Abstract
Background
Survivors of pediatric medulloblastoma experience long-term morbidity associated with the toxic effects of postoperative radiotherapy (RT). Proton RT limits radiation dose to normal tissues thereby reducing side effects of treatment while maintaining high cure rates. However, long-term data on disease outcomes and long-term effects of proton RT remain limited.
Methods
One hundred seventy-eight pediatric medulloblastoma patients treated with proton RT between 2002 and 2016 at the Massachusetts General Hospital comprise the cohort of patients who were treated with surgery, radiation therapy, and chemotherapy. We evaluated event-free survival (EFS), overall survival (OS), and local control using the Kaplan-Meier method. The cumulative incidence of brainstem injury and secondary malignancies was assessed.
Results
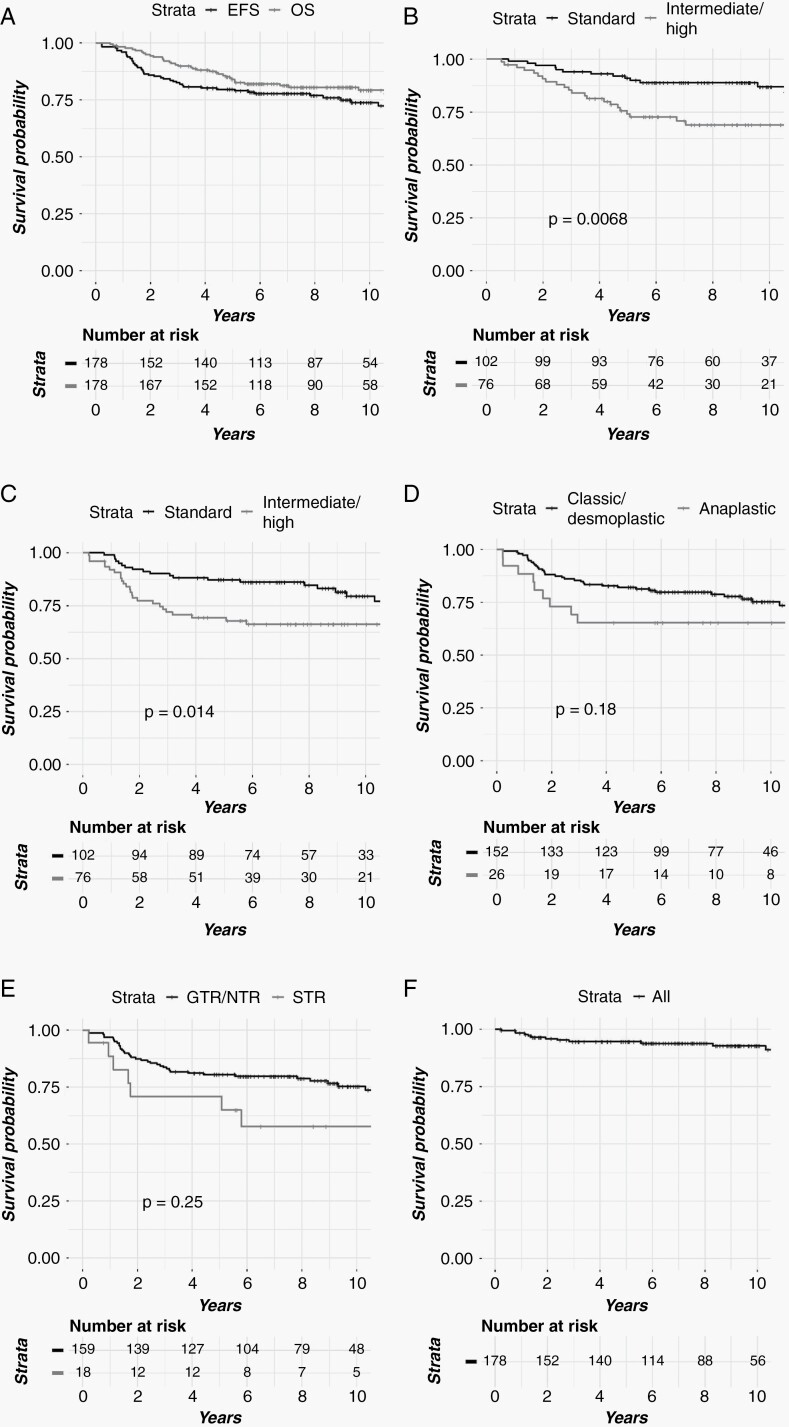
Median follow-up was 9.3 years. One hundred fifty-nine patients (89.3%) underwent a gross total resection (GTR). The 10-year OS for the entire cohort, standard-risk (SR), and intermediate/high-risk (IR/HR) patients was 79.3%, 86.9%, and 68.9%, respectively. The 10-year EFS for the entire cohort, SR, and IR/HR cohorts was 73.8%, 79.5%, and 66.2%. The 10-year EFS and OS for patients with GTR/NTR were 75.3% and 81.0% vs 57.7% and 61.0% for subtotal resection (STR). On univariate analysis, IR/HR status was associated with inferior EFS, while both anaplastic histology and IR/HR status were associated with worse OS. The 10-year cumulative incidence of secondary tumors and brainstem injury was 5.6% and 2.1%, respectively.
Conclusions
In this cohort study of pediatric medulloblastoma, proton RT was effective, and disease outcomes were comparable to historically treated photon cohorts. The incidence of secondary malignancies and brainstem injury was low in this cohort with mature follow-up.
Keywords: pediatric medulloblastoma, proton, radiation
Key Points.
Proton radiotherapy for pediatric medulloblastoma is a safe and effective treatment modality.
Disease outcomes are comparable with those previously reported from photon-treated patients.
The rate of brainstem injury and secondary malignancies was low.
Importance of the Study.
