Abstract
Purpose:
Dysphagia is a common sequela of Parkinson disease (PD) and is associated with malnutrition, aspiration pneumonia, and mortality. This review article synthesized evidence regarding the effectiveness of interventions for dysphagia in PD.
Method:
Electronic searches were conducted in Ovid MEDLINE, Embase, Cochrane Central Register of Controlled Trials, CINAHL, and speechBITE. Of the 2,015 articles identified, 26 met eligibility criteria: interventional or observational studies with at least five or more participants evaluating dysphagia interventions in adults with PD-related dysphagia, with outcomes measured using videofluoroscopic swallowing study (VFSS), fiberoptic endoscopic evaluation of swallowing (FEES), or electromyography (EMG). Risk of bias (RoB) was evaluated using the Evidence Project tool and predetermined criteria regarding the rigor of swallowing outcome measures.
Results:
Interventions were classified as follows: pharmacological (n = 11), neurostimulation (n = 8), and behavioral (n = 7). Primary outcome measures varied across studies, including swallowing timing, safety, and efficiency, and were measured using VFSS (n = 17), FEES (n = 6), and EMG (n = 4). Critical appraisal of study findings for RoB, methodological rigor, and transparency showed the majority of studies failed to adequately describe contrast media used, signal acquisition settings, and rater blinding to time point. Low certainty evidence generally suggested improved swallow timing with exercises with biofeedback and deep brain stimulation (DBS), improved safety with DBS and expiratory muscle strength training, and improved efficiency with the Lee Silverman Voice Treatment and levodopa.
Conclusions:
Studies with lower RoB and greater experimental rigor showed potential benefit in improving swallowing efficiency but not safety. Further research investigating discrete changes in swallowing pathophysiology post-intervention is warranted to guide dysphagia management in PD.
Supplemental Material:
Parkinson disease (PD) is one of the most common neurological disorders internationally, with a rising prevalence with age (De Rijk et al., 1995; Pringsheim et al., 2014; von Campenhausen et al., 2005). In the context of increasing life expectancies globally, a steady increase in PD is anticipated, with almost 9 million people affected by 2030 (Dorsey et al., 2007; Pringsheim et al., 2014; Suttrup & Warnecke, 2016). This debilitating condition is known to affect the central and peripheral nervous systems, with the most salient histopathological feature being the presence of α-synuclein aggregates (Lewy bodies and Lewy neurites; Braak et al., 2004; Mu et al., 2013). Disrupted neural signaling in PD is also attributed to neuroinflammatory processes, mitochondrial dysfunction, and altered apoptosis pathways (Rocha et al., 2018). Although PD primarily involves degeneration of the nigrostriatal dopaminergic pathway (Braak et al., 2004; Chaudhuri et al., 2006; Mu et al., 2013; Pringsheim et al., 2014), it also impacts other neural systems, causing neuromediator dysfunctions, which, in turn, result in complex functional deficits (Jellinger, 1991; Mu et al., 2013). Bulbar dysfunctions (including dysphagia, hypophonia, dysarthria, and sialorrhea) are frequently noted in PD and are equally, if not more, debilitating as the hallmark features (Braak et al., 2004; Chaudhuri et al., 2006; Leopold & Kagel, 1997; Miller et al., 2006; Nilsson et al., 1996; Potulska et al., 2003). In particular, dysphagia is significantly associated with malnutrition and aspiration pneumonia in PD, with the latter being a leading cause of death in this population (Baijens & Speyer, 2009; Beyer et al., 2001; Johnston et al., 1995; Kalf et al., 2012; Wang et al., 2002). Dysphagia also negatively impacts quality of life, with patients reporting restricted participation in social activities involving eating and drinking (Andersson & Sidenvall, 2001; Clarke et al., 1998; Ekberg et al., 2002; Farri et al., 2007; Plowman-Prine et al., 2009).
There is limited evidence regarding the pathophysiological mechanisms underlying oropharyngeal dysphagia in PD. Treatments frequently involve a combination of rehabilitative and compensatory approaches (Smith et al., 2012). Rehabilitative approaches include resistance exercises for the laryngeal, respiratory, and orofacial muscles. Compensatory strategies aim to make eating and drinking safer without inducing longer lasting changes in swallowing physiology.
The majority of previously published systematic reviews examining the relative effectiveness of dysphagia treatments date back to 2014 or earlier (Baijens & Speyer, 2009; Deane et al., 2001; Smith et al., 2012; Van Hooren et al., 2014) and lack comprehensive consideration of different treatment modalities (i.e., pharmacological, neurostimulation, and behavioral approaches). Additionally, these historical reviews, together with a more recent review by López-Liria et al. (2020), display unevenness in the appraisal of study quality, rigor, and transparency. Thus, the purpose of this systematic review was to identify and evaluate literature regarding the efficacy of pharmacological, neurostimulation, and behavioral interventions as distinct categories for the treatment of dysphagia in patients with PD as well as to carefully scrutinize and critically appraise study findings, methodological rigor, and transparency in order to guide evidence-informed clinical decision-making.
With respect to experimental rigor and transparency, we were particularly interested to review details regarding the instrumental methods that were used to measure treatment outcomes. Videofluoroscopic swallowing study (VFSS) and fiberoptic endoscopic evaluation of swallowing (FEES) are widely accepted as gold standard approaches for dysphagia diagnosis in clinical practice. However, even these procedures have been criticized for a lack of standards and poor interrater agreement (Kuhlemeier et al., 1998; McCullough et al., 2001; Ott, 1998; Plowman & Humbert, 2018; Swan et al., 2019; Tohara et al., 2010). Several recent papers note that nonstandardized VFSS practices persist in clinical practice, both in the United States and internationally (Boaden et al., 2020, 2021; Martin-Harris et al., 2021). Accordingly, we felt it was important to appraise the rigor with which the methods of these instrumental examinations were performed and reported in research studies measuring treatment outcomes for dysphagia in PD. Variations that may impact diagnostic accuracy and measures of swallowing physiology include, but are not limited to, variations in signal acquisition settings and frame rate (e.g., Bonilha et al., 2013; Peladeau-Pigeon & Steele, 2013, 2015), contrast media concentration (e.g., Steele et al., 2013), the consistencies studied (e.g., Steele, Peladeau-Pigeon, et al., 2019), bolus volume (e.g., Butler et al., 2011), and whether or not participants were instructed to wait for a cue before initiating a swallow (e.g., Daniels et al., 2007; Nagy et al., 2013). It is important to understand not only the protocols that were used but also how the data were processed. For example, in a protocol containing several sips of thin liquid barium, it is critical to know whether the resulting data represent the mean value across task repetitions within participants, reflect data for all swallows (with appropriate handling of repeated measures), or reflect data for a particular swallow (e.g., the first bolus or the bolus showing the worst score on a particular parameter). Furthermore, given that studies suggest that penetration–aspiration and swallowing physiology may vary within an individual across repeated sips of thin liquid (Steele, Mukherjee, et al., 2019) or across tasks of different consistencies and volumes (Hazelwood et al., 2017), an important aspect of rigor in reporting is to understand the number of boluses of each consistency and volume that were included in a protocol. For this purpose, we developed an a priori criterion-based set of 10 quality indicators based on questions proposed for the assessment of study quality and rigor in two recent reviews exploring dysphagia treatment outcomes (Bahia & Lowell, 2020; Mancopes et al., 2020). As listed in Table 1, these included questions regarding the number of boluses and consistencies tested, bolus volumes, contrast media, recording settings, the time point of rating, rater blinding, and reliability.
Table 1.
Questions used in the appraisal of rigor in instrumental evaluations of swallowing.
| Was more than one bolus tested? |
| Was more than one consistency tested? |
| Were details regarding volume reported? |
| If used, were details regarding barium (or other contrast) concentration reported? |
| Were details regarding recording settings reported (specifically signal acquisition rate)? |
| Were ratings made post hoc from recorded signals (as opposed to online)? |
| Were raters blinded to participant ID/group assignment? |
| Were raters blinded to time point/condition? |
| Were interrater reliability statistics reported? |
| Were intrarater reliability statistics reported? |
Method
Literature Search
A comprehensive literature search was carried out by a trained health information specialist in May 2019. The search was conducted according to the Cochrane Handbook for Systematic Reviews of Interventions (Higgins et al., 2019) and the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) statement (Moher et al., 2009). Electronic database searches were conducted in Ovid MEDLINE, Embase, CINAHL, speechBITE, and Cochrane Central Register of Controlled Trials, with keywords and subject headings related to swallowing, dysphagia, and PD. The full search strategy can be found in the Appendix. The search was limited to peer-reviewed English-language human studies published from database inception to May 2019. Reference lists of all articles included for synthesis were hand-searched for additional relevant articles.
Selection Criteria
Studies were eligible if they included adult (over 18 years of age) patients with idiopathic PD and associated oropharyngeal dysphagia as well as examined the effect of a dysphagia-targeted intervention with pre- and posttreatment comparison. Studies describing individuals with non-idiopathic parkinsonian syndromes were excluded. Studies were required to report outcomes using one or more of the following instrumental methods: VFSS, FEES, and/or electromyography (EMG). Studies were excluded if they did not report primary data (i.e., editorials, systematic reviews, book chapters), were single-case reports, or were limited to interventions for esophageal dysphagia with no oropharyngeal component. Conference proceedings and other gray literature were also excluded. Two reviewers independently screened the titles and abstracts of identified citations, followed by a full-text review of potentially eligible studies. Disagreements regarding inclusion were resolved by consensus.
Data Extraction and Quality Appraisal
Two reviewers performed data extraction independently and in duplicate using data extraction forms. The information extracted included study characteristics; patient demographics; characterization of PD based on severity and duration; intervention type, intensity, and duration; and reported swallowing outcomes. Risk of bias (RoB) was evaluated according to a tool developed by the Evidence Project (Kennedy et al., 2019), which has been validated across both randomized and nonrandomized studies. This tool includes eight criteria, each of which is rated as being present (yes) or not (no), not reported (em dash), or not applicable (blank cell). The tool assesses whether (a) a cohort of participants was followed over time and included multiple assessments with the same participants, (b) intervention outcomes were compared against a control or comparison group, (c) pre- and post-intervention data were reported, (d) there was random assignment of participants to the intervention, (e) participants were randomly selected for enrollment from an available pool of candidates, (f) the study group had a follow-up rate of 80% or more, (g) the comparison groups were equivalent on sociodemographic factors, and (h) comparison groups were equivalent at baseline on the selected outcome measures. Overall RoB was classified as high if more than 80% of the criteria were scored as absent or not reported and low if at least 80% or more of the criteria were rated as being present. In cases where particular criteria were not applicable, the denominator was adjusted to reflect the number of articles for which the criterion applied. The rigor and reporting transparency of the instrumental methods were appraised using the criteria in Table 1, including questions regarding the number of boluses and consistencies tested, bolus volumes, contrast media, recording settings, the time point of rating, rater blinding, and reliability.
Data Synthesis
Where sufficient data were available, a meta-analysis was planned. Given heterogeneity in study designs and a paucity of “poolable” data for the outcomes of interest, the method and results were summarized descriptively for all reported videofluoroscopic, endoscopic, and electromyographic measures, and overall findings were summarized narratively.
