Abstract
Background
The study aimed to evaluate whether simplified chemotherapy followed by dose-reduced irradiation was effective for treating patients (ages 3–21 years) with localized germinoma. The primary endpoint was 3-year progression-free survival (PFS) rate.
Methods
Patients with a complete response to chemotherapy with carboplatin and etoposide received 18 Gy WVI + 12 Gy boost to the tumor bed. Patients with partial response proceeded to 24 Gy WVI + 12 Gy. Longitudinal cognitive functioning was evaluated prospectively on ALTE07C1 and was a primary study aim.
Results
One hundred and fifty-one patients were enrolled; 137 were eligible. Among 90 evaluable patients, 74 were treated with 18 Gy and 16 with 24 Gy WVI. The study failed to demonstrate noninferiority of the 18 Gy WVI regimen compared to the design threshold of 95% 3-year PFS rate, where, per design, patients who could not be assessed for progression at 3 years were counted as failures. The Kaplan-Meier (KM)-based 3-year PFS estimates were 94.5 ± 2.7% and 93.75 ± 6.1% for the 18 Gy and 24 Gy WVI cohorts, respectively. Collectively, estimated mean IQ and attention/concentration were within normal range. A lower mean attention score was observed at 9 months for patients treated with 24 Gy. Acute effects in processing speed were observed in the 18 Gy cohort at 9 months which improved at 30-month assessment.
Conclusions
While a failure according to the prospective statistical noninferiority design, this study demonstrated high rates of chemotherapy responses, favorable KM-based PFS and OS estimates in the context of reduced irradiation doses and holds promise for lower long-term morbidities for patients with germinoma.
Keywords: braintumor, germinoma, response-based radiation
Key Points.
The 2-drug chemotherapy regimen, not requiring hyperhydration, resulted in high response rates (92.31%) in germinoma.
No ventricular failures were observed with reduced whole ventricular radiation (18 Gy).
The Kaplan-Meier based 3-years PFS and OS was 94.5 ± 2.7% and 100%, respectively.
Importance of the Study.
This is the first and largest prospective study of a dose reduction strategy in localized germinoma, a rare disease in North America and Europe. It is a multicentre study through the COG consortium, on 137 eligible patients across USA, Canada, and Australia. This trial demonstrated a Kaplan-Meier based 3-year PFS of 94.5 ± 2.7% with reduced dose of whole ventricular irradiation (WVI of 18 Gy) in patients aged 3–21 years. Furthermore, the mandated prospective evaluation of cognitive abilities within this study suggests improved functioning in patients who received reduced WVI, paving the way of minimizing deleterious effects of irradiation on the neurocognitive outcome in young patients.
Primary central nervous system (CNS) germ cell tumors (GCT) comprise approximately 3-5% of primary brain tumors in children and young adults in Western countries. Germinomas account for about two-thirds of GCT.1,2 These tumors may secrete low levels of human chorionic gonadotropin-beta (hCGβ) detectable in the blood and cerebrospinal fluid (CSF) and are considered “pure” in the absence of teratomatous tissue and any hCGβ elevation.3 An international consensus on the level of mild elevation of hCGβ has yet to be achieved.4 Germinomas don’t secrete alpha fetoprotein (AFP).
Historically patients with germinomas were treated with radiation therapy alone; however, concerning late effects were observed such as decreased neurocognitive function, vasculopathies, endocrine dysfunction, and development of secondary malignancies.5–9 Systemic chemotherapy followed by involved-field radiotherapy (IFR) has been used but the observation of relapses within the ventricles and outside the IFR in several studies prompted the International Society of Pediatric Oncology (SIOP), French Society of Pediatric Oncology (SFOP), and Japanese Brain Tumor clinical trial groups to change from IFR to whole ventricular irradiation (WVI) following chemotherapy for localized germinoma.10–13
Various chemotherapy regimens used for germinoma, have produced similar survival rates. We hypothesized that a response-based reduced dose of WVI and a boost to the tumor bed after a simplified 2-drug chemotherapy regimen not necessitating hyperhydration12,14–16 would not compromise survival and decrease the risk of neurocognitive late effects. Herein, we present the results of COG ACNS1123, Stratum 2, a phase II prospective study for patients with localized germinoma.
Patients and Methods
Study Objectives
The primary objective of Stratum 2 of ACNS1123 for localized germinoma was to determine the 3-year progression-free survival (PFS) rate and describe patterns of failure of a simplified chemotherapy regimen followed by dose-reduced radiotherapy of 18 Gy, in patients with complete response at the end of induction, aged 3 to ≤ 21 years with serum and/or CSF hCGβ ≤ 50 IU/L. Another primary objective was to longitudinally evaluate the cognitive, social, and behavioral functioning of children and young adults with co-enrollment on the COG ALTE07C1 study (NCT00772200). Secondary objectives included estimating the PFS and overall survival (OS) distribution of localized germinoma patients with serum and/or CSF hCGβ ≤ 50 IU/L and serum and/or CSF hCGβ > 50 IU/L and ≤ 100 IU/L, respectively.
The study was approved by NCI’s Pediatric Central IRB and local institutional review boards as applicable. All patients or their legal guardians provided written informed consent and assent prior to enrollment.
