Abstract
Objective
Hemodialysis patients show an approximately threefold higher prevalence of cognitive impairment compared to the age‐matched general population. Impaired microcirculatory function is one of the assumed causes. Dynamic retinal vessel analysis is a quantitative method for measuring neurovascular coupling and microvascular endothelial function. We hypothesize that cognitive impairment is associated with altered microcirculation of retinal vessels.
Methods
152 chronic hemodialysis patients underwent cognitive testing using the Montreal Cognitive Assessment. Retinal microcirculation was assessed by Dynamic Retinal Vessel Analysis, which carries out an examination recording retinal vessels' reaction to a flicker light stimulus under standardized conditions.
Results
In unadjusted as well as in adjusted linear regression analyses a significant association between the visuospatial executive function domain score of the Montreal Cognitive Assessment and the maximum arteriolar dilation as response of retinal arterioles to the flicker light stimulation was obtained.
Conclusion
This is the first study determining retinal microvascular function as surrogate for cerebral microvascular function and cognition in hemodialysis patients. The relationship between impairment in executive function and reduced arteriolar reaction to flicker light stimulation supports the involvement of cerebral small vessel disease as contributing factor for the development of cognitive impairment in this patient population and might be a target for noninvasive disease monitoring and therapeutic intervention.
Keywords: cerebral small vessel disease, cognitive impairment, dialysis, retinal vessels
1. INTRODUCTION
Cognitive impairment in hemodialysis patients attracts more and more clinical and scientific attention throughout the last years. An approximately three‐fold higher prevalence compared to the general population as well as adverse outcomes including increased mortality have been shown recently (Murray et al., 2006; Kurella Tamura et al., 2009; Drew et al., 2019; O'Lone et al., 2016; Kallenberg et al., 2016). However, knowledge about the underlying pathogenesis of cognitive impairment in hemodialysis patients is still limited. An involvement of cerebrovascular rather than neurodegenerative disease has been supported predominantly by findings of imaging studies: In a study by Drew et al. (2013) using magnet resonance imaging, hemodialysis patients had more severe white matter disease, cerebral atrophy including reduced hippocampal size and a higher prevalence of both small and large vessel infarcts than controls. Atrophy of grey matter especially in frontotemporal brain areas was described in several other studies including End Stage Renal Disease (ESRD)‐ but also Chronic Kidney Disease populations (Drew et al., 2017a; Savazzi et al., 2001; Tsuruya et al., 2015; Yoshimitsu et al., 2000). The latter brain areas are related to executive functions, which have been demonstrated to be impaired early in the course of dementia in hemodialysis patients (Drew et al., 2017b; Kurella Tamura et al., 2010), which is also the case in patients with vascular dementia (Wallin et al., 2018). Vascular contributions to cognitive impairment/dementia include endothelial dysfunction and impaired neurovascular coupling (Toth et al., 2017). In healthy individuals, neurovascular coupling and proper endothelial function ensure sufficient cerebral blood flow by adapting vessel diameters depending on the metabolic demand of the neuronal area.
Examining and quantifying these mechanisms in patients usually requires invasive techniques or is restricted to animal studies. Retinal imaging technologies offer the unique possibility to measure neurovascular coupling and endothelial dysfunction of arterioles and venules non‐invasively. Retinal vessels share a common embryonic origin and are therefore anatomically and physiologically comparable to cerebral small vessels (Park, 2007). Dynamic Retinal Vessel Analysis (DVA; Houben et al., 2017) monitors quantitative changes of retinal vessel diameters following flicker light stimuli in video sequences (Figure 1). The flickering light physiologically induces dilation of arterioles and venules via NO release, which is mediated by neurovascular coupling (Newman, 2013). Based on this involvement of NO‐signaling presumably mediated by endothelial cells, parameters of Dynamic Vessel Analyzer (DVA) are considered as potential biomarkers for microvascular endothelial dysfunction (Dorner et al., 2003; Houben et al., 2017). Dilation of retinal vessels following flickering light is known to be altered by factors like age, hypertension, hyperlipidemia, diabetes mellitus, weight and heart failure (Lim et al., 2013; Nagele et al., 2018; Sorensen et al., 2016). Previously, we were able to show, that reduced dilation of retinal venules is an independent risk factor for mortality in our cohort of hemodialysis patients. By applying machine learning methods, we could further demonstrate a role of the retinal arteriolar signal in cardiovascular mortality in those patients (Gunthner et al., 2019; Werfel et al., 2021).
FIGURE 1.
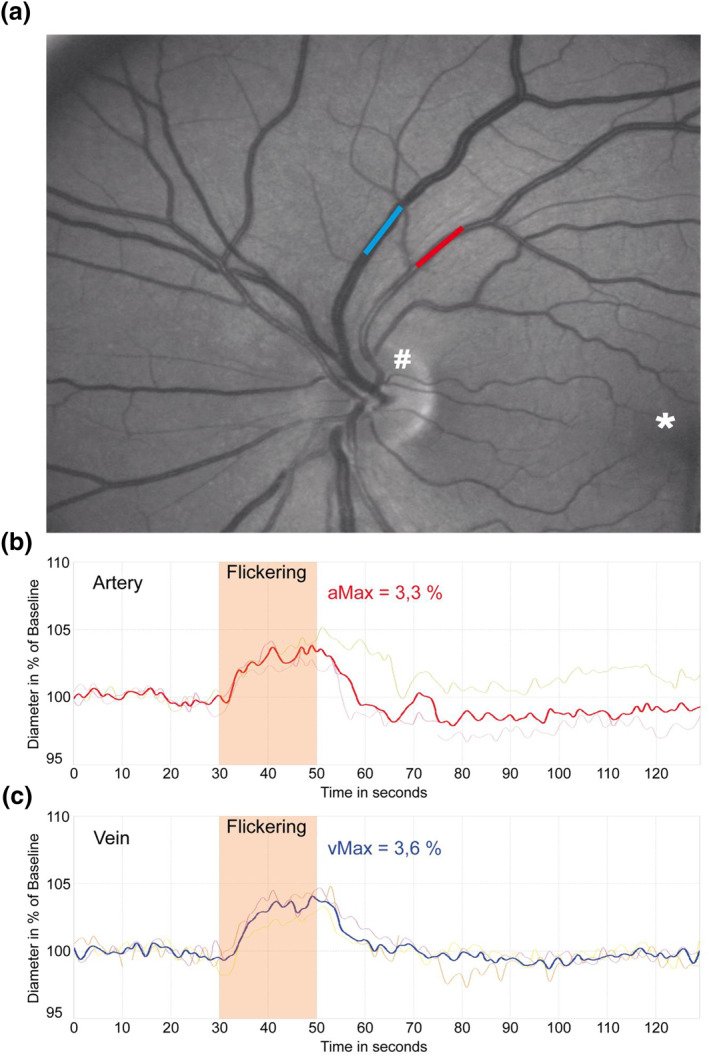
a: Screenshot of dynamic retinal vessel analysis with examined arteriole (red), venule (blue), as well as optic nerve head (#) and macula (*). b and c: Example for median time‐diameter curves of arteriolar and venular diameters in one of the examined patients. aMax and vMax are detected near the end of flickering episode. Thick lines represent median of three measurements; thin lines represent single measurements
Regarding patients with cognitive impairment, we could already show alterations of retinal arterioles' response to the flicker light stimulus in patients with Alzheimer's disease and mild cognitive impairment compared to healthy controls (Kotliar et al., 2017).