Here we report on a large cohort of pediatric medulloblastoma patients treated with proton radiotherapy at a mature median follow-up time of 9.3 years. We demonstrate favorable disease outcomes comparable to reported photon-treated cohorts. In addition, patterns of relapse were similar to that reported in photon-treated cohorts, with distant failure being the most common form of relapse. Intermediate/high-risk status was associated with inferior EFS, while both anaplastic histology and intermediate/high-risk status were associated with worse OS. There was a low incidence of secondary tumors (5.6%) and brainstem injury (2.1%) at 10 years. The rate of second malignant tumors (2.1%) at 10 years was lower than that reported previously in photon-treated patients. The excellent disease and toxicity outcomes reported in this prospective trial provide a strong rationale to utilize proton radiotherapy as an optimal treatment option in pediatric medulloblastoma.
Medulloblastoma is the most common embryonal tumor of childhood and arises in the posterior fossa (PF). Optimal management in children ≥3 years consists of a combination of surgical resection, cranio-spinal irradiation (CSI) with involved field (IF) boost, and chemotherapy. With this approach, 5-year OS is over 80% in patients with standard-risk disease.1 While survival outcomes have significantly improved, pediatric brain tumor survivors continue to experience long-term effects of their therapy, including cardiac, pulmonary, endocrine, thyroid, and secondary malignancies that contribute to long-term mortality.2–5 Proton radiotherapy (RT), by virtue of its minimal exit dose, limits dose to normal tissues and therefore may result in a reduction in the incidence and severity of late effects. The use of proton RT for CSI has been shown to reduce dose to the cochlea, and eliminate dose to the thorax, abdomen, and pelvis.6 In addition, preliminary results from ACNS 0331 have challenged the traditional paradigm of the PF boost, in lieu of an IF approach which may further reduce toxicity for patients with standard-risk disease. Few studies to date have reported on long-term disease and toxicity outcomes in pediatric patients treated with proton RT for medulloblastoma. In this study, we aimed to assess long-term disease and toxicity outcomes in a cohort of patients treated with proton RT for pediatric medulloblastoma.
Materials and Methods
Patient Selection
All pediatric patients in this cohort were treated at the Massachusetts General Hospital (MGH) and prospectively enrolled in two clinical trials or a prospective pediatric radiation registry, and a small number of patients were identified from a departmental pediatric radiation oncology database. One hundred thirty patients (73.0%) were treated on two prospective clinical trials (NCT00105560 and NCT01063114), an additional 34 patients (19.1%) are prospectively followed on our consented Pediatric Proton/Photon Consortium Registry (PPCR) (NCT01696721), and the remaining patients 14 patients (7.9%) were identified from our internal pediatric radiation database.
This study was approved by the Mass General Brigham institutional review board. All tumor pathology was locally reviewed. All patients with histologically confirmed standard-risk (SR), intermediate-risk (IR), or high-risk (HR) medulloblastoma treated with proton RT at the MGH between 2002 and 2016 were included in the study. All but one patient had an attempt at surgical resection of their primary tumor followed by concurrent and/or adjuvant chemotherapy and proton RT. Patients underwent an MRI of the brain before and after surgical resection and were staged with lumbar cerebrospinal fluid (CSF) cytology.
Patients were categorized as either SR, IR, or HR. SR was defined as patients who had a gross total resection (GTR) or minimal volume of residual disease (≤1.5 cm2) and no evidence of metastatic disease in the brain, CSF, or spine. IR was defined as patients with minimal volume residual disease, without metastatic disease, and with diffuse or large cell or anaplastic histology. All other patients were considered high risk.
Radiation Planning and Chemotherapy
RT typically started within 35 days of surgical resection and was delivered with either passively scattered or pencil beam scanning (PBS) proton RT. A radiation planning CT simulation scan with 2.5 mm slices in either the prone or supine position was performed from the top of the head through the bottom of the pelvis. For the CSI phase of treatment, the clinical target volume (CTV) included the entire subarachnoid space, the nerve roots, and the entire vertebral body for skeletally immature patients as previously described by our group.7 The RT dose was prescribed in gray relative biological equivalents (GyRBE) using an RBE value of 1.1. For patients with SR disease, the CSI RT dose was typically 23.4 GyRBE (18.0-34.2) at 1.8 GyRBE per fraction. A minority of patients received 18 GyRBE treated on (or per) the ACNS 0331 protocol testing the lower CSI dose in younger patients. HR patients typically received 36 GyRBE of CSI, but IR patients received CSI doses between 23.4 GyRBE and 36 GyRBE depending on age, family preference, and chemotherapy dose intensity. The boost was delivered either with a whole PF or an IF boost to 54-55.8 GyRBE and in accordance with contemporary Children’s Oncology Group (COG) guidelines for target delineation and coverage.
All patients in our cohort were treated with chemotherapy. The most common chemotherapy regimens were dictated by the COG SR and HR protocols (ACNS 0331 and 0332), but 11% of the patients were treated with pre-RT chemotherapy regimens including high-dose chemotherapy on the Head Start or COG infant protocols. After completion of RT, patients were followed in accordance with the recommendations set forth in the COG protocols, which includes MRI of the brain and spine every 3 months for the first year, every 6 months for years 1-3, and then annually after 3 years. Most patients underwent routine post-treatment follow-up at their home institution at the end of treatment. Physician notes and MRI were reviewed by the treating radiation oncologist, although in cases of concern for disease relapse or brainstem injury, the scans were centrally reviewed in our multidisciplinary pediatric CNS tumor board by a neuro-radiologist.
Outcomes
The variables analyzed in this study include age, sex, histological subtype, extent of resection (EOR), proton treatment modality, CSI dose, disease stage, total RT dose, surgery to RT interval, and radiation treatment time. The disease control endpoints evaluated were overall survival (OS), event-free survival (EFS), and location of failure (PF vs brain and/or spine). OS was defined as the time from the start of RT to the time of death from any cause and was censored at the date of last follow-up. EFS was defined as the time from the start of RT to either death, progression of disease, or development of a secondary tumor and was censored at the time of last follow-up. Secondary tumors were defined as any benign or malignant neoplasm in the irradiated field and secondary malignancies were only included for patients with second malignant tumors. We recorded local failure (LF), defined as a recurrence in the tumor bed or within the PF, and distant failure (DF), defined as recurrence elsewhere in the supratentorial brain, within the CSF, or spine.
