Abstract
Background
Veledimex (VDX)-regulatable interleukin-12 (IL-12) gene therapy in recurrent glioblastoma (rGBM) was reported to show tumor infiltration of CD8+ T cells, encouraging survival, but also up-regulation of immune checkpoint signaling, providing the rationale for a combination trial with immune checkpoint inhibition.
Methods
An open-label, multi-institutional, dose-escalation phase I trial in rGBM subjects (NCT03636477) accrued 21 subjects in 3 dose-escalating cohorts: (1) neoadjuvant then ongoing nivolumab (1mg/kg) and VDX (10 mg) (n = 3); (2) neoadjuvant then ongoing nivolumab (3 mg/kg) and VDX (10 mg) (n = 3); and (3) neoadjuvant then ongoing nivolumab (3 mg/kg) and VDX (20 mg) (n = 15). Nivolumab was administered 7 (±3) days before resection of the rGBM followed by peritumoral injection of IL-12 gene therapy. VDX was administered 3 hours before and then for 14 days after surgery. Nivolumab was administered every two weeks after surgery.
Results
Toxicities of the combination were comparable to IL-12 gene monotherapy and were predictable, dose-related, and reversible upon withholding doses of VDX and/or nivolumab. VDX plasma pharmacokinetics demonstrate a dose-response relationship with effective brain tumor tissue VDX penetration and production of IL-12. IL-12 levels in serum peaked in all subjects at about Day 3 after surgery. Tumor IFNγ increased in post-treatment biopsies. Median overall survival (mOS) for VDX 10 mg with nivolumab was 16.9 months and for all subjects was 9.8 months.
Conclusion
The safety of this combination immunotherapy was established and has led to an ongoing phase II clinical trial of immune checkpoint blockade with controlled IL-12 gene therapy (NCT04006119).
Keywords: clinical trial, controlled gene expression, gene therapy, glioblastoma, immunotherapy
Key Points
Controlled IL-12 gene therapy with nivolumab was safe in recurrent GBM patients.
This combination immunotherapy increased tumor IFNγ, suggesting immune activation.
Importance of the Study.
Combining two immunotherapies, one involving controlled IL-12 gene therapy and the second nivolumab, was well tolerated in patients with recurrent GBM.
Glioblastoma (GBM) is a deadly adult cancer with a median overall survival in newly diagnosed patients of 14.5 months.1 Current first-line treatments include surgical resection, chemoradiation, and tumor-treating fields.2 Additional FDA-approved treatments include intracavitary chemotherapy (Gliadel) and bevacizumab. Recurrent GBM (rGBM) can be treated with additional surgical resection. Survival after relapse is 6 to 9 months.3 Immunotherapy has not been successful for rGBM, due to the immunosuppressive microenvironment of this tumor with a paucity of tumor-infiltrating lymphocytes (TILs) targetable by immune checkpoint blockade (ICB).4
Interleukin-12 (IL-12) enhances natural and adaptive immunity, potently stimulates production of interferon-γ (IFNγ), and changes the TME from Th0 to Th 1.5–10 Clinical trials of systemic IL-12 had to be discontinued because of poor subject tolerance.10–13 To minimize systemic toxicity, a ligand-inducible expression switch [RheoSwitch Therapeutic System® (RTS®)] was developed to control production of intratumoral IL-12. Transcription of the IL-12 transgene occurs in the presence of the activator ligand, veledimex (VDX).14,15 An adenoviral vector was engineered to deliver the IL-12 transgene controlled by the RTS promoter (Ad-RTS-hIL-12 or Ad) upon the oral administration of veledimex (VDX) termed “Controlled IL-12” (previously, “Regulatable IL-12”).16
In a phase I clinical trial (NCT02026271) in humans whose rGBM were surgically resected,16 we showed that: (1) Ad-RTS-hIL-12 could be injected peritumorally, (2) a dose-response existed between VDX dose and plasma/rGBM concentrations, indicating VDX crossed the blood-brain-barrier, (3) a dose-response existed between VDX dose and IL-12’s downstream effector, IFNγ, (4) a dose-response existed between VDX dose and toxicity, including a cytokine release-like syndrome (CRS), and (5) the optimal dose of daily oral VDX was at 20 mg. Median overall survival (mOS) in the cohort of subjects that received 20 mg VDX was 12.7 months.
Post-treatment biopsies from three patients on the phase I clinical trial of Controlled IL-12 gene therapy demonstrated that rGBM was indeed infiltrated with more CD8+ T cells for months after treatment with increased intra-tumor levels of IFNγ, validating the original hypothesis.16 However, there was also increased expression of PD-1 and PD-L1 in rGBM immune cell infiltrates. This led us to hypothesize that rGBM immune-evasion from the IL-12 immuno-gene therapy could occur via PD-1/ PD-L1 immune checkpoint signaling. This provides the rationale for the current trial of combining controlled IL-12 gene therapy with immune checkpoint inhibition.
In this first-in-human phase I clinical trial of two immunotherapies for rGBM we thus dose-escalated VDX and the immune checkpoint inhibitor (ICI), nivolumab, separately in the first two cohorts to ensure subject tolerability, before expanding to the final cohort of subjects where both VDX and nivolumab were administered at previously established maximum tolerated doses. Based on the report by Cloughesy et al.,17 nivolumab was administered before and after tumor resection and controlled IL-12 immuno-gene therapy. We report that the combination of these two immunotherapies was well tolerated with toxicities that were promptly reversible upon discontinuation of VDX and were comparable to our previously published single IL-12 gene therapy clinical trial.16 The finding of safety of this combination has led to a phase II clinical trial combining controlled IL-12 immuno-gene therapy with ICI in rGBM.
Methods
Study Design
A single-arm, dose-escalating, open-label, and multi-institutional phase I study was performed (NCT03636477, clinicaltrials.gov) to evaluate the safety and tolerability of the combination of nivolumab (at 1 and 3 mg/kg every two weeks) and Ad-RTS-hIL-12 (peritumoral injection, 2 x 1011 viral particles) with two oral VDX dose levels (10 and 20 mg) in rGBM subjects scheduled for tumor resection. The primary endpoint was safety assessment for combined nivolumab and Ad-RTS-hIL-12 + VDX. Secondary endpoints included overall survival, VDX concentration, and correlative immune responses. The trial began accrual on June 14, 2018 and was completed on October 17, 2019. Adverse events were evaluated based on National Cancer Institute’s Common Terminology Criteria for Adverse Events (NCI CTCAE), v 5. A cytokine release syndrome (CRS) working definition was assessed, as published.16
Subjects
Participants were selected based on age (18 to 75 years old), diagnosis of supratentorial, histologically confirmed GBM (World Health Organization (WHO) Grade IV), and evidence of recurrence as determined by MRI according to Response Assessment in Neuro-Oncology Criteria (RANO) after receiving standard initial therapy.18,19 Eligible subjects had: a Karnofsky performance status ≥70, ability to undergo MRI with contrast, failed previous standard-of-care treatment, recovered from previous treatments, normal ECG and peripheral oxygen saturation (SpO2) ≥90%. Washout periods from previous therapies were: nitrosureas–6 weeks; other cytotoxic agents–4 weeks; antiangiogenic agents, including bevacizumab–4weeks; targeted agents, including small molecule tyrosine kinase inhibitors–2 weeks; and vaccine-based therapy–3 months. Eligibility based on preoperative laboratories were: Hemoglobin ≥9 g/L; Lymphocytes >500/mm3; Absolute neutrophil count ≥1500/mm3; Platelets ≥100 000/mm3; Serum creatinine ≤1.5 x upper limit of normal (ULN), Aspartate transaminase (AST) and alanine transaminase (ALT) ≤2.5 x ULN and for subjects with documented liver metastases, ALT and AST ≤5 x ULN; Total bilirubin < 1.5 x ULN; aPTT or International normalized ratio (INR) within normal institutional limits. Patients were excluded if they had: radiotherapy within 4 weeks of VDX dosing, previous treatment with immune checkpoint inhibitors or other agents specifically targeting T cells, clinically significant increased intracranial pressure or uncontrolled seizures, known immunosuppressive disease, autoimmune conditions, and/or chronic viral infections, acute clinically significant infection within 2 weeks of first VDX dose or were being treated for chronic infections, fever, treatment with enzyme-inducing antiepileptic drugs within 7 days prior to the first dose of VDX, except for levetiracetam (Keppra®), other concurrent clinically active malignant disease, requiring treatment, with the exception of nonmelanoma cancers of the skin or carcinoma in situ of the cervix or nonmetastatic prostate cancer, nursing or pregnancy, prior exposure to VDX, use of medications that induce, inhibit, or are substrates of cytochrome P450 (CYP450) 3A4 within 7 days prior to VDX dosing, contraindication for a neurosurgical procedure, and unstable or clinically significant concurrent medical conditions. rGBM that exhibited multifocal, multicentric or multiple FLAIR/T2 abnormalities were not excluded from eligibility. Steroid use was also permitted.
