Abstract
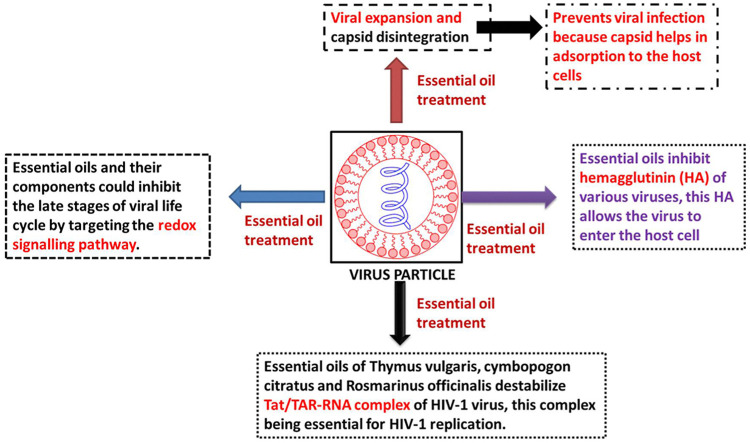
Essential oils and their chemical constituents have been reported with well documented antimicrobial effects against a range of bacterial, fungal and viral pathogens. By definition, essential oils are a complex mixture of volatile organic compounds which are synthesized naturally in different parts of the plant as part of plants secondary metabolism. The chemical composition of the essential oils is dominated by the presence of a range of compounds including phenolics, terpenoids, aldehydes, ketones, ethers, epoxides and many others inferring that essential oils must be effective against a wide range of pathogens. This review article mainly focuses on the antiviral potential of essential oils and their chemical constituents especially against influenza and coronaviruses. Essential oils have been screened against several pathogenic viruses, including influenza and other respiratory viral infections. The essential oils of cinnamon, bergamot, lemongrass, thyme, lavender have been reported to exert potent antiviral effects against influenza type A virus. The essential oil of Citrus reshni leaves has been shown to be effective against H5N1 virus. The essential oil of Lippia species at a concentration of 11.1 μg/mL has been shown to induce 100% inhibition of yellow fever virus in Vero cells. Essential oils and oleoresins have been shown through in vitro and in vivo experiments to induce antiviral effects against Coronavirus infectious bronchitis virus. A study reported 221 phytochemical compounds and essential oils to be effective against severe acute respiratory syndrome associated coronavirus (SARS-CoV) using a cell-based assay measuring SARS-CoV-induced cytopathogenic effect on Vero E6 cells. The main mechanism of antiviral effects of essential oils has been found to cause capsid disintegration and viral expansion which prevents the virus to infect host cells by adsorption via the capsid. Essential oils also inhibit hemagglutinin (an important membrane protein of various viruses) of certain viruses; this membrane protein allows the virus to enter the host cell. Many essential oils and their components could inhibit the late stages of viral life cycle by targeting the redox signalling pathway. Essential oils of Thymus vulgaris, cymbopogon citratus and Rosmarinus officinalis have been found to destabilize the Tat/TAR-RNA complex of HIV-1 virus, this complex being essential for HIV-1 replication. Being lipophilic in nature, essential oils can penetrate viral membranes easily leading to membrane disintegration. The current comprehensive review will facilitate researchers to find chemical entities from plant sources as possible inhibitory agents against various viruses.
Keywords: Essential oils, Antiviral, Terpenes, Severe acute respiratory syndrome, Influenza
Graphical abstract
1. Introduction
The consumption of plant and plant-extracted natural products for cosmetic, religious and medicinal uses has an existence dating back to the rise of human civilizations [1]. Plants and their derived natural products show enormous chemical compositions which serve as leads in the field of drug development, design and research [2]. Medicinal plants bear bio-nutrients and phytochemicals in high concentrations and show remarkable bioactivities. Phytochemicals have been identified with enormous potential to act against a huge number of human ailments including chronic diseases like coronary heart disease, diabetes and cancer [3]. The role of these phytochemicals has been highly investigated in plants and it is well established that these chemicals are generated in the demand of self-protection. Besides, phytochemicals give plants a protective cover against damages and diseases and also contribute towards plant flavor, aroma and color [4]. The medicinal value of aromatic plants is generally credited to the presence of essential oils. These essential oils consist of a huge number of plant secondary metabolites. The use of essential oils has been predominantly linked to perfumes, cosmetics and food flavoring due to the presence of high aroma. Besides this, continuous researches have demonstrated immense potential of essential oils and their constituent chemical species in the management, protection and cure several human diseases [5,6]. Since last 3 or 4 decades, the uses of essential oils have gained strong attention in aromatherapy and phytomedicine. Therefore, essential oils have been widely explored by researchers in basic research, especially their antioxidant, antimicrobial and antitumor activities. Essential oils generally comprise of diversity of chemical mixtures which bears tens to hundreds of varieties of molecules, mostly the mixture of benzene derivatives and terpenes. These terpenes and benzene derivatives contribute to the rich bioactivity of essential oils [7,8]. Essential oils and terpenes look similar at first glance as their bear similarity of being from plant origin and both are aromatic. Many people believed that they both serve similar purposes. Similarities between essential oils and terpenes have created confusion of weather they are same or different, but these two are different [9].
The demand for aromatic and medicinal plants is growing continuously due to enhancement in consumers and interest in their medicinal, culinary and other anthropogenic applications. Consumers globally are gaining more information regarding food, nutrition and health issues, alongside they are too becoming aware of health benefits of using aromatic, medicinal plants and plant metabolites [10]. Essential oils are among the secondary metabolites generated by the plants. The use of essential oils has remained mostly confined to the perfumery and cosmetic domains besides bear rich chemical composition. Essential oils are worthy of getting better understanding into their biological characteristics and rich chemistry. More attention is needed to explore individual components or different extracts of essential oils for applications in human health, environment and agriculture [11]. Essential oils could be explored as an operative substitutes or complements to synthetic compounds of the chemical industry, without inducing the identical secondary effects.
2. Essential oils, chemical composition and uses in different health care products
2.1. Definition
An abundance of practical definitions exists for the term volatile or essential oils. Essential oils are highly concentrated oily and hydrophobic volatile liquids generated by different plant materials including roots, fruits, wood, herbs, bark, twigs, leaves, seeds, buds, rhizomes, peels, flowers and entire plant belonging to a distinct botanic species [[12], [13], [14]]. Strong odor is one of the prominent characteristic features of essential oils. Moreover, they display almost similar chemical composition and biological activities when extracted from a single botanical species growing under similar climate, edaphic conditions and common harvest season [15]. The presence of plant essence in these oils earns them the name “Essential Oils”. Mostly essentials oils are plant derived but few of them are generated by microorganisms and animals too [16]. Fungi, seaweeds, liverworts and mosses have also been recognized of producing essential oils [17]. They are soluble in weakly polar and nonpolar organic, low in density than water (excluding exceptions) solvents, rarely colored and limpid [18]. Essential oils are mostly colorless in appearance when fresh, but some may be green (bergamot, Citrus bergamia), orange (Sweet orange, Citrus sinensis), blue (chamomile, Matricaria chamomilla) and pale yellow (yellow mandarin) [19]. These oils undergo easy oxidation with age by exposure to heat light or air, which causes color darkening in essential oils [20]. Therefore, to avoid such oxidation, essential oils needs to be stored in a cool and dark place and most suitably with the amber glass containers. Plant terpenes and essential oils are differentiated on the bases of chemical constituents as essentials oils accommodate both terpenes and other natural class of compounds as well.
2.2. Distribution and uses of essential oils
Several factors are responsible in deciding the quality and quantity of essential oils in different plant materials like plant organ, chemotypes, methods of preparation, stage of plant harvest, age, soil type and texture and climate. There are numerous plant derived essential oils used in one and the other way in different fields, approximately 3000 essential oils from about 2000 plants [20,22]. Quite often all the plants have the tendency to generate essential oils but few produce in traces and few in healthy amounts. The plants producing essential oil which is of commercial importance are known as essential oil plants [23]. Essential oils generally are present in higher plants with around 17,500 known species but they are present in healthy amounts in some specific plant families including Zingiberaceae, Poaceae, Apiaceae, Umbelliferae, Asteraceae, Lamiaceae, Rutaceae, Compositae, Lauraceae, Cupressaceae, Acoraceae, Rosaceae, Oleaceae, Myristricaceae, Myrtaceae, etc. [[24], [25], [26], [27]]. Mostly, the bioactivities of these essential oils remain obscure. Essential oils are currently a subject of high importance in research and several industries as well due to their remarkable potential of pharmacological activities and tendency of acting as natural preservatives [28]. The ecological role of essential oils have been extensively studied and established. The well-known are plant-animal interactions for repelling and protection against predators, plant interactions like germination inhibition and allelopathic agents and attraction of insects which assist in pollination [29]. Industries always look for antimicrobial safety in cosmetics because the microbial degradation can pose threat to customer's health. Essential oils have strong antimicrobial property and hence can serve as natural ingredients in cosmetics for microbial protection. Drugs bearing essential oils and essential oils are of remarkable importance in agriculture as insecticides, food technology as flavor for drinks, food, spices and preservatives, perfumery as perfume, pharmacy as healer and in aromatherapy [30]. The importance and vitality of essential oils is being highly valued in plant chemotaxonomy [31,32].
2.3. Composition of essentials oils
EOs are in general a very complex mixture (60–300) of nonpolar and semipolar lipophilic components of low molecular weight, at different concentrations with two or three appearing to be major ones: terpenoids, straight-chain compounds not containing any side chain, aromatic and phenolic components and Sulfured derivatives [33,34]. The difference in taste and odor of essential oils highly depends on the plant type, season of harvesting, geographical location, methods of drying and the techniques involved in extraction [[35], [36], [37]]. The primary volatile constituents present in essential oils are classified into terpenoids and polypropanoids [[38], [39], [40]]. Here we will consider terpenoids only.
2.4. Terpenes and terpenoids
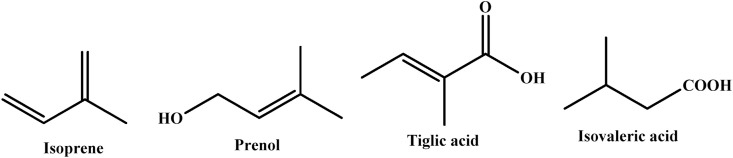
Terpenes are the plant secondary metabolites having carbon backbone of isoprene (2-methylbuta-1,3-diene) units in their structure [41]. Mevalonic acid pathway in cytoplasm of plant cells results in the formation of terpenes. Terpenoids are generated by different biochemical modifications like rearrangement and oxidation of terpenes. These biochemical modifications form terpenoids of oxygenated derivatives of terpenes like acids, alcohols, ketones, aldehydes, esters and ethers. Terpenoids are the largest class of phytochemicals comprising of nearly 40,000 different compounds of primary and secondary metabolism have been identified [41]. Every year new members get added to this class of natural products. Terpenoids can generally be classified into four different groups including true terpenes, saponins, steroids and cardiac glycosides. These four groups of compounds are present in almost every living system, primarily in plant derived essential oils. Therefore, these are considered as largest and structurally more diverse group of plant derived natural products [42]. Hemiterpenoids, monoterpenoids and sesquiterpenoids are the only sufficient volatile constituents of essential oils. As widely acknowledged, the composition of EOs is mainly represented by mono-, sesqui-, and even diterpene hydrocarbons and their respective oxygenated derivatives [[43], [44], [45]]. Essential oils bear structurally low molecular weight constituents making them highly volatile. The major constituents of essential oils are terpenes [46]. These terpenes are formed by the systematic assembly of isoprene units (5 carbon base units). Heat to tail condensation of these units gives rise to huge number terpenes. The structural and chemical diversity of terpenes are a result of repetition, cyclization, and rearrangements of these isoprene units. Main constituents of essential oils include monoterpenes with C10 skeleton, sesquiterpenes with C15 skeleton, diterpenes with C20 skeleton and triterpenes with C30 skeleton. Higher terpenes and their oxygenated derivatives are present in very low concentrations in essential oils [47].
2.5. Hemiterpenes
Hemiterpenes are the minor constituents of terpene content of essential oils. These generally are the 2-methylbutane skeleton bearing ester, aldehyde and alcoholic groups [48]. The number of hemiterpenes in essential oils is very low (approx. Less than 100) [49]. Recently, the leaves of Prinsepia utilis have been recognized with chlorinated hemiterpenes (Fig. 1 ) [50].
Fig. 1.
Some of the hemiterpenes constituents of essential oils.
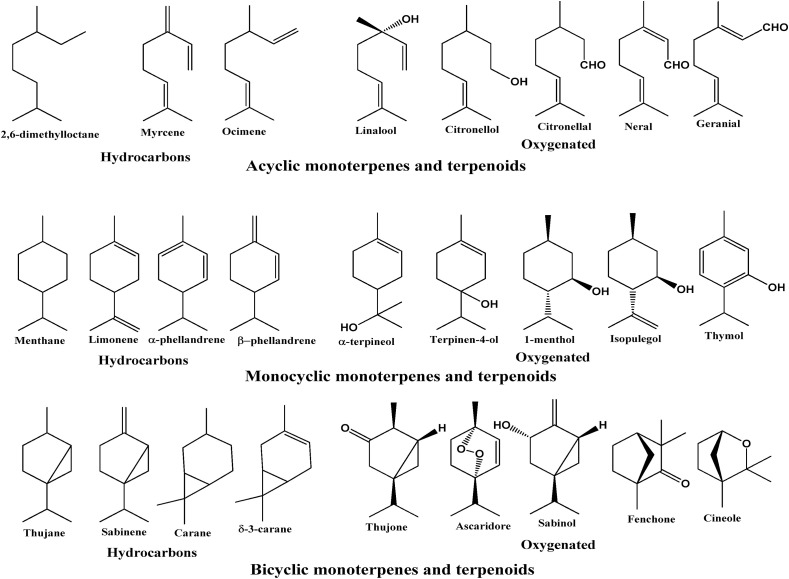
2.6. Monoterpenes
The linkage of head to tail combination of two isoprene units gives rise to regular monoterpenes (C10). Monoterpenes are the low molecular weight molecules present in higher concentrations (>90%) in some of the essential oils. Therefore, these molecules contribute in forming specific smell by a plant [51,52]. Monoterpenes bear a double bond in their structural moiety and are almost present in all plant essential oils. These monoterpenes are generally involved in the plant to plant interaction and plant to animal interactions like fruit dissemination, seed dissemination pollination and allelopathic agents. There are more than 30 basic skeletons for monoterpenes and hence can be subdivided into three subgroups: acyclic, monocyclic and bicyclic. Different monoterpenes both true and oxygenated terpenes have been shown in Fig. 2 .