Aim of the Study: By applying dynamic retinal vessel analysis, we aimed to evaluate a potential relationship between retinal microcirculation as surrogate for cerebral microcirculation and cognitive function in hemodialysis patients for the first time.
2. METHODS
2.1. Patients, in‐ and exclusion criteria
Recruitment from hemodialysis centers in Munich and surrounding area took place between June 2011 and July 2013. Inclusion criteria were age above 18 years and hemodialysis vintage of at least three months. General exclusion criteria consisted of systemic infection, electrolyte disorder, malignancy and pregnancy. To rule out interference with cognitive testing, any motoric disorder of the dominant hand, a history of aphasia or amaurosis and other first language than German were defined as further exclusion criteria. All patients with MoCA and DVA assessments were included in this study.
This study is part of the risk stratification in end‐stage renal disease study, which is an observational study to evaluate the use of non‐invasive markers of autonomic function and micro‐ and macrocirculation to predict mortality and cardiovascular endpoints in ESRD patients. For a more detailed study protocol we refer to our previous publication (Schmaderer et al., 2016). Cognitive and DVA assessments were part of a sub study and have therefore not been carried out in all participating dialysis centers (see flow chart “data acquisition process” in Supplementary Figure 1).
2.2. Standard protocol approvals, registrations, and patient consents
The study protocol was submitted to the ethics committees of the Faculty of Medicine of the Technische Universität München and of the Landesärztekammer of Bavaria; both raised no objections. All clinical investigations were conducted in accordance with the principles of the Declaration of Helsinki, sixth revision. Written informed consent was obtained from all participants before any study specific procedure. The study is registered at ClinicalTrials.gov (Identifier number: NCT01152892).
2.3. Cognitive testing
For evaluation of cognitive function, the Montreal Cognitive Assessment (MoCA; Nasreddine et al., 2005) was chosen for the current study. Testing was performed under standardized conditions (before dialysis in a separate room) on a midweek dialysis day (Tholen et al., 2014). Executive functions were identified to be impaired early in the course of dementia (Drew et al., 2017b; Kurella Tamura et al., 2010) and to be the predominantly affected domain in hemodialysis patients (Sarnak et al., 2013). Executive functions are covered by the MoCA on a five‐point scale on the basis of three different tasks, called Visuospatial Executive VSE‐domain. For the present analyses MoCA scores without the potential additional point for lower educational level were used (educational level was added as independent variable instead). Furthermore the MoCA‐cut‐off was set at 24 points, which was confirmed to be more suitable for hemodialysis patients by two independent studies (Angermann et al., 2017; Tiffin‐Richards et al., 2014).
2.4. Demographic/clinical characteristics and laboratory parameters
Demographics including age, sex, educational level (according to the cut‐off of 12 years predefined in the MoCA test), cardiovascular risk factors and other comorbidities were derived from medical reports or obtained by patient interviews. Arterial hypertension was defined by the regular intake of antihypertensive medication and hypercholesterolemia by the regular intake of statins or total cholesterol levels above 200 mg/dl in the most recent laboratory examination. Concerning dialysis data, dialysis vintage was calculated as the cumulative time of patients requiring hemodialysis; the ultrafiltration rate was obtained from the protocol of the patients' most recent dialysis session. Blood pressure measurements were performed at the beginning of hemodialysis session. hsCRP was determined as previously described (Lorenz et al., 2018).
2.5. Retinal vessel analysis
Similar to cognitive assessment, eye examinations took place before a midweek dialysis session. If performed on the same day, cognitive assessment was carried out shortly before the eye examination, as for the dynamic measurement mydriasis was induced by instillation of one drop tropicamide (MydriaticumStulln: Pharma Stulln Ltd, Germany) in the eye.
Dynamic vessel analysis was performed in the eye with the dilated pupil by recording retinal vessel diameter changes within short segments (0.5–1 mm) approximately two disc diameters away from the optic disc in the upper‐ or lower‐temporal direction (Figure 1). The diameters of two previously marked vessels (one arteriole and one venule) were continuously measured for 350 s using the DVA (IMEDOS Systems, Jena, Germany). Baseline measurement lasted 50 s, followed by three episodes of flicker light stimulation, where each cycle consisted of 20 s flickering light and 80 s of non‐flicker light recording (Kotliar et al., 2011).
Mean maximum arteriolar and venular dilation (aMax and vMax) were determined in a window 4 s before and 1 s after the end of flickering light stimulation. The maximum arteriolar and venular dilation as primary parameters were chosen as these are the best‐established functional retinal biomarkers and have been evaluated in recently published landmark studies (Nagele et al., 2018; Sorensen et al., 2016). For quality control arteriolar and venular measurements were evaluated by the first observer based on a cumulative scoring method as previously published (Kotliar et al., 2011). The quality score ranged from 0 (“poor quality”) to 5 (“excellent quality”) and criteria included artefacts, noise, gaps within the curve and completeness of data points during flicker episodes. Measurements with a quality score result below 2.5 were discussed with an independent, second observer and excluded whenever necessary.
