Abstract
Objectives
Greater dietary self‐monitoring adherence is associated with weight loss, however, the dietary self‐monitoring adherence criteria that predict weight loss are unknown. The criteria used to define adherence to dietary self‐monitoring in obesity treatment tend to vary, particularly in studies that include dietary self‐monitoring via mobile applications (apps). The objectives of this study were to (a) determine weight change outcomes related to app‐based dietary self‐monitoring and (b) determine the associations between the frequency, consistency, and completeness of dietary self‐monitoring and weight change.
Methods
In this single‐arm uncontrolled prospective study, employees at a large, urban health system who had overweight or obesity self‐monitored dietary intake for 8 weeks using the Calorie Counter by FatSecret app. A paired sample t‐test examined the association of app‐based dietary self‐monitoring and weight change; linear regression examined the associations of frequent, consistent, and complete dietary self‐monitoring and weight change.
Results
A significant mean difference [t (89) = 6.59, p < 0.001] was found between baseline and 8‐week weight (M = −1.5 ± 2.1 kg) in the sample (N = 90). Linear regression revealed a significant association [F (1, 88) = 7.18, p = 0.009] between total weeks of consistent dietary self‐monitoring (M = 4.4 ± 2.8) and percent weight loss (M = −1.54% ± 2.26%), and a significant association [F (1, 88) = 6.42, p = 0.013] between dietary self‐monitoring frequency (M = 50.1% ± 33.3%) and percent weight loss. The total weeks of complete dietary self‐monitoring (M = 3.42 ± 2.87) was not associated [F (1, 88) = 3.57, p = 0.062] with percent weight loss.
Conclusions
Consistent and frequent app‐based dietary self‐monitoring were associated with short‐term weight loss. Emphasizing these aspects of self‐monitoring may be an avenue for decreasing the burden of self‐monitoring.
Keywords: dietary, dietary adherence, monitoring, obesity, overweight, weight loss
1. INTRODUCTION
Standard obesity treatment includes dietary self‐monitoring (DSM), which entails documenting the details of one's dietary intake with calorie amounts and timing of consumption. 1 DSM with a paper diary is a common behavior modification technique, 1 , 2 and greater self‐monitoring adherence is associated with weight loss. 1 , 2 More frequent self‐monitoring of dietary intake, 2 increased consistency of self‐monitoring, 3 , 4 and increased completeness of self‐monitoring 5 have been associated with weight loss. Adherence to DSM declines over time, however, and barriers to using a paper diary include the tedious nature of self‐monitoring, diary misplacement, manually writing DSM entries, and no immediate feedback. 2 DSM via mobile device applications (apps) is an appealing alternative to conventional self‐monitoring techniques. 6 After recording DSM data into an app, immediate feedback regarding calories, nutrient intake, and progress toward goal achievement is commonly provided. 2 Most Americans (97%) own a mobile phone, and 85% own a smartphone capable of downloading health‐related apps. 7
A recent review found a positive relationship between self‐monitoring via digital modes 2 ; however, less is known about app‐based DSM compared to the large body of knowledge on paper‐based DSM, 8 particularly the relationship between adherence to DSM and weight loss. 9 The criteria used to define adherence to DSM in obesity treatment tend to vary. 10 Adherence to app‐based DSM has been operationally defined in weight loss interventions as a specific recorded amount of calorie intake, the frequency of DSM, or combinations of both. 11 For example, the frequency, consistency, and completeness of DSM are common indicators of adherence. 3 Frequency has been defined as the total number of days and percentage of days over the course of a study that participants were adherent to DSM, 2 , 11 , 12 , 13 , 14 , 15 , 16 while consistency has been defined as documenting ≥three daily DSM records per week. 3 , 4 Regarding completeness, individuals who recorded 50% or more of their weekly calorie goal were considered adherent, while those who recorded less than 50% of their goal were non‐adherent. 17 Considering the lack of consensus on assessment of adherence, 10 , 11 and that the DSM adherence criteria associated with weight loss is unknown, 9 studying multiple indicators 18 of adherence to app‐based DSM, and their respective relationships with weight loss is warranted.
To date, no studies have simultaneously examined the relationships between the frequency, consistency, and completeness of app‐based DSM and weight change in adults with obesity or overweight to determine which DSM adherence indicators may be beneficial for weight loss. The aims of this study were to (a) determine weight change outcomes related to app‐based DSM, and (b) determine the associations between the frequency, consistency, and completeness of DSM and weight change.
2. METHODS
2.1. Research design, setting, and sample
This single‐arm uncontrolled prospective study utilized the Calorie Counter by FatSecret app (Calorie Counter) 18 to examine the relationship between DSM adherence and weight change at 8 weeks among employees at a large health system in an urban Midwestern community. 19 Calorie Counter is the only free commercial publicly available DSM app with a counterpart, Fatsecret Professional, that permits monitoring and export of client data by a health professional. 20 Self‐reported baseline weight, height, and sex were entered into each participant's Calorie Counter account by the research team, and the activity level was set to sedentary, per manufacturer recommendations. 18 Each participant's weekly weight loss goal was set to 0.45 kg (1 pound [lb.]) per week 21 ; thus, the end‐of‐study goal weight was 3.63 kg (8 lbs) less than baseline weight. Calorie Counter then calculated a recommended daily maximum calorie intake goal that displayed on the participant's app dashboard.
