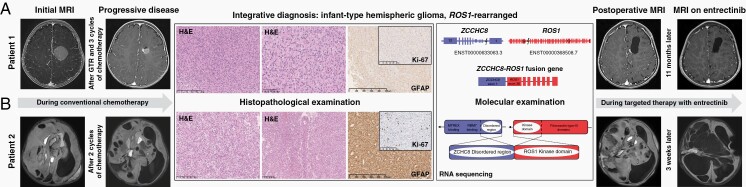
Infant hemispheric high-grade gliomas (iHGGs) constitute a biologically and clinically distinct subgroup of pediatric HGGs. One defining hallmark of iHGG is rearrangement of receptor tyrosine kinase either ALK, ROS1, NTRK1,2,3, or MET. The ROS1-positive iHGGs are an exceptionally rare tumor subtype with a relatively poor prognosis.1 The most typical ROS1 fusion partner for such tumors is GOPC.2 Here we describe two extremely rare cases of iHGG with ROS1 fused with the Zinc Finger CCHC-Type Containing 8 (ZCCHC8) gene. Although these patients had major differences in clinical presentation, MRI characteristics, and histopathology, both children were successfully treated with the NTRK/ROS1/ALK inhibitor entrectinib. To date, only two ZCCHC8:ROS1-positive gliomas were described; one of the patients received targeted therapy.3,4
In our first patient, a 1-month-old female, the tumor was diagnosed as an intracranial mass on routine neurosonography. MRI demonstrated a well-demarcated left frontoparietal solid 1.5 × 1.6 × 1.7 cm mass, which had homogeneous contrast enhancement (Figure 1A). The initial choice was a watch-and-wait strategy, but in 2 months, the tumor size reached 2.6 × 2.4 × 2.9 cm. A gross-total resection was performed with minimal blood loss and no neurologic complications. Histopathological examination was consistent with HGG with epithelioid and rhabdoid features. The RNA sequencing revealed a ZCCHC8:ROS1 fusion transcript (ZCCHC8: ENST00000633063.3, exon1; ROS1:ENST00000368508.7, exon36); the integrative diagnosis was infant-type hemispheric glioma, ROS1-rearranged. The patient received chemotherapy according to the BabyPOG protocol.5 After 3 cycles of chemotherapy, MRI showed local recurrence. The patient then underwent a near-total resection and histopathological examination demonstrated similar morphologic features. We considered two ROS1 kinase inhibitors, crizotinib and entrectinib. The preference was given to entrectinib for its ability to penetrate the blood-brain barrier.6,7 Entrectinib (supplied by Roche via a compassionate use program) was administered in 300 mg daily doses. To date, the patient remains clinically stable on entrectinib for 11 months, with no signs of recurrence on MRI and no treatment-related adverse events.
Fig. 1.
Clinical course for the two cases [Patient 1-panel (A), Patient 2-panel (B)], with corresponding MRI, histopathology, immunohistochemistry (IHC), and molecular data. IHC panel included CD34, chromogranin A, EMA, GFAP, Ki-67, myelin basic protein, NeuN CC2, neurofilament, p53, S100, INI1, H3K27me3. Cells of both tumors were immunopositive for S100, GFAP, INI1, p53, H3K27me3.
The second newborn girl presented with increasing head circumference and left hemiparesis. MRI revealed a large heterogeneous hypervascular mass in the right hemisphere, 12.5 × 10 × 9.6 cm, with cystic components and signs of intratumoral hemorrhage (Figure 1B). It was decided to proceed without a biopsy due to the risks of life-threatening hemorrhage. Chemotherapy according to the HIT-MED Guidance protocol (ClinicalTrials.gov NCT02417324) was initiated. MRI performed after 2 cycles of chemotherapy showed progressive disease, associated with neurological deterioration. A biopsy was finally performed, complicated by severe bleeding. Histopathological examination revealed a glial tumor with rare astroblastic pseudorosettes. The ZCCHC8:ROS1 fusion transcript was identified, identical to described in the first case. Entrectinib (90 mg daily) was administered as a salvage therapy. MRI performed after 3 weeks of the targeted therapy revealed a rapid dramatic response with regression of the solid component of the tumor and no apparent side effects. The patient continues on entrectinib for 5 months and is clinically stable without neurological deficits.
Only two reports on ZCCHC8:ROS1-positive tumors have been published, and only one was given the targeted therapy. ZCCHC8 as a fusion partner of ROS1 was firstly identified in a case of congenital glioblastoma; the patient died of intraoperative hemorrhage.3 Another patient, a 4-month-old girl diagnosed with ZCCHC8:ROS1-positive glioblastoma, received chemotherapy, multiple resections, anti-MEK targeted therapy, and ultimately was treated with entrectinib. The patient improved neurologically and demonstrated tumor shrinkage on MRI.4 After 11 months of the anti-ROS1 therapy, resistance occurred resulting in the fatal disease progression (Dr Ku, personal communication).
ROS1 rearrangements were described in a variety of pediatric malignancies, including HGGs, glioneuronal tumors, atypical meningioma, inflammatory myofibroblastic tumors, and pleuropulmonary blastoma.2,7–9 The efficacy of targeted therapy against ROS1 fusion proteins was evaluated in the STARTRK-NG trial. However, only two patients with gliomas harboring GOPC:ROS1 and EEF1G:ROS1 fusions were enrolled in the trial; both of them demonstrated partial tumor response.10 Additionally, two single cases of targeted therapy of ROS1:ARCN1 and ZCCHC8:ROS1-rearranged gliomas represented resistance to entrectinib after 4 and 11 months of therapy, respectively.4,6 Mechanisms of resistance may involve secondary point mutations in the ROS1 kinase domain (F2004C/I and G2032R) or collateral signaling pathway activation.7 The resistance could theoretically be overcome by a combination of several modalities (radiation therapy, different kinase inhibitors) or next-generation ROS1 inhibitors, although further clinical testing is needed.
