Summary
Background
COVID-19 has affected the African region in many ways. We aimed to generate robust information on the transmission dynamics of COVID-19 in this region since the beginning of the pandemic and throughout 2022.
Methods
For each of the 47 countries of the WHO African region, we consolidated COVID-19 data from reported infections and deaths (from WHO statistics); published literature on socioecological, biophysical, and public health interventions; and immunity status and variants of concern, to build a dynamic and comprehensive picture of COVID-19 burden. The model is consolidated through a partially observed Markov decision process, with a Fourier series to produce observed patterns over time based on the SEIRD (denoting susceptible, exposed, infected, recovered, and dead) modelling framework. The model was set up to run weekly, by country, from the date the first infection was reported in each country until Dec 31, 2021. New variants were introduced into the model based on sequenced data reported by countries. The models were then extrapolated until the end of 2022 and included three scenarios based on possible new variants with varying transmissibility, severity, or immunogenicity.
Findings
Between Jan 1, 2020, and Dec 31, 2021, our model estimates the number of SARS-CoV-2 infections in the African region to be 505·6 million (95% CI 476·0–536·2), inferring that only 1·4% (one in 71) of SARS-CoV-2 infections in the region were reported. Deaths are estimated at 439 500 (95% CI 344 374–574 785), with 35·3% (one in three) of these reported as COVID-19-related deaths. Although the number of infections were similar between 2020 and 2021, 81% of the deaths were in 2021. 52·3% (95% CI 43·5–95·2) of the region's population is estimated to have some SARS-CoV-2 immunity, given vaccination coverage of 14·7% as of Dec 31, 2021. By the end of 2022, we estimate that infections will remain high, at around 166·2 million (95% CI 157·5–174·9) infections, but deaths will substantially reduce to 22 563 (14 970–38 831).
Interpretation
The African region is estimated to have had a similar number of COVID-19 infections to that of the rest of the world, but with fewer deaths. Our model suggests that the current approach to SARS-CoV-2 testing is missing most infections. These results are consistent with findings from representative seroprevalence studies. There is, therefore, a need for surveillance of hospitalisations, comorbidities, and the emergence of new variants of concern, and scale-up of representative seroprevalence studies, as core response strategies.
Funding
None.
Introduction
The COVID-19 pandemic has had a substantial impact on health, societies, and economies worldwide, including in the African region. As of Dec 31, 2021, the 47 countries of the WHO African region had reported 7·1 million infections and 155 000 deaths, accounting for 2·5% and 2·9% of the global burden of COVID-19, respectively.1 When the already increased disease burden and restricted capacity of health systems across countries in the region are considered, this reported burden represents a larger problem than the numbers suggest. The burden is uneven across the region, with relative numbers of infections per 100 000 population ranging from 29·46 (Niger) to 25 061·16 (Seychelles) and deaths from 1·07 (Chad) to 151·66 (South Africa).1 The variation in the number of infections within and across countries is driven by socioecological factors, and the number of deaths by biophysical factors.2, 3 The socioecological factors, primarily population density, age, and hygiene, influence the rate of transmission.4, 5 In addition, public health response measures, specifically lockdowns and safe hygiene practices, contribute to minimising the role of these socioecological factors on the rate of SARS-CoV-2 transmission.5 By contrast, the biophysical factors, primarily comorbidities such as hypertension, diabetes (type 2), chronic obstructive pulmonary disease, HIV, and obesity, increase the severity and risk of mortality after infection.6, 7, 8 The prevalence of these comorbidities varies in Africa, and in some instances they afflict a substantial portion of the population—up to 27% for HIV/AIDS, 22·1% for diabetes, 22·1% for chronic obstructive pulmonary disease, and 30·8% for hypertension.9 Health system responses, such as surveillance and effective case management, aim to mitigate against the effects of these biophysical factors. These are complemented by the use of available vaccines, which enhance antibody development and thereby reduce the severity of disease.
Research in context.
Evidence before this study
The COVID-19 pandemic has had widespread effects on all countries. Globally, and particularly in the African region, the reported impact (in terms of infections and deaths) is known to underestimate the actual impact, given that countries are not able to test all suspected cases of infection during widespread community transmission. Knowledge of the true burden of COVID-19 relies on having a comprehensive mortality surveillance framework and regular seroprevalence studies, neither of which are reliably available in most countries of the African region. According to SeroTracker—a dashboard for SARS-CoV-2 serosurveys that systematically monitors and synthesises SARS-CoV-2 serological studies—as of Dec 31, 2021, 211 seroprevalence surveys (of 2812 globally) had been conducted in the WHO African region since the beginning of the pandemic. Only 11 of the 47 countries in this region have conducted seroprevalence surveys that were nationally representative, with only five countries conducting seroprevalence surveys with low risk of bias. Reporting of deaths is similarly underestimated; an assessment of health information systems shows only 10% of deaths in the WHO Africa region were being registered before the COVID-19 pandemic, compared with a global average of 62%. Reliable sources for understanding the true burden of COVID-19, therefore, remain insufficient in the WHO African region.
Added value of this study
Our findings consolidate the available data from the region, together with epidemiological evidence about COVID-19, to statistically build a picture of the true burden of COVID-19 transmission and outcomes in each country of the region. This modelling study offers an analysis of a more comprehensive picture of the progression and true burden of SARS-CoV-2 infection and COVID-19-related deaths, allowing for better understanding of the patterns so far seen in the region.
Implications of all the available evidence
These findings provide information on the variable impact of the pandemic across countries in the WHO African region. We provide an empirical basis to enable better preparedness and response to similar pandemics in the future. Moving forward, the predictions from this study could help to guide policy and practice in terms of where and how to plan response actions to the COVID-19 pandemic in the medium term. Our findings also provide a mechanism for other health programmes to generate evidence for policy when data is incomplete.
Misinformation related to the COVID-19 response activities, together with the negative socioeconomic implications of mitigation measures, has negatively affected the applicability of these interventions across countries.5 Among these, delivery of essential health services were especially impacted, with widespread disruptions to service continuity.10 Weak existing capacities for surveillance and diagnostics further contributed to the nature of the pandemic response in most countries in the region, including under-reporting of infections and deaths.11
Among and within countries in the region, the unique socioecological and biophysical factors among populations have contributed to the varying patterns in transmission and mortality observed. The introduction of vaccines (which reduce the susceptible population) and the emergence of variants of concern, which can influence transmission and disease severity, have also contributed to these unique trends.12, 13 The alpha (B.1.1.7), beta (B.1.351), delta (B.1.617.2), and omicron (B.1.1.529) variants reported in the region have had greater rates of transmission than the reference strain, driving spikes in transmission as they emerge.14 22 of the 47 countries in the region had reported the delta variant in mid 2021, which is postulated to have fuelled the observed third transmission wave and the associated increased mortality. By the end of 2021, the omicron variant dominated—accounting for 71·43% of sequenced samples by Dec 31, 2021. Variants increase the potential for immune escape, potentially leading to increased mortality as they negate protection gained from earlier infections or vaccinations (or both).15
Seroprevalence studies suggest that the reported COVID-19 infections and deaths represent a small fraction of the actual burden of COVID-19 in the WHO African region.16, 17 As of Dec 31, 2021, there were 3179 seroprevalence studies across 134 countries and territories tracked.18 Among the 47 countries of the WHO African region, 31 had seroprevalence studies, of which 11 were nationally representative and five—in Ethiopia, Kenya, Senegal, Sierra Leone, and South Africa—were at low risk of bias, as categorised in the SeroTracker.18 Looking at these countries, their reported infections at the time of the seroprevalence studies represented a small proportion (one in 50 to one in 200) of their actual burden of COVID-19.19, 20
It is, therefore, important to decipher what has happened in the region since the COVID-19 pandemic started. Other efforts have been made to understand the true COVID-19 burden; however, these have been restricted in scope to a country or subregion, based on scarce primary information, or do not reflect country-specific adaptive factors.21, 22 Consequently, the outputs produced tended to be implausible, and not correlated with other known realities in the region. Given the challenges of health information availability in the region, it is important to leverage dynamic, adaptive approaches that make use of existing primary data, and to validate these data with quality information, such as from seroprevalence studies and available mortality statistics. We aimed to present such an approach to derive best estimates of the country-specific burden of COVID-19 in the WHO African region since the pandemic began, and of anticipated patterns for 2022.
Methods
Model conceptualisation
We used a standard epidemiological SEIRD (denoting susceptible, exposed, infected, recovered, and dead) compartmental model framework with modifications to include partially observed Markov processes for each of the 47 countries in the WHO African region.23 Trends in the data were explored by country, using time plots, from the first infection (in each country) to Dec 31, 2021, before the model fitting. All individuals, regardless of age, were included.
First, we assumed that all the countries recorded widespread, sustained community transmission of COVID-19 and face a potential for emergence of new variants. However, due to different capacities and policies for detection and reporting, we assume that the officially reported infections and deaths only represent an observed layer of COVID-19, beneath which are unreported or undetected infections and deaths (unobserved layer). To account for these, we conceptualised the SEIRD model with modification to include a partially observed Markov decision process, which is a statistical approach whereby the hidden states are assumed to have the Markov property and emit symbols or observations. For the compartment model (SEIRD), the hidden states are the number of individuals in the compartments.23
Second, the susceptible population, which was increased by the population birth rates and diminished by the population death rates, was divided into immune and non-immune states to reflect different paths for each population subgroup. The immune population was those who had been vaccinated or who had recovered from COVID-19. Equation (1) was used to model growth in vaccination rates up to the achieved vaccination coverage, and this draws from the total susceptible population.