Treatment Agents
Ad-RTS-hIL-12 is a replication-deficient adenoviral serotype 5 vector encoding the human interleukin-12 p70 (hIL-12) transgene under control of the RTS promoter.14,20 Veledimex (VDX) is an orally active small molecule activator ligand (R)-N’-(3,5-dimethylbenzoyl)-N’-(2,2-dimethylhexan-3-yl)-2-ethyl-3-methoxybenzohydrazide.14,15 Nivolumab (Opdivo®) is an FDA-approved PD-1 inhibitor (Bristol Myers Squibb, New York, NY, USA).
Imaging and Tumor Evaluation
Focality of disease was determined at study entry from review of MRI during screening. Unifocal disease was defined as a single enhancing lesion. Multifocal disease was defined as more than 1 noncontiguous enhancing lesion.21 Separate FLAIR/T2 changes were also evaluated. Following resection, an MRI was performed within 72 hours. Imaging assessments. MRI-based tumor imaging assessments were performed using RANO/iRANO criteria,18,19 by measuring the sum of the products of perpendicular bi-dimensional diameters (SPD) at 2 weeks (Day 14), 4 weeks (Day 28 ± 7 days), 8 weeks (Day 56 ± 7 days), and every 8 weeks thereafter, until the occurrence of confirmed tumor progression.
Measurement of VDX
rGBM tissue and plasma were analyzed for VDX using a validated liquid chromatography/mass spectrometry method.15
Cytokine Analyses
Serum IL-12 and IFNγ were measured by ELISA (R&D Systems, Inc., Minneapolis, MN, Cat # IL-12, D1200; IFNγ, DIF50). IL-12 and IFNγ were measured by electrochemiluminescence immunoassay (human “V-PLEX” custom kit, Meso Scale Discovery MSD, Cat # K151A0H-01). Assays were run according to manufacturers’ guidelines in duplicate.
Peripheral Blood Immunophenotyping
Flow cytometry analysis of peripheral blood lymphocytes was performed using standard methods and a phenotyping panel focusing on T cell and NK cell profiling (PPD Laboratories, Highland Heights, KY).
Histopathology
Hematoxylin and eosin (H&E) stained sections were evaluated as part of the standard of care at clinical sites.
Tumor Immunoprofiling
Formalin-fixed, paraffin-embedded tumor samples were prepared from resected rGBMs pre- and post-treatment (at time of suspected progression). H&E stained slides were reviewed (NeoGenomics) to confirm tumor (500 malignant cells). Fifteen to 30 regions of interest (ROI) per slide were manually selected from a virtual H&E stain using tissue autofluorescence in the Cy2 (FITC) channel on the serial section to be immunolabeled. Multiplexed immunofluorescence labeling was performed using MultiOmyx™, a high-order multiplexing direct immunofluorescence methodology (NeoGenomics), to label multiple immunomarkers on a single tissue section. The panel included antibodies with specificities for CD3, CD4, CD8, CD20, CD34, CD45RO, CD56, CD68, PD-1, PD-L1, CTLA-4, FOXP3, LAG3, TIM3, c-caspase-3, Ki-67, and GFAP. Each of nine cycles of labeling was performed using a pair of antibodies directly conjugated either to Cy3 or Cy5, followed by imaging using an INCell Analyzer 2200 (GE Healthcare Life Sciences, DBA Cytavita, Marlborough, MA) and then dye inactivation.22,23 Twenty-five to eighty thousand nucleated cells per sample were analyzed with proprietary software (NeoGenomics), using the DAPI channel to align markers. Exploratory image analyses were performed using proprietary algorithms to quantify expression of markers, detect and classify cells by immunophenotype, and construct heat maps of density of positive cells per unit area by immunophenotype.
Statistical Analysis
Safety of Ad-RTS-hIL-12 + VDX and nivolumab was qualitatively reported by AE severity and frequency for each study drug and in combination. rGBM and plasma VDX concentration and time course of cytokine (IL-12 and IFNγ) concentrations are expressed as means ± SEM. Statistical analysis for VDX and cytokine concentration was one-way analysis of variance (ANOVA), and when appropriate (when a comparison between specific treatment groups was needed), an unpaired t-test was performed. The Kaplan-Meier method was used to estimate OS. Differences were considered significant at P < .05. The term “CI” denotes a (95%) confidence interval.
Study Approval
Institutional review boards approved the study and informed consent was obtained from subjects before enrollment. Participating institutions included Brigham and Women’s Hospital/Dana Farber Cancer Institute (Boston, MA) (DFCI 18–209), Northwestern Memorial Hospital (Chicago, IL), the University of Texas MD Anderson Hospital (Houston, TX), and the University of Minnesota Hospitals Medical Center (Minneapolis, MN).
Results
Schedule of Combination Immunotherapy
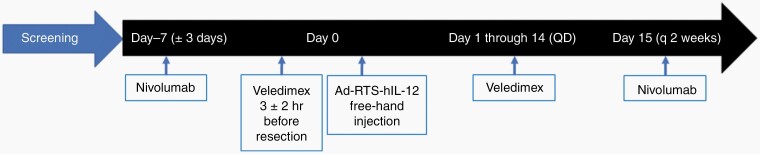
In this phase I dose-escalation study, we first wanted to determine if the combination of the 2 immunotherapies was tolerated in subjects. Previous trials had determined the tolerable doses for each immunotherapy, singly.16,24 Therefore, we escalated the dose of each treatment separately before proceeding to the full dose for both immunotherapies. Three cohorts of rGBM subjects were accrued sequentially. The first (n = 3) was treated with nivolumab at 1 mg/kg and VDX at 10 mg. Upon determination of tolerability (1/3 subjects or less experiencing a dose limiting toxicity), the second cohort (n = 3) was treated with nivolumab at 3 mg/kg and VDX at 10 mg. Upon determination of tolerability, the third cohort (n = 3) was treated and then further expanded by an additional 12 (15 total) subjects with nivolumab at 3 mg/kg and VDX at 20 mg. Eligible subjects were administered one intravenous dose of nivolumab, 7 (±3) days before scheduled craniotomy(Figure 1). Three (±2) hours before craniotomy, subjects were administered one dose of VDX. Peripheral blood was collected at surgery (Day 0). Intraoperatively, freehand injections of Ad-RTS-hIL-12 to two noncontiguous peritumoral sites for a total volume of 0.1 mL were performed. The type and mode of injection has been previously described.25–28 Subjects began daily single-dose oral VDX for days 1–14 after surgery. Nivolumab (1mg/kg or 3 mg/kg) was administered every 2 weeks with the first dose 14 days after surgery and continued until confirmed progression, unacceptable toxicity, or subject withdrawal. This schedule was thus completed in all 15 accrued subjects.
Fig. 1.
Upon screening and determination of eligibility, each accrued subject underwent an intravenous infusion of nivolumab at either 1 mg/kg (cohort 1) or 3mg/kg (cohorts 2 and 3), seven days (±3 days) before scheduled craniotomy. The activator ligand, veledimex (VDX), was administered 3 hours (±2 hours) before planned craniotomy for tumor resection at either 10 mg (cohort 1 and 2) or 20 mg (cohort 3). Pericavitary injection of the IL-12 immunogene therapy vector (Ad-RTS-hIL-12) was then carried out as previously described.16 Subjects then orally took VDX daily for 14 days postoperatively at 10 mg (cohorts 1 and 2) or 20 mg (cohort 3). Nivolumab was then administered every 2 weeks at either 1 mg/kg (cohort 1) or 3mg/kg (cohorts 2 and 3).
