Abstract
OBJECTIVES
High-grade tumours are observed even in Stage I lung adenocarcinomas. Tumour spread through air spaces (STAS) is a risk factor for recurrence after resection. However, there is no ideal predictive biomarker for STAS in high-grade Stage I lung adenocarcinoma. This study assessed the prognostic impact of the preoperative peripheral monocyte count in lung adenocarcinoma.
METHODS
We retrospectively analysed the data of 444 patients with resected Stage I lung adenocarcinoma during 2006–2016. Univariable and multivariable Cox proportional analyses of recurrence-free probability (RFP) and overall survival (OS) were used to analyze preoperative complete peripheral blood cell count data. Since monocyte count was associated with poor prognosis, the relationship between preoperative peripheral monocyte count and clinicopathological factors, including STAS, was assessed. In addition, immunohistochemical CD68 staining was performed to evaluate tumour-associated macrophages (TAMs).
RESULTS
A higher preoperative peripheral monocyte count was a predictor of lower RFP (P = 0.004) and lower OS (P < 0.001). In multivariable analysis, a higher peripheral monocyte count was an independent prognostic factor for RFP and OS (hazard ratio: 1.88, 95% confidence interval: 1.07–3.31, P = 0.029; hazard ratio: 2.13, 95% confidence interval: 1.22–3.75, P = 0.008, respectively). A higher peripheral monocyte count was associated with a higher frequency of STAS (P = 0.017) and higher number of CD68+ TAMs (P = 0.013).
CONCLUSIONS
A higher preoperative peripheral monocyte count was an independent marker for a poor prognosis in Stage I lung adenocarcinoma and was associated with a higher frequency of STAS.
Keywords: Preoperative peripheral monocyte count, Spread through air spaces, Risk of recurrence, Prognostic marker, Lung adenocarcinoma
The most beneficial therapy for early-stage lung adenocarcinoma is surgery; however, more than 10% of patients with pathological Stage IA non-small cell lung cancer (NSCLC) exhibit postoperative recurrence after undergoing curative resection [1].
INTRODUCTION
The most beneficial therapy for early-stage lung adenocarcinoma is surgery; however, more than 10% of patients with pathological Stage IA non-small cell lung cancer (NSCLC) exhibit postoperative recurrence after undergoing curative resection [1]. This indicates the existence of heterogeneity, as seen in high-grade tumours, even in patients with early-stage NSCLC.
Spread through air spaces (STAS) is a risk factor for recurrence in patients with early-stage NSCLC who have undergone resection. It is defined as the spread of tumour cells through air spaces into the lung parenchyma adjacent to the edge of the tumour. STAS has been shown to be a significant risk factor for disease recurrence in patients with Stage I lung adenocarcinoma treated with limited resection [2]. It has an insidious invasive pattern that is not visible to pathologists on gross examination, and its predictors remain unknown. Identification of the predictors of STAS in patients with early-stage lung cancer could facilitate optimization of treatment strategies, including selection of limited surgery.
Previously, we reported that a higher-than-control density of CD68+ tumour-associated macrophages (TAMs) is an independent predictor of an increased rate of STAS [3]. Therefore, in this study, we focused on determining whether peripheral monocytes, which are possible progenitor cells of TAMs, can serve as biomarkers for predicting the incidence of STAS. An elevated preoperative peripheral monocyte count has recently been shown to predict poor prognosis in various types of malignancies, including hepatic cell carcinoma and malignant lymphomas [4–6]. In NSCLC [6, 7], this parameter has been reported to indicate a poor prognosis; however, it is not specifically associated with the incidence of STAS and Stage I lung adenocarcinoma. Therefore, the optimal biomarker to predict the incidence of STAS and high-grade Stage I lung adenocarcinoma remains unclear. In this study, we assessed the prognostic influence of preoperative peripheral monocyte count on the incidence of STAS and postoperative recurrence in patients with Stage I lung adenocarcinoma who underwent curative resection.
PATIENTS AND METHODS
Ethics statement
This retrospective study was conducted in compliance with the Declaration of Helsinki and approved by the Institutional Review Board of Kagawa University (Approval Number: 2016-030) on 29 June 2016. The need for informed consent was waived by the Institutional Review Board owing to the retrospective nature of the study.
Patients
We reviewed patients (n = 444) with therapy-naive Stage I lung adenocarcinoma who underwent surgical resection with systematic lymph node dissection at Kagawa University between 2006 and 2016. Patients with multifocal invasive carcinomas were excluded from the study. The initial computed tomography (CT) scan was performed 3 months after surgery; thereafter, chest and abdomen CT scans were routinely performed every 6 months. Among these patients, we excluded those showing clinical evidence of infections, other inflammations, haematological diseases and those who used drugs that might influence their haematological data.
Clinical data were collected from a prospectively maintained lung carcinoma database. Disease recurrence was confirmed by clinical, radiological and pathological assessments. The TNM stage was assigned based on the eighth edition of the American Joint Committee on Cancer TNM Staging Manual [8].
