Abstract
Background
Brain metastases (BM) are responsible for neurological decline and poor overall survival. Although the pro-metastatic roles of glial cells, and the acquisition of neuronal attributes in established BM tumors have been described, there are no studies that investigate the initial interplay between neurons and brain-seeking tumor cells. The aim of this study was to characterize early tumor-neuron interactions and the induced CNS-adaptive changes in tumor cells prior to macro-colonization
Methods
Utilizing pure neuronal cultures and brain-naïve and patient-derived BM tumor cells, we surveyed the early induction of mediators of neurotransmitter (NT) and synaptic signaling in breast and lung tumor cells. Reliance on microenvironmental GABA in breast-to-brain metastatic cells (BBMs) was assessed in vitro and in vivo.
Results
Coculture with neurons induces early expression of classical NT receptor genes (HTR4, GRIA2, GRIN2B, GRM4, GRM8, DRD1) and neuronal synaptic mediators (CNR1, EGR2, ARC, NGFR, NRXN1) in breast and lung cancer cells. NT-dependent classification of tumor cells within the neuronal niche shows breast cancer cells become GABAergic responsive brain metastases (GRBMs) and transition from relying on autocrine GABA, to paracrine GABA from adjacent neurons; while autocrine Dopaminergic breast and lung tumor cells persist. In vivo studies confirm reliance on paracrine GABA is an early CNS-acclimation strategy in breast cancer. Moreover, neuronal contact induces early resurgence in Reelin expression in tumor cells through epigenetic activation, facilitating CNS adaptation.
Conclusion
Tumor-neuron interactions allow for CNS adaptation early in the course of brain metastasis.
Keywords: brain metastasis, neurons, neurotransmitter, synaptic mediators, tumors
Key Points.
Neuronal contact induces mediators of NT and synaptic signaling in tumor cells.
Breast cancer cells become GABAergic responsive brain metastases on neuron exposure.
Neuron-mediated Reelin resurgence in tumor cells facilitates early CNS adaptation in vitro.
Importance of the Study.
Brain metastases continue to remain a significant complication of advanced carcinomas. Although neuronal attributes have been reported in established brain metastases, few studies have characterized early interactions of tumor cells with neurons prior to CNS colonization. Our study shows that early dynamic interplay between tumor cells and neurons induces CNS-adaptive changes in tumor cells that may facilitate enhanced survival within the brain-metastatic niche. The findings from this study will facilitate the identification of new actionable targets and biomarkers for effective management of brain-metastatic progression.
Brain metastases (BMs) are the most common cause of intracranial neoplasms and related morbidity in cancer patients. Based on the “seed and soil” hypothesis, it has now been established that once tumor cells extravasate from systemic circulation into the brain parenchyma, they can respond to microenvironmental cues and alter cellular landscape in order to favorably adapt and develop a CNS-metastatic niche.1 For example, the pro-metastatic role of astrocytes and microglia in the successful establishment of brain metastases has been investigated before.2,3 Moreover, previous studies have reported the acquisition of neuronal characteristics in brain-metastatic tumors, that are not observed in primary tumor masses.4 Others have demonstrated the upregulation of NMDA receptors in aggressive primary breast tumors, which primes them for brain metastasis.5 These studies examine inherent transcriptomic and proteomic attributes in tumor cells that are already selected for effective CNS colonization. However, there are currently no reports characterizing neuron-mediated CNS-acclimation in tumor cells, early in the brain-metastatic cascade.
Neurons contribute to a significant portion of cellular signaling within the brain through electro-chemical communication mediated by classical neurotransmitters (NT) including GABA, Glutamate, Acetylcholine, Dopamine, and Serotonin.6 NT-induced responses in neurons and their recipient cells are integral to synaptic plasticity in the central nervous system (CNS).7 We postulate that initial interactions between brain-seeking tumor cells and neurons induces similar CNS adaptation in tumor cells and facilitates the establishment of BMs.
In this study, we recapitulated early tumor-neuron interaction in the brain through cocultures of primary neurons and brain-naïve/brain-metastatic breast and lung cancer cells. We characterized the induction of neurotransmitter and synaptic signaling in tumor cells upon exposure to neurons, and examined NT-dependent classification of tumor cells within the neuronal microenvironment. Furthermore, we assessed the effect of neuronal cues on reactivation of dormant breast cancer cells, which have been implicated in the eventual establishment of clinical brain metastases.8 The current study thus provides insight into the effects of neuronal input in facilitating microenvironmental adaptation in tumor cells early in brain metastasis, before macro-colonization.
Materials and Methods
Cell Culture
Low passage Patient-derived Brain-metastatic (BM) breast (BBM 3.1) and lung (LuBM5) cells were propagated as previously described.9 MD-MB-231Br-Her2, BT474, SKBR3, and lung (A549) cell lines were maintained in Advanced DMEM-F12, 10%FBS, 1% glutamine, 1%Antibacterial-Antimycotic. RELN gene was knocked down lentivirally in BT474 cells using shRNA (Genecopoiea CS-HSH015094-LVRU6MP).
Primary Neuron Cultures
Neural cells were isolated from whole brain tissue of postnatal day 1–4 mice as described previously.10 Mixed neural cell cultures were treated with Cytosine Arabinoside (Sigma, C6645-25MG), to eliminate dividing cells, leaving behind pure neuronal cultures. 50% media change with complete neuron culture medium (Supplementary Materials and Methods) was performed every 3 days to maintain healthy pure neurons.
Establishment of Dormant Breast Cancer Cells
SKBR3 cells were acclimated to dormancy medium over one week. Dormancy media composition was as follows: (DMEM with no glucose, no glutamine, Thermo A1413004) supplemented with 5% Fetal Bovine Serum (FBS), and 1%Antibacterial-Antimycotic agent (Sigma, 15240062).
Tumor-Neuron Cocultures
For protein expression studies, tumor cells were resuspended in complete neuron culture medium and seeded onto established neuronal cultures in a 1:60 (tumor:neuron) ratio. For mRNA expression studies, pure neuronal cultures were established on PDL-coated plates. Tumor cells were then plated onto 0.4uM trans-wells and placed on top of the plated neurons in a 1:60 (tumor:neuron) ratio. At 48 hours, tumor cells were trypsinized (Thermo, 25300-120) and collected for further qPCR analysis.
Valproic Acid (VPA) Chemosensitivity Assays
MDA-MB-231BR (231-BR) cells were acclimated to 2mM GABA in growth medium over one week, and levels of cytotoxic effect of VPA at IC50 concentration in control and GABA-treated cells was determined using the LIVE/DEAD Viability/Cytotoxicity Kit (Thermo, L3224).
