Abstract
Implanted biomaterials elicit a series of distinct immune and repair-like responses that are collectively known as the foreign body reaction (FBR). These include processes involving innate immune inflammatory cells and wound repair cells that contribute to the encapsulation of biomaterials with a dense collagenous and largely avascular capsule. Numerous studies have shown that the early phase is dominated by macrophages that fuse to form foreign body giant cells that are considered a hallmark of the FBR. With the advent of more precise cell characterization techniques, specific macrophage subsets have been identified and linked to more or less favorable outcomes. Moreover, studies comparing synthetic- and natural-based polymer biomaterials have allowed the identification of macrophage subtypes that distinguish between fibrotic and regenerative responses. More recently, cells associated with adaptive immunity have been shown to participate in the FBR to synthetic polymers. This suggests the existence of cross-talk between innate and adaptive immune cells that depends on the nature of the implants. However, the exact participation of adaptive immune cells, such as T and B cells, remains unclear. In fact, contradictory studies suggest either the independence or dependence of the FBR on these cells. Here, we review the evidence for the involvement of adaptive immunity in the FBR to synthetic polymers with a focus on cellular and molecular components. In addition, we examine the possibility that such biomaterials induce specific antibody responses resulting in the engagement of adaptive immune cells.
Keywords: Biocompatibility, foreign body, polymers, adaptive immunity
1. Introduction
1.1. Biomaterials and the FBR
Biomaterials are widely used in medical applications, ranging from implants and drug delivery devices to various regenerative medicine applications. However, when implanted, they can cause an unwanted response called the foreign body reaction (FBR), which involves a series of overlapping cellular and molecular events. The first step involves protein adsorption, where proteins from blood and interstitial fluid accumulate near and on the biomaterial [1]. At this stage, interactions include aspects of coagulation and induce changes in protein conformation. Subsequently, cellular interactions resemble those observed in acute and chronic inflammation but with elements that are unique to the FBR [2], [136]. Finally, there is an encapsulation phase, where a collagenous capsule forms around the biomaterial via extracellular matrix (ECM) deposition [1]. Composed primarily of foreign body giant cells (FBGC) and components of wound-like responses, such as macrophages, the FBR is key in determining the fate of biomaterials. The release of various cytokines from activated macrophages largely influences the recruitment of other cells and the extent of inflammation as well as ECM deposition and remodeling. It should be noted that recent reports have described polymer-based materials as eliciting no or minimal FBR in the traditional sense, suggesting that the standard for biocompatibility should be reconsidered [137]. Nevertheless, understanding the FBR is critical as it can impact the ability of biomaterials to perform their functions. Importantly, elucidating the temporal and spatial participation of cell types and molecules present at the various stages is critical for the design of strategies to mitigate the FBR for specific applications. For example, extensive polymeric libraries have been thoroughly investigated in efforts to dampen inflammatory responses, and thus reduce fibrosis and the FBR [3]. In addition, multiple approaches involving polymer modifications, as well as controlled release of immunomodulators, are being pursued [136].
1.2. Polymers
Biomaterials come in many forms and from multiple sources and predominantly consist of metals, ceramics, or polymers [4]. Displaying wide variability and versatility, polymers can be classified based on many factors such as morphology (amorphous, semicrystalline, or crystalline), chain architecture (linear, branched, or cross-linked), and molecular forces (covalent bonds or noncovalent interactions) [4]. Polymers can also be engineered to degrade in response to certain chemical, biological, mechanical, thermal, or photo triggers [4]. One main classifier is the source of the polymers: natural or synthetic [5]. Natural polymers have the advantage of eliciting little FBR, having low toxicity, and aiding in biological responses such as interaction with adhesive receptors and cell signaling [5]. Examples of natural polymers include silk, collagen, hyaluronic acid, and alginate. On the other hand, synthetic polymers are advantageous as they are easily manufactured and processed into different shapes and structures. Examples of synthetic polymers include polylactic acid (PLA), polyethylene glycol (PEG), polyglycolic acid (PGA), and polycaprolactone (PCL). Some processing techniques include electrospinning, 3D printing, and solid freeform fabrication [4]. These techniques allow the manipulation of properties so that they can be tailored for specific applications [4]. For example, synthetic polymers can be modified to achieve ideal biomechanical properties by altering their elastic modulus, stiffness, and shape. Another property is the wettability of the polymer. Depending on whether the polymer is hydrophobic or hydrophilic, proteins will have different affinities to the biomaterial, leading to distinct responses [7]. Porosity is also important as several studies have shown distinct FBR depending on pore size [1]. Specifically, porous scaffolds have been shown to elicit less severe inflammation responses than non-porous scaffolds. More porous biomaterials tend to have a thinner fibrous encapsulation than solid biomaterials. The difference caused by porosity is likely due to the increased surface area to volume ratio in more porous materials. The topography of the biomaterial can also have an effect on the FBR, specifically in relation to macrophage behavior [7].
1.3. Cell types in the FBR
Historically, it has been appreciated that inflammatory cells, as part of the innate immune system, are the predominant drivers of FBR. One of the first cells to respond in the FBR are neutrophils, which are recruited to the implantation site and release cytokines in response to being unable to phagocytose the biomaterial [1, 8]. Tissue-resident mast cells join neutrophils in this early stage of FBR and contribute cytokines as well as other molecules [8]. Subsequently, macrophages are recruited and activated in a manner that is unique to the FBR and distinct from wound healing. Macrophages then attempt to phagocytose the biomaterial, releasing enzymes and other factors that contribute to persistent inflammation [1]. Following their accumulation on surfaces, macrophages fuse to form FBGC, which is a hallmark of the FBR [1, 8]. There have been many descriptions of specific phenotypes of macrophages in the FBR, as both pro-inflammatory (M1) and anti-inflammatory (M2) phenotypes have been implicated in the response [1]. Consequently, the relative levels of M1 and M2 phenotypes in response to biomaterials have been studied as a predictor of tissue remodeling success or failure. However, the complex roles and heterogeneity of macrophage phenotypes in the context of the FBR have been highlighted in a recent review [138].
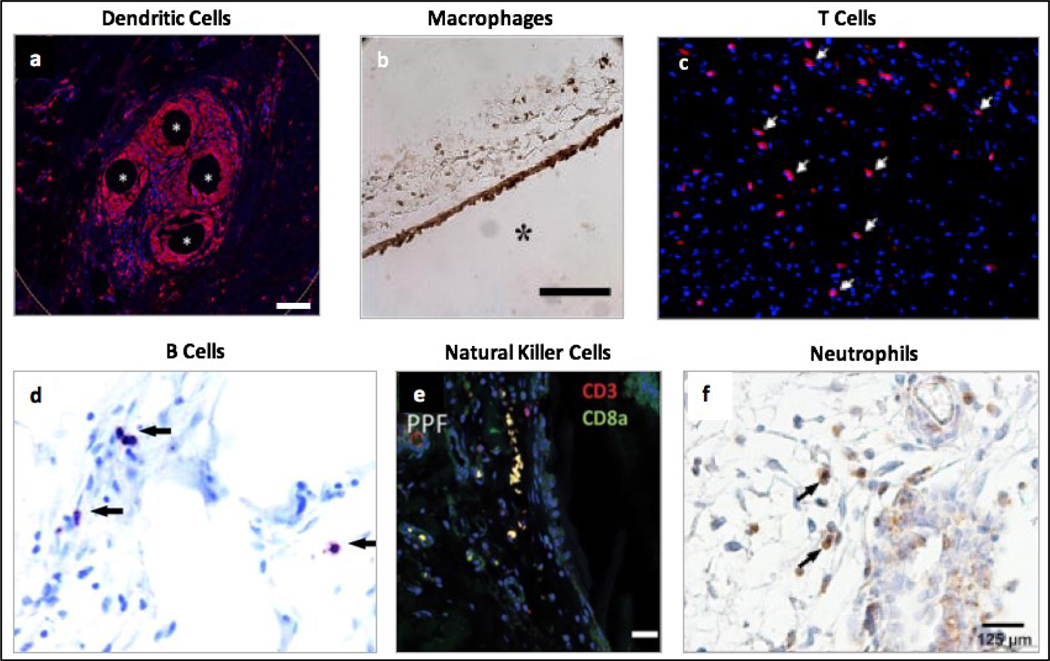
Dendritic cells (DCs), which bridge the gap between adaptive and innate immunity, have also been detected in the FBR. Specifically, they contribute to antigen presentation, T cell priming, T cell tolerance, and activation of regulatory T cells (Tregs) [9]. T cells are responsible for secreting specific cytokines, depending on the T cell type present [9]. One specific T cell type implicated in the inflammatory response of FBR is TH17 cells, which secrete interleukin (IL)-17 [10]. Furthermore, natural killer (NK) cells also play roles through activating macrophages via the secretion of interferon-γ (INF-γ) in the FBR [7]. NK cells additionally secrete alarmins that activate DCs [11]. Finally, B cells are recruited by several factors including CXCL13, a chemokine secreted by macrophages [12]. In the fibrotic cascade, B cells have been shown to enhance fibrosis through their relationship with myofibroblast recruitment [12]. Figure 1 provides examples of various immune cells detected in the FBR to polymers.
Figure 1.
Different cell types in the foreign body reaction to implanted polymeric biomaterials. a) Presence of dendritic cells shown by CD11b (red) staining of tissue samples from humans, approximately 1 year after surgery to repair an abdominal wall hernia using polypropylene mesh. Scale bar = 100 μm. Reprinted from Dievernich et al. Hernia, 2021 under the terms of the Creative Commons CC-BY License [13]. b) Presence of macrophages shown by mac3 (brown) staining of tissue samples from immunocompetent C57BL/6 mice, 4 weeks after a subcutaneous implantation of a poly-ethylene glycol hydrogel. Scale bar = 100 μm. Reprinted with permission from Lynn et al. J Biomed Mater Res A, 2011, 96: 621–631 (John Wiley & Sons) [14]. c) Presence of T cells shown by CD3 (red) staining of tissue samples from Sprague-Dawley rats, 3 weeks after a subcutaneous implantation of a polyurethane-encapsulated biosensor. Reprinted with permission from Ward et al. J Biomater Sci Polym Ed, 2008, 19: 1065–1072 (Taylor & Francis) [15]. d) Presence of B cells shown by B220 (purple) staining of tissue samples from specific pathogen-free female C57BL/6 mice, 4 weeks after a subcutaneous materials injection of nylon mesh. Reprinted with permission from Higgins et al. Am J Pathol, 2009, 175: 161–170 (Elsevier) [16]. e) Presence of natural killer cells shown by CD8a (green) staining of tissue samples from immunocompetent Sprague-Dawley rats, 6 weeks after an intradermal implantation of a polypropylene fumarate coated implant. Scale bar = 20 μm. Reprinted with permission from Bracaglia et al. J Biomed Mater Res A, 2019, 107: 494–504 (John Wiley & Sons) [17]. f) Presence of neutrophils shown by anti- myeloperoxidase antibody (brown) staining of tissue samples from C57BL/6 mice, 14 days after implantation of Dacron protheses in striated muscle tissue. Reprinted with permission from Moussavian et al. J Vasc Surgery, 2016, 64: 1815–1824 (Elsevier) [18].
1.4. Molecules in the FBR
Many molecules have been shown to play key roles in the FBR, specifically in relation to interaction with the biomaterial surface, inflammation, and encapsulation response. For example, several proteins including fibrinogen, vitronectin, and serum amyloid P have been identified on the surface of biomaterials shortly after implantation [19]. Fibronectin and vitronectin have been shown to promote fusion of macrophages [8]. During the early inflammatory phase, various cytokines, chemokines, and growth factors such as transforming growth factor (TGF-β), platelet-derived growth factor (PDGF), and IL-1 play multiple roles including recruitment of macrophages [8]. Macrophages, in turn, secrete more growth factors and chemokines such as PDGF, tumor necrosis factor (TNF-α), and IL-6, which contribute to the attraction of additional macrophages [8]. IL-4, from the degranulation of mast cells and perhaps T cells, has been shown to induce the fusion of macrophages to form FBGCs [8]. Finally, high levels of matrix metalloproteinase (MMP)-9 have also been implicated in inflammation and the encapsulation of biomaterials [20].
Strategies to attenuate the FBR require in-depth knowledge of the cells and molecules involved in the FBR. Therefore, it is critical to elucidate specific processes of the FBR and the corresponding cells and signaling cascades. For instance, the role of oxidative stress, potential byproducts of cell and material interactions, in augmenting inflammation and adaptive immune responses suggests that various aspects of synthetic biomaterials may be functionalized to attenuate FBR, including material composition, surface properties, and degradation products [143, 144]. Thus, one understudied area of the FBR is the adaptive immune response to synthetic polymer biomaterials. With the prevalence of synthetic polymers in medical applications, characterizing the role of the adaptive immune response can serve to develop anti-FBR strategies and inform polymeric device design.
2. Polymers and the FBR
Polymeric materials are commonly used in biomedical devices because of their chemical versatility, physical properties, and biocompatibility. While extensive research in this field has led to the discovery of polymeric scaffolds, implantable devices, drug delivery systems, surgical meshes, and more, the FBR to synthetic polymers is not entirely understood. A better understanding of cell and protein interactions with polymers may allow for future development of materials that reduce the inflammation, tissue damage, and fibrous encapsulation often associated with the FBR.
2.1. Polymer properties and their influence on the FBR
Several studies have shown that a variety of physical and chemical properties of biomaterials are implicated in the FBR [21, 22, 145]. Architecture and topography are of particular interest because the first stage of the FBR begins with protein adsorption to the biomaterial surface. Consequently, altering surface properties could influence the composition of proteins that adhere, which in turn could affect the types and activation state of cells that interact with the biomaterial surface.