Third, the exposed population was again subdivided into multiple states to reflect different COVID-19 variants of concern, since each has a unique epidemiology. The exposure to the different variants of concern was factored in by considering their respective incubation periods as the input into the model (appendix p 2). The overall effect in the model was a weighted average of the variants of concerns' inverse of the latent periods, with the weights being the proportions of their prevalence.
Fourth, the individuals with COVID-19 were subdivided into observed and unobserved or hidden states using the partially observed Markov decision process, with the observed infections being a function of the force of infection for both the immune and non-immune populations, respectively.
Finally, the infected population was captured depending on the different states' severity of illness. This fifth stage involved determining the outcome of illness and represented the final states arising from both immune and non-immune subpopulations, which was determined by the infectious periods for either subpopulation. The framework assumes that all individuals who recover will rejoin the immune subpopulation and are at risk of being exposed again, although this risk is mitigated by the reduced risk of severe disease due to immunity gained, either through vaccination against or recovery from COVID-19. Once a person enters a state, their projection is defined subsequently based on the outcome.
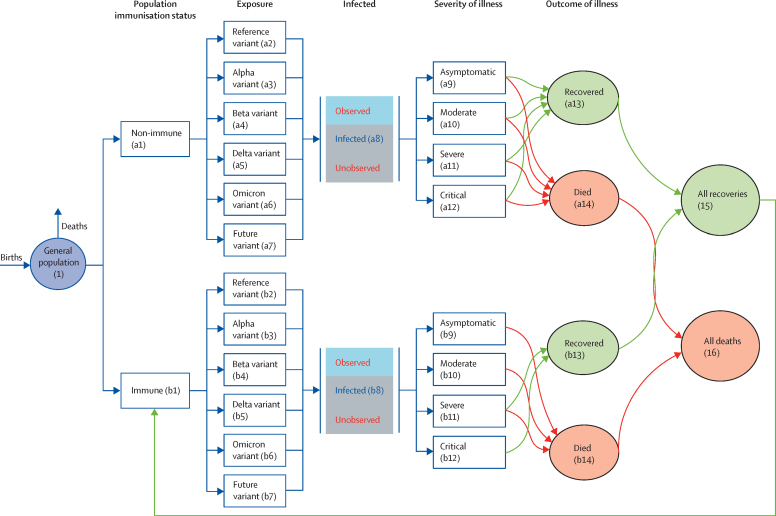
Figure 1 shows the model framework and different transition states. 14 Markov states are defined for either the non-immune or immune subpopulations, reflecting the two situations every individual is in. Either of these groups could be infected with a circulating variant at a given timepoint. This infection could be recorded and reported (observed) or missed (hidden) depending on the testing approaches and capacity available. The final outcome of an infection is represented by four mutually exclusive Markov states: asymptomatic, moderate, severe, or critical. These were derived using evidence from the existing evidence base (appendix p 2). We did not consider interstate transition—only the final state—due to absence of quality information on their magnitude. Each of these four states can progress to either recovery or death. All deaths from both immune and non-immune subpopulations represent an absorbing Markov state, whereas all the recovered individuals return to the immune pool to continue the cycle after a time, depending on the level of immunity accrued. The probability of transitioning from one of the compartments (states) to another was derived from published evidence and is listed in the appendix (p 2).
Figure 1.
Adapted POMP model for SARS-CoV-2 transitions and outcomes
POMP=partially observed Markov decision process.
Evidence used to parametrise the model
We used the following datasets: country population estimates, total population, mortality rates, birth rates for the different years from the UN Population Division; COVID-19 infections, deaths, and vaccination rates from WHO's COVID-19 tracker;1 and data on seroprevalence surveys from the WHO unity studies.18
We searched PubMed and institutional websites to obtain the latest evidence on the different transition probabilities which were a function of the respective incubation periods (appendix p 2). We searched for articles published, in English, between Jan 1, 2020, and Dec 31, 2021, using the search terms “COVID-19”, “variants”, “rates”, “transmission”, and “probabilities”. A synthesis of the obtained information and evidence for the various data sources led to the following suggested incubation periods: 5 days for the reference strain and beta, 3·5 days for alpha, 4 days for delta, and 3 days for omicron.24, 25 On the probability of infection, immunisation reduces the severity of infections by 87·5%, risk of infection by 69·0%, mortality by 86·5%, and reinfection by 90·4%.26, 27, 28, 29, 30, 31, 32 Immunisation levels and variant severity were used to determine the outcome of the infections.
Empirical framework for the conceptual model
Determining the susceptible population
The model was designed to run in each of the 47 countries independently. The susceptible population was taken as the whole country population in 2020, allowing this to evolve over time based on the country's birth and mortality rate. These rates were estimated from the UN Population Division census data.
Determining population immunisation status
The population was compartmentalised into non-immune and immune subpopulations on the basis of the recoveries from the dynamic model and the vaccination rate at given timepoints. We use the term immune to denote the population expected to have developed immunity (either from vaccination or natural infection) and use vaccinated to describe the population that has received a vaccine.
The logistic equation used to estimate the progression of the vaccinated population was:
| (1) |
where φ(t) is the proportion of the population vaccinated at time t, φ(0) is the proportion of the vaccinated population at the beginning of the process (t=0), K is the maximum proportion of the immune individuals in the population at the end of the time duration, and r is the intrinsic growth rate of the immune population.
The vaccinated population at any given timepoint aggregates with the recovered population to form the immune population. The difference between the total estimated population and the immune population gives the total number of individuals who are not immune at any timepoint.
Determining exposure status
The susceptible population (non-immune and immune) were exposed to the variants of concern at a rate proportional to the inverse of their incubation periods. This exposure was weighted by the proportion of infections being caused by each variant in each of the countries.
Determining infected states
The probability of infection (ie, transitioning to the infected compartment) is given by:
| (2) |
where the transmission rate β(t) is a function of contact rate and variant-weighted transmission probability, I is the number of infections, and ι is the mean number of imported infections, used as described by Sun and colleagues.33 α is a mixing parameter and set to 1 because of the homogeneous assumption and N(t) is the population size. The population sizes in each of the countries is extrapolated from the census data. The rate β(t) in equation (2) allows for waves experienced and takes the form:
| (3) |
where a and b in equation (3) are the Fourier coefficients and
for any period P and are estimated from the observed data.
The probability of infection with SARS-CoV-2 is a function of the specific variant's infectivity, together with the socioecological factors at play in each country. For each country, a specific estimated transmission adjustor (contact rate) was derived. This contact rate was conceptualised as a function of population density, mobility, personal perceptions of safety, and urbanisation, and was modulated by the intensity with which public health measures were applied, specifically lockdowns and hygiene improvements. On analysis, the following were found to have a relationship with the patterns of transmission and were used to determine the contact rate: population age, population density, population in formal employment, populations living in slums or in urban areas, connectivity per square km, personal safety, and access to basic sanitation The country-specific contact rates vary, from a low of 1·24 (Burkina Faso), to 21·00 (São Tomé and Príncipe) representing the varied effects of socioecological environment on COVID-19 transmission across the region (appendix p 6). To estimate the true incidence, which is hindered by incomplete data, the partially observed Markov decision process was used as previously described.23 This process recognises that a proportion of overall transmission in a given country is unobserved, leading to incompleteness in reported data for infections and deaths. The appendix (pp 5–6) includes more details on the process used and country-specific outcomes.
Determining the severity and outcome of illness
The severity of COVID-19 illness was modelled as a function of the severity of symptoms associated with variants of concern and levels of immunity.
Deaths rates by country were derived based on the biophysical factors that statistically influence the severity of illness and mortality—namely, different population cohorts for age variability and the burden of hypertension, type 2 diabetes, obesity, HIV, and tuberculosis (vulnerability index).34
Running the model
To date, the observed transmission data show a cyclical pattern of COVID-19 infection in countries. The weekly reported data from each country was used to initialise the model to derive the patterns of transmission (waves) by fitting a Fourier series to the data. This pattern assumed that changes in reported infections or deaths represent real changes in transmission patterns. The reported data on infections and deaths were derived from official WHO statistics.1 The model was constructed independently in Microsoft Excel and in R software (version 4.1.2) for internal validation. Both were set up to run weekly from the date the first infection was reported in each country until Dec 31, 2021. New variants were introduced into the model based on sequenced data reported to WHO by countries. The models were then extrapolated until Dec 31, 2022, and three additional scenarios were generated to explore the effects of new variants: a 200% increase in (1) transmissibility, (2) severity, or (3) reinfection rates.
Sensitivity analysis
Sensitivity analyses were conducted to test the effect of assumptions on the results and the overall robustness of the model. Structural uncertainty was tested to see if results behaved in a predictable manner. For example, an increase in the transmissibility of a variant should lead to increased infections in the short term. Additionally, parameter uncertainty was incorporated using 95% CI. The assumptions were run with higher and lower limits to generate overall model credibility at 95% confidence.
Internal consistency and model validation
Building the model independently on two different platforms (Microsoft Excel and R) helped to achieve internal consistency, as well as catering for users' preference of tools. The emerging results were consistent from both models. The results for deaths were compared with reported deaths in seven countries that have reliable and complete mortality reporting.35 These countries—South Africa, Cape Verde, Botswana, Eswatini, Seychelles, Namibia, and Equatorial Guinea—were used for comparisons with model outcomes of reported deaths. Representative seroprevalence data were also used to validate cases from the model outputs. The R software model is hosted on a GitHub repository.
Presentation of results
The model generates weekly information on infections, deaths, and immunity status from the first COVID-19 infection in a country until Dec 31, 2021, and produces projections until Dec 31, 2022. In the results, we present the annual summations for 2020 and 2021, contrasted with the reported data from countries. Findings were presented by country and other policy-relevant disaggregations, including income groups (according to the World Bank classification) and African regional economic communities to discern patterns by location or wealth. Data are presented in both absolute and relative figures (per 100 000 population) to adjust for population differences.
