Abstract
Background
Clinically, the low expression of wild-type aryl hydrocarbon receptor-interacting protein (AIP) in patients with sporadic growth hormone (GH)-secreting pituitary adenoma (GHPA) is associated with a more aggressive phenotype. However, the mechanism by which AIP expression is regulated in GHPA remains unclear. Herein, we investigated a transcription factor that regulates AIP expression and explored its role in tumor phenotypes.
Methods
General transcription factor IIB (GTF2B) was predicted by several bioinformatic tools to regulate AIP expression transcriptionally. Regulation by GTF2B was evaluated using chromatin immunoprecipitation (ChIP), reverse transcription PCR, luciferase reporter, and western blot experiments in SH-SY5Y cells. Furthermore, the 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyl-2H-tetrazolium bromide (MTT) assay, transwell invasive assay, ELISA, western blot, immunohistochemical staining, and terminal deoxynucleotidyl transferase dUTP nick end labeling were performed to investigate the effects of GTF2B and AIP on tumor cell proliferation, apoptosis, growth hormone secretion, and invasiveness in GH3 cells and mouse xenograft models. Moreover, correlations between GTF2B and AIP expression were explored in GHPA cases.
Results
ChIP and luciferase reporter studies demonstrated that the regulation of AIP expression by GTF2B was dependent on the intergenic-5′ untranslated region element of AIP and the initial residual S65 of GTF2B. In vitro and in vivo experiments indicated that GTF2B regulated AIP expression to impact the GHPA phenotype; this was confirmed by data from 33 GHPA cases.
Conclusions
We determined the regulation by GTF2B of AIP transcription in GHPA and its impact on tumor phenotype. Our findings suggest that GTF2B may be a potential therapeutic target for GHPA with low AIP expression.
Keywords: AIP, GTF2B, somatotropinoma, transcriptional regulation
Key Points.
GTF2B promotes AIP transcription to suppress GHPA cell proliferation, invasiveness, and GH hypersecretion.
Transcriptional regulation of AIP by GTF2B is dependent on the intergenic-5U region of AIP.
Importance of the Study.
This work explored the transcriptional mechanism by which GTF2B regulates the tumor suppressor gene AIP in GHPA. GTF2B promoted AIP expression by binding the intergenic-5′ untranslated region element of AIP, thus inhibiting GHPA tumor development. Our findings suggest the therapeutic potential of targeting GTF2B in GHPAs with low AIP expression, which always present as refractory.
Epidemiologically, due to severe complications (hypertension, headaches, visual disturbances, insulin resistance, cardiac hypertrophy, sleep apnea syndrome, and other types of tumors), patients with somatotropinoma or growth hormone-secreting pituitary adenoma (GHPA) face increased morbidity and mortality risks compared with the normal population over the medium- and long-term.1 Moreover, some patients with these pathologically benign tumors may undergo tumor recurrence, as well as elevated levels of GH and/or insulin-like growth factors-1 (IGF-1) or glucose control deterioration despite conventional therapy (tumor resection by surgery, medicine such as somatostatin analogs, or radiotherapy), which results in very poor prognosis.2,3
The aryl hydrocarbon receptor-interacting protein (AIP) gene is considered a tumor suppressor in the pituitary; loss-of-function mutations in this gene predispose individuals to familial isolated pituitary adenomas or sporadic pituitary adenomas with invasive characteristics4–6 and a relatively poor response to somatostatin analogs (SSAs).7,8 However, GHPA cases with a familial background account for just 5% of pituitary adenomas, and most cases are sporadic.9 Moreover, the prevalence of germline AIP mutations in patients with apparently sporadic pituitary adenomas is reportedly very low.5,10,11 In addition, it has been confirmed that low wild-type AIP protein expression in patients with sporadic GHPA is associated with a more aggressive phenotype12 and a worse response to SSAs,13,14 which leads to a poorer prognosis.
Although several studies have investigated how AIP expression might be regulated in GHPA, the underlying mechanism remains unclear. One study revealed that miR-34a may be responsible for lower AIP expression in GHPAs with an invasive phenotype and SSA resistance,15 which indicates that epigenetic regulation may play an important role in AIP protein expression, such as via long noncoding RNA, transcriptional factor regulation, and post-translational modifications.
Several promoter regions within a gene (which can be up to several hundred kilobases away) serve as modifiers, ensuring its accurate expression by recruiting transcription activating or silencing factors.16 Over the last two decades, the development of multiple prediction algorithms has made it possible to determine transcription factor binding sites in silico.17 In the present study, we used several bioinformatic tools to predict that a transcription factor, general transcription factor IIB (GTF2B), may regulate AIP expression transcriptionally by binding a noncoding evolutionary conserved region (ncECR) within the 5′ untranslated region (UTR) of AIP. Furthermore, we confirmed the transcriptional regulation effects of GTF2B on AIP expression in both clinical samples and cell lines, and investigated the influence of GTF2B on GHPA phenotypes in association with its effect on AIP expression.
Materials and Methods
Bioinformatics Analysis
The ECR Browser (http://ecrbrowser.dcode.org/) was used to generate a conservation profile by aligning the human AIP gene with its rat counterpart in a pair-wise fashion, and the selection parameters were established as >100 base pairs (bp) in length and >75% identity for the identification of ncECRs and the conserved SNPs in the identified ncECRs. The VISTA Browser (http://pipeline.lbl.gov/cgi-bin/gateway2) was complementary to the ECR Browser for ncECR confirmation. The UCSC Genome Browser on Human December 2013 (GRCh38/hg38) Assembly (https://genome.ucsc.edu/cgi-bin/hgGateway) was consulted for the AIP gene sequence (ncECR regions on Chr.11q13.1 with upstream or downstream sequences, for the cloning and luciferase experiments). The proprietary database MatInspector (Genomatix, Munich, Germany; https://www.genomatix.de) was used for all predictions of possible transcription factors and their interactions with the AIP promoter region.
Cell Culture
The human neuroblastoma cell line SH-SY5Y was purchased from ATCC (CCL-2266; Manassas, VA, USA). The rat somatomammotroph tumor cell line GH3 was purchased from the Cell Resource Center of the Chinese Academy of Medical Sciences (Beijing, China). Cells were seeded onto poly-L-lysine-coated plates and passaged when they reached 60–70% confluence. For the initial plating of cells in experiments, manual cell counts were performed using a hemocytometer. Culture medium details are described in the Supplementary Material.
Plasmids and Constructs
Complementary DNA (cDNA) encoding some proteins was generated by reverse transcription (RT) PCR of total RNA extracted from some cell lines. Additionally, expression vectors were created using site-directed mutagenesis or purchased from Santa Cruz Biotechnology (Santa Cruz, CA, USA). Details regarding the methods and conditions of transfection are available in the Supplementary Material.