Peripheral venous blood sample was collected from each patient upon admission to the hospital before surgery. Blood tests were performed in our hospital with Sysmex XN-3000 (Sysmex Corporation, Kobe, Japan), a fully automated blood cell counting system. The neutrophil-to-lymphocyte ratio and the lymphocyte-to-monocyte ratio of all included patients were calculated.
Evaluation of clinicopathological factors
The following clinical characteristics were retrieved from the available clinical records: age, sex, smoking history, clinical tumour status, pathological tumour status, lymphovascular invasion, STAS, peripheral leucocyte count, peripheral monocyte count, peripheral lymphocyte count, peripheral neutrophil count, neutrophil-to-lymphocyte ratio and lymphocyte-to-monocyte ratio. CT tumour size was measured as a solid component of tumour.
Histological evaluation
Two pathologists blinded to the clinical outcomes of the patients reviewed haematoxylin and eosin (H&E)-stained tissue sections using an Olympus BX53 upright microscope (Olympus Corporation, Japan) with a standard 22-mm-diameter eyepiece. The tumours were classified according to the 2015 WHO classification of lung carcinomas [9]. Lymphatic and vascular invasion were recorded if at least 1 tumour cell cluster was visible.
STAS was defined by the presence of small clusters of tumour cell nests within air spaces in the lung parenchyma beyond the edge of the main tumour and were composed of a micropapillary pattern, solid nests or single cells [2, 9, 10]. The edge of the main tumour was defined as the outer border of the tumour, which was typically observed through low-power histological examination. STAS was considered present when tumour cells were identified beyond the edge of the main tumour, even if they existed only in the first alveolar layer from the tumour edge.
Immunohistochemistry
Formalin-fixed, paraffin-embedded tumour specimens from patients who met the inclusion criteria were used for tissue microarray construction. We marked 1 representative tumour area on the haematoxylin and eosin-stained tissue sections. Next, using a tissue arrayer (Tissue Microprocessor KIN-2; Azumaya, Japan), we arrayed a cylindrical 3-mm tissue core from the corresponding paraffin block into a recipient block. In total, there were 444 available cases with adequate cores for immunohistochemical analysis.
The immunohistochemical analysis was performed to evaluate TAMs in the primary tumour. We obtained 4-µm-thick sections from the tissue microarray blocks and then stained them with anti-CD68 antibody (clone KP1, Dako; 1:50) using a BenchMark ULTRA automated immunohistochemical slide staining system (Ventana Medical Systems, Inc.). For immunohistochemically stained tissue sections with immune markers, the 3 tumour areas with the highest density of immune cell infiltration (hot spots) were photographed using an Olympus BX53 microscope equipped with a DP22 digital camera (Olympus Corporation, Japan) using a 20× objective. CD68+ TAMs were counted on each of the 3 photographs using the manual counting method. The average count of 3 areas was considered as the number of CD68+ TAMs for each patient.
Statistical analysis
Associations between variables were analysed using Fisher’s exact test and chi-square tests for categorical variables and Mann–Whitney U test for continuous variables. Patients were classified into the following quartile groups based on their complete peripheral blood cell count: score 1, ≤25th percentile; score 2, >25th percentile and ≤50th percentile; score 3, >50th percentile and ≤75th percentile; and score 4, >75th percentile. Recurrence-free probability (RFP) was defined as the time from surgical resection to the date of disease recurrence. Overall survival (OS) was defined as the time from surgical resection to the date of death or the last follow-up. RFP and OS were estimated using the Kaplan–Meier method, and their associations with factors were analysed using the log-rank test. Multivariable analyses were performed using the Cox proportional hazards regression model. Multivariable models were constructed to include only preoperative factors that were significant in the univariable analysis to examine predictive power as a preoperative marker. Associations between pathological factors were investigated, and when strong associations were observed, only 1 factor was included in the model. All statistical tests were 2-sided with a significance level of 5%. Statistical analyses were conducted using IBM SPSS Statistics for Windows (version 23.0; IBM Corporation, Armonk, NY, USA).
RESULTS
Clinicopathological characteristics of patients
The clinicopathological characteristics of the patients are shown in Table 1. The median age of the 444 patients was 70 (range, 38–92) years, and more than half of the patients were men (n = 238; 54%). With respect to surgical procedures, 333 (75%) patients underwent lobectomy and 111 (25%) underwent limited resection (segmentectomy or wedge resection). According to Japanese lung cancer practice guidelines, 26 patients received tegafur-uracil–adjuvant therapy. One of these patients underwent segmentectomy and received postoperative radiotherapy as adjuvant therapy because the resection margin was insufficient. During the study period, 55 patients experienced recurrence, and 56 patients died. Six of the patients who received tegafur-uracil–adjuvant therapy relapsed, and 4 of them experienced distant recurrences. The median follow-up period for patients who were alive at the time of the last follow-up was 60 (mean ± standard deviation, 63 ± 33) months.
