Abstract
It has been shown that the coexistence of methanogenesis and reductive acetogenesis in the hindgut of the wood-feeding termite Reticulitermes flavipes is based largely on the radial distribution of the respective microbial populations and relatively high hydrogen partial pressures in the gut lumen. Using Clark-type microelectrodes, we showed that the situation in Cubitermes orthognathus and other soil-feeding members of the subfamily Termitinae is different and much more complex. All major compartments of agarose-embedded hindguts were anoxic at the gut center, and high H2 partial pressures (1 to 10 kPa) in the alkaline anterior region rendered the mixed segment and the third proctodeal segment (P3) significant sources of H2. Posterior to the P3 segment, however, H2 concentrations were generally below the detection limit (<100 Pa). All hindgut compartments turned into efficient hydrogen sinks when external H2 was supplied, but methane was formed mainly in the P3/4a and P4b compartments, and in the latter only when H2 or formate was added. Addition of H2 to the gas headspace stimulated CH4 emission of living termites, indicating that endogenous H2 production limits methanogenesis also in vivo. At the low H2 partial pressures in the posterior hindgut, methanogens would most likely outcompete homoacetogens for this electron donor. This might explain the apparent predominance of methanogenesis over reductive acetogenesis in the hindgut of soil-feeding termites, although the presence of homoacetogens in the anterior, highly alkaline region cannot yet be excluded. In addition, the direct contact of anterior and posterior hindgut compartments in situ permits a cross-epithelial transfer of H2 or formate, which would not only fuel methanogenesis in these compartments, but would also create favorable microniches for reductive acetogenesis. In situ rates and spatial distribution of H2-dependent acetogenic activities are addressed in a companion paper (A. Tholen and A. Brune, Appl. Environ. Microbiol. 65:4497–4505, 1999).
In the metabolic reactions involved in lignocellulose degradation in termite hindguts, hydrogen appears to be the key intermediate linking the fermentative breakdown of carbohydrates with methanogenesis and reductive acetogenesis. In lower termites, H2 formation is mainly attributed to the dense populations of cellulolytic flagellates which are characteristic for this group. In higher termites, symbiotic flagellates are absent, and the reactions responsible for the formation of H2 are not known. Reductive acetogenesis and methanogenesis, however, occur in all termites investigated to date and are considered typical for the strictly anaerobic metabolic activities in termite guts (for reviews, see references 9–11).
In earlier studies, it was generally assumed that the H2 partial pressure in termite guts is very low. Since methanogens would be expected to outcompete homoacetogens for H2 in a homogeneous well-mixed system simply for thermodynamic reasons (31), it was enigmatic why reductive acetogenesis apparently predominates over methanogenesis as the major hydrogen sink reaction in the guts of many wood-feeding termites (7, 9). However, recent studies with microsensors have revealed that termite hindguts are by far not homogeneous anoxic fermentors, but are highly structured environments characterized by steep gradients of O2, H2, and pH (12, 13, 17). In the case of the wood-feeding lower termite Reticulitermes flavipes, the hindgut paunch exhibits luminal H2 partial pressures as high as 5 kPa (17). The methanogenic microbial population of this termite is located almost exclusively within the microoxic periphery of the hindgut, i.e., directly at the gut epithelium (21), where it represents a major hydrogen sink and gives rise to steep H2 gradients towards the gut wall (17). The homoacetogenic microorganisms, however, are most likely located within the gut lumen, where they would benefit from the high H2 partial pressure (17, 22). On the basis of these results, it was postulated that the coexistence of homoacetogens and methanogens within the hindgut of this termite is not based on direct competition for a limited substrate, but rather on resource partitioning effected by the spatial distribution of the different H2-consuming populations within the gut (11, 17). It is not clear whether the situation in Reticulitermes flavipes can be generalized for other wood-feeding termites.
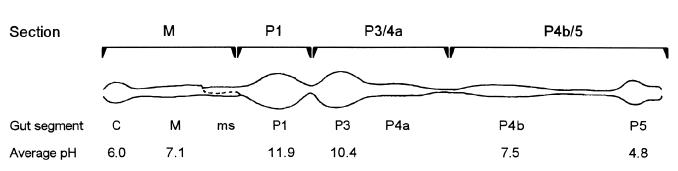
In contrast to lower termites, the majority of members of the most abundant and ecologically important family of higher termites (Termitidae) are soil feeding (38). A humivorous mode of nutrition is attributed to the majority of all genera in three of the four subfamilies, Apicotermitinae (95%), Termitinae (74%), and Nasutitermitinae (44%), whereas no soil feeders are found among the fungus-cultivating members of the subfamily Macrotermitinae (24). The hindguts of soil-feeding termites are highly compartmentalized and characterized by an unusually high pH in their anterior region (2–4) (Fig. 1). In soil-feeding Termitinae, the luminal pH increases sharply in the mixed segment, a gut region located between the neutral midgut and the first proctodeal dilation (P1), which possesses the highest alkalinity ever observed in biological systems (pH >12 [12]).
