Abstract
Background
Medicare fee-for-service (FFS) claims data are increasingly leveraged for dementia research. Few studies address the validity of recent claim data to identify dementia, or carefully evaluate characteristics of those assigned the wrong diagnosis in claims.
Methods
We used claims data from 2014 to 2018, linked to participants administered rigorous, annual dementia evaluations in 5 cohorts at the Rush Alzheimer’s Disease Center. We compared prevalent dementia diagnosed through the 2016 cohort evaluation versus claims identification of dementia, applying the Bynum-standard algorithm.
Results
Of 1 054 participants with Medicare Parts A and B FFS in a 3-year window surrounding their 2016 index date, 136 had prevalent dementia diagnosed during cohort evaluations; the claims algorithm yielded 217. Sensitivity of claims diagnosis was 79%, specificity 88%, positive predictive value 50%, negative predictive value 97%, and overall accuracy 87%. White participants were disproportionately represented among detected dementia cases (true positive) versus cases missed (false negative) by claims (90% vs 75%, respectively, p = .04). Dementia appeared more severe in detected than missed cases in claims (mean Mini-Mental State Exam = 15.4 vs 22.0, respectively, p < .001; 28% with no limitations in activities of daily living versus 45%, p = .046). By contrast, those with “over-diagnosis” of dementia in claims (false positive) had several worse health indicators than true negatives (eg, self-reported memory concerns = 51% vs 29%, respectively, p < .001; mild cognitive impairment in cohort evaluation = 72% vs 44%, p < .001; mean comorbidities = 7 vs 4, p < .001).
Conclusions
Recent Medicare claims perform reasonably well in identifying dementia; however, there are consistent differences in cases of dementia identified through claims than in rigorous cohort evaluations.
Keywords: Dementia, Diagnosis, Medicare
Over 6 million adults in the United States have Alzheimer’s dementia, and this is predicted to double in the next 30 years (1); total costs associated with Alzheimer’s disease and related disorders (ADRD) are projected to exceed $1 trillion in 2050 (1). Clearly, a wide range of research is critical to fully address the impact of ADRD on individual and public health—from better understanding the epidemiology of ADRD, to evaluating interventions for prevention and treatment, to estimating associated health care and economic costs.
Medicare fee-for-service (FFS) claims data are increasingly leveraged for both epidemiologic and health services research on dementia, primarily because claims data represent the large majority of Americans age 65 years and older. Although several previous cohort studies with linked Medicare claims data have assessed the validity of dementia identification in claims versus “gold standard” neurologic evaluations (2–6), even the most recent primarily include Medicare claims from approximately a decade in the past (2–5). There have been many changes in health care over the past 10 years—such as Medicare coverage of cognitive screening as part of Wellness visits in 2011, and adoption of 10th revision of the International Statistical Classification of Diseases (ICD-10) codes in 2015 to increase the accuracy of diagnostic coding. Furthermore, besides focusing on older claims data, many existing studies have utilized brief neuropsychologic testing, have implemented cognitive testing at long intervals (ie, missing cases with rapid progression to death), or utilize electronic health records rather than uniform participant evaluations; thus, the “gold standard” dementia diagnosis in some cohorts may itself lack sensitivity and/or specificity.
To more fully understand how claims data over the last decade may perform in identifying those with dementia, we leveraged five ongoing cohorts of older women and men in the Rush Alzheimer’s Disease Center, all of whom receive extensive annual, harmonized neuropsychologic and neurologic assessments. We compared prevalent dementia identified by the cohorts in 2016 versus dementia identification in linked Medicare FFS claims, available for participants in a 1-year window (6 months before/after) or 3-year window (18 months before/after) surrounding the 2016 index cohort assessment.
Method
To compare comprehensive clinical evaluation of dementia versus dementia identification in Medicare FFS claims data, we included participants in 5 cohorts at the Rush Alzheimer’s Disease Center (RADC). All studies were approved by an institutional review board of Rush University Medical Center, and informed consent (including for Medicare linkage) was obtained from all participants. The request for Medicare claims was reviewed and approved by the Centers for Medicare and Medicaid Services Privacy Board, and research identifiable files were shared through a signed data use agreement with CMS. Medicare claims data are currently available for participants from 1991 through 2018. For the linkage, most participants provided their Medicare beneficiary identification number, which was used along with their name, sex, date of birth, and social security number if available, to link with Medicare claims files; we were able to link 99% of participants who provided consent.