Study design was informed by Social Cognitive Theory which posits that human behavior is the outcome of the interaction of personal, behavioral, and environmental influences. 22 An established self‐regulation skill set, achieved through the process of self‐monitoring, goal setting, feedback, self‐reward, and social support, is one of the best predictors of weight loss. 23 , 24 The accuracy and consistency of self‐monitoring are postulated as requirements to change health behavior. 23 , 25
Potential participants accessed the study flier via the employee intranet and provided informed consent via Qualtrics (Version July 2018), an online survey platform. Participants consented to self‐monitoring all food and fluid intake consumed on a daily basis for 8 weeks, as well as their body weight on a weekly basis. Study enrollment took place from March 2019 to June 2019; Figure 1 shows the flow of participants through the study. The sample consisted of 90 adults who met the eligibility criteria including (a) age ≥ 18 years with a body mass index (BMI) ≥ 27.0 kg/m2; (b) the ability to read, write, and understand the English language; (c) access to a mobile device with internet service capable of downloading and using the Calorie Counter app; (d) interest in losing weight; (e) willing to use Calorie Counter to self‐monitor weight and dietary intake; and (f) no weight loss or gain >11.34 kg (25 lbs) during the past 6 months. Exclusion criteria included (a) diagnosed medical conditions that influence weight (e.g., ascites, diabetes, eating disorder, renal failure, schizophrenia, congestive heart failure, cancer); (b) previous bariatric surgery; (c) pregnant, breastfeeding, or planning to become pregnant in the next eight weeks; (d) change in the following medications in the past 6 months‐ Sertraline, corticosteroids (e.g., Prednisone), atypical antipsychotics (e.g., Quetiapine); or (e) currently taking weight loss supplements or Food and Drug Administration (FDA) approved weight loss medications (e.g., phentermine).
FIGURE 1.
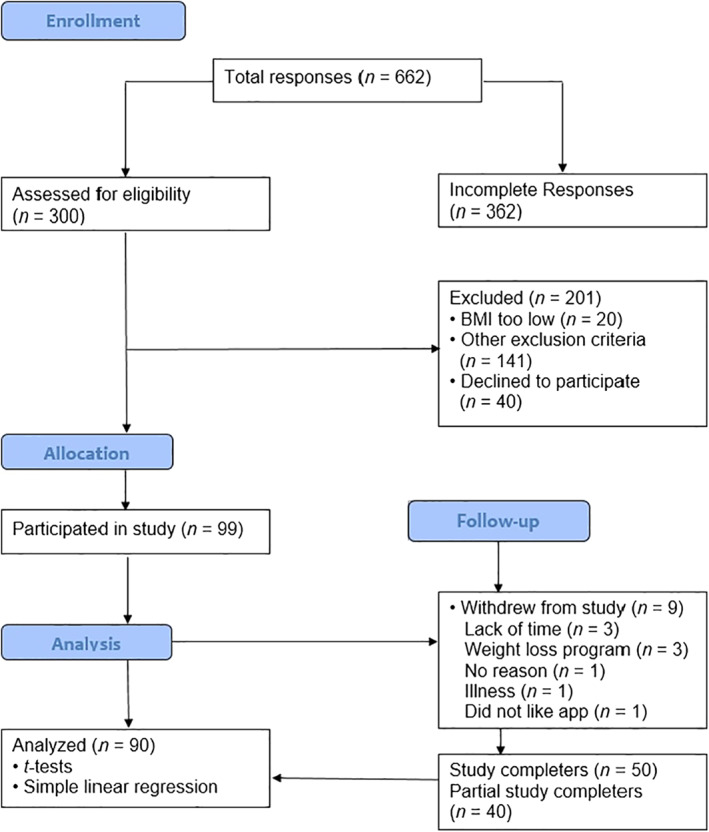
Participant flow chart following the Consolidated Standards of Reporting Trials guidelines; adapted from Schulz 52
Study email accounts created via Google Suite facilitated Calorie Counter setup and email delivery of Focus on Nutrition, 26 a free nutrition and wellness newsletter from Harvard Medical School. Each participant received a welcome email with the Calorie Counter and study email login information and step‐by‐step directions for downloading and logging into Calorie Counter on both Android and Apple devices. Participants could watch a pre‐existing video about the basics of how to use Calorie Counter (Fat Secret App Demo) if interested. 27 Participants received a follow‐up email if no data were recorded after seven days. Participants who only recorded a baseline weight after their first week in the study received an email reminder to record weight on a weekly basis. Participants also automatically received an e‐mail from Calorie Counter every two weeks reminding them to record their weight. Beginning of their third week, participants were instructed how to access the 6‐part Focus on Nutrition email newsletters that were sent during weeks three through eight. The protocol was approved by the Mount Carmel Health Institutional Review Board.
2.2. Data collection
Participant demographic data (sex, race, ethnicity, highest educational level completed, employment status, age, and marital or partner status) were collected via a Qualtrics survey, and self‐monitoring data were collected via Fatsecret Professional. Fatsecret Professional automatically emailed the principal investigator weekly and daily reports of self‐monitoring data recorded by each participant in Calorie Counter. The weekly report included the total number of days during the week that DSM was recorded, the amount of calories consumed per day, and the specific day(s) that weight was recorded with the weight measurement. 18 The daily report included each participant's self‐reported body weight and total intake of calories for the day. 18 Participants received a $20 Amazon e‐gift card via e‐mail after completing self‐reported weight measures at baseline and at the end of eight weeks. At study end, each participant was given the option to keep their Calorie Counter account, which was then disconnected from Fatsecret Professional.
2.3. Measures
Baseline weight was self‐reported in pounds, and baseline height was self‐reported in feet and inches. Self‐reported height and weight have been found to be valid 28 and reliable. 29 BMI was calculated using the formula: weight (pounds) divided by height (inches) 2 multiplied by the conversion factor 703 and expressed in kg/m2. 30 Overweight was defined as a BMI of 25.0 to 29.9 kg/m2, and obesity was defined as a BMI ≥ 30 kg/m2. 30 Final weight was defined as the last weight observation that each participant recorded after the baseline weight. Percent weight change from baseline to 8 weeks was the outcome variable of interest.