Patients
Patients between 3 and 21 years of age with newly diagnosed localized primary germinoma with tumors located in the suprasellar, pineal, bifocal (pineal + suprasellar) and ventricles were eligible including those with unilateral contiguous parenchymal extension. No histological confirmation was required if hCGβ in serum and/or CSF was 5–50 IU/L for unifocal tumors or ≤ 100 IU/L for bifocal tumors. Patients with hCGβ > 50 IU/L and ≤ 100 IU/L in serum and/or CSF required histological confirmation. Patients with histologically proven germinoma but hCGβ > 100 IU/L were excluded. These patients enrolled on stratum 1 of the study ACNS1123, results of which were published by Fangusaro.17 Patients with tumors in basal ganglia or thalamus, and patients with metastatic disease were excluded. Enrollment on the ALTE07C1 was required for participation on the therapeutic study.
Required Evaluations
MRI of brain and spine with and without contrast, serum and CSF tumor markers (AFP, hCGβ), and lumbar CSF cytology (unless medically contraindicated) were performed at baseline and at completion of chemotherapy and completion of irradiation. Neurocognitive assessments were completed at three time points: 9 (±3) months; 30 (±6) months, and 60 (±12) months after diagnosis. At the time of this report, data collection for the 3rd time point was still ongoing.
Response Criteria
Response assessments were based on MRI and tumor markers performed after cycles 2 and 4 of chemotherapy, and within 4–6 weeks of completion of radiation therapy and are detailed in Supplementary Table 1. Confirmation of negative tumor markers at completion of chemotherapy, even if tumor markers were negative at diagnosis, was mandatory for response evaluation. A retrospective central radiology review of all evaluable patients was performed by two radiologists (D.S; A.B).
Treatment
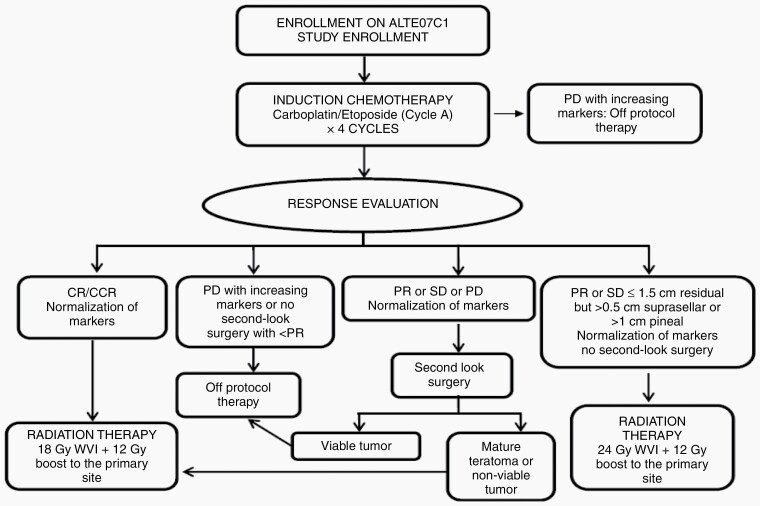
Chemotherapy consisted of carboplatin (600 mg/m2) on day 1 and etoposide (150 mg/m2) on days 1–3 for four 21-day cycles. Following chemotherapy, patients with complete response (CR) received 18 Gy WVI with a boost of 12 Gy to the tumor bed (subsequently referred to 18 Gy WVI). Second-look surgery was required for patients with partial response (PR), stable disease (SD) with > 1.5 cm residual, or progressive disease (PD) with normalization of hCGβ to remain on therapy. Those with SD or PR with < 1.5 cm residual received 24 Gy WVI with a boost of 12 Gy to the tumor bed (subsequently referred to 24 Gy WVI). Patients who did not achieve CR or PR with or without second-look surgery or failed to achieve normal levels of tumor markers following 4 cycles of chemotherapy were taken off protocol therapy (Figure 1). Patients could receive proton or photon-based radiotherapy.
Fig. 1.
Treatment schematic.
Statistical Design
All eligible and evaluable patients who received 18 Gy WVI were included in the primary analysis. The primary objective was to evaluate if the previously reported 3-year PFS rate of 95% could be maintained with the proposed reduced-intensity treatment.10,18 A one-sample exact binomial test and a noninferiority margin of 8% (null hypothesis P ≤ .87) led to a sample size of 79 patients with 90% power and 5% type 1 error. For the primary analysis, all patients who could not be evaluated for progression at 3 years were counted as failures. For success, at least 73 patients needed to be progression-free at 3 years. Kaplan-Meier (KM) estimates were used to estimate PFS distribution as a secondary endpoint. PFS was calculated from the date of treatment initiation to the date of disease progression, death from any cause, or date of last follow-up.
Neurocognitive evaluations focused on three ALTE07C1 outcomes: processing speed, attention/concentration, and estimated IQ based on Block Design and Vocabulary,19 as assessed by the age-appropriate version of the Wechsler Intelligence Scales. Outcomes from 9- and 30-month assessments of cognitive functioning are available and included in this report. Prior to analyses, raw scores were age-adjusted using general population normative data in published manuals. Paired t-tests were used to investigate change over time. Two sample t-tests were used to assess differences between two independent samples such as patients treated with 18 Gy versus 24 Gy. The p-values provided are for two-tailed tests unless otherwise specified and .05 was used as significance threshold without multiplicity adjustments.