Cohort Studies
Participants age 65 years and older were enrolled in one of the following clinical cohort studies of aging: the Religious Orders Study (7), the Rush Memory and Aging Project (7), the Minority Aging Research Study (MARS) (8), the Rush African American Clinical Core (9), and the Rush Latino Core (10). Participants in the Religious Orders Study are older Catholic nuns, priests, and brothers from across the United States who have been followed since 1994. The Rush Memory and Aging Project started in 1997 and consisted of older lay-persons who are recruited from across the Chicago metropolitan area. The MARS and the Rush African American Clinical Core both consist of older African Americans recruited from churches, senior buildings, and social clubs and organizations that cater to diverse populations in the Chicago metropolitan area; MARS was initiated in 2004 and the Clinical Core in 2008. Finally, the Rush Latino Core began in 2015 and includes older adults in the Chicago area who self-identify as Latinx. All studies recruited participants without known dementia at baseline who agreed to annual clinical evaluations. All have a rolling admission with new participants recruited each year and evaluated annually, until death or withdrawal from the study. Follow-up is high, ranging between 85% and 90% across all the cohorts.
Dementia Assessments
Cohort evaluations
Clinical assessments were conducted every year in all cohorts, with procedures harmonized across all cohorts. Briefly, participants were administered a uniform, structured, clinical evaluation including a battery of 21 cognitive tests, of which 18 were common to all cohorts. Using these 18 tests, the severity of impairment was rated for 5 cognitive domains (11). A neuropsychologist, blinded to participant demographics, reviewed the impairment ratings and other clinical information and rendered a clinical judgment regarding the presence of dementia. An experienced clinician then reviewed all available data, examined the participant (thus the clinician was not blinded to participant demographics), and rendered a final diagnostic classification. Clinical diagnosis of dementia and Alzheimer’s dementia was based on criteria of the joint working group of the National Institute of Neurological and Communicative Disorders and Stroke and the Alzheimer’s Disease and Related Disorders Association (NINCDS/ADRDA) (12,13).
Medicare claims algorithm
To identify dementia from Medicare claims data, we applied the Bynum-standard algorithm (14), which uses Parts A and B FFS claims data (since claims data are not uniformly available for those in Medicare Advantage). Details are in Supplementary Materials; briefly, as per the Bynum-standard algorithm, we searched for any claims with relevant ADRD ICD-9 or ICD-10 codes in the MedPAR, Carrier, Home Health, and Hospice files. To ensure full identification of ADRD claims, the algorithm also includes claims by underserved populations who received care from Federally Qualified Health Centers, Rural Health Centers, or Critical Access Hospitals under payment option II, which appear in the Outpatient File rather than the Carrier file. Overall, ADRD from Medicare claims were identified according to 2 conditions: (1) at least 1 ADRD claim from MedPAR, Home Health, or Hospice files or (2) at least 2 ADRD claims in the Carrier or outpatient files that were at least 7 days apart, to account for potential misclassification resulting from “rule out” diagnoses.
Characteristics of Participants
Demographic and health-related covariates were self-reported at each cohort evaluation (with the exception of educational attainment, sex, and race/ethnicity that were reported only at baseline). Education was measured in years. In addition, score on the Mini-Mental State Exam (MMSE) was determined at each annual cohort assessment. To assess self-reported memory complaints, participants were asked, “About how often do you have trouble remembering things,” with 5 response options ranging from never to very frequently. In addition, all participants were asked, “Compared to 10 years ago, would you say that your memory is much better, a little better, the same, a little worse, or much worse,” and these were also scored from 1 to 5 (15). We added together both items, and those with a score of 8–10 were considered to have memory concerns. Mild cognitive impairment (MCI) was also determined annually at the cohort evaluation; briefly, diagnosis of MCI required cognitive impairment in the absence of full criteria for dementia (11,16). Disabilities in activities of daily living (ADL) were identified via the Katz scale as the number of items on which a participant reported the need for assistance in walking across a small room, bathing, dressing, eating, transferring from a bed to a chair, and toileting. Disability in instrumental activities of daily living (IADL) was identified by asking participants to report the need for assistance in using the telephone, preparing meals, doing light housekeeping, doing heavy housekeeping, handling medications, handling finances, traveling within the community, and shopping. Finally, we also used the Medicare claims data to identify 2 health-related variables; we summed the number of chronic medical conditions using the Elixhauser (17) comorbidity score, a count of the number of comorbid conditions, and we also identified hospitalizations in the claims.
Statistical Analysis
In primary analyses, we defined each participant’s 2016 cohort evaluation as our index date (2016 was the most recent year for which we could create the “observation windows” before and after the index date in claims data, as done in the Bynum-standard claims algorithm (14)). We then considered prevalent dementia as diagnosis at any cohort evaluation from study enrollment through the 2016 index date. In the claims data, we separately considered dementia identification in 2 observation windows, as per the Bynum-standard algorithm; we used a 1-year window (ie, 6 months before/after) and a 3-year window (ie, 18 months before/after) around the 2016 index date.