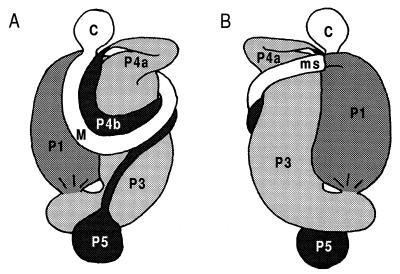
FIG. 1.
Gut morphology of a Cubitermes sp. worker termite, also representative of other soil-feeding species of the subfamily Termitinae. The gut was drawn in its unraveled state to illustrate the various segments: C, crop; M, midgut; ms, mixed segment; P1 to -5, proctodeal segments 1 to 5, respectively. The average luminal pH was determined for the indicated gut regions in Cubitermes speciosus by using intact guts and glass pH microelectrodes (12). When gut sections were used, the guts were separated at the indicated positions.
Soil-feeding termites generally show higher methane emission rates and lower potential rates of H2-dependent acetogenesis than wood-feeding species, indicating that in this feeding guild, methanogenesis represents the major hydrogen sink reaction in the hindgut (7, 29). In view of the enormous biomass of termites and their keystone role in decomposition processes in tropical ecosystems, it is not astonishing that their CH4 emissions are considered to contribute significantly to the global fluxes of this atmospherically relevant trace gas (for a review, see references 6 and 30). It is therefore important to understand the physiological and autecological factors that regulate the coexistence of the H2-oxidizing populations in the gut microbial community of these termites. To date, it is completely unknown whether H2 accumulates to significant concentrations in any of the hindgut compartments, whether homoacetogens coexist with methanogens in the same compartment(s), or whether each group is localized in different gut regions. Since the existing studies have so far compared CH4 emission of living termites with H2-dependent acetogenesis in gut homogenates, it also cannot be excluded that the importance of homoacetogens in soil-feeding termites has been underestimated.
In this study, we used Clark-type microelectrodes to characterize the axial and radial profiles of O2 and H2 partial pressure in the gut of soil-feeding Cubitermes spp., focusing on the distribution of hydrogen sources and potential hydrogen sinks among the different gut compartments with respect to the localization of the methanogenic activities. In a parallel study described in a companion paper, we used a newly developed technique involving microinjection of radiotracers to determine in situ rates of reductive acetogenesis and the exact location of homoacetogens within the gut (33).
MATERIALS AND METHODS
Termites.
Cubitermes orthognathus Emerson and Cubitermes umbratus Williams were collected near Busia (Kenya) and in the Shimba Hills Natural Reserve (Kenya), respectively; Thoracotermes macrothorax (Sjöstedt), Noditermes indoensis Sjöstedt, and Procubitermes sp. (all Termitidae: Termitinae) were collected in the Mayombe rain forest (Congo-Brazzaville). Anoplotermes sp. (Termitidae: Apicotermitinae) was collected in a coastal rain forest in southern Brazil and tentatively identified as A. pacificus Fr. Müller on the basis of the close association of the nest with tree roots (19). The termites were brought to the laboratory in polypropylene containers together with nest fragments and soil of the original collection site; measurements were generally performed within 1 to 2 months of collection. Reticulitermes flavipes (Kollar) (Rhinotermitidae) and Nasutitermes arborum (Smeathman) (Termitidae: Nasutitermitinae) were from birch-fed laboratory cultures. Worker caste termites were used for all experiments.
Microelectrode measurements.
Clark-type oxygen microelectrodes with guard cathodes (28) were constructed in our laboratory and calibrated as described previously (13). Hydrogen microelectrodes had the same principal design (37), except that the working electrode was platinum coated as described by Ebert and Brune (17). Testing and calibration procedures were as previously described (17). Both types of microelectrodes had tip diameters of 10 to 20 μm.
The experimental setup for the measurements was essentially as described previously (13). The bottom of a glass-faced microchamber (17) was filled 2 to 4 mm deep with a layer of agarose (1%) made up with modified insect Ringer’s solution (13). A freshly dissected termite gut was placed flat and fully extended onto the agarose, and was quickly covered with a shallow layer (1 to 2 mm) of identical agarose (40°C), which cooled and solidified immediately. Both the microchamber and the agarose were electrically grounded for static discharge. Experiments with defined gas headspace were performed by using a glass bell placed over the microchamber, which was continuously flushed with the desired gas mixture (setup described in detail in reference 17). Microelectrodes were positioned with a manual micromanipulator (MM33; Märzhäuser, Wetzlar, Germany); all measurements were performed at room temperature (20 to 22°C).
