Summary
Background
Human Papillomavirus (HPV) self-collection offered by community health workers (CHWs) during home visits has been hampered by low levels of triage Pap among HPV-positive women. We investigated effectiveness of a mHealth intervention to increase adherence to triage Pap.
Methods
We conducted a hybrid type I cluster randomised effectiveness-implementation trial in Jujuy, Argentina. CHWs (clusters) were eligible if actively offering HPV self-collection and served at least 26 women aged 30 years and over. Women were eligible if they conducted self-collection and provided a mobile phone number. 260 CHWs were randomly allocated (3:2 ratio) to a multi-component intervention (Up to four SMS messages sent to HPV-positive women, and one SMS message to CHWs to prompt a visit of women with no triage Pap 60 days after a positive-test), or control group (Usual care: Women instructed to attend their health centre 30 days after HPV self-collection to pick-up results). The primary effectiveness outcome was percentage of HPV-positive women with triage 120 days after the HPV-test result. We evaluated implementation of the intervention using the RE-AIM framework.
Findings
221 CHWs (132 intervention, 89 control group) contacted 5389 women; and 5351 agreed to participate (3241 intervention, 2110 control group). In total 314/445 (70·5%) HPV-positive women of the intervention group had triage at 120 days after the HPV result, compared to 163/292 (55·1%) in the control group: 15·5% point improvement; 95%CI: 6·8–24·1; relative risk: 1·28; 95%CI: 1·11–1·48. 97·2% of women accepted the intervention and 86·9% of CHWs agreed to its adoption.
Interpretation
The multicomponent mHealth intervention was effective in increasing the percentage of HPV-positive women who had triage Pap, allowing for many more women at risk of cervical cancer to receive timely follow-up.
Funding
National Cancer Institute of the National Institutes of Health (USA) under Award Number R01CA218306.
Keywords: mHealth, HPV Self-collection, Community health workers, Argentina, Implementation Science, Cervical cancer, Prevention
Research in context ATICA Study.
Evidence before this study
Relevant publications with the key terms “cervical cancer”, “SMS messages”, “mobile health”, “cervical cancer screening”, “clinical trials”, “self-collection”, “triage”, “follow-up” were reviewed for quality and relevance. The initial search to define the study protocol covered PubMed between 2010 and 2016. The search was extended to April 2021 when writing the manuscript. Only studies written in English and Spanish were considered.
Added value of this study
Our main hypothesis was that mHealth interventions are an effective strategy to increase triage among HPV-positive women who perform HPV self-collection. It was based on previous evidence indicating that sending SMS messages to patients as reminders is effective in a variety of settings and for different health problems. We were interested in this approach because HPV self-collection offered by community health workers during home visits has been shown to dramatically increase screening uptake among women with lower access to the health care system, but adherence to triage after a positive HPV-self collected test in programmatic contexts of low-middle income settings remains low. A mobile health (mHealth) intervention has the potential to increase triage adherence among HPV-positive women without being heavily dependent on scarce human resources. Although some RCTs have evaluated effectiveness of SMS messages to increase screening uptake, we did not find any RCT evaluating effectiveness of sending SMS messages to women and CHWs to increase triage or follow-up among HPV-positive women with self-collected tests.
Implications of all available evidence
The data from the ATICA study demonstrate that sending SMS messages to women and community health workers is a highly effective to increase triage after an HPV-positive, self-collected test. The intervention decreased the time to triage, allowing for more women at risk of cervical cancer to receive timely follow-up. The intervention was accepted by women and community health workers and had few implementation problems. Our results have strong implications for middle- and low-income countries implementing or considering implementing HPV self-collection with mHealth interventions to ensure high follow-up adherence and make progress toward the WHO targets to eliminate cervical cancer.
Alt-text: Unlabelled box
Introduction
Cervical cancer (CC) is one of the leading causes of cancer death among women from low- and middle-income countries (LMIC).1 Paradoxically, the disease is preventable with existing knowledge and technologies, and that is why the World Health Organization (WHO) has launched a global initiative to eliminate CC through vaccination, screening and treatment.2 Human papillomavirus (HPV) DNA testing is a highly effective screening method3 and allows women to self-collect samples. HPV self-collection has been shown to increase screening uptake,4 especially when offered during home visits by community health workers (CHWs),5 with great potential to contribute to the WHO proposed elimination goal (90% of girls fully HPV vaccinated, 70% of women screened by the age of 30, 90% of women with precancer and cancer treated/managed).2
In an HPV self-collection screening program, triage of HPV-positive women is a key step to identify who will need further diagnostic and treatment procedures. While several triage methods are available for detecting precancerous lesions, cytology is one of the recommended methods per WHO guidelines6 and screening policies of several high- and middle-income countries.7,8 Cytologic triage involves HPV-positive women attending health centres to have a Papanicolaou (Pap) smear performed by a health professional. However, low adherence to follow-up after positive screening is a widespread and long-standing problem for CC programs in LMICs.9 Though self-collection has been deemed as particularly appropriate to increase screening among socially disadvantaged women, it may actually further decrease adherence to follow-up, as women with reduced contact with the healthcare system are less likely to adhere to screening/follow-up recommendations.10
Yet another major barrier is the delivery of test results after an abnormal screening result.11 In several studies that have evaluated HPV self-collection in LMICs, efforts to assure that women know their result and are aware of the need to continuing the process with a triage Pap have included home visits by CHWs5 or trained nurses,12 or phone calls performed by study staff.13 However, these strategies are difficult to sustain in real-world programmatic conditions. For example, in Argentina, around 13%8 of all screened women will be HPV-positive and would need to be contacted. Effective interventions aimed at improving adherence to triage are needed.
Using mobile phone text messages (SMS messages) to communicate with patients and providers has proven to be effective in a variety of settings and health problems.14 SMS messages can be automated, requiring less staff time, and in many LMICs, have improved clinic attendance rates.15 It is therefore safe to assume that mobile health (mHealth) interventions have the potential to increase triage adherence among HPV-positive women without being heavily dependent on human resources. In addition, in the context of the COVID-19 pandemic/post pandemic, combining HPV self-collection with mHealth strategies could be an effective strategy to overcoming challenges faced by both women and the health system to assure that the screening process continues.16
Little data exist about SMS message effectiveness in CC prevention.17 Regarding adherence to follow-up, one trial in Tanzania18 found that sending women SMS message did not increase follow-up screening after a visual inspection (VIA) based screen and treat approach. Given the dearth of evidence, additional evidence is needed to assess the effectiveness of mHealth interventions to increase triage after HPV-self-collection.
In Argentina, programmatic HPV self-collection offered by CHWs during home visits was introduced in 2014 to increase uptake among socially disadvantaged women and at present 7 provinces have implemented it. However, adherence to triage remains a challenge,8,10 with an estimated 25% of women attending triage at 120 days after the HPV result.10 We hypothesized that a real difference could be made in adherence to triage in CC screening by combining HPV self-collection and a multi-component mHealth intervention (SMS messages sent to HPV-positive women and SMS message sent to CHWs to visit those HPV+ women who, at 60 days since the HPV result, have not attended triage).19 This paper presents results from the ATICA study (Application of Information and Communication Technologies to HPV Self-collection, for its initials in Spanish), a hybrid type I trial set in Jujuy, Argentina.
Methods
Design overview
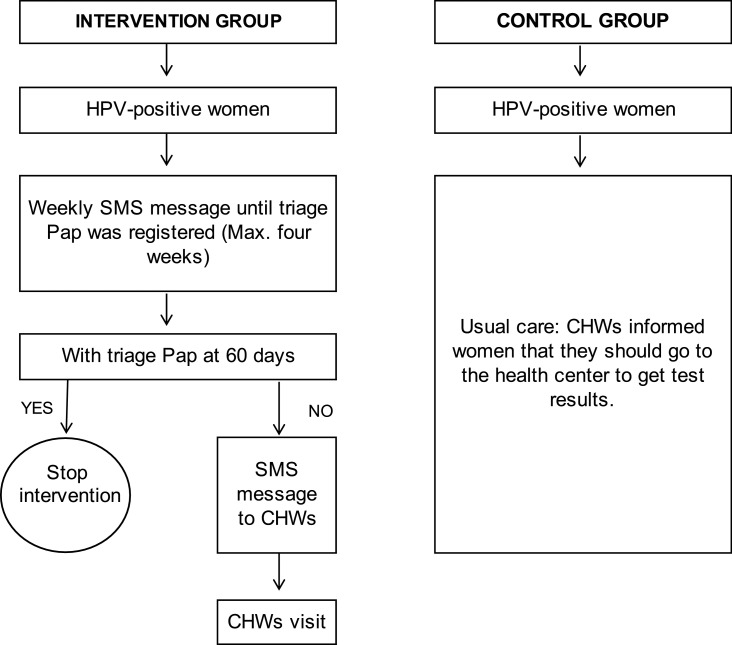
Methods of the ATICA study have been extensively described elsewhere.19 ATICA is an effectiveness-implementation hybrid type I trial, combining a cluster randomised controlled trial (RCT) to evaluate the effectiveness of a multi-component mHealth intervention (Figure 1) with a mixed-methods approach involving quantitative and qualitative evaluations of the implementation. In this report we present results from the cluster RCT and the quantitative evaluation of the implementation using the Reach, Effectiveness, Adoption, Implementation and Maintenance (RE-AIM) framework, specifically developed to assess interventions beyond efficacy across multiple public health criteria.20 RE-AIM framework was integrated in all stages of the research process, including conceptualization (e.g., selecting implementation processes that would be evaluated), data collection and analysis.19
Figure 1.
