Abstract
Purpose of Review:
This article discusses the central disorders of hypersomnolence, a group of disorders resulting in pathologic daytime sleepiness, particularly narcolepsy type 1 and narcolepsy type 2, idiopathic hypersomnia, and Kleine-Levin syndrome. Disease features, diagnostic testing, epidemiology, pathophysiology, and treatment are reviewed.
Recent Findings:
Increasing evidence implicates autoimmunity in narcolepsy type 1, including a strong association with human leukocyte antigen–DQB1*06:02, association with a polymorphism in the T-cell receptor alpha locus in genome-wide association, and the identification of autoreactive T cells in patients with this type of narcolepsy. In contrast, the cause or causes of narcolepsy type 2 and idiopathic hypersomnia are unknown. Multiple treatment options exist, including two medications approved by the US Food and Drug Administration (FDA) in 2019. These include solriamfetol, a dopamine- and norepinephrine-reuptake inhibitor, and pitolisant, an H3-inverse agonist/antagonist that increases histaminergic neurotransmission.
Summary:
The central disorders of hypersomnolence all cause severe sleepiness but can be differentiated based on ancillary symptoms, diagnostic testing, and pathophysiology. It is important that these disorders are identified because multiple treatments are available to improve functioning and quality of life.
INTRODUCTION
The central disorders of hypersomnolence are marked by pathologic daytime sleepiness. For most of these disorders, the underlying pathophysiology is unknown. Narcolepsy type 1 is now thought likely to be an autoimmune disorder resulting in the loss of orexin (hypocretin) neurons in people who are genetically susceptible. Although daytime sleepiness is the hallmark of all these disorders, clinical features such as cataplexy, sleep duration, and episodic or continuous symptoms, can help subdivide them. Diagnostic testing involves an assessment of daytime sleepiness with subjective and objective measures and the exclusion of other causes. Various treatments are available for narcolepsy type 1 and narcolepsy type 2, which are used off-label for the other disorders.
SYMPTOMATOLOGY
The International Classification of Sleep Disorders, Third Edition (ICSD-3) classifies eight different central disorders of hypersomnolence: narcolepsy type 1, narcolepsy type 2, idiopathic hypersomnia, Kleine-Levin syndrome, hypersomnia associated with a psychiatric disorder, hypersomnia due to a medical disorder, hypersomnia due to a medication or substance, and insufficient sleep syndrome.1 In the last three conditions, excessive daytime sleepiness can be clearly attributed to a comorbid medical disorder, a substance, or chronically short sleep durations. [KP 1] Hypersomnia associated with a psychiatric disorder is similar, in that excessive daytime sleepiness occurs in the presence of a psychiatric disorder. However, it is distinct in that the excessive daytime sleepiness is not necessarily caused by the comorbid psychiatric disease; they merely need to be comorbid with each other. [KP 2] In contrast, for the other four disorders, sleepiness and associated symptoms are manifestations of the hypersomnia disorder itself, not a consequence or comorbidity of another condition. This article focuses on these four disorders.
By definition, in the ICSD-3, excessive daytime sleepiness manifests as either an irrepressible need to sleep or episodes of daytime sleep.1 However, the experience of daytime sleepiness may qualitatively differ across diagnoses. Classically, people with narcolepsy type 1 experience sudden “attacks” of a need to sleep,1 while people with idiopathic hypersomnia more often describe their sleepiness as a state of persistently low vigilance.2
In addition to sleepiness, core features of narcolepsy type 1 include cataplexy, sleep paralysis, sleep-related hallucinations, and disrupted nocturnal sleep (Table 3-1). [KP 3] Cataplexy is the sudden loss of muscle tone induced by emotion, most commonly strong positive emotions and especially laughter (Table 3-2).3 It is thought to represent the intrusion of rapid eye movement (REM)-sleep muscle atonia into wakefulness.4 In some cases, cataplexy involves the whole body and can result in falls, although injury is rare. More commonly, attacks are partial, resulting in only transient neck, face, or limb weakness. Attacks are bilateral, but one side may be more affected.4 Consciousness is maintained. Episodes typically last for seconds to minutes. Presentation in children can be quite distinct from that in adults, with episodes of positive motor phenomena near the onset of disease, including tongue thrusting and other dyskinesias.4 Pseudocataplexy, a psychogenic nonepileptic spell, has been reported in those with and without narcolepsy type 1.5,6 Narcolepsy type 1 is the only hypersomnia disorder to manifest cataplexy, and cataplexy is the clinical feature that distinguishes narcolepsy type 1 and narcolepsy type 2. [KP 4] Cataplexylike symptoms are reported in a small number of other neurologic disorders, including Norrie disease and Niemann-Pick type C,7,8 but otherwise, cataplexy is quite specific for narcolepsy type 1.
Table 3-1.
Clinical Features and Diagnostic Criteria
| Narcolepsy Type 1 | Narcolepsy Type 2 | Idiopathic Hypersomnia | Kleine-Levin Syndrome | |
|---|---|---|---|---|
| Symptoms | ||||
| Excessive daytime sleepiness | Must be present | Must be present | Must be present | Must be present |
| Cataplexy | Common | Never | Never | Never |
| Sleep hallucinations | Common | Less common | Least common (but sometimes present) | Not applicable (NA) |
| Sleep paralysis | Common | Less common | Least common (but sometimes present) | NA |
| Disrupted nocturnal sleep | Common | Less than narcolepsy type 1 | Less than narcolepsy type 1, not typical | NA |
| Long sleep times | Not typical | May be present | Common | Very prolonged during an episode |
| Pronounced sleep inertia | Rare | May be present | Common | NA |
|
| ||||
| Diagnostic criteria (International Classification of Sleep Disorders, Third Edition) | ||||
| Multiple sleep latency test (MSLT) mean sleep latency for diagnosis | ≤8 minutes | ≤8 minutes | ≤8 minutes | MSLT not necessary for diagnosis |
| Number of sleep-onset REM periods for diagnosis | 2 or more | 2 or more | 0–1 | NA |
| Orexin (hypocretin) levels in CSF (if tested) | Low | Normal | Normal | Normal |
| Necessary criteria for diagnosis, in addition to excessive daytime sleepiness | (1) Cataplexy and typical MSLT findings OR (2) orexin (hypocretin) deficiency | (1) Typical MSLT findings AND (2) no other cause | (1) Typical MSLT findings OR (2) >11 hours sleep on 24-hour polysomnography OR (3) >11 hours sleep on average over at least 1 week ofactigraphy, AND (4) no other cause | (1) Episodic sleepiness accompanied by cognitive dysfunction, altered perception, altered eating disinhibition AND (2) no other cause |
CSF = cerebrospinal fluid.
Table 3-2.
Phenomenology of Cataplexy
| Clinical Aspect | Typical Presentation of Cataplexy |
|---|---|
| Duration | Seconds to 2 minutes |
| Typical muscle groups involved | Neck, face, limbs |
| Laterality | Bilateral |
| Effects on consciousness | Consciousness retained |
| Tone | Atonic |
| Reflexes | Reduced/absent in affected muscles |
| Trigger | Strong positive emotions |
| Positive phenomena | Phasic muscle twitching of the face may be present; more dyskinetic movements can be seen in children |
Sleep paralysis occurs when REM atonia persists briefly upon awakening, such that the person is awake but unable to move voluntary muscles except for the eyes. It is commonly experienced in narcolepsy type 1 (53% to 69%) and relatively uncommon in idiopathic hypersomnia (20%).9 People with narcolepsy type 2 have rates intermediate to these other two groups (35%).9 Unlike cataplexy, sleep paralysis is very nonspecific, present, at least occasionally, in approximately 10% of the general population.10
Sleep-related hallucinations include hallucinations upon falling asleep (hypnogogic) or waking up (hypnopompic). As with sleep paralysis, sleep-related hallucinations are most common in people with narcolepsy type 1 (63% to 77%), least common in people with idiopathic hypersomnia (25%), and intermediate in people with narcolepsy type 2 (42%).9,11 Sleep-related hallucinations commonly co-occur with sleep paralysis. The narcolepsy tetrad of excessive daytime sleepiness, cataplexy, sleep paralysis, and sleep-related hallucinations is seen in complete form in only 45% of people with narcolepsy type 1, and typically, all four features are not present at initial presentation.11
Disrupted nocturnal sleep is common in narcolepsy type 1, manifesting on sleep studies as a higher arousal index12 and longer time awake after sleep onset.13 Patients with narcolepsy type 1 report significantly more nocturnal awakenings than patients with idiopathic hypersomnia.14 People with narcolepsy type 1 have a higher number of arousals and lower sleep efficiency than those with either narcolepsy type 2 or idiopathic hypersomnia (Figure 3-1).15
Figure 3-1.