Indicators to adherence of DSM for each participant were defined and calculated as follows: frequency—The total number of days that any DSM occurred over the duration of the study, and then as the percentage of days that any DSM occurred over the duration of the study 2 , 11 , 12 , 13 , 14 , 15 , 16 ; consistency—Documentation of greater than or equal to three daily DSM records per week (consistent) or the recording of less than three days of DSM per week (inconsistent) 3 , 4 and then cumulatively defined based on the sum of “consistent” weeks of self‐monitoring (0–8 weeks); completeness—The recording of 50% or more of one's weekly calorie goal (complete) or less than 50% of one's weekly calorie goal (incomplete) for each week. 17 Completeness was then cumulatively defined for each participant as the sum of complete weeks of self‐monitoring (0–8 weeks). The PI also compared the Fatsecret Professional weekly reports for weeks 1, 3, 5, and 7 to the corresponding Fatsecret Professional daily reports for every participant to assess for backlogging of dietary intake. Backlogging was defined as the recording of any DSM data for a date prior to the current day, however this phenomenon was not part of our definitions of frequency, consistency, or completeness.
2.4. Power analysis and sample size
A priori power analysis was conducted with G*Power 31 version 3.1.9.2, using linear multiple regression: fixed model, R 2 deviation from zero. To achieve a power of 0.80, with an alpha level of 0.05, medium effect size of 0.15, three predictor variables, and a two‐tailed test, 77 participants were needed for the study. A previous app‐based DSM weight loss study of three months duration experienced a 24% withdrawal rate. 32 Thus, the ideal sample size for this study was 96 participants to compensate for possible withdrawal. The actual withdraw rate was 9.1%, and post hoc calculated power for this study was 0.87.
2.5. Statistical analysis
The Statistical Package for the Social Sciences (SPSS) Version 25.0 (IBM Corp) was used to analyze the data. The study completers (n = 50) were participants who recorded a baseline weight, any dietary intake, and a weight during the final week eight; partial completers (n = 40) were participants who recorded a baseline weight, any dietary intake, and their last weight observation between baseline and the end of week seven. Withdrawals (n = 9) were participants who wished to discontinue participating in the study; data from those who withdrew were not used in the final analysis. The flow of participants though the study is outlined in Figure 1. The demographics of participants who self‐monitored dietary intake and weight were compared to the demographics of participants who withdrew of the study. The Mann‐Whitney U test revealed no significant differences in age (U = 280.5, z = −1.52, p = 0.129), baseline weight (U = 325.0, z = −0.974, p = 0.330), or baseline BMI (U = 336.0, z = −0.842, p = 0.40), and Fisher's exact test revealed no significant differences in sex (odds ratio [OR] = 0.967, 95% confidence interval [CI] = 0.930, 1.0004, p = 1.0), ethnicity (OR = 0.967, 95% CI = 0.930, 1.0004, p = 1.0), or education (OR = 0.365, CI = 0.086, 1.554, p = 0.18) between participants and withdrawals.
Data analysis for all study participants who did not formally withdrawal were performed with intention‐to‐treat analysis using the last observation carried forward for partial completers. 17 Statistical significance was determined with a p value set at <0.05 (two‐tailed test). 24 Statistical analysis controlled for age and education; older age has been significantly correlated to the recording of more complete days of self‐monitoring 32 and adoption of diet‐related self‐monitoring behaviors may differ according to education level. 33 A dependent samples t‐test was used to explore mean weight change between baseline weight and final weight. 34 Boxplots, normality plots, and casewise diagnostics detected three percent weight change outliers (over two standard deviations from the mean); these outliers were participants who lost a higher percentage of weight compared to the rest of the sample. One outlier was discovered in the consistency variable; the case had no consistent weeks of DSM. Analyses were performed with and without these outliers.
The consistency and completeness of DSM were coded as binary variables (consistent or inconsistent and complete or incomplete, respectively). The frequency of DSM was a continuous variable. The association between each independent variable and percent weight change at eight weeks was first analyzed with Pearson correlations and then simple linear regression. Each independent variable was then entered into the multiple regression equation simultaneously; however, the independent variables were all highly correlated with each other (r > 0.907), which violated the assumption of multicollinearity. Thus, only simple linear regression results are presented for each predictor individually.
3. RESULTS
Tables 1, 2, 3 provide descriptive data of demographic, outcome, and independent variables for the entire sample (N = 90), respectively. The sample had obesity (n = 69; 77%) or overweight (n = 21; 23%), was fully employed, age 42 years on average (±10.1 years), predominantly female (96.7%), White (90%), and not Hispanic or Latino (96.7%). The majority completed a Bachelor's or Associate's degree (61%). Nearly two‐thirds of the sample was married (63%). Over half of the participants (n = 50, 56%) were study completers. The sample had an average BMI of 35.1 kg/m2 (± 6.2 kg/m2), consistent with obesity, and an average baseline weight of 96.4 (± 4.6) kg. Participants self‐monitored diet 28 (±18) days out of 56 days on average. Figure 2 presents the average frequency (days) of DSM per week. Most of the sample (n = 80, 89%) backlogged one or more days of DSM during the study. Most of the partial study completers (75%) recorded a final weight during the first five weeks of the study.
TABLE 1.