Reelin Treatment
BT474 cells (scrambled and Reelin knockdown) were treated with 2.5nM Human recombinant Reelin (R&D systems, #8546-MR) for 24 hours for qPCR analysis.
Animals
Female athymic mice on BalbC background purchased from Jackson Labs. Animal procedures were performed under approved IACUC protocols and guidelines. 1 × 105 MDA-MB231Br cells (2 ul volume) were injected intracranially into the cerebellum. Injected animals in the study were divided into three groups: untreated (n = 5), and animals treated with early administration of Valproic acid (VPA; n = 5), vs. late administration of VPA (n = 5). Mice were treated with 200mg/kg VPA intraperitoneally for 14 days post-transplantation.
RNA Isolation and qPCR
RNA isolation and qPCR methods used as previously described.10 All human primers used for qPCR analysis were purchased from IDT (Supplementary Table 1). Human Neurotransmitter receptor (330231 PAHS-060ZA) and Synaptic Plasticity (330231 PAHS-126Z) arrays were obtained from Qiagen.
Immunofluorescence
Human primary tumor tissue microarrays were obtained from Biomax US (Breast PM2a-ER, Lung LC241l). Patient-consented surgically resected brain-metastatic tumor tissues acquired via USC Neurosurgery were formalin-fixed, paraffin-embedded, and processed into 10um thick sections by the histology core at USC. Standard immunofluorescence protocol was followed for paraffin-embedded tissue sections and fixed cells (Supplementary Methods). Primary antibody information is detailed in Supplementary Table 2. All secondary antibodies (fluorophore-conjugated) were purchased from Jackson ImmunoResearch and used at 1:300 (Goat Anti-rabbit Cy3, Goat Anti-rabbit 647, Donkey Anti-Goat 488). Phalloidin (488 or 647 conjugated) was used to stain cellular actin where necessary. Stained cells and tissue sections were mounted in Prolong Gold Antifade reagent with DAPI (Invitrogen).
Microscopy and Quantification
Confocal imaging was performed using the Leica SP8 microscope. Quantification from confocal imaging is detailed in Supplementary Methods.
Bioinformatics
Gene expression data from TCGA datasets (normal tissue, primary tumor, metastatic tissue) (Breast: TARGET GTEx, n = 1212; and Lung: TARGET GTEx, n = 1122) were visualized and analyzed using UCSC Xena, a platform for multi-omic clinical/phenotypic data (https://xena.ucsc.edu).
Kaplan-Meier survival analysis on breast and lung cancer datasets—TCGA (overall survival) and Bos-Massague (morbidity from brain relapse)- for RELN was performed using SurvExpress, an online biomarker validation and survival analysis tool (http://bioinformatica.mty.itesm.mx:8080/Biomatec/SurvivaX.jsp). High vs. Low gene expression thresholds were determined internally by the SurvExpress data analysis software.
Gene expression values from the 84-gene arrays were visualized as clustergrams using Heatmapper, a web-based interactive data visualization tool (http://heatmapper.ca). For clustergrams, Ct values from qPCR analysis were normalized to the same housekeeping gene and plotted in Heatmapper as Log10 of absolute expression values. Row Z scores for each map were based on internal controls from Heatmapper.
Venn diagrams were constructed using a freely available web tool designed by the Bioinformatics and Evolutionary Genomics (BEG) department at Ghent University, Belgium (http://bioinformatics.psb.ugent.be/webtools/Venn/).
Statistics
Statistics were performed using GraphPad Prism. t-tests were used to compare expression between two different cell types/conditions. One/Two-way Anova was used to compare more than 2 conditions/groups. Statistical significance and Hazard Ratio for survival data from in vivo experiments was calculated using Log-Rank Test.
Results
Neuronal Exposure Induces Expression of NT Receptors and Synaptic Mediators in Tumor Cells
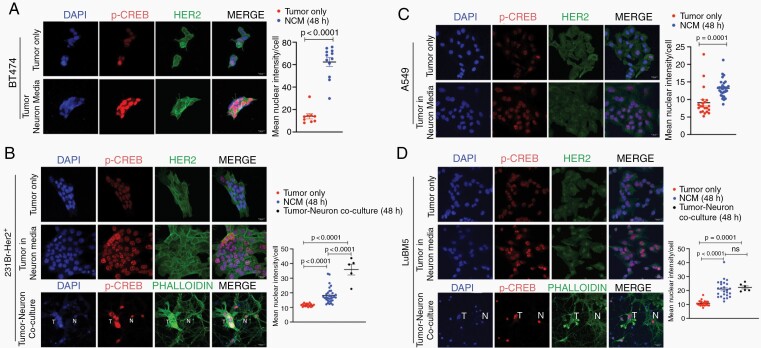
cAMP Response Element Binding Protein (CREB) is a well-studied regulator of neuronal gene expression and synaptic plasticity in the brain e.g., BDNF, EGR2, ARC, FOS, GABRB1.11 Ser133-phosphorylated CREB can bind to CRE elements on target genes to promote their transcription. Therefore, we first wanted to determine whether neuronal exposure activates CREB in tumor cells. Immunofluorescence (IF) showed enhanced Ser133-phosphorylated CREB staining in both non-BM and BM breast and lung cancer cells upon neuronal exposure (Figure 1A–D), indicating increased CREB activity and target gene transcription in the neuronal microenvironment.
Fig. 1.
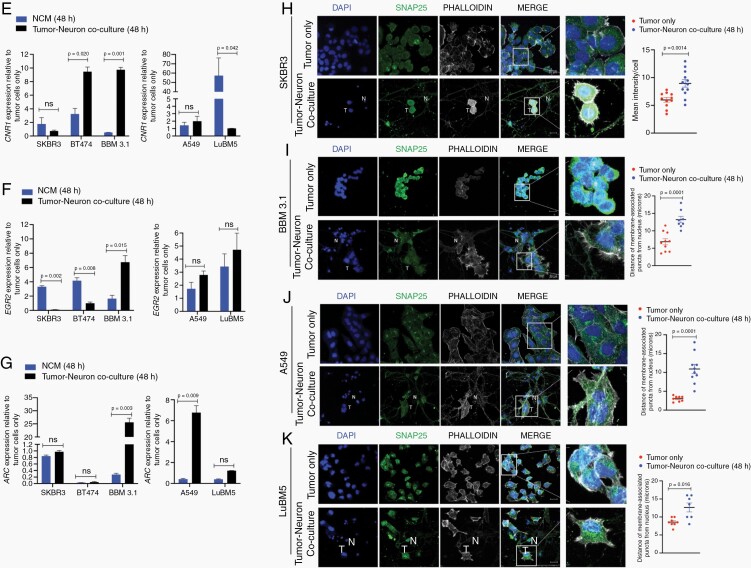
Neuronal exposure induces expression of NT receptors and synaptic mediators in tumor cells. Ser133-phosphorylated CREB protein expression and quantification in BT474 (A) and 231Br-Her2+ (B) breast cancer, and A549 (C) and LuBM5 (D) lung cancer cells in three conditions: tumor cells only, NCM-treated, tumor-neuron coculture (T: tumor cell; N: neuron; Magnification 63x; Scale 20 μm). (E-G) qPCR target validation to investigate differences in mRNA expression in tumor cells on neuronal exposure (NCM = blue bars, Coculture = black bars). Data for each cell line represented as fold change in expression relative to tumor only. SNAP25 protein immunofluorescence and quantification in breast cancer SKBR3 (H) and BBM 3.1(I), and lung cancer A549 (J) and LuBM5 (K) cells in tumor only, and tumor-neuron coculture (T: tumor cell; N: neuron; Magnification 63x).