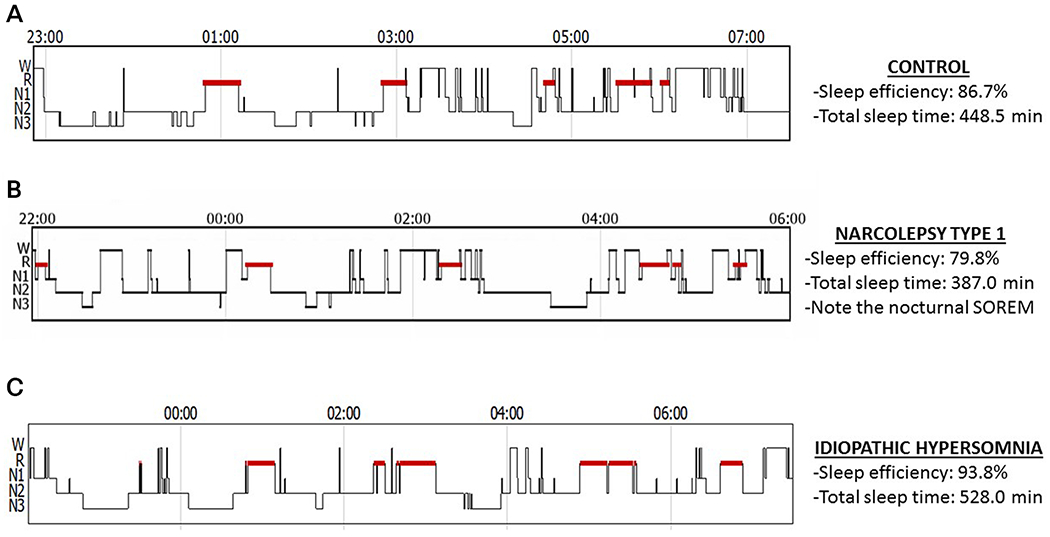
Differences in sleep efficiency and duration by diagnosis. Hypnograms from a nonsleepy control (A), a patient with narcolepsy type 1 (B), and a patient with idiopathic hypersomnia (C). The y-axis indicates the stage of sleep (wake [W], REM [R], N1, N2, and N3), while the x-axis is the time of night. People with narcolepsy type 1 have reduced sleep efficiency compared with controls and may have sleep-onset REM periods (ie, REM sleep within 15 minutes of sleep onset). People with idiopathic hypersomnia have high sleep efficiency and may have long sleep times but without sleep-onset REM periods (SOREM).
In addition to excessive daytime sleepiness, core features of idiopathic hypersomnia include long sleep durations and pronounced sleep inertia. [KP 5] While people with narcolepsy type 1 typically sleep less than 9 to 10 hours in a 24-hour period,16,17 it is common (but not mandatory) for people with idiopathic hypersomnia to experience long nocturnal sleep of more than 10 to 11 hours, along with very long daytime naps. This extended duration of sleep is typically not refreshing. Rates of long sleep in narcolepsy type 2 are not fully characterized, but a long sleep phenotype occurs in approximately 18% of patients with narcolepsy, most of whom have narcolepsy type 2.17
Another common and problematic symptom seen primarily in idiopathic hypersomnia is pronounced sleep inertia. Immediately upon awakening, the period of sleepiness, desire to return to sleep, and cognitive dysfunction is known as sleep inertia. This state is brief in healthy, well-rested individuals awakening during their circadian day,18 but in people with idiopathic hypersomnia, the period of sleep inertia is markedly prolonged and more severe, classically referred to as sleep drunkenness.19 It is not uncommon for people with idiopathic hypersomnia to require the assistance of another person to awaken,2 and they can take several hours to reach their full alertness. Sleep drunkenness is clearly more common in idiopathic hypersomnia than narcolepsy type 1, although its frequency in narcolepsy type 2 is not well studied.20 In a minority of patients, the sleep drunkenness is the most problematic symptom, even overshadowing the daytime sleepiness itself.19
People with narcolepsy type 2 manifest excessive daytime sleepiness, with variable rates of sleep paralysis, sleep-related hallucinations, and disrupted nocturnal sleep. Sleep times are sometimes prolonged, and sleep inertia may be present. Thus, narcolepsy type 2 may look more similar to either narcolepsy type 1 or idiopathic hypersomnia, depending on the patient. [KP 6] No clinical features are unique to narcolepsy type 2.
Kleine-Levin syndrome differs considerably from these other central disorders of hypersomnolence, most notably, in that it is episodic rather than persistent. People with Kleine-Levin syndrome experience recurrent attacks of profound sleepiness and long sleep times. During attacks, patients will sleep an average of 18 hours per day.21 In addition to episodic sleepiness, a diagnosis of Kleine-Levin syndrome requires the presence of at least one of the following: cognitive dysfunction, altered perception, disordered eating patterns, or disinhibited behavior during the attacks.1 [KP 7] Derealization and apathy are among the most common manifestations, while hypersexuality and hyperphagia are each present in approximately 50% of patients with Kleine-Levin syndrome.22 At least two separate attacks, with normal baseline in between, must occur for a Kleine-Levin syndrome diagnosis. Attacks typically last 2 days to 5 weeks, although they may be longer and occur at least once every 18 months.
DIAGNOSIS
Diagnosis generally requires a combination of clinical evaluation, exclusion of other disorders, and objective testing within the sleep laboratory (Table 3-1). The exception is Kleine-Levin syndrome, which is diagnosed based on clinical features without sleep laboratory testing. The mainstay of sleep laboratory testing for the other disorders is the overnight polysomnogram immediately followed by the multiple sleep latency test (MSLT). The overnight polysomnogram accomplishes several goals: (1) exclusion of nocturnal causes of excessive daytime sleepiness (eg, obstructive sleep apnea); (2) measurement of initial REM latency from the time of sleep onset, which if less than 15 minutes is used toward the diagnosis of narcolepsy; and (3) exclusion of acute sleep restriction, which could give a false-positive MSLT result. A minimum of 6 hours of sleep should be obtained on this test, although 7 hours is preferred.
The MSLT is a daytime test in which patients are given five nap opportunities, spaced 2 hours apart and beginning 1.5 hours after waking in the morning. During each nap opportunity, patients are asked to lie in bed, close their eyes, and try to fall asleep. Two main diagnostic measures are obtained from the MSLT: the mean sleep latency and number of sleep-onset REM periods.
The mean sleep latency is the average time to the first epoch (30-second scoring window) of any stage of sleep, across all nap opportunities. For narcolepsy type 1, a mean sleep latency of ≤8 minutes shows a good balance of sensitivity and specificity.23 For convenience, this 8-minute threshold has been extended to the diagnoses of narcolepsy type 2 and idiopathic hypersomnia. It is clear that higher values may be seen in sleepy patients,1 and the 8-minute threshold does not perform as well in disorders other than narcolepsy type 1.24,25
A sleep-onset REM period is the onset of REM sleep within 15 minutes of falling asleep. It does not require a transition directly from wake to REM but rather the appearance of REM sleep close to (within 15 minutes of) sleep onset. Considering both the initial REM latency on the nocturnal polysomnogram and each of the five MSLT nap opportunities, an individual can have anywhere from zero to six sleep-onset REM periods. [KP 8] The presence of two or more sleep-onset REM periods supports the diagnosis of either kind of narcolepsy and excludes the diagnosis of idiopathic hypersomnia (Case 3-1).
Case 3-1
A 28-year-old woman presented for evaluation of excessive daytime sleepiness. She thought her sleepiness started in high school but had since gradually worsened. She went to bed around 10:00 pm, fell asleep within 5 minutes, and woke to an alarm at 6:30 am. She had to hit her snooze button 2 times, getting out of bed around 7:00 am. On non-workdays, she kept the same bedtime but slept as late as 10:00 am. She never had any unusual physical responses to laughter or anger or episodes of transient muscle weakness, but she did occasionally wake up paralyzed for a few seconds. Because of her sleepiness, her primary care doctor suspected depression and prescribed fluoxetine. She took this for 6 months with no change in symptoms, so her primary care physician gradually weaned her off this medication. She was on no medications at the time of presentation.
She completed a sleep log and underwent actigraphy and polysomnography/multiple sleep latency test (MSLT). Actigraphy and the sleep log were generally concordant with the sleep durations she reported in clinic, averaging 8 hours of estimated sleep per night on weekdays and 10.5 hours on weekends. Several daytime naps of 2 hours each were additionally present during the weekend. Polysomnogram showed a nocturnal sleep latency of 7 minutes, REM latency of 10 minutes, sleep efficiency of 90%, total sleep time of 445 minutes, apnea-hypopnea index of 0, and periodic limb movement index of 7. Her urine drug screen was negative. Her MSLT results from the next day are listed in the grid below.
| Nap | Sleep Latency | REM Latency |
|---|---|---|
| 1 | 3 minutes | No REM |
| 2 | 5 minutes | No REM |
| 3 | 3 minutes | 13 min |
| 4 | 4 minutes | No REM |
| 5 | 10 minutes | No REM |
Based on her clinical presentation and sleep testing, she was diagnosed with narcolepsy type 2.