Statistical Analysis
Patient characteristics were summarized using descriptive statistics and displayed as percentages or ranges and medians as appropriate. Rates of OS, EFS, and local control were estimated using the Kaplan-Meier method, and the effect of patient characteristics and treatment factors on patient outcomes were evaluated using the log-rank test. Hazard ratios were estimated through univariate analyses using a Cox proportional hazards model or the Fine-Gray model for competing risks. The cumulative incidence and rates of LF were calculated using death and DF as competing risks. For analyses, the IR and HR groups were analyzed together given the relatively low number of patients classified as intermediate risk. The cumulative incidence of secondary tumors, secondary malignancies, secondary benign tumors, and brainstem necrosis were calculated using death as a competing risk. Median follow-up time was calculated using the reverse Kaplan-Meier method. All P-values are reported on 2-sided tests. All analyses were performed using R Statistical Package v3.5.
Results
Patient Characteristics
One hundred seventy-eight proton-treated medulloblastoma patients comprise the study cohort. Of these, 102 (57.3%) were SR, and 76 were IR or HR (42.7%). The median follow-up was 9.3 years for the entire cohort (range 0.5-17.2). The patient demographic, tumor, and treatment-related characteristics are reported in Table 1. Patients were predominantly treated with a passive scatter approach (95.5%).
Table 1.
Clinical Patient Characteristics of Pediatric Medulloblastoma Patients Treated with Proton Radiotherapy
| Characteristics | N = 178 |
|---|---|
| Median follow-up (y) | 9.3 (0.5–17.2) |
| Age at RT (y) | 8.1 (2.5–24.1) |
| ≤8 Y | 88 (49.4) |
| >8 Y | 90 (50.6) |
| Gender | |
| Male | 103 (57.9) |
| Female | 75 (42.1) |
| Histological subtype | |
| Classic and or desmoplastic | 152 (85.4) |
| Anaplastic or large cell variant | 26 (14.6) |
| Extent of resection | |
| GTR/near-GTR | 159 (89.3) |
| STR | 18 (10.1) |
| Biopsy only | 1 (0.6) |
| Risk | |
| Standard risk | 102 (57.3) |
| Intermediate | 16 (9.0) |
| High risk | 60 (33.7) |
| Chemotherapy | |
| No | 0 (0) |
| Yes | 178 (100.0) |
| Chemo timing | |
| Prior | 29 (16.3) |
| During | 139 (78.1) |
| After | 73 (41.0) |
| Modality | |
| PBS | 7 (3.9) |
| Passive scatter | 170 (95.5) |
| Both | 1 (0.6) |
| Craniospinal radiation dose (GyRBE)a | 23.4 (18.0–36.0) |
| 18.0 to <23.4 | 8 (4.6) |
| 23.4 | 107 (61.1) |
| >23.4 to <36.0 | 13 (7.4) |
| 36.0 | 47 (26.9) |
| Stage | |
| M0 | 131 (73.6) |
| M1 | 5 (2.8) |
| M2 | 16 (9.0) |
| M3 | 26 (14.6) |
| Total dose | 54 (50.4–59.4) |
| ≤ 54 | 170 (95.5) |
| > 54 | 8 (4.5) |
| Surgery to RT interval (days) | 32.0 (18.0–662.0) |
| ≤ 35 Days | 97 (54.5) |
| >35 Days | 81 (45.5) |
| Median radiation treatment time in days (range) | 42.0 (21.0–51.0) |
| ≤ 45 Days | 151 (84.8) |
| >45 Days | 27 (15.2) |
Abbreviations: CSI, cranio-spinal irradiation; GTR, gross total resection; GyRBE, gray relative biological equivalents; PBS, pencil beam scanning; RT, radiotherapy; STR, subtotal resection.
aExcludes 3 who did not receive CSI.
Treatment Outcomes
At last follow-up, 36 patients had died and 38 patients had progressed. The 10-year OS for the entire cohort, SR, and IR/HR patients was 79.3% (95% CI: 73.1-85.9), 86.9% (95% CI: 79.9-94.4), and 68.9% (95% CI: 58.7-80.8), respectively (Figure 1A and B). The 10-year EFS for the entire cohort, SR and IR/HR cohorts was 73.8%, (95% CI: 67.1-81.1) and 79.5% (95% CI: 71.1-88.9) vs 66.2% (95% CI: 56.3-78.0) (Figure 1C). The 10-year EFS and OS for patients with GTR/NTR (near total resection) were 75.3% (95% CI: 68.3-83.0) and 81.0% (95% CI: 74.7-87.8) vs 57.7% (95% CI: 38.0-87.7) and 61.0% (95% CI: 40.3-92.2) for subtotal resection (STR) (Figure 1E). On univariate analysis, IR/HR status was associated with inferior EFS (Table 2; hazard ratio: 2.1 (1.1-3.7), P = .014) and OS (Table 3; hazard ratio: 2.5 (95% CI: 1.3-4.8), P = .0068), while anaplastic histology was associated with inferior OS (Table 3; hazard ratio: 2.3 (95% CI: 1.1-4.8), P = .030) (Table 3).
Fig. 1.
Clinical outcomes by (A) EFS and OS. (B) OS by risk. (C) EFS by risk. (D) EFS by histology. (E) EFS by extent of resection. (F) LC. Abbreviations: EFS, event-free survival; LC, local control; OS, overall survival.
Table 2.