Estimation of hydrogen fluxes.
Gut segments were approximated as endless cylinders, and the H2 flux from or into each segment was estimated from the slope of the radial H2 concentration profiles directly above the gut, by using Fick’s first law of diffusion: J = −2πrlφDs δC(r)/δr (16), where J is the total flux of molecules through the surface per unit of time, r and l are segment radius and length, respectively, φ is the porosity of the agarose, Ds is the apparent diffusion coefficient, and δC(r)/δr is the slope of the concentration gradient at radius r. The calculation details were exactly as previously described (17).
Gas emission by termites and gut sections.
Between 10 and 20 termites (60 for A. pacificus) were placed into small glass vials (10 ml). In the case of C. orthognathus, 20 guts were also dissected and separated into five sections representing the major gut compartments (Fig. 1), which were carefully placed onto filter paper strips (Whatman no. 1) soaked with buffered salt solution (BSS [35]). The paper strips were then placed into separate vials containing a shallow layer of BSS. The P3 and the P4a segments were not separated to avoid leaking of the gut contents. For the measurements under anoxic conditions, the complete preparation procedure was performed in an anoxic glove box with a N2 atmosphere. All vials were closed with rubber septa; when indicated, the gas headspace was exchanged by flushing with the desired gas mixture for 1 min. The vials were incubated at room temperature (22 ± 1°C). For a period of 1.5 to 2 h, headspace samples (300 μl) were taken at regular intervals and immediately replaced with an equal volume of the original headspace mixture, using a syringe equipped with a gas-tight valve. CH4 and H2 concentrations were determined by gas chromatography on a molecular sieve column by using flame-ionization detection (27) and an HgO-reduction trace gas analyzer (18), respectively; the gas emission rates were corrected for the dilution effects caused by the sampling procedure. All gases were supplied by SWF, Friedrichshafen, Germany, and were 99.999% pure.
RESULTS
Axial profiles.
Freshly prepared guts of soil-feeding Termitinae embedded in agarose-Ringer’s solution remained physiologically active for more than 1 h, as indicated by the stable oxygen and hydrogen gradients between the hindgut and agarose surface, which usually reached a steady state within 5 to 10 min, and by (although infrequent) hindgut peristalsis, mainly in the posterior segments. In this respect, the results were similar to those previously obtained with wood-feeding termites (13, 17). Only the results with Cubitermes orthognathus are presented in detail, although axial and radial profiles of Cubitermes umbratus and Thoracotermes macrothorax guts gave essentially the same results.
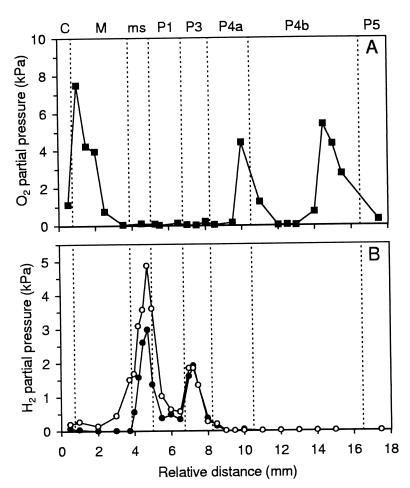
Axial oxygen profiles revealed that all gut compartments except the tubular midgut and the less-dilated regions of the posterior hindgut (Fig. 1) were anoxic at the gut center (Fig. 2A). Hydrogen accumulated only in the anterior gut region, with the highest H2 partial pressures in the mixed segment and P3 (Fig. 2B). In the P4a and P4b segments, H2 was always below the detection limit (100 Pa). The low values obtained in the rectum (P5) may have to be regarded with caution, since the termites frequently voided their rectal contents during dissection. When the measurements were performed under anoxic conditions, the H2 partial pressures in the anterior gut regions increased significantly; only in P3 was this effect less pronounced.
FIG. 2.
Axial profiles of oxygen (A) and hydrogen (B) partial pressure in agarose-embedded guts of Cubitermes orthognathus. H2 measurements were performed under air (●) or under N2 headspace (○). The graphs represent typical profiles obtained with freshly fed termites. All values are for the gut center. The borders between gut segments are indicated by vertical dotted lines; see Fig. 1 for definitions of abbreviations.
We found that H2 partial pressures in the anterior hindgut decreased progressively with time already during the first weeks after collection; this phenomenon was most pronounced in case of P3. However, high H2 partial pressures in the P3 segment of Cubitermes orthognathus, which ranged from 0.1 to 1 kPa in starved individuals, could be restored by feeding topsoil from the collection site to small batches of termites. Within 24 h, the H2 partial pressure in the P3 rose to maximum values (1.5 to 9 kPa), which were even higher than those measured 2 weeks after collection. Apparently, intestinal H2 production in soil-feeding Termitinae is severely affected by the starvation situation with which the termites are inevitably faced in the laboratory. Unfortunately, all long-term feeding attempts by us, and to our knowledge by others, have so far been unsuccessful.