Results
Patient Characteristics
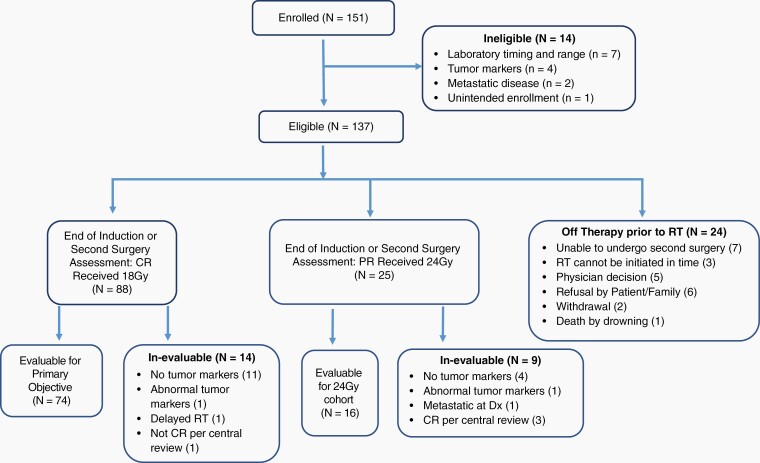
Between May 2012, and June 2018, 151 patients were enrolled; 137 were eligible and 74 were evaluable for the primary objective and treated with 18 Gy WVI (Figure 2). Patient characteristics are shown in Table 1.
Fig. 2.
CONSORT diagram.
Table 1.
Characteristics of 137 Eigible and 74 Evaluable Patients Meeting Criteria for 18 Gy WVI
| Characteristics | Number of patients (%) | |
|---|---|---|
| Evaluable | Eligible | |
| Gender | ||
| Female | 20 (27) | 37 (27) |
| Male | 54 (73) | 100 (73) |
| Age | ||
| Median (range), years | 14.28 (7.05–20.59) | 14.09 (4.95–21.46) |
| Race | ||
| Asian | 7 (9) | 14 (10) |
| Black or African American | 5 (7) | 9 (7) |
| Native Hawaiian or Pacific Islander | 4 (5) | 8 (6) |
| White | 49 (66) | 86 (63) |
| Multi | 1 (1) | 1 (1) |
| Unknown | 8 (8) | 19 (14) |
| All patients | 74 (100) | 137 (100) |
Seventy of 137 eligible patients (51%) had negative hCGβ in serum and CSF at diagnosis. Negative markers were defined according to institutional norm values or in the absence hereof as < 5 IU/L). Most patients with positive hCGβ (n = 67; 49%) at diagnosis showed marker elevation within CSF only (n = 62); 112 patients (81.75%) had normal serum hCGβ level. Only 6 patients had hCGβ elevation between 50 and 100 IU/L and underwent histological verification of germinoma. Of the 74 evaluable patients for 18 Gy WVI, 54 were male (72.97%). The median age at enrollment was 14.28 (7.05–20.59) years. The most common tumor locations were pineal in 30 (40.54%) and suprasellar in 22 (29.73%), with a male (28/30; 93%) and female (15/22; 68%) predominance in the pineal and suprasellar location, respectively. Fifteen (20.27%) patients had bifocal tumors and 7 (9.45%) had ventricular-based lesions (Table 2).
Table 2.
Tumor Location of 137 Eligible and 74 Evaluable Patients Respectively Treated With 18 Gy WVI Based on Institutional Review
| Bifocal | Pineal | Suprasellar | Ventricle | All | |
|---|---|---|---|---|---|
| Gender | |||||
| Female | 3/3 | 7/2 | 26/15 | 1/0 | 37/20 (27%) |
| Male | 16/12 | 58/28 | 17/7 | 9/7 | 100/54 (73%) |
| All Patients | 19/15 | 65/30 | 43/22 | 10/7 | 137/74 (100%) |
Sixteen of the 74 evaluable patients did not have surgery/biopsy at baseline and were enrolled either based on elevated hCGβ in serum and/or CSF (n = 11), or due to bifocal location (n = 5). Twenty-two of the 74 evaluable patients with positive hCGβ at baseline had surgery/biopsy, and 3 had hCGβ levels exceeding 50 IU/L requiring histological confirmation.
Sixteen of 25 eligible patients who achieved a PR following chemotherapy and were treated with 24 Gy WVI were evaluable with a median age of 13.96 (7.13–19.62) years.
