Abstract
Non-invasive masks are designed based on generic facial models; therefore, difficulties in fitting patients’ unique characteristics are common. A poor fit of the mask may have consequences such as air leaks or pressure ulcers. It is possible to optimize the fit of interfaces by adapting them to a patient’s face. Our objective is to design an individualized silicone mask for non-invasive ventilation for a premature phantom using a three-dimensional (3D) scanner and bioprinter. The facial surface of the manikin was scanned with a 3D scanner in a supine position, in an incubator with a sliding mattress and in <2 min. We printed the tailor-made mask in 3 h with biocompatible and hypoallergenic silicone. When applied under a simulated clinical scenario, the mask possessed good structural reliability after post-processing and optimal mechanical features. We observed adequate thoracic excursion and 14% reduction in air leaks when the manikin was ventilated with the customized mask with a neonatal ventilator. We ink the edges of personalized and standard masks. After fitting them to phantoms, personalized mask showed better pressure distribution. Our subsequent research direction is to test the viability of personalizing non-invasive ventilation masks for very preterm infants of our department.
Keywords: Premature infant, Non-invasive ventilation, Mask, Pressure ulcer, 3D printing
1. Introduction
The application of three-dimensional (3D) printing for medical purposes has become more common in recent years, and it’s application has been further accelerated due to the coronavirus disease 2019 (COVID-19) pandemic[1]. 3D printing is a technology that allows printing solid objects in different materials by overlapping layers in cross sections. The 3D models can be created de novo or by replicating an existing object with the use of a 3D scanner.
Although the literature related to 3D printing in medicine remains scarce, disciplines such as dentistry, reconstructive surgery, neurosurgery, and orthopedic surgery are among the top disciplines that have proven the utility of 3D printing. Although the applications of 3D printing are still limited in neonatology, a specialty that has undergone a significant technological advancement in recent decades, it is expected that this technology will contribute to the individualization of neonatal care[2]. Recent publications have demonstrated the viability to create individualized masks for occupational health, aerosol therapy or non-invasive ventilation (NIV) in adults and children[3-7].
NIV is used in neonatology departments worldwide. NIV requires the use of an interface that connects the ventilator to the patient. There are different types of interfaces; one of the most used in neonatology is nasal mask. NIV masks are designed based on generic facial models; therefore, difficulties in fitting patients’ unique facial characteristics are quite inevitable, especially in younger patients or in patients with peculiar facial features[3,8]. A poor fit of the mask may have relevant clinical consequences such as air leaks, making it difficult to achieve proper ventilation, and oxygenation[9]. The great diversity of human faces’ geometries, especially in newborns during the 1st days of life or in growing infants, may represent a limitation for standardized masks[5]. An individualized mask was recently designed for the application of nocturnal continuous positive airway pressure (CPAP) in a pediatric patient with Treacher-Collins syndrome and obstructive sleep apnea[3]. With the use of this individualized mask, Morrison et al. described an air leakage reduction of 74% and a greater adherence of the patient to therapy compared to the standard mask.
In addition, skin in-contact with the mask can suffer pressure ulcers, especially if the mask does not fit properly. Mask-related pressure ulcers cause pain, discomfort and loss of function, resulting in extended hospital stays and increased costs[4], and are recognized as preventable adverse events in healthcare[10,11]. Despite the application of best practices to prevent medical device-related pressure ulcers, the Skin Adverse Events’ Working Group of the Hospital Clínic reported an incidence of 30.7% of pressure ulcers related to the use of NIV masks in very preterm infants during the 1st week of life (65% of these patients will suffer from a recurrence)[12]. Other complications related to these ulcers can range from local/systemic infections to long-term scars.
More effective strategies to prevent pressure ulcers related to the use of medical devices are urgently needed[4]. There is no report regarding individualized neonatal masks for NIV. We believe that 3D printing can make an important contribution to a more personalized neonatal care in the near future.
Before a planned test with premature patients, we checked with the present work the feasibility of the process of creating and printing a 3D customized mask for NIV for a manikin. Our primary objective is to design an individualized prototype of a biocompatible silicone nasal mask for NIV for the Premature Anne manikin from Laerdel® using a 3D printer. The secondary objectives are:
(i) To assist the manikin achieve adequate thoracic excursion using the customized mask;
(ii) To compare the reduction in air leakage between the customized mask and standardized mask suitable for the aforementioned manikin; and
(iii) To compare the pressure distribution at the skin contact areas between the customized mask and standardized mask suitable for the aforementioned manikin.