Fig. 2.
Some of the monoterpene and monoterpenoid constituents of essential oils.
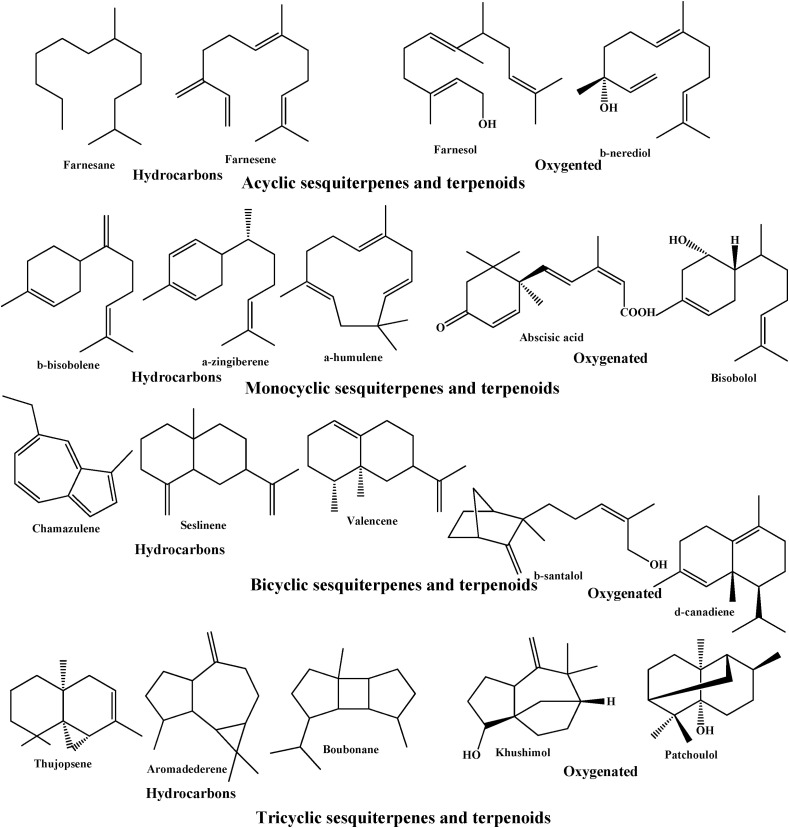
2.7. Sesquiterpenes
Sesquiterpenes are another major class of major constituent terpenes present in essential oils and are less in volatility than monoterpenes. These are formed by combination of three isoprene units which makes them more versatile in structural diversity. Sesquiterpenes exist in different forms as generated by the combination of these three isoprene units like linear, mono-, bi-, and tri-cyclic. These are the most diverse group of terpenoids found in plants and essential oils (Fig. 3 ).
Fig. 3.
Some of the sesquiterpenes and sesquiterpenoid constituents of essential oils.
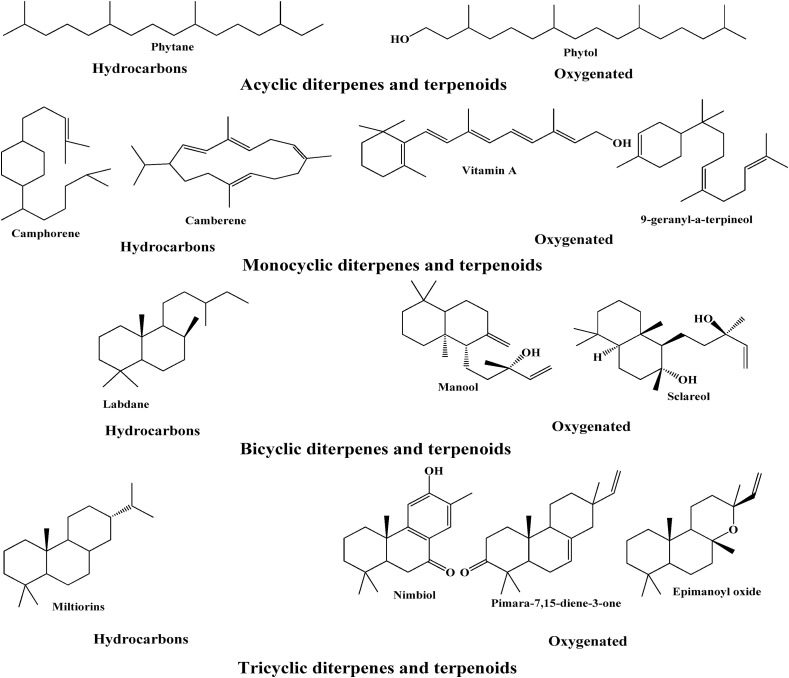
2.8. Diterpenes
Diterpenes are more complex chemically and structurally mostly found associated with plant resin and few times encountered as by-products during essential oil extraction. These are of high molecular weight due to presence of C20 skeleton which makes them less volatile and less abundant than mono- and Sesquiterpenes. As a result of high molecular weight these diterpenes are difficult to isolate in essential oils through distillation process. If diterpenes are present in essential oils, they usually are in miniscule amounts. However, the traditional distillation process assists in identification and separation of these diterpenes from essential oils [53].
Generally, molecules with molecular masses greater than 300 uma can be seen as symbol of inadequate extraction settings or adulteration. Diterpenes that are typically found in essential oils include camphorene, cafestol, kahweol, cambrene, and taxideme (Fig. 4 ).
Fig. 4.
Some of the diterpene and diterpenoid constituents of essential oils.
2.9. Biological activity of essential oils
The pharmacological applications of essential oils have been extensively evaluated in the view to search for possible alternative medicine. Since past few decades essential oils have been searched upon extensively for their possible role in an array of biological systems [54]. Essential oils present remarkable potency of having wide range of bioactivities.
2.10. Antioxidant activity
Antioxidant activity of essential oils is one of their most intensively investigated properties. Oxidation of many biological substances causes cellular damage and may metabolic diseases to name a few: Alzheimer's disease, Parkinson's disease, inflammation, arthritis, diabetes and cancer [[55], [56], [57], [58], [59]]. Essential oils are well known for their rich antioxidants that can be investigated to inhibit oxidative damage [60]. The antioxidants species are those which tend to delay the oxidation of substrate or inhibit it [61]. The volatile components present in essential oils not only produce protective cover against oxidative stress but also acts as prooxidants, by targeting cellular redox potential and inducing damage to intracellular biomolecules. As prooxidants, essential oils may cause damage to DNA and cellular proteins. Besides antioxidant potential of essential oils, prooxidant activity must also be considered when it comes to antioxidant studies of essential oils.
The phenolic content of essential oils has been highly regarded for their antioxidant potency. Recent studies have also reported that the volatile constituents of essential oils can also contribute to antioxidant property of essential oils as individually or in mixture. The essential oil extracted from Melissa officinalis L. (lemon balm) has been reported of higher antioxidant capacity than that of BHT. The GC-MS analysis of this oil has showed the primary constituents were geranial, neral and citronellal with percentage yields of 23.4%, 16.5%, 13.7%, respectively [62].
2.11. Anticancer activity
Cancer is a leading global health threat with 18.1 million patients being diagnosed each year. This disease is highly lethal and was accounted for a mortality of 9.6 million lives alone in the year of 2018. Out of these deaths, 70% of deaths belonged to low- and meddle-income countries [63]. The currently available chemotherapeutic drugs for cancer treatment include Taxol, Camptothecin, Vincristine and Vinblastine [64]. There are several studies that have reported the anticancer activity of essential oils. An approximate estimation of 500 research papers has been published on anticancer activity of essential oils [[65], [66], [67], [68]]. Although, there are no clear cut studies that have reported that aromatherapy could prevent or treat cancer. Higher number of studies has reported anticancer activities of essential oils against different cancer cell line in vitro using petri dishes. Essential oils have been reported with strong anti-inflammatory potential which leads to the fact that these could also have anticancer effects as there is a direct relationship between reactive oxygen species at the origination of oxidation and inflammation. From the past 10 years there has been an increased interest of researchers in the anticancer activity of essential oils which lead to investigation of more than 100 essential oils belonging to more than 20 families against 20 distinct types of cancers [69]. Bourgou and collaborators shown that essential oil derived from seeds of Nigella sativa L. (black cumin) inhibits the growth of A-549 and DLD-1 cancer cell lines with IC50 values of 43.0 and 46.0 μg/mL, respectively [70]. Wang and co-workers have reported the toxicological potential of essential oil from Rosamarinus officinalis L. and its constituents including 1,8-ceneole, α-pinene and β-pinene. The investigation was carried out on three different human cancer cell lines including SK-OV-3, HO-8910 and Bel-7402, the essential oil showed IC50 values of 0.025%).076% and 0.13% (v/v), respectively [71].
2.12. Antimicrobial activity
Essential oils possess strong potential as antimicrobial agents; this property of theirs has been well established and reported by several research works. The antimicrobial action of essential oils not only depends of phytochemical constituents but also interactions among different components leading to synergistic or antagonistic activities. The factors deciding the antimicrobial actions of essentials oils include chemical content, concentration, interactions among primary active components and the vulnerability of microorganisms [72,73]. Their inactive constituents may influence the rate of reaction, resorption along with biological effects of active constituents. Therefore, the presence of both minor and major constituents of essential oils may lead to modification of activity by posing synergistic or antagonistic effects [74,75]. The essential oils derived from thyme, oregano and cinnamon have been reported of strong antibacterial activities against Pseudomonas fluorescens, Listeria monocytogenes, Bacillus thermosphacta and Escherichia coli [76]. The increasing order of some of the essential oils in terms of antimicrobial activities is reported as follows: sage (Salvia officinalis) < mustard (Sinapis alba) < rosemary (Salvia rosmarinus) < mint (Mentha) < thyme (Thymus vulgaris) < cinnamon (Cinnamomum cassia) < coriander (Coriandrum sativum) < clove (Syzygium aromaticum) < oregano (Origanum vulgare) [77]. The resistance developed by infectious bacteria to antibiotics is most serious issue regarding human health, globally. The bacteria that result in posing huge clinical burdens includes Escherichia coli, Pseudomonas aeruginosa, Acinetobacter baumannii, Clostridium difficile, Enterococcus faecium, Staphylococcus aureus, Enterobacter species and Klebsiella [78]. Due to the existence of peptidoglycan layer outside the cellular membrane in gram-positive bacteria, essential oils show maximum activity against them. The gram-negative bacteria bear a bilayer phospholipid membrane outside the inner membrane and are connected to the inner membrane with lipopolysaccharides [79]. There are several studies which have reported the bioactivity of essential oils against bacteria and fungi using distinct pathogen microorganisms [[80], [81], [82], [83]]. Some of the earlier studies have reported that the gram-negative bacterial strains of E. coli and P. aeruginosa are strongly targeted by the essential oils from fruits of Piper guineense and roots of P. caldense [84,85]. An in vitro study carried out by Thanissery et al. showed inhibition of Campylobacter and Salmonella enterica by the essential oils of clove, rosemary and mixed thyme-orange oils [86]. Thyme oil revealed maximum inhibition against Salmonella in comparison to other two essential oils with zones of inhibition (ZI) of 18.5 mm. The essential oils from oregano, thyme, rosemary, clove, pimento and cinnamon has been recognized of antibacterial activities against Pseudomonas aeruginosa, Staphylococcus aureus and Salmonella typhi [87]. Clove oil was most active among all these tested essential oils. The antimicrobial activity of these essential oils was attributed to their rich chemical content including p-cymene, eugenol, cinnamic aldehyde, thymol and carvacrol. The essential oils derived from medicinal and aromatic plants are rich in eugenol, carvacrol and thymol and all of these show potential inhibition of food-borne pathogens including Vibrio vulnificus, Listeria monocytogenes, Salmonella typhimurium, and Escherichia coli [88]. An in vitro analysis carried out by Sutili et al. showed strong inhibition effects of essential oils derived from Hesperozygis ringens, Ocimum gratissimum and Ocimum americanum against Aeromonas hydrophila [89]. Escherichia coli (E. coli) and Staphylococcus aureus (S. aureus) are two opportunistic pathogens that cause severe and life-threatening infections in immunocompromised patients. The essential oil derived from Eucalyptus globulus have been reported of inducing strong growth inhibiting effects on Gram-positive (E. coli) and Gram-negative bacteria (S. aureus) [90]. Further, essential oils from oregano and thyme have been reported to inhibit the growth of moulds including Aspergillus niger and A. flaous [91]. Clove essential oils has been reported of remarkable inhibition against Botrytis cinerea (gray mold) [92].
2.13. Anti-inflammatory activity
Essential oils have been reported of strong anti-inflammatory potential. Most of them were firstly recognized and involved in the treatment of oxidative and inflammatory diseases. The most popular herb Cymbopogon citratus has been used as anti-inflammatory and analgesic agent. The underlying mechanism of action was found to be suppression of COX-2 expressions by this essential oil. Citral was the primary and major constituent of this essential oil and has been considered as active constituent for its COX-2 suppression and PPAR α and γ activation [93].