Table 1:
Clinicopathological characteristics of patients
| Variables | N |
|---|---|
| Age, years median (range) | 70 (38–92) |
| Sex, male/female | 238/206 |
| Smoking history (never/ever) | 237/207 |
| Comorbidity and past history | |
| COPD | 73 |
| ILD | 8 |
| Diabetes mellitus | 25 |
| Autoimmune disease | 13 |
| Heart disease | 42 |
| Chronic kidney disease | 10 |
| Cerebrovascular disease | 22 |
| Other malignant tumour | 110 |
| Others | 15 |
| Surgical procedure (Lob/Seg/Wedge) | 333/56/55 |
| Pathological T status (Tis/T1/T2) | 27/351/66 |
| Lymphovascular invasion (absent/present) | 325/119 |
| Visceral pleural invasion (absent/present) | 400/44 |
| Histologic subtype | |
| AIS + MIA | 104 |
| Lepidic | 72 |
| Acinar | 26 |
| Papillary | 177 |
| Micropapillary | 10 |
| Solid | 25 |
| Others | 30 |
| STAS (absent/present) | 309/135 |
| Leucocyte counts, mean ± SD, mm−3 | 5755 ± 1743 |
| Neutrophil counts, mean ± SD, mm−3 | 3505 ± 1310 |
| Monocyte counts, mean ± SD, mm−3 | 330 ± 149 |
| Lymphocyte counts, mean ± SD, mm−3 | 1726 ± 802 |
| LMR, mean ± SD, mm−3 | 5.78 ± 2.75 |
| NLR, mean ± SD, mm−3 | 2.43 ± 2.64 |
| Postoperative adjuvant chemotherapy | 27 |
| Recurrences of lung adenocarcinoma | |
| Local or regional | 25 |
| Distant | 30 |
COPD: chronic obstructive pulmonary disease; ILD, interstitial lung disease; Lob, lobectomy; Seg, segmentectomy; Wedge, wedge resection; STAS, spread through air spaces; LMR, lymphocyte to monocyte ratio; NLR, neutrophil-to-lymphocyte ratio; SD: standard deviation; AIS: adenocarcinoma in situ; MIA: minimally invasive adenocarcinoma.
Evaluation of the prognostic influence of complete peripheral blood cell count
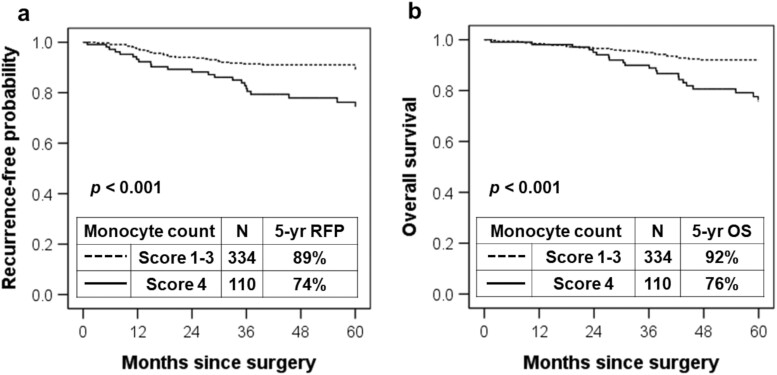
Kaplan–Meier analysis was performed to determine whether complete peripheral blood cell count was associated with RFP and OS. The RFP of the group with a peripheral monocyte count score of 4 was significantly lower (5-year RFP, 74%) than that of the groups with scores of 1, 2 and 3 (5-year RFP, 90%, 92% and 85%, respectively; P = 0.004). The OS of the group with a peripheral monocyte count score of 4 was significantly lower (5-year OS, 76%) than that of the groups with scores of 1, 2 and 3 (5-year OS, 89%, 96% and 91%, respectively; P < 0.001; Supplementary Material, Table S1). Furthermore, when peripheral monocyte count scores 1, 2 and 3 were combined, the RFP of patients with a peripheral monocyte count score of 4 was lower than the combined RFP of patients with peripheral monocyte count scores of 1–3 (5-year RFP, 74% vs 89%; P < 0.001; Fig. 1A). The OS of patients with a peripheral monocyte count score of 4 was lower than that of patients with peripheral monocyte count scores of 1–3 (5-year OS, 76% vs 92%; P < 0.001; Fig. 1B). Based on the association between poor prognosis and peripheral monocyte count, all subsequent statistical analyses focused on peripheral monocyte count.
Figure 1.
(A) Association between recurrence-free probability and peripheral monocyte count. The 5-year recurrence-free probability associated with the peripheral monocyte count score of 4 was lower than that associated with the peripheral monocyte count scores of 1–3 (5-year recurrence-free probability, 74% vs 89%, respectively; P < 0.001). (B) Association between overall survival and peripheral monocyte count. The 5-year overall survival associated with the peripheral monocyte count score of 4 was lower than that associated with the peripheral monocyte count scores of 1–3 (5-year overall survival, 76% vs 92%; P < 0.001). RFP: recurrence-free probability.