Results
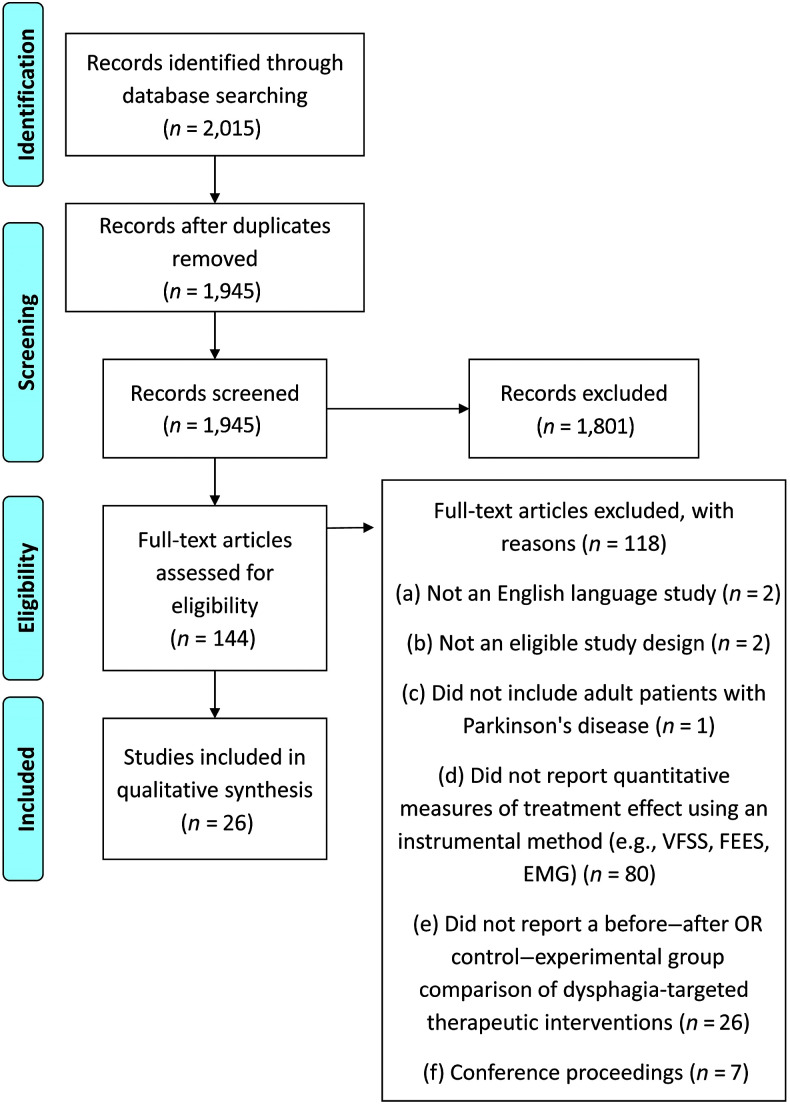
Figure 1 shows the PRISMA diagram summarizing the search strategy and results for this review article. Of the 2,015 citations identified by the search, 1,945 were screened for eligibility after duplicates were removed. Of these, 144 studies were considered potentially eligible, requiring full-text review, and 26 were found to meet all criteria for inclusion and synthesis (Alfonsi et al., 2017; Argolo et al., 2013; Athukorala et al., 2014; Baijens et al., 2013; Bushmann et al., 1989; Ciucci et al., 2008; El Sharkawi et al., 2002; Fuh et al., 1997; Hirano et al., 2015; Hunter et al., 1997; Khedr et al., 2019; Kondo et al., 2017; Kulneff et al., 2013; Lengerer et al., 2012; Michou et al., 2014; Miles et al., 2017; Monte et al., 2005; Pitts et al., 2009; Stegemöller et al., 2017; Sundstedt, Holmén, et al., 2017; Sundstedt et al., 2012; Tawadros et al., 2012; Tison et al., 1996; Troche et al., 2010; Warnecke et al., 2016; Xie et al., 2015). Interrater agreement between two reviewers was calculated, using the kappa statistic, to be .59 (moderate agreement) at the title and abstract screening stage and .71 (substantial agreement) at the full-text review stage (McHugh, 2012).
Figure 1.
Preferred Reporting Items for Systematic Reviews and Meta-Analyses diagram showing the process followed for selecting articles for inclusion in the review. VFSS = videofluoroscopic swallowing study; FEES = fiberoptic endoscopic evaluation of swallowing; EMG = electromyography.
Study Characteristics
A summary of study characteristics and participant demographics for the included studies can be found in Supplemental Material S1. The majority of studies were interventional. Eleven studies were randomized controlled trials (RCTs). Nine studies were before–after trials. Four studies were prospective cohort studies, and two studies were retrospective cohort studies. All studies were single-center studies and were conducted across 12 countries, including Australia, Brazil, France, Germany, Italy, Japan, the Netherlands, New Zealand, Sweden, Taiwan, the United Kingdom, and the United States of America.
Sample sizes varied widely, ranging from six to 90 participants, with a mean age ranging from 49.3 to 75.0 years. Of the 24 studies reporting descriptive statistics regarding the age of participants with PD, 21 reported a mean or median age of over 60 years for the patient participants (Alfonsi et al., 2017; Athukorala et al., 2014; Bushmann et al., 1989; Ciucci et al., 2008; El Sharkawi et al., 2002; Fuh et al., 1997; Hirano et al., 2015; Hunter et al., 1997; Khedr et al., 2019; Kulneff et al., 2013; Michou et al., 2014; Miles et al., 2017; Monte et al., 2005; Pitts et al., 2009; Stegemöller et al., 2017; Sundstedt et al., 2012; Tawadros et al., 2012; Tison et al., 1996; Troche et al., 2010; Warnecke et al., 2016; Xie et al., 2015). The remaining three studies had participants with ages between 45 and 60 years (Argolo et al., 2013; Lengerer et al., 2012; Sundstedt, Holmén, et al., 2017). Across the 21 studies reporting the gender distribution of included participants, the mean proportion of male participants was 70% (Alfonsi et al., 2017; Argolo et al., 2013; Athukorala et al., 2014; Baijens et al., 2013; Bushmann et al., 1989; Ciucci et al., 2008; El Sharkawi et al., 2002; Hirano et al., 2015; Hunter et al., 1997; Kondo et al., 2017; Kulneff et al., 2013; Lengerer et al., 2012; Michou et al., 2014; Miles et al., 2017; Monte et al., 2005; Pitts et al., 2009; Stegemöller et al., 2017; Sundstedt, Holmén, et al., 2017; Sundstedt et al., 2012; Troche et al., 2010; Xie et al., 2015).
Of the 13 studies reporting the criteria used to confirm the diagnosis of PD, 10 used the United Kingdom Parkinson's Disease Society Brain Bank Clinical Diagnostic Criteria (Alfonsi et al., 2017; Argolo et al., 2013; Hirano et al., 2015; Hunter et al., 1997; Khedr et al., 2019; Lengerer et al., 2012; Michou et al., 2014; Pitts et al., 2009; Troche et al., 2010; Warnecke et al., 2016). Of the remaining three studies, one based eligibility on a neurologist-confirmed diagnosis (Athukorala et al., 2014), one required participants to have at least two symptoms from a set of three (resting tremor, rigidity, and/or bradykinesia; Fuh et al., 1997), and one determined eligibility using criteria outlined in a textbook (Tison et al., 1996). Of the 19 studies reporting the severity of PD, 16 used the Hoehn and Yahr scale (Argolo et al., 2013; Athukorala et al., 2014; Baijens et al., 2013; Bushmann et al., 1989; Ciucci et al., 2008; El Sharkawi et al., 2002; Fuh et al., 1997; Hirano et al., 2015; Hoehn & Yahr, 1967; Khedr et al., 2019; Michou et al., 2014; Monte et al., 2005; Pitts et al., 2009; Sundstedt, Holmén, et al., 2017; Tison et al., 1996; Troche et al., 2010; Warnecke et al., 2016). The remaining three studies (Lengerer et al., 2012; Sundstedt et al., 2012; Xie et al., 2015) used the Unified Parkinson's Disease Rating Scale, Part III (Martinez-Martin et al., 1994).
The majority of studies (n = 17) measured outcomes using VFSS (Argolo et al., 2013; Baijens et al., 2013; Bushmann et al., 1989; Ciucci et al., 2008; El Sharkawi et al., 2002; Fuh et al., 1997; Hirano et al., 2015; Hunter et al., 1997; Khedr et al., 2019; Lengerer et al., 2012; Michou et al., 2014; Miles et al., 2017; Monte et al., 2005; Pitts et al., 2009; Tison et al., 1996; Troche et al., 2010; Xie et al., 2015). FEES was used to measure swallowing outcomes in six studies (Baijens et al., 2013; Kondo et al., 2017; Kulneff et al., 2013; Sundstedt, Holmén, et al., 2017; Sundstedt et al., 2012; Warnecke et al., 2016). The four remaining studies reported outcomes measured using EMG, of which three used surface EMG (sEMG; Athukorala et al., 2014; Stegemöller et al., 2017; Tawadros et al., 2012) and one used intramuscular EMG (Alfonsi et al., 2017).
Reported Results
Supplemental Material S1 also summarizes the intervention approaches used and the reported results for the included studies. These will be briefly described by intervention type.
Pharmacological Interventions
Across the 26 studies included for synthesis, nine explored the effects of dopamine agonist medications on swallowing (e.g., levodopa, carbidopa, apomorphine, domperidone, rotigotine). Bushmann et al. (1989) found that administering levodopa with carbidopa led to partial improvements in swallowing efficiency in the form of faster transit times and reduced residue. Mixed results were reported by Fuh et al. (1997), with improvements seen in six of 12 patients receiving levodopa with benserazide, including reductions in pharyngeal residue. This was concordant with results from the work of Warnecke et al. (2016), who demonstrated improvement in swallowing efficiency and residue with levodopa administration in seven of 15 patients. By contrast, Monte et al. (2005) observed no improvements in swallowing efficiency with levodopa. Similarly, Tawadros et al. (2012) found no influence of levodopa on submental sEMG burst parameters, laryngeal parameters, or number of swallows at any volume.
Two studies reported shorter oral preparatory phase durations and shorter pharyngeal transit times after the administration of apomorphine (Hunter et al., 1997; Tison et al., 1996). The Tison et al. (1996) study also reported reductions in residue and piecemeal swallowing in seven of eight patients and improvements in airway protection in two of three patients with laryngeal penetration at baseline. Hirano et al. (2015) investigated the effectiveness of a rotigotine patch in improving swallowing efficiency and reported shorter pharyngeal transit times in all six patients.
Other pharmacological interventional studies included a single study that explored the effect of botulinum toxin injections on opening of the upper esophageal sphincter (UES) in a mixed sample of patients with neurological diagnoses, including 12 with PD (Alfonsi et al., 2017). Among these 12 patients, six were reported to show a strong response after a first injection, with four more showing partial response. Finally, Kondo et al. (2017) explored the effects of applying capsaicin ointment to the external auditory canal, with the goal of stimulating the vagus nerve. The experimental group (n = 10) included one participant with PD. They reported groupwise improvements in glottal closure, timing, and efficiency in the experimental group compared with no changes in the placebo group, which included two participants with PD.
Neurostimulation Interventions
Several studies explored the impact of neurostimulation approaches to intervention, with six studying the impact of deep brain stimulation (DBS). Ciucci et al. (2008) reported improvements in swallowing timing and pharyngeal composite score. Lengerer et al. (2012) also reported improvements in swallowing timing and latency. Xie et al. (2015) reported that 60-Hz stimulation reduced the frequency of aspiration by 57%, with benefits persisting at a 6-week follow-up assessment. On the contrary, Sundstedt et al. (2012) found that initial reductions of premature spillage were not maintained 1 year post. The same group replicated these results in 2017, noting no changes in premature spillage, penetration–aspiration, or pharyngeal residue (Sundstedt, Holmén, et al., 2017). Similarly, Kulneff et al. (2013) found no significant effect of DBS on FEES parameters, including secretions, premature spillage, penetration–aspiration, and residue. Within this category, a single study described the impact of transcutaneous neuromuscular electrical stimulation (VitalStim; Baijens et al., 2013), showing no differences for any visuoperceptual measures on FEES or VFSS. Similarly, a single study explored the impact of repetitive transcranial magnetic stimulation (rTMS) on swallowing safety and efficiency (Khedr et al., 2019) but found no differences in penetration–aspiration or residue between sham and real rTMS groups.
Behavioral Interventions
The remaining seven studies in this review article explored the effects of behavioral interventions. Of these, two measured the effect of the Lee Silverman Voice Treatment (LSVT) program. El Sharkawi et al. (2002) found the LSVT to be effective in shortening timing measures and reducing oral residue for 3- and 5-ml liquid swallows posttreatment. Miles et al. (2017) showed improvements after the LSVT in the form of reduced pharyngeal residue and significantly increased duration and maximal opening of the UES. Two studies described the effects of expiratory muscle strength training (EMST; Pitts et al., 2009; Troche et al., 2010), with both reporting significant improvements in penetration–aspiration after training. The three remaining studies described exercise-based interventions, as follows:
Argolo et al. (2013) employed an exercise program targeting “strength and range of motion of the mouth, larynx and pharyngeal structures, coordination between breathing and swallowing, and airway protection.”
Athukorala et al. (2014) used sEMG biofeedback to train skills in generating submental muscle contractions with precise timing and amplitude.
Stegemöller et al. (2017) studied the effects of a therapeutic singing intervention.
Neither the Argolo et al. study nor the Stegemöller et al. study observed any improvements in swallowing after the intervention. Athukorala et al. noted some changes in timing measures of submental muscle contraction for dry swallows.