Cloning and Luciferase Assays
All ncECRs identified in section Bioinformatics Analysis of the Methods were cloned into specific reporter vectors (pGL3 luciferase reporter vector system) and their luciferase activities were measured after being transfected into SH-SY5Y cells. Primers with specific restriction sites (e.g., KpnI, BglII, or XhoI) were designed (Supplementary Table S1). The intergenic-5′ untranslated region (IG-5U)AIP elements with or without single nucleotide polymorphisms (SNPs) were also cloned into the firefly luciferase-expressing vector psiCHECK-2 (Promega, Madison, WI, USA) to confirm the binding sites in IG-5UAIP elements. Further details are given in the Supplementary Materials.
Chromatin Immunoprecipitation (ChIP) and Quantitative Real-time PCR Assay
SH-SY5Y cells were transfected with wild- or mut-GTF2B, or with AIP siRNA overexpression vectors for the ChIP test. The ChIP real-time PCR assay was used to detect and quantify the enrichment of GTF2B at the binding site of IG-5UAIP of AIP. All experiments are detailed in the Supplementary Material and Supplementary Table S2.
Real-time PCR
RT-PCR was then performed using the Light Cycler 2.0 (Roche, USA) and SYBR Premix Ex Taq II (Takara Bio, Shiga, Japan; DRR081A). Experimental details, including the primers and PCR conditions, are listed in the Supplementary Material and Supplementary Table S2.
Cell Proliferation Assay
Cell proliferation assays were performed using the MTT assay (Sigma-Aldrich, M5655) for various treatments at different time points after transfection (24 h, 48 h, and 72 h). The absorbance of cell supernatant aliquots at 490 nm was measured using an automatic plate analyzer (Bio-Rad Laboratories, Hercules, CA, USA).
Transwell Invasion Assay
For the invasion assay, 8 µm Transwells in a 24-well plate were coated with 150 µL of a mixture of culture medium and reconstituted Matrigel (BD Biosciences, Franklin Lakes, NJ, USA) at a ratio of 2:1. Absorbance at OD 560 nm was then measured using a plate reader. Further details are available in the Supplementary Material.
Protein Extraction and Western Blot Analysis
The experiment included cell harvesting, protein extraction and separation, immunoblot incubation, and antibody probing. Antibody information is listed in Supplementary Table S3, and the steps of the experiment are described in the Supplementary Material.
Animal Care and Ethics Statement
Six-week-old female BALB/c nude mice were housed in laminar flow cabinets under specific pathogen-free conditions with free access to food and water. All animal handling and procedures were performed according to a protocol that was approved by the institutional animal care and use committee of Zhejiang University.
GH3 cells (1 × 108/L) with various transduction treatments were injected with Matrigel Matrix (Corning, Corning, NY. USA; No. 354262) into the right axilla of mice at random to make up various groups in the animal experiment. Next, overexpression plasmids and/or siRNA vectors (blank vectors were used as negative controls (NC)) in various treatments dissolved with diluted water were intratumorally injected into the animals (5 nmol/kg) every 3 days for 6 weeks.18 After 12 days of injections, the tumor volumes were calculated using the following formula: volume (mm3) = (L × W2) × 0.54; where L = the longest diameter and W = the shortest diameter. In addition, the tumor volume of each mouse was recorded once every 3 days. All mice were sacrificed upon completion of the 42-day experiment, followed by tumor excision, measuring, and refrigeration for next studies.
Immunohistochemical (IHC) Analysis
Formalin-fixed paraffin-embedded xenograft tumor sections were collected for IHC experiments, which are detailed in the Supplementary Material. Antibody information is listed in Supplementary Table S4.
Terminal Deoxynucleotidyl Transferase dUTP Nick End Labeling (TUNEL) Staining of GH3 Xenografts
Tissue harvested from GH3 xenografts was fixed in 4% formaldehyde and embedded in paraffin for TUNEL staining. The staining was performed using a TUNEL kit (Roche, 11684817910) as per the manufacturer’s instructions. The reactivity intensity was measured and quantified using a DS-Ri2.
ELISA
The levels of GH in cell culture supernatant were measured using a rat ELISA kit (LifeSpan BioSciences, Seattle, WA, USA; LS-F27456) according to the manufacturer’s protocol. Absorbance was read at 450 nm using an ELISA plate reader (Thermo Fisher Scientific). To measure GH and IGF-1 levels in mice with tumor models, blood was drawn from the retro-orbital sinus. According to the manufacturer’s instructions, GH levels were measured using a rat ELISA kit (LifeSpan BioSciences, LS-F27456), while IGF-1 levels were assessed using a mouse ELISA kit (Abcam, ab108874).
Patients and Tissue Samples
All patients in the present study were confirmed to have somatotroph adenomas by IHC, according to the 2017 WHO pathological classification of pituitary adenomas. Based on the criteria by Trouillas et al.19 and the definition suggested by Sav et al.20 “invasion” in this study was considered to be histological/radiological signs of the tumor and/or surgical detection of the tumor in the cavernous or sphenoid sinus, while “proliferation” was defined by the presence of at least two of the following three criteria: Ki-67 ≥ 3%; mitoses: n > 2/10 high-power fields; and p53: >10 positive nuclei/10 high-power fields. All tumors were then classified into five grades: 1a (non-invasion), 1b (non-invasion and proliferation), 2a (invasion), 2b (invasion and proliferation), and 3 (malignant). They were then further classified into two groups: low grade (grades 1a, 1b, and 2a) and high grade (grades 2b and 3). To explore the correlations between clinical features and GTF2B and AIP mRNA expression, we enrolled 33 patients (33 samples) with somatotroph adenomas (all from the Department of Neurosurgery, The Second Affiliated Hospital of Zhejiang University, from April 2016 to December 2019). All patients were diagnosed with pituitary adenoma for the first time and did not receive any treatment before surgery. Once removed from the body, all tissue fragments of the tumor samples were immediately frozen in liquid nitrogen and stored at −80°C until molecular analysis.
This study was approved by the ethics committee of The Second Affiliated Hospital of Zhejiang University, according to the 3rd edition of the Guidelines on the Practice of Ethical Committees in Medical Research (issued by the Royal College of Physicians of London). Written informed consent was obtained from each patient in accordance with the Declaration of Helsinki.
Statistical Analysis
Statistical analysis was performed using IBM SPSS Statistics Version 26 (IBM Corp., Armonk, NY, USA). Data are expressed as the mean ± SD. Mann–Whitney U tests and two-tailed Student’s t-tests were used for comparisons between two groups. Spearman’s correlation analysis was used to analyze the correlations between two variables. A value of P < .05 was considered statistically significant.