To quantify the comparisons of cohort versus claims identification of prevalent dementia, we calculated sensitivity, specificity, positive and negative predictive values, accuracy, and the kappa statistic for overall agreement. In addition, to characterize the 4 groups of “true positives” (dementia in cohort and claims), “true negatives” (no dementia in cohort or claims), “false negatives” (dementia in cohort but not in claims), and “false positives” (no dementia in cohort but dementia in claims), we compared a range of demographic (eg, age, race) and cognitive or physical health-related factors (eg, MMSE score, memory concerns, ADL disabilities) in 2016 across the groups. For comparing the number of comorbidities and the hospitalizations across groups, we utilized claims data during the 1- or 3-year window around the 2016 index date, as described above. We used t-tests (for continuous variables) or chi-square tests (for categorical variables) for statistical comparisons across true-positive versus false-negative groups, and separately false-positive versus true-negative groups.
Secondarily, we repeated all of these analyses, considering the 2012 cohort visit as the index date, instead of 2016, to test a recent but earlier time period that did not have any overlap with our observation windows for 2016; we also note that analyses with the 2016 index date use primarily ICD-10 codes, whereas the 2012 analyses include only ICD-9 codes.
Results
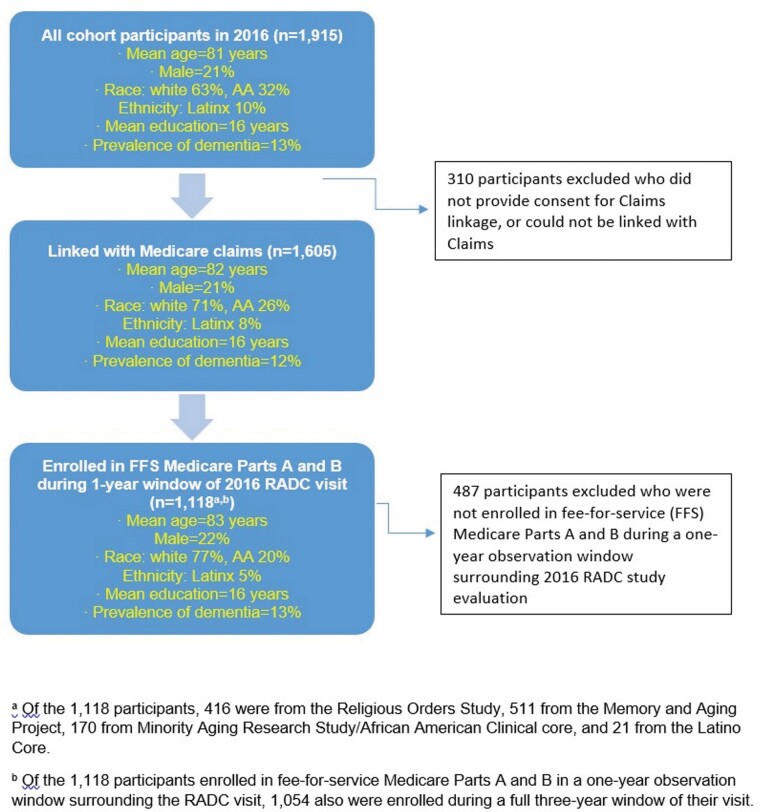
In 2016, there were 1 915 active participants across the five cohorts (Figure 1). On average, participants were 81 years of age, and 21% were male. Furthermore, 63% were White and 32% African American; 10% were Latinx. Overall, they had high levels of education (mean = 16 years), and the prevalence of dementia as of 2016 was 13%. For these analyses, we excluded 310 who did not provide consent to link their cohort data with Medicare claims, or who could not be linked with their claims files. Among the remaining 1 605, our analytic sample included the 1 118 participants who were enrolled in FFS Medicare Parts A and B in the full 1-year window surrounding their 2016 cohort index date. In this analytic sample, 416 participants were from the Religious Orders Study, 511 from the Memory and Aging Project, 170 from MARS/African American Clinical Core, and 21 from the Latino Core.
Figure 1.
Flow chart of eligibility for analyses comparing dementia diagnosis in Rush Alzheimer’s Disease Center (RADC) cohorts in 2016 and in Medicare claims.
There were few differences in all active participants compared to the analytic sample (Figure 1). In the analytic sample, mean age was 83 years, and 22% were male. The analytic sample had less racial and ethnic diversity than the overall cohorts; 77% were White, 20% were African American, and 5% were Latinx. This is because the African American and Latinx participants were more likely than White participants to be enrolled in Medicare Advantage (as has been observed in the general population as well (18)). Importantly, the prevalence of dementia was 13% in the analytic sample, identical to that among all active participants. Finally, most participants enrolled in FFS Medicare Parts A and B during the 1-year observation window were also enrolled for the full 3-year window surrounding their cohort visit (n = 1 054), and findings were similar regarding characteristics (data not shown).
