, Proske U and Stacey MJ. Morphological identification and intrafusal distribution of the endings of static fusimotor axons in the cat. J Physiol 230: 405–427, 1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Barliya A, Omlor L, Giese MA and Flash T. An analytical formulation of the law of intersegmental coordination during human locomotion. Exp Brain Res 193: 371–385, 2009. [DOI] [PubMed] [Google Scholar]
- 64.Barthelemy D, Grey MJ, Nielsen JB and Bouyer L. Involvement of the corticospinal tract in the control of human gait. Prog Brain Res 192: 181–197, 2011. [DOI] [PubMed] [Google Scholar]
- 65.Barthelemy D and Nielsen JB. Corticospinal contribution to arm muscle activity during human walking. J Physiol 588: 967–979, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Basu C, Wilson AM and Hutchinson JR. The locomotor kinematics and ground reaction forces of walking giraffes. J Exp Biol 222: 2019. [DOI] [PubMed] [Google Scholar]
- 67.Bays PM and Wolpert DM. Computational principles of sensorimotor control that minimize uncertainty and variability. J Physiol 578: 387–396, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Beloozerova IN and Rossignol S. Antidromic discharges in dorsal roots of decerebrate cats. II: studies during treadmill locomotion. Brain Res 996: 227–236, 2004. [DOI] [PubMed] [Google Scholar]
- 69.Beloozerova IN, Stout EE and Sirota MG. Distinct Thalamo-Cortical Controls for Shoulder, Elbow, and Wrist during Locomotion. Front Comput Neurosci 7: 62, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Ben-Arie N, Bellen HJ, Armstrong DL, McCall AE, Gordadze PR, Guo Q, Matzuk MM and Zoghbi HY. Math1 is essential for genesis of cerebellar granule neurons. Nature 390: 169–172, 1997. [DOI] [PubMed] [Google Scholar]
- 71.Ben-Arie N, Hassan BA, Bermingham NA, Malicki DM, Armstrong D, Matzuk M, Bellen HJ and Zoghbi HY. Functional conservation of atonal and Math1 in the CNS and PNS. Development 127: 1039–1048, 2000. [DOI] [PubMed] [Google Scholar]
- 72.Berkley KJ, Budell RJ, Blomqvist A and Bull M. Output systems of the dorsal column nuclei in the cat. Brain Res 396: 199–225, 1986. [DOI] [PubMed] [Google Scholar]
- 73.Bernard G, Bouyer L, Provencher J and Rossignol S. Study of cutaneous reflex compensation during locomotion after nerve section in the cat. J Neurophysiol 97: 4173–4185, 2007. [DOI] [PubMed] [Google Scholar]
- 74.Berniker M, Jarc A, Bizzi E and Tresch MC. Simplified and effective motor control based on muscle synergies to exploit musculoskeletal dynamics. Proc Natl Acad Sci U S A 106: 7601–7606, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Bernstein N. The co-ordination and regulation of movements. Oxford: Pergamon Press, 1967. [Google Scholar]
- 76.Bessou P, Dupui P, Cabelguen JM, Joffroy M, Montoya R and Pages B. Discharge patterns of gamma motoneurone populations of extensor and flexor hindlimb muscles during walking in the thalamic cat. Prog Brain Res 80: 37–45, 1989. [DOI] [PubMed] [Google Scholar]
- 77.Bessou P, Joffroy M, Montoya R and Pages B. Evidence of the co-activation of alpha-motoneurones and static gamma-motoneurones of the sartorius medialis muscle during locomotion in the thalamic cat. Exp Brain Res 82: 191–198, 1990. [DOI] [PubMed] [Google Scholar]
- 78.Betley JN, Wright CV, Kawaguchi Y, Erdelyi F, Szabo G, Jessell TM and Kaltschmidt JA. Stringent specificity in the construction of a GABAergic presynaptic inhibitory circuit. Cell 139: 161–174, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Bewick GS and Banks RW. Mechanotransduction in the muscle spindle. Pflugers Arch 467: 175–190, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Bewick GS, Reid B, Richardson C and Banks RW. Autogenic modulation of mechanoreceptor excitability by glutamate release from synaptic-like vesicles: evidence from the rat muscle spindle primary sensory ending. J Physiol 562: 381–394, 2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Bianchi L, Angelini D, Orani GP and Lacquaniti F. Kinematic coordination in human gait: relation to mechanical energy cost. J Neurophysiol 79: 2155–2170, 1998. [DOI] [PubMed] [Google Scholar]
- 82.Blaszczyk J and Loeb GE. Why cats pace on the treadmill. Physiol Behav 53: 501–507, 1993. [DOI] [PubMed] [Google Scholar]
- 83.Blemker SS and Delp SL. Three-dimensional representation of complex muscle architectures and geometries. Ann Biomed Eng 33: 661–673, 2005. [DOI] [PubMed] [Google Scholar]
- 84.Bloedel JR and Courville J. Cerebellar afferent systems. In: Handbook of Physiology Vol. II, Motor Control, Part 2, edited by Brooks VB. Bethesda: American Physiological Society, 1981, p. 735–829. [Google Scholar]
- 85.Blouin J, Bard C, Teasdale N, Paillard J, Fleury M, Forget R and Lamarre Y. Reference systems for coding spatial information in normal subjects and a deafferented patient. Exp Brain Res 93: 324–331, 1993. [DOI] [PubMed] [Google Scholar]
- 86.Blum KP, Campbell KS, Horslen BC, Nardelli P, Housley SN, Cope TC and Ting LH. Diverse and complex muscle spindle afferent firing properties emerge from multiscale muscle mechanics. Elife 9: 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Blum KP, Nardelli P, Cope TC and Ting LH. Elastic tissue forces mask muscle fiber forces underlying muscle spindle Ia afferent firing rates in stretch of relaxed rat muscle. J Exp Biol 222: 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Boesten AJ and Voogd J. Projections of the dorsal column nuclei and the spinal cord on the inferior olive in the cat. J Comp Neurol 161: 215–237, 1975. [DOI] [PubMed] [Google Scholar]
- 89.Bolton DA and Misiaszek JE. Contribution of hindpaw cutaneous inputs to the control of lateral stability during walking in the cat. J Neurophysiol 102: 1711–1724, 2009. [DOI] [PubMed] [Google Scholar]
- 90.Borisyuk R, Merrison-Hort R, Soffe SR, Koutsikou S and Li WC. To swim or not to swim: A population-level model of Xenopus tadpole decision making and locomotor behaviour. Biosystems 161: 3–14, 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Bosco G and Poppele RE. Proprioception from a spinocerebellar perspective. Physiol Rev 81: 539–568, 2001. [DOI] [PubMed] [Google Scholar]
- 92.Bourane S, Grossmann KS, Britz O, Dalet A, Del Barrio MG, Stam FJ, Garcia-Campmany L, Koch S and Goulding M. Identification of a spinal circuit for light touch and fine motor control. Cell 160: 503–515, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Bouyer LJ and Rossignol S. Contribution of cutaneous inputs from the hindpaw to the control of locomotion. I. Intact cats. J Neurophysiol 90: 3625–3639, 2003. [DOI] [PubMed] [Google Scholar]
- 94.Bouyer LJ and Rossignol S. Contribution of cutaneous inputs from the hindpaw to the control of locomotion. II. Spinal cats. J Neurophysiol 90: 3640–3653, 2003. [DOI] [PubMed] [Google Scholar]
- 95.Bouyer LJ, Whelan PJ, Pearson KG and Rossignol S. Adaptive locomotor plasticity in chronic spinal cats after ankle extensors neurectomy. J Neurosci 21: 3531–3541, 2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.BOYD IA. The histological structure of the receptors in the knee-joint of the cat correlated with their physiological response. J Physiol 124: 476–488, 1954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Boyd IA, Gladden MH, McWilliam PN and Ward J. Control of dynamic and static nuclear bag fibres and nuclear chain fibres by gamma and beta axons in isolated cat muscle spindels. J Physiol 265: 133–162, 1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Bras H, Jankowska E, Noga B and Skoog B. Comparison of Effects of Various Types of NA and 5-HT Agonists on Transmission from Group II Muscle Afferents in the Cat. Eur J Neurosci 2: 1029–1039, 1990. [DOI] [PubMed] [Google Scholar]
- 99.Bretzner F and Drew T. Contribution of the motor cortex to the structure and the timing of hindlimb locomotion in the cat: a microstimulation study. J Neurophysiol 94: 657–672, 2005. [DOI] [PubMed] [Google Scholar]
- 100.Brock LG, Coombs JS and Eccles JC. The recording of potentials from motoneurones with an intracellular electrode. J Physiol 117: 431–460, 1952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Brown AG. Organization in the spinal cord - the anatomy and physiology of identified neurones. London: Springer-Verlag, 1981. [Google Scholar]
- 102.Brown AG. The spinocervical tract. Prog Neurobiol 17: 59–96, 1981. [DOI] [PubMed] [Google Scholar]
- 103.Brown AG and Fyffe RE. Direct observations on the contacts made between Ia afferent fibres and alpha-motoneurones in the cat’s lumbosacral spinal cord. J Physiol 313: 121–140, 1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Brown AG and Iggo A. A quantitative study of cutaneous receptors and afferent fibres in the cat and rabbit. J Physiol 193: 707–733, 1967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Brown MC, Engberg I and Matthews PB. The relative sensitivity to vibration of muscle receptors of the cat. J Physiol 192: 773–800, 1967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Brown TG. The intrinsic factors in the act of progression in the mammal. Proc R Soc B 84: 308–319, 1911. [Google Scholar]
- 107.Brownstone RM, Gossard JP and Hultborn H. Voltage-dependent excitation of motoneurones from spinal locomotor centres in the cat. Exp Brain Res 102: 34–44, 1994. [DOI] [PubMed] [Google Scholar]
- 108.Brownstone RM and Wilson JM. Strategies for delineating spinal locomotor rhythm-generating networks and the possible role of Hb9 interneurones in rhythmogenesis. Brain Res Rev 57: 64–76, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Buchanan TS, Almdale DP, Lewis JL and Rymer WZ. Characteristics of synergic relations during isometric contractions of human elbow muscles. J Neurophysiol 56: 1225–1241, 1986. [DOI] [PubMed] [Google Scholar]
- 110.Buford JA and Smith JL. Adaptive control for backward quadrupedal walking. III. Stumbling corrective reactions and cutaneous reflex sensitivity. J Neurophysiol 70: 1102–1114, 1993. [DOI] [PubMed] [Google Scholar]
- 111.Bui TV, Akay T, Loubani O, Hnasko TS, Jessell TM and Brownstone RM. Circuits for grasping: spinal dI3 interneurons mediate cutaneous control of motor behavior. Neuron 78: 191–204, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Bullinger KL, Nardelli P, Pinter MJ, Alvarez FJ and Cope TC. Permanent central synaptic disconnection of proprioceptors after nerve injury and regeneration. II. Loss of functional connectivity with motoneurons. J Neurophysiol 106: 2471–2485, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Burgess PR, Howe JF, Lessler MJ and Whitehorn D. Cutaneous receptors supplied by myelinated fibers in the cat. II. Number of mechanoreceptors excited by a local stimulus. J Neurophysiol 37: 1373–1386, 1974. [DOI] [PubMed] [Google Scholar]
- 114.Burgess PR, Petit D and Warren RM. Receptor types in cat hairy skin supplied by myelinated fibers. J Neurophysiol 31: 833–848, 1968. [DOI] [PubMed] [Google Scholar]
- 115.Burke D, Gandevia SC and McKeon B. The afferent volleys responsible for spinal proprioceptive reflexes in man. J Physiol 339: 535–552, 1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Burke D, Gandevia SC and McKeon B. Monosynaptic and oligosynaptic contributions to human ankle jerk and H-reflex. J Neurophysiol 52: 435–448, 1984. [DOI] [PubMed] [Google Scholar]
- 117.Burke RE. John Eccles’ pioneering role in understanding central synaptic transmission. Prog Neurobiol 78: 173–188, 2006. [DOI] [PubMed] [Google Scholar]
- 118.Burke RE, Jankowska E and ten BG. A comparison of peripheral and rubrospinal synaptic input to slow and fast twitch motor units of triceps surae. J Physiol 207: 709–732, 1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Butt SJ, Harris-Warrick RM and Kiehn O. Firing properties of identified interneuron populations in the mammalian hindlimb central pattern generator. J Neurosci 22: 9961–9971, 2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Caggiano V, Leiras R, Goni-Erro H, Masini D, Bellardita C, Bouvier J, Caldeira V, Fisone G and Kiehn O. Midbrain circuits that set locomotor speed and gait selection. Nature 553: 455–460, 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Caicoya AG, Illert M and Janike R. Monosynaptic Ia pathways at the cat shoulder. J Physiol 518 (Pt 3): 825–841, 1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Capaday C and Stein RB. Amplitude modulation of the soleus H-reflex in the human during walking and standing. J Neurosci 6: 1308–1313, 1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Capaday C and Stein RB. Difference in the amplitude of the human soleus H reflex during walking and running. J Physiol 392: 513–522, 1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Capelli P, Pivetta C, Soledad EM and Arber S. Locomotor speed control circuits in the caudal brainstem. Nature 551: 373–377, 2017. [DOI] [PubMed] [Google Scholar]
- 125.Capogrosso M, Wenger N, Raspopovic S, Musienko P, Beauparlant J, Bassi LL, Courtine G and Micera S. A computational model for epidural electrical stimulation of spinal sensorimotor circuits. J Neurosci 33: 19326–19340, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Cappellini G, Ivanenko YP, Poppele RE and Lacquaniti F. Motor patterns in human walking and running. J Neurophysiol 95: 3426–3437, 2006. [DOI] [PubMed] [Google Scholar]
- 127.Carlson-Kuhta P, Trank TV and Smith JL. Forms of forward quadrupedal locomotion. II. A comparison of posture, hindlimb kinematics, and motor patterns for upslope and level walking. J Neurophysiol 79: 1687–1701, 1998. [DOI] [PubMed] [Google Scholar]
- 128.Carp JS, Tennissen AM, Chen XY, Schalk G and Wolpaw JR. Long-term spinal reflex studies in awake behaving mice. J Neurosci Methods 149: 134–143, 2005. [DOI] [PubMed] [Google Scholar]
- 129.Carp JS, Tennissen AM, Chen XY and Wolpaw JR. H-reflex operant conditioning in mice. J Neurophysiol 96: 1718–1727, 2006. [DOI] [PubMed] [Google Scholar]
- 130.Catavitello G, Ivanenko YP and Lacquaniti F. Planar Covariation of Hindlimb and Forelimb Elevation Angles during Terrestrial and Aquatic Locomotion of Dogs. PLoS One 10: e0133936, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Caterina MJ. Transient receptor potential ion channels as participants in thermosensation and thermoregulation. Am J Physiol Regul Integr Comp Physiol 292: R64–R76, 2007. [DOI] [PubMed] [Google Scholar]
- 132.Cavagna GA, Heglund NC and Taylor CR. Mechanical work in terrestrial locomotion: two basic mechanisms for minimizing energy expenditure. Am J Physiol 233: R243–R261, 1977. [DOI] [PubMed] [Google Scholar]
- 133.Cazalets JR, Borde M and Clarac F. Localization and organization of the central pattern generator for hindlimb locomotion in newborn rat. J Neurosci 15: 4943–4951, 1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Cazalets JR, Sqalli-Houssaini Y and Clarac F. Activation of the central pattern generators for locomotion by serotonin and excitatory amino acids in neonatal rat. J Physiol 455: 187–204, 1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Chamberlin ME, Fulwider BD, Sanders SL and Medeiros JM. Does fear of falling influence spatial and temporal gait parameters in elderly persons beyond changes associated with normal aging? J Gerontol A Biol Sci Med Sci 60: 1163–1167, 2005. [DOI] [PubMed] [Google Scholar]
- 136.Chambers WW and Sprague JM. Functional localization in the cerebellum. II. Somatotopic organization in cortex and nuclei. AMA Arch Neurol Psychiatry 74: 653–680, 1955. [DOI] [PubMed] [Google Scholar]
- 137.Chanaud CM, Pratt CA and Loeb GE. Functionally complex muscles of the cat hindlimb. II. Mechanical and architectural heterogenity within the biceps femoris. Exp Brain Res 85: 257–270, 1991. [DOI] [PubMed] [Google Scholar]
- 138.Chapin JK, Sorensen SM and Woodward DJ. Acute ethanol effects on sensory responses of single units in the somatosensory cortex of rats during different behavioral states. Pharmacol Biochem Behav 25: 607–614, 1986. [DOI] [PubMed] [Google Scholar]
- 139.Chapin JK and Woodward DJ. Somatic sensory transmission to the cortex during movement: gating of single cell responses to touch. Exp Neurol 78: 654–669, 1982. [DOI] [PubMed] [Google Scholar]
- 140.Chapin JK and Woodward DJ. Somatic sensory transmission to the cortex during movement: phasic modulation over the locomotor step cycle. Exp Neurol 78: 670–684, 1982. [DOI] [PubMed] [Google Scholar]
- 141.Charles JP, Cappellari O, Spence AJ, Wells DJ and Hutchinson JR. Muscle moment arms and sensitivity analysis of a mouse hindlimb musculoskeletal model. J Anat 229: 514–535, 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Chen HH, Hippenmeyer S, Arber S and Frank E. Development of the monosynaptic stretch reflex circuit. Curr Opin Neurobiol 13: 96–102, 2003. [DOI] [PubMed] [Google Scholar]
- 143.Chen HH, Tourtellotte WG and Frank E. Muscle spindle-derived neurotrophin 3 regulates synaptic connectivity between muscle sensory and motor neurons. J Neurosci 22: 3512–3519, 2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Chen XY and Wolpaw JR. Operant conditioning of H-reflex in freely moving rats. J Neurophysiol 73: 411–415, 1995. [DOI] [PubMed] [Google Scholar]
- 145.Chen Y, Chen XY, Jakeman LB, Chen L, Stokes BT and Wolpaw JR. Operant conditioning of H-reflex can correct a locomotor abnormality after spinal cord injury in rats. J Neurosci 26: 12537–12543, 2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Chen Y, Chen XY, Jakeman LB, Schalk G, Stokes BT and Wolpaw JR. The interaction of a new motor skill and an old one: H-reflex conditioning and locomotion in rats. J Neurosci 25: 6898–6906, 2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Chesler AT, Szczot M, Bharucha-Goebel D, Ceko M, Donkervoort S, Laubacher C, Hayes LH, Alter K, Zampieri C, Stanley C, Innes AM, Mah JK, Grosmann CM, Bradley N, Nguyen D, Foley AR, Le Pichon CE and Bonnemann CG. The Role of PIEZO2 in Human Mechanosensation. N Engl J Med 375: 1355–1364, 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Cheung VC, d’Avella A, Tresch MC and Bizzi E. Central and sensory contributions to the activation and organization of muscle synergies during natural motor behaviors. J Neurosci 25: 6419–6434, 2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Cheung VCK, Cheung BMF, Zhang JH, Chan ZYS, Ha SCW, Chen CY and Cheung RTH. Plasticity of muscle synergies through fractionation and merging during development and training of human runners. Nat Commun 11: 4356, 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Chowdhury RH, Tresch MC and Miller LE. Musculoskeletal geometry accounts for apparent extrinsic representation of paw position in dorsal spinocerebellar tract. J Neurophysiol 118: 234–242, 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Christie BP, Charkhkar H, Shell CE, Burant CJ, Tyler DJ and Triolo RJ. Ambulatory searching task reveals importance of somatosensation for lower-limb amputees. Sci Rep 10: 10216, 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Chung B, Bacque-Cazenave J, Cofer DW, Cattaert D and Edwards DH. The effect of sensory feedback on crayfish posture and locomotion: I. Experimental analysis of closing the loop. J Neurophysiol 113: 1763–1771, 2015. [DOI] [PubMed] [Google Scholar]
- 153.Chvatal SA, Macpherson JM, Torres-Oviedo G and Ting LH. Absence of postural muscle synergies for balance after spinal cord transection. J Neurophysiol 110: 1301–1310, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Chvatal SA and Ting LH. Voluntary and reactive recruitment of locomotor muscle synergies during perturbed walking. J Neurosci 32: 12237–12250, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Clark FJ, Burgess RC, Chapin JW and Lipscomb WT. Role of intramuscular receptors in the awareness of limb position. J Neurophysiol 54: 1529–1540, 1985. [DOI] [PubMed] [Google Scholar]
- 156.Clarke KA and Still J. Gait analysis in the mouse. Physiol Behav 66: 723–729, 1999. [DOI] [PubMed] [Google Scholar]
- 157.Clayton HM and Hobbs SJ. A Review of Biomechanical Gait Classification with Reference to Collected Trot, Passage and Piaffe in Dressage Horses. Animals (Basel) 9: 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Cofer D, Cymbalyuk G, Reid J, Zhu Y, Heitler WJ and Edwards DH. AnimatLab: a 3D graphics environment for neuromechanical simulations. J Neurosci Methods 187: 280–288, 2010. [DOI] [PubMed] [Google Scholar]
- 159.Cohen O, Harel R, Aumann TD, Israel Z and Prut Y. Parallel processing of internal and external feedback in the spinocerebellar system of primates. J Neurophysiol 118: 254–266, 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Cole JD and Katifi HA. Evoked potentials in a man with a complete large myelinated fibre sensory neuropathy below the neck. Electroencephalogr Clin Neurophysiol 80: 103–107, 1991. [DOI] [PubMed] [Google Scholar]
- 161.Collins WF III, Mendell LM and Munson JB. On the specificity of sensory reinnervation of cat skeletal muscle. J Physiol 375: 587–609, 1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Conway BA, Hultborn H and Kiehn O. Proprioceptive input resets central locomotor rhythm in the spinal cat. Exp Brain Res 68: 643–656, 1987. [DOI] [PubMed] [Google Scholar]
- 163.Cooke JD, Larson B, Oscarsson O and Sjolund B. Organization of afferent connections to cuneocerebellar tract. Exp Brain Res 13: 359–377, 1971. [DOI] [PubMed] [Google Scholar]
- 164.Cooke JD, Larson B, Oscarsson O and Sjolund B. Origin and termination of cuneocerebellar tract. Exp Brain Res 13: 339–358, 1971. [DOI] [PubMed] [Google Scholar]
- 165.Cooper S. The responses of the primary and secondary endings of muscle spindles with intact motor innervation during applied stretch. Q J Exp Physiol Cogn Med Sci 46: 389–398, 1961. [DOI] [PubMed] [Google Scholar]