Responses
Response assessment after completion of 4 cycles of chemotherapy was available in 130 of the 137 eligible patients. Of the remaining 7 patients, 2 underwent second-look surgery showing nonviable tumor, 1 withdrew consent, and 4 refused further treatment at the discretion of parents or the treating physician. At the end of chemotherapy evaluation, 81/130 had CR (62.31%), 39/130 (30.00%) had PR, and 10/130 (7.69%) had SD. Elevated hCGβ at diagnosis of 45 of 67 (67.16%) patients had already normalized after 2 cycles of chemotherapy; all but 5 patients had normalized marker after 4 cycles. Those 5 patients had serum AFP (ng/ml) and CSF hCGβ (IU/ml) levels below 10 and 5 respectively but were evaluated as positive by institutional norms. No patient with normal hCGβ at diagnosis developed elevated abnormal levels after chemotherapy. The number of patients with complete response increased to 88 patients after second surgery but 14 of 88 patients were deemed in-evaluable. Primary reason for in-evaluability was inadequate assessment of tumor marker at completion of chemotherapy.
Of 74 patients evaluable for the primary efficacy objective and undergoing 18 Gy WVI, 68 (91.89%) had a CR at the end of chemotherapy on institutional and central radiology review. One of the 6 patients not considered CR had no institutional radiological response evaluation but was considered CR on central radiology review. This patient underwent second-look surgery due to cystic residual and histology showed nonviable tumor. Of the remaining five patients, 2 had PR and 3 SD; all had second-look surgery and pathology revealed mature teratoma (n = 3, SD) or nonviable tumor (n = 2, PR). No cases of growing teratoma syndrome occurred. All 11 patients with second-look surgery due to incomplete response to chemotherapy, showed fibrosis/scar or mature teratoma and nonviable germ cell tumor.
Central radiology review of eligible patients who received 24 Gy WVI, revealed 3 patients with CR after chemotherapy and one patient with metastatic disease at diagnosis. Hence only 16 patients with PR were evaluable for primary efficacy assessments.
Treatment Failure/Recurrent Patients
Primary outcome analysis revealed 10 failures within the first 3 years, among the 74 evaluable patients: 4 progressed, 4 were lost to follow-up (23.1–31.6 months from treatment initiation) and 2 withdrew consent (at 18.6 and 23.7 months). Thus, the study failed to meet its primary efficacy threshold and closed short of the planned sample size of 79. The estimated 3-year PFS rate based on the dichotomous endpoint was 86.5% (95% exact CI: 76.5–93.3%) which failed to demonstrate noninferiority of the 18 Gy WVI regimen compared to design threshold of 95%. All 4 progressions occurred outside the radiation field at a median time of 8.91 (2.98 to 19.44) months postcompletion of 18 Gy WVI. There were 2 spinal and 2 parenchymal relapses; both parenchymal relapses were along the diagnostic biopsy track combined with an external drain (EVD) and third ventriculostomy (ETV) respectively.
A total of 8 relapses occurred among the 137 eligible patients. Three of 8 (37.5 %) relapses occurred along the biopsy tract combined with ETV (n = 2) or EVD (n = 1). The patients underwent diagnostic surgery for hCGβ negative germinoma (n = 2) and for a germinoma with CSF hCGβ of 80 IU/L according to study protocol. Characteristics of relapsed patients are listed in Table 3.
Table 3.
Characteristics of Relapsed Patients
| Patient | Age at dx (years) | Gender | Tumor Location | hCGβ (IU/L) in Serum/CSF at Diagnosis | CT Response* | Relapse Location | Time to Relapse From Start of RT (months) | Dose of WVI (Gy) | Evaluability |
|---|---|---|---|---|---|---|---|---|---|
| 1 | 20.21 | Male | Brain Ventricle | 1/3 | CCR | Spine | 9.98 | 18 | Evaluable |
| 2 | 9.86 | Female | Suprasellar | 3/2 | CCR | Primary site | 24.92 | 24 | Not evaluable No tumor markers after completion of CT |
| 3 | 10.92 | Female | Pineal Gland | 2/medically contraindicated | PR | Positive CSF | 1.98 | 24 | Evaluable |
| 4 | 7.52 | Female | Suprasellar | 27/26.8 | CCR | Left parietal | 11.40 | 18 |
Not evaluable
No tumor markers after completion of CT |
| 5 | 17.00 | Male | Bifocal | 2.4/1 | CR | Spine | 9.71 | 18 | Evaluable |
| 6 | 9.61 | Male | Pineal Gland | 2/6.3 | CCR | Right parietal | 4.89 | 18 | Evaluable |
| 7 | 13.82 | Male | Bifocal | 6.3/6.8 | CCR | Right frontal | 21.32 | 18 | Evaluable |
| 8 | 17.48 | Male | Pineal Gland | 46/80 | CCR | Right frontal, pineal, spinal | 23.40 | 24 | Evaluable |
Bifocal, pineal and suprasellar location; CCR, continued complete response; CR, complete response; PR, partial response; CT, chemotherapy; RT, radiation therapy.
Toxicity
There were no unexpected toxicities. The most common toxicities included hematological and electrolyte abnormalities, including hyponatremia. No toxic deaths occurred (Supplementary Table 2).
Outcome on Cognitive Functioning as Evaluated by ALTE07C1
Processing speed, attention/concentration, and estimated IQ results for the first 2 planned time points (9 ± 3 m and 30 ± 6 m postdiagnosis) for patients who received irradiation on this study are reported here (N = 113), regardless of evaluability for the primary efficacy outcome. The groups treated with 18 vs. 24 Gy WVI were seen for their 9-month assessment, on average at 5.10 (SD:2.35) months and 5.42 (SD:3.18) months after starting radiation, respectively.
