Abstract
Three-dimensional (3D) bioprinting is an emerging technology that is widely used in regenerative medicine. With the continuous development of the technology, it has attracted great attention and demonstrated promising prospects in ophthalmologic applications. In this paper, we review the three main types of 3D bioprinting technologies: Vat polymerization-based bioprinting, extrusion-based bioprinting, and jetting-based bioprinting. We also present in this review the analysis of the usage of both natural and synthesized hydrogels as well as the types of cells adopted for bioinks. Cornea and retina are the two main types of ocular tissues developed in bioprinting, while other device and implants were also developed for the ocular disease treatment. We also summarize the advantages and limitations as well as the future prospects of the current bioprinting technologies based on systematic reviews.
Keywords: Bioprinting, Ophthalmology, Bioinks, Biomaterials, Ocular bioprinting
1. Introduction
Bioprinting is an additive biofabrication method that prints target tissue engineering structures automatically by depositing bioinks in a layer-by-layer manner[1]. The technology attempts to produce original-tissue-like constructs through the precise combination of living cells, natural or synthesized biomaterials, crosslinkers, and/or other functional factors[2]. Since the emergence of the invention[3,4], bioprinting has been subject to persistent development mostly in the last decades and is used in a wide range of medical applications nowadays[5].
In ophthalmology, three-dimensional (3D) printing is utilized for diverse purposes, varying from the fabrication of preoperative eye models[6], personalized lens[7,8], glasses[9,10] and to other implants[9]. However, in tissue engineering and regenerative medicine, the use of bioprinting in fabricating ocular tissues and preserving relevant biological functions still need to be further studied.
In this article, we review the main 3D bioprinting techniques, summarize the bioinks adopted in 3D bioprinting, and discuss the applications of bioprinting in ophthalmology. We also present the advantages and limitations of bioprinting in the ocular tissue engineering as well as the future directions that will translate the technologies to personalized therapeutic and pharmaceutical tools in ophthalmology.
At present, vat polymerization (VP)-based, material extrusion-based, and material jetting-based printing strategies are the three principal printing techniques used in biological and medical applications (Figure 1). Due to the distinct working principles, each approach demonstrates different strengths and drawbacks.
Figure 1.
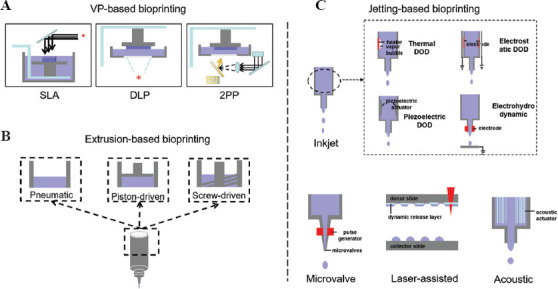
Three major categories of bioprinting technologies. (A) VP-based bioprinting. (B) Extrusion-based bioprinting. (C) Jetting-based bioprinting. DLP: Digital light processing; DOD: Drop-on-demand; SLA: Stereolithography; VP: Vat polymerization; 2PP: Two-photon polymerization.
1.1. VP-based bioprinting
The VP-based bioprinting refers to the process that fabricates the 3D structures through photo-polymerization reaction with photo-curable liquid bio-resin radiated by the light at specific wavelength[11]. The developed techniques include stereolithography (SLA), digital light processing (DLP), and two-photon polymerization (2PP) (Figure 1A).
As shown in Figure 1A, the SLA system cures the bio-resin when the laser source is refracted and scanned the materials in a vat. Depending on the position of the laser source, SLA can print the structures either in a top-down or bottom-up manner[11]. In the top-down method, the system prints the support and target structures together to form a precise designed construct. On the contrary, the bottom-up method scanned and cured the biomaterials with the light source from the bottom side. During the bottom-up printing, the build platform is raised above the vat for each peeling step between layers, thus resulting in a slower printing speed than the top-down method with continuous light scanning. To attain a successful printing, it is critical for both methods to have a good bonding between the printed structure and build-platform. Immediately after printing, the support structures need to be removed manually. The advantages of SLA techniques include the high lateral and vertical printable resolution (about 20–50 μm and 25–100 μm, respectively)[12], a wide range of printable viscosities (up to 5 Pa.s)[13], high printable cell density (could be 108 cells per ml), and great potential to formulate highly complex structures with the aid of support structures. However, its applications are limited in bioprinting due to the harmful effect of UV rays (shorter wavelength results in more DNA damage) and cytotoxic effect from the increased light intensity and photo-initiator concentration. To reduce DNA damage, the usage of visible light source as well as biocompatible photo-initiators is necessary.
Different from the SLA, the DLP prints the structures with the use of a digital micro-mirror device (DMD) to crosslink the photo-curable bio-resin in a vat. With the aid of DMD, DLP can speed up the printing process by crosslinking a layer of bio-resin rather than a single dot in SLA[14]. By controlling the light source power and exposure time, the cure depth of the bio-resin can be determined in the DLP system. Compared to the SLA, the DLP is more suitable in printing large-scale structures, which could be attained in micron resolutions.
The 2PP system adopts a near-infrared femtosecond laser light source (about 740 nm wavelength) and fabricates microstructures in a nanoscale resolution (up to sub-100 nm)[15]. The femtosecond laser is tightly focused to the bio-resin, and controlled the movement through an oil-immersed objective lens. The polymerization process in 2PP is initiated by exciting the molecules through two-photon absorption. The process can occur in an extremely short period (mm/seconds of scanning speed) and fabricate highly complex structure at any spatial position. However, the application of 2PP in bioprinting is also limited by a few drawbacks, including the material degradation caused by high laser power and bubble damage[16].
1.2. Extrusion-based bioprinting
The extrusion-based bioprinting is the most widely used technique because it has fast printing speed and can work with a broad range of printable bioinks[17]. In general, the material can be extruded from the cartridges either through pneumatic pressure or mechanical forces (piston-driven or screw-driven) (Figure 1B). The extrusion-based bioprinting can print bioinks with high cell densities (107 cells per ml) and print multiple types of cells at same time to fabricate a heterogenous structure. However, it also has limitations on the cell viability due to the damage with shear stress and nozzle clogging. To improve the performance of extrusion-based bioprinting, it is necessary to optimize the bioink design, select nozzle of suitable size, and choose suitable materials. The adjustment of printing parameters (e.g., pressure, speed, layer thickness) is required before printing to achieve a better performance.
1.3. Jetting-based bioprinting
As shown in Figure 1C, the jetting-based bioprinting represents a big group of techniques, including inkjet bioprinting, microvalve bioprinting, laser-assisted bioprinting, and acoustic bioprinting[18]. An advantage of the jetting-based printing technique is the drop-on-demand (DOD) patterning of different types of cells and biomaterials in a noncontact profile. Using this method, the droplets can be generated either by the heater-vapored bubbles in a thermal style[19], the deformation under the electrode pressure in an electrostatic style[20,21], the vibration of a piezoelectric actuator in piezoelectric style[22,23], or the high voltage filed energy from the nozzle electrodes in electrohydrodynamic style[24,25]. Different from the traditional continuous method, the DOD inkjet bioprinting ejects droplets only when the signal meets the demanded levels, which makes the droplet to formulate specific patterns as designed. Besides, as the diameter of inkjet nozzle is comparable to the size of a cell (50 μm), the inkjet printing could also be used for single cell printing. The high-resolution printing helps fabricate smaller tissues/organs, and the unique printing patterns could enhance the cell-cell and cell-matrix interactions. The inkjet bioprinting would contribute to cell damages by shear stress or pressure during printing or collision with the substrate after ejection. However, the damage only occurs to a low proportion of the cells; the efficiency and high-throughput features of inkjet printing still outweigh its drawbacks.