Subject Characteristics
For the trial, we accrued subjects who had failed standard chemoradiation and had recurred one or more times. There was no exclusion for number of failures, for multifocal or multicentric disease, or for tumors that had more than one area of FLAIR/T2 change. Accrued rGBM patients suffered a first (76%) or ≥ second (24%) recurrence (mean previous treatments = 1.6) (Table 1). 71% of subjects had unifocal disease and 29% had multifocal disease. In the VDX 10 mg dose cohorts (nivolumab at 1 or 3 mg/kg, n = 6), 83% had unifocal and 17% had multifocal disease. In the VDX 20 mg dose cohorts (nivolumab at 3 mg/kg, n = 15), 67% had unifocal and 33% had multifocal disease. 90% of rGBM were IDH wild-type and 52% were unmethylated for the MGMT promoter. 81% of subjects were treated with a cumulative dose of dexamethasone less than or equal to 20 mg over the 14-day postoperative period. 76% of subjects receiving all planned VDX doses. One subject in cohort 1 missed one dose at day 3, while in cohort 3, 2 subjects missed one day each (day 6 and day 4), one missed 2 days (day 0 and 6) and one missed 3 days (day 6, 13, and 14). The mean number of VDX doses was 14.62 (minimum of 12 and maximum of 15). The mean number of doses of nivolumab was 8.1 (min 1, max 42) with dosing ongoing in 1 subject. These results thus showed that this was a heavily pretreated population where the relatively high incidence of multifocal disease not only portends a poorer prognosis but also excludes patients from most available clinical trials.
Table 1.
Subject Demographics
| Characteristics1 | Cohort 1: Ad+VDX 10 mg | Cohort 2: Ad+VDX 10 mg | Cohort 3: Ad+VDX 20 mg | Expansion Cohort Ad+VDX 20 mg | Total |
|---|---|---|---|---|---|
| Nivolumab (1 mg/kg) | Nivolumab (3 mg/kg) | Nivolumab (3 mg/kg) | Nivolumab (3 mg/kg) | ||
| n = 3 | n = 3 | n = 3 | n = 12 | n = 21 | |
| Age in years Mean (Range) | 42.9 (30, 63) | 59.4 (52, 66) | 60.1 (47, 76) | 59.7 (24, 78) | 57.3 (24, 78) |
| Gender | |||||
| Male | 1 (33%) | 1 (33%) | 1 (33%) | 6 (50%) | 9 (43%) |
| Female | 2 (67%) | 2 (67%) | 2 (67%) | 6 (50%) | 12 (57%) |
| Disease status2 | |||||
| Unifocal | 2 (67%) | 3 (100%) | 3 (100%) | 7 (58%) | 15 (71%) |
| Multifocal | 1 (33%) | 0 | 0 | 5 (42%) | 6 (29%) |
| Number of lesions3 | |||||
| 1 | 1 (33%) | 2 (67%) | 2 (67%) | 5 (42%) | 10 (48%) |
| 2 | 1 (33%) | 1 (33%) | 1 (33%) | 4 (33%) | 7 (33%) |
| 3+ | 1 (33%) | 0 | 0 | 3 (25%) | 4 (19%) |
| Number of recurrences | |||||
| 1st recurrence | 2 (67%) | 3 (100%) | 2 (67%) | 8 (67%) | 16 (76%) |
| ≥2 recurrence | 1 (33%) | 0 | 1 (33%) | 4 (33%) | 5 (24%) |
| Prior lines of treatment mean (Range) | 1 (1, 1) | 1 (1, 1) | 1.7 (1, 3) | 1.6 (1, 4) | 1.6 (1, 4) |
| IDH Status, n (%) | |||||
| Mutated | 1 (33%) | 0 | 1 (33%) | 0 | 2 (10%) |
| Wild-type | 2 (67%) | 3 (100%) | 2 (67%) | 12 (100%) | 19 (90%) |
| Methylation status, n (%) | |||||
| Methylated | 2 (67%) | 1 (33%) | 0 | 6 (50%) | 9 (43%) |
| Unmethylated | 1 (33%) | 2 (67%) | 3 (100%) | 5 (42%) | 11 (52%) |
| Undetermined | 0 | 0 | 0 | 1 (8%) | 1 (5%) |
| KPS at Screening, n (%) | |||||
| ≥70–90 | 0 | 0 | 2 (67%) | 2 (17%) | 4 (19%) |
| ≥ 90 | 3 (100%) | 3 (100%) | 1 (33%) | 10 (83%) | 17 (81%) |
| Cumulative Steroid Use | |||||
| Days 0–14 (mg) | 65.07 | 3.3 | 0 | 29.7 | 26.7 |
| Mean (Range) | (0, 116) | (0, 10) | (0, 0) | (0, 280) | (0, 280) |
| Dexamethasone (total, Days 0–14) | |||||
| ≤20 mg | 1 (33%) | 3 (100%) | 3 (100%) | 10 (83%) | 17 (81%) |
| >20 mg | 2 (67%) | 0 | 0 | 2 (17%) | 4 (19%) |
| Veledimex dosing compliance (Days 0–14) | |||||
| 100 % | 2 (67%) | 3 (100%) | 2 (67%) | 9 (75%) | 16 (76%) |
| <100%4 | 1 (33%) | 0 | 1 (33%) | 3 (25%) | 5 (24%) |
| Nivolumab doses, mean (Range) | 6.3 (6, 7) | 19.7 (2, 42) | 8.7 (6, 14) | 5.5 (1, 11) | 8.1 (1, 42) |
1Data collection and cleaning are ongoing as of data cutoff (16 October 2020).
2At study entry with unifocal defined as 1 enhancing lesion and multifocal as 2 or more enhancing lesions on MRI.
3At study entry including enhancing lesions and also FLAIR/T2 changes on MRI.
4Number of doses missed explained in text; Ad, Ad-RTS-hIL-12; VDX, veledimex.
Safety and Adverse Events
The main objective of the trial was to determine if there was a combined dose of VDX (that controlled IL-12 gene therapy) and of nivolumab that was tolerated. Table 2 lists the grade 3 or greater adverse events and serious adverse events considered related to the controlled IL-12 gene therapy (Ad + V), nivolumab, or the combination. Nine subjects (42.9%) experienced twelve Grade 3 or higher toxicities considered related to controlled IL-12 immuno-gene therapy: decreased lymphocyte count (28.6%), brain edema (9.5%), increased ALT, cold type hemolytic anemia, cytokine release syndrome, and decreased WBC count (4.8%). Nine subjects (42.9%) experienced eleven related Grade 3 or higher toxicities related to nivolumab: decreased lymphocyte count and brain edema (14.3%), cold type hemolytic anemia, cytokine release syndrome, knee arthralgia, increased lipase and decreased WBC count (4.8%). Over 60% of treated subjects suffered a grade 2 cytokine release syndrome, defined as having at least one or more of the following: pyrexia, lymphopenia, thrombocytopenia, neutropenia, and/or elevated ALT/AST. Upon cessation of VDX and/or nivolumab all of the above adverse events reversed back to baseline. One of the more serious adverse events occurred for one patient in cohort 1 with clinically symptomatic brain edema 28 days after craniotomy after the second dose of nivolumab (day 15) and the last dose of VDX (day 14), requiring hospitalization. A third dose of nivolumab was withheld and the symptomatic edema was treated with dexamethasone and also low dose bevacizumab. By day 37, the clinical symptoms associated with the edema had resolved. It was judged that the edema was possibly related to the nivolumab rather than the IL-12 gene therapy. For other AEs and SAEs, return to baseline occurred within days after cessation of VDX and/or nivolumab. Resumption of VDX and/or nivolumab was based on the severity of the AE or SAE, evaluation of relatedness, and timing for return to baseline. For grade 3 or higher AEs that were possibly related, VDX and/or nivolumab were not resumed. For lower-grade AEs that occurred within the 14-day period of VDX administration and judged to be possibly related, VDX was continued unless there was concern for the AE increasing in severity. In fact, for the 5/21 subjects with less than 100% compliance with the full course of VDX treatment (Table 1), 1/21 with ALT increase (cohort 1) did not resume VDX, 2/21 with lymphocyte decrease (cohort 2) continued to a full course of VDX while 1/21 with lymphocyte decrease (cohort 3) did not, and then 1/21 with cold type hemolytic anemia, 1/21 with a cytokine release syndrome, and 1/21 with decrease in lymphocytes (expansion cohort) did not finish the full course of VDX. In summary, these data thus show that AEs were predictable, dose-related and promptly reversible upon withholding VDX and/or nivolumab with no drug-related deaths.
Table 2.