Flow diagram of the mHealth intervention.
CHW: community health worker, HPV: DNA human papillomavirus.
The study was approved by the Bioethics Review Committee of Jujuy's Ministry of Health and Institutional Review Boards of CEMIC, Harvard T.H Chan School of Public Health and Rutgers University, and the Deakin University Human Research Ethics Committee. Women signed an informed consent, as well as CHWs participating in the CHW survey. The trial is registered in ClinicalTrials.gov (NCT03478397).
Setting
Jujuy province is located in northwest Argentina and 85% of its population live in urban areas.19 The primary health care (PHC) system has 270 health centres and employs about 700 full-time CHWs who visit approximately 110,000 households twice a year for health-related tasks.
Since 2012, HPV-testing has been the primary CC screening test, available for women aged 30 years or older attending public health centres. Women are screened with HPV-testing every five years. Since 2014, HPV self-collection is offered during the CHW routine home visits. Women self-collect samples with a cervical sampler kit (Qiagen, Gaithersburg, MD, USA), which is comprised of a cervical brush, specimen container, and transport medium. Community health workers transport specimens at room temperature to health centres; from where they are sent to the provincial HPV laboratory to be analysed for 13 high-risk HPV types using hybrid-capture 2, following the manufacturer's instructions. According to the national guidelines,21 HPV-positive women with self-collected tests must go through cytology (triage Pap), and those HPV-positive women whose triage Paps are classified as atypical squamous cells of undetermined significance or worse (ASCUS+) are referred to colposcopy, then to biopsy if colposcopy images are classified as abnormal following the International Federation of Cervical Pathology and Colposcopy (IFCPC) classification.22 Women with histologically confirmed cervical intraepithelial neoplasia (CIN) of grade 2 or worse are referred to treatment.
All HPV-testing/diagnoses/treatments of women screened in the public health system are registered on the national screening information system (SITAM, for its initials in Spanish). Results of HPV tests and triage Paps are instantly available online to providers at public health establishments.
Participant eligibility and recruitment
We regarded CHWs as clusters and judged them eligible if they were actively offering HPV self-collection, that is, they had offered HPV self-collection during the three years preceding the study (2014-2017). We also required that eligible CHWs served a population of at least 26 target women (aged 30 years and older with no HPV testing in the last five years). The rationale for this was the following: Assuming approximately 13% of women with self-collected tests would be positive8 and conservatively assuming that 60% of these women would be willing to participate in the study, a CHW serving 26 women aged 30 years and over would contribute, in average, with at least two HPV-positive women to the study.
A woman was eligible if she performed HPV self-collection during the CHW home visit; and was able to provide a mobile phone number.19 All women satisfying the eligibility criteria were invited to participate by the CHW and signed a written informed consent.
Randomisation and masking
Eligible CHWs were classified into four groups according to gender and the area where they worked (urban/rural areas), and a stratified sample of 260 CHWs was randomly selected with allocation proportional to strata. The set of randomly selected CHWS which were already randomly allocated to the study arms were invited by the primary health care authorities to participate in the trial and attend the first training session. The invitation did not disclose the group allocation. During the first training session, they were informed that participation was voluntary, and they could opt out with no consequences. They were then informed about group allocation and explained the study procedures. 85% and 86% of the invited CHWs in the intervention and control group, respectively, completed the training and participated in the study. The sample of 260 CHWs were randomly allocated to intervention or control (3:2 ratio) within strata defined by gender and urban/rural area. The study statistician produced a computer-generated random number lists for the CHWs random selection and intervention allocation.19 All CHWs were assigned to the trial arms at the same time guaranteeing allocation concealment. Regarding women's consent, the CHWs were instructed to invite the woman and seek consent before disclosing their group allocation. Blinding of intervention and outcome assessments were not feasible due to the characteristics of the study.
Sample size and power considerations
The unit of randomisation was the CHW, and the unit of observation was the woman. We expected an average of two HPV-positive women per CHWs (240 in the intervention and 160 in the control group).19 For the primary outcome (triage Pap percentage at 120 days), the target sample size had 97% power to detect a 20% absolute difference between the two groups when the triage in the control group is 30% (two-sided test, alpha = 0.05, intraclass correlation coefficient (ICC) 0.10). For the secondary outcome (triage Pap percentage at 60 days), the proposed sample size had 90% power to detect a 10% absolute difference when the control group had a 15% triage by day 60.
Intervention development
The intervention was rooted in the Health Belief Model (HBM) as described elsewhere,19 anticipating that the SMS messages would serve as specific cues to action and stimulate triage behaviour. The CHW visit would be an additional cue to action to non-adherent women, as well as an opportunity to provide counselling and support which will change women's perceived benefits of and barriers to screening, as well as the perceived threat of CC. We expected both components of the intervention to increase adherence to triage in the intervention group.19,23,24 In addition, the HBM guided formative research carried out to inform the design of the multicomponent intervention23,24: Firstly, focus group (FGs) included discussions with women to incorporate in the SMS messages a persuasive element that would reinforce the SMS message as a cue to action from women point of view.23 Secondly, formative research provided information on women´s individual beliefs regarding CC, HPV testing and triage24 that was used to train CHWs about messages and support to be provided during their visit of positive women who had not been to triage at 60 days.
We also developed an automated messaging system (MATYS) that connected asynchronously with SITAM to identify women and CHWs to whom messages should be sent. MATYS was designed to register data on delivery and receipt of SMS messages; a phone number was considered valid if MATYS did not kick back an error notification signalling the number was non-existent.
The intervention was planned in consultation with staff from the National Program on Cervical Cancer Prevention (NPCCP), the Jujuy Ministry of Health, the HPV laboratory at Pablo Soria Hospital, and women (through formative research and pilot testing)23 with the goals of high acceptability and feasibility, and widespread usability. The intervention was also designed to reduce the number of CHW visits aimed at providing women with counselling and support.
Procedures
CHWs from both groups identified eligible women and invited them to participate during their routine home visits. Once women have performed HPV self-collection, they checked the eligibility criteria and invited them to participate in the study. Once women consented, the CHWs described the procedures according to the group the CHW was allocated to.
Women with HPV-positive self-collected tests recruited by CHWs allocated to the intervention group received a multi-component intervention (Figure 1). Those women with an HPV-negative test received one SMS message stating that HPV results were available at the health centre.
Intervention first component
HPV-positive women received one weekly SMS message over a four-week period, notifying that HPV results were available and that they should attend the health centre.19 The final number of SMS messages delivered to HPV-positive women depended on triage Pap adherence, MATYS stopped sending SMS messages when a triage Pap was registered in SITAM.
Intervention second component
CHWs received an SMS message to visit HPV-positive women who did not have a triage Pap registered 60 days after the HPV result. Although initially we had planned to send an e-mail to the CHWs, during the training sessions we learned that many CHWs did not use their e-mail account. Therefore, MATYS delivered both an SMS message and an e-mail. The SMS message and e-mail included a secure link to MATYS giving access to information regarding the non-adherent women. Then, they should visit those women to provide counselling about the importance of triage and encourage them to attend the health centre for triage in the following 30 days. The intervention evaluation only includes outcomes related to SMS messages.
Once women had performed HPV self-collection, CHWs allocated to intervention explained the sequence and content of SMS messages, and that they might come back for a personal visit. Women with HPV-positive self-collected tests participating in the control group received usual care: once they performed HPV self-collection offered by CHWs during the home visits, women were instructed by CHWs to attend their health centre within 30 days after the HPV self-collection, to pick-up results.