Results
Prediction and Investigation of Functional ncECRs and the Corresponding Transcription Factors Within the AIP Genomic Locus
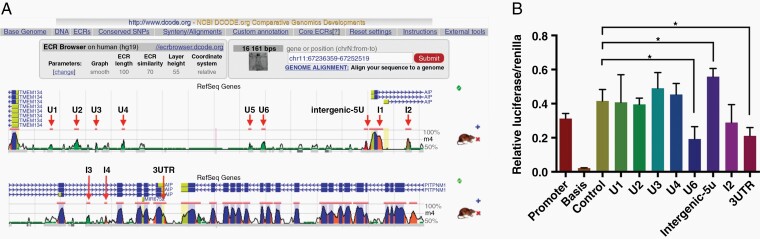
Using comparative genomics in the ECR Browser (http://ecrbrowser.dcode.org/), 13 ncECRs were identified within the AIP genomic region on human chromosome 11q13.1 that were conserved between humans and rats (Figure 1A). Six of these DNA sequences (U1–6) were located upstream of the AIP gene, four were intronic (I1–4), and one was intergenic. Both UTRs (5′UTR and 3′UTR) were also indicated as ncECRs. Because the intergenic region is very close to 5′UTR, both were merged as one ncECR (the intergenic-5U, chr11:67250220–67250629, 410bp) to be tested in the next step. All of the identified ncECRs were evaluated to investigate their activity as enhancers or silencers of AIP transcription using the pGL3 luciferase reporter system in the human neuroblastoma SH-SY5Y cell line. Four ncECRs (U5, I1, I3, and I4) were unable to be effectively amplified by PCR under various experimental conditions. Of the other ncECRs, intergenic-5U had the most significant increase in expression of the luciferase reporter gene (P < .05, vs. control group). In contrast, U6 and 3′UTR had reduced expression of the reporter gene (P < .05, vs. control group) (Figure 1B). These data indicate that some ncECRs in the AIP gene may play a regulatory role in AIP transcription.
Fig. 1.
Investigation functional ncECRs within the AIP genomic locus. A. Panel shows human–rat pair-wise comparison of Human genome (hg19) and Rat genome (rn4). Pink marked lines represent ncECRs, blue marked peaks represent coding exons, green marked peaks represent transposons and simple repeats, salmon marked peaks represent intronic regions, red marked peaks represent intergenic regions, yellow marked peaks represent the untranslated region (UTR: 5′UTR and 3′UTR) of AIP. U1–U6 are conserved regions upstream of AIP, I1–I4 are intragenic conserved regions, while intergenic-5U is the merged as one region by intergenic and 5′UTR sequences. B. Luciferase assay results of ncECRs of the AIP genomic locus. (*P < .05, vs. control.). The experiments were repeated three times with consistent results. The two-tailed Student’s t-tests were used. Bar represents mean ± SD. See online supplementary material for a color version of this figure.
Next, we performed a prediction analysis of transcription factors and transcription factor binding sites (TFBSs) in the intergenic-5U element with MatInspector database that is a software tool for TFBSs description to locate matches in DNA sequences. As well as the original human–rat comparison that was used to identify ncECRs, sequences from the mouse, cow, and dog were also added in the analysis. Because the intergenic-5U element had significantly increased expression of the luciferase reporter gene, the models for screening transcription factors were designed as follows: the transcription factors needed to be enriched in the pituitary gland and be indicated as an enhancer of AIP transcription; TFBSs in the intergenic-5U elements needed to be present in all five species in the same orientation; and there needed to be at least three match sites for each transcription factor in one species with P < .05. Hence, only INRE (described as hypothetical protein B1J92_L12848g [Candida glabrata] by the National Center for Biotechnology Information [NCBI]) and GTF2B (also known as TF2B) conformed to these criteria. Here, GTF2B was considered an intriguing candidate for the following research because it had greater significance in the Match model (a lower P-value) and its protein sequence in Homo sapiens was available, unlike that of INRE (Supplementary Figure S1A). Moreover, the Graphical View displayed two TFBSs to GTF2B within the intergenic-5U region of AIP in H. sapiens (67250480–67250486 and 67250551–67250557), and the latter site was common to all five species (Supplementary Figure S1B). Furthermore, two conserved SNPs were identified within the intergenic-5U region by ECR Browser. One SNP (rs561050596[C/T]); 67250470) was located adjacent to one TFBS (67250480–67250486), and another SNP (rs377565228 [C/G]; 67250553) was located within another TFBS (67250551–67250557) (Supplementary Figure S1C). Alignment work using UniProt (www.uniprot.org) revealed that the protein sequences of GTF2B (or TF2B) were almost the same between humans and rats (identity: 99.684%, with one similar position, 302 amino acid [aa]) (Supplementary Figure S1D).
Evaluation of Whether GTF2B Transcription Activity on AIP Expression Depends on the Intergenic-5U Element
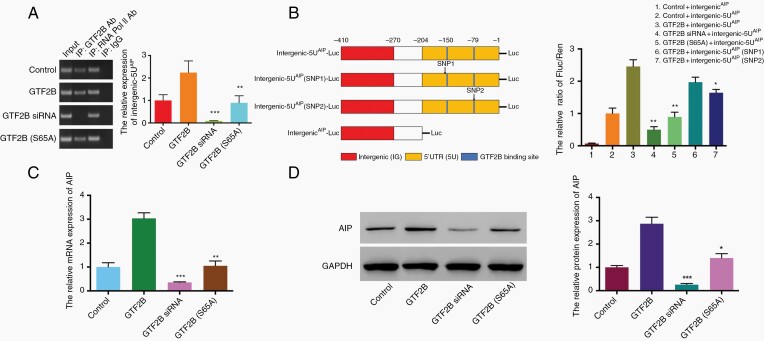
Here, SH-SY5Y cells were used. It has been reported that the phosphorylation of GTF2B at serine 65 is a critical event in transcription, linking the gene promoter and terminator21 ChIP results demonstrated that GTF2B, but not GTF2B (S65A) (serine at 65 aa replaced by alanine), mediated the fold enrichment of the intergenic-5U element (chr11:67250220-67250629, 410bp) of the AIP promoter; this effect was decreased by GTF2B siRNA (Figure 2A). Furthermore, to confirm the activity that was dependent on the binding of GTF2B to the intergenic-5U element of AIP, a pGL3 luciferase reporter system was designed. Fluorescence intensity was strongest in the group with co-transfection of GTF2B and the intergenic-5U element of AIP with luciferase (GTF2B + intergenic-5UAIP-Luc), while intensity was relatively weak in the group with GTF2B siRNA overexpression (P < .01), and was not observed in the group with only the intergenic element of AIP (intergenicAIP-Luc). Luciferase intensity in the group transfected with GTF2B (S65A) (GTF2B(S65A) + intergenic-5UAIP-Luc) was also weaker than GTF2B + intergenic-5UAIP-Luc (P < .01). Additionally, compared with GTF2B + intergenic-5UAIP-Luc, the intensity was mildly decreased in the group transfected with intergenic-5UAIP-Luc with SNP2 (rs377565228), but not with SNP1 (rs561050596) (Figure 2B). Finally, GTF2B overexpression in SH-SY5Y cells induced AIP transcription and protein expression, while GTF2B siRNA transfection inhibited AIP expression. Conversely, GTF2B (S65A) transfection did not seem to affect AIP expression (Figure 2C and D).