2.14. Miscellaneous activities
Several research papers have been published in the context of insect repellent activity of essential oils. The essential oils from Lavandula angustifolia (Miller), Hyptis suaveolens L. and Hyptis spicigera L. have been reported with strong repellent activity against Sitophilus zeamais adults [94]. The antileishmanial activity was evaluated against promastigotes and intracellular amastigotes, and cytotoxicity was performed with J774. G8, which were incubated with different concentrations of E. bracteolate. Promastigote forms showed E. bracteolate EO IC50 value of 7.945 μg/mL (24 h) [95]. Furthermore, essential oils have been shown to possess significant antinematocidal activity. A study carried out by Barbosa et al. has reported the antinematocidal activities of essential oils derived from Thymus caespititius, Thymbra capitata, Satureja montana, Origanum vulgare and Chamaespartium tridentatum against the Bursaphelenchus xylophilus (pinewood nematode) [96]. Essential oils derived from the leaves of Cymbopogon citratus (lemon grass) and Tithonia diversifolia (Mexican sunflower) were investigated for their antinematocidal activity against Caenorhabditis elegans, Panagrellus redivivus, Meloidogyne incognita and Bursaphelenchus xylophilus. Out of these nematodes, both the essential oils showed higher activity against M. incognita with IC50 values of 1.068 μg/mL and 0.747 μg/mL [97].
Several studies have shown that essential oils inhibit the growth of zoonotic Anisakis. In a similar study, essential oil derived from Origanum compactum was reported to induce 100% mortality in Anisakis simplex L3 larvae isolated from Micromesistius poutassou [98]. In an another study Origanum syriacum essential oil was reported to induce protective effects against the gastrointestinal parasite Anisakis simplex and mosquito vector Culex quinquefasciatus [99]. Gómez-Rincón and coworkers have shown that essential oils of Melaleuca alternifolia (tea tree) inhibit the growth of L3 larvae of Anisakis simplex [100].
2.15. Antiviral activity of essential oils and their chemical constituents
From literature survey, it has been well documented that essential oils possess strong antiviral action against several DNA and RNA viruses like herpes simplex virus type-1 and -2 (HSV-1 and HSV-2), poliovirus, influenza virusadeno type 3, dengue virus type-2, coxsackievirus B-1 and Junin virus [101]. Bioactive plant-based and pure natural products are a novel source of rich antiviral drugs due to the presence of genetically higher chemical diversities. Viral diseases are of great concern to human health, globally. Till date, only a hand full of drugs has been found active against several viruses, this creates a need for lead drugs or molecules that can produce strong antiviral actions. The essential oils from oregano and clove has been reported of remarkable antiviral property against a number of non-enveloped DNA and RNA viruses including adeno virus type-3, coxsackie virus B-1 and polio virus [102,103]. The essential oil from Melaleuca alternifolia has been found to produce remarkable antivirus activity against Tobacco Mosaic Virus (TMV) and also resulted in suppressing the lesion formation of Nicotiana glutinosa within 10 days of administration [104]. An in vitro study has shown that essential oil of tea, eucalyptus and thyme and their monoterpenes content has been found substantially active against HSV-1. These essential oils produced inhibition of HSV-1 by almost 96% and the monoterpenes showed about 80% inhibition against the virus [105]. The antiherpes potential of essential oils and their chemical constituents from plant source has been reported earlier [106]. The essential oils from thyme oil, manuka oil, Australian eucalyptus oil and tea tree oil have been already reported of antiherpes activities [[107], [108], [109]]. Several constituents of essential oils like phenylpropanes, sesquiterpenes and triterpenes, have been approved for their strong antiviral activity against rhinovirus and herpes viruses [[110], [111], [112], [113]]. There are very few researches which have reported the ability of monoterpenes to suppress the viral multiplication, they have not been systematically analyzed for their antiviral efficacy. Isoborneol has been reported as a potent antiviral agent against the HSV but there is a lack of research in the field of antiviral activities of terpenes, their role of inhibiting the viral multiplication cycle and modes of action [114]. Many in vitro studies have reported that essential oils retard the ability of HSV-1 to multiply under experimental conditions [115]. The different forms of primary viral infections caused by HSV-1 in human beings are mucocutaneous herpes infection, neo natal herpes, herpetic keratitis and herpetic encephalitis. The essential oils from Glechon spathulata, Artemisia arborescens and Glechon marifoia have been shown with potent antiviral activity against HSV-1 [116]. The essential oil from Melissa officinalis bears two major constituents citronellal and citral which could have the potential to inhibit the replication of HSV-2 [117]. Essential oils from the Eupatorium patens and Artemisia douglasiana have been found active against the dengue virus [118]. Further, the Lippia junelliana and Lippia turbinate essential oils showed strong antiviral activity against the Junin virus. The essential oil constituents of Pogostemoncablin have been found active against the H2N2 influenza-A virus [119]. The anti-avian influenza virus H5N1 activity was reported by the essential oils from leaves and fruits of Fortune llamargarita [120]. The Trachyspermum oil has been reported of producing remarkable antiviral potential against the Japanese encephalitis virus (JEV). A study by Zeedan et al. has shown the anti-ORF virus (aparapox virus) activity of the essential oil from Achillea fragrantissima [121]. Recently, an in vitro study was carried out by Pour ghan bari et al., evaluating the antiviral potency of lemon balm (M. officinalis) essential oil and noseltamivir on H9N2 avian influenza virus and also analyzed their synergistic efficacy against the virus [122]. They have reported that the lemon balm essential oil at various concentrations retarded the process of replication in H9N2 virus. However, these replication inhibition effects were enhanced on co-administration with seltamivir. The essential oils derived from Colombian MAPs like Ocimum campechianum, Hyptismutabilis, Lepechinia vulcanicola, Minthostachys mollis and Lepechinia salviifolia, have been shown with great efficacy against the herpes viruses type-1 and -2 [123]. These essential oils were reported to inhibit the viral infections at early stages. Therefore, plant derived essential oils have a great tendency to be developed as antiviral agents against different viral diseases in human and could be alternatives to replace synthetic antiviral drugs. Cermelli et al. have investigated the volatile oils of Myrtaceaen species, Eucalyptus globulus Labill in particular, against a number of respiratory viruses and bacteria. They demonstrated the activity of E. globulus volatile oil against a strain of animal virus, a strain of infectious disease virus, 10 isolates of Stenotrophomonas maltophilia, 10 isolates of enteric bacteria pneumonia, 30 isolates of H. parainfluenzae, 40 isolates of Haemophilus influenzae, 20 isolates of S. aureus, 40 isolates of S. agalactiae and 120 isolates of Streptococcus pyogenes. Furthermore, a gentle toxicity was reported for the epidemic parotitis virus [124,125].
Antiviral activities of essential oils and their extracted components have been listed in Table 1, Table 2 , respectively.
Table 1.
The antiviral actives of some essential oils of plant origin [126].
| S.no. | Source of essential oils | Target viruses | Mechanism type | SI | IC50 |
|---|---|---|---|---|---|
| 1 | Osmunda regalis (Tunisian fern) | Coxsackie virus B4 | ND | 789.8 | 2.2 μg/mL |
| 2 | Dysphania ambrosioides | Coxsackie virus B4 | ND | 74.3 | 21.7 μg/mL |
| 3 | Cymbopogon nardus | HIV-1 | ND | ND | 1.2 mg/mL |
| 4 | Thymus vulgarisvulgaris, Cymbopogon citratus, Rosmarinus officinalis | HIV-1 | ND | 1.13–3.6 | 0.05–0.83 μg/mL |
| 5 | Fortunella margarita fruit | Avian influenza virus A (H5N1) | ND | ND | 6.8 μg/mL |
| 6 | Citrus reshni ripe fruit peel | Avian influenza virus A (H5N1) | ND | 8.7 | 2.5 μg/mL |
| 7 | Pelargonium graveolens | IFV-A (H1N1) | Intercellular | >21 | <3.1 μL/mL |
| 8 | Eucalyptus globulus | IFV-A (H1N1) | Intercellular | >0.5 | <50 μL/mL |
| 9 | Lavandula officinalis | IFV-A (H1N1) | Intercellular | >8 | <3.1 μL/mL |
| 10 | Thymus vulgaris | IFV-A (H1N1) | Intercellular | >4 | <3.1 μL/mL |
| 11 | Cymbopogon flexuosus | IFV-A (H1N1) | ND | >4 | <3.1 μL/mL |
| 12 | Citrus bergamia | IFV-A (H1N1) | Intercellular | >5 | <3.1 μL/mL |
| 13 | Cinnamomum zeylanicum | IFV-A (H1N1) | Intercellular | >4 | <3.1 μL/mL |
| 14 | Patchouli | IFV-A (H1N1) | ND | 1.15 | 0.088 mg/mL |
| 15 | Mexican oregano (Lippia graveolens) | Acyclovir-resistant HSV-1 | Intercellular | 13.1 | 55.9 μg/mL |
| 16 | Salvia desoleana | Acyclovir-resistant HSV-2 | Intercellular and intracellulara | 55.2 | 28.6 μg/mL |
| 17 | Thymus capitatus | HSV-2 | Intercellular | 6.0 | 18.6 μg/mL |
| 18 | Thymus capitatus | HSV-1 | Intercellular | 6.9 | 17.6 μg/mL |
| 19 | Rosmarinus officinalis | HSV-1 | ND | 46.1 | 0.006% |
| 20 | Satureja hotensis | HSV-1 | ND | 32.2 | 0.008% |
| 21 | Artemisia kermanensis | HSV-1 | ND | 66.4 | 0.004% |
| 22 | Eucalyptus caesia | HSV-1 | ND | 38.8 | 0.007% |
| 23 | Zataria multiflora | HSV-1 | ND | 55.4 | 0.003% |
| 24 | Mexican oregano (Lippia graveolens) | HSV-1 | Intercellular | 7.4 | 99.6 μg/mL |
| 25 | Pulicaria vulgaris | HSV-1 | Intercellular | 1 | 0.001% |
| 26 | Lallemantia royleana | HSV-1 | Intercellular | 6.4 | 0.011% |
| 27 | Star Anise | HSV-1 | Intercellular | 160 | 1 μg/mL |
| 28 | Mentha suaveolens | HSV-1 | Intracellular | 67 | 5.1 μg/mL |
| 29 | Australian tea tree | HSV-1 | Intracellular | 43 | 13.2 μg/mL |
| 30 | Sinapis arvensis | HSV-1 | Intercellular | 1.5 | 0.035% |
Note: SI = selectivity index, ND = not defined, HIV = human immunodeficiency viruses, HSV = human herpes viruses, Intracellular = effects on intercellular events of life cycle in viruses, Intercellular = effects on virus surface pre or post adsorption and “a” = indicates later stages of viral lifecycle.
Table 2.
Antiviral activity of some individual essential oil compounds [126].
| S.no. | Compounds | Target viruses | Mechanism type | SI | IC50 |
|---|---|---|---|---|---|
| 1 | Camphor | BVDV | Intercellular | 13.9 | 318.51 μg/mL |
| 2 | 1, 8-Cineole | BVDV | Intercellular | 9.1 | 331.17 μg/mL |
| 3 | Germacrone | IFV-A (H1N1) | Intercellular and intracellularb | >41 | 6.03 μM |
| 4 | β-Santalol | IFV-A (H3N2) | Intracellulara | 10–100 μg/mL | |
| 5 | Eugenol | IFV-A (H1N1) | ND | ND | <3.1 μL/mL |
| 6 | Carvacrol | IFV-A (H1N1) | ND | <0.15 | 2.6 μg/mL |
| 7 | Carvacrol | Acyclovir-resistant HSV-1 | Intracellular | 8.7 | 28.6 μg/mL |
| 8 | Limonene | HSV-1 | Intercellular | 10.2 | 5.9 μg/mL |
| 9 | β-Pinene | HSV-1 | Intercellular | 24.3 | 3.5 μg/mL |
| 10 | Carvacrol | HSV-1 | Intercellular | 43 | 7 μM |
| 11 | Thymol | HSV-1 | Intercellular | 43 | 7 μM |
| 12 | Carvacrol | HSV-1 | Intracellular | 5.1 | 48.6 μg/mL |
| 13 | p-Cymene | HSV-1 | Intercellular | ND | >0.1% |
| 14 | Carvacrol | HSV-1 | Intercellular | 1.4 | 0.037% |
| 15 | Thymol | HSV-1 | Intercellular | 7 | 0.002% |
| 16 | Eugenol | HSV-1 | Intercellular | 2.4 | 35 μg/mL |
| 17 | Trans-anethole | HSV-1 | Intercellular | 5 | 20 μg/mL |
| 18 | β-Eudesmol | HSV-1 | Intercellular | 5.8 | 6 μg/mL |
| 19 | Farnesol | HSV-1 | Intercellular | 11.4 | 3.5 μg/mL |
| 20 | β-Caryophyllene | HSV-1 | Intercellular | 140 | 0.25 μg/mL |
Note: SI = selectivity index, ND = not defined, BVDV = Bovine viral diarrhea virus, HIV = human immunodeficiency viruses, HSV = human herpes viruses, Intracellular = effects on intercellular events of life cycle in viruses, Intercellular = effects on virus surface pre or post adsorption, “a” = later stages of viral lifecycle and “b” = initial stages of viral lifecycle.
