Abstract
BACKGROUND
Severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2)–specific antibodies have been detected in human milk up to 6 weeks post–coronavirus disease 2019 (COVID-19) vaccination. We evaluated SARS-CoV-2-specific antibodies, neutralization activity, effect of pasteurization, and persistence through 6 months after vaccination.
METHODS
This prospective longitudinal study enrolled 30 pregnant or lactating women. SARS-CoV-2 antibodies and neutralization capacity were analyzed using an enzyme-linked immunosorbent assay compared at prevaccination and 1, 3, and 6 months postvaccination, and through Holder pasteurization.
RESULTS
Human milk SARS-CoV-2-specific IgG levels peaked at 1 month postvaccination and persisted above prevaccination levels for at least 6 months (P = .005). SARS-CoV-2-specific IgA was detected at 1 and 3 months (both P < .001) but waned by 6 months compared with baseline (P = .07). Milk SARS-CoV-2-specific IgG and IgA correlated with serum IgG at the same time point (R2 = 0.37, P < .001 and R2 = 0.19, P < .001). Neutralization activity was seen in 83.3%, 70.4%, and 25.0% of milk samples at 1, 3, and 6 months postvaccination. Neutralization most strongly correlated with SARS-CoV-2-specific IgG (R2 = 0.57, P < .001). Pre- and postpasteurization samples showed similar IgG (0.84 vs 1.07, P = .36) and neutralizing activity (57.7% vs 58.7% inhibition, P = .27), but lower IgM and IgA levels postpasteurization (0.09 vs 0.06, P = .004 and 0.21 vs 0.18, P = .043).
CONCLUSIONS
The data suggest that human milk SARS-CoV-2-specific antibodies may be available to milk-fed infants for up to 6 months. In addition, donor milk from vaccinated mothers retain IgG and neutralizing activity.
What’s Known on This Subject:
Data suggests that maternal COVID-19 messenger RNA (mRNA) vaccination stimulates the presence of SARS-CoV-2 antibodies in human milk up to 6 weeks, but less is known about the duration of antibody neutralization ability and persistence after pasteurization.
What This Study Adds:
In this longitudinal prospective study of 30 lactating women, vaccination induced a strong SARS-CoV-2-specific antibody response in human milk which lasted at least 6 months and correlated with neutralizing activity that is not significantly reduced with pasteurization methods.
The United States Centers for Disease Control and Prevention (CDC) have recently strengthened recommendations for coronavirus disease 2019 (COVID-19) vaccinations in pregnant and lactating individuals.1 Although clinical trials for the current vaccines with emergency use authorization excluded lactating and pregnant women, increasing evidence in human and animal studies shows that benefits outweigh risks of vaccination in both pregnant and lactating populations.2–4 There is no theoretical or biological evidence to suggest that vaccine components would be transmitted in human milk or harm a human milk-fed infant.5,6 Studies have shown that mothers who are pregnant or lactating are more likely to decline a COVID-19 vaccine because of fear of harm caused to themselves or their infant, with a significantly higher level of hesitancy among non-White, non-Asian, and non-Hispanic populations.7 Conversely, data showing potential benefits to the human milk-fed infant may support a decision to vaccinate.
Reports early in the pandemic show that mothers who were naturally infected with severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) have antibodies present in their milk.8–12 The antibodies present in human milk post–natural infection can neutralize SARS-CoV-2 activity.10 More recent reports have shown that lactating mothers who have received the COVID-19 messenger RNA (mRNA) vaccination series also produced anti-SARS-CoV-2 antibodies in their milk, suggesting that vaccinated mothers may confer some protection to the infant through breastfeeding.13–17 One study of breastfeeding mother-infant dyads found that anti-SARS-CoV-2 immunoglobulin (Ig) G was present in the oral mucosa of infants after breastfeeding.18 Current studies have evaluated the duration of antibody presence through 6 weeks, but more data on the duration of antibody persistence in milk is needed.
As vaccination recommendations for pregnant and lactating women strengthen, it is likely that an increasing proportion of human milk donated to milk banks will be from vaccinated women. One research group found that Holder pasteurization, a standard practice for milk banking to neutralize most pathogenic microorganisms,19 had no significant effect on the relative level of SARS-CoV-2-specific IgA antibodies in human milk from women who had recovered from a SARS-CoV-2 infection.20 However, the effect of pasteurization on the level and protective capability of vaccine-induced antibodies in human milk is still unreported. The objectives of this study were to determine the presence of antibodies in human milk after vaccination, assess the duration of antibody persistence, analyze neutralization capacity, and evaluate the impact of pasteurization on these antibodies.