Safety Results1
| Adverse event2 relationship | Cohort 1: Ad+VDX 10 m) | Cohort 2: Ad+VDX 10 mg | Cohort 3: Ad+VDX 20 mg | Expansion Cohort: Ad+VDX 20 mg | Total | |||||
|---|---|---|---|---|---|---|---|---|---|---|
| Nivolumab (1 mg/kg) | Nivolumab (3 mg/kg) | Nivolumab (3 mg/kg) | Nivolumab (3 mg/kg) | |||||||
| n = 3 | n = 3 | n = 3 | n = 12 | n = 21 | ||||||
| Ad+V | Nivo | Ad+V | Nivo | Ad+V | Nivo | Ad+V | Nivo | Ad+V | Nivo | |
| Related ≥ Grade 3 AEs | ||||||||||
| ALT increased | 1 (33.3%) | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 (4.8%) | 0 |
| Brain edema | 0 | 1 (33.3%) | 0 | 0 | 0 | 0 | 2 (16.7%) | 2 (16.7%) | 2 (9.5%) | 3 (14.3%) |
| Cold type hemolytic anemia | 0 | 0 | 0 | 0 | 0 | 0 | 1 (8.3%) | 1 (8.3%) | 1 (4.8%) | 1 (4.8%) |
| Cytokine release syndrome | 0 | 0 | 0 | 0 | 0 | 0 | 1 (8.3%) | 1 (8.3%) | 1 (4.8%) | 1 (4.8%) |
| Knee arthralgia | 0 | 0 | 0 | 1 (33.3%) | 0 | 0 | 0 | 0 | 0 | 1 (4.8%) |
| Lipase increased | 0 | 0 | 0 | 1 (33.3%) | 0 | 0 | 0 | 0 | 0 | 1 (4.8%) |
| Lymphocyte count decreased3 | 0 | 1 (33.3%) | 2 (67.7%) | 1 (33.3%) | 1 (33%) | 0 | 3 (25.0%) | 1 (8.3%) | 6 (28.6%) | 3 (14.3%) |
| WBC count decreased | 0 | 0 | 0 | 0 | 0 | 0 | 1 (8.3%) | 1 (8.3%) | 1 (4.8%) | 1 (4.8%) |
| Related SAEs | ||||||||||
| Cerebral edema | 0 | 1 (33.3%) | 0 | 0 | 0 | 0 | 1 (8.3%) | 1 (8.3%) | 1 (4.8%) | 2 (9.5%) |
| Cold type hemolytic anemia | 0 | 0 | 0 | 0 | 0 | 0 | 1 (8.3%) | 1 (8.3%) | 1 (4.8%) | 1 (4.8%) |
| Cytokine release syndrome | 0 | 0 | 0 | 0 | 0 | 0 | 1 (8.3%) | 1 (8.3%) | 1 (4.8%) | 1 (5%) |
| Lymphocyte count decreased3 | 0 | 0 | 0 | 0 | 0 | 0 | 1 (8.3%) | 0 | 1 (4.8%) | 0 |
| Cytokine release syndrome 4 | ||||||||||
| Grade 2 | 3 (100.0%) | 3 (100.0%) | 2 (66.7%) | 5 (41.7%) | 13 (61.9%) | |||||
| Grade 3 | 0 | 0 | 0 | 0 | 0 |
1Data collection and cleaning are ongoing as of data cutoff (16 October 2020)
2CTCAE v5 as applicable
3One ≥ Grade 3 AE of Lymphocyte count decreased was considered related to both Ad+V and nivolumab
4Ziopharm Cytokine Release Syndrome Working Definition.
Abbreviations and acronyms: Ad, Ad-RTS-hIL-12; and VDX, veledimex.
Pharmacokinetic/Pharmacodynamic Studies
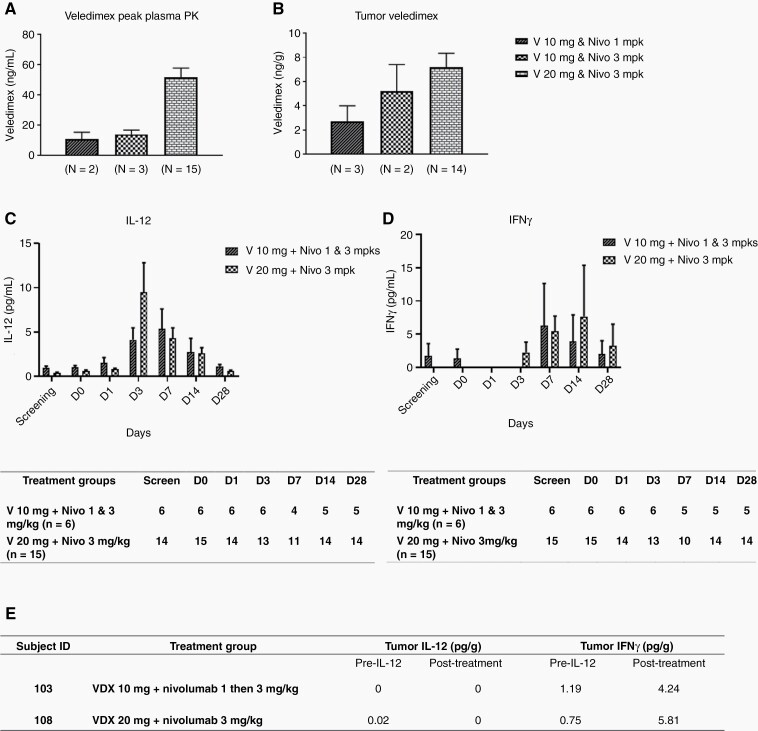
The availability of blood samples from subjects before and after treatment allowed the study of VDX, IL-12, and IFNγ kinetics. There was a dose-response between peak VDX plasma and tumor concentrations (Figure 2A and B). A serum dose-response of cytokine IL-12 and IFNγ was seen between VDX 10 mg and 20 mg, independent of nivolumab dose (Figure 2C and D). Peak level of serum IL-12 was observed on Day 3 at 9.5 ± 3.3 pg/mL in VDX 20 mg plus nivolumab 3 mg/kg cohort and on Day 7 at 5.4 ± 2.2 pg/mL in VDX 10 mg plus nivolumab 1 & 3 mg/kg cohorts. Levels of serum IFN-γ generally peaked after IL-12 across cohorts. These cytokines then decreased back to baseline over the ensuing 2 weeks. These data thus showed that VDX led to IL-12 and, indirectly, IFNγ generation even in the presence of immune checkpoint inhibition.
Fig. 2.
A. Veledimex peak plasma PK results. (Left, VDX 10 mg and nivolumab 1 mg/kg; middle, VDX 10 mg and nivolumab 3 mg/kg; and right, VDX 20 mg and nivolumab 3 mg/kg.). There is a dose-response relationship for plasma veledimex concentration. The VDX 10 mg and nivolumab 1 mg/kg cohort has a similar veledimex plasma concentration to VDX 10 mg and nivolumab 3 mg/kg cohort, and for the VDX 20 mg and nivolumab 3 mg/kg cohort, the veledimex plasma concentration is more than double the level of the VDX 10 mg with nivolumab 1 or 3 mg/kg cohorts (mean ± SEM). B. Veledimex tumor pharmacokinetics (PK). Veledimex tumor concentration is similar to the pattern observed on plasma except for VDX 10 mg and nivolumab 3 mg/kg cohort is slightly higher than VDX 10 mg and nivolumab 1 mg/kg cohort. C&D. Serum cytokine levels. A dose-response of serum cytokine IL-12 and IFN-γ levels was seen between VDX 10 mg and 20 mg dosing with the nivolumab dose combinations. Peak levels of serum IL-12 were observed on Day 3 and Day 7 in VDX 20 mg with nivolumab 3 mg/kg cohort and VDX 10 mg with nivolumab 1or 3 mg/kg cohorts, respectively. Peak levels of serum IFNγ were observed on Day 7 and Day 14 in VDX 10 mg with nivolumab 1or 3 mg/kg cohorts and the VDX 20 mg with nivolumab 3 mg/kg cohort, respectively. (Data are presented as mean ± SEM by day of collection.) The tables below the panels show the number of subjects whose blood samples were available for analyses at indicated timepoints. E. Tumor cytokine levels. Tumor IL-12 and IFNγ pre-IL-12 (though post an adjuvant dose of nivolumab) and post-treatment (Ad + VDX and nivolumab) in subject 103 from the VDX 10 mg and nivolumab initially 1 mg/kg dose-escalated to 3 mg/kg and subject 108 from the VDX 20 mg and nivolumab 3 mg/kg cohorts, respectively.