Relationship of the peripheral monocyte count with clinicopathological factors
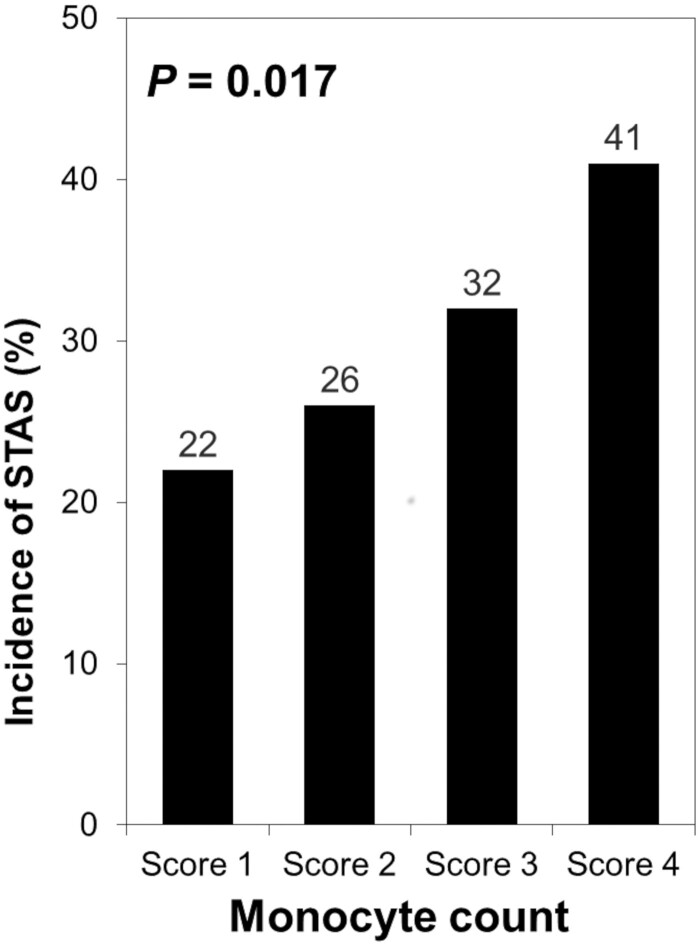
The relationships between peripheral monocyte count and clinicopathological factors are shown in Table 2. A higher peripheral monocyte count was significantly associated with sex, smoking history, lymphovascular invasion, visceral pleural invasion and STAS. The incidence of STAS was higher in patients with a higher peripheral monocyte count score (incidence of patients with STAS, 22% for score 1, 26% for score 2, 32% for score 3 and 41% for score 4; P = 0.017; Fig. 2).
Table 2:
Patient characteristics and preoperative peripheral monocyte counts
| Variables | Monocyte counts |
P-value | |||
|---|---|---|---|---|---|
| Score 1–3 |
Score 4 |
||||
| N = 334 (%) | N = 110 (%) | ||||
| Age, years | 0.46 | ||||
| ≤65 | 112 | 33.5 | 32 | 29.1 | |
| >65 | 222 | 66.5 | 78 | 70.9 | |
| Sex | <0.001 | ||||
| Female | 185 | 55.4 | 21 | 19.1 | |
| Male | 149 | 44.6 | 89 | 80.9 | |
| Smoking history | <0.001 | ||||
| Never | 202 | 60.5 | 35 | 31.8 | |
| Ever | 132 | 39.5 | 75 | 68.2 | |
| Respiratory comorbidity | |||||
| COPD | 0.19 | ||||
| With | 284 | 85.0 | 87 | 79.1 | |
| Without | 50 | 15.0 | 23 | 20.9 | |
| ILD | 1 | ||||
| With | 328 | 98.2 | 108 | 98.2 | |
| Without | 6 | 1.8 | 2 | 1.8 | |
| Surgical procedure | 0.042 | ||||
| Lobectomy | 259 | 77.5 | 74 | 67.3 | |
| Sublobar resection | 75 | 22.5 | 36 | 32.7 | |
| Recurrences of lung cancer | 0.005 | ||||
| Never | 302 | 90.4 | 87 | 79.1 | |
| Local or regional | 16 | 4.8 | 9 | 8.2 | |
| Distant | 16 | 4.8 | 14 | 12.7 | |
| CT tumour size | 0.13 | ||||
| ⩽2 cm | 223 | 66.8 | 64 | 58.2 | |
| >2 cm | 111 | 33.2 | 46 | 41.8 | |
| Pathological invasive tumour size | 0.12 | ||||
| <2 cm | 206 | 61.7 | 58 | 52.7 | |
| >2 cm | 128 | 38.3 | 52 | 47.3 | |
| Lymphovascular invasion | 0.047 | ||||
| Absent | 253 | 75.7 | 72 | 65.5 | |
| Present | 81 | 24.3 | 38 | 34.5 | |
| Visceral pleural invasion | 0.005 | ||||
| Absent | 309 | 92.5 | 91 | 82.7 | |
| Present | 25 | 7.5 | 19 | 17.3 | |
| STAS | 0.008 | ||||
| Absent | 244 | 73.1 | 65 | 59.1 | |
| Present | 90 | 26.9 | 45 | 40.9 | |
Significant P-values are shown in bold.