Methods
Study Design and Participants
Pregnant or lactating women who planned to receive COVID-19 vaccination were recruited through posted fliers and word of mouth. At enrollment, participants completed a study questionnaire to collect demographic information, preexisting conditions, and history of previous COVID-19 infection. Previous COVID-19 infection status was confirmed by analyzing prevaccination serum and milk for the presence of SARS-CoV-2-specific IgG in all participants. Date and brand of the vaccines and adverse effects were ascertained after each vaccine dose. The study was approved by the institutional review board at Children’s Hospital Los Angeles, and written informed consent was obtained from each participant.
Specimen Collection
Paired blood and human milk samples from lactating mothers were collected at baseline (prevaccination) and at 1, 3, and 6 months after the first COVID-19 vaccine dose. Human milk was collected at each visit until the participant stopped lactating. Participants were instructed to self-collect 30 ml of human milk into a clean sterile container or sterile milk collection bag using an electrical or manual pump at home. If the human milk was not freshly pumped, the milk was frozen in the participant’s home freezer until transported to the laboratory. All milk samples were stored at −80°C until antibody testing.
SARS-CoV-2-Specific Serology
Serum IgG and IgA antibodies specific for the SARS-CoV-2 receptor binding domain (RBD) epitope within the spike protein were measured using a previously described enzyme-linked immunosorbent assay (ELISA).21 A positive cutoff OD490 value of 0.20 for IgG and 0.10 for IgA was used for RBD on the basis of the published protocol and the mean of the negative control values plus 3 standard deviations from 20 blood samples collected between 2017 and 2019.
SARS-CoV-2-Specific IgM, IgG, and IgA Antibody Assay in Human Milk
We measured human milk IgM, IgG, and IgA antibodies using a modified ELISA.8,21 Briefly, human milk samples were thawed and centrifuged at 1000 G for 10 minutes twice to remove cells and fat. High binding 96-well plates (Corning) were coated at a concentration of 2 µg/mL of recombinant SARS-CoV-2 RBD protein (kindly provided by Aubree Gordon, PhD, University of Michigan) in phosphate-buffered saline (PBS) and incubated overnight at 4°C. After washing 3 times with PBS-1% Tween20 (PBS-T), the plates were incubated with 200 µL blocking solution consisting of PBS-T+ 0.5% milk powder + 3% goat serum) for 1 hour at room temperature. The plates were incubated with human milk samples at a dilution of 1:10 for 2 hours. After washing the plates, secondary antibodies for IgM, IgG, or IgA (Rockland) were added at a dilution of 1:3000 in PBS+1% bovine serum albumin. After incubation, 100 µl O-phenylenediamine dihydrochloride solution (Sigma-Aldrich) was added to each well, and the plates were incubated for 20 minutes before quenching with 50 µL of 3M HCl. Optical density (OD) values were measured at 490 nm immediately after adding HCl. Positive cutoff OD490 values of 0.14, 0.20, and 0.21 were determined for IgM, IgG, and IgA, respectively, on the basis of the mean of the negative control values plus 3 standard deviations from 20 archived human milk samples collected between 2017 to 2019. Negative controls were included with each run.
Neutralizing Activity in Human Milk
Human milk neutralization activity against SARS-CoV-2 was measured by using a commercially available surrogate virus neutralization test kit (sVNT, GenScript, New Jersey). The result from the sVNT was highly correlated with the 90% plaque reduction neutralization test titer assay (Pearson R = 0.84, P < .01).22 The protocol was modified to optimize testing for neutralizing antibodies in human milk. Briefly, undiluted human milk was mixed with an equal volume of horseradish peroxidase conjugated to the recombinant SARS-CoV-2 RBD protein in micro cluster tubes and incubated for 30 minutes at 37°C. Then, 100 μL of each mixture was added to each well on the angiotensin-converting enzyme-2-coated microtiter plate. After incubation for 15 minutes at 37°C, the plates were washed with wash solution 4 times. To each well, 100 μL of 3,3′,5,5′-tetramethylbenzidine solution was added, and the plate was incubated in the dark at 22 to 25°C for 15 minutes. The reaction was stopped by the addition of 50 μL stop solution, and the absorbance was read immediately at 450 nm in an ELISA microplate reader. The assay was performed in duplicate. A positive and negative control was included with each run. The percent inhibition of each sample was calculated as inhibition (%) = (1 - sample OD value/negative control OD value × 100). The cutoff value for positive neutralization was percent inhibition ≥25%, based on the mean percent inhibition of the negative control samples plus 3 standard deviations from 20 archived healthy individuals from 2017 to 2019.
Holder Pasteurization
Human milk samples were thawed and then pasteurized in an ACE HMP2070-40HC compact automated pasteurizer (Andover, UK) using the Holder pasteurization method at the University of California Health Milk Bank. Human milk samples in 2 mL aliquots were fixed inside water-filled 50 mL milk bottles. Dual validation of the pasteurizer water bath and milk bottle temperatures during the cycle confirmed reaching and maintaining at least 62.5°C for 30 minutes. After pasteurization, the human milk samples were cooled within 1 hour and refrozen to −20°C per standard milk bank procedures until antibody and neutralization assay testing. The assays were performed in duplicate.