Comment
Diagnosis of narcolepsy type 2 requires daily excessive daytime sleepiness that has been present for at least 3 months, an absence of cataplexy, and polysomnogram/MSLT showing an MSLT mean sleep latency of ≤8 minutes and at least two sleep-onset REM periods. This patient’s mean sleep latency was 5 minutes, with one sleep-onset REM period on the polysomnogram (initial REM latency of 10 minutes) and one sleep-onset REM period on the MSLT (REM during nap 3). This case exemplifies the use of a nocturnal sleep-onset REM period plus a single MSLT sleep-onset REM period to meet the requirement for at least two sleep-onset REM periods for the diagnosis of narcolepsy. Prior diagnostic criteria considered only sleep-onset REM periods during the MSLT, but the inclusion of nocturnal sleep-onset REM periods in the current diagnostic criteria reflects recent data showing specificity of a nocturnal sleep-onset REM period for narcolepsy.
Thus, for the diagnosis of either type of narcolepsy, an MSLT-based diagnosis requires a mean sleep latency ≤8 minutes and at least two sleep-onset REM periods, while an MSLT-based diagnosis of idiopathic hypersomnia requires a mean sleep latency of ≤8 minutes and zero or one sleep-onset REM period.
Several clinical scenarios must be considered before ordering a polysomnogram /MSLT. First, sleep deprivation can result in false-positive results, and so this testing should be ordered only for patients who are routinely obtaining sufficient sleep (ie, at least 7 to 9 hours in adults).26,27 Sleep logs should be collected for at least 1 to 2 weeks leading up to the polysomnogram/MSLT, supplemented by actigraphy when available.
Second, multiple medications can affect MSLT results. Medications that are sedating or alerting can shorten or prolong the mean sleep latency, respectively, in addition to impacting the clinical symptoms of the patient. If medications are suspected to be contributing to the daytime sleepiness, these should be withdrawn or replaced with less-sedating treatments whenever possible and then sleepiness reassessed. Sleep-onset REM periods can also be suppressed by medications, most notably serotonergic antidepressants.28,29 Ideally, REM-suppressant medications would be withdrawn prior to testing. A 2-week medication-free period is recommended,26 although this may be too short for medications with very long half-lives (eg, fluoxetine). [KP 9] In practice, it is sometimes not appropriate to withdraw REM-suppressing medications, in which case the interpretation of results must consider the possibility that sleep-onset REM periods have been artificially suppressed.
Third, illicit drugs may also affect sleep latency, sleep-onset REM periods, or both, and so urine drug testing is often performed the morning of the MSLT. [KP 10]
Both narcolepsy type 1 and idiopathic hypersomnia can also be diagnosed by using objective confirmatory testing other than the MSLT (Table 3-1). Narcolepsy type 1 may be diagnosed by testing orexin (hypocretin) levels within CSF. [KP 11] Orexin (hypocretin) levels are low or absent (<110 pg/mL) in people with narcolepsy type 1 but normal (>200 pg/mL) in those with other hypersomnias. Together with sleepiness, orexin (hypocretin) deficiency is sufficient to diagnose narcolepsy type 1, even in the absence of other clinical features or MSLT findings. As of 2019, orexin (hypocretin) testing is commercially available as a send-out to the Mayo Clinic laboratory.30
Testing for human leukocyte antigen (HLA)-DQB1*06:02 may also be considered, as this allele is present in 98% of people with orexin (hypocretin)–deficient narcolepsy type 1.31 However, it is also present in many controls (up to 30%) and, therefore, is more useful when it is negative than when it is positive. It may be particularly useful when considering lumbar puncture for orexin (hypocretin) testing because if HLA is negative, orexin (hypocretin) levels are very unlikely to be abnormal and lumbar puncture may be avoided. [KP 12]
Diagnosis of idiopathic hypersomnia can be made by documentation of long sleep durations, in lieu of MSLT, either by an extended polysomnogram of up to 24 hours or ambulatory actigraphy of at least 7 days. [KP 13] A cutoff of 11 hours is used for both extended polysomnogram and actigraphy. Multiple protocols for measuring 24-hour sleep time by polysomnogram exist in Europe,24,32 but payer policies in the United States typically do not cover this testing. Some laboratories within the United States extend overnight polysomnography or the final MSLT nap or both to try to capture at least 11 hours of sleep time, but these protocols await validation and require laboratory staffing models that are not always available.
Actigraphy uses motion, typically at the wrist, to estimate sleep-wake state. Some devices incorporate other signals, such as light or bioimpedance, into sleep-wake algorithms. These devices have been reasonably well validated for insomnia but have quite limited validation for the central disorders of hypersomnolence.33,34 Despite this, actigraphy was incorporated into ICSD-3 diagnostic criteria for idiopathic hypersomnia.
Actigraphy is also recommended for 1 to 2 weeks prior to polysomnogram/MSLT, whenever possible, to help exclude insufficient sleep durations.1 The use of actigraphy is limited by cost and limited payer reimbursement. Further, these actigraphy recommendations refer only to clinical actigraphs, not direct-to-consumer devices. At present, these consumer tools are not recommended for the diagnosis of any sleep disorder.35
No additional tests beyond polysomnogram/MSLT are available for the diagnosis of narcolepsy type 2. HLA-DQB1*06:02 may be present but is frequently absent, and orexin (hypocretin) levels are (by definition) normal in patients with narcolepsy type 2. If a patient suspected of having narcolepsy type 2 based on clinical grounds undergoes CSF testing and is found to be orexin (hypocretin) deficient, the diagnosis changes to narcolepsy type 1.
EPIDEMIOLOGY
Population-based estimates for the central disorders of hypersomnolence are most readily available for narcolepsy type 1 because the combination of cataplexy and excessive daytime sleepiness is reasonably specific even in the absence of a polysomnogram /MSLT. The prevalence of narcolepsy type 1 is approximately 1 in 2000 (ie, 0.05%).36 Regional and/or ethnic differences exist, with considerably higher rates in Japan and considerably lower rates in Israel.36 Age of onset is bimodal, with peaks at approximately 15 and 35 years of age.37 Diagnostic delay from the onset of symptoms is more than 10 years on average.11
It is more difficult to obtain population-based estimates of narcolepsy type 2 because the nonspecific nature of sleepiness and absence of cataplexy make MSLT necessary for diagnosis. In the Wisconsin Sleep Cohort, a population-based cohort of adults, the prevalence of narcolepsy type 2 was estimated at 0.20%, approximately 3 times more common than narcolepsy type 1 (0.07%) in the same cohort.38 Using a medical claims database of 8.4 million people, other investigators found lower rates of both disorders but similarly found narcolepsy type 2 (0.07%) to be several times more common than narcolepsy type 1 (0.014%).39 [KP 14]
Idiopathic hypersomnia is classically taught to be a rare disease, in the absence of population-based data with objective measurement of sleepiness or sleep duration. Using subjective reports, Ohayon and colleagues40 found population frequencies of long 24-hour sleep times (>9 hours) of 8.4%, long sleep plus associated distress of 1.6%, and Diagnostic and Statistical Manual of Mental Disorders, Fifth Edition (DSM-5)41 hypersomnia disorder of 0.5%. Hypersomnia disorder, similar but not identical in definition to idiopathic hypersomnia, was much more common than narcolepsy type 1, which had a prevalence of 0.04% in the same study.