Training of CHWs
CHWs participated in three training sessions carried out between July and December 2018. Training sessions were run separately for CHWs allocated to the intervention and control group. Training included presentations about study design and methods, recruiting women, obtaining informed consent, data collection, filling in study forms, and ethical considerations. In Jujuy province HPV self-collection is routinely offered by CHWs during home visits. All CHWs periodically trained by the national and provincial programs on cervical cancer prevention on how to communicate with women regarding the cervical cancer prevention process and how to provide counselling and support to HPV-positive women. Therefore, the training sessions for the CHWs selected to participate in the trial included a refresher module about these two topics (providing information and counselling). These sessions were delivered by staff from NPCCP using programmatic materials on counselling.22,25 Training of CHWs from the intervention group also included modules to address women´s beliefs that may negatively influence their adherence to triage Pap and promote behavioural change. Different techniques were used to train CHWs about how to proceed during the visit to non-adherent women: oral presentations by health communication specialists, role playing, and videos specifically produced for the ATICA study showing different scenarios of women´s beliefs and strategies to overcome possible barriers.
Regarding the second component of the intervention, home visits to those women who did not have triage Paps at 60 days, CHWs were instructed to inquire about the reasons why they did not have triage Paps. This would allow them to identify individual beliefs and barriers to triage Pap. CHW were also instructed to provide them with information about the importance and benefits of triage and support to help them reduce barriers. For example, if the problem was related to a subjective barrier (e.g, the women being afraid of the HPV result), CHWs would reinforce the idea that HPV is a highly prevalent infection and that a positive HPV result does not mean cancer. They would also stress that the triage Pap is necessary to identify a pre-cancerous lesion that can be easily treated, but that it can develop into CC over time, if not treated.
Sensitisation of key stakeholders
Before the trial started, we held a meeting with directors of health centres, PHC supervisors and gynaecologists. The objective of this meeting was to present the ATICA study, sensitize these key stakeholders about women´s beliefs and barriers faced by HPV positive women and discuss possible facilitators. Thus, we stressed the importance of reducing barriers mentioned by women and facilitating their access to triage (e.g., by performing the triage Pap on the same day that women attended the health centre after receiving the HPV-test result if possible, and if not, scheduling an appointment as soon as possible).
Data collection
Effectiveness trial women recruitment took place between December 4, 2018 and July 31, 2019. CHWs from both groups used an ad-hoc trial form to collect data on women's characteristics: age, education level, household with children younger than five, over-crowding, health insurance, screening in the last ten years, use of cell phone, and computers/internet/social media. Data on HPV testing, triage, diagnosis, and treatment were extracted from SITAM.
End of follow-up was March 4, 2020, when lock down was established in Argentina due to the COVID-19 pandemic.
RCT outcome measures
The primary outcome was the percentage of HPV-positive women with triage 120 days after the HPV result. The secondary outcome was the percentage of HPV-positive women with triage 60 days after the HPV result, which measures the effect of the first component of the intervention - i.e., SMS messages sent to women. Date of HPV result was defined as the date the result was uploaded to SITAM.
In addition, we are presenting the following additional data on the care continuum (screening, diagnosis, and treatment), which is essential for effective disease prevention:
-
a.
The percentage of HPV-positive women with abnormal triage Paps (ASCUS+) who had colposcopy and biopsy.
-
b.
The percentage of women with histologically diagnosed CIN2+ who received treatment.
Evaluation of the intervention implementation
We evaluated the implementation of the intervention using the RE-AIM framework,20 (Outcome definitions and data sources presented in Table 1). Although planned semi-structured interviews with stakeholders19 to evaluate their acceptability of the intervention and perspectives on how to build and enhance project sustainability were delayed due to the COVID-19 pandemic, we present data on current activities being carried out with the Argentinean National Cancer Institute (ARG-NCI) to scale-up the ATICA strategy as a measure of the Maintenance dimension. RE-AIM measures were derived using data from the trial, a survey of HPV-positive women19 and a self-administered survey of CHWs.19 Data regarding delivery and receipt of SMS message were extracted from MATYS.19
Table 1.
Measurements and data sources for the implementation evaluation based on RE-AIM.
| REAIM dimensions | Definition | Data sources |
|---|---|---|
| REACH: Representativeness of women reached by the intervention | % of eligible women who accepted to participate in the trial | Trial database |
| EFFECTIVENESS of the intervention in increasing women's adherence to triage |
% of HPV-positive women with triage at 120 days (primary outcome) and 60 days (secondary outcome) in the intervention group vs. control group (Risk ratios and differences in percentage) | Trial database |
| IMPLEMENTATION of intervention activities according to protocol |
% of randomized CHWs in the intervention group that participated in training | Trial database |
| % of randomized CHWs that participated in trainings and enrolled at least one eligible woman to the intervention group | Trial database | |
| % of SMS messages that reached an HPV-positive womens’ valid phone number | Automated messaging system (MATYS) | |
| % of SMS messages that reached a CHWs´ valid phone number | Automated messaging system (MATYS) | |
| Receipt of SMS messages reported by women: | ||
| ▪ % of women who received at least one SMS message | Women survey | |
| ▪ % of phone number wrongly registered | ||
| ▪ % of women not having received or not remembering having received the SMS message | ||
| Acceptability: % of women who agreed with the statement “An SMS message is a good communication channel to be informed that the HPV self-collection result is available at the health centre” | Women survey | |
| ADOPTION of the intervention (intention to use an innovation or evidence-based practice) | Adoption: % of CHWs that visited at least one HPV-positive woman after receiving the SMS message. | Trial database |
| Acceptability: % of CHWs that agreed with programmatic incorporation of the mHealth intervention | CHW survey | |
| MAINTENANCE of the intervention (extent to which the intervention becomes institutionalised or part of the routine organisation practices and policies | Qualitative information about programmatic incorporation of the intervention | Meetings with stakeholders |
Women survey
All HPV-positive women from the intervention group were contacted by trained interviewers for an in-person/phone interview.19 The questionnaire included open and closed questions related to women's experiences and perceptions, including receipt, acceptability and relevance of SMS message frequency, time and content, the perceived effect of the multiple components of the intervention on their adherence, and reasons for adhering/not adhering to triage. Interviews took place between December 2019 and October 2020. 370 (83%) HPV-positive women were interviewed (59 women could not be reached, 3 had died and 13 refused to answer the survey). We report results on acceptability and number of SMS messages effectively received. Acceptability was measured through agreement (highly agree or agree) with the statement “An SMS message is a good communication channel to be informed that the HPV self-collection result is available at the health centre” (options: Highly agree/agree, disagree, highly disagree, nor agree or disagree).
CHWs survey
Ten weeks after the recruitment of women was completed, we held a workshop as a closing activity to present preliminary results to CHWs and health authorities. During this workshop, CHWs were asked to complete an anonymous, self-administered semi-structured survey to evaluate their acceptability of the intervention, and barriers and facilitators in implementing and adopting the intervention.19 122 CHWs from the intervention group completed the survey. We report results on acceptability (percentage of CHWs that agreed with programmatic incorporation of the mHealth intervention).
Data analysis
Baseline characteristics of CHWs (gender, rural/urban area) were compared between trial groups using the Chi-squared test to assess potential imbalances due to the fact that not all the randomly selected and randomized CHWs ultimately participated in the study (85% in the intervention group and 86% in the control group). Baseline characteristics of HPV-positive women were compared between groups using generalized estimating equations (GEE) models which account for clustering, with distribution and link function selected based on the type of variable. Groups were compared to investigate potential biases in the recruitment and/or consent process as CHWs might have disclosed the group allocation before inviting women to participate in the study. All analyses were conducted on an intention-to-treat approach. The effectiveness of the intervention on the two outcomes, triage percentage at 120 and 60 days, was estimated using generalized estimating equations (GEE) with log link and binomial distribution. An exchangeable working correlation matrix was assumed to account for the clustering induced by the CHWs. We report estimated triage percentages for each group, difference in percentages and risk ratios along with their 95% confidence intervals (CIs). Effect modification by baseline factors (age, health insurance, education level, rural/urban area) was explored using the same GEE model including group (intervention/control), the potential effect modifier and the interaction modifier by group. Cox models for the outcome time to triage Pap, with a robust sandwich covariance matrix, were fitted to estimate the hazard ratios of intervention relative to control overall and by rural/urban status. Plots of the Kaplan-Meier estimates for each group and according to rural/urban area are presented. Descriptive statistics were used to report data on other RE-AIM outcomes.