2. Bioinks
Compared with the traditional methods, bioprinting can be used to design complex 3D structures. It prints bioinks layer by layer to control the spatial distribution of cells and is used to fabricate the specific structure of tissues. The success of using bioprinting depends on printability and bioactivity of the bioinks[26]. In ophthalmology, bioinks of either cell-free hydrogel or cell-laden biomaterials were developed for different purposes.
2.1. Biomaterials
Biocompatibility, printability, and mechanical properties are the three main requirements for bioinks[27]. To formulate a highly biocompatible environment for the cells, decellularized extracellular matrix (dECM), and nature-derived or semi-synthesized hydrogels are widely used in ophthalmic applications.
(1) dECM
The ECM is important for cell nutrition, protection, and tissue function[28]. The ECM network is rich in molecules of collagen, elastin, microfibrillar proteins, adhesive glycoproteins and proteoglycans, providing support and crucial cues for cell adhesion, engraftment, and functions[29]. The dECM was developed for bioink bioprinting to mimic an optimized microenvironment for specific tissue engineering[30]; for instance, cornea- and retina-specific dECMs were developed for ocular tissue regeneration[31-33]. In corneal engineering, dECMs present great advantage in maintaining the keratocyte morphology and transparency by preventing the transdifferentiation of corneal fibroblasts[34]. To prepare the cornea-specific dECM bioink for bioprinting, Kim et al. decellularized the ECM from the bovine corneal stroma and lyophilized the cornea-derived dECM (Co-dECM) samples[31]. When printing, the Co-dECM powder was solubilized in acidic solutions and adjusted to form printable gel. The removal of the cells helps to reduce immune rejection response in tissue grafting. Moreover, the Co-dECM bioink has comparable levels of collagen and glycosaminoglycans as the natural cornea. The thin collagen fibrils in the bioink have a larger amount of proteoglycans, which make it possible to pass through the greater amount of light and maintain the transparency property as the native cornea. In addition, the Co-dECM bioink presented no toxicity in animal experiments and showed a good therapeutic potential in corneal diseases[35].
(2) Hydrogels
Hydrogels are structured networks of crosslinked polymers that are capable of absorbing and retaining large quantities of water. They can be engineered to support the cellular growth, migration, and tissue formation[36,37]. Hydrogels present strong biomimetic advantages in the clinical translational applications attributed to their hydrophilic nature, good biocompatibility, biodegradability, controllable responsiveness to external stimuli, and tunable physical and chemical properties such as adhesion or low mechanical properties. Both naturally derived and synthetically derived hydrogels are widely used in bioprinting.
A series of natural hydrogels have been used for bioprinting due to their similarity to the cell microenvironment. The corneal stroma is rich in collagen, proteoglycan, and matrix metalloproteinase, which play an important role in the mechanical strength and transparency of the cornea. Due to the good biocompatibility and low immunogenicity, collagen is widely used as a bioink in 3D bioprinting[38]. However, low mechanical property of the pure collagen is the main limitation of using it as the bioink to form a stable structure. Crosslinking using different methods (e.g., chemical, physical, or biological) or a mixture with other components (e.g., fibrin, agarose, and alginate) can be performed to improve the properties of collagen bioinks[39-41]. Depending on the types of strategies selected, the bioinks can be tuned to prepare suitable low-viscosity solutions for jetting-based printing[42,43] or hydrogels with increased storage modules for extrusion-based printing[38]. In addition, synthesized peptide-based collagen is considered a good option to reduce the batch-to-batch variation effect and improve the mechanical properties in bioprinting[41].
Hyaluronic acid, which is another natural component of ECM, is abundant in the subretinal space. The hyaluronic acid hydrogel provides a biocompatible environment for the culture of retinal cells but the physiological property needs to be modified to fulfill the need of the native retina and printing requirements. Crosslinking the hydrogel with methacrylate groups could mimic the mechanical properties of retina and contributed to a good survival rate for the retinal pigment epithelial cells and differentiation of the fetal retinal progenitor cells (fRPCs)[44].
Gelatin is a type of hydrogel which shows good adhesive properties in the oculus usage and is considered a good option for cornea engineering[45]. It is biocompatible, transparent and non-toxic. Besides, it can be easily processed and has the reasonable mechanical properties to mimic the ECM. Gelatin can gain different viscoelastic and mechanical properties to facilitate the functions of oculus.
In addition to the natural hydrogels, synthetic bioinks are also widely used in bioprinting. Gelatin methacrylate (GelMA) is a photopolymerized hydrogel comprising of modified natural ECM components and can be prepared from the water-soluble gelatin. Gelatin is a temperature-sensitive polymer that transforms between the solid and liquid states when the temperature changes. With the addition of methacryloyl component, GelMA can improve the thermal stability of gelatin, demonstrating properties of both physicochemical strength and biocompatibility[46]. Both simulation of the matrix microenvironment and 3D printing of GelMA hydrogels to produce artificial matrices with high transparency, biocompatibility, and stability are the important pillars in the development of bioprinting in ophthalmology[47].
2.2. Types of cells
To facilitate their application in printing, the cells can be presented as cell-laden biomaterials, cell suspension or tissue spheroids for different printing technologies.
(1) Cell composition
The eyeball is a complex organ and composed of a variety of tissues. The cornea and retina are the two main types of tissues obtaining great attention in the ocular regenerative medicine and tissue engineering.
The cornea is located at the anterior section of the eye and formed by five layers: epithelium, bowman membrane, stroma, Descemet’s membrane, and endothelium. To form the stroma equivalent, the corneal keratocytes can be isolated from the stromal cells of cadaverous human corneal tissues[48] and purified to print together with the biomaterials[49]. Besides, the corneal epithelium cells and endothelium cells also triggered the attention from the researchers to be bio-printed[50,51]. The human corneal epithelial cells and endothelial cells can be harvested from the donor corneas and made laden with matrix gel to form bioinks.
The retina is a complex stratified structure, which is located at the posterior section of the eye. It embodies more than 60 types of cells and nerve fibers that are difficult to be regenerated when damaged[52]. The cells isolated from the rat retinal tissues can be prepared as cell suspensions and printed independently with inkjet printing method onto a substrate matrix[53,54]. Besides, the fetal human retinal progenitor cells have been adopted in bioprinting, and have successfully differentiated into photoreceptors after printing[44].
(2) Stem cell induction
The latest advances in the research of induced pluripotent stem cell have paved the way for the production of patient-specific cells that are ideal for autologous cell replacement therapies in the treatment of various alternative diseases. Adipose-derived stem cells (ADSCs) are fat-derived stem cells with self-renewal, proliferation, and differentiation potentials. ADSCs can differentiate into corneal cells both in vivo and in vitro, and as reported in the previous studies, other researchers have printed differentiated ADSCs with corneal characteristics using biomaterials, thereby successfully printing a layered corneal tissue that mimics the natural cornea[28]. In addition, the mesenchymal stem cells derived from the turbinate can also use to produce cells of keratocyte lineage that can be used for corneal regeneration[31].
3. Function and applications
In a general sense, bioprinting refers to 3D printing for medical applications, which can be divided into four stages of development[55]. Stage I is to print non-biocompatible structures that can be used as the models for surgery planning. Stage II is to print biocompatible but non-biodegradable products, such as implanted prothesis. Stage III is to print biocompatible and biodegradable products, which can be used as scaffolds to improve tissue damage repair or regeneration. Stage IV is to print biomimic 3D structures with cells. In the narrow sense, bioprinting can also be defined as cellular printing[27]. In the present review, our definition of bioprinting encompasses all four stages of development.