Role of the funding source
No funding was received.
Results
The outputs generated from both Microsoft Excel and R software were identical, suggesting consistency between the two platforms and minimal entry errors. The model uploaded on the GitHub repository has also been run by three independent experts external to the study, all of whom have been able to reproduce the model outputs. With regards to validity, mortality results for the countries with robust mortality data collection systems had at least 95% concurrence with the model outcomes.
We present two sets of results: country-specific infections and deaths between Jan 1, 2020, and Dec 31, 2021, and projections for 2022 with case scenarios.
Table 1 shows country-specific and combined estimates of reported infections and deaths, together with vaccination and immunisation status, up to Dec 31, 2021. Of the 1·14 billion individuals in the WHO African region, our model estimates that 505·6 million (95% CI 476·0–536·2) were infected with SARS-CoV-2 in this time period. 55% of these infections were in 2020 and 45% were in 2021. Compared with the 7·1 million infections reported, our findings suggest that only 1·4% (one in 71) of SARS-CoV-2 infections were reported via official channels. Our model estimates that 439 500 (95% CI 344 374–574 785) deaths occurred in this time period, of which 19% were in 2020 and 81% in 2021. Compared with the 155 248 deaths reported, 35·3% (one in three) of COVID-19-related deaths were reported as such via official channels. Reported versus estimated infections and deaths are compared, by country, in the appendix (p 6). Up to 52·3% (95% CI 43·5–95·2) of the population has developed SARS-CoV-2 immunity, with 14·7% having been vaccinated.1
Table 1.
SARS-CoV-2 infections and COVID-19-related deaths across the 47 countries in the WHO African region, Jan 1, 2020–Dec 31, 2021
|
Infections with SARS-CoV-2 |
Deaths from COVID-19 | Fully vaccinated (%) | Immune population (%) | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Cumulative total (95% CI) | Total in 2020 | Total in 2021 | Reported | Reported, % of cumulative total | Total for 2020–21 (95% CI) | Total in 2020 | Total in 2021 | Reported | Reported, % of cumulative total | |||
| Algeria | 19 594 780 (18 358 126–20 831 434) | 10 762 115 (10 195 315–11 328 915) | 8 832 665 (8 162 811–9 502 519) | 218 037 | 1·1% | 21 491 (17 037–26 536) | 4137 (2387–6449) | 17 354 (14 650–20 086) | 6275 | 29·2% | 12·8% | 51·1% |
| Angola | 14 792 564 (13 675 036–15 911 979) | 8 129 958 (7 617 793–8 643 023) | 6 662 606 (6 057 244–7 268 956) | 76 791 | 0·5% | 10 890 (8628–14 231) | 2256 (1408–3788) | 8634 (7220–10 443) | 1765 | 16·2% | 12·3% | 50·5% |
| Benin | 5 427 175 (5 152 007–5 703 030) | 2 977 908 (2 851 669–3 104 342) | 2 449 267 (2 300 338–2 598 689) | 25 361 | 0·5% | 1471 (1257–1836) | 304 (239–471) | 1167 (1018–1365) | 161 | 10·9% | 10·9% | 49·7% |
| Botswana | 1 337 147 (1 171 681–1 520 744) | 747 102 (671 235–831 250) | 590 045 (500 446–689 493) | 212 482 | 15·9% | 3586 (2848–4965) | 795 (542–1427) | 2791 (2306–3538) | 2439 | 68·0% | 44·0% | 75·2% |
| Burkina Faso | 9 246 522 (8 714 176–9 779 047) | 4 932 877 (4 688 846–5 176 951) | 4 313 645 (4 025 330–4 602 096) | 17 632 | 0·2% | 6752 (5099–9388) | 1318 (774–2526) | 5433 (4325–6861) | 320 | 4·7% | 3·1% | 44·8% |
| Burundi | 5 332 997 (4 997 194–5 669 710) | 2 924 541 (2 770 454–3 078 868) | 2 408 456 (2 226 740–2 590 842) | 29 919 | 0·6% | 149 (137–238) | 43 (39–83) | 107 (98–155) | 14 | 9·4% | 0·0% | 43·5% |
| Cameroon | 11 922 360 (11 262 810–12 584 727) | 6 540 934 (6 238 332–6 844 519) | 5 381 426 (5 024 478–5 740 208) | 108 451 | 0·9% | 8242 (6717–9867) | 1531 (886–2276) | 6711 (5830–7591) | 1851 | 22·5% | 2·5% | 45·2% |
| Cape Verde | 295 548 (277 499–313 622) | 162 461 (154 190–170 745) | 133 087 (123 309–142 877) | 40 738 | 13·8% | 383 (303–497) | 71 (41–123) | 312 (262–374) | 356 | 93·0% | 46·0% | 74·4% |
| Central African Republic | 2 123 225 (1 996 144–2 250 766) | 1 144 738 (1 086 474–1 203 194) | 978 487 (909 670–1 047 572) | 8569 | 0·4% | 1009 (849–1359) | 171 (124–331) | 838 (725–1027) | 101 | 10·0% | 8·8% | 48·2% |
| Chad | 7 177 459 (6 737 215–7 617 954) | 3 773 397 (3 571 637–3 975 290) | 3 404 062 (3 165 578–3 642 663) | 5707 | 0·1% | 9908 (7857–13 556) | 1806 (1122–3478) | 8102 (6735–10 078) | 181 | 1·8% | 0·6% | 42·8% |
| Comoros | 393 751 (375 243–412 347) | 214 249 (205 783–222 772) | 179 502 (169 459–189 575) | 6657 | 1·7% | 333 (271–438) | 52 (31–100) | 281 (240–338) | 158 | 47·4% | 28·3% | 60·1% |
| Congo (Brazzaville) | 2 471 580 (2 357 008–2 586 518) | 1 354 332 (1 301 882–1 407 012) | 1 117 248 (1 055 125–1 179 506) | 20 089 | 0·8% | 1560 (1257–1933) | 257 (144–428) | 1303 (1112–1505) | 371 | 23·8% | 10·6% | 49·6% |
| Côte d'Ivoire | 11 775 339 (11 189 013–12 361 773) | 6 457 651 (6 188 876–6 726 433) | 5 317 688 (5 000 137–5 635 340) | 68 146 | 0·6% | 4953 (4027–6087) | 925 (573–1444) | 4028 (3454–4643) | 712 | 14·4% | 8·2% | 48·2% |
| DR Congo | 40 070 320 (37 920 838–42 219 897) | 21 992 682 (21 007 459–22 977 905) | 18 077 638 (16 913 379–19 241 992) | 76 021 | 0·2% | 27 415 (21 874–34 145) | 5647 (3690–8732) | 21 767 (18 184–25 413) | 1205 | 4·4% | 0·1% | 43·5% |
| Equatorial Guinea | 642 309 (607 635–677 375) | 351 636 (335 748–367 708) | 290 673 (271 886–309 667) | 13 714 | 2·1% | 362 (287–477) | 73 (51–126) | 288 (237–351) | 175 | 48·4% | 14·5% | 52·4% |
| Eritrea | 1 568 640 (1 466 880–1 670 470) | 858 901 (812 274–905 573) | 709 739 (654 606–764 897) | 7982 | 0·5% | 1129 (965–1494) | 184 (134–352) | 945 (830–1143) | 131 | 11·6% | 0·0% | 43·6% |
| Eswatini | 601 538 (566 287–638 268) | 339 353 (322 999–356 188) | 262 185 (243 288–282 080) | 65 834 | 10·9% | 1334 (1044–1695) | 272 (163–437) | 1063 (881–1258) | 1307 | 98·0% | 26·1% | 64·0% |
| Ethiopia | 51 611 475 (49 248 944–53 979 577) | 28 346 909 (27 263 242–29 432 289) | 23 264 566 (21 985 702–24 547 288) | 415 447 | 0·8% | 34 734 (27 651–44 190) | 7170 (4168–11 504) | 27 564 (23 483–32 686) | 6926 | 19·9% | 3·5% | 45·7% |
| Gabon | 1 039 165 (976 345–1 102 374) | 570 670 (541 852–599 641) | 468 495 (434 493–502 733) | 41 073 | 4·0% | 323 (252–415) | 54 (29–96) | 270 (224–320) | 288 | 89·1% | 8·6% | 50·3% |
| The Gambia | 1 088 735 (994 824–1 183 297) | 595 285 (552 343–638 626) | 493 450 (442 481–544 671) | 10 176 | 0·9% | 1446 (1170–1866) | 328 (227–520) | 1118 (943–1346) | 343 | 23·7% | 9·8% | 49·3% |
| Ghana | 13 953 560 (13 225 596–14 682 366) | 7 674 756 (7 341 220–8 008 792) | 6 278 804 (5 884 376–6 673 574) | 141 295 | 1·0% | 4941 (3945–6123) | 968 (573–1510) | 3973 (3372–4613) | 1287 | 26·0% | 7·5% | 48·2% |
| Guinea | 5 882 987 (5 601 751–6 164 250) | 3 222 288 (3 093 401–3 351 200) | 2 660 699 (2 508 350–2 813 050) | 31 645 | 0·5% | 2776 (2221–3495) | 611 (383–941) | 2164 (1838–2554) | 421 | 15·2% | 7·8% | 48·0% |