Statistics
Nonparametric continuous variables were analyzed using Wilcoxon matched-pairs signed-rank tests. Correlations were computed using Pearson correlation coefficient. χ2 or Fisher’s exact test were used to compare proportions. Statistical analyses were performed using GraphPad Prism version 9 (San Diego, CA) and R Studio v4.0.3 (Boston, MA). All tests were 2-tailed with P < .05 considered significant.
Results
Participants
A total of 30 lactating or pregnant participants enrolled between December 2020 and August 2021 (Table 1). We collected a total of 94 human milk and 88 blood samples at prevaccination and 1, 3 and 6 months postvaccination; 6 participants provided only milk samples at the prevaccination time point. One participant had self-reported history of SARS-CoV-2 infection 6 months before enrollment. Two participants showed elevated SARS-CoV-2-specific IgG consistent with an unknown previous infection in their prevaccination serum sample. Of these, only 1 showed elevated IgG and IgA in human milk prevaccination. One additional individual had reverse transcription polymerase chain reaction (RT-PCR)–confirmed SARS-CoV-2 infection 15 days after the first COVID-19 vaccination dose. Samples from the participant with high baseline and the participant with postvaccination infection were excluded from the longitudinal statistical analyses. One participant who received Ad26.COV2.S (Janssen/Johnson & Johnson) was excluded to focus on recipients of COVID-19 mRNA vaccines.
TABLE 1.
Participant Characteristics at Enrollment
| Characteristics (n = 30) | n (%) or Mean (Range) |
|---|---|
| Age, mean (range), y | 34.9 (27.1–43.3) |
| Race, n (%) | |
| Asian | 10 (33.3) |
| White | 20 (66.7) |
| Ethnicity, n (%) | |
| Hispanic | 3 (10.0) |
| Non-Hispanic | 27 (20.0) |
| Highest level of education, n (%) | |
| College, Associate degree | 1 (3.3) |
| College, Bachelor’s degree | 14 (46.7) |
| Postgraduate degree | 15 (50.0) |
| Comorbid condition, n (%) | 8 (26.7) |
| Asthma | 3 (10.0) |
| Cardiovascular | 2 (6.7) |
| Renal | 1 (3.3) |
| Gestational diabetes with last pregnancy, resolved | 3 (10.0) |
| Other endocrine | 1 (3.3) |
| Obese (BMI >30) | 6 (20.0) |
| Pregnant at enrollment, n (%) | 3 (10.0) |
| Gestational age at delivery, mean (range), wk | 39 (34–41) |
| Infant sex, male, n (%) | 11 (36.7) |
| Exclusive breastfeeding, n (%) | 25 (83.3) |
| Previous SARS-CoV-2 infection, n (%) | 4 (13.3) |
| Vaccine Manufacturer, n (%) | |
| BNT162b2 (Pfizer-BioNTech) | 26 (86.7) |
| mRNA-1273 (Moderna) | 3 (10.0) |
| Ad26.COV2.S (Janssen/Johnson and Johnson) | 1 (3.3) |
Of the 27 participants included in the longitudinal analysis, 25 participants (92.6%) received BNT162b2 (Pfizer) vaccine, and 2 (7.4%) received mRNA-1273 (Moderna) vaccine. All received both doses of the mRNA vaccine to complete the primary series. Local and systemic adverse effects after first and second vaccine doses were similar to those reported in clinical trials (Supplemental Table 1).23,24 No severe adverse effects were reported.
Human Milk SARS-CoV-2-Specific IgM, IgG, and IgA
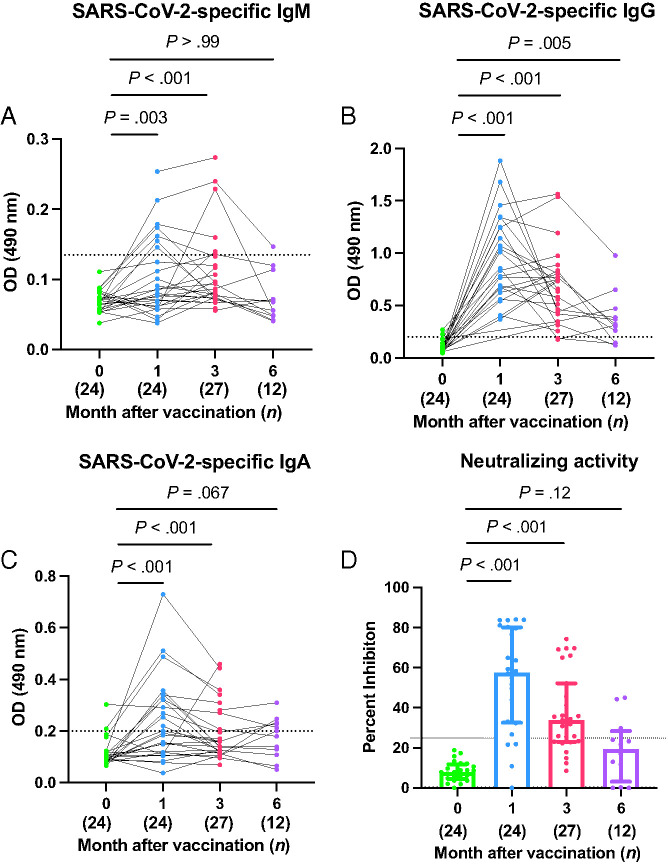
Only 7 of 24 (29.1%) and 6 of 27 (22.2%) mothers were positive for SARS-CoV-2-specific IgM at the 1- and 3-month time points, respectively (Table 2). However, the median IgM OD of prevaccination human milk significantly increased from 0.07 (IQR: 0.05–0.08) to 0.09 (IQR: 0.06–0.15) at 1 month and 0.09 (IQR: 0.07–0.13) at 3 months (P = .011 and P < .001, respectively) (Fig 1A). By 6 months postvaccination, SARS-CoV-2-specific IgM (0.06 [IQR: 0.05–0.10]) was not detectable in milk at significant levels above baseline (P > .99).