Fig. 2.
The transcriptional effect of GTF2B on AIP expression in SH-SY5Y cell line. A. ChIP experiment verifies GTF2B can bind intergenic-5U element. The relative expression of intergenic-5U by RT-PCR represents difference of binding activity between various treatments (**P < .01 and ***P < .001, vs. GTF2B group). B. Binding activity dependent on TFBSs located within 5U segment. Luciferase activity normalized for Renilla luciferase activity and expressed relative to the activity of the control + intergenci-5UAIP group. Different luciferase reporter constructs are design as left diagram; SNP1: rs561050596, C > T; SNP2: rs377565228 C > G; (*P < .05, **P < .01, vs. GTF2B + intergenic-5UAIP group). C and D. Effects of various transfections on the expression of AIP mRNA and protein in SH-SY5Ycells, analyzed by qRT-PCR and western blotting (*P < .05, **P < .01, and ***P < .001, vs. GTF2B group). Three independent experiments were repeated for each result. The two-tailed Student’s t-tests were used. Bar represents mean ± SD.
Effects of GTF2B on the AIP-Dependent Phenotypes of GH3 Cells
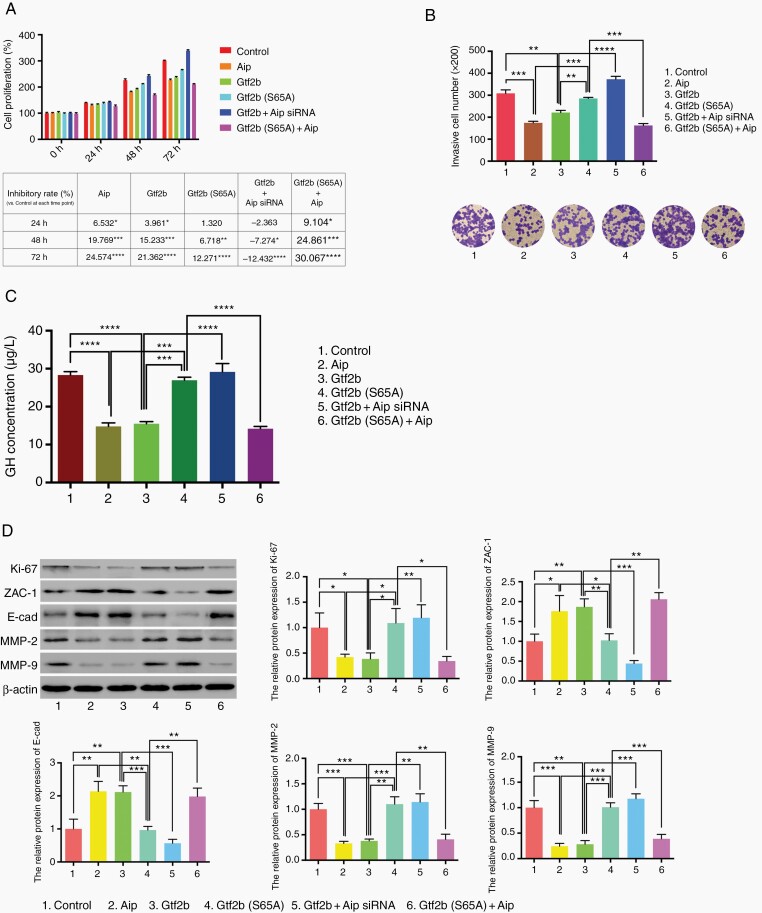
Because both GTF2B and AIP are highly conserved between humans and rats, the rat GH pituitary adenoma cell line GH3 was used to elucidate the influence of GTF2B and AIP on cell phenotypes through cell proliferation, invasiveness, and GH secretion and proteins expression analysis. It is known that AIP inhibits tumor growth; similarly, in the present study, Gtf2b also inhibited GH3 cell proliferation from the beginning (at 24 h point), which could be reversed by Aip siRNA treatment. However, Gtf2b (S65A) could restrain cell proliferation later (at 48 h point) with lower inhibitory rates, and the addition of Aip (Gtf2b (S65A) + Aip) could improve inhibitory rate for Gtf2b (S65A)(Figure 3A). Moreover, in Figure 3B and C, GH3 cells with Gtf2b (column 3) or −Aip (column 2) overexpression had a more invasive and GH-secreting phenotype than those with control (column 1) or Gtf2b (S65A) (column 4) transfection. Conversely, Aip siRNA overexpression (column 5) led to a complete reversal of the function of Gtf2b in tumor cells. GH3 cells with Gtf2b (S65A) + Aip overexpression (column 6) had weaker invasiveness and GH secretion than cells with Gtf2b (S65A). Western blotting results displayed that the Gtf2b overexpression group, like Aip overexpression group, had less expression of Ki-67 (cell proliferation) and matrix metallopeptidase 2/9 (MMP2/9, invasiveness promotion), but more zinc-finger protein 1 (ZAC1) and E-cadherin (E-cad) (invasiveness inhibition) expression, compared to control or Gtf2b (S65A) group. The expression pattern of proteins in the Gtf2b overexpression group could be reversed by Aip siRNA, while that in the Gtf2b (S65A) group could be reversed by Aip overexpression. These findings indicate that the inhibition by GTF2B of GHPA cell proliferation and invasiveness and GH secretion depends on the promotion by GTF2B of AIP expression transcriptionally.
Fig. 3.
Effect of GTF2B on tumor cell phenotype of GH3 cell line. A. Proliferation analysis of tumor cell with different transfection by MTT assay. OD value of control group at 0 h was normalized as 100% and all relative cell proliferation value at different time points are shown in column diagram above; the table below displays the relative inhibitory rate vs. control group at each time point. B. Transwell array was used to assess GH3 cell invasiveness with various treatments (original magnification, ×200). C. ELISA was performed to test GH secretion of GH3 cell with various treatments. D. Western blot analysis of Ki-67, ZAC-1, E-cadherin, MMP2 and MMP9 expression in GH3 cell line with various treatments. Each condition was tested in triplicate. The two-tailed Student’s t-tests were used. Bar represents mean ± SD, *P < .05, **P < .01, ***P < .001, and ****P < .0001.