| Guinea-Bissau | 877 530 (834 314–920 816) | 480 574 (460 753–500 414) | 396 956 (373 561–420 403) | 6476 | 0·7% | 942 (754–1233) | 153 (96–287) | 789 (658–946) | 149 | 15·8% | 1·2% | 44·2% |
| Kenya | 24 226 574 (23 090 189–25 365 544) | 13 297 373 (12 776 462–13 819 401) | 10 929 201 (10 313 726–11 546 143) | 292 237 | 1·2% | 17 403 (13 743–22 235) | 3774 (2262–5989) | 13 629 (11 480–16 246) | 5376 | 30·9% | 7·9% | 48·5% |
| Lesotho | 978 338 (898 147–1 063 680) | 542 435 (505 624–581 550) | 435 903 (392 523–482 130) | 28 408 | 2·9% | 700 (545–904) | 138 (81–232) | 562 (464–672) | 673 | 96·1% | 34·3% | 64·1% |
| Liberia | 2 242 665 (2 110 345–2 375 096) | 1 221 943 (1 161 277–1 282 641) | 1 020 722 (949 068–1 092 455) | 6656 | 0·3% | 3264 (2628–4137) | 525 (307–925) | 2739 (2321–3212) | 287 | 8·8% | 19·5% | 54·3% |
| Madagascar | 12 262 540 (11 577 017–12 949 623) | 6 610 303 (6 296 344–6 925 216) | 5 652 237 (5 280 673–6 024 407) | 50 335 | 0·4% | 9822 (7597–12 971) | 1816 (1002–3259) | 8006 (6595–9711) | 1027 | 10·5% | 2·0% | 44·2% |
| Malawi | 8 595 493 (8 161 497–9 033 871) | 4 716 221 (4 516 692–4 917 144) | 3 879 272 (3 644 805–4 116 727) | 74 201 | 0·9% | 10 493 (8063–13 757) | 2084 (1211–3580) | 8409 (6852–10 177) | 2355 | 22·4% | 3·6% | 45·8% |
| Mali | 9 046 812 (8 590 414–9 503 283) | 4 958 423 (4 749 225–5 167 639) | 4 088 389 (3 841 189–4 335 644) | 20 671 | 0·2% | 10 647 (8539–13 170) | 2324 (1547–3480) | 8323 (6992–9689) | 666 | 6·3% | 2·0% | 44·5% |
| Mauritania | 2 117 914 (2 013 524–2 223 356) | 1 166 273 (1 118 164–1 214 601) | 951 641 (895 361–1 008 755) | 41 160 | 1·9% | 1083 (807–1565) | 343 (260–564) | 740 (547–1002) | 863 | 79·7% | 19·8% | 55·4% |
| Mauritius | 616 380 (558 594–680 772) | 342 028 (315 148–371 541) | 274 352 (243 446–309 231) | 68 310 | 11·1% | 871 (718–1398) | 139 (96–381) | 731 (622–1017) | 786 | 90·3% | 71·5% | 85·3% |
| Mozambique | 14 116 565 (13 241 912–14 997 275) | 7 763 989 (7 363 019–8 167 648) | 6 352 576 (5 878 893–6 829 627) | 175 648 | 1·2% | 6222 (5066–7765) | 1269 (783–1976) | 4953 (4284–5789) | 1976 | 31·8% | 19·0% | 54·5% |
| Namibia | 1 302 837 (1 213 785–1 394 446) | 705 023 (664 350–747 010) | 597 814 (549 435–647 436) | 146 726 | 11·3% | 3676 (2894–4849) | 893 (558–1431) | 2783 (2336–3418) | 3620 | 98·5% | 13·5% | 57·1% |
| Niger | 10 874 074 (10 341 589–11 406 594) | 5 958 076 (5 714 024–6 202 148) | 4 915 998 (4 627 565–5 204 446) | 7371 | 0·1% | 14 222 (10 721–19 315) | 3206 (1933–5540) | 11 017 (8788–13 775) | 274 | 1·9% | 4·0% | 45·5% |
| Nigeria | 91 737 574 (85 871 675–97 604 199) | 50 367 030 (47 678 653–53 055 900) | 41 370 544 (38 193 022–44 548 299) | 241 607 | 0·3% | 44 340 (34 479–55 598) | 7385 (3559–12 545) | 36 956 (30 920–43 054) | 3034 | 6·8% | 2·2% | 44·6% |
| Rwanda | 5 870 486 (5 595 866–6 145 605) | 3 208 343 (3 082 554–3 334 439) | 2 662 143 (2 513 312–2 811 166) | 110 648 | 1·9% | 1866 (1426–2581) | 444 (291–772) | 1421 (1135–1809) | 1349 | 72·3% | 42·0% | 67·6% |
| São Tomé and Príncipe | 101 760 (96 836–106 811) | 55 167 (52 898–57 482) | 46 593 (43 938–49 329) | 4029 | 4·0% | 76 (67–98) | 14 (12–25) | 61 (54–73) | 59 | 78·1% | 23·4% | 58·3% |
| Senegal | 7 530 875 (7 161 980–7 899 826) | 4 128 927 (3 959 824–4 298 030) | 3 401 948 (3 202 156–3 601 797) | 74 870 | 1·0% | 6066 (4602–8004) | 1200 (629–2089) | 4866 (3973–5916) | 1890 | 31·2% | 5·6% | 47·0% |
| Seychelles | 75 524 (75 524–75 524) | 44 635 (44 635–44 635) | 30 889 (30 889–30 889) | 24 661 | 32·7% | 136 (136–136) | 38 (38–38) | 98 (98–98) | 126 | 92·4% | 79·6% | 95·2% |
| Sierra Leone | 3 507 473 (3 291 914–3 723 096) | 1 893 789 (1 794 994–1 992 616) | 1 613 684 (1 496 920–1 730 480) | 6983 | 0·2% | 2029 (1732–2635) | 298 (223–576) | 1730 (1509–2059) | 123 | 6·1% | 4·8% | 45·8% |
| South Africa | 30 333 409 (27 896 307–32 787 235) | 16 856 012 (15 732 736–17 980 682) | 13 477 397 (12 163 572–14 806 553) | 3 446 540 | 11·4% | 92 118 (72 596–116 570) | 19 748 (11 685–30 956) | 72 370 (60 911–85 615) | 91 061 | 98·9% | 26·8% | 63·8% |
| South Sudan | 4 941 483 (4 724 232–5 158 905) | 2 709 343 (2 609 737–2 808 995) | 2 232 140 (2 114 495–2 349 910) | 15 248 | 0·3% | 1752 (1464–2191) | 486 (385–687) | 1266 (1079–1504) | 143 | 8·2% | 1·7% | 44·4% |
| Tanzania | 26 279 966 (23 228 497–29 355 927) | 13 965 661 (12 566 690–15 375 477) | 12 314 305 (10 661 808–13 980 451) | 29 306 | 0·1% | 33 243 (24 450–54 526) | 2210 (1069–11 965) | 31 032 (23 381–42 561) | 737 | 2·2% | 2·3% | 44·0% |
| Togo | 3 707 521 (3 544 534–3 870 802) | 2 035 172 (1 960 395–2 110 009) | 1 672 349 (1 584 139–1 760 793) | 30 169 | 0·8% | 995 (822–1256) | 220 (160–339) | 776 (662–917) | 248 | 24·9% | 12·3% | 50·7% |
| Uganda | 20 552 111 (19 501 269–21 605 945) | 11 288 989 (10 807 073–11 771 996) | 9 263 122 (8 694 196–9 833 949) | 120 404 | 0·6% | 19 972 (15 374–26 681) | 4203 (2356–7278) | 15 769 (13 018–19 404) | 2910 | 14·6% | 3·1% | 45·3% |
| Zambia | 8 529 905 (8 092 312–8 973 502) | 4 696 947 (4 495 249–4 900 262) | 3 832 958 (3 597 063–4 073 240) | 249 199 | 2·9% | 6080 (4773–8083) | 1343 (824–2261) | 4737 (3949–5822) | 3730 | 61·4% | 3·4% | 47·0% |
| Zimbabwe | 6 841 779 (6 490 885–7 199 329) | 3 763 003 (3 601 594–3 926 880) | 3 078 776 (2 889 291–3 272 449) | 211 728 | 3·1% | 6295 (4819–8433) | 1391 (835–2371) | 4905 (3984–6063) | 4999 | 79·4% | 21·1% | 56·8% |
| All countries | 505 616 764 (474 999 886–536 207 062) | 276 850 422 (262 796 503–290 860 956) | 228 766 342 (212 203 384–245 346 107) | 7 125 357 | 1·4% | 439 500 (344 374–574 785) | 84 620 (49 893–146 650) | 354 880 (294 481–428 136) | 155 248 | 35·3% | 14·7% | 52·3% |
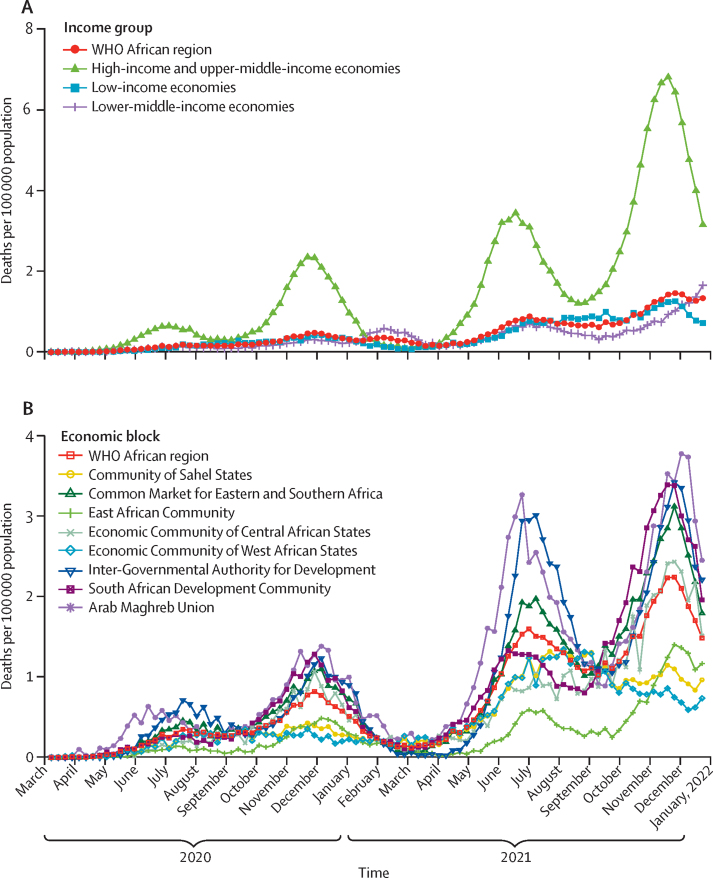
There have been four distinct waves of deaths, occurring in June–August, 2020, and between November, 2020, and January, 2021 (figure 2). Our findings highlight how the effects of the COVID-19 pandemic varied, on average, among countries when grouped by income and location, as represented by regional economic communities (appendix pp 6–7). High-income or upper-middle-income countries (n=8) had 1·0 (95% CI 0·7–1·1) deaths per 100 000 population, compared with 0·4 (95% CI 0·3–0·5) per 100 000 for both lower middle-income (n=17) and low-income countries (n=22). Lower-middle-income countries had the highest mortality rates in the WHO African region in February–April 2021, before dropping and having the lowest rates from July–Dec, 2021. Looking at regional economic communities, the highest mortality in the region was in the South African Development Community (0·8 per 100 000 population [95% CI 0·5–0·9]), Common Market for Eastern and Southern Africa (0·5 [0·3–0·5]), the Arab Maghreb Union (0·4 [0·3–0·5), Community of Sahel States (0·4 [0·3–0·5]), and Economic Community of West African States (0·4 [0·3–0·5]), followed (in order) by the Inter-Governmental Authority for Development (0·3 [0·3–0·4]), Economic Community of Central African States (0·3 [0·2–0·4]), and the East African Community (0·3 [0·2–0·4]). Pandemic peaks vary by regional economic community and in intensity.