TABLE 2.
Breast Milk SARS-CoV-2 Specific Antibody and Neutralization Activity by Timepoint
| Prevaccination (n = 24), n (%) | Postvaccination, n (%) | ||||
|---|---|---|---|---|---|
| 1 mo (n = 24) | 3 mo (n = 27) | 6 mo (n = 12) | |||
| Pre-HP | Post-HP | ||||
| IgM positive | 0 (0) | 7 (29.1) | 3 (12.5) | 6 (22.2) | 1 (8.3) |
| IgG positive | 2 (8.3) | 24 (100) | 24 (100) | 25 (92.6) | 9 (75.0) |
| IgA positive | 1 (4.2) | 12 (50.0) | 9 (37.5) | 7 (25.9) | 5 (41.7) |
| Neutralization activity | 0 (0) | 20 (83.3) | 22 (91.7) | 19 (70.4) | 3 (25.0) |
Positive activity is defined as result above the cutoff value determined from negative controls obtained before the COVID-19 pandemic. HP, Holder pasteurization.
FIGURE 1.
Human milk SARS-CoV-2-specific IgM, IgG and IgA levels. Optical density (OD) levels of SARS-CoV-2-specific IgM, IgG, and IgA of prevaccinated human milk were compared 1, 3, and 6 months after vaccination (A, B, and C, respectively). The dotted lines indicate the positive cutoff OD490 values of 0.14, 0.20, and 0.21 for IgM, IgG, and IgA, respectively. Neutralizing activities in prevaccinated human milk was compared at 1, 3, and 6 months after the first dose of vaccination (D). The dotted line indicates the positive cutoff of 25% neutralizing activity. Wilcoxon matched-pairs signed-rank tests were used for statistical analysis. Error bars indicate interquartile range.
All human milk samples (24 of 24 [100%]) collected at 1 month and the majority collected at 3 months (25 of 27 [92.6%]) and 6 months (9 of 12 [75.0%]) were above the positive cutoff value for SARS-CoV-2-specific IgG (Table 2 and Fig 1B). The median OD value for SARS-CoV-2-specific IgG significantly increased from 0.08 (IQR: 0.06–0.12) prevaccination to 0.84 (IQR: 0.63–1.25) at the 1-month postvaccination time point (P < .001). At 3 months postvaccination, the median SARS-CoV-2-specific IgG remained significantly higher, at 0.66 (IQR: 0.42–0.81), compared to the prevaccination time point (P < .001). IgG level decreased at the 6-month time point (0.32 [IQR: 0.17–0.45]) but remained significantly higher than prevaccination IgG level (P = .005).
Only 12 lactating mothers of 24 (50%) at 1 month and 7 of 27 (25.9%) at 3 months were positive for SARS-CoV-2-specific IgA (Table 2 and Fig 1C). The median OD for SARS-CoV-2-specific IgA significantly increased from prevaccination (0.10 [IQR: 0.08– 0.12]) to 1 month (0.21 [IQR: 0.13–0.34]) and 3 months (0.14 [IQR: 0.12–0.27]) postvaccination (both P < .001). SARS-CoV-2-specific IgA levels were indistinguishable from baseline levels of 0.19 (IQR: 0.11–0.23) at 6 months (P = .067).