Demographic and clinical variable descriptive data (N = 90)
| Variable | Whole sample (N = 90) n (%) | Study completers (n = 50) n (%) | Partial study completers (n = 40) n (%) |
|---|---|---|---|
| Gender | |||
| Male | 3 (3.3) | 3 (6) | 0 |
| Female | 87 (96.7) | 47 (94) | 40 (100) |
| Race | |||
| Black | 7 (7.8) | 3 (6) | 4 (10) |
| White | 81 (90) | 47 (94) | 34 (85) |
| Other | 2 (2.2) | 0 | 2 (5) |
| Ethnicity | |||
| Not Hispanic/Latino | 87 (96.7) | 47 (98) | 38 (95) |
| Hispanic/Latino | 3 (3.3) | 1 (2) | 2 (5) |
| Education | |||
| High school | 7 (7.8) | 3 (6) | 4 (10) |
| Some college | 7 (7.8) | 3 (6) | 4 (10) |
| Associate degree | 24 (26.6) | 8 (16) | 12 (30) |
| Bachelor's degree | 31 (34.4) | 18 (36) | 13 (32.5) |
| Master's degree | 17 (18.9) | 11 (22) | 6 (15) |
| Professional | 3 (3.3) | 3 (6) | 0 |
| Doctoral degree | 1 (1.1) | 0 | 1 (2.5) |
| Marital status | |||
| Never married | 21 (23.3) | 14 (28) | 7 (17.5) |
| Married | 57 (63.3) | 32 (64) | 25 (62.5) |
| Widowed | 1 (1.1) | 1 (2) | 0 |
| Domestic partner | 2 (2.2) | 2 (4) | 0 |
| Divorced | 9 (10) | 1 (2) | 8 (20) |
| Employment | |||
| Working now | 90 (100) | 50 (100) | 40 (100) |
| Weight category | |||
| Overweight | 21 (23) | 15 (30) | 6 (15) |
| Obesity | 69 (77) | 35 (70) | 34 (85) |
| Backlogging a | 80 (89) | 45 (90) | 35 (87.5) |
| Variable (range) | Whole sample (N = 90) M (SD) 95% CI | Study completers (n = 50) M (SD) 95% CI | Partial study completers (n = 40) M (SD) 95% CI |
|---|---|---|---|
| Age (years) | 42.8 (10.1) | 43.1 (9.1) | 42.4 (11.4) |
| (23–69) | 40.7, 44.9 | 40.1, 45.7 | 38.7, 46 |
| Baseline BMI (kg/m2) | 35.1 (6) | 33.6 (5.5) | 36.9 (6.7) |
| (27–52) | 33.8, 36.4 | 32, 35.2 | 34.8, 39.1 |
| Baseline weight (kg) | 96.4 (4.6) | 91.8 (17.2) | 102.1 (20.9) |
| (55.3–145.2) | 92.3, 100.5 | 86.9, 96.7 | 95.4, 108.8 |
Abbreviations: BMI, body mass index; CI, confidence interval; kg, kilograms; kg/m2, kilograms/meter squared; M, mean; SD, standard deviation.
Backlogging = the recording of any self‐monitoring data for a date prior to the current day.
TABLE 2.
Weight change outcomes by completion status (N = 90)
| Variable (range) | Whole sample (N = 90) M (SD) 95% CI | Study completers (n = 50) M (SD) 95% CI | Partial study completers (n = 40) M (SD) 95% CI |
|---|---|---|---|
| Final weight (kilograms) range = 54.8–143.4 | 94.9 (19.5) | 89.8 (16.9) | 101.4 (20.6) |
| 90.9, 99.0 | 85.0, 94.6 | 94.8, 108.0 | |
| Change in weight (kilograms) range = −6.7–2.3 | −1.5 (2.1) | −2.1 (2.4) | −0.8 (1.4) |
| −1.0, −1.9 | −1.4, −2.8 | −0.3, −1.2 | |
| Percent weight change range = −7.51–2.75 | −1.54 (2.26) | −2.19 (2.58) | −0.73 (1.45) |
| −1.07, −2.02 | −1.46, −2.93 | −0.26, −1.19 | |
| Final BMI (kg/m2) range = 26.2–50.5 | 34.6 (6.2) | 32.9 (5.5) | 36.7 (6.4) |
| 33.3, 35.9 | 31.3, 34.4 | 34.6, 38.7 |
Abbreviations: BMI, body mass index; CI, confidence interval; kg/m2, kilograms/meter squared; M, mean; SD, standard deviation.
TABLE 3.
Adherence outcomes for dietary self‐monitoring (DSM) by completion status (N = 90)
| Variable Range a | Whole sample (N = 90) M (SD) 95% CI | Study completers (n = 50) M (SD) 95% CI | Partial study completers (n = 40) M (SD) 95% CI |
|---|---|---|---|
| Frequency (days) b | 28.1 (18.4) | 39.3 (15.5) | 14 (10.5) |
| Range = 1–56 | 24.2, 32 | 34.9, 43.8 | 10.7, 17.4 |
| Frequency (percent) c , d | 50.1 (33.3) | 70.2 (27.7) | 25 (18.7) |
| Range = 1.8–100 | 43.2, 57 | 62.3, 78 | 19.1, 31 |
| Consistency (weeks) e | 4.4 (2.8) | 6.1 (2.3) | 2.4 (1.7) |
| Range = 0–8 | 3.9, 5 | 5.4, 6.8 | 1.8, 2.9 |
| Completeness (weeks) f | 3.4 (2.9) | 4.9 (2.8) | 1.6 (1.6) |
| Range = 0–8 | 2.8, 4 | 4.1, 5.7 | 1.1 (2.1) |
Abbreviations: CI, confidence interval; M, mean; SD, standard deviation.
Range = actual range.
Frequency (days) = the total number of days that any DSM occurred over 8 weeks.
Frequency (percent) = the percentage of days that any DSM occurred over 8 weeks.
Frequency denominator = 56 days.
Consistency = documentation of ≥ three daily DSM records per week.
Completeness = the recording of ≥ 50% of one's weekly calorie goal.
FIGURE 2.
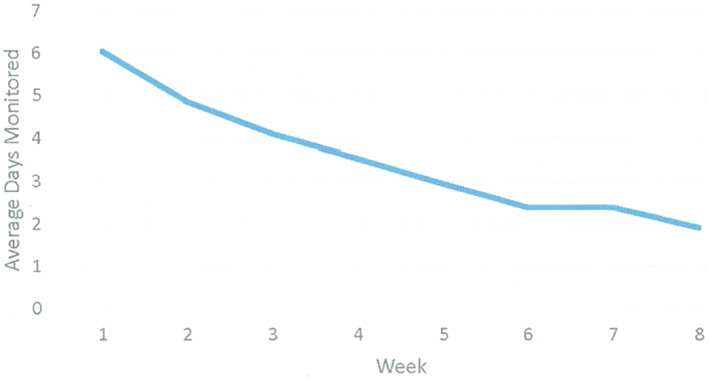
The average frequency (days) of dietary self‐monitoring per week
3.1. Percent weight loss
Change in weight (Table 2) ranged from weight loss of 6.7 kg to weight gain of 2.3 kg, and percent weight change ranged from a 7.51% weight loss to a 2.75% weight gain. Dependent‐samples t‐tests with and without percent weight change outliers revealed no significant differences in results. Thus, results are reported using data from the entire sample (N = 90). Controlling for age and education, a significant [t (89) = −6.59, p < 0.001] mean difference (M = −1.5 ± 2.1 kg, 95% CI = −1, −1.9 kg) was found between baseline weight (M = 96.4, ± 4.6 kg, 95% CI 92.3, 100.5 kg) and final, 8‐week weight (M = 94.9 ± 19.5 kg, 95% CI 90.9, 99 kg) that corresponded to a percent weight of M = −1.54 ± 2.6 (95% CI = −1.07, −2.02). Partial completers (n = 40) achieved an average 0.73% ± 1.45% weight loss.