3. Antiviral activity against coronaviruses
3.1. What are coronaviruses?
Coronaviruses have been listed under a different sub-family (coronavirinae) of family coronaviridae. The members of the family Coronaviridae, a monophyletic cluster in the order Nidovirales, are enveloped, positive stranded RNA viruses of three classes of vertebrates: fish (bafiniviruses), birds (coronaviruses) and mammals (toro– and caronaviruses). On the study of unrooted and rooted phylogenetic trees projected for variant genomic regions, four different CoV clusters are eminent. Three clusters correspond to already found unofficial groups 1, 2 and 3 that are now classified officially as genera, Alpha-, Beta- and Gamma-CoV's, respectively. The fourth cluster is different from the farmer three and found in birds. By all the standards this fourth cluster has been listed under a different genus (unofficial, yet to be approved) tentatively termed as “Delta-CoV's”. Moreover, in case of Beta- CoV's further four divergent linages can be distinguished, assigned as A through D, that correspond to former subgroups 2 A through D, respectively (Fig. 5 ).
Fig. 5.
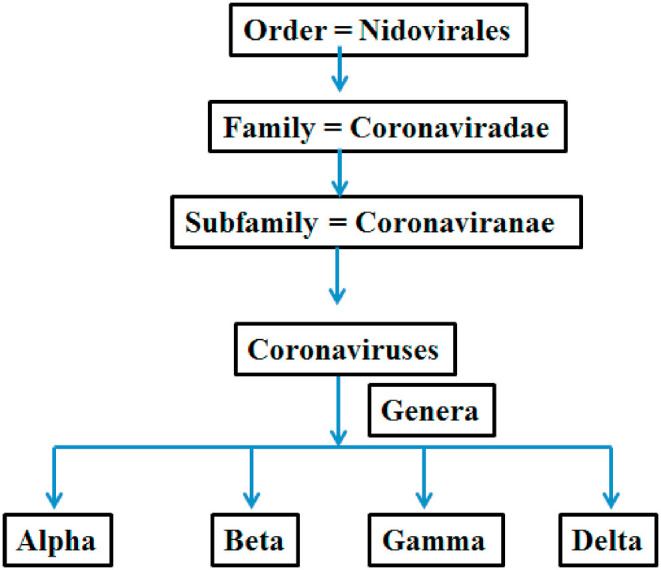
Classification of coronaviruses.
Across Coronavirinae, virons are spherical in shape and a size of 120–160 nm, bacilliform, 170–200 × 75–88 nm (Bafinivirus) or both, having a characteristically bent bacilliform into crescents (Torovirus). These particles are decked with typically large, petal- or club-shaped surface projections (spikes), creating an image of solar corona (Fig. 6 ) under electron microscopy. The CoV's bear helical nucleocapsids and can been released from virion on the application of detergents. The nucleocapsids of coronavirus appear to be loosely-wound and in Torovirinae are characteristically tubular. Coronoviridae are the large size RNA viruses discovered so far in terms of genetic complexity and genome size, except rivaled by okaviruses (belonging to the family of Roniviridae, okaviruses are large invertebrate nidoviruses).
Fig. 6.
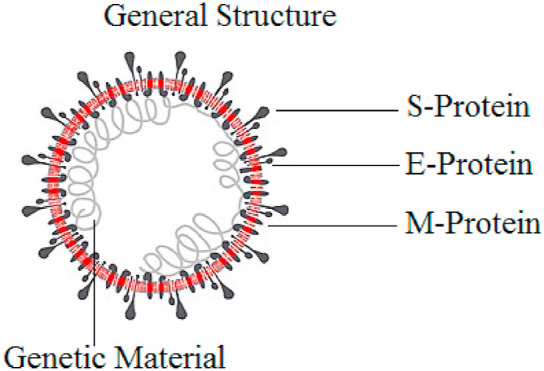
General structure of coronaviruses.
3.2. Anti-coronavirus activities of essential oils
Essential oils have been shown with tremendous antiviral potential against different types of disease causing viruses in humans. The antiviral action of oleoresins and essential oils mixture from different aromatic herbs and medicinal plants was evaluated against coronavirus infectious bronchitis virus (IBV) both in vivo and in vitro [127]. This mixture used in avian IBV treatment resulted in decreasing of virus titer in two different host systems, embryonating eggs and Vero E6 cells. The effectiveness of this mixture against IBV was also investigated in chickens. The essential oils and oleoresins mixture was administrated to the chickens through spraying at 1:20 dilution and it was reported that before 2 h of IBV exposure, the mixture produced remarkable results against IBV. This type treatment showed substantial effects of reducing seriousness of signs and clinical lesions in birds along with it reduced the amount of viral RNA in the trachea of birds. The birds administrated with this essential oils and oleoresins mixture showed protection against IBV for a time period of 4 days and also reduced the clinical symptoms and transmission of the viral infection for over two weeks. This mixture produced virucidal action as it proved more beneficial prior to virus entry and attachment. Therefore, these results indicated that this treatment mixture is more active against cell free virus and hence could be active against other types of viruses as well including other human coronaviruses. In an another study carried out by Wen et al. evaluating the effects of 221 phytochemicals and essential oils components against SARS-CoV (severe acute respiratory syndrome coronavirus [128]. They used cell-based assay investigating cytopathogenic effects on SARS-CoV infected Vero E6 cells. Out of all the experimental components, 10 terpenoids, 2 sesquiterpenoids and 2 triterpenoids showed maximum inhibition at doses ranging from 3.3 to 10 μM. The researchers measured the concentrations of 22 compounds which showed 50% inhibition of proliferation in Vero E6 cells (CC50) and replication (EC50). This was the first research involving the essential oil derived phytochemicals showing that they could potentially inhibit the SARS-CoV and provided a novel approach towards the formation of anti-SARS-CoV drugs. The essential oils derived from Citrus sinesis, Anthemis hyalina and Nigella sativa, have been reported of significant activity against replication of coronaviruses and the levels of TRP gene expressions [129]. To assess the influence of extracts on the replication of coronavirus (CoV) and on the manifestation of TRP genes throughout Coronavirus contagion, HeLa-CEACAM1a (HeLa-epithelial carcinoembryonic antigen-related cell adhesion molecule 1a) cells were injected with MHV-A59 (mouse hepatitis virus–A59) was found to be the nontoxic active dose. TCID 50/mL (Tissue Culture Infectious Dose that will yield a cytopathic impact in 50% of the injected tissue culture cells) was found for treatments to determine the viral loads. The levels of inflammatory cytokine IL-8 were found to enhance after 24 h and 48 h of extract exposure. TRPA1, TRPV4, TRPM6, TRPC4, TRPM7, and TRPM8 were the genes which expression levels altered considerably post extract treatments. The virus load reduced once extracts were added to the CoV infested cells. All the extract treatments had an effect on TRP gene expression, IL-8 secretion, and virus load after CoV infection and could be the best candidate in our hands that contains potential treatment molecule(s).
The essential oil of Laurus nobilus consists of β-pinene, α-pinene, 1,8-cineole and b-ocimene, as major constituents. The oil has been reported to possess strong antiviral activity against SARS-CoV-1 with a selective index and IC50 values of 4.6 and 120 mg/mL, respectively [130]. The Juniperus oxycedrus essential oil has been previously reported of antiviral activities. A total of 48 compounds have been recognized in this oil with major constituents as 2.2% δ-cadinene, 2.3% epibicycloses-quiphellandren, 6.7% limonene and α-phellandrene. All of these compounds showed remarkable inhibitory activity against the SARS-CoV-1 coronavirus [130]. The essential oil from Theileria orientalis is rich in its chemical composition. A total number of 43 compounds have been identified from this oil, which constitutes a total of 86.6% of oil. The major constituents of the oil were 9.55% α-cedrol, 9.48% δ-3-carene and 35.72% α-pinene. All these active phytochemicals were reported of inhibitory activity on SARS-CoV-1 coronavirus [130].
4. Anti-SARS-CoV-2 (COVID-19) activities of essential oils and their chemical constituents
4.1. The disastrous COVID-19 pandemic
Coronavirus disease of 2019 has emerged as a lethal health threat and continues to grow with a rapid pace. The disease is caused by severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2 virus). Signs and symptoms of coronavirus disease 2019 may appear 2–14 days after exposure. The time period after exposure and before having symptoms is known as incubation period. Common signs and symptoms may include: fever, cough, pneumonia, tiredness difficulty in breathing and multiple organ failure. This coronavirus outbreak, which was later named as COVID-19, originated in Wuhan city of China from where it spread throughout the world. The disease disbursed to several parts of the world quickly due to the outbreak in the month of December, considered as global month of festivals. Till September 26, 2020, there were more than 32, 344, 734 cases and 984,902 deaths reported of COVID-19, globally [131]. Some of the country's worst hit by COVID-19 pandemic includes United States of America, India, Brazil, Russian Federation, Colombia, Peru, Mexico, Spain, Argentina, South Africa, France, Chile, Iran (Islamic Republic of), The United Kingdom, Bangladesh, Iraq, Saudi Arabia, Turkey, etc. There are only a few drugs approved for SARS-CoV-2 infection and its inflammatory symptoms. No vaccine has been developed against this disease, besides there are more than 20 vaccines in development, globally. The first 10 countries with number of COVID-19 cases and deaths are listed in Table 3 .
Table 3.
The number of cases and deaths in the worst hit countries by COVID-19 in the world [131].
| S. no. | Country | Number of cases | Number of deaths |
|---|---|---|---|
| 1 | United States of America | 6,910,082 | 201,634 |
| 2 | India | 5,903,932 | 93,379 |
| 3 | Brazil | 4,657,702 | 139,808 |
| 4 | Russian Federation | 1,136,048 | 20,056 |
| 5 | Colombia | 790,823 | 24,924 |
| 6 | Peru | 788,930 | 31,938 |
| 7 | Mexico | 715,457 | 75,439 |
| 8 | Spain | 704,209 | 31,118 |
| 9 | Argentina | 678,266 | 14,766 |
| 10 | South Africa | 667,049 | 16,283 |
4.2. Antiviral activity of essential oils against SARS-CoV-2 and their mechanism of action
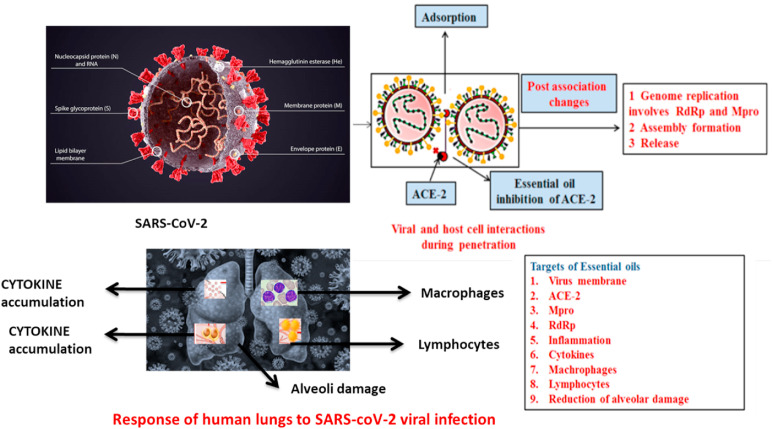
Essential oils are well known for their antiviral, immunomodulatory, anti-inflammatory and bronchodilatory activities. Essential oils and their chemical constituents have been proposed for SARS-CoV-2 infections owing to their lipophilic nature. These oils have a potential to enter the viral membrane leading to membrane disruption. Essential oils and its constituent phytochemicals disturb viral replication and also benefit the host respiratory system via mucus lysis and bronchodilation. The data available till date on anti-COVID-19 activity of essential oils is mostly based on in vitro studies and computer aided docking techniques.
Since ages, garlic oil has been used to treat several human ailments including influenza, cold and other infections. GC-MS analysis of garlic oil has identified 18 coumpounds. The main compounds were 6.5% diallyl tetrasulphide, 6.7% allyl methyl trisulphide, 8.2% allyl (E)-1-propenyl disulphide, 22.8% allyl trisulphide and 28.4% allyl disulhide. Out of the 18 components, 17 were investigated for their action against viral main protease Mpro/6LU7 and ACE-2 protein of SARS-CoV-2. ACE-2 is an enzyme paving the way for virus entrance into host cell and Mpro aids in viral replication. The 17 compounds investigated showed remarkable interactions with Mpro and ACE-2, hence revealing the strong potency of garlic oil to treat COVID-19 [132]. Oxidative stress produced by virus in host cells play a leading role throughout the life cycle and pathogenesis of diseases caused by viruses. Due to oxidative stress, antioxidant pathways are activated in host cells including nuclear factor erythroid 1p45-related factor 2 (Nrf2) [133]. Nrf2 transcription factor is well known to modulate the levels of expressions of several genes related to antiviral actions [134]. It has been reported that a compound PB125® has a strong potential to stimulate the Nrf2 which resulted in downregulation of TMPRSS2 mRNA and ACE-2 levels in HepG2 human liver derived cells. The ACE-2 and TMPRSS2 mRNA are both involved in the penetration of SARS-CoV-2 into host cell. Additionally, PB125® was reported to alter the expressions of about 36 genes related to cytokines in primary human pulmonary artery endothelial cells recognized as cytokine storm in the course of severe COVID-19 cases. It was suggested that activation of Nrf2 could inhibit the cytokine storm in SARS-CoV-2 patients [134]. Study performed by Ho et al. and Patel et al. showed that diallyl sulphide, one of the active constituents of garlic oil, could potentially activation of Nrf2 in lung MRC-5 cells. Translocation of Nrf2 into nucleus post activation triggered p38/ERK-signalling and hence inhibited the lung injury through suppression of oxidative stress [135,136].