Kleine-Levin syndrome is the rarest of the central disorders of hypersomnolence, with an estimated prevalence of 1 to 5 cases per 1 million.21 [KP 15] The majority of people with Kleine-Levin are male, with a typical onset between 12 and 20 years of age. Symptoms typically remit with time, with a median duration of 15 years.21
PATHOPHYSIOLOGY
Narcolepsy type 1 is a disorder of orexin (hypocretin) deficiency. Orexin (hypocretin) is a hypothalamic peptide that promotes wakefulness and stabilizes wake/non-REM/REM state. Human narcolepsy type 1 is due to loss of orexin (hypocretin)–producing neurons,42 although in dogs, genetic alterations within the orexin (hypocretin) receptor cause a similar phenotype.43 Neuronal loss is very specific to orexin (hypocretin) neurons within the hypothalamus, sparing the neighboring melanin-concentrating hormone neurons.42
Narcolepsy type 1 has long been suspected to be an autoimmune disorder. As early as the 1980s, narcolepsy was shown to be associated with specific HLA alleles, and DQB1*06:02 is now known to be the most strongly, but not only, associated allele.44 A genome-wide association study of narcolepsy type 1 identified associations between several polymorphisms in the T-cell receptor alpha locus, thus implicating both components of T-cell antigen presentation and recognition.45 Seasonal peaks in narcolepsy type 1 onset, associations with both H1N1 infection and some forms of H1N1 vaccines, and associations with other infections have provided additional clues to an autoimmune origin.44,46,47 Most recently, autoreactive T cells have been identified in people with narcolepsy type 1.48 Taken together, these findings strongly suggest that narcolepsy type 1 is an autoimmune disease occurring in genetically susceptible (ie, HLA-DQB1*06:02–positive) individuals. [KP 16]
The cause of narcolepsy type 2 is currently unknown. A subset (approximately 20%) of people initially diagnosed with narcolepsy type 2 have or will develop orexin (hypocretin) deficiency,31 changing the diagnosis to narcolepsy type 1. For the remaining cases of narcolepsy type 2, orexin (hypocretin) levels are normal, and a distinct pathophysiology must be present. Other dysfunction of the orexin (hypocretin) system might be causal, as suggested by a postmortem examination of a patient with narcolepsy type 2 found to have partial loss of orexin (hypocretin) neurons within the posterior hypothalamus.49
The cause or causes of idiopathic hypersomnia also remain unknown. Similar to narcolepsy type 2, orexin (hypocretin) levels are normal in idiopathic hypersomnia. A variety of pathophysiologic hypotheses have been proposed across multiple systems. One theory involves abnormal activation of the soporific γ-aminobutyric acid (GABA)-ergic system. CSF from people with hypersomnia disorders (primarily idiopathic hypersomnia and narcolepsy type 2), applied to GABAA receptors in vitro, resulted in greater potentiation of receptor activity than did CSF from controls, implying the presence of a positive allosteric modulator of GABAA receptors in patients with hypersomnia.50 Using very different methods, another group was unable to replicate this finding.51 However, the known role of GABA in promotion of sleep provides biological plausibility to this hypothesis, as do randomized controlled trial and clinical observations that medications that modulate or antagonize GABAA receptors decrease sleepiness in patients with these disorders.52,53
Autonomic dysfunction in idiopathic hypersomnia is suggested by a greater frequency of autonomic symptoms in patients with idiopathic hypersomnia than in controls.2,54 Additionally, a single study evaluated heart rate variability in a group of patients with idiopathic hypersomnia and found a relative increase in parasympathetic to sympathetic activity in the patients with idiopathic hypersomnia versus the controls.55
Although circadian rhythm disorders have to be excluded as the cause of sleepiness in patients diagnosed with any of the central hypersomnia disorders,1 several studies have suggested circadian rhythm dysfunction in patients with idiopathic hypersomnia. Assessment of the peripheral circadian system in skin fibroblasts has demonstrated a longer circadian period length56 and decreased amplitude of expression of circadian genes BMAL1, PER1, and PER2.57
It is presently unclear whether narcolepsy type 2 and idiopathic hypersomnia share a common pathophysiology and, if so, to what extent. Clinically, these two disorders can be very similar, and the only diagnostic feature that separates them is the number of sleep-onset REM periods on an MSLT. Multiple groups have shown that MSLT-based diagnosis frequently changes on repeat testing in patients with these disorders,58–60 making it difficult to convincingly separate the two with current tools.
The cause of Kleine-Levin syndrome is also unknown. It is most commonly a sporadic disorder, but a familial form also occurs in fewer than 10% of cases.61 Single-photon emission computed tomography (SPECT) imaging in Kleine-Levin syndrome has demonstrated abnormalities during both the symptomatic and the asymptomatic periods, despite a clinical picture of relative normalcy in between bouts. In particular, during symptomatic spells, hypoperfusion of the right dorsomedial prefrontal cortex and right parietotemporal junction is seen, potentially resulting in the apathy and derealization commonly seen during episodes.62 Both during and between episodes, compared with controls, additional hypoperfusion of orbitofrontal cortex, superior temporal cortex, hypothalamus, posterior thalamus, caudate, and anterior cingulate is seen.
TREATMENT
Various treatment options are available for these disorders, although evidence in support of their use varies considerably by intervention and by disorder.
Nonpharmacologic Approaches
Nonpharmacologic treatments for the central disorders of hypersomnolence have not been well studied. Scheduled naps are often recommended for people with narcolepsy type 1 because short naps are usually restorative. In a controlled study, only the combination of scheduled naps with regular nocturnal sleep scheduling, rather than either behavioral approach alone, provided significant benefit to standard pharmacologic treatment.63 People with idiopathic hypersomnia often find it difficult to limit the duration of naps, and even long naps do not feel restorative, so scheduled naps are less useful. [KP 17]
Pharmacologic Treatment of Sleepiness
Nonpharmacologic treatments are typically not sufficient as monotherapy for the treatment of these disorders. Multiple medications have been approved by the US Food and Drug Administration (FDA) for the treatment of narcolepsy. The FDA does not currently distinguish between narcolepsy type 1 and narcolepsy type 2 in its approval process, and studies supporting the efficacy of these medications have frequently included patients with both disorders. In contrast, no medications are approved by the FDA for the treatment of idiopathic hypersomnia or Kleine-Levin syndrome. Therefore, all treatments prescribed for these latter two disorders are off-label. Medications used for the treatment of sleepiness in the narcolepsies and idiopathic hypersomnia include modafinil/armodafinil, traditional psychostimulants, sodium oxybate, solriamfetol, and pitolisant.
Modafinil/Armodafinil.
Modafinil is a racemic mix of two enantiomers. Its full mechanism of action is not known, but a major mechanism is enhancement of dopaminergic neurotransmission.64 It also has actions on glutamatergic and orexin (hypocretin) systems.64 In a meta-analysis of narcolepsy studies, modafinil decreased Epworth Sleepiness Scale scores (by 2.73 points more than placebo), prolonged sleep latency on the MSLT (by 1.1 minutes) and maintenance of wakefulness test (by 2.8 minutes), and improved quality of life (Case 3-2).65 A single randomized controlled trial of modafinil limited to people with idiopathic hypersomnia showed significant improvements in Epworth Sleepiness Scale scores (median decrease by 4.5 points more with modafinil than placebo) but not in the maintenance of wakefulness test (median improvement of 2.8 minutes more with modafinil), although the analysis was underpowered.66 [KP 18] Armodafinil is the R-enantiomer of modafinil. Unlike modafinil, which better controls sleepiness when prescribed in two divided doses,67 armodafinil is usually taken as a single morning dose.
Serious side effects of modafinil and armodafinil include Stevens-Johnson syndrome and other related conditions, angioedema, psychosis, mania, hallucinations, suicidal ideation, and dependency or abuse.68 Additionally, modafinil and armodafinil may interact with hormonal birth control, reducing effectiveness and requiring an additional or alternate form of birth control. [KP 19] More commonly observed side effects include headache and anxiety. Insomnia may be seen, especially with dosing either medication too close to bedtime. However, these medications are generally somewhat better tolerated than the traditional psychostimulants.64
Traditional psychostimulants.
Traditional psychostimulants are frequently used in the treatment of narcolepsy and idiopathic hypersomnia, with FDA approval for narcolepsy treatment with some formulations of methylphenidate, dextroamphetamine, amphetamine, and combination dextroamphetamine/amphetamine. Relatively limited randomized controlled trial data are available for these agents in the treatment of narcolepsy, but long clinical experience supports their use69 and available studies do support the effectiveness of methylphenidate, dextroamphetamine, and methamphetamine.70,71 Methamphetamine is rarely used, while combination amphetamine/dextroamphetamine is more often used in practice. Small clinical series of people with idiopathic hypersomnia suggest that these psychostimulants are effective in 25% to 41%.72,73
Traditional psychostimulants carry a risk of dependence and abuse, making these medications FDA schedule II. Other rare but serious side effects are psychosis, behavior changes, mood changes, arrhythmias, hypertension, cardiac disease, seizures, hepatotoxicity, pancytopenia, and erythema multiforme. More common side effects include anxiety, irritability, insomnia, tachycardia/palpitations, and blood pressure increases. In the author’s experience, most of these medications typically require dosing 2 times a day, with the exception of lisdexamfetamine (not approved for narcolepsy but used off-label) and some extended-release preparations. Some patients prefer a combination of immediate- and extended-release medication; the extended release helps maintain a baseline level of alertness, and immediate release helps with morning awakening and acts as a “rescue” medication when sleepiness is particularly severe or optimal alertness is needed (eg, when driving).
Sodium oxybate.
Sodium oxybate is the sodium salt of γ-hydroxybutyrate and is FDA approved for the treatment of both sleepiness and cataplexy in people with narcolepsy. It is taken at bedtime and again after 2.5 to 4.0 hours and promotes sleep consolidation and deep sleep.74 [KP 20] In a meta-analysis of narcolepsy studies, sodium oxybate improved the ability to remain awake during the maintenance of wakefulness test (by 5.2 minutes more than placebo) and improved clinical global impression of change.75 Unlike modafinil/armodafinil and the traditional psychostimulants, sodium oxybate also significantly decreases the frequency of cataplexy events (by a mean of 8 attacks per week).75 Although sodium oxybate is FDA approved for the treatment of either type of narcolepsy, some clinical trial data suggest the benefit may be greater in those with narcolepsy type 1.76 No published randomized controlled trials for sodium oxybate in idiopathic hypersomnia are presently available, but a series of approximately 40 people with idiopathic hypersomnia suggests similar effectiveness of sodium oxybate in idiopathic hypersomnia and narcolepsy type 1, as well as a reduction in sleep drunkenness in most idiopathic hypersomnia patients.77
Serious side effects of sodium oxybate include obtundation/central nervous system depression, clinically significant respiratory depression, abuse/dependence, depression, suicidal ideation, and psychosis. Because γ-hydroxybutyrate is a drug of abuse, there is a potential for sodium oxybate diversion or abuse; in cases of misuse, sodium oxybate may result in seizures, coma, or death. Common reactions include nausea, bed-wetting, and dizziness. Sodium oxybate must be dispensed under an FDA Risk Evaluation and Mitigation Strategy Program and is dispensed directly through a centralized pharmacy.78 [KP 21]
Case 3-2
A 24-year-old woman returned in follow-up for narcolepsy type 2, diagnosed 6 months before based on typical clinical history and findings on polysomnogram/multiple sleep latency test. Upon diagnosis, she was prescribed modafinil, initially as 100 mg every morning and then increased by 100 mg once a week to a maximum dose of 400 mg every morning. She was on no other medications. She reported that modafinil was very helpful for her daytime sleepiness and she did not notice any side effects. However, she found that her sleepiness returned by late afternoon, and she was concerned about her ability to drive home safely after work. Her modafinil dosing was changed to 200 mg every morning and 200 mg at noon, which allowed her to remain alert into the evening without developing sleepiness during her commute.