- 166.Cooper SE, Martin JH and Ghez C. Effects of inactivation of the anterior interpositus nucleus on the kinematic and dynamic control of multijoint movement. J Neurophysiol 84: 1988–2000, 2000. [DOI] [PubMed] [Google Scholar]
- 167.Cope TC, Bonasera SJ and Nichols TR. Reinnervated muscles fail to produce stretch reflexes. J Neurophysiol 71: 817–820, 1994. [DOI] [PubMed] [Google Scholar]
- 168.Cope TC and Clark BD. Motor-unit recruitment in self-reinnervated muscle. J Neurophysiol 70: 1787–1796, 1993. [DOI] [PubMed] [Google Scholar]
- 169.Cote MP and Gossard JP. Task-dependent presynaptic inhibition. J Neurosci 23: 1886–1893, 2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Courtine G and Schieppati M. Human walking along a curved path. II. Gait features and EMG patterns. Eur J Neurosci 18: 191–205, 2003. [DOI] [PubMed] [Google Scholar]
- 171.Crago PE, Houk JC and Rymer WZ. Sampling of total muscle force by tendon organs. J Neurophysiol 47: 1069–1083, 1982. [DOI] [PubMed] [Google Scholar]
- 172.Cronin NJ, Prilutsky BI, Lichtwark GA and Maas H. Does ankle joint power reflect type of muscle action of soleus and gastrocnemius during walking in cats and humans? J Biomech 46: 1383–1386, 2013. [DOI] [PubMed] [Google Scholar]
- 173.Crowe A and Matthews PB. The effects of stimulation of static and dynamic fusimotor fibres on the response to stretching of the primary endings of muscle spindles. J Physiol 174: 109–131, 1964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.D’Angelo G, Thibaudier Y, Telonio A, Hurteau MF, Kuczynski V, Dambreville C and Frigon A. Modulation of phase durations, phase variations and temporal coordination of the four limbs during quadrupedal split-belt locomotion in intact adult cats. J Neurophysiol 112: 1825–1837, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.d’Avella A, Saltiel P and Bizzi E. Combinations of muscle synergies in the construction of a natural motor behavior. Nat Neurosci 6: 300–308, 2003. [DOI] [PubMed] [Google Scholar]
- 176.Dacko SM, Sokoloff AJ and Cope TC. Recruitment of triceps surae motor units in the decerebrate cat. I. Independence of type S units in soleus and medial gastrocnemius muscles. J Neurophysiol 75: 1997–2004, 1996. [DOI] [PubMed] [Google Scholar]
- 177.Dagg AI. The locomotion of the camel (Camelus dromedarius). Journal of Zoology 174: 67–78, 1974. [Google Scholar]
- 178.Dambreville C, Labarre A, Thibaudier Y, Hurteau MF and Frigon A. The spinal control of locomotion and step-to-step variability in left-right symmetry from slow to moderate speeds. J Neurophysiol 114: 1119–1128, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.Danner SM, Shevtsova NA, Frigon A and Rybak IA. Computational modeling of spinal circuits controlling limb coordination and gaits in quadrupeds. Elife 6: 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180.Danner SM, Zhang H, Shevtsova NA, Borowska-Fielding J, Deska-Gauthier D, Rybak IA and Zhang Y. Spinal V3 Interneurons and Left-Right Coordination in Mammalian Locomotion. Front Cell Neurosci 13: 516, 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181.Dasen JS, De CA, Wang B, Tucker PW and Jessell TM. Hox repertoires for motor neuron diversity and connectivity gated by a single accessory factor, FoxP1. Cell 134: 304–316, 2008. [DOI] [PubMed] [Google Scholar]
- 182.Dasen JS, Tice BC, Brenner-Morton S and Jessell TM. A Hox regulatory network establishes motor neuron pool identity and target-muscle connectivity. Cell 123: 477–491, 2005. [DOI] [PubMed] [Google Scholar]
- 183.Dassule HR, Lewis P, Bei M, Maas R and McMahon AP. Sonic hedgehog regulates growth and morphogenesis of the tooth. Development 127: 4775–4785, 2000. [DOI] [PubMed] [Google Scholar]
- 184.de Guzman CP, Roy RR, Hodgson JA and Edgerton VR. Coordination of motor pools controlling the ankle musculature in adult spinal cats during treadmill walking. Brain Res 555: 202–214, 1991. [DOI] [PubMed] [Google Scholar]
- 185.Degtyarenko AM, Simon ES and Burke RE. Differential modulation of disynaptic cutaneous inhibition and excitation in ankle flexor motoneurons during fictive locomotion. J Neurophysiol 76: 2972–2985, 1996. [DOI] [PubMed] [Google Scholar]
- 186.Degtyarenko AM, Simon ES, Norden-Krichmar T and Burke RE. Modulation of oligosynaptic cutaneous and muscle afferent reflex pathways during fictive locomotion and scratching in the cat. J Neurophysiol 79: 447–463, 1998. [DOI] [PubMed] [Google Scholar]
- 187.Delhaye BP, Long KH and Bensmaia SJ. Neural Basis of Touch and Proprioception in Primate Cortex. Compr Physiol 8: 1575–1602, 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 188.Deliagina TG, Musienko PE and Zelenin PV. Nervous mechanisms of locomotion in different directions. Curr Opin Physiol 8: 7–13, 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 189.Delp SL, Anderson FC, Arnold AS, Loan P, Habib A, John CT, Guendelman E and Thelen DG. OpenSim: open-source software to create and analyze dynamic simulations of movement. IEEE Trans Biomed Eng 54: 1940–1950, 2007. [DOI] [PubMed] [Google Scholar]
- 190.Descherevsky VI. Kinetic model of muscle contraction. In: Biological and biochemical oscillators, edited by Chance B, Ghosh AK, Pye EK and Hess BA. Academic Press, 1973, p. 311–328. [Google Scholar]
- 191.Desrochers E, Harnie J, Doelman A, Hurteau MF and Frigon A. Spinal control of muscle synergies for adult mammalian locomotion. J Physiol 597: 333–350, 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 192.Dietz V, Fouad K and Bastiaanse CM. Neuronal coordination of arm and leg movements during human locomotion. Eur J Neurosci 14: 1906–1914, 2001. [DOI] [PubMed] [Google Scholar]
- 193.Dietz V, Quintern J and Berger W. Corrective reactions to stumbling in man: functional significance of spinal and transcortical reflexes. Neurosci Lett 44: 131–135, 1984. [DOI] [PubMed] [Google Scholar]
- 194.Dietz V, Quintern J and Sillem M. Stumbling reactions in man: significance of proprioceptive and pre-programmed mechanisms. J Physiol 386: 149–163, 1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 195.Dietz V, Zijlstra W and Duysens J. Human neuronal interlimb coordination during split-belt locomotion. Exp Brain Res 101: 513–520, 1994. [DOI] [PubMed] [Google Scholar]
- 196.DiGiovanna J, Dominici N, Friedli L, Rigosa J, Duis S, Kreider J, Beauparlant J, van den Brand R, Schieppati M, Micera S and Courtine G. Engagement of the Rat Hindlimb Motor Cortex across Natural Locomotor Behaviors. J Neurosci 36: 10440–10455, 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 197.Dominguez-Rodriguez LE, Stecina K, Garcia-Ramirez DL, Mena-Avila E, Milla-Cruz JJ, Martinez-Silva L, Zhang M, Hultborn H and Quevedo JN. Candidate Interneurons Mediating the Resetting of the Locomotor Rhythm by Extensor Group I Afferents in the Cat. Neuroscience 450: 96–112, 2020. [DOI] [PubMed] [Google Scholar]
- 198.Dominici N, Ivanenko YP, Cappellini G, d’Avella A, Mondi V, Cicchese M, Fabiano A, Silei T, Di PA, Giannini C, Poppele RE and Lacquaniti F. Locomotor primitives in newborn babies and their development. Science 334: 997–999, 2011. [DOI] [PubMed] [Google Scholar]
- 199.Donelan JM, McVea DA and Pearson KG. Force regulation of ankle extensor muscle activity in freely walking cats. J Neurophysiol 101: 360–371, 2009. [DOI] [PubMed] [Google Scholar]
- 200.Donelan JM and Pearson KG. Contribution of force feedback to ankle extensor activity in decerebrate walking cats. J Neurophysiol 92: 2093–2104, 2004. [DOI] [PubMed] [Google Scholar]
- 201.Donelan JM and Pearson KG. Contribution of sensory feedback to ongoing ankle extensor activity during the stance phase of walking. Can J Physiol Pharmacol 82: 589–598, 2004. [DOI] [PubMed] [Google Scholar]
- 202.Dorn TW, Schache AG and Pandy MG. Muscular strategy shift in human running: dependence of running speed on hip and ankle muscle performance. J Exp Biol 215: 1944–1956, 2012. [DOI] [PubMed] [Google Scholar]
- 203.Dorofeev IY, Avelev VD, Shcherbakova NA and Gerasimenko YP. The role of cutaneous afferents in controlling locomotion evoked by epidural stimulation of the spinal cord in decerebrate cats. Neurosci Behav Physiol 38: 695–701, 2008. [DOI] [PubMed] [Google Scholar]
- 204.Dostal WF and Andrews JG. A three-dimensional biomechanical model of hip musculature. J Biomech 14: 803–812, 1981. [DOI] [PubMed] [Google Scholar]
- 205.Drew T. Functional organization within the medullary reticular formation of the intact unanesthetized cat. III. Microstimulation during locomotion. J Neurophysiol 66: 919–938, 1991. [DOI] [PubMed] [Google Scholar]
- 206.Drew T, Cabana T and Rossignol S. Responses of medullary reticulospinal neurones to stimulation of cutaneous limb nerves during locomotion in intact cats. Exp Brain Res 111: 153–168, 1996. [DOI] [PubMed] [Google Scholar]
- 207.Drew T, Dubuc R and Rossignol S. Discharge patterns of reticulospinal and other reticular neurons in chronic, unrestrained cats walking on a treadmill. J Neurophysiol 55: 375–401, 1986. [DOI] [PubMed] [Google Scholar]
- 208.Drew T and Rossignol S. Phase-dependent responses evoked in limb muscles by stimulation of medullary reticular formation during locomotion in thalamic cats. J Neurophysiol 52: 653–675, 1984. [DOI] [PubMed] [Google Scholar]
- 209.Drew T and Rossignol S. A kinematic and electromyographic study of cutaneous reflexes evoked from the forelimb of unrestrained walking cats. J Neurophysiol 57: 1160–1184, 1987. [DOI] [PubMed] [Google Scholar]
- 210.Dubuc R, Cabelguen JM and Rossignol S. Rhythmic antidromic discharges of single primary afferents recorded in cut dorsal root filaments during locomotion in the cat. Brain Res 359: 375–378, 1985. [DOI] [PubMed] [Google Scholar]
- 211.Duenas SH, Loeb GE and Marks WB. Monosynaptic and dorsal root reflexes during locomotion in normal and thalamic cats. J Neurophysiol 63: 1467–1476, 1990. [DOI] [PubMed] [Google Scholar]
- 212.Durkovic RG. Classical conditioning, sensitization and habituation in the spinal cat. Physiol Behav 14: 297–304, 1975. [DOI] [PubMed] [Google Scholar]
- 213.Durkovic RG. Classical conditioning of the flexion reflex in spinal cat: features of the reflex circuitry. Neurosci Lett 39: 155–160, 1983. [DOI] [PubMed] [Google Scholar]
- 214.Durkovic RG and Damianopoulos EN. Forward and backward classical conditioning of the flexion reflex in the spinal cat. J Neurosci 6: 2921–2925, 1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 215.Dutto DJ, Hoyt DF, Cogger EA and Wickler SJ. Ground reaction forces in horses trotting up an incline and on the level over a range of speeds. J Exp Biol 207: 3507–3514, 2004. [DOI] [PubMed] [Google Scholar]
- 216.Duysens J. Reflex control of locomotion as revealed by stimulation of cutaneous afferents in spontaneously walking premammillary cats. J Neurophysiol 40: 737–751, 1977. [DOI] [PubMed] [Google Scholar]
- 217.Duysens J and Loeb GE. Modulation of ipsi- and contralateral reflex responses in unrestrained walking cats. J Neurophysiol 44: 1024–1037, 1980. [DOI] [PubMed] [Google Scholar]
- 218.Duysens J and Pearson KG. The role of cutaneous afferents from the distal hindlimb in the regulation of the step cycle of thalamic cats. Exp Brain Res 24: 245–255, 1976. [DOI] [PubMed] [Google Scholar]
- 219.Duysens J and Pearson KG. Inhibition of flexor burst generation by loading ankle extensor muscles in walking cats. Brain Res 187: 321–332, 1980. [DOI] [PubMed] [Google Scholar]
- 220.Duysens J, Tax AA, Murrer L and Dietz V. Backward and forward walking use different patterns of phase-dependent modulation of cutaneous reflexes in humans. J Neurophysiol 76: 301–310, 1996. [DOI] [PubMed] [Google Scholar]
- 221.Duysens J, Tax AA, Trippel M and Dietz V. Increased amplitude of cutaneous reflexes during human running as compared to standing. Brain Res 613: 230–238, 1993. [DOI] [PubMed] [Google Scholar]
- 222.Duysens J, Tax AA, van der Doelen B, Trippel M and Dietz V. Selective activation of human soleus or gastrocnemius in reflex responses during walking and running. Exp Brain Res 87: 193–204, 1991. [DOI] [PubMed] [Google Scholar]
- 223.Duysens J and Van de Crommert HW. Neural control of locomotion; The central pattern generator from cats to humans. Gait Posture 7: 131–141, 1998. [DOI] [PubMed] [Google Scholar]
- 224.Duysens J, Van Wezel BM, Van de Crommert HW, Faist M and Kooloos JG. The role of afferent feedback in the control of hamstrings activity during human gait. Eur J Morphol 36: 293–299, 1998. [DOI] [PubMed] [Google Scholar]
- 225.Eccles JC, Eccles RM and Lundberg A. Synaptic actions on motoneurones caused by impulses in Golgi tendon organ afferents. J Physiol 138: 227–252, 1957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 226.Eccles JC, Eccles RM and Lundberg A. The convergence of monosynaptic excitatory afferents on to many different species of alpha motoneurones. J Physiol 137: 22–50, 1957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 227.Eccles JC, Eccles RM and MAGNI F. Central inhibitory action attributable to presynaptic depolarization produced by muscle afferent volleys. J Physiol 159: 147–166, 1961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 228.Eccles JC, HUBBARD JI and Oscarsson O. Intracellular recording from cells of the ventral spinocerebellar tract. J Physiol 158: 486–516, 1961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 229.Eccles JC, KOSTYUK PG and Schmidt RF. Central pathways responsible for depolarization of primary afferent fibres. J Physiol 161: 237–257, 1962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 230.Eccles JC, Nicoll RA, Schwarz WF, Taborikova H and Willey TJ. Reticulospinal neurons with and without monosynaptic inputs from cerebellar nuclei. J Neurophysiol 38: 513–530, 1975. [DOI] [PubMed] [Google Scholar]
- 231.Eccles JC, Oscarsson O and Willis WD. Synaptic action of group I and II afferent fibres of muscle on the cells of the dorsal spinocerebellar tract. J Physiol 158: 517–543, 1961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 232.Eccles JC, Schmidt R and Willis WD. Pharmacological studies on studies on presynaptic inhibition. J Physiol 168: 500–530, 1963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 233.Eccles RM and Lundberg A. Integrative pattern of Ia synaptic actions on motoneurones of hip and knee muscles. J Physiol 144: 271–298, 1958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 234.Edgley S, Jankowska E and McCrea D. The heteronymous monosynaptic actions of triceps surae group Ia afferents on hip and knee extensor motoneurones in the cat. Exp Brain Res 61: 443–446, 1986. [DOI] [PubMed] [Google Scholar]
- 235.Edgley SA. Organisation of inputs to spinal interneurone populations. J Physiol 533: 51–56, 2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 236.Edgley SA and Aggelopoulos NC. Short latency crossed inhibitory reflex actions evoked from cutaneous afferents. Exp Brain Res 171: 541–550, 2006. [DOI] [PubMed] [Google Scholar]
- 237.Edgley SA and Jankowska E. An interneuronal relay for group I and II muscle afferents in the midlumbar segments of the cat spinal cord. J Physiol 389: 647–674, 1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 238.Edgley SA and Jankowska E. Field potentials generated by group II muscle afferents in the middle lumbar segments of the cat spinal cord. J Physiol 385: 393–413, 1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 239.Edgley SA and Jankowska E. Information processed by dorsal horn spinocerebellar tract neurones in the cat. J Physiol 397: 81–97, 1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 240.Edgley SA and Wallace NA. A short-latency crossed pathway from cutaneous afferents to rat hindlimb motoneurones. J Physiol 411: 469–480, 1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 241.Edwards DH and Prilutsky BI. Sensory feedback in the control of posture and locomotion. In: Neurobiology of motor control: Fundamental concepts and new directions, edited by Hooper SL and Buschges A. New York: Wiley, 2017, p. 263–304. [Google Scholar]
- 242.Ekeberg O and Grillner S. Simulations of neuromuscular control in lamprey swimming. Philos Trans R Soc Lond B Biol Sci 354: 895–902, 1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 243.Ekeberg O and Pearson K. Computer simulation of stepping in the hind legs of the cat: an examination of mechanisms regulating the stance-to-swing transition. J Neurophysiol 94: 4256–4268, 2005. [DOI] [PubMed] [Google Scholar]
- 244.Ekerot CF and Larson B. The dorsal spino-olivocerebellar system in the cat. I. Functional organization and termination in the anterior lobe. Exp Brain Res 36: 201–217, 1979. [DOI] [PubMed] [Google Scholar]
- 245.Ellaway PH, Taylor A and Durbaba R. Muscle spindle and fusimotor activity in locomotion. J Anat 227: 157–166, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 246.Emonet-Denand F, Jami L and Laporte Y. Skeleto-fusimotor axons in the hind-limb muscles of the cat. J Physiol 249: 153–166, 1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 247.Eng JJ and Hoffer JA. Regional variability of stretch reflex amplitude in the cat medial gastrocnemius muscle during a postural task. J Neurophysiol 78: 1150–1154, 1997. [DOI] [PubMed] [Google Scholar]
- 248.Engberg I and Lundberg A. An electromyographic analysis of muscular activity in the hindlimb of the cat during unrestrained locomotion. Acta Physiol Scand 75: 614–630, 1969. [DOI] [PubMed] [Google Scholar]
- 249.English AW. An electromyographic analysis of forelimb muscles during overground stepping in the cat. J Exp Biol 76: 105–122, 1978. [DOI] [PubMed] [Google Scholar]
- 250.English AW and Weeks OI. An anatomical and functional analysis of cat biceps femoris and semitendinosus muscles. J Morphol 191: 161–175, 1987. [DOI] [PubMed] [Google Scholar]
- 251.Farrell BJ, Bulgakova MA, Beloozerova IN, Sirota MG and Prilutsky BI. Body stability and muscle and motor cortex activity during walking with wide stance. J Neurophysiol 112: 504–524, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 252.Favorov OV, Nilaweera WU, Miasnikov AA and Beloozerova IN. Activity of somatosensory-responsive neurons in high subdivisions of SI cortex during locomotion. J Neurosci 35: 7763–7776, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 253.Fedirchuk B, Stecina K, Kristensen KK, Zhang M, Meehan CF, Bennett DJ and Hultborn H. Rhythmic activity of feline dorsal and ventral spinocerebellar tract neurons during fictive motor actions. J Neurophysiol 109: 375–388, 2013. [DOI] [PubMed] [Google Scholar]
- 254.Feldman AG and Orlovsky GN. Activity of interneurons mediating reciprocal 1a inhibition during locomotion. Brain Res 84: 181–194, 1975. [DOI] [PubMed] [Google Scholar]
- 255.Feldman EL, Callaghan BC, Pop-Busui R, Zochodne DW, Wright DE, Bennett DL, Bril V, Russell JW and Viswanathan V. Diabetic neuropathy. Nat Rev Dis Primers 5: 41, 2019. [DOI] [PubMed] [Google Scholar]
- 256.Feng-Chen KC and Wolpaw JR. Operant conditioning of H-reflex changes synaptic terminals on primate motoneurons. Proc Natl Acad Sci U S A 93: 9206–9211, 1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 257.Fenn WO and Marsh BS. Muscular force at different speeds of shortening. J Physiol 85: 277–297, 1935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 258.Fields HL, Clanton CH and Anderson SD. Somatosensory properties of spinoreticular neurons in the cat. Brain Res 120: 49–66, 1977. [DOI] [PubMed] [Google Scholar]
- 259.Fink AJ, Croce KR, Huang ZJ, Abbott LF, Jessell TM and Azim E. Presynaptic inhibition of spinal sensory feedback ensures smooth movement. Nature 509: 43–48, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 260.Fischer MS, Lehmann SV and Andrada E. Three-dimensional kinematics of canine hind limbs: in vivo, biplanar, high-frequency fluoroscopic analysis of four breeds during walking and trotting. Sci Rep 8: 16982, 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 261.Fitzsimmons NA, Lebedev MA, Peikon ID and Nicolelis MA. Extracting kinematic parameters for monkey bipedal walking from cortical neuronal ensemble activity. Front Integr Neurosci 3: 3, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 262.Flash T and Hogan N. The coordination of arm movements: an experimentally confirmed mathematical model. J Neurosci 5: 1688–1703, 1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 263.Flynn JR, Graham BA, Galea MP and Callister RJ. The role of propriospinal interneurons in recovery from spinal cord injury. Neuropharmacology 60: 809–822, 2011. [DOI] [PubMed] [Google Scholar]
- 264.Formento E, Minassian K, Wagner F, Mignardot JB, Le Goff-Mignardot CG, Rowald A, Bloch J, Micera S, Capogrosso M and Courtine G. Electrical spinal cord stimulation must preserve proprioception to enable locomotion in humans with spinal cord injury. Nat Neurosci 21: 1728–1741, 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 265.Forssberg H. Stumbling corrective reaction: a phase-dependent compensatory reaction during locomotion. J Neurophysiol 42: 936–953, 1979. [DOI] [PubMed] [Google Scholar]
- 266.Forssberg H, Grillner S and Halbertsma J. The locomotion of the low spinal cat. I. Coordination within a hindlimb. Acta Physiol Scand 108: 269–281, 1980. [DOI] [PubMed] [Google Scholar]
- 267.Forssberg H, Grillner S, Halbertsma J and Rossignol S. The locomotion of the low spinal cat. II. Interlimb coordination. Acta Physiol Scand 108: 283–295, 1980. [DOI] [PubMed] [Google Scholar]
- 268.Forssberg H, Grillner S and Rossignol S. Phase dependent reflex reversal during walking in chronic spinal cats. Brain Res 85: 103–107, 1975. [DOI] [PubMed] [Google Scholar]
- 269.Forssberg H, Grillner S and Rossignol S. Phasic gain control of reflexes from the dorsum of the paw during spinal locomotion. Brain Res 132: 121–139, 1977. [DOI] [PubMed] [Google Scholar]