An in silico study by Kulkarni et al. investigated the potential of different plant derived essential oils belonging to different families against S1 (also known as receptor binding domain) subunit in spike proteins of COVID-19 virus [137]. S1 subunit of the spike proteins is involved in the interaction of virus with host cell through ACE-2 receptors. Out of the essential oils used, pulegone, thymol, L-4-terpineol, cinnamyl acetate, geraniol, carvacrol and cinnamaldehyde, anethole, showed potential inhibition of S1 subunit in silico [137]. A similar docking study was carried out by Elfiky demonstrating the efficacy of thymoquinone and cinnamaldehyde against RdRps (SARS-CoV RNA-dependent RNA polymerase) and COVID-19. Both the compounds were found to possess lower binding affinities towards RdRps [138]. It was concluded by Elifiky that cinnamaldehyde could target viral attachment of SARS-CoV-2, provided further in vivo and in vitro studies are required to establish this fact. The Huang et al. evaluated the protective effects of cinnamaldehyde against acute lung injury induced through lipopolysaccharide in xenografted mice models [139]. Cinnamaldehyde produced beneficial effects on mice lungs through reduction of pulmonary oedema and wet/dry ratio. Further, cinnamaldehyde suppressed macrophages, neutrophils and total cell count in bronchoalveolar lavage fluid. Additionally, it reduced the production of cytokines involved in inflammation including IL-1β, IL-13, IL-6 and TNF-α [139]. This in silico data suggested that cinnamaldehyde could play a vital role against COVID-19 provide more in vivo and in vitostudies are required.
The essential oils derived from Eucalyptus globulus have been used to treat several respiratory diseases like pharyngitis, sinusitis and bronchitis. One of the active constituents of eucalyptus oil, 1,8-cineole has been reported of muscle relaxant effects through reduction in contraction of smooth muscles caused by different agents [140,141]. Further, studies have reported that cineole inhalation produced analgesic and anti-inflammatory effects; therefore, it could be used as COPD and for asthmatic patients [142]. The Eucalyptus essential oil has been reported of strong antiviral activities against HSV-1 and HSV-2 herpes simplex viruses and enveloped mumps viruses [143]. The strong evidences supporting the antiviral activities of essential oil and its constituents from Eucalyptus has led to researchers to investigate its activity against SARS-CoV-2 through in vitro assays and docking. One of the active constituent of Eucalyptus oil, jensenone, has been investigated for its activity against viral proteinase Mpro/3CLpro by Sharma et al. The results of the study revealed that jensenone molecule produced a complex by reacting with Mpro through hydrophobic interactions with PRO184, TRY126, LEU29, TRP207, PRO52, and ALA7, ionic interactions with HIS163, ARG38, ASP34 and LYS3, and hydrogen bond interactions with T16, D10, L30, V18, and M4 [144]. Sharma et al. have performed a molecular docking analysis on one of the other active constituents of eucalyptus oil, eucalyptol (1,8-cineole) and showed that it could bind with Mpro thereby inhibiting viral replication. The eucalyptol/Mpro complexes have been reported of forming strong ionic interactions, hydrogen bond interactions and hydrophobic interactions [145]. However, it was suggested by the authors that the validity of these results should be verified through in vitro enzyme assays against SARS-CoV-2. A study was carried out by Merad et al. showing that almost all of the SARS-CoV-2 patients develop lung abnormalities. The deaths and severity in COVID-19 patients have been attributed to the overactive and abnormal inflammatory responses. This storm of inflammatory responses is a reason of enhanced levels of cytokines considerable mononuclear cell infilitration and profound lymphopaenia in lungs and other effected organs like kidneys, lymph nodes, spleen and heart. The cytokine profile witnessed in the SARS-CoV-2 patients showed enhanced levels of cytokines like TNF, IL-6 and -7 and other inflammatory cytokines [146]. Different ex vivo and in vitro studies have been carried out to investigate the effects of eucalyptus oil and its active constituent eucalyptol on macrophage and monocytes recruitment during lung infections and inflammation. Data of these studies determine marked immunomodulatory properties of both eucalyptus oil and its vigorous ingredient, i.e. eucalyptol. Both treatments decreased the discharge of pro-inflammatory cytokines from macrophages and monocytes, but their phagocytic properties were not halted [147]. Taken together, data from clinical and preclinical trials indicated remarkable anti-COVID-19 potential in eucalyptus oil and its active components. Therefore, more investigations are immediately needed in this regard.
Silva et al. screened 171 essential oils and their constituent compounds against different proteins in SARS-CoV-2 including spike proteins, Rdrp, ADP-ribose-1-phosphatase, endoribonuclease, human angiotensin-converting enzyme and viral proteases Mpro, through molecular docking [148]. Among the 171 compounds only few showed higher efficacy of binding to Mpro including (E)-nerolidol, (E,E)-farnesol, and (E,E)-α-farnesene. The results revealed that on administrating these essential oil components in mixture or alone could inhibit the viral replication. The main proteins involved in the viral infection of SARS-CoV include endoribonuclease and non-structural protein 15 (Nsp15) [149]. The compounds (E)-nerolidol, (E,E)-farnesol, (E,E)-α-farnesene, and (E)-β-farnesene, have showed remarkable binding affinity towards Nsp15. The viral replication in RNA viruses is catalyzed by RdRp and is a primary and vital step in viral replication. Therefore becomes a leading target for antiviral therapies against RNA viruses [150]. (E,E)-farnesol has been reported of best docking scores against RDRp. The presence of spike proteins on SARS-CoV-2 virus aids it to enter the host cell by interacting with ACE-2 present on the membrane of host cell [151]. Hence, ACE-2 was considered as one of the leading target of management therapies against COVID-19. Several essential oil component have been reported of higher affinity towards ACE-2 including α-bulnesene, eremanthin, (Z)-spiroether, β-sesquiphellandrene, (E)-nerolidol, (E,E)-farnesol, (E)-β-farnesene, and (E,E)-α-farnesene. The compounds geranyl formate, (E,E)-farnesol, (E)-β-farnesene, (E,E)-α-farnesene, eremanthin, and (E)-cinnamyl acetate, showed higher efficacy against spike proteins of SARS-CoV-2. Fig. 7 demonstrates the possible activities of essential oils and their chemical constituents in SARS-CoV-2 infection.
Fig. 7.
Representation of different possible modes of action of essential oils and their constituents against SARS-CoV-2.
A molecular docking study was performed by Silva and co-workers to analyze the efficacies of carvacrol, menthol and eugenol (major constituents of essential oils) against several proteins in SARS-CoV-2. These compounds were reported of greater affinities towards ACE-2, RdRp, Mpro and spike proteins. Similar, an in silico study carried out by Kumar et al. evaluating the binding potential of carvacrol with main protease protein in SARS-CoV-2 [152]. Results showed that carvacrol showed remarkable suppression of viral replication through inhibition of Mpro. Menthol has been reported of rich pharmacological activities including gastroprotective, anti-inflammatory, and immunomodulatory properties in rat models. Treatment with menthol was found to considerably decrease the expressions and levels of pro-inflammatory cytokines, i.e. interleukin-1, tumour necrosis factor-α (TNF-α), and interleukin-23 in the treated rats [153,154]. Eugenol has been shown to have antiviral activities against HSV-1 and HSV-2, respectively [155]. The eugenol treatment has been reported of significant recruitment of leukocytes and suppressing the inflammatory storm in lungs via suppression of cytokines (TNF-alpha and IL-6) [156]. Games et al. have performed an in vivo study assessing the impact of three molecules including carvacrol in elastase-induced pulmonary emphysema mice model [157]. Their results indicated that cavacrol inhibited the macrophage recruitment, alveoli enlargement and the expressions of IL-1β, IL-6, IL-8, and IL-17 of the fluid in bronchoalveolar lavage. The inflammatory responses were low in carvacrol treated-mice in comparison to disease control group. In summary, the data based on in vivo and in silico studies pointed out at potential role of carvacrol, menthol and eugenol against COVID-19. To further establish these results against SARS-CoV-2, more in vitro and in vivo studies are required. Geranium and lemon oils displayed significant ACE2 inhibitory effects in epithelial cell [158]. Fig. 8 depicts the possible targets of essential oils and their active constituents against SARS-CoV-2 infection.
Fig. 8.
The biochemical pathways as possible targets for essential oils and their chemical constituents against SARS-CoV-2. The events post SARS-CoV-2 infection in lungs and role of essential oils to suppress its adverse symptoms.
4.3. Future prospects
It is required to carry on the development of potential antiviral therapeutics based on natural products especially essential oils and their chemical constituents against coronaviruses because they are cost-effective and also cause minimum side effects in subjects. There are no potential vaccines developed against several viruses including SARS-CoV-1 and SARS-CoV-2. Therefore, overcoming these infectious viruses seems to be problematic and tough. However, plant derived essential oils and their chemical constituents have been reported of remarkable antiviral properties and based on docking studies they could prove beneficial against coronaviruses as well. The main drawback of antiviral drugs against coronaviruses is targeting specified viral genes and proteins is the rapid genomic mutations like as observed in HIV, HSV and SARS-CoV-2 [159,160]. The studies performed on antiviral properties of essential oils are at initial stages. Therefore, identification of active substances, the description of underlying mechanisms, as well as the analysis of effectiveness and apparent in vivo applications is suggested in order to back the probe of potent antiviral chemotherapeutics from essential oils. Essential oils and their chemical constituents have been reported of targeting several potential bimolecular pathways in different viruses including coronaviruses [161]. Hence, more research should be performed to explore the probability of combatting COVID-19 by combination therapies with other natural ingredients or prescribed medicine. Essential oils are rich in chemical ingredients that are of remarkable pharmacological importance. These molecules should be investigated for their antiviral actions against SARS-CoV-2. Essential oils do possess a great potential of targeting several potential pathways in viruses and it is believed that they could produce the same against COVID-19. Essential oils inhalation could prove adjuvant therapy for COVID-19 patients as they are rich in active volatile constituents including β-ocimene, 1,8-cineole, β-pinene, α-pinene, carnosic acid and rosmarinic acid. Volatile molecules show high vapor pressure and this property makes lungs to exile them easily post ingestion. Moreover, the easy intake and exile of volatile constituents of essential oils could be used in pharmacological point of view for drug delivery. Like so, a hypothetical molecule for COVID-19 should have to maintain a proper balance between intake and exhalation by lungs along with its pharmacological action. In this regard essential oils are advantageous in use against COVID-19 as they could be administrated both orally and in aerosolized form. We trust that natural remedies, such as aromatic herbs and essential oils derived from medicinal plants, will continue to play an imperative role and contribute in the development and advancement of anti-coronavirus drugs.
5. Conclusion
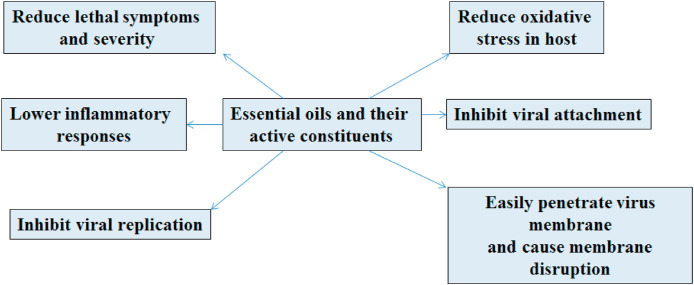
In conclusion, the literature survey on antiviral effects of essential oils and their chemical constituents indicate that they could prove as leads in drug development and design against COVID-19. They bear strong potential to target vital pathways during pathogenesis of viral life cycle. Essential oils can easily penetrate the virus membrane thereby causing membrane disruption. Essential oils have a remarkable potential of inhibiting virus attachment (by inhibition of ACE-2) and replication (by inhibition of Mpro). Further, essential oils have a strong tendency to suppress the cytokine storm generated during SARS-CoV-2 infection and hence inhibit the alveolar inflammation. Moreover, these essential oils inhibit the macrophages recruitment to the damaged site. Owing to these activities of essential oils, it is believed that they could prove beneficial for COVID-19 patients and also could suppress the severe symptoms caused by the infection.
Declaration of competing interest
The authors declare that there is no conflict of interest to indicate.