All analyses were performed with SAS software, version 9.4 (SAS Institute, Cary, NC).
Role of the funding source
The funder had no role in study design, data collection, analysis, or writing of the report. The corresponding author had full access to all data in the study and had final responsibility for the decision to submit for publication.
Results
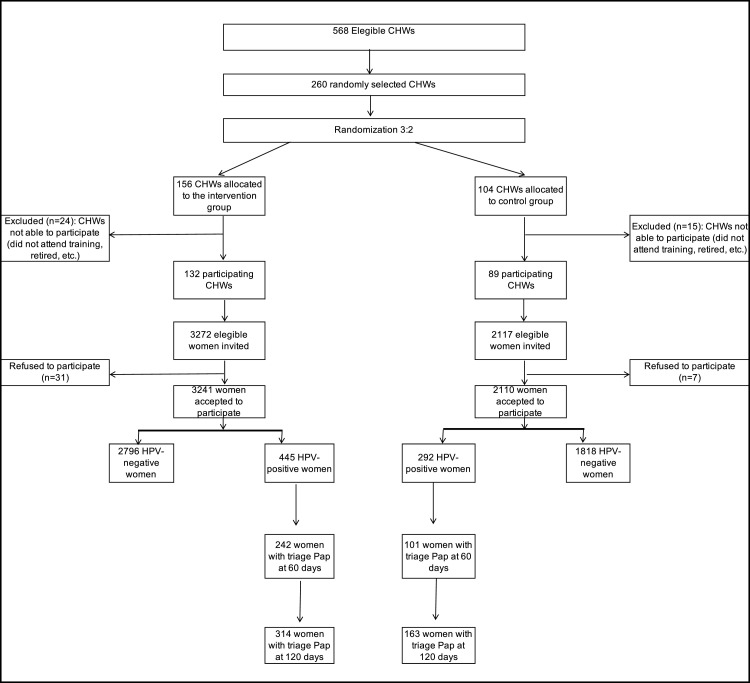
Figure 2 shows the study flow chart. Of the 260 randomised CHWs, 221 participated in the study. CHWs who participated were predominantly women (n=181, 81·9%) and worked in urban area (n=165, 74·7%; Table 2). No difference was noted in sex (p=0·68) or urban/rural area (p=0·32) between participating and non-participating (84·6% women; 82·1% urban area) CHWs.
Figure 2.
Trial profile.
CHWs: community health workers; HPV: human papillomavirus.
Table 2.
Characteristics of Community Health Workers and HPV-positive women.
| Total population | Intervention group | Control group | p-value | |
|---|---|---|---|---|
| CHWs characteristics1 | ||||
| Total (n) | 221 | 132 | 89 | |
| Gender | ||||
| Male | 40 (18·1%) | 26 (19·7) | 14 (15·7%) | 0·45 |
| Female | 181 (81·9%) | 106 (80·3%) | 75 (84·3%) | |
| Area | ||||
| Urban | 165 (74·7%) | 99 (75·0%) | 66 (74·2%) | 0·88 |
| Rural | 56 (25·3%) | 33 (25·0%) | 23 (25·8%) | |
| HPV-positive women characteristics2 | ||||
| Total (n) | 737 | 445 | 292 | |
| Age (years) | ||||
| Mean (SD) | 42·44 (11·01) | 42·38 (11·03) | 42·52 (10·97) | 0.87 |
| 30-39 | 360 (48·8%) | 218 (49%) | 142 (48·6%) | 0.66 |
| 40-49 | 185 (25·1%) | 110 (24·7%) | 75 (25·7%) | |
| 50-64 | 153 (20·8%) | 95 (21·3%) | 58 (19·9%) | |
| 65+ | 39 (5·3%) | 22 (4·9%) | 17 (5·8%) | |
| Area | ||||
| Urban | 600 (81·4%) | 353 (79·3%) | 247 (84·6%) | 0.77 |
| Rural | 137 (18·6%) | 92 (20·7%) | 45 (15·4%) | |
| Education (c) | ||||
| Never went to school/Primary (incomplete) | 54 (7·3%) | 40 (9%) | 14 (4·8%) | 0.83 |
| Primary / Secondary (incomplete) | 251 (34·1%) | 143 (32·1%) | 108 (37%) | |
| Secondary /Tertiary (incomplete/complete) | 432 (58·6%) | 262 (58·9%) | 170 (58·2%) | |
| Overcrowding (>3 people/room) | ||||
| Yes | 128 (17·4%) | 82 (18·4%) | 46 (15·8%) | 0·38 |
| No | 609 (82·6%) | 363 (81·6%) | 246 (84·2%) | |
| Household with children younger than 5 | ||||
| Yes | 290 (39·3%) | 172 (38·7%) | 118 (40·4%) | 0·60 |
| No | 447 (60·7%) | 273 (61·3%) | 174 (59·6%) | |
| Health insurance | ||||
| Public | 618 (83·9%) | 371 (83·4%) | 247 (84·6%) | 0.68 |
| Private/social security | 119 (16·1%) | 74 (16·6%) | 45 (15·4%) | |
| Screening in the last 10 years | ||||
| No | 322 (43·7%) | 189 (42·5%) | 133 (45·5%) | 0.44 |
| Yes | 415 (56·3%) | 256 (57·5%) | 159 (54·5%) | |
| Shared phones with other family members | ||||
| Yes | 94 (12·8%) | 60 (13·5%) | 34 (11·6%) | 0·59 |
| No | 643 (87·2%) | 385 (86·5%) | 258 (88·4%) | |
| Mobile phone plan3 | ||||
| Pre-paid plan | 375 (51·2%) | 221 (49·8%) | 154 (52·7%) | 0·53 |
| Monthly plan | 359 (48·8%) | 223 (50·2%) | 136 (46·6%) | |
| Had Personal Computer (≥1 per household) | ||||
| Yes | 317 (43·2%) | 177 (39·8%) | 140 (48·4%) | 0·086 |
| No | 417 (56·8%) | 268 (60·2%) | 149 (51·6%) | |
| Had Internet access at home4 | ||||
| Yes | 410 (55·7%) | 243 (54·7%) | 167 (57·2%) | 0·90 |
| No | 326 (44·3%) | 201 (45·3%) | 125 (42·8%) | |
| Mobile phone with internet access | ||||
| Yes | 642 (87·1%) | 387 (87%) | 255 (87·3%) | 0·92 |
| No | 95 (12·9%) | 58 (13%) | 37 (12·7%) | |
| Use of social networks | ||||
| Yes | 656 (89·0%) | 396 (89·0%) | 260 (89·0%) | 0·86 |
| No | 81 (11·0%) | 49 (11·0%) | 32 (11·0%) |
p-value, Chi-square test.
p-value from a generalized estimating equation model controlling for CHWs clustering, binary distribution or multinomial distribution according to outcome distribution.
3 missing.
1 missing.
CHWs: community health workers.
The 221 participating CHWs (132 in the intervention and 89 in the control group) invited 5389 eligible women, of whom 5351 (99·3%) agreed to participate (3241 in the intervention and 2110 in the control group).
A total of 737 women had an HPV-positive result, 445 in the intervention (13·7%) and 292 in the control group (13·8%). Of them, 322 (43·7%) had no screening in the last 10 years, and 94 (12·8%) shared phones with other family members (Table 2). There were no significant differences between groups regarding sociodemographic characteristics.
In total, 314 (70·5%) HPV-positive women of the intervention group had a triage Pap at 120 days after the HPV result, compared to 163 (55·1%) in the control group (Table 3). Regarding the intervention first component (SMS messages sent to women), in total, 242 (53·9%) HPV-positive women in the intervention group and 101 (33·4%) in the control group had a triage Pap at day 60 (Table 3).
Table 3.
Effectiveness of the intervention on triage Paps at 60 and 120 days after HPV-positive test result.
| No. women with Pap (%) | Difference in percentage (95%CI) | RR (95%CI) | p-value | ||
|---|---|---|---|---|---|
| At 120 days | |||||
| Control group (292 HPV-positive women, 89 CHWs) |
Usual care | 163 (55·1%) |
– | – | |
| Intervention group (445 HPV-positive women, 132 CHWs) |
Multicomponent mHealth intervention | 314 (70·5%) |
15·5 (6·8–24·1) |
1·28 (1·11–1·48) |
0·0005 |
| At 60 days | |||||
| Control group (292 HPV-positive women, 89 CHWs) |
Usual care | 101 (33·4%) |
– | – | |
| Intervention group (445 HPV-positive women, 132 CHWs) |
Only SMS messages |
242 (53·9%) |
20·5 (12·0–29·0) |
1·61 (1·29–2·02) |
<0·0001 |
CHWs: community health workers. RR: Risk Ratio. CI: confidence intervals.