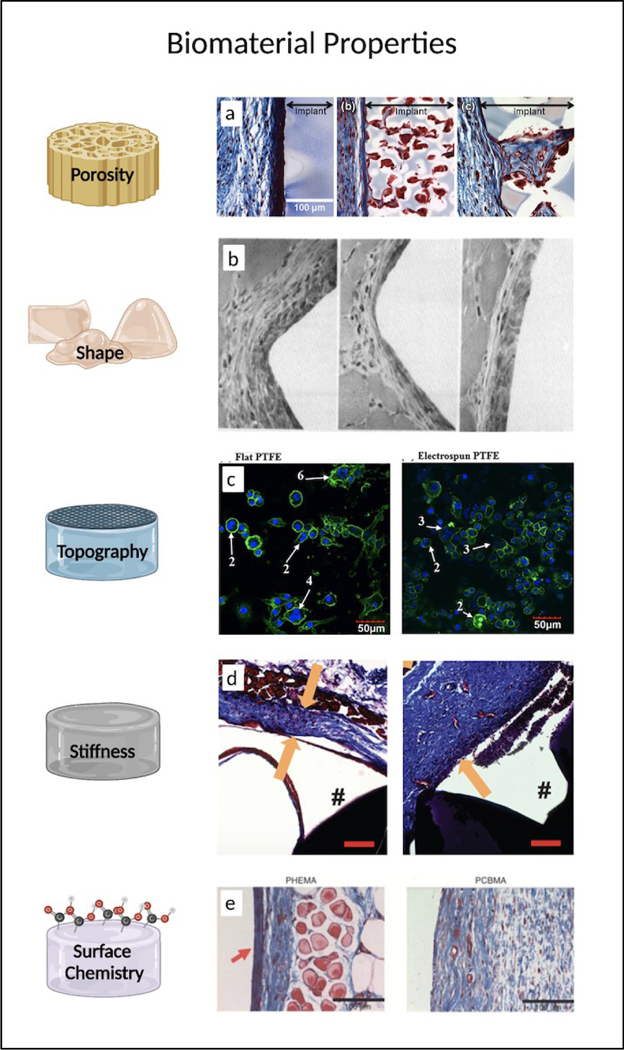
Biomaterial porosity is also believed to be a major implicator in the FBR since it has been shown to influence both the behavior of proteins and cells as they physically interact with the surface [23]. The ideal pore size depends on biomaterial as well as the properties of the cells and tissues in the environment. In general, pores ranging from 20–1500 μm have been investigated in cell migration, spreading, proliferation, and vascularization studies [24–27]. For example, porous poly(2-hydroxyethyl methacrylate) (pHEMA) scaffolds of 34 μm diameter have been shown to induce greater vascularization, up to 63% greater expression in M1 markers, and up to 85% reduction in M2 markers. In the same study, histological photomicrographs reveal that that larger pore sizes not only reduced the dense fibrosis at the implant edge but also increased cellular infiltrate within the pores (Fig 2a) [28]. In another study, however, PCL scaffolds of 40 μm box-shaped pores similarly caused macrophage polarization, with the anti-inflammatory, pro-healing M2 macrophages being the dominant phenotype [29]. Beyond possible differences in cell types, polymer chemistry, and mechanical properties, pore shapes may also be a contributing factor explaining such stark differences in macrophage polarization.
Figure 2.
The influence of physical and chemical properties of synthetic polymeric biomaterials on the foreign body response. a) Pore size of pHEMA scaffolds affects cellular (red) and collagen (blue) composition of the FBR. Reprinted with permission from Sussman et al. Ann Biomed Eng, 2013, 42: 1508–16 (Springer Nature) [28]. b) H&E stained sections of muscle tissue 14 days after implantation of PVC rods with triangle, pentagon, and circular cross sections. Reprinted with permission from Matlaga et al. J Biomed Mater Res, 1976, 10: 391–7 (John Wiley & Sons) [139]. c) Representative confocal microscopy of foreign body giant cells on flat and electrospun PTFE on day 21. Reprinted with permission from Lamichhane et al. J Biomed Mater Res A, 2017, 105: 244150 (John Wiley & Sons) [33]. d) Representative images revealing fibrous capsule thickness 28 days after PEG hydrogels of low stiffness (left) and high stiffness (right) were implanted subcutaneously in mice. Scale bar = 100um. Reprinted with permission from Jansen et al. Biomacromolecules, 2018, 19: 2880–80 (American Chemical Society) [36]. e) Masson’s trichrome staining of collagen surrounding pHEMA and pCBMA hydrogels after three months implantation. Reprinted with permission from Zhang et al. Nat Biotechnol, 2013, 31: 553–556 (Springer Nature) [140]. Created with BioRender.com.
The shape of the whole biomaterial device or implant is another factor that may affect the foreign body response. In a study involving the implantation of six different polymeric rods with pentagon, triangular, or circular shaped cross sections in rat gluteal muscle, researchers noted more cellular enzyme activity and tissue response in the sharper shaped rods (Fig 2b) [139]. Consequently, in all implantable devices, the minimization of any acute angles and sharp textures is generally recommended in order to avoid any additional tissue abrasion and inflammatory response.
Beyond differences in polymer chemistry [30] and shape, polymer processing and fiber orientation are also factors influencing macrophage behavior [31–33]. In the case of human tendon fibroblasts cultured on PCL mats, it was determined that highly aligned electrospun fibers resulted in less inflammatory response via downregulation of MMP-1 when compared to randomly oriented scaffolds [32]. In vivo experiments further supported the idea that disorganized orientation of PCL fibers promoted a pro-inflammatory response among macrophages and tendon fibroblasts [31]. Moreover, in another study where murine macrophages were plated on various topographies, microscopy images reveal decreased FBGC on electrospun polytetrafluoroethylene (PTFE) compared to the flat PTFE and control polystyrene (Fig 2c) [33]. Overall, these results highlight the innate sensitivity and phenotypic responses of cells to biomaterial topography.
Elastic modulus of the biomaterial is another factor that has been considered, and a recent study has shown that mechanical matching of biomaterials minimizes the FBR by reducing inflammation and fibrosis [34]. Specifically, the association of more robust FBR appears to implicate fibroblasts and macrophages that displayed higher concentration of nuclear Yes-associated protein (YAP) [34, 35]. YAP, an mechanosensitive transcriptional regulator, was found to be in higher concentration in stiff implants as opposed to implants coated with a soft polyacrylamide or silicone layer [34]. Similarly, lowering the modulus of a zwitterionic-PEG hydrogel reduced the density of adhered macrophages and was associated with lessened FBR (Fig 2d) [36].
Surface chemistry of biomaterials such as charge characteristics, hydrophobicity, and presence of different chemical moieties can also influence tissue-material interactions [37]. For example, in one study involving pHEMA and pCBMA hydrogels of similar stiffness implanted subcutaneously (SC) in mice, researchers determined that the thinner collagen capsule surrounding the pHEMA hydrogels could be attributed to its zwitterionic chemistry resulting in resistance against nonspecific protein adsorption (Fig 2e) [140]. As mentioned earlier, hydrophobicity plays a significant role in the degradation of biodegradable materials. Hydrophobicity also plays a role in determining the adsorption of proteins on the biomaterial. In one study, the addition of a hydrophilic carboxylic acid moiety resulted in increased affinity to albumin, constituting up to 45% of the total adsorbed protein, and increased the expression of anti-inflammatory cytokines by M2 macrophages [38]. Conversely, the addition of a hydrophobic methyl moiety increased affinity to the opsonin IgG2 and thus increased expression of pro-inflammatory cytokines by M1 macrophages, which play an important role in activating the innate immune response. In another study, nanoscale chemical modifications resulted in reduced MMP-9 expression in neutrophils and reduced IL-6 and IL-1β expression in macrophages [39]. Because MMP-9 is actively involved in collagen degradation, and therefore impacts tissue remodeling, the enzyme has largely been studied for its role in activating macrophage fusion and FBGC formation. Thus, surface modification approaches have been extensively pursued in order to modulate protein absorption and evade the immune system [40].
2.2. Protein and cell interactions with polymeric materials
Within seconds to minutes after implantation, host proteins immediately adhere to biomaterial surfaces. Typically, the process begins with diffusion of proteins toward the biomaterial surface. Commonly adsorbed proteins include albumin, immunoglobulin, complement, fibrinogen, fibronectin, and others [41]. Next, the proteins anchor themselves onto the interface via directional interactions with the surface or with adjacent water molecules. Through a stepwise mechanism involving altering spatial orientation and surface interactions to determine the most energetically favorable positioning, the proteins then “lock-down” onto the biomaterial surface [42]. Studies have shown that upon binding to select surfaces, fibrinogen can unfold and expose cryptic binding sites for interactions with other proteins, platelets [43], and leukocytes [44]. In fact, for biomaterials in contact with blood, the extent of platelet adhesion is correlated not with the concentration of fibrinogen adsorbed onto the protein layer but on the degree of adsorption-induced unfolding [43]. Moreover, the protein layer that forms on biomaterials is often multilayered, and the dynamic, competitive nature of protein adsorption and desorption is referred to as the Vroman Effect [45].
Consequently, cells that interact with the biomaterial surface are drawn in by interactions with the absorbed protein layer, such as through integrin binding. Acute inflammation typically begins with the migration of neutrophils, which is short-lived but upholds the important role of phagocytosing debris. Shortly thereafter, chemotactic factors including histamines recruit monocytes and macrophages, and these phagocytic cells may remain at the injury/implantation site for weeks to months. In polyethylene terephthalate (PET) implants, the administration of both H1 and H2 histamine receptor antagonists was shown to reduce the recruitment of macrophages/monocytes and neutrophils to the implantation site [46]. In the longer term, the FBR is characterized by a more heterogeneous accumulation of immune cells. As mentioned above, macrophages are considered central mediators of the FBR, playing key roles in the phagocytosis of biomaterial particles and cell debris and influencing key processes such as ECM deposition and vascularization. Depending on the composition of proteins adhered onto the biomaterial surface, the M0 macrophages are polarized to the proinflammatory M1 state or the anti-inflammatory M2 state. Although evidence suggests that increased M2 macrophage density and higher ratios of M2:M1 macrophages are associated with more favorable tissue remodeling [47], there is a delicate balance to be struck between the two phenotypes. It is also important to note that the traditional view of the inflammation dominated by M1 versus M2 macrophage activation is an over-simplification, as the phenotypes of neutrophils and macrophages are much more dynamic than previously thought [48, 49].
Unlike the role of the innate immunity in the FBR, little is known about the role of humoral/adaptive immunity. This is due, in part, to the absence of known polymer-based antigens. However, hypersensitivity reactions to PEG polymers appear to implicate the complement pathway as a bridge between innate and adaptive immunity [50]. DCs also serve as a mediator between innate and adaptive immunity, with studies suggesting that biomaterial factors such as hydrophobicity, surface chemistry, and protein adsorption affect dendritic cell phenotypes, thus potentially affecting T cell phenotypes [51]. Given the lack of consensus surrounding the role of lymphocytes or DCs in the FBR, there has been increased motivation to elucidate the role of adaptive immunity.
2.3. The innate and adaptive immune system
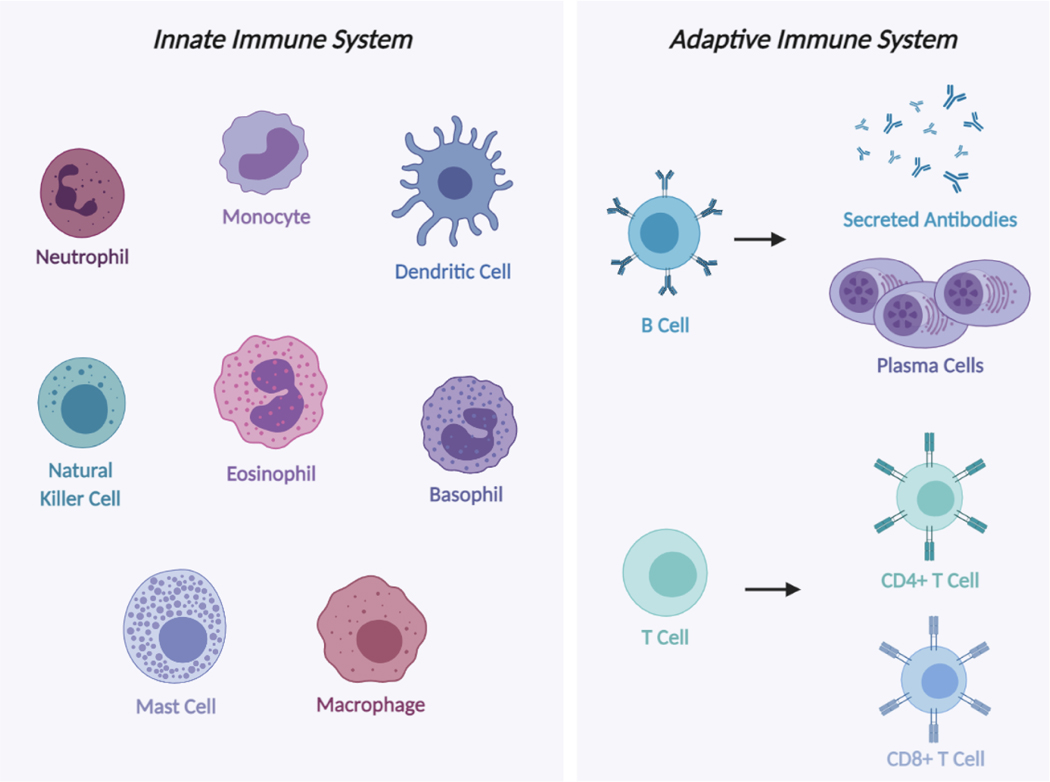
The immune system is made up of organs, cells, and molecules that work together to prevent and fight infections. It is responsible for helping the body deal with diverse types of threats including pathogens, cancer cells, and injuries. Dysfunction of the immune system can lead to serious complications including establishment of chronic infections, allergies, or autoimmune responses. Thus, it is essential for the immune system to be able to recognize self from non-self in order to respond to invading harmful substances and pathogens but not attack the host’s own tissues [52]. Different molecules and cell types of the innate and adaptive immune system can be seen in Figure 3.