Dementia Identification in Cohort Evaluation and in Claims Data
In 2016, in the analytic sample for the 1-year window, there were 144 cases of prevalent dementia (Table 1) determined by participants’ cohort evaluations (n = 135 of these were classified as Alzheimer’s dementia, data not shown in table). When using the claims-based algorithm, we identified 158 participants with dementia during the 1-year observation window; we note that 99% of these were identified with ICD-10 codes. This resulted in a sensitivity of 64% for the claims-based algorithm compared to the gold standard cohort evaluation. Specificity was high, at 93%. In parallel, the positive predictive value was 58%, and the negative predictive value was 95%. The overall accuracy (ie, correct classification as either dementia or no dementia) was 89%, and the overall kappa between the cohort and claims-based diagnoses was 0.55.
Table 1.
Performance of Medicare Claims-Based Algorithm for Identifying Dementia Compared With Rush Alzheimer’s Disease Center Dementia Evaluations, 2016a,b
| Dementia Status in Cohort Evaluation | Sensitivity (95% CI) | Specificity (95% CI) | Positive Predictive Value (95% CI) | Negative Predictive Value (95% CI) | Accuracy (95% CI) | Kappa (95% CI) | |||
|---|---|---|---|---|---|---|---|---|---|
| Dementia status in claims | Yes | No | Total | ||||||
| 1-y windowb | 64% (56, 72%) | 93% (92, 95%) | 58% (51, 66%) | 95% (93, 96%) | 89% (88, 91%) | 0.55 (0.48, 0.62) | |||
| Yes | 92 | 66 | 158 | ||||||
| No | 52 | 908 | 960 | ||||||
| Total | 144 | 974 | 1 118 | ||||||
| 3-y windowb | 79% (73, 86%) | 88% (86, 90%) | 50% (43, 56%) | 97% (95, 98%) | 87% (85, 89%) | 0.54 (0.47, 0.61) | |||
| Yes | 108 | 109 | 217 | ||||||
| No | 28 | 809 | 837 | ||||||
| Total | 136 | 918 | 1 054 |
aDementia status in cohort evaluations represents all prevalent dementia cases identified as of 2016 who met standard clinical criteria as part of annual neurologic/neuropsychologic assessments.
bDementia status in claims represents prevalent cases of dementia identified using a validated algorithm in Medicare claims files. Dementia status was defined either using claims within a 1-y window of the 2016 Cohort evaluation (ie, ±6 mo) or using claims within a 3-y window (ie, ±18 mo).
When we extended the window of observation for the claims data to 3 years rather than 1 year (Table 1), we identified 217 participants with dementia using the claims-based algorithm (of which 97% were identified with ICD-10 codes). As expected, the sensitivity of the claims algorithm was substantially higher (79%) in the 3-year window. At the same time, specificity was slightly lower (88%). The positive predictive value, negative predictive value, overall accuracy, and the kappa statistic (50%, 97%, 87%, and 0.54, respectively) were all fairly similar to those observed using the 1-year window.
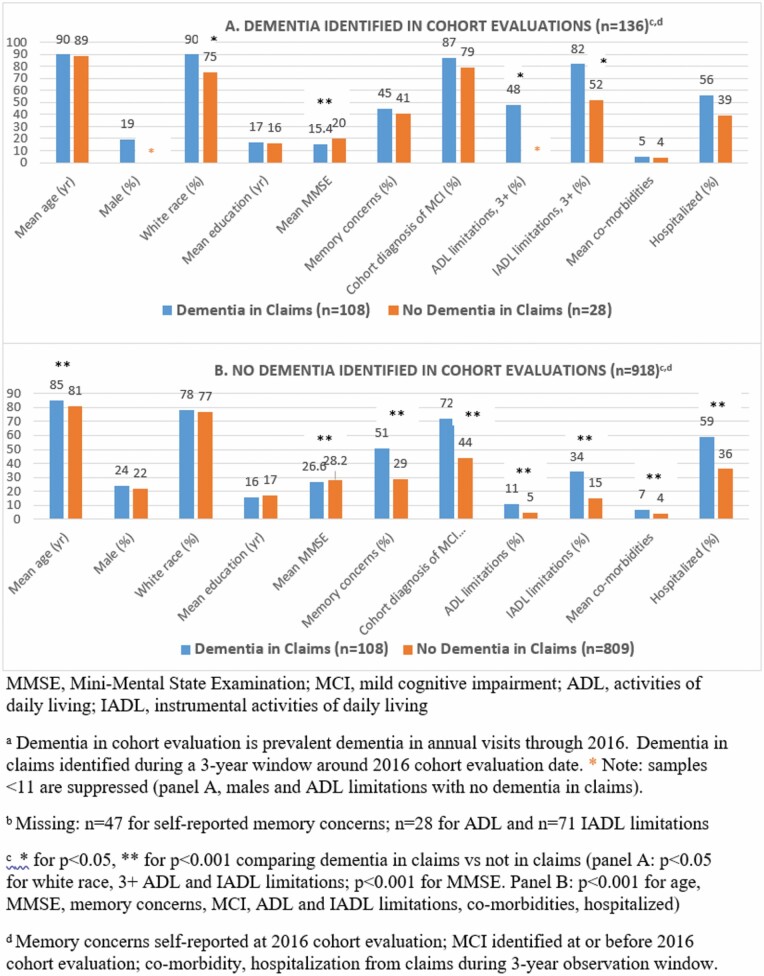
To begin to understand potential factors that may be related to false negative and to false positive determination of dementia in the claims data (Figure 2), we conducted 2 sets of analyses. These analyses focus on the 3-year claims observation window, since most other studies of dementia in cohort versus claims data have used a claims observation window of 3 years or more.