Summaries of the RoB evaluations performed using the Evidence Project tool can be found in Table 2. The study by Baijens et al. (2013) included separate reporting and analysis of outcomes measured using VFSS and FEES and is therefore included twice, reflecting separate appraisals of these two portions of the study. Common concerns with respect to bias included failure to report any information regarding whether participants were randomly selected for assessment (17 of 27 assessments; Alfonsi et al., 2017; Athukorala et al., 2014; Baijens et al., 2013; Bushmann et al., 1989; Ciucci et al., 2008; Fun et al., 1997; Hunter et al., 1997; Kondo et al., 2017; Lengerer et al., 2012; Michou et al., 2014; Pitts et al., 2009; Stegemöller et al., 2017; Tawadros et al., 2012; Tison et al., 1996; Troche et al., 2010; Xie et al., 2015). Of the 10 studies where this item was reported, none used random selection during participant recruitment (Argolo et al., 2013; El Sharkawi et al., 2002; Hirano et al., 2015; Khedr et al., 2019; Kulneff et al., 2013; Miles et al., 2017; Monte et al., 2005; Sundstedt, Holmén, et al., 2017; Sundstedt et al., 2012; Warnecke et al., 2016). Attrition rates were below 20% across all studies, except two (Monte et al., 2005; Warnecke et al., 2016). Overall, a high RoB was identified in 22 assessments (Alfonsi et al., 2017; Argolo et al., 2013; Athukorala et al., 2014; Bushmann et al., 1989; Ciucci et al., 2008; El Sharkawi et al., 2002; Fuh et al., 1997; Hirano et al., 2015; Hunter et al., 1997; Kondo et al., 2017; Kulneff et al., 2013; Lengerer et al., 2012; Miles et al., 2017; Monte et al., 2005; Pitts et al., 2009; Stegemöller et al., 2017; Sundstedt, Holmén, et al., 2017; Sundstedt et al., 2012; Tawadros et al., 2012; Tison et al., 1996; Warnecke et al., 2016; Xie et al., 2015).
Table 2.
Risk-of-bias evaluation using the Evidence Project tool.
| Study | Cohort study? | Control/ comparison group? | Pre- and post-intervention data reported? | Random assignment of participants to intervention? | Random selection of participants for enrollment? | Follow-up rate of 80% or more? | Comparison groups equivalent on sociodemographics? | Comparison groups equivalent at baseline on disclosure? | Overall risk-of-bias score |
|---|---|---|---|---|---|---|---|---|---|
| Alfonsi et al. (2017) | Yes | No | No | No | — | Yes | No | No | 2/8 = 25.0% |
| Argolo et al. (2013) | Yes | No | Yes | No | Yes | 2/5 = 40.0% | |||
| Athukorala et al. (2014) | Yes | No | Yes | — | Yes | 2/5 = 40.0% | |||
| Baijens et al. (2013): VFSS arm | Yes | Yes | Yes | Yes | — | Yes | Yes | Yes | 7/8 = 87.5% |
| Baijens et al. (2013): FEES arm | Yes | Yes | Yes | Yes | — | Yes | Yes | Yes | 7/8 = 87.5% |
| Bushmann et al. (1989) | Yes | Yes | Yes | — | Yes | Yes | No | 5/7 = 71.4% | |
| Ciucci et al. (2008) | Yes | No | Yes | — | Yes | 3/5 = 60.0% | |||
| El Sharkawi et al. (2002) | Yes | No | Yes | No | Yes | 3/5 = 60.0% | |||
| Fuh et al. (1997) | Yes | No | Yes | — | Yes | 3/5 = 60.0% | |||
| Hirano et al. (2015) | Yes | No | Yes | No | Yes | 3/5 = 60.0% | |||
| Hunter et al. (1997) | Yes | No | Yes | — | Yes | 3/6 = 60.0% | |||
| Khedr et al. (2019) | Yes | Yes | Yes | Yes | No | Yes | Yes | Yes | 7/8 = 87.5% |
| Kondo et al. (2017) | No | Yes | Yes | Yes | — | Yes | Yes | Yes | 6/8 = 75.0% |
| Kulneff et al. (2013) | Yes | No | Yes | No | Yes | 3/5 = 60.0% | |||
| Lengerer et al. (2012) | Yes | No | Yes | — | Yes | 3/5 = 60.0% | |||
| Michou et al. (2014) | Yes | Yes | Yes | — | Yes | Yes | Yes | 6/7 = 85.7% | |
| Miles et al. (2017) | Yes | No | Yes | No | Yes | 3/5 = 60.0% | |||
| Monte et al. (2005) | Yes | Yes | No | No | No | No | Yes | No | 3/8 = 37.5% |
| Pitts et al. (2009) | Yes | No | Yes | — | Yes | 3/5 = 60.0% | |||
| Stegemöller et al. (2017) | Yes | Yes | Yes | No | — | Yes | Yes | Yes | 6/8 = 75.0% |
| Sundstedt et al. (2012) | Yes | No | Yes | No | Yes | 3/5 = 60.0% | |||
| Sundstedt, Holmén, et al. (2017) | Yes | No | Yes | No | Yes | 3/5 = 60.0% | |||
| Tawadros et al. (2012) | Yes | Yes | Yes | — | Yes | Yes | No | 5/7 = 71.4% | |
| Tison et al. (1996) | Yes | No | Yes | — | Yes | 3/5 = 60.0% | |||
| Troche et al. (2010) | Yes | Yes | Yes | Yes | — | Yes | Yes | Yes | 7/8 = 87.5% |
| Warnecke et al. (2016) | Yes | No | Yes | No | No | 2/5 = 40.0% | |||
| Xie et al. (2015) | Yes | No | Yes | — | Yes | 3/5 = 60.0% |
Note. Em dashes indicate data not reported. VFSS = videofluoroscopic swallowing study; FEES = fiberoptic endoscopic evaluation of swallowing.
Figure 2 shows the results of the appraisal of rigor in the performance and reporting of instrumental measures of swallowing; these results reveal several shortcomings of the selected studies. Four studies reported outcomes based on swallowing of only a single bolus (Alfonsi et al., 2017; Kondo et al., 2017; Pitts et al., 2009; Tison et al., 1996). Seven studies reported results for only a single bolus consistency (Alfonsi et al., 2017; Kondo et al., 2017; Michou et al., 2014; Pitts et al., 2009; Tawadros et al., 2012; Tison et al., 1996; Troche et al., 2010). By contrast, all of the selected studies, with the exception of one (El Sharkawi et al., 2002), reported details regarding the bolus volumes tested. A methodological detail that was inadequately reported in multiple studies (n = 15) was the identification of the brands, concentrations, or preparation methods of barium or other contrast agents used (Argolo et al., 2013; Bushmann et al., 1989; El Sharkawi et al., 2002; Fuh et al., 1997; Hirano et al., 2015; Hunter et al., 1997; Khedr et al., 2019; Kondo et al., 2017; Kulneff et al., 2013; Lengerer et al., 2012; Monte et al., 2005; Sundstedt, Holmén, et al., 2017; Sundstedt et al., 2012; Warnecke et al., 2016; Xie et al., 2015). Similarly, details regarding recording settings and signal acquisition rates were missing from 15 studies (Argolo et al., 2013; Athukorala et al., 2014; Bushmann et al., 1989; El Sharkawi et al., 2002; Fuh et al., 1997; Hirano et al., 2015; Khedr et al., 2019; Kondo et al., 2017; Kulneff et al., 2013; Pitts et al., 2009; Sundstedt, Holmén, et al., 2017; Sundstedt et al., 2012; Tawadros et al., 2012; Tison et al., 1996; Warnecke et al., 2016).
Figure 2.
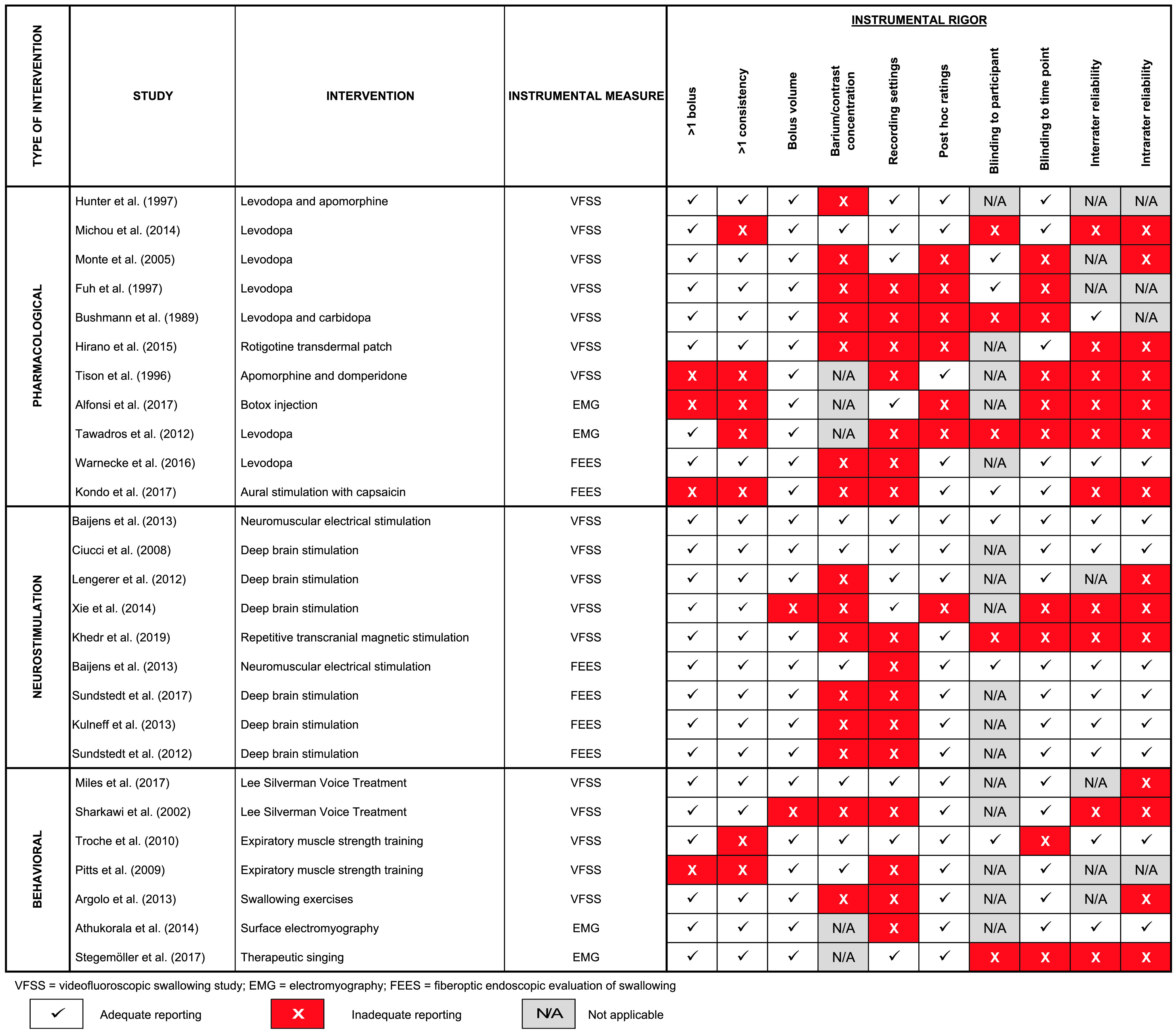
Appraisal of rigor used in instrumental measures of swallowing. Included studies are grouped by intervention type and listed in descending order of instrumental rigor.