Figure 3.
Illustration of innate and adaptive immune system cells. Innate immune responses are mediated by macrophages, mast cells, natural killer cells, dendritic cells, monocytes, and granulocytes such as neutrophils, eosinophils and basophils. Adaptive immune responses are mediated by T and B cells. T cells such as CD4+ and CD8+ T cells recruit macrophages to sites of infection to eliminate microbes while B cells differentiate into antibody-secreting cells, plasma cells, and secrete antibodies, which destroy microbes. Created with BioRender.com.
Innate immunity is the first line of defense against foreign materials, infections, or injuries and provides an immediate, non-specific response [53]. It supports the functions of physical barriers such as the skin, mucous membranes, and epithelial cell layers, which help prevent infections by blocking entry of microbes. Epithelial cilia sweep away mucus layers allowing the removal of invading pathogens [54]. If pathogens do breach the epithelial cell layers, phagocytes, NK cells, and complement proteins become activated to eliminate them. Innate immune responses depend on cells and proteins that recognize features of pathogens and become activated to attack them [55]. Small molecules and proteins that are physiologically present in tissues or released from activated cells all aid in the innate immune response. Some components of the innate immune system are constitutively active while other components are activated by interactions of cells, cell receptors, and proteins with invading pathogens [56]. Additional important functions of the system are to prevent responses to benign invaders and distinguish between different types of pathogens to induce the appropriate adaptive immune response.
While the innate immune system response is immediate, the adaptive immune response develops over time [57]. Also, unlike the innate immune system, which targets pathogens non-specifically, the adaptive immune system involves antigen-specific responses and provides long-term protection. Any substance that can elicit an adaptive immune response is considered an antigen and recognized by immune cells that specifically attack it. At the cellular level, lymphocytes (T cells and B cells) are the primary drivers of adaptive immunity and mount cell-mediated and humoral (antibody) responses [58]. When induced, antibody responses involve secretion of immunoglobulins from B cells that circulate in the bloodstream. When they encounter the antigen responsible for their production, they neutralize it by binding and preventing it from interacting with corresponding targets. For example, antibodies bind to surface proteins on viruses and prevent them from interacting with host cells. Antibody binding can also serve as a molecular tag to target invaders for destruction by phagocytic cells; a process known as opsonization. When antibodies bind a target, they can activate complement, which can further help opsonization.
In cell-mediated immunity, activated T cells interact with antigens that are presented on the surface of cells. For example, host cells infected with viruses can display antigen of viral origin on their surface, which leads to recognition and elimination of the infected cells by T cells. In addition, antigen-specific cells amplify their response by recruiting innate effector mechanisms. Moreover, adaptive immunity develops memory from previous encounters with specific pathogens so that it can develop faster, stronger future responses when exposed to the same pathogen [59].
2.4. Cells of the adaptive immune system
Lymphocytes are white blood cells made in the bone marrow and found in blood and lymph nodes. They are the only cells that generate clonally distributed receptors that are specific to an antigen [60]. As mentioned previously, lymphocytes can recognize foreign antigens and trigger adaptive immune responses. When naive lymphocytes that have not previously encountered antigen recognize foreign or pathogenic antigens, lymphocytes specific to the antigen proliferate and differentiate into effector cells and memory cells [61]. Unlike naive lymphocytes, activated effector lymphocytes produce molecules needed to eliminate antigens.
Lymphocytes can be categorized as either T cells, which are principally responsible for mediating cell-mediated immunity, or B cells, which are predominantly responsible for mediating humoral immunity [56]. T and B cells consist of many different clones, where each clone of cells expresses the same distinct antigen receptor. T and B cells differ in their specificity [62]. While most T cells can only recognize peptide fragments of cell surface antigens, B cells can recognize many different types of molecules. In the adaptive immune system, different kinds of lymphocytes recognize specific types of antigens and differentiate into effector cells, which eliminate the antigens.
2.4.1. T Cells
T cells mature in the thymus and migrate to peripheral lymphoid tissues or circulate in blood to interact with specific antigens. Their functions include activating other immune cells such as phagocytes, killing infected host cells, and regulating the immune response. They are divided in two main categories, helper T (TH) and cytotoxic T cells. TH cells, typically expressing CD4, produce cytokines that aid the function of other cells, including those of the innate immune system. In addition, they can induce activation of B cells and macrophages and stimulate inflammation [63]. Cytotoxic T cells, typically expressing CD8, can kill cells infected with pathogens as well as tumor cells. T cells can also be categorized in more specialized subtypes based on the expression of surface proteins such as CD3, CD4, CD8, CD25, and CD45 or defined by specific cytokines produced and functions performed, including TH1, TH2, TH9, TH17, and Treg cells [64].
2.4.2. B Cells
B cells, typically expressing CD45, CD19, CD20, CD24, CD38, and CD22, mature in the bone marrow and circulate in the lymphatic system or blood [56]. They produce antibodies that mediate humoral immunity. They have membrane-bound antibodies that act as cell surface receptors that recognize antigens. Antigens on the surface of pathogens can bind to B cell receptors. B cells differentiate into plasma cells, which are effector cells that secrete antibodies when they are activated by binding of an antigen [65].
2.5. Humoral and cell-mediated immunity
Adaptive immunity involves humoral and cell-mediated responses, which involve distinct but overlapping cells and molecules. Humoral immunity is mediated by cells and molecules in extracellular fluid such as secreted antibodies and complement proteins. B cells produce these antibodies, which enter systemic circulation and are present in extracellular fluids and the lumen of mucosal tissues. Antibodies block pathogens from invading tissue cells, neutralize toxins made by pathogens, and enhance the uptake of pathogens by phagocytes. However, some pathogens can survive and replicate inside cells including phagocytes [65]. Antibodies are ineffective at attacking pathogens once they have entered cells, which is when cell-mediated immunity takes over and plays an important role [56]. Cellular immunity is mediated by T cells, which recognize foreign or pathogenic antigens on the surface of the host cell. Some T cells directly kill any cell that contains pathogens while others recruit phagocytes to the site of infection to attack extracellular pathogens.
Comprehensive reviews on aspects of the adaptive immune system within the context of biomedical engineering applications and implantassociated fibrosis have been published [9, 56].
3. Animal models and the FBR
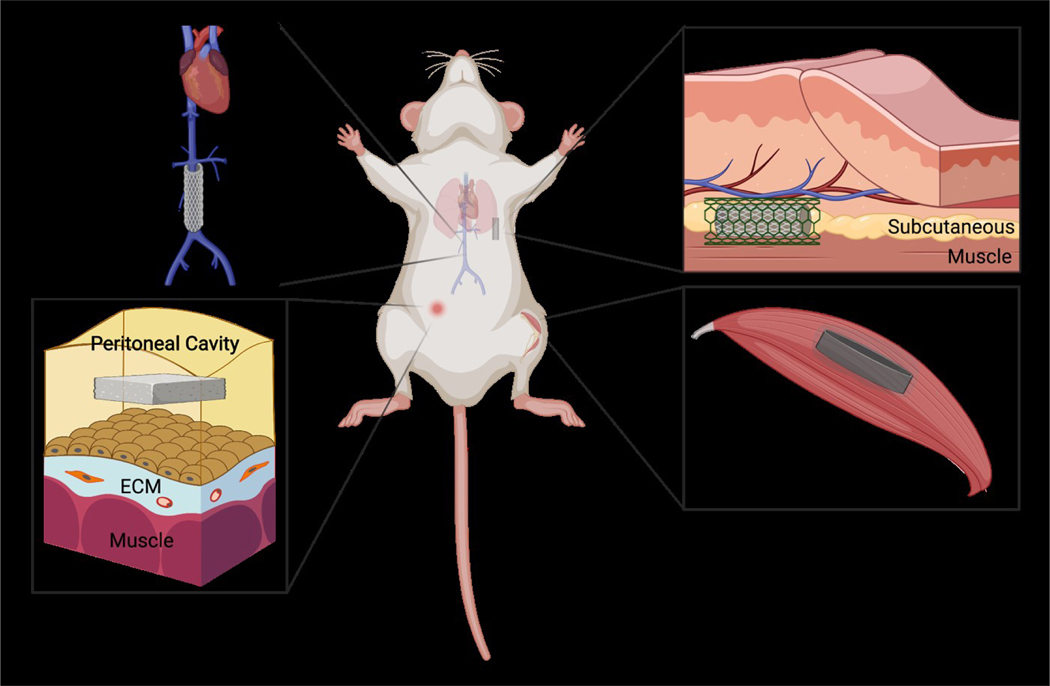
A number of experimental animal models have been employed to probe the participation of immune cells and molecules in the FBR. As mentioned above, the bulk of such studies have focused on innate immunity. However, mice and rats deficient in cells or molecules linked to adaptive immunity have also been studied for their response to synthetic polymers. In this section, we review FBR studies involving animals deficient in components of adaptive immunity. Figure 4 illustrates several common biomaterial implantation sites in rodents. Furthermore, table 1 summarizes the FBR outcomes measured in genetically compromised rodents implanted with various synthetic polymers.
Figure 4.
Common biomaterial implantation models in rodents. Evaluation of immune responses typically involves implantation of biomaterials into mice or rats and subsequent analyses of cellular and molecular interactions. Biomaterials are most often implanted SC, sometimes enclosed in a metal cage. Other sites include muscle or the vascular system in the case of blood-contacting biomaterials. Intraperitoneal image reprinted and modified from Liappas et al. Biomed Research International 2015 under the terms of the Creative Commons Attribution License [146]. Created with Biorender.com.
Table 1.
Animal models deficient in adaptive immunity and FBR
| Animal Model | Polymer | Implantation Model | Duration | Compared to WT? | Measured Outcomes | Reference |
|---|---|---|---|---|---|---|
|
| ||||||
|
hypogammaglobulinemic CB-17 SCID mice |
PET | intraperitoneal | 16 hours | Y | No change (leukocyte accumulation) | [86] |
|
B cells & T cells (deficient) CB-17 SCID mice |
PGA-P(CL/LA) & PLLA-P(CL/LA) | inferior vena cava & aorta | 21–42 days | N | No change (leukocyte accumulation, FBGC formation, collagen deposition, vascularization) | [84] |
|
T cells (deficient) BALB/C nude mice |
PEU & PET & SR | subcutaneous (cage implant) | 7–21 days | Y | No change (leukocyte accumulation*, FBGC formation) *Lower adherent cell densities in nude mice with PET at day 7 & PEU at day 21. |
[80] |
|
T cells (deficient) BALB/C nude mice |
PCL-Gelatin-HA | subcutaneous | 28–56 days | N | No change (fibrous encapsulation) | [82] |
|
NK cells (deficient) C57BL/6J mice |
||||||
|
NKT cells (deficient) BALB/CJ mice |
||||||
|
IL-4Ra (deficient) BALB/CJ mice |
PEU & PET | subcutaneous | 14–28 days | Y | No change (leukocyte accumulation, FBGC formation) | [83] |
|
mast cells (deficient) C57BL/6J mice |
||||||
|
B cells & T cells (deficient) C57BL/6J SCID mice |
||||||
|
T cells (deficient) BALB/C nude mice |
PCL-CaP | subcutaneous | 4–14 days | Y | No change (collagen deposition), vascularization ↑ | [81] |
|
IFN-γ (KO) C57BL/6 mice |
PE-PUR | subcutaneous | 7 days | Y | leukocyte accumulation ↓, vascularization ↓, pro-inflammatory cytokines ↓, anti-inflammatory cytokine ↑, collagen deposition ↓ | [97] |
|
MyD88 (KO) B6.129P2(SJL)- MyD 8 8 <tm 1.1Defr>/J mice |
PEG | subcutaneous | 2–28 days | Y | leukocyte accumulation ↓, fibrous encapsulation ↓, vascularization ↓ | [96] |
|
T cells (deficient) C57BL/6 nude mice |
PDMS | subcutaneous | 21 –42 days | Y | 0 |am, Ra: No change (leukocyte accumulation & fibrous encapsulation) 40 ^m, Ra: leukocyte accumulation ↑ & fibrous encapsulation ↑ | [141] |
|
IL-17A (KO) & IL-17RA (KO) C57BL/6J mice |
PCL | intramuscular | 42–84 days | Y | leukocyte accumulation ↓, fibrosis-related gene expression ↓, collagen deposition ↓, fibrous encapsulation ↓, fiber diameter ↓ | [10] |
|
B cells (KO) C57BL/6J muMt-mice |
PCL | intramuscular | 7–42 days | Y | No change (leukocyte accumulation), fibrosis-related gene expression ↓, collagen deposition ↓ | [79] |
|
B cells & T cells (deficient) CB-17 NOD SCID mice |
PVA | subcutaneous | 2–14 days | Y | No change (leukocyte accumulation) | [85] |
|
T cells (deficient) CBH-rnu/Arc nude rats |
PA-Gelatin | subcutaneous | 7–90 days | N | No change (leukocyte accumulation, vascularization, collagen deposition, fibrotic tissue ingrowth) | [110], [111] |
|
T cells (deficient) Sprague-Dawley nude rats |
PPF-pericardium | subcutaneous | 42 days | Y | No change (leukocyte accumulation) | [17] |
3.1. FBR in mice strains lacking components of adaptive immunity
Lymphocytes including T cells and B cells, alongside NK cells possessing adaptive immune features, have been shown to promote macrophage adhesion and fusion in vitro [8, 9, 66–70]. Yet, very few in vivo studies have been published elucidating the relationship between lymphocytes and FBR. Previously implicated in wound healing [71–73] and fibrosis [74–78], B cells have been investigated for their contribution to the FBR towards synthetic materials in animal models. Specifically, in a recent study, after 6 weeks following intramuscular implantation of PCL particulates into muMt- (B cell knockout (KO)) mice, collagen matrix deposition as well as expression of fibrosis-associated inflammatory genes for S100a4, Col1a1, Col3a1, TGF-β, p21, and IL-23 significantly decreased [79]. Clearly, the lack of mature B cells markedly reduced fibrotic responses to the synthetic implants. However, no statistical differences were observed between the wild-type (WT) and KO animals for CD4+ or CD8+ T cell counts in the injured quad tissue as well as expression of cytokines related to the PCL pro-fibrotic response, IL-4 and IL-17f. Interestingly, the synthetic polymers in WT animals delayed infiltration and increased antigen-presentation-associated phenotypes of B cells. Overall, the role of B cells in modulating FBR was evident.