Comment
Although modafinil can be taken as a single morning dose, it is common for patients to report it wearing off too early when it is dosed this way, and dividing the 400 mg into two doses of 200 mg, one in the morning and one around lunchtime, can better control afternoon and evening sleepiness.67 Alternatively, because armodafinil retains higher drug concentrations in the afternoon than does modafinil79 changing her prescription to the equivalent dosage of armodafinil (250 mg every morning) would be another reasonable option. Although other medication classes could be considered, her excellent response to modafinil in the morning and early afternoon and absence of side effects favor trying to optimize treatment with modafinil/armodafinil before changing medication classes.
Solriamfetol.
In March 2019, the FDA approved a novel wake-promoting medication, solriamfetol, for the treatment of sleepiness associated with narcolepsy or obstructive sleep apnea. [KP 22] It is a dopamine- and norepinephrine-reuptake inhibitor. In the phase 3 randomized controlled trial of solriamfetol for narcolepsy, daily doses of 75 mg, 150 mg, and 300 mg all significantly decreased Epworth Sleepiness Scale scores (by 2.2 points, 3.8 points, and 4.8 points more than placebo, respectively), and daily doses of 150 mg and 300 mg significantly improved the ability to remain awake during the maintenance of wakefulness test (by 7.7 minutes and 10.2 minutes more than placebo, respectively).80 The FDA approved a starting dose of 75 mg for narcolepsy (lower for sleep apnea) and a maximum dose of 150 mg, despite benefit from the 300-mg dose in clinical trials. To date, solriamfetol has not been studied for the treatment of sleepiness associated with idiopathic hypersomnia, but off-label prescribing might be reasonable for people with idiopathic hypersomnia refractory to other agents.
Serious side effects of solriamfetol include elevation of heart rate or blood pressure and development of psychiatric symptoms. It has abuse potential and is a schedule IV drug. Common side effects of solriamfetol include headache, nausea, appetite suppression, insomnia, and anxiety.81
Pitolisant.
Pitolisant, a histamine H3 inverse agonist/antagonist that promotes wakefulness by increasing central nervous system histaminergic transmission, has been approved for the treatment of narcolepsy by the European Medicines Agency for several years. It was approved by the FDA for the treatment of sleepiness due to narcolepsy in August 2019. [KP 23] In a phase 3 randomized controlled trial of patients with narcolepsy type 1 or narcolepsy type 2, it significantly decreased Epworth Sleepiness Scale scores (by 3.0 points more than placebo) but was not shown to be not inferior to modafinil (modafinil decreased Epworth Sleepiness Scale scores by 0.1 points more than pitolisant).82 Pitolisant has not been tested for idiopathic hypersomnia in a randomized controlled trial, but a small case series suggests it is beneficial in about one-third of people with treatment-refractory idiopathic hypersomnia.83
Serious side effects of pitolisant include prolongation of the QT interval. Pitolisant may lessen the efficacy of hormonal birth control, requiring an additional or alternate form of birth control. Common side effects include insomnia, nausea, and anxiety. Pitolisant is currently the only unscheduled treatment for excessive daytime sleepiness in narcolepsy.84
Pharmacologic Treatment of Cataplexy
Some patients with narcolepsy type 1 will need pharmacologic treatment for cataplexy in addition to treatment for sleepiness.
Sodium oxybate, antidepressants, and pitolisant.
Sodium oxybate is the only medication that is FDA approved for the treatment of cataplexy. As such, it allows control of multiple symptoms of narcolepsy, including daytime sleepiness, disrupted nocturnal sleep, cataplexy, and other REM-dyscontrol symptoms, with a single agent.69 However, because of potential side effects or drug-drug interactions, some patients will prefer or need other treatment options for cataplexy. REM-suppressing antidepressants are frequently used off-label for cataplexy treatment. This practice is based on long clinical experience rather than randomized controlled trial data.69 In our center’s experience, venlafaxine and fluoxetine are particularly useful agents for cataplexy. In a randomized controlled trial, pitolisant reduced weekly cataplexy frequency by 75% (versus 38% in the placebo group).85 [KP 24] However, pitolisant is currently FDA approved only for sleepiness, not for cataplexy.84
Pharmacologic Treatment of Kleine-Levin Syndrome
Because of its distinct clinical presentation with recurrent hypersomnia, Kleine-Levin syndrome requires a somewhat different treatment approach. No published randomized controlled trial of Kleine-Levin syndrome exists, so data come from patient series. During attacks of sleepiness, treatment with wake-promoting medications can be attempted. However, data suggest that this has limited effectiveness, with clear benefit in only 20% of patients treated with methylphenidate.86 Amphetamines may be more helpful, reducing hypersomnia in 71% of patients, but not necessarily improving other disease manifestations.86 Instead, efforts are often directed at preventing relapses or shortening their duration and severity. In a series of 130 patients seen at a single site (a national referral center for Kleine-Levin syndrome), 71 patients who chose lithium treatment and 49 patients who opted for no treatment were followed for nearly 2 years on average.87 Those who opted for lithium treatment had significant improvements compared with those with no treatment, including reductions in the duration of the episode, severity of the episode, and number of episodes. [KP 25] Other treatments attempted in smaller series of patients with Kleine-Levin syndrome with at least some benefit include the macrolide antibiotic clarithromycin,88,89 antiepileptic medications (carbamazepine, valproate, phenobarbital, and phenytoin)86 and IV methylprednisolone.90
CONCLUSION
The central disorders of hypersomnolence can be distinguished based on clinical symptoms, objective testing, and, in the case of narcolepsy type 1, pathophysiology. Knowledge of the biology and optimal treatment of these disorders has increased considerably in recent years, including approval of two new medications for narcolepsy treatment in 2019, but important questions remain. Assessment and treatment for the full range of clinical symptoms, including but not limited to excessive daytime sleepiness, can help optimize functioning and quality of life for patients with these disorders.
KEY POINTS.
-
[KP 1]
Diagnosis of hypersomnia due to a medical disorder, hypersomnia due to a medication or substance, or insufficient sleep syndrome requires that the excessive daytime sleepiness is believed to be caused by a diagnosed medical or neurologic disorder, a medication or substance, or short sleep durations.
-
[KP 2]
The diagnosis of hypersomnia associated with a psychiatric disease does not imply that the psychiatric disease is necessarily caused by sleepiness or vice versa, just that the two conditions coexist.
-
[KP 3]
The five core clinical features of narcolepsy type 1 are excessive daytime sleepiness, cataplexy, sleep paralysis, sleep-related hallucinations, and disrupted nocturnal sleep. Many patients will not have all five symptoms.
-
[KP 4]
Cataplexy is very specific to narcolepsy type 1 and is not seen in the other hypersomnia disorders. Clinically, the presence or absence of cataplexy differentiates narcolepsy type 1 and narcolepsy type 2.
-
[KP 5]
The core clinical features of idiopathic hypersomnia are excessive daytime sleepiness, long sleep durations, and prominent sleep inertia, although not all symptoms are present in all patients.
-
[KP 6]
The phenotype of narcolepsy type 2 is intermediate between narcolepsy type 1 and idiopathic hypersomnia and has features of each.
-
[KP 7]
Kleine-Levin syndrome manifests as recurrent, severe hypersomnolence that is associated with cognitive dysfunction, altered perception, altered eating, or disinhibition.
-
[KP 8]
The two main diagnostic outputs of the multiple sleep latency test (MSLT) are the mean sleep latency and the number of sleep-onset rapid eye movement (REM) periods. The REM latency from the preceding night study should be counted toward the total sleep-onset REM period count.
-
[KP 9]
Sleep-onset REM periods can also be suppressed by medications, most notably serotonergic antidepressants. Ideally, REM-suppressant medications would be withdrawn prior to testing. A 2-week medication-free period is recommended, although this may be too short for medications with very long half-lives (eg, fluoxetine).
-
[KP 10]
Short habitual sleep times, medications, and illicit drugs may all affect MSLT results and must be considered prior to ordering and interpreting the MSLT.
-
[KP 11]
Excessive daytime sleepiness and CSF orexin (hypocretin) deficiency are sufficient to diagnose narcolepsy type 1, even in the absence of cataplexy.