COPD: chronic obstructive pulmonary disease; CT: computed tomography; ILD: interstitial lung disease; STAS: spread through air spaces.
Figure 2.
Association between the incidence of spread through air spaces and the peripheral monocyte count. The incidence of spread through air spaces was higher in patients with a higher peripheral monocyte count score (incidence of patients with spread through air spaces, 22% for score 1, 26% for score 2, 32% for score 3 and 41% for score 4; P = 0.017). STAS: spread through air spaces.
Association between patient outcomes and the peripheral monocyte count
For RFP, the univariable analysis identified 8 significant risk factors: sex, smoking history, surgical procedure, CT tumour size, pathological invasive tumour size, lymphovascular invasion, visceral pleural invasion, STAS and high peripheral monocyte count (Table 3). We performed a multivariable analysis with only preoperative factors to examine the predictive power of STAS as a preoperative marker. In the multivariable analysis of the preoperative factors, a higher peripheral monocyte count was shown to be a statistically significant independent predictor of RFP [hazard ratio (HR): 1.88, 95% confidence interval (CI): 1.07–3.31, P = 0.029]. The other independent prognostic factors were the CT tumour size (HR: 3.30, 95% CI: 1.90–5.71, P < 0.001) and surgical procedure (HR: 2.40, 95% CI: 1.38–4.17, P = 0.002; Table 4).
Table 3:
Univariable associations of patient outcome with clinicopathologic factors and preoperative monocyte counts
| Variables | N | 5-year RFP | P | 5-year OS | P-value |
|---|---|---|---|---|---|
| Age, years | 0.99 | 0.004 | |||
| ≤65 | 144 | 86% | 92% | ||
| >65 | 300 | 85% | 80% | ||
| Sex | 0.012 | 0.002 | |||
| Female | 206 | 91% | 91% | ||
| Male | 238 | 81% | 78% | ||
| Smoking history | 0.020 | 0.032 | |||
| Never | 237 | 90% | 88% | ||
| Ever | 207 | 81% | 79% | ||
| Surgical procedure | 0.003 | <0.001 | |||
| Lobectomy | 333 | 88% | 89% | ||
| Sublobar resection | 111 | 76% | 65% | ||
| CT tumour size | <0.001 | 0.012 | |||
| ⩽2 cm | 287 | 91% | 88% | ||
| >2 cm | 157 | 75% | 77% | ||
| Pathological invasive tumour size | <0.001 | 0.002 | |||
| ⩽2 cm | 264 | 91% | 89% | ||
| >2 cm | 180 | 78% | 77% | ||
| Lymphovascular invasion | <0.001 | <0.001 | |||
| Absent | 325 | 92% | 89% | ||
| Present | 119 | 68% | 69% | ||
| Visceral pleural invasion | <0.001 | <0.001 | |||
| Absent | 400 | 88% | 86% | ||
| Present | 44 | 64% | 67% | ||
| STAS | <0.001 | <0.001 | |||
| Absent | 309 | 94% | 88% | ||
| Present | 135 | 66% | 74% | ||
| Monocyte counts | <0.001 | <0.001 | |||
| Score 1–3 | 334 | 89% | 92% | ||
| Score 4 | 110 | 74% | 76% |
Significant P-values are shown in bold.
CT: computed tomography; OS: overall survival; RFP: recurrence-free probability; STAS: spread through air spaces.
Table 4:
Multivariable analysis
| Variables | Category | HR | 95% CI |
|---|---|---|---|
| Recurrence-Free Probability | |||
| Sex | Male vs. female | 1.18 | 0.53 – 2.63 |
| Smoking history | Ever vs. never | 1.41 | 0.67 – 2.93 |
| CT tumor size | > 2cm vs. < 2cm | 3.30 | 1.90 – 5.71 |
| Surgical procedure | Sublobar vs. lobectomy | 2.40 | 1.38 – 4.17 |
| Monocyte counts | Score 4 < Score 1-3 | 1.88 | 1.07 – 3.31 |
| Overall survival | |||
| Age | > 65 vs. < 65 | 1.01 | 1.00 – 1.02 |
| Sex | Male vs. female | 1.51 | 0.67 – 3.42 |
| Smoking history | Ever vs. never | 1.06 | 0.52 – 2.17 |
| CT tumor size | > 2cm vs. < 2cm | 2.01 | 1.18 – 3.42 |
| Surgical procedure | Sublobar vs. lobectomy | 3.78 | 2.21 – 6.47 |
| Monocyte counts | Score 4 < Score 1-3 | 2.13 | 1.22 – 3.75 |
Significant p-values are shown in bold.