Compared to B cell-mediated effects, the modulatory impacts of T cells on FBR have been more actively examined. In one study, PEU, PET, or SR disks were placed SC in a cage-implant model in T cell-deficient nude mice and normal mice [80]. Analysis of the implants showed no differences with respect to pro-fibrotic IL-13 levels in exudate supernatants, macrophage adherent density, and FBGC formation on the biomaterial surfaces after 21 days. Although adherent cell density was substantially reduced for the case of PEU implantation in nude mice versus WT at day 21, no significant trend could be established across the time points and FBGC induction was still comparable between the two groups. This study indicated that T cells are not required for FBR and that they are not the only reservoirs of IL-4 and/or IL-13 necessary for macrophage fusion in vivo, although the possibility was not excluded that T cells may still support FBGC formation via unknown mechanisms. Another study developed and employed infrared-excited nonlinear microscopy to characterize the FBR to 3D-electrospun mPCL-CaP scaffolds implanted SC in GFP-expressing athymic nude mice [81]. When monitored longitudinally through intravital multiphoton imaging, the mice exhibited dense networks of perfused neovessels as well as robust accumulation of bundled fibrillar collagen at 14 days post-implantation throughout the implantation site, which were comparable to those observed in WT mice and thus confirmed the dispensable role of T cells in mediating the FBR. Interestingly, neovascularization was promoted to a greater degree in the nude mice compared to control. Although FBR-associated inflammatory tissue remodeling was apparent, the scaffold fibers demonstrated neither resorption nor infiltration by cells. In congruence with these findings, PCL-comprising scaffolds, blended with non-crosslinked gelatin and hydroxyapatite (HA), also triggered abundant FBRs in immunocompromised nude mice [82]. Histological analysis revealed the formation of a thin fibrotic capsule bordering the nanocomposite scaffolds following 8 weeks of SC implantation. Despite sporadic distribution of inflammatory cells, neither tissue ingrowth nor infiltration was detected. It should be noted that in this study, all the experiments were conducted in nude mice and were not compared to immunocompetent control mice. More recently, an interesting study involving silicone breast implantation examined the FBR as a function of implant surface architecture in a rabbit model, followed by investigation of a causal relationship between the anti-fibrotic effects of optimized surface topography and presence of T cells in a mouse model [141]. Among silicone breast implants of various surface topographies (0 to 90 μm roughness) placed SC in rabbits for 3 weeks, full-scale SmoothSilk implants (4 μm roughness) showed minimal fibrotic encapsulation with genetic signature indicating reduced accumulation of CD68+CD11b+ macrophages and elevated recruitment of FOXP3+ immunoinhibitory Tregs. Using miniaturized SmoothSilk implant mimics and quantitative FACS, a subsequent experiment revealed the role of adaptative immunity in modulating FBR. More specifically, compared to those with 0 μm roughness, the miniaturized 4 μm roughness silicone implants induced diminished fibrotic capsule formation and CD68+CD11b+ macrophage presence in the capsule tissues in WT but not in nude mice (T-cell-deficient), at 3 weeks post-surgery. The observed attenuation in fibrotic and immune responses indicated the active involvement of T cells during FBR. Furthermore, a comprehensive, multiplex gene expression array analysis of 6-week explants demonstrated decreased levels of anti-inflammatory cytokines and immunoinhibitory Tregs-response transcripts for the 4 um roughness implants in nude mice, confirming the significance of T cells in suppressing inflammation and fibrosis in this model.
FBR was also evaluated in mice lacking NK cells, NKT cells, or mast cells and in SCID mice lacking both B and T cells in response to SC implantation of PEU or PET polymers for up to 28 days [83]. FBGC formation on explanted polymer surfaces, as assessed by histological staining as well as quantitative and qualitative image analyses, was comparable between the genetically deficient strains and their respective background controls, indicating that all the deficient models tested could elicit macrophage responses. These observations suggested that NK cells, NKT cells, and mast cells were not the only sources of IL-4 or IL-13 for macrophage fusion and that other additional cytokines may have actively promoted FBGC development. There was also a clear implication that redundant innate mechanisms could adequately compensate for the absence of primary fusion-inducing pathways to trigger FBRs, which did not require normal numbers of T or B cells. Similarly, a typical FBR was elicited when biodegradable tubular scaffolds, PGA-P(CL/LA) and PLLA-P(CL/LA), were implanted as inferior vena cava and aortic interposition grafts, respectively, in SCID/bg mice lacking mature T and B cells [84]. The scaffolds were hybrid constructs where P(CL/LA) copolymer solution interconnected the fibers of PGA or PLLA nonwoven felts. By week 3 post-surgery, there were strong indications of macrophage infiltration, multinucleated giant cell formation, and collagen deposition throughout the walls and internal lumens of both PGA-P(CL/LA) venous grafts and PLLA-P(CL/LA) arterial grafts as assessed by histological staining, with external collagen encapsulation surrounding the PLLA-P(CL/LA) scaffolds. By week 6, organized vascular neotissue formation was pronounced within the lumens of both scaffolds that comprised partial endothelialization by vWF+ endothelial cells, medial layer generation by αSMA+ myofibroblasts, and dense collagen deposition as evaluated by histochemical staining. It was evident that although the SCID/bg mice completely lacked functional adaptive immunity, their innate immune system was sufficient for generating FBR comparable to immunocompetent mice. Consistent with these observations, SC implantation of PVA sponges for up to 14 days into NOD SCID mice, which were deficient in functional T and B cells, resulted in normal leukocyte recruitment [85]. However, detailed FACS analysis revealed differences in the subpopulations of recruited cells with decreased levels of CD40, CD86, and MHCII, which are implicated in antigen presentation and myeloid cell differentiation. In contrast, levels of macrophage and granulocyte markers, F4/80 and Gr1, were increased in comparison to control. Parallel profiling of extracellular vesicles present in the sponges showed a similar decrease in key proteins linked to antigen presentation including MHCII in NOD SCID mice. These findings indicated that lack of functional adaptive immunity did not impact the inflammatory cascade typical of early FBR, although more critical readouts such as vessel formation, collagen deposition, and fibrotic encapsulation were not examined. In contrast, a separate study hinted at the antigen-dependent nature of the FBR against a synthetic polymer [10]. Specifically, when lethally-irradiated WT mice were infused with both CD45.1 (WT donor) and CD45.2 (OTII-Rag−/− donor) bone marrow and subsequently implanted with PCL particles in muscle wounds for 1 week, IL-17 was only produced by WT-specific CD4+ T cells but not ovalbumin (OVA)-specific Rag−/− CD4+ T cells in the tissues and draining lymph nodes. Moreover, CD4+ T cells were predominantly from the WT donor mice. This observation indicated that IL-17 expression by CD4+ T cells was antigen-specific, given that Rag−/− CD4+ T cells could still undergo normal TH17 differentiation in vitro as validated in a follow-up experiment and were thus equally capable of IL-17 production. Furthermore, the adjuvanticity of the synthetic material was revealed with greater OVA-specific T cell growth and IL-17 secretion for OTII splenocytes cultured with PCL or PE than for those cultured without any synthetic materials for 48 hours.
Since synthetic polymers are generally not efficient in stimulating antigenic responses, the literature describing participation of antibodies in the FBR is scarce. In one study, the potential role of IgG adsorption was evaluated following the intraperitoneal implantation of PET disks in SCID mice that have less than 1% of the normal plasma IgG levels [86]. These hypogammaglobulinemic mice displayed near-normal recruitment as well as adherence of neutrophils and monocytes/macrophages, as quantified by intracellular enzyme activities of MPO and nonspecific esterase from the extracted implant, respectively, indicating that IgG adsorption was not required for the early inflammatory reaction. In a separate study, mice lacking MyD88, which is a signaling adaptor protein induced by most toll-like receptors (TLRs) to mediate T cell activation [87–92] and also expressed by B cells for antibody generation [93–95], were probed in the context of FBR [96]. Specifically, PEG hydrogel disks were implanted SC in MyD88-deficient mice for up to 28 days, after which the kinetics of inflammatory cell accumulation and fibrous encapsulation were examined via histological analysis. The MyD88-deficient mice showed diminished recruitment of inflammatory cells around the implants with reduced cell layer thickness, which comprised mostly macrophages and less frequently neutrophils and monocytes. In addition, compared to WT mice, the MyD88-lacking mice exhibited a substantial decrease in collagenous fibrous capsule formation as well as reduced vascularization. These findings are consistent with the ability of MyD88 to promote both innate and adaptive immune responses. Based on the prior evidence for the association of a TH17/IL-17 immune signature with fibrotic responses to human breast implants, IL-17 as an adaptive immune factor and its receptor IL-17RA were further investigated in the context of FBR using KO animal models [10]. Specifically, when IL-17A-deficient or IL-17RA-deficient mice were implanted intramuscularly with PCL particles, gene expressions for fibrosis-associated markers including TGF-β, S100a4, Col I, and Col III decreased in comparison to WT mice 6 weeks post-implantation. Reductions in the pro-fibrotic mRNA levels were consistent with diminished collagen matrix, attenuated fibrotic capsule formation, and thinner fiber diameter as measured by histochemical staining and with decrease in fibrosis-related protein α-SMA expression as measured by immunofluorescence staining for both IL-17-deficient mice and IL-17RA-deficient mice 12 weeks post-implantation. The accumulation of neutrophils and macrophages around the PCL implants was also lower in the KO strains than in WT mice. In a separate study, the contribution of IFN-γ to FBR was evaluated [97], given that the molecule is an important regulator of effector cells during adaptive immune responses [98–104]. Specifically, when polyether polyurethane (PE-PUR) sponge disks were implanted SC in IFN-γ KO mice for 7 days, key parameters of the FBR were attenuated relative to WT animals. These included decreases in angiogenic readouts such as hemoglobin content and blood vessel counts from H&E staining, VEGF levels (ELISA), and blood flow measurements with laser Doppler perfusion imaging. In addition, neutrophil and macrophage accumulations as respectively quantified by MPO and NAG enzymatic activities decreased. At the molecular level, these changes were associated with reduced levels of the pro-inflammatory cytokines TNF-α and CXCL1, an increase in the cytokine IL-10, and down-regulation of iNOS gene expression. Overall, IFNγ was found to be a major orchestrator of FBR, with its absence resulting in diminished progression of inflammation, angiogenesis, and fibrosis. Secreted predominantly by effector CD4+ TH2 cells [105–107] and implicated in B cell maturation for humoral immunity [105, 106, 108], IL-4 has been also evaluated on its ability to regulate FBR in vivo [83, 109]. In one study, direct injection of IL-4-neutralizing antibodies substantially reduced FBGC density on PEUU A’ film in cage implant model mice at 7 days post-implantation, whereas injection of recombinant IL-4 resulted in increased FBGC formation [109]. In another study, however, KO mice deficient in IL-4 receptor (IL-4Ra) exhibited normal levels of FBGC generation on PEU or PET polymers at 28 days following SC implantation [83]. Therefore, FBGC formation required IL-4 but not necessarily IL-4Ra-mediated signaling in the context of synthetic polymer implantation.
3.2. FBR in rat strains lacking components of adaptive immunity
Inflammatory events and the FBR were also investigated in several immunodeficient rat models. The 6-week-long SC implantation of PPF-coated pericardial scaffolds into athymic rats lacking mature T cell populations yielded robust immune cell infiltration at the implant site, with accumulation of CD68+ macrophages, CD11b/c+ DCs, and CD8+/CD3a+ NK cells as assessed by immunohistochemical analyses [17]. TNF-α was also detected via ELISA in the homogenized samples of the implants and surrounding tissues. Compared to untreated conditions, PPF-coating was shown to induce less inflammation overall by preventing the rapid enzymatic breakdown and thus better controlling the exposure of unaltered ECM molecules from the pericardium. The roughly similar magnitude of early FBR instigated against the PPF-coated scaffold in both athymic and immunocompetent rats, as determined by macrophage density and cell infiltration, indicated that T cells were not necessary for the FBR. Synthetic polymers coated with natural materials also demonstrated normal FBR in T cell-deficient rats [110, 111]. Specifically, when gelatin-coated polyamide (PA) meshes were implanted SC in immunocompromised nude rats, vigorous FBR characterized by leukocyte filtration, neovascularization, and fibrosis was observed 90 days post-implantation [110]. Immunofluorescence staining revealed the accumulation of CD68+ macrophages with both CCR7+ pro-inflammatory M1 subtypes and CD163+ anti-inflammatory M2 subtypes as well as αSMA+ vessel formation around the mesh filaments. In addition, histological staining revealed the robust deposition of collagen around the mesh implants, which was confirmed by birefringence analyses showing well-aligned, thick, dense collagen fiber bundles. A follow-up study by the same group also noted the abundant presence of collagen and elastin fibers around the gelatin-coated PA mesh at 90 days post-implantation in nude rats, with blood vessel formation and minimal bridging fibrosis between the mesh fibers [111]. Furthermore, SEM analysis demonstrated fibrotic tissue ingrowth in the implants.
In summary, despite the existence of contradictory findings, adaptive immune components generally have not been shown to play essential roles for initiation and progression of synthetic-polymer-elicited FBRs in genetically compromised animal models. Discrepant reports with regards to the involvement and importance of lymphocytes or related molecules during FBR can be explained by types of synthetic polymer and implant model. Conceivably, variability in these parameters could influence the nature of host protein adsorption to the implant surface, DAMP signaling, and differential expression of molecules linked to adaptive immunity.