Performance scores at 9- and 30-month assessment of children treated with irradiation collectively (18 Gy or 24 Gy) did not differ from general population norms for attention/concentration (P = .4773 and P = .3262) or estimated IQ (P = .2315 and P = .4154), but were significantly lower for processing speed at 9 months (mean: 91.82, SD: 16.95, P < .001) and 30 months (mean: 95.74, SD: 13.92, P = .0139). A significant difference in neurocognitive performance between those treated with 18 Gy vs. 24 Gy was the lower attention/concentration (P = .0282) in the 24 Gy group at 9 months. The mean estimated IQ for children treated with 18 Gy at 9 months and 30 months was 100.3 (95%CI: 96.73–103.81) and 104.2 (95%CI: 100.11–108.36), while the mean estimated IQ for those treated with 24 Gy was 92.80 (95% CI: 85.35–100.25) and 95.00 (95% CI: 84.33–105.67), respectively (Supplementary Tables 3 and 4).
Results of longitudinal change in neurocognitive scores in the collective cohort (18 Gy or 24 Gy) from 9- to 30-month assessment showed no significant differences in attention/concentration; however, processing speed (P = .0340) and estimated IQ (P = .0119) scores significantly increased at time 2. The processing speed and estimated IQ at 30 months was higher than 9-month scores (P = .0464 and P = .0321, respectively) in the 18 Gy patients, but no difference was detected in patients who were treated with 24 Gy (P = .4979 and P = .1880) (Supplementary Table 5).
Progression-Free and Overall Survival
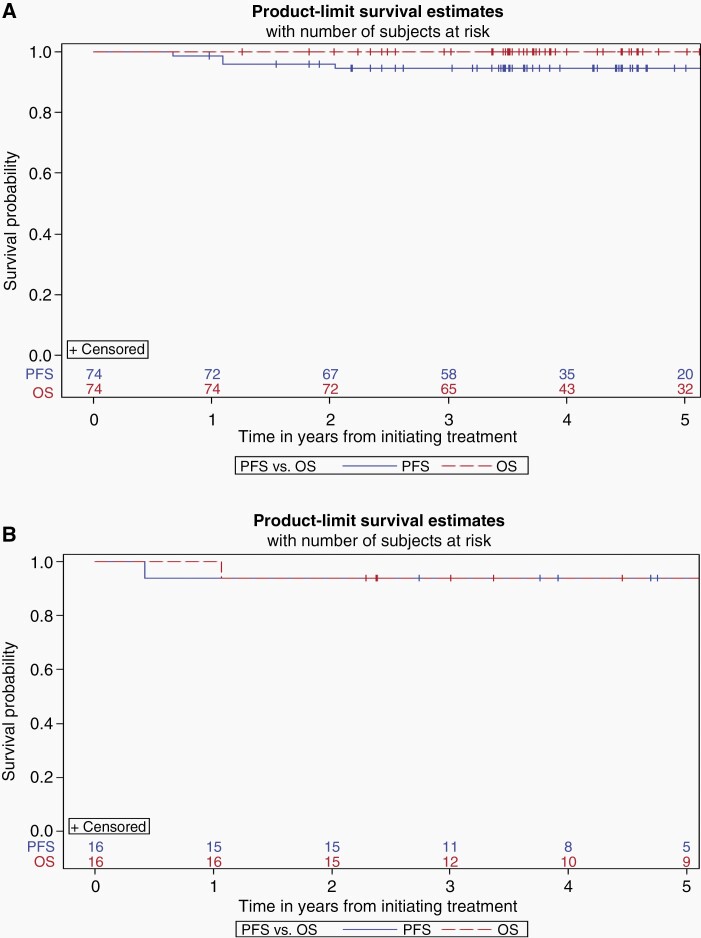
Using the Kaplan-Meier approach where patients lost to follow-up were treated as censored, at a median follow-up of 4.08 years (range 0.98–6.09), the 3-year estimated PFS and OS of the 74 evaluable patients was 94.5 ± 2.7% and 100% respectively (Figure 3A). At a median follow-up of 4.46 years (range 2.29–5.90), the 3-year estimated PFS and OS of the 16 evaluable patients who received 24 Gy WVI plus boost of 12 Gy to the primary tumor bed was 93.75 ± 6.1% and 93.75 ± 6.1% respectively (Figure 3B). Supplementary KM curves demonstrate the 3-year estimated PFS and OS for evaluable and in-evaluable patients treated with 18 Gy and 24 Gy WVI respectively (Supplementary Figure 1A–D) as well as for patients who went off treatment early (Supplementary Figure 2).
Fig. 3.
(A, B) KM curves for 18 and 24 Gy WVI, respectively.