CI, confidence interval; CT, computed tomography; HR, hazard ratio.
The univariable analysis showed 9 significant risk factors for OS: age, sex, smoking history, surgical procedure, CT tumour size, pathological invasive tumour size, lymphovascular invasion, visceral pleural invasion, STAS and high peripheral monocyte count. We performed a multivariable analysis including only preoperative factors to examine the predictive power as a preoperative marker. The multivariable analysis revealed that a higher peripheral monocyte count was a statistically significant independent prognostic factor for OS (HR: 2.13, 95% CI: 1.22–3.75, P = 0.008). The other independent prognostic factors were the CT tumour size (HR: 2.01, 95% CI: 1.18–3.42, P = 0.011) and surgical procedure (HR: 3.78, 95% CI: 2.21–6.47, P < 0.001; Table 4).
Correlation between the number of CD68+ tumour-associated macrophages in tumours and the peripheral monocyte count
The number of CD68+ TAMs in tumours was 18 ± 23 (median: 11, range: 0–163). The number of CD68+ TAMs was higher in the group with a peripheral monocyte count score of 4 than in the group with peripheral monocyte count scores of 1–3 (median: 13, range: 0–107 vs median: 10, range: 0–163, P = 0.013).
DISCUSSION
In the present study, peripheral monocyte count was found to be an independent predictive factor for postoperative recurrence in pathological Stage I lung adenocarcinoma, and it was associated with the incidence of STAS. Monocytes contribute to the inflammatory process through their differentiation into macrophages or dendritic cells in the tissue microenvironment [11]. Monocytes can also stimulate cancer cell migration and inhibit anti-tumour immunity [12, 13]. Hamilton et al. [14] reported that macrophages derived from monocytes interact with circulating tumour cells to release cytokines, chemokines and growth factors, resulting in aggressive circulating tumour cell invasion behaviour in small cell lung cancer. TAMs are also known to stimulate tumour cell proliferation, promote angiogenesis and favour invasion and metastasis by producing growth and angiogenic factors. Thus, a high monocyte count may lead to tumour progression [15].
Peripheral monocytes and myeloid progenitor cells grow into TAMs when they enter tumours [16]. TAMs are classified into 2 phenotypes, namely, classically activated type 1 macrophages (M1) and alternatively activated type 2 macrophages (M2) [17]. These macrophages are polarized in the M1 pathway and are affected by bacterial moieties such as lipopolysaccharides and cytokines including interferon-gamma. Activated M1 macrophages promote an anti-tumour response, eliminate tumour cells, present antigens to T cells for an adaptive immune response and produce cytokines [17, 18]. In contrast, exposure to Th2 and tumour-derived cytokines such as interleukin-4, interleukin-10, interleukin-13, transforming growth factor-beta and prostaglandin E2, promotes M2 polarization [19]. M2 macrophages, which promote tumour development, express high levels of class A scavenger receptors (CD204) and mannose receptors (CD163) [20, 21], suppress Th1-mediated inflammation through IL-10 and IL-1b production and promote angiogenesis via vascular endothelial growth factor production [22–24]. In this study, TAMs were stained with CD68, which is known as a pan-macrophage marker, but they were not stained using a specific anti-CD163 antibody, which is a distinct M2 macrophage marker. However, as described in a previous study [25], M2 macrophages primarily constitute TAMs in the distinct tumour microenvironment. This finding supports the hypothesis that the prognostic and biological significance of CD68+ total macrophages is at least partly consistent with that of CD163+ M2 macrophages.
In this study, a higher peripheral monocyte count was significantly associated with a poor prognosis in Stage I lung adenocarcinoma. Furthermore, the peripheral monocyte count correlated with STAS and the number of CD68+ TAMs. Patients with a higher peripheral monocyte count were more likely to harbour STAS-positive tumours. STAS is a form of lung cancer spread defined as the presence of tumour cells within air spaces in the lung parenchyma beyond the edge of the main tumour [18, 26, 27]. Therefore, it is an infiltrative form of lung cancer and is widely known to be a poor prognostic factor. In a previous study, we demonstrated using univariable analysis that the high density of CD68+ TAMs was an independent predictor of a high STAS rate and associated with a high risk of recurrence. Although the reason for the relationship between STAS and the peripheral monocyte count and the CD68+ TAM count is unclear, these results suggest that the incidence of STAS may be associated with the immunological functions of monocyte-derived TAMs and may indicate a complex relationship between peripheral monocytes and TAMs in lung adenocarcinoma. Further research on the distribution of monocytes around tumours is required to elucidate the underlying mechanisms.
In this study, a higher peripheral monocyte count was significantly associated with sex and smoking history. Cigarette smoking has been reported to increase the peripheral monocyte count [28, 29]. Systemic inflammation is associated with impaired lung function, particularly in patients who smoke cigarette extensively [30]. The proportion of smokers among men is known to be higher than that among women [30]. Thus, although the peripheral monocyte count is typically higher in male smokers, the monocyte count remains a significant prognostic factor after adjustment for smoking history and patient sex.