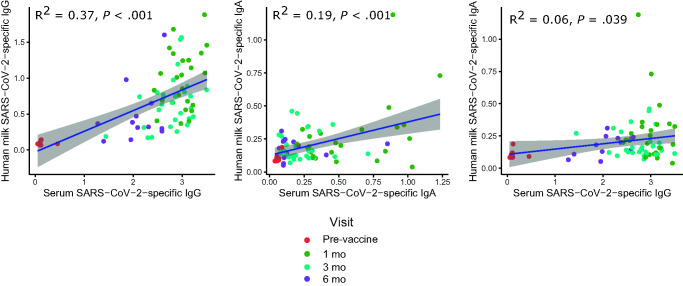
The levels of SARS-CoV-2-specific IgG and IgA in human milk positively correlated with SARS-CoV-2-specific IgG and IgA in blood collected at the same time point (R2 = 0.37, P < .001 and R2 = 0.19, P < .001, respectively) (Fig 2). The level of SARS-CoV-2-specific IgA in human milk had a small but significant correlation with the level of SARS-CoV-2-specific IgG in blood (R2 = 0.06, P = .039). At 6 months, serum SARS-CoV-2-specific IgG remained above the positive cutoff in all 18 participants who had reached the 6-month follow-up, including the 12 who donated human milk (median OD 2.02 [IQR: 1.51–2.36]); 6 had stopped lactating.
FIGURE 2.
Correlation of human milk SARS-CoV-2-specific IgG and IgA compared to serum IgG at the same time point (n = 75). The colors represent different visit time points. SARS-CoV-2-specific IgG and IgA in human milk showed positive correlations with the same isotypes in blood. The level of SARS-CoV-2-specific IgA in human milk showed a small but significant correlation with the level of SARS-CoV-2-specific IgG in blood. Correlations were analyzed using Pearson correlation coefficient.
Neutralizing Activity in Human Milk
Most human milk samples (20 of 24 [83.3%]) showed neutralizing capacity at 1 month; two-thirds (19 of 27 [70.4%]) displayed neutralization activity at 3 months, but only one-quarter (3 of 12 [25.0%]) retained activity at 6 months (Table 2 and Fig 1D). Human milk showed a median neutralizing activity of 57.7% (IQR: 32.6%–80.13%) inhibition at 1 month postvaccination, significantly increased from prevaccination samples (7.9% [IQR: 4.8%–12.1%], P < .001). After 3 and 6 months postvaccination, percent inhibition decreased to 33.8% (IQR: 23.0%–52.2%) and 19.4% (IQR: 3.2%–28.4%), remaining significantly higher than prevaccination inhibition levels at 3 months but not 6 months psotvaccination (P < .001 and P = .12, respectively).
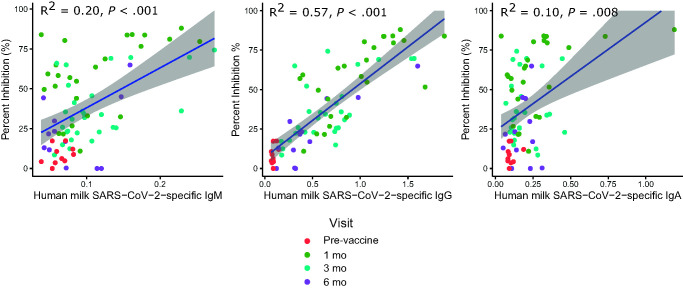
SARS-CoV-2-specific IgG showed the strongest correlation with neutralizing activity in milk (R2 = 0.57, P < .001) (Fig 3). The level of SARS-CoV-2-specific IgM and IgA also showed small but statistically significant positive correlations with neutralizing activity (R2 = 0.20, P < .001 and R2 = 0.10, P < .008, respectively).
FIGURE 3.
SARS-CoV-2 neutralization activity of human milk correlate with SARS-CoV-2-specific IgM, IgG and IgA (n = 86). The colors represent different visit time points. SARS-CoV-2-specific IgG showed the strongest correlation with neutralizing activity in milk. The level of SARS-CoV-2-specific IgM and IgA also showed small but statistically significant positive correlations. Correlations were analyzed by using Pearson correlation coefficient.
Human Milk Pasteurization
We tested for SARS-CoV-2-specific antibody levels and neutralizing activity in human milk before and after Holder pasteurization in 24 samples at 1-month postvaccination. After pasteurization, 100% milk samples retained SARS-CoV-2-specific IgG above the cutoff. However, fewer samples retained IgM and IgA above cutoff values (Table 2). Evaluation of OD levels showed no statistical difference between pre and postpasteurization human milk samples in SARS-CoV-2-specific IgG (0.84 [IQR: 0.63–1.25] vs 1.07 [IQR: 0.60–1.42], P = .36) (Fig 4). SARS-CoV-2-specific IgM (0.09 [IQR: 0.06–0.15] vs 0.06 [IQR: 0.05–0.08], P = .004) and IgA (0.21 [IQR: 0.13–0.34] vs 0.18 [IQR: 0.10–0.28], P = .043) significantly dropped from pre to postpasteurization. After pasteurization, the median neutralizing activity was 58.7% inhibition (IQR: 34.3–76.0), which was not significantly changed compared to before pasteurization (57.7% [IQR: 32.5–80.1], P = .27).