Paired tumor samples pre- and post-Ad-RTS-hIL-12 treatment were available for four subjects who underwent re-resection (Supplementary Table 1). Two of these (subject ID 103 and 108) had evaluable tumor IL-12 and IFNγ results at both timepoints. There was no detectable increase in IL-12 levels in tumors, as expected due to the period of time elapsed after injection. However, there was a sustained increase of IFNγ levels from immune activation in post-treatment samples16 (Figure 2E). There was a significant increase in the percentage of CD3+CD8+ T cells in peripheral blood, from Day 0 to Day 28 (P = .02) in the VDX 20 mg and nivolumab 3 mg/kg dose group but there was no change in CD3+CD4+ T cell numbers (Supplementary Figure 1) or NK after therapy (data not shown). These data thus suggested that the combination may have increased peripheral CD8+ T cells but also intratumoral IFNγ.
Imaging Studies
Tumor progression is visible by MRI when there is increased gadolinium contrast uptake in tumor bed. However, inflammatory responses can also cause this, a process termed pseudoprogression. MRIs were initially suggestive of progressive disease after start of Ad-RTS-hIL-12 + VDX and PD-1 checkpoint inhibitor therapy in at least two patients but there was a subsequent decrease in this enhancement (Supplementary Figure 2). This was consistent with pseudoprogression (PsP) with partial responses at Week 12
Tumor Immunoprofiling
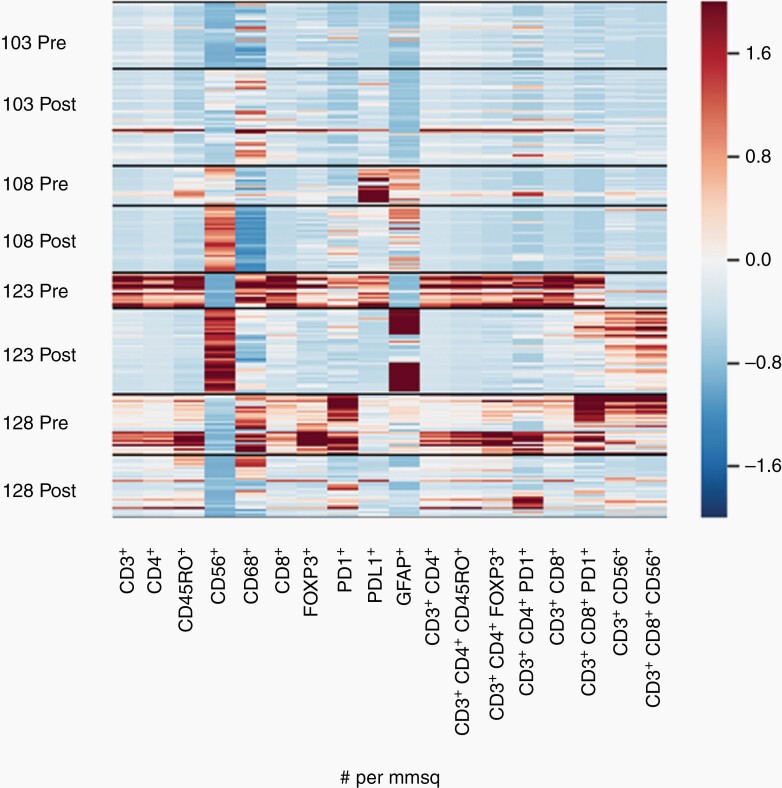
Four subjects with rGBM which had been treated with Ad-RTS-hIL-12 + VDX and ongoing nivolumab appeared to recur with new contrast enhancement and consented to another tumor resection. These were subject 103, 108, 123, and 128 whose injected tumors were re-resected 108, 93, 149, and 148 days after Ad-RTS-hIL-12 injection (Supplementary Table 1). This provided matched pre- and post-tissues that could be studied by quantitative multiplex immunofluorescence. Figure 3 is a heatmap from quantitative image analyses counting cells positive for each immunofluorescence marker per mm2. Each row is composed of several bands that represent the region of interest of the slide that was analyzed. For subject 123 and 128 there was a visibly significant decrease in the number of PD1+ cells pre- and postinjection and this was also true for subject 108 and 103, albeit less so. Similar findings could be visualized for PD-L1, especially for subjects 108 and 123. These data suggest that the addition of the immune checkpoint inhibitor did reduce PD-1+/PD-L1+ up-regulation. Somewhat surprisingly, though, there were also decreases in activated TILs, particularly comparing the pre- and post-tissue samples in subject 123 and 128. The significance of this finding will be expanded in the discussion.
Fig. 3.
Heat Map constructed from quantitative image analyses of multiplex immunofluorescence (MultiOmyx™ High-Order Multiplexing) images from 4 matched pairs of tumor samples pre- and post-treatment. Ratios are expressed as positive cells per mm2 of tissue (density). The panel consists of markers covering the following immunophenotypes: CD3+ (pan T cells), CD3+ CD4+ (helper T cells), CD3+ CD8+ (cytotoxic T cells), CD3+ CD8+ CD56+ (NKT cells), CD3+ CD4+ FOXP3+ (regulatory T cells), and PD-1+ and PD-L1+ total immune cells. CD56+ cells may include NK cells but have been confounded by expression of NCAM on tumor cells (not shown). Structural marker Glial Fibrillary Acidic Protein (GFAP) expression is expected to be higher in tumor than normal adjacent brain tissue or nontumor gliosis. The apparent row width varies because the number of bands depends on the number of regions of interest (ROI) analyzed per sample, as compared with conventional heat maps for a homogenized tumor sample or liquid specimen (see Materials and Methods). Refer to Supplementary Table 1 for corresponding subject characteristics and the treatment group assigned.
Efficacy
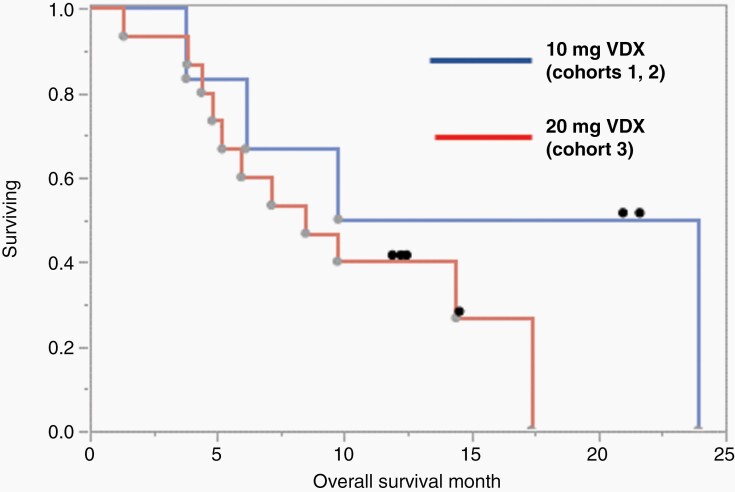
Like a typical phase I trial, this trial was not designed or powered to measure efficacy. However, overall survival curves are still presented. As of the data cutoff date (16 October 2020), dosing was ongoing in 1 subject and 6 subjects were being followed. Median overall survival (mOS) for VDX 10 mg in combination with nivolumab at 1 or 3 mg/kg (n = 6) was 16.9 months (3.8, 24.0 months, 95% CI) (Figure 4, blue line). Two subjects in the VDX 10 mg dose group and four subjects in the VDX 20 mg dose group were alive at data cutoff date. The mOS for VDX 20 mg with nivolumab (n = 15) was 8.5 months (4.4, 17.4 months, 95% CI) (Figure 4, red line). The mOS among all subjects (N = 21) was 9.8 months (5.2,17.4 months, 95% CI). In conclusion, subject overall survival in the 10 mg VDX cohorts (cohorts 1 and 2) was similar to that observed in the previous controlled IL-12 monotherapy trial16 and somewhat superior to the mOS of 9.8 months, reported for single nivolumab administration.24 However, the 20 mg VDX cohort (cohort 3) exhibited reduced survival when compared to the monotherapy IL-12 gene therapy trial.16 The significance of this finding is discussed below.
Fig. 4.