Discussion
The study failed its primary objective to achieve 3-year PFS rate of 95% with the reduced irradiation strategy of 18 Gy WVI and hence failed to demonstrate noninferiority compared to historical controls.10,18 It should be noted however that neither of the historical studies counted patients who withdrew consent or were lost to follow-up within the 3-year window as failures. Hence using a comparison of KM-based PFS and OS, where patients are censored when lost to follow-up may represent a more appropriate comparison between the studies. The observed KM-based 3-year PFS and OS estimates based on 74 evaluable patients treated with 18 Gy WVI was 94.5 ± 2.7% and 100%, respectively (Figure 3A) on our study, which compares favorably to the 5-year PFS and OS of 91 ± 3.9% and 93.7% ± 3.6%, respectively in the radiation only MAKEI study18 and 88% ± 4% and 96% ± 3% respectively in the combined chemotherapy and irradiation SIOP CNS GCT 96 trial.10 Given that recurrences typically happen early, within first 2–3 years, we believe our 3-year results are promising. We do acknowledge however that late recurrences have been reported in retrospective institutional series20,21 and ideally results of germinoma trials should be updated with longer follow-up. Moreover, the irradiation doses utilized in the present trial were 10–20 Gy lower than irradiation doses used in the historical studies. Furthermore, the simplified 2-drug chemotherapy regimen was tolerated well and resulted in response rates of 92.31% (CR+PR) in 130 eligible patients at the completion of chemotherapy. Historical studies, which are typically small, describe response rates in the range of 63–84% to different chemotherapeutic regimens22,23 but no information on response rates to chemotherapy has been reported thus far in large prospective studies.10,11
Patients with residual disease more than 1.5 cm after chemotherapy were required to undergo surgical resection in order to proceed to reduced irradiation and to remain on ACNS1123 study. None of the 11 study patients with second-look surgery showed any viable malignant germ cells. The SIOP working group concluded, based on their trial results published in 2013 (2 years after the opening of ACNS1123) that residual disease at the end of treatment was not associated with a worse prognosis for germinoma patients.10 In view of this conclusion as well as our study findings, the question arises whether risks associated with surgical resection of residual lesions in the precarious suprasellar and pineal regions outweigh any potential benefit. The fact that 18 of the 24 patients were taken off therapy prior to irradiation due to inability/unwillingness to undergo second-look surgery and/or physician decision, may be reflective of this dilemma (Figure 2). These 24 patients were treated outside ACNS1123 and no irradiation information was collected; in retrospect a regrettable omission precluding important insights. However, patients were followed for PFS and OS and no progressions were reported.
The study suffered from a high rate of in-evaluability likely related to the rarity of the disease and the fact that it was the very first Germinoma trial within COG. The reasons for postchemotherapy in-evaluability in 23 (14 + 9) patients included discrepant central radiology review and inadequate tumor marker re-evaluation (Figure 2). A common issue was inadequate re-evaluation of serum and/or CSF markers after completion of chemotherapy in patients with initial marker negative biopsy-proven germinoma which rendered those patients in-evaluable for study purposes. The next Germinoma trial will incorporate real time central radiology and marker review after completion of chemotherapy as a result of the experience from this study.
The prospective evaluation of cognitive functioning revealed possible therapy-related acute effects in processing speed at 9 months following diagnosis, with improved functioning at the 30-month assessment in children who received reduced WVI to 18 Gy. However, current data on neurocognitive functioning need to be interpreted with caution due to small sample size, lack of analysis of potential confounding variables (surgical complications, hydrocephalus), and limited long-term follow-up data. Thus, ongoing, systematic collection of neurocognitive data is of utmost importance to better identify the benefits of irradiation reduction strategies.
Treatment Failure and Recurrence Pattern
None of the 74 evaluable patients treated with 18 Gy WVI relapsed within the ventricular field or in the primary tumor region. In the SIOP CNS GCT 96 study, seven of the 65 patients who received chemotherapy and focal radiation relapsed (6 ventricular, 1 spinal), within 34 months of diagnosis. Relapse of various types of brain tumors within or along the biopsy/surgical site has been described.24–28 This study’s observation of 50% (2 of 4) relapses within the surgical/CSF diversion tract—undertaken at diagnosis—is concerning. Interestingly, none of the evaluable patients (6/74) undergoing (second) surgery due to a residual lesion larger than 1.5 cm after completion of chemotherapy, relapsed. Future studies may need to determine if there is a need for inclusion of diagnostic surgical tracts into the radiation field. Also, the risk of recurrence should be taken into consideration when evaluating the need for a biopsy in cases with classical clinical (e.g. diabetes insipidus) and radiological germinoma characteristics, negative AFP, and mild elevation of hCGβ. Notably, 16 of 74 evaluable patients did not undergo diagnostic surgery at baseline, based on study criteria of elevated tumor markers (11/16) and bifocal location (5/16) and remain free of progression. However, the observed difference in number of relapses in biopsied (4/58) versus nonbiopsied (0/16) patients is not statistically significant (P = .57) and could be by chance alone.
This is the first and largest prospective study of a dose reduction strategy in localized germinoma, using 18 Gy WVI to evaluate outcome, pattern of failures, and neurocognitive effects of this irradiation strategy. While a failure according to the prospective statistical noninferiority design, this study demonstrated high rates of chemotherapy responses, favorable KM-based PFS and OS estimates in the context of reduced irradiation doses without ventricular failures and holds promise for lower long-term morbidities for patients with germinoma.
Supplementary Material
Contributor Information
Ute Bartels, Department of Paediatrics, The Hospital for Sick Children, Toronto, Ontario, Canada.