In seven studies, rating was described as being performed online as opposed to post hoc from recorded signals (Alfonsi et al., 2017; Bushmann et al., 1989; Fuh et al., 1997; Hirano et al., 2015; Monte et al., 2005; Tawadros et al., 2012; Xie et al., 2015). Rating by multiple individuals was used in 20 of the selected studies (Alfonsi et al., 2017; Athukorala et al., 2014; Baijens et al., 2013; Bushmann et al., 1989; Ciucci et al., 2008; El Sharkawi et al., 2002; Hirano et al., 2015; Khedr et al., 2019; Kondo et al., 2017; Kulneff et al., 2013; Michou et al., 2014; Stegemöller et al., 2017; Sundstedt, Holmén, et al., 2017; Sundstedt et al., 2012; Tawadros et al., 2012; Tison et al., 1996; Troche et al., 2010; Warnecke et al., 2016; Xie et al., 2015). Notably, interrater reliability statistics were not reported in half (i.e., 10) of these studies (Alfonsi et al., 2017; El Sharkawi et al., 2002; Hirano et al., 2015; Khedr et al., 2019; Kondo et al., 2017; Michou et al., 2014; Stegemöller et al., 2017; Tawadros et al., 2012; Tison et al., 1996; Xie et al., 2015). In five studies, raters were not blinded to participant identity or group assignment (Bushmann et al., 1989; Khedr et al., 2019; Michou et al., 2014; Stegemöller et al., 2017; Tawadros et al., 2012). Rater blinding to the important detail of the time point when data were collected relative to the intervention was employed in 15 studies (Argolo et al., 2013; Athukorala et al., 2014; Baijens et al., 2013; Ciucci et al., 2008; El Sharkawi et al., 2002; Hirano et al., 2015; Hunter et al., 1997; Kondo et al., 2017; Lengerer et al., 2012; Miles et al., 2017; Pitts et al., 2009; Sundstedt, Holmén, et al., 2017; Sundstedt et al., 2012; Warnecke et al., 2016).
Additional information regarding protocols across the 17 studies that used VFSS as an outcome measure is summarized in Table 3. Of these, two studies did not describe patient positioning/view (Hirano et al., 2015; Pitts et al., 2009), 11 performed the study in lateral view only (Argolo et al., 2013; Baijens et al., 2013; Bushmann et al., 1989; Khedr et al., 2019; Ciucci et al., 2008; El Sharkawi et al., 2002; Hunter et al., 1997; Lengerer et al., 2012; Michou et al., 2014; Monte et al., 2005; Troche et al., 2010), and four performed the study in both lateral and anterior–posterior views (Fuh et al., 1997; Miles et al., 2017; Tison et al., 1996; Xie et al., 2015). Seven studies did not report the videofluoroscopy frame rate. Where frame rate was reported, a single study performed VFSS at 15 frames per second (fps; Lengerer et al., 2012), two studies performed VFSS at 25 fps (Baijens et al., 2013; Hunter et al., 1997), and seven studies performed VFSS at 30 fps (Ciucci et al., 2008; Fuh et al., 1997; Michou et al., 2014; Miles et al., 2017; Monte et al., 2005; Troche et al., 2010; Xie et al., 2015). A summary of the protocols used for FEES and EMG is available in Tables 4 and 5, respectively.
Table 3.
Additional details regarding videofluoroscopy protocols used in the selected studies.
| Study | Intervention | Position | Equipment | Protocol | Analysis/blinding | Frames per second | Consistency/volume/barium |
|---|---|---|---|---|---|---|---|
| Argolo et al. (2013) | Swallowing exercises | Lateral | — | Thin liquid, thick liquid, puree, and soft solids. | Randomized and analyzed frame by frame by SLP blinded to the time point of measurement (pre- vs. posttherapy). | — |
|
| Baijens et al. (2013) | Neuromuscular electrical stimulation | Lateral | Philips Diagnost 97 system and a Panasonic AG-DVC30 mini-DV camcorder | Low-density barium (40% [wt/vol]), thickened barium, and crackers coated with barium paste. | Randomized. SLPs blinded to group, to time point of measurement (pre- vs. posttherapy), and to each other's ratings. | 25 |
|
| Bushmann et al. (1989) | Levodopa and carbidopa | Lateral | — | Thin liquid, thick liquid, custard, cookie, and usual medications. After baseline VFSS, patients took usual dose of levodopa. Second VFSS repeated after 90 min or subjective response. Non-PD participants only had single VFSS. | Independently rated by 2 SLPs, one of whom was blind to diagnosis. | — |
|
| Ciucci et al. (2008) | Deep brain stimulation | Lateral | Philips Universal R/F EasyDiagnost Eleva and Regis program | Single time point ≥ 3 months after surgery. VFSS with DBS-On and DBS-Off. 1 hr between conditions. Counterbalanced order. Standard clinical procedures were used. Instruction: “Swallow as you would typically.” | — | 30 |
|
| El Sharkawi et al. (2002) | Lee Silverman Voice Treatment | Lateral | VHS video recorder | VFSS before and after 1 month of the LSVT using a standard protocol. | Clinician was blinded to the time point of measurement (pre- vs. posttherapy). | — |
|
| Fuh et al. (1997) | Levodopa | Lateral + frontal position | Super VHS tape recorder | After a baseline VFSS examination, patients took 200 mg of levodopa (in combination with 50 mg of benserazide). A second VFSS examination was begun 60–90 min later. | Rated by one observer who was blinded to symptom severity but not to the time the drugs were taken. | 30 |
|
| Hirano et al. (2015) | Rotigotine transdermal patch | — | — | Screen with diluted solution of barium × 2. If swallowing was not severely impaired, concentrated solution of barium × 1 (unrestricted volume). Barium mixed with jelly was then swallowed. | One SLP and one neurologist who were blinded to all clinical details. Rating according to a scale established by the Japanese Society of Dysphagia Rehabilitation and the DOSS. | — |
|
| Hunter et al. (1997) | Levodopa and apomorphine | Lateral | Shimadzu image intensifier and a Panasonic Super VHS recorder | VFSS performed according to a standard protocol. | Evaluated independently by two SLPs blinded to the patient and timing of the swallow in relation to the dopaminergic challenge. | 25 |
|
| Khedr et al. (2019) | Repetitive transcranial magnetic stimulation | Lateral | GE Prestige II | VFSS was performed before and after rTMS sessions while patients were on levodopa therapy. Cued swallows. | — | — |
|
| Lengerer et al. (2012) | Deep brain stimulation | Lateral | Siemens Polystar X-ray machine | Three different consistencies across three conditions (preoperative, postoperative DBS-On, and postoperative DBS-Off). Participants took usual dopaminergic medication. Mean of 20.3 months and an SD of 8.6 between the pre- and postoperative exams. About 10 min between the postoperative conditions (DBS-On and DBS-Off). | VFSS images were blindly rated under the supervision of an experienced linguist. | 15 |
|
| Michou et al. (2014) | Levodopa | Lateral | Siemens Fluorospot Compact imaging system, Siemens Sireskop SX X-ray unit, and a Videomed DI-TV system (Sony DHR-1000) | Baseline VFSS, then usual first levodopa dose. After an hour of rest, pharyngeal catheter inserted. Cortical and cranial nerve stimulation administered. Catheter removed, and a second VFSS was performed. | SLP blinded to time point and medication status. | 30/25 | Thin: 6 swallows of 5-mL thin liquid barium (60% [wt/vol], E-Z-HD) |
| Miles et al. (2017) | Lee Silverman Voice Treatment | Lateral and anterior–posterior | Toshiba DF-323H videofluoroscope and Horita VS-50 Video Stopwatch | Lateral view: thin liquid barium: 20 ml, then 100 ml by straw. Instruction: “Drink the whole cup in your own time but without stopping.” Then, 5-ml barium paste. AP view: 20-ml thin bolus. Instruction: “Drink all in one go.” If residue seen, participant asked to perform a dry swallow. |
Authors blinded to participant and time point. | 30 |
|
| Monte et al. (2005) | Levodopa | Lateral | Super VHS tape recorder | 1. Thin barium × 2. Instruction to swallow all the bolus volume at once. 2. Bread × 2. Tap water rinses. On-drug, between 1 and 2 hr after last dose of levodopa. |
Performed by an examiner blinded to patient identity. | 30 |
|
| Pitts et al. (2009) | Expiratory muscle strength training | — | Kay Elemetrics Digital Swallowing Workstation (Model 7200) | 30-m thin bolus, swallowed in a continuous manner. | SLP blinded to experimental condition. | — |
|
Note. Em dashes indicate data not reported. SLP(s) = speech-language pathologist(s); VFSS = videofluoroscopic swallowing study; PD = Parkinson disease; DBS = deep brain stimulation; VHS = Video Home System; LSVT = Lee Silverman Voice Treatment; DOSS = Dysphagia Outcome and Severity Scale; rTMS = repetitive transcranial magnetic stimulation; AP = anterior–posterior.
Table 4.
Additional details regarding fiberoptic endoscopic evaluation of swallowing (FEES) protocols used in selected studies.
| Study | Intervention | Equipment | Protocol | Analysis/blinding | Consistency |
|---|---|---|---|---|---|
| Baijens et al. (2013) | VitalStim | PENTAX FNL-10RP3, Alphatron Stroboview ACLS camera, Alphatron light source, IVACX computerized video archiving system; recorded on a DVD | 10-ml thin liquid × 3, 10-ml thick liquid × 3, bite-sized crackers × 3 | Judges blinded to group, to time point of measurement (pre- vs. posttherapy), and to each other's ratings. | Thin liquid: water dyed with 5% methylene blue Thick liquid: applesauce dyed with 5% methylene blue |
| Kondo et al. (2017) | Aural stimulation with capsaicin ointment to the external auditory canal | PENTAX VNL-100S endoscope (3.1 mm in diameter) | Standard FEES protocol of The Oto-Rhino-Laryngological Society of Japan. Tested 5, 30, and 60 min after a single application of 0.5 g of 0.025% capsaicin or placebo ointment to the right external auditory canal. | Video images evaluated using endoscopic swallowing scoring and the SMRC scale by a second otolaryngologist blinded to clinical data and original ratings. | Water (3 ml) dyed with blue food coloring |
| Kulneff et al. (2013) | Deep brain stimulation | Olympus ENF-P4 transnasal flexible endoscope and a Wolf 5502 endocam | One solid and four different liquid consistencies. Started with thin liquid, then thicker and solid consistencies, and finished with water. | — |
|
| Sundstedt et al. (2012) | Deep brain stimulation | Olympus ENF-P4 transnasal flexible endoscope and a Wolf 5502 endocam | One solid and four different liquid consistencies. Started with thin liquid, then thicker and solid consistencies, and finished with water. | Video recordings were de-identified and randomly ordered. Scored according to a predefined protocol. |
|
| Sundstedt, Holmén, et al. (2017) | Deep brain stimulation | Olympus ENF-P4 transnasal flexible endoscope and a Wolf 5502 endocam. In later examinations, an Olympus ENF-VH flexible video endoscope and an Olympus CV-170 light source system. | One solid and four different liquid consistencies. For the paper, only the final 2 consistencies were analyzed. | Raters blinded to patient status, time point, DBS status, and swallowing function. |
|
| Warnecke et al. (2016) | Oral levodopa administration | Olympus ENF-P4 flexible fiberoptic rhinolaryngoscope (3.1 mm in diameter), a Storz endovision telecam SL PAL 20212020 light source, a Storz endovision telecam SL PAL 20212030 camera, a Sony DVM 14M2MDE color monitor, and a Sony SVO9500MDP video recorder |
|
Independently scoring in random order by two raters, blinded to patient and assessment conditions. |
|
Note. The em dash indicates data not reported. SMRC = sensory, motion, reflex, clearance; DBS = deep brain stimulation.
Table 5.
Additional details regarding electromyography (EMG) protocols used in selected studies.
| Author, year | Intervention | EMG protocol | Equipment | Consistencies |
|---|---|---|---|---|
| Alfonsi et al. (2017) | Botox | Three-channel recording: (a) suprahyoid/submental muscles (sEMG), (b) cricopharyngeus muscle (needle EMG), and (c) piezoelectric transducer signal collected on neck surface over cricothyroid membrane. Water (3 ml) administered via syringe and swallowed. | ||
| Athukorala et al. (2014) | Skill training therapy | Submental sEMG. Five saliva and five 10-mL water swallows with task types randomized within and between participants. Instructions to, “Hold the water/ saliva in your mouth and when you hear the go signal, swallow as quickly as possible.” Average premotor time, preswallow time, duration of submental muscle contraction calculated for each task, at each session, per participant. | KayPENTAX Digital Swallowing Workstation | |
| Stegemöller et al. (2017) | Singing | Right and left submental and laryngeal sEMG. Amplitude and timing measures. Three swallows each for thin and thick stimuli. EMG amplitudes were not normalized. | Delsys Trigno EMG sensors, The Motion Monitor soft-ware (Innovative Sports Training, Inc.) | Thin: 10 ml of water; Thick: 10 ml of pudding |
| Tawadros et al. (2012) | Levodopa | Data collected in morning in off-levodopa state and repeated 1 hr after self-administration of regular morning medication. Submental sEMG was filtered and laryngeal accelerometry signals collected. Baseline measurements made during rest. A 9-s postswallow clearing phase also measured. EMG parameters included peak amplitude, burst area and duration, and rise time and fall time. Time between onset of submental and laryngeal burst was also collected. Duration and peak amplitude of accelerometry signals calculated. |
2g piezo-electric accelerometer (IC Sensors model 3145), GrassTM 15LT Astro-Med, Inc, National Instruments™ BNC-2120, LabVIEW 7 | Six water boluses (3, 5, 10, 15, 20, and 25 ml). Three repetitions of each. A subset of participants also drank a 100-ml “stress test” bolus. |
EMG = electromyography; sEMG = surface electromyography; N/A = not applicable.