Differences, RR, CI, and p values calculated under a generalized estimation equation approach accounting for clustering induced by CHWs.
Women follow-up
Median total follow-up until March 4, 2020, was 306 days (range 187–447).
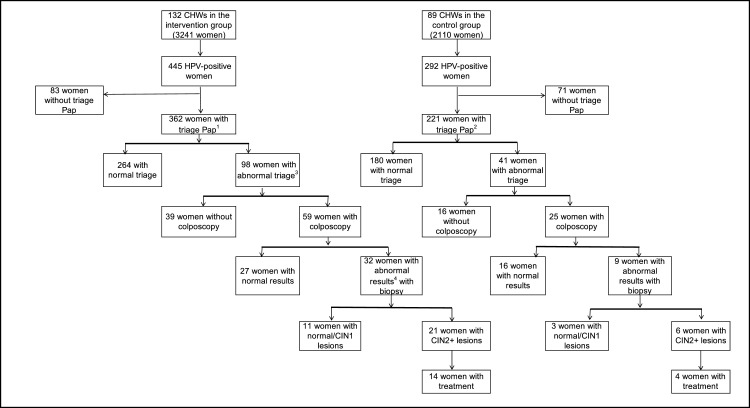
Of 98 women with abnormal triage Pap in the intervention group, 59 (60·2%) attended colposcopy during the total follow-up period and 32 (54.2%) had an abnormal result (Figure 3). Biopsy was performed in 100% of abnormal colposcopies, in which 21 CIN2+ cases were detected, and 14 (66·7%) had been treated by March 4, 2020.
Figure 3.
Follow up of HPV-positive women.
1 Includes 314 women with triage Pap at 120 days and 48 with triage Pap in the remaining follow-up period (day 121-447).
2 Includes 163 women with triage Pap at 120 days and 58 with triage Pap in the remaining follow-up period (day 121-447).
3 Abnormal triage Pap is defined as atypical squamous cells of undetermined significance or worse (ASCUS+).
3 Colposcopy is classified as abnormal following the International Federation of Cervical Pathology and Colposcopy (IFCPC) classification.22.
HPV: human papilomavirus; CIN1: cervical intraepithelial neoplasia of grade 1; CIN2+: cervical intraepithelial neoplasia of grade 2 or worse.
Of 41 women from the control group with abnormal triage Pap, 25 (60·9%) had colposcopy during the study follow-up period. Nine (36.0%) had abnormal findings and biopsy was performed in all of them. Six women had CIN2+ disease detected and four (66·7%) had been treated by March 4, 2020.
The average time between receiving the HPV result and colposcopy was shorter in the intervention group (134 days, SD 74·3) compared to the control group (173 days, SD 89·5; p=0·058).
Evaluation of the intervention implementation
Effectiveness
The multicomponent intervention produced a 15·5% point improvement in adherence to triage Pap by day 120 compared to usual care (95%CI: 6·8–24·1) (Table 3). The relative risk was 1·28 (95%CI: 1·11–1·48), indicating that women in the intervention group were 28·0% more likely to have a triage Pap by day 120 relative to the control group. The intervention first component significantly increased adherence to triage Pap at day 60 with a 20·5% point improvement in the intervention group compared to the control group (95%CI: 12·0–29·0) and a relative risk of 1·61 (CI: 1·29–2·02) indicating that women in the intervention group were 61·0% more likely to have a triage Pap by day 60 relative to the control group.
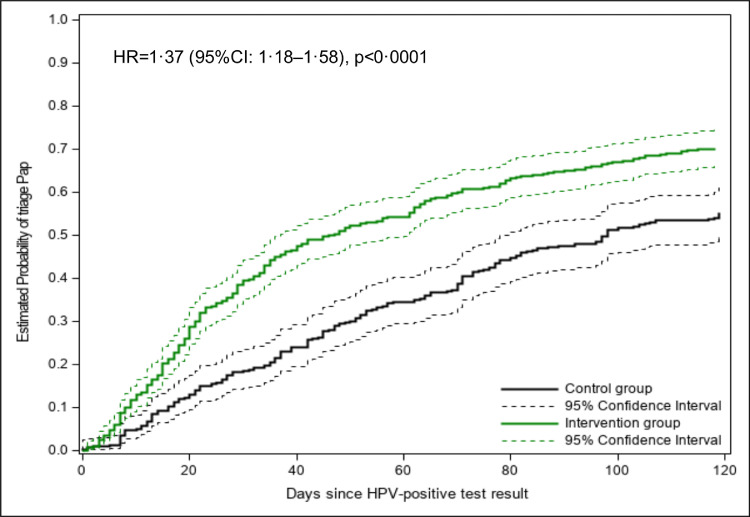
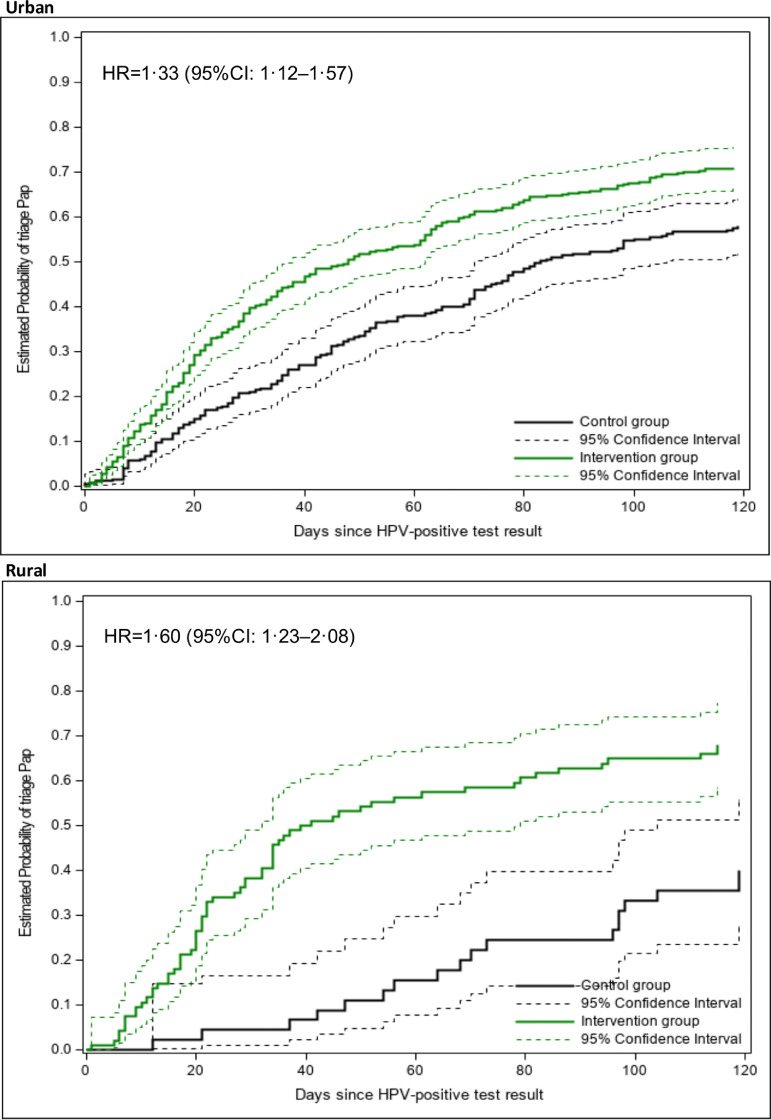
Figure 4 shows the estimated probability of having a triage Pap at a given time for each group and the estimated hazard ratio (HR) for the intervention group relative to the control group. The intervention significantly decreased the time to triage (p < 0·0001). For example, a triage level of 50% was reached around day 50 in the intervention group and around day 100 in the control group. The intervention effect was stronger among women from rural areas (Figure 5). However, the interaction urban/rural area × group was not significant (Cox model, p=0·244).
Figure 4.
Estimated probability of having a triage Pap by group.
HR: hazard ratio. CI: confidence interval. HR and CI estimated under a Cox model with robust sandwich covariance matrix to account for clustering.
Figure 5.
Estimated probability of having a triage Pap by rural/urban area and group.
HR: hazard ratio. CI: confidence interval. HR and CI estimated under a Cox model with robust sandwich covariance matrix to account for clustering.