References
- 1.Jamshidi-Kia F., Lorigooini Z., Amini-Khoei H. Medicinal plants: past history and future perspective. J. herbmed pharmacol. 2018;7(1) [Google Scholar]
- 2.Mathur S., Hoskins C. Drug development: lessons from nature. Biomed. Rep. 2017 Jun 1;6(6):612–614. doi: 10.3892/br.2017.909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Saxena M., Saxena J., Nema R., Singh D., Gupta A. Phytochemistry of medicinal plants. J. Pharmacogn. Phytochem. 2013;1(6):168–182. [Google Scholar]
- 4.Rao N. Bioactives phytochemicals in Indian foods and their potential in health promotion and disease prevention. Asia Pac. J. Clin. Nutr. 2003;12(1):9–22. [PubMed] [Google Scholar]
- 5.Caputi L., Aprea E. Use of terpenoids as natural flavouring compounds in food industry. Recent Patents on Food. Nutrition & Agriculture. 2011;24:9–16. doi: 10.2174/2212798411103010009. [DOI] [PubMed] [Google Scholar]
- 6.Djilani A., Dicko A. In: Bouayed J., Bohn T., editors. vol. 7. IntechOpen; Rijeka: 2012. The therapeutic benefits of essential oils; pp. 155–179. (Nutrition, Well-Being and Health). [Google Scholar]
- 7.Astani A., Reichling J., Schniztler P. Comparative study on the antiviral activity of selected monoterpenes derived from essential oils. Phytother Res. 2010;24:673–679. doi: 10.1002/ptr.2955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Almeida R.N., Fatima A.M., Souto M.F., Sousa D.P. Essential oils and their constituents: anticonvulsant activity. Molecules. 2011;16:2726–2742. doi: 10.3390/molecules16032726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kumari S., Pundhir S., Priya P., Jeena G., Punetha A., Chawla K., et al. EssoilDB: a database of essential oils reflecting terpene composition and variability in the plant kingdom. Database. 2014;14:1–12. doi: 10.1093/database/bau120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Inoue M., Hayashi S., Craker L.E. Role of medicinal and aromatic plants: past, present, and future. Pharmacognosy Med. Plants. 2019 Feb 4:1–13. doi: 10.5772/intechopen.82497. http://creativecommons.org/licenses7by3.0 InTechOpen. [DOI] [Google Scholar]
- 11.Awuchi C.G. Medicinal plants: the medical, food, and nutritional biochemistry and uses. Int. J. Adv. Acad. Res. 2019;5(11):220–241. [Google Scholar]
- 12.Kumari S., Pundhir S., Priya P., Jeena G., Punetha A., Chawla K., et al. EssoilDB: a database of essential oils reflecting terpene composition and variability in the plant kingdom. Database. 2014;14:1–12. doi: 10.1093/database/bau120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Palazzolo E., Laudicina V.A., Germanà M.A. Current and potential use of Citrus essential oils. Curr. Org. Chem. 2013;17:3042–3049. [Google Scholar]
- 14.Costa M.A., Zia Z.Q., Davin L.B., Lewis N.G. In: Romeo J.T., editor. vol. 33. Springer Science+Business Media Kluwer Academic/Plenum Publishers; New York: 1999. Chapter four: toward engineering the metabolic pathways of cancer-preventing lignans in cereal grains and other crops; pp. 67–87. (Recent Advances in Phytochemistry, Phytochemicals in Human Health Protection, Nutrition, and Plant Defense). [Google Scholar]
- 15.Sirousmehr A., Arbabi J., Asgharipour M.R. Effect of drought stress levels and organic manures on yield, essential oil content and some morphological characteristics of sweet basil (Ocimum basilicum) Biology. 2014;8(4):880–885. [Google Scholar]
- 16.Surburg H., Panten J. Common Fragrance and Flavor Materials. Preparation, Properties and Uses. sixth ed. Wiley-VCH Verlag GmbH Co; Germany: 2016. pp. 84–85. [Google Scholar]
- 17.Stephane F.F., Jules B.K. IntechOpen; 2020 May 14. Terpenoids as Important Bioactive Constituents of Essential Oils. InEssential Oils-Bioactive Compounds, New Perspectives and Applications. [Google Scholar]
- 18.Gupta V., Mittal P., Bansal P., Khokra S.L., Kaushik D. Pharmacological potential of Matricaria reticula. Int. J. Pharmaceut. Drug Res. 2010;2:67–71. [Google Scholar]
- 19.Martin A., Varona S., Navarette A., Cocero M.J. Encapsulation and co-precipitation processes with supercritical fluids: applications with essential oils. Open Chem. Eng. J. 2010;4:31–41. [Google Scholar]
- 20.Skold M., Karlberg A.T., Matura M., Borje A. The fragrance chemical β-caryophyllene-air oxidation and skin sensitization. Food Chem. Toxicol. 2006;44:538–545. doi: 10.1016/j.fct.2005.08.028. [DOI] [PubMed] [Google Scholar]
- 22.Raut J.S., Karuppayil S.M. A status review on the medicinal properties of essential oils. Industrial Crops. 2014;62:250–264. [Google Scholar]
- 23.Chlodwin F., Novak J. In: Handbook of Essential Oils: Science, Technology, and Applications. KHC B., Buchbauer G., editors. Taylor and Francis Group; London, UK: 2010. Sources of essential oils. [Google Scholar]
- 24.Ebadollahi A. Essential oils from Myrtaceae family as natural insecticides. Annu. Rev. Res. Biol. 2013;3(3):148–175. [Google Scholar]
- 25.Butnariu M., Sarac I. Essential oils from plants. J. Biotechnol. Biomed. Sci. 2018;1(4):35–43. [Google Scholar]
- 26.Nieto G. Biological activities of three essential oils of the Lamiaceae family. Medecines. 2017;4:63–72. doi: 10.3390/medicines4030063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ur-Rahman A. vol. 3. Bentham eBooks; Saif Zone, Saif, Sharjah, United Arab Emirates: 2016. p. 141. (Frontiers in Clinical Drug Research—Antiinfectives). [Google Scholar]
- 28.Dreger M., Wielgus K. Application of essential oils as natural cosmetic preservatives. Herba Pol. 2013;59:142–156. [Google Scholar]
- 29.Harrewijn P., Oosten V.A.M., Piron P.G.M. Kluwer Academic Publishers; Dordrecht, The Netherlands: 2001. Natural Terpenoids as Messengers. [Google Scholar]
- 30.Fernández-López J., Viuda-Martos M. Introduction to the special issue: application of essential oils in food systems. Foods. 2018;7:56. doi: 10.3390/foods7040056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Vetter S., Franz C., Glasl S., Kastner U., Saukel J., Jurenitsch J. Inheritance of sesquiterpene lactone types within the Achillea millefolium complex (Compositae) Plant Breed. 1997;116:79–82. [Google Scholar]
- 32.de Cn G., Quintero A., Orellana R.C. Chemotaxonomic value of essential oil compounds in Citrus species. Acta Hortic. 2002;576:49–55. [Google Scholar]
- 33.Masango P. Cleaner production of essential oils by steam distillation. J. Clean. Prod. 2005;13:833–839. [Google Scholar]
- 34.Sell CS. The Chemistry of Fragrance. From Perfumer to Consumer. second ed. Cambridge, UK: The Royal Society of Chemistry. p. 329.
- 35.Hussaina A.I., Anwar F., Sherazi S.T.H., Przybylski R. Chemical composition, antioxidant and antimicrobial activities of basil (Ocimum basilicum) essential oils depends on seasonal variations. Food Chem. 2008;108:986–995. doi: 10.1016/j.foodchem.2007.12.010. [DOI] [PubMed] [Google Scholar]
- 36.Taiz L., Zeiger E. Plant Physiology. fifth ed. Sinauer Associates Inc., Publishers Sunderland; MA, USA: 2010. p. 782. [Google Scholar]
- 37.Dima C., Dima S. Essential oils in foods: extraction, stabilization and toxicity. Current Opinion in Food Science. 2015;5:29–35. [Google Scholar]
- 38.Andrade E.H.A., Alves C.N., Guimaraes E.F., Carreira L.M.M., Maia J.G.S. Variability in essential oil composition of Piper dilatatum LC rich. Biochem. Systemat. Ecol. 2011;39:669–675. [Google Scholar]
- 39.Griffin S.G., Wyllie S.G., Markam J.L., Leach D.N. The role of structure and molecular properties of terpenoids in determining their antimicrobial activity. Flavour Fragrance J. 1999;14:322–332. [Google Scholar]
- 40.Sangwan N.S., Farooqi A.H.A., Shabih F., Sangwan R.S. Regulation of essential oil production in plants. Plant Growth Regul. 2001;34:3–21. [Google Scholar]
- 41.Lichtfouse E. vol. 12. Springer Nature; France: 2013. p. 233. (Suistanable Agriculture Reviews.). [Google Scholar]
- 42.Harborne J.B., Tomas-Bardenan F.A. Clarendon; Oxford: 1991. Ecological Chemistry and Biochemistry of Plant Terpenoids. [Google Scholar]
- 43.Surburg H., Panten J. Common Fragrance and Flavor Materials. Preparation, Properties and Uses. fifth ed. Wiley-VCH; Weinheim: 2006. [Google Scholar]
- 44.Reineccius G.A. In: Flavour and Fragrances: Chemistry, Bioprocessing and Sustainability. Berger R.G., editor. Springer-Verlag: Heidelberg; 2007. Flavour-isolation of essential oils; pp. 409–426. [Google Scholar]
- 45.Baharum S.N., Bunawan H., Ghani M.A., Mustapha W.A., Noor N.M. Analysis of the chemical composition of the essential oil of Polygonum minus Huds. Using two-dimensional gas chromatography-time-of-fligth mass spectrometry (GC-TOF MS) Molecules. 2010;15:7006–7015. doi: 10.3390/molecules15107006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Chizolla R. In: Naturals Products. Ramawat K.G., Merillon J.M., editors. Pringer-Verlag; Berlin Heidelberg: 2013. Regular monoterpenes and sesquiterpenes (essential oils) pp. 2973–3008. [Google Scholar]
- 47.Ruberto G., Baratta M.T. Antioxidant activity of selected essential oils components in two lipid model systems. Food Chem. 2000;69:167–174. [Google Scholar]
- 48.Sell C. In: Handbook of Essential Oils: Sciences, Technology, and Applications. KHC B., Buchbauer G., editors. CRC Press/Taylor & Francis Press; Boca Raton, Florida: 2010. Chemistry of essential oils; p. 131. [Google Scholar]
- 49.Ludwiczuk A., Skalicka-Wozniak K., Georgiev M.I. In: Pharmacognosy. Badal S., Delgoda R., editors. Academic Press; Boston, MA, USA: 2017. Chapter 11 - terpenoids; pp. 233–266. [Google Scholar]
- 50.Ying-Qian X., Zhi Y., Jun-Yi H., Jie T., Yoshihisa T., Hong-Quan D. Immunosuppressive terpenes from Prinsepia utilis. J. Asian Nat. Prod. Res. 2007;7:637–642. doi: 10.1080/10286020600979589. [DOI] [PubMed] [Google Scholar]
- 51.Bakkali F., Averbeck S., Averbeck D., Idaomar M. Biological effects of essential oils: a review. Food Chem. Toxicol. 2008;46:446–475. doi: 10.1016/j.fct.2007.09.106. [DOI] [PubMed] [Google Scholar]
- 52.Loza-Ravera H. Monoterpenes in essential oils: biosynthesis and properties. Adv. Exp. Med. Biol. 1999;464:49–62. doi: 10.1007/978-1-4615-4729-7_5. [DOI] [PubMed] [Google Scholar]
- 53.Mansoor K., Lockwood G.B. Encyclopedia of Separation Science. Academic Press; 2007. Chromatography/terpenoids. [Google Scholar]
- 54.Shabaan H.A.E., El-Ghorab A.H., Shibamoto T. Bioactivity of essential oils and their volatile aroma components: Review. J. Essent. Oil Res. 2012;24(2):203–212. [Google Scholar]
- 55.Moreira P., Smith M.A., Zhu X., Honda K., Lee H.G., Aliev G., et al. Since oxidative damage is a key phenomenon in Alzheimer's disease, treatment with antioxidants seems to be a promising approach for slowing disease profession. Oxidative damage and Alzheimer's disease: are antioxidant therapies useful? Drug News Perspect. 2005;18:13. doi: 10.1358/dnp.2005.18.1.877164. [DOI] [PubMed] [Google Scholar]
- 56.Naito Y., Uchiyama K., Yoshikama T. Oxidative stress involvement in diabetic nephropathy and its prevention by astaxanthin. Oxidative Stress Disease. 2006;21:235–242. [Google Scholar]
- 57.Lui J., Mori A. Oxidative damage hypothesis of stress associated aging acceleration: neuroprotective effects of natural and nutritional antioxidants. Res. Commun. Biol. Psychol. Psychiat. Neurosci. 2005;30–31:1–16. [Google Scholar]
- 58.Beal M.F. Mitochondrial, oxidative damage, and inflammation in Parkinson's disease. Ann. N. Y. Acad. Sci. 2003;991:120–131. doi: 10.1111/j.1749-6632.2003.tb07470.x. [DOI] [PubMed] [Google Scholar]
- 59.Mimica-Dukic N., Bozin B., Sokovic M., Simin N. Antimicrobial and antioxidant activities of Melissa officinalis L. (Lamiaceae) essential oil. J. Agric. Food Chem. 2004;52:2485–2489. doi: 10.1021/jf030698a. [DOI] [PubMed] [Google Scholar]
- 60.Yanishlieva-Maslarova N. In: Antioxidant in Food: Practical Applications. Yanishlieva N., Pokorny J., Gordor M., editors. Woodhead Publishing Ltd; Cambridge: 2001. Sources of natural antioxidants: vegetables, fruits, herbs, spices and teas; pp. 201–249. [Google Scholar]
- 61.Halliwell B. The antioxidant paradox. Lancet. 2000;355(9210):1179–1180. doi: 10.1016/S0140-6736(00)02075-4. [DOI] [PubMed] [Google Scholar]
- 62.Mimica-Dukic N., Orcic D., Lesjak M., Sibul F. Medicinal and Aromatic Crops: Production, Phytochemistry, and Utilization. Washington, DC, USA. ACS; 2016. Essential oils as powerful antioxidants: misconception or scientific fact? pp. 187–208. [Google Scholar]
- 63.International Agency for Research on Cancer (Iarc) vol. 263. Press release No; 2018. p. 3. (World Health Organization). [Google Scholar]
- 64.Wall M.E., Wani M.C. Camptothecin and Taxol: from discovery to clinic. J. Ethnopharmacol. 1996;51:239–254. doi: 10.1016/0378-8741(95)01367-9. [DOI] [PubMed] [Google Scholar]