Confirmation of GTF2B Inhibition of Tumor Growth via AIP in vivo
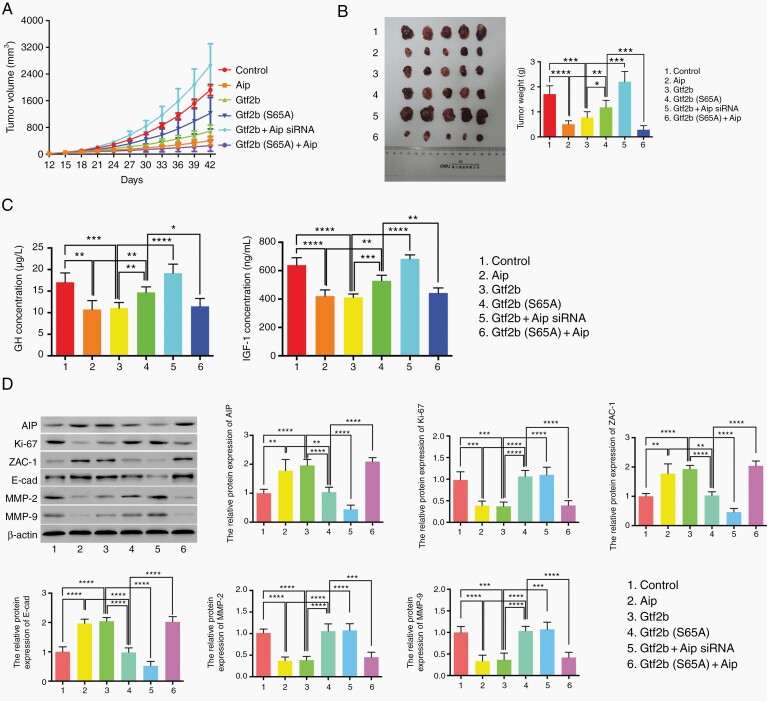
Because nude mice are immune-deficient and amenable to xenografts, GHPA models were established via transplantation of GH3 with variant transduction treatments subcutaneously into the flanks of nude mice. The tumor size and weight in the Aip, Gtf2b, and Gtf2b (S65A) + Aip groups were significantly smaller than those in the control group, while those of the Gtf2b (S65A) and Gtf2b + Aip siRNA groups were much larger (P < .05) (Figure 4A and B). To detect the effects of various treatments on GH and IGF-1 secretion in GH3 xenograft tumor-bearing mice, blood serum was examined, and both GH and IGF-1 levels in the Aip, Gtf2b, and Gtf2b (S65A) + Aip groups were much lower than in the control group (Figure 4C). Likewise, IHC and western blot results revealed that tumor samples from mice bearing GH3 with Aip, Gtf2b, and Gtf2b (S65A) + Aip had a lower Ki-67 index and lower MMP-2 and MMP-9 expression, but higher AIP, Zac-1, and E-cadherin expression (Figure 4D and Supplementary Figure S2). Furthermore, to test apoptotic rates of these groups, TUNEL staining was performed on paraffin-embedded section of GH3 xenograft tumors treated with various transduction. As shown in Supplementary Figure S3, tumor tissue from the Aip and Gtf2b groups had more TUNEL-positive cells (apoptotic cells) than the Gtf2b (S65A). Gtf2b + Aip siRNA group could decrease the number of apoptotic cells compared with Aip or Gtf2b group, while Gtf2b (S65A) + Aip led to the reversal of the lower tumor apoptotic rate in the Gtf2b (S65A) group. These in vivo results suggest that GTF2B impedes pituitary tumor growth via AIP regulation of tumor cell proliferation, apoptosis, invasiveness, and GH/IGF-1 secretion.
Fig. 4.
The inhibition of GTF2B on tumor growth in subcutaneous GHPA models. A. Tumor volume of mice with various treatments once daily since the 12th day after tumor cell transplantation, measured for 42 days. B. Macroscopic image of tumor resected at the 42nd day (left) and the tumor weight comparison between various treatments (right). C. ELISA assays to test GH (left) and IGF-1 (right) secretion from mice with various treatments. D. Western blot analysis of Ki-67, ZAC-1, E-cadherin, MMP2 and MMP9 expression in tumor models with various treatments. Each group contained five mice, the two-tailed Student’s t-tests were used. Bar represents mean ± SD, *P < .05, **P < .01, ***P < .001, and ****P < .0001.
Correlations Between GTF2B and AIP Expression in GHPA Samples
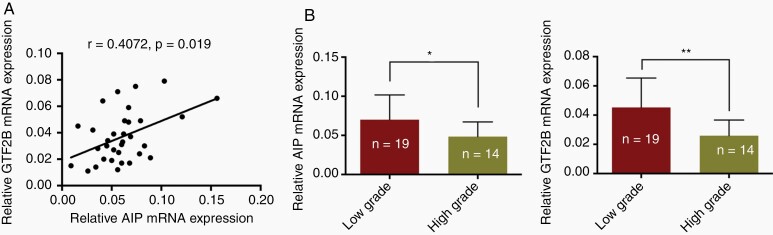
To evaluate the correlations between GTF2B and AIP mRNA expression in GHPA samples, we enrolled 33 GHPA patients (male:14, female:19), and the age ranged from 24 to 57 years (mean ± SD, 40.97 ± 8.33; median, 42). No patient had GTF2B or AIP mutations or the two SNPs (rs561050596 and rs377565228) in either germline or tumor cells. Patients with high-grade GHPAs accounted for 42.42% (14/33) of the group (Table 1). There was a significant positive correlation between GTF2B and AIP mRNA expression levels in the GHPA samples (Spearman’s correlation analysis; P = .019) (Figure 5A). In addition, in the high-grade group, GTF2B and AIP mRNA expression levels were lower than those in the low-grade group (Figure 5B). Chi-square test indicated that neither GTF2B nor AIP mRNA expression levels were related to patient age or sex; however, most of the GHPAs with low GTF2B or AIP mRNA levels were in the high-grade group or Ki-67 ≥ 3% group (Table 1).
Table 1.