2.6. Statistical analyses
IBM SPSS Statistics for Windows, version 23 (IBM Corp., Armonk, N.Y., USA) was used for statistical tests. As in our previous publications, MoCA raw scores (not adjusted for educational level) were used for all calculations, as we believe that one extra point on the basis of patients' formal education is not enough to adjust for this important risk factor for cognitive impairment. Instead, educational level was included in corresponding analyses as independent variable.
Demographic and clinical characteristics were obtained by means of descriptive statistics. Patients were divided on the basis of their MoCA score in a “cognitively normal” and a “cognitively impaired” group for the illustration of differences in their demographic and clinical characteristics as well as in their DVA results.
Given the discrepancy between the number of patients, who underwent the MoCA (n = 242) only, compared to those, who participated in the DVA sub‐study and underwent both the MoCA and the DVA (n = 152), we carried out a Chi‐Square‐test for categorical variables (gender, educational level, cardiovascular risk factors) and a Mann‐Whitney‐test for numerical variables (age, Body mass index (BMI), dialysis vintage, MoCA score and subcategories).
To identify influencing factors on the MoCA total score and the subcategories, Spearman rho correlation analyses with age, gender, educational level and the cardiovascular risk factors were calculated (Table 2). These parameters are associated with an increased risk of dementia in the general population and especially the cardiovascular risk factors may play the most prominent role in the pathogenesis of dementia among patients with ESRD, as cognitive impairment is assumed to be related to microvascular brain damage in this population (Drew et al., 2017a; Savazzi et al., 2001; Tsuruya et al., 2015; Yoshimitsu et al., 2000).
TABLE 2.
MoCA score and Visuospatial Executive VSE domain and their correlation to demographical and clinical features (n = 152)
| MoCA score | VSE | |||
|---|---|---|---|---|
| Rho a | p‐value | Rho a | p‐value | |
| Demographics | ||||
| Age | −0.497 | <0.001 | −0.395 | <0.001 |
| Educational level ≤12 years | −0.392 | <0.001 | −0.311 | <0.001 |
| Sex, male | −0.072 | 0.375 | −0.103 | 0.207 |
| Comorbidities and hemodynamics | ||||
| Arterial hypertension | −0.060 | 0.462 | −0.044 | 0.588 |
| Systolic arterial pressure, mmHg | −0.199 | 0.014 | −0.177 | 0.029 |
| Diastolic arterial pressure, mmHg | 0.096 | 0.238 | 0.096 | 0.240 |
| Mean arterial pressure, mmHg | −0.039 | 0.630 | 0.001 | 0.997 |
| Diabetes mellitus | −0.314 | <0.001 | −0.223 | 0.006 |
| Hypercholesterolemia | −0.140 | 0.086 | −0.134 | 0.101 |
| Nicotine abuse | 0.183 | 0.024 | 0.083 | 0.309 |
| BMI, kg/m2 | −0.072 | 0.379 | −0.068 | 0.405 |
| Dialysis‐specific data | ||||
| Dialysis vintage (months on dialysis) | 0.198 | 0.014 | 0.117 | 0.153 |
| Ultrafiltration volume, liters | −0.035 | 0.674 | −0.088 | 0.297 |
Abbreviations: BMI: Body mass index, MoCA: Montreal Cognitive Assessment, VSE: Visuo spatial executive.
calculation with Spearman correlation.
To identify influencing factors on the DVA parameters correlation analyses with age, cardiovascular risk factors, BMI and hsCRP were calculated (Supplementary Table 2). These factors have been demonstrated to have an impact on changes in retinal microcirculation in previous studies (Gepstein et al., 2012; Nagel et al., 2004; Nguyen et al., 2008; Wong et al., 2004).
Addressing the primary aim of this study the associations between the MoCA total score and the VSE domain with parameters of retinal microcirculation (aMax, vMax) were calculated twofold: firstly, correlation analyses between MoCA total score and the VSE domain and aMax and vMax were calculated, secondly, linear regression analyses using the DVA parameters as dependent variable, MoCA score and VSE domain as independent parameters, were calculated controlled for confounding variables. All variables with an association with MoCA scores and VSE domain scores of p < 0.05 in the previous correlation analyses, namely age, educational level, systolic arterial pressure, nicotine abuse and dialysis vintage were included but not diabetes given the systematic selection of this parameter (see results below, Supplementary Table 1).
3. RESULTS
In 152 mainly Caucasian patients, data for DVA and MoCA were available, respectively. The comparison between patients with a MoCA test only (n = 90) to those with a MoCA and DVA examination (n = 152) revealed a systematic selection of patients included in the current DVA‐analyses for diabetes (p = 0.004), hypercholesterolemia (p = 0.027) and for the MoCA score (p = 0.021) and thus several subcategories. Patients with higher prevalence of diabetes and hypercholesterolemia as well as with lower MoCA scores were in the MoCA only group (Supplementary Table 1). Mean difference for MoCA scores between groups was 1.26 points.
Median MoCA value for the examined cohort was 25. Dividing the score in subcategories showed reduced scores for VSE function (4/5 points), language/verbal fluency (2/3 points) and memory function/recall (3/4 points).
After dividing patients on the basis of their MoCA score in a “cognitively normal” and a “cognitively impaired” group, 67 patients (44.1%) showed cognitive impairment (Table 1).
TABLE 1.
