Abstract
Mitral valve replacement in infants is challenging and there are limited alternative valves available. Since the Boston group published their first report on alternative valves for mitral valve replacement in infants, there has been a growth in the literature on the topic, mostly based on the use of a stented bovine jugular vein graft (Melody® valve). The challenges of the Melody valve are firstly in its length of 28 mm unexpanded, which has the potential to cause left ventricular outflow tract obstruction, and secondly, the valve needs mechanical dilatation, which is laborious. A modified No-React® Injectable Biopulmonic™ Prosthesis (Bio Integral Surgical, Inc., Mississauga, ON, Canada) which is shorter (19 mm) and simpler in that it is self-expanding was implanted in a 14-month-old child to replace her mitral valve. The operation was successful and the short-term function of the prosthesis is good.
Keywords: Congenital valve disease, Mitral valve replacement, Mitral regurgitation
The challenge of mitral valve replacement in a child is finding a valve that is flexible and malleable allowing for a comfortable insertion without causing obstruction, yet has the potential to expand in a growing child.
INTRODUCTION
The challenge of mitral valve replacement in a child is finding a valve that is flexible and malleable allowing for a comfortable insertion without causing obstruction, yet has the potential to expand in a growing child. The aim of our report is to describe the use of a modified No-React® Injectable Biopulmonic™ Prosthesis as an option in mitral valve replacement in young children.
CASE REPORT
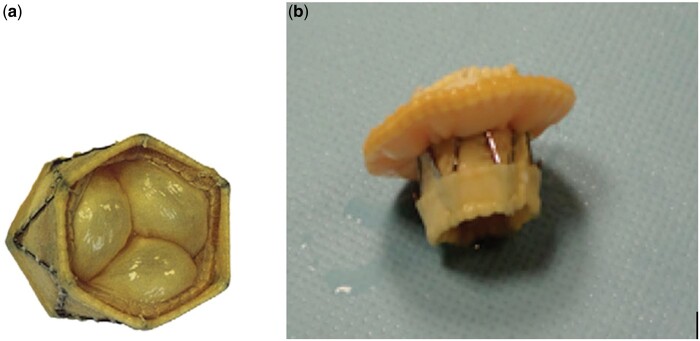
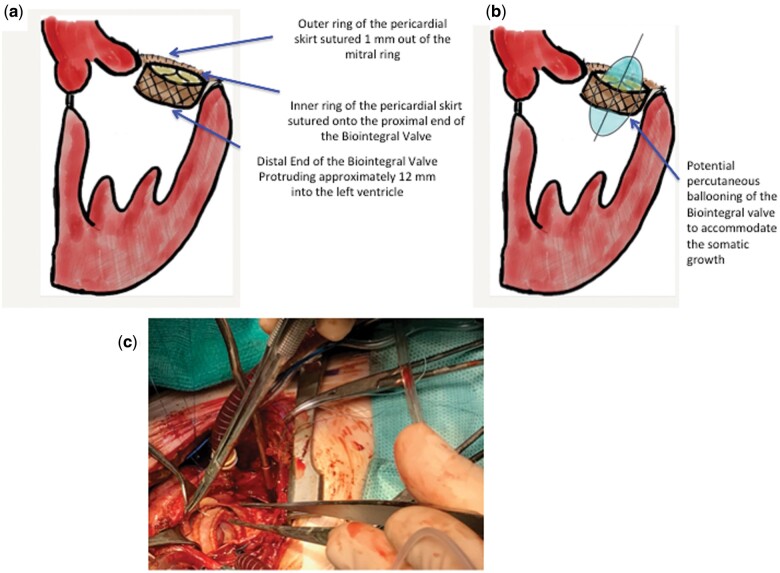
A 14-month-old child, with a complete atrioventricular septal defect, underwent mitral valve re-repair after 2 previous repair attempts. The preoperative transthoracic echocardiogram showed dilated left atrium (diameter 30 mm, area 10 cm2), severe mitral valve regurgitation due to a retracted anterior leaflet, an annulus diameter of 17 mm and a preserved ventricular function. After an unsatisfactory further attempt of valve repair, the valve was replaced using a modified 19 mm No-React Injectable Biopulmonic valve (Fig. 1B). The prosthesis consists of a porcine pulmonic valve covered with a bovine pericardium sleeve, mounted on a self-expandable nitinol stent. The valve was attached to the mitral annulus using a bovine pericardial skirt with a running suture of polypropylene monofilament. The inner ring of the skirt was sutured to the proximal end of the prosthetic valve, while the outer ring was sutured 1 mm outside the mitral annulus on the atrial side. This leaves ∼12 mm of the valve protruding into the left ventricle (Fig. 2A and C). Coming off bypass, the transoesophageal echocardiogram revealed a good function of the valve with a trivial residual regurgitation. The patient was discharged uneventfully. At the 1-year follow-up, the transthoracic echocardiogram showed a mean transvalvular gradient of 8 mmHg, no left ventricle outflow tract gradient and a normal left ventricular function. At the 3-year follow-up, the mitral transvalvular gradient was 6 mmHg, a mild degree of mitral valve regurgitation was noticed, the pulmonary pressure was 20 mmHg and the left ventricular outflow remained unobstructed.