Figure 2.
Characteristics of cohort participants, according to dementia status from cohort evaluations versus Medicare claims-based algorithm over a 3-y observation window for claims data, 2016.
First, to evaluate “under-diagnosis” of dementia in the claims, we compared characteristics of participants across the 2 categories of true positives versus false negatives. Mean age was very similar (p = .4) in the true positives (mean = 90 years) and false negatives (mean = 89 years). We observed significantly more White participants in the true-positive group than in the false-negative group (90% vs 75%, p = .04), indicating that dementia is disproportionately recognized by the health care system in White individuals. Furthermore, several indicators consistently suggested that the strongest factors in distinguishing true positives versus false negatives in the claims data were related to disease severity. Mean MMSE score was substantially lower in true positives than false negatives (mean = 15.4 and mean = 22.0, respectively, p < .0001). ADL limitations were also more common among those with their dementia identified in claims (28% had no limitations) than not identified (45% had no limitations, p = .046), as were IADL limitations (true positive: 82% had 3+ limitations vs false negative: 52%, p = .01).
In the second set of analyses, to better understand the false-positive group (or “over-diagnosis”), we compared the false positives versus those with no dementia in either the cohort or claims data (Figure 2). Mean age was significantly older among those identified with dementia only in the claims (mean = 85 years) compared to the true negatives (mean = 81, p < .0001), although sex, race, and mean education were all virtually identical in the false-positive and true-negative groups. Multiple variables representing health status were strongly related to “over-diagnosis,” such that those with markers of worse cognitive health and worse overall health were more likely to be in the false-positive group. Specifically, participants with no cohort diagnosis of dementia but with dementia in claims data had lower MMSE scores than the true negative group (mean = 26.0 and mean = 28.1, respectively, p < .0001). They had substantially more self-reported memory concerns (51% vs 29%, respectively, p < .0001), and higher prevalence of MCI as determined by their annual cohort evaluations (72% vs 44%, respectively, p < .0001). Similarly, the false-positive group had more ADL and IADL limitations than the true-negative group (both p < .0001). Finally, during the claims observation window, comparing the false-positive and true-negative groups, those with dementia in claims data had nearly twice as many comorbidities (mean comorbidities = 7 vs 4, respectively, p < .0001) and were more likely to have been hospitalized (59% vs 36%, respectively, p < .0001); these may indicate both worse health and greater interaction with the health care system. We also secondarily examined the cohort evaluations in the 1–2 years following the 2016 index evaluation, and a minority of this false-positive group was subsequently given a dementia diagnosis (16% at 1 year and 30% at 2 years, among those still alive, data not shown).
When we examined the 1-year claims observation window, results regarding characteristics of the true positives versus false negatives, and false positives versus true negatives, were all highly consistent with our findings using the 3-year window (Supplementary Table 1).
Secondary Analyses
We also conducted analyses using the 2012 cohort evaluation as the index date (Supplementary Table 2). In 2012, we included the 1 182 participants enrolled in FFS Medicare Parts A and B during the 1-year observation window, and 1 089 during the 3-year window surrounding their 2012 index date. In total, there were 151 prevalent dementia cases diagnosed during the cohort evaluations as of 2012 (ie, similar prevalence of dementia to 2016). In claims data, during the 1-year observation window, we identified 143 participants with dementia, and during the 3-year window, 212 cases were identified. Results were consistent with analyses using 2016 as the index date, although sensitivity was somewhat worse. For the 1-year observation window, the sensitivity was 54%, specificity was 94%, positive predictive value was 57%, negative predictive value was 93%, accuracy was 89%, and the kappa statistic was 0.50. As we found in the 2016 data, for the 3-year observation window, sensitivity was better (70%), specificity was slightly lower (88%), and positive predictive value was slightly lower (48%), whereas negative predictive value (95%), overall accuracy (86%), and the kappa statistic were similar (0.49).