Demographic and clinical characteristics (n = 152) stratified by MoCA score
| Demographics | MoCA >24 (n = 85) | MoCA ≤24 (n = 67) | p‐value |
|---|---|---|---|
| Age (years), mean ± SD | 58.93 ± 15.34 | 70.79 ± 11.48 | <0.001 |
| Educational level ≤12 years, n (%) | 40 (47.1) | 56 (83.6) | <0.001 |
| Sex m/f, n (%) | 56 (65.9)/29 (34.1) | 50(74.6)/17(25.4) | 0.244 |
| Comorbidities | |||
| Arterial hypertension, n (%) | 80 (94.1) | 64 (95.5) | 0,700 |
| Diabetes mellitus, n (%) | 19 (22.4) | 29 (43.3) | 0.006 |
| Hypercholesterolemia, n (%) | 46 (54.1) | 46 (68.7) | 0.069 |
| Nicotine abuse, n (%) | 22 (25.9) | 8 (11.9) | 0.032 |
| BMI, mean ± SD | 25.50 ± 5.61 | 26.45 ± 5.42 | 0.161 |
| Aetiology of ESRD | |||
| Diabetic nephropathy, n (%) | 8 (9.4) | 12 (17.9) | 0.124 |
| Hypertensive nephropathy, n (%) | 5 (5.9) | 11 (16.4) | 0.036 |
| Glomerulonephritis, n (%) | 25 (29.4) | 15 (22.4) | 0.329 |
| Other causes, n (%) | 35 (41.2) | 18 (26.9) | 0.066 |
| Unknown, n (%) | 12 (14.1) | 11 (16.4) | 0.694 |
| Dialysis‐specific data | |||
| Dialysis vintage (months), mean ± SD | 70.34 ± 69.08 | 55.32 ± 56.22 | 0.190 |
| Ultrafiltration volume (liters), mean ± SD | 2.21 ± 1.11 | 2.40 ± 1.02 | 0.292 |
| Microcirculatory function ‐ DVA | |||
| aMax | 2.2 ± 2.5 | 1.6 ± 2.1 | 0.172 |
| vMax | 3.6 ± 2.4 | 2.9 ± 2.0 | 0.024 |
Abbreviations: aMax: maximum arteriolar dilation; DVA: dynamic vessel analysis, ESRD: end stage renal disease, m/f: male/female, vMax: maximum venular dilation.
Marked numerical differences when comparing the two groups (MoCA ≤24 vs > 24) were present for age, educational level, gender, diabetes, hypercholesterolemia, nicotine abuse and dialysis vintage (Table 1). Patients in the group with a MoCA score ≤24 points (classified as impaired) were older, predominantly male, less educated and had a higher cardiovascular burden except for smoking. With respect to dialysis vintage patients with preserved cognitive function overruled patients with cognitive impairment.
Correlation analyses of MoCA scores with demographics, comorbidities and dialysis‐specific parameters confirmed statistically significant associations with age, educational level, diabetes, nicotine abuse and dialysis vintage (Table 2). The VSE subcategory showed similar significant associations except for nicotine abuse and dialysis vintage.
Flickering light stimulation of the retina measured by DVA revealed a median dilation of the arteriole of 1.9 ± 2.3% above baseline diameter (aMax). Venular dilation (vMax) was 3.3 ± 2.3% above baseline, accordingly. Comparing aMax and vMax between the “cognitively impaired” and “cognitively normal” group showed reduced aMax and vMax for the patients with MoCA score ≤24 (Table 1). However, further analysis also showed a negative correlation of age with aMax and vMax (Supplementary Table 2). Additionally, arterial hypertension assessed as a comorbidity was significantly associated with reduced aMax and (without statistical significance) with reduced vMax. Further laboratory parameters, which were measured to determine systemic inflammation and calcium‐phosphate homeostasis as well as dialysis‐specific parameters did not show significant associations with aMax and vMax.
To explore the associations of the retinal microcirculation and cognitive impairment, the correlation of DVA parameters with MoCA score and subcategories were analyzed (Table 3).
TABLE 3.
Unadjusted and adjusted associations between MoCA parameters and Dynamic Vessel Analyzer (DVA)
| aMax | vMax | aMax (adjusted) b | vMax (adjusted) b | |||||
|---|---|---|---|---|---|---|---|---|
| MoCA parameters | Rho a | p | Rho a | p | Beta | p | Beta | p |
| MoCA score | 0.122 | 0.135 | 0.185 | 0.023 | 0.046 | 0.637 | 0.016 | 0.866 |
| ‐ VSE | 0.201 | 0.013 | 0.164 | 0.044 | 0.183 | 0.047 | 0.031 | 0.733 |
| ‐ Naming | −0.074 | 0.366 | −0.036 | 0.663 | ‐ | ‐ | ||
| ‐ Attention | −0.026 | 0.753 | 0.059 | 0.471 | ‐ | ‐ | ||
| ‐ Language | 0.050 | 0.544 | 0.094 | 0.253 | ‐ | ‐ | ||
| ‐ Abstraction | 0.043 | 0.602 | −0.013 | 0.876 | ‐ | ‐ | ||
| ‐ Memory | 0.019 | 0.821 | 0.115 | 0.160 | ‐ | ‐ | ||
| ‐ Orientation | 0.030 | 0.713 | −0.039 | 0.630 | ‐ | ‐ | ||
Abbreviations: aMax: maximum arteriolar dilation; MoCA: Montreal Cognitive Assessment, vMax: maximum venular dilation, VSE: Visuospatial executive.
calculation with Spearman correlation.
adjustment in linear regression for age, education level, systolic arterial pressure, nicotine abuse and dialysis vintage.
Patients with lower MoCA score showed attenuated dilation of arteriole and venule (statistically significant for vMax, Figure 2). Analysing the subcategories of MoCA revealed that only the VSE domain was significantly associated with aMax as well as vMax. This relationship, which was stronger for the maximum arteriolar dilation, is depicted in the box plot diagram (Figure 2c), where patients were categorized on the basis of their VSE results and the corresponding maximum arteriolar dilation: Higher VSE scores are related to a better response of retinal arterioles to the flicker light stimulus.
FIGURE 2.