Limitations
Our study has several limitations. First, the presence of other subtypes (6.8%) of adenocarcinoma as minor components may have influenced the results of this study. Second, we were unable to assess the epidermal growth factor receptor status of the patients, which may affect the prognosis of those with lung adenocarcinoma. Third, the retrospective design of our study is a key limitation that is liable to introduce selection bias and potentially distort the observed associations. Further prospective studies are required to confirm the present findings and to clarify the mechanism by which peripheral monocytes influence clinical outcomes.
Conclusion
In conclusion, our study demonstrated that a higher preoperative peripheral monocyte count is an independent marker for poorer prognosis in Stage I lung adenocarcinoma and is associated with a higher frequency of STAS. Peripheral monocyte count, which can be obtained from a complete blood count before surgery, may be one of the simplest prognostic markers for Stage I lung adenocarcinoma. High preoperative peripheral monocyte counts would preclude certain surgical procedures for Stage I lung adenocarcinoma, such as sublobar resection. The preoperative identification of poor prognostic factors such as STAS for Stage I lung adenocarcinoma could help clinicians make better clinical decisions regarding surgical procedures, such as lobectomy, and evaluate the indications for multidisciplinary treatment before surgery.
SUPPLEMENTARY MATERIAL
Supplementary material is available at ICVTS online.
Funding
This work was supported, in part, by JSPS KAKENHI [grant number JP20K07392].
Conflict of interest: none declared.
Data availability statement
We confirm that the data supporting the findings of this study are available within the article and its supplementary materials.
Author contributions
Chihiro Yoshida: Data curation; Formal analysis; Investigation; Visualization; Writing—original draft. Kyuichi Kadota: Conceptualization; Funding acquisition; Methodology; Writing—review & editing. Ryo Ishikawa: Resources. Tetsuhiko Go: Validation. Reiji Haba: Project administration. Hiroyasu Yokomise: Supervision.
Reviewer information
Interactive CardioVascular and Thoracic Surgery thanks David G. Healy, Calvin S.H. Ng and the other anonymous reviewer(s) for their contribution to the peer review process of this article.
Supplementary Material
ABBREVIATIONS
- CI
Confidence interval
- CT
Computed tomography
- HR
Hazard ratio
- NSCLC
Non-small cell lung cancer
- OS
Overall survival
- RFP
Recurrence-free probability
- STAS
Spread through air spaces
- TAM
Tumour-associated macrophage
REFERENCES
- 1. Okami J, Shintani Y, Okumura M, Ito H, Ohtsuka T, Toyooka S. et al. ; Japanese Joint Committee of Lung Cancer Registry. Demographics, safety and quality, and prognostic information in both the seventh and eighth editions of the TNM classification in 18,973 surgical cases of the Japanese Joint Committee of Lung Cancer Registry Database in 2010. J Thorac Oncol 2019;14:212–22. [DOI] [PubMed] [Google Scholar]
- 2. Kadota K, Nitadori JI, Sima CS, Ujiie H, Rizk NP, Jones DR. et al. Tumor spread through air spaces is an important pattern of invasion and impacts the frequency and location of recurrences after limited resection for small stage I lung adenocarcinomas. J Thorac Oncol 2015;10:806–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Yoshida C, Kadota K, Ikeda T, Ibuki E, Go T, Haba R. et al. Tumor-associated macrophage infiltration is associated with a higher rate of tumor spread through air spaces in resected lung adenocarcinomas. Lung Cancer 2021;158:91–6. [DOI] [PubMed] [Google Scholar]
- 4. Shen SL, Fu SJ, Huang XQ, Chen B, Kuang M, Li SQ. et al. Elevated preoperative peripheral blood monocyte count predicts poor prognosis for hepatocellular carcinoma after curative resection. BMC Cancer 2014;14:744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Porrata LF, Ristow K, Colgan JP, Habermann TM, Witzig TE, Inwards DJ. et al. Peripheral blood lymphocyte/monocyte ratio at diagnosis and survival in classical Hodgkin’s lymphoma. Haematologica 2012;97:262–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Kumagai S, Marumo S, Shoji T, Sakuramoto M, Hirai T, Nishimura T. et al. Prognostic impact of preoperative monocyte counts in patients with resected lung adenocarcinoma. Lung Cancer 2014;85:457–64. [DOI] [PubMed] [Google Scholar]