FIGURE 4.
Human milk SARS-CoV-2-specific antibody levels and neutralizing activity persist after Holder pasteurization (n = 24). The median level of SARS-CoV-2-specific IgM, IgG, and IgA in human milk collected 1 month after vaccination were compared pre- and postpasteurization (A, B, and C, respectively). Neutralization activity was compared pre- and postpasteurization (D). Wilcoxon matched-pairs signed-rank tests were used for statistical analysis. Error bars indicate interquartile range.
Discussion
COVID-19 vaccination induced SARS-CoV-2-specific antibodies in human milk that peaked at 1 month and were durable for at least 6 months. We found that the most abundant isotype for SARS-CoV-2-specific antibodies in milk was IgG. Although IgG levels decreased from the 1-month postvaccination peak, SARS-CoV-2-specific IgG persisted above prevaccination levels at the half-year mark, and 75% of milk tested remained positive at 6 months. Compared with the prevaccination baseline, levels of SARS-CoV-2-specific IgA in human milk increased through 3 months postvaccination. However, IgA levels were lower than IgG and increased above the positive cutoff in only one-half of the study participants, even at the 1-month peak. Most participants showed neutralizing activity against SARS-CoV-2 in human milk at 1 month which remained significantly higher than the prevaccination baseline through 3 months. There was significant interindividual variability as neutralizing activity persisted through 6 months in human milk in only one-quarter of the participants. Neutralizing activity against SARS-CoV-2 was correlated with both IgG and IgA but showed the strongest correlation with spike-specific IgG levels in the human milk.
This study adds to the current published literature that has reported early presence of SARS-CoV-2-specific antibodies in human milk through 6 weeks after COVID-19 mRNA vaccination and a small study that showed waning antibodies by 4 months after an inactivated SARS-CoV-2 vaccine.13–17 In one report, all prenatally vaccinated mothers showed SARS-CoV-2-specific antibody presence in umbilical cord blood and human milk samples at higher titers than those who experienced a SARS-CoV-2 infection during pregnancy.6 Of antibodies analyzed in human milk postvaccination, it has been observed that IgA and IgG are the main isotypes25–27 and IgG has been described as the dominant isotype,14,28 which is consistent with our findings. Antibodies against SARS-CoV-2 found in the milk of women after COVID-19 resolution showed strong neutralizing activity postinfection in one study,10 similar to our findings postvaccination. Yet, others have found no correlation between neutralizing capacity and levels of IgA isotype antibodies in human milk.20
In our study, we also aimed to elucidate the effects of pasteurization on the immunologic profile of human milk, particularly because donated human milk is an important source of nutrition for preterm infants and other infants in the ICU. It is worth noting that the presence of immunologic components in pasteurized donor milk, and particularly neutralization activity, may represent a significant benefit to infants who may be at increased risk for respiratory infections. A previous study investigating Holder pasteurization of human milk from previously SARS-CoV-2 infected mothers found a significant decrease in SARS-CoV-2-specific secretory IgA postpasteurization, although there was no significant decrease in the total (nonspecific) amount of IgA postpasteurization.20 Other studies have found decreases in all antibody isotypes with Holder pasteurization, with varying susceptibility by isotype, and with IgM being the most thermosensitive isotype.19 Our study shows that vaccine-induced IgG in human milk persisted and retained neutralizing activity against SARS-CoV-2 after pasteurization. However, both IgM and IgA levels decreased significantly in the pasteurization process.
The study findings are promising but must be interpreted with caution. First, there is no known correlation of protection against SARS-CoV-2 infection at the present time.29 We used the human milk from 20 mothers collected before the initial SARS-CoV-2 outbreak in December 2019 to establish a baseline and positive cutoff value for our antibody detection assay. Although this optimizes the interpretation, we cannot be certain that this represents a protective cutoff value. The assays were set up and optimized as qualitative tests using negative controls but without a standard positive titration with each run; thus, quantitative comparison of the antibody levels from assay to assay is not strictly possible, and these results must be interpreted cautiously. Second, it is unclear whether neutralizing IgG is biologically active in consumed human milk to the same extent as IgA. IgA in milk has been previously described for its ability to provide protection at mucosal barriers.30 The prevailing theory is that human milk consumed by neonates is regurgitated in the airways, coating the infant’s respiratory path with protective IgA.31 It is unclear whether IgG can provide protection in the same way, or whether degradation of IgG due to the digestive environment of the stomach, which has been noted to be ∼48%, reduces protection.32 The digestive effect of the gastric environment has been noted for IgA and IgM isotypes, although these isotypes diminished by lower levels, with IgM showing no significant decreases between infant gastric and nongastric human milk samples.32 Additional studies have detected SARS-CoV-2-specific IgG in saliva samples of infants postbreastfeeding from vaccinated mothers, but no similar antibodies were detected in the same infant serum samples.18 Third, our assay measured total SARS-CoV-2-specific IgA and did not distinguish IgA from dimeric secretory IgA. Previous studies conducted in populations of naturally infected mothers by SARS-CoV-2 found that the majority of IgA in human milk is presented in its secretory form and have shown high correlation between IgA and secretory antibody.8
The SARS-CoV-2-specific antibodies, especially the IgG subtype, in human milk induced by COVID-19 mRNA vaccination persists for at least 6 months, and neutralization persists for at least 3 months. Human milk pasteurization does not impact IgG levels or neutralization activity. This data reinforces evidence for breastfeeding recommendations in mothers after vaccination and helps inform milk bank policies concerning donations from vaccinated women, because milk-delivered antibodies could offer human milk-fed children protection against SARS-CoV-2 infection.