Number of women in each stratum: Urban: intervention (353), control (247); Rural: intervention (92), control (45).
Reach
In total 5351 (99·3%) eligible women were included in the study: mean age was 44·1 (SD 11·4); 2745 (51·3%) had secondary and tertiary education level (complete/incomplete); and 4472 (83·4%) had public health insurance (Table 4).
Table 4.
Reach, Effectiveness, Implementation Adoption and Maintenance measurements.
| REAIM dimension | Definition | Result |
|---|---|---|
| REACH | % of eligible women who accepted to participate in the trial. | 99·3% (n=5351/5389) |
| EFFECTIVENESS | % of HPV-positive women with triage at 120 days in the intervention group vs. control group % of HPV-positive women with triage at 60 days in the intervention group vs. control group |
70·5% vs 55·1% 53·9% vs. 33·4% |
| IMPLEMENTATION1 |
% of randomized CHWs in the intervention group that participated in training | 89·1% (n=139/156) |
| % of randomized CHWs that participated in trainings and enrolled at least one eligible woman to the intervention group | 94·9 % (n=132/139) | |
| % of SMS messages that reached a HPV-positive womens´ valid phone number2 | 94·2% (419/445) | |
| % of SMS messages that reached a CHWs´ valid phone number | 96·2% (227/2373) | |
| Receipt of SMS messages reported by women4: | ||
| ▪ % of women who received at least one SMS message | 78·1% (289/370) | |
| ▪ % of phone number wrongly registered | 7·0% (26/370) | |
| ▪ % of women not having received or not remembering having received the SMS message | 14·8% (55/370) | |
| Acceptability5: % of women who agreed with the statement “An SMS message is a good communication channel to be informed that the HPV self-collection result is available at the health centre” | 97·2% (278/286) | |
| ADOPTION |
Adoption: % of CHWs that visited at least one HPV-positive woman after receiving the SMS message. | 90·8% (89/98) |
| Acceptability: % of CHWs that agreed with programmatic incorporation of the mHealth intervention | 86·9% (n=106/122) | |
| MAINTENANCE | Programmatic incorporation of the intervention (qualitative data) | Collaborative work with the Argentinean NCI to plan the scaling-up of the ATICA strategy began in November 2021, its implementation is planned for 2022 |
CHWs: community health workers. HPV: Human Papillomavirus.
Percentages in the Implementation section refer to CHWs and HPV-positive women from the Intervention Group.
Phone numbers were considered valid if they did not kick back the error notification.
This figure includes 34 HPV-positive women who had their triage Pap registered in SITAM after day 60.
Number of women included in the denominator corresponds to women who answered the survey.
Number of women included in the denominator corresponds to women who answered the acceptability section of the survey.
Implementation
89·1% of CHWs in the intervention group participated in training (139 of 156) (Table 4). Among them, 132 (94·9%) had at least one woman enrolled in the study. In the first component of the intervention, MATYS reached 419 valid phone numbers of the 445 HPV-positive women. Cases which it did not reach a phone number were due to registration of an invalid phone number (8) or to problems in MATYS-SITAM communication (18). 78·1% of the HPV-positive women interviewed from the intervention group reported receiving at least one SMS message (289 of 370); 7·0% reported that their number was registered incorrectly (26 of 370), and 14·8% reported that they did not receive or did not remember receiving the SMS message (55 of 370). Regarding the second component of the intervention, in total, 96.2% (227 of 237) of SMS messages sent to CHWs to alert them to visit women with no triage Pap registered in SITAM at day 60 reached a valid phone number.
Acceptability by women was high: 97·2% of women agreed with the statement “An SMS message is a good communication channel to be informed that the HPV self-collection result is available at the health centre” (278 of 286).
Adoption of the intervention by CHWs
For the second component of the intervention, 90·8% (89/98) of CHWs with women with no triage Pap registered in SITAM at day 60, visited at least one HPV-positive woman after receiving the SMS message (Table 4). 86·9% (106 of 122) of interviewed CHWs from the intervention group agreed that the intervention should be incorporated in routine screening program activities.
Maintenance
Collaborative work with the Argentinean NCI to plan the scaling-up of the ATICA strategy began in November 2021 and the implementation is planned for 2022.
Discussion
This is, to our knowledge, one of the first trials demonstrating the effectiveness of an mHealth intervention to increase triage of HPV-positive women with self-collected tests. The ATICA trial was implemented in the province of Jujuy, Argentina, a low resource setting where HPV-testing has been routinely offered in the public health sector since 2012. SMS messages were sent by an automated system (MATYS) connected with SITAM, the national information system on screening. Thus, our results demonstrate what can be achieved when an mHealth intervention is rooted in programmatic, real-world conditions.
Our intervention resulted in a 15·5% increase in the percentage of women with triage Pap at 120 days after the HPV result, showing that the mHealth multicomponent intervention was effective in improving triage adherence. When we considered triage at 60 days (which captures only the effect of sending SMS messages to the women), the positive effect of the intervention was higher, showing a 20·5% increase compared to the control group. Our results contrast with those of a study carried out in Tanzania18 showing no effect of SMS messages to increase attendance at follow-up by HPV-positive women screened with a VIA-based screen-and-treat approach. In that study, HPV-positive women who received SMS message included women already treated, and VIA-negative women for evaluation of HPV-testing clinical performance, which might have limited the effect of the intervention. The distinction is important, because SMS message is a tool to convey a message, and analysis of its impact cannot be separated from the health system challenges they address.26 CC screening entails multiple steps (screening/triage/diagnosis/treatment), each of them presenting specific challenges and barriers. Our intervention was intended to facilitate communication between HPV-positive women and the health system regarding triage, an essential step of the diagnosis/treatment process, and this might have contributed to its effectiveness. Also, mHealth interventions grounded in health behaviour theories are generally more effective than those with no theoretical foundation.15 Whereas we used the HBM to design and anticipate the effect of the intervention, in the Tanzanian study the lack of a framework might have influenced the impact of the intervention on follow-up.18
The multicomponent intervention increased the proportion of women with triage Pap at 120 days. However, the effect was smaller than that of the first component, despite the high level of adoption (90·8%) of the visit to HPV-positive women who had not triage at day 60 by CHWs. This is probably a result of two concurrent factors. The first one is time, as a main effect of the intervention was to decrease the time to triage Pap. Thus, the difference between the intervention and control group in the first 60-day period was reduced as time went by, with women in the control group slowly catching up. This is coincident with routine programmatic data from Argentina showing that adherence to triage increased with time, from 18·0% at 60 days to 42·9% at 18 months.10 Secondly, many women who did not have triage at day 60 might be facing barriers that could not be solved by CHW visits (e.g., socio-structural barriers such as work, domestic or childcare barriers), reducing the effect of the second component on the effectiveness of the multicomponent intervention. Evidence has shown that socio-structural barriers are more difficult to address by the health system, needing intersectoral action and social participation.27 The first component probably resulted in improved and earlier communication of results, especially among women whose reduced adherence was mainly linked to gaps in the women's health system communication process. As no human resources are involved in the first component, in settings where no CHW visit is possible, only sending SMS messages to HPV-positive women will significantly increase adherence to triage without over-burdening scarce health staff. Also, the first component of the intervention might be implemented in settings where self-collection is not offered during home visits. Thus, SMS messages could be sent to HPV-positive women who have performed self-collection, irrespective of the method used to offer it (i.e., invitation letters, offer at health centres by health providers, during home visits, etc.).
In our study, the decrease in time from the HPV result to triage Pap had also an impact on the time to colposcopy, which was shorter in the intervention group. Although this reduction was marginally statistically significant, this might be due to the study not being designed to detect this difference. Longer times to colposcopy have been associated with a decreased lifetime benefit of screening and a decrement in life years gained.28 Meanwhile, shorter times to colposcopy will probably have an impact on time to treatment, given that colposcopy, and biopsy, when needed, are the diagnostic procedures to confirm high grade intraepithelial lesions or higher and refer women for treatment. Thus, our mHealth intervention can contribute to increase the quality of the entire screening/diagnosis/treatment process.
Our study provides some evidence that the intervention might be more effective for rural women although we could not demonstrate a significative interaction between rural/urban status and intervention. This was probably due to the small number of rural women in our sample (137). Our result corroborates those by Erwin29 who found that, compared to urban women, rural women who received SMS or SMS + voucher for transportation were more likely to perform clinician-collected HPV-testing at health centres. For Erwin et al,29 a lower level of baseline knowledge among rural women could have amplified the effects of the SMS message in the rural area.