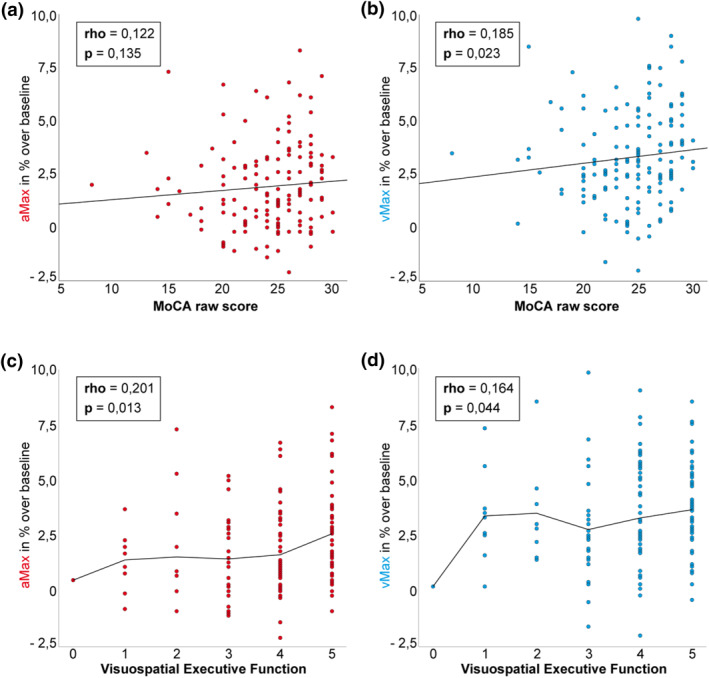
Relationship between MoCA and Visuospatial Executive VSE results and parameters of dynamic retinal vessel analysis. Scatter‐Dot Plots for MoCA score with aMax (a) and vMax (b), as well as VSE functions with aMax (c) and vMax (d). Spearman rho is displayed for each association. Lines represent linear regression (a,b) and mean for each category (c,d). MoCA: Montreal Cognitive Assessment, VSE: Visuospatial Executive (0–5 points attainable), aMax: maximum arteriolar dilation, vMax: maximum venular dilation
On the basis of the significant association between the VSE domain and both dynamic parameters linear regression analyses were carried out: In unadjusted analyses only the relationship between the VSE domain and aMax was significant (p = 0.024, corrected R 2 = 0.027 for aMax and p = 0.191, corrected R 2 = 0.005 for vMax, respectively). To show an effect independently of demographics, hemodynamics and dialysis‐specific factors, the subsequent regression analysis was adjusted to age, educational level, systolic arterial pressure, nicotine abuse and dialysis vintage. After adjustment only the maximum arteriolar dilation remained significantly associated to the VSE domain (p = 0.047, Table 3). The association between the overall score and the maximum venular dilation could not be maintained in adjusted analyses.
4. DISCUSSION
In the current study cognitive function and particularly visuospatial and executive functions, measured by the MoCA test, and their relationship to retinal microcirculatory dysfunction as surrogate for cerebral small vessel damages were analyzed in a cohort of 152 hemodialysis patients. For the maximum arteriolar dilation, which represents the response of retinal arterioles to flicker light stimuli, an independent association was demonstrated to the VSE domain of the MoCA test.
The major novelty of the current study consisted of this significant association between patients' performance in executive functions measured by the VSE domain of the MoCA test and the maximum arteriolar dilation, one standard parameter of the DVA. In this context in unadjusted as well as in adjusted analyses the VSE domain and the maximum arteriolar dilation were positively correlated meaning that a higher reaction of retinal arterioles to the flicker light stimulus is linked to better performance in executive functions and vice versa. To the best of our knowledge this is the first study evaluating cognitive function and changes in retinal microcirculation in a cohort of hemodialysis patients. There are several potential explanations for our findings: As already mentioned above, retinal arteriolar dilation as response to the flicker light stimulus reflects intact neurovascular coupling, a phenomenon existing in retinal as well as cerebral microcirculation. Neurovascular coupling consists of complex interactions between neurons, astrocytes, the endothelium and smooth muscle cells of arterioles to ensure an adequate nutritional supply for increased neuronal activity via NO‐mediated vasodilatation. Age‐mediated neurovascular uncoupling is likely to contribute to neuronal dysfunction especially by reduced NO‐bioavailability leading to cognitive impairment, which is supported by studies in aged rodents as well as in the general elderly population (Fabiani et al., 2014; Seidelmann et al., 2016; Sorond et al., 2005; Toth et al., 2013; Zhang et al., 1995). Which part of the neurovascular unit is affected (neurons and endothelial cells both exhibit NO‐synthases) and in what way hemodialysis might contribute to this uncoupling process has still to be elucidated.
Furthermore, our results point to an involvement of microvascular endothelial dysfunction of retinal and thus presumably also cerebral microvasculature in the pathogenesis of cognitive impairment in hemodialysis patients. Similar as for neurovascular (un‐)coupling NO‐mediated vasodilatation is supposedly impaired contributing to regional cerebral hypoperfusion and thus to neuronal damage and cognitive impairment (Sabayan et al., 2014; Wang et al., 2016). Further possible mechanisms involved include increased platelet adherence and aggregation, leucocyte adhesion and infiltration as well as proliferation of smooth muscle cells promoted by reduced NO‐release (Katusic & Austin, 2016). In previous studies predominantly the traditional cardiovascular risk factors and especially arterial hypertension were identified to play an important role in the development of microvascular endothelial dysfunction (as recently reviewed [Gimbrone & Garcia‐Cardena, 2016]), which is also the case in the current study. Being associated with cardiovascular risk factors as well as pre‐diabetes (Sorensen et al., 2016), therapeutic strategies could be derived on the basis of DVA examinations. However, if interventions like lifestyle modifications, blood sugar control or medical treatment for example, with antihypertensive agents especially angiotensin‐converting enzyme inhibitors or statins will be beneficial also in hemodialysis patients still has to be evaluated by future research.
A few limitations of the current study have to be addressed: Patients, who took part in the eye examination, were healthier in terms of cardiovascular comorbidity and cognitive impairment compared to those, who underwent cognitive testing only. This is, at least in part, explained by the higher demands of the eye examinations with respect to patients' compliance, which is also supported by the number of examinations, which could not be included in the current analyses due to poor quality (Supplementary Figure 1). Therefore, associations between retinal microcirculatory dysfunction and cognitive impairment probably have been underestimated in the current cohort especially regarding the relationship between DVA and global cognition assessed by the MoCA score. Furthermore, our results might also be attenuated, because data acquisition and thus analysis was limited to patients with mild to moderate cognitive impairment. Patients with advanced stages of cognitive impairment were not included due to their incapacity in providing written informed consent.
Another limitation consists of the cognitive testing instrument applied throughout the study. The MoCA was chosen due to feasibility reasons dictated by the modality of cognitive testing (before dialysis) chosen for the current study. Thus, no detailed neuropsychological test battery and especially no additional tests focusing on the evaluation of executive functions were carried out.