Neuronal responsiveness resulting from neurotransmitter (NTs) chemical inputs constitutes synaptic activity.12,13 Therefore, we characterized tumor cells as responders to NT and synaptic cues and determine changes that lead to adaptation to the neural microenvironment. We first conducted a wide survey of NT receptor and synaptic mediator mRNA expression in brain-metastatic (BM) and primary (non-BM) breast and lung cancer cells that were either cocultured with neurons, or acclimated to neuron-conditioned media (NCM). Non-BM breast (SKBR3, BT474) and lung (A549) cell lines, as well as patient-derived BM breast (BBM 3.1) and lung (LuBM5) cells were used for our study. Results showed upregulation of several NT and synaptic signaling targets, many regulated by CREB (Supplementary Figure 1A and B). We then identified NT receptor and synaptic mediator mRNAs (CNR1, HTR3A, BDNF, CEBPD, TIMP1) commonly upregulated in both BM and non-BM breast and lung cancer cells on exposure to the neuronal microenvironment (Supplementary Figure 1C–F). Subsequently, we validated selected target mRNA to confirm whether their regulation was reliant on NCM exposure or tumor-neuron coculture.
GRIA2 and GRIN2B encoding for ionotropic AMPA- and NMDA-type Glutamate receptor subunits respectively,14,15 displayed significantly enhanced expression in tumor cells when cocultured with neurons only (Supplementary Figure 1G and H). GRMs 4 and 8, encoding metabotropic Glutamate receptors16 were upregulated significantly in BM cells exposed to NCM and in coculture with neurons (Supplementary Figure 1I and J). GABRB1, a GABA receptor type-A subunit B1,17 showed significant increase in BT474 and BBM3.1 in NCM only (Supplementary Figure 1K). Although Serotonin receptor subunit HTR418 was upregulated significantly in tumor cells in both conditions, this induction was enhanced in neuronal coculture (Supplementary Figure 1L). DRD1 (Supplementary Figure 1M), a D1-type Dopamine GPCR that regulates learning and memory,19 remained unchanged in lung cancer cells. However, there was DRD1 expression enhanced in: a) SKBR3 in NCM, BBM3.1, and neuron cocultures, c) and BT474 in both NCM and neuron cocultures.
Induction of CNR1 (Figure 1E), the main endocannabinoid receptor involved in long- and short-term synaptic activity,20 was significantly higher in BT474 and BBM 3.1 in neuronal cocultures, and LUBM5 in NCM. EGR2 (Figure 1F), a transcription factor in early synaptic development,21 was upregulated in tumor cells in both NCM and neuron cocultures. ARC (Figure 1G), a master regulator of synaptic plasticity22 showed significant upregulation in BBM 3.1 and A549 cancer cells only in neuronal coculture, and was downregulated in tumor cells in NCM. NGFR (Supplementary Figure 1N) encoding for a low-affinity receptors for nerve growth factors showed significant upregulation in all but BBM3.1 tumor cells, in NCM and neuronal cocultures.
MMP9 (Supplementary Figure 1O), a metalloprotease involved in promoting tumor invasiveness23 and with novel functions in regulating pathological glutamate-dependent neuronal activity,24 was significantly upregulated in all breast cancer cells treated with NCM. In contrast, MMP9 was significantly downregulated in A549 cells and remained unchanged in LuBM5 cells in neuronal cocultures, highlighting differential responses of breast and lung cancer cells within the neuronal microenvironment. NRXN1, a cell adhesion molecule and synaptic mediator critical to Glutamate and GABA-mediated activity,25 was upregulated in SKBR3 in NCM; while in neuronal cocultures there was an increase of NRXN1 in SKBR3 and BBM3.1 (Supplementary Figure 1P). Taken together, these results indicate that mediators of NT and synaptic signaling are initiated early in tumor cells, particularly in neuronal cocultures, during the establishment of BMs.
Given that tumor cells have distinct NT responsiveness within the neuronal microenvironment, we next examined the expression of regulators of neurotransmission in tumor cells in coculture. Specifically, SNAP25 is a neuronal vesicle protein that facilitates NT release at chemical synapses and NT-receptor trafficking at the plasma membrane.26,27 We asked whether neuronal exposure alters SNAP25 expression and localization in tumor cells. Although we observed endogenous SNAP25 in all tumor cells, this signal was peri-nuclear (Figure 1H–K). However, in neuronal cocultures, there was not only not only a significant increase in SNAP25 signal in SKBR3 cells (Figure 1H), but a translocation to its normal plasma membrane location in BBM3.1, A549, and LuBM5 cells (Figure 1I–K). We next assessed the expression of PSD-95 a critical scaffolding protein that regulates excitatory postsynaptic structure and synaptic strength.28 Intensity of PSD-95 puncta in neurons was enhanced in areas of direct contact with tumor filopodia in coculture with BT474 and BBM 3.1 cells (Supplementary Figure 1QI and II), indicating neuronal responsiveness to tumor cells within the CNS microenvironment. Taken together, direct interaction with neurons induces membrane localization of NT-vesicle and synaptic scaffolding proteins in tumors—indicating direct neuron-tumor communication.
Breast cancer cells display enhanced reliance on paracrine microenvironmental GABA in presence of neurons:
In the brain, neurons are classified by their utilization of the 5 classical NTs Glutamate, GABA, dopamine, serotonin, and acetylcholine. Therefore, we investigated whether acquisition of neuronal attributes in tumors is by autocrine NT signaling, or by responses to paracrine neurotransmitters from neurons. In breast cancer cells endogenous dopamine expression in breast cancer cells remained unchanged upon coculture with neurons; suggesting autocrine expression (Supplementary Figure 2A and B). Dopamine signal was enhanced in lung cancer cells upon neuronal exposure (Supplementary Figure 2C and D). Basal levels of Serotonin were detected in tumor cells alone, which was reduced or remained unchanged upon coculture with neurons (Supplementary Figure 2E–H).