Kaplan-Meier survival analysis for overall survival in VDX 10 mg (blue) and 20 mg (red) dose groups. Median overall survival (mOS) for VDX 10 mg in combination with nivolumab either at 1 or 3 mg/kg (n = 6) was 16.9 months (3.8, 24.0 months, CI) whereas for VDX 20 mg in combination with nivolumab mOS (n = 15) was 8.5 months (4.4, 17.4 months, CI). mOS among all subjects (n = 21) was 9.8 months (5.2,17.4 months, CI). Censored (alive) subjects at data cutoff are shown as a black dot. The data cutoff was 16 October 2020.
Discussion
The GBM microenvironment is immunosuppressive,29 rendering immune checkpoint inhibition alone ineffective.24,30 GBM is a “cold tumor” largely devoid of tumor-infiltrating lymphocytes. Patients have global immune dysfunction with decreased levels of circulating cytotoxic T cells, a situation potentially worsened by standard treatments such as chemotherapy and dexamethasone.31,32 The biologic basis for this phase I combination trial of nivolumab with IL-12 gene therapy was the observation of increased TILs but also increased expression of programmed cell death protein 1 (PD-1) and programmed cell death ligand 1 (PD-L1) in rGBM in a phase I controlled IL-12 immuno-gene therapy trial.16 In the current trial, four subjects with matched pre- and post-treatment biopsies had a decrease in PD-1 and/or PD-L1 positive cells. This validates the hypothesis that nivolumab would reduce rGBM immune checkpoint signaling induced after controlled IL-12 gene therapy.
Another important consideration for this trial is that combining two immunotherapies in humans with the potential for increased brain inflammation and toxicity has not been studied well before. In particular, cerebral edema due to inflammation was a consideration. As presented in the results, one grade 3–4 cerebral edema occurred in one patient, requiring hospitalization, treatment with steroids, and bevacizumab. Attribution of adverse events to one of these two immunotherapies, to postsurgical events, to progression of events, or to a combination of the above is difficult: in the case presented in the results, attribution was judged to be due to nivolumab because of the temporality of the event with respect to immune checkpoint administration. For cytokine release syndromes, attribution always was to the combination since both therapies have been reported to cause this. In spite of this, the combination of these two immunotherapies was well tolerated, with AEs comparable to those observed with the IL-12 gene monotherapy study, although there were 5/21 subjects with brain edema and 13/21 with grade 2 CRS in this study, while there no brain edema and 6/21 subjects with CRS reported in the monotherapy study at the 10 or 20 mg doses.16 This safety profile justifies an ongoing phase II trial of controlled IL-12 gene therapy in combination with an ICI (NCT 04006119, clinicaltrials.gov). Based on the safety profile, this phase II trial also employed a neoadjuvant schedule for the administration of the ICI (i.e. one week before surgery and then every 3 weeks after). The ICI was switched to cemiplimab (350 mg, Regeneron, Inc.), because of a clinical supply agreement reached in 2018 by the sponsor with Regeneron, Inc. Other clinical trials for GBM using replicating, oncolytic adenoviruses33 in combination with ICI are ongoing, such as NCT02798406 (clinicaltrials.gov). It is still not known how different this approach will be compared to the one in this paper in terms of clinical safety and efficacy.
Plasma and tumor PK demonstrate that oral VDX crosses the blood-brain barrier with drug levels proportional to dosing in serum and rGBM. Quantitively, tumor VDX levels were approximately 10% of peak plasma levels. In Figure 3B, there was slightly more tumor VDX in the VDX/ Nivo 3 mg cohort 2 compared to VDX/Nivo 1 mg cohort 3. Since the number of patients in each of these cohorts is small, it is difficult to attribute much significance to this particularly since the peak plasma levels in either cohort were similar (see Figure 2A). In panels C and D of Figure 2 we grouped cohorts 1 and 2 together, since these subjects were administered the dame dose of the activator ligand, VDX. Serum IL-12 was detected in all subjects following initiation of VDX, followed by a transient increase in downstream serum IFNγ, consistent with the previous trial,16 although the peak levels of IL-12 were lower in this trial when compared with the monotherapy trial.16 Perhaps, this also led to a reduced number of TILs in the pre- vs. post-treatment TILs observed in the multiple immunofluorescence analysis. In fact, the multiple immunofluorescence analyses of immune cell markers before and after treatment (Figure 3) provided some unexpected results. With the caveat that only 4/21 patients in this trial were able to undergo another craniotomy with tumor resection, one would have expected to see reduction of PD-1+ and PD-L1+ immunofluorescent cells when comparing tumor before and after injection as shown for at least 3 of these subjects (108, 123, and 128). The relatively unexpected finding though was there was also a reduction in markers of TILs in tumors comparing before and after injection. It is unlikely that the one dose of preoperative nivolumab led to increases of TILs, PD-1+ and PD-L1+ cells in tumors before injection. It is more likely that the multiple doses of nivolumab during the treatment led to the observed changes. This would suggest that timing of nivolumab with IL-12 gene therapy may matter. In mouse models of infection, neoadjuvant anti-PD1 has also been reported to be deleterious to immune-activation,34 leading us to speculate if this could be a reason for the reduction in post-treatment TILs. The ongoing phase II trial with more patients will help elucidate whether the timing of immune checkpoint inhibition influenced IL-12 immuno-activation. It is also unlikely that nivolumab competed with the PD-1+ immunofluorescence antibody and, even if it did, this would not explain the differences in PD-L1+ and other lymphocyte markers.
Another unexpected finding consistent with the observations above was that, although there was elevated tumor IFNγ after combination therapy like we had seen in the monotherapy study, the levels were much lower than what we had observed with the monotherapy study, again suggesting a somewhat deleterious effect of Nivolumab on the IL-12 immunologic effect.
Overall survival in this combination trial showed some differences with that in patients in the monotherapy trial,16 where patients in the monotherapy VDX 20 mg cohort (n = 15) had a mOS of 12.7 months and subjects on a lower dose of VDX (10 mg, n = 6) had a mOS that was less (7.6 months).35 Interestingly, in this phase I combination trial, this was reversed: the VDX 10 mg cohort (with nivolumab) had a mOS of 16.9 months while the VDX 20 mg cohort (with nivolumab) had a mOS of 8.5 months, suggesting that the PD-1 inhibitor only benefited survival with the lower VDX dose. The benefit of PD-1 inhibition was lost in the 20 mg VDX cohort when compared to the monotherapy study, although differences in outcome may reflect a lower percentage of multifocal tumor patients in the monotherapy study (7%) compared to the combination study (33%). Another possible explanation is that patients in the 20 mg VDX cohorts had less than 100% compliance (4/15) with VDX due to AEs/ SAEs when compared to those in the 10 mg VDX cohorts (1/6). Further, subjects in the 10 mg cohorts had more nivolumab doses when compared to those in the 20 mg cohorts. Taken in combination, all three of these factors (unifocal disease, VDX compliance, and number of nivolumab doses) may have contributed to the increased survival of the 10 mg vs. 20 mg cohorts.
In this combination trial the potential prognostic influence of multiple covariates was limited by sample size. We also did not collect extent of tumor resection data because the published monotherapy trial did not show a difference between gross total vs. subtotally resected patients (see Figure 5 of ref.16). In addition, in this trial some eligible patients had multifocal tumors to start off with and subtotal resections were allowed. In the previously published monotherapy study, there was a deleterious effect of dexamethasone, where subjects (VDX 20 mg) treated with less than 20 mg of dexamethasone over a 14-day postoperative period had a mOS of 17.8 months as compared with a mOS of 6.0 months in subjects treated with greater than 20 mg cumulative exposure to dexamethasone.16 In this combination trial, we did not limit dexamethasone use, which is likely to also have confounded some of the discrepancies in survival data between the monotherapy and this combined immunotherapy study as well as between the combination cohorts. Steroid requirement is an exclusionary criterion for the current phase II trial of controlled IL-12 gene therapy (NCT 04006119).
Some patients receiving this combination immunotherapy have MRIs showing increased enhancement. Unfortunately, perfusion or MR spectroscopic imaging was not available for these cases. Subsequently, the imaging shows a decrease in enhancement, suggestive of pseudoprogression. This is one of several reports for immunotherapy that link MRI findings to pseudoprogression based on biopsy data.36–38
In summary, this study supports the tolerability of two immunotherapies working in combination for rGBM. A phase II study of Controlled IL-12 in combination with neoadjuvant anti-PD-1 has completed accrual (NCT 04006119). As data matures from this study, it will provide further information on whether this combination should be pursued further, whether the timing of anti-PD-1 may be re-evaluated or whether other means to block immune checkpoint signaling should be pursued.39
Supplementary Material
Acknowledgments
We thank the study subjects and their families for their participation, as well as the research staff for their dedication to the trial.