The Correlation Between Clinical Features of GHPAs and the Expression of GTF2B and AIP mRNA
| Clinical features | Number A (%, A/33) | Low GTF2B mRNA expression (n = 16) a | Low AIP mRNA expression (n = 16) b | ||
|---|---|---|---|---|---|
| Number B (%, B/A) | P value c | Number C (%, C/A) | P value c | ||
| Age, years | |||||
| <42 | 16(48.48) | 8(43.75) | 0.849 | 9(37.50) | 0.387 |
| ≥42 | 17(51.52) | 8(47.06) | 7(41.18) | ||
| Sex | |||||
| Male | 14(42.42) | 9(57.14) | 0.247 | 7(50.00) | 0.881 |
| Female | 19(57.58) | 7(36.84) | 9(47.37) | ||
| Ki-67 (%) | |||||
| <3 | 21(63.64) | 7(33.33) | 0.021 | 6(28.57) | 0.002 |
| ≥3 | 12(36.36) | 9(75.00) | 10(83.33) | ||
| High graded | |||||
| No | 19(57.58) | 5(26.32) | 0.010 | 6(31.58) | 0.024 |
| Yes | 14(42.42) | 11(78.57) | 10(71.43) |
aThe median of relative GTF2B mRNA expression level is 0.033, so the number of low GTF2B mRNA expression is 16 (<0.033).
bThe median of relative AIP mRNA expression level is 0.06, so the number of low AIP mRNA expression is 16 (<0.06).
cAnalysis by chi-square test.
dThe grade classification is based on the criteria by Trouillas et al.19.
Fig. 5.
The correlation between GTF2B and AIP mRNA level and their effects on tumor features in GHPA samples. A. Spearman correlation analysis was used to test the correlation between GTF2B and AIP mRNA expression level in 33 GHPAs patients. B. qRT-PCR analysis of GTF2B and AIP mRNA expression between low grade group (n = 19) and high-grade group (n = 14) of GHPA samples. The Mann–Whitney U test was used. Bar represents mean ± SD, *P < .05, **P < .01, and ***P < .001.
Discussion
AIP is a tumor suppressor gene, and AIP gene mutations are present in approximately 20% of patients with familial isolated pituitary adenomas.5,22 Generally, patients with AIP germline mutations are younger and have more aggressive tumors with worse responses to somatostatin receptor ligands (SRLs; octreotide and lanreotide).8,22 In patients with sporadic GHPAs without AIP mutations, low AIP protein expression has been observed in 50% of those with SRL resistance.13 Moreover, research by Kasuki et al. has indicated that AIP is a better marker of invasiveness in somatotropinomas than Ki-67 or p53.12 It has recently been demonstrated that there is an altered microenvironment in AIP-mutated pituitary tumors; some tumor-derived factors interact with macrophages, resulting in infiltration, epithelial-to-mesenchymal transition, and a more aggressive phenotype.23 These results indicate that AIP expression is important for the prognosis of the GHPA patients. Thus, the present study focused on identifying the transcription mechanism that impacts AIP gene expression.
It has been reported that ncECRs constitute an ordered combination of overlapping TFBSs24 or are strongly enriched for overlapping TFBSs.25,26 To investigate transcriptional regulation of the AIP region, a complementary approach was used in this study. The ECR Browser was used to identify all possible ncECRs of the AIP gene region and was followed by the screening of candidate regions using luciferase reporter systems. A single ncECR, intergenic-5U (the intergenic region and 5′UTR), of AIP was identified as a potential transcription element; this was confirmed by ChIP and luciferase assays. Furthermore, two SNPs (rs561050596 and rs377565228) within the region were designed as vectors, and experiments indicated that the intergenic-5U region is very important for the binding of the transcription factors. In fact, it has been suggested that some SNPs in these regions may modulate the transcriptional patterns of genes,27 and that these noncoding variants may be associated with common diseases and traits.28 Although we confirmed that one SNP (rs377565228)in the intergenic-5U region of AIP was able to affect the binding of transcription factors and its subsequent transcription in vitro, none of the 33 patients in our study had either of the two SNPs. Unfortunately, there is no information about the associations between either variant and clinical presentation, or about variant frequency, in the Database of Single Nucleotide Polymorphisms of the NCBI (https://www.ncbi.nlm.nih.gov/snp/). In a study that analyzed 49 GHPA patients and 167 healthy controls, the metastasis-suppressor KiSS-1 c.-145delA (rs5780218) promoter polymorphism was reported to have a possible association with somatotropinoma incidence.29 Furthermore, a larger study (2542 patients vs. 2788 controls) identified three new susceptibility loci associated with sporadic pituitary adenoma in a Chinese Han population cohort: 10p12.31 rs2359536, 10q21.1 rs10763170, and 13q12.13 rs17083838.30 Thus, when considering rs561050596 and rs377565228 (or other SNPs within or near the region), more GHPA cases should be enrolled to explore the effects of both SNPs on AIP transcription and GHPA phenotypes in further clinical studies.
In the present study, MatInspector (Genomatix, Munich, Germany) analysis predicted that the GTF2B transcription factor was most likely to bind to the IG-5U region with the highest fold change in the luciferase assay. This database has been widely used for genetic studies. For example, Sterling et al. reported that the synuclein alpha (SNCA) gene has some cis-regulatory regions for its transcription and protein expression that are associated with the Parkinson’s disease process.31 It was also predicted by Song et al. that some potential binding motifs for transcription factors exist within the promoter regions of miR-378; one such factor was a highly conserved binding site for nuclear respiratory factor 1 (NRF1) that repressed miR-378 promoter activity.32 GTF2B, which is required for transcription initiation by RNA polymerase II, forms a complex with GTF2D and GTF2A in the nucleus for promoter sequence recognition and transcription initiation.33 It has previously been demonstrated that GTF2B is involved in the proliferation of hepatocellular carcinoma by counteracting the transcriptional activation of hepatitis B virus X protein.34 In addition, a new paradigm for GTF2B functionality was recently established that has potent anti-viral immunity.35 In the current study, GTF2B affected AIP transcription and protein expression and influenced GHPA tumor phenotypes by binding to the ncECR intergenic-5U in AIP. However, the possibility cannot be excluded that other transcription factors and binding sites in these high evolutionary conserved regions, or other intronic or intergenic regions, are involved in AIP transcription modulation. These “parameters” may function as a regulatory network for AIP expression with their own weight coefficient, which should be studied using more clinical data in the future.
Previous studies have reported that the transcription/expression of genes is not only driven by their promoter regions; other cis-acting genomic regions also play a potential regulatory role in gene expression levels. Korbonits et al. confirmed that both miR-10736 and miR-34a15 negatively regulate AIP protein expression; moreover, miR-34a may respond to the low AIP expression in GHPAs with an invasive phenotype and SSA resistance.15 Other than microRNAs (miRNAs), long noncoding RNAs have also been linked to gene regulation affecting cellular homeostasis and even malignant transformation.37 Circular RNAs (circRNAs) are another conserved element that are deregulated in cancer via their functional role—having a sponge effect on miRNAs.38 Recently, circTVF25 has been demonstrated to act as an miRNA sponge that sequesters miR107 and promotes proliferation and migration in bladder cancer.39 Hence, AIP expression may be regulated through the circTVE25–miR107 pathway; this should be investigated in a future study. Furthermore, it should be considered that noncoding RNA networks may play a key molecular role in AIP expression.