Discussion
Summary of Findings
Of the 26 studies included for synthesis, 11 described pharmacological interventions, eight investigated the effects of neurostimulation, and seven described outcomes of behavioral interventions. Although several studies concluded that posttreatment improvements were seen in swallowing safety, efficiency, and timing measures, findings were inconsistent, and the quality of the evidence was generally low based on high RoB and low instrumental rigor ratings. Notwithstanding these concerns, overall, low certainty evidence across more than one study suggested the following trends:
DBS and exercises guided using sEMG biofeedback may lead to improvements in swallow timing/latency (Athukorala et al., 2014; Ciucci et al., 2008; Lengerer et al., 2012).
EMST and DBS may lead to improvements in swallowing safety (Pitts et al., 2009; Troche et al., 2010; Xie et al., 2015).
The LSVT and pharmacological intervention with levodopa may lead to improvements in swallowing efficiency (Bushmann et al., 1989; El Sharkawi et al., 2002; Michou et al., 2014; Miles et al., 2017; Warnecke et al., 2016).
As a group, those studies rated to have a lower RoB and greater experimental rigor reported improvements in swallowing efficiency but little effect on swallowing safety (Hunter et al., 1997; Khedr et al., 2019; Kulneff et al., 2013; Michou et al., 2014; Miles et al., 2017; Sundstedt, Holmén, et al., 2017; Sundstedt et al., 2012; Troche et al., 2010). However, mild baseline impairment was identified to be a common limitation (Lengerer et al., 2012; Miles et al., 2017; Troche et al., 2010) and may have introduced a ceiling effect that obscured signs of improvement. Additionally, given that inadequate reporting of methodological details about contrast media, signal acquisition settings, and rater blinding to time point was identified as a concern in the majority of the selected studies, these apparent trends in results should be interpreted with caution. Pooling of data across studies was determined to be inappropriate given the significant heterogeneity seen across studies in videofluoroscopy, endoscopy, and EMG protocols and methods of measurement.
Limitations Associated With Instrumental Rigor
In addition to mild baseline impairment limiting generalizability, the appraisal of rigor in instrumental evaluations revealed several other concerns regarding the selected studies. A number of studies included participants with significant differences in baseline dysphagia and PD severities within groups, with no subgroup analyses to distinguish outcomes based on severity. The generalizability of outcomes to individuals with both mild and severe baseline impairment in these cases is questionable. Additionally, several studies demonstrated limitations in their instrumental protocols when evaluating effectiveness of treatments by basing their conclusions on trials involving single fluid consistencies (Alfonsi et al., 2017; Kondo et al., 2017; Pitts et al., 2009; Tawadros et al., 2012; Tison et al., 1996; Troche et al., 2010) and single boluses (Alfonsi et al., 2017; Kondo et al., 2017; Pitts et al., 2009; Tison et al., 1996). The results of these studies must therefore be interpreted with caution, given that findings may not be replicable, at both within-participant and across-participants levels. Furthermore, of the 20 studies with multiple assessors, 10 failed to explicitly report on interrater reliability (Alfonsi et al., 2017; El Sharkawi et al., 2002; Hirano et al., 2015; Khedr et al., 2019; Kondo et al., 2017; Michou et al., 2014; Stegemöller et al., 2017; Tawadros et al., 2012; Tison et al., 1996; Xie et al., 2015). Given the subjectivity associated with rating instrumental outcomes, such reliability is important to ensure reproducibility and accuracy of findings.
Findings in Context
Our findings are concordant with and build on those of another recent systematic review by López-Liria et al. (2020). Both reviews emphasize the lack of substantial scientific evidence comparing the effectiveness of the various techniques described, highlighting that more work needs to be done to establish or define what types of rehabilitation techniques, maneuvers, and exercises are effective for dysphagia management in PD. However, our systematic review goes beyond this to highlight important limitations with regard to the methodological rigor and RoB in the included studies and emphasizes that findings must be interpreted in the context of overall low certainty evidence. In the López-Liria et al. review, RoB was evaluated using the Jadad scale (Jadad et al., 1996). According to this scale, studies meeting three or more of the following criteria are rated as having a low RoB: (a) The study is described as randomized, (b) an appropriate method of generating the randomization sequence is described, (c) the study employed and describes appropriate blinding, and (d) participant dropouts and loss to follow-up are fully reported. Using these criteria, López-Liria et al. found only one study to be of low quality. Our results are discordant with this appraisal, identifying serious RoB and poor experimental rigor as concerns in the majority of studies reviewed. Using the Evidence Project tool, which is specifically designed to capture RoB across a range of study designs, our review highlights additional gaps in methodological rigor related to the inclusion of control/comparison groups, the equivalence of groups on sociodemographics and that at baseline, and random selection of participants from eligible pools. In addition, our review involves further scrutiny of study for important rigor and transparency criteria pertinent to dysphagia clinical practice, particularly with respect to descriptions of the instrumental examination protocols used for measuring outcomes. Evaluation of these additional domains provides a further nuanced appraisal of the effectiveness of these interventions. By emphasizing these limitations, our review encourages end-user clinicians to interpret findings with caution and to critically appraise the interventions implemented in their clinical practice. In addition, these limitations may shed light on the reasons for significant practice variation.
Previous efforts to synthesize evidence regarding the effectiveness of pharmacological, neurostimulation, and behavioral interventions for dysphagia in PD have yielded limited results or have limited their scope to specific intervention approaches, study designs, search periods, and/or databases (Ashford et al., 2009; Baijens et al., 2009; Battel et al., 2021; Chang et al., 2021; Deane et al., 2001; Grimes et al., 2019; Smith et al., 2012; Van Hooren et al., 2014). Conclusions from these systematic reviews highlight the lack of sufficient evidence to support or refute swallowing therapies in PD due to a limited number of well-designed studies (Baijens et al., 2009; Chang et al., 2021; Deane et al., 2001), with some attributing the inconsistency of results across previous studies to the inclusion of different stages of PD and the use of different evaluation tools for dysphagia in each study (Chang et al., 2021). Most of these reviews concluded that further investigations are warranted, including large, randomized sham-controlled trials (Chang et al., 2021; Van Hooren et al., 2014).
This systematic review represents an effort to synthesize and compare evidence in a comprehensive manner across three types of intervention, utilizing a broad search strategy and no limits in terms of study design and date of publication. Overall, our findings concur with the findings of previous reviews, suggesting very low certainty evidence to guide practice.
Currently, there is a lack of formal guidance around treatment for oropharyngeal dysphagia in people with PD in North American professional practice guidelines. Guidelines from other countries provide weak recommendations for EMST (Grimes et al., 2019) and the LSVT based on very low certainty evidence (Kalf et al., 2011; Ministry of Health, Social Services, and Equality & Institute of Health Sciences of Aragon, 2014). Beyond this, current guidelines provide little direction regarding treatment choices for dysphagia in PD. This reflects a situation of clinical equipoise as well as a limited and low certainty evidence base, which precludes our ability to make recommendations to guide clinical practice (Kamal et al., 2012).
Limitations of the Review
Our review had several limitations. First, instrumental outcomes were limited to VFSS, FEES, and EMG; while outcomes evaluated by manometry and other instrumental techniques may provide additional data regarding PD-related dysphagia interventions, we chose the three most commonly used instrumental measures. Second, only studies with quantitative measures of effect from before–after or parallel-arm comparisons were included; qualitative descriptions of effect or single-arm studies were excluded, but these may provide additional insight into the benefits and harms of available interventions. Third, we found that the Evidence Project tool does not capture some elements of quality that other tools assess. For example, the Cochrane RoB tool (Higgins et al., 2019) includes blinding of participants and personnel, blinding of outcome assessment, and selective reporting, all of which are key domains in RoB assessment. However, given that not all included studies were RCTs, this tool was not used. Additionally, the lack of a numeric RoB summary score representing the overall quality across included articles poses a challenge to succinctly summarizing the overall RoB in the review. Finally, interventions targeting cough strength, respiratory function, and overall physiologic reserve were beyond the scope of this review article.
Conclusions
Future research is needed to elucidate the effects of pharmacological, neurostimulation, and behavioral interventions for dysphagia in PD by implementing standard protocols targeting specific physiological mechanisms related to swallowing safety, efficiency, and timing and rigorous measurement of outcomes using videofluoroscopy, endoscopy, EMG, or other tools. Specifically, there is a need to design future studies with the following considerations:
Given the lack of clear evidence that the aforementioned interventions impact the frequency of penetration–aspiration, studies should expand their focus to measuring changes in other physiological parameters related to airway protection. This could be done by studying parameters that capture the integrity of laryngeal vestibule closure and the time needed to achieve laryngeal vestibule closure (Curtis et al., 2020a, 2020b).
Future studies investigating improvements in swallowing efficiency and timing as outcomes should measure parameters related to pharyngeal constriction, pharyngeal shortening, and UES opening (Curtis et al., 2020a, 2020b).
Future studies need to provide much greater detail regarding the methods used to collect and interpret instrumental measures of swallowing. Thorough descriptions of methods that permit replication and provide evidence of experimental rigor are needed. In particular, protocols should include trials across various consistencies of food and fluids, a sufficient number of swallowing tasks to account for variability within a person, details about the type and concentration of barium used, and equipment used for data collection to be specified for replicability purposes.
In order to provide strong evidence of treatment effect, studies should strive to compare study groups that are equivalent on sociodemographic factors and baseline swallowing function, with confirmation that baseline function is unequivocally impaired on the parameters of interest.
Robust studies with due consideration to these elements are warranted to guide evidence-based clinical practice.
Author Contributions
Pooja Gandhi: Conceptualization (Equal), Methodology (Lead), Investigation (Equal), Formal analysis (Lead), Validation (Equal), Writing - original draft (Lead), Writing - review & editing (Equal). Catriona M. Steele: Conceptualization (Equal), Investigation (Equal), Data curation (Lead), Validation (Equal), Writing - review & editing (Equal).
Supplementary Material
Acknowledgments
This work was supported by National Institute on Deafness and Other Communication Disorders Grant R01DC011020, awarded to Catriona M. Steele. The funding body was not involved in the design of the study; in the collection, analysis, and interpretation of data; and in writing this review article.