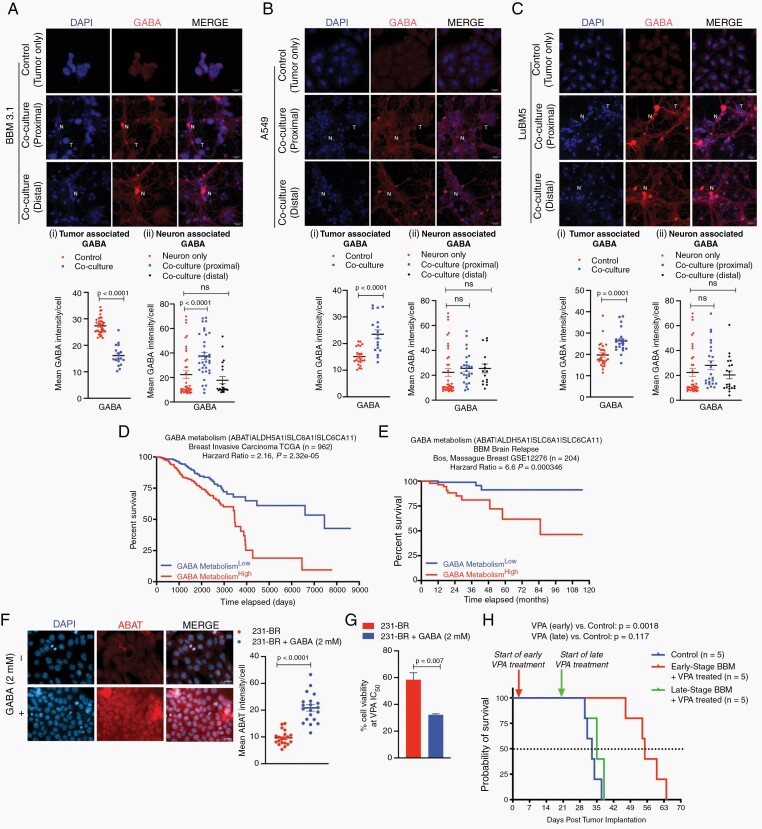
We observed an inverse effect for inhibitory NT GABA in tumor cells interacting with neurons. Specifically, endogenous GABA levels in BBM3.1 cells were significantly reduced when cocultured with neurons (Figure 2Ai). Correspondingly, expression of GABA synthetic enzyme GAD1 was significantly reduced in BBM 3.1 cells cocultured with neurons, suggesting a diminished autocrine GABA response in tumor cells in the neuronal niche (Supplementary Figure 3A). Considering this, we postulated whether neurons can be a paracrine source of GABA for these brain-trophic tumor cells. Since paracrine and synaptic signaling occurs between cells located in proximity, we quantified GABA signal in neurons adjacent to tumor cells, as well as in those neurons located distally within the coculture. Results showed an increase in GABA intensity in neurons in immediate proximity of BBM 3.1 cells, compared to neurons that were situated distally (Figure 2Aii). In contrast, both non-BM (A549) and BM (LuBM5) lung cancer cells showed an increase in autocrine GABA intensity on coculture with neurons), suggesting increased reliance on autocrine GABA in these cells (Figure 2Bi and Ci). GAD1 expression remained unchanged in A549 cells and showed reduction in LuBM5 cells, upon neuronal cocultures (Supplementary Figure 3B). Moreover, neurons both proximal and distant to lung cancer cells in coculture showed no change in GABA intensity (Figure 2Bii and Cii). Overall, an increase of neuronal GABA in proximity to breast cancers, concomitantly with enhanced GABA receptor expression in tumors upon neuronal exposure, as previously described (Figure 1), indicates that breast cancers become GABAergic responsive brain metastases (GRBMs).
Fig. 2.
Breast cancer display enhanced reliance on paracrine microenvironmental GABA in presence of neurons. Immunofluorescence of GABA in breast cancer (A) and lung cancer (B,C) in 2 conditions: tumor only, tumor neuron coculture (T: tumor cell; N: neuron; Magnification 63x; Scale 20 μm). Mean GABA intensity was measured for tumor cells (Ai, Bi, Ci), and for neurons proximal and distant to tumor cells (Aii, Bii, Cii) in all cocultures. Intensity/cell was plotted for all groups (mean and SEM). Kaplan-Meier survival analysis in primary breast cancer (D) and breast-to-brain metastasis (E) by mRNA expression of genes involved in GABA metabolism (ABAT, ALDH5A1, SLC6A1, SLC6A11). Analysis was performed using publicly available TCGA and Bos-Massague datasets for breast cancer respectively. Low gene expression (blue) and high gene expression (red) were plotted on the graph (Y-axis = % patient survival (100-0), and X-axis = time of data collection). (D) Overall survival, and (E) Probability of mortality from brain relapse in breast cancer patients, both categorized by differential expression of GABA shunt mediator genes. Hazard Ratios were calculated for each KS plot, significance was calculated using Log-Rank test. (F) IF and quantification for ABAT in 231-BR cells in 2 conditions (control, 2mM GABA-treated). (G) Quantification of cell viability in 231-BR cells treated with IC50 concentration of VPA in two conditions (control, 2mM GABA-treated). (H) Kaplan-Meier survival analysis for in vivo CNS colonization in breast cancer. Blue, red and green lines represent survival data from control (untreated; n = 5), early-stage VPA treated (n = 5), and late-stage VPA treated groups (n = 5), respectively.