2. Methods
This experimental observational study was carried out in the Neonatology Department of the Hospital Clínic Barcelona in 2020 and 2021.
The research team made up of two physicians and six nurses who are part of the Skin Adverse Events’ Working Group of our hospital. The project was also a collaboration with an engineer from the Biomedical Engineering Department of the Hospital Clínic who is used to work on 3D printing models in resin for orthopedic and reconstructive surgery, and a collaboration with Avinent Implant System S.L.U. (Santpedor, Spain) and Elkem Silicones France SAS companies.
The followings steps were proposed to obtain the tailor-made mask:
(i) Image capture. Scan of the facial surface of the manikin was made using the 3D Peel 2 CAD scanner with LED light. The scanner has a mesh resolution of 0.25 mm and a scan area of 380 mm × 380 mm. This step necessitates the use of a device that does not need contact with the manikin, can be used inside the incubator and does not use harmful radiations.
(ii) Image processing. Once the points’ cloud of the scanned surface was obtained, we converted it into a.STL file (standard data transmission format that transformed the scanned volumetric surface to a solid reconstruction using triangle-faces modeling). Next, we redesigned the standard mask model by adapting the part that is in contact with the manikin so that it matches the exact physiognomy of the scanned face.
(iii) 3D mask printing. The mask was printed using the Liquid Deposition Modeling technique, which consists of deposition by extrusion of liquid silicone at room temperature with a curing process. The printed mask had a resolution of 200 μm thick layer and was extruded through a nozzle orifice with diameter of 234 μm. Avinent Implant System S.L.U. (Santpedor, Spain) Company is the owner of the bioprinter. The chosen material for this purpose was from the AMSil™ Silicone Elastomers series. Elkem Silicones France SAS Company is the manufacturer of the silicone. In addition to the already known properties, other properties of silicone include resistance to oxidation processes, thermal stability, and resistance to compression and friction; these properties justify the wide application of silicone in the health sector. Moreover, the printable silicone is hypoallergenic when it is in contact with the skin.
We compared air leaks detected by the ventilator between the customized mask and the standard mask that corresponds to the size of the manikin according to our interface selection protocol for non-invasive neonatal ventilation. The standard mask used was the CareFusion® Infant Flow LP Nasal CPAP System. The manikin was modified to minimize leakage through the mouth.
The edges of both masks were marked with ink on the face of the manikin to qualitatively compare their support areas.
Since the current prototype was developed on a base and tested on the face of a mannequin, an ethical approval was not necessary and was therefore not sought from the ethics committee of our center.
3. Results
3.1. Image capture
The facial surface of the manikin was scanned with a 3D Peel 2 CAD scanner, as shown in Figure 1. The manikin was placed in a supine position, in an incubator with a sliding mattress and with three spatial reference points. The procedure lasted 10 min, with a direct light to manikin’s eyes in 1.5 – 2 min.
Figure 1.
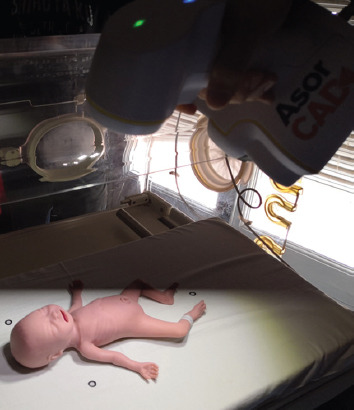
Scan of the facial surface of the manikin.
3.2. Image processing
We use Mimics Medical 24.0 program from Materialize (approved for designing medical and surgical devices for medical use) to customize a mask that perfectly fits the geometry of the manikin with smooth contact boundaries. The customized mask was designed to rest on the nasal bone, nasolabial sulcus and philtrum, without contacting the alas of the nose nor obstructing the nostrils. In the manikin that was used to design the prototype of customized mask, its size would correspond to the XS–S size of commercial masks. The work related to image processing and subsequent design corrections under medical guidelines lasted 2 h (Figure 2).
Figure 2.
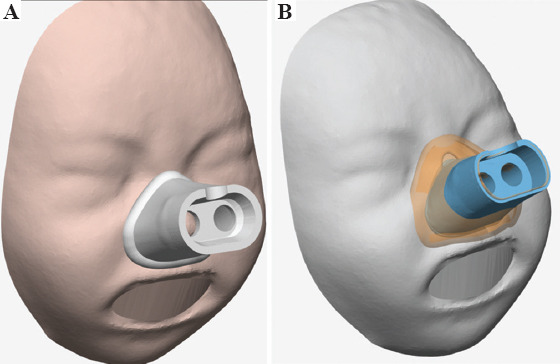
(A) Customized mask design. (B) Comparison of the contact surface with the standardized mask (in orange) and with the customized mask (in blue).