Figure 2.
Trends in SARS-CoV-2 infections and COVID-19-related deaths, Jan 1, 2020–Dec 31, 2021
COVID-19 mortality trends for different country-income levels (A) and regional economic communities (B) in the WHO African region.
Table 2 shows the predicted COVID-19 burden in 2022. We anticipate 166·2 million (157·5–174·9) infections and 22 563 (14 970–38 831) deaths if current variants and transmission dynamics remain constant. New variants will only marginally change the expected SARS-CoV-2 infections, but will significantly affect the number of COVID-19-related deaths. Modelling three example scenarios showed that the greatest change in mortality is observed for a severe variant, not for a more infectious or transmissible variant. For example, for the WHO African region, a variant that is 200% more reinfectious would cause a 0·88% increase in infections, whereas a variant that is 200% more severe would cause a 212% increase in deaths to 70 489 (95% CI 44 987–118 412).
Table 2.
Projected estimates of SARS-CoV-2 infections and COVID-19-related deaths in 2022, and in different scenarios
|
Estimated burden in 2022 (95% CI) |
Scenario 1: Variant 200% more severe (95% CI) |
Scenario 2: Variant 200% more reinfectious (95% CI) |
Scenario 3: Variant 200% more transmissible (95% CI) |
|||||
|---|---|---|---|---|---|---|---|---|
| Infections | Deaths | Infections | Deaths | Infections | Deaths | Infections | Deaths | |
| Algeria | 6 063 865 (5 737 078–6 390 652) | 835 (450–1368) | 6 069 547 (5 741 186–6 397 908) | 2832 (1638–4386) | 6 115 322 (5 796 038–6 434 620) | 1040 (630–1589) | 6 066 428 (5 702 001–6 430 855) | 1006 (634–1510) |
| Angola | 4 568 793 (4 255 882–4 881 852) | 681 (480–1154) | 4 577 415 (4 264 294–4 890 684) | 1731 (1026–3076) | 4 632 742 (4 312 479–4 953 196) | 644 (407–1113) | 4 607 724 (4 393 896–4 821 705) | 592 (385–1052) |
| Benin | 1 714 418 (1 638 510–1 790 394) | 64 (55–133) | 1 716 620 (1 640 692–1 792 621) | 208 (165–335) | 1 729 583 (1 656 343–1 802 877) | 88 (75–157) | 1 725 139 (1 659 111–1 791 214) | 48 (43–96) |
| Botswana | 181 027 (164 821–197 638) | 98 (66–178) | 180 154 (163 933–197 145) | 272 (179–511) | 189 909 (171 194–209 929) | 116 (83–207) | 188 103 (175 895–200 685) | 108 (74–185) |
| Burkina Faso | 3 251 906 (3 071 033–3 432 807) | 260 (192–561) | 3 253 256 (3 077 248–3 429 285) | 959 (498–2022) | 3 277 264 (3 096 985–3 457 567) | 240 (184–568) | 3 269 972 (3 139 617–3 400 336) | 300 (211–662) |
| Burundi | 1 891 938 (1 780 973–2 003 044) | 14 (14–32) | 1 894 635 (1 783 320–2 006 430) | 14 (13–51) | 1 914 881 (1 808 635–2 021 347) | 7 (7–26) | 1 906 029 (1 829 896–1 982 303) | 7 (7–22) |
| Cameroon | 4 144 864 (3 949 913–4 340 193) | 349 (206–567) | 4 135 825 (3 941 308–4 330 601) | 932 (500–1525) | 4 178 293 (3 987 708–4 369 373) | 367 (213–610) | 4 143 931 (3 904 221–4 384 073) | 362 (214–559) |
| Cape Verde | 43 726 (41 633–45 825) | 11 (9–21) | 43 818 (41 725–45 919) | 32 (24–54) | 43 291 (41 344–45 239) | 11 (10–20) | 43 668 (41 677–45 659) | 13 (11–23) |
| Central African Republic | 681 717 (644 433–719 046) | 29 (25–89) | 682 709 (645 184–720 287) | 143 (113–274) | 703 495 (667 286–739 751) | 76 (68–144) | 700 573 (669 450–731 762) | 29 (25–91) |
| Chad | 2 531 166 (2 388 282–2 674 081) | 310 (251–930) | 2 553 767 (2 410 932–2 696 622) | 1342 (923–2646) | 2 530 088 (2 391 234–2 668 984) | 516 (400–1197) | 2 638 252 (2 524 042–2 752 489) | 206 (161–804) |
| Comoros | 97 713 (93 709–101 736) | 7 (7–19) | 97 791 (93 764–101 834) | 35 (27–65) | 98 864 (94 845–102 897) | 4 (3–17) | 98 734 (94 795–102 706) | 7 (7–18) |
| Congo (Brazzaville) | 787 607 (756 982–818 246) | 86 (71–138) | 787 142 (756 498–817 800) | 207 (135–335) | 795 497 (765 774–825 260) | 68 (52–128) | 788 564 (750 315–826 852) | 54 (42–116) |
| Côte d'Ivoire | 3 883 627 (3 719 225–4 048 058) | 239 (192–379) | 3 880 957 (3 716 764–4 045 164) | 601 (360–964) | 3 917 173 (3 757 886–4 076 497) | 233 (164–386) | 3 879 678 (3 672 068–4 087 299) | 220 (169–362) |
| DR Congo | 14 412 681 (13 751 804–15 073 597) | 1349 (819–2255) | 14 415 247 (13 753 756–15 076 755) | 3983 (2410–6489) | 14 573 309 (13 929 097–15 217 564) | 1517 (1009–2446) | 14 434 067 (13 607 890–15 260 246) | 1496 (1065–2409) |
| Equatorial Guinea | 192 559 (183 393–201 821) | 12 (10–27) | 192 438 (183 289–201 674) | 72 (56–112) | 193 606 (185 222–202 068) | 17 (15–33) | 192 742 (185 133–200 423) | 19 (16–34) |
| Eritrea | 556 208 (522 285–590 141) | 67 (58–141) | 554 858 (520 924–588 815) | 192 (139–349) | 559 528 (525 286–593 807) | 67 (59–121) | 562 015 (541 169–582 866) | 75 (69–134) |
| Eswatini | 125 274 (119 573–131 206) | 48 (33–81) | 124 821 (119 091–130 618) | 149 (95–235) | 126 752 (121 323–132 267) | 52 (38–86) | 124 792 (117 960–131 765) | 52 (37–82) |
| Ethiopia | 17 757 737 (17 064 546–18 451 191) | 1919 (1154–3017) | 17 706 550 (17 012 847–18 400 914) | 5369 (3035–8687) | 17 898 442 (17 234 376–18 563 074) | 1693 (861–2901) | 17 743 573 (16 882 514–18 604 919) | 1396 (619–2553) |
| Gabon | 321 296 (305 487–337 125) | 16 (13–29) | 321 920 (305 944–337 948) | 52 (38–81) | 323 368 (308 080–338 694) | 12 (10–24) | 319 295 (300 255–338 415) | 18 (15–30) |
| The Gambia | 330 947 (305 952–356 008) | 66 (55–117) | 334 398 (309 480–359 368) | 206 (132–363) | 338 951 (314 133–363 877) | 66 (55–127) | 340 801 (319 944–361 680) | 61 (49–124) |
| Ghana | 4 562 751 (4 361 585–4 763 931) | 219 (152–352) | 4 572 633 (4 369 517–4 775 762) | 725 (422–1 120) | 4 598 596 (4 404 521–4 792 789) | 216 (113–390) | 4 567 447 (4 312 869–4 822 119) | 219 (144–353) |
| Guinea | 1 936 995 (1 858 763–2 015 236) | 42 (34–133) | 1 935 965 (1 857 662–2 014 276) | 302 (204–539) | 1 954 943 (1 878 788–2 031 098) | 134 (107–235) | 1 943 343 (1 857 215–2 029 479) | 91 (72–204) |
| Guinea-Bissau | 309 146 (296 036–322 265) | 27 (24–77) | 308 766 (295 713–321 844) | 120 (91–234) | 311 722 (298 708–324 747) | 44 (38–83) | 311 232 (298 657–323 825) | 27 (22–80) |
| Kenya | 7 849 880 (7 541 715–8 158 136) | 721 (437–1279) | 7 861 122 (7 553 893–8 168 453) | 2655 (1552–4277) | 7 940 638 (7 643 019–8 238 799) | 721 (377–1309) | 7 890 514 (7 513 323–8 267 786) | 853 (505–1356) |