Univariate Analysis of Clinical and Pathological Variables Associated With Event-Free Survival
| Variable | N | Events | Event-Free survival (%) | |||
|---|---|---|---|---|---|---|
| Years | HR | P | ||||
| 5 | 10 | |||||
| Age at RT | ||||||
| ≤8 Y | 88 | 27 | 77.0 (68.7–86.4) | 70.7 (61.3–81.6) | ||
| >8 Y | 90 | 19 | 82.2 (74.7–90.5) | 77.2 (68.3–87.2) | 0.7 (0.4–1.3) | .22 |
| Histological subtype | ||||||
| Classic/desmoplastic | 152 | 37 | 82.1 (76.2–88.4) | 75.2 (68.1–83.2) | ||
| Anaplastic | 26 | 9 | 65.4 (49.4–86.5) | 65.4 (49.4–86.5) | 1.6 (0.8–3.4) | .18 |
| Sex | ||||||
| Male | 103 | 32 | 76.7 (69.0–85.3) | 69.2 (60.0–79.7) | ||
| Female | 75 | 14 | 83.7 (75.7–92.6) | 80.1 (71.2–90.2) | 0.6 (0.3–1.1) | .072 |
| Extent of resectiona | ||||||
| GTR/NTR | 159 | 39 | 80.5 (74.5–86.9) | 75.3 (68.3–83.0) | ||
| STR | 18 | 7 | 70.8 (52.3–96.0) | 57.7 (38.0–87.7) | 1.9 (0.8–4.2) | .12 |
| RT duration (days) | ||||||
| ≤45 | 151 | 40 | 80.0 (73.8–86.6) | 72.8 (65.3–81.1) | ||
| >45 | 27 | 6 | 77.8 (63.6–95.2) | 77.8 (63.6–95.2) | 0.8 (0.3–1.8) | .54 |
| Risk | ||||||
| Standard | 102 | 20 | 87.2 (81.0–94.0) | 79.5 (71.1–88.9) | ||
| Intermediate/High | 76 | 26 | 69.3 (59.7–80.6) | 66.2 (56.3–78.0) | 2.1 (1.1–3.7) | .014 |
Abbreviations: GTR, gross total resection; HR, hazard ratio; NTR, near total resection; RT, radiotherapy; STR, subtotal resection.
aThis analysis excludes 1 patient with biopsy only.
Table 3.
Univariate Analysis of Clinical and Pathological Variables Associated with Overall Survival
| Variable | N | Deaths | Overall Survival (%) | |||
|---|---|---|---|---|---|---|
| Years | HR | P | ||||
| 5 | 10 | |||||
| Age at RT | ||||||
| ≤8 Y | 88 | 22 | 80.4 (72.4–89.2) | 75.8 (66.9–85.8) | ||
| >8 Y | 90 | 14 | 87.3 (80.5–94.6) | 83.0 (75.2–91.7) | 0.6 (0.3–1.2) | .17 |
| Histological subtype | ||||||
| Classic/desmoplastic | 152 | 27 | 87.2 (82.0–92.7) | 81.7 (75.3–88.7) | ||
| Anaplastic | 26 | 9 | 65.0 (48.9–86.4) | 65.0 (48.9–86.4) | 2.3 (1.1–4.8) | .030 |
| Sex | ||||||
| Male | 103 | 26 | 80.1 (72.6–88.3) | 73.2 (64.3–83.2) | ||
| Female | 75 | 10 | 89.1 (82.3–96.5) | 87.7 (80.4–95.6) | 0.5 (0.2–1.0) | .058 |
| Extent of resectiona | ||||||
| GTR/NTR | 159 | 30 | 84.6 (79.2–90.5) | 81.0 (74.7–87.8) | ||
| STR | 18 | 6 | 76.0 (58.0–99.6) | 61.0 (40.3–92.2) | 2.0 (0.8–4.8) | .11 |
| RT duration (days) | ||||||
| ≤45 | 151 | 32 | 83.1 (77.3–89.4) | 78.5 (71.7–85.9) | ||
| >45 | 27 | 4 | 88.6 (77.2–100.0) | 84.1 (70.9–99.8) | 0.7 (0.2–1.8) | .41 |
| Risk | ||||||
| Standard | 102 | 14 | 91.0 (85.6–96.8) | 86.9 (79.9–94.4) | ||
| Intermediate/high | 76 | 22 | 74.1 (64.7–84.9) | 68.9 (58.7–80.8) | 2.5 (1.3–4.8) | .0068 |
Abbreviations: GTR, gross total resection; HR, hazard ratio; NTR, near total resection; RT, radiotherapy; STR, subtotal resection.
aThis analysis excludes 1 patient with biopsy only.
Patterns of Failure and Toxicity
A total of 38 patients experienced either a local and/or a DF at a median time of 1.6 years (0.22-10.3). Three of the 38 patients (7.9%) experienced an isolated LF. Nine patients developed concurrent LF and DF, and 26 patients failed distantly while the primary site was locally controlled. The 10-year cumulative incidence of LF was 6.6% (95% CI: 3.5-11.2) (Figure 1F). The 10-year local control (LC) was 93.5% (95% CI: 87.0-97.4) for SR and 93.3% (95% CI: 86.2-97.6) for IR/HR patients (hazard ratio: 1.0 (95% CI: 0.3-3.1), P = 1.0). On univariate analysis, neither EOR nor risk group were predictive of LF (Table 4).
Table 4.
Univariate Analysis of Clinical and Pathological Variables Associated With Local Failure
| Variable | N | LF | Local Failure (%) | |||
|---|---|---|---|---|---|---|
| Years | HR | P | ||||
| 5 | 10 | |||||
| Age at RT | ||||||
| ≤8 Y | 88 | 8 | 94.3 (88.0–97.9) | 91.4 (83.9–96.3) | ||
| >8 (y) | 90 | 4 | 95.6 (89.8–98.6) | 95.6 (89.8–98.6) | 0.5 (0.2–1.6) | 0.26 |
| Histological subtype | ||||||
| Classic/desmoplastic | 152 | 9 | 96 (92.0–98.4) | 94.2 (89.4–97.4) | ||
| Anaplastic | 26 | 3 | 88.5 (72.9–97.2) | 88.5 (72.9–97.2) | 2.1 (0.6–7.7) | 0.27 |
| Sex | ||||||
| Male | 103 | 5 | 97.1 (92.4–99.2) | 95.5 (89.6–98.6) | ||
| Female | 75 | 7 | 91.9 (84.2–96.7) | 90.4 (82.2–95.8) | 2.0 (0.6–6.2) | 0.23 |
| Extent of resectiona | ||||||
| GTR/NTR | 159 | 12 | 94.3 (90.0–97.2) | 92.6 (87.6–96.1) | ||
| STR | 18 | 0 | 100 (–) | 100 (–) | 3.8e−5 (1.8e−5 –8.0e−5) | 0.23 |
| RT duration (days) | ||||||
| ≤45 | 151 | 12 | 94 (89.4–97.1) | 92.1 (86.8–95.9) | ||
| >45 | 27 | 0 | 100 (–) | 100 (–) | 3.4e−5 (1.7e−5–6.7e−05) | 0.12 |
| Risk | ||||||
| Standard | 102 | 7 | 96.1 (91.0–98.7) | 93.5 (87.0–97.4) | ||
| Intermediate/high | 76 | 5 | 93.3 (86.2–97.6) | 93.3 (86.2–97.6) | 1.0 (0.3–3.1) | 1 |
Abbreviations: GTR, gross total resection; HR, hazard ratio; LF, local failure; NTR, near total resection; RT, radiotherapy; STR, subtotal resection.
aThis analysis excludes 1 patient with biopsy only.