Previous studies show breast cancer utilize GABA through the GABA metabolic shunt for proliferative advantage.4 TCGA datasets results show primary breast cancer patients with high GABA shunt mRNA expression (ABAT, ALDH5A1, SLC6A1, SLC6A11)) are associated with significantly worse overall survival with 2.16 hazard ratio (Figure 2D). Furthermore, enhanced expression of GABA shunt mediators correlates with significantly worse prognosis in brain metastasis relapse, with 6.6-fold greater risk of death (Figure 2E). Thus, we postulated that inhibiting GABA availability in brain-trophic tumor cells will lead to better prognostic index. We first examined GABA utilization by breast cancer cells in vitro. Brain-trophic 231-BR cells were exposed to 2mM GABA over one week to recapitulate early exposure to microenvironmental GABA. GABA-treated cells showed significant enhancement of GABA transaminase (ABAT) (Figure 2F), suggesting increased GABA utilization. Valproic Acid (VPA) used in the treatment of neurological disorders and as an adjuvant in cancer therapy, has been shown to block GABA metabolism by inhibiting ABAT and ALDH5A1.29 231-BR cells treated with exogenous GABA showed significant decrease in cell viability when treated with IC50 VPA (9.937mM), compared to control cells for 48 hours (Figure 2G, Supplementary Figure 3C). Furthermore, GABA treatment alone did not affect cell viability (Supplementary Figure 3D). Our results suggest VPA blocks GABA metabolism in breast cancer cells resulting in enhanced cell death in vitro. To validate these in vitro findings and to determine whether breast cancer cells become GABAergic responsive brain metastases, BBM xenografts were treated with VPA. Results show that xenografts with early VPA regimen (3 days post-transplantation) have significantly enhanced overall survival compared to both untreated and late-VPA treated (21 days post-transplantation) groups (Figure 2H); With late-VPA treatment group show no significant difference in survival compared to untreated group. Overall, our results indicate breast cancers become GABAergic responsive brain metastases early-on through their reliance on paracrine GABA and its metabolic utilization, and that targeting this property earlier in disease progression provides significant survival benefit.
Neuron and Neurotransmitter Exposure Induces Resurgence of Reelin Expression in Tumor Cells
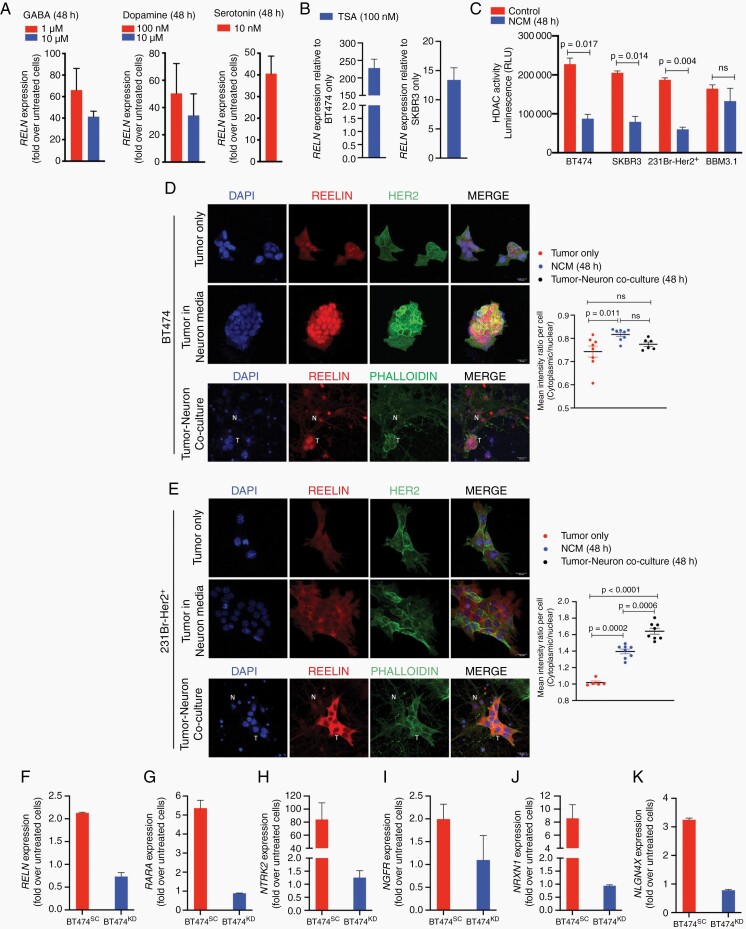
Cancer is a heterogenous disease with distinct molecular features, proliferative potential, and ability to adapt to foreign microenvironments.30 Furthermore, a sub-population of slow-growing dormant tumor cells (DTCs) can interact with their microenvironment at distant sites, and can be reactivated to eventually form metastases.31 We thus asked whether DTCs can respond to neurotransmitters. First, we established dormant SKBR3 cells32 in vitro (Supplementary Figure 4A–C), and then treated them with exogeneous GABA, dopamine, and serotonin. Results show that tumor cells remained dormant post-NT exposure (Supplementary Figure 4D–F).
Therefore, we next asked whether neurotransmitter treatment affects tumor-neuron interaction. Results show in DTCs, a dose-dependent increase expression of RELN, an extracellular matrix glycoprotein essential in neuronal synaptic communication and in regulating tumor migration and invasiveness in epithelial cancers33 (Figure 3A). Additionally, previous results from synaptic mediator mRNA analysis revealed significantly increase in RELN expression in both breast and lung cancer cells upon neuronal coculture (Supplementary Figure 1B). We thus postulated that neuron exposure stimulates Reelin expression in tumor cells early in brain metastasis to facilitate CNS acclimation. Although astrocyte-mediated Reelin has been shown to regulate Her2+ breast cancer proliferation in the brain,34 there are currently no studies that evaluate the role of neuronal input in inducing Reelin expression in tumor cells for CNS acclimation. RELN expression was significantly reduced in primary and metastatic breast and lung tumors compared to that in normal tissue (Supplementary Figure 5A and B), consistent with previous reports that loss of tumor-derived RELN expression promotes invasiveness in epithelial tumors35,36 and correspondingly, negligible endogenous RELN expression seen in non-BM BT474, SKBR3, and A549 cells; With BBM 3.1 and lung LuBM5 showed significantly enhanced endogenous RELN expression relative to their non-BM counterparts (Supplementary Figure 5C and D). Immunofluorescence confirmed the presence of Reelin in brain-metastatic breast and lung cancer, with the protein being distinctly expressed, and localized towards the plasma membrane in these tumors (Supplementary Figure 5E and F). We next determined whether Reelin expression was prognostic in breast and lung cancer. Elevated expression of mediators of the Reelin signaling cascade (RELN, LRP8, DAB1) was significantly associated with worse prognosis from brain metastases relapse from breast cancer with a hazard ratio of 3.68 (Supplementary Figure 5G). Moreover, while not prognostic in lung cancer, in breast cancer increased RELN expression was significantly associated with worse overall survival and patients with high expression were at 1.8 times greater risk of death (Supplementary Figure 5H and I).
Fig. 3.