- 7. Hai Y, Chen N, Wu W, Wang Z, Lin F, Guo C. et al. High postoperative monocyte indicates inferior Clinicopathological characteristics and worse prognosis in lung adenocarcinoma or squamous cell carcinoma after lobectomy. BMC Cancer 2018;18:1011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Amin MB, Edge S, Greene F, Byrd DR, Brookland RK, Washington MK. et al. AJCC Cancer Staging Manual. 8th edn. New York, NY: Springer, 2017, 431–56. [Google Scholar]
- 9. Travis WD, Brambilla E, Burke AP, Marx A, Nicholson AG.. WHO Classification of Tumors of the Lung, Pleura, Thymus and Heart. Lyon: International Agency for Research on Cancer, 2015. [DOI] [PubMed] [Google Scholar]
- 10. Warth A, Muley T, Kossakowski CA, Goeppert B, Schirmacher P, Dienemann H. et al. Prognostic impact of intra-alveolar tumor spread in pulmonary adenocarcinoma. Am J Surg Pathol 2015;39:793–801. [DOI] [PubMed] [Google Scholar]
- 11. Shi C, Pamer EG.. Monocyte recruitment during infection and inflammation. Nat Rev Immunol 2011;11:762–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Coussens LM, Werb Z.. Inflammation and cancer. Nature 2002;420:860–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Mantovani A, Allavena P, Sica A, Balkwill F.. Cancer-related inflammation. Nature 2008;454:436–44. [DOI] [PubMed] [Google Scholar]
- 14. Hamilton G, Rath B, Klameth L, Hochmair MJ.. Small cell lung cancer: recruitment of macrophages by circulating tumor cells. Oncoimmunology 2016;5:e1093277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Mantovani A, Marchesi F, Malesci A, Laghi L, Allavena P.. Tumour-associated macrophages as treatment targets in oncology. Nat Rev Clin Oncol 2017;14:399–416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Srivastava MK, Andersson A, Zhu L, Harris-White M, Lee JM, Dubinett S. et al. Myeloid suppressor cells and immune modulation in lung cancer. Immunotherapy 2012;4:291–304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Mantovani A, Sozzani S, Locati M, Allavena P, Sica A.. Macrophage polarization: tumor-associated macrophages as a paradigm for polarized M2 mononuclear phagocytes. Trends Immunol 2002;23:549–55. [DOI] [PubMed] [Google Scholar]
- 18. Nielsen SR, Schmid MC.. Macrophages as key drivers of cancer progression and metastasis. Mediators Inflamm 2017;2017:9624760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Siveen KS, Kuttan G.. Role of macrophages in tumour progression. Immunol Lett 2009;123:97–102. [DOI] [PubMed] [Google Scholar]
- 20. Komohara Y, Ohnishi K, Kuratsu J, Takeya M.. Possible involvement of the M2 anti-inflammatory macrophage phenotype in growth of human gliomas. J Pathol 2008;216:15–24. [DOI] [PubMed] [Google Scholar]
- 21. Kawamura K, Komohara Y, Takaishi K, Katabuchi H, Takeya M.. Detection of M2 macrophages and colony-stimulating factor 1 expression in serous and mucinous ovarian epithelial tumors. Pathol Int 2009;59:300–5. [DOI] [PubMed] [Google Scholar]
- 22. Heusinkveld M, van der Burg SH.. Identification and manipulation of tumor associated macrophages in human cancers. J Transl Med 2011;9:216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Gordon S, Martinez FO.. Alternative activation of macrophages: mechanism and functions. Immunity 2010;32:593–604. [DOI] [PubMed] [Google Scholar]
- 24. Heusinkveld M, de Vos van Steenwijk PJ, Goedemans R, Ramwadhdoebe TH, Gorter A, Welters MJ. et al. M2 macrophages induced by prostaglandin E2 and IL-6 from cervical carcinoma are switched to activated M1 macrophages by CD4+ Th1 cells. J Immunol 2011;187:1157–65. [DOI] [PubMed] [Google Scholar]
- 25. Cao L, Che X, Qiu X, Li Z, Yang B, Wang S. et al. M2 macrophage infiltration into tumor islets leads to poor prognosis in non-small-cell lung cancer. Cancer Manag Res 2019;11:6125–38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Qian BZ, Pollard JW.. Macrophage diversity enhances tumor progression and metastasis. Cell 2010;141:39–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Dirkx AE, Oude Egbrink MG, Wagstaff J, Griffioen AW.. Monocyte/macrophage infiltration in tumors: modulators of angiogenesis. J Leukoc Biol 2006;80:1183–96. [DOI] [PubMed] [Google Scholar]
- 28. Bridges RB, Wyatt RJ, Rehm SR.. Effect of smoking on peripheral blood leukocytes and serum antiproteases. Eur J Respir Dis Suppl 1985;139:24–33. [PubMed] [Google Scholar]
- 29. Bridges RB, Wyatt RJ, Rehm SR.. Effects of smoking on inflammatory mediators and their relationship to pulmonary dysfunction. Eur J Respir Dis Suppl 1986;146:145–52. [PubMed] [Google Scholar]
- 30. Walter RE, Wilk JB, Larson MG, Vasan RS, Keaney JF Jr, Lipinska I. et al. Systemic inflammation and COPD: the Framingham Heart Study. Chest 2008;133:19–25. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
We confirm that the data supporting the findings of this study are available within the article and its supplementary materials.