Finally, also DVA as a method to assess retinal microcirculatory function might be considered another limitation: Dialysis treatment and its efficacy have an impact on vessel reaction, since vascular reactivity is influenced by potassium and H+ ions (Newman, 2013; Takir et al., 2015). To meet this demand, DVA was strictly carried out under the standardized conditions mentioned above (before dialysis on a midweek dialysis day).
In conclusion, this is the first time that a relationship between parameters of retinal microcirculation and cognitive impairment in hemodialysis patients has been demonstrated. This association supports the supposed pathogenesis of microvascular damage and especially neurovascular uncoupling and microvascular endothelial dysfunction being among the causes for the excessively high prevalence of cognitive impairment in hemodialysis patients.
CONFLICT OF INTEREST
Nothing to report.
Supporting information
Supplementary Material
ACKNOWLEDGEMENTS
We thank all dialysis centers including physicians, nurses and patients for their participation in our study. Funding of the study includes Else‐Kroener Fresenius Foundation (P27/10//A33/10) and Servier Research Grant in Hypertension (111014/ASD/StgDM).
Open access funding enabled and organized by Projekt DEAL.
Angermann, S. , Günthner, R. , Hanssen, H. , Lorenz, G. , Braunisch, M. C. , Steubl, D. , Matschkal, J. , Kemmner, S. , Hausinger, R. , Block, Z. , Haller, B. , Heemann, U. , Kotliar, K. , Grimmer, T. , & Schmaderer, C. (2022). Cognitive impairment and microvascular function in end‐stage renal disease. International Journal of Methods in Psychiatric Research, 31(2), e1909. 10.1002/mpr.1909
Susanne Angermann and Roman Günthner should be considered joint first author.
Timo Grimmer and Christoph Schmaderer should be considered joint senior author.
DATA AVAILABILITY STATEMENT
The data that supports the findings of this study are available in the supplementary material of this article. Further raw data are available from the corresponding author upon reasonable request.
REFERENCES
- Angermann, S. , Baumann, M. , Steubl, D. , Lorenz, G. , Hauser, C. , Suttmann, Y. , Reichelt, A.‐L. , Satanovskij, R. , Sonntag, F. , Heemann, U. , Grimmer, T. , & Schmaderer, C. (2017). Cognitive impairment in hemodialysis patients: Implementation of cut‐off values for the Montreal Cognitive Assessment (MoCA)‐test for feasible screening. PLoS One, 12, e0184589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dorner, G. T. , Garhofer, G. , Kiss, B. , Polska, E. , Polak, K. , Riva, C. E. , & Schmetterer, L. (2003). Nitric oxide regulates retinal vascular tone in humans. American Journal of Physiology ‐ Heart and Circulatory Physiology, 285, H631–H636. [DOI] [PubMed] [Google Scholar]
- Drew, D. A. , Bhadelia, R. , Tighiouart, H. , Novak, V. , Scott, T. M. , Lou, K. V. , Shaffi, K. , Weiner, D. E. , & Sarnak, M. J. (2013). Anatomic brain disease in hemodialysis patients: A cross‐sectional study. American Journal of Kidney Diseases: The Official Journal of the National Kidney Foundation, 61, 271–278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drew, D. A. , Koo, B. B. , Bhadelia, R. , Weiner, D. E. , Duncan, S. , la Garza, M. M.‐D. , Gupta, A. , Tighiouart, H. , Scott, T. , & Sarnak, M. J. (2017). White matter damage in maintenance hemodialysis patients: A diffusion tensor imaging study. BMC Nephrology, 18, 213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drew, D. A. , Weiner, D. E. , & Sarnak, M. J. (2019). Cognitive impairment in CKD: Pathophysiology, management, and prevention. American Journal of Kidney Diseases: The Official Journal of the National Kidney Foundation, 74, 782–790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drew, D. A. , Weiner, D. E. , Tighiouart, H. , Duncan, S. , Gupta, A. , Scott, T. , & Sarnak, M. J. (2017). Cognitive decline and its risk factors in prevalent hemodialysis patients. American Journal of Kidney Diseases: The Official Journal of the National Kidney Foundation, 69, 780–787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fabiani, M. , Gordon, B. A. , Maclin, E. L. , Pearson, M. A. , Brumback‐Peltz, C. R. , Low, K. A. , McAuley, E. , Sutton, B. P. , Kramer, A. F. , & Gratton, G. (2014). Neurovascular coupling in normal aging: A combined optical, ERP and fMRI study. NeuroImage, 85(Pt 1), 592–607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gepstein, R. , Rosman, Y. , Rechtman, E. , Koren‐Morag, N. , Segev, S. , Assia, E. , & Grossman, E. (2012). Association of retinal microvascular caliber with blood pressure levels. Blood Pressure, 21, 191–196. [DOI] [PubMed] [Google Scholar]
- Gimbrone, M. A., Jr , & Garcia‐Cardena, G. (2016). Endothelial cell dysfunction and the pathobiology of atherosclerosis. Circulation Research, 118, 620–636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gunthner, R. , Hanssen, H. , Hauser, C. , Angermann, S. , Lorenz, G. , Kemmner, S. , et al. (2019). Impaired retinal vessel dilation predicts mortality in end‐stage renal disease. Circulation Research, 124, 1796‐1807. [DOI] [PubMed] [Google Scholar]
- Houben, A. , Martens, R. J. H. , & Stehouwer, C. D. A. (2017). Assessing microvascular function in humans from a chronic disease perspective. Journal of the American Society of Nephrology, 28, 3461–3472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kallenberg, M. H. , Kleinveld, H. A. , Dekker, F. W. , van Munster, B. C. , Rabelink, T. J. , van Buren, M. , & Mooijaart, S. P. (2016). Functional and cognitive impairment, frailty, and adverse health outcomes in older patients reaching ESRD‐A systematic review. Clinical Journal of the American Society of Nephrology: CJASN, 11, 1624–1639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Katusic, Z. S. , & Austin, S. A. (2016). Neurovascular protective function of endothelial nitric oxide‐ recent advances. Circulation Journal, 80, 1499–1503. [DOI] [PubMed] [Google Scholar]