3.1. Treatment device and prosthesis
(1) Contact lens and scleral cover shell prothesis
Contact lens are used for vision correction or other therapeutic or cosmetic purposes. In addition, they can be incorporated with sensors for disease diagnosis and management (e.g., measure glucose composite in tears or monitor intraocular pressure for glaucoma)[56]. Through 3D printing technology, different sensor components have been designed and printed to make cost-efficient and smart contact lens[7,8,57]. The nanostructures patterned by direct laser writing help to detect the disease at early stages, while techniques still need to be further developed for commercial utilization.
The scleral cover shell prosthesis is used to correct the eye diagram in pathological conditions. It covers the corneal and adjacent scleral areas during the treatment. In the pilot study, Sanchez-Tena et al. printed a prosthesis with polylactic acid (PLA) using fused filament fabrication printing[58]. Further attempts using biocompatible materials to simulate the corneoscleral profile fitting are necessary.
(2) Intraocular lenses (IOLs)
IOLs are common personalized devices to treat cataract in clinic. The products mimic the shape and dimensions of crystalline lens and provide the substitute optical functions. Studies have been performed to reproduce the IOLs with a patented 3D printing technology called Print Optical. In the method, the photopolymer material (e.g., Luxexcel polymer) was deposited onto a poly(methylmethacrylate) (PMMA) substrate and cured with ultraviolet[59,60]. The printed IOLs with PMMA-like materials had a good level of transparency and showed the properties of biconvex lens, but were still limited by the surface roughness and optical performance (e.g., wavefront aberrations) in comparison to the qualified implants[59]. Similar results were also observed in the study by John et al., which demonstrated higher levels of surface roughness, figure errors, and wavefront deformations than the control product[60]. A better performance on the curvature radii was obtained on smaller lenses when printed. Although printing technology has great advantage over the manufacture of irregular and asymmetric products, it still has room for improvement in the future replication of IOLs. Further improvement on the surface figure is needed to make the printed lens fulfill the requirements of clinical implant.
(3) Choroidal models
The choroid is a soft membrane layer formed mainly by blood vessels between retina and sclera. It is the main blood supply for the retina and the surrounding ocular tissues. The reduction of choroidal vessels due to tumor or other ocular diseases will result in the loss or death of tissues in the supply areas. To evaluate the structural changes of choroidal vessels, Maloca et al. printed 3D models of choroidal vessels based on the optical coherence tomography images from normal eyes and pigmented choroidal tumors[6]. The group segmented the choroidal vessels in the interested areas by a threshold filter, and printed the 3D models with transparent resin by SLA printing or gypsum power by additive fused deposition modeling. The resolution of the models was limited to 1 mm in wall thickness, which was able to characterize the choroidal vessels in details. This helps to better understand the architecture of choroidal vessels and their interactions with the adjacent tissues and tumors.
(4) Artificial eyes
In the study by Xie et al., an eye model with different ametropia state was produced using 3D printing technology for the fundus viewing system[10]. The optical parameters of printed models are adjustable and the models can simulate the optical performance of human eye. The technique developed by Xie et al. could be a useful tool for fundus range viewing research and training for fundus examination in future. Besides, conformer shells are clear plastic lens fitted to support the shape of the eye socket and maintain the natural appearance without glasses after an enucleation/eye removal surgery. 3D printing of conformer shells makes the production of individualized shells possible.
A lot of attempts have also been pursued to generate “bionic eyes” using 3D printing technology. By printing the semiconductive polymer onto a hemispherical surface using a customized extrusion printer, Park et al. fabricated the polymer-based photodetectors that can generate electricity from the light stimuli[61]. The production of electronic devices by 3D printing confirms that the design of light receptors is a more flexible and efficient method than the traditional manufacturing. Indirectly, this might facilitate the development of wearable and implantable material that can restore the vision in future.
(5) Orbital implant
3D printing is a flexible and low-cost method for designing customized complex orbital reconstruction implants[62-65]. Based on the digital images from the orbital tumor resection or fracture, the researchers can reconstruct the structure model, design the implant templates according to the orbital structure of the intact part, and print the 3D models to serve as stencil for the actual implant material[62]. The application of 3D printing technology reduces the need to adjust and manipulate the Medpor-titanium implant during the operation and could improve various surgical indicators (e.g., reduced tissue damage, shortened surgical duration, improved clinical outcomes, and cost effectiveness)[62,64]. Besides, 3D printing technology also demonstrated advantages in implant exchange or dermis fat graft secondary to the orbital implants[63]. This technique can be used to design the exact shape of the implant and center the implants in patients with recurrent implant migration.
3.2. Drug delivery systems
Due to the existence of blood-retinal barrier and blood-aqueous barrier, it is difficult to deliver the drug to the back of the eye for treatment. Chitosan is one of the hemi-synthetic, highly biocompatible, and biodegradable hydrogels considered suitable for ocular drug delivery[66-68]. Chitosan nanoparticles could prolong drug delivery, facilitate penetration through the physiological barriers, and enhance mucoadhesive properties[69,70]. Meanwhile, preparation of nanogels of personalized medication using 3D printing technology has begun to gain traction[70].
3.3. Graft tissue and organs
(1) Cornea
The cornea is the outermost protective layer of the eye and is responsible for the transmission and refraction of incident light. Although corneal diseases are the causal factor of visual impairment and blindness worldwide[71], the percentage of corneal transplantation undertaken in individuals with treatable corneal blindness is still very low (approximately 1.4%)[72]. By estimation, more than 12.7 million patients are on the waiting list of a keratoplasty[73]. The shortage of donated cornea in eye banks against the increasing cases of corneal blindness is the main driver for the development of artificial cornea. Compared with the traditional technologies for artificial cornea production, 3D bioprinting provides a fast method to reconstruct individual-specific tissues and organs with high reproducibility.
Bioprinting offers the possibility of producing artificial cornea. The human corneal scanning model is used to print artificial cornea with complex structure through bioprinting, but the tissue function of artificial cornea still needs to be further validated in clinical trials[31]. The challenge in bioprinting the cornea lies in the transparency, microporosity and specific shape properties of the structure[74,75].
The natural cornea consists of three cellular layers (epithelium, stroma and endothelium) and two acellular layers (Bowman’s and Descemet’s membranes) (Figure 2)[76]. Stroma forms the major part of the cornea, accounting for 90% of the corneal thickness (approximate 500 µm)[75]. A series of studies have presented feasible strategies to bioprint the stroma equivalents[35,49,77-79]. Among the bioprinting technologies, the extrusion-based method is the most widely used, whereas the jetting method also demonstrates some advantages (Table 1). With the aid of a supportive gelatin scaffold, Isaacson et al. successfully printed the cornea-shaped structure with optimized cell-laden collagen/alginate bioinks using pneumatic extrusion 3D bioprinter[49]. A high cell viability of corneal keratocytes was observed on both day 1 (92%) and day 7 (83%) post-printing. Without using supportive scaffold, Campos et al. adopted an electromagnetic micro-valve, rather than micro-extrusion, to directly print the corneal structure with collagen hydrogel in a DOD manner[77]. The transparency and optical density of the printed structure was comparable to those of the native cornea, and the keratocyte cells assumed the typical dendritic morphology on day 7 post-printing. GelMA is another good option for stroma printing; however, in GelMA-only printed structure, the keratocytes stayed in round shapes after days[35]. The specially designed alignment of collagen fibrils is critical to the transparency of the cornea. To mimic the sophisticated topological alignment of the stroma structure, Kong et al. fabricated the 3D construct with poly (e-caprolactone)-poly (ethylene glycol) (PECL) microfibrous scaffold, which is a modified type of poly (e-caprolactone) (PCL) with improved hydrophilicity, and GelMA hydrogel by direct writing[78]. The construction provided a good environment for the survival and phenotype maintenance of keratocyte cells. In another study, shear stress was induced by printing nozzles of different sizes in order to organize the printed collagen fibrils in the lattice pattern[79].