Discussion
Compared with the classification of dementia in cohort evaluations, we found that a Medicare claims algorithm performed reasonably well in identifying prevalent dementia among older individuals. In particular, when allowing a 3-year observation window for the FFS claims data, sensitivity of claims dementia diagnosis was 79%, specificity was 88%, and overall accuracy for classifying those with and without dementia was 87%. These findings for the Bynum-standard algorithm (12) are particularly reassuring given the rigorous cognitive assessments performed every year in these cohorts, likely leading to relatively early cohort diagnosis of dementia, and thus potentially a lower sensitivity comparing claims to cohort data than might be observed in other studies with brief neuropsychologic assessments and/or less-frequent evaluations.
Several other studies with fairly recent claims data (2–6,14) have compared cohort assessment of dementia with Medicare claims. Most of these (2–4) include claims from approximately a decade in the past (ie, linked claims data through 2008–2012); one recent study presented data from 2016 to 2018 (6), using ICD-10 codes, and found good concordance of dementia in Medicare claims versus a “gold standard” diagnosis (although this study was clinic based and utilized the information available in electronic health records as the gold standard dementia diagnosis). Overall then, our results—based on recent Medicare claims combined with rigorous and uniform cognitive evaluations in community-based cohorts—provide new and relevant information for future research. Still, in the previous studies, applying a claims observation window of 2–5 years (2-6,14), sensitivity has ranged from approximately 50%–70%, with specificity of approximately 85%–95%. Our observed sensitivity and specificity, with a comparable 3-year claims observation window, were similar or even higher, whether using 2016 or the earlier 2012 index date for prevalent dementia in our cohorts. Although it is difficult to directly compare results across differing participant groups and differing cohorts, evidence may suggest that identification of dementia in claims data is improving—as has been reported in earlier time periods (19,20) as well.
Interestingly, regardless of differences between our methods as well as follow-up period compared with existing studies, we found quite similar patterns of missed dementia diagnosis in the claims data as have been reported in previous research—including studies using claims going back to the early 2000s (21). For example, most studies reported that dementia diagnosis is disproportionately missed by claims data among African Americans (2,3,14,21,22), as we also found here; this points to potential challenges using claims data in African Americans, as well as a continuing need to address these disparities in health care. Furthermore, studies consistently have observed that various indicators of more severe dementia (eg, lower cognitive test scores, greater functional limitations) are more frequent among participants in whom dementia was identified in claims data (3,4,14,21); this suggests dementia research in claims data will more strongly reflect those with more severe disease. Overall, despite apparent improvements in detection of dementia in claims data, the barriers to diagnosis, and the potential biases when utilizing claims data, remain remarkably steady.
Less research has considered characteristics of the “false positives,” or those “over-diagnosed” in claims versus cohort identification of dementia, despite this group being large in our study and others (eg, the Health and Retirement Study [HRS] reported positive predictive values of approximately 50%–60%, meaning that around half of those with dementia in claims did not have dementia in the cohort evaluation (2,14)). Given the particularly rigorous dementia evaluations in our cohorts—with extensive diagnostic testing and thus a high level of confidence in diagnostic classification—we were well-positioned to assess this group. Interestingly, the majority of individuals in the false-positive group reported memory concerns (51%) on their cohort survey, and nearly three-quarters had prevalent MCI (72%) in their cohort evaluations. Moreover, the false-positive group had among the highest number of comorbidities (mean = 7 comorbid conditions), and hospitalization was common (59% had been hospitalized during the observation period). Together, these findings indicate that the combination of memory concerns with mild cognitive impairment and particularly poor overall health could lead to practical challenges in distinguishing clinical dementia from preclinical disease by the health care system. Although we found that a minority of these false positives (16%–30%) went on to a cohort dementia diagnosis in the 1–2 years after the index date, nonetheless, it also seems plausible that the inclusion of these “false positives” in claims-based dementia research may not cause substantial bias, at least in some contexts, since most are not cognitively healthy.
There are limitations to consider. In particular, the RADC cohorts were not designed as nationally representative samples; thus, we cannot necessarily generalize our results to the entire United States. However, participants in all research studies usually diverge from the general population in various ways. For example, our participants are more educated than the general U.S. population; at the same time, our analytic sample included 20% African American participants, which is higher than the 10% African American enrollment in Medicare in 2016 (18). In addition, our participants were somewhat older than many other cohorts (mean age = 83 years in RADC cohorts and mean = 77–82 years in existing publications (2–4,12)), and some data have suggested that older age is related to better sensitivity of claims identification of dementia (3,12). Finally, about 60% of participants reside in the Chicago area, and it is possible that Medicare identification of dementia may differ by region due to differences in clinical practice or health care infrastructure.