Arzu Onar-Thomas, Department of Biostatistics, St Jude Children’s Research Hospital, Memphis, Tennessee, USA.
Sunita K Patel, City of Hope National Medical Center, Departments of Population Sciences and Supportive Care Medicine, Duarte, California, USA.
Dennis Shaw, Department of Radiation Oncology, Cleveland Clinic, Cleveland, Ohio, USA.
Jason Fangusaro, Department of Pediatrics, Children’s Healthcare of Atlanta and Emory University School of Medicine, Atlanta, Georgia, USA.
Girish Dhall, Children’s of Alabama, University of Alabama at Birmingham (UAB), Birmingham, Alabama, USA.
Mark Souweidane, Department of Neurological Surgery, Weill Cornell Medicine and Memorial Sloan-Kettering Cancer Center, New York, New York, USA.
Aashim Bhatia, Department of Radiology, Children’s Hospital of Pittsburgh of UPMC, Pittsburgh, New York, USA.
Leanne Embry, Department of Pediatrics, University of Texas Health Science Center at San Antonio, San Antonio, Texas, USA.
Christine L Trask, Rhode Island Hospital, Warren Alpert Medical School of Brown University, Providence, Rhode Island, USA.
Erin S Murphy, Department of Pediatrics, Children’s Hospital and Regional Medical Center, Seattle, Washington, USA.
Shannon MacDonald, Department of Radiation Oncology, Massachusetts General Hospital Cancer Center, Boston, Massachusetts, USA.
Shengjie Wu, Department of Biostatistics, St Jude Children’s Research Hospital, Memphis, Tennessee, USA.
James M Boyett, Department of Biostatistics, St Jude Children’s Research Hospital, Memphis, Tennessee, USA.
Sarah Leary, Department of Pediatrics, Children’s Hospital and Regional Medical Center, Seattle, Washington, USA.
Maryam Fouladi, Department of Pediatrics, Nationwide Children’s Hospital, Columbus, Ohio, USA.
Amar Gajjar, Department of Pediatric Medicine, St Jude Children’s Research Hospital, Memphis, Tennessee, USA.
Soumen Khatua, Department of Pediatric Hematology-Oncology, Mayo Clinic, Rochester, Minnesota, USA.
Funding
This work was supported by National Clinical Trials Network Operations Center Grant U10CA180886, Statistics and Data Center Grant U10CA180899, National Cancer Institute Community Oncology Research Program Grant UG1CA189955, and St. Baldrick’s Foundation
Conflict of interest statement. None.
Authorship statement. Following authors listed on the manuscript have contributed significantly to the experimental design (UB, SK, JF, GD, JB, AG) and its implementation (UB, SK, JF, GD, AOT, AG). All authors have been involved in the analysis and interpretation of the data, writing of the manuscript at draft and any revision stages, and have read and approved the final version.
Disclaimer
The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
References
- 1. Fetcko K, Dey M. Primary central nervous system germ cell tumors: a review and update. Med Res Arch. 2018; 6(3):1719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Echevarría ME, Fangusaro J, Goldman S. Pediatric central nervous system germ cell tumors: a review. Oncologist. 2008;13(6):690–699. [DOI] [PubMed] [Google Scholar]
- 3. Ogino H, Shibamoto Y, Takanaka T, et al. . CNS germinoma with elevated serum human chorionic gonadotropin level: clinical characteristics and treatment outcome. Int J Radiat Oncol Biol Phys. 2005;62(3):803–808. [DOI] [PubMed] [Google Scholar]
- 4. Murray MJ, Bartels U, Nishikawa R, Fangusaro J, Matsutani M, Nicholson JC. Consensus on the management of intracranial germ-cell tumours. Lancet Oncol. 2015;16(9):e470–e477. [DOI] [PubMed] [Google Scholar]
- 5. Chemaitilly W, Sklar CA. Endocrine complications in long-term survivors of childhood cancers. Endocr Relat Cancer. 2010;17(3):R141–R159. [DOI] [PubMed] [Google Scholar]
- 6. Mabbott DJ, Spiegler BJ, Greenberg ML, Rutka JT, Hyder DJ, Bouffet E. Serial evaluation of academic and behavioral outcome after treatment with cranial radiation in childhood. J Clin Oncol. 2005;23(10):2256–2263. [DOI] [PubMed] [Google Scholar]
- 7. Bowers DC, Liu Y, Leisenring W, et al. . Late-occurring stroke among long-term survivors of childhood leukemia and brain tumors: a report from the Childhood Cancer Survivor Study. J Clin Oncol. 2006;24(33):5277–5282. [DOI] [PubMed] [Google Scholar]
- 8. Clement SC, Schouten-van Meeteren AY, Boot AM, et al. . Prevalence and risk factors of early endocrine disorders in childhood brain tumor survivors: a nationwide, multicenter study. J Clin Oncol. 2016;34(36):4362–4370. [DOI] [PubMed] [Google Scholar]
- 9. Morris EB, Gajjar A, Okuma JO, et al. . Survival and late mortality in long-term survivors of pediatric CNS tumors. J Clin Oncol. 2007;25(12):1532–1538. [DOI] [PubMed] [Google Scholar]