- 270.Francis JT, Xu S and Chapin JK. Proprioceptive and cutaneous representations in the rat ventral posterolateral thalamus. J Neurophysiol 99: 2291–2304, 2008. [DOI] [PubMed] [Google Scholar]
- 271.Freeman AW and Johnson KO. A model accounting for effects of vibratory amplitude on responses of cutaneous mechanoreceptors in macaque monkey. J Physiol 323: 43–64, 1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 272.Freeman AW and Johnson KO. Cutaneous mechanoreceptors in macaque monkey: temporal discharge patterns evoked by vibration, and a receptor model. J Physiol 323: 21–41, 1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 273.Freeman MA and Wyke B. The innervation of the knee joint. An anatomical and histological study in the cat. J Anat 101: 505–532, 1967. [PMC free article] [PubMed] [Google Scholar]
- 274.Frigon A. Central pattern generators of the mammalian spinal cord. Neuroscientist 18: 56–69, 2012. [DOI] [PubMed] [Google Scholar]
- 275.Frigon A. The neural control of interlimb coordination during mammalian locomotion. J Neurophysiol 117: 2224–2241, 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 276.Frigon A. Fundamental contributions of the cat model to the neural control of locomotion. In: The neural control of movement, edited by Whelan PJ and Sharples SA. London: Academic Press, 2020, p. 315–348. [Google Scholar]
- 277.Frigon A, Barriere G, Leblond H and Rossignol S. Asymmetric changes in cutaneous reflexes after a partial spinal lesion and retention following spinalization during locomotion in the cat. J Neurophysiol 102: 2667–2680, 2009. [DOI] [PubMed] [Google Scholar]
- 278.Frigon A, Carroll TJ, Jones KE, Zehr EP and Collins DF. Ankle position and voluntary contraction alter maximal M waves in soleus and tibialis anterior. Muscle Nerve 35: 756–766, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 279.Frigon A, D’Angelo G, Thibaudier Y, Hurteau MF, Telonio A, Kuczynski V and Dambreville C. Speed-dependent modulation of phase variations on a step-by-step basis and its impact on the consistency of interlimb coordination during quadrupedal locomotion in intact adult cats. J Neurophysiol 111: 1885–1902, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 280.Frigon A, Desrochers E, Thibaudier Y, Hurteau MF and Dambreville C. Left-right coordination from simple to extreme conditions during split-belt locomotion in the chronic spinal adult cat. J Physiol 595: 341–361, 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 281.Frigon A and Gossard JP. Asymmetric control of cycle period by the spinal locomotor rhythm generator in the adult cat. J Physiol 587: 4617–4628, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 282.Frigon A and Gossard JP. Evidence for specialized rhythm-generating mechanisms in the adult mammalian spinal cord. J Neurosci 30: 7061–7071, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 283.Frigon A, Hurteau MF, Thibaudier Y, Leblond H, Telonio A and D’Angelo G. Split-belt walking alters the relationship between locomotor phases and cycle duration across speeds in intact and chronic spinalized adult cats. J Neurosci 33: 8559–8566, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 284.Frigon A and Rossignol S. Plasticity of reflexes from the foot during locomotion after denervating ankle extensors in intact cats. J Neurophysiol 98: 2122–2132, 2007. [DOI] [PubMed] [Google Scholar]
- 285.Frigon A and Rossignol S. Adaptive changes of the locomotor pattern and cutaneous reflexes during locomotion studied in the same cats before and after spinalization. J Physiol 586: 2927–2945, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 286.Frigon A and Rossignol S. Locomotor and reflex adaptation after partial denervation of ankle extensors in chronic spinal cats. J Neurophysiol 100: 1513–1522, 2008. [DOI] [PubMed] [Google Scholar]
- 287.Frigon A and Rossignol S. Short-latency crossed inhibitory responses in extensor muscles during locomotion in the cat. J Neurophysiol 99: 989–998, 2008. [DOI] [PubMed] [Google Scholar]
- 288.Frigon A, Sirois J and Gossard JP. Effects of ankle and hip muscle afferent inputs on rhythm generation during fictive locomotion. J Neurophysiol 103: 1591–1605, 2010. [DOI] [PubMed] [Google Scholar]
- 289.Frigon A, Thibaudier Y and Hurteau MF. Modulation of forelimb and hindlimb muscle activity during quadrupedal tied-belt and split-belt locomotion in intact cats. Neuroscience 290: 266–278, 2015. [DOI] [PubMed] [Google Scholar]
- 290.Frigon A, Thibaudier Y, Johnson MD, Hurteau MF and Heckman CJ. Cutaneous inputs from the back abolish locomotor-like activity and reduce spastic-like activity in the adult cat following complete spinal cord injury. Exp Neurol 235: 588–598, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 291.Fritz N, Illert M, de la Motte S, Reeh P and Saggau P. Pattern of monosynaptic Ia connections in the cat forelimb. J Physiol 419: 321–351, 1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 292.Fung J and Macpherson JM. Determinants of postural orientation in quadrupedal stance. J Neurosci 15: 1121–1131, 1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 293.Fung J and Macpherson JM. Attributes of quiet stance in the chronic spinal cat. J Neurophysiol 82: 3056–3065, 1999. [DOI] [PubMed] [Google Scholar]
- 294.Galvez-Lopez E, Maes LD and Abourachid A. The search for stability on narrow supports: an experimental study in cats and dogs. Zoology (Jena) 114: 224–232, 2011. [DOI] [PubMed] [Google Scholar]
- 295.Gambaryan PP. How mammals run: Anatomical adaptations. New York: John Wiley & Sons, 1974. [Google Scholar]
- 296.Ganley KJ and Powers CM. Intersegmental dynamics during the swing phase of gait: a comparison of knee kinetics between 7 year-old children and adults. Gait Posture 23: 499–504, 2006. [DOI] [PubMed] [Google Scholar]
- 297.Garcia-Ramirez DL, Calvo JR, Hochman S and Quevedo JN. Serotonin, dopamine and noradrenaline adjust actions of myelinated afferents via modulation of presynaptic inhibition in the mouse spinal cord. PLoS One 9: e89999, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 298.Garwicz M. Spinal reflexes provide motor error signals to cerebellar modules--relevance for motor coordination. Brain Res Brain Res Rev 40: 152–165, 2002. [DOI] [PubMed] [Google Scholar]
- 299.Garwicz M, Levinsson A and Schouenborg J. Common principles of sensory encoding in spinal reflex modules and cerebellar climbing fibres. J Physiol 540: 1061–1069, 2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 300.Gatto G, Smith KM, Ross SE and Goulding M. Neuronal diversity in the somatosensory system: bridging the gap between cell type and function. Curr Opin Neurobiol 56: 167–174, 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 301.Geertsen SS, Stecina K, Meehan CF, Nielsen JB and Hultborn H. Reciprocal Ia inhibition contributes to motoneuronal hyperpolarisation during the inactive phase of locomotion and scratching in the cat. J Physiol 589: 119–134, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 302.Gellman R, Gibson AR and Houk JC. Inferior olivary neurons in the awake cat: detection of contact and passive body displacement. J Neurophysiol 54: 40–60, 1985. [DOI] [PubMed] [Google Scholar]
- 303.Gellman R, Houk JC and Gibson AR. Somatosensory properties of the inferior olive of the cat. J Comp Neurol 215: 228–243, 1983. [DOI] [PubMed] [Google Scholar]
- 304.Gibson AR, Horn KM and Pong M. Activation of climbing fibers. Cerebellum 3: 212–221, 2004. [DOI] [PubMed] [Google Scholar]
- 305.Gill ML, Grahn PJ, Calvert JS, Linde MB, Lavrov IA, Strommen JA, Beck LA, Sayenko DG, Van Straaten MG, Drubach DI, Veith DD, Thoreson AR, Lopez C, Gerasimenko YP, Edgerton VR, Lee KH and Zhao KD. Neuromodulation of lumbosacral spinal networks enables independent stepping after complete paraplegia. Nat Med 24: 1677–1682, 2018. [DOI] [PubMed] [Google Scholar]
- 306.Gillis GB and Biewener AA. Hindlimb muscle function in relation to speed and gait: in vivo patterns of strain and activation in a hip and knee extensor of the rat (Rattus norvegicus). J Exp Biol 204: 2717–2731, 2001. [DOI] [PubMed] [Google Scholar]
- 307.Gillis GB, Flynn JP, McGuigan P and Biewener AA. Patterns of strain and activation in the thigh muscles of goats across gaits during level locomotion. J Exp Biol 208: 4599–4611, 2005. [DOI] [PubMed] [Google Scholar]
- 308.Giuliani CA and Smith JL. Stepping behaviors in chronic spinal cats with one hindlimb deafferented. J Neurosci 7: 2537–2546, 1987. [PMC free article] [PubMed] [Google Scholar]
- 309.Goldberger ME and Murray M. Restitution of function and collateral sprouting in the cat spinal cord: the deafferented animal. J Comp Neurol 158: 37–53, 1974. [DOI] [PubMed] [Google Scholar]
- 310.Goldfinger MD and Fukami Y. Distribution, density and size of muscle receptors in cat tail dorsolateral muscles. J Anat 135: 371–384, 1982. [PMC free article] [PubMed] [Google Scholar]
- 311.Goldring JM, Kuno M, Nunez R and Snider WD. Reaction of synapses on motoneurones to section and restoration of peripheral sensory connexions in the cat. J Physiol 309: 185–198, 1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 312.Goode DJ and Van HJ. Loss of patellar and Achilles tendon reflexes in classical ballet dancers. Arch Neurol 39: 323, 1982. [DOI] [PubMed] [Google Scholar]
- 313.Gorassini M, Eken T, Bennett DJ, Kiehn O and Hultborn H. Activity of hindlimb motor units during locomotion in the conscious rat. J Neurophysiol 83: 2002–2011, 2000. [DOI] [PubMed] [Google Scholar]
- 314.Gordon AM, Huxley AF and Julian FJ. The variation in isometric tension with sarcomere length in vertebrate muscle fibres. J Physiol 184: 170–192, 1966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 315.Gosgnach S, Quevedo J, Fedirchuk B and McCrea DA. Depression of group Ia monosynaptic EPSPs in cat hindlimb motoneurones during fictive locomotion. J Physiol 526 Pt 3: 639–652, 2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 316.Goslow GE Jr., Reinking RM and Stuart DG. The cat step cycle: hind limb joint angles and muscle lengths during unrestrained locomotion. J Morphol 141: 1–41, 1973. [DOI] [PubMed] [Google Scholar]
- 317.Goslow GE Jr., Seeherman HJ, Taylor CR, McCutchin MN and Heglund NC. Electrical activity and relative length changes of dog limb muscles as a function of speed and gait. J Exp Biol 94: 15–42, 1981. [DOI] [PubMed] [Google Scholar]
- 318.Gossard JP. Control of transmission in muscle group IA afferents during fictive locomotion in the cat. J Neurophysiol 76: 4104–4112, 1996. [DOI] [PubMed] [Google Scholar]
- 319.Gossard JP, Brownstone RM, Barajon I and Hultborn H. Transmission in a locomotor-related group Ib pathway from hindlimb extensor muscles in the cat. Exp Brain Res 98: 213–228, 1994. [DOI] [PubMed] [Google Scholar]
- 320.Gossard JP, Cabelguen JM and Rossignol S. Intra-axonal recordings of cutaneous primary afferents during fictive locomotion in the cat. J Neurophysiol 62: 1177–1188, 1989. [DOI] [PubMed] [Google Scholar]
- 321.Gossard JP, Cabelguen JM and Rossignol S. Phase-dependent modulation of primary afferent depolarization in single cutaneous primary afferents evoked by peripheral stimulation during fictive locomotion in the cat. Brain Res 537: 14–23, 1990. [DOI] [PubMed] [Google Scholar]
- 322.Gossard JP, Cabelguen JM and Rossignol S. An intracellular study of muscle primary afferents during fictive locomotion in the cat. J Neurophysiol 65: 914–926, 1991. [DOI] [PubMed] [Google Scholar]
- 323.Gossard JP and Rossignol S. Phase-dependent modulation of dorsal root potentials evoked by peripheral nerve stimulation during fictive locomotion in the cat. Brain Res 537: 1–13, 1990. [DOI] [PubMed] [Google Scholar]
- 324.Gossard JP, Sirois J, Noue P, Cote MP, Menard A, Leblond H and Frigon A. Chapter 2--the spinal generation of phases and cycle duration. Prog Brain Res 188: 15–29, 2011. [DOI] [PubMed] [Google Scholar]
- 325.Granit R. The functional role of the muscle spindles--facts and hypotheses. Brain 98: 531–556, 1975. [DOI] [PubMed] [Google Scholar]
- 326.Grau JW. Learning from the spinal cord: how the study of spinal cord plasticity informs our view of learning. Neurobiol Learn Mem 108: 155–171, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 327.Grau JW, Salinas JA, Illich PA and Meagher MW. Associative learning and memory for an antinociceptive response in the spinalized rat. Behav Neurosci 104: 489–494, 1990. [DOI] [PubMed] [Google Scholar]
- 328.Gray J. Animal Locomotion (World Naturalist). Norton, New York: Littlehampton Book Services Ltd, 1968. [Google Scholar]
- 329.Gregor RJ, Maas H, Bulgakova MA, Oliver A, English AW and Prilutsky BI. Time course of functional recovery during the first 3 mo after surgical transection and repair of nerves to the feline soleus and lateral gastrocnemius muscles. J Neurophysiol 119: 1166–1185, 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 330.Gregor RJ, Roy RR, Whiting WC, Lovely RG, Hodgson JA and Edgerton VR. Mechanical output of the cat soleus during treadmill locomotion: in vivo vs in situ characteristics. J Biomech 21: 721–732, 1988. [DOI] [PubMed] [Google Scholar]
- 331.Gregor RJ, Smith DW and Prilutsky BI. Mechanics of slope walking in the cat: quantification of muscle load, length change, and ankle extensor EMG patterns. J Neurophysiol 95: 1397–1409, 2006. [DOI] [PubMed] [Google Scholar]
- 332.Gregory JE, Brockett CL, Morgan DL, Whitehead NP and Proske U. Effect of eccentric muscle contractions on Golgi tendon organ responses to passive and active tension in the cat. J Physiol 538: 209–218, 2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 333.Gregory JE, McIntyre AK and Proske U. Tendon organ afferents in the knee joint nerve of the cat. Neurosci Lett 103: 287–292, 1989. [DOI] [PubMed] [Google Scholar]
- 334.Grey MJ, Mazzaro N, Nielsen JB and Sinkjaer T. Ankle extensor proprioceptors contribute to the enhancement of the soleus EMG during the stance phase of human walking. Can J Physiol Pharmacol 82: 610–616, 2004. [DOI] [PubMed] [Google Scholar]
- 335.Grey MJ, Nielsen JB, Mazzaro N and Sinkjaer T. Positive force feedback in human walking. J Physiol 581: 99–105, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 336.Griffin TM, Kram R, Wickler SJ and Hoyt DF. Biomechanical and energetic determinants of the walk-trot transition in horses. J Exp Biol 207: 4215–4223, 2004. [DOI] [PubMed] [Google Scholar]
- 337.Grillner S. Control of locomotion in bipeds, tetrapods, and fish. In: Handbook of Physiology, The Nervous System, Motor Control, edited by Brooks VB. Bethesda, MD: 1981, p. 1179–1236. [Google Scholar]
- 338.Grillner S and Dubuc R. Control of locomotion in vertebrates: spinal and supraspinal mechanisms. Adv Neurol 47: 425–453, 1988. [PubMed] [Google Scholar]
- 339.Grillner S and El Manira A. Current Principles of Motor Control, with Special Reference to Vertebrate Locomotion. Physiol Rev 100: 271–320, 2020. [DOI] [PubMed] [Google Scholar]
- 340.Grillner S, Halbertsma J, Nilsson J and Thorstensson A. The adaptation to speed in human locomotion. Brain Res 165: 177–182, 1979. [DOI] [PubMed] [Google Scholar]
- 341.Grillner S, Hongo T and Lund S. Descending monosynaptic and reflex control of gamma-motoneurones. Acta Physiol Scand 75: 592–613, 1969. [DOI] [PubMed] [Google Scholar]
- 342.Grillner S and Rossignol S. On the initiation of the swing phase of locomotion in chronic spinal cats. Brain Res 146: 269–277, 1978. [DOI] [PubMed] [Google Scholar]
- 343.Grillner S, Wallen P, Saitoh K, Kozlov A and Robertson B. Neural bases of goal-directed locomotion in vertebrates--an overview. Brain Res Rev 57: 2–12, 2008. [DOI] [PubMed] [Google Scholar]
- 344.Grillner S and Zangger P. On the central generation of locomotion in the low spinal cat. Exp Brain Res 34: 241–261, 1979. [DOI] [PubMed] [Google Scholar]
- 345.Grillner S and Zangger P. The effect of dorsal root transection on the efferent motor pattern in the cat’s hindlimb during locomotion. Acta Physiol Scand 120: 393–405, 1984. [DOI] [PubMed] [Google Scholar]
- 346.Guertin P, Angel MJ, Perreault MC and McCrea DA. Ankle extensor group I afferents excite extensors throughout the hindlimb during fictive locomotion in the cat. J Physiol 487 (Pt 1): 197–209, 1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 347.Gurfinkel VS and Pal’tsev EI. [Effect of the segmentary apparatus of the spinal cord on the execution of simple movement reactions]. Biofizika 10: 855–860, 1965. [PubMed] [Google Scholar]
- 348.Haftel VK, Bichler EK, Wang QB, Prather JF, Pinter MJ and Cope TC. Central suppression of regenerated proprioceptive afferents. J Neurosci 25: 4733–4742, 2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 349.Hagbarth KE. Excitatory and inhibitory skin areas for flexor and extensor motoneurons. Acta Physiol Scand Suppl 26: 1–58, 1952. [PubMed] [Google Scholar]
- 350.Halbertsma JM. The stride cycle of the cat: the modelling of locomotion by computerized analysis of automatic recordings. Acta Physiol Scand Suppl 521: 1–75, 1983. [PubMed] [Google Scholar]
- 351.Hammar I, Bannatyne BA, Maxwell DJ, Edgley SA and Jankowska E. The actions of monoamines and distribution of noradrenergic and serotoninergic contacts on different subpopulations of commissural interneurons in the cat spinal cord. Eur J Neurosci 19: 1305–1316, 2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 352.Hammar I, Chojnicka B and Jankowska E. Modulation of responses of feline ventral spinocerebellar tract neurons by monoamines. J Comp Neurol 443: 298–309, 2002. [DOI] [PubMed] [Google Scholar]
- 353.Han S, Chu JU, Kim H, Park JW and Youn I. Multiunit Activity-Based Real-Time Limb-State Estimation from Dorsal Root Ganglion Recordings. Sci Rep 7: 44197, 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 354.Haridas C and Zehr EP. Coordinated interlimb compensatory responses to electrical stimulation of cutaneous nerves in the hand and foot during walking. J Neurophysiol 90: 2850–2861, 2003. [DOI] [PubMed] [Google Scholar]
- 355.Haridas C, Zehr EP and Misiaszek JE. Context-dependent modulation of interlimb cutaneous reflexes in arm muscles as a function of stability threat during walking. J Neurophysiol 96: 3096–3103, 2006. [DOI] [PubMed] [Google Scholar]
- 356.Harischandra N, Knuesel J, Kozlov A, Bicanski A, Cabelguen JM, Ijspeert A and Ekeberg O. Sensory feedback plays a significant role in generating walking gait and in gait transition in salamanders: a simulation study. Front Neurorobot 5: 3, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 357.Harkema SJ, Hurley SL, Patel UK, Requejo PS, Dobkin BH and Edgerton VR. Human lumbosacral spinal cord interprets loading during stepping. J Neurophysiol 77: 797–811, 1997. [DOI] [PubMed] [Google Scholar]
- 358.Harnie J, Audet J, Klishko AN, Doelman A, Prilutsky BI and Frigon A. The spinal control of backward locomotion. J Neurosci 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 359.Harnie J, Audet J, Klishko AN, Doelman A, Prilutsky BI and Frigon A. The Spinal Control of Backward Locomotion. J Neurosci 41: 630–647, 2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 360.Harnie J, Doelman A, de VE, Audet J, Desrochers E, Gaudreault N and Frigon A. The recovery of standing and locomotion after spinal cord injury does not require task-specific training. Elife 8: 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 361.Harrison PJ and Jankowska E. Sources of input to interneurones mediating group I non-reciprocal inhibition of motoneurones in the cat. J Physiol 361: 379–401, 1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 362.Hart BL. Facilitation by strychnine of reflex walking in spinal dogs. Physiol Behav 6: 627–628, 1971. [DOI] [PubMed] [Google Scholar]
- 363.Hasan Z. A model of spindle afferent response to muscle stretch. J Neurophysiol 49: 989–1006, 1983. [DOI] [PubMed] [Google Scholar]
- 364.Hatze H. A general myocybernetic control model of skeletal muscle. Biol Cybern 28: 143–157, 1978. [DOI] [PubMed] [Google Scholar]
- 365.Hatze H. A three-dimensional multivariate model of passive human joint torques and articular boundaries. Clin Biomech (Bristol, Avon) 12: 128–135, 1997. [DOI] [PubMed] [Google Scholar]
- 366.He J, Levine WS and Loeb GE. Feedback gains for correcting small perturbations to standing posture. IEEE Transactions on Automatic Control 36: 322–332, 1991. [Google Scholar]
- 367.Heglund NC, Taylor CR and McMahon TA. Scaling stride frequency and gait to animal size: mice to horses. Science 186: 1112–1113, 1974. [DOI] [PubMed] [Google Scholar]
- 368.Hicheur H, Pham QC, Arechavaleta G, Laumond JP and Berthoz A. The formation of trajectories during goal-oriented locomotion in humans. I. A stereotyped behaviour. Eur J Neurosci 26: 2376–2390, 2007. [DOI] [PubMed] [Google Scholar]
- 369.Hicheur H, Terekhov AV and Berthoz A. Intersegmental coordination during human locomotion: does planar covariation of elevation angles reflect central constraints? J Neurophysiol 96: 1406–1419, 2006. [DOI] [PubMed] [Google Scholar]
- 370.Hiebert GW and Pearson KG. Contribution of sensory feedback to the generation of extensor activity during walking in the decerebrate Cat. J Neurophysiol 81: 758–770, 1999. [DOI] [PubMed] [Google Scholar]
- 371.Hiebert GW, Whelan PJ, Prochazka A and Pearson KG. Contribution of hind limb flexor muscle afferents to the timing of phase transitions in the cat step cycle. J Neurophysiol 75: 1126–1137, 1996. [DOI] [PubMed] [Google Scholar]
- 372.Higgin D, Krupka A, Maghsoudi OH, Klishko AN, Nichols TR, Lyle MA, Prilutsky BI and Lemay MA. Adaptation to slope in locomotor-trained spinal cats with intact and self-reinnervated lateral gastrocnemius and soleus muscles. J Neurophysiol 123: 70–89, 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 373.Hildebrand M. Symmetrical gaits of dogs in relation to body build. J Morphol 124: 353–360, 1968. [DOI] [PubMed] [Google Scholar]