| Lesotho | 213 186 (197 120–229 487) | 22 (16–40) | 214 211 (197 901–230 851) | 72 (45–121) | 216 732 (201 106–232 592) | 22 (15–43) | 216 061 (205 066–227 409) | 28 (23–45) |
| Liberia | 639 226 (607 052–671 424) | 126 (106–238) | 639 704 (607 557–671 865) | 342 (246–642) | 642 738 (610 348–675 153) | 103 (85–220) | 648 082 (620 791–675 387) | 80 (71–191) |
| Madagascar | 4 363 475 (4 148 954–4 578 207) | 355 (192–768) | 4 376 990 (4 160 951–4 593 526) | 1115 (560–2216) | 4 370 389 (4 160 310–4 580 686) | 424 (233–907) | 4 343 938 (4 154 190–4 533 864) | 326 (162–739) |
| Malawi | 2 928 205 (2 803 593–3 053 231) | 495 (325–873) | 2 935 579 (2 811 257–3 060 302) | 1649 (986–2764) | 2 956 083 (2 834 755–3 077 737) | 426 (236–864) | 2 949 544 (2 835 020–3 064 464) | 641 (450–1019) |
| Mali | 3 194 175 (3 056 324–3 332 027) | 461 (339–845) | 3 197 760 (3 059 591–3 335 929) | 1846 (1146–2814) | 3 222 083 (3 089 140–3 355 035) | 684 (479–1102) | 3 198 446 (3 021 411–3 375 481) | 632 (456–1039) |
| Mauritania | 593 131 (568 589–617 736) | 87 (69–142) | 592 807 (568 260–617 417) | 237 (173–391) | 598 411 (574 215–622 703) | 58 (41–113) | 593 109 (568 879–617 357) | 53 (43–90) |
| Mauritius | 55 415 (51 156–59 966) | 18 (15–32) | 54 137 (50 042–58 559) | 57 (46–96) | 55 747 (51 228–60 664) | 16 (14–31) | 53 774 (49 864–57 857) | 21 (19–34) |
| Mozambique | 4 014 053 (3 800 801–4 227 899) | 253 (167–423) | 4 020 640 (3 807 659–4 234 038) | 699 (403–1180) | 4 078 650 (3 874 142–4 283 996) | 262 (162–445) | 4 047 865 (3 889 713–4 206 393) | 253 (159–413) |
| Namibia | 332 384 (314 463–351 200) | 129 (73–239) | 329 303 (311 637–347 799) | 452 (275–789) | 338 569 (321 417–356 061) | 135 (76–247) | 341 839 (324 439–359 873) | 119 (65–234) |
| Niger | 3 774 224 (3 614 921–3 933 531) | 816 (662–1496) | 3 772 178 (3 612 116–3 932 248) | 3206 (2505–4821) | 3 809 670 (3 656 178–3 963 167) | 874 (704–1628) | 3 777 538 (3 576 262–3 978 823) | 699 (582–1481) |
| Nigeria | 32 331 254 (30 561 690–34 100 861) | 2188 (1128–3654) | 32 349 490 (30 579 284–34 119 881) | 5711 (2394–10 118) | 32 683 499 (30 940 986–34 426 123) | 2590 (1340–4345) | 32 357 963 (30 406 517–34 309 482) | 2252 (1159–3673) |
| Rwanda | 1 190 418 (1 140 919–1 240 072) | 59 (44–108) | 1 191 179 (1 142 019–1 240 506) | 171 (107–318) | 1 206 246 (1 158 811–1 253 817) | 60 (43–117) | 1 198 195 (1 148 258–1 248 267) | 54 (40–106) |
| São Tomé and Príncipe | 26 228 (25 194–27 271) | 3 (3–8) | 26 260 (25 214–27 311) | 3 (3–12) | 26 483 (25 465–27 514) | 6 (5–10) | 26 350 (25 255–27 453) | 2 (2–7) |
| Senegal | 2 514 720 (2 406 229–2 623 232) | 272 (157–474) | 2 519 149 (2 410 124–2 628 174) | 738 (407–1345) | 2 544 614 (2 440 850–2 648 435) | 190 (101–412) | 2 523 082 (2 418 222–2 627 977) | 276 (169–478) |
| Seychelles | 946 (946–946) | 0 (0–0) | 946 (946–946) | 0 (0–0) | 983 (983–983) | 0 (0–0) | 1032 (1032–1032) | 0 (0–0) |
| Sierra Leone | 1 168 068 (1 103 950–1 232 204) | 40 (36–152) | 1 167 413 (1 102 952–1 231 890) | 159 (123–401) | 1 178 840 (1 117 022–1 240 676) | 40 (34–184) | 1 201 541 (1 146 956–1 256 142) | 99 (90–221) |
| South Africa | 6 537 582 (6 106 899–6 974 234) | 2960 (1586–4930) | 6 430 195 (6 016 260–6 847 505) | 9002 (4998–14 786) | 6 403 026 (6 003 741–6 803 536) | 2930 (1564–4994) | 6 434 731 (6 019 709–6 851 543) | 3025 (1707–4961) |
| South Sudan | 1 744 556 (1 679 295–1 809 832) | 102 (93–183) | 1 745 640 (1 680 275–1 811 011) | 179 (147–365) | 1 759 629 (1 697 264–1 822 017) | 64 (57–150) | 1 749 861 (1 667 785–1 831 966) | 64 (56–153) |
| Tanzania | 9 450 843 (8 382 143–10 522 204) | 4724 (3911–7742) | 9 436 837 (8 396 249–10 480 452) | 15 993 (13 100–26 665) | 9 544 827 (8 527 240–10 567 140) | 5201 (4283–9066) | 9 490 155 (9 007 302–9 975 201) | 4768 (3989–7441) |
| Togo | 1 153 943 (1 110 440–1 197 480) | 49 (45–91) | 1 156 413 (1 112 948–1 199 893) | 130 (97–217) | 1 167 764 (1 126 297–1 209 256) | 54 (45–96) | 1 158 396 (1 105 618–1 211 185) | 67 (60–107) |
| Uganda | 7 114 151 (6 792 989–7 435 582) | 1292 (800–2192) | 7 058 092 (6 736 492–7 379 868) | 3549 (2163–6175) | 7 196 341 (6 886 425–7 506 514) | 1376 (928–2321) | 7 116 621 (6 815 964–7 417 498) | 1140 (689–2079) |
| Zambia | 2 809 320 (2 689 420–2 930 275) | 275 (160–496) | 2 805 923 (2 686 055–2 926 588) | 896 (538–1545) | 2 840 772 (2 722 028–2 960 039) | 281 (171–506) | 2 814 294 (2 698 678–2 930 741) | 280 (174–505) |
| Zimbabwe | 1 828 189 (1 748 626–1 908 639) | 368 (238–630) | 1 831 479 (1 752 307–1 911 813) | 1149 (748–1901) | 1 841 555 (1 762 436–1 921 370) | 369 (250–641) | 1 821 533 (1 736 199–1 907 290) | 420 (285–683) |
| All countries | 166 175 533 (157 464 380–174 900 821) | 22 563 (14 970–38 831) | 166 054 679 (157 386 115–174 736 154) | 70 489 (44 987–118 412) | 167 629 908 (159 171 709–176 100 555) | 24 111 (15 845–42 361) | 166 550 566 (157 936 016–175 173 116) | 22 562 (15 045–38 557) |
Discussion
In this modelling analysis, we present a comprehensive picture of the COVID-19 pandemic in the WHO African region. The use of a dynamic modelling approach that incorporates reported data from multiple sources is crucial to developing such a picture, given the variation in completeness of data (including mortality records) among countries in the region.22 Although few countries have nationally representative seroprevalence, mortality, and socioecological data, our model has brought these data together (using a coherent epidemiological framework and statistical tools) to provide estimates of the additional infections that have gone unreported in the region, and projections for what infections and deaths might look like by the end of 2022.
Our use of a generated country-specific contact rate as a function of the multiple socioecological drivers of the differentiated transmission helped to address the challenge of knowing which of these factors are having the impacts seen in different countries—a notable obstacle in many other models. The contact rate here represents the total effect of these socioecological factors, without necessitating an understanding of how they each are functioning and influencing each other.