3.2. Associations between the indicators of self‐monitoring adherence and weight loss
Correlational analyses revealed significant low‐to medium‐level associations between frequency (r = −0.261, p = 0.007), consistency (r = −0.275, p = 0.004), and completeness (r = −0.197, p = 0.031) of DSM with percent weight change (M = −1.54 ± 2.26, 95% CI = −2.02, −1.07). Table 3 presents descriptive data of the independent variables and Table 4 presents the simple linear regression results. Controlling for age and education, simple linear regression analyses revealed a significant [F (1, 88) = 6.42, 95% CI = −0.032, −0.004, p = 0.013] association (R 2 = 0.068 and adjusted R 2 = 0.057) between the frequency of DSM (M = 50.14 ± 32.94, 95% CI = 43.24, 57.04) and percent weight change. Controlling for age and education, a significant [F (1, 88) = 7.18, 95% CI = −0.391, −0.058, p = 0.009] association (R 2 = 0.075, adjusted R 2 = 0.065) between the total weeks of consistent DSM (M = 4.44 ± 2.77, 95% CI = 3.87, 5.02) and percent weight change was also noted. Each 1% increase in frequency of DSM was associated with a 0.02% decrease in weight. Each week of consistent DSM was associated with a 0.23% decrease in weight. Total weeks of complete DSM (M = 3.42 ± 2.87, 95% CI = 2.82, 4.02) was not significantly associated with weight change [F (1, 88) = 3.57, 95% CI = −0.319, 0.008, p = 0.062]. When the three percent weight change outliers were removed, one regression model revealed a significant [F (1, 85) = 5.29, 95% CI = −0.310, −0.023 p = 0.024] association (R 2 = 0.039, adjusted R 2 = 0.028) between total weeks of complete DSM (M = 3.44 ± 2.9) and percent weight change (M = −1.34 ± 2.01, 95% CI = −1.77, −0.91).
TABLE 4.
Regressions of associations between self‐monitoring adherence and weight change (N = 90)
| Variable | B | SE (b) | B | t | p | 95% CI |
|---|---|---|---|---|---|---|
| Frequency | −0.018 | 0.007 | −0.261 | −2.53 | 0.013 | −0.032, −0.004 |
| Consistency | −0.225 | 0.084 | −0.275 | −2.68 | 0.009 | −0.391, −0.058 |
| Completeness | −0.155 | 0.082 | −0.197 | −1.89 | 0.062 | −0.319, 0.008 |
Abbreviations: B, standardized beta coefficient; CI, confidence interval; p, significance level; SE, (b); standard, error of the unstandardized beta coefficient; t, t test value.
3.3. Study completer results
Among study completers (n = 50), a significant [t (49) = 5.96, p < 0.001] mean difference (M = −2.1 ± 2.4 kg, 95% CI = −1.4, −2.8 kg) was found between baseline weight (M = 91.8 ± 17.2 kg, 95% CI = 86.9, 96.7 kg) and final weight (M = 89.8 ± 16.9 kg, 95% CI = 85, 94.6 kg) that corresponded to a percent weight change of M = −2.19% ± 2.58% (95% CI = −1.46, −2.93). Study completers self‐monitored diet an average of 70.2% (± 27.7%) of the days in the study. Study completers consistently self‐monitored diet an average of 6.1 (± 2.3) weeks and completely self‐monitored diet an average of 4.9 (± 2.8) weeks. Correlational analysis revealed weak associations between the frequency (r = −0.047, p = 0.748), consistency (r = −0.082, p = 0.569), and completeness (r = −0.023, p = 0.857) of DSM that were not significantly associated with weight change. Regression analysis of study completers revealed that frequency [F (1, 48) = 0.105, 95% CI = −0.031, 0.023, p = 0.748], consistency [F (1, 48) = 0.328, 95% CI = −0.414, 0.230, p = 0.569], and completeness [F (1, 48) = 0.033, 95% CI = −0.290, 0.242, p = 0.857] were not significantly associated with percent weight change.
4. DISCUSSION
This theory‐informed study tested the relationship between app‐based DSM and weight change in adults with obesity and/or overweight and is among the first to simultaneously examine the associations between three indicators of adherence to app‐based DSM and weight change. The use of app‐based DSM, and the frequency and consistency of DSM, were significantly associated with weight loss; completeness of DSM was not significantly associated with weight change in an intent to treat analysis. However, a study completer analysis yielded a significant association between the use of app‐based DSM and weight loss as well. Study completers lost a modestly higher percentage of weight and also self‐monitored dietary intake more frequently, consistently, and completely than the whole sample, yet these indicators of adherence to DSM and weight change were not significantly related to weight loss, potentially due to low power or the phenomenon of backlogging.