Appendix
Full Details of the Search Strategy Used in This Review Article
Ovid MEDLINE ALL <1946 to May 20, 2019>
Search history sorted by search number ascending
| No. | Searches | Results |
|---|---|---|
| 1 | exp Parkinsonian Disorders | 74,758 |
| 2 | Parkinson Disease | 61,685 |
| 3 | parkinson*.tw,kf,jn. | 109,844 |
| 4 | (lewy adj2 bod*).tw,kf. | 8,623 |
| 5 | paralysis agitans.tw,kf. | 1,172 |
| 6 | 1 or 2 or 3 or 4 or 5 | 121,588 |
| 7 | exp Deglutition Disorders | 50,198 |
| 8 | Deglutition | 9,223 |
| 9 | dysphag*.tw,kf,jn. | 26,666 |
| 10 | deglut*.tw,kf. | 4,407 |
| 11 | swallow*.tw,kf. | 27,696 |
| 12 | 7 or 8 or 9 or 10 or 11 | 84,845 |
| 13 | 6 and 12 | 1,064 |
| 14 | 13 not (exp animals/ not exp humans/) | 1,049 |
| 15 | 14 not ((exp infants/ or exp children/) not exp adults/) | 1,039 |
| 16 | limit 15 to english language | 888 |
Cochrane Central Register of Controlled Trials <2014 to Present>
Search history sorted by search number ascending
| No. | Searches | Results |
|---|---|---|
| 1 | exp Parkinsonian Disorders | 3,805 |
| 2 | Parkinson Disease | 3,621 |
| 3 | parkinson*.tw,kf,jn. | 9,069 |
| 4 | (lewy adj2 bod*).tw,kf. | 354 |
| 5 | paralysis agitans.tw,kf. | 10 |
| 6 | 1 or 2 or 3 or 4 or 5 | 9,442 |
| 7 | exp Deglutition Disorders | 2,559 |
| 8 | Deglutition | 352 |
| 9 | dysphag*.tw,kf,jn. | 3,239 |
| 10 | deglut*.tw,kf. | 127 |
| 11 | swallow*.tw,kf. | 4,129 |
| 12 | 7 or 8 or 9 or 10 or 11 | 8,085 |
Embase Classic + Embase <1947 to May 20, 2019>
| No. | Searches | Results |
|---|---|---|
| 1 | parkinsonism | 30,678 |
| 2 | Parkinson disease | 143,921 |
| 3 | parkinson*.tw,kw,jn. | 162,520 |
| 4 | (lewy adj2 bod*).tw,kw. | 13,453 |
| 5 | paralysis agitans.tw,kw. | 494 |
| 6 | 1 or 2 or 3 or 4 or 5 | 200,869 |
| 7 | dysphagia | 66,007 |
| 8 | swallowing | 23,295 |
| 9 | dysphag*.tw,kw,jn. | 47,989 |
| 10 | deglut*.tw,kw. | 6,600 |
| 11 | swallow*.tw,kw. | 45,782 |
| 12 | 7 or 8 or 9 or 10 or 11 | 109,644 |
| 13 | 6 and 12 | 3,116 |
| 14 | 13 not ((exp animals/ or exp animal experimentation/ or nonhuman/) not exp human/) | 3,044 |
| 15 | 14 not ((exp embryo/ or exp fetus/ or exp juvenile/) not exp adult/) | 2,979 |
| 16 | limit 15 to (conference abstract or conference paper or “conference review”) | 1,010 |
| 17 | 15 not 16 | 1,969 |
| 18 | 17 not medline.cr. | 1,799 |
| 19 | limit 18 to english language | 1,622 |
CINAHL With Full Text (May 21, 2019)
| No. | Query | Limiters/expanders | Results |
|---|---|---|---|
| S1 | (MH “Parkinsonian Disorders+”) | Search modes – Boolean/Phrase | 19,196 |
| S2 | (MH “Parkinson Disease”) | Search modes – Boolean/Phrase | 17,952 |
| S3 | TI parkinson* OR AB parkinson* OR SO parkinson* | Search modes – Boolean/Phrase | 23,479 |
| S4 | AB (lewy n2 bod*) OR TI (lewy n2 bod*) | Search modes – Boolean/Phrase | 1,768 |
| S5 | TI paralysis agitans OR AB paralysis agitans | Search modes – Boolean/Phrase | 10 |
| S6 | S1 OR S2 OR S3 OR S4 OR S5 | Search modes – Boolean/Phrase | 27,457 |
| S7 | (MH “Deglutition Disorders”) | Search modes – Boolean/Phrase | 7,139 |
| S8 | (MH “Deglutition”) | Search modes – Boolean/Phrase | 3,196 |
| S9 | TI dysphag* OR AB dysphag* OR SO dysphag* | Search modes – Boolean/Phrase | 7,915 |
| S10 | TI deglut* OR AB deglut* | Search modes – Boolean/Phrase | 388 |
| S11 | TI swallow* OR AB swallow* | Search modes – Boolean/Phrase | 7,506 |
| S12 | S7 OR S8 OR S9 OR S10 OR S11 | Search modes – Boolean/Phrase | 15,320 |
| S13 | S6 AND S12 | Search modes – Boolean/Phrase | 416 |
| S14 | S13 | Limiters – English Language search modes – Boolean/Phrase |
405 |
speechBITE – Advanced Search (May 22, 2019)
| No. | Searches | Results |
|---|---|---|
| 1 | Keyword: Parkinson* SLP Practice Area: Swallowing | 41 |
| 2 | Keyword: Parkinson* SLP Practice Area: Dysphagia | 35 (no new articles added) |
| 3 | Keyword: Parkinson* Dysphagia | 36 (1 new article added) |
Funding Statement
This work was supported by National Institute on Deafness and Other Communication Disorders Grant R01DC011020, awarded to Catriona M. Steele.
References
- Alfonsi, E. , Restivo, D. A. , Cosentino, G. , De Icco, R. , Bertino, G. , Schindler, A. , Todisco, M. , Fresia, M. , Cortese, A. , Prunetti, P. , Ramusino, M. C. , Moglia, A. , Sandrini, G. , & Tassorelli, C. (2017). Botulinum toxin is effective in the management of neurogenic dysphagia. Clinical-electrophysiological findings and tips on safety in different neurological disorders. Frontiers in Pharmacology, 8, 80. https://doi.org/10.3389/fphar.2017.00080 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andersson, I. , & Sidenvall, B. (2001). Case studies of food shopping, cooking and eating habits in older women with Parkinson's disease. Journal of Advanced Nursing, 35(1), 69–78. https://doi.org/10.1046/j.1365-2648.2001.01823.x [DOI] [PubMed] [Google Scholar]
- Argolo, N. , Sampaio, M. , Pinho, P. , Melo, A. , & Nóbrega, A. C. (2013). Do swallowing exercises improve swallowing dynamic and quality of life in Parkinson's disease? NeuroRehabilitation, 32(4), 949–955. https://doi.org/10.3233/NRE-130918 [DOI] [PubMed] [Google Scholar]
- Ashford, J. , McCabe, D. , Wheeler-Hegland, K. , Frymark, T. , Mullen, R. , Musson, N. , Schooling, T. , & Hammond, C. S. (2009). Evidence-based systematic review: Oropharyngeal dysphagia behavioral treatments. Part III—Impact of dysphagia treatments on populations with neurological disorders. Journal of Rehabilitation Research & Development, 46(2), 222–215. https://doi.org/10.1682/JRRD.2008.08.0093 [PubMed] [Google Scholar]
- Athukorala, R. P. , Jones, R. D. , Sella, O. , & Huckabee, M. L. (2014). Skill training for swallowing rehabilitation in patients with Parkinson's disease. Archives of Physical Medicine and Rehabilitation, 95(7), 1374–1382. https://doi.org/10.1016/j.apmr.2014.03.001 [DOI] [PubMed] [Google Scholar]
- Bahia, M. M. , & Lowell, S. Y. (2020). A systematic review of the physiological effects of the effortful swallow maneuver in adults with normal and disordered swallowing. American Journal of Speech-Language Pathology, 29(3), 1655–1673. https://doi.org/10.1044/2020_AJSLP-19-00132 [DOI] [PubMed] [Google Scholar]
- Baijens, L. W. , & Speyer, R. (2009). Effects of therapy for dysphagia in Parkinson's disease: Systematic review. Dysphagia, 24(1), 91–102. https://doi.org/10.1007/s00455-008-9180-1 [DOI] [PubMed] [Google Scholar]
- Baijens, L. W. , Speyer, R. , Passos, V. L. , Pilz, W. , van der Kruis, J. , Haarmans, S. , & Desjardins-Rombouts, C. (2013). Surface electrical stimulation in dysphagic Parkinson patients: A randomized clinical trial. The Laryngoscope, 123(11), E38–E44. https://doi.org/10.1002/lary.24119 [DOI] [PubMed] [Google Scholar]
- Battel, I. , Calvo, I. , & Walshe, M. (2021). Interventions involving biofeedback to improve swallowing in people with Parkinson disease and dysphagia: A systematic review. Archives of Physical Medicine and Rehabilitation, 102(2), 314–322. https://doi.org/10.1016/j.apmr.2020.06.033 [DOI] [PubMed] [Google Scholar]
- Beyer, M. K. , Herlofson, K. , Årsland, D. , & Larsen, J. P. (2001). Causes of death in a community-based study of Parkinson's disease. Acta Neurologica Scandinavica, 103(1), 7–11. https://doi.org/10.1034/j.1600-0404.2001.00191.x [DOI] [PubMed] [Google Scholar]
- Boaden, E. , Nightingale, J. , Bradbury, C. , Hives, L. , & Georgiou, R. (2020). Clinical practice guidelines for videofluoroscopic swallowing studies: A systematic review. Radiography, 26(2), 154–162. https://doi.org/10.1016/j.radi.2019.10.011 [DOI] [PubMed] [Google Scholar]
- Boaden, E. , Nightingale, J. , Hives, L. , Bradbury, C. , Benfield, J. , Patel, T. , & Georgiou, R. (2021). Current videofluoroscopy practice in the United Kingdom: A survey of imaging professionals. Radiography, 27(2), 499–504. https://doi.org/10.1016/j.radi.2020.11.004 [DOI] [PubMed] [Google Scholar]
- Bonilha, H. S. , Blair, J. , Carnes, B. , Huda, W. , Humphries, K. , McGrattan, K. , & Martin-Harris, B. (2013). Preliminary investigation of the effect of pulse rate on judgments of swallowing impairment and treatment recommendations. Dysphagia, 28(4), 528–538. https://doi.org/10.1007/s00455-013-9463-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- Braak, H. , Ghebremedhin, E. , Rüb, U. , Bratzke, H. , & Del Tredici, K. (2004). Stages in the development of Parkinson's disease-related pathology. Cell and Tissue Research, 318(1), 121–134. https://doi.org/10.1007/s00441-004-0956-9 [DOI] [PubMed] [Google Scholar]
- Bushmann, M. , Dobmeyer, S. M. , Leeker, L. , & Perlmutter, J. S. (1989). Swallowing abnormalities and their response to treatment in Parkinson's disease. Neurology, 39(10), 1309–1314. https://doi.org/10.1212/WNL.39.10.1309 [DOI] [PubMed] [Google Scholar]
- Butler, S. G. , Stuart, A. , Case, L. D. , Rees, C. , Vitolins, M. , & Kritchevsky, S. B. (2011). Effects of liquid type, delivery method, and bolus volume on penetration–aspiration scores in healthy older adults during flexible endoscopic evaluation of swallowing. Annals of Otology, Rhinology & Laryngology, 120(5), 288–295. https://doi.org/10.1177/000348941112000502 [DOI] [PubMed] [Google Scholar]
- Chang, M. C. , Park, J. S. , Lee, B. J. , & Park, D. (2021). Effectiveness of pharmacologic treatment for dysphagia in Parkinson's disease: A narrative review. Neurological Sciences, 42(2), 513–519. https://doi.org/10.1007/s10072-020-04865-w [DOI] [PubMed] [Google Scholar]
- Chaudhuri, K. R. , Healy, D. G. , & Schapira, A. H. (2006). Non-motor symptoms of Parkinson's disease: Diagnosis and management. The Lancet Neurology, 5(3), 235–245. https://doi.org/10.1016/S1474-4422(06)70373-8 [DOI] [PubMed] [Google Scholar]
- Ciucci, M. R. , Barkmeier-Kraemer, J. M. , & Sherman, S. J. (2008). Subthalamic nucleus deep brain stimulation improves deglutition in Parkinson's disease. Movement Disorders, 23(5), 676–683. https://doi.org/10.1002/mds.21891 [DOI] [PubMed] [Google Scholar]
- Clarke, C. E. , Gullaksen, E. , Macdonald, S. , & Lowe, F. (1998). Referral criteria for speech and language therapy assessment of dysphagia caused by idiopathic Parkinson's disease. Acta Neurologica Scandinavica, 97(1), 27–35. https://doi.org/10.1111/j.1600-0404.1998.tb00605.x [DOI] [PubMed] [Google Scholar]
- Curtis, J. A. , Molfenter, S. M. , & Troche, M. S. (2020a). Pharyngeal area changes in Parkinson's disease and its effect on swallowing safety, efficiency, and kinematics. Dysphagia, 35(2), 389–398. https://doi.org/10.1007/s00455-019-10052-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Curtis, J. A. , Molfenter, S. M., & Troche, M. S. (2020b). Predictors of residue and airway invasion in Parkinson's disease. Dysphagia, 35(2), 220–230. https://doi.org/10.1007/s00455-019-10014-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daniels, S. K. , Schroeder, M. F. , DeGeorge, P. C. , Corey, D. M. , & Rosenbek, J. C. (2007). Effects of verbal cue on bolus flow during swallowing. American Journal of Speech-Language Pathology, 16(2), 140–147. https://doi.org/10.1044/1058-0360(2007/018 [DOI] [PubMed] [Google Scholar]