In our study, SMS messages reached the vast majority of HPV-positive women and CHWs. Frequent changes in phone numbers or loss of cell phones have been signalled as a barrier for using SMS messages to communicate with patients,30 but the fact that SMS messages were sent to women during a short period of time (four weeks) immediately after the HPV result was available might have limited these problems. Women's acceptability of the intervention was high, and most considered that an SMS message was a good communication channel to be informed that the HPV self-collection result was available at the health centre. Message content and tone were carefully designed, which is a key element for the success of mHealth interventions.31 We conducted formative research and pilot testing with key stakeholders23 to convey the health system's intention of caring for the population. Although the protocol initially stipulated that the SMS message would include the HPV result, our previous formative research indicated that women preferred not to receive it, as they raised concerns about confidentiality, and considered that result delivery was a responsibility of a health professional. Therefore, the SMS message only mentioned the term “self-collection”, and informed women about the test result availability and the importance of attending the health centre.23 This is similar to results by Moodley et al 32 in South Africa showing that women rejected receiving abnormal screening results through SMS messages and asked to be told “please come to the clinic” to get the results from a health professional. Two studies carried out in Africa that analysed women´s preferences regarding SMS messages showed that most women did not prefer to receive their CC screening result through SMS messages (range 7-32%).13,33 In our study, omitting the result and the term HPV-testing in the SMS message might have reduced worries related to privacy and increased the intervention acceptability. Importantly, our study was carried out before the change in the use of mHealth interventions produced by the COVID-19 pandemic.34 Additional evidence will be necessary to analyse how this change has modified the acceptability of including the actual result in SMS messages, or in newer social media technologies (i.e. WhatsApp and other instant messaging apps).
In our study, the CHWs selected had at least 26 women as their target population for HPV self-collection. Those CHWs with lower amounts of target women and, therefore not eligible for inclusion in the study, are mainly located in very small rural areas. Effectiveness of the intervention might have been different if women from these small rural areas had been included. However, since our results show that the intervention effect was stronger among women from rural areas, effectiveness would not be decreased if CHWs and women from very small rural settings had been included but, if anything, this caveat would likely have an additive influence in the presented effects.
In conclusion, sending SMS messages to HPV-positive women after using self-collection, and to CHWs informing them to visit those women who were not triaged at day 60 is effective in increasing adherence to triage Pap, allowing for many more women at risk of CC to receive timely follow-up. These results are particularly important in the midst of the COVID-19 pandemic (and post pandemic), as using mHealth strategies has been signalled as a key intervention that may help health workers to contact individuals needing follow-up more efficiently while minimizing person-to-person contact.14 In summary, our study provides key evidence on the effectiveness of digital tools to improve the quality and effectiveness of the screening process associated with HPV-self collection, which will highly contribute to the CC elimination goal.
Contributors
SA had the original idea for the trial, was the principal investigator and study coordinator, and led writing of the report. MP was the project manager, made substantial contributions to study design, contributed to the data analysis and interpretation, and produced the figures and tables (in consultation with co-authors). VSA was responsible for formative research, contributed with interpretation of findings and a critical revision of the manuscript. LT made a substantial contribution to the conception and design of the study, led training of community health workers (with MC) and did critical revision of the manuscript. RK made a substantial contribution to the conception and design of the study and made a critical revision of the manuscript. MC was responsible for design of communication and training materials and led training of community health workers (with LT). LF and VS were largely involved with project implementation and contributed with interpretation of findings and a critical revision of the manuscript. KV made substantial contributions to the design and implementation of the study and made a critical revision of the manuscript. LO made substantial contributions to the design and implementation of the study, developed the statistical plan, conducted the data analysis, and made a critical revision of the manuscript. SA, MP and LO accessed and verified all the data in the study. All authors read and approved the final manuscript.
Data sharing
The study protocol is published and available in an open-access article.19 De-identified individual participant data on which summary statistics and tables are based will be made available from the point of, and up to five years after the acceptance for publication of the main findings. These data can be requested to the Principal Investigator (Dr. Silvina Arrossi) and only under a data-sharing agreement. Other materials such as forms and questionnaires will be made freely available upon request from the point of, and up to five years after the acceptance for publication of the trial main findings. Requests can be made to the Principal Investigator.
Declaration of Interests
The authors declare that there is no conflict of interest. The ATICA study was funded by the National Cancer Institute of the National Institutes of Health under Award Number R01CA218306.
Acknowledgments
The ATICA study was funded by the National Cancer Institute of the National Institutes of Health under Award Number R01CA218306. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health. ATICA study was led by the Center for the Study of State and Society, in association with the Dana-Farber Cancer Institute/Harvard T.H. Chan School of Public Health, USA, Deakin University, Australia, and Rutgers University, USA, with support and in collaboration from the Argentinean National Cancer Institute and the Jujuy Ministry of Health. The authors would like to thank the Direction of the National Cancer Institute, the National Program on Cervical Cancer Prevention, the Jujuy Ministry of Health (especially the Direction of Primary Health Care, and the Jujuy Program on Cervical Cancer Prevention), for their support. We also thank the interviewers who carried out the women survey, and Laura Livieri, Isabel Tripodi and Nora Villagra for their administrative support, and Agustín Oliveto for his support. Finally, we especially thank the women and community health workers who participated in the trial (the complete list of CHWs can be found at https://www.proyectoatica.com.ar/en/community-health-workers/).
Footnotes
Supplementary material associated with this article can be found in the online version at doi:10.1016/j.lana.2022.100199.
Appendix A. †ATICA study team
Silvina Arrossi, Fernando Binder, Paula Frejdkes, Leonardo Ghigliani, Alejandro Hunt; Rosa Laudi, Victoria Sánchez Antelo, Juan David Mazzadi, Melisa Paolino, Ethel Terreno (Centro de Estudios de Estado y Sociedad/ Consejo Nacional de Investigaciones Científicas y Técnicas, Argentina); Kasisomayajula Viswanath (Harvard University, USA); Racquel E. Kohler (Rutgers University, USA); Liliana Orellana (Deakin University, Australia); Gladis Apaza, Alicia Campanera, Mauricio Cucchiaro, Liliana Flores, Carlos Ibarra, Pablo Luzcubir, Natalia Martiarena, Verónica Serra, Marcos Ugarte (Ministry of Health of Jujuy, Argentina); Oscar Marin, Guillermo Preusse (Hospital Pablo Soria, Jujuy, Argentina); Ana Laura Echenique (Facultad de Humanidades y Ciencias Sociales, Universidad Nacional de Jujuy, Argentina); Milca Cuberli, Mariana Curotto, Laura Thouyaret, Leonardo Valdés, Julieta Zalacaín Colombo (Argentinian National Cancer Institute).