3.3. 3D printing
The created design was sent for printing. The process of printing the mask with selected biocompatible and hypoallergenic silicone (AMSil™ Silbione™ 24501-50 TRS A-B) took 3 h and consumed about 2 g of the material. The first mask obtained, as shown in Figure 3, had no macroscopic defects. An adhesive silicone strip type Silbione RTGel 4642 A&B (which does not damage the skin after removal) was added to the edge of the mask in contact with the manikin to optimize its adherence.
Figure 3.
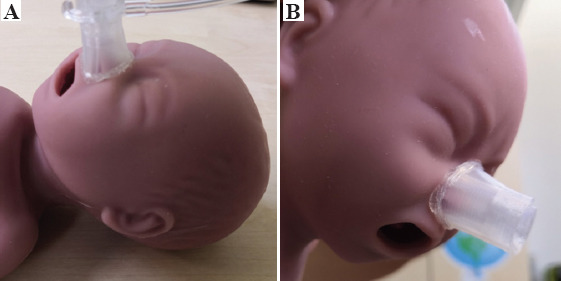
(A) Checking the fit of printed nasal mask on the face of the manikin (B) checking the adherence of printed nasal mask to the face (without subjection system).
The manikin was ventilated with non-invasive ventilator and the printed mask with adequate thoracic excursion.
Since air leakage percentage was not displayed on our neonatal non-invasive ventilators, we created a “leak-free circuit” with an invasive ventilator. For this purpose, the manikin leaks were blocked with a plasticine mold in the mouth and printed mask was connected to inspiratory and expiratory tubing. Under the same conditions, with a constant flow of air, invasive ventilator measured 78% of air leakage from standardized mask, and 64% from individualized mask.
For evaluating how the mask was fitted to the manikin’s face, we marked with ink the edges of both masks. We fitted both mask to two manikin’s face prints during 30 s. Pressure distribution is shown in Figure 4.
Figure 4.
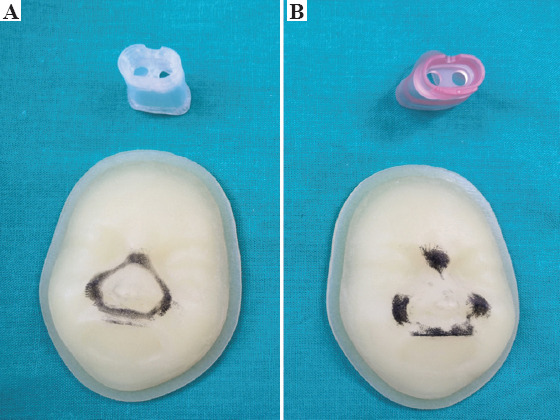
(A) Support areas of the personalized nasal mask. (B) Support areas of the standard nasal mask to two manikin’s face prints. Material used for customized mask was AMSil™ Silbione™ 24501-50 TRS A-B from Elkem Silicones France SAS. Material used for standardized mask was Medical Grade Liquid Silicone Rubber from CareFusion ® and for printed faces was Agilus30Clear from Stratasys TM (PolyJet® technology).
We estimated that the whole process might involve 6 h and cost 80 euros. This price also accounted for the time spent by the team of engineers, who designed the prototype and made the subsequent corrections under medical criteria, and the materials used.
4. Discussion
This is a pilot study that demonstrated that a customized NIV mask for a manikin (a manikin that emulates the size of an extremely premature patient) can be 3D printed using a 3D scanner imaging system. The customized nasal mask was designed to mimic existing nasal masks but has a tailor-made shape to fit the manikin’s face. The evaluation of the process of designing a mask from scan data, the organization of its manufacture and the printing of the prototype described in this study are valuable experiences for future reference.
To the best of our knowledge, although this is not the first study that demonstrated effectiveness of 3D scanning and printing technology in healthcare[6,8], this is the first article reporting the manufacture of a 3D individualized mask for neonatal population and the application of silicone certified for medical use.