- Deane, K. H. , Jones, D. , Ellis-Hill, C. , Clarke, C. E. , Playford, E. D. , & Ben-Shlomo, Y. (2001). Physiotherapy for Parkinson's disease: A comparison of techniques. Cochrane Database of Systematic Reviews, 1, CD002815. https://doi.org/10.1002/14651858.CD002815 [DOI] [PubMed] [Google Scholar]
- De Rijk, M. C. , Breteler, M. M. B. , Graveland, G. A. , Ott, A. , Grobbee, D. E. , Van der Meche, F. G. A. , & Hofman, A. (1995). Prevalence of Parkinson's disease in the elderly: The Rotterdam Study. Neurology, 45(12), 2143–2146. https://doi.org/10.1212/WNL.45.12.2143 [DOI] [PubMed] [Google Scholar]
- Dorsey, E. R. , Constantinescu, R. , Thompson, J. P. , Biglan, K. M. , Holloway, R. G. , Kieburtz, K. , Ravina, B. M. , Schifitto, G. , Siderowf, A. , & Tanner, C. M. (2007). Projected number of people with Parkinson disease in the most populous nations, 2005 through 2030. Neurology, 68(5), 384–386. https://doi.org/10.1212/01.wnl.0000247740.47667.03 [DOI] [PubMed] [Google Scholar]
- Ekberg, O. , Hamdy, S. , Woisard, V. , Wuttge-Hannig, A. , & Ortega, P. (2002). Social and psychological burden of dysphagia: Its impact on diagnosis and treatment. Dysphagia, 17(2), 139–146. https://doi.org/10.1007/s00455-001-0113-5 [DOI] [PubMed] [Google Scholar]
- El Sharkawi, A. , Ramig, L. , Logemann, J. A. , Pauloski, B. R. , Rademaker, A. W. , Smith, C. H. , Pawlas, A. , Baum, S. , & Werner, C. (2002). Swallowing and voice effects of Lee Silverman Voice Treatment (LSVT®): A pilot study. Journal of Neurology, Neurosurgery & Psychiatry, 72(1), 31–36. https://doi.org/10.1136/jnnp.72.1.31 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farri, A. , Accornero, A. , & Burdese, C. (2007). Social importance of dysphagia: Its impact on diagnosis and therapy. Acta Otorhinolaryngologica Italica, 27(2), 83–86. [PMC free article] [PubMed] [Google Scholar]
- Fuh, J. L. , Lee, R. C. , Wang, S. J. , Lin, C. H. , Wang, P. N. , Chiang, J. H. , & Liu, H. C. (1997). Swallowing difficulty in Parkinson's disease. Clinical Neurology and Neurosurgery, 99(2), 106–112. https://doi.org/10.1016/S0303-8467(97)80006-6 [DOI] [PubMed] [Google Scholar]
- Grimes, D. , Fitzpatrick, M. , Gordon, J. , Miyasaki, J. , Fon, E. A. , Schlossmacher, M. , Suchowersky, O. , Rajput, A. , Lafontaine, A. L. , Mestre, T. , Appel-Cresswell, S. , Kalia, S. K. , Schoffer, K. , Zurowski, M. , Postuma, R. B. , Udow, S. , Fox, S. , Barbeua, P. , & Hutton, B. (2019). Canadian guideline for Parkinson disease. Canadian Medical Association Journal, 191(36), E989–E1004. https://doi.org/10.1503/cmaj.181504 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hazelwood, R. , Armeson, K. E. , Hill, E. G. , Bonilha, H. S. , & Martin-Harris, B. (2017). Identification of swallowing tasks from a modified barium swallow study that optimize the detection of physiological impairment. Journal of Speech, Language, and Hearing Research, 60(7), 1855–1863. https://doi.org/10.1044/2017_JSLHR-S-16-0117 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Higgins, J. P. , Thomas, J. , Chandler, J. , Cumpston, M. , Li, T. , Page, M. J. , & Welch, V. A. . (Eds.). (2019). Cochrane handbook for systematic reviews of interventions. Cochrane; Wiley. https://doi.org/10.1002/9781119536604 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirano, M. , Isono, C. , Sakamoto, H. , Ueno, S. , Kusunoki, S. , & Nakamura, Y. (2015). Rotigotine transdermal patch improves swallowing in dysphagic patients with Parkinson's disease. Dysphagia, 30(4), 452–456. https://doi.org/10.1007/s00455-015-9622-5 [DOI] [PubMed] [Google Scholar]
- Hoehn, M. M. , & Yahr, M. D. (1967). Parkinsonism: Onset, progression and mortality. Neurology, 17(5), 427–442. https://doi.org/10.1212/WNL.17.5.427 [DOI] [PubMed] [Google Scholar]
- Hunter, P. C. , Crameri, J. , Austin, S. , Woodward, M. C. , & Hughes, A. J. (1997). Response of parkinsonian swallowing dysfunction to dopaminergic stimulation. Journal of Neurology, Neurosurgery & Psychiatry, 63(5), 579–583. https://doi.org/10.1136/jnnp.63.5.579 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jadad, A. R. , Moore, R. A. , Carroll, D. , Jenkinson, C. , Reynolds, D. J. M. , Gavaghan, D. J. , & McQuay, H. J. (1996). Assessing the quality of reports of randomized clinical trials: Is blinding necessary? Controlled Clinical Trials, 17(1), 1–12. https://doi.org/10.1016/0197-2456(95)00134-4 [DOI] [PubMed] [Google Scholar]
- Jellinger, K. A. (1991). Pathology of Parkinson's disease. Molecular and Chemical Neuropathology, 14(3), 153–197. https://doi.org/10.1007/BF03159935 [DOI] [PubMed] [Google Scholar]
- Johnston, B. T. , Li, Q. , Castell, J. A. , & Castell, D. O. (1995). Swallowing and esophageal function in Parkinson's disease. American Journal of Gastroenterology (Springer Nature), 90(10), 1741–1746. [PubMed] [Google Scholar]
- Kalf, J. G. , de Swart, B. J. M. , Bloem, B. R. , & Munneke, M. (2012). Prevalence of oropharyngeal dysphagia in Parkinson's disease: A meta-analysis. Parkinsonism & Related Disorders, 18(4), 311–315. https://doi.org/10.1016/j.parkreldis.2011.11.006 [DOI] [PubMed] [Google Scholar]
- Kalf, J. G. , de Swart, B. J. M. , Bonnier-Baars, M. , Kanters, J. , Hofman, M. , Kocken, J. , Miltenburg, M. , Bloem, B. R. , & Munneke, M. (2011). Guidelines for speech-language therapy in Parkinson's disease. ParkinsonNet/National Parkinson Foundation (NPF). [Google Scholar]
- Kamal, R. M. , Ward, E. , & Cornwell, P. (2012). Dysphagia training for speech-language pathologists: Implications for clinical practice. International Journal of Speech-Language Pathology, 14(6), 569–576. https://doi.org/10.3109/17549507.2012.713394 [DOI] [PubMed] [Google Scholar]
- Kennedy, C. E. , Fonner, V. A. , Armstrong, K. A. , Denison, J. A. , Yeh, P. T. , O'Reilly, K. R. , & Sweat, M. D. (2019). The Evidence Project risk of bias tool: Assessing study rigor for both randomized and non-randomized intervention studies. Systematic Reviews, 8(1), 3–10. https://doi.org/10.1186/s13643-018-0925-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khedr, E. M. , Mohamed, K. O. , Soliman, R. K. , Hassan, A. M. , & Rothwell, J. C. (2019). The effect of high-frequency repetitive transcranial magnetic stimulation on advancing Parkinson's disease with dysphagia: Double blind randomized clinical trial. Neurorehabilitation and Neural Repair, 33(6), 442–452. https://doi.org/10.1177/1545968319847968 [DOI] [PubMed] [Google Scholar]
- Kondo, E. , Jinnouchi, O. , Nakano, S. , Ohnishi, H. , Kawata, I. , Okamoto, H. , & Takeda, N. (2017). Aural stimulation with capsaicin ointment improved swallowing function in elderly patients with dysphagia: A randomized, placebo-controlled, double-blind, comparative study. Clinical Interventions in Aging, 12, 1921–1928. https://doi.org/10.2147/CIA.S138357, 1928 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuhlemeier, K. V. , Yates, P. , & Palmer, J. B. (1998). Intra- and interrater variation in the evaluation of videofluorographic swallowing studies. Dysphagia, 13(3), 142–147. https://doi.org/10.1007/PL00009564 [DOI] [PubMed] [Google Scholar]
- Kulneff, L. , Sundstedt, S. , Olofsson, K. , van Doorn, J. , Linder, J. , Nordh, E. , & Blomstedt, P. (2013). Deep brain stimulation—Effects on swallowing function in Parkinson's disease. Acta Neurologica Scandinavica, 127(5), 329–336. https://doi.org/10.1111/ane.12019 [DOI] [PubMed] [Google Scholar]
- Lengerer, S. , Kipping, J. , Rommel, N. , Weiss, D. , Breit, S. , Gasser, T. , Plewnia, C. , Kruger, R. , & Wächter, T. (2012). Deep-brain-stimulation does not impair deglutition in Parkinson's disease. Parkinsonism & Related Disorders, 18(7), 847–853. https://doi.org/10.1016/j.parkreldis.2012.04.014 [DOI] [PubMed] [Google Scholar]
- Leopold, N. A. , & Kagel, M. C. (1997). Laryngeal deglutition movement in Parkinson's disease. Neurology, 48(2), 373–375. https://doi.org/10.1212/WNL.48.2.373 [DOI] [PubMed] [Google Scholar]
- López-Liria, R. , Parra-Egeda, J. , Vega-Ramírez, F. A. , Aguilar-Parra, J. M. , Trigueros-Ramos, R. , Morales-Gázquez, M. J. , & Rocamora-Pérez, P. (2020). Treatment of dysphagia in Parkinson's disease: A systematic review. International Journal of Environmental Research and Public Health, 17(11), 4104. https://doi.org/10.3390/ijerph17114104 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mancopes, R. , Smaoui, S. , & Steele, C. M. (2020). Effects of expiratory muscle strength training on videofluoroscopic measures of swallowing: A systematic review. American Journal of Speech-Language Pathology, 29(1), 335–356. https://doi.org/10.1044/2019_AJSLP-19-00107 [DOI] [PubMed] [Google Scholar]
- Martinez-Martín, P. , Gil-Nagel, A. , Gracia, L. M. , Gómez, J. B. , Martinez-Sarries, J. , Bermejo, F. , & The Cooperative Multicentric Group. (1994). Unified Parkinson's disease rating scale characteristics and structure. Movement Disorders, 9(1), 76–83. https://doi.org/10.1002/mds.870090112 [DOI] [PubMed] [Google Scholar]
- Martin-Harris, B. , Bonilha, H. S. , Brodsky, M. B. , Francis, D. O. , Fynes, M. M. , Martino, R. , O'Rourke, A. K. , & Rogus-Pulia, N. M. (2021). The modified barium swallow study for oropharyngeal dysphagia: Recommendations from an interdisciplinary expert panel. Perspectives of the ASHA Special Interest Groups, 1–10. https://doi.org/10.1044/2021_PERSP-20-00303 [Google Scholar]
- McCullough, G. H. , Wertz, R. T. , Rosenbek, J. C. , Mills, R. H. , Webb, W. G. , & Ross, K. B. (2001). Inter- and intrajudge reliability for videofluoroscopic swallowing evaluation measures. Dysphagia, 16(2), 110–118. https://doi.org/10.1007/PL00021291 [DOI] [PubMed] [Google Scholar]
- McHugh, M. L. (2012). Interrater reliability: The kappa statistic. Biochemia Medica, 22(3), 276–282. https://doi.org/10.11613/BM.2012.031 [PMC free article] [PubMed] [Google Scholar]
- Michou, E. , Hamdy, S. , Harris, M. , Vania, A. , Dick, J. , Kellett, M. , & Rothwell, J. (2014). Characterization of corticobulbar pharyngeal neurophysiology in dysphagic patients with Parkinson's disease. Clinical Gastroenterology and Hepatology, 12(12), 2037–2045.e4. https://doi.org/10.1016/j.cgh.2014.03.020 [DOI] [PubMed] [Google Scholar]
- Miles, A. , Jardine, M. , Johnston, F. , de Lisle, M. , Friary, P. , & Allen, J. (2017). Effect of Lee Silverman Voice Treatment (LSVT LOUD®) on swallowing and cough in Parkinson's disease: A pilot study. Journal of the Neurological Sciences, 383, 180–187. https://doi.org/10.1016/j.jns.2017.11.015 [DOI] [PubMed] [Google Scholar]