There are important strengths to our study as well. As we noted earlier, the detailed annual neuropsychologic and neurologic evaluations in our cohorts ensure highly valid identification of dementia, including those experiencing even rapid cognitive decline and death. The very high follow-up rates reduce potential for differential bias as well. Importantly, our cohorts not only include diverse populations, but African American and Latinx participants are recruited from similar geographic areas as most of the White participants, minimizing possible bias from regional differences in health care or clinical practice (although there may still be more local differences).
Overall, the particular weaknesses and strengths of using claims data for health research will vary depending on the scientific question, but continued evaluation and understanding of characteristics of individuals both with and without dementia in claims data is critical to advancing health and health care in our rapidly aging population.
Supplementary Material
Acknowledgments
The sponsor had no role in the design, methods, data collection, analysis, and preparation of this manuscript.
Contributor Information
Francine Grodstein, Rush Alzheimer’s Disease Center, Chicago, Illinois, USA; Department of Internal Medicine, Rush University Medical Center, Chicago, Illinois, USA.
Chiang-Hua Chang, Institute for Healthcare Policy and Innovation, University of Michigan, Ann Arbor, Michigan, USA; Department of Internal Medicine, University of Michigan, Ann Arbor, Michigan, USA.
Ana W Capuano, Rush Alzheimer’s Disease Center, Chicago, Illinois, USA; Department of Neurological Sciences, Rush University Medical Center, Chicago, Illinois, USA.
Melinda C Power, Department of Epidemiology, Milken Institute School of Public Health, George Washington University, Washington, District of Columbia, USA.
David X Marquez, Rush Alzheimer’s Disease Center, Chicago, Illinois, USA; Department of Kinesiology and Nutrition, University of Illinois at Chicago, Chicago, Illinois, USA.
Lisa L Barnes, Rush Alzheimer’s Disease Center, Chicago, Illinois, USA; Department of Neurological Sciences, Rush University Medical Center, Chicago, Illinois, USA.
David A Bennett, Rush Alzheimer’s Disease Center, Chicago, Illinois, USA; Department of Neurological Sciences, Rush University Medical Center, Chicago, Illinois, USA.
Bryan D James, Rush Alzheimer’s Disease Center, Chicago, Illinois, USA; Department of Internal Medicine, Rush University Medical Center, Chicago, Illinois, USA.
Julie P W Bynum, Institute for Healthcare Policy and Innovation, University of Michigan, Ann Arbor, Michigan, USA; Department of Internal Medicine, University of Michigan, Ann Arbor, Michigan, USA.
Funding
This research was supported by grants from the National Institute on Aging at the National Institutes of Health (AG P30AG10161, P30AG72975, R01AG17917, RF1AG22018, R01AG062711, P01AG019783) and grant K01AG050823 to B.D.J.
Conflict of Interest
None declared.
Author Contributions
F.G.: Conceptualization; Data curation; Investigation; Methodology; Writing—original draft. C.-H.C.: Conceptualization; Data curation; Analysis; Investigation; Methodology; Writing—review and editing. A.W.C.: Data curation; Investigation; Analysis; Methodology; Writing—review and editing. M.C.P.: Investigation; Methodology; Writing—review and editing. D.X.M.: Data curation; Investigation; Methodology; Funding Acquisition; Supervision; Writing—review and editing. L.L.B.: Data curation; Investigation; Methodology; Funding Acquisition; Supervision; Writing—review and editing. D.A.B.: Data curation; Investigation; Methodology; Funding Acquisition; Supervision; Writing—review and editing. B.D.J.: Conceptualization; Data curation; Investigation; Methodology; Project Administration; Writing—review and editing. J.P.W.B.: Funding acquisition: Conceptualization; Data curation; Investigation; Methodology; Project Administration; Supervision; Writing—review and editing.