Among the 178 patients, 8 patients (4.5%) developed a secondary (benign or malignant) tumor at a median follow-up of 9.1 years (4.4-13.3), and 3 patients (1.7%) developed a second malignancy. The 10-year cumulative incidence (CI) of any second tumors, second malignancies, and second benign tumors was 5.6% (95% CI: 2.2-11.3), 2.1% (95% CI: 0.6-5.8), and 3.4% (95% CI: 0.9-8.9), respectively. The in-field second tumors were glioblastoma (n = 2), meningioma (n = 2), high-grade glioma (n = 1). The remaining patients (n = 3) developed an ovarian fibroma (out of field and the patient had DICER1 mutations), plexiform fibro-myxoma of the esophagus (penumbral dose), and a papillary thyroid carcinoma (mean dose: 0.02 Gy, max: 1.95 Gy). The 2 patients with glioblastoma died.
Four patients developed brainstem injury at a median of 4.2 years (0.7-11.0). The 5- and 10-year cumulative incidence of brainstem injury were 1.1% (95% CI: 0.2-3.7) and 1.9% (95% CI: 0.5-5.1).
Discussion
With a median follow-up of 9.3 years, this is the largest study to date to evaluate mature oncologic outcomes in a pediatric medulloblastoma cohort treated with proton therapy. The strengths of this study, including the enrollment of the majority of patients on a prospective protocol and the ability to follow patients longitudinally over many years, provide several critical insights into long-term disease outcomes, patterns of failure, and the rate of secondary tumors that have not been previously reported in the literature for a proton-treated cohort of medulloblastoma.
The favorable long-term disease outcomes in our study should quell any concerns regarding the possibility of increased disease relapse in proton-treated patients. The OS and EFS outcomes in our study are similar to those that have been previously published for photon RT. For example, in the St. Jude Medulloblastoma-86 study, which used 3-dimensional conformal RT (3D-CRT), 5-year EFS for the SR and HR group was 83% and 70%, respectively, for patients treated with photons, comparable to the 5-year EFS of 87.3% and 68.9% reported in our study.8 Similarly, in the COG A9961 Phase III study, which randomized SR patients to 2 adjuvant chemotherapy regimens, the 5-year EFS was 81%, and the data were most recently updated to demonstrate a 10-year EFS of 75.8%,9,10 which is comparable to our cohort at 79.6% EFS. Our outcomes are also comparable to the more modern ACNS 0331 trial, which demonstrated a 5-year EFS of 82.2% in the SR patients who received IF RT.1 The similar OS and EFS seen in our study in comparison to photon-treated cohorts, suggests that an RBE value of 1.1 is reasonable for prescribing dose in proton therapy.
Our study provides additional insights regarding the importance of several prognostic factors in medulloblastoma. EOR has been shown to be prognostic for survival in several older prospective and retrospective studies.11–13 In our study, STR trended toward inferior EFS and OS, although it did not reach statistical significance. However, more recent studies have questioned the prognostic relevance of EOR, particularly when considering molecular subtypes.14,15 Our study also confirms the importance of anaplastic histology, with an inferior OS for patients with anaplastic histology compared to classic/desmoplastic subtype. This is consistent with several prior studies, including a large meta-analysis demonstrating poor oncologic outcomes for patients with anaplasia.16,17
The patterns of relapse in our study are consistent with what has been reported by COG and other retrospective studies which have focused on a predominantly photon-treated population.10 In our cohort, the predominant pattern of failure was distant, with relatively few failures in the tumor bed. In COG A9961, while a significant number of patients failed distantly (66%), approximately 33% of patients failed in the PF, in comparison to 28% in our study. In a multi-institutional study of CRT led by the St. Jude team, Merchant et al reported 30.7% of all failures were local, while 23% were in the spine, again consistent with the failures seen in our study.18 There has been speculation of a theoretical difference in patterns of failure of protons vs photons due to variations in the linear energy transfer (LET) and relative biological effectiveness (RBE). Our group previously evaluated 16 relapsed medulloblastoma patients who were treated with protons and found no correlation between lower LET values and location of recurrence.19 Instead, emerging data suggest that the pattern of relapse is most likely dependent on molecular subtype and therapy delivered, with group 3 and group 4 tumors most likely to present with a distant relapse.20,21 Collectively, these studies demonstrate that the pattern of failure in proton-treated cohorts remains similar to those seen in photon-treated patients.
The overall rate of second tumors in our medulloblastoma cohort are comparable to those from historically photon-treated cohorts.9,22–25However, the rate of second malignancies in our proton-treated cohort was lower than has been reported in the photon literature. A recent meta-analysis demonstrated a 10-year cumulative incidence of 6.1% of secondary tumors, comparable to the 10-year cumulative incidence of secondary tumors of 5.6% found in our proton-treated cohort.26 The pooled analysis showed an overall 10-year CI of secondary malignant neoplasms of 3.7%, nearly double what was found in our proton cohort. In addition, in the photon cohorts, nearly 40% of the secondary malignancies occurred outside of the central nervous system, and of those 43% were either thyroid carcinoma or adenoma, similar to what was found in our study. Despite the lack of exit dose with protons, 1 patient developed a papillary thyroid carcinoma with a mean thyroid dose of 0.02 Gy. A recent oral abstract presented at the American Society of Radiation Oncology (ASTRO) 2019 meeting from Paulino et al evaluated 103 patients treated with passive scatter proton therapy at a median follow-up of 78.5 months and demonstrated a 5 and 10 actuarial secondary malignancy rate of 2.6% and 6.0%, comparable to photon cohorts.27 Notably, no patients in the proton cohort developed a secondary malignancy in the region of exit dose, although their median follow-up was shorter. The development of secondary malignancies, even in a proton-treated cohort, reflects the large volume of tissue irradiated with CSI and suggests that protons may never completely eliminate this risk. In addition, several recent studies have shown that patients with medulloblastoma carry cancer predisposition genes which may increase their risk of secondary malignancy at baseline. One medulloblastoma-specific study found that 6% of patients had a cancer predisposition gene.28 Another study of all pediatric cancer patients found that approximately 9% of pediatric cancer patients have an underlying germline cancer predisposition gene and likely warrants screening in the future to enhance surveillance of those found to have a gene placing them at higher risk for subsequent malignancies.29 Nevertheless, it is encouraging to see a low incidence of secondary malignant neoplasms with protons.