Supplementary Material
Acknowledgments
We thank the study mothers for their enthusiastic participation and Lauren Turner, Shirley Mendieta, Jaycee Jumarang, and Jennifer Del Valle for their recruitment and data collection contributions.
Glossary
- CDC
Centers for Disease Control and Prevention
- COVID-19
coronavirus disease 2019
- ELISA
enzyme-linked immunosorbent assay
- Ig
Immunoglobulins
- IQR
interquartile range
- mRNA
messenger RNA
- OD
optical density
- PBS
phosphate-buffered saline
- PBS-T
PBS-1%Tween20
- RBD
receptor binding domain
- SARS-CoV-2
severe acute respiratory syndrome coronavirus 2
- sVNT
surrogate virus neutralization test
Footnotes
Ms Perez and Mr Luna Centeno collected data and drafted the initial manuscript; Mr Cheng and Dr Marentes Ruiz designed the data collection instruments and collected data; Dr Lee analyzed the data and reviewed and revised the manuscript; Mr Congrave-Wilson and Dr Stellwagen collected data and reviewed and revised the manuscript; Dr Powell helped design analysis methods and reviewed and revised the manuscript; Dr Pannaraj conceptualized and designed the study, supervised the data collection and analysis, and reviewed and revised the manuscript for important intellectual content; and all authors approved the final manuscript as submitted and agree to be accountable for all aspects of the work.
FUNDING: This work was partially supported by the National Institute of Allergy and Infectious Diseases grant U01AI144616-02S1. Funded by the National Institutes of Health (NIH).
References
- 1. Centers for Disease Control and Prevention . COVID-19: Pregnancy or Breastfeeding. 2021. Available at: https://www.cdc.gov/coronavirus/2019-ncov/vaccines/recommendations/pregnancy.html. Updated August 11, 2021. Accessed August 20, 2021
- 2. Stafford IA, Parchem JG, Sibai BM. The coronavirus disease 2019 vaccine in pregnancy: risks, benefits, and recommendations. Am J Obstet Gynecol. 2021;224(5):484–495 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Costantine MM, Landon MB, Saade GR. Protection by exclusion: another missed opportunity to include pregnant women in research during the coronavirus disease 2019 (COVID-19) pandemic. Obstet Gynecol. 2020;136(1):26–28 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Pierce-Williams RAM, Burd J, Felder L, et al. Clinical course of severe and critical coronavirus disease 2019 in hospitalized pregnancies: A United States cohort study. Am J Obstet Gynecol MFM. 2020;2(3):100134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Golan Y, Prahl M, Cassidy A, et al. Evaluation of messenger RNA from COVID-19 BTN162b2 and mRNA-1273 vaccines in human milk. JAMA Pediatr. 2021;175(10):1069–1071 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Gray KJ, Bordt EA, Atyeo C, et al. Coronavirus disease 2019 vaccine response in pregnant and lactating women: a cohort study. Am J Obstet Gynecol. 2021;225(3):303.e1–303.e17 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Sutton D, D’Alton M, Zhang Y, et al. COVID-19 vaccine acceptance among pregnant, breastfeeding, and nonpregnant reproductive-aged women. Am J Obstet Gynecol MFM. 2021;3(5):100403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Fox A, Marino J, Amanat F, et al. Robust and specific secretory IgA against SARS-CoV-2 detected in human milk. iScience. 2020;23(11):101735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Chambers C, Krogstad P, Bertrand K, et al. Evaluation for SARS-CoV-2 in breast milk from 18 infected women. JAMA. 2020;324(13):1347–1348 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Pace RM, Williams JE, Järvinen KM, et al. Characterization of SARS-CoV-2 RNA, Antibodies, and Neutralizing Capacity in Milk Produced by Women with COVID-19. MBio. 2021;12(1):e03192-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Tam PCK, Ly KM, Kernich ML, et al. Detectable severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) in human breast milk of a mildly symptomatic patient with coronavirus disease 2019 (COVID-19). Clin Infect Dis. 2021;72(1):128–130 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Dong Y, Chi X, Hai H, et al. Antibodies in the breast milk of a maternal woman with COVID-19. Emerg Microbes Infect. 