The use of NIV changed the way neonatologists treated patients with respiratory failure, thereby minimizing the risks and complications associated with endotracheal intubation and invasive ventilation. There are between 350,000 and 400,000 births each year in Spain; about 7 – 13% are preterm births[13]. The majority of very preterm infants (born before week 32 of gestation) need NIV, especially during the first few days or weeks of life. Around 100 very preterm infants are born in our hospital each year; more than 70% of them will require NIV for a mean period of 8 days. NIV needs an interface that links the ventilator with the patient. There are different types of interfaces, for example, prongs or nasal masks (the current project is based on nasal masks). Unfortunately, the use of these interfaces has raised other problems such as air leaks and pressure ulcers[14]. It is possible to optimize the fit of these interfaces by adapting them to the specific characteristics of each patient[6,15].
Given the vulnerability of the target population (very premature infants), the development conditions of the mask are very demanding. For this reason, we proposed a preliminary study in which we worked with a manikin (subject of the present publication).
Requested requirements for the first step of the described process, that is, facial image capture, were accomplished. We worked without touching the manikin (scanning the face instead of using a mold), keeping it inside the incubator (avoiding extra mobilizations), and without using harmful radiation or long light exposures (maximum 2 min). It is known that long or intense light exposures are detrimental to the neurodevelopment of premature patients[16]. AsorCAD Creafom gamma Academia 3D scanner has given us these advantages as well as an adequate image resolution for the size of the manikin’s face. This step lasted less than 10 min, similar to that described by Willox et al. in their customized masks’ tests on adult patients[8]. This form of facial image capture has a limitation that it can be affected by the patient’s gestures, so a neutral posture of the face should be acquired[8].
The 3D printing of the mask has been made with a biocompatible and hypoallergenic silicone thanks to the participation of the companies Avinent Implant System S.L.U. (Santpedor, Spain) and Elkem Silicones France SAS. 3D printing with silicone is a strong point of our project, because despite its universal use in healthcare, the application of this material in 3D printing is quite novel. Its most notable characteristics are low allergenicity, durability, non-deformability and stability at high temperatures (in case of sterilization procedures). The transparency of the mask is a point to improve. A breakthrough in the field of 3D printing materials is expected in the near future.
The personalized mask perfectly fits the facial features of the manikin compared to standardized masks. We believe that this perfect fit reduces air leaks and avoids an excessively firm fixation as indicated in our results. If the mask is too tightly fitted, the pressure on the skin leads to the appearance of skin lesions. Through the qualitative tests with ink, we observed that the support area of the personalized mask on the face was homogeneous and the pressure was better distributed, but even so, there is no guarantee that the personalized mask will not cause pressure injuries in patients. This is an issue to be explored in further studies. The standardized mask discontinuously rested on manikin’s face; high pressure points on the nasal bridge and the upper lip were detected. These areas are susceptible to frequent occurrence of pressure ulcers[12].
Finally, the physical characteristics of the 3D printed prototype allow the incorporation of an adhesive silicone strip in the contour that rests on manikin; this strip does not damage the skin when it is removed. This extra adherence may reduce damage caused by friction mechanisms, instead of pressure mechanisms. Willox et al. added granuflexv to their printed prototype to cushion the edges as the mask seems more rigid[8]. The adhesive silicone strip could also be used in commercially available masks.
The use of a resuscitation manikin in this study enabled us to connect a ventilation system to the manikin to check the correct thoracic excursion with the use of a tailor-made mask. However, due to such use of the phantom, other ventilatory parameters could not be tested.
3D printing can also become a potentially useful tool for both patients and health personnel in the background of the COVID-19 pandemic. Individualized printed masks represent an improvement in NIV therapies for patients affected by respiratory failure. On the other hand, customized masks could also play a role in the protection of healthcare personnel; some studies have demonstrated the application of 3D printing in the development of personal protective equipment and respiratory protective devices[7,17-19].
At the end of the study, we estimated that the price of making an individualized mask was 80 euros at the time. To prevent NIV-related skin ulcerations, our hospital’s protocol recommends the alternative use of two generic nasal masks of different sizes in every infant (these masks need to be renewed every 5 days). The total NIV days in our department are around 600 days/year, corresponding to the use of approximately 250 standardized masks. This price is, in general terms, higher than that of standardized masks (in our case, it is 4 times higher), but we believe that the cost can be substantially reduced by automating the design phase and by having the printer available in the hospital. Potential clinical benefits from tailor-made masks can lead to a favorable cost/benefit ratio.