References
- 1. et al. Alzheimer’s disease facts and figures. [Ebook]. Chicago, 2021. Retrieved from https://alz.org/media/Documents/alzheimers-facts-and-figures-2021-r.pdf
- 2. Chen Y, Tysinger B, Crimmins E, Zissimopoulos JM. Analysis of dementia in the US population using Medicare claims: insights from linked survey and administrative claims data. Alzheimers Dement (N Y). 2019;5:197–207. doi: 10.1016/j.trci.2019.04.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Amjad H, Roth DL, Sheehan OC, Lyketsos CG, Wolff JL, Samus QM. Underdiagnosis of dementia: an observational study of patterns in diagnosis and awareness in US older adults. J Gen Intern Med. 2018;33(7):1131–1138. doi: 10.1007/s11606-018-4377-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Lee E, Gatz M, Tseng C, et al. Evaluation of medicare claims data as a tool to identify dementia. J Alzheimers Dis. 2019;67(2):769–778. doi: 10.3233/JAD-181005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Jain S, Rosenbaum PR, Reiter JG, et al. Using medicare claims in identifying Alzheimer’s disease and related dementias. Alzheimer’s Dement. 2021;17:515–524. Doi: 10.1002/alz.12199 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Moura LMVR, Festa N, Price M, et al. Identifying medicare beneficiaries with dementia. J Am Geriatr Soc. 2021;69(8):2240–2251. doi: 10.1111/jgs.17183 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Bennett DA, Buchman AS, Boyle PA, Barnes LL, Wilson RS, Schneider JA. Religious Orders Study and Rush Memory and Aging Project. J Alzheimers Dis. 2018;64(s1):S161–S189. doi: 10.3233/JAD-179939 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Barnes LL, Shah RC, Aggarwal NT, Bennett DA, Schneider JA. The Minority Aging Research Study: ongoing efforts to obtain brain donation in African Americans without dementia. Curr Alzheimer Res. 2012;9(6):734–745. doi: 10.2174/156720512801322627 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Schneider JA, Aggarwal NT, Barnes L, Boyle P, Bennett DA. The neuropathology of older persons with and without dementia from community versus clinic cohorts. J Alzheimers Dis. 2009;18(3):691–701. doi: 10.3233/JAD-2009-1227 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Marquez DX, Glover CM, Lamar M, et al. Representation of older Latinxs in cohort studies at the Rush Alzheimer’s Disease Center. Neuroepidemiology. 2020;54(5):404–418. doi: 10.1159/000509626 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Bennett DA, Wilson RS, Schneider JA, et al. Natural history of mild cognitive impairment in older persons. Neurology. 2002;59(2):198–205. doi: 10.1212/wnl.59.2.198 [DOI] [PubMed] [Google Scholar]
- 12. McKhann G, Drachman D, Folstein M, Katzman R, Price D, Stadlan EM. Clinical diagnosis of Alzheimer’s disease: report of the NINCDS-ADRDA Work Group under the auspices of Department of Health and Human Services Task Force on Alzheimer’s disease. Neurology. 1984;34(7):939–944. doi: 10.1212/wnl.34.7.939 [DOI] [PubMed] [Google Scholar]
- 13. Bennett DA, Schneider JA, Aggarwal NT, et al. Decision rules guiding the clinical diagnosis of Alzheimer’s disease in two community-based cohort studies compared to standard practice in a clinic-based cohort study. Neuroepidemiology. 2006;27(3):169–176. doi: 10.1159/000096129 [DOI] [PubMed] [Google Scholar]
- 14. McCarthy EP, Chang C-H, Tilton N, Langa KM, Bynum JPW. Validation of claims algorithms to identify Alzheimer’s disease and related dementias. J. Gerontol. A Biol. Sci. Med. Sci. 2021:glab373. doi: 10.1093/gerona/glab373 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Arvanitakis Z, Leurgans SE, Fleischman DA, et al. Memory complaints, dementia, and neuropathology in older blacks and whites. Ann Neurol. 2018;83(4):718–729. doi: 10.1002/ana.25189 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Boyle PA, Yu L, Schneider JA, Wilson RS, Bennett DA. Scam awareness related to incident Alzheimer dementia and mild cognitive impairment: a prospective cohort study. Ann Intern Med. 2019;170(10):702–709. doi: 10.7326/M18-2711 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Elixhauser A, Steiner C, Harris DR, Coffey RM. Comorbidity measures for use with administrative data. Med Care. 1998;36(1):8–27. doi: 10.1097/00005650-199801000-00004 [DOI] [PubMed] [Google Scholar]
- 18. America’s Health Insurance Plans. Medicare Advantage Demographics Report, 2016. Washington DC, 2019. Retrieved from https://www.ahip.org/medicare-advantage-demographics-report-2016 [Google Scholar]
- 19. Taylor DH Jr, Sloan FA, Doraiswamy PM. Marked increase in Alzheimer’s disease identified in Medicare claims records between 1991 and 1999. J Gerontol A Biol Sci Med Sci. 2004;59(7):762–766. doi: 10.1093/gerona/59.7.m762 [DOI] [PubMed] [Google Scholar]
- 20. Zhu Y, Chen Y, Crimmins EM, Zissimopoulos JM. Sex, race, and age differences in prevalence of dementia in Medicare claims and survey data. J Gerontol B Psychol Sci Soc Sci. 2021;76(3):596–606. doi: 10.1093/geronb/gbaa083 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Power MC, Gianattasio KZ, Ciarleglio A. Implications of the use of algorithmic diagnoses or Medicare claims to ascertain dementia. Neuroepidemiology. 2020;54(6):462–471. doi: 10.1159/000510753 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Gianattasio KZ, Prather C, Glymour MM, Ciarleglio A, Power MC. Racial disparities and temporal trends in dementia misdiagnosis risk in the United States. Alzheimers Dement (N Y). 2019;5:891–898. doi: 10.1016/j.trci.2019.11.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.