- 374.Hildebrand M. The quadrupedal gaits of vertebrates. BioScience 39: 766–775, 1989. [Google Scholar]
- 375.Hill AV. The heat of shortening and the dynamic constants of muscle. Proc R Soc B 126: 136–195, 1938. [Google Scholar]
- 376.Hines ML and Carnevale NT. The NEURON simulation environment. Neural Comput 9: 1179–1209, 1997. [DOI] [PubMed] [Google Scholar]
- 377.Hippenmeyer S, Vrieseling E, Sigrist M, Portmann T, Laengle C, Ladle DR and Arber S. A developmental switch in the response of DRG neurons to ETS transcription factor signaling. PLoS Biol 3: e159, 2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 378.Hishinuma M and Yamaguchi T. Modulation of reflex responses during fictive locomotion in the forelimb of the cat. Brain Res 482: 184–188, 1989. [DOI] [PubMed] [Google Scholar]
- 379.Hof AL, Gazendam MG and Sinke WE. The condition for dynamic stability. J Biomech 38: 1–8, 2005. [DOI] [PubMed] [Google Scholar]
- 380.Hoffer JA, Caputi AA, Pose IE and Griffiths RI. Roles of muscle activity and load on the relationship between muscle spindle length and whole muscle length in the freely walking cat. Prog Brain Res 80: 75–85, 1989. [DOI] [PubMed] [Google Scholar]
- 381.Hoffer JA, Loeb GE, Sugano N, Marks WB, O’Donovan MJ and Pratt CA. Cat hindlimb motoneurons during locomotion. III. Functional segregation in sartorius. J Neurophysiol 57: 554–562, 1987. [DOI] [PubMed] [Google Scholar]
- 382.Hollerbach MJ and Flash T. Dynamic interactions between limb segments during planar arm movement. Biol Cybern 44: 67–77, 1982. [DOI] [PubMed] [Google Scholar]
- 383.Holmqvist B and Lundberg A. Differential supraspinal control of synaptic actions evoked by volleys in the flexion reflex afferents in alpha motoneurones. Acta Physiol Scand Suppl 186: 1–15, 1961. [PubMed] [Google Scholar]
- 384.Holmqvist B, Oscarsson O and ROSEN I. Functional organization of the cuneocrebellar tract in the cat. Acta Physiol Scand 58: 216–235, 1963. [DOI] [PubMed] [Google Scholar]
- 385.Honeycutt CF, Gottschall JS and Nichols TR. Electromyographic responses from the hindlimb muscles of the decerebrate cat to horizontal support surface perturbations. J Neurophysiol 101: 2751–2761, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 386.Honeycutt CF, Nardelli P, Cope TC and Nichols TR. Muscle spindle responses to horizontal support surface perturbation in the anesthetized cat: insights into the role of autogenic feedback in whole body postural control. J Neurophysiol 108: 1253–1261, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 387.Honeycutt CF and Nichols TR. Disruption of cutaneous feedback alters magnitude but not direction of muscle responses to postural perturbations in the decerebrate cat. Exp Brain Res 203: 765–771, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 388.Honeycutt CF and Nichols TR. The mechanical actions of muscles predict the direction of muscle activation during postural perturbations in the cat hindlimb. J Neurophysiol 111: 900–907, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 389.Hongo T, Jankowska E and Lundberg A. The rubrospinal tract. 3. Effects on primary afferent terminals. Exp Brain Res 15: 39–53, 1972. [DOI] [PubMed] [Google Scholar]
- 390.Hongo T, Lundberg A, Phillips CG and Thompson RF. The pattern of monosynaptic Ia-connections to hindlimb motor nuclei in the baboon: a comparison with the cat. Proc R Soc Lond B Biol Sci 221: 261–289, 1984. [DOI] [PubMed] [Google Scholar]
- 391.Hooper SL. Body size and the neural control of movement. Curr Biol 22: R318–R322, 2012. [DOI] [PubMed] [Google Scholar]
- 392.Hoover DM and Carlton WW. The subacute neurotoxicity of excess pyridoxine HCl and clioquinol (5-chloro-7-iodo-8-hydroxyquinoline) in beagle dogs. II. Pathology. Vet Pathol 18: 757–768, 1981. [DOI] [PubMed] [Google Scholar]
- 393.Horak FB, Diener HC and Nashner LM. Influence of central set on human postural responses. J Neurophysiol 62: 841–853, 1989. [DOI] [PubMed] [Google Scholar]
- 394.Horn KM, Deep A and Gibson AR. Progressive limb ataxia following inferior olive lesions. J Physiol 591: 5475–5489, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 395.Houk J and Henneman E. Responses of Golgi tendon organs to active contractions of the soleus muscle of the cat. J Neurophysiol 30: 466–481, 1967. [DOI] [PubMed] [Google Scholar]
- 396.Houk J and Simon W. Responses of Golgi tendon organs to forces applied to muscle tendon. J Neurophysiol 30: 1466–1481, 1967. [DOI] [PubMed] [Google Scholar]
- 397.Housley SN, Nardelli P, Carrasco DI, Rotterman TM, Pfahl E, Matyunina LV, McDonald JF and Cope TC. Cancer Exacerbates Chemotherapy-Induced Sensory Neuropathy. Cancer Res 80: 2940–2955, 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 398.Hoyt DF and Taylor CR. Gait and the energetics of locomotion in horses. Nature 292: 239–240, 1981. [Google Scholar]
- 399.Hreljac A. Determinants of the gait transition speed during human locomotion: kinematic factors. J Biomech 28: 669–677, 1995. [DOI] [PubMed] [Google Scholar]
- 400.Hu Y, Hu S, Wang W, Wu X, Marshall FB, Chen X, Hou L and Wang C. Earliest evidence for commensal processes of cat domestication. Proc Natl Acad Sci U S A 111: 116–120, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 401.Hughes DI, Mackie M, Nagy GG, Riddell JS, Maxwell DJ, Szabo G, Erdelyi F, Veress G, Szucs P, Antal M and Todd AJ. P boutons in lamina IX of the rodent spinal cord express high levels of glutamic acid decarboxylase-65 and originate from cells in deep medial dorsal horn. Proc Natl Acad Sci U S A 102: 9038–9043, 2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 402.Hulliger M. The mammalian muscle spindle and its central control. Rev Physiol Biochem Pharmacol 101: 1–110, 1984. [DOI] [PubMed] [Google Scholar]
- 403.Hultborn H, Conway BA, Gossard JP, Brownstone R, Fedirchuk B, Schomburg ED, Enriquez-Denton M and Perreault MC. How do we approach the locomotor network in the mammalian spinal cord? Ann N Y Acad Sci 860: 70–82, 1998. [DOI] [PubMed] [Google Scholar]
- 404.Hultborn H and Nielsen JB. Spinal control of locomotion--from cat to man. Acta Physiol (Oxf) 189: 111–121, 2007. [DOI] [PubMed] [Google Scholar]
- 405.Hunt SP and Mantyh PW. The molecular dynamics of pain control. Nat Rev Neurosci 2: 83–91, 2001. [DOI] [PubMed] [Google Scholar]
- 406.Hurteau MF and Frigon A. A spinal mechanism related to left-right symmetry reduces cutaneous reflex modulation independent of speed during split-belt locomotion. J Neurosci 38: 10314–10328, 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 407.Hurteau MF, Thibaudier Y, Dambreville C, Chraibi A, Desrochers E, Telonio A and Frigon A. Non-linear modulation of cutaneous reflexes with increasing speed of locomotion in spinal cats. J Neurosci 37: 3896–3912, 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 408.Hurteau MF, Thibaudier Y, Dambreville C, Danner SM, Rybak I and Frigon A. Intralimb and interlimb cutaneous reflexes during locomotion in the intact cat. J Neurosci 38: 4104–4122, 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 409.Hurteau MF, Thibaudier Y, Dambreville C, Desaulniers C and Frigon A. Effect of stimulating the lumbar skin caudal to a complete spinal cord injury on hindlimb locomotion. J Neurophysiol 113: 669–676, 2015. [DOI] [PubMed] [Google Scholar]
- 410.Hutchison DL, Roy RR, Hodgson JA and Edgerton VR. EMG amplitude relationships between the rat soleus and medial gastrocnemius during various motor tasks. Brain Res 502: 233–244, 1989. [DOI] [PubMed] [Google Scholar]
- 411.Huxley AF. Muscle structure and theories of contraction. Prog Biophys Biophys Chem 7: 255–318, 1957. [PubMed] [Google Scholar]
- 412.Hyvarinen J and Poranen A. Receptive field integration and submodality convergence in the hand area of the post-central gyrus of the alert monkey. J Physiol 283: 539–556, 1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 413.Ijspeert AJ. Biorobotics: using robots to emulate and investigate agile locomotion. Science 346: 196–203, 2014. [DOI] [PubMed] [Google Scholar]
- 414.Illich PA, Salinas JA and Grau JW. Latent inhibition, overshadowing, and blocking of a conditioned antinociceptive response in spinalized rats. Behav Neural Biol 62: 140–150, 1994. [DOI] [PubMed] [Google Scholar]
- 415.Imai F and Yoshida Y. Molecular mechanisms underlying monosynaptic sensory-motor circuit development in the spinal cord. Dev Dyn 247: 581–587, 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 416.Inglis JT, Horak FB, Shupert CL and Jones-Rycewicz C. The importance of somatosensory information in triggering and scaling automatic postural responses in humans. Exp Brain Res 101: 159–164, 1994. [DOI] [PubMed] [Google Scholar]
- 417.Inglis JT and Macpherson JM. Bilateral labyrinthectomy in the cat: effects on the postural response to translation. J Neurophysiol 73: 1181–1191, 1995. [DOI] [PubMed] [Google Scholar]
- 418.Ishikawa M, Komi PV, Grey MJ, Lepola V and Bruggemann GP. Muscle-tendon interaction and elastic energy usage in human walking. J Appl Physiol (1985) 99: 603–608, 2005. [DOI] [PubMed] [Google Scholar]
- 419.Ishizuka N, Mannen H, Hongo T and Sasaki S. Trajectory of group Ia afferent fibers stained with horseradish peroxidase in the lumbosacral spinal cord of the cat: three dimensional reconstructions from serial sections. J Comp Neurol 186: 189–211, 1979. [DOI] [PubMed] [Google Scholar]
- 420.Ito M. Cerebellar circuitry as a neuronal machine. Prog Neurobiol 78: 272–303, 2006. [DOI] [PubMed] [Google Scholar]
- 421.Ivanenko YP, Cappellini G, Dominici N, Poppele RE and Lacquaniti F. Coordination of locomotion with voluntary movements in humans. J Neurosci 25: 7238–7253, 2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 422.Ivanenko YP, Cappellini G, Dominici N, Poppele RE and Lacquaniti F. Modular control of limb movements during human locomotion. J Neurosci 27: 11149–11161, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 423.Ivanenko YP, Cappellini G, Poppele RE and Lacquaniti F. Spatiotemporal organization of alpha-motoneuron activity in the human spinal cord during different gaits and gait transitions. Eur J Neurosci 27: 3351–3368, 2008. [DOI] [PubMed] [Google Scholar]
- 424.Ivanenko YP, d’Avella A, Poppele RE and Lacquaniti F. On the origin of planar covariation of elevation angles during human locomotion. J Neurophysiol 99: 1890–1898, 2008. [DOI] [PubMed] [Google Scholar]
- 425.Ivanenko YP, Poppele RE and Lacquaniti F. Spinal cord maps of spatiotemporal alpha-motoneuron activation in humans walking at different speeds. J Neurophysiol 95: 602–618, 2006. [DOI] [PubMed] [Google Scholar]
- 426.Ivashko DG, Prilutsky BI, Markin SN, Chapin JK and Rybak IA. Modeling the spinal cord neural circuitry controlling cat hindlimb movement during locomotion. Neurocomputing 52–54: 621–629, 2003. [Google Scholar]
- 427.Iwahara T, Atsuta Y, Garcia-Rill E and Skinner RD. Spinal cord stimulation-induced locomotion in the adult cat. Brain Res Bull 28: 99–105, 1992. [DOI] [PubMed] [Google Scholar]
- 428.Iwamura Y. Hierarchical somatosensory processing. Curr Opin Neurobiol 8: 522–528, 1998. [DOI] [PubMed] [Google Scholar]
- 429.Jacobs R and van Ingen Schenau GJ. Control of an external force in leg extensions in humans. J Physiol 457: 611–626, 1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 430.Jalaleddini K, Minos NC, Chakravarthi RS, Joon SW, Loeb GE, Sanger TD and Valero-Cuevas FJ. Neuromorphic meets neuromechanics, part II: the role of fusimotor drive. J Neural Eng 14: 025002, 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 431.Jami L. Golgi tendon organs in mammalian skeletal muscle: functional properties and central actions. Physiol Rev 72: 623–666, 1992. [DOI] [PubMed] [Google Scholar]
- 432.Janig W, Schmidt RF and Zimmermann M. Single unit responses and the total afferent outflow from the cat’s foot pad upon mechanical stimulation. Exp Brain Res 6: 100–115, 1968. [DOI] [PubMed] [Google Scholar]
- 433.Jankowska E. Interneuronal relay in spinal pathways from proprioceptors. Prog Neurobiol 38: 335–378, 1992. [DOI] [PubMed] [Google Scholar]
- 434.Jankowska E. Spinal interneuronal networks in the cat: elementary components. Brain Res Rev 57: 46–55, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 435.Jankowska E. On the distribution of information from muscle spindles in the spinal cord; how much does it depend on random factors? J Anat 227: 184–193, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 436.Jankowska E, Bannatyne BA, Stecina K, Hammar I, Cabaj A and Maxwell DJ. Commissural interneurons with input from group I and II muscle afferents in feline lumbar segments: neurotransmitters, projections and target cells. J Physiol 587: 401–418, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 437.Jankowska E and Edgley SA. Functional subdivision of feline spinal interneurons in reflex pathways from group Ib and II muscle afferents; an update. Eur J Neurosci 32: 881–893, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 438.Jankowska E and Hammar I. Spinal interneurones; how can studies in animals contribute to the understanding of spinal interneuronal systems in man? Brain Res Brain Res Rev 40: 19–28, 2002. [DOI] [PubMed] [Google Scholar]
- 439.Jankowska E and Hammar I. Interactions between spinal interneurons and ventral spinocerebellar tract neurons. J Physiol 591: 5445–5451, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 440.Jankowska E, Jukes MG, Lund S and Lundberg A. The effect of DOPA on the spinal cord. 5. Reciprocal organization of pathways transmitting excitatory action to alpha motoneurones of flexors and extensors. Acta Physiol Scand 70: 369–388, 1967. [DOI] [PubMed] [Google Scholar]
- 441.Jankowska E, Jukes MG, Lund S and Lundberg A. The effect of DOPA on the spinal cord. 6. Half-centre organization of interneurones transmitting effects from the flexor reflex afferents. Acta Physiol Scand 70: 389–402, 1967. [DOI] [PubMed] [Google Scholar]
- 442.Jeneskog T and Johansson H. The rubro-bulbospinal path. A descending system known to influence dynamic fusimotor neurones and its interaction with distal cutaneous afferents in the control of flexor reflex afferent pathways. Exp Brain Res 27: 161–179, 1977. [DOI] [PubMed] [Google Scholar]
- 443.Jessell TM, Surmeli G and Kelly JS. Motor neurons and the sense of place. Neuron 72: 419–424, 2011. [DOI] [PubMed] [Google Scholar]
- 444.Jiang J, Azim E, Ekerot CF and Alstermark B. Direct and indirect spino-cerebellar pathways: shared ideas but different functions in motor control. Front Comput Neurosci 9: 75, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 445.Johansson H. Role of knee ligaments in proprioception and regulation of muscle stiffness. J Electromyogr Kinesiol 1: 158–179, 1991. [DOI] [PubMed] [Google Scholar]
- 446.Johansson H, Sjolander P and Sojka P. Actions on gamma-motoneurones elicited by electrical stimulation of joint afferent fibres in the hind limb of the cat. J Physiol 375: 137–152, 1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 447.Johansson H, Sjolander P and Sojka P. A sensory role for the cruciate ligaments. Clin Orthop Relat Res 161–178, 1991. [PubMed] [Google Scholar]
- 448.Johansson RS and Westling G. Roles of glabrous skin receptors and sensorimotor memory in automatic control of precision grip when lifting rougher or more slippery objects. Exp Brain Res 56: 550–564, 1984. [DOI] [PubMed] [Google Scholar]
- 449.Johnson KO. The roles and functions of cutaneous mechanoreceptors. Curr Opin Neurobiol 11: 455–461, 2001. [DOI] [PubMed] [Google Scholar]
- 450.Jordan LM. Factors determining motoneuron rhythmicity during fictive locomotion. Symp Soc Exp Biol 37: 423–444, 1983. [PubMed] [Google Scholar]
- 451.Josset N, Roussel M, Lemieux M, Lafrance-Zoubga D, Rastqar A and Bretzner F. Distinct Contributions of Mesencephalic Locomotor Region Nuclei to Locomotor Control in the Freely Behaving Mouse. Curr Biol 28: 884–901, 2018. [DOI] [PubMed] [Google Scholar]
- 452.Kaas JH, Nelson RJ, Sur M, Dykes RW and Merzenich MM. The somatotopic organization of the ventroposterior thalamus of the squirrel monkey, Saimiri sciureus. J Comp Neurol 226: 111–140, 1984. [DOI] [PubMed] [Google Scholar]
- 453.Katonis P, Papoutsidakis A, Aligizakis A, Tzanakakis G, Kontakis GM and Papagelopoulos PJ. Mechanoreceptors of the posterior cruciate ligament. J Int Med Res 36: 387–393, 2008. [DOI] [PubMed] [Google Scholar]
- 454.Kiehn O. Locomotor circuits in the mammalian spinal cord. Annu Rev Neurosci 29: 279–306, 2006. [DOI] [PubMed] [Google Scholar]
- 455.Kiehn O. Decoding the organization of spinal circuits that control locomotion. Nat Rev Neurosci 17: 224–238, 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 456.Kiehn O and Butt SJ. Physiological, anatomical and genetic identification of CPG neurons in the developing mammalian spinal cord. Prog Neurobiol 70: 347–361, 2003. [DOI] [PubMed] [Google Scholar]
- 457.Kiehn O, Dougherty KJ, Hagglund M, Borgius L, Talpalar A and Restrepo CE. Probing spinal circuits controlling walking in mammals. Biochem Biophys Res Commun 396: 11–18, 2010. [DOI] [PubMed] [Google Scholar]
- 458.Kiehn O and Kjaerulff O. Spatiotemporal characteristics of 5-HT and dopamine-induced rhythmic hindlimb activity in the in vitro neonatal rat. J Neurophysiol 75: 1472–1482, 1996. [DOI] [PubMed] [Google Scholar]
- 459.Kim JH, Ebner TJ and Bloedel JR. Comparison of response properties of dorsal and ventral spinocerebellar tract neurons to a physiological stimulus. Brain Res 369: 125–135, 1986. [DOI] [PubMed] [Google Scholar]
- 460.Kim JH, Wang JJ and Ebner TJ. Climbing fiber afferent modulation during treadmill locomotion in the cat. J Neurophysiol 57: 787–802, 1987. [DOI] [PubMed] [Google Scholar]
- 461.Kim SS, Gomez-Ramirez M, Thakur PH and Hsiao SS. Multimodal Interactions between Proprioceptive and Cutaneous Signals in Primary Somatosensory Cortex. Neuron 86: 555–566, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 462.Kjaerulff O and Kiehn O. Distribution of networks generating and coordinating locomotor activity in the neonatal rat spinal cord in vitro: a lesion study. J Neurosci 16: 5777–5794, 1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 463.Klein TJ and Lewis MA. A physical model of sensorimotor interactions during locomotion. J Neural Eng 9: 046011, 2012. [DOI] [PubMed] [Google Scholar]
- 464.Klishko AN, Akyildiz A, Mehta-Desai R and Prilutsky BI. Common and distinct muscle synergies during level and slope locomotion in the cat. J Neurophysiol 2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 465.Klishko AN, Farrell BJ, Beloozerova IN, Latash ML and Prilutsky BI. Stabilization of cat paw trajectory during locomotion. J Neurophysiol 112: 1376–1391, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 466.Knox CK, Kubota S and Poppele RE. A determination of excitability changes in dorsal spinocerebellar tract neurons from spike-train analysis. J Neurophysiol 40: 626–646, 1977. [DOI] [PubMed] [Google Scholar]
- 467.Knusel J, Crespi A, Cabelguen JM, Ijspeert AJ and Ryczko D. Reproducing Five Motor Behaviors in a Salamander Robot With Virtual Muscles and a Distributed CPG Controller Regulated by Drive Signals and Proprioceptive Feedback. Front Neurorobot 14: 604426, 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 468.Kriellaars DJ, Brownstone RM, Noga BR and Jordan LM. Mechanical entrainment of fictive locomotion in the decerebrate cat. J Neurophysiol 71: 2074–2086, 1994. [DOI] [PubMed] [Google Scholar]
- 469.Krinke G, Heid J, Bittiger H and Hess R. Sensory denervation of the plantar lumbrical muscle spindles in pyridoxine neuropathy. Acta Neuropathol 43: 213–216, 1978. [DOI] [PubMed] [Google Scholar]
- 470.Krishnan V, Rosenblatt NJ, Latash ML and Grabiner MD. The effects of age on stabilization of the mediolateral trajectory of the swing foot. Gait Posture 38: 923–928, 2013. [DOI] [PubMed] [Google Scholar]
- 471.Krouchev N, Kalaska JF and Drew T. Sequential activation of muscle synergies during locomotion in the intact cat as revealed by cluster analysis and direct decomposition. J Neurophysiol 96: 1991–2010, 2006. [DOI] [PubMed] [Google Scholar]
- 472.Krylova O, Herreros J, Cleverley KE, Ehler E, Henriquez JP, Hughes SM and Salinas PC. WNT-3, expressed by motoneurons, regulates terminal arborization of neurotrophin-3-responsive spinal sensory neurons. Neuron 35: 1043–1056, 2002. [DOI] [PubMed] [Google Scholar]
- 473.LaBella LA, Niechaj A and Rossignol S. Low-threshold, short-latency cutaneous reflexes during fictive locomotion in the “semi-chronic” spinal cat. Exp Brain Res 91: 236–248, 1992. [DOI] [PubMed] [Google Scholar]
- 474.Lacquaniti F, Terzuolo C and Viviani P. The law relating the kinematic and figural aspects of drawing movements. Acta Psychol (Amst) 54: 115–130, 1983. [DOI] [PubMed] [Google Scholar]
- 475.Laflamme OD and Akay T. Excitatory and inhibitory crossed reflex pathways in mice. J Neurophysiol 120: 2897–2907, 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 476.Lajoie Y, Teasdale N, Cole JD, Burnett M, Bard C, Fleury M, Forget R, Paillard J and Lamarre Y. Gait of a deafferented subject without large myelinated sensory fibers below the neck. Neurology 47: 109–115, 1996. [DOI] [PubMed] [Google Scholar]
- 477.Laliberte AM, Goltash S, Lalonde NR and Bui TV. Propriospinal Neurons: Essential Elements of Locomotor Control in the Intact and Possibly the Injured Spinal Cord. Front Cell Neurosci 13: 512, 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 478.Lam T and Pearson KG. Proprioceptive modulation of hip flexor activity during the swing phase of locomotion in decerebrate cats. J Neurophysiol 86: 1321–1332, 2001. [DOI] [PubMed] [Google Scholar]