- 65.Bhalla Y., Gupta V.K., Jaitak V. Anticancer activity of essential oils: a review. J. Sci. Food Agric. 2013;93:3643–3653. doi: 10.1002/jsfa.6267. [DOI] [PubMed] [Google Scholar]
- 66.Magalhaes H.I.F., De Sousa E.B.V. In: Bioactive Essential Oils and Cancer. de Sousa D.P., editor. Springer International Publishing; Switzerland: 2015. Antitumor essential oils; pp. 135–175. [Google Scholar]
- 67.Prashar A., Locke I.C., Evans C.S. Cytotoxicity of lavender oil and its major components to human skin cells. Cell Prolif. 2004;37:221–229. doi: 10.1111/j.1365-2184.2004.00307.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Legault J., Pichette A. Potentiating effect of beta-caryophyllene on anticancer activity of alpha-humulene, isocaryophyllene and paclitaxel. J. Pharm. Pharmacol. 2007;59:1643–1647. doi: 10.1211/jpp.59.12.0005. [DOI] [PubMed] [Google Scholar]
- 69.B Bassole I.H.N., Scifo R., Gnoula C., Morel L., Lobaccaro J.M., et al. Anticancer activity of essential oils and their chemical components- a review. Am. J. Cancer Res. 2014;4(6):591–607. [PMC free article] [PubMed] [Google Scholar]
- 70.Bourgou S., Pichette A., Marzouk B., Legault J. Bioactivities of black cumin essential oil and its main terpenes from Tunisia. South Afr. J. Bot. 2010;76:210–216. [Google Scholar]
- 71.Wang W., Li N., Luo M., Zu Y., Efferth T. Antimicrobial activity and anticancer activity of Rosmarinus officinalis L. essential oils compared to that of its main components. Molecules. 2012;17:2704–2713. doi: 10.3390/molecules17032704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Basollé I.H.N., Juliani H.R. Essential oils in combination and their antimicrobial properties. Molecules. 2012;17:3989–4006. doi: 10.3390/molecules17043989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Gallucci M.N., Olivia M., Casero C., Dambolena J., Luna A., Zygadlo J., et al. Antimicrobial combined action of terpenes against the food-borne microorganisms Escherichia coli, Staphylococcus aureus and Bacillus cereus. Flavour Fragrance J. 2009;24(6):348–354. [Google Scholar]
- 74.Pandey A.K., Singh P., Tripathi N.N. Chemistry and bioactivities of essential oils of some Ocimum species: an overview. Asian Pacific J. Tropical Biomed. 2014;4:682–694. [Google Scholar]
- 75.Nascimiento M.N.G., Junqueira J.G.M., Terezan A.P., Severino R.P., Silva T.S., Martins C.H.G., et al. Chemical composition and antimicrobial activity of essential oils from Xylopia aromatic (Annonaceae) flowers and leaves. Revista Virtual de Quimica. 2018;10(5):1578–1590. [Google Scholar]
- 76.Mith H., Dure R., Delcenserie V., Zhiri A., Daube A., Clinquart Antimicrobial activities of commercial essential oils and their components against food-borne pathogens and food spoilage bacteria. Food Sci. Nutr. 2014;4:403–416. doi: 10.1002/fsn3.116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Burt S. Essential oils: their antibacterial properties and potential applications in foods—a review. Int. J. Food Microbiol. 2004;94:223–253. doi: 10.1016/j.ijfoodmicro.2004.03.022. [DOI] [PubMed] [Google Scholar]
- 78.Espinoza J., Urzua A., Sanhueza W.M., Fincheira P., Muñoz M.L., Wilkens M. Essential oil, extracts, and sesquiterpenes obtained from the heartwood of Pilgerodendron uviferum act as potential inhibitors of the Staphylococcus aureus NorA multidrug efflux pump. Front. Microbiol. 2019;40:337–351. doi: 10.3389/fmicb.2019.00337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Bhavaniramya S., Vanajothi R., Vishnupriya S., Al-Aboody M.S., Vijayakumar R., Baskaran D. Computational characterization of deleterious SNPs in Toll-like receptor gene that potentially cause mastitis in dairy cattle. Biocatalysis Agric. Biotechnol. 2019;19:101151. [Google Scholar]
- 80.Hammer K.A., Carson C.F., Dunstan J.A., Hale J., Lehmann H., Robinson C.J., et al. Antimicrobial an anti-inflammatory activity of five Taxandria fragrans oils in vitro. Microbiol. Immunol. 2008;52:522–530. doi: 10.1111/j.1348-0421.2008.00070.x. [DOI] [PubMed] [Google Scholar]
- 81.Assiri A.M.A., Elbanna K., Al-Thubiani A., Ramadan M.F. Cold-pressed oregano (Orangium vulgare) oil: a rich source of bioactive lipids with novel antioxidant and microbial properties. Eur. Food Res. Technol. 2016;242:1013–1023. [Google Scholar]
- 82.Rahman A., Al-Reza S.M., Kang S.C. Antifungal activity of essential oil and extracts of Piper chaba hunter against phytopathogenic fungi. JAOCS (J. Am. Oil Chem. Soc.) 2011;88:573–579. [Google Scholar]
- 83.Zore G.B., Thakre A.D., Jadhav S., Karuppayil S.M. Terpenoids inhibit Candida albicans growth by affecting membrane integrity and arrest of cell cycle. Phytomedicine. 2011;18:1181–1190. doi: 10.1016/j.phymed.2011.03.008. [DOI] [PubMed] [Google Scholar]
- 84.Oyedeji O.A., Adeniyi B.A., Ayayi O., König W.A. Essential oil composition of Piper guineense and its antimicrobial activity. Another chemotype from Nigeria. Phytother Res. 2005;19:362–364. doi: 10.1002/ptr.1679. [DOI] [PubMed] [Google Scholar]
- 85.Rocha D.S., Silva J.M., Navarro D.M., Camara C.A.G., Lira C.S., Ramos C.S. Potential antimicrobial and chemical composition of essential oils from Pipper caldense tissues. J. Mexican Chem. Soc. 2016;60(3):148–151. [Google Scholar]
- 86.Thanissery R., Kathariou S., Smith D.P. Rosemary oil, clove oil, and a mix of thyme-orange essential oils inhibit Salmonella and Campylobacter in vitro. J. Appl. Poultry Res. 2014 Jun 1;23(2):221–227. [Google Scholar]
- 87.Conner . In: Naturally Occurring Compounds,” in Antimicrobials in Foods. Davidison P.M., Branen A.L., editors. Marcel Dekker; New York, NY, USA: 1993. pp. 441–468. [Google Scholar]
- 88.Kim J., Marshall M.R., Wei C.-I. Antibacterial activity of some essential oil components against five foodborne pathogens. J. Agric. Food Chem. 1995;43(11):2839–2845. [Google Scholar]
- 89.Sutili F.J., de Lima Silva L., Gressler L.T., Gressler L.T., Battisti E.K., Heinzmann B.M., de Vargas A.C., Baldisserotto B. Plant essential oils against A eromonas hydrophila: in vitro activity and their use in experimentally infected fish. J. Appl. Microbiol. 2015 Jul;119(1):47–54. doi: 10.1111/jam.12812. [DOI] [PubMed] [Google Scholar]
- 90.Bachir R.G., Benali M. Antibacterial activity of the essential oils from the leaves of Eucalyptus globulus against Escherichia coli and Staphylococcus aureus. Asian Pacific J. Tropical Biomed. 2012 Sep;2(9):739. doi: 10.1016/S2221-1691(12)60220-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Paster N., Juven B.J., Shaaya E., Menasherov M., Nitzan R., Weisslowicz H., Ravid U. Inhibitory effect of oregano and thyme essential oils on moulds and foodborne bacteria. Lett. Appl. Microbiol. 1990 Jul;11(1):33–37. [Google Scholar]
- 92.Aguilar-González A.E., Palou E., López-Malo A. Antifungal activity of essential oils of clove (Syzygium aromaticum) and/or mustard (Brassica nigra) in vapor phase against gray mold (Botrytis cinerea) in strawberries. Innovat. Food Sci. Emerg. Technol. 2015 Dec 1;32:181–185. [Google Scholar]
- 93.Katsukawa M., Nakata R., Takizawa Y., Hori K., Takahashi S., Inoue H. Citral, a component of lemongrass oil, activates PPARα and γ and suppresses COX-2 expression. Biochim. Biophys. Acta Mol. Cell Biol. Lipids. 2010;1801(11):1214–1220. doi: 10.1016/j.bbalip.2010.07.004. [DOI] [PubMed] [Google Scholar]
- 94.Conti B., Cnale A., Cioni P.L., Flamini G. Repellence of essential oils from tropical and Mediterranean Lamiaceae against Sitophilus zeamais. Bull. Insectol. 2010;63(2):197–202. [Google Scholar]
- 95.Rottini M.M., Amaral A.C.F., Ferreira J.L.P., Oliviera E.S.C., Siva J.R., Taniwaki N.N., et al. Endlicheria bracteolate (Meisn.) essential oil as a weapon against Leishmania amazonensis: in vitro assay. Molecules. 2019;24:2525–2538. doi: 10.3390/molecules24142525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Barbosa P., Lima A.S., Vieira P., Dias L.S., Tinoco M.T., Barroso J.G., Pedro L.G., Figueiredo A.C., Mota M. Nematicidal activity of essential oils and volatiles derived from Portuguese aromatic flora against the pinewood nematode, Bursaphelenchus xylophilus. J. Nematol. 2010 Mar;42(1):8. [PMC free article] [PubMed] [Google Scholar]
- 97.Wei-xuan W.A., Kui W.A., Jian-mei X.U., Li-gang Z.H. Antinematodal activity of the leaf essential oils of Cymbopogon citratus and Tithonia diversifolia. Nat. Prod. Res. Dev. 2016 Aug 30;28(8):1266. [Google Scholar]
- 98.López V., Cascella M., Benelli G., Maggi F., Gómez-Rincón C. Green drugs in the fight against Anisakis simplex—larvicidal activity and acetylcholinesterase inhibition of Origanum compactum essential oil. Parasitol. Res. 2018 Mar 1;117(3):861–867. doi: 10.1007/s00436-018-5764-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.López V., Pavela R., Gómez-Rincón C., Les F., Bartolucci F., Galiffa V., Petrelli R., Cappellacci L., Maggi F., Canale A., Otranto D. Efficacy of Origanum syriacum essential oil against the mosquito vector Culex quinquefasciatus and the gastrointestinal parasite Anisakis simplex, with insights on Acetylcholinesterase inhibition. Molecules. 2019 Jan;24(14):2563. doi: 10.3390/molecules24142563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Gómez-Rincón C., Langa E., Murillo P., Valero M.S., Berzosa C., López V. Activity of tea tree (Melaleuca alternifolia) essential oil against L3 larvae of Anisakis simplex. BioMed Res. Int. 2014 May 25:2014. doi: 10.1155/2014/549510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Allahverdiyev A., Duran N., Ozguven M., Koltas andS. Antiviral activity of the volatile oils of Melissa officinalis L. against Herpessimplexvirustype-2. Phytomedicine. 2004;11(7–8):657–661. doi: 10.1016/j.phymed.2003.07.014. [DOI] [PubMed] [Google Scholar]
- 102.Reichling J., Schnitzler P., Suschke U., Saller R. Essential oils of aromatic plants with antibacterial, antifungal, antiviral, andcytotoxicproperties-anoverview. ForschendeKomplementarmedizin. 2009;16(2):79–90. doi: 10.1159/000207196. [DOI] [PubMed] [Google Scholar]
- 103.Wagstaff A.J., Faulds D., Goa K.L. Aciclovir:areappraisal of its antiviral activity, pharmacokinetic properties and therapeutic efficacy. Drugs. 1994;47(1):153–205. doi: 10.2165/00003495-199447010-00009. [DOI] [PubMed] [Google Scholar]
- 104.Bishop Chris D. Antiviral activity of the essential oil of Melaleuca alternfolia (maiden & betche) cheel (tea tree) against Tobacco mosaic virus. J. Essent. Oil Res. Nov/Dec 1995;7:641–644. [Google Scholar]
- 105.Astani Akram, Reichling Jürgen, Paul Schnitzler. Comparative study on the antiviral activity of selected monoterpenes derived from essential oils. J. Phytother. Res. 2010;24:673–679. doi: 10.1002/ptr.2955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Farag R.S., Shalaby A.S., El-Baroty G.A., Ibrahim N.A., Ali M.A., Hassan E.M. Chemical and biological evaluation of the essential oils of different Melaleuca species. Phytother Res. 2004;18:30–35. doi: 10.1002/ptr.1348. [DOI] [PubMed] [Google Scholar]
- 107.Schnitzler P., Schön K., Reichling J. Antiviral activity of Australian tea tree oil and eucalyptus oil against herpes simplex virus in cell culture. Pharmazie. 2001;56:343–347. [PubMed] [Google Scholar]
- 108.Reichling J., Koch C., Stahl-Biskup E., Sojka C., Schnitzler P. Virucidal activity of a beta-triketone-rich essential oil of Leptospermum scoparium (manuka oil) against HSV-1 and HSV-2 in cell culture. Planta Med. 2005;71:1123–1127. doi: 10.1055/s-2005-873175. [DOI] [PubMed] [Google Scholar]
- 109.Schnitzler P., Koch C., Reichling J. Susceptibility of drug resistant clinical HSV-1 strains to essential oils of ginger, thyme, hyssop and sandal wood. Antimicrob. Agents Chemother. 2007;51:1859–1862. doi: 10.1128/AAC.00426-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Hayashi K., Hayashi T., Ujita K., Takaishi Y. Characterization of antiviral activity of a sesquiterpene, triptofordin C-2. J. Antimicrob. Chemother. 1996;37:759–768. doi: 10.1093/jac/37.4.759. [DOI] [PubMed] [Google Scholar]
- 111.Niedermeyer T.H., Lindequist U., Mentel R., et al. Antiviral terpenoid constituents of Ganoderma pfeifferi. J. Nat. Prod. 2005;68:1728–1731. doi: 10.1021/np0501886. [DOI] [PubMed] [Google Scholar]
- 112.Benencia F., Courrèges M.C. Antiviral activity of sandal wood oil against herpes simplex viruses-1 and -2. Phytomedicine. 1999;6:119–123. doi: 10.1016/S0944-7113(99)80046-4. [DOI] [PubMed] [Google Scholar]
- 113.Tragoolpua Y., Jatisatieur A. Anti-herpes simplex virus activities of Eugenia caryophyllus (Spreng.) Bullock & S. G. Harrison and essential oil, eugenol. Phytother Res. 2007;21:1153–1158. doi: 10.1002/ptr.2226. [DOI] [PubMed] [Google Scholar]