Conclusion
Our research demonstrated that the transcription factor GTF2B was able to mediate AIP expression and influence GHPA phenotypes, such as tumor growth, tumor invasion, and GH hypersecretion. These effects were based on the TFBS located at the intergenic-5U region in AIP. It is suggested that GTF2B may be a potential therapeutic target for GHPA with low AIP expression. Moreover, other epigenetic mechanisms will also be considered in further researches.
Supplementary Material
Acknowledgments
The authors thank Bronwen Gardner, Ph.D., from Liwen Bianji (Edanz) (www.liwenbianji.cn/), for editing the English text of a draft of this manuscript.
Contributor Information
Feng Cai, Department of Neurosurgery, The Second Affiliated Hospital of Zhejiang University School of Medicine, Hangzhou, Zhejiang Province, P.R. China.
Shasha Chen, Department of Geriatrics, The Second Affiliated Hospital of Zhejiang University School of Medicine, Hangzhou, Zhejiang Province, P.R. China.
Xuebin Yu, Department of Neurosurgery, Shaoxing People’s Hospital (Shaoxing Hospital, Zhejiang University School of Medicine), Shaoxing, Zhejiang Province, P.R. China.
Jing Zhang, Department of Geriatrics, Zhejiang Provincial Key Lab of Geriatrics, Zhejiang Hospital, Hangzhou, Zhejiang Province, P.R. China.
Weiwei Liang, Department of Endocrinology, The Second Affiliated Hospital of Zhejiang University School of Medicine, Hangzhou, Zhejiang Province, P.R. China.
Yan Zhang, Department of Medical Oncology, The Second Affiliated Hospital of Zhejiang University School of Medicine, Hangzhou, Zhejiang Province, P.R. China.
Yike Chen, Department of Neurosurgery, The Second Affiliated Hospital of Zhejiang University School of Medicine, Hangzhou, Zhejiang Province, P.R. China.
Sheng Chen, Department of Neurosurgery, The Second Affiliated Hospital of Zhejiang University School of Medicine, Hangzhou, Zhejiang Province, P.R. China.
Yuan Hong, Department of Neurosurgery, The Second Affiliated Hospital of Zhejiang University School of Medicine, Hangzhou, Zhejiang Province, P.R. China.
Wei Yan, Department of Neurosurgery, The Second Affiliated Hospital of Zhejiang University School of Medicine, Hangzhou, Zhejiang Province, P.R. China.
Wei Wang, Department of Neurosurgery, The Second Affiliated Hospital of Zhejiang University School of Medicine, Hangzhou, Zhejiang Province, P.R. China.
Jianmin Zhang, Department of Neurosurgery, The Second Affiliated Hospital of Zhejiang University School of Medicine, Hangzhou, Zhejiang Province, P.R. China.
Qun Wu, Department of Neurosurgery, The Second Affiliated Hospital of Zhejiang University School of Medicine, Hangzhou, Zhejiang Province, P.R. China.
Funding
This study was supported by the National Natural Science Foundation of China (81502321 and 81870964).
Conflict of interest statement. No conflict of interest.
Authorship statement. Conception or design of the study: F.C., X.Y., and Q.W. Laboratory practice: F.C., Sha.C., Jin.Z., Y.Z., and Y.C. Drafting the article: F.C., Sha.C., Y.Z., Sha.C., H.Y., W.Y., and W.W. Subject recruitment: X.Y., W.L., She.C., Y.H., W.Y., W.W., Jin.Z., and Q.W. Data analysis and interpretation: F.C., Sha.C., Jin.Z., W.L., and Y.Z. Critical revision: F.C., Sha.C., X.Y., Jia.Z., and W.L. All authors critically reviewed the article and approved the final manuscript.
References
- 1. Katznelson L, LawsER, Jr., Melmed S, et al. Acromegaly: an endocrine society clinical practice guideline. J Clin Endocrinol Metab. 2014; 99(11):3933–3951. [DOI] [PubMed] [Google Scholar]
- 2. Melmed S, Bronstein MD, Chanson P, et al. A consensus statement on acromegaly therapeutic outcomes. Nat Rev Endocrinol. 2018;14(9):552–561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Gadelha MR, Bronstein MD, Brue T, et al. Pasireotide versus continued treatment with octreotide or lanreotide in patients with inadequately controlled acromegaly (PAOLA): a randomised, phase 3 trial. Lancet Diabetes Endocrinol. 2014;2(11):875–884. [DOI] [PubMed] [Google Scholar]
- 4. Leontiou CA, Gueorguiev M, van der Spuy J, et al. The role of the aryl hydrocarbon receptor-interacting protein gene in familial and sporadic pituitary adenomas. J Clin Endocrinol Metab. 2008;93(6):2390–2401. [DOI] [PubMed] [Google Scholar]
- 5. Cai F, Zhang YD, Zhao X, et al. Screening for AIP gene mutations in a Han Chinese pituitary adenoma cohort followed by LOH analysis. Eur J Endocrinol. 2013;169(6):867–884. [DOI] [PubMed] [Google Scholar]
- 6. Vierimaa O, Georgitsi M, Lehtonen R, et al. Pituitary adenoma predisposition caused by germline mutations in the AIP gene. Science. 2006;312(5777):1228–1230. [DOI] [PubMed] [Google Scholar]
- 7. Oriola J, Lucas T, Halperin I, et al. Germline mutations of AIP gene in somatotropinomas resistant to somatostatin analogues. Eur J Endocrinol. 2013;168(1):9–13. [DOI] [PubMed] [Google Scholar]
- 8. Daly AF, Tichomirowa MA, Petrossians P, et al. Clinical characteristics and therapeutic responses in patients with germ-line AIP mutations and pituitary adenomas: an international collaborative study. J Clin Endocrinol Metab. 2010;95(11):E373–E383. [DOI] [PubMed] [Google Scholar]
- 9. Beckers A, Daly AF. The clinical, pathological, and genetic features of familial isolated pituitary adenomas. Eur J Endocrinol. 2007;157(4):371–382. [DOI] [PubMed] [Google Scholar]
- 10. Cazabat L, Bouligand J, Salenave S, et al. Germline AIP mutations in apparently sporadic pituitary adenomas: prevalence in a prospective single-center cohort of 443 patients. J Clin Endocrinol Metab. 2012;97(4):E663–E670. [DOI] [PubMed] [Google Scholar]
- 11. Preda V, Korbonits M, Cudlip S, Karavitaki N, Grossman AB. Low rate of germline AIP mutations in patients with apparently sporadic pituitary adenomas before the age of 40: a single-centre adult cohort. Eur J Endocrinol. 2014;171(5):659–666. [DOI] [PubMed] [Google Scholar]
- 12. Kasuki Jomori de Pinho L, Vieira Neto L, Armondi Wildemberg LE, et al. Low aryl hydrocarbon receptor-interacting protein expression is a better marker of invasiveness in somatotropinomas than Ki-67 and p53. Neuroendocrinology. 2011;94(1):39–48. [DOI] [PubMed] [Google Scholar]