2020;9(1):1467–1469 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Perl SH, Uzan-Yulzari A, Klainer H, et al. SARS-CoV-2-specific antibodies in breast milk after COVID-19 vaccination of breastfeeding women. JAMA. 2021;325(19):2013–2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Gray KJ, Bordt EA, Atyeo C, et al. Coronavirus disease 2019 vaccine response in pregnant and lactating women: a cohort study. Am J Obstet Gynecol. 2021;225(3):303.e1–303.e17 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Esteve-Palau E, Gonzalez-Cuevas A, Guerrero ME, et al. Quantification of specific antibodies against SARS-CoV-2 in breast milk of lactating women vaccinated with an mRNA vaccine. JAMA Netw Open. 2021;4(8):e2120575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Jakuszko K, Kościelska-Kasprzak K, Żabińska M, et al. Immune response to vaccination against COVID-19 in breastfeeding health workers. Vaccines (Basel). 2021;9(6):663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Calil VMLT, Palmeira P, Zheng Y, Krebs VLJ, Carvalho WB, Carneiro-Sampaio M. CoronaVac can induce the production of anti-SARS-CoV-2 IgA antibodies in human milk. Clinics (São Paulo). 2021;76:e3185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Schwartz A, Nir O, Toussia-Cohen S, et al. Presence of SARS-CoV-2 antibodies in lactating women and their infants following BNT162b2 messenger RNA vaccine. Am J Obstet Gynecol. 2021;225(5):577–579 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Rodríguez-Camejo C, Puyol A, Fazio L, et al. Impact of holder pasteurization on immunological properties of human breast milk over the first year of lactation. Pediatr Res. 2020;87(1):32–41 [DOI] [PubMed] [Google Scholar]
- 20. van Keulen BJ, Romijn M, Bondt A, et al. Human milk from previously COVID-19-infected mothers: the effect of pasteurization on specific antibodies and neutralization capacity. Nutrients. 2021;13(5):1645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Stadlbauer D, Amanat F, Chromikova V, et al. SARS-CoV-2 seroconversion in humans: a detailed protocol for a serological assay, antigen production, and test setup. Curr Protoc Microbiol. 2020;57(1):e100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Perera RAPM, Ko R, Tsang OTY, et al. Evaluation of a SARS-CoV-2 surrogate virus neutralization test for detection of antibody in human, canine, cat, and hamster sera. J Clin Microbiol. 2021;59(2):e02504-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Baden LR, El Sahly HM, Essink B, et al. Efficacy and safety of the mRNA-1273 SARS-CoV-2 vaccine. N Engl J Med. 2021;384(5):403–416 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Polack FP, Thomas SJ, Kitchin N, et al. ; C4591001 Clinical Trial Group . Safety and efficacy of the BNT162b2 mRNA Covid-19 vaccine. N Engl J Med. 2020;383(27):2603–2615 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Low JM, Gu Y, Ng MSF, et al. Codominant IgG and IgA expression with minimal vaccine mRNA in milk of BNT162b2 vaccinees. NPJ Vaccines. 2021;6(1):105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Juncker HG, Mulleners SJ, van Gils MJ, et al. The levels of SARS-CoV-2 specific antibodies in human milk following vaccination. J Hum Lact. 2021;37(3):477–484 [DOI] [PubMed] [Google Scholar]
- 27. Friedman MR, Kigel A, Bahar Y, et al. BNT162b2 COVID-19 mRNA vaccine elicits a rapid and synchronized antibody response in blood and milk of breastfeeding women. medRxiv. 2021:2021.2003.2006.21252603 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Fox A, Norris C, Amanat F, Zolla-Pazner S, Powell RL. The vaccine-elicited immunoglobulin profile in milk after COVID-19 mRNA-based vaccination is IgG-dominant and lacks secretory antibodies. medRxiv. 2021:2021.2003. 2022.21253831 [Google Scholar]
- 29. Krammer F. A correlate of protection for SARS-CoV-2 vaccines is urgently needed. Nat Med. 2021;27(7):1147–1148 [DOI] [PubMed] [Google Scholar]
- 30. Hanson LA. Breastfeeding provides passive and likely long-lasting active immunity. Ann Allergy Asthma Immunol. 1998;81(6):523–533; quiz 533–524, 537 [DOI] [PubMed] [Google Scholar]
- 31. Vassilopoulou E, Feketea G, Koumbi L, Mesiari C, Berghea EC, Konstantinou GN. Breastfeeding and COVID-19: from nutrition to immunity. Front Immunol. 2021;12(946):661806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Demers-Mathieu V, Underwood MA, Beverly RL, Nielsen SD, Dallas DC. Comparison of human milk immunoglobulin survival during gastric digestion between preterm and term infants. Nutrients. 2018;10(5):E631. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.