Figure 1:
Original (a) and Modified Biopulmonic valve (b).
Figure 2:
Intraoperative view (a) Biopulmonic Valve in Mitral position, (b) Potential ballooning, (c) intraoperative field.
DISCUSSION
There are few options when replacing the mitral valve in a child. Replacement with mechanical valves in small children is limited by their dependence on long-term anticoagulation and the need to re-operate in order to accommodate somatic growth. The use of biological prostheses is limited by early degeneration and by the potential for left ventricular outflow tract obstruction. The use of percutaneous pulmonary valves like the Melody® valve [2, 4] (Melody Valve, Medtronic, Minneapolis, MN, USA) or the Edwards Sapien® Valve (Edwards Lifesciences, LLC, Irvine, CA, USA) has been recently proposed as an option in young patients [1, 3]. Our hesitation in using these prostheses lies in that the melody valve is potentially obstructive due to its length (28 mm unexpanded and 23.6 mm expanded), while the Sapien valve has a strong radial force that may interfere with the coronary patency [1, 3].
The indication for BioIntegral valve implantation is the transventricular, off-pump, treatment of pulmonary regurgitation; it is also approved for use in the atrioventricular valve position in compassionate cases, in accordance with the European Health Authorities. Due to the shorter length protruding into the left ventricle, it is potentially less obstructive than percutaneous stented valves. Compared to a mechanical prosthesis, the Biointegral valve has the potential to be expanded later, accommodating for the somatic growth of the patient (Fig. 2B). In terms of durability, the latest generation of Biointegral Injectable Valves has shown a lower propensity towards calcification and degeneration due to its treatment with glutaraldehyde and detoxification by the No-React process [5]. Theoretically, the presence of a soft ring should reduce the pressure on the atrioventricular valve annulus and on the coronaries. To our knowledge, this is the first report of a BioIntegral valve implantation in the mitral position in a small child. It proved to be a safe alternative to the conventional and non-conventional prostheses utilized so far. The long-term outcome of using a Biointegral valve for mitral valve replacement in young children and infants is unknown; however, our early results are promising.
INSTITUTIONAL REVIEW BOARD OR ETHICS COMMITTEE APPROVAL
The Hospital Institutional Review Board or the Ethics Committee does not scrutinize case reports.
Conflict of interest: none declared.
Reviewer information
Interactive CardioVascular and Thoracic Surgery thanks Masakazu Nakao, Kevin M. Veen and the other anonymous reviewers for their contribution to the peer review process of this article.
REFERENCES
- 1. Abdullah I, Ramirez FB, McElhinney DB, Lock JE, del Nido PJ, Emani S.. Modification of a stented bovine jugular vein conduit (melody valve) for surgical mitral valve replacement. Ann Thorac Surg 2012;94:e97–8. [DOI] [PubMed] [Google Scholar]
- 2. Pluchinotta FR, Piekarski BL, Milani V, Kretschmar O, Burch PT, Hakami L. et al. Surgical atrioventricular valve replacement with melody valve in infants and children a multicenter study. Circ Cardiovasc Interv 2018;11:e007145. [DOI] [PubMed] [Google Scholar]
- 3. Trezzi M, Cetrano E, Iacobelli R, Carotti A.. Edwards Sapien 3: valve for mitral replacement in a child after melody valve endocarditis. Ann Thorac Surg 2017;104:e429–30. [DOI] [PubMed] [Google Scholar]
- 4. Bonhoeffer P, Boudjemline Y, Saliba Z, Merckx J, Aggoun Y, Bonnet D. et al. Percutaneous replacement of pulmonary valve in a right-ventricle to pulmonary-artery prosthetic conduit with valve dysfunction. Lancet 2000;356:1403–5. [DOI] [PubMed] [Google Scholar]
- 5. Marianeschi SM, Iacona GM, Seddio F, Abella RF, Condoluci C, Cipriani A. et al. Shelhigh No-React porcine pulmonic valve conduit: a new alternative to the homograft. Ann Thorac Surg 2001;71:619–23. [DOI] [PubMed] [Google Scholar]