The next step of our research team is to test the viability of the process in very preterm infants admitted in our department. This will include the previously mentioned steps (3D facial scan, image processing, and 3D printing process) by following the same methodology. To make sure very preterm infants could eventually benefit from these tailor-made masks as soon as possible, the team is dedicated to shortening the time that it currently takes to produce each mask by automating the design phase. This new proposed project will be presented to the ethics committee of our hospital. Once ethical approval is granted by the research ethics committee, we will conduct a clinical study to test the resulting tailor-made, 3D-printed NIV masks and compare their effectiveness and safety versus the standardized masks. The key endpoints of the project are related to the ability of the tailor-made masks in optimizing patient’s ventilation, and especially their ability to reduce skin ulcerations.
4. Conclusions
Our research team has tested and validated the technical viability of designing and printing silicone tailor-made 3D facial masks for NIV to a very preterm manikin (Anne Premature from Laerdel®). The customization of these non-invasive masks allows perfect fit of the specific anatomic features of any given patient’s face. In near future, these tailor-made masks could reduce air leaks and allow for a homogeneous distribution of pressure on the skin, thereby optimizing NIV and reducing the risk for skin ulcers, respectively.
Acknowledgments
The authors would like to thank Avinent Implant System S.L.U. and Elkem Silicones France SAS, especially Lauriane Depernon (Technical Service Manager Skin Adhesives - Compentence Center – ATRiON), for their technological support and knowledge in the development of this project. The authors would like also to thank to the Skin Adverse Events’ Working Group of the Neonatology Department of Hospital Clínic for their collaboration, their expertise has been fundamental.
Funding
Our project was granted by research aid Adolfo Valls-Respisurf of the Spanish Society of Neonatology (edition 2018).
Conflict of interest
Authors Cristina Borràs-Novell, Mario García Causapié and Oscar García-Algar do not have conflicts of interest. Authors Maria Murcia and Damien Djian work in Elkem Company as Sales Manager HC&IAM Iberia and Sales Expert 3D printing Europa and R&I – Compentence Center – ATRiON respectively. The mentioned companies had altruist collaboration in the current project, within the framework of Research & Development.
Author contributions
C.B.N. literature review, image capture, prototype design corrections, prototype tests, data management, data interpretation, draft manuscript, consolidation of coauthor comments, and paper finalization. M.G.C. image capture, prototype design, prototype tests, data interpretation, and manuscript review. M.M prototype design and manuscript review. D.D. prototype design, prototype printing, and manuscript review. O.G.A. conceptualization, work coordination, and manuscript review.
References
- 1.Choong YY, Tan HW, Patel DC, et al. The Global Rise of 3D Printing During the COVID-19 Pandemic. Nat Rev Mater. 2020;5:637–9. doi: 10.1038/s41578-020-00234-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Aufieri R, Picone S, Gente M, et al. 3D Printing in Neonatal Care. Ital J Pediatr. 2015;41:A1. http://doi.org/10.1186/1824-7288-41-s1-a1. [Google Scholar]
- 3.Morrison RJ, VanKoevering KK, Nasser HB, et al. Conference:Combined Otolaryngology Spring Meetings. Boston: 2015. Personalized 3D-Printed CPAP Masks Improve CPAP Effectiveness in Children with OSA and Craniofacial Anomalies Robert'. http://doi.org/10.13140/RG.2.1.2328.1687. [Google Scholar]
- 4.Hsu DY, Cheng YL, Bien MY, et al. Development of a Method for Manufacturing Customized Nasal Mask Cushion for CPAP Therapy. Australas Phys Eng Sci Med. 2015;38:657–66. doi: 10.1007/s13246-015-0383-0. http://doi.org/10.1007/s13246-015-0383-0. [DOI] [PubMed] [Google Scholar]
- 5.Sela M, Toledo N, Honen Y, et al. Customized Facial Constant Positive Air Pressure (CPAP) Masks. 2016. [Last accessed on 2021 Sep 23]. Available from: http://arxiv.org/abs/1609.07049 .