Recently, there has been increasing concern regarding the possibility of increased brainstem injury in proton-treated patients, due to changes in the RBE and LET near the distal portion of the spread-out Bragg peak (SOBP).30,31 COG has adjusted brainstem constraints for proton-treated patients in ACNS 0831 to reflect the aforementioned issues with brainstem dosimetry, which underscores the importance of understanding biologic proton dosimetry. Nevertheless, brainstem injury in our cohort was quite low and comparable to prior studies from photon-treated brain tumor patients.32,33 Our study definitively demonstrates that with careful radiation dosimetry planning, including limiting hot spots in the brainstem, and judicious selection of radiation beam angles, the incidence of brainstem injury in proton patients can be kept below 2%.
There are several limitations of this study that must be acknowledged. First, molecular subgrouping was unavailable for our cohort and has recently been shown to have a prognostic role in medulloblastoma. However, outcomes are comparable to other photon cohorts published in the last decade where genetic subtyping was also not available. Given the large numbers, this is confirmatory that the subtype distribution is similar to those found in other pediatric cohorts already published. Next, while this proton cohort has the longest follow-up of disease outcomes reported for a proton-treated cohort, other long-term toxic effects such as cardiac, ototoxicity, pulmonary, endocrine, and neurocognitive deficits were not reported in this study but is planned to be the topic of another manuscript. Finally, the relatively small patient numbers made it difficult to perform any multi-variate analysis to determine the impact of clinical and pathological variables on OS and EFS. A collaborative multi-institutional approach using the PPCR may better elucidate the magnitude of impact of proton RT in pediatric brain tumor survivors.34–37
The majority of patients in this study were treated with a passive scatter proton technique. Proton technology has significantly evolved over the last decade with the introduction of intensity-modulated proton therapy (IMPT) and PBS, which allow for modulation of the individual beamlets and dose painting to further increase the therapeutic ratio in pediatric tumors. Although photon technology continues to evolve with the introduction of volumetric modulated arc therapy (VMAT), allowing for improved cardiac sparing, the pattern of dose deposition and the reduction in integral dose still make protons a better treatment option with regards to a probable reduction in normal tissue toxicity, although data on late effects in these patients are scant. Recently, a study by Xiang et al demonstrated a reduction in second cancer risk for protons compared to intensity modulated radiation therapy (odds ratio: 0.31, P < .001).38
As evidence for the benefits of proton therapy continue to grow, addressing inequity and potential barriers to access will be crucial. Odei et al demonstrated that only 15% of patients in the United States with pediatric CNS tumors received proton therapy in 2012.39 In addition, patients with private insurance and higher income brackets were more likely to receive proton therapy. Recent data suggest that the number receiving proton therapy is over 50%, especially for patients with rhabdomyosarcoma, medulloblastomas, ependymomas, and atypical teratoid rhabdoid tumors.40 Bitterman et al demonstrated that black pediatric patients enrolled on COG trials were less likely to receive proton RT.41 As potential indications for proton therapy expand, it will be important for all stakeholders, including insurance companies and treating institutions to ensure equitable care for all patients, regardless of race or socioeconomic status.
To our knowledge, this is the largest and most mature published study of a proton-treated medulloblastoma cohort. It definitively demonstrates disease outcomes comparable to those previously established in the photon literature. In addition, the low rates of second malignant neoplasms, particularly outside of the CNS, is promising, and will require continued long-term follow-up. Continued long-term follow-up of the toxic effects of treatment, particularly neurocognitive, cardiac, ototoxicity, pulmonary, and endocrine sequelae, will be necessary to establish protons as the standard of care in pediatric medulloblastoma. However, there is already a growing body of literature demonstrating benefits in some of these domains.7,42 Collectively, the excellent disease and toxicity outcomes reported provide a strong rationale to utilize proton RT as an optimal treatment option in pediatric medulloblastoma.
Contributor Information
Sujith Baliga, Department of Radiation Oncology, The Ohio State University Wexner Medical Center, Columbus, Ohio, USA.
Sara Gallotto, Department of Radiation Oncology, Massachusetts General Hospital/Harvard Medical School, Boston, Massachusetts, USA.
Benjamin Bajaj, Department of Radiation Oncology, Massachusetts General Hospital/Harvard Medical School, Boston, Massachusetts, USA.
Jacqueline Lewy, Department of Radiation Oncology, Massachusetts General Hospital/Harvard Medical School, Boston, Massachusetts, USA.
Elizabeth Weyman, Department of Radiation Oncology, Massachusetts General Hospital/Harvard Medical School, Boston, Massachusetts, USA.
Miranda P Lawell, Department of Radiation Oncology, Massachusetts General Hospital/Harvard Medical School, Boston, Massachusetts, USA.
Beow Y Yeap, Department of Radiation Oncology, Massachusetts General Hospital/Harvard Medical School, Boston, Massachusetts, USA.
David E Ebb, Department of Pediatric Hematology-Oncology, Massachusetts General Hospital, Boston, Massachusetts, USA.
Mary Huang, Department of Pediatric Hematology-Oncology, Massachusetts General Hospital, Boston, Massachusetts, USA.
Paul Caruso, Department of Pediatric Neuroradiology, Massachusetts General Hospital, Boston, Massachusetts, USA.