Figure 2.
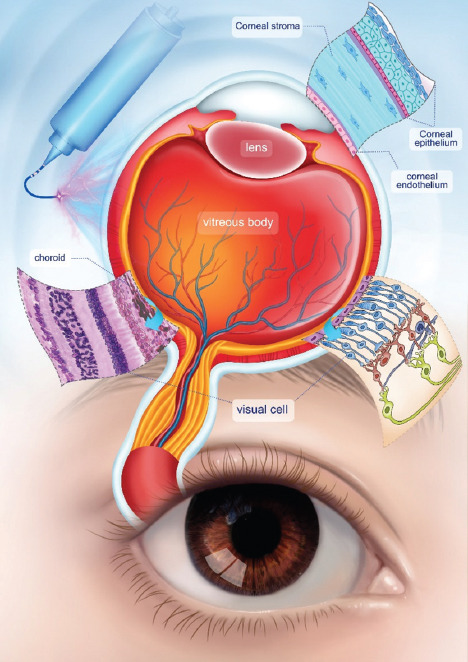
Roles of bioprinting in ophthalmology.
Table 1.
Application of 3D bioprinting in ophthalmology
| Ocular tissues | Tissue layer | Bioprinting approaches | Biomaterials | Cell types | References |
|---|---|---|---|---|---|
| Cornea | Epithelium | Extrusion | GelMa bioink/GelMA dome-shaped mold | CEpCs/LECs, Human CEpCs line | [50,86] |
| Stroma | Extrusion, Inkjet, Laser-assisted, SLA | Matrigel-COL I bioink/LN-COL IV support sheet, ALG-COL I bioink/FRESH support, COL I-AG bioink/ no support, GelMa bioink/reinforced with PEG-PCL fibers, GelMA bioink/ no support, cornea-derived dECM bioink/no spport, ALG/GEL bioink/resin support | CSKs/LSSCs, human LECs+ADSCs, human CSKs, rat LSSCs, human TDMSCs with keratocyte induction, HCKs | [28,31,35,49,79,80,86-88] | |
| Endothelium | Extrusion | Gelatin-RGD bioink/amniotic membrane dECM support | CECs, human CECs | [51,86] | |
| Retina | Retinal pigment epithelium | Laser-assisted, Microvalve jetting | HA-GM and PEG-RGDs, DMEM:F12/ALG and Pluronic | RPEs, human fetal retinal progenitor cells, ARPE-19, human retinoblastoma cell line (Y79) | [44,89] |
| Retinal ganglion cells | Piezoelectric inkjet, 2PP, thermal inkjet combined with electrospinning | DMEM, ITO-coated glass, alginate and culture Medium/PLA/HEIP and matrigel for electrospinning | Retinal granlion cell neurons, retinal glial cells, human iPSC, retinal ganglion cells | [54,83,90] |
ADSCs: Adipose-derived stem cells; AG: Agarose; ALG: Alginate; CECs: Corneal endothelial cells; ARPE-19: Adult retinal pigmented epithelial cell line-19; CEpCs: Corneal epithelial cells; COL: Collagen; CSK: Corneal stromal keratocytes; dECM: Decellularized extracellular matrix; DMEM: Dubelcco’s Modified Eagle’s medium; FRESH: Freeform reversible embedding of suspended hydrogels; GelMA: Gelatin methacrylate; HA-GM: Hyaluronic acid with methacrylation by glycidyl-hydroxyl reaction; HCKs: Human corneal keratocytes; HEIP: Hexafluoroisopropanol; iPSC: Induced pluripotent stem cell; ITO: Indium tin oxide; LECs: Limbal epithelial cells; LN: Laminin; LSSCs: Limbal stromal stem cells; PEG-PCL: Polyethylene glycol-polycaprolactone; PEG-RGDs: Arg-Gly-Asp-Ser peptide; PLA: Polylactic acid; RPE: Retinal pigment epithelial cells; SLA: Stereolithography; TDMSCs: Turbinate-derived mesenchymal stem cells; 2PP: Two-photon polymerization
Besides, researchers also attempted to print the endothelial and epithelial components of the cornea (Table 1)[45]. For instance, Sorkio et al. printed the stratified corneal epithelium and stroma construct with the stem cell-laden laminin/collagen bioink using laser-assisted technology[28]. Jin et al. printed the cells of corneal epithelium using DLP technology coupled with extrusion printing[50]. Kim et al. printed the corneal endothelium cells using extrusion printing[51]. The results of these studies strengthened the possibility that corneal substitute can be rapidly generated. Nevertheless, further in vitro and vivo studies are still needed for clinical validation.
Hence, 3D bioprinting technologies embody a great potential and show promising prospects in the fabrication of artificial cornea. Efforts have been made to improve the printing of corneal cells for personalized medicine. However, printing multilayers of the cornea and maintaining the physiological and mechanical properties and functions is still an immense challenge. Using natural macromolecules in the ECM, rather than synthetic materials, can reduce the risk of corneal transplantation, but the degree of immune response elicited by these constructs remains to be investigated[12,80].
(2) Retina
The retina is situated at the back of the eye and contains cells of completely different morphologies and functions arranged in a stratified and vascularized manner[52,81]. It forms distinct circuits to generate visual output; the loss of retinal neurons could lead to visual impairment or blindness. Compared with the cornea, the retina has a more complex multilayered structure (Figure 2). It is composed of different types of cells in nine layers, that is, the pigment epithelial cells in the retinal pigment epithelium (RPE) layer, rod, and cone photoreceptor cells in the photoreceptors layer, Müller cells in the outer nuclear layer, horizontal cells in the outer plexiform layer, bipolar cells in the inner nuclear layer, amacrine cells in the inner plexiform layer, retinal ganglion cells (RGCs) and glial cells in the ganglion cell layer and nerve fiber layer, and internal limiting membranes. Those cells interact and form circuits to work together in transmitting and converting the light signals from the environment into electrical signals in the brain. The structure is about 400 mm in thickness and contains more than 130 million cells[82].
Researchers have attempted to regenerate the retinal tissues using 3D bioprinting technologies and achieved proof of principle in certain types of cells. Lorber et al. was first to successfully print the RGCs and glia directly using piezoelectric inkjet printing technology[83]. The cells demonstrated a good degree of viability post-printing but were unable to form a complex cellular structure in multilayers. The challenges in creating a functional retina through printing include achieving sufficient number of cells and maintaining the cell phenotype and viability post-printing[53]. Validation of the functionalities of printed retina is also very important to create transplantable tissues, while the construction of blood vessels is critical to ensure the supply of nutrients and oxygen to the tissues. In combination with the degradable scaffold prepared with electrospinning technology, Kador et al. printed the RGCs together with brain-derived neurotrophic factor and ciliary neurotrophic factor onto the scaffold using the thermal inkjet printing technique[54]. The RGCs demonstrated a good degree of viability, possessed normal electrophysiological functions, and presented radial axon outgrowth on the scaffold. According to other reports, attempts have been made to print photoreceptors and RPE, which are other types of retinal cells[44,84,85]. Incorporated with the matched hyaluronic acid hydrogel, a bilayer structure of human fRPCs and RPE were printed and the fRPCs differentiated to mutant photoreceptors in 2 weeks[44].