- 479.Lam T and Pearson KG. Sartorius muscle afferents influence the amplitude and timing of flexor activity in walking decerebrate cats. Exp Brain Res 147: 175–185, 2002. [DOI] [PubMed] [Google Scholar]
- 480.Lamont EV and Zehr EP. Earth-referenced handrail contact facilitates interlimb cutaneous reflexes during locomotion. J Neurophysiol 98: 433–442, 2007. [DOI] [PubMed] [Google Scholar]
- 481.Larson B, Miller S and Oscarsson O. A spinocerebellar climbing fibre path activated by the flexor reflex afferents from all four limbs. J Physiol 203: 641–649, 1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 482.Latash EM, Lecomte CG, Danner SM, Frigon A, Rybak IA and Molkov YI. On the Organization of the Locomotor CPG: Insights From Split-Belt Locomotion and Mathematical Modeling. Front Neurosci 14: 598888, 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 483.Latash ML, Scholz JP and Schoner G. Toward a new theory of motor synergies. Motor Control 11: 276–308, 2007. [DOI] [PubMed] [Google Scholar]
- 484.Lavoie S, McFadyen B and Drew T. A kinematic and kinetic analysis of locomotion during voluntary gait modification in the cat. Exp Brain Res 106: 39–56, 1995. [DOI] [PubMed] [Google Scholar]
- 485.Lavrov I, Courtine G, Dy CJ, van den Brand R, Fong AJ, Gerasimenko Y, Zhong H, Roy RR and Edgerton VR. Facilitation of stepping with epidural stimulation in spinal rats: role of sensory input. J Neurosci 28: 7774–7780, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 486.Lawson SN and Waddell PJ. Soma neurofilament immunoreactivity is related to cell size and fibre conduction velocity in rat primary sensory neurons. J Physiol 435: 41–63, 1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 487.Lay AN, Hass CJ and Gregor RJ. The effects of sloped surfaces on locomotion: a kinematic and kinetic analysis. J Biomech 39: 1621–1628, 2006. [DOI] [PubMed] [Google Scholar]
- 488.Leblond H, L’Esperance M, Orsal D and Rossignol S. Treadmill locomotion in the intact and spinal mouse. J Neurosci 23: 11411–11419, 2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 489.Lechner SG and Lewin GR. Hairy sensation. Physiology (Bethesda) 28: 142–150, 2013. [DOI] [PubMed] [Google Scholar]
- 490.Lemieux M, Josset N, Roussel M, Couraud S and Bretzner F. Speed-Dependent Modulation of the Locomotor Behavior in Adult Mice Reveals Attractor and Transitional Gaits. Front Neurosci 10: 42, 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 491.Lemon RN and Griffiths J. Comparing the function of the corticospinal system in different species: organizational differences for motor specialization? Muscle Nerve 32: 261–279, 2005. [DOI] [PubMed] [Google Scholar]
- 492.Leurs F, Bengoetxea A, Cebolla AM, De SC, Dan B and Cheron G. Planar covariation of elevation angles in prosthetic gait. Gait Posture 35: 647–652, 2012. [DOI] [PubMed] [Google Scholar]
- 493.Li L, Rutlin M, Abraira VE, Cassidy C, Kus L, Gong S, Jankowski MP, Luo W, Heintz N, Koerber HR, Woodbury CJ and Ginty DD. The functional organization of cutaneous low-threshold mechanosensory neurons. Cell 147: 1615–1627, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 494.Li L, Zhang S and Dobson J. The contribution of small and large sensory afferents to postural control in patients with peripheral neuropathy. J Sport Health Sci 8: 218–227, 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 495.Lidierth M and Apps R. Gating in the spino-olivocerebellar pathways to the c1 zone of the cerebellar cortex during locomotion in the cat. J Physiol 430: 453–469, 1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 496.Lin CC and Crago PE. Structural model of the muscle spindle. Ann Biomed Eng 30: 68–83, 2002. [DOI] [PubMed] [Google Scholar]
- 497.Liu Q, Tang Z, Surdenikova L, Kim S, Patel KN, Kim A, Ru F, Guan Y, Weng HJ, Geng Y, Undem BJ, Kollarik M, Chen ZF, Anderson DJ and Dong X. Sensory neuron-specific GPCR Mrgprs are itch receptors mediating chloroquine-induced pruritus. Cell 139: 1353–1365, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 498.Llinas R, Walton K, Hillman DE and Sotelo C. Inferior olive: its role in motor learing. Science 190: 1230–1231, 1975. [DOI] [PubMed] [Google Scholar]
- 499.Lloyd DP. Integrative pattern of excitation and inhibition in two-neuron reflex arcs. J Neurophysiol 9: 439–444, 1946. [DOI] [PubMed] [Google Scholar]
- 500.Loeb GE. Somatosensory unit input to the spinal cord during normal walking. Can J Physiol Pharmacol 59: 627–635, 1981. [DOI] [PubMed] [Google Scholar]
- 501.Loeb GE. The distal hindlimb musculature of the cat: interanimal variability of locomotor activity and cutaneous reflexes. Exp Brain Res 96: 125–140, 1993. [DOI] [PubMed] [Google Scholar]
- 502.Loeb GE, Bak MJ and Duysens J. Long-term unit recording from somatosensory neurons in the spinal ganglia of the freely walking cat. Science 197: 1192–1194, 1977. [DOI] [PubMed] [Google Scholar]
- 503.Loeb GE and Duysens J. Activity patterns in individual hindlimb primary and secondary muscle spindle afferents during normal movements in unrestrained cats. J Neurophysiol 42: 420–440, 1979. [DOI] [PubMed] [Google Scholar]
- 504.Loeb GE and Hoffer JA. Activity of spindle afferents from cat anterior thigh muscles. II. Effects of fusimotor blockade. J Neurophysiol 54: 565–577, 1985. [DOI] [PubMed] [Google Scholar]
- 505.Loeb GE, Hoffer JA and Marks WB. Activity of spindle afferents from cat anterior thigh muscles. III. Effects of external stimuli. J Neurophysiol 54: 578–591, 1985. [DOI] [PubMed] [Google Scholar]
- 506.Loeb GE, Hoffer JA and Pratt CA. Activity of spindle afferents from cat anterior thigh muscles. I. Identification and patterns during normal locomotion. J Neurophysiol 54: 549–564, 1985. [DOI] [PubMed] [Google Scholar]
- 507.Loeb GE, Tsianos GA, Fishel JA, Wettels N and Schaal S. Understanding haptics by evolving mechatronic systems. Prog Brain Res 192: 129–144, 2011. [DOI] [PubMed] [Google Scholar]
- 508.Lomeli J, Quevedo J, Linares P and Rudomin P. Local control of information flow in segmental and ascending collaterals of single afferents. Nature 395: 600–604, 1998. [DOI] [PubMed] [Google Scholar]
- 509.Loram ID, Maganaris CN and Lakie M. Paradoxical muscle movement in human standing. J Physiol 556: 683–689, 2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 510.Loutit AJ, Vickery RM and Potas JR. Functional organization and connectivity of the dorsal column nuclei complex reveals a sensorimotor integration and distribution hub. J Comp Neurol 2020. [DOI] [PubMed] [Google Scholar]
- 511.Lovely RG, Gregor RJ, Roy RR and Edgerton VR. Effects of training on the recovery of full-weight-bearing stepping in the adult spinal cat. Exp Neurol 92: 421–435, 1986. [DOI] [PubMed] [Google Scholar]
- 512.Lovely RG, Gregor RJ, Roy RR and Edgerton VR. Weight-bearing hindlimb stepping in treadmill-exercised adult spinal cats. Brain Res 514: 206–218, 1990. [DOI] [PubMed] [Google Scholar]
- 513.Lund RD and Webster KE. Thalamic afferents from the dorsal column nuclei. An experimental anatomical study in the rat. J Comp Neurol 130: 301–312, 1967. [DOI] [PubMed] [Google Scholar]
- 514.Lund RD and Webster KE. Thalamic afferents from the spinal cord and trigeminal nuclei. An experimental anatomical study in the rat. J Comp Neurol 130: 313–328, 1967. [DOI] [PubMed] [Google Scholar]
- 515.Lundberg A. Function of the ventral spinocerebellar tract. A new hypothesis. Exp Brain Res 12: 317–330, 1971. [DOI] [PubMed] [Google Scholar]
- 516.Lundberg A. Multisensory control of spinal reflex pathways. Prog Brain Res 50: 11–28, 1979. [DOI] [PubMed] [Google Scholar]
- 517.Lundberg A, Malmgren K and Schomburg ED. Role of joint afferents in motor control exemplified by effects on reflex pathways from Ib afferents. J Physiol 284: 327–343, 1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 518.Lundberg A, Malmgren K and Schomburg ED. Reflex pathways from group II muscle afferents. 2. Functional characteristics of reflex pathways to alpha-motoneurones. Exp Brain Res 65: 282–293, 1987. [DOI] [PubMed] [Google Scholar]
- 519.Lyle MA, Prilutsky BI, Gregor RJ, Abelew TA and Nichols TR. Self-reinnervated muscles lose autogenic length feedback, but intermuscular feedback can recover functional connectivity. J Neurophysiol 116: 1055–1067, 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 520.Lynn B. The form and distribution of the receptive fields of Pacinian corpuscles found in and around the cat’s large foot pad. J Physiol 217: 755–771, 1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 521.Ma Q, Fode C, Guillemot F and Anderson DJ. Neurogenin1 and neurogenin2 control two distinct waves of neurogenesis in developing dorsal root ganglia. Genes Dev 13: 1717–1728, 1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 522.Maas H, Gregor RJ, Hodson-Tole EF, Farrell BJ and Prilutsky BI. Distinct muscle fascicle length changes in feline medial gastrocnemius and soleus muscles during slope walking. J Appl Physiol 106: 1169–1180, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 523.Maas H and Sandercock TG. Are skeletal muscles independent actuators? Force transmission from soleus muscle in the cat. J Appl Physiol (1985) 104: 1557–1567, 2008. [DOI] [PubMed] [Google Scholar]
- 524.MacLeod TD, Hreljac A and Imamura R. Changes in the preferred transition speed with added mass to the foot. J Appl Biomech 30: 95–103, 2014. [DOI] [PubMed] [Google Scholar]
- 525.Macpherson JM. Strategies that simplify the control of quadrupedal stance. I. Forces at the ground. J Neurophysiol 60: 204–217, 1988. [DOI] [PubMed] [Google Scholar]
- 526.Macpherson JM. Strategies that simplify the control of quadrupedal stance. II. Electromyographic activity. J Neurophysiol 60: 218–231, 1988. [DOI] [PubMed] [Google Scholar]
- 527.Macpherson JM and Fung J. Weight support and balance during perturbed stance in the chronic spinal cat. J Neurophysiol 82: 3066–3081, 1999. [DOI] [PubMed] [Google Scholar]
- 528.Macpherson JM, Horak FB, Dunbar DC and Dow RS. Stance dependence of automatic postural adjustments in humans. Exp Brain Res 78: 557–566, 1989. [DOI] [PubMed] [Google Scholar]
- 529.Madey SM, Cole KJ and Brand RA. Sensory innervation of the cat knee articular capsule and cruciate ligament visualised using anterogradely transported wheat germ agglutinin-horseradish peroxidase. J Anat 190 (Pt 2): 289–297, 1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 530.Maltenfort MG and Burke RE. Spindle model responsive to mixed fusimotor inputs and testable predictions of beta feedback effects. J Neurophysiol 89: 2797–2809, 2003. [DOI] [PubMed] [Google Scholar]
- 531.Mann MD. Clarke’s column and the dorsal spinocerebellar tract: a review. Brain Behav Evol 7: 34–83, 1973. [DOI] [PubMed] [Google Scholar]
- 532.Mantle-St John LA and Tracey DJ. Somatosensory nuclei in the brainstem of the rat: independent projections to the thalamus and cerebellum. J Comp Neurol 255: 259–271, 1987. [DOI] [PubMed] [Google Scholar]
- 533.Manuel M, Chardon M, Tysseling V and Heckman CJ. Scaling of Motor Output, From Mouse to Humans. Physiology (Bethesda) 34: 5–13, 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 534.Manuel M and Zytnicki D. Alpha, beta and gamma motoneurons: functional diversity in the motor system’s final pathway. J Integr Neurosci 10: 243–276, 2011. [DOI] [PubMed] [Google Scholar]
- 535.Marey É-J. Le mouvement. Paris: G. Masson, 1894. [Google Scholar]
- 536.Margaria R. Positive and negative work performances and their efficiencies in human locomotion. Int Z Angew Physiol 25: 339–351, 1968. [DOI] [PubMed] [Google Scholar]
- 537.Markin SN, Klishko AN, Shevtsova NA, Lemay MA, Prilutsky BI and Rybak IA. A neuromechanical model of spinal control of locomotion. In: Neuromechanical Modeling of Posture and Locomotion, edited by Prilutsky BI and Edwards DH. New York: Springer, 2016, p. 21–68. [Google Scholar]
- 538.Markin SN, Lemay MA, Prilutsky BI and Rybak IA. Motoneuronal and muscle synergies involved in cat hindlimb control during fictive and real locomotion: a comparison study. J Neurophysiol 107: 2057–2071, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 539.Maro GS, Vermeren M, Voiculescu O, Melton L, Cohen J, Charnay P and Topilko P. Neural crest boundary cap cells constitute a source of neuronal and glial cells of the PNS. Nat Neurosci 7: 930–938, 2004. [DOI] [PubMed] [Google Scholar]
- 540.Marple-Horvat DE and Armstrong DM. Central regulation of motor cortex neuronal responses to forelimb nerve inputs during precision walking in the cat. J Physiol 519 Pt 1: 279–299, 1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 541.Mastinu E, Engels LF, Clemente F, Dione M, Sassu P, Aszmann O, Branemark R, Hakansson B, Controzzi M, Wessberg J, Cipriani C and Ortiz-Catalan M. Neural feedback strategies to improve grasping coordination in neuromusculoskeletal prostheses. Sci Rep 10: 11793, 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 542.Matsushita M and Xiong G. Projections from the cervical enlargement to the cerebellar nuclei in the rat, studied by anterograde axonal tracing. J Comp Neurol 377: 251–261, 1997. [DOI] [PubMed] [Google Scholar]
- 543.Matsuyama K and Drew T. Vestibulospinal and reticulospinal neuronal activity during locomotion in the intact cat. I. Walking on a level surface. J Neurophysiol 84: 2237–2256, 2000. [DOI] [PubMed] [Google Scholar]
- 544.Matsuyama K and Drew T. Vestibulospinal and reticulospinal neuronal activity during locomotion in the intact cat. II. Walking on an inclined plane. J Neurophysiol 84: 2257–2276, 2000. [DOI] [PubMed] [Google Scholar]
- 545.Matthews PB. The differentiation of two types of fusimotor fibre by their effects on the dynamic response of muscle spindle primary endings. Q J Exp Physiol Cogn Med Sci 47: 324–333, 1962. [DOI] [PubMed] [Google Scholar]
- 546.Matthews PB. Muscle spindles and their motor control. Physiol Rev 44: 219–288, 1964. [DOI] [PubMed] [Google Scholar]
- 547.Matthews PB. Mammalian muscle receptors and their central actions. London: Edward Arnold, 1972. [Google Scholar]
- 548.Matthews PB. Evolving views on the internal operation and functional role of the muscle spindle. J Physiol 320: 1–30, 1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 549.Matthews PB. Where Anatomy led, Physiology followed: a survey of our developing understanding of the muscle spindle, what it does and how it works. J Anat 227: 104–114, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 550.Maunz RA, Pitts NG and Peterson BW. Cat spinoreticular neurons: locations, responses and changes in responses during repetitive stimulation. Brain Res 148: 365–379, 1978. [DOI] [PubMed] [Google Scholar]
- 551.Maxwell DJ and Soteropoulos DS. The mammalian spinal commissural system: properties and functions. J Neurophysiol 123: 4–21, 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 552.Mayer WP and Akay T. Stumbling corrective reaction elicited by mechanical and electrical stimulation of the saphenous nerve in walking mice. J Exp Biol 221: 2018. [DOI] [PubMed] [Google Scholar]
- 553.Mayer WP, Murray AJ, Brenner-Morton S, Jessell TM, Tourtellotte WG and Akay T. Role of muscle spindle feedback in regulating muscle activity strength during walking at different speed in mice. J Neurophysiol 120: 2484–2497, 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 554.McAllister JP and Wells J. The structural organization of the ventral posterolateral nucleus in the rat. J Comp Neurol 197: 271–301, 1981. [DOI] [PubMed] [Google Scholar]
- 555.McAndrew Young PM, Wilken JM and Dingwell JB. Dynamic margins of stability during human walking in destabilizing environments. J Biomech 45: 1053–1059, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 556.McCall AA, Miller DM, DeMayo WM, Bourdages GH and Yates BJ. Vestibular nucleus neurons respond to hindlimb movement in the conscious cat. J Neurophysiol 116: 1785–1794, 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 557.McCrea DA. Spinal circuitry of sensorimotor control of locomotion. J Physiol 533: 41–50, 2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 558.McCrea DA and Perreault MC. PAD and modulation of group II-evoked flexion reflexes during MLR-evoked fictive locomotion. In: Presynaptic inhibition and neural control, edited by Rudomin P, Romo R and Mendell L. Oxford University Press, 1997, p. 366–384. [Google Scholar]
- 559.McCrea DA and Rybak IA. Organization of mammalian locomotor rhythm and pattern generation. Brain Res Rev 57: 134–146, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 560.McCrea DA, Shefchyk SJ, Stephens MJ and Pearson KG. Disynaptic group I excitation of synergist ankle extensor motoneurones during fictive locomotion in the cat. J Physiol 487 (Pt 2): 527–539, 1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 561.McFadyen BJ, Lavoie S and Drew T. Kinetic and energetic patterns for hindlimb obstacle avoidance during cat locomotion. Exp Brain Res 125: 502–510, 1999. [DOI] [PubMed] [Google Scholar]
- 562.McGuigan MP, Yoo E, Lee DV and Biewener AA. Dynamics of goat distal hind limb muscle-tendon function in response to locomotor grade. J Exp Biol 212: 2092–2104, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 563.McIntyre AK, Proske U and Tracey DJ. Afferent fibres from muscle receptors in the posterior nerve of the cat’s knee joint. Exp Brain Res 33: 415–424, 1978. [DOI] [PubMed] [Google Scholar]
- 564.McVea DA, Donelan JM, Tachibana A and Pearson KG. A role for hip position in initiating the swing-to-stance transition in walking cats. J Neurophysiol 94: 3497–3508, 2005. [DOI] [PubMed] [Google Scholar]
- 565.Mears SC and Frank E. Formation of specific monosynaptic connections between muscle spindle afferents and motoneurons in the mouse. J Neurosci 17: 3128–3135, 1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 566.Mehta R and Prilutsky BI. Task-dependent inhibition of slow-twitch soleus and excitation of fast-twitch gastrocnemius do not require high movement speed and velocity-dependent sensory feedback. Front Physiol 5: 410, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 567.Menard A, Leblond H and Gossard JP. The modulation of presynaptic inhibition in single muscle primary afferents during fictive locomotion in the cat. J Neurosci 19: 391–400, 1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 568.Menard A, Leblond H and Gossard JP. Sensory integration in presynaptic inhibitory pathways during fictive locomotion in the cat. J Neurophysiol 88: 163–171, 2002. [DOI] [PubMed] [Google Scholar]
- 569.Mendell LM, Taylor JS, Johnson RD and Munson JB. Rescue of motoneuron and muscle afferent function in cats by regeneration into skin. II. Ia-motoneuron synapse. J Neurophysiol 73: 662–673, 1995. [DOI] [PubMed] [Google Scholar]
- 570.Mendelsohn AI, Simon CM, Abbott LF, Mentis GZ and Jessell TM. Activity Regulates the Incidence of Heteronymous Sensory-Motor Connections. Neuron 87: 111–123, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 571.Merlet AN, Harnie J, Macovei M, Doelman A, Gaudreault N and Frigon A. Mechanically stimulating the lumbar region inhibits locomotor-like activity and increases the gain of cutaneous reflexes from the paws in spinal cats. J Neurophysiol 123: 1026–1041, 2020. [DOI] [PubMed] [Google Scholar]
- 572.Merlet AN, Harnie J, Macovei M, Doelman A, Gaudreault N and Frigon A. Cutaneous inputs from perineal region facilitate spinal locomotor activity and modulate cutaneous reflexes from the foot in spinal cats. J Neurosci Res 2021. [DOI] [PubMed] [Google Scholar]
- 573.Mileusnic MP, Brown IE, Lan N and Loeb GE. Mathematical models of proprioceptors. I. Control and transduction in the muscle spindle. J Neurophysiol 96: 1772–1788, 2006. [DOI] [PubMed] [Google Scholar]
- 574.Mileusnic MP and Loeb GE. Mathematical models of proprioceptors. II. Structure and function of the Golgi tendon organ. J Neurophysiol 96: 1789–1802, 2006. [DOI] [PubMed] [Google Scholar]
- 575.Miller CT, Hale ME, Okano H, Okabe S and Mitra P. Comparative Principles for Next-Generation Neuroscience. Front Behav Neurosci 13: 12, 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 576.Miller DM, DeMayo WM, Bourdages GH, Wittman SR, Yates BJ and McCall AA. Neurons in the pontomedullary reticular formation receive converging inputs from the hindlimb and labyrinth. Exp Brain Res 235: 1195–1207, 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 577.Miller S, Reitsma DJ and Van der Meche FG. Functional organization of long ascending propriospinal pathways linking lumbo-sacral and cervical segments in the cat. Brain Res 62: 169–188, 1973. [DOI] [PubMed] [Google Scholar]
- 578.Miller S, Ruit JB and Van der Meche FG. Reversal of sign of long spinal reflexes dependent on the phase of the step cycle in the high decerebrate cat. Brain Res 128: 447–459, 1977. [DOI] [PubMed] [Google Scholar]
- 579.Minetti AE, Ardigo LP and Saibene F. The transition between walking and running in humans: metabolic and mechanical aspects at different gradients. Acta Physiol Scand 150: 315–323, 1994. [DOI] [PubMed] [Google Scholar]
- 580.Misiaszek JE. The H-reflex as a tool in neurophysiology: its limitations and uses in understanding nervous system function. Muscle Nerve 28: 144–160, 2003. [DOI] [PubMed] [Google Scholar]
- 581.Misiaszek JE. Control of frontal plane motion of the hindlimbs in the unrestrained walking cat. J Neurophysiol 96: 1816–1828, 2006. [DOI] [PubMed] [Google Scholar]
- 582.Misiaszek JE, De Serres SJ, Stein RB, Jiang W and Pearson KG. Stretch and H reflexes in triceps surae are similar during tonic and rhythmic contractions in high decerebrate cats. J Neurophysiol 83: 1941–1950, 2000. [DOI] [PubMed] [Google Scholar]