- 6.Shikama M, Nakagami G, Noguvhi H, et al. Development of Personalized Fitting Device with 3-Dimensional Solution for Prevention of Niv Oronasal Mask-Related Pressure Ulcers. Respir Care. 2018;63:1024–32. doi: 10.4187/respcare.05691. http://doi.org/10.4187/respcare.05691. [DOI] [PubMed] [Google Scholar]
- 7.Makowski K, Okrasa M. Application of 3D Scanning and 3D Printing for Designing and Fabricating Customized Half-mask Facepieces:A Pilot Study. Work. 2019;63:125–35. doi: 10.3233/WOR-192913. http://doi.org/10.3233/WOR-192913. [DOI] [PubMed] [Google Scholar]
- 8.Willox M, Metherall P, Jeays-Ward K, et al. Custom-made 3D Printed Masks for Children Using Non-invasive Ventilation:A Feasibility Study of Production Method and Testing of Outcomes in Adult Volunteers. J Med Eng Technol. 2020;44:213–23. doi: 10.1080/03091902.2020.1769759. http://doi.org/10.1080/03091902.2020.1769759. [DOI] [PubMed] [Google Scholar]
- 9.Barker N, Willox M, Elphick H. A Review of the Benefits, Challenges and the Future for Interfaces for Long Term Non-Invasive Ventilation in Children. Int J Respir Pulm Med. 2018;5:77. http://doi:10.23937/2378-3516/1410077. [Google Scholar]
- 10.Apold J, Rydrych D. Preventing Device-related Pressure Ulcers:Using Data to Guide Statewide Change. J Nurs Care Qual. 2012;27:28–34. doi: 10.1097/NCQ.0b013e31822b1fd9. http://doi.org/10.1097/NCQ.0b013e31822b1fd9. [DOI] [PubMed] [Google Scholar]
- 11.Murray JS, Noonan C, Quigley S, et al. Medical Device-related Hospital-acquired Pressure Ulcers in Children:An Integrative Review. J Pediatr Nurs. 2013;28:585–95. doi: 10.1016/j.pedn.2013.05.004. http://doi:10.1016/j.pedn.2013.05.004. [DOI] [PubMed] [Google Scholar]
- 12.Gracia MC, Muñoz A, Vidal M. Incidence and risk factors associated with the appearance of pressure injuries in premature less than 34 weeks with non-invasive ventilation. Rev Enferm. 2021;44:23–9. [Google Scholar]
- 13.Instituto Nacional de Estadística. Estadística de Nacimientos. 2020. [Last accessed on 2020 Jul 03]. Available from: https://www.ine.es/dyngs/INEbase/es/ operacion.htm?c=Estadistica_C&cid=1254736177007 &menu=ultiDatos&idp=1254735573002 .
- 14.Pittman J, Beeson T, Kitterman J, et al. Medical Device-related Hospital-Acquired Pressure Ulcers:Development of an Evidence-based Position Statement. J Wound Ostomy Continence Nurs. 2015;42:151–4. doi: 10.1097/WON.0000000000000113. http://doi.org/10.1097/WON.0000000000000113. [DOI] [PubMed] [Google Scholar]
- 15.Ma Z, Drinnan M, Hyde P, et al. Mask Interface for Continuous Positive Airway Pressure Therapy:Selection and Design Considerations. Exp Rev Med Dev. 2018;15:725–33. doi: 10.1080/17434440.2018.1525291. http://doi.org/10.1080/17434440.2018.1525291. [DOI] [PubMed] [Google Scholar]
- 16.Santos J, Pearce SE, Stroustrup A. Impact of Hospital-based Environmental Exposures on Neurodevelopmental Outcomes of Preterm Infants. Curr Opin Pediatr. 2015;27:254–60. doi: 10.1097/MOP.0000000000000190. http://doi.org/10.1097/MOP.0000000000000190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Celik H K, Kose O, Ulmeanu M, et al. Design and Additive Manufacturing of Medical Face Shield for Healthcare Workers Battling Coronavirus (COVID-19) Int J Bioprint. 2020;6:286. doi: 10.18063/ijb.v6i4.286. http://doi.org/10.18063/ijb.v6i4.286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Sherborne C, Claeyssens F. Considerations Using Additive Manufacture of Emulsion Inks to Produce Respiratory Protective Filters Against Viral Respiratory Tract Infections Such as the COVID-19 Virus. Int J Bioprint. 2021;7:316. doi: 10.18063/ijb.v7i1.316. http://doi.org/10.18063/ijb.v7i1.316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Bishop EG, Leigh SJ. Using Large-Scale Additive Manufacturing as a Bridge Manufacturing Process in Response to Shortages in Personal Protective Equipment during the COVID-19 Outbreak. Int J Bioprint. 2020;6:281. doi: 10.18063/ijb.v6i4.281. http://doi.org/10.18063/ijb.v6i4.281. [DOI] [PMC free article] [PubMed] [Google Scholar]