4. Prospects
In recent years, the application of bioprinting technology has considerably expanded to the field of ophthalmology. This technology has a broad range of applications in ophthalmology, including the production of treatment device and prosthesis as well as the establishment of a system to deliver drug to transplantable tissues and organs. Further development of the technology is still needed to enhance functionalities of the printed structures to fulfill the requirements for treating ocular diseases.
Funding
This work is supported by grants from the Visiting Scholar Foundation of Key Laboratory of Biorheological Science and Technology (Chongqing University), Ministry of Education (CQKLBST-2020-003 to YW), the Sichuan Applied Basic Research Project (2018JY0402 to HL) and Luzhou Municipal People’s Government-Southwest Medical University Science and Technology Strategic Cooperation Project (2018LZXNYD-ZK19 to HL), National Natural Science Foundation of China (NSFC 31972915 to WHH), the Science and Technology Project of Guangdong Province (2016B090917001to WHH), and Sanming Project of Medicine in Shenzhen (SZSM201612019 to WHH).
Conflict of interest
The authors declare that they have no conflicts of interest with the contents of this article.
Author contributions
Y.W., J.W., Z.J., W.Y., H.Z., and H.L. reviewed the literature and wrote the manuscript. Y.W., J.W., and Z.J. prepared the literature and figured. H.L. and W.H. conceived the idea of the review and revised the manuscript. All authors approved the final manuscript for publication.
References
- 1.Di Marzio N, Eglin D, Serra T, et al. Bio-Fabrication:Convergence of 3D Bioprinting and Nano-Biomaterials in Tissue Engineering and Regenerative Medicine. Front Bioeng Biotechnol. 2020;8:326. doi: 10.3389/fbioe.2020.00326. https://doi.org/10.3389/fbioe.2020.00326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Moroni L, Burdick JA, Highley C, et al. Biofabrication Strategies for 3D In Vitro Models and Regenerative Medicine. Nature reviews. Materials. 2018;3:21–37. doi: 10.1038/s41578-018-0006-y. https://doi.org/10.1038/s41578-018-0006-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Hull CW, Uvp I. Apparatus for Production of Three-dimensional Objects by Stereolithography. Patent US-6027324-A 1986 [Google Scholar]
- 4.Klebe RJ. Cytoscribing:A Method for Micropositioning Cells and the Construction of Two and Three-dimensional Synthetic Tissues. Exp Cell Res. 1988;179:362–73. doi: 10.1016/0014-4827(88)90275-3. https://doi.org/10.1016/0014-4827(88)90275-3. [DOI] [PubMed] [Google Scholar]
- 5.Castilho M, de Ruijter M, Beirne S, et al. Multitechnology Biofabrication:A New Approach for the Manufacturing of Functional Tissue Structures? Trends Biotechnol. 2020;38:1316–28. doi: 10.1016/j.tibtech.2020.04.014. https://doi.org/10.1016/j.tibtech.2020.04.014. [DOI] [PubMed] [Google Scholar]
- 6.Maloca PM, Tufail A, Hasler PW, et al. 3D Printing of the Choroidal Vessels and Tumours Based on Optical Coherence Tomography. Acta Ophthalmol. 2019;97:e313–6. doi: 10.1111/aos.13637. https://doi.org/10.1111/aos.13637. [DOI] [PubMed] [Google Scholar]
- 7.AlQattan B, Yetisen A Kand Butt H. Direct laser writing of nanophotonic structures on contact lenses. ACS Nano. 2018;12:5130–40. doi: 10.1021/acsnano.8b00222. https://doi.org/10.1021/acsnano.8b00222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Park J, Ahn D B, Kim J, et al. Printing of Wirelessly Rechargeable Solid-state Supercapacitors for Soft, Smart Contact Lenses with Continuous Operations. Sci Adv. 2019;5:eaay0764. doi: 10.1126/sciadv.aay0764. https://doi.org/10.1126/sciadv.aay0764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Sommer AC, Blumenthal EZ. Implementations of 3D Printing in Ophthalmology. Graefes Arch Clin Exp Ophthalmol. 2019;257:1815–22. doi: 10.1007/s00417-019-04312-3. https://doi.org/10.1007/s00417-019-04312-3. [DOI] [PubMed] [Google Scholar]
- 10.Xie P, Hu Z, Zhang X, et al. Application of 3-dimensional Printing Technology to Construct an Eye Model for Fundus Viewing Study. PLoS One. 2014;9:e109373. doi: 10.1371/journal.pone.0109373. https://doi.org/10.1371/journal.pone.0109373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ng WL, Lee JM, Zhou M, et al. Vat Polymerization-based Bioprinting-Process, Materials, Applications and Regulatory Challenges. Biofabrication. 2020;12:022001. doi: 10.1088/1758-5090/ab6034. https://doi.org/10.1088/1758-5090/ab6034. [DOI] [PubMed] [Google Scholar]
- 12.Zhao W, Qin P, Zhang D, et al. Long Non-coding RNA PVT1 Encapsulated in Bone Marrow Mesenchymal Stem Cell-derived Exosomes Promotes Osteosarcoma Growth and Metastasis by Stabilizing ERG and Sponging miR-183-5p. Aging (Albany NY) 2019;11:9581–96. doi: 10.18632/aging.102406. https://doi.org/10.18632/aging.102406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hinczewski C, Corbel S, Chartier T. Ceramic Suspensions Suitable for Stereolithography. J Eur Ceram Soc. 1998;18:583–90. https://doi.org/10.1016/S0955-2219(97)00186-6. [Google Scholar]
- 14.Zhu W, Ma X, Gou M, et al. 3D Printing of Functional Biomaterials for Tissue Engineering. Curr Opin Biotechnol. 2016;40:103–12. doi: 10.1016/j.copbio.2016.03.014. https://doi.org/10.1016/j.copbio.2016.03.014. [DOI] [PubMed] [Google Scholar]
- 15.Xing JF, Zheng ML, Duan XM. Two-photon Polymerization Microfabrication of Hydrogels:An Advanced 3D Printing Technology For Tissue Engineering and Drug Delivery. Chem Soc Rev. 2015;44:5031–9. doi: 10.1039/c5cs00278h. https://doi.org/10.1039/c5cs00278h. [DOI] [PubMed] [Google Scholar]
- 16.Nguyen AK, Narayan RJ. Two-photon Polymerization for Biological Applications. Mater Today. 2017;20:314–22. https://doi.org/10.1016/j.mattod.2017.06.004. [Google Scholar]
- 17.Jiang T, Munguia-Lopez JG, Flores-Torres S, et al. Extrusion Bioprinting of Soft Materials:An Emerging Technique for Biological Model Fabrication. Appl Phys Rev. 2019;6:011310. https://doi.org/10.1063/1.5059393. [Google Scholar]
- 18.Li X, Liu B, Pei B, et al. Inkjet Bioprinting of Biomaterials. Chem Rev. 2020;120:10793–833. doi: 10.1021/acs.chemrev.0c00008. [DOI] [PubMed] [Google Scholar]