Appendix B. Supplementary materials
References
- 1.Bray F., Ferlay J., Soerjomataram I., Siegel R.L., Torre L.A., Jemal A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2018;68:394–424. doi: 10.3322/caac.21492. [DOI] [PubMed] [Google Scholar]
- 2.Das M. WHO launches strategy to accelerate elimination of cervical cancer. Lancet Oncol. 2021;22:20–21. doi: 10.1016/S1470-2045(20)30729–4. [DOI] [PubMed] [Google Scholar]
- 3.Sankaranarayanan R., Nene B.M., Shastri S.S., et al. HPV screening for cervical cancer in rural India. N Engl J Med. 2009;360:1385–1394. doi: 10.1056/NEJMoa0808516. [DOI] [PubMed] [Google Scholar]
- 4.Arbyn M., Smith S.B., Temin S., Sultana F., Castle P., Collaboration on Self-Sampling and HPV Testing Detecting cervical precancer and reaching underscreened women by using HPV testing on self-samples: updated meta-analyses. BMJ. 2018;363:k4823. doi: 10.1136/bmj.k4823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Arrossi S., Thouyaret L., Herrero R., et al. Effect of self-collection of HPV DNA offered by community health workers at home visits on uptake of screening for cervical cancer (the EMA study): a population-based cluster-randomised trial. Lancet Glob Health. 2015 doi: 10.1016/S2214-109X(14)70354-7. [DOI] [PubMed] [Google Scholar]
- 6.World Health Organization. Comprehensive cervical cancer control: a guide to essential practice: 2014 https://apps.who.int/iris/bitstream/handle/10665/144785/9789241548953_eng.pdf;jsessionid=29BEC2F0B65A8742657234351B986EDC?sequence=1. Accessed 20 May 2021. [PubMed]
- 7.Cuschieri K., Ronco G., Lorincz A., et al. Eurogin roadmap 2017: triage strategies for the management of HPV-positive women in cervical screening programs. Int J Cancer. 2018 doi: 10.1002/ijc.31261. [DOI] [PubMed] [Google Scholar]
- 8.Arrossi S., Paolino M., Laudi R., et al. Programmatic human papillomavirus testing in cervical cancer prevention in the Jujuy Demonstration Project in Argentina: a population-based, before-and-after retrospective cohort study. Lancet Glob Health. 2019;7:e772–e783. doi: 10.1016/S2214-109X(19)30048-8. [DOI] [PubMed] [Google Scholar]
- 9.Murillo R., Almonte M., Pereira A., et al. Cervical cancer screening programs in Latin America and the Caribbean. Vaccine. 2008 doi: 10.1016/j.vaccine.2008.06.013. [DOI] [PubMed] [Google Scholar]
- 10.Paolino M., Gago J., Pera A.L., Cinto O., Thouyaret L., Arrossi S. Adherence to triage among women with HPV-positive self-collection: a study in a middle-low income population in Argentina. Ecancermedicalscience. 2020;14:1138. doi: 10.3332/ecancer.2020.1138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Zapka J., Taplin S.H., Price R.A., Cranos C., Yabroff R. Factors in quality care- the case of follow-up to abnormal cancer screening tests - problems in the steps and interfaces of care. J Natl Cancer Inst Monogr. 2010 doi: 10.1093/jncimonographs/lgq009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lazcano Ponce E., Lorincz A.T., Cruz-Valdez A., et al. Self-collection of vaginal specimens for human papillomavirus testing in cervical cancer prevention (MARCH): a community-based randomised controlled trial. Lancet. 2011 doi: 10.1016/S0140-6736(11)61522-5. [DOI] [PubMed] [Google Scholar]
- 13.Nakalembe M., Makanga P., Kambugu A., Laker-Oketta M., Huchko M.J., Martin J. A public health approach to cervical cancer screening in Africa through community-based self-administered HPV testing and mobile treatment provision. Cancer Med. 2020;9:8701–8712. doi: 10.1002/cam4.3468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Yang Q., Van Stee S.K. The Comparative Effectiveness of Mobile Phone Interventions in Improving Health Outcomes: Meta-Analytic Review. JMIR Mhealth Uhealth. 2019;7:e11244. doi: 10.2196/11244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Beratarrechea A., Lee A.G., Willner J.M., Jahangir E., Ciapponi A., Rubinstein A. The impact of mobile health interventions on chronic disease outcomes in developing countries: a systematic review. Telemed J E Health. 2014 doi: 10.1089/tmj.2012.0328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Basu P., Alhomoud S., Taghavi K., Carvalho A.L., Lucas E., Baussano I. Cancer Screening in the Coronavirus Pandemic Era: Adjusting to a New Situation. JCO Glob Oncol. 2021;7:416–424. doi: 10.1200/GO.21.00033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Zhang D., Advani S., Waller J., et al. Mobile technologies and cervical cancer screening in low- and middle-income countries: a systematic review. JCO Glob Oncol. 2020;6:617–627. doi: 10.1200/JGO.19.00201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Linde D.S., Andersen M.S., Mwaiselage J., Manongi R., Kjaer S.K., Rasch V. Effectiveness of one-way text messaging on attendance to follow-up cervical cancer screening among human papillomavirus-positive tanzanian women (Connected2Care): parallel-group randomized controlled Trial. J Med Internet Res. 2020;22:e15863. doi: 10.2196/15863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Arrossi S., Paolino M., Orellana L., Thouyaret L., Kohler R.E., Viswanath K. Mixed-methods approach to evaluate an mHealth intervention to increase adherence to triage of human papillomavirus-positive women who have performed self-collection (the ATICA study): study protocol for a hybrid type I cluster randomized effectiveness-implementation trial. Trials. 2019;20:148. doi: 10.1186/s13063-019-3229-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Glasgow R.E., Vogt T.M., Boles S.M. Evaluating the public health impact of health promotion interventions: the RE-AIM framework. Am J Public Health. 1999;89:1322–1327. doi: 10.2105/ajph.89.9.1322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Arrossi S, Thouyaret L, Paul L. Recomendaciones para el tamizaje, seguimiento y tratamiento de mujeres para la prevención del cáncer cervico-uterino en el marco de la incorporación de la prueba de VPH. Buenos Aires: Ministerio de Salud de la Nación; 2015. [cited 2021 September]. Available from: https://bancos.salud.gob.ar/recurso/recomendaciones-para-el-tamizaje-seguimiento-y-tratamiento-de-mujeres-para-la-prevencion. Accessed 20 May 2021
- 22.Arrossi S., Curotto M., Thouyaret L., Paolino M., Cuberli M., Laudi R. Manual para la implementación del test de VPH en contexto programático. Buenos Aires: Instituto Nacional del Cáncer (INC); 2016 https://bancos.salud.gob.ar/recurso/manual-para-la-implementacion-del-test-de-vph-en-contexto-programatico Available from: [Google Scholar]
- 23.Sanchez Antelo V., Kohler R.E., Curotto M., Viswanath K.V., Paolino M., Arrossi S. Developing SMS Content to Promote Papanicolaou Triage Among Women Who Performed HPV Self-collection Test: Qualitative Study. JMIR Form Res. 2020;4:e14652. doi: 10.2196/14652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Sanchez Antelo V., Kohler R.K., Szwarc L., Paolino M., Viswanath K., Arrossi S. Knowledge and perceptions regarding triage among human papillomavirus-tested women: A qualitative study of perspectives of low-income women in Argentina. Womens Health (Lond) 2020;16 doi: 10.1177/1745506520976011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Cuberli M., Arrossi S. Instituto Nacional del Cáncer (INC); Buenos Aires: 2013. Consejería para la prevención del cáncer de cuello de útero. Propuestas para una mejor comunicación con las mujeres durante el tamizaje, seguimiento y tratamiento. [Google Scholar]
- 26.Labrique A., Vasudevan L., Weiss W., Wilson K. Establishing standards to evaluate the impact of integrating digital health into health systems. Glob Health Sci Pract. 2018;6(suppl 1):S5–S17. doi: 10.9745/GHSP-D-18-00230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.de Andrade L.O., Pellegrini Filho A., Solar O., et al. Social determinants of health, universal health coverage, and sustainable development: case studies from Latin American countries. Lancet. 2015;385(9975):1343–1351. doi: 10.1016/S0140-6736(14)61494-X. [DOI] [PubMed] [Google Scholar]
- 28.Doubeni C.A., Gabler N.B., Wheeler C.M., et al. Timely follow-up of positive cancer screening results: A systematic review and recommendations from the PROSPR Consortium. CA Cancer J Clin. 2018;68:199–216. doi: 10.3322/caac.21452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Erwin E., Aronson K.J., Day A., et al. SMS behaviour change communication and eVoucher interventions to increase uptake of cervical cancer screening in the Kilimanjaro and Arusha regions of Tanzania: a randomised, double-blind, controlled trial of effectiveness. BMJ Innov. 2019;5:28–34. doi: 10.1136/bmjinnov-2018-000276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Cormick G., Ciganda A., Cafferata M.L., et al. Text message interventions for follow up of infants born to mothers positive for Chagas disease in Tucumán, Argentina: a feasibility study. BMC Res Notes. 2015;8:508. doi: 10.1186/s13104-015-1498-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Head K.J., Noar S.M., Iannarino N.T., Grant Harrington N. Efficacy of text messaging-based interventions for health promotion: a meta-analysis. Soc Sci Med. 2013;97:41–48. doi: 10.1016/j.socscimed.2013.08.003. [DOI] [PubMed] [Google Scholar]
- 32.Moodley J., Constant D., Botha M.H., van der Merwe F.H., Edwards A., Momberg M. Exploring the feasibility of using mobile phones to improve the management of clients with cervical cancer precursor lesions. BMC Womens Health. 2019;19:2. doi: 10.1186/s12905-018-0702-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Huchko M.J., Saduma I., Blat C., Oketch S., Bukusi E.A. How Providing Cervical Cancer Screening Results via Cell Phone Affects Patient Follow-Up Rates in Western Kenya. J Glob Oncol. 2019;5:1–8. doi: 10.1200/JGO.18.00264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Budd J., Miller B.S., Manning E.M., et al. Digital technologies in the public-health response to COVID-19. Nat Med. 2020;26:1183–1192. doi: 10.1038/s41591-020-1011-4. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.