Alisa Perry, Department of Radiation Oncology, Massachusetts General Hospital/Harvard Medical School, Boston, Massachusetts, USA.
Robin M Jones, Department of Pediatric Neurology, Massachusetts General Hospital, Boston, Massachusetts, USA.
Shannon M MacDonald, Department of Radiation Oncology, Massachusetts General Hospital/Harvard Medical School, Boston, Massachusetts, USA.
Nancy J Tarbell, Department of Radiation Oncology, Massachusetts General Hospital/Harvard Medical School, Boston, Massachusetts, USA.
Torunn I Yock, Department of Radiation Oncology, Massachusetts General Hospital/Harvard Medical School, Boston, Massachusetts, USA.
Funding
None.
Conflict of interest statement. MIM Software, Inc. gave in-kind support.
Authorship statement. The following authors contributed to data collection: S.B., S.G., B.B., J.L., E.W., and T.I.Y. The following authors contributed to data analysis and interpretation: S.B., S.G., B.Y.Y., B.B., T.I.Y., D.E.E., M.H., P.C., A.P., R.M.J., S.M.M., and N.J.T. All authors have seen and approved the manuscript.
References
- 1. Michalski J, Vezina G, Burger P, et al. MB-109 preliminary results of COG ACNS0331: a phase III trial of involved field radiotherapy (IFRT) and low dose craniospinal irradiation (LD-CSI) with chemotherapy in average risk medulloblastoma: a report from the Children’s Oncology Group. Neuro Oncol. 2016;18(suppl_3):iii122. [Google Scholar]
- 2. Armstrong GT, Chen Y, Yasui Y, et al. Reduction in late mortality among 5-year survivors of childhood cancer. N Engl J Med. 2016;374(9):833–842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Chemaitilly W, Sklar CA. Endocrine complications in long-term survivors of childhood cancers. Endocr Relat Cancer. 2010;17(3):R141–R159. [DOI] [PubMed] [Google Scholar]
- 4. van der Pal HJ, van Dalen EC, van Delden E, et al. High risk of symptomatic cardiac events in childhood cancer survivors. J Clin Oncol. 2012;30(13):1429–1437. [DOI] [PubMed] [Google Scholar]
- 5. Armstrong GT, Liu Q, Yasui Y, et al. Long-term outcomes among adult survivors of childhood central nervous system malignancies in the Childhood Cancer Survivor Study. J Natl Cancer Inst. 2009;101(13):946–958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Yuh GE, Loredo LN, Yonemoto LT, et al. Reducing toxicity from craniospinal irradiation: using proton beams to treat medulloblastoma in young children. Cancer J. 2004;10(6):386–390. [DOI] [PubMed] [Google Scholar]
- 7. Yock TI, Yeap BY, Ebb DH, et al. Long-term toxic effects of proton radiotherapy for paediatric medulloblastoma: a phase 2 single-arm study. Lancet Oncol. 2016;17(3):287–298. [DOI] [PubMed] [Google Scholar]
- 8. Gajjar A, Chintagumpala M, Ashley D, et al. Risk-adapted craniospinal radiotherapy followed by high-dose chemotherapy and stem-cell rescue in children with newly diagnosed medulloblastoma (St Jude Medulloblastoma-96): long-term results from a prospective, multicentre trial. Lancet Oncol. 2006;7(10):813–820. [DOI] [PubMed] [Google Scholar]
- 9. Packer RJ, Zhou T, Holmes E, Vezina G, Gajjar A. Survival and secondary tumors in children with medulloblastoma receiving radiotherapy and adjuvant chemotherapy: results of Children’s Oncology Group trial A9961. Neuro Oncol. 2013;15(1):97–103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Packer RJ, Gajjar A, Vezina G, et al. Phase III study of craniospinal radiation therapy followed by adjuvant chemotherapy for newly diagnosed average-risk medulloblastoma. J Clin Oncol. 2006;24(25):4202–4208. [DOI] [PubMed] [Google Scholar]
- 11. del Charco JO, Bolek TW, McCollough WM, et al. Medulloblastoma: time-dose relationship based on a 30-year review. Int J Radiat Oncol Biol Phys. 1998;42(1):147–154. [DOI] [PubMed] [Google Scholar]
- 12. Hughes EN, Shillito J, Sallan SE, Loeffler JS, Cassady JR, Tarbell NJ. Medulloblastoma at the joint center for radiation therapy between 1968 and 1984. The influence of radiation dose on the patterns of failure and survival. Cancer. 1988;61(10):1992–1998. [DOI] [PubMed] [Google Scholar]
- 13. Berry MP, Jenkin RD, Keen CW, Nair BD, Simpson WJ. Radiation treatment for medulloblastoma. A 21-year review. J Neurosurg. 1981;55(1):43–51. [DOI] [PubMed] [Google Scholar]
- 14. Thompson EM, Hielscher T, Bouffet E, et al. Prognostic value of medulloblastoma extent of resection after accounting for molecular subgroup: a retrospective integrated clinical and molecular analysis. Lancet Oncol. 2016;17(4):484–495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Thompson EM, Bramall A, Herndon JE 2nd, Taylor MD, Ramaswamy V. The clinical importance of medulloblastoma extent of resection: a systematic review. J Neurooncol. 2018;139(3):523–539. [DOI] [PubMed] [Google Scholar]
- 16. Rutkowski S, von Hoff K, Emser A, et al. Survival and prognostic factors of early childhood medulloblastoma: an international meta-analysis. J Clin Oncol. 2010;28(33):4961–4968. [DOI] [PubMed] [Google Scholar]
- 17. Giangaspero F, Wellek S, Masuoka J, Gessi M, Kleihues P, Ohgaki H. Stratification of medulloblastoma on the basis of histopathological grading. Acta Neuropathol. 2006;112(1):5–12. [DOI] [PubMed] [Google Scholar]
- 18. Merchant TE, Kun LE, Krasin MJ, et al. Multi-institution prospective trial of reduced-dose craniospinal irradiation (23.4 Gy) followed by conformal posterior fossa (36 Gy) and primary site irradiation (55.8 Gy) and dose-intensive chemotherapy for average-risk medulloblastoma. Int J Radiat Oncol Biol Phys. 2008;70(3):782–787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Sethi RV, Giantsoudi D, Raiford M, et al. Patterns of failure after proton therapy in medulloblastoma; linear energy transfer distributions and relative biological effectiveness associations for relapses. Int J Radiat Oncol Biol Phys. 2014;88(3):655–663. [DOI] [PubMed] [Google Scholar]