- 19.Cui X, Boland T, D'Lima DD, et al. Thermal Inkjet Printing in Tissue Engineering and Regenerative Medicine. Recent Pat Drug Deliv Formul. 2012;6:149–155. doi: 10.2174/187221112800672949. https://doi.org/10.2174/1−1112800672949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Nishiyama Y, Nakamura M, Henmi C, et al. Development of a Three-dimensional Bioprinter:Construction of Cell Supporting Structures Using Hydrogel and State-of-the-art Inkjet Technology. J Biomech Eng. 2009;131:035001. doi: 10.1115/1.3002759. https://doi.org/10.1115/1.3002759. [DOI] [PubMed] [Google Scholar]
- 21.Nakamura M, Iwanaga S, Henmi C, et al. Biomatrices and Biomaterials for Future Developments of Bioprinting and Biofabrication. Biofabrication. 2010;2:0141. doi: 10.1088/1758-5082/2/1/014110. https://doi.org/10.1088/1758-5082/2/1/014110. [DOI] [PubMed] [Google Scholar]
- 22.Wijshoff H. The Dynamics of the Piezo Inkjet Printhead Operation. Phys Rep. 2010;491:77–177. https://doi.org/10.1016/j.physrep.20https://doi.org/10.03.003. [Google Scholar]
- 23.Christensen K, Xu C, Chai W, et al. Freeform Inkjet Printing of Cellular Structures with Bifurcations. Biotechnol Bioeng. 2015;112:1047–55. doi: 10.1002/bit.25501. https://doi.org/10.1002/bit.25501. [DOI] [PubMed] [Google Scholar]
- 24.Park JU, Hardy M, Kang SJ, et al. High-resolution Electrohydrodynamic Jet Printing. Nat Mater. 2007;6:782–9. doi: 10.1038/nmat1974. https://doi.org/10.1038/nmat1974. [DOI] [PubMed] [Google Scholar]
- 25.Poellmann MJ, Barton KL, Mishra S, et al. Patterned Hydrogel Substrates for Cell Culture with Electrohydrodynamic Jet Printing. Macromol Biosci. 2011;11:1164–8. doi: 10.1002/mabi.201100004. https://doi.org/10.1002/mabi.201100004. [DOI] [PubMed] [Google Scholar]
- 26.Dorishetty P, Dutta NK, Choudhury NR. Bioprintable Tough Hydrogels for Tissue Engineering Applications. Adv Colloid Interface Sci. 2020;281:102163. doi: 10.1016/j.cis.2020.102163. https://doi.org/10.1016/j.cis.2020.102163. [DOI] [PubMed] [Google Scholar]
- 27.Gu Z, Fu J, Lin H, et al. Development of 3D Bioprinting:From Printing Methods to Biomedical Applications. Asian J Pharm Sci. 2019;15:529–557. doi: 10.1016/j.ajps.2019.11.003. https://doi.org/10.1016/j.ajps.2019.11.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Sorkio A, Koch L, Koivusalo L, et al. Human Stem Cell Based Corneal Tissue Mimicking Structures Using Laser-assisted 3D Bioprinting and Functional Bioinks. Biomaterials. 2018;171:57–71. doi: 10.1016/j.biomaterials.2018.04.034. https://doi.org/10.1016/j.biomaterials.2018.04.034. [DOI] [PubMed] [Google Scholar]
- 29.Mecham RP. Overview of Extracellular Matrix. Curr Protoc Cell Biol, Chapter 10:Unit 10 11. 2012 doi: 10.1002/0471143030.cb1001s00. https://doi.org/10.1002/0471143030.cb1001s57. [DOI] [PubMed] [Google Scholar]
- 30.Pati F, Jang J, Ha DH, et al. Printing Three-dimensional Tissue Analogues with Decellularized Extracellular Matrix Bioink. Nat Commun. 2014;5:3935. doi: 10.1038/ncomms4935. https://doi.org/10.1038/ncomms4935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kim H, Park MN, Kim J, et al. Characterization of Cornea-specific Bioink:High transparency, Improved In Vivo Safety. J Tissue Eng. 2019;10:2041731418823382. doi: 10.1177/2041731418823382. https://doi.org/10.1177/2041731418823382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Maqueda M, Mosquera JL, Garcia-Arumi J, et al. Repopulation of Decellularized Retinas with hiPSC-derived Retinal Pigment Epithelial and Ocular Progenitor Cells Shows Cell Engraftment, Organization and Differentiation. Biomaterials. 2021;276:121049. doi: 10.1016/j.biomaterials.2021.121049. https://doi.org/10.1016/j.biomaterials.2021.121049. [DOI] [PubMed] [Google Scholar]
- 33.Wang F, Shi W, Li H, et al. Decellularized Porcine Cornea-derived Hydrogels for the Regeneration of Epithelium and Stroma in Focal Corneal Defects. Ocul Surf. 2020;18:748–60. doi: 10.1016/j.jtos.2020.07.020. https://doi.org/10.1016/j.jtos.2020.07.020. [DOI] [PubMed] [Google Scholar]
- 34.Chameettachal S, Prasad D, Parekh Y, et al. Prevention of Corneal Myofibroblastic Differentiation In Vitro Using a Biomimetic ECM Hydrogel for Corneal Tissue Regeneration. ACS Appl Bio Mater. 2021;4:533–44. doi: 10.1021/acsabm.0c01112. https://doi.org/10.1021/acsabm.0c01112. [DOI] [PubMed] [Google Scholar]
- 35.Bektas CK, Hasirci V. Cell Loaded 3D Bioprinted GelMA Hydrogels for Corneal Stroma Engineering. Biomater Sci. 2020;8:438–49. doi: 10.1039/c9bm01236b. https://doi.org/10.1039/C9BM01236B. [DOI] [PubMed] [Google Scholar]
- 36.Leijten J, Seo J, Yue K, et al. Spatially and Temporally Controlled Hydrogels for Tissue Engineering. Mater Sci Eng R Rep. 2017;119:1–35. doi: 10.1016/j.mser.2017.07.001. https://doi.org/10.1016/j.mser.2017.07.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Lee KY, Mooney DJ. Hydrogels for Tissue Engineering. Chem Rev. 2001;101:1869–79. doi: 10.1021/cr000108x. https://doi.org/10.1021/cr000108x. [DOI] [PubMed] [Google Scholar]
- 38.Osidak EO, Karalkin PA, Osidak MS, et al. Viscoll Collagen Solution as a Novel Bioink for Direct 3D Bioprinting. J Mater Sci Mater Med. 2019;30:31. doi: 10.1007/s10856-019-6233-y. https://doi.org/10.1007/s10856-019-6233-y. [DOI] [PubMed] [Google Scholar]
- 39.Stepanovska J, Otahal M, Hanzalek K, et al. pH Modification of High-Concentrated Collagen Bioinks as a Factor Affecting Cell Viability, Mechanical Properties, and Printability. Gels. 2021;7:252. doi: 10.3390/gels7040252. https://doi.org/10.3390/gels7040252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Wu Z, Liu J, Lin J, et al. Novel Digital Light Processing Printing Strategy Using a Collagen-Based Bioink with Prospective Cross-Linker Procyanidins. Biomacromolecules. 2022;23:240–52. doi: 10.1021/acs.biomac.1c01244. https://doi.org/10.1021/acs.biomac.1c01244. [DOI] [PubMed] [Google Scholar]
- 41.Lee JM, Suen SK, Ng WL, et al. Bioprinting of Collagen:Considerations, Potentials, and Applications. Macromol Biosci. 2021;21:e2000280. doi: 10.1002/mabi.202000280. https://doi.org/10.1002/mabi.202000280. [DOI] [PubMed] [Google Scholar]
- 42.Roth EA, Xu T, Das M, et al. Inkjet printing for high-throughput cell patterning. Biomaterials. 2004;25:3707–15. doi: 10.1016/j.biomaterials.2003.10.052. https://doi.org/10.1016/j.biomaterials.2003.10.052. [DOI] [PubMed] [Google Scholar]
- 43.Ng WL, Lee JM, Yeong WY, et al. Microvalve-based Bioprinting process, Bio-inks and Applications. Biomater Sci. 2017;5:632–47. doi: 10.1039/c6bm00861e. https://doi.org/10.1039/c6bm00861e. [DOI] [PubMed] [Google Scholar]