4. Adaptive immunity and the FBR
As discussed above, the FBR involves a variety of immune and repair cells and it is widely accepted that macrophage activation, FBGC formation, and fibroblast-mediated ECM deposition remodeling are main effectors [81]. In addition, NK cells, DCs, innate lymphoid cells (ILCs), neutrophils, basophils, eosinophils, and monocytes participate in various stages and pathways of FBR [112, 113]. These cells have been shown to cross talk with T and B cells in various injury/repair processes and bridge innate and adaptive immunity [114]. It is therefore important to consider the evidence for the presence of adaptive immune cells and relevant molecules in the FBR.
4.1. Participation of adaptive immune cells in the FBR
Several studies have provided evidence for the presence of adaptive immune cells and associated molecules after implantation of biomaterials (Table 2). T cells are reported to play a role in FBR, as multiple studies have shown their recruitment to implantation sites. In terms of the temporal nature of their recruitment, they have been shown to interact with macrophages as early as 2 hours after implantation [67]. In addition, long term studies have suggested their continuous participation. In one study, T cell subtypes were analyzed 7 days after SC ECM implantation in mice by flow cytometry, and TH cells were found to be the largest subtype [115]. In another study using cage implantation of PEU, PET, and SR polymers in rats, the recruitment of T cell subsets (CD8+, CD4+, and CD4+/CD25+) was evaluated following successive implantations that were two weeks apart [116]. Following the first implantation, 60% of T cells were CD4+ and 40% were CD8+. In addition, CD4+CD25+ T cells were also detected. Two weeks after the second implantation, an increase in T cells and inflammatory cells was observed and that included the empty cage control. A difference in T cell subtypes was not detected, which prompted the investigators to conclude that the increase was due to non-specific recruitment and did not involve memory or other features of adaptive immunity. More detailed information about T cell subtypes in the FBR has been obtained from the analysis of tissues surrounding PP meshes. In a study involving urogynecology procedures, patients were divided into responders and nonresponders based on pain scores following removal (1–6 years implantation) [117]. Analysis included detection of CD8, CD4 (TH) and FOXP3 (Tregs) as well as measurements of fibrosis and cytokines/growth factors. Responders were found to have more FOXP3 (Tregs) and lower TGF-β1. In a separate study involving analysis of seven PP meshes used in hernia repair (median implant duration of 1 year), immunofluorescence microscopy was used to detect CD3+, CD4+ TH, CD8+, and FOXP3+ Treg cells [13]. Specifically, approximately 20% CD4+ TH and 25% FOXP3+ Treg were observed in clusters at the mesh tissue-interface.
Table 2.
Adaptive immune cells and molecules involved in FBR
| Polymer | Tissue | Outcomes post implantation | Model | Duration | Reference | |
|---|---|---|---|---|---|---|
|
| ||||||
| Cells | ||||||
| DC | PLGA PLG |
in vitro subcutaneous |
enhanced DC maturation enrichment of DC around the implant |
in vitro C57BL/6J mice |
24 hours 7 days |
[119] [120] |
| CD3+ T cell | PP mesh | human abdominal wall hernia repair | aggregation around the implant | Clinical | 0.6–6 years | [13] |
| ECM | subcutaneous | ↑ cell level | C57BL/6J mice | 7 days | [115] | |
| CD4+ Th | PEU, PET, SR | subcutaneous | ↑ cell level | Sprague-Dawley rats | 7, 14, 21 days | [116] |
| PP mesh | human abdominal wall hernia repair | aggregation around the implant | Clinical | 0.6–6 years | [13] | |
| CD25+ CD4+ T cell | PEU, PET, SR | subcutaneous | ↑ cell level | Sprague-Dawley rats | 7, 14, 21 days | [116] |
| FOXP3+ Treg | PP mesh PP mesh |
human abdominal wall hernia repair pelvic mesh |
aggregation around the implant recruitment around the implant along with CD8+ T cells |
Clinical Clinical |
0.6–6 years 1 –6 years |
[13] [117], [124] |
| PEU, PET, SR | subcutaneous | ↑ cell level | Sprague-Dawley rats | 7, 14, 21 days | [116] | |
| CD8+ cytotoxic T cell | PDMS (breast implant) | subcutaneous (mammary fat tissue) | ↑ cell level in less textured surface (SmoothSilk) | New Zealand White rabbits, C57BL/6J mice | 3 weeks - 6 months | [141] |
| PP mesh | human abdominal wall hernia repair | aggregation around the implant | Clinical | 0.6–6 years | [13] | |
| PDMS (breast implant) | subcutaneous (mammary fat tissue) | ↑ cell level in highly textured surface | New Zealand White rabbits | 3 weeks - 6 months (up to 1 year) | [141] | |
| NKT cell | PE-PUR | subcutaneous | ↑ cell recruitment | C57BL/6 mice | 7 days | [97] |
| ECM, PCL | muscle | ↑ cell recruitment | C57BL/6J mice | 5 days - 3 weeks | [79] | |
| CD19+ B cell | alginate hydrogel microsphere, polystyrene | intraperitoneal | ↑ cell recruitment | C57BL/6J mice | 28 days | [12] |
| PDMS (breast implant) | subcutaneous (mammary fat tissue) | ↑ cell level in highly textured surface | C57BL/6J mice | 3 weeks - 6 months | [141] | |
| CD20+ B cell | PP meshes | human abdominal wall hernia repair | cluster around foreign body granuloma | Clinical | 0.6–6 years | [13] |
|
| ||||||
| Molecules | ||||||
| IFN-γ | PE-PUR PDMS (breast implant) |
subcutaneous subcutaneous (mammary fat tissue) |
impaired angiogenesis in IFN-γ-deficient mice ↑ expression in highly textured surface |
C57BL/6 mice C57BL/6J mice |
7 days 3 weeks - 6 months |
[97] [141] |
| TNF-α | acetaminophen | intraperitoneal | ↑ expression | C57BL/6 & male BALB/c mice | 1 day | [132] |
| PCL | subcutaneous | ↑ expression, promotes IL-17 production | C57BL/6 mice | 6 weeks | [10] | |
| IL-2 | PLGA | intraperitoneal | enhanced activation of CD8+ T cells | C57BL/6 (B6) mice | 60 hours | [133] |
| IL-4 | PEU, PET, SR PDMS (breast implant) |
subcutaneous subcutaneous (mammary fat tissue) |
↑ expression ↑ expression in less textured surface (SmoothSilk) |
Sprague-Dawley rats New Zealand White rabbits |
14 days 3 weeks - 6 months (up to 1 year) |
[80] [141] |
| IL-6 | alginate hydrogel microsphere, polystyrene | intraperitoneal | ↑ expression in plasma | C57BL/6 mice | 1 day | [12], [132] |
| PEU, PET, SR | subcutaneous | ↑ expression | Sprague-Dawley rats | 14 days | [80] | |
| IL-10 | PDMS (breast implant) | subcutaneous (mammary fat tissue) | ↑ expression in less textured surface (SmoothSilk) | New Zealand White rabbits | 3 weeks - 6 months (up to 1 year) | [141] |
| IL-12 | PEI, PLL, cationic dextran, cationic gelatin | intraperitoneal | ↑ expression mediated by TLR-4 in macrophages and spleen cells | C57BL/6J mice | 24 hours | [134] |
| PEU, PET, SR | subcutaneous | ↑ expression | Sprague-Dawley rats | 14 days | [80] | |
| IL-13 | amphiphilic PUR | subcutaneous | ↑ expression | Sprague-Dawley rats | 21 days | [15] |
| PDMS (breast implant) | subcutaneous (mammary fat tissue) | ↑ expression in less textured surface (SmoothSilk) | New Zealand White rabbits | 3 weeks - 6 months (up to 1 year) | [141] | |
| IL-17 | PCL silicone breast implant |
subcutaneous subcutaneous |
chronic secretion of IL-17, promotes fibrosis ↑ expression |
C57BL/6 mice Clinical |
12 weeks 41 months |
[10] [10] |
| TGF-β1 | nylon mesh PP mesh |
subcutaneous pelvic mesh |
↑ expression ↑ expression |
C57BL/6 mice Clinical |
2–10 weeks 1 –6 years | [16] [117] |
| IL-36γ IL-23 |
PCL PCL |
subcutaneous subcutaneous |
↑ expression, associated with IL-17 and F2 macrophages ↑ expression, promotes IL-17 production |
C57BL/6 mice C57BL/6 mice |
12 weeks 6 weeks |
[125] [10] |
| CXCL13 | alginate hydrogel microsphere, polystyrene | intraperitoneal | ↑ expression | C57BL/6 mice, non-human primates (NHPs) | 14 days | [12] |
| CXCL10 | PDMS (breast implant) | subcutaneous (mammary fat tissue) | ↑ expression in highly textured surface | C57BL/6J mice | 3 weeks - 6 months | [141] |
| STAT1 | PDMS (breast implant) | subcutaneous (mammary fat tissue) | ↑ expression in highly textured surface | C57BL/6J mice | 3 weeks - 6 months | [141] |
| CSF1R | alginate hydrogel microsphere, polystyrene | intraperitoneal / subcutaneous | ↑ expression with infiltrating macrophages and FBGCs adjacent to the implantation | C57BL/6 mice, non-human primates (NHPs) | 28 days | [12] |
Immunosuppression was also detected around breast implant materials with specific topologies in both rabbit and mouse models [141]. According to Doloff et al. (2021), FOXP3+ Treg cells increased around the SmoothSilk (4μm) breast implants using poly(dimethylsiloxane) (PDMS) in New Zealand White rabbits after up to 6 months of implantation in mammary fat pad tissues, when compared to materials with higher surface roughness. Experiments showed similar results in C57BL/6 wild-type mice. Up to 6 months after implantation of miniaturized SmoothSilk breast implant materials (4μm) into the subcutaneous mammary fat pad space of mice, showed significantly increased level of FOXP3+ Treg cell expression, with reduced fibrosis. When T cell and B cell responses to different surface topography in mice were examined, increased and decreased proportions of T cells and B cells were found to be associated with highly textured surfaces (90μm) and less textured surfaces (4μm), respectively. Specifically, CD3+CD8+ cytotoxic T cells were increased in response to highly textured implants. While the exact mechanism(s) of T cell activation and overall participation in the FBR remain unknown and the quantification of cells is inconsistent, it is possible to conclude that T cells participate in both the early and late stages of the FBR.
B cells are known to participate in wound healing but their participation in the FBR has not been extensively reported. Recently, studies have provided evidence of increased B cell recruitment in FBR. Specifically, a study showed increased B cell number in tissue at 5 days and 1–3 weeks post implantation of polyester PCL in comparison to saline-treated mice [79]. In patients implanted with PP mesh for hernia repair (1 year median), CD20+ B cells were observed by immunofluorescence at the mesh-tissue interface but at much lower frequency than T cells [13]. In a separate study, CD19+ B cells were detected in a mouse model 28 days following SC implantation of alginate microspheres and polystyrene materials [12].
In addition to T cells and B cells that are the major cellular components of adaptive immune response, DCs that primarily function in innate immune response are also essential for adaptive immune response initiation especially in stimulating naïve T cells following uptake, processing and presenting antigens [118]. Studies have also shown enhanced DC maturation and enrichment around implanted PLGA and PLG in both cell culture (5 days post introduction) and a mice model with SC implantation (7 days post procedure), indicating that DCs bridge innate and adaptive immunity [119, 120]. The possible role of DCs in the FBR has been discussed in recent reviews [51, 121].
4.2. Participation of adaptive immune molecules in the FBR
Several molecular determinants of adaptive immune responses have been identified in the FBR to polymers (Table 2). Key molecules in the adaptive immune response, including IL-2, IL-4, IL-6, IL-10, IL-12, IL-13, IL-17, IL-23, and TGF-β1 have been shown to be induced in the FBR, suggesting their possible involvement [9]. For example, IL-6 participates in the cross-talk of naive CD4+ T cells with TH17 cells and has been shown to be increased in sarcoidosis patients that also display increased CD4+ T cells compared to healthy controls [122]. IL-23 functions in a similar manner and has been detected in induced activated CD4+ T cells from mice spleen tissue [123]. IL-10, which can be produced by TH17 cells, has been detected in the FBR to PEU, PET and SR implants in rats at 14 days [80]. Other factors implicated in adaptive immunity and observed in the FBR include IL-4, IFN-γ, and TGF-β. Specifically, IL-4 and IL-13 were detected in the FBR in numerous studies and have been shown to be critical for macrophage fusion. In one study, IL-4 and IL-13 expression level increased after PEU, PET, and SR implantation over 14 days [80]. Results from another study also revealed that both IL-13 and connective tissue growth factor (CTGF) levels increased upon SC implantation of amphiphilic PUR [15]. IFN-γ has complex functions in immunity and can be produced by TH1, B cells, and NKT cells and has been detected in the introduction of PE-PUR sponges, while mice deficient in IFN-γ also showed impaired angiogenesis [97]. Similarly, TGF-β can be produced by multiple cell types including Treg and has been induced in PP mesh implants of patients [117] as well as nylon mesh implants in mice [16]. Clinical histology of patients with 3–5 years of implanted pelvic mesh further demonstrated the recruitment of FOXP3+ Treg and TGF-β1 on the mesh-tissue boundary though not as much as CD4+ T cells, indicating the regulation of inflammation in FBR [124].
Immunoinhibitory cytokines, including IL-4, IL-10, IL-13, IL-25 were shown to be increased in response to SmoothSilk (4μm) breast implants in New Zealand White rabbits after 6 months by NanoString multiplexed gene-expression analysis [141]. On the other hand, pro-inflammatory cytokines such as IL-1, IL-6, IL-26, and TNF-α were detected to have reduced levels in comparison to highly rough implants (90 μm). Consistent with the findings in rabbits, implants with 90 μm features induced higher expression of pro-inflammatory transcription factor STAT1, various cytokines such as IFN-γ, and chemokine CXCL10 according to single-cell RNA seq results. T cell stimulatory receptor gene IL-27ra was also upregulated in both rabbits and mice implanted with highly textured implants.