App‐based DSM in the current study was significantly associated with an average weight loss of 1.54% (−1.5 kg) of baseline weight. Comparison of our results with app‐based DSM studies is somewhat difficult secondary to heterogeneity of study designs and how adherence has been defined 9 , 11 ; however, relationships between app‐based DSM and weight change are inconsistent. The amount of weight loss associated with app‐based DSM in the current study is similar to a study that implemented the Lose It! app [−1.59 kg (−3.5 ± 1.0 lbs)]. 32 Results of the current study are also consistent with the results of studies that found significant associations between app‐based DSM and weight loss, 2 , 14 , 16 but contrast with studies that found no significant associations between app‐based DSM and weight change. 35 , 36 The average weight loss of 1.54% in this study was not clinically significant (≥ 5.0% of baseline weight), 37 , 38 , 39 , 40 which may be related to decreased DSM over the course of the study and short study duration of 8 weeks. Nonetheless, results of the current study suggest that app‐based DSM may aid short‐term weight loss and support the idea that the relationship between app‐based DSM and weight loss may be similar to the relationship between paper‐based DSM and weight loss.
Participants self‐monitored dietary intake an average of 28 (50%) of the total number of study days, which was significantly associated with weight loss. Frequency of DSM in the current study is similar to previous app‐based DSM studies, 6 , 13 , 16 , 41 , 42 , 43 , 44 which found that participants adhered to DSM 45% 13 to 58% 43 of possible study days. DSM frequency in the current study explained only 6.8% of the variance in weight loss, which is low compared to a study that found self‐monitoring frequency explained 27% of the variance in weight loss. 9 The low amount of explained variance suggests that other behavioral and social characteristics may be associated with weight loss. Greater frequency of app‐based DSM has been significantly associated with greater weight loss, 15 , 45 and our results suggest that DSM with an app for at least half the time may be associated with short‐term weight loss. However, it is unclear how frequent self‐monitoring needs to be to result in clinically significant weight loss. Nonetheless, potential may exist to decrease the burden associated with self‐monitoring every day.
Participants self‐monitored at least one food or beverage on at least 3 days per week for an average of 4.4 weeks, which was significantly associated with weight loss. Among the adherence variables examined, consistency of DSM explained the most variance (7.5%) in weight loss. This significant association is similar to evidence in other studies, 46 , 47 however, these studies required more days of DSM per week to be considered consistent. Moderate (3–4 days per week) and high (>5 days per week) consistency of self‐monitoring has been associated with clinically significant weight loss. 4 Thus, consistently self‐monitoring dietary intake 3 days per week for 4.4 weeks or more is associated with moderate weight loss, but the precise amount of consistent DSM needed for weight loss that is clinically significant is unclear. If the minimum amount of app‐based DSM required to lose weight was 3 days per week for 4–4.5 weeks, then this may be another avenue to reduce the DSM burden.
The sample completely self‐monitored ≥50% of their respective weekly calorie goals an average of 3.42/8 weeks, which was not significantly associated with weight change. Completeness had the weakest correlation with percent weight loss. Assessment of the association between completeness of DSM and weight change can be difficult, as completeness can be combined and confounded with other variables, such as recording body weight and frequency of meal consumption. 48 Consistent with the results of the current study, Peterson et al. (2014) 3 did not find a significant association between completeness of DSM and weight change. Contrasting with the findings of the current study, Yon et al. 49 found a significant association between self‐monitoring adherence to weekly individualized calorie goals and weight loss with personal digital assistants. Results of the current study suggest that completely self‐monitoring ≥50% of an individualized weekly calorie goal may not help with short‐term weight loss. Further investigation of the relationship between completely self‐monitoring dietary intake and weight loss is needed.
Backlogging is a phenomenon encountered in weight loss studies 40 and was prevalent in the current study. DSM diaries are intended to be used in real time, but diet recording can occur long after dietary consumption. Backlogging DSM data can make diary entries appear as if they were recorded in real time when this may not have occurred. 40 A significant correlation between percent weight loss and recording dietary intake within 15 min of opening an electronic paper diary was found in a previous study. 50 Thus, it is possible that weight loss might have been greater if DSM occurred in a timelier manner. Examination of the association between backlogging of DSM data and weight change is outside the scope of the current study, however further investigation of the effect of backlogging self‐monitoring data may be warranted.
This study has several strengths and some limitations. This investigation used a free commercial DSM app with a professional database, which allowed objective collection of all participant self‐monitoring data over the course of the study. All study components were remotely delivered, which facilitated recruitment and retention of a robust size of participants. Limitations should also be noted. The only recruitment method used was the employee intranet, which posed a risk of selection bias. 51 This study was eight weeks, descriptive, and uncontrolled, thus findings apply to the short‐term, and no causal relationships can be inferred. All data were subject to self‐report bias. The sample was small, and consisted of mostly White, middle‐aged, educated women working in a large, urban health system, limiting generalizability.
The associations between the indicators of adherence examined here with long‐term weight loss should be investigated in large, randomized trials of longer duration. Perhaps a less stringent self‐monitoring requirement, such as 3 days of app‐based DSM per week, positively effects adherence and weight loss, including clinically significant weight loss. Comparing the amount of weight change between consistent and inconsistent self‐monitors with and without backlogging of dietary data may reveal the effects of backlogging on weight change.
5. CONCLUSION
App‐based DSM was associated with short‐term weight loss in adults with obesity or overweight who were interested in losing weight, suggesting that the relationship between app‐based DSM and weight loss is similar to the relationship between paper‐based DSM and weight loss. Results suggest that consistent (≥3 days/week) and frequent (≥50% of days) app‐based DSM are associated with weight loss. Clinicians may wish to consider emphasizing frequent and consistent app‐based self‐monitoring when providing weight loss counseling.
CONFLICT OF INTEREST
The authors declared no conflict of interest.
AUTHOR CONTRIBUTIONS
Jason Payne, Melanie Turk, Melissa Kalarchian, and Christine Pellegrini conceived and developed the study design. Jason Payne and Melanie Turk obtained grant funding and implemented the study. Jason Payne collected data of the study. Jason Payne and Melanie Turk analyzed the study data and wrote the manuscript. Jason Payne, Melanie Turk, Melissa Kalarchian, and Christine Pellegrini interpreted results, critiqued and revised the manuscript, and approved the submitted manuscript.