- 44.Wang P, Li X, Zhu W, et al. 3D Bioprinting of Hydrogels for Retina Cell Culturing. Bioprinting (Amsterdam, Netherlands) 2018;11:e00029. doi: 10.1016/j.bprint.2018.e00029. https://doi.org/10.1016/j.bprint.2018.e00029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Khalili M, Asadi M, Kahroba H, et al. Corneal Endothelium Tissue Engineering:An Evolution of Signaling Molecules, Cells, and Scaffolds toward 3D Bioprinting and Cell Sheets. J Cell Physiol. 2020;236:3275–303. doi: 10.1002/jcp.30085. https://doi.org/10.1002/jcp.30085. [DOI] [PubMed] [Google Scholar]
- 46.Ashammakhi N, Ahadian S, Xu C, et al. Bioinks and Bioprinting Technologies to Make Heterogeneous and Biomimetic Tissue Constructs. Materials Today Bio. 2019;1:100008. doi: 10.1016/j.mtbio.2019.100008. https://doi.org/10.1016/j.mtbio.2019.100008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.West-Mays JA, Dwivedi DJ. The Keratocyte:Corneal Stromal Cell with Variable Repair Phenotypes. Int J Biochem Cell Biol. 2006;38:1625–31. doi: 10.1016/j.biocel.2006.03.010. https://doi.org/10.1016/j.biocel.2006.03.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Gouveia RM, Connon CJ. The Effects of Retinoic Acid on Human Corneal Stromal Keratocytes Cultured In Vitro Under Serum-Free Conditions. Investig Ophthalmol Visual Sci. 2013;54:7483–91. doi: 10.1167/iovs.13-13092. https://doi.org/10.1167/iovs.13-13092. [DOI] [PubMed] [Google Scholar]
- 49.Isaacson A, Swioklo S, Connon CJ. 3D Bioprinting of a Corneal Stroma Equivalent. Exp Eye Res. 2018;173:188–93. doi: 10.1016/j.exer.2018.05.010. https://doi.org/10.1016/j.exer.2018.05.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Jin K, Wang S, Zhang Y, et al. Long Non-coding RNA PVT1 Interacts with MYC and its Downstream Molecules to Synergistically Promote Tumorigenesis. Cell Mol Life Sci. 2019;76:4275–89. doi: 10.1007/s00018-019-03222-1. https://doi.org/10.1007/s00018-019-03222-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Kim KW, Lee SJ, Park SH, et al. Ex Vivo Functionality of 3D Bioprinted Corneal Endothelium Engineered with Ribonuclease 5-Overexpressing Human Corneal Endothelial Cells. Adv Healthc Mater. 2018;7:1800398. doi: 10.1002/adhm.201800398. https://doi.org/10.1002/adhm.201800398. [DOI] [PubMed] [Google Scholar]
- 52.Masland RH. Cell Populations of the Retina:The Proctor Lecture. Investig Ophthalmol Visual Sci. 2011;52:4581–91. doi: 10.1167/iovs.10-7083. https://doi.org/10.1167/iovs.10-7083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Lorber B, Hsiao WK, Martin KR. Three-dimensional Printing of the Retina. Curr Opin Ophthalmol. 2016;27:262–7. doi: 10.1097/ICU.0000000000000252. https://doi.org/10.1097/ICU.0000000000000252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Kador KE, Grogan SP, Dorthé EW, et al. Control of Retinal Ganglion Cell Positioning and Neurite Growth:Combining 3D Printing with Radial Electrospun Scaffolds. Tissue Eng Part A. 2016;22:286–94. doi: 10.1089/ten.tea.2015.0373. https://doi.org/10.1089/ten.TEA.2015.0373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Yong HE, Qing GA, Liu A, et al. 3D Bioprinting:From Structure to Function. J Zhejiang Univ. 2019;53:407–19. https://doi.org/10.3785/j.issn.1008-973X.2019.03.001. [Google Scholar]
- 56.Farandos NM, Yetisen AK, Monteiro MJ, et al. Contact Lens Sensors in Ocular Diagnostics. Adv Healthc Mater. 2015;4:792–8. doi: 10.1002/adhm.201400504. https://doi.org/10.1002/adhm.201400504. [DOI] [PubMed] [Google Scholar]
- 57.Tang H, Alqattan B, Jackson T, et al. Cost-Efficient Printing of Graphene Nanostructures on Smart Contact Lenses. ACS Applied Materials &Interfaces. 2020;12(9):10820–10828. doi: 10.1021/acsami.9b21300. https://doi.org/10.1021/acsami.9b21300. [DOI] [PubMed] [Google Scholar]
- 58.Sanchez-Tena MA, Alvarez-Peregrina C, Santos-Arias F, et al. Application of 3D Printing Technology in Scleral Cover Shell Prosthesis. J Med Syst. 2019;43:149. doi: 10.1007/s10916-019-1280-y. https://doi.org/10.1007/s10916-019-1280-y. [DOI] [PubMed] [Google Scholar]
- 59.Debellemanière G, Flores M, Montard M, et al. Three-dimensional Printing of Optical Lenses and Ophthalmic Surgery:Challenges and Perspectives. J Refract Surg (Thorofare NJ:1995) 2016;32:201–4. doi: 10.3928/1081597X-20160121-05. https://doi.org/10.3928/1081597X-20160121-05. [DOI] [PubMed] [Google Scholar]
- 60.John G, Michal EP, Tomasz ST. Quantitative Evaluation of Performance of Three-dimensional Printed Lenses. Opt Eng. 2017;56:1–13. doi: 10.1117/1.OE.56.8.084110. https://doi.org/10.1117/1.OE.56.8.084110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Park SH, Su R, Jeong J, et al. 3D Printed Polymer Photodetectors. Adv Mater (Deerfield Beach, Fla.) 2018;30:e1803980. doi: 10.1002/adma.201803980. https://doi.org/10.1002/adma.201803980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Callahan AB, Campbell AA, Petris C, et al. Low-Cost 3D Printing Orbital Implant Templates in Secondary Orbital Reconstructions. Ophthalmic Plastic Reconstr Surg. 2017;33:376–80. doi: 10.1097/IOP.0000000000000884. https://doi.org/10.1097/IOP.0000000000000884. [DOI] [PubMed] [Google Scholar]
- 63.Dave TV, Gaur G, Chowdary N, et al. Customized 3D Printing:A Novel Approach to Migrated Orbital Implant. Saudi J Ophthalmol. 2018;32:330–3. doi: 10.1016/j.sjopt.2018.03.003. https://doi.org/10.1016/j.sjopt.2018.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Fan B, Chen H, Sun YJ, et al. Clinical Effects of 3-D Printing-assisted Personalized Reconstructive Surgery for Blowout Orbital Fractures. Graefes Arch Clin Exp Ophthalmol. 2017;255:2051–7. doi: 10.1007/s00417-017-3766-y. https://doi.org/10.1007/s00417-017-3766-y. [DOI] [PubMed] [Google Scholar]
- 65.Kang S, Kwon J, Ahn CJ, et al. Generation of Customized Orbital Implant Templates Using 3-dimensional Printing for Orbital Wall Reconstruction. Eye (London, England) 2018;32:1864–70. doi: 10.1038/s41433-018-0193-1. https://doi.org/10.1038/s41433-018-0193-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Zamboulis A, Nanaki S, Michailidou G, et al. Chitosan and its Derivatives for Ocular Delivery Formulations:Recent Advances and Developments. Polymers. 2020;12:1519. doi: 10.3390/polym12071519. https://doi.org/10.3390/polym12071519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Silva MM, Calado R, Marto J, et al. Chitosan Nanoparticles as a Mucoadhesive Drug Delivery System for Ocular Administration. Mar Drugs. 2017;15:370. doi: 10.3390/md15120370. https://doi.org/10.3390/md15120370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Başaran E, Yazan Y. Ocular Application of Chitosan. Exp Opin Drug Deliv. 2012;9:701–12. doi: 10.1517/17425247.2012.681775. https://doi.org/10.1517/17425247.2012.681775. [DOI] [PubMed] [Google Scholar]