Neuron and neurotransmitter exposure induces resurgence of Reelin expression in tumor cells. (A) RELN expression in dormant SKBR3 cells treated for 48 hours with GABA, Dopamine, and Serotonin. (B) RELN expression in BT474, SKBR3 cells treated with TSA (100nM). Data displayed as fold increase in RELN over untreated cells. (C) HDAC activity (luminescence) in BT474, SKBR3, 231Br-Her2+, BBM 3.1 cells in tumor cells only (control), and tumor cells grown in NCM for 48 hours. Immunofluorescence for Reelin (Red) in breast cancer (D) BT474, (E) 231Br-Her2+ cells in tumor alone, tumor cells grown in NCM, tumor cells in coculture with neurons. (T: tumor cell; N: neuron; Magnification 63x; Scale 20 μm). (F-K) BT474-control (red) and RELN knockdown (blue) cells were treated with exogenous human recombinant Reelin (2.5nM). Change in mRNA expression of Reelin response genes RELN (F), RARA (G) and synaptic mediator genes NTRK2 (H), NGFR (I), NRXN1 (J), NLGN4X (K). Data displayed as fold change in mRNA over untreated cells.
RELN is known to be epigenetically suppressed in primary cancers37 and we postulated that exposure to neurons will reverse this blockade. We first confirmed the epigenetic modulation of RELN in non-BM tumor cells by treating BT474, SKBR3, and A549 cells with HDAC inhibitor Trichostatin A (TSA). Results show a large increases in RELN expression in these cells post-TSA treatment (Figure 3B; Supplementary Figure 5J). Furthermore, HDAC activity revealed that exposure to neurons for 48 hours was able to significantly reduce Histone De-Acetylase activity in breast and lung cancer cells (Figure 3C; Supplementary Figure 5K) indicating that neuron exposure can reverse epigenetic suppression in brain metastatic tumor cells, allowing global increase in gene expression including RELN.
Correspondingly, non-BM BT474 cells showed enhanced Reelin upon exposure to the neuronal microenvironment for 48 hours (Figure 3D). Brain-trophic MDA-MB-231Br-Her2 + cells (Figure 3E) and non-BM A549 cells (Supplementary Figure 5L) showed significant increases in Reelin expression in NCM and neuronal cocultures. Moreover, LuBM5 showed significant increase in Reelin expression in NCM but not in coculture (Supplementary Figure 5M). Overall, our results indicate neuronal exposure enhances Reelin expression in tumor cells.
Reelin released by GABAergic and glutamatergic neurons in the adult brain is necessary for normal neurotransmission and synaptic plasticity and is thus ubiquitous in the neuronal microenvironment.38 Thus, we asked whether paracrine Reelin from the brain microenvironment influences acquisition of neuronal characteristics in metastatic breast tumor cells. To determine this, we first knocked down RELN in BT474 cells (BT474KD) to mitigate the effect of tumor-derived Reelin (Supplementary Figure 5N). BT474control and BT474KD cells were then treated with 2.5nM Reelin. Results show that BT474control cells upregulate endogenous RELN and its target gene RARA (Figure 3F and G). Moreover, in BT474control cells, there was a reduction of reduced gene expression involved in epithelial-to-mesenchymal transition (MMP9, VIM, SNAI1; Supplementary Figure 5O–Q), and upregulation of synaptic mediators (NTRK2, NGFR, NRXN1, NLGN4XFigure 3H–K); indicating their ability to upregulate autocrine Reelin signaling upon stimulation with paracrine Reelin. In contrast, BT474KD cells were unable to respond to exogenous Reelin. These results suggest that although loss of Reelin in the primary tumor mediates metastasis, the presence of microenvironmental Reelin together with the neuron-mediated resurgence of Reelin expression and signaling in tumor cells is crucial in breast-to-brain metastasis.
Discussion
The investigation of initial interactions of tumor cells with the CNS microenvironmental niche is imperative in understanding the mediators of tumor colonization and brain metastases progression. Most studies have focused on tumor masses that have already successfully established themselves in the brain. But initial interactions of tumor cells with neurons and the ensuing CNS-adaptive responses in these tumor cells are largely unexplored. Given that primary tumors show varying proclivity to the CNS, it is essential to determine commonalities and differences in their interaction with the neuronal milieu.
Overall, we provide evidence that neurons promote the expression of CNS-specific genes in tumor cells, which are crucial for successful metastatic adaptation in the brain. We show neuronal contact induces enhanced neurotransmitter responsiveness in both breast and lung cancer cells, highlighting the acclimatory effects of early direct interactions between tumor cells and neurons within the CNS. Furthermore, our results corroborate previous observation of GRIN2B expression in established BBMs.5 However, we now show that it is the early neuronal contact that significantly increases GRIN2B expression in breast and lung cancer cells.
In neurons, the change in synapse strength according to stimuli can induce memory through short-term (STP) or long-term potentiation (LTP). Neuronal synaptic mediators have been previously identified, and their role in STP/LTP have been determined (e.g BDNF, Reelin, CREB, Glutamate, and GABA receptors, metalloproteases like MMP9, ADAMs).12,39 In the current study we demonstrate that exposure to neurons enhances expression of mediators involved in neuronal LTP and STP in both breast and lung cancer cells. Moreover, in neuronal cocultures, tumor cells show upregulation of ARC, an immediate-early neuronal LTP mediator present on dendritic spines and postsynaptic densities,40 suggesting neurons may regulate tumor cells by transferring signals at direct points of contact. Whether these tumor-neuron interactions form true-like memory state as seen in neuron-neuron interactions remains to be further elucidated.
We observed in tumor cells increased plasma membrane localization of neuronal-associated synaptic vesicle protein SNAP25 upon direct neuronal contact. Plasma membrane targeting and increase in local concentration of SNAP25 is essential for neurotransmission via exocytosis41 as well as receptor trafficking,26 suggesting that tumor cells participate in neuronal signaling and response within the neuronal microenvironment.
Our results show reduced GABA in breast cancer cells upon neuronal contact, accompanied by enhanced GABA levels in tumor-adjacent neurons, which corroborates the reliance of brain-trophic breast cancer cells on microenvironmental GABA within the CNS.4 Reduced GABA levels in breast cancer cells has been associated with worse prognosis.42 Thus, GABA levels in breast cancer cells in the neuronal microenvironment could be correlated to their aggressiveness and ability to form clinical metastases. Furthermore, we show in vivo that only early administration of VPA (3 days post tumor implantation) delays CNS colonization of breast cancer and provides significant survival benefit. This could be attributed in part to inhibition of GABA metabolism mediators ABAT and ALDH5A1 by VPA.29 Once brain metastases have been established (by 21 days post tumor implantation), the inability of pharmacological intervention in prolonging survival may be due to enhanced proliferative properties in the tumor cells, and reduced reliance on the microenvironment once CNS-acclimation is accomplished. Interestingly, recent topographical analysis from patient brain metastasis show breast cancers have an increased proclivity to the cerebellum –a GABAergic hub in the brain.43 Thus, providing evidence that these tumors early on become GABAergic responsive brain metastases. Accordingly, early chemotherapeutic regimen with VPA as an adjuvant may be clinically beneficial to potentially prevent CNS metastasis in patients with advanced breast cancer.