- 13. Kasuki L, Vieira Neto L, Wildemberg LE, et al. AIP expression in sporadic somatotropinomas is a predictor of the response to octreotide LAR therapy independent of SSTR2 expression. Endocr Relat Cancer. 2012;19(3):L25–L29. [DOI] [PubMed] [Google Scholar]
- 14. Kasuki L, Colli LM, Elias PC, Castro M, Gadelha MR. Resistance to octreotide LAR in acromegalic patients with high SSTR2 expression: analysis of AIP expression. Arq Bras Endocrinol Metabol. 2012;56(8):501–506. [DOI] [PubMed] [Google Scholar]
- 15. Denes J, Kasuki L, Trivellin G, et al. Regulation of aryl hydrocarbon receptor interacting protein (AIP) protein expression by MiR-34a in sporadic somatotropinomas. PLoS One. 2015;10(2):e0117107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Liu J, Francke U. Identification of cis-regulatory elements for MECP2 expression. Hum Mol Genet. 2006;15(11):1769–1782. [DOI] [PubMed] [Google Scholar]
- 17. Cartharius K, Frech K, Grote K, et al. MatInspector and beyond: promoter analysis based on transcription factor binding sites. Bioinformatics. 2005;21(13):2933–2942. [DOI] [PubMed] [Google Scholar]
- 18. Sang LJ, Ju HQ, Liu GP, et al. LncRNA CamK-a regulates Ca(2+)-signaling-mediated tumor microenvironment remodeling. Mol Cell. 2018;72(1):71–83 e77. [DOI] [PubMed] [Google Scholar]
- 19. Trouillas J, Roy P, Sturm N, et al. A new prognostic clinicopathological classification of pituitary adenomas: a multicentric case-control study of 410 patients with 8 years post-operative follow-up. Acta Neuropathol. 2013;126(1):123–135. [DOI] [PubMed] [Google Scholar]
- 20. Sav A, Rotondo F, Syro LV, et al. Invasive, atypical and aggressive pituitary adenomas and carcinomas. Endocrinol Metab Clin North Am. 2015;44(1):99–104. [DOI] [PubMed] [Google Scholar]
- 21. Wang Y, Fairley JA, Roberts SG. Phosphorylation of TFIIB links transcription initiation and termination. Curr Biol. 2010;20(6):548–553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Igreja S, Chahal HS, King P, et al. Characterization of aryl hydrocarbon receptor interacting protein (AIP) mutations in familial isolated pituitary adenoma families. Hum Mutat. 2010;31(8):950–960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Barry S, Carlsen E, Marques P, et al. Tumor microenvironment defines the invasive phenotype of AIP-mutation-positive pituitary tumors. Oncogene. 2019;38(27):5381–5395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Loots GG, Ovcharenko I, Pachter L, Dubchak I, Rubin EM. rVista for comparative sequence-based discovery of functional transcription factor binding sites. Genome Res. 2002;12(5):832–839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Warnefors M, Hartmann B, Thomsen S, Alonso CR. Combinatorial gene regulatory functions underlie ultraconserved elements in drosophila. Mol Biol Evol. 2016;33(9):2294–2306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Viturawong T, Meissner F, Butter F, Mann M. A DNA-centric protein interaction map of ultraconserved elements reveals contribution of transcription factor binding hubs to conservation. Cell Rep. 2013;5(2):531–545. [DOI] [PubMed] [Google Scholar]
- 27. Sadee W, Hartmann K, Seweryn M, et al. Missing heritability of common diseases and treatments outside the protein-coding exome. Hum Genet. 2014;133(10):1199–1215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Maurano MT, Humbert R, Rynes E, et al. Systematic localization of common disease-associated variation in regulatory DNA. Science. 2012;337(6099):1190–1195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Amorim P, Grande IPP, Batista RL, et al. Association between KISS1 rs5780218 promoter polymorphism and onset of growth hormone secreting pituitary adenoma. Ann Endocrinol (Paris). 2019;80(2):96–100. [DOI] [PubMed] [Google Scholar]
- 30. Ye Z, Li Z, Wang Y, et al. Common variants at 10p12.31, 10q21.1 and 13q12.13 are associated with sporadic pituitary adenoma. Nat Genet. 2015;47(7):793–797. [DOI] [PubMed] [Google Scholar]
- 31. Sterling L, Walter M, Ting D, Schule B. Discovery of functional non-coding conserved regions in the alpha-synuclein gene locus. F1000Res. 2014;3:259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Zhang T, Zhao X, Steer CJ, Yan G, Song G. A negative feedback loop between microRNA-378 and Nrf1 promotes the development of hepatosteatosis in mice treated with a high fat diet. Metabolism. 2018;85:183–191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Elsby LM, O’Donnell AJ, Green LM, Sharrocks AD, Roberts SG. Assembly of transcription factor IIB at a promoter in vivo requires contact with RNA polymerase II. EMBO Rep. 2006;7(9):898–903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Li L, Zhang A, Cao X, et al. General transcription factor IIb overexpression and a potential link to proliferation in human hepatocellular carcinoma. Pathol Oncol Res. 2013;19(2):195–203. [DOI] [PubMed] [Google Scholar]
- 35. Haas DA, Meiler A, Geiger K, et al. Viral targeting of TFIIB impairs de novo polymerase II recruitment and affects antiviral immunity. PLoS Pathog. 2018;14(4):e1006980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Trivellin G, Igreja S, Garcia E, et al. MiR-107 inhibits the expression of aryl hydrocarbon receptor interacting protein (AIP) and is potentially involved in pituitary tumorigenesis. Paper presented at: Society for Endocrinology BES 20112011.
- 37. Huarte M. The emerging role of lncRNAs in cancer. Nat Med. 2015;21(11):1253–1261. [DOI] [PubMed] [Google Scholar]
- 38. Anastasiadou E, Jacob LS, Slack FJ. Non-coding RNA networks in cancer. Nat Rev Cancer. 2018;18(1):5–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Zhong Z, Lv M, Chen J. Screening differential circular RNA expression profiles reveals the regulatory role of circTCF25-miR-103a-3p/miR-107-CDK6 pathway in bladder carcinoma. Sci Rep. 2016;6:30919. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.