- 69.Lynch C, Kondiah PP, Choonara YE, et al. Advances in Biodegradable Nano-Sized Polymer-Based Ocular Drug Delivery. Polymers. 2019;11:1371. doi: 10.3390/polym11081371. https://doi.org/10.3390/polym11081371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Cho H, Jammalamadaka U, Tappa K. Nanogels for Pharmaceutical and Biomedical Applications and Their Fabrication Using 3D Printing Technologies. Materials (Basel, Switzerland) 2018;11:302. doi: 10.3390/ma11020302. https://doi.org/10.3390/ma11020302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Flaxman SR, Bourne RR, Resnikoff S, et al. Global Causes of Blindness and Distance Vision Impairment 1990–2020:A Systematic Review and meta-analysis. Lancet Global Health. 2017;5:e1221–34. doi: 10.1016/S2214-109X(17)30393-5. https://doi.org/10.1016/S2214-109X(17)30393-5. [DOI] [PubMed] [Google Scholar]
- 72.Mathews PM, Lindsley K, Aldave AJ, et al. Etiology of Global Corneal Blindness and Current Practices of Corneal Transplantation:A Focused Review. Cornea. 2018;37:1198–203. doi: 10.1097/ICO.0000000000001666. https://doi.org/10.1097/ICO.0000000000001666. [DOI] [PubMed] [Google Scholar]
- 73.Gain P, Jullienne R, He Z, et al. Global Survey of Corneal Transplantation and Eye Banking. JAMA Ophthalmol. 2016;134:167–73. doi: 10.1001/jamaophthalmol.2015.4776. https://doi.org/10.1001/jamaophthalmol.2015.4776. [DOI] [PubMed] [Google Scholar]
- 74.Zhang B, Xue Q, Li J, et al. 3D bioprinting for Artificial Cornea:Challenges and Perspectives. Med Eng Phys. 2019;71:68–78. doi: 10.1016/j.medengphy.2019.05.002. https://doi.org/10.1016/j.medengphy.2019.05.002. [DOI] [PubMed] [Google Scholar]
- 75.Fuest M, Yam GH, Mehta JS, et al. Prospects and Challenges of Translational Corneal Bioprinting. Bioengineering. 2020;7:71. doi: 10.3390/bioengineering7030071. https://doi.org/10.3390/bioengineering7030071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Faye PA, Poumeaud F, Chazelas P, et al. Focus on Cell Therapy to Treat Corneal Endothelial Diseases. Exp Eye Res. 2021;204:108462. doi: 10.1016/j.exer.2021.108462. https://doi.org/10.1016/j.exer.2021.108462. [DOI] [PubMed] [Google Scholar]
- 77.Campos DF, Rohde M, Ross M, et al. Corneal Bioprinting Utilizing Collagen-based Bioinks and Primary Human Keratocytes. J Biomed Mater Res Part A. 2019;107:1945–53. doi: 10.1002/jbm.a.36702. https://doi.org/10.1002/jbm.a.36702. [DOI] [PubMed] [Google Scholar]
- 78.Kong B, Chen Y, Liu R, et al. Fiber Reinforced GelMA Hydrogel to Induce the Regeneration of Corneal Stroma. Nat Commun. 2020;11:1435–5. doi: 10.1038/s41467-020-14887-9. https://doi.org/10.1038/s41467-020-14887-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Kim H, Jang J, Park J, et al. Shear-induced Alignment of Collagen Fibrils Using 3D Cell Printing for Corneal Stroma Tissue Engineering. Biofabrication. 2019;11:035017. doi: 10.1088/1758-5090/ab1a8b. https://doi.org/10.1088/1758-5090/ab1a8b. [DOI] [PubMed] [Google Scholar]
- 80.Holland G, Pandit A, Sanchez-Abella L, et al. Artificial Cornea:Past, Current, and Future Directions. Front Med (Lausanne) 2021;8:770780. doi: 10.3389/fmed.2021.770780. https://doi.org/10.3389/fmed.2021.770780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Hoon M, Okawa H, Santina LD, et al. Functional Architecture of the Retina:Development and Disease. Prog Retin Eye Res. 2014;42:44–84. doi: 10.1016/j.preteyeres.2014.06.003. https://doi.org/10.1016/j.preteyeres.2014.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Ruiz-Alonso S, Villate-Beitia I, Gallego I, et al. Current Insights Into 3D Bioprinting:An Advanced Approach for Eye Tissue Regeneration. Pharmaceutics. 2021;13:308. doi: 10.3390/pharmaceutics13030308. https://doi.org/10.3390/pharmaceutics13030308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Lorber B, Hsiao WK, Hutchings IM, et al. Adult Rat Retinal Ganglion Cells and Glia can be Printed by Piezoelectric Inkjet Printing. Biofabrication. 2014;6:015001. doi: 10.1088/1758-5082/6/1/015001. https://doi.org/10.1088/1758-5082/6/1/015001. [DOI] [PubMed] [Google Scholar]
- 84.Masaeli E, Forster V, Picaud S, et al. Tissue Engineering of Retina Through High Resolution 3-Dimensional Inkjet Bioprinting. Biofabrication. 2020;12:025006. doi: 10.1088/1758-5090/ab4a20. https://doi.org/10.1088/1758-5090/ab4a20. [DOI] [PubMed] [Google Scholar]
- 85.Masaeli E, Marquette C. Direct-Write Bioprinting Approach to Construct Multilayer Cellular Tissues. Front Bioeng Biotechnol. 2020;7:478. doi: 10.3389/fbioe.2019.00478. https://doi.org/10.3389/fbioe.2019.00478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Meek KM, Knupp C. Corneal Structure and Transparency. Prog Retin Eye Res. 2015;49:1–16. doi: 10.1016/j.preteyeres.2015.07.001. https://doi.org/10.1016/j.preteyeres.2015.07.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Kutlehria S, Dinh TC, Bagde A, et al. High-throughput 3D Bioprinting of Corneal Stromal Equivalents. J Biomed Mater Res B Appl Biomater. 2020;108:2981–94. doi: 10.1002/jbm.b.34628. https://doi.org/10.1002/jbm.b.34628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Mahdavi SS, Abdekhodaie MJ, Kumar H, et al. Stereolithography 3D Bioprinting Method for Fabrication of Human Corneal Stroma Equivalent. Ann Biomed Eng. 2020;48:1955–70. doi: 10.1007/s10439-020-02537-6. https://doi.org/10.1007/s10439-020-02537-6. [DOI] [PubMed] [Google Scholar]
- 89.Shi P, Edgar TY, Yeong WY, et al. Hybrid Three-dimensional (3D) Bioprinting of Retina Equivalent for Ocular Research. Int J Bioprint. 2017;3:8. doi: 10.18063/IJB.2017.02.008. https://doi.org/10.18063/IJB.2017.02.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Worthington KS, Wiley LA, Kaalberg EE, et al. Two-photon Polymerization for Production of Human iPSC-derived Retinal Cell Grafts. Acta Biomater. 2017;55:385–95. doi: 10.1016/j.actbio.2017.03.039. https://doi.org/10.1016/j.actbio.2017.03.039. [DOI] [PMC free article] [PubMed] [Google Scholar]