- Miller, N. , Noble, E. , Jones, D. , & Burn, D. (2006). Hard to swallow: Dysphagia in Parkinson's disease. Age and Ageing, 35(6), 614–618. https://doi.org/10.1093/ageing/afl105 [DOI] [PubMed] [Google Scholar]
- Ministry of Health, Social Services, and Equality & Institute of Health Sciences of Aragon. (2014). Clinical practice guideline for the management of patients with Parkinson's disease. https://portal.guiasalud.es/wpcontent/uploads/2018/12/GPC_546_Parkinson_IACS_comp_en.pdf
- Moher, D. L. , Liberati, A. , Tetzlaff, J. , & Altman, D. G. (2009). Preferred Reporting Items for Systematic Reviews and Meta-Analyses: The PRISMA Statement. BMJ, 339, b2535. https://doi.org/10.1136/bmj.b2535 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Monte, F. S. , da Silva-Júnior, F. P. , Braga-Neto, P. , Nobre e Souza, M. Â. , & Sales de Bruin, V. M. (2005). Swallowing abnormalities and dyskinesia in Parkinson's disease. Movement Disorders, 20(4), 457–462. https://doi.org/10.1002/mds.20342 [DOI] [PubMed] [Google Scholar]
- Mu, L. , Sobotka, S. , Chen, J. , Su, H. , Sanders, I. , Adler, C. H. , Shill, H. A. , Caviness, J. N. , Samanta, J. E. , Beach, T. G. , & Arizona Parkinson's Disease Consortium. (2013). Alpha-synuclein pathology and axonal degeneration of the peripheral motor nerves innervating pharyngeal muscles in Parkinson disease. Journal of Neuropathology & Experimental Neurology, 72(2), 119–129. https://doi.org/10.1097/NEN.0b013e3182801cde [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nagy, A. , Leigh, C. , Hori, S. F. , Molfenter, S. M. , Shariff, T. , & Steele, C. M. (2013). Timing differences between cued and noncued swallows in healthy young adults. Dysphagia, 28(3), 428–434. https://doi.org/10.1007/s00455-013-9456-y [DOI] [PubMed] [Google Scholar]
- Nilsson, H. , Ekberg, O. , Olsson, R. , & Hindfelt, B. (1996). Quantitative assessment of oral and pharyngeal function in Parkinson's disease. Dysphagia, 11(2), 144–150. https://doi.org/10.1007/BF00417905 [DOI] [PubMed] [Google Scholar]
- Ott, D. J. (1998). Observer variation in evaluation of videofluoroscopic swallowing studies: A continuing problem. Dysphagia, 13(3), 148–150. [PubMed] [Google Scholar]
- Peladeau-Pigeon, M. , & Steele, C. M. (2013). Technical aspects of a videofluoroscopic swallowing study. Canadian Journal of Speech-Language Pathology and Audiology, 37(3), 216–226. [Google Scholar]
- Peladeau-Pigeon, M. , & Steele, C. M. (2015). Understanding image resolution and quality in videofluoroscopy. SIG 13 Perspectives on Swallowing and Swallowing Disorders (Dysphagia), 24(3), 115–124. https://doi.org/10.1044/sasd24.3.115 [Google Scholar]
- Pitts, T. , Bolser, D. , Rosenbek, J. , Troche, M. , Okun, M. S. , & Sapienza, C. (2009). Impact of expiratory muscle strength training on voluntary cough and swallow function in Parkinson disease. Chest, 135(5), 1301–1308. https://doi.org/10.1378/chest.08-1389 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Plowman, E. K. , & Humbert, I. A. (2018). Elucidating inconsistencies in dysphagia diagnostics: Redefining normal. International Journal of Speech-Language Pathology, 20(3), 310–317. https://doi.org/10.1080/17549507.2018.1461931 [DOI] [PubMed] [Google Scholar]
- Plowman-Prine, E. K. , Sapienza, C. M. , Okun, M. S. , Pollock, S. L. , Jacobson, C. , Wu, S. S. , & Rosenbek, J. C. (2009). The relationship between quality of life and swallowing in Parkinson's disease. Movement Disorders, 24(9), 1352–1358. https://doi.org/10.1002/mds.22617 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Potulska, A. , Friedman, A. , Królicki, L. , & Spychala, A. (2003). Swallowing disorders in Parkinson's disease. Parkinsonism & Related Disorders, 9(6), 349–353. https://doi.org/10.1016/S1353-8020(03)00045-2 [DOI] [PubMed] [Google Scholar]
- Pringsheim, T. , Jette, N. , Frolkis, A. , & Steeves, T. D. (2014). The prevalence of Parkinson's disease: A systematic review and meta-analysis. Movement Disorders, 29(13), 1583–1590. https://doi.org/10.1002/mds.25945 [DOI] [PubMed] [Google Scholar]
- Rocha, E. M. , De Miranda, B. , & Sanders, L. H. (2018). Alpha-synuclein: Pathology, mitochondrial dysfunction and neuroinflammation in Parkinson's disease. Neurobiology of Disease, 109, 249–257. https://doi.org/10.1016/j.nbd.2017.04.004 [DOI] [PubMed] [Google Scholar]
- Smith, S. K. , Roddam, H. , & Sheldrick, H. (2012). Rehabilitation or compensation: Time for a fresh perspective on speech and language therapy for dysphagia and Parkinson's disease? International Journal of Language & Communication Disorders, 47(4), 351–364. https://doi.org/10.1111/j.1460-6984.2011.00093.x [DOI] [PubMed] [Google Scholar]
- Steele, C. M. , Molfenter, S. M. , Peladeau-Pigeon, M. , & Stokely, S. L. (2013). Challenges in preparing contrast media for videofluoroscopy. Dysphagia, 28(3), 464–467. https://doi.org/10.1007/s00455-013-9476-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steele, C. M. , Mukherjee, R. , Kortelainen, J. M. , Pölönen, H. , Jedwab, M. , Brady, S. L. , Theimer, K. B. , Langmore, S. , Riquelme, L. F. , Swigert, N. B. , Bath, P. M. , Goldstein, L. B. , Hughes, R. L. , Leifer, D. , Lees, K. R. , Meretoja, A. , & Muehlemann, N. (2019). Development of a non-invasive device for swallow screening in patients at risk of oropharyngeal dysphagia: Results from a prospective exploratory study. Dysphagia, 34(5), 698–707. https://doi.org/10.1007/s00455-018-09974-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steele, C. M. , Peladeau-Pigeon, M. , Barbon, C. E. A. , Guida, B. T. , Namasivayam-MacDonald, A. M. , Nascimento, W. V. , Smaoui, S. , Tapson, M. S. , Valenzano, T. J. , Waito, A. A. , & Wolkin, T. S. (2019). Reference values for healthy swallowing across the range from thin to extremely thick liquids. Journal of Speech, Language, and Hearing Research, 62(5), 1338–1363. https://doi.org/10.1044/2019_JSLHR-S-18-0448 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stegemöller, E. L. , Hibbing, P. , Radig, H. , & Wingate, J. (2017). Therapeutic singing as an early intervention for swallowing in persons with Parkinson's disease. Complementary Therapies in Medicine, 31, 127–133. https://doi.org/10.1016/j.ctim.2017.03.002 [DOI] [PubMed] [Google Scholar]
- Sundstedt, S. , Holmén, L. , Rova, E. , Linder, J. , Nordh, E. , & Olofsson, K. (2017). Swallowing safety in Parkinson's disease after zona incerta deep brain stimulation. Brain and Behavior, 7(6), e00709. https://doi.org/10.1002/brb3.709 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sundstedt, S. , Nordh, E. , Linder, J. , Hedström, J. , Finizia, C. , & Olofsson, K. (2017). Swallowing quality of life after zona incerta deep brain stimulation. Annals of Otology, Rhinology & Laryngology, 126(2), 110–116. https://doi.org/10.1177/0003489416675874 [DOI] [PubMed] [Google Scholar]
- Sundstedt, S. , Olofsson, K. , van Doorn, J. , Linder, J. , Nordh, E. , & Blomstedt, P. (2012). Swallowing function in Parkinson's patients following zona incerta deep brain stimulation. Acta Neurologica Scandinavica, 126(5), 350–356. https://doi.org/10.1111/j.1600-0404.2012.01658.x [DOI] [PubMed] [Google Scholar]
- Suttrup, I. , & Warnecke, T. (2016). Dysphagia in Parkinson's disease. Dysphagia, 31(1), 24–32. https://doi.org/10.1007/s00455-015-9671-9 [DOI] [PubMed] [Google Scholar]
- Swan, K. , Cordier, R. , Brown, T. , & Speyer, R. (2019). Psychometric properties of visuoperceptual measures of videofluoroscopic and fibre-endoscopic evaluations of swallowing: A systematic review. Dysphagia, 34(1), 2–33. https://doi.org/10.1007/s00455-018-9918-3 [DOI] [PubMed] [Google Scholar]
- Tawadros, P. B. , Cordato, D. , Cathers, I. , & Burne, J. A. (2012). An electromyographic study of parkinsonian swallowing and its response to levodopa. Movement Disorders, 27(14), 1811–1815. https://doi.org/10.1002/mds.25262 [DOI] [PubMed] [Google Scholar]
- Tison, F. , Wiart, L. , Guatterie, M. , Fouillet, N. , Lozano, V. , Henry, P. , & Barat, M. (1996). Effects of central dopaminergic stimulation by apomorphine on swallowing disorders in Parkinson's disease. Movement Disorders, 11(6), 729–732. https://doi.org/10.1002/mds.870110622 [DOI] [PubMed] [Google Scholar]
- Tohara, H. , Nakane, A. , Murata, S. , Mikushi, S. , Ouchi, Y. , Wakasugi, Y. , Takashima, M. , Chiba, Y. , & Uematsu, H. (2010). Inter- and intra-rater reliability in fibroptic endoscopic evaluation of swallowing. Journal of Oral Rehabilitation, 37(12), 884–891. https://doi.org/10.1111/j.1365-2842.2010.02116.x [DOI] [PubMed] [Google Scholar]
- Troche, M. S. , Okun, M. S. , Rosenbek, J. C. , Musson, N. , Fernandez, H. H. , Rodriguez, R. , Romrell, J. , Pitts, T. , Wheeler-Hegland, K. M. , & Sapienza, C. M. (2010). Aspiration and swallowing in Parkinson disease and rehabilitation with EMST: A randomized trial. Neurology, 75(21), 1912–1919. https://doi.org/10.1212/WNL.0b013e3181fef115 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Hooren, M. R. A. , Baijens, L. W. J. , Voskuilen, S. , Oosterloo, M. , & Kremer, B. (2014). Treatment effects for dysphagia in Parkinson's disease: A systematic review. Parkinsonism & Related Disorders, 20(8), 800–807. https://doi.org/10.1016/j.parkreldis.2014.03.026 [DOI] [PubMed] [Google Scholar]
- von Campenhausen, S. , Bornschein, B. , Wick, R. , Bötzel, K. , Sampaio, C. , Poewe, W. , Oertel, W. , Siebert, U. , Berger, K. , & Dodel, R. (2005). Prevalence and incidence of Parkinson's disease in Europe. European Neuropsychopharmacology, 15(4), 473–490. https://doi.org/10.1016/j.euroneuro.2005.04.007 [DOI] [PubMed] [Google Scholar]
- Wang, X. , You, G. , Chen, H. , & Cai, X. (2002). Clinical course and cause of death in elderly patients with idiopathic Parkinson's disease. Chinese Medical Journal, 115(9), 1409–1411. [PubMed] [Google Scholar]
- Warnecke, T. , Suttrup, I. , Schröder, J. B. , Osada, N. , Oelenberg, S. , Hamacher, C. , Suntrup, S. , & Dziewas, R. (2016). Levodopa responsiveness of dysphagia in advanced Parkinson's disease and reliability testing of the FEES-Levodopa-test. Parkinsonism & Related Disorders, 28, 100–106. https://doi.org/10.1016/j.parkreldis.2016.04.034 [DOI] [PubMed] [Google Scholar]
- Xie, T. , Vigil, J. , MacCracken, E. , Gasparaitis, A. , Young, J. , Kang, W. , Bernard, J. , Warnke, P. , & Kang, U. J. (2015). Low-frequency stimulation of STN-DBS reduces aspiration and freezing of gait in patients with PD. Neurology, 84(4), 415–420. https://doi.org/10.1212/WNL.0000000000001184 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.



 This work is licensed under a
This work is licensed under a