-
[KP 12]
Patients with hypersomnia who test negative for human leukocyte antigen–DQB1*06:02 are very unlikely to be orexin (hypocretin) deficient, such that the usefulness of lumbar puncture for orexin (hypocretin) is very low in this group.
-
[KP 13]
The MSLT may be normal in a substantial number of patients suspected of having idiopathic hypersomnia. In these patients, idiopathic hypersomnia may alternately be diagnosed by recording at least 11 hours of sleep per 24 hours, either by polysomnography or estimated by wrist activity over at least 7 days.
-
[KP 14]
The population prevalence of narcolepsy type 1 is approximately 1 per 2000. Narcolepsy type 2 may be 3 to 4 times more common than narcolepsy type 1, based on a large population-based MSLT study and insurance database claims.
-
[KP 15]
Kleine-Levin syndrome is rare, with a prevalence estimated at 1 to 5 cases per 1 million.
-
[KP 16]
Narcolepsy type 1 is strongly suspected to be autoimmune, with multiple alterations within T-cell pathways implicated.
-
[KP 17]
Scheduled naps are likely to be more useful for people with narcolepsy type 1 than with idiopathic hypersomnia.
-
[KP 18]
Modafinil has been shown in randomized controlled trials to improve sleepiness in people with narcolepsy type 1 and narcolepsy type 2 and idiopathic hypersomnia.
-
[KP 19]
Modafinil, armodafinil, and pitolisant may all decrease the efficacy of hormonal birth control pills. Additional or alternate birth control methods should be used while taking these medications and for 21 days after their discontinuation.
-
[KP 20]
Sodium oxybate is dosed at bedtime and in the middle of the night and improves sleep consolidation and deep sleep.
-
[KP 21]
A sodium oxybate prescription requires enrollment in an FDA Risk Evaluation and Mitigation Strategy program because of the potential for serious risks.
-
[KP 22]
Solriamfetol is a dopamine- and norepinephrine-reuptake inhibitor approved by the FDA for narcolepsy treatment in 2019. It reduces sleepiness in patients with narcolepsy.
-
[KP 23]
Pitolisant increases histaminergic neurotransmission and was approved by the FDA for narcolepsy treatment in 2019. It reduces sleepiness and cataplexy in people with narcolepsy and may reduce sleepiness in people with idiopathic hypersomnia.
-
[KP 24]
Cataplexy can be treated with sodium oxybate, antidepressants, and pitolisant.
-
[KP 25]
Lithium may aid in the prevention of symptomatic episodes and reduce the severity of episodes in people with Kleine-Levin syndrome.
ACKNOWLEDGMENT
This work was supported by grants from the National Institutes of Health (NS083748 and NS111280).
Relationship Disclosure:
Dr Trotti has served on the editorial boards for Current Sleep Medicine Reports, Journal of Clinical Sleep Medicine, and Sleep and has received compensation/honoraria from the American Academy of Neurology, the American Academy of Sleep Medicine, Associated Professional Sleep Societies, the Kentucky Sleep Society, Medscape CME, Oakstone CME, and the Society of Behavioral Sleep Medicine and research/grant support from the American Academy of Sleep Medicine Foundation and the National Institutes of Health/National Institute of Neurological Disorders and Stroke (NS083748, NS111280, and NS113912).
Footnotes
Unlabeled Use of Products/Investigational Use Disclosure: Dr Trotti discusses the unlabeled/investigational use of antiepileptic medications (carbamazepine, phenobarbital, phenytoin, and valproate), lithium, clarithromycin, methylprednisolone, modafinil/armodafinil, pitolisant, sodium oxybate, solriamfetol, and traditional psychostimulants for the treatment of idiopathic hypersomnia or Kleine-Levin syndrome; antidepressants, lisdexamfetamine, and methamphetamine for the treatment of narcolepsy; and pitolisant for cataplexy.
REFERENCES
- 1.American Academy of Sleep Medicine. International Classification of Sleep Disorders. 3rd ed. Darien, IL: American Academy of Sleep Medicine; 2014. [Google Scholar]
- 2.Vernet C, Leu-Semenescu S, Buzare MA, Arnulf I. Subjective symptoms in idiopathic hypersomnia: beyond excessive sleepiness. J Sleep Res 2010;19(4):525–534. doi: 10.1111/j.1365-2869.2010.00824.x. [DOI] [PubMed] [Google Scholar]
- 3.Cataplexy Guilleminault C.. Narcolepsy. Vol 3. New York, NY: Spectrum Publications; 1976. [Google Scholar]
- 4.Dauvilliers Y, Siegel JM, Lopez R, et al. Cataplexy—clinical aspects, pathophysiology and management strategy. Nat Rev Neurol 2014;10(7):386–395. doi: 10.1038/nrneurol.2014.97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Plazzi G, Khatami R, Serra L, et al. Pseudocataplexy in narcolepsy with cataplexy. Sleep Med 2010;11(6):591–594. doi: 10.1016/j.sleep.2010.03.004. [DOI] [PubMed] [Google Scholar]
- 6.Shankar R, Jalihal V, Walker M, Zeman A. Pseudocataplexy and transient functional paralysis: a spectrum of psychogenic motor disorder. J Neuropsychiatry Clin Neurosci 2010;22(4):445–450. doi: 10.1176/appi.neuropsych.22.4.445. [DOI] [PubMed] [Google Scholar]
- 7.Smit LS, Lammers GJ, Catsman-Berrevoets CE. Cataplexy leading to the diagnosis of Niemann-Pick disease type C. Pediatr Neurol 2006;35(1):82–84. doi: 10.1016/j.pediatrneurol.2005.12.012. [DOI] [PubMed] [Google Scholar]
- 8.Vossler DG, Wyler AR, Wilkus RJ, et al. Cataplexy and monoamine oxidase deficiency in Norrie disease. Neurology 1996;46(5):1258–1261. doi: 10.1212/wnl.46.5.1258. [DOI] [PubMed] [Google Scholar]
- 9.Khan Z, Trotti LM. Central disorders of hypersomnolence: focus on the narcolepsies and idiopathic hypersomnia. Chest 2015;148(1):262–273. doi: 10.1378/chest.14-1304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Denis D, French CC, Gregory AM. A systematic review of variables associated with sleep paralysis. Sleep Med Rev 2018;38:141–157. doi: 10.1016/j.smrv.2017.05.005. [DOI] [PubMed] [Google Scholar]
- 11.Luca G, Haba-Rubio J, Dauvilliers Y, et al. Clinical, polysomnographic and genome-wide association analyses of narcolepsy with cataplexy: a European Narcolepsy Network study. J Sleep Res 2013;22(5):482–495. doi: 10.1111/jsr.12044. [DOI] [PubMed] [Google Scholar]
- 12.Frauscher B, Gschliesser V, Brandauer E, et al. Motor disturbances during non-REM and REM sleep in narcolepsy-cataplexy: a video-polysomnographic analysis. J Sleep Res 2011;20(4):514–521. doi: 10.1111/j.1365-2869.2011.00906.x. [DOI] [PubMed] [Google Scholar]
- 13.Roth T, Dauvilliers Y, Mignot E, et al. Disrupted nighttime sleep in narcolepsy. J Clin Sleep Med 2013;9(9):955–965. doi: 10.5664/jcsm.3004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Bruck D, Parkes JD. A comparison of idiopathic hypersomnia and narcolepsy-cataplexy using self report measures and sleep diary data. J Neurol Neurosurg Psychiatry 1996;60(5):576–578. doi: 10.1136/jnnp.60.5.576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Takei Y, Komada Y, Namba K, et al. Differences in findings of nocturnal polysomnography and multiple sleep latency test between narcolepsy and idiopathic hypersomnia. Clin Neurophysiol 2012;123(1):137–141. doi: 10.1016/j.clinph.2011.05.024. [DOI] [PubMed] [Google Scholar]
- 16.Rogers AE, Aldrich MS, Caruso CC. Patterns of sleep and wakefulness in treated narcoleptic subjects. Sleep 1994;17(7):590–597. doi: 10.1093/sleep/17.7.590. [DOI] [PubMed] [Google Scholar]
- 17.Vernet C, Arnulf I. Narcolepsy with long sleep time: a specific entity? Sleep 2009;32(9): 1229–1235. doi: 10.1093/sleep/32.9.1229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Tassi P, Muzet A. Sleep inertia. Sleep Med Rev 2000;4(4):341–353. doi: 10.1053/smrv.2000.0098. [DOI] [PubMed] [Google Scholar]
- 19.Roth B, Nevsimalova S, Rechtschaffen A. Hypersomnia with “sleep drunkenness.” Arch Gen Psychiatry 1972;26(5):456–462. doi: 10.1001/archpsyc.1972.01750230066013. [DOI] [PubMed] [Google Scholar]
- 20.Trotti LM. Waking up is the hardest thing I do all day: sleep inertia and sleep drunkenness. Sleep Med Rev 2017;35:76–84. doi: 10.1016/j.smrv.2016.08.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Arnulf I, Groos E, Dodet P. Kleine-Levin syndrome: a neuropsychiatric disorder. Rev Neurol (Paris) 2018;174(4):216–227. doi: 10.1016/j.neurol.2018.03.005. [DOI] [PubMed] [Google Scholar]
- 22.Kleine-Levin syndrome Arnulf I.. In: Kryger MH, ed. Principles and practice of sleep medicine. Amsterdam, Netherlands: Elsevier; 2017. [Google Scholar]
- 23.Arand D, Bonnet M, Hurwitz T, et al. The clinical use of the MSLT and MWT. Sleep 2005;28(1): 123–144. doi: 10.1093/sleep/28.1.123. [DOI] [PubMed] [Google Scholar]
- 24.Vernet C, Arnulf I. Idiopathic hypersomnia with and without long sleep time: a controlled series of 75 patients. Sleep 2009;32(6):753–759. doi: 10.1093/sleep/32.6.753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Pizza F, Moghadam KK, Vandi S, et al. Daytime continuous polysomnography predicts MSLT results in hypersomnias of central origin. J Sleep Res 2013;22(1):32–40. doi: 10.1111/j.1365-2869.2012.01032.x. [DOI] [PubMed] [Google Scholar]
- 26.Littner MR, Kushida C, Wise M, et al. Practice parameters for clinical use of the multiple sleep latency test and the maintenance of wakefulness test. Sleep 2005;28(1):113–121. doi: 10.1093/sleep/28.1.113. [DOI] [PubMed] [Google Scholar]
- 27.Consensus Conference Panel, Watson NF, Badr MS, et al. Recommended amount of sleep for a healthy adult: a joint consensus statement of the American Academy of Sleep Medicine and Sleep Research Society. J Clin Sleep Med 2015;11(6):591–592. doi: 10.5664/jcsm.4758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Wichniak A, Wierzbicka A, Jernajczyk W. Sleep as a biomarker for depression. Int Rev Psychiatry 2013;25(5):632–645. doi: 10.3109/09540261.2013.812067. [DOI] [PubMed] [Google Scholar]
- 29.Wang YQ, Li R, Zhang MQ, et al. The neurobiological mechanisms and treatments of REM sleep disturbances in depression. Curr Neuropharmacol 2015;13(4):543–553. doi: 10.2174/1570159x13666150310002540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Orexin-A/yypocretin-1, spinal fluid. mayocliniclabs.com/test-catalog/Clinical+and+Interpretive/604230. Accessed March 16, 2020.