- 10. Calaminus G, Kortmann R, Worch J, et al. . SIOP CNS GCT 96: final report of outcome of a prospective, multinational nonrandomized trial for children and adults with intracranial germinoma, comparing craniospinal irradiation alone with chemotherapy followed by focal primary site irradiation for patients with localized disease. Neuro Oncol. 2013;15(6):788–796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Alapetite C, Brisse H, Patte C, et al. . Pattern of relapse and outcome of non-metastatic germinoma patients treated with chemotherapy and limited field radiation: the SFOP experience. Neuro Oncol. 2010;12(12):1318–1325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Finlay J, da Silva NS, Lavey R, et al. . The management of patients with primary central nervous system (CNS) germinoma: current controversies requiring resolution. Pediatr Blood Cancer. 2008;51(2):313–316. [DOI] [PubMed] [Google Scholar]
- 13. Matsutani M. Treatment of Intracranial germ cell tumors: the second phase II study of Japanese GCT study group. Neuro-oncology. 2008; 10(3): 420–421. [Google Scholar]
- 14. Khatua S, Dhall G, O’Neil S, et al. . Treatment of primary CNS germinomatous germ cell tumors with chemotherapy prior to reduced dose whole ventricular and local boost irradiation. Pediatr Blood Cancer. 2010;55(1):42–46. [DOI] [PubMed] [Google Scholar]
- 15. Cheng S, Kilday JP, Laperriere N, et al. . Outcomes of children with central nervous system germinoma treated with multi-agent chemotherapy followed by reduced radiation. J Neurooncol. 2016;127(1):173–180. [DOI] [PubMed] [Google Scholar]
- 16. Afzal S, Wherrett D, Bartels U, et al. . Challenges in management of patients with intracranial germ cell tumor and diabetes insipidus treated with cisplatin and/or ifosfamide based chemotherapy. J Neurooncol. 2010;97(3):393–399. [DOI] [PubMed] [Google Scholar]
- 17. Fangusaro J, Wu S, MacDonald S, et al. . Phase II trial of response-based radiation therapy for patients with localized CNS nongerminomatous germ cell tumors: a children’s oncology group study. J Clin Oncol. 2019;37(34):3283–3290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Bamberg M, Kortmann RD, Calaminus G, et al. . Radiation therapy for intracranial germinoma: results of the German cooperative prospective trials MAKEI 83/86/89. J Clin Oncol. 1999;17(8):2585–2592. [DOI] [PubMed] [Google Scholar]
- 19. Sattler JM. Resource guide to accompany assessment of children: cognitive foundations. Fifth edition. La Mesa, CA, USA: Jerome M. Sattler, Publisher, Inc.; 2008. [Google Scholar]
- 20. Hong KT, Lee DH, Kim BK, et al. . Treatment outcome and long-term follow-up of central nervous system germ cell tumor using upfront chemotherapy with subsequent photon or proton radiation therapy: a single tertiary center experience of 127 patients. BMC Cancer. 2020;20(1):979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Kamoshima Y, Sawamura Y, Ikeda J, Shirato H, Aoyama H. Late recurrence and salvage therapy of CNS germinomas. J Neurooncol. 2008;90(2):205–211. [DOI] [PubMed] [Google Scholar]
- 22. Allen JC, DaRosso RC, Donahue B, Nirenberg A. A phase II trial of preirradiation carboplatin in newly diagnosed germinoma of the central nervous system. Cancer. 1994;74(3):940–944. [DOI] [PubMed] [Google Scholar]
- 23. Balmaceda C, Heller G, Rosenblum M, et al. . Chemotherapy without irradiation–a novel approach for newly diagnosed CNS germ cell tumors: results of an international cooperative trial. The First International Central Nervous System Germ Cell Tumor Study. J Clin Oncol. 1996;14(11):2908–2915. [DOI] [PubMed] [Google Scholar]
- 24. Lüdemann WO, Obler R, Tatagiba M, Samii M. Seeding of malignant meningioma along a surgical trajectory on the scalp. Case report and review of the literature. J Neurosurg. 2002;97(3):683–686. [DOI] [PubMed] [Google Scholar]
- 25. Chang H, Ding Y, Wang P, Wang Q, Lin Y, Li B. Cutaneous metastases of the glioma. J Craniofac Surg. 2018;29(1):e94–e96. [DOI] [PubMed] [Google Scholar]
- 26. Schmalisch K, Beschorner R, Psaras T, Honegger J. Postoperative intracranial seeding of craniopharyngiomas–report of three cases and review of the literature. Acta Neurochir (Wien). 2010;152(2):313–9; discussion 319. [DOI] [PubMed] [Google Scholar]
- 27. Choi UK, Cha SH, Song GS, et al. . Recurrent intracranial germinoma along the endoscopic ventriculostomy tract. Case report. J Neurosurg. 2007;107(1 Suppl):62–65. [DOI] [PubMed] [Google Scholar]
- 28. Haw C, Steinbok P. Ventriculoscope tract recurrence after endoscopic biopsy of pineal germinoma. Pediatr Neurosurg. 2001;34(4):215–217. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.