- Kotliar, K. , Hauser, C. , Ortner, M. , Muggenthaler, C. , Diehl‐Schmid, J. , Angermann, S. , Hapfelmeier, A. , Schmaderer, C. , & Grimmer, T. (2017). Altered neurovascular coupling as measured by optical imaging: A biomarker for alzheimer's disease. Scientific Reports, 7, 12906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kotliar, K. E. , Lanzl, I. M. , Schmidt‐Trucksass, A. , Sitnikova, D. , Ali, M. , Blume, K. , Halle, M. , & Hanssen, H. (2011). Dynamic retinal vessel response to flicker in obesity: A methodological approach. Microvascular Research, 81, 123–128. [DOI] [PubMed] [Google Scholar]
- Kurella Tamura, M. , Covinsky, K. E. , Chertow, G. M. , Yaffe, K. , Landefeld, C. S. , & McCulloch, C. E. (2009). Functional status of elderly adults before and after initiation of dialysis. New England Journal of Medicine, 361, 1539–1547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kurella Tamura, M. , Larive, B. , Unruh, M. L. , Stokes, J. B. , Nissenson, A. , Mehta, R. L. , & Chertow, G. M. (2010). Prevalence and correlates of cognitive impairment in hemodialysis patients: The frequent hemodialysis network trials. Clinical Journal of the American Society of Nephrology: CJASN, 5, 1429–1438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lim, M. , Sasongko, M. B. , Ikram, M. K. , Lamoureux, E. , Wang, J. J. , Wong, T. Y. , & Cheung, C. Y. (2013). Systemic associations of dynamic retinal vessel analysis: A review of current literature. Microcirculation, 20, 257–268. [DOI] [PubMed] [Google Scholar]
- Lorenz, G. , Schmalenberg, M. , Kemmner, S. , Haller, B. , Steubl, D. , Pham, D. , Schreiegg, A. , Bachmann, Q. , Schmidt, A. , Haderer, S. , Huber, M. , Angermann, S. , Günthner, R. , Braunisch, M. , Hauser, C. , Reichelt, A.‐L. , Matschkal, J. , Suttmann, Y. , Moog, P. , … Schmaderer, C. (2018). Mortality prediction in stable hemodialysis patients is refined by YKL‐40, a 40‐kDa glycoprotein associated with inflammation. Kidney International, 93, 221–230. [DOI] [PubMed] [Google Scholar]
- Murray, A. M. , Tupper, D. E. , Knopman, D. S. , Gilbertson, D. T. , Pederson, S. L. , Li, S. , Smith, G. E. , Hochhalter, A. K. , Collins, A. J. , & Kane, R. L. (2006). Cognitive impairment in hemodialysis patients is common. Neurology, 67, 216–223. [DOI] [PubMed] [Google Scholar]
- Nagele, M. P. , Barthelmes, J. , Ludovici, V. , Cantatore, S. , von Eckardstein, A. , Enseleit, F. , Lüscher, T. F. , Ruschitzka, F. , Sudano, I. , & Flammer, A. J. (2018). Retinal microvascular dysfunction in heart failure. European Heart Journal, 39, 47–56. [DOI] [PubMed] [Google Scholar]
- Nagel, E. , Vilser, W. , & Lanzl, I. (2004). Age, blood pressure, and vessel diameter as factors influencing the arterial retinal flicker response. Investigative Ophthalmology & Visual Science, 45, 1486–1492. [DOI] [PubMed] [Google Scholar]
- Nasreddine, Z. S. , Phillips, N. A. , Bedirian, V. , Charbonneau, S. , Whitehead, V. , Collin, I. , Cummings, J. L. , & Chertkow, H. (2005). The montreal cognitive assessment, MoCA: A brief screening tool for mild cognitive impairment. Journal of the American Geriatrics Society, 53, 695–699. [DOI] [PubMed] [Google Scholar]
- Newman, E. A. (2013). Functional hyperemia and mechanisms of neurovascular coupling in the retinal vasculature. Journal of Cerebral Blood Flow and Metabolism: Official Journal of the International Society of Cerebral Blood Flow and Metabolism, 33, 1685–1695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nguyen, T. T. , Wang, J. J. , Sharrett, A. R. , Islam, F. M. A. , Klein, R. , Klein, B. E. K. , Cotch, M. F. , & Wong, T. Y. (2008). Relationship of retinal vascular caliber with diabetes and retinopathy: The multi‐ethnic study of atherosclerosis (MESA). Diabetes Care, 31, 544–549. [DOI] [PubMed] [Google Scholar]
- O'Lone, E. , Connors, M. , Masson, P. , Wu, S. , Kelly, P. J. , Gillespie, D. , et al. (2016). Cognition in people with end‐stage Kidney disease treated with hemodialysis: A systematic review and meta‐analysis. American Journal of Kidney Diseases, 67, 925–935. [DOI] [PubMed] [Google Scholar]
- Park, S. (2007). Duane’s foundations of clinical ophtalmology. Lippincott Williams&Williams.The anatomy and cell biology of the retina. [Google Scholar]
- Sabayan, B. , Westendorp, R. G. , Grond, J. , Stott, D. J. , Sattar, N. , van Osch, M. J. P. , van Buchem, M. A. , & de Craen, A. J. M. (2014). Markers of endothelial dysfunction and cerebral blood flow in older adults. Neurobiology of Aging, 35, 373–377. [DOI] [PubMed] [Google Scholar]
- Sarnak, M. J. , Tighiouart, H. , Scott, T. M. , Lou, K. V. , Sorensen, E. P. , Giang, L. M. , Drew, D. A. , Shaffi, K. , Strom, J. A. , Singh, A. K. , & Weiner, D. E. (2013). Frequency of and risk factors for poor cognitive performance in hemodialysis patients. Neurology, 80, 471–480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Savazzi, G. M. , Cusmano, F. , & Musini, S. (2001). Cerebral imaging changes in patients with chronic renal failure treated conservatively or in hemodialysis. Nephron, 89, 31–36. [DOI] [PubMed] [Google Scholar]