- 583.Mitra P and Brownstone RM. An in vitro spinal cord slice preparation for recording from lumbar motoneurons of the adult mouse. J Neurophysiol 107: 728–741, 2012. [DOI] [PubMed] [Google Scholar]
- 584.Monzee J, Lamarre Y and Smith AM. The effects of digital anesthesia on force control using a precision grip. J Neurophysiol 89: 672–683, 2003. [DOI] [PubMed] [Google Scholar]
- 585.Moraud EM, Capogrosso M, Formento E, Wenger N, DiGiovanna J, Courtine G and Micera S. Mechanisms Underlying the Neuromodulation of Spinal Circuits for Correcting Gait and Balance Deficits after Spinal Cord Injury. Neuron 89: 814–828, 2016. [DOI] [PubMed] [Google Scholar]
- 586.Moraud EM, von ZJ, Miehlbradt J, Wurth S, Formento E, DiGiovanna J, Capogrosso M, Courtine G and Micera S. Closed-loop control of trunk posture improves locomotion through the regulation of leg proprioceptive feedback after spinal cord injury. Sci Rep 8: 76, 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 587.Mori S, Matsui T, Kuze B, Asanome M, Nakajima K and Matsuyama K. Cerebellar-induced locomotion: reticulospinal control of spinal rhythm generating mechanism in cats. Ann N Y Acad Sci 860: 94–105, 1998. [DOI] [PubMed] [Google Scholar]
- 588.Mori S, Matsui T, Kuze B, Asanome M, Nakajima K and Matsuyama K. Stimulation of a restricted region in the midline cerebellar white matter evokes coordinated quadrupedal locomotion in the decerebrate cat. J Neurophysiol 82: 290–300, 1999. [DOI] [PubMed] [Google Scholar]
- 589.Mori S, Reynolds PJ and Brookhart JM. Contribution of pedal afferents to postural control in the dog. Am J Physiol 218: 726–734, 1970. [DOI] [PubMed] [Google Scholar]
- 590.Morrison KM, Miesegaes GR, Lumpkin EA and Maricich SM. Mammalian Merkel cells are descended from the epidermal lineage. Dev Biol 336: 76–83, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 591.Muir GD and Whishaw IQ. Ground reaction forces in locomoting hemi-parkinsonian rats: a definitive test for impairments and compensations. Exp Brain Res 126: 307–314, 1999. [DOI] [PubMed] [Google Scholar]
- 592.Muller H and Sternad D. A randomization method for the calculation of covariation in multiple nonlinear relations: illustrated with the example of goal-directed movements. Biol Cybern 89: 22–33, 2003. [DOI] [PubMed] [Google Scholar]
- 593.Muniak MA, Ray S, Hsiao SS, Dammann JF and Bensmaia SJ. The neural coding of stimulus intensity: linking the population response of mechanoreceptive afferents with psychophysical behavior. J Neurosci 27: 11687–11699, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 594.Murphy PR. Tonic and phasic discharge patterns in toe flexor gamma-motoneurons during locomotion in the decerebrate cat. J Neurophysiol 87: 286–294, 2002. [DOI] [PubMed] [Google Scholar]
- 595.Murphy PR and Hammond GR. Gamma-motoneurone discharge patterns during fictive locomotion in the decerebrate cat. Exp Physiol 75: 107–110, 1990. [DOI] [PubMed] [Google Scholar]
- 596.Murphy PR and Hammond GR. The role of cutaneous afferents in the control of gamma-motoneurones during locomotion in the decerebrate cat. J Physiol 434: 529–547, 1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 597.Murphy PR and Martin HA. Fusimotor neurone responses to medial plantar nerve stimulation in the decerebrate cat. J Physiol 482 (Pt 1): 167–177, 1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 598.Musienko PE, Bogacheva IN and Gerasimenko YP. Significance of peripheral feedback in the generation of stepping movements during epidural stimulation of the spinal cord. Neurosci Behav Physiol 37: 181–190, 2007. [DOI] [PubMed] [Google Scholar]
- 599.Musienko PE, Lyalka VF, Gorskii OV, Merkulyeva N, Gerasimenko YP, Deliagina TG and Zelenin PV. Comparison of operation of spinal locomotor networks activated by supraspinal commands and by epidural stimulation of the spinal cord in cats. J Physiol 598: 3459–3483, 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 600.Nardone A, Corna S, Turcato AM and Schieppati M. Afferent control of walking: are there distinct deficits associated to loss of fibres of different diameter? Clin Neurophysiol 125: 327–335, 2014. [DOI] [PubMed] [Google Scholar]
- 601.Nashner LM. Adapting reflexes controlling the human posture. Exp Brain Res 26: 59–72, 1976. [DOI] [PubMed] [Google Scholar]
- 602.Nichols TR. A biomechanical perspective on spinal mechanisms of coordinated muscular action: an architecture principle. Acta Anat (Basel) 151: 1–13, 1994. [DOI] [PubMed] [Google Scholar]
- 603.Nichols TR. Receptor mechanisms underlying heterogenic reflexes among the triceps surae muscles of the cat. J Neurophysiol 81: 467–478, 1999. [DOI] [PubMed] [Google Scholar]
- 604.Nichols TR. Distributed force feedback in the spinal cord and the regulation of limb mechanics. J Neurophysiol 119: 1186–1200, 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 605.Nichols TR and Cope TC. Cross-bridge mechanisms underlying the history-dependent properties of muscle spindles and stretch reflexes. Can J Physiol Pharmacol 82: 569–576, 2004. [DOI] [PubMed] [Google Scholar]
- 606.Nichols TR, Cope TC and Abelew TA. Rapid spinal mechanisms of motor coordination. Exerc Sport Sci Rev 27: 255–284, 1999. [PubMed] [Google Scholar]
- 607.Nielsen J, Crone C and Hultborn H. H-reflexes are smaller in dancers from The Royal Danish Ballet than in well-trained athletes. Eur J Appl Physiol Occup Physiol 66: 116–121, 1993. [DOI] [PubMed] [Google Scholar]
- 608.Nielsen JB and Sinkjaer T. Afferent feedback in the control of human gait. J Electromyogr Kinesiol 12: 213–217, 2002. [DOI] [PubMed] [Google Scholar]
- 609.Nilsson J, Thorstensson A and Halbertsma J. Changes in leg movements and muscle activity with speed of locomotion and mode of progression in humans. Acta Physiol Scand 123: 457–475, 1985. [DOI] [PubMed] [Google Scholar]
- 610.O’Donovan MJ, Bonnot A, Wenner P and Mentis GZ. Calcium imaging of network function in the developing spinal cord. Cell Calcium 37: 443–450, 2005. [DOI] [PubMed] [Google Scholar]
- 611.Ogihara N, Kikuchi T, Ishiguro Y, Makishima H and Nakatsukasa M. Planar covariation of limb elevation angles during bipedal walking in the Japanese macaque. J R Soc Interface 9: 2181–2190, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 612.Ogihara N, Oku T, Andrada E, Blickhan R, Nyakatura JA and Fischer MS. Planar covariation of limb elevation angles during bipedal locomotion in common quails (Coturnix coturnix). J Exp Biol 217: 3968–3973, 2014. [DOI] [PubMed] [Google Scholar]
- 613.Ogihara N and Yamazaki N. Generation of human bipedal locomotion by a bio-mimetic neuro-musculo-skeletal model. Biol Cybern 84: 1–11, 2001. [DOI] [PubMed] [Google Scholar]
- 614.Olausson H, Lamarre Y, Backlund H, Morin C, Wallin BG, Starck G, Ekholm S, Strigo I, Worsley K, Vallbo AB and Bushnell MC. Unmyelinated tactile afferents signal touch and project to insular cortex. Nat Neurosci 5: 900–904, 2002. [DOI] [PubMed] [Google Scholar]
- 615.Oliver KM, Florez-Paz DM, Badea TC, Mentis GZ, Menon V and de Nooij JC. Molecular correlates of muscle spindle and Golgi tendon organ afferents. Nat Commun 12: 1451, 2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 616.Ollivier-Lanvin K, Krupka AJ, Auyong N, Miller K, Prilutsky BI and Lemay MA. Electrical stimulation of the sural cutaneous afferent nerve controls the amplitude and onset of the swing phase of locomotion in the spinal cat. J Neurophysiol 105: 2297–2308, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 617.Olree KS and Vaughan CL. Fundamental patterns of bilateral muscle activity in human locomotion. Biol Cybern 73: 409–414, 1995. [DOI] [PubMed] [Google Scholar]
- 618.Orlovskii GN. [Relations between reticulo-spinal neurons and locomotor regions of the brain stem]. Biofizika 15: 171–178, 1970. [PubMed] [Google Scholar]
- 619.Orlovskii GN. [Work of reticulo-spinal neurons during locomotion]. Biofizika 15: 728–737, 1970. [PubMed] [Google Scholar]
- 620.Orlovskii GN. [Activity of Purkinje cells during locomotion]. Biofizika 17: 891–896, 1972. [PubMed] [Google Scholar]
- 621.Orlovskii GN and Fel’dman AG. [Classification of the neurons of the lumbo-sacral region of the spinal cord in accordance with their discharge patterns during provoked locomotion]. Neirofiziologiia 4: 410–417, 1972. [PubMed] [Google Scholar]
- 622.Orlovsky GN. Activity of rubrospinal neurons during locomotion. Brain Res 46: 99–112, 1972. [DOI] [PubMed] [Google Scholar]
- 623.Orlovsky GN. Activity of vestibulospinal neurons during locomotion. Brain Res 46: 85–98, 1972. [DOI] [PubMed] [Google Scholar]
- 624.Orlovsky GN. The effect of different descending systems on flexor and extensor activity during locomotion. Brain Res 40: 359–371, 1972. [DOI] [PubMed] [Google Scholar]
- 625.Orlovsky GN, Deliagina TG and Grillner S. Neural control of locomotion: from mollusc to man. New York: Oxford University Press, 1999. [Google Scholar]
- 626.Osborn CE and Poppele RE. The extent of polysynaptic responses in the dorsal spinocerebellar tract to stimulation of group I afferent fibers in gastrocnemius-soleus. J Neurosci 8: 316–319, 1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 627.Oscarsson O. THREE ASCENDING TRACTS ACTIVATED FROM GROUP I AFFERENTS IN FORELIMB NERVES OF THE CAT. Prog Brain Res 12: 179–196, 1964. [DOI] [PubMed] [Google Scholar]
- 628.Oscarsson O. Functional organization of the spino- and cuneocerebellar tracts. Physiol Rev 45: 495–522, 1965. [DOI] [PubMed] [Google Scholar]
- 629.Oscarsson O. Termination and functional organization of the dorsal spino-olivocerebellar path. J Physiol 200: 129–149, 1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 630.Oscarsson O and Uddenberg N. Identification of a spinocerebellar tract activated from forelimb afferents in the cats. Acta Physiol Scand 62: 125–136, 1964. [DOI] [PubMed] [Google Scholar]
- 631.Ota M, Tateuchi H, Hashiguchi T and Ichihashi N. Verification of validity of gait analysis systems during treadmill walking and running using human pose tracking algorithm. Gait Posture 85: 290–297, 2021. [DOI] [PubMed] [Google Scholar]
- 632.Paixao S, Loschek L, Gaitanos L, Alcala MP, Goulding M and Klein R. Identification of Spinal Neurons Contributing to the Dorsal Column Projection Mediating Fine Touch and Corrective Motor Movements. Neuron 104: 749–764, 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 633.Pandy MG. Moment arm of a muscle force. Exerc Sport Sci Rev 27: 79–118, 1999. [PubMed] [Google Scholar]
- 634.Pandy MG, Kumar V, Berme N and Waldron KJ. The dynamics of quadrupedal locomotion. J Biomech Eng 110: 230–237, 1988. [DOI] [PubMed] [Google Scholar]
- 635.Pantall A, Hodson-Tole EF, Gregor RJ and Prilutsky BI. Increased intensity and reduced frequency of EMG signals from feline self-reinnervated ankle extensors during walking do not normalize excessive lengthening. J Neurophysiol 115: 2406–2420, 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 636.Park H, Latash EM, Molkov YI, Klishko AN, Frigon A, DeWeerth SP and Prilutsky BI. Cutaneous sensory feedback from paw pads affects lateral balance control during split-belt locomotion in the cat. J Exp Biol 222: 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 637.Patterson MM, Cegavske CF and Thompson RF. Effects of a classical conditioning paradigm on hind-limb flexor nerve response in immobilized spinal cats. J Comp Physiol Psychol 84: 88–97, 1973. [DOI] [PubMed] [Google Scholar]
- 638.Pearcey GEP and Zehr EP. We Are Upright-Walking Cats: Human Limbs as Sensory Antennae During Locomotion. Physiology (Bethesda) 34: 354–364, 2019. [DOI] [PubMed] [Google Scholar]
- 639.Pearcey GEP and Zehr EP. What lies beneath the brain: Neural circuits involved in human locomotion. In: The neural control of movement, edited by Whelan PJ and Sharples SA. London: Academic Press, 2020, p. 385–418. [Google Scholar]
- 640.Pearson K, Ekeberg O and Buschges A. Assessing sensory function in locomotor systems using neuro-mechanical simulations. Trends Neurosci 29: 625–631, 2006. [DOI] [PubMed] [Google Scholar]
- 641.Pearson KG. Generating the walking gait: role of sensory feedback. Prog Brain Res 143: 123–129, 2004. [DOI] [PubMed] [Google Scholar]
- 642.Pearson KG. Role of sensory feedback in the control of stance duration in walking cats. Brain Res Rev 57: 222–227, 2008. [DOI] [PubMed] [Google Scholar]
- 643.Pearson KG, Acharya H and Fouad K. A new electrode configuration for recording electromyographic activity in behaving mice. J Neurosci Methods 148: 36–42, 2005. [DOI] [PubMed] [Google Scholar]
- 644.Pearson KG and Collins DF. Reversal of the influence of group Ib afferents from plantaris on activity in medial gastrocnemius muscle during locomotor activity. J Neurophysiol 70: 1009–1017, 1993. [DOI] [PubMed] [Google Scholar]
- 645.Pearson KG, Fouad K and Misiaszek JE. Adaptive changes in motor activity associated with functional recovery following muscle denervation in walking cats. J Neurophysiol 82: 370–381, 1999. [DOI] [PubMed] [Google Scholar]
- 646.Pearson KG, Misiaszek JE and Fouad K. Enhancement and resetting of locomotor activity by muscle afferents. Ann N Y Acad Sci 860: 203–215, 1998. [DOI] [PubMed] [Google Scholar]
- 647.Pearson KG, Misiaszek JE and Hulliger M. Chemical ablation of sensory afferents in the walking system of the cat abolishes the capacity for functional recovery after peripheral nerve lesions. Exp Brain Res 150: 50–60, 2003. [DOI] [PubMed] [Google Scholar]
- 648.Pearson KG and Rossignol S. Fictive motor patterns in chronic spinal cats. J Neurophysiol 66: 1874–1887, 1991. [DOI] [PubMed] [Google Scholar]
- 649.Pedotti A. A study of motor coordination and neuromuscular activities in human locomotion. Biol Cybern 26: 53–62, 1977. [DOI] [PubMed] [Google Scholar]
- 650.Perciavalle V, Bosco G and Poppele R. Correlated activity in the spinocerebellum is related to spinal timing generators. Brain Res 695: 293–297, 1995. [DOI] [PubMed] [Google Scholar]
- 651.Perell KL, Gregor RJ, Buford JA and Smith JL. Adaptive control for backward quadrupedal walking. IV. Hindlimb kinetics during stance and swing. J Neurophysiol 70: 2226–2240, 1993. [DOI] [PubMed] [Google Scholar]
- 652.Perez MA, Lundbye-Jensen J and Nielsen JB. Task-specific depression of the soleus H-reflex after cocontraction training of antagonistic ankle muscles. J Neurophysiol 98: 3677–3687, 2007. [DOI] [PubMed] [Google Scholar]
- 653.Perreault MC, Angel MJ, Guertin P and McCrea DA. Effects of stimulation of hindlimb flexor group II afferents during fictive locomotion in the cat. J Physiol 487 (Pt 1): 211–220, 1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 654.Perreault MC, Drew T and Rossignol S. Activity of medullary reticulospinal neurons during fictive locomotion. J Neurophysiol 69: 2232–2247, 1993. [DOI] [PubMed] [Google Scholar]
- 655.Perreault MC, Enriquez-Denton M and Hultborn H. Proprioceptive control of extensor activity during fictive scratching and weight support compared to fictive locomotion. J Neurosci 19: 10966–10976, 1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 656.Perreault MC, Rossignol S and Drew T. Microstimulation of the medullary reticular formation during fictive locomotion. J Neurophysiol 71: 229–245, 1994. [DOI] [PubMed] [Google Scholar]
- 657.Perreault MC, Shefchyk SJ, Jimenez I and McCrea DA. Depression of muscle and cutaneous afferent-evoked monosynaptic field potentials during fictive locomotion in the cat. J Physiol 521 Pt 3: 691–703, 1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 658.Perret C and Berthoz A. Evidence of static and dynamic fusimotor actions on the spindle response to sinusoidal stretch during locomotor activity in the cat. Exp Brain Res 18: 178–188, 1973. [DOI] [PubMed] [Google Scholar]
- 659.Perret C and Buser P. Static and dynamic fusimotor activity during locomotor movements in the cat. Brain Res 40: 165–169, 1972. [DOI] [PubMed] [Google Scholar]
- 660.Perret C and Cabelguen JM. Main characteristics of the hindlimb locomotor cycle in the decorticate cat with special reference to bifunctional muscles. Brain Res 187: 333–352, 1980. [DOI] [PubMed] [Google Scholar]
- 661.Perret C, Cabelguen JM and Orsal D. Analysis of the pattern of activity in “knee flexor” motoneurons during locomotion in the cat. In: Stance and Motion: Facts and Concepts, edited by Gurfinkel VS, Ioffe ME, Massion J and Roll JP. New York: Plenum Press, 1988, p. 133–141. [Google Scholar]
- 662.Perret C, Millanvoye M and Cabelguen JM. [Ascending spinal messages during fictitious locomotion in curarized cats]. J Physiol (Paris) 65: Suppl, 1972. [PubMed] [Google Scholar]
- 663.Peterson BW, Franck JI and Daunton NG. Changes in responses of medial pontomedullary reticular neurons during repetitive cutaneous, vestibular, cortical, and tectal stimulation. J Neurophysiol 39: 564–581, 1976. [DOI] [PubMed] [Google Scholar]
- 664.Petit D and Burgess PR. Dorsal column projection of receptors in cat hairy skin supplied by myelinated fibers. J Neurophysiol 31: 849–855, 1968. [DOI] [PubMed] [Google Scholar]
- 665.Petras JM. Spinocerebellar neurons in the rhesus monkey. Brain Res 130: 146–151, 1977. [DOI] [PubMed] [Google Scholar]
- 666.Petras JM and Cummings JF. The origin of spinocerebellar pathways. II. The nucleus centrobasalis of the cervical enlargement and the nucleus dorsalis of the thoracolumbar spinal cord. J Comp Neurol 173: 693–716, 1977. [DOI] [PubMed] [Google Scholar]
- 667.Pfau T, Hinton E, Whitehead C, Wiktorowicz-Conroy A and Hutchinson JR. Temporal gait parameters in the alpaca and the evolution of pacing and trotting locomotion in the Camelidae. Journal of Zoology 283: 193–202, 2011. [Google Scholar]
- 668.Pham QC, Hicheur H, Arechavaleta G, Laumond JP and Berthoz A. The formation of trajectories during goal-oriented locomotion in humans. II. A maximum smoothness model. Eur J Neurosci 26: 2391–2403, 2007. [DOI] [PubMed] [Google Scholar]
- 669.Philippson M. L’autonomie et la centralisation dans le système nerveux des animaux. Trav Lab Physiol Inst Solvay (Bruxelles) 7: 1–208, 1905. [Google Scholar]
- 670.Pierotti DJ, Roy RR, Gregor RJ and Edgerton VR. Electromyographic activity of cat hindlimb flexors and extensors during locomotion at varying speeds and inclines. Brain Res 481: 57–66, 1989. [DOI] [PubMed] [Google Scholar]
- 671.Pietravalle P, Morano S, Cristina G, Mancuso M, Valle E, Annulli MA, Tomaselli M, Pozzessere G and Di MU. Early complications in type 1 diabetes: central nervous system alterations preceded kidney abnormalities. Diabetes Res Clin Pract 21: 143–154, 1993. [DOI] [PubMed] [Google Scholar]
- 672.Pijpers A, Winkelman BH, Bronsing R and Ruigrok TJ. Selective impairment of the cerebellar C1 module involved in rat hind limb control reduces step-dependent modulation of cutaneous reflexes. J Neurosci 28: 2179–2189, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 673.Piliavskii AI, Iakhnitsa IA and Bulgakova NV. [Antidromic impulsation of the dorsal roots at the time of real locomotion in rats]. Neirofiziologiia 20: 579–585, 1988. [PubMed] [Google Scholar]
- 674.Pillai S, Wang Y, Wolpaw JR and Chen XY. Effects of H-reflex up-conditioning on GABAergic terminals on rat soleus motoneurons. Eur J Neurosci 28: 668–674, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 675.Pires NJ, Lay BS and Rubenson J. Joint-level mechanics of the walk-to-run transition in humans. J Exp Biol 217: 3519–3527, 2014. [DOI] [PubMed] [Google Scholar]
- 676.Pompeiano O and Brodal A. Spinovestibular fibers in the cat; an experimental study. J Comp Neurol 108: 353–381, 1957. [DOI] [PubMed] [Google Scholar]
- 677.Pompeiano O and Swett JE. Actions of graded cutaneous and muscular afferent volleys on brain stem units in the decrebrate, cerebellectomized cat. Arch Ital Biol 101: 552–583, 1963. [PubMed] [Google Scholar]
- 678.Pratt CA, Chanaud CM and Loeb GE. Functionally complex muscles of the cat hindlimb. IV. Intramuscular distribution of movement command signals and cutaneous reflexes in broad, bifunctional thigh muscles. Exp Brain Res 85: 281–299, 1991. [DOI] [PubMed] [Google Scholar]
- 679.Pratt CA and Loeb GE. Functionally complex muscles of the cat hindlimb. I. Patterns of activation across sartorius. Exp Brain Res 85: 243–256, 1991. [DOI] [PubMed] [Google Scholar]
- 680.Prilutsky BI. Coordination of two- and one-joint muscles: functional consequences and implications for motor control. Motor Control 4: 1–44, 2000. [DOI] [PubMed] [Google Scholar]
- 681.Prilutsky BI. Muscle coordination: the discussion continues. Motor Control 4: 97–116, 2000. [DOI] [PubMed] [Google Scholar]
- 682.Prilutsky BI and Gregor RJ. Swing- and support-related muscle actions differentially trigger human walk-run and run-walk transitions. J Exp Biol 204: 2277–2287, 2001. [DOI] [PubMed] [Google Scholar]
- 683.Prilutsky BI, Gregor RJ and Ryan MM. Coordination of two-joint rectus femoris and hamstrings during the swing phase of human walking and running. Exp Brain Res 120: 479–486, 1998. [DOI] [PubMed] [Google Scholar]
- 684.Prilutsky BI, Herzog W and Allinger TL. Force-sharing between cat soleus and gastrocnemius muscles during walking: explanations based on electrical activity, properties, and kinematics. J Biomech 27: 1223–1235, 1994. [DOI] [PubMed] [Google Scholar]
- 685.Prilutsky BI, Isaka T, Albrecht AM and Gregor RJ. Is coordination of two-joint leg muscles during load lifting consistent with the strategy of minimum fatigue? J Biomech 31: 1025–1034, 1998. [DOI] [PubMed] [Google Scholar]
- 686.Prilutsky BI, Klishko AN, Weber DJ and Lemay MA. Computing motion dependent afferent activity during cat locomotion using a forward dynamics musculoskeletal model. In: Neuromechanical Modeling of Posture and Locomotion, edited by Prilutsky BI and Edwards DH. New York: Springer, 2016, p. 273–307. [Google Scholar]