- 114.Armaka M., Papanikolaou E., Sivropoulou A., Arsenakis M. Antiviral properties of isoborneol, a potent inhibitor of herpes simplex virus type 1. Antivir. Res. 1999;43:79–92. doi: 10.1016/s0166-3542(99)00036-4. [DOI] [PubMed] [Google Scholar]
- 115.Schnitzler P., Astani A., Reichling J. Screening for antiviral activities of isolated compounds from essential oils. Evid. Based Complement Altern. Med. 2011;2011:2536438. doi: 10.1093/ecam/nep187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Venturi C.R., Danielli L.J., Klein F., et al. Chemical analysis and in vitro antiviral and antifungal activities of essential oils fromGlechonspathulata and Glechonmarifolia. Pharmaceut. Biol. 2015;53(5):682–688. doi: 10.3109/13880209.2014.936944. [DOI] [PubMed] [Google Scholar]
- 117.Allahverdiyev A., Duran N., Ozguven M., Koltas S. Antiviral activity of the volatile oils of Melissa officinalis L. against Herpes simplex virus type-2. Phytomedicine. 2004;11(7–8):657–661. doi: 10.1016/j.phymed.2003.07.014. [DOI] [PubMed] [Google Scholar]
- 118.Garcıa C.C., Talarico L., Almeida N., Colombres S., Duschatzky C., Damonte E.B. Virucidal activity of essential oils from aromatic plants of San Luis,Argentina. Phytother Res. 2003;17(9):1073–1075. doi: 10.1002/ptr.1305. [DOI] [PubMed] [Google Scholar]
- 119.Swamy M.K., Sinniah U.R. A comprehensive review on the phytochemical constituents and pharmacological activities of Pogostemon cablin Benth.: an aromatic medicinal plant of industrial importance. Molecules. 2015;20(5):8521–8547. doi: 10.3390/molecules20058521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Ibrahim N.A., El-Hawary S.S., Mohammed M.M.D., et al. Chemical composition, antiviral against avian influenza (H5N1) virus and antimicrobial activities of the essential oils of the leaves and fruits of Fortunella margarita, lour. swingle, growing in Egypt. J. Appl. Pharmaceut. Sci. 2015;5(1):6–12. [Google Scholar]
- 121.Zeedan G.S.G., Abdalhamed A.M., Ottai M.E., Abdelshafy S., Abdeen E. Antimicrobial, antiviral activity and GC-MS analysis of essential oil extracted from Achillea fragrantissima plant growing in Sinai Peninsula, Egypt. J. Microb. Biochem. Technol. 2014;8:6. [Google Scholar]
- 122.Pourghanbari G., Nili H., Moattari A., Mohammadi A., Iraji A. Antiviral activity of the oseltamivir and Melissa officinalis L.essential oil against avian influenza virus (H9N2) Virus Disease. 2016;27(2):170–178. doi: 10.1007/s13337-016-0321-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Brand Y.M., Roa-Linares V.C., Betancur-Galvis L.A., Durán-Garcıa D.C., Stashenko E. Antiviral activity of Colombian Labiatae and Verbenaceae family essential oils and monoterpenes on Human Herpes viruses. J. Essent. Oil Res. 2016;28(2):130–137. [Google Scholar]
- 124.Cermelli Claudio, et al. Effect of eucalyptus essential oil on respiratory bacteria and viruses. Curr. Microbiol. 2008;56(1):89–92. doi: 10.1007/s00284-007-9045-0. [DOI] [PubMed] [Google Scholar]
- 125.Schnitzler P., Wiesenhofer K., Reichling J. Comparative study on the cytotoxicity of different Myrtaceae essential oils on cultured Vero and RC-37 cells. Die Pharmazie-An International Journal of Pharmaceutical Sciences. 2008;63(11):830–835. [PubMed] [Google Scholar]
- 126.Ma L., Yao L. Antiviral effects of plant-derived essential oils and their components: an updated review. Molecules. 2020 Jan;25(11):2627. doi: 10.3390/molecules25112627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Jackwood M.W., Rosenbloom R., Petteruti M., Hilt D.A., McCall A.W., Williams S.M. Avian coronavirus infectious bronchitis virus susceptibility to botanical oleoresins and essential oils in vitro and in vivo. Virus Res. 2010;149:86–94. doi: 10.1016/j.virusres.2010.01.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Wen C.C., Kuo Y.H., Jan J.T., Liang P.H., Wang S.Y., Liu H.G., et al. Specific plant terpenoids and lignoids possess potent antiviral activities against severe acute respiratory syndrome coronavirus. J. Med. Chem. 2007;50:4087–4095. doi: 10.1021/jm070295s. [DOI] [PubMed] [Google Scholar]
- 129.Ulasli M., Gurses S.A., Bayraktar R., Yumrutas O., Oztuzcu S., Igci M., et al. The effects of Nigella sativa (ns), Anthemis hyalina (ah) and Citrus sinensis (Cs) extracts on the replication of coronavirus and the expression of TRP genes family. Mol. Biol. Rep. 2014;41:1703–1711. doi: 10.1007/s11033-014-3019-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Loizzo M.R., Saab A.M., Tundis R., Statti G.A., Menichini F., Lampronti I., Gambari R., Cinatl J., Doerr H.W. Phytochemical analysis and in vitro antiviral activities of the essential oils of seven Lebanon species. Chem. Biodivers. 2008;5(3):461–470. doi: 10.1002/cbdv.200890045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.World Health Organisation WHO coronavirus disease (COVID-19) dashboard. https://covid19.who.int/ Accessed 2020/9/26. Available at.
- 132.Thuy B.T.P., et al. Investigation into SARS-CoV-2 resistance of compounds in garlic essential oil. ACS Omega. 2020;5:8312–8320. doi: 10.1021/acsomega.0c00772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Lee C. Therapeutic modulation of virus-induced oxidative stress via the Nrf2-dependent antioxidative pathway. Oxid Med Cell Longev. 2018:6208067. doi: 10.1155/2018/6208067. 2018:6208067–. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.McCord J.M., Hybertson B.M., Cota-Gomez A., Gao B. 2020. Nrf2 Activator PB125 ® as a Potential Therapeutic Agent against COVID-19. bioRxiv:2020.2005.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Ho C.Y., Cheng Y.T., Chau C.F., Yen G.C. Effect of diallyl sulfide on in vitro and in vivo Nrf2-mediated pulmonic antioxidant enzyme expression via activation ERK/p38 signaling pathway. J. Agric. Food Chem. 2012;60:100–107. doi: 10.1021/jf203800d. [DOI] [PubMed] [Google Scholar]
- 136.Patel V.J., Biswas Roy S., Mehta H.J., Joo M., Sadikot R.T. Alternative and natural therapies for acute lung injury and acute respiratory distress syndrome. BioMed Res. Int. 2018:2476824. doi: 10.1155/2018/2476824. 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Kulkarni S.A., Nagarajan S.K., Ramesh V., Palaniyandi V., Selvam S.P., Madhavan T. Computational evaluation of major components from plant essential oils as potent inhibitors of SARSCoV-2 spike protein. J. Mol. Struct. 2020;1221:128823. doi: 10.1016/j.molstruc.2020.128823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Elfiky A.A. SARS-CoV-2 RNA dependent RNA polymerase (RdRp) targeting: an in silico perspective. J. Biomol. Struct. Dyn. 2020 doi: 10.1080/07391102.2020.1761882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Huang H., Wang Y. The protective effect of cinnamaldehyde on lipopolysaccharide induced acute lung injury in mice. Cell. Mol. Biol. 2017;63:58–63. doi: 10.14715/cmb/2017.63.8.13. [DOI] [PubMed] [Google Scholar]
- 140.Bastos V.P., et al. Inhibitory effect of 1,8-cineole on Guinea-pig airway challenged with ovalbumin involves a preferential action on electromechanical coupling. Clin. Exp. Pharmacol. Physiol. 2009;36:1120–1126. doi: 10.1111/j.1440-1681.2009.05189.x. [DOI] [PubMed] [Google Scholar]
- 141.Coelho-de-Souza L., Leal-Cardoso J., Matos F., Saad L., Magalhães P. Relaxant effects of the essential oil of Eucalyptus tereticornis and its main constituent 1,8-cineole on Guinea-pig tracheal smooth muscle. Planta Med. 2006;71:1173–1175. doi: 10.1055/s-2005-873173. [DOI] [PubMed] [Google Scholar]
- 142.Juergens L.J., Worth H., Juergens U.R. New perspectives for mucolytic, anti-inflammatory and adjunctive therapy with 1,8-cineole in COPD and asthma: review on the new therapeutic approach. Adv. Ther. 2020;37:1737–1753. doi: 10.1007/s12325-020-01279-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Lau S.K.P., et al. Coexistence of different genotypes in the same bat and serological characterization of Rousettus bat coronavirus HKU9 belonging to a novel Betacoronavirus subgroup. J. Virol. 2010;84:11385–11394. doi: 10.1128/JVI.01121-10. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 144.Sharma A.D., Kaur I. Jensenone from eucalyptus essential oil as a potential inhibitor of COVID 19 corona virus infection. Res Rev Biotech Biosci. 2020;7:59–66. [Google Scholar]
- 145.Sharma A.D., Kaur I. Eucalyptol (1,8 cineole) from eucalyptus essential oil a potential inhibitor of COVID 19 corona virus infection by molecular docking studies. Preprints. 2020:2020030455. [Google Scholar]
- 146.Merad M., Martin J.C. Pathological inflammation in patients with COVID-19: a key role for monocytes and macrophages. Nat. Rev. Immunol. 2020;20:355–362. doi: 10.1038/s41577-020-0331-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Sadlon A.E., Lamson D.W. Immune-modifying and antimicrobial effects of eucalyptus oil and simple inhalation devices. Altern Med Rev J Clin Ther. 2010;15:33–47. [PubMed] [Google Scholar]
- 148.Silva J., Figueiredo P., Byler K., Setzer W. Essential oils as antiviral agents. Potential of essential oils to treat SARS-CoV-2 infection: an in-silico investigation. Int. J. Mol. Sci. 2020;21:3426. doi: 10.3390/ijms21103426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Bhardwaj K., Sun J., Holzenburg A., Guarino L.A., Kao C.C. RNA recognition and cleavage by the SARS coronavirus endoribonuclease. J. Mol. Biol. 2006;361:243–256. doi: 10.1016/j.jmb.2006.06.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Shuai C., et al. An overall picture of SARS coronavirus (SARSCoV) genome-encoded major proteins: structures, functions and drug development. Curr. Pharmaceut. Des. 2006;12:4539–4553. doi: 10.2174/138161206779010459. [DOI] [PubMed] [Google Scholar]
- 151.Zhang H., Penninger J.M., Li Y., Zhong N., Slutsky A.S. Angiotensin-converting enzyme 2 (ACE2) as a SARS-CoV-2 receptor: molecular mechanisms and potential therapeutic target. Intensive Care Med. 2020;46:586–590. doi: 10.1007/s00134-020-05985-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Kumar A., Choudhir G., Shukla S.K., Sharma M., Tyagi P., Bhushan A., Rathore M. Identification of phytochemical inhibitors against main protease of COVID-19 using molecular modeling approaches. J. Biomol. Struct. Dyn. 2020 doi: 10.1080/07391102.2020.1772112. Pubmed. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Bastaki S.M., Adeghate E., Amir N., Ojha S., Oz M. Menthol inhibits oxidative stress and inflammation in acetic acid-induced colitis in rat colonic mucosa. Am J Transl Res. 2018;10:4210–4222. [PMC free article] [PubMed] [Google Scholar]
- 154.Rozza A.L., Meira de Faria F., Souza Brito A.R., Pellizzon C.H. The gastroprotective effect of menthol: involvement of anti-apoptotic, antioxidant and anti-inflammatory activities. PloS One. 2014;9 doi: 10.1371/journal.pone.0086686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Benencia F., Courrèges M.C. In vitro and in vivo activity of eugenol on human herpesvirus. Phytotherapy Res PTR. 2000;14:495–500. doi: 10.1002/1099-1573(200011)14:7<495::aid-ptr650>3.0.co;2-8. [DOI] [PubMed] [Google Scholar]
- 156.Barboza J.N., da Silva Maia Bezerra Filho C., Silva R.O., Medeiros J.V.R., de Sousa D.P. An overview on the anti-inflammatory potential and antioxidant profile of eugenol. Oxid Med Cell Longev. 2018:3957262. doi: 10.1155/2018/3957262. 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Games E., et al. Structurally related monoterpenes p-cymene, carvacrol and thymol isolated from essential oil from leaves of Lippia sidoides Cham. (Verbenaceae) protect mice against elastaseinduced emphysema. Molecules. 2016;21:1390. doi: 10.3390/molecules21101390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Senthil Kumar K.J., Gokila Vani M., Wang C.S., Chen C.C., Chen Y.C., Lu L.P., Huang C.H., Lai C.S., Wang S.Y. Geranium and lemon essential oils and their active compounds downregulate angiotensin-converting enzyme 2 (ACE2), a SARS-CoV-2 spike receptor-binding domain, in epithelial cells. Plants. 2020 Jun;9(6):770. doi: 10.3390/plants9060770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.McMahon M.A., Siliciano J.D., Lai J., Liu J.O., Stivers J.T., Siliciano R.F., Kohli R.M. The antiherpetic drug acyclovir inhibits HIV replication and selects the V75I reverse transcriptase multidrug resistance mutation. J. Biol. Chem. 2008;283:31289–31293. doi: 10.1074/jbc.C800188200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Li Q., Wu J., Nie J., Zhang L., Hao H., Liu S., Zhao C., Zhang Q., Liu H., Nie L., Qin H. The impact of mutations in SARS-CoV-2 spike on viral infectivity and antigenicity. Cell. 2020 Sep 3;182(5):1284–1294. doi: 10.1016/j.cell.2020.07.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Hudson J.B. CRC Press; Boston, MA, USA: 1990. Antiviral Compounds from Plants. [Google Scholar]