Recently, pro-inflammatory cytokine IL-17 which is secreted by TH17 was identified to mediate inflammation and fibrosis in FBR after PCL implantation, and results showed that chronic IL-17 secretion promotes fibrosis. Associated with IL-17, IL-36γ produced by macrophages is also a biomarker for FBR, which was shown to increase after PCL implantation [125]. Other inflammation biomarkers such as CXCL13 and colony stimulating factor-1 receptor (CSF1R) are known to increase significantly after alginate hydrogel or polystyrene implantation in both mice models and non-human primates, which leads to the B cell adaptive immune response as mentioned above [12].
4.3. Involvement of antibodies in the FBR to polymers
B cells contribute to adaptive immunity by secreting antibodies and their production in response to polymers has been argued for several decades. Early studies, now considered erroneous, suggested the formation of antibodies specific for silicone breast implants [126]. The production of less specific antibodies, termed anti-polymer antibodies (APAs) has also been suggested but their characterization is limited and their existence has not been confirmed [127]. In contrast to these mostly unsubstantiated reports, antibodies specific for polymeric PEG have been well described in respect to its structure and mechanism [50, 128]. Studies showed that PEG-drug formulations can induce the formation of anti-PEG antibodies that contribute to accelerated drug clearance and cause hypersensitivity reactions [129]. It is therefore important to consider the antigenic structures in PEG and the possible presence of similar moieties in other polymers. PEGs are flexible polymers that can be used to form linear or branched structures with variable end groups to facilitate linkage to other molecules. Their antigenicity depends on conjugation to other macromolecules and the Ab response involves a combination of differential responses against the individual components of the whole PEGylated nanostructure. Interestingly, free soluble PEG is not immunogenic and there no reports suggesting formation of anti-PEG Abs following implantation of bulk material. Moreover, backbone epitopes and terminal end-groups induce the production of antibodies with weaker and stronger binding affinities, respectively. Finally, it is also known that PEGylated drugs and PEGylated nanoparticles induce immune responses via different mechanisms and these differ from those induced by protein or liposome-based vaccines.
It is intriguing to consider the impact of PEG-specific antibodies on health and whether similar observations have been made in patients exposed to other polymers. At this time, there are no reports that include characterization of specific antibody responses towards implanted polymers, suggesting that PEG might represent a unique occurrence. However, the induction of anti-PEG antibodies in a conformation-specific manner raises the possibility that degradation of bulk polymers could result in the release of byproducts capable of inducing antibody formation. Polymer degradation has been linked to increased innate immune responses [147], but an association with adaptive responses has yet to be made. In the case of PEG-antibodies, hypersensitivity reactions were the most significant clinical outcome and they were similar to those observed in response to liposomal drugs and a variety of other molecules. It appears that similar responses have been reported in response to adhesive components (isobornyl acrylate (IBOA)) found in the skin-contacting parts of glucose sensors and insulin pumps. At this time, there are no reports that include characterization of specific antibody responses towards implanted polymers, suggesting that PEG might represent a unique occurrence.
5. Conclusions and future perspectives
Adaptive immune cells and relevant molecules have been detected in the FBR to polymers in humans and experimental animal models, but their contribution to this process remains poorly understood. It is also unclear if their presence necessarily implies their participation as well as induction of adaptive immunity. Studies in mice lacking specific cell types or molecules have produced mixed results with some showing normal responses despite almost complete elimination of adaptive immune cells. However, the development of advanced cell characterization techniques such as multi-plex flow cell sorting and single-cell RNA sequencing has allowed the identification of T cell subtypes that hint at induction of adaptive immunity. Most compelling evidence has been produced by implantation of PCL microparticles in muscle or SC in a mouse model and includes the lack of CD4+ T cells in chimeric animals with OTII-Rag−/− T cells [10]. In the same study, sequential implantation of PCL in muscle and 1 week later in skin resulted in enhanced FBR, which prompted the investigators to suggest the existence of immune memory. Obvious unanswered questions are the nature of the antigenic response as well as the identity of the antigen(s). Perhaps, as suggested by the investigators, it involved low-level chemical derivatization of self-protein by the synthetic material. This is reminiscent of the conformational changes in surface-adsorbed fibrinogen that was shown to display cryptic sites adhesive for macrophages [130]. If this is true, changes in protein conformation induced by polymers could explain the induction of both innate and adaptive immune responses in the FBR. It is intriguing to consider whether polymer properties can influence the nature of the antigenic response.
Despite compelling evidence in animal models, the reality is that millions of patients are exposed to polymers more than once in their lifetimes. Examples include insulin pumps, continuous glucose monitoring sensors, polymer-coated stents, breast implants, pacemaker wires, and many others. Evidence for induction of adaptive immunity and formation of specific IgG molecules in such patients is lacking. More importantly, severe adverse effects following repeated implantations have not been reported. In line with these observations, there are no reports of reduced FBR in immunosuppressed patients. In fact, meta-analysis of patients with dental implants revealed no significant effect of immunocompromised conditions on implant survival [131]. On the other hand, it has been suggested that in individuals with breast implants that leak, silicones can induce autoimmune/inflammatory syndrome by adjuvants leading to increased incidence of allergies, autoimmune diseases, immune deficiencies and lymphomas [135]. Therefore, these observations raise some critical questions that remain to be addressed. First, if exposure to polymer-based implants leads to IgG production what is the nature of the antigen(s). Second, does the site of implantation or the properties of polymer dictate the antigenic response. For example, reduced FBR in an immune privileged site such as the uterine cavity has been described in a mouse model [142]. Last, will attenuation of adaptive immunity in patients improve FBR outcomes such as reduction of inflammation, FBGC formation, and fibrosis. Answering these questions will enhance our understanding of this complex process and could lead to the development of efficient strategies to improve the function and lifespan of polymer-based biomaterials.
Acknowledgements
This work was supported by NIH grant DK115969 (T R K). We thank Dr. Aaron Morris (U. of Michigan) for critical reading of the manuscript and helpful suggestions and comments. Figures 2, 3, and 4 were created with BioRender software.
Abbreviations
- APAs
anti-polymer antibodies
- ASC
Apoptosis-associated speck-like protein containing a C-terminal caspase recruitment domain
- αSMA
alpha smooth muscle actin
- bFGF
basic fibroblast growth factor
- CCL
chemokine (C-C motif) ligand
- CD
cluster of differentiation
- Col
collagen
- CSF1R
colony stimulating factor-1 receptor
- CTGF
connective tissue growth factor
- CXCL
chemokine (C-X-C motif) ligand
- DAMP
damage-associated molecular pattern
- DCs
dendritic cells
- ECM
extracellular matrix
- ELISA
enzyme-linked immunosorbent assay
- FACS
fluorescence-activated cell sorting
- FBGC
foreign body giant cell
- FBR
foreign body reaction
- FOXP3
Forkhead box protein P3
- GFP
green fluorescent protein
- HA
hydroxyapatite
- H&E
hematoxylin and eosin
- IBOA
isobornyl acrylate
- IgG
immunoglobulin
- IL
interleukin
- ILCs
innate lymphoid cells
- IFN
interferon
- iNOS
Inducible nitric oxide synthase
- IVC
inferior vena cava
- KO
knockout
- MHC
major histocompatibility complex
- MMP
matrix metalloproteinases
- MPO
Myeloperoxidase
- MyD88
myeloid differentiation primary response gene 88
- mPCL-CaP
medical grade polycaprolactone-tricalcium phosphate
- NAG
N-acetylglucosaminidase
- NK
natural killer
- NOD
non-obese diabetic
- OVA
ovalbumin
- PA
polyamide
- PCL
polycaprolactone
- PDGF
platelet-derived growth factor
- PE
polyether
- PEG
polyethylene glycol
- PET
polyethylene terephthalate
- PEU
polyether urethane
- PEUU A’
poly(etherurethane urea) PGA: polyglycolic acid
- pHEMA
poly(2-hydroxyethyl methacrylate)
- PLA
polylactic acid
- PLL
polylysine
- PLLA
poly-L-lactic acid
- PLG
polylactide-co-glycolide
- PLGA
poly(lactic-co-glycolic acid)
- PP
polypropylene
- PPF
poly(propylene fumarate)
- PTFE
Polytetrafluoroethylene
- PUR
polyurethane
- PVA
polyvinyl alcohol
- SC
subcutaneous(ly)
- SCID
severe combined immunodeficient
- SDS-PAGE
sodium dodecyl sulphate–polyacrylamide gel electrophoresis
- SEM
scanning electron microscopy
- SR
silicon rubber
- TGF
transforming growth factor
- TH
T helper cell
- TLR
toll-like receptor
- Tregs
regulatory T cells
- TNF
tumor necrosis factor
- VEGF
vascular endothelial growth factor
- VWF
von Willebrand factor
- YAP
Yes-associated protein
- WT
wild-type
References
- [1].Yu T, Tutwiler VJ and Spiller K 2015. The Role of Macrophages in the Foreign Body Response to Implanted Biomaterials Biomaterials in Regenerative Medicine and the Immune System (Cham: Springer International Publishing; ) [Google Scholar]
- [2].Klopfleisch R and Jung F 2017. The pathology of the foreign body reaction against biomaterials J Biomed Mater Res A. 105 927–40 [DOI] [PubMed] [Google Scholar]
- [3].Ma M, Liu WF, Hill PS, Bratlie KM, Siegwart DJ, Chin J, Park M, Guerreiro J and Anderson DG 2011. Development of cationic polymer coatings to regulate foreign-body responses Adv Mater. 23 H189–94 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Simionescu BC and Ivanov D 2014. Handbook of Bioceramics and Biocomposites (Cham: Springer International Publishing; ) [Google Scholar]
- [5].Festas A, Ramos A and Davim J 2020. Medical devices biomaterials – A review Proceedings of the Institution of Mechanical Engineers, Part L: Journal of Materials: Design and Applications. 234 218–28 [Google Scholar]
- [6].Manam NS, Harun WSW, Shri DNA, Ghani SAC, Kurniawan T, Ismail MH and Ibrahim MHI 2017. Study of corrosion in biocompatible metals for implants: A review J Alloy Compd. 701 698–715 [Google Scholar]
- [7].Sheikh Z, Brooks PJ, Barzilay O, Fine N and Glogauer M 2015. Macrophages, Foreign Body Giant Cells and Their Response to Implantable Biomaterials Materials (Basel). 8 5671–701 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Anderson JM, Rodriguez A and Chang DT 2008. Foreign body reaction to biomaterials Semin Immunol. 20 86–100 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Adusei KM, Ngo TB and Sadtler K 2021. T lymphocytes as critical mediators in tissue regeneration, fibrosis, and the foreign body response Acta Biomater. [DOI] [PubMed]
- [10].Chung L et al. 2020. Interleukin 17 and senescent cells regulate the foreign body response to synthetic material implants in mice and humans Sci Transl Med. 12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Babensee JE 2008. Interaction of dendritic cells with biomaterials Semin Immunol. 20 101–8 [DOI] [PubMed] [Google Scholar]
- [12].Doloff JC et al. 2017. Colony stimulating factor-1 receptor is a central component of the foreign body response to biomaterial implants in rodents and non-human primates Nat Mater. 16 671–80 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Dievernich A, Achenbach P, Davies L and Klinge U 2021. Characterization of innate and adaptive immune cells involved in the foreign body reaction to polypropylene meshes in the human abdomen Hernia. [DOI] [PMC free article] [PubMed]
- [14].Lynn AD, Blakney AK, Kyriakides TR and Bryant SJ 2011. Temporal progression of the host response to implanted poly(ethylene glycol)-based hydrogels J Biomed Mater Res A. 96 621–31 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Ward WK, Li AG, Siddiqui Y, Federiuk IF and Wang XJ 2008. Increased expression of Interleukin-13 and connective tissue growth factor, and their potential roles during foreign body encapsulation of subcutaneous implants J Biomater Sci Polym Ed. 19 1065–72 [DOI] [PubMed] [Google Scholar]
- [16].Higgins DM, Basaraba RJ, Hohnbaum AC, Lee EJ, Grainger DW and Gonzalez-Juarrero M 2009. Localized immunosuppressive environment in the foreign body response to implanted biomaterials Am J Pathol. 175 161–70 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Bracaglia LG, Winston S, Powell DA and Fisher JP 2019. Synthetic polymer coatings diminish chronic inflammation risk in large ECM-based materials J Biomed Mater Res A. 107 494–504 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Moussavian MR, Laschke MW, Schlachtenberger G, Von Heesen M, Wagner M, Glanemann M and Menger MD 2016. Perigraft vascularization and incorporation of implanted Dacron prostheses are affected by rifampicin coating J Vasc Surg. 64 1815–24 [DOI] [PubMed] [Google Scholar]
- [19].Kim J 2020. Systematic approach to characterize the dynamics of protein adsorption on the surface of biomaterials using proteomics Colloids Surf B Biointerfaces. 188 110756 [DOI] [PubMed] [Google Scholar]
- [20].Franz S, Rammelt S, Scharnweber D and Simon JC 2011. Immune responses to implants - a review of the implications for the design of immunomodulatory biomaterials Biomaterials. 32 6692–709 [DOI] [PubMed] [Google Scholar]
- [21].Andorko JI and Jewell CM 2017. Designing biomaterials with immunomodulatory properties for tissue engineering and regenerative medicine Bioeng Transl Med. 2 139–55 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Zhang D, Chen Q, Shi C, Chen M, Ma K, Wan J and Liu R 2021 Dealing with the Foreign-Body Response to Implanted Biomaterials: Strategies and Applications of New Materials Adv Funct Mater. 2021, 31, 2007226. [Google Scholar]
- [23].Jordan SW, Fligor JE, Janes LE and Dumanian GA 2017. Implant Porosity and the Foreign Body Response Plast Reconstr Surg. 141 103e–12e [DOI] [PubMed] [Google Scholar]
- [24].Matena J et al. 2015. SLM produced porous titanium implant improvements for enhanced vascularization and osteoblast seeding Int J Mol Sci. 16 7478–92 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Loh QL and Choong C 2013. Three-dimensional scaffolds for tissue engineering applications: role of porosity and pore size Tissue Eng Part B Rev. 19 485–502 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Wang X, Lou T, Zhao W, Song G, Li C and Cui G 2016. The effect of fiber size and pore size on cell proliferation and infiltration in PLLA scaffolds on bone tissue engineering J Biomater Appl. 30 1545–51 [DOI] [PubMed] [Google Scholar]
- [27].Chiu YC, Cheng MH, Engel H, Kao SW, Larson JC, Gupta S and Brey EM 2011. The role of pore size on vascularization and tissue remodeling in PEG hydrogels Biomaterials. 32 6045–51 [DOI] [PubMed] [Google Scholar]
- [28].Sussman EM, Halpin MC, Muster J, Moon RT and Ratner BD 2013. Porous implants modulate healing and induce shifts in local macrophage polarization in the foreign body reaction Ann Biomed Eng. 42 1508–16 [DOI] [PubMed] [Google Scholar]
- [29].Tylek T, Blum C, Hrynevich A, Schlegelmilch K, Schilling T, Dalton PD and Groll J 2019. Precisely defined fiber scaffolds with 40 mum porosity induce elongation driven M2-like polarization of human macrophages Biofabrication. 12 025007 [DOI] [PubMed] [Google Scholar]
- [30].Rostam HM et al. 2020. Immune-Instructive Polymers Control Macrophage Phenotype and Modulate the Foreign Body Response In Vivo Matter. 2 1564–81 [Google Scholar]
- [31].Schoenenberger AD et al. 2020. Macromechanics and polycaprolactone fiber organization drive macrophage polarization and regulate inflammatory activation of tendon in vitro and in vivo Biomaterials. 249 120034 [DOI] [PubMed] [Google Scholar]
- [32].Schoenenberger AD, Foolen J, Moor P, Silvan U and Snedeker JG 2018. Substrate fiber alignment mediates tendon cell response to inflammatory signaling Acta Biomater. 71 306–17 [DOI] [PubMed] [Google Scholar]
- [33].Lamichhane S, Anderson JA, Vierhout T, Remund T, Sun H and Kelly P 2017. Polytetrafluoroethylene topographies determine the adhesion, activation, and foreign body giant cell formation of macrophages J Biomed Mater Res A. 105 2441–50 [DOI] [PubMed] [Google Scholar]
- [34].Carnicer-Lombarte A. Mechanical matching of implant to host minimises foreign body reaction. bioRxiv. 829648. 2019.