- 687.Prilutsky BI, Maas H, Bulgakova M, Hodson-Tole EF and Gregor RJ. Short-term motor compensations to denervation of feline soleus and lateral gastrocnemius result in preservation of ankle mechanical output during locomotion. Cells Tissues Organs 193: 310–324, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 688.Prilutsky BI and Zatsiorsky VM. Optimization-based models of muscle coordination. Exerc Sport Sci Rev 30: 32–38, 2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 689.Prochazka A. Sensorimotor gain control: a basic strategy of motor systems? Prog Neurobiol 33: 281–307, 1989. [DOI] [PubMed] [Google Scholar]
- 690.Prochazka A. Quantifying proprioception. Prog Brain Res 123: 133–142, 1999. [PubMed] [Google Scholar]
- 691.Prochazka A and Ellaway P. Sensory systems in the control of movement. Compr Physiol 2: 2615–2627, 2012. [DOI] [PubMed] [Google Scholar]
- 692.Prochazka A and Gorassini M. Ensemble firing of muscle afferents recorded during normal locomotion in cats. J Physiol 507 (Pt 1): 293–304, 1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 693.Prochazka A and Gorassini M. Models of ensemble firing of muscle spindle afferents recorded during normal locomotion in cats. J Physiol 507 (Pt 1): 277–291, 1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 694.Prochazka A, Hulliger M, Trend P, Llewellyn M and Durmuller N. Muscle afferent contribution to control of paw shakes in normal cats. J Neurophysiol 61: 550–562, 1989. [DOI] [PubMed] [Google Scholar]
- 695.Prochazka A, Hulliger M, Zangger P and Appenteng K. ‘Fusimotor set’: new evidence for alpha-independent control of gamma-motoneurones during movement in the awake cat. Brain Res 339: 136–140, 1985. [DOI] [PubMed] [Google Scholar]
- 696.Prochazka A, Trend P, Hulliger M and Vincent S. Ensemble proprioceptive activity in the cat step cycle: towards a representative look-up chart. Prog Brain Res 80: 61–74, 1989. [DOI] [PubMed] [Google Scholar]
- 697.Prochazka A, Westerman RA and Ziccone SP. Discharges of single hindlimb afferents in the freely moving cat. J Neurophysiol 39: 1090–1104, 1976. [DOI] [PubMed] [Google Scholar]
- 698.Prochazka A, Westerman RA and Ziccone SP. Ia afferent activity during a variety of voluntary movements in the cat. J Physiol 268: 423–448, 1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 699.Proske U and Gandevia SC. The proprioceptive senses: their roles in signaling body shape, body position and movement, and muscle force. Physiol Rev 92: 1651–1697, 2012. [DOI] [PubMed] [Google Scholar]
- 700.Proske U, Morgan DL and Gregory JE. Muscle history dependence of responses to stretch of primary and secondary endings of cat soleus muscle spindles. J Physiol 445: 81–95, 1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 701.Proske U, Tsay A and Allen T. Muscle thixotropy as a tool in the study of proprioception. Exp Brain Res 232: 3397–3412, 2014. [DOI] [PubMed] [Google Scholar]
- 702.Putnam CA. Sequential motions of body segments in striking and throwing skills: descriptions and explanations. J Biomech 26 Suppl 1: 125–135, 1993. [DOI] [PubMed] [Google Scholar]
- 703.Quevedo J, Fedirchuk B, Gosgnach S and McCrea DA. Group I disynaptic excitation of cat hindlimb flexor and bifunctional motoneurones during fictive locomotion. J Physiol 525 Pt 2: 549–564, 2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 704.Quevedo J, Stecina K, Gosgnach S and McCrea DA. Stumbling corrective reaction during fictive locomotion in the cat. J Neurophysiol 94: 2045–2052, 2005. [DOI] [PubMed] [Google Scholar]
- 705.Quevedo J, Stecina K and McCrea DA. Intracellular analysis of reflex pathways underlying the stumbling corrective reaction during fictive locomotion in the cat. J Neurophysiol 94: 2053–2062, 2005. [DOI] [PubMed] [Google Scholar]
- 706.Raible DW and Ungos JM. Specification of sensory neuron cell fate from the neural crest. Adv Exp Med Biol 589: 170–180, 2006. [DOI] [PubMed] [Google Scholar]
- 707.Ranade SS, Woo SH, Dubin AE, Moshourab RA, Wetzel C, Petrus M, Mathur J, Begay V, Coste B, Mainquist J, Wilson AJ, Francisco AG, Reddy K, Qiu Z, Wood JN, Lewin GR and Patapoutian A. Piezo2 is the major transducer of mechanical forces for touch sensation in mice. Nature 516: 121–125, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 708.Rexed B. A cytoarchitectonic atlas of the spinal cord in the cat. J Comp Neurol 100: 297–379, 1954. [DOI] [PubMed] [Google Scholar]
- 709.Rho MJ, Lavoie S and Drew T. Effects of red nucleus microstimulation on the locomotor pattern and timing in the intact cat: a comparison with the motor cortex. J Neurophysiol 81: 2297–2315, 1999. [DOI] [PubMed] [Google Scholar]
- 710.Richmond FJ and Abrahams VC. What are the proprioceptors of the neck? Prog Brain Res 50: 245–254, 1979. [DOI] [PubMed] [Google Scholar]
- 711.Riddell JS and Hadian M. Interneurones in pathways from group II muscle afferents in the lower-lumbar segments of the feline spinal cord. J Physiol 522 Pt 1: 109–123, 2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 712.Riener R and Edrich T. Identification of passive elastic joint moments in the lower extremities. J Biomech 32: 539–544, 1999. [DOI] [PubMed] [Google Scholar]
- 713.Rifkin JT, Todd VJ, Anderson LW and Lefcort F. Dynamic expression of neurotrophin receptors during sensory neuron genesis and differentiation. Dev Biol 227: 465–480, 2000. [DOI] [PubMed] [Google Scholar]
- 714.Robinson FR, Houk JC and Gibson AR. Limb specific connections of the cat magnocellular red nucleus. J Comp Neurol 257: 553–577, 1987. [DOI] [PubMed] [Google Scholar]
- 715.Romanes GJ. The motor cell columns of the lumbo-sacral spinal cord of the cat. J Comp Neurol 94: 313–363, 1951. [DOI] [PubMed] [Google Scholar]
- 716.Romanes GJ. The motor pools of the spinal cord. Prog Brain Res 11: 93–119, 1964. [DOI] [PubMed] [Google Scholar]
- 717.Ross KT and Nichols TR. Heterogenic feedback between hindlimb extensors in the spontaneously locomoting premammillary cat. J Neurophysiol 101: 184–197, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 718.Rossignol S, Dubuc R and Gossard JP. Dynamic sensorimotor interactions in locomotion. Physiol Rev 86: 89–154, 2006. [DOI] [PubMed] [Google Scholar]
- 719.Rossignol S and Frigon A. Recovery of locomotion after spinal cord injury: some facts and mechanisms. Annu Rev Neurosci 34: 413–440, 2011. [DOI] [PubMed] [Google Scholar]
- 720.Rossignol S, Saltiel P, Perreault MC, Drew T, Pearson K and Belanger M. Intralimb and interlimb coordination in the cat during real and fictive rhythmic motor programs. Seminars in neuroscience 5: 67–75, 1993. [Google Scholar]
- 721.Rotterman TM, Nardelli P, Cope TC and Alvarez FJ. Normal distribution of VGLUT1 synapses on spinal motoneuron dendrites and their reorganization after nerve injury. J Neurosci 34: 3475–3492, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 722.Rudjord T. A second order mechanical model of muscle spindle primary endings. Kybernetik 6: 205–213, 1970. [DOI] [PubMed] [Google Scholar]
- 723.Rudomin P, Jimenez I, Solodkin M and Duenas S. Sites of action of segmental and descending control of transmission on pathways mediating PAD of Ia- and Ib-afferent fibers in cat spinal cord. J Neurophysiol 50: 743–769, 1983. [DOI] [PubMed] [Google Scholar]
- 724.Rudomin P, Lomeli J and Quevedo J. Tonic differential supraspinal modulation of PAD and PAH of segmental and ascending intraspinal collaterals of single group I muscle afferents in the cat spinal cord. Exp Brain Res 159: 239–250, 2004. [DOI] [PubMed] [Google Scholar]
- 725.Rudomin P and Schmidt RF. Presynaptic inhibition in the vertebrate spinal cord revisited. Exp Brain Res 129: 1–37, 1999. [DOI] [PubMed] [Google Scholar]
- 726.Rudomin P, Solodkin M and Jimenez I. PAD and PAH response patterns of group Ia- and Ib-fibers to cutaneous and descending inputs in the cat spinal cord. J Neurophysiol 56: 987–1006, 1986. [DOI] [PubMed] [Google Scholar]
- 727.Rushmer DS, Roberts WJ and Augter GK. Climbing fiber responses of cerebellar Purkinje cells to passive movement of the cat forepaw. Brain Res 106: 1–20, 1976. [DOI] [PubMed] [Google Scholar]
- 728.Rushmer DS, Woollacott MH, Robertson LT and Laxer KD. Somatotopic organization of climbing fiber projections from low threshold cutaneous afferents to pars intermedia of cerebellar cortex in the cat. Brain Res 181: 17–30, 1980. [DOI] [PubMed] [Google Scholar]
- 729.Rybak IA, Shevtsova NA, Lafreniere-Roula M and McCrea DA. Modelling spinal circuitry involved in locomotor pattern generation: insights from deletions during fictive locomotion. J Physiol 577: 617–639, 2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 730.Rybak IA, Stecina K, Shevtsova NA and McCrea DA. Modelling spinal circuitry involved in locomotor pattern generation: insights from the effects of afferent stimulation. J Physiol 577: 641–658, 2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 731.Ryczko D and Dubuc R. The multifunctional mesencephalic locomotor region. Curr Pharm Des 19: 4448–4470, 2013. [DOI] [PubMed] [Google Scholar]
- 732.Saal HP and Bensmaia SJ. Touch is a team effort: interplay of submodalities in cutaneous sensibility. Trends Neurosci 37: 689–697, 2014. [DOI] [PubMed] [Google Scholar]
- 733.Sainburg RL, Ghez C and Kalakanis D. Intersegmental dynamics are controlled by sequential anticipatory, error correction, and postural mechanisms. J Neurophysiol 81: 1045–1056, 1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 734.Sainburg RL, Ghilardi MF, Poizner H and Ghez C. Control of limb dynamics in normal subjects and patients without proprioception. J Neurophysiol 73: 820–835, 1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 735.Saltiel P and Rossignol S. Critical points in the forelimb fictive locomotor cycle and motor coordination: effects of phasic retractions and protractions of the shoulder in the cat. J Neurophysiol 92: 1342–1356, 2004. [DOI] [PubMed] [Google Scholar]
- 736.Sandercock TG and Heckman CJ. Force from cat soleus muscle during imposed locomotor-like movements: experimental data versus Hill-type model predictions. J Neurophysiol 77: 1538–1552, 1997. [DOI] [PubMed] [Google Scholar]
- 737.Sandkuhler J, Chen JG, Cheng G and Randic M. Low-frequency stimulation of afferent Adelta-fibers induces long-term depression at primary afferent synapses with substantia gelatinosa neurons in the rat. J Neurosci 17: 6483–6491, 1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 738.Sandkuhler J and Liu X. Induction of long-term potentiation at spinal synapses by noxious stimulation or nerve injury. Eur J Neurosci 10: 2476–2480, 1998. [DOI] [PubMed] [Google Scholar]
- 739.Santuz A, Akay T, Mayer WP, Wells TL, Schroll A and Arampatzis A. Modular organization of murine locomotor pattern in the presence and absence of sensory feedback from muscle spindles. J Physiol 597: 3147–3165, 2019. [DOI] [PubMed] [Google Scholar]
- 740.Schaafsma A, Otten E and Van Willigen JD. A muscle spindle model for primary afferent firing based on a simulation of intrafusal mechanical events. J Neurophysiol 65: 1297–1312, 1991. [DOI] [PubMed] [Google Scholar]
- 741.Schieppati M, Schmid M and Sozzi S. Rapid processing of haptic cues for postural control in blind subjects. Clin Neurophysiol 125: 1427–1439, 2014. [DOI] [PubMed] [Google Scholar]
- 742.Schillings AM, Van Wezel BM and Duysens J. Mechanically induced stumbling during human treadmill walking. J Neurosci Methods 67: 11–17, 1996. [DOI] [PubMed] [Google Scholar]
- 743.Schmidt A and Fischer MS. Arboreal locomotion in rats - the challenge of maintaining stability. J Exp Biol 213: 3615–3624, 2010. [DOI] [PubMed] [Google Scholar]
- 744.Scholz JP and Schoner G. The uncontrolled manifold concept: identifying control variables for a functional task. Exp Brain Res 126: 289–306, 1999. [DOI] [PubMed] [Google Scholar]
- 745.Schomburg ED and Behrends HB. The possibility of phase-dependent monosynaptic and polysynaptic is excitation to homonymous motoneurones during fictive locomotion. Brain Res 143: 533–537, 1978. [DOI] [PubMed] [Google Scholar]
- 746.Schomburg ED, Meinck HM, Haustein J and Roesler J. Functional organization of the spinal reflex pathways from forelimb afferents to hindlimb motoneurones in the cat. Brain Res 139: 21–33, 1978. [DOI] [PubMed] [Google Scholar]
- 747.Schomburg ED, Petersen N, Barajon I and Hultborn H. Flexor reflex afferents reset the step cycle during fictive locomotion in the cat. Exp Brain Res 122: 339–350, 1998. [DOI] [PubMed] [Google Scholar]
- 748.Schomburg ED, Roesler J and Meinck HM. Phase-dependent transmission in the excitatory propriospinal reflex pathway from forelimb afferents to lumbar motoneurones during fictive locomotion. Neurosci Lett 4: 249–252, 1977. [DOI] [PubMed] [Google Scholar]
- 749.Schomburg ED, Steffens H and Warneke G. Functional organization of the spinal reflex pathways from forelimb afferents to hindlimb motoneurones in the cat. II. Conditions of the interneuronal connections. Brain Res 375: 280–290, 1986. [DOI] [PubMed] [Google Scholar]
- 750.Segers V, Lenoir M, Aerts P and De CD. Influence of M. tibialis anterior fatigue on the walk-to-run and run-to-walk transition in non-steady state locomotion. Gait Posture 25: 639–647, 2007. [DOI] [PubMed] [Google Scholar]
- 751.Segundo JP, Takenaka T and Encabo H. Somatic sensory properties of bulbar reticular neurons. J Neurophysiol 30: 1221–1238, 1967. [DOI] [PubMed] [Google Scholar]
- 752.Seki K and Yamaguchi T. Cutaneous reflex activity of the cat forelimb during fictive locomotion. Brain Res 753: 56–62, 1997. [DOI] [PubMed] [Google Scholar]
- 753.Serbedzija GN, Fraser SE and Bronner-Fraser M. Pathways of trunk neural crest cell migration in the mouse embryo as revealed by vital dye labelling. Development 108: 605–612, 1990. [DOI] [PubMed] [Google Scholar]
- 754.Serrano-Gotarredona R, Oster M, Lichtsteiner P, Linares-Barranco A, Paz-Vicente R, Gomez-Rodriguez F, Camunas-Mesa L, Berner R, Rivas-Perez M, Delbruck T, Liu SC, Douglas R, Hafliger P, Jimenez-Moreno G, Civit BA, Serrano-Gotarredona T, Acosta-Jimenez AJ and Linares-Barranco B. CAVIAR: a 45k neuron, 5M synapse, 12G connects/s AER hardware sensory-processing- learning-actuating system for high-speed visual object recognition and tracking. IEEE Trans Neural Netw 20: 1417–1438, 2009. [DOI] [PubMed] [Google Scholar]
- 755.Severin FV. [Role of the gamma-motor system in activation of extensor alpha-motor neurons during controlled locomotion]. Biofizika 15: 1096–1102, 1970. [PubMed] [Google Scholar]
- 756.Severin FV, Orlovskii GN and Shik ML. [Work of muscle receptors during controlled locomotion]. Biofizika 12: 502–511, 1967. [PubMed] [Google Scholar]
- 757.Shakya SS, Bannatyne BA, Jankowska E, Hammar I, Nilsson E and Maxwell DJ. Inhibitory inputs to four types of spinocerebellar tract neurons in the cat spinal cord. Neuroscience 226: 253–269, 2012. [DOI] [PubMed] [Google Scholar]
- 758.Shefchyk SJ and Jordan LM. Excitatory and inhibitory postsynaptic potentials in alpha-motoneurons produced during fictive locomotion by stimulation of the mesencephalic locomotor region. J Neurophysiol 53: 1345–1355, 1985. [DOI] [PubMed] [Google Scholar]
- 759.Shefchyk SJ, Stein RB and Jordan LM. Synaptic transmission from muscle afferents during fictive locomotion in the mesencephalic cat. J Neurophysiol 51: 986–997, 1984. [DOI] [PubMed] [Google Scholar]
- 760.Sherrington CS. The integrative action of the nervous system. New Haven, CT: Yale University Press, 1906. [Google Scholar]
- 761.Sherrington CS. Flexion-reflex of the limb, crossed extension-reflex, and reflex stepping and standing. J Physiol 40: 28–121, 1910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 762.Shevtsova NA, Talpalar AE, Markin SN, Harris-Warrick RM, Kiehn O and Rybak IA. Organization of left-right coordination of neuronal activity in the mammalian spinal cord: insights from computational modelling. J Physiol 594: 6117–6131, 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 763.Shik ML, Severin FV and Orlovskii GN. [Control of walking and running by means of electric stimulation of the midbrain]. Biofizika 11: 659–666, 1966. [PubMed] [Google Scholar]
- 764.Shimamura M and Akert K. Peripheral nervous relations of propriospinal and spino-bulbo-spinal reflex systems. Jap J Physiol 15: 638–647, 1965. [Google Scholar]
- 765.Shimamura M and Aoki M. Effects of spino-bulbo-spinal reflex volleys on flexor motoneurons of hindlimb in the cat. Brain Res 16: 333–349, 1969. [DOI] [PubMed] [Google Scholar]
- 766.Shimamura M and Kogure I. Discharge patterns of reticulospinal neurons corresponding with quadrupedal leg movements in thalamic cats. Brain Res 260: 27–34, 1983. [DOI] [PubMed] [Google Scholar]
- 767.Shimamura M, Kogure I and Wada S. Three types of reticular neurons involved in the spino-bulbo-spinal reflex of cats. Brain Res 186: 99–113, 1980. [DOI] [PubMed] [Google Scholar]
- 768.Shin HC and Chapin JK. Movement induced modulation of afferent transmission to single neurons in the ventroposterior thalamus and somatosensory cortex in rat. Exp Brain Res 81: 515–522, 1990. [DOI] [PubMed] [Google Scholar]
- 769.Shin HC, Park HJ and Chapin JK. Differential phasic modulation of short and long latency afferent sensory transmission to single neurons in the ventroposterolateral thalamus in behaving rats. Neurosci Res 17: 117–125, 1993. [DOI] [PubMed] [Google Scholar]
- 770.Shin HC, Park HJ and Chapin JK. Differential phasic modulation of short and long latency afferent sensory transmission to single neurons in the primary somatosensory cortex in behaving rats. Neurosci Res 19: 419–425, 1994. [DOI] [PubMed] [Google Scholar]
- 771.Shrestha SS, Bannatyne BA, Jankowska E, Hammar I, Nilsson E and Maxwell DJ. Excitatory inputs to four types of spinocerebellar tract neurons in the cat and the rat thoracolumbar spinal cord. J Physiol 590: 1737–1755, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 772.Shroyer NF, Helmrath MA, Wang VY, Antalffy B, Henning SJ and Zoghbi HY. Intestine-specific ablation of mouse atonal homolog 1 (Math1) reveals a role in cellular homeostasis. Gastroenterology 132: 2478–2488, 2007. [DOI] [PubMed] [Google Scholar]
- 773.Siegel JM and Tomaszewski KS. Behavioral organization of reticular formation: studies in the unrestrained cat. I. Cells related to axial, limb, eye, and other movements. J Neurophysiol 50: 696–716, 1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 774.Silverman J, Garnett NL, Giszter SF, Heckman CJ, Kulpa-Eddy JA, Lemay MA, Perry CK and Pinter M. Decerebrate mammalian preparations: unalleviated or fully alleviated pain? A review and opinion. Contemp Top Lab Anim Sci 44: 34–36, 2005. [PubMed] [Google Scholar]
- 775.Simonsen EB and Dyhre-Poulsen P. Amplitude of the human soleus H reflex during walking and running. J Physiol 515 (Pt 3): 929–939, 1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 776.Sinkjaer T, Andersen JB, Ladouceur M, Christensen LO and Nielsen JB. Major role for sensory feedback in soleus EMG activity in the stance phase of walking in man. J Physiol 523 Pt 3: 817–827, 2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 777.Sinkjaer T, Andersen JB and Larsen B. Soleus stretch reflex modulation during gait in humans. J Neurophysiol 76: 1112–1120, 1996. [DOI] [PubMed] [Google Scholar]
- 778.Sirois J, Frigon A and Gossard JP. Independent control of presynaptic inhibition by reticulospinal and sensory inputs at rest and during rhythmic activities in the cat. J Neurosci 33: 8055–8067, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 779.Sjostrom A and Zangger P. Muscle spindle control during locomotor movements generated by the deafferented spinal cord. Acta Physiol Scand 97: 281–291, 1976. [DOI] [PubMed] [Google Scholar]
- 780.SKOGLUND S. Anatomical and physiological studies of knee joint innervation in the cat. Acta Physiol Scand Suppl 36: 1–101, 1956. [PubMed] [Google Scholar]
- 781.Slawinska U, Majczynski H, Dai Y and Jordan LM. The upright posture improves plantar stepping and alters responses to serotonergic drugs in spinal rats. J Physiol 590: 1721–1736, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 782.Smith JL, Betts B, Edgerton VR and Zernicke RF. Rapid ankle extension during paw shakes: selective recruitment of fast ankle extensors. J Neurophysiol 43: 612–620, 1980. [DOI] [PubMed] [Google Scholar]
- 783.Smith JL, Carlson-Kuhta P and Trank TV. Forms of forward quadrupedal locomotion. III. A comparison of posture, hindlimb kinematics, and motor patterns for downslope and level walking. J Neurophysiol 79: 1702–1716, 1998. [DOI] [PubMed] [Google Scholar]
- 784.Smith JL, Chung SH and Zernicke RF. Gait-related motor patterns and hindlimb kinetics for the cat trot and gallop. Exp Brain Res 94: 308–322, 1993. [DOI] [PubMed] [Google Scholar]
- 785.Smith JL, Smith LA, Zernicke RF and Hoy M. Locomotion in exercised and nonexercised cats cordotomized at two or twelve weeks of age. Exp Neurol 76: 393–413, 1982. [DOI] [PubMed] [Google Scholar]
- 786.Smith SS and Chapin JK. The estrous cycle and the olivo-cerebellar circuit. II. Enhanced selective sensory gating of responses from the rostral dorsal accessory olive. Exp Brain Res 111: 385–392, 1996. [DOI] [PubMed] [Google Scholar]
- 787.Song Z, Banks RW and Bewick GS. Modelling the mechanoreceptor’s dynamic behaviour. J Anat 227: 243–254, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 788.Spagna JC, Goldman DI, Lin PC, Koditschek DE and Full RJ. Distributed mechanical feedback in arthropods and robots simplifies control of rapid running on challenging terrain. Bioinspir Biomim 2: 9–18, 2007. [DOI] [PubMed] [Google Scholar]