- Schmaderer, C. , Tholen, S. , Hasenau, A. L. , Hauser, C. , Suttmann, Y. , Wassertheurer, S. , Mayer, C. C. , Bauer, A. , Rizas, K. D. , Kemmner, S. , Kotliar, K. , Haller, B. , Mann, J. , Renders, L. , Heemann, U. , & Baumann, M. (2016). Rationale and study design of the prospective, longitudinal, observational cohort study “rISk strAtification in end‐stage renal disease” (ISAR) study. BMC Nephrology, 17, 161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seidelmann, S. B. , Claggett, B. , Bravo, P. E. , Gupta, A. , Farhad, H. , Klein, B. E. , Klein, R. , Di Carli, M. , & Solomon, S. D. (2016). Retinal vessel calibers in predicting long‐term cardiovascular outcomes: The atherosclerosis risk in communities study. Circulation, 134, 1328–1338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sorensen, B. M. , Houben, A. J. , Berendschot, T. T. , Schouten, J. S. A. G. , Kroon, A. A. , van der Kallen, C. J. H. , Henry, R. M. A. , Koster, A. , Sep, S. J. S. , Dagnelie, P. C. , Schaper, N. C. , Schram, M. T. , & Stehouwer, C. D. A. (2016). Prediabetes and type 2 diabetes are associated with generalized microvascular dysfunction: The maastricht study. Circulation, 134, 1339–1352. [DOI] [PubMed] [Google Scholar]
- Sorond, F. A. , Khavari, R. , Serrador, J. M. , & Lipsitz, L. A. (2005). Regional cerebral autoregulation during orthostatic stress: Age‐related differences. The Journals of Gerontology. Series A Biological Sciences and Medical Sciences, 60, 1484–1487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takir, S. , Uydes‐Dogan, B. S. , & Ozdemir, O. (2015). Retina derived relaxation is mediated by K(ir) channels and the inhibition of Ca(2+) sensitization in isolated bovine retinal arteries. Experimental Eye Research, 132, 240–248. [DOI] [PubMed] [Google Scholar]
- Tholen, S. , Schmaderer, C. , Kusmenkov, E. , Chmielewski, S. , Förstl, H. , Kehl, V. , Heemann, U. , Baumann, M. , & Grimmer, T. (2014). Variability of cognitive performance during hemodialysis: Standardization of cognitive assessment. Dementia and Geriatric Cognitive Disorders, 38, 31–38. [DOI] [PubMed] [Google Scholar]
- Tiffin‐Richards, F. E. , Costa, A. S. , Holschbach, B. , Frank, R. D. , Vassiliadou, A. , Krüger, T. , Kuckuck, K. , Gross, T. , Eitner, F. , Floege, J. , Schulz, J. B. , & Reetz, K. (2014). The Montreal Cognitive Assessment (MoCA) ‐ a sensitive screening instrument for detecting cognitive impairment in chronic hemodialysis patients. PLoS One, 9, e106700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Toth, P. , Csiszar, A. , Tucsek, Z. , Sosnowska, D. , Gautam, T. , Koller, A. , Schwartzman, M. L. , Sonntag, W. E. , & Ungvari, Z. (2013). Role of 20‐HETE, TRPC channels, and BKCa in dysregulation of pressure‐induced Ca2+ signaling and myogenic constriction of cerebral arteries in aged hypertensive mice. American Journal of Physiology ‐ Heart and Circulatory Physiology, 305, H1698–H1708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Toth, P. , Tarantini, S. , Csiszar, A. , & Ungvari, Z. (2017). Functional vascular contributions to cognitive impairment and dementia: Mechanisms and consequences of cerebral autoregulatory dysfunction, endothelial impairment, and neurovascular uncoupling in aging. American Journal of Physiology ‐ Heart and Circulatory Physiology, 312, H1–H20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsuruya, K. , Yoshida, H. , Haruyama, N. , Fujisaki, K. , Hirakata, H. , & Kitazono, T. (2015). Clinical significance of fronto‐temporal gray matter atrophy in executive dysfunction in patients with chronic Kidney disease: The VCOHP study. PLoS One, 10, e0143706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wallin, A. , Roman, G. C. , Esiri, M. , Kettunen, P. , Svensson, J. , Paraskevas, G. P. , & Kapaki, E. (2018). Update on vascular cognitive impairment associated with subcortical small‐vessel disease. Journal of Alzheimer's Disease, 62, 1417–1441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang, N. , Allali, G. , Kesavadas, C. , Noone, M. L. , Pradeep, V. G. , Blumen, H. M. , & Verghese, J. (2016). Cerebral small vessel disease and motoric cognitive risk syndrome: Results from the Kerala‐einstein study. Journal of Alzheimer's Disease, 50, 699–707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Werfel, S. , Gunthner, R. , Hapfelmeier, A. , Hanssen, H. , Kotliar, K. , Heemann, U. , & Schmaderer, C. (2021). Identification of cardiovascular high risk groups from dynamic retinal vessel signals using untargeted machine learning. Cardiovascular Research, 118, 612‐621. [DOI] [PubMed] [Google Scholar]
- Wong, T. Y. , Duncan, B. B. , Golden, S. H. , Klein, R. , Couper, D. J. , Klein, B. E. K. , Hubbard, L. D. , Sharrett, A. R. , & Schmidt, M. I. (2004). Associations between the metabolic syndrome and retinal microvascular signs: The atherosclerosis risk in communities study. Investigative Ophthalmology & Visual Science, 45, 2949–2954. [DOI] [PubMed] [Google Scholar]
- Yoshimitsu, T. , Hirakata, H. , Fujii, K. , Kanai, H. , Hirakata, E. , Higashi, H. , et al. (2000). Cerebral ischemia as a causative mechanism for rapid progression of brain atrophy in chronic hemodialysis patients. Clinical Nephrology, 53, 445–451. [PubMed] [Google Scholar]
- Zhang, F. , Xu, S. , & Iadecola, C. (1995). Role of nitric oxide and acetylcholine in neocortical hyperemia elicited by basal forebrain stimulation: Evidence for an involvement of endothelial nitric oxide. Neuroscience, 69, 1195–1204. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Material
Data Availability Statement
The data that supports the findings of this study are available in the supplementary material of this article. Further raw data are available from the corresponding author upon reasonable request.