ACKNOWLEDGMENTS
The authors wish to thank Mount Carmel Health System for assistance in implementation, Mr. Bruce Johnson (MSc) for assistance with data analysis, and the participants for their participation in this study. This project was supported in part by a research grant from the Ohio Nurses Foundation (ONF), the foundation of the Ohio Nurses Association (ONA).
Payne JE, Turk MT, Kalarchian MA, Pellegrini CA. Adherence to mobile‐app‐based dietary self‐monitoring—Impact on weight loss in adults. Obes Sci Pract. 2022;8(3):279‐288. 10.1002/osp4.566
REFERENCES
- 1. Wadden TA, Tronieri JS, Butryn ML. Lifestyle modification approaches for the treatment of obesity in adults. Am Psychol. 2020;75(2):235‐251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Patel ML, Wakayama LN, Bennett GG. Self‐monitoring via digital health in weight loss interventions: a systematic review among adults with overweight or obesity. Obes. 2021;29(3):478‐499. [DOI] [PubMed] [Google Scholar]
- 3. Peterson ND, Middleton KR, Nackers LM, Medina KE, Milsom VA, Perri MG. Dietary self‐monitoring and long‐term success with weight management. Obes. 2014;22(9):1962‐1967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Hutchesson MJ, Tan CY, Morgan P, Callister R, Collins C. Enhancement of self‐monitoring in a web‐based weight loss program by extra individualized feedback and reminders: randomized trial. J Med Internet Res. 2016;18(4):e82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Baker RC, Kirschenbaum DS. Self‐monitoring may be necessary for successful weight control. Behav Ther. 1993;24(3):377‐394. [Google Scholar]
- 6. Ross KM, Wing RR. Impact of newer self‐monitoring technology and brief phone‐based intervention on weight loss: a randomized pilot study. Obes. 2016;24(8):1653‐1659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Pew Research Center . Mobile fact sheet. Pew Research Center Internet & Technology website. April 7, 2021. Accessed May 23, 2021. https://www.pewresearch.org/internet/fact‐sheet/mobile/
- 8. Holzmann SL, Holzapfel C. A scientific overview of smartphone applications and electronic devices for weight management in adults. J Personalized Med. 2019;9(2):1–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Turner‐McGrievy GM, Dunn CG, Wilcox S, et al. Defining adherence to mobile dietary self‐monitoring and assessing tracking over time: tracking at least two eating occasions per day is best marker of adherence within two different mobile health randomized weight loss interventions. J Acad Nutr Diet. 2019;119(9):1516‐1524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. DiFilippo KN, Huang W‐H, Andrade JE, Chapman‐Novakofski KM. The use of mobile apps to improve nutrition outcomes: a systematic literature review. J Telemed Telecare. 2015;21(5):243‐253. [DOI] [PubMed] [Google Scholar]
- 11. Payne JE, Turk MT, Kalarchian MA, Pellegrini CA. Defining adherence to dietary self‐monitoring using a mobile app: a narrative review. J Acad Nutr Diet. 2018;118(11):2094‐2119. [DOI] [PubMed] [Google Scholar]
- 12. Allen JK, Stephens J, Dennison Himmelfarb CR, Stewart KJ, Hauck S. Randomized controlled pilot study testing use of smartphone technology for obesity treatment. J Obes. 2013;2013:151597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Carter MC, Burley VJ, Cade JE. Weight loss associated with different patterns of self‐monitoring using the mobile phone app My Meal Mate. JMIR mHealth uHealth. 2017;5(2):e8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Dunn CG, Turner‐McGrievy GM, Wilcox S, Hutto B. Dietary self‐monitoring through calorie tracking but not through a digital photography app is associated with significant weight loss: the 2SMART pilot study‐a 6‐month randomized trial. J Acad Nutr Diet. 2019;119(9):1525‐1532. [DOI] [PubMed] [Google Scholar]
- 15. Goldstein S, Goldstein C, Bond D, Raynor H, Wing R, Thomas J. Associations between self‐monitoring and weight change in behavioral weight loss interventions. Health Psychol. 2019;38(12):1128–1136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Turner‐McGrievy GM, Wilcox S, Boutte A, et al. The dietary intervention to enhance tracking with mobile devices (DIET mobile) study: a 6‐Month randomized weight loss trial. Obes. 2017;25(8):1336‐1342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Burke LE, Styn MA, Sereika SM, et al. Using mHealth technology to enhance self‐monitoring for weight loss: a randomized trial. Am J Prev Med. 2012;43(1):20‐26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. FatSecret . FatSecret. Fatsecret Professional website. Accessed March 16, 2018. https://professional.fatsecret.com/index.html
- 19. Payne JE, Turk MT, Pellegrini CA, Kalarchian MA. Abstract P506: dietary self‐monitoring using an app: are frequency, consistency and completeness related to weight loss? Circ. 2020;141(Suppl_1):AP506. [Google Scholar]
- 20. Google . Google. Calorie Counter by FatSecret. Google Play Store website. Accessed March 16, 2018. https://play.google.com/store/apps/details?id=com.fatsecret.android
- 21. Perreault L. Obesity in adults: Behavioral therapy. UpToDate website. Published 2018. Accessed June 27, 2018. https://www.uptodate.com/contents/obesity‐in‐adults‐behavioral‐therapy
- 22. Bandura A. Social Cognitive Theory: an agentic perspective. Annu Rev Psychol. 2001;52:1‐26. [DOI] [PubMed] [Google Scholar]
- 23. Teixeira PJ, Carraca EV, Marques MM, et al. Successful behavior change in obesity interventions in adults: a systematic review of self‐regulation mediators. BMC Med. 2015;13:84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Hartmann‐Boyce J, Johns DJ, Jebb SA, Aveyard P. Effect of behavioural techniques and delivery mode on effectiveness of weight management: systematic review, meta‐analysis and meta‐regression. Obes Rev. 2014;15(7):598‐609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Burke LE, Wang J, Sevick MA. Self‐monitoring in weight loss: a systematic review of the literature. J Am Diet Assoc. 2011;111(1):92‐102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Harvard Health Publishing . Harvard Medical School. Focus on Nutrition. HEALTHbeat website. Published 2019. Accessed January 20, 2019. https://www.health.harvard.edu/healthbeat/co_reg