- [35].Meli VS et al. 2020. YAP-mediated mechanotransduction tunes the macrophage inflammatory response Sci Adv. 6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [36].Jansen LE, Amer LD, Chen EY, Nguyen TV, Saleh LS, Emrick T, Liu WF, Bryant SJ and Peyton SR 2018. Zwitterionic PEG-PC Hydrogels Modulate the Foreign Body Response in a Modulus-Dependent Manner Biomacromolecules. 19 2880–88 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [37].Mariani E, Lisignoli G, Borzi RM and Pulsatelli L 2019. Biomaterials: Foreign Bodies or Tuners for the Immune Response? Int J Mol Sci. 20 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [38].Visalakshan RM et al. 2019. Biomaterial Surface Hydrophobicity-Mediated Serum Protein Adsorption and Immune Responses ACS Appl Mater Interfaces. 11 27615–23 [DOI] [PubMed] [Google Scholar]
- [39].Christo SN, Bachhuka A, Diener KR, Mierczynska A, Hayball JD and Vasilev K 2016. The Role of Surface Nanotopography and Chemistry on Primary Neutrophil and Macrophage Cellular Responses Adv Healthc Mater. 5 956–65 [DOI] [PubMed] [Google Scholar]
- [40].Ngo BKD and Grunlan MA 2017. Protein Resistant Polymeric Biomaterials ACS Macro Letters. 6 992–1000 [DOI] [PubMed] [Google Scholar]
- [41].Andrade JD and Hlady V 1987. Plasma protein adsorption: the big twelve Ann N Y Acad Sci. 516 158–72 [DOI] [PubMed] [Google Scholar]
- [42].Penna MJ, Mijajlovic M and Biggs MJ 2014. Molecular-level understanding of protein adsorption at the interface between water and a strongly interacting uncharged solid surface J Am Chem Soc. 136 5323–31 [DOI] [PubMed] [Google Scholar]
- [43].Sivaraman B and Latour RA 2009. The relationship between platelet adhesion on surfaces and the structure versus the amount of adsorbed fibrinogen Biomaterials. 31 832–9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [44].Lishko VK, Kudryk B, Yakubenko VP, Yee VC and Ugarova TP 2002. Regulated unmasking of the cryptic binding site for integrin alpha M beta 2 in the gamma Cdomain of fibrinogen Biochemistry. 41 12942–51 [DOI] [PubMed] [Google Scholar]
- [45].Horbett TA and Latour RA 2020. 2.1.2 - Adsorbed Proteins on Biomaterials Biomaterials Science: An Introduction to Materials in Medicine (Amsterdam: Academic Press; ) [Google Scholar]
- [46].Zdolsek J, Eaton JW and Tang L 2007. Histamine release and fibrinogen adsorption mediate acute inflammatory responses to biomaterial implants in humans J Transl Med. 5 31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [47].Brown BN, Londono R, Tottey S, Zhang L, Kukla KA, Wolf MT, Daly KA, Reing JE and Badylak SF 2011. Macrophage phenotype as a predictor of constructive remodeling following the implantation of biologically derived surgical mesh materials Acta Biomater. 8 978–87 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [48].Witherel CE, Abebayehu D, Barker TH and Spiller KL 2019. Macrophage and Fibroblast Interactions in Biomaterial-Mediated Fibrosis Adv Healthc Mater. 8 e1801451 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [49].Moore LB and Kyriakides TR 2015. Molecular Characterization of Macrophage-Biomaterial Interactions Adv Exp Med Biol. 865 109–22 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [50].Kozma GT, Shimizu T, Ishida T and Szebeni J 2020. Anti-PEG antibodies: Properties, formation, testing and role in adverse immune reactions to PEGylated nanobiopharmaceuticals Adv Drug Deliv Rev. 154–155 163–75 [DOI] [PubMed]
- [51].Keselowsky BG and Lewis JS 2017. Dendritic cells in the host response to implanted materials Semin Immunol. 29 33–40 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [52].Abramson J and Husebye ES 2016. Autoimmune regulator and self-tolerance - molecular and clinical aspects Immunol Rev. 271 127–40 [DOI] [PubMed] [Google Scholar]
- [53].Iwasaki A and Medzhitov R 2015. Control of adaptive immunity by the innate immune system Nat Immunol. 16 343–53 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [54].Chaplin DD 2010. Overview of the immune response J Allergy Clin Immunol. 125 S3–23 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [55].Murphy K and Weaver C 2017. Janeway’s Immunobiology, 9th Edition (New York: Garland Science; ) [Google Scholar]
- [56].Keselowsky BG, Acharya A and Lewis JS 2020. 2.2.3 - Innate and Adaptive Immunity: The Immune Response to Foreign Materials Biomaterials Science: An Introduction to Materials in Medicine (Amsterdam: Academic Press; ) [Google Scholar]
- [57].Marshall JS, Warrington R, Watson W and Kim HL 2018. An introduction to immunology and immunopathology Allergy Asthma Clin Immunol. 14 49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [58].Nicholson LB 2016. The immune system Essays Biochem. 60 275–301 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [59].Farber DL, Netea MG, Radbruch A, Rajewsky K and Zinkernagel RM 2016. Immunological memory: lessons from the past and a look to the future Nat Rev Immunol. 16 124–8 [DOI] [PubMed] [Google Scholar]
- [60].Yatim KM and Lakkis FG 2015. A brief journey through the immune system Clin J Am Soc Nephrol. 10 1274–81 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [61].Medina KL 2016. Overview of the immune system Handb Clin Neurol. 133 61–76 [DOI] [PubMed] [Google Scholar]
- [62].Abbas AK, Lichtman AH and Pillai S 2020. Basic Immunology: Functions and Disorders of the Immune System (Philadelphia: Elsevier; ) [Google Scholar]
- [63].Goswami R and Awasthi A 2020. Editorial: T Cell Differentiation and Function in Tissue Inflammation Front Immunol. 11 289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [64].Biron C 2016. Chapter 4 - Innate Immunity: Recognizing and Responding to Foreign Invaders—No Training Needed Viral Pathogenesis: From Basics to Systems Biology (Amsterdam: Academic Press; ) [Google Scholar]
- [65].Vanguri VK 2014. The Adaptive Immune System Pathobiology of Human Disease: A Dynamic Encyclopedia of Disease Mechanisms (Amsterdam: Academic Press; ) [Google Scholar]
- [66].Xia Z and Triffitt JT 2006. A review on macrophage responses to biomaterials Biomed Mater. 1 R1–9 [DOI] [PubMed] [Google Scholar]
- [67].Brodbeck WG, Macewan M, Colton E, Meyerson H and Anderson JM 2005. Lymphocytes and the foreign body response: lymphocyte enhancement of macrophage adhesion and fusion J Biomed Mater Res A. 74 222–9 [DOI] [PubMed] [Google Scholar]
- [68].Macewan MR, Brodbeck WG, Matsuda T and Anderson JM 2005. Monocyte/lymphocyte interactions and the foreign body response: in vitro effects of biomaterial surface chemistry J Biomed Mater Res A. 74 285–93 [DOI] [PubMed] [Google Scholar]
- [69].Chang DT, Jones JA, Meyerson H, Colton E, Kwon IK, Matsuda T and Anderson JM 2008. Lymphocyte/macrophage interactions: biomaterial surface-dependent cytokine, chemokine, and matrix protein production J Biomed Mater Res A. 87 676–87 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [70].Anderson JM and Mcnally AK 2011. Biocompatibility of implants: lymphocyte/macrophage interactions Semin Immunopathol. 33 221–33 [DOI] [PubMed] [Google Scholar]
- [71].Iwata Y et al. 2009. CD19, a response regulator of B lymphocytes, regulates wound healing through hyaluronan-induced TLR4 signaling Am J Pathol. 175 649–60 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [72].Landen NX, Li D and Stahle M 2016. Transition from inflammation to proliferation: a critical step during wound healing Cell Mol Life Sci. 73 3861–85 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [73].Debes GF and Mcgettigan SE 2019. Skin-Associated B Cells in Health and Inflammation J Immunol. 202 1659–66 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [74].Bhogal RK and Bona CA 2005. B cells: no longer bystanders in liver fibrosis J Clin Invest. 115 2962–5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [75].Wynn TA 2008. Cellular and molecular mechanisms of fibrosis J Pathol. 214 199–210 IL-4 secretion [DOI] [PMC free article] [PubMed] [Google Scholar]
- [76].Zhu F, Bai X and Chen X 2017. B lymphocytes in renal interstitial fibrosis J Cell Commun Signal. 11 213–8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [77].Pillai S 2019. T and B lymphocytes in fibrosis and systemic sclerosis Curr Opin Rheumatol. 31 576–81 [DOI] [PubMed] [Google Scholar]
- [78].Della-Torre E et al. 2020. B lymphocytes directly contribute to tissue fibrosis in patients with IgG4-related disease J Allergy Clin Immunol. 145 968–81 e14 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [79].Moore EM, Maestas DR, Cherry CC, Garcia JA, Comeau HY, Huyer LD, Blosser RL, Rosson GD and Elisseeff JH 2021. Biomaterials direct functional B cell response in a material specific manner bioRxiv. 2021.01.12.426347 [DOI] [PMC free article] [PubMed]
- [80].Rodriguez A, Macewan SR, Meyerson H, Kirk JT and Anderson JM 2009. The foreign body reaction in T-cell-deficient mice J Biomed Mater Res A. 90 106–13 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [81].Dondossola E, Holzapfel BM, Alexander S, Filippini S, Hutmacher DW and Friedl P 2016. Examination of the foreign body response to biomaterials by nonlinear intravital microscopy Nat Biomed Eng. 1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [82].Yang X, Yang F, Walboomers XF, Bian Z, Fan M and Jansen JA 2010. The performance of dental pulp stem cells on nanofibrous PCL/gelatin/nHA scaffolds J Biomed Mater Res A. 93 247–57 [DOI] [PubMed] [Google Scholar]
- [83].ang J, Jao B, Mcnally AK and Anderson JM 2014. In vivo quantitative and qualitative assessment of foreign body giant cell formation on biomaterials in mice deficient in natural killer lymphocyte subsets, mast cells, or the interleukin-4 receptoralpha and in severe combined immunodeficient mice J Biomed Mater Res A. 102 2017–23 [DOI] [PubMed] [Google Scholar]
- [84].Roh JD et al. 2008. Small-diameter biodegradable scaffolds for functional vascular tissue engineering in the mouse model Biomaterials. 29 1454–63 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [85].Qian J, Park DJ, Perrott S, Patel P and Eliceiri BP 2021. Genetic Background and Kinetics Define Wound Bed Extracellular Vesicles in a Mouse Model of Cutaneous Injury Int J Mol Sci. 22 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [86].Tang L, Lucas AH and Eaton JW 1993. Inflammatory responses to implanted polymeric biomaterials: role of surface-adsorbed immunoglobulin G J Lab Clin Med. 122 292–300 [PubMed] [Google Scholar]
- [87].Jung A, Kato H, Kumagai Y, Kumar H, Kawai T, Takeuchi O and Akira S 2008. Lymphocytoid choriomeningitis virus activates plasmacytoid dendritic cells and induces a cytotoxic T-cell response via MyD88 J Virol. 82 196–206 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [88].Rahman AH, Cui W, Larosa DF, Taylor DK, Zhang J, Goldstein DR, Wherry EJ, Kaech SM and Turka LA 2008. MyD88 plays a critical T cell-intrinsic role in supporting CD8 T cell expansion during acute lymphocytic choriomeningitis virus infection J Immunol. 181 3804–10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [89].Zhou S, Kurt-Jones EA, Cerny AM, Chan M, Bronson RT and Finberg RW 2009. MyD88 intrinsically regulates CD4 T-cell responses J Virol. 83 1625–34 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [90].Loures FV, Pina A, Felonato M, Feriotti C, De Araujo E F and Calich VL 2011. MyD88 signaling is required for efficient innate and adaptive immune responses to Paracoccidioides brasiliensis infection Infect Immun. 79 2470–80 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [91].Deguine J and Barton GM 2014. MyD88: a central player in innate immune signaling F1000Prime Rep. 6 97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [92].Oliveira AC et al. 2017. Crucial role for T cell-intrinsic IL-18R-MyD88 signaling in cognate immune response to intracellular parasite infection Elife. 6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [93].Kang SM, Yoo DG, Kim MC, Song JM, Park MK, O E, Quan FS, Akira S and Compans RW 2011. MyD88 plays an essential role in inducing B cells capable of differentiating into antibody-secreting cells after vaccination J Virol. 85 11391–400 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [94].Matsushita K and Yoshimoto T 2014. B cell-intrinsic MyD88 signaling is essential for IgE responses in lungs exposed to pollen allergens J Immunol. 193 5791–800 [DOI] [PubMed] [Google Scholar]