Our data indicates that induction of Reelin expression in tumor cells on neuronal exposure is important for CNS acclimation. In the brain, Reelin is secreted by GABAergic neurons,38 indicating its importance in cells that utilize GABA as a neurotransmitter. We show in breast cancer cells, enhanced expression of Reelin is concomitant with reduced Epithelial-Mesenchymal Transition and increased neuronal synaptic mediators in these tumor cells. This indicates that upregulation of Reelin facilitates breast cancer cells transition back to a more epithelial phenotype and establish themselves in the brain. This data could be correlated with the higher mortality from brain relapse in breast cancer patients who show enhanced activity of the Reelin signaling pathway. Furthermore, we also show that dormant breast cancer cells can respond to neurotransmitter signals to activate RELN transcription. Reactivation of quiescent dormant cells in a fertile microenvironment contributes to their progress to macro-metastases. It remains to be seen whether this Reelin expression aids in the reactivation of dormant cells in the CNS-metastatic niche.
In conclusion, our results demonstrate that tumor-neuron interaction is important in facilitating CNS acclimation in tumor cells. Thus, exploiting the reliance of incoming tumor cells on the neuronal microenvironment can lead to potentially effective control of brain metastasis in patients.
Supplementary Material
Acknowledgments
We would like to acknowledge patient advocates (Ms. Michele Rakoff, Ms. Michelle Atlan, Ms. Andrea Hutton and Ms. Sharon Schlesinger) and all breast cancer patients/survivors/family members for the invaluable role they have played in our research over the years.
Contributor Information
Krutika Deshpande, Department of Neurological Surgery, University of Southern California, Los Angeles, California, USA; USC Brain Tumor Center, University of Southern California, Los Angeles, California, USA; Keck School of Medicine, University of Southern California, Los Angeles, California, USA.
Vahan Martirosian, Department of Neurological Surgery, University of Southern California, Los Angeles, California, USA; USC Brain Tumor Center, University of Southern California, Los Angeles, California, USA; Keck School of Medicine, University of Southern California, Los Angeles, California, USA.
Brooke Naomi Nakamura, Department of Medicine, Division of Gastrointestinal and Liver Diseases, University of Southern California, Los Angeles, California, USA; USC Brain Tumor Center, University of Southern California, Los Angeles, California, USA.
Mukund Iyer, Department of Neurological Surgery, University of Southern California, Los Angeles, California, USA; USC Brain Tumor Center, University of Southern California, Los Angeles, California, USA; Keck School of Medicine, University of Southern California, Los Angeles, California, USA.
Alex Julian, Department of Neurological Surgery, University of Southern California, Los Angeles, California, USA; USC Brain Tumor Center, University of Southern California, Los Angeles, California, USA; Keck School of Medicine, University of Southern California, Los Angeles, California, USA.
Rachel Eisenbarth, Department of Neurological Surgery, University of Southern California, Los Angeles, California, USA; USC Brain Tumor Center, University of Southern California, Los Angeles, California, USA; Keck School of Medicine, University of Southern California, Los Angeles, California, USA.
Ling Shao, Department of Medicine, Division of Gastrointestinal and Liver Diseases, University of Southern California, Los Angeles, California, USA; Keck School of Medicine, University of Southern California, Los Angeles, California, USA.
Frank Attenello, Department of Neurological Surgery, University of Southern California, Los Angeles, California, USA; USC Brain Tumor Center, University of Southern California, Los Angeles, California, USA; Keck School of Medicine, University of Southern California, Los Angeles, California, USA.
Josh Neman, Department of Neurological Surgery, University of Southern California, Los Angeles, California, USA; USC Brain Tumor Center, University of Southern California, Los Angeles, California, USA; Keck School of Medicine, University of Southern California, Los Angeles, California, USA.
Funding
This work was supported by Susan G Komen Career Catalyst Grant (CCR15332673), National Institutes of Health/National Cancer Institute (1R01CA223544-01A1), and Department of Defense BCRP (BC141728).
Conflict of interest statement. None declared.
Authorship statement. Experimental design: K.D., J.N., and V.M.; implementation, analysis, and interpretation of data: K.D., J.N., B.N.N., M.I., A.J., R.E., L.S., and F.A.; writing and revision: K.D. and J.N.
References
- 1. Achrol AS, Rennert RC, Anders C, et al. . Brain metastases. Nat Rev Dis Primers. 2019; 5(1):5. [DOI] [PubMed] [Google Scholar]
- 2. Neman J, Choy C, Kowolik CM, et al. . Co-evolution of breast-to-brain metastasis and neural progenitor cells. Clin Exp Metastasis. 2013; 30(6):753–768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Schulz M, Salamero-Boix A, Niesel K, Alekseeva T, Sevenich L. Microenvironmental regulation of tumor progression and therapeutic response in brain metastasis. Front Immunol. 2019; 10(1713):1–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Neman J, Termini J, Wilczynski S, et al. . Human breast cancer metastases to the brain display GABAergic properties in the neural niche. Proc Natl Acad Sci USA. 2014; 111(3):984–989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Zeng Q, Michael IP, Zhang P, et al. . Synaptic proximity enables NMDAR signalling to promote brain metastasis. Nature 2019; 573(7775):526–531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Hyman SE. Neurotransmitters. Curr Biol. 2005; 15(5):R154–R158. [DOI] [PubMed] [Google Scholar]
- 7. Huang EP, Stevens CF. Neurotransmitter release and synaptic plasticity. In: Bittar EE, ed. Advances in Organ Biology. Vol 2: Elsevier; 1997:171–191. [Google Scholar]
- 8. Flüh C, Mafael V, Adamski V, Synowitz M, Held-Feindt J. Dormancy and NKG2D system in brain metastases: Analysis of immunogenicity. Int J Mol Med. 2020; 45(2):298–314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Martirosian V, Deshpande K, Lin M, et al. . Utilization of discarded surgical tissue from ultrasonic aspirators to establish patient-derived metastatic brain tumor cells: a guide from the operating room to the research laboratory. Curr Protoc 2021; 1(6):e140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Deshpande K, Saatian B, Martirosian V, et al. . Isolation of neural stem cells from whole brain tissues of adult mice. Curr Protoc Stem Cell Biol 2019; 49(1):e80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Sakamoto K, Karelina K, Obrietan K. CREB: a multifaceted regulator of neuronal plasticity and protection. J Neurochem. 2011; 116(1):1–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Citri A, Malenka RC. Synaptic plasticity: multiple forms, functions, and mechanisms. Neuropsychopharmacology. 2008; 33(1):18–41. [DOI] [PubMed] [Google Scholar]