Contributor Information
E Antonio Chiocca, Brigham and Women’s Hospital and Harvard Medical School, Boston, Massachusetts, USA.
Arnold B Gelb, Ziopharm Oncology, Inc., Boston, Massachusetts, USA.
Clark C Chen, University of Minnesota, Minneapolis, Minnesota, USA.
Ganesh Rao, Baylor College of Medicine, Houston, Texas, USA.
David A Reardon, Dana-Farber Cancer Institute, Boston, Massachusetts, USA.
Patrick Y Wen, Dana-Farber Cancer Institute, Boston, Massachusetts, USA.
Wenya Linda Bi, Brigham and Women’s Hospital and Harvard Medical School, Boston, Massachusetts, USA.
Pierpaolo Peruzzi, Brigham and Women’s Hospital and Harvard Medical School, Boston, Massachusetts, USA.
Christina Amidei, Northwestern Memorial Hospital, Chicago, Illinois, USA.
Dan Triggs, Brigham and Women’s Hospital and Harvard Medical School, Boston, Massachusetts, USA.
Leah Seften, Brigham and Women’s Hospital and Harvard Medical School, Boston, Massachusetts, USA.
Grace Park, Brigham and Women’s Hospital and Harvard Medical School, Boston, Massachusetts, USA.
James Grant, Brigham and Women’s Hospital and Harvard Medical School, Boston, Massachusetts, USA.
Kyla Truman, Brigham and Women’s Hospital and Harvard Medical School, Boston, Massachusetts, USA.
Jill Y Buck, Ziopharm Oncology, Inc., Boston, Massachusetts, USA.
Nira Hadar, Ziopharm Oncology, Inc., Boston, Massachusetts, USA.
Nathan Demars, Ziopharm Oncology, Inc., Boston, Massachusetts, USA.
John Miao, Ziopharm Oncology, Inc., Boston, Massachusetts, USA.
Taylor Estupinan, Ziopharm Oncology, Inc., Boston, Massachusetts, USA.
John Loewy, Ziopharm Oncology, Inc., Boston, Massachusetts, USA.
Kamal Chadha, Ziopharm Oncology, Inc., Boston, Massachusetts, USA.
Joseph Tringali, Ziopharm Oncology, Inc., Boston, Massachusetts, USA.
Laurence Cooper, Ziopharm Oncology, Inc., Boston, Massachusetts, USA.
Rimas V Lukas, Northwestern Memorial Hospital, Chicago, Illinois, USA.
Funding
The clinical trial was sponsored by Ziopharm Oncology, Inc. E.A.C. received support from grants 2P01CA163205 and CA069246-20 from the National Institutes of Health.
Conflict of Interest statement. E.A.C. is currently an advisor to Candel Therapeutics, Insightec, Inc., DNAtrix Inc., Voyager Therapeutics, and GSK and has equity interest in DNAtrix, Seneca Therapeutics, and immunomic Therapeutics; he has also advised Oncorus, Merck, Tocagen, Ziopharm, Stemgen, NanoTx., Ziopharm Oncology, Cerebral Therapeutics, Genenta. Merck, Janssen, Karcinolysis, Shanghai Biotech. He has received research support from NIH, US Department of Defense, American Brain Tumor Association, National Brain Tumor Society, Alliance for Cancer Gene Therapy, Neurosurgical Research Education Foundation, Advantagene, NewLink Genetics and Amgen. He also is a named inventor on patents related to oncolytic HSV1. R.V.L. has received NIH funding via NCI Brain Tumor SPORE P50CA221747, has served on the advisory boards for Monteris and Ziopharm, and has received consulting fees from AbbVie, NewLink Genetics, and ReNeuron and travel support from Genentech-Roche to present at a meeting, honoraria for medical editing for MedLink Neurology, honoraria for review of content for medical accuracy for EBSCO Publishing, and honoraria for creating and presenting content for CME board review courses for American Physician Institute. He has received personal fees from AbbVie for internal presentations. D.A.R. reports research support from Acerta Pharmceuticals, Agenus, Celldex, EMD Serono, Incyte, Inovio, Omniox, and Tragara. He has also received personal fees from Abbvie, Advantagene, Agenus, Amgen, Bayer, Bristol Myers Squibb, Celldex, DelMar, EMD Serono, Genentech/Roche, Imvax, Inovio, Medicenna Biopharma, Inc., Merck, Merck KGaA, Monteris, Novocure, Oncorus, Oxigene, Regeneron, Stemline, Sumitono Dainippon Pharma, and Taiho Oncology Inc. PYW received research support from Agios, Astra Zeneca/Medimmune, Bayer, Beigene, Celgene, Eli Lily, Genentech/Roche, Kazia, MediciNova, Merck, Novartis, Nuvation Bio, Oncoceutics, Vascular Biogenics, VBI Vaccines. He also received personal fees from Agios, Astra Zeneca, Bayer, Boston Pharmaceuticals, CNS Pharmaceuticals, Elevate Bio, Elsevier, Immunomic Therapeutics, Imvax, Karyopharm, Merck, Novartis, Nuvation Bio, UpToDate, Vascular Biogenics, VBI Vaccines, Voyager, and QED. J.Y.B., N.H., N.D., J.M., T.E., J.T., J.L., K.C., A.B.G., and L.J.N.C., F.L. are employees of, own equity interest in and/or have assigned intellectual property rights to Ziopharm Oncology, Inc. In addition, A.B.G. has equity ownership in Merck. In addition, LJNC has received royalties from City of Hope National Medical Center and Immatics, holds receipts of intellectual property rights from Sangamo BioSciences and MD Anderson Cancer Center, and Ziopharm Oncology, and also has equity ownership interest in Targazyme, Ziopharm Oncology, Immatics, Kiadis/Sanofi, CellChorus, Secure Transfusion Services, and AuraVax. CCC., P.W., W.L.B., P.P., C.A., D.T., and L.S. declare they have no competing interests.
Authorship statement. E.A.C., R.V.L., C.C.C., G.R., D.A.R., P.W., W.L.B., P.P., C.A., D.T., L.S., G.P., J.G., and K.T. were responsible for the clinical trial at each institution, data acquisition, data analysis, and/or manuscript drafting. J.Y.B., N.H., N.D., J.M., T.E., J.T., J.L., K.C., L.J.N.C., and A.B.G., were responsible for trial sponsorship, data analyses and manuscript drafting. E.A.C. and A.G. were responsible for final manuscript editing.
Data Availability Statement
Trial Registration Number: NCT03636477. All data associated with this study are present in the paper or supplementary materials. Original clinical data are available via Mr. Nate Demars, Ziopharm Oncology, Inc., One First Avenue, Parris Building 34, Navy Yard Plaza, Charlestown, Massachusetts Boston, MA, USA, 02129, NDemars@ziopharm.com.