- 31.Han F, Lin L, Schormair B, et al. HLA DQB 1*06:02 negative narcolepsy with hypocretin/orexin deficiency. Sleep 2014;37(10):1601–1608. doi: 10.5665/sleep.4066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Evangelista E, Lopez R, Barateau L, et al. Alternative diagnostic criteria for idiopathic hypersomnia: a 32-hour protocol. Ann Neurol 2018;83(2):235–247. doi: 10.1002/ana.25141. [DOI] [PubMed] [Google Scholar]
- 33.Smith MT, McCrae CS, Cheung J, et al. Use of actigraphy for the evaluation of sleep disorders and circadian rhythm sleep-wake disorders: an American Academy of Sleep Medicine systematic review, meta-analysis, and GRADE assessment. J Clin Sleep Med 2018;14(7):1209–1230. doi: 10.5664/jcsm.7228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Cook JD, Eftekari SC, Leavitt LA, et al. Optimizing actigraphic estimation of sleep duration in suspected idiopathic hypersomnia. J Clin Sleep Med 2019;15(4):597–602. doi: 10.5664/jcsm.7722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Khosla S, Deak MC, Gault D, et al. Consumer sleep technology: an American Academy of Sleep Medicine position statement. J Clin Sleep Med 2018;14(5):877–880. doi: 10.5664/jcsm.7128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Genetic Mignot E. and familial aspects of narcolepsy. Neurology 1998;50(2 Suppl 1):S16–S22. doi: 10.1212/wnl.50.2_suppl_1.s16. [DOI] [PubMed] [Google Scholar]
- 37.Dauvilliers Y, Montplaisir J, Molinari N, et al. Age at onset of narcolepsy in two large populations of patients in France and Quebec. Neurology 2001;57(11):2029–2033. doi: 10.1212/wnl.57.11.2029. [DOI] [PubMed] [Google Scholar]
- 38.Goldbart A, Peppard P, Finn L, et al. Narcolepsy and predictors of positive MSLTs in the Wisconsin Sleep Cohort. Sleep 2014;37(6):1043–1051. doi: 10.5665/sleep.3758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Scheer D, Schwartz SW, Parr M, et al. Prevalence and incidence of narcolepsy in a US health care claims database, 2008–2010. Sleep 2019;42(7). pii:zsz091. doi: 10.1093/sleep/zsz091. [DOI] [PubMed] [Google Scholar]
- 40.Ohayon MM, Reynolds CF 3rd, Dauvilliers Y. Excessive sleep duration and quality of life. Ann Neurol 2013;73(6):785–794. doi: 10.1002/ana.23818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.American Psychiatric Association. Diagnostic and statistical manual of mental disorders, 5th ed (DSM-5). Washington, DC: American Psychiatric Association, 2013. [Google Scholar]
- 42.Thannickal TC, Moore RY, Nienhuis R, et al. Reduced number of hypocretin neurons in human narcolepsy. Neuron 2000;27(3):469–474. doi: 10.1016/s0896-6273(00)00058-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Lin L, Faraco J, Li R, et al. The sleep disorder canine narcolepsy is caused by a mutation in the hypocretin (orexin) receptor 2 gene. Cell 1999;98(3):365–376. doi: 10.1016/s0092-8674(00)81965-0. [DOI] [PubMed] [Google Scholar]
- 44.Bassetti CLA, Adamantidis A, Burdakov D, et al. Narcolepsy: clinical spectrum, aetiopathophysiology, diagnosis and treatment. Nat Rev Neurol 2019;15(9):519–539. doi: 10.1038/s41582-019-0226-9. [DOI] [PubMed] [Google Scholar]
- 45.Hallmayer J, Faraco J, Lin L, et al. Narcolepsy is strongly associated with the T-cell receptor alpha locus. Nat Genet 2009;41(6):708–711. doi: 10.1038/ng.372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Wu H, Zhuang J, Stone WS, et al. Symptoms and occurrences of narcolepsy: a retrospective study of 162 patients during a 10-year period in eastern China. Sleep Med 2014;15(6):607–613. doi: 10.1016/j.sleep.2013.12.012. [DOI] [PubMed] [Google Scholar]
- 47.Ahmed SS, Schur PH, MacDonald NE, Steinman L. Narcolepsy, 2009 A(H1N1) pandemic influenza, and pandemic influenza vaccinations: what is known and unknown about the neurological disorder, the role for autoimmunity, and vaccine adjuvants. J Autoimmun 2014;50:1–11. doi: 10.1016/j.jaut.2014.01.033. [DOI] [PubMed] [Google Scholar]
- 48.Latorre D, Kallweit U, Armentani E, et al. T cells in patients with narcolepsy target self-antigens of hypocretin neurons. Nature 2018;562(7725):63–68. doi: 10.1038/s41586-018-0540-1. [DOI] [PubMed] [Google Scholar]
- 49.Thannickal TC, Nienhuis R, Siegel JM. Localized loss of hypocretin (orexin) cells in narcolepsy without cataplexy. Sleep 2009;32(8):993–998. doi: 10.1093/sleep/32.8.993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Rye DB, Bliwise DL, Parker K, et al. Modulation of vigilance in the primary hypersomnias by endogenous enhancement of GABAA receptors. Sci Transl Med 2012;4(161):161ra51. doi: 10.1126/scitranslmed.3004685. [DOI] [PubMed] [Google Scholar]
- 51.Dauvilliers Y, Evangelista E, Lopez R, et al. Absence of y-aminobutyric acid-a receptor potentiation in central hypersomnolence disorders. Ann Neurol 2016;80(2):259–268. doi: 10.1002/ana.24710. [DOI] [PubMed] [Google Scholar]
- 52.Trotti LM, Saini P, Bliwise DL, et al. Clarithromycin in γ-aminobutyric acid-related hypersomnolence: a randomized, crossover trial. Ann Neurol 2015;78(3):454–465. doi: 10.1002/ana.24459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Trotti LM, Saini P, Koola C, et al. Flumazenil for the treatment of refractory hypersomnolence: clinical experience with 153 patients. J Clin Sleep Med 2016;12(10):1389–1394. doi: 10.5664/jcsm.6196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Miglis MG, Schneider L, Kim P, Cheung J, Trotti LM. Frequency and severity of autonomic symptoms in idiopathic hypersomnia [published online February 20, 2020]. J Clin Sleep Med. doi: 10.5664/jcsm.8344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Sforza E, Roche F, Barthélémy JC, Pichot V. Diurnal and nocturnal cardiovascular variability and heart rate arousal response in idiopathic hypersomnia. Sleep Med 2016;24:131–136. doi: 10.1016/j.sleep.2016.07.012. [DOI] [PubMed] [Google Scholar]
- 56.Materna L, Halfter H, Heidbreder A, et al. Idiopathic hypersomnia patients revealed longer circadian period length in peripheral skin fibroblasts. Front Neurol 2018;9:424. doi: 10.3389/fneur.2018.00424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Lippert J, Halfter H, Heidbreder A, et al. Altered dynamics in the circadian oscillation of clock genes in dermal fibroblasts of patients suffering from idiopathic hypersomnia. PLoS One 2014;9(1):e85255. doi: 10.1371/journal.pone.0085255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Trotti LM, Staab BA, Rye DB. Test-retest reliability of the multiple sleep latency test in narcolepsy without cataplexy and idiopathic hypersomnia. J Clin Sleep Med 2013;9(8):789–795. doi: 10.5664/jcsm.2922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Ruoff C, Pizza F, Trotti LM, et al. The MSLT is repeatable in narcolepsy type 1 but not narcolepsy type 2: a retrospective patient study. J Clin Sleep Med 2018;14(1):65–74. doi: 10.5664/jcsm.6882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Lopez R, Doukkali A, Barateau L, et al. Test-retest reliability of the multiple sleep latency test in central disorders of hypersomnolence. Sleep 2017;40(12). doi: 10.1093/sleep/zsx164. [DOI] [PubMed] [Google Scholar]