Contributor Information
Ludmila Papusha, Dmitry Rogachev National Medical Research Center of Pediatric Hematology, Oncology and Immunology, Moscow, Russian Federation.
Margarita Zaytseva, Dmitry Rogachev National Medical Research Center of Pediatric Hematology, Oncology and Immunology, Moscow, Russian Federation.
Agnesa Panferova, Dmitry Rogachev National Medical Research Center of Pediatric Hematology, Oncology and Immunology, Moscow, Russian Federation.
Alexander Druy, Dmitry Rogachev National Medical Research Center of Pediatric Hematology, Oncology and Immunology, Moscow, Russian Federation.
Andge Valiakhmetova, Dmitry Rogachev National Medical Research Center of Pediatric Hematology, Oncology and Immunology, Moscow, Russian Federation.
Anton Artemov, Dmitry Rogachev National Medical Research Center of Pediatric Hematology, Oncology and Immunology, Moscow, Russian Federation.
Ekaterina Salnikova, Dmitry Rogachev National Medical Research Center of Pediatric Hematology, Oncology and Immunology, Moscow, Russian Federation.
Alexey Kislyakov, Dmitry Rogachev National Medical Research Center of Pediatric Hematology, Oncology and Immunology, Moscow, Russian Federation; Morozov City Children Hospital, Moscow, Russian Federation.
Evgeny Imyanitov, St. Petersburg Pediatric Medical University, St. Petersburg, Russian Federation; N.N. Petrov Institute of Oncology, St. Petersburg, Russian Federation.
Alexander Karachunsky, Dmitry Rogachev National Medical Research Center of Pediatric Hematology, Oncology and Immunology, Moscow, Russian Federation.
Alexey Maschan, Dmitry Rogachev National Medical Research Center of Pediatric Hematology, Oncology and Immunology, Moscow, Russian Federation.
Eugene I Hwang, Division of Oncology, Children’s National Hospital, Washington, DC, USA; Brain Tumor Institute, Washington, DC, USA.
Galina Novichkova, Dmitry Rogachev National Medical Research Center of Pediatric Hematology, Oncology and Immunology, Moscow, Russian Federation.
Roger J Packer, Division of Oncology, Children’s National Hospital, Washington, DC, USA; Brain Tumor Institute, Washington, DC, USA; Center for Neuroscience and Behavioral Medicine, Washington, DC, USA.
Funding
The study was supported by the Foundation for support and development in the field of pediatric hematology, oncology and immunology “Science for Children” and the Russian Science Foundation [grant number 21-75-30015].
Conflict of interest statement. All authors declare no conflict of interest.
Authorship statement. L.P., M.Z., and A.D. developed the concept; L.P., M.Z., A.D., and A.V. wrote the manuscript; A.K.I. conducted histological examination; A.P., A.D., and E.I. performed molecular studies; L.P., A.V., A.A., E.S., A.K.I., and A.M. collected, summarized, and analyzed clinical data; A.K.A., A.M., E.I.H., G.N., and R.J.P. expertly supervised the interpretation and writing. All authors have critically read the manuscript and approved the final version.
References
- 1. Stucklin ASG, Ryall S, Fukuoka K, et al. Alterations in ALK/ROS1/NTRK/MET drive a group of infantile hemispheric gliomas. Nat Commun. 2019;10(1):4343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Sievers P, Stichel D, Sill M, et al. GOPC:ROS1 and other ROS1 fusions represent a rare but recurrent drug target in a variety of glioma types. Acta Neuropathol. 2021;142:1065–1069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Coccé MC, Mardin BR, Bens S, et al. Identification of ZCCHC8 as fusion partner of ROS1 in a case of congenital glioblastoma multiforme with a t(6;12)(q21;q24.3). Genes Chromosomes Cancer. 2016;55(9):677–687. [DOI] [PubMed] [Google Scholar]
- 4. Ku DTL, Shing MMK, Chan GCF, et al. ROS1 inhibitor entrectinib use in relapse/refractory infantile glioblastoma with positive ROS1 fusion—a case report with promising response. Neuro Oncol. 2020;22(Suppl 3):352. [Google Scholar]
- 5. Duffner PK, Horowitz ME, Krischer JP, et al. Postoperative chemotherapy and delayed radiation in children less than three years of age with malignant brain tumors. N Engl J Med. 1993;328(24):1725–1731. [DOI] [PubMed] [Google Scholar]
- 6. Mayr L, Guntner AS, Madlener S, et al. Cerebrospinal fluid penetration and combination therapy of entrectinib for disseminated ROS1/NTRK-fusion positive pediatric high-grade glioma. J Pers Med. 2020;10(4):290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. García-Pardo M, Calles A. ROS-1 NSCLC therapy resistance mechanism. Precis Cancer Med. 2021;4:16–31. [Google Scholar]
- 8. Rossing M, Westmose YC, Sehested A, et al. Genomic diagnostics leading to the identification of a TFG-ROS1 fusion in a child with possible atypical meningioma. Cancer Genet. 2017;212–213:32–37. [DOI] [PubMed] [Google Scholar]
- 9. Meng Z, Chen P, Zang F, et al. A patient with classic biphasic pulmonary blastoma harboring CD74-ROS1 fusion responds to crizotinib. Onco Targets Ther. 2017;11:157–161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Robinson G, Desai A, Basu E, et al. Entrectinib in recurrent or refractory solid tumors including primary CNS tumors: updated data in children and adolescents. Neuro Oncol. 2020;22(Suppl 3):iii344. [Google Scholar]