Comparison of the estimated burden emerging with information from nationally representative seroprevalence studies proved difficult. These studies all had different designs, study populations, and results. First, they focused on specific age groups (usually adults), with none including children; our model included individuals of all ages. Second, they each used different sampling approaches; for example, Sierra Leone had more participants than Senegal (despite Senegal having double its population), making cross comparison difficult. Studies in Kenya and South Africa were based on blood donor data only. Third, none had a population structure like the actual country population—ie, in age, sex, or geographical location. Due to these challenges, many existing studies majorly underestimated the actual seroprevalence. For example, a seroprevalence study in Sierra Leone suggested actual prevalence was 43-times higher than reported infections. South Africa and Senegal had seroprevalence estimates only marginally above those from this study for the same time period (47·4% and 28·8% vs 35·6–41·2% and 21·4–23·2% respectively). Conversely, in Kenya, Ethiopia, and Sierra Leone, the seroprevalence estimates were substantially lower than the study estimates (7·9%, 3·5%, and 2·6% vs 15·7–16·9%, 11·3–12·1%, and 25·2–28·1%). There is still substantial work needed in developing standard seroprevalence studies across the region.
We have also compared our model estimates with those of other models, specifically those from a 2022 publication by the Institute for Health Metrics and Evaluation estimating excess mortality due to COVID-19 in 74 countries.22 They suggest a five-times higher estimate of deaths in the WHO African region than reported—a 10% increase in all-cause mortality in the region. Such a substantial increase in mortality is implausible. There are various methodological assumptions that could have contributed to the overestimate. First, the authors use time-series seasonal and secular trends for input mortality data that were corrected for anomalies, reporting lag and under-reporting by predictive regression models, which had distributional structural changes since the pandemic began. Second, the authors' assumption of similar weekly mortality patterns in 2020–21 to that in 2019 (the reference year) is faulty, because these patterns were disrupted by the pandemic. Finally, there was no incorporation of virus characteristics driven by different variants, instead making use of exogenous covariates to the virus. The major drivers of mortality—namely rate of transmission, variant virulence, and personal biophysical characteristics—are all downplayed, leading to overestimations of mortality numbers.
Looking at the emerging reported infections, we note that the low rate of detected infections is not unique to Africa, with studies conducted in France, South Korea, and the USA suggesting that, at varying timepoints, up to 90%, 42%, and 75% of infections (respectively) have gone unreported.36, 37, 38 During widespread community transmission, it is impractical to document every infection—thus low reporting is not necessarily a good measure of detection capacity. Within the 47 countries in this study, on average, there are lower rates and capacity for testing, less tendency to seek care, and lower availability of health services than other regions and countries of the world, as well as quality of care gaps;39 all of which make the identification of COVID-19-positive individuals less likely than in countries with higher incomes.35 The true burden of the pandemic in 2020, at 24% of the population infected and 84 620 deaths, is within the range of an earlier prediction of 22% (95% CI 16–25) of the population infected and 150 078 (82 735–189 579) deaths.31 This similitude suggests that capacity exists for making accurate predictions, and is particularly strong when local information is used to build the models—as compared with extrapolation of data from other locations.
With the deaths, however, the reported numbers are closer to the true burden, which is a reflection of the relative difficulty in missing mortality statistics compared with missing infections, many of which present with non-troubling symptoms. The estimated deaths represent an infection fatality rate of 0·09% (0·03% for 2020 and 0·16% for 2021), which is comparable to the value of 0·09% predicted for locations with COVID-19 population mortality rates less than the global average (<118 deaths per million).40
To put the mortality estimates in context across the region, the 47 countries we included had an estimated combined crude death count of 7 000 000 in 2019,41 implying that the estimated COVID-19 deaths in 2020 and 2021 represent increases of 1·2% and 5·0% in mortality, respectively, when assuming the same levels of mortality for other causes. When using the 2019 cause of death statistical estimates, COVID-19 is the 22nd major cause of death in 2020 in the region, just below hypertensive heart disease (86 387 deaths); using 2021 statistics, COVID-19 would be the seventh major cause of death, below malaria (357 238 deaths). The four-times increase in COVID-19-related deaths in 2021 is in line with anecdotal information from countries suggesting many more such deaths in 2021 than in 2020. This increase in the number of deaths from the same numbers of infections aligns with the introduction of the more transmissible delta variant of SARS-CoV-2, suggesting delta was more infectious to vulnerable individuals or caused more severe disease for everyone (or both).
The high numbers of individuals with SARS-CoV-2 immunity compared with the numbers who have received vaccines reflects the delay in initiating vaccination in the African region. The effects of this delay are difficult to decipher. The current evidence is mixed, regarding the strength and duration of the antibody response from vaccination versus natural infection, with reputable (yet conflicting) evidence for both natural immunity and vaccination being stronger.42 Irrespective of the source of immunity, the potential severity of subsequent SARS-CoV-2 infections is presumed to be reduced in those with immunity.42 However, less than 50% of the region's population have some immunity against SARS-CoV-2, highlighting the continued risk in the region and the need to scale-up efforts to boost overall population immunity through vaccination.
The variations seen in mortality by location and income level are equally unique. Variations by income categorisations could be attributed to the increased prevalence of comorbidities (particularly hypertension, diabetes, and obesity) and ageing populations associated with increases in income level.5 The variations by location require further research to decipher, particularly the highest burdens in the South African Development Community—which might have been magnified by the substantial burden in South Africa—and the lowest burden indicated in the East African Community, Economic Community of Central African States, and Inter-Governmental Authority for Development.
Finally, our predictions suggest that 2022 will have 73% of the number of SARS-CoV-2 infections seen in in 2021, but with 6·4% of the deaths. However, should there be a new variant with significant immune escape, this pattern could change. An increasing proportion of infections will be among individuals with previous exposure to SARS-CoV-2, among whom the disease severity is likely to be decreased.42 However, this picture of a persisting high number of infections, but with fewer deaths, calls for some policy changes that emphasise surveillance to identify and target individuals most at risk of developing severe disease.
The approach taken in this modelling study represents a novel way to generate knowledge when primary data are scarce; however, there are several limitations. First, the latent disease process in the community was modelled using partial observations: reported infections and deaths. Partial observations are a consequence of either measurement noise, entirely unmeasured latent variables, or both. A key limitation of this analysis is, therefore, the inability of the model to estimate the measurement noise levels and the reporting rates (as influenced by testing capacities), which widely varied across the included countries. Without this information, we are unaware of whether the latent process is consistent with the observed data. The effect of this limitation was mitigated by optimally determining contact rates, specific for each of the countries from the data. The timing, duration, and depth of the pandemic waves were also determined from primary data reported by countries, and these data were often batch reported—thus affecting the stochasticity of the data in some cases.
Second, the model also assumed homogeneous mixing of the population and that the future evolution of the process depended only on the current state, together with future randomness. Each individual is assumed to have an equal chance of infection. We recognise the possibility of interactions among variants—as well as waning immunity by variants of concern and vaccine types—but this information was not available to input into the model. We also acknowledge the presence of a time lag between the introduction and identification of variants of concern due to differing capacities for genomic sequencing and surveillance among countries. For model simplification, the type of vaccine administered was not considered.
Third, although seroprevalence studies are considered the gold standard for understanding the spread of COVID-19, we recognise the specific challenges of using data from seroprevalence studies conducted in Africa, which are often based on specific population subgroups (eg, blood donors, urban populations, or adults). Appropriate seroprevalence studies would be fully representative of the population at national level; however, such studies are scarce in the region, given their resource intensive requirements. We used the few representative studies available, as well as some of the studies that were not fully representative, as the closest empirical information that existed.
Finally, the quality of the information from the model is dependent on the quality of the primary data. Not all countries were reporting COVID-19 statistics at the same frequency and quality. The statistical approach to have a country-specific contact rate helped to minimise this bias, although we recognise it cannot be entirely eradicated.
In summary, the findings of our model suggest that the WHO African region is estimated to have had a similar number of COVID-19 infections to the rest of the world, but with fewer deaths. Current methods of determining impact—specifically case surveillance and seroprevalence studies—are not able to provide comprehensive information on the situation. Models can complement the information available, provided they are based on locally available information from countries. Moving forward, there is a need to strengthen the focus on identification and targeting of interventions for those populations most at risk. Enhanced surveillance— particularly for comorbidities, community surveillance for the emergence of new variants of concern, and for changes in seroprevalence and in-hospital surveillance to monitor suspected cases of COVID-19—should form the basis of the response in the region.
Data sharing
All data used in this study are available in the public domain and were accessed by the authors from the publicly available datasets. The codes used in the model framework and the data analysis have been made available on a GitHub repository.
Declaration of interests
We declare no competing interests.
Acknowledgments
Acknowledgments
The authors are from the Regional Office for Africa of the World Health Organization. We thank the Member States of the Africa region and the WHO Country Offices for sharing information and providing the enabling environment needed to interpret and derive policy-relevant recommendations for their action.
Contributors
This model conceptualisation and analysis is a result of a multidisciplinary team. JWC, LM, and MRM conceptualised the study. HCK, HKK, RT-O, JAA, JKM, and BD designed the methodology. T-O, SNK, HKK, JM, and JAA collected and curated data. JKM, JAA, and HKK managed the software used. BD, HCK, JAA, JWC, TB, ASG, and LM analysed the data. TB, ASG, and HCK accessed and validated the underlying data. JWC, ASG, and LM supervised the study. JM, HKK, RT-O, ABWS, SNK, and JAA led the data visualisation. HCK, RT-O, HKK, BD, and SNK wrote the original draft of the manuscript. All authors reviewed and edited the final draft.