- 789.Srinivasan M and Ruina A. Computer optimization of a minimal biped model discovers walking and running. Nature 439: 72–75, 2006. [DOI] [PubMed] [Google Scholar]
- 790.Stantcheva KK, Iovino L, Dhandapani R, Martinez C, Castaldi L, Nocchi L, Perlas E, Portulano C, Pesaresi M, Shirlekar KS, de Castro RF, Paparountas T, Bilbao D and Heppenstall PA. A subpopulation of itch-sensing neurons marked by Ret and somatostatin expression. EMBO Rep 17: 585–600, 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 791.Stapley PJ, Ting LH, Hulliger M and Macpherson JM. Automatic postural responses are delayed by pyridoxine-induced somatosensory loss. J Neurosci 22: 5803–5807, 2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 792.Stecina K, Fedirchuk B and Hultborn H. Information to cerebellum on spinal motor networks mediated by the dorsal spinocerebellar tract. J Physiol 591: 5433–5443, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 793.Stecina K, Quevedo J and McCrea DA. Parallel reflex pathways from flexor muscle afferents evoking resetting and flexion enhancement during fictive locomotion and scratch in the cat. J Physiol 569: 275–290, 2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 794.Stenum J and Choi JT. Neuromuscular effort predicts walk-run transition speed in normal and adapted human gaits. J Exp Biol 219: 2809–2813, 2016. [DOI] [PubMed] [Google Scholar]
- 795.Stuart DG, Goslow GE, Mosher CG and Reinking RM. Stretch responsiveness of Golgi tendon organs. Exp Brain Res 10: 463–476, 1970. [DOI] [PubMed] [Google Scholar]
- 796.Stuart DG and Hultborn H. Thomas Graham Brown (1882--1965), Anders Lundberg (1920-), and the neural control of stepping. Brain Res Rev 59: 74–95, 2008. [DOI] [PubMed] [Google Scholar]
- 797.Stuart DG and Pierce PA. The academic lineage of Sir John Carew Eccles (1903–1997). Prog Neurobiol 78: 136–155, 2006. [DOI] [PubMed] [Google Scholar]
- 798.Stubbs PW and Mrachacz-Kersting N. Short-latency crossed inhibitory responses in the human soleus muscle. J Neurophysiol 102: 3596–3605, 2009. [DOI] [PubMed] [Google Scholar]
- 799.Surmeli G, Akay T, Ippolito GC, Tucker PW and Jessell TM. Patterns of spinal sensory-motor connectivity prescribed by a dorsoventral positional template. Cell 147: 653–665, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 800.Suzuki S, Kano T, Ijspeert AJ and Ishiguro A. Sprawling Quadruped Robot Driven by Decentralized Control With Cross-Coupled Sensory Feedback Between Legs and Trunk. Front Neurorobot 14: 607455, 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 801.Szczecinski NS, Bockemuhl T, Chockley AS and Buschges A. Static stability predicts the continuum of interleg coordination patterns in Drosophila. J Exp Biol 221: 2018. [DOI] [PubMed] [Google Scholar]
- 802.Takeoka A and Arber S. Functional Local Proprioceptive Feedback Circuits Initiate and Maintain Locomotor Recovery after Spinal Cord Injury. Cell Rep 27: 71–85, 2019. [DOI] [PubMed] [Google Scholar]
- 803.Takeoka A, Vollenweider I, Courtine G and Arber S. Muscle spindle feedback directs locomotor recovery and circuit reorganization after spinal cord injury. Cell 159: 1626–1639, 2014. [DOI] [PubMed] [Google Scholar]
- 804.Talbot WH, Darian-Smith I, Kornhuber HH and Mountcastle VB. The sense of flutter-vibration: comparison of the human capacity with response patterns of mechanoreceptive afferents from the monkey hand. J Neurophysiol 31: 301–334, 1968. [DOI] [PubMed] [Google Scholar]
- 805.Talpalar AE, Bouvier J, Borgius L, Fortin G, Pierani A and Kiehn O. Dual-mode operation of neuronal networks involved in left-right alternation. Nature 500: 85–88, 2013. [DOI] [PubMed] [Google Scholar]
- 806.Taylor A, Durbaba R, Ellaway PH and Rawlinson S. Static and dynamic gamma-motor output to ankle flexor muscles during locomotion in the decerebrate cat. J Physiol 571: 711–723, 2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 807.Thompson AK, Chen XY and Wolpaw JR. Acquisition of a simple motor skill: task-dependent adaptation plus long-term change in the human soleus H-reflex. J Neurosci 29: 5784–5792, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 808.Thompson AK, Pomerantz FR and Wolpaw JR. Operant conditioning of a spinal reflex can improve locomotion after spinal cord injury in humans. J Neurosci 33: 2365–2375, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 809.Thompson AK and Wolpaw JR. Restoring walking after spinal cord injury: operant conditioning of spinal reflexes can help. Neuroscientist 21: 203–215, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 810.Thornell LE, Carlsson L, Eriksson PO, Liu JX, Osterlund C, Stal P and Pedrosa-Domellof F. Fibre typing of intrafusal fibres. J Anat 227: 136–156, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 811.Thorstensson A and Roberthson H. Adaptations to changing speed in human locomotion: speed of transition between walking and running. Acta Physiol Scand 131: 211–214, 1987. [DOI] [PubMed] [Google Scholar]
- 812.Ting LH and Macpherson JM. Ratio of shear to load ground-reaction force may underlie the directional tuning of the automatic postural response to rotation and translation. J Neurophysiol 92: 808–823, 2004. [DOI] [PubMed] [Google Scholar]
- 813.Ting LH and Macpherson JM. A limited set of muscle synergies for force control during a postural task. J Neurophysiol 93: 609–613, 2005. [DOI] [PubMed] [Google Scholar]
- 814.Todd AJ, Hughes DI, Polgar E, Nagy GG, Mackie M, Ottersen OP and Maxwell DJ. The expression of vesicular glutamate transporters VGLUT1 and VGLUT2 in neurochemically defined axonal populations in the rat spinal cord with emphasis on the dorsal horn. Eur J Neurosci 17: 13–27, 2003. [DOI] [PubMed] [Google Scholar]
- 815.Tokuhiro A, Nagashima H and Takechi H. Electromyographic kinesiology of lower extremity muscles during slope walking. Arch Phys Med Rehabil 66: 610–613, 1985. [PubMed] [Google Scholar]
- 816.Torres-Oviedo G, Macpherson JM and Ting LH. Muscle synergy organization is robust across a variety of postural perturbations. J Neurophysiol 96: 1530–1546, 2006. [DOI] [PubMed] [Google Scholar]
- 817.Tourtellotte WG and Milbrandt J. Sensory ataxia and muscle spindle agenesis in mice lacking the transcription factor Egr3. Nat Genet 20: 87–91, 1998. [DOI] [PubMed] [Google Scholar]
- 818.Tresch MC, Saltiel P and Bizzi E. The construction of movement by the spinal cord. Nat Neurosci 2: 162–167, 1999. [DOI] [PubMed] [Google Scholar]
- 819.Tripodi M, Stepien AE and Arber S. Motor antagonism exposed by spatial segregation and timing of neurogenesis. Nature 479: 61–66, 2011. [DOI] [PubMed] [Google Scholar]
- 820.Tsianos GA, Rustin C and Loeb GE. Mammalian muscle model for predicting force and energetics during physiological behaviors. IEEE Trans Neural Syst Rehabil Eng 20: 117–133, 2012. [DOI] [PubMed] [Google Scholar]
- 821.Turksen K, Kupper T, Degenstein L, Williams I and Fuchs E. Interleukin 6: insights to its function in skin by overexpression in transgenic mice. Proc Natl Acad Sci U S A 89: 5068–5072, 1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 822.Tuthill JC and Azim E. Proprioception. Curr Biol 28: R194–R203, 2018. [DOI] [PubMed] [Google Scholar]
- 823.Udo M, Matsukawa K, Kamei H, Minoda K and Oda Y. Simple and complex spike activities of Purkinje cells during locomotion in the cerebellar vermal zones of decerebrate cats. Exp Brain Res 41: 292–300, 1981. [DOI] [PubMed] [Google Scholar]
- 824.Usoskin D, Furlan A, Islam S, Abdo H, Lonnerberg P, Lou D, Hjerling-Leffler J, Haeggstrom J, Kharchenko O, Kharchenko PV, Linnarsson S and Ernfors P. Unbiased classification of sensory neuron types by large-scale single-cell RNA sequencing. Nat Neurosci 18: 145–153, 2015. [DOI] [PubMed] [Google Scholar]
- 825.Vallbo AB, Hagbarth KE, Torebjork HE and Wallin BG. Somatosensory, proprioceptive, and sympathetic activity in human peripheral nerves. Physiol Rev 59: 919–957, 1979. [DOI] [PubMed] [Google Scholar]
- 826.van der Zouwen CI, Boutin J, Fougere M, Flaive A, Vivancos M, Santuz A, Akay T, Sarret P and Ryczko D. Freely Behaving Mice Can Brake and Turn During Optogenetic Stimulation of the Mesencephalic Locomotor Region. Front Neural Circuits 15: 639900, 2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 827.van Deursen RW and Simoneau GG. Foot and ankle sensory neuropathy, proprioception, and postural stability. J Orthop Sports Phys Ther 29: 718–726, 1999. [DOI] [PubMed] [Google Scholar]
- 828.van Kan PL, Gibson AR and Houk JC. Movement-related inputs to intermediate cerebellum of the monkey. J Neurophysiol 69: 74–94, 1993. [DOI] [PubMed] [Google Scholar]
- 829.Van Wezel BM, Ottenhoff FA and Duysens J. Dynamic control of location-specific information in tactile cutaneous reflexes from the foot during human walking. J Neurosci 17: 3804–3814, 1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 830.VanderHorst VG and Holstege G. Organization of lumbosacral motoneuronal cell groups innervating hindlimb, pelvic floor, and axial muscles in the cat. J Comp Neurol 382: 46–76, 1997. [PubMed] [Google Scholar]
- 831.Varoqui H, Schafer MK, Zhu H, Weihe E and Erickson JD. Identification of the differentiation-associated Na+/PI transporter as a novel vesicular glutamate transporter expressed in a distinct set of glutamatergic synapses. J Neurosci 22: 142–155, 2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 832.Vaughan C, Davis B and O’Connor J. Dynamics of human gait. Cape Town: Kiboho Publishers, 1999. [Google Scholar]
- 833.Viala G and Buser P. [Inhibition of spinal locomotor activity by a special method of somatic stimulation in rabbits]. Exp Brain Res 21: 275–284, 1974. [DOI] [PubMed] [Google Scholar]
- 834.Viala G, Orsal D and Buser P. Cutaneous fiber groups involved in the inhibition of fictive locomotion in the rabbit. Exp Brain Res 33: 257–267, 1978. [DOI] [PubMed] [Google Scholar]
- 835.Vincent JA, Gabriel HM, Deardorff AS, Nardelli P, Fyffe REW, Burkholder T and Cope TC. Muscle proprioceptors in adult rat: mechanosensory signaling and synapse distribution in spinal cord. J Neurophysiol 118: 2687–2701, 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 836.Vincent JA, Wieczerzak KB, Gabriel HM, Nardelli P, Rich MM and Cope TC. A novel path to chronic proprioceptive disability with oxaliplatin: Distortion of sensory encoding. Neurobiol Dis 95: 54–65, 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 837.Voorhoeve PE and van KR. Reflex behaviour of fusimotor neurones of the cat upon electrical stimulation of various afferent fibers. Acta Physiol Pharmacol Neerl 10: 391–407, 1962. [PubMed] [Google Scholar]
- 838.Vrieseling E and Arber S. Target-induced transcriptional control of dendritic patterning and connectivity in motor neurons by the ETS gene Pea3. Cell 127: 1439–1452, 2006. [DOI] [PubMed] [Google Scholar]
- 839.Wagner FB, Mignardot JB, Le Goff-Mignardot CG, Demesmaeker R, Komi S, Capogrosso M, Rowald A, Seanez I, Caban M, Pirondini E, Vat M, McCracken LA, Heimgartner R, Fodor I, Watrin A, Seguin P, Paoles E, Van Den Keybus K, Eberle G, Schurch B, Pralong E, Becce F, Prior J, Buse N, Buschman R, Neufeld E, Kuster N, Carda S, von ZJ, Delattre V, Denison T, Lambert H, Minassian K, Bloch J and Courtine G. Targeted neurotechnology restores walking in humans with spinal cord injury. Nature 563: 65–71, 2018. [DOI] [PubMed] [Google Scholar]
- 840.Wakeling JM, Tijs C, Konow N and Biewener AA. Modeling muscle function using experimentally determined subject-specific muscle properties. J Biomech 117: 110242, 2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 841.Wand P, Prochazka A and Sontag KH. Neuromuscular responses to gait perturbations in freely moving cats. Exp Brain Res 38: 109–114, 1980. [DOI] [PubMed] [Google Scholar]
- 842.Wang Y, Pillai S, Wolpaw JR and Chen XY. Motor learning changes GABAergic terminals on spinal motoneurons in normal rats. Eur J Neurosci 23: 141–150, 2006. [DOI] [PubMed] [Google Scholar]
- 843.Wang Y, Pillai S, Wolpaw JR and Chen XY. H-reflex down-conditioning greatly increases the number of identifiable GABAergic interneurons in rat ventral horn. Neurosci Lett 452: 124–129, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 844.Watson AH and Bazzaz AA. GABA and glycine-like immunoreactivity at axoaxonic synapses on 1a muscle afferent terminals in the spinal cord of the rat. J Comp Neurol 433: 335–348, 2001. [DOI] [PubMed] [Google Scholar]
- 845.Weber DJ, Stein RB, Everaert DG and Prochazka A. Decoding sensory feedback from firing rates of afferent ensembles recorded in cat dorsal root ganglia in normal locomotion. IEEE Trans Neural Syst Rehabil Eng 14: 240–243, 2006. [DOI] [PubMed] [Google Scholar]
- 846.Weber DJ, Stein RB, Everaert DG and Prochazka A. Limb-state feedback from ensembles of simultaneously recorded dorsal root ganglion neurons. J Neural Eng 4: S168–S180, 2007. [DOI] [PubMed] [Google Scholar]
- 847.Wercberger R and Basbaum AI. Spinal cord projection neurons: a superficial, and also deep, analysis. Curr Opin Physiol 11: 109–115, 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 848.Westling G and Johansson RS. Factors influencing the force control during precision grip. Exp Brain Res 53: 277–284, 1984. [DOI] [PubMed] [Google Scholar]
- 849.Wetzel MC, Atwater AE, Wait JV and Stuart DG. Kinematics of locomotion by cats with a single hindlimb deafferented. J Neurophysiol 39: 667–678, 1976. [DOI] [PubMed] [Google Scholar]
- 850.Whelan PJ. Control of locomotion in the decerebrate cat. Prog Neurobiol 49: 481–515, 1996. [DOI] [PubMed] [Google Scholar]
- 851.Whelan PJ, Hiebert GW and Pearson KG. Stimulation of the group I extensor afferents prolongs the stance phase in walking cats. Exp Brain Res 103: 20–30, 1995. [DOI] [PubMed] [Google Scholar]
- 852.Whelan PJ and Pearson KG. Comparison of the effects of stimulating extensor group I afferents on cycle period during walking in conscious and decerebrate cats. Exp Brain Res 117: 444–452, 1997. [DOI] [PubMed] [Google Scholar]
- 853.Whelan PJ and Pearson KG. Plasticity in reflex pathways controlling stepping in the cat. J Neurophysiol 78: 1643–1650, 1997. [DOI] [PubMed] [Google Scholar]
- 854.Wilmink RJ and Nichols TR. Distribution of heterogenic reflexes among the quadriceps and triceps surae muscles of the cat hind limb. J Neurophysiol 90: 2310–2324, 2003. [DOI] [PubMed] [Google Scholar]
- 855.Wilshin S, Reeve MA, Haynes GC, Revzen S, Koditschek DE and Spence AJ. Longitudinal quasi-static stability predicts changes in dog gait on rough terrain. J Exp Biol 220: 1864–1874, 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 856.Wilson RJ, Chersa T and Whelan PJ. Tissue PO2 and the effects of hypoxia on the generation of locomotor-like activity in the in vitro spinal cord of the neonatal mouse. Neuroscience 117: 183–196, 2003. [DOI] [PubMed] [Google Scholar]
- 857.Wilson VJ. Ipsilateral excitation of extensor motoneurones. Nature 198: 290–291, 1963. [DOI] [PubMed] [Google Scholar]
- 858.Wilson VJ, Kato M, Peterson BW and Wylie RM. A single-unit analysis of the organization of Deiters’ nucleus. J Neurophysiol 30: 603–619, 1967. [DOI] [PubMed] [Google Scholar]
- 859.Wilson VJ, Kato M, Thomas RC and Peterson BW. Excitation of lateral vestibular neurons by peripheral afferent fibers. J Neurophysiol 29: 508–529, 1966. [DOI] [PubMed] [Google Scholar]
- 860.Windhorst U. Muscle proprioceptive feedback and spinal networks. Brain Res Bull 73: 155–202, 2007. [DOI] [PubMed] [Google Scholar]
- 861.Winter DA. Moments of force and mechanical power in jogging. J Biomech 16: 91–97, 1983. [DOI] [PubMed] [Google Scholar]
- 862.Winter DA. Biomechanics and Motor Control of Human Movement. Wiley, 2009. [Google Scholar]
- 863.Wisleder D, Zernicke RF and Smith JL. Speed-related changes in hindlimb intersegmental dynamics during the swing phase of cat locomotion. Exp Brain Res 79: 651–660, 1990. [DOI] [PubMed] [Google Scholar]
- 864.Wolpaw JR. What can the spinal cord teach us about learning and memory? Neuroscientist 16: 532–549, 2010. [DOI] [PubMed] [Google Scholar]
- 865.Wolpaw JR. The negotiated equilibrium model of spinal cord function. J Physiol 596: 3469–3491, 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 866.Wolpaw JR and Lee CL. Memory traces in primate spinal cord produced by operant conditioning of H-reflex. J Neurophysiol 61: 563–572, 1989. [DOI] [PubMed] [Google Scholar]
- 867.Wolpaw JR and Tennissen AM. Activity-dependent spinal cord plasticity in health and disease. Annu Rev Neurosci 24: 807–843, 2001. [DOI] [PubMed] [Google Scholar]
- 868.Woo SH, Lumpkin EA and Patapoutian A. Merkel cells and neurons keep in touch. Trends Cell Biol 25: 74–81, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 869.Woo SH, Ranade S, Weyer AD, Dubin AE, Baba Y, Qiu Z, Petrus M, Miyamoto T, Reddy K, Lumpkin EA, Stucky CL and Patapoutian A. Piezo2 is required for Merkel-cell mechanotransduction. Nature 509: 622–626, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 870.Wu H, Petitpre C, Fontanet P, Sharma A, Bellardita C, Quadros RM, Jannig PR, Wang Y, Heimel JA, Cheung KKY, Wanderoy S, Xuan Y, Meletis K, Ruas J, Gurumurthy CB, Kiehn O, Hadjab S and Lallemend F. Distinct subtypes of proprioceptive dorsal root ganglion neurons regulate adaptive proprioception in mice. Nat Commun 12: 1026, 2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 871.Wu SX, Koshimizu Y, Feng YP, Okamoto K, Fujiyama F, Hioki H, Li YQ, Kaneko T and Mizuno N. Vesicular glutamate transporter immunoreactivity in the central and peripheral endings of muscle-spindle afferents. Brain Res 1011: 247–251, 2004. [DOI] [PubMed] [Google Scholar]
- 872.Xerri C, Benelhadj M and Harlay F. Deficits and recovery of body stabilization during acrobatic locomotion after focal lesion to the somatosensory cortex: a kinematic analysis combined with cortical mapping. Arch Ital Biol 142: 217–236, 2004. [PubMed] [Google Scholar]
- 873.Xu Y, Sladky JT and Brown MJ. Dose-dependent expression of neuronopathy after experimental pyridoxine intoxication. Neurology 39: 1077–1083, 1989. [DOI] [PubMed] [Google Scholar]
- 874.Yakovenko S, Gritsenko V and Prochazka A. Contribution of stretch reflexes to locomotor control: a modeling study. Biol Cybern 90: 146–155, 2004. [DOI] [PubMed] [Google Scholar]
- 875.Yakovenko S, McCrea DA, Stecina K and Prochazka A. Control of locomotor cycle durations. J Neurophysiol 94: 1057–1065, 2005. [DOI] [PubMed] [Google Scholar]
- 876.Yakovenko S, Mushahwar V, VanderHorst V, Holstege G and Prochazka A. Spatiotemporal activation of lumbosacral motoneurons in the locomotor step cycle. J Neurophysiol 87: 1542–1553, 2002. [DOI] [PubMed] [Google Scholar]
- 877.Yamaguchi T. The central pattern generator for forelimb locomotion in the cat. Prog Brain Res 143: 115–122, 2004. [DOI] [PubMed] [Google Scholar]
- 878.Yang JF, Lam T, Pang MY, Lamont E, Musselman K and Seinen E. Infant stepping: a window to the behaviour of the human pattern generator for walking. Can J Physiol Pharmacol 82: 662–674, 2004. [DOI] [PubMed] [Google Scholar]
- 879.Yang JF, Stein RB and James KB. Contribution of peripheral afferents to the activation of the soleus muscle during walking in humans. Exp Brain Res 87: 679–687, 1991. [DOI] [PubMed] [Google Scholar]
- 880.Yang Q, Logan D and Giszter SF. Motor primitives are determined in early development and are then robustly conserved into adulthood. Proc Natl Acad Sci U S A 116: 12025–12034, 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 881.Yang X, Arber S, William C, Li L, Tanabe Y, Jessell TM, Birchmeier C and Burden SJ. Patterning of muscle acetylcholine receptor gene expression in the absence of motor innervation. Neuron 30: 399–410, 2001. [DOI] [PubMed] [Google Scholar]
- 882.Young RP, Scott SH and Loeb GE. The distal hindlimb musculature of the cat: multiaxis moment arms at the ankle joint. Exp Brain Res 96: 141–151, 1993. [DOI] [PubMed] [Google Scholar]
- 883.Zajac FE. Muscle and tendon: properties, models, scaling, and application to biomechanics and motor control. Crit Rev Biomed Eng 17: 359–411, 1989. [PubMed] [Google Scholar]
- 884.Zatsiorsky VM. Kinetics of human motion. Champaign: Human Kinetics, 2002. [Google Scholar]
- 885.Zehr EP. Considerations for use of the Hoffmann reflex in exercise studies. Eur J Appl Physiol 86: 455–468, 2002. [DOI] [PubMed] [Google Scholar]
- 886.Zehr EP, Barss TS, Dragert K, Frigon A, Vasudevan EV, Haridas C, Hundza S, Kaupp C, Klarner T, Klimstra M, Komiyama T, Loadman PM, Mezzarane RA, Nakajima T, Pearcey GE and Sun Y. Neuromechanical interactions between the limbs during human locomotion: an evolutionary perspective with translation to rehabilitation. Exp Brain Res 234: 3059–3081, 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 887.Zehr EP, Komiyama T and Stein RB. Cutaneous reflexes during human gait: electromyographic and kinematic responses to electrical stimulation. J Neurophysiol 77: 3311–3325, 1997. [DOI] [PubMed] [Google Scholar]
- 888.Zehr EP and Loadman PM. Persistence of locomotor-related interlimb reflex networks during walking after stroke. Clin Neurophysiol 123: 796–807, 2012. [DOI] [PubMed] [Google Scholar]
- 889.Zehr EP and Stein RB. What functions do reflexes serve during human locomotion? Prog Neurobiol 58: 185–205, 1999. [DOI] [PubMed] [Google Scholar]
- 890.Zeisel A, Hochgerner H, Lonnerberg P, Johnsson A, Memic F, van der Zwan J, Haring M, Braun E, Borm LE, La MG, Codeluppi S, Furlan A, Lee K, Skene N, Harris KD, Hjerling-Leffler J, Arenas E, Ernfors P, Marklund U and Linnarsson S. Molecular Architecture of the Mouse Nervous System. Cell 174: 999–1014, 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 891.Zelenin PV, Deliagina TG, Grillner S and Orlovsky GN. Postural control in the lamprey: A study with a neuro-mechanical model. J Neurophysiol 84: 2880–2887, 2000. [DOI] [PubMed] [Google Scholar]