- 27. Bodies by Design Personal Training Studios . Fat Secret App Demo [Video]. YouTube. Published September 15, 2015. Accessed April 2019, 2018. https://www.youtube.com/watch?v=m2NplOJ8‐so
- 28. Bonn SE, Trolle Lagerros Y, Balter K. How valid are web‐based self‐reports of weight? J Med Internet Res. 2013;15(4):e52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Celis‐Morales C, Livingstone KM, Woolhead C, et al. How reliable is internet‐based self‐reported identity, socio‐demographic and obesity measures in European adults? Genes Nutr. 2015;10(5):28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Centers for Disease Control and Prevention (CDC) Division of Nutrition, Physical Activity, and Obesity . Calculating BMI using the English System. Growth Chart Training website. Published May 9, 2014. Accessed June 9, 2018. https://www.cdc.gov/nccdphp/dnpao/growthcharts/training/bmiage/page5_2.html
- 31. Faul F, Erdfelder E, Buchner A, Lang AG. Statistical power analyses using G*Power 3.1: tests for correlation and regression analyses. Behav Res Methods. 2009;41:1149‐1160. [DOI] [PubMed] [Google Scholar]
- 32. Wharton CM, Johnston CS, Cunningham BK, Sterner D. Dietary self‐monitoring, but not dietary quality, improves with use of smartphone app technology in an 8‐week weight loss trial. J Nutr Educ Behav. 2014;46(5):440‐444. [DOI] [PubMed] [Google Scholar]
- 33. Kong A, Beresford SA, Imayama I, et al. Adoption of diet‐related self‐monitoring behaviors varies by race/ethnicity, education, and baseline binge eating score among overweight‐to‐obese postmenopausal women in a 12‐month dietary weight loss intervention. Nutr Res (NY). 2012;32(4):260‐265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Polit DF. Statistics and Data Analysis for Nursing Research. 2nd ed. Pearson; 2010. [Google Scholar]
- 35. Banerjee P, Mendu VVR, Korrapati D, Gavaravarapu SM. Calorie counting smart phone apps: effectiveness in nutritional awareness, lifestyle modification and weight management among young Indian adults. J Health Inform. 2020;26(2):816–828. [DOI] [PubMed] [Google Scholar]
- 36. Hutchesson MJ, Callister R, Morgan PJ, et al. A targeted and tailored ehealth weight loss program for young women: the be positive be healthe randomized controlled trial. Healthc (Basel, Switzerland). 2018;6(2):1–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Hamman RF, Wing RR, Edelstein SL, et al. Effect of weight loss with lifestyle intervention on risk of diabetes. Diabetes Care. 2006;29(9):2102‐2107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Douketis JD, Macie C, Thabane L, Williamson DF. Systematic review of long‐term weight loss studies in obese adults: clinical significance and applicability to clinical practice. Int J Obes. 2005;29(10):1153‐1167. [DOI] [PubMed] [Google Scholar]
- 39. Knowler WC, Barrett‐Connor E, Fowler SE, et al. Reduction in the incidence of type 2 diabetes with lifestyle intervention or metformin. N Engl J Med. 2002;346(6):393‐403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Wing RR, Lang W, Wadden TA, et al. Benefits of modest weight loss in improving cardiovascular risk factors in overweight and obese individuals with type 2 diabetes. Diabetes Care. 2011;34(7):1481‐1486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Spring B, Pellegrini CA, Pfammatter A, et al. Effects of an abbreviated obesity intervention supported by mobile technology: the ENGAGED randomized clinical trial. Obes. 2017;25(7):1191‐1198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Carter MC, Burley VJ, Nykjaer C, Cade JE. Adherence to a smartphone application for weight loss compared to website and paper diary: pilot randomized controlled trial. J Med Internet Res. 2013;15(4):e32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Burke LE, Zheng Y, Ma Q, et al. The SMARTER pilot study: testing feasibility of real‐time feedback for dietary self‐monitoring. Prev Med Rep. 2017;6:278‐285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Serrano KJ, Coa KI, Yu M, Wolff‐Hughes DL, Atienza AA. Characterizing user engagement with health app data: a data mining approach. Trans Behav Med. 2017;7(2):277‐285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Lee K‐W, Kim H‐B, Lee S‐H, Ha H‐K. Changes in weight and health‐related behavior using smartphone applications in patients with colorectal polyps. J Nutr Educ Behav. 2019;51(5):539‐546. [DOI] [PubMed] [Google Scholar]
- 46. Painter SL, Ahmed R, Hill JO, et al. What matters in weight loss? An in‐depth analysis of self‐monitoring. J Med Internet Res. 2017;19(5):e160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Patel ML, Hopkins CM, Bennett GG. Early weight loss in a standalone mHealth intervention predicting treatment success. Obes Sci Pract. 2019;5(3):231‐237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Thomas JG, Wing RR. Health‐e‐call, a smartphone‐assisted behavioral obesity treatment: pilot study. JMIR mHealth uHealth. 2013;1(1):e3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Yon BA, Johnson RK, Harvey‐Berino J, Gold BC, Howard AB. Personal digital assistants are comparable to traditional diaries for dietary self‐monitoring during a weight loss program. J Behav Med. 2007;30(2):165‐175. [DOI] [PubMed] [Google Scholar]
- 50. Burke LE, Sereika SM, Music E, Warziski M, Styn MA, Stone A. Using instrumented paper diaries to document self‐monitoring patterns in weight loss. Contemp Clin Trials. 2008;29(2):182‐193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Pannucci CJ, Wilkins EG. Identifying and avoiding bias in research. Plast Reconstr Surg. 2010;126(2):619‐625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Schulz KF, Altman DG, Moher D. CONSORT 2010 statement: updated guidelines for reporting parallel group randomised trials. BMJ Clin Res. 2010;340:c332. [DOI] [PMC free article] [PubMed] [Google Scholar]