References
- 1. Marenco-Hillembrand L, Wijesekera O, Suarez-Meade P, et al. Trends in glioblastoma: outcomes over time and type of intervention: a systematic evidence based analysis. J Neurooncol. 2020;147(2):297–307. [DOI] [PubMed] [Google Scholar]
- 2. Wen PY, Weller M, Lee EQ, et al. Glioblastoma in adults: a Society for Neuro-Oncology (SNO) and European Society of Neuro-Oncology (EANO) consensus review on current management and future directions. Neuro Oncol. 2020;22(8):1073–1113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Kamiya-Matsuoka C, Gilbert MR. Treating recurrent glioblastoma: an update. CNS Oncol. 2015;4(2):91–104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Tomaszewski W, Sanchez-Perez L, Gajewski TF, Sampson JH. Brain tumor microenvironment and host state: implications for immunotherapy. Clin Cancer Res. 2019;25(14):4202–4210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Lasek W, Zagożdżon R, Jakobisiak M. Interleukin 12: still a promising candidate for tumor immunotherapy? Cancer Immunol Immunother. 2014;63(5):419–435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Lotze MT. Interleukin 12: cellular and molecular immunology of an important regulatory cytokine. Introduction. Ann N Y Acad Sci. 1996;795:xiii–xix. [DOI] [PubMed] [Google Scholar]
- 7. Trinchieri G. Interleukin-12 and the regulation of innate resistance and adaptive immunity. Nat Rev Immunol. 2003;3(2):133–146. [DOI] [PubMed] [Google Scholar]
- 8. Curtsinger JM, Johnson CM, Mescher MF. CD8 T cell clonal expansion and development of effector function require prolonged exposure to antigen, costimulation, and signal 3 cytokine. J Immunol. 2003;171(10):5165–5171. [DOI] [PubMed] [Google Scholar]
- 9. Micallef MJ, Ohtsuki T, Kohno K, et al. Interferon-gamma-inducing factor enhances T helper 1 cytokine production by stimulated human T cells: synergism with interleukin-12 for interferon-gamma production. Eur J Immunol. 1996;26(7):1647–1651. [DOI] [PubMed] [Google Scholar]
- 10. Leonard JP, Sherman ML, Fisher GL, et al. Effects of single-dose interleukin-12 exposure on interleukin-12-associated toxicity and interferon-gamma production. Blood. 1997;90(7):2541–2548. [PubMed] [Google Scholar]
- 11. Car BD, Eng VM, Lipman JM, Anderson TD. The toxicology of interleukin-12: a review. Toxicol Pathol. 1999;27(1):58–63. [DOI] [PubMed] [Google Scholar]
- 12. Atkins MB, Robertson MJ, Gordon M, et al. Phase I evaluation of intravenous recombinant human interleukin 12 in patients with advanced malignancies. Clin Cancer Res. 1997;3(3):409–417. [PubMed] [Google Scholar]
- 13. Rodolfo M, Colombo MP. Interleukin-12 as an adjuvant for cancer immunotherapy. Methods. 1999;19(1):114–120. [DOI] [PubMed] [Google Scholar]
- 14. Barrett JA, Cai H, Miao J, et al. Regulated intratumoral expression of IL-12 using a RheoSwitch Therapeutic System® (RTS®) gene switch as gene therapy for the treatment of glioma. Cancer Gene Ther. 2018;25(5-6):106–116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Cai H, Sun L, Miao J, Krishman S, Lebel F, Barrett JA. Plasma pharmacokinetics of veledimex, a small-molecule activator ligand for a proprietary gene therapy promoter system, in healthy subjects. Clin Pharmacol Drug Dev. 2017;6(3):246–257. [DOI] [PubMed] [Google Scholar]
- 16. Chiocca EA, Yu JS, Lukas RV, et al. Regulatable interleukin-12 gene therapy in patients with recurrent high-grade glioma: results of a phase 1 trial. Sci Transl Med. 2019;11(505): eaaw5680. doi:10.1126/scitranslmed.aaw5680 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Cloughesy TF, Mochizuki AY, Orpilla JR, et al. Neoadjuvant anti-PD-1 immunotherapy promotes a survival benefit with intratumoral and systemic immune responses in recurrent glioblastoma. Nat Med. 2019;25(3):477–486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Wen PY, Macdonald DR, Reardon DA, et al. Updated response assessment criteria for high-grade gliomas: response assessment in neuro-oncology working group. J Clin Oncol. 2010;28(11):1963–1972. [DOI] [PubMed] [Google Scholar]
- 19. Okada H, Weller M, Huang R, et al. Immunotherapy response assessment in neuro-oncology: a report of the RANO working group. Lancet Oncol. 2015;16(15):e534–e542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Komita H, Zhao X, Katakam AK, et al. Conditional interleukin-12 gene therapy promotes safe and effective antitumor immunity. Cancer Gene Ther. 2009;16(12):883–891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Shakur SF, Bit-Ivan E, Watkin WG, Merrell RT, Farhat HI. Multifocal and multicentric glioblastoma with leptomeningeal gliomatosis: a case report and review of the literature. Case Rep Med. 2013;2013:132679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Gerdes MJ, Sevinsky CJ, Sood A, et al. Highly multiplexed single-cell analysis of formalin-fixed, paraffin-embedded cancer tissue. Proc Natl Acad Sci U S A. 2013;110(29):11982–11987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Sood A, Miller AM, Brogi E, et al. Multiplexed immunofluorescence delineates proteomic cancer cell states associated with metabolism. JCI Insight. 2016;1(6): e87030. doi:10.1172/jci.insight.87030 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Reardon DA, Brandes AA, Omuro A, et al. Effect of nivolumab vs bevacizumab in patients with recurrent glioblastoma: the CheckMate 143 phase 3 randomized clinical trial. JAMA Oncol. 2020;6(7):1003–1010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Chiocca EA, Abbed KM, Tatter S, et al. A phase I open-label, dose-escalation, multi-institutional trial of injection with an E1B-Attenuated adenovirus, ONYX-015, into the peritumoral region of recurrent malignant gliomas, in the adjuvant setting. Mol Ther. 2004;10(5):958–966. [DOI] [PubMed] [Google Scholar]
- 26. Chiocca EA, Smith KM, McKinney B, et al. A phase I trial of Ad.hIFN-β gene therapy for glioma. Mol Ther. 2008;16(3):618–626. [DOI] [PubMed] [Google Scholar]
- 27. Cloughesy TF, Landolfi J, Hogan DJ, et al. Phase 1 trial of vocimagene amiretrorepvec and 5-fluorocytosine for recurrent high-grade glioma. Sci Transl Med. 2016;8(341):341ra375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Wheeler LA, Manzanera AG, Bell SD, et al. Phase II multicenter study of gene-mediated cytotoxic immunotherapy as adjuvant to surgical resection for newly diagnosed malignant glioma. Neuro Oncol. 2016;18(8):1137–1145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Reardon DA, Wucherpfennig K, Chiocca EA. Immunotherapy for glioblastoma: on the sidelines or in the game? Discov Med. 2017;24(133):201–208. [PubMed] [Google Scholar]
- 30. Omuro A, Vlahovic G, Lim M, et al. Nivolumab with or without ipilimumab in patients with recurrent glioblastoma: results from exploratory phase I cohorts of CheckMate 143. Neuro Oncol. 2018;20(5):674–686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Mirzaei R, Sarkar S, Yong VW. T cell exhaustion in glioblastoma: intricacies of immune checkpoints. Trends Immunol. 2017;38(2):104–115. [DOI] [PubMed] [Google Scholar]
- 32. Filley AC, Henriquez M, Dey M. Recurrent glioma clinical trial, CheckMate-143: the game is not over yet. Oncotarget. 2017;8(53):91779–91794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Lang FF, Conrad C, Gomez-Manzano C, et al. Phase I study of DNX-2401 (Delta-24-RGD) oncolytic adenovirus: replication and immunotherapeutic effects in recurrent malignant glioma. J Clin Oncol. 2018;36(14):1419–1427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Pauken KE, Godec J, Odorizzi PM, et al. The PD-1 pathway regulates development and function of memory CD8+ T cells following respiratory viral infection. Cell Rep. 2020;31(13):107827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Chiocca EA, Lukas RV, Yu J, et al. Final results of controlled IL-12 monotherapy in adults with grade III or IV gliomas. Am Soc Clin Oncol Annual Meeting. 2021; 38(15_suppl):3040. doi:10.1200/JCO.2020.38.15_suppl.3040 [Google Scholar]
- 36. Roth P, Valavanis A, Weller M. Long-term control and partial remission after initial pseudoprogression of glioblastoma by anti-PD-1 treatment with nivolumab. Neuro Oncol. 2017;19(3):454–456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Qin L, Li X, Stroiney A, et al. Advanced MRI assessment to predict benefit of anti-programmed cell death 1 protein immunotherapy response in patients with recurrent glioblastoma. Neuroradiology. 2017;59(2):135–145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Ranjan S, Quezado M, Garren N, et al. Clinical decision making in the era of immunotherapy for high grade-glioma: report of four cases. BMC Cancer. 2018;18(1):239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Mineo M, Lyons SM, Zdioruk M, et al. Tumor interferon signaling is regulated by a lncRNA INCR1 transcribed from the PD-L1 locus. Mol Cell. 2020;78(6):1207–1223 e1208. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Trial Registration Number: NCT03636477. All data associated with this study are present in the paper or supplementary materials. Original clinical data are available via Mr. Nate Demars, Ziopharm Oncology, Inc., One First Avenue, Parris Building 34, Navy Yard Plaza, Charlestown, Massachusetts Boston, MA, USA, 02129, NDemars@ziopharm.com.