Supplementary Material
References
- 1.WHO WHO coronavirus (COVID-19) dashboard. https://covid19.who.int
- 2.Kong JD, Tekwa EW, Gignoux-Wolfsohn SA. Social, economic, and environmental factors influencing the basic reproduction number of COVID-19 across countries. PLoS One. 2021;16 doi: 10.1371/journal.pone.0252373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Gesesew HA, Koye DN, Fetene DM, et al. Risk factors for COVID-19 infection, disease severity and related deaths in Africa: a systematic review. BMJ Open. 2021;11 doi: 10.1136/bmjopen-2020-044618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Tsori Y, Granek R. Epidemiological model for the inhomogeneous spatial spreading of COVID-19 and other diseases. PLoS One. 2021;16 doi: 10.1371/journal.pone.0246056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Zhang F, Karamagi H, Nsenga N, et al. Predictors of COVID-19 epidemics in countries of the World Health Organization African region. Nat Med. 2021;27:2041–2047. doi: 10.1038/s41591-021-01491-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Nachega JB, Kapata N, Sam-Agudu NA, et al. Minimizing the impact of the triple burden of COVID-19, tuberculosis and HIV on health services in sub-Saharan Africa. Int J Infect Dis. 2021;113(suppl 1):S16–S21. doi: 10.1016/j.ijid.2021.03.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bigna JJ, Noubiap JJ. The rising burden of non-communicable diseases in sub-Saharan Africa. Lancet Glob Health. 2019;7:e1295–e1296. doi: 10.1016/S2214-109X(19)30370-5. [DOI] [PubMed] [Google Scholar]
- 8.Adeloye D, Basquill C, Papana A, Chan KY, Rudan I, Campbell H. An estimate of the prevalence of COPD in Africa: a systematic analysis. COPD. 2015;12:71–81. doi: 10.3109/15412555.2014.908834. [DOI] [PubMed] [Google Scholar]
- 9.Adeloye D. An estimate of the incidence and prevalence of stroke in Africa: a systematic review and meta-analysis. PLoS One. 2014;9 doi: 10.1371/journal.pone.0100724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.WHO Third round of the global pulse survey on continuity of essential health services during the COVID-19 pandemic. Feb 7, 2022. https://www.who.int/publications/i/item/WHO-2019-nCoV-EHS_continuity-survey-2022.1
- 11.Aborode AT, Hasan MM, Jain S, et al. Impact of poor disease surveillance system on COVID-19 response in Africa: time to rethink and rebuilt. Clin Epidemiol Glob Health. 2021;12 doi: 10.1016/j.cegh.2021.100841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Tenforde MW, Self WH, Adams K, et al. Association between mRNA vaccination and COVID-19 hospitalization and disease severity. JAMA. 2021;326:2043–2054. doi: 10.1001/jama.2021.19499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bahl A, Johnson S, Maine G, et al. Vaccination reduces need for emergency care in breakthrough COVID-19 infections: a multicenter cohort study. Lancet Reg Health Am. 2021;4 doi: 10.1016/j.lana.2021.100065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lauring AS, Hodcroft EB. Genetic variants of SARS-CoV-2—what do they mean? JAMA. 2021;325:529–531. doi: 10.1001/jama.2020.27124. [DOI] [PubMed] [Google Scholar]
- 15.Hu J, Peng P, Cao X, et al. Increased immune escape of the new SARS-CoV-2 variant of concern omicron. Cell Mol Immunol. 2022;19:293–295. doi: 10.1038/s41423-021-00836-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Li R, Pei S, Chen B, et al. Substantial undocumented infection facilitates the rapid dissemination of novel coronavirus (SARS-CoV2) Science. 2020;368:489–493. doi: 10.1126/science.abb3221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Usuf E, Roca A. Seroprevalence surveys in sub-Saharan Africa: what do they tell us? Lancet Glob Health. 2021;9:e724–e725. doi: 10.1016/S2214-109X(21)00092-9. [DOI] [PubMed] [Google Scholar]
- 18.Arora RK, Joseph A, Van Wyk J, et al. SeroTracker: a global SARS-CoV-2 seroprevalence dashboard. Lancet Infect Dis. 2021;21:e75–e76. doi: 10.1016/S1473-3099(20)30631-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Uyoga S, Adetifa IMO, Otiende M, et al. Prevalence of SARS-CoV-2 antibodies from a national serosurveillance of Kenyan blood donors, January-March 2021. JAMA. 2021;326:1436–1438. doi: 10.1001/jama.2021.15265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Barrie MB, Lakoh S, Kelly JD, et al. SARS-CoV-2 antibody prevalence in Sierra Leone, March 2021: a cross-sectional, nationally representative, age-stratified serosurvey. BMJ Glob Health. 2021;6 doi: 10.1136/bmjgh-2021-007271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Murray J. Forecasting COVID-19 impact on hospital bed-days, ICU-days, ventilator days and deaths by US state in the next 4 months. medRxiv. 2020 doi: 10.1101/2020.03.27.20043752. published online March 30. [DOI] [Google Scholar]
- 22.Wang H, Paulson KR, Pease SA, et al. Estimating excess mortality due to the COVID-19 pandemic: a systematic analysis of COVID-19-related mortality, 2020–21. Lancet. 2022;399:1513–1536. doi: 10.1016/S0140-6736(21)02796-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.King AA, Nguyen D, Ionides EL. Statistical inference for partially observed Markov processes via the R package pomp. arXiv. 2015 doi: 10.48550/arXiv.1509.00503. published online Sept 1. [DOI] [Google Scholar]
- 24.Snell LB, Wang W, Alcolea-Medina A, et al. Descriptive comparison of admission characteristics between pandemic waves and multivariable analysis of the association of the alpha variant (B.1.1.7 lineage) of SARS-CoV-2 with disease severity in inner London. BMJ Open. 2022;12 doi: 10.1136/bmjopen-2021-055474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Cheng L, Song S, Fan Q, et al. Cross-neutralization of SARS-CoV-2 Kappa and Delta variants by inactivated vaccine-elicited serum and monoclonal antibodies. Cell Discov. 2021;7:112. doi: 10.1038/s41421-021-00347-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Akkiz H. Implications of the novel mutations in the SARS-CoV-2 genome for transmission, disease severity, and the vaccine development. Front Med (Lausanne) 2021;8 doi: 10.3389/fmed.2021.636532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Jara A, Undurraga EA, González C, et al. Effectiveness of an inactivated SARS-CoV-2 vaccine in Chile. N Engl J Med. 2021;385:875–884. doi: 10.1056/NEJMoa2107715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Levine-Tiefenbrun M, Yelin I, Katz R, et al. Initial report of decreased SARS-CoV-2 viral load after inoculation with the BNT162b2 vaccine. Nat Med. 2021;27:790–792. doi: 10.1038/s41591-021-01316-7. [DOI] [PubMed] [Google Scholar]
- 29.Marfe G, Perna S, Shukla AK. Effectiveness of COVID-19 vaccines and their challenges (Review) Exp Ther Med. 2021;22 doi: 10.3892/etm.2021.10843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Tregoning JS, Flight KE, Higham SL, Wang Z, Pierce BF. Progress of the COVID-19 vaccine effort: viruses, vaccines and variants versus efficacy, effectiveness and escape. Nat Rev Immunol. 2021;21:626–636. doi: 10.1038/s41577-021-00592-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Vasireddy D, Vanaparthy R, Mohan G, Malayala SV, Atluri P. Review of COVID-19 variants and COVID-19 vaccine efficacy: what the clinician should know? J Clin Med Res. 2021;13:317–325. doi: 10.14740/jocmr4518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kojima N, Klausner JD. Protective immunity after recovery from SARS-CoV-2 infection. Lancet Infect Dis. 2022;22:12–14. doi: 10.1016/S1473-3099(21)00676-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Sun P, Lu X, Xu C, Sun W, Pan B. Understanding of COVID-19 based on current evidence. J Med Virol. 2020;92:548–551. doi: 10.1002/jmv.25722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Cabore JW, Karamagi HC, Kipruto H, et al. The potential effects of widespread community transmission of SARS-CoV-2 infection in the World Health Organization African region: a predictive model. BMJ Glob Health. 2020;5 doi: 10.1136/bmjgh-2020-002647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.WHO SCORE for Health Data Technical Package. Global report on health data systems and capacity. Jan 31, 2021. https://www.who.int/publications/i/item/9789240018709
- 36.Pullano G, Di Domenico L, Sabbatini CE, et al. Underdetection of cases of COVID-19 in France threatens epidemic control. Nature. 2021;590:134–139. doi: 10.1038/s41586-020-03095-6. [DOI] [PubMed] [Google Scholar]
- 37.Lee C, Apio C, Park T. Estimation of undetected asymptomatic COVID-19 cases in South Korea using a probabilistic model. Int J Environ Res Public Health. 2021;18 doi: 10.3390/ijerph18094946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Mahajan A, Solanki R, Sivadas N. Estimation of undetected symptomatic and asymptomatic cases of COVID-19 infection and prediction of its spread in the USA. J Med Virol. 2021;93:3202–3210. doi: 10.1002/jmv.26897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Karamagi HC, Tumusiime P, Titi-Ofei R, et al. Towards universal health coverage in the WHO African region: assessing health system functionality, incorporating lessons from COVID-19. BMJ Glob Health. 2021;6 doi: 10.1136/bmjgh-2020-004618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Ioannidis JPA. Infection fatality rate of COVID-19 inferred from seroprevalence data. Bull World Health Organ. 2021;99:19–33F. doi: 10.2471/BLT.20.265892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.WHO World Health Statistics 2021. Monitoring health for the SDGs, sustainable development goals. May 20, 2021. https://www.who.int/publications/i/item/9789240027053
- 42.Abu-Raddad LJ, Chemaitelly H, Bertollini R. Severity of SARS-CoV-2 reinfections as compared with primary infections. N Engl J Med. 2021;385:2487–2489. doi: 10.1056/NEJMc2108120. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
All data used in this study are available in the public domain and were accessed by the authors from the publicly available datasets. The codes used in the model framework and the data analysis have been made available on a GitHub repository.