- 20. Hill RM, Richardson S, Schwalbe EC, et al. Time, pattern, and outcome of medulloblastoma relapse and their association with tumour biology at diagnosis and therapy: a multicentre cohort study. Lancet Child Adolesc Health. 2020;4(12):865–874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Michalski JM, Janss AJ, Vezina LG, et al. Children’s Oncology Group Phase III trial of reduced-dose and reduced-volume radiotherapy with chemotherapy for newly diagnosed average-risk medulloblastoma. J Clin Oncol. 2021;39(24):2685–2697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Christopherson KM, Rotondo RL, Bradley JA, et al. Late toxicity following craniospinal radiation for early-stage medulloblastoma. Acta Oncol. 2014;53(4):471–480. [DOI] [PubMed] [Google Scholar]
- 23. Stavrou T, Bromley CM, Nicholson HS, et al. Prognostic factors and secondary malignancies in childhood medulloblastoma. J Pediatr Hematol Oncol. 2001;23(7):431–436. [DOI] [PubMed] [Google Scholar]
- 24. Hoff KV, Hinkes B, Gerber NU, et al. Long-term outcome and clinical prognostic factors in children with medulloblastoma treated in the prospective randomised multicentre trial HIT’91. Eur J Cancer. 2009;45(7):1209–1217. [DOI] [PubMed] [Google Scholar]
- 25. Tsui K, Gajjar A, Li C, et al. Subsequent neoplasms in survivors of childhood central nervous system tumors: risk after modern multimodal therapy. Neuro Oncol. 2015;17(3):448–456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Bavle A, Tewari S, Sisson A, Chintagumpala M, Anderson M, Paulino AC. Meta-analysis of the incidence and patterns of second neoplasms after photon craniospinal irradiation in children with medulloblastoma. Pediatr Blood Cancer. 2018;65(8):e27095. [DOI] [PubMed] [Google Scholar]
- 27. Paulino AC, Grosshans DR, Ludmir EB, et al. Preliminary report of secondary malignant neoplasm incidence in pediatric patients receiving proton versus photon craniospinal irradiation for medulloblastoma. Int J Radiat Oncol Biol Phys. 2019;105(1):S109–S110. [Google Scholar]
- 28. Waszak SM, Northcott PA, Buchhalter I, et al. Spectrum and prevalence of genetic predisposition in medulloblastoma: a retrospective genetic study and prospective validation in a clinical trial cohort. Lancet Oncol. 2018;19(6):785–798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Zhang J, Walsh MF, Wu G, et al. Germline mutations in predisposition genes in pediatric cancer. N Engl J Med. 2015;373(24):2336–2346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Haas-Kogan D, Indelicato D, Paganetti H, et al. National cancer institute workshop on proton therapy for children: considerations regarding brainstem injury. Int J Radiat Oncol Biol Phys. 2018;101(1):152–168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Indelicato DJ, Flampouri S, Rotondo RL, et al. Incidence and dosimetric parameters of pediatric brainstem toxicity following proton therapy. Acta Oncol. 2014;53(10):1298–1304. [DOI] [PubMed] [Google Scholar]
- 32. Merchant TE, Li C, Xiong X, Kun LE, Boop FA, Sanford RA. Conformal radiotherapy after surgery for paediatric ependymoma: a prospective study. Lancet Oncol. 2009;10(3):258–266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Murphy ES, Merchant TE, Wu S, et al. Necrosis after craniospinal irradiation: results from a prospective series of children with central nervous system embryonal tumors. Int J Radiat Oncol Biol Phys. 2012;83(5):e655–e660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Lawell MP, Indelicato DJ, Paulino AC, et al. An open invitation to join the Pediatric Proton/Photon Consortium Registry to standardize data collection in pediatric radiation oncology. Br J Radiol. 2020;93(1107):20190673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Hess CB, Indelicato DJ, Paulino AC, et al. An update from the Pediatric Proton Consortium Registry. Front Oncol. 2018;8:165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Kasper HB, Raeke L, Indelicato DJ, et al. The pediatric proton consortium registry: a multi-institutional collaboration in U.S. proton centers. Int J Part Ther. 2014;1(2):323–333. [Google Scholar]
- 37. Berrington de Gonzalez A, Vikram B, Buchsbaum JC, et al. A clarion call for large-scale collaborative studies of pediatric proton therapy. Int J Radiat Oncol Biol Phys. 2017;98(5):980–981. [DOI] [PubMed] [Google Scholar]
- 38. Xiang M, Chang DT, Pollom EL. Second cancer risk after primary cancer treatment with three-dimensional conformal, intensity-modulated, or proton beam radiation therapy. Cancer. 2020;126(15):3560–3568. [DOI] [PubMed] [Google Scholar]
- 39. Odei B, Frandsen JE, Boothe D, Ermoian RP, Poppe MM. Patterns of care in proton radiation therapy for pediatric central nervous system malignancies. Int J Radiat Oncol Biol Phys. 2017;97(1):60–63. [DOI] [PubMed] [Google Scholar]
- 40. Journy N, Indelicato DJ, Withrow DR, et al. Patterns of proton therapy use in pediatric cancer management in 2016: an international survey. Radiother Oncol. 2019;132:155–161. [DOI] [PubMed] [Google Scholar]
- 41. Bitterman DS, Bona K, Laurie F, et al. Race disparities in proton radiotherapy use for cancer treatment in patients enrolled in Children’s Oncology Group trials. JAMA Oncol. 2020;6(9):1465–1468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Kahalley LS, Peterson R, Ris MD, et al. Superior intellectual outcomes after proton radiotherapy compared with photon radiotherapy for pediatric medulloblastoma. J Clin Oncol. 2020;38(5):454–461. [DOI] [PMC free article] [PubMed] [Google Scholar]