- 61.Nguyen QT, Groos E, Leclair-Visonneau L, et al. Familial Kleine-Levin syndrome: a specific entity? Sleep 2016;39(8):1535–1542. doi: 10.1093/sleep/zsx164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Kas A, Lavault S, Habert MO, Arnulf I. Feeling unreal: a functional imaging study in patients with Kleine-Levin syndrome. Brain 2014;137(Pt 7):2077–2087. doi: 10.1093/brain/awu112. [DOI] [PubMed] [Google Scholar]
- 63.Rogers AE, Aldrich MS, Lin X. A comparison of three different sleep schedules for reducing daytime sleepiness in narcolepsy. Sleep 2001;24(4):385–391. doi: 10.1093/sleep/24.4.385. [DOI] [PubMed] [Google Scholar]
- 64.Murillo-Rodriguez E, Barciela Veras A, Barbosa Rocha N, et al. An overview of the clinical uses, pharmacology, and safety of modafinil. ACS Chem Neurosci 2018;9(2):151–158. doi: 10.1021/acschemneuro.7b00374. [DOI] [PubMed] [Google Scholar]
- 65.Golicki D, Bala MM, Niewada M, Wierzbicka A. Modafinil for narcolepsy: systematic review and meta-analysis. Medical Sci Monit 2010;16(8):Ra177–Ra186. [PubMed] [Google Scholar]
- 66.Mayer G, Benes H, Young P, et al. Modafinil in the treatment of idiopathic hypersomnia without long sleep time—a randomized, double-blind, placebo-controlled study. J Sleep Res 2015;24(1): 74–81. doi: 10.1111/jsr.12201. [DOI] [PubMed] [Google Scholar]
- 67.Schwartz JR, Feldman NT, Bogan RK. Dose effects of modafinil in sustaining wakefulness in narcolepsy patients with residual evening sleepiness. J Neuropsychiatry Clin Neurosci 2005;17(3):405–412. doi: 10.1176/jnp.17.3.405. [DOI] [PubMed] [Google Scholar]
- 68.Modafinil. Prescribing information. Cephalon, Inc; 2004. Accessed March 16, 2020. modafinil.com/prescribe/index.html. [Google Scholar]
- 69.Morgenthaler TI, Kapur VK, Brown T, et al. Practice parameters for the treatment of narcolepsy and other hypersomnias of central origin. Sleep 2007;30(12):1705–1711. doi: 10.1093/sleep/30.12.1705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Mitler MM, Hajdukovic R, Erman MK. Treatment of narcolepsy with methamphetamine. Sleep 1993;16(4):306–317. doi: 10.1093/sleep/16.4.306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Mitler MM, Hajdukovic R. Relative efficacy of drugs for the treatment of sleepiness in narcolepsy. Sleep 1991;14(3):218–220. doi: 10.1093/sleep/14.3.218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Ali M, Auger RR, Slocumb NL, Morgenthaler T. Idiopathic hypersomnia: clinical features and response to treatment. J Clin Sleep Med 2009;5(6):562–568. [PMC free article] [PubMed] [Google Scholar]
- 73.Anderson KN, Pilsworth S, Sharples LD, et al. Idiopathic hypersomnia: a study of 77 cases. Sleep 2007;30(10):1274–1281. doi: 10.1093/sleep/30.10.1274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Black J, Pardi D, Hornfeldt CS, Inhaber N. The nightly use of sodium oxybate is associated with a reduction in nocturnal sleep disruption: a double-blind, placebo-controlled study in patients with narcolepsy. J Clin Sleep Med 2010;6(6):596–602. [PMC free article] [PubMed] [Google Scholar]
- 75.Alshaikh MK, Tricco AC, Tashkandi M, et al. Sodium oxybate for narcolepsy with cataplexy: systematic review and meta-analysis. J Clin Sleep Med 2012;8(4):451–458. doi: 10.5664/jcsm.2048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Black J, Swick T, Bogan R, et al. Impact of sodium oxybate, modafinil, and combination treatment on excessive daytime sleepiness in patients who have narcolepsy with or without cataplexy. Sleep Med 2016;24:57–62. doi: 10.1016/j.sleep.2016.07.010. [DOI] [PubMed] [Google Scholar]
- 77.Leu-Semenescu S, Louis P, Arnulf I. Benefits and risk of sodium oxybate in idiopathic hypersomnia versus narcolepsy type 1: a chart review. Sleep Med 2016;17:38–44. doi: 10.1016/j.sleep.2015.10.005. [DOI] [PubMed] [Google Scholar]
- 78.Xyrem. Package insert. Jazz Pharmaceuticals; 2018. [Google Scholar]
- 79.Darwish M, Kirby M, Hellriegel ET, Robertson P Jr. Armodafinil and modafinil have substantially different pharmacokinetic profiles despite having the same terminal half-lives: analysis of data from three randomized, single-dose, pharmacokinetic studies. Clin Drug Investig 2009;29(9):613–623. doi: 10.2165/11315280-000000000-00000. [DOI] [PubMed] [Google Scholar]
- 80.Thorpy MJ, Shapiro C, Mayer G, et al. A randomized study of solriamfetol for excessive sleepiness in narcolepsy. Ann Neurol 2019;85(3):359–370. doi: 10.1002/ana.25423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Sunosi (solriamfetol). Package insert. Jazz Pharmaceuticals; 2019. Accessed March 16, 2020. sunosihcp.com.
- 82.Dauvilliers Y, Bassetti C, Lammers GJ, et al. Pitolisant versus placebo or modafinil in patients with narcolepsy: a double-blind, randomised trial. Lancet Neurol 2013;12(11):1068–1075. doi: 10.1016/S1474-4422(13)70225-4. [DOI] [PubMed] [Google Scholar]
- 83.Leu-Semenescu S, Nittur N, Golmard JL, Arnulf I. Effects of pitolisant, a histamine H3 inverse agonist, in drug-resistant idiopathic and symptomatic hypersomnia: a chart review. Sleep Med 2014;15(6):681–687. doi: 10.1016/j.sleep.2014.01.021. [DOI] [PubMed] [Google Scholar]
- 84.Wakix (pitolisant). Package insert. Harmony Biosciences, LLC; 2019. Accessed March 16, 2020. wakix.com. [Google Scholar]
- 85.Szakacs Z, Dauvilliers Y, Mikhaylov V, et al. Safety and efficacy of pitolisant on cataplexy in patients with narcolepsy: a randomised, double-blind, placebo-controlled trial. Lancet Neurol 2017;16(3):200–207. doi: 10.1016/S1474-4422(16)30333-7. [DOI] [PubMed] [Google Scholar]
- 86.Arnulf I, Zeitzer JM, File J, et al. Kleine-Levin syndrome: a systematic review of 186 cases in the literature. Brain 2005;128(Pt 12):2763–2776. doi: 10.1093/brain/awh620. [DOI] [PubMed] [Google Scholar]
- 87.Leu-Semenescu S, Le Corvec T, Groos E, et al. Lithium therapy in Kleine-Levin syndrome: an open-label, controlled study in 130 patients. Neurology 2015;85(19):1655–1662. doi: 10.1212/WNL.0000000000002104. [DOI] [PubMed] [Google Scholar]
- 88.Rezvanian E, Watson NF. Kleine-Levin syndrome treated with clarithromycin. J Clin Sleep Med 2013;9(11):1211–1212. doi: 10.5664/jcsm.3176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Trotti LM, Bliwise DL, Rye DB. Further experience using clarithromycin in patients with Kleine-Levin syndrome. J Clin Sleep Med 2014;10(4):457–458. doi: 10.5664/jcsm.3634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Léotard A, Groos E, Chaumereuil C, et al. IV steroids during long episodes of Kleine-Levin syndrome. Neurology 2018;90(17):e1488–e1492. doi: 10.1212/WNL.0000000000005349. [DOI] [PubMed] [Google Scholar]