- [95].Tian M et al. 2018. B Cell-Intrinsic MyD88 Signaling Promotes Initial Cell Proliferation and Differentiation To Enhance the Germinal Center Response to a Virus-like Particle J Immunol. 200 937–48 [DOI] [PubMed] [Google Scholar]
- [96].Amer LD, Saleh LS, Walker C, Thomas S, Janssen WJ, Alper S and Bryant SJ 2019. Inflammation via myeloid differentiation primary response gene 88 signaling mediates the fibrotic response to implantable synthetic poly(ethylene glycol) hydrogels Acta Biomater. 100 105–17 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [97].Cassini-Vieira P, De Carvalho Santuchi M, Da Silva R F, Russo RC, Araujo FA, Dos Santos R a S, Andrade SP, Teixeira MM and Barcelos LS 2018. Lack of interferon-gamma attenuates foreign body reaction to subcutaneous implants in mice J Biomed Mater Res A. 106 2243–50 [DOI] [PubMed] [Google Scholar]
- [98].Schroder K, Hertzog PJ, Ravasi T and Hume DA 2004. Interferon-gamma: an overview of signals, mechanisms and functions J Leukoc Biol. 75 163–89 [DOI] [PubMed] [Google Scholar]
- [99].Schoenborn JR and Wilson CB 2007. Regulation of interferon-gamma during innate and adaptive immune responses Adv Immunol. 96 41–101 [DOI] [PubMed] [Google Scholar]
- [100].Robb RJ and Hill GR 2012. The interferon-dependent orchestration of innate and adaptive immunity after transplantation Blood. 119 5351–8 [DOI] [PubMed] [Google Scholar]
- [101].Kak G, Raza M and Tiwari BK 2018. Interferon-gamma (IFN-gamma): Exploring its implications in infectious diseases Biomol Concepts. 9 64–79 [DOI] [PubMed] [Google Scholar]
- [102].Castro F, Cardoso AP, Goncalves RM, Serre K and Oliveira MJ 2018. Interferon-Gamma at the Crossroads of Tumor Immune Surveillance or Evasion Front Immunol. 9 847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [103].Lee AJ and Ashkar AA 2018. The Dual Nature of Type I and Type II Interferons Front Immunol. 9 2061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [104].Alspach E, Lussier DM and Schreiber RD 2019. Interferon gamma and Its Important Roles in Promoting and Inhibiting Spontaneous and Therapeutic Cancer Immunity Cold Spring Harb Perspect Biol. 11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [105].Paul WE 2015. History of interleukin-4 Cytokine. 75 3–7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [106].Yoshimoto T 2018. The Hunt for the Source of Primary Interleukin-4: How We Discovered That Natural Killer T Cells and Basophils Determine T Helper Type 2 Cell Differentiation In Vivo Front Immunol. 9 716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [107].Gieseck RL 3rd, Wilson MS and Wynn TA 2018. Type 2 immunity in tissue repair and fibrosis Nat Rev Immunol. 18 62–76 [DOI] [PubMed] [Google Scholar]
- [108].Heeb LEM, Egholm C and Boyman O 2020. Evolution and function of interleukin-4 receptor signaling in adaptive immunity and neutrophils Genes Immun. 21 143–9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [109].Kao WJ, Mcnally AK, Hiltner A and Anderson JM 1995. Role for interleukin-4 in foreign-body giant cell formation on a poly(etherurethane urea) in vivo J Biomed Mater Res. 29 1267–75 [DOI] [PubMed] [Google Scholar]
- [110].Ulrich D et al. 2014. Human endometrial mesenchymal stem cells modulate the tissue response and mechanical behavior of polyamide mesh implants for pelvic organ prolapse repair Tissue Eng Part A. 20 785–98 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [111].Edwards SL, Ulrich D, White JF, Su K, Rosamilia A, Ramshaw JA, Gargett CE and Werkmeister JA 2015. Temporal changes in the biomechanical properties of endometrial mesenchymal stem cell seeded scaffolds in a rat model Acta Biomater. 13 286–94 [DOI] [PubMed] [Google Scholar]
- [112].Moore EM, Maestas DR Jr., Comeau HY and Elisseeff JH 2021. The Immune System and Its Contribution to Variability in Regenerative Medicine Tissue Eng Part B Rev. 27 39–47 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [113].Shanley LC, Mahon OR, Kelly DJ and Dunne A 2021. Harnessing the innate and adaptive immune system for tissue repair and regeneration: Considering more than macrophages Acta Biomater. [DOI] [PubMed]
- [114].Chu C, Liu L, Rung S, Wang Y, Ma Y, Hu C, Zhao X, Man Y and Qu Y 2020. Modulation of foreign body reaction and macrophage phenotypes concerning microenvironment J Biomed Mater Res A. 108 127–35 [DOI] [PubMed] [Google Scholar]
- [115].Sadtler K, Allen BW, Estrellas K, Housseau F, Pardoll DM and Elisseeff JH 2017. The Scaffold Immune Microenvironment: Biomaterial-Mediated Immune Polarization in Traumatic and Nontraumatic Applications Tissue Eng Part A. 23 1044–53 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [116].Rodriguez A, Voskerician G, Meyerson H, Macewan SR and Anderson JM 2008. T cell subset distributions following primary and secondary implantation at subcutaneous biomaterial implant sites J Biomed Mater Res A. 85 556–65 [DOI] [PubMed] [Google Scholar]
- [117].Artsen AM, Liang R, Meyn L, Rytel M, Palcsey S, Abramowitch SD and Moalli PA 2020. T regulatory cells and TGF-beta1: Predictors of the host response in mesh complications Acta Biomater. 115 127–35 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [118].Fu C and Jiang A 2018. Dendritic Cells and CD8 T Cell Immunity in Tumor Microenvironment Front Immunol. 9 3059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [119].Park J and Babensee JE 2012. Differential functional effects of biomaterials on dendritic cell maturation Acta Biomater. 8 3606–17 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [120].Kim J, Li WA, Sands W and Mooney DJ 2014. Effect of pore structure of macroporous poly(lactide-co-glycolide) scaffolds on the in vivo enrichment of dendritic cells ACS Appl Mater Interfaces. 6 8505–12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [121].Zhu FJ, Tong YL, Sheng ZY and Yao YM 2019. Role of dendritic cells in the host response to biomaterials and their signaling pathways Acta Biomater. 94 132–44 [DOI] [PubMed] [Google Scholar]
- [122].Celada LJ et al. 2018. PD-1 up-regulation on CD4(+) T cells promotes pulmonary fibrosis through STAT3-mediated IL-17A and TGF-beta1 production Sci Transl Med. 10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [123].Aggarwal S, Ghilardi N, Xie MH, De Sauvage F J and Gurney AL 2003 Interleukin-23 promotes a distinct CD4 T cell activation state characterized by the production of interleukin-17 J Biol Chem. 278 1910-4 [DOI] [PubMed] [Google Scholar]
- [124].Artsen AM, Rytel M, Liang R, King GE, Meyn L, Abramowitch SD and Moalli PA 2019. Mesh induced fibrosis: The protective role of T regulatory cells Acta Biomater. 96 203–10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [125].Sommerfeld SD et al. 2019. Interleukin-36gamma-producing macrophages drive IL-17-mediated fibrosis Sci Immunol. 4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [126].Vojdani A, Brautbar N and Campbell AW 1994. Antibody to silicone and native macromolecules in women with silicone breast implants Immunopharmacol Immunotoxicol. 16 497–523 [DOI] [PubMed] [Google Scholar]
- [127].De Jong W H, Kallewaard M, Goldhoorn CA, Verhoef CM, Bijlsma JW, Schouten JS and Van Loveren H 2004. Long-term exposure to silicone breast implants does not induce antipolymer antibodies Biomaterials. 25 1095–103 [DOI] [PubMed] [Google Scholar]
- [128].Huckaby CS, Conneely OM, Beattie WG, Dobson AD, Tsai MJ and O’malley BW 1987. Structure of the chromosomal chicken progesterone receptor gene Proc Natl Acad Sci U S A. 84 8380–4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [129].Lee CC, Su YC, Ko TP, Lin LL, Yang CY, Chang SS, Roffler SR and Wang AH 2020. Structural basis of polyethylene glycol recognition by antibody J Biomed Sci. 27 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [130].Hu WJ, Eaton JW, Ugarova TP and Tang L 2001. Molecular basis of biomaterial-mediated foreign body reactions Blood. 98 1231–8 [DOI] [PubMed] [Google Scholar]
- [131].Duttenhoefer F, Fuessinger MA, Beckmann Y, Schmelzeisen R, Groetz KA and Boeker M 2019. Dental implants in immunocompromised patients: a systematic review and meta-analysis Int J Implant Dent. 5 43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [132].Masubuchi Y, Sugiyama S and Horie T 2009. Th1/Th2 cytokine balance as a determinant of acetaminophen induced liver injury Chem Biol Interact. 179 273–9 [DOI] [PubMed] [Google Scholar]
- [133].Steenblock ER and Fahmy TM 2008. A comprehensive platform for ex vivo T-cell expansion based on biodegradable polymeric artificial antigen-presenting cells Mol Ther. 16 765–72 [DOI] [PubMed] [Google Scholar]
- [134].Chen H, Li P, Yin Y, Cai X, Huang Z, Chen J, Dong L and Zhang J 2010. The promotion of type 1 T helper cell responses to cationic polymers in vivo via toll-like receptor-4 mediated IL-12 secretion Biomaterials. 31 8172– 80 [DOI] [PubMed] [Google Scholar]
- [135].Cohen Tervaert J W, Colaris MJ and Van Der Hulst R R 2017. Silicone breast implants and autoimmune rheumatic diseases: myth or reality Curr Opin Rheumatol. 29 348–54 [DOI] [PubMed] [Google Scholar]
- [136].Whitaker R, Hernaez-Estrada B, Hernandez RM, Santos-Vizcaino E and Spiller KL 2021. Immunomodulatory Biomaterials for Tissue Repair Chem Rev. 121 11305–35 [DOI] [PubMed] [Google Scholar]
- [137].Crawford L, Wyatt M, Bryers J and Ratner B 2021. Biocompatibility Evolves: Phenomenology to Toxicology to Regeneration Adv Healthc Mater. 10 e2002153 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [138].Martin KE and Garcia AJ 2021. Macrophage phenotypes in tissue repair and the foreign body response: Implications for biomaterial-based regenerative medicine strategies Acta Biomater. 133 4–16 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [139].Matlaga BF, Yasenchak LP and Salthouse TN 1976. Tissue response to implanted polymers: the significance of sample shape J Biomed Mater Res. 10 391–7 [DOI] [PubMed] [Google Scholar]
- [140].Zhang L, Cao Z, Bai T, Carr L, Ella-Menye JR, Irvin C, Ratner BD and Jiang S 2013. Zwitterionic hydrogels implanted in mice resist the foreign-body reaction Nat Biotechnol. 31 553–6 [DOI] [PubMed] [Google Scholar]
- [141].Doloff JC et al. 2021. The surface topography of silicone breast implants mediates the foreign body response in mice, rabbits and humans Nat Biomed Eng. 5 1115–30 [DOI] [PubMed] [Google Scholar]
- [142].Hernandez JL, Park J, Yao S, Blakney AK, Nguyen HV, Katz BH, Jensen JT and Woodrow KA 2021. Effect of tissue microenvironment on fibrous capsule formation to biomaterial-coated implants Biomaterials. 273 120806 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [143].Marrazzo P and O’Leary C 2020. Repositioning Natural Antioxidants for Therapeutic Applications in Tissue Engineering Bioengineering. 7 104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [144].Mouthuy PA, Snelling SJB, Dakin SG, Milkovic L, Gasparovic AC, Carr AJ and Zarkovic N 2016. Biocompatibility of implantable materials: An oxidative stress viewpoint Biomaterials. 109 55–68 [DOI] [PubMed] [Google Scholar]
- [145].Jurak M, Wiacek AE, Ladniak A, Przykaza K and Szafran K 2021. What affects the biocompatibility of polymers? Advances in Colloid and Interface Science. 294 102451 [DOI] [PubMed] [Google Scholar]
- [146].Liappas G et al. 2015 T Helper 17/Regulatory T Cell Balance and Experimental Models of Peritoneal Dialysis-Induced Damage Biomed Res Int. 2015. 416480 [DOI] [PMC free article] [PubMed]
- [147].Li C, Guo C, Fitzpatrick V, Ibrahim A, Zwierstra MJ, Hanna P, Lechtig A, Nazarian A, Lin SJ and Kaplan DL 2020. Design of biodegradable, implantable devices towards clinical translation. Nat Rev Mater. 5 61–81 [Google Scholar]