- 13. Lovinger DM. Neurotransmitter roles in synaptic modulation, plasticity and learning in the dorsal striatum. Neuropharmacology 2010; 58(7):951–961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Isaac JT, Ashby MC, McBain CJ. The role of the GluR2 subunit in AMPA receptor function and synaptic plasticity. Neuron 2007; 54(6):859–871. [DOI] [PubMed] [Google Scholar]
- 15. Akashi K, Kakizaki T, Kamiya H, et al. . NMDA receptor GluN2B (GluRε2/NR2B) subunit is crucial for channel function, postsynaptic macromolecular organization, and actin cytoskeleton at hippocampal CA3 synapses. J Neurosci. 2009; 29(35):10869–10882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Crupi R, Impellizzeri D, Cuzzocrea S. Role of metabotropic glutamate receptors in neurological disorders. Front Mol Neurosci. 2019; 12(12):20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Duka T, Nikolaou K, King SL, et al. . GABRB1 single nucleotide polymorphism associated with altered brain responses (but not performance) during measures of impulsivity and reward sensitivity in human adolescents. Front Behav Neurosci. 2017; 11:24-24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Rebholz H, Friedman E, Castello J. Alterations of expression of the serotonin 5-HT4 receptor in brain disorders. Int J Mol Sci . 2018; 19(11):3581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Mishra A, Singh S, Shukla S. Physiological and functional basis of dopamine receptors and their role in neurogenesis: possible implication for parkinson’s disease. J Exp Neurosci 2018; 12(12):1179069518779829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Heifets BD, Castillo PE. Endocannabinoid signaling and long-term synaptic plasticity. Annu Rev Physiol. 2009; 71:283–306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Poirier R, Cheval H, Mailhes C, et al. . Distinct functions of egr gene family members in cognitive processes. Front Neurosci. 2008; 2(1):47–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Korb E, Finkbeiner S. Arc in synaptic plasticity: from gene to behavior. Trends Neurosci. 2011; 34(11):591–598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Li H, Qiu Z, Li F, Wang C. The relationship between MMP-2 and MMP-9 expression levels with breast cancer incidence and prognosis. Oncol Lett 2017; 14(5):5865–5870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Lepeta K, Kaczmarek L. Matrix metalloproteinase-9 as a novel player in synaptic plasticity and schizophrenia. Schizophr Bull. 2015; 41(5):1003–1009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Graf ER, Zhang X, Jin SX, Linhoff MW, Craig AM. Neurexins induce differentiation of GABA and glutamate postsynaptic specializations via neuroligins. Cell 2004; 119(7):1013–1026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Antonucci F, Corradini I, Fossati G, et al. . SNAP-25, a known presynaptic protein with emerging postsynaptic functions. Front Synaptic Neurosci. 2016; 8(7). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Fan HP, Fan FJ, Bao L, Pei G. SNAP-25/syntaxin 1A complex functionally modulates neurotransmitter gamma-aminobutyric acid reuptake. J Biol Chem. 2006; 281(38):28174–28184. [DOI] [PubMed] [Google Scholar]
- 28. Chen X, Nelson CD, Li X, et al. . PSD-95 is required to sustain the molecular organization of the postsynaptic density. J Neurosci. 2011; 31(17):6329–6338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Johannessen CU, Johannessen SI. Valproate: past, present, and future. CNS Drug Rev. 2003; 9(2):199–216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Marusyk A, Polyak K. Tumor heterogeneity: causes and consequences. Biochim Biophys Acta. 2010; 1805(1):105–117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Neophytou CM, Kyriakou TC, Papageorgis P. Mechanisms of metastatic tumor dormancy and implications for cancer therapy. Int J Mol Sci . 2019; 20(24). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Yadav AS, Pandey PR, Butti R, et al. . The biology and therapeutic implications of tumor dormancy and reactivation. Front Oncol. 2018; 8:72–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Khialeeva E, Carpenter EM. Nonneuronal roles for the reelin signaling pathway. Dev Dyn. 2017; 246(4):217–226. [DOI] [PubMed] [Google Scholar]
- 34. Jandial R, Choy C, Levy DM, Chen MY, Ansari KI. Astrocyte-induced Reelin expression drives proliferation of Her2(+) breast cancer metastases. Clin Exp Metastasis. 2017; 34(2):185–196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Stein T, Cosimo E, Yu X, et al. . Loss of reelin expression in breast cancer is epigenetically controlled and associated with poor prognosis. Am J Pathol. 2010; 177(5):2323–2333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Serrano-Morales JM, Vázquez-Carretero MD, Peral MJ, Ilundáin AA, García-Miranda P. Reelin-Dab1 signaling system in human colorectal cancer. Mol Carcinog. 2017; 56(2):712–721. [DOI] [PubMed] [Google Scholar]
- 37. Chen Y, Sharma RP, Costa RH, Costa E, Grayson DR. On the epigenetic regulation of the human reelin promoter. Nucleic Acids Res. 2002; 30(13):2930–2939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Campo CG, Sinagra M, Verrier D, Manzoni OJ, Chavis P. Reelin secreted by GABAergic neurons regulates glutamate receptor homeostasis. PLoS One. 2009; 4(5):e5505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Ho VM, Lee JA, Martin KC. The cell biology of synaptic plasticity. Science 2011; 334(6056):623–628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Messaoudi E, Kanhema T, Soulé J, et al. . Sustained Arc/Arg3.1 synthesis controls long-term potentiation consolidation through regulation of local actin polymerization in the dentate gyrus in vivo. J Neurosci: Off J Soc Neurosci. 2007; 27(39):10445–10455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Koticha DK, McCarthy EE, Baldini G. Plasma membrane targeting of SNAP-25 increases its local concentration and is necessary for SNARE complex formation and regulated exocytosis. J Cell Sci. 2002; 115(Pt 16):3341-3351. [DOI] [PubMed] [Google Scholar]
- 42. Brzozowska A, Burdan F, Duma D, Solski J, Mazurkiewicz M. γ-amino butyric acid (GABA) level as an overall survival risk factor in breast cancer. Ann Agric Environ Med. 2017; 24(3):435–439. [DOI] [PubMed] [Google Scholar]
- 43. Neman J, Franklin M, Madaj Z, et al. . Use of predictive spatial modeling to reveal that primary cancers have distinct central nervous system topography patterns of brain metastasis. J Neurosurg. 2021; 136(1):88–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.