Radial profiles.
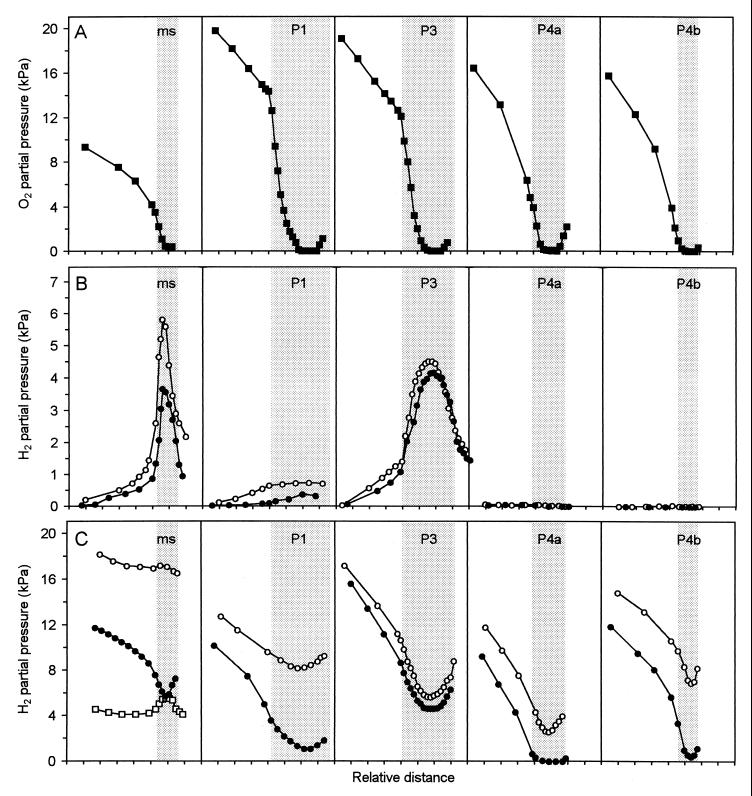
The negative slopes of the O2 profiles from the agarose surface toward the gut wall indicated that all hindgut regions were significant oxygen sinks (Fig. 3A). The gradients were quasilinear at a distance, but due to the radial symmetry of the system, increased in curvature close to the gut wall, which was most obvious when the gut diameter was small. The sharp changes in slope at the perimeter of the P1 and the P3 segments, however, which were previously observed as well with Cubitermes speciosus (20), indicate a smaller diffusion coefficient in these gut regions, probably caused by a lower porosity of the tightly packed gut contents. In the large proctodeal compartments P1 and P3, O2 often penetrated significantly into the gut, especially in starved termites, rendering only the gut center completely anoxic.
FIG. 3.
Radial profiles of the oxygen (A) and hydrogen partial pressure (B and C) around and within different hindgut segments of Cubitermes orthognathus. The guts were embedded in agarose; the shaded areas indicate the position of the gut proper in the surrounding agarose, represented by the white background. One axis mark represents a distance of 250 μm. Oxygen profiles (A) were measured under an air headspace, and hydrogen profiles were measured under air (●) or under N2 (○), without (B) or with (C) the addition of H2 (20 kPa) to the headspace gas. In the case of the mixed segment (ms), an N2 headspace with 5 kPa of H2 was also used (□). The graphs represent typical sets of similar profiles obtained in numerous experiments with different guts.
The highest hydrogen partial pressures were always found at the center of the mixed segment and the P3 segment (Fig. 3B). The steep concentration profiles indicated strong fluxes of H2 directed towards the gut periphery, which generally continued into the agarose. In the case of the mixed segment, the H2 efflux from the gut increased together with the luminal H2 partial pressure when the guts were incubated under anoxic conditions. Hydrogen accumulation and H2 fluxes from the gut proper into the agarose were less pronounced in P1, and were never observed in the posterior compartments, P4a and P4b.
Uptake of exogenously supplied hydrogen.
When exogenous H2 was provided via the headspace gas, all hindgut regions turned into hydrogen sinks, especially those which accumulated no or only small amounts of H2 from internal sources (Fig. 3C). Consumption of H2 was generally more pronounced when it was supplied in the presence of air than under nitrogen, and in the hindgut compartments posterior to the P3 compartment, external H2 was often completely consumed already in the gut periphery when O2 was present. Only in the case of the mixed segment incubated under anoxic conditions, endogenous H2 production caused H2 concentrations at the gut center despite the presence of exogenous H2; a comparison with the profile under air indicates a mainly aerobic nature of the H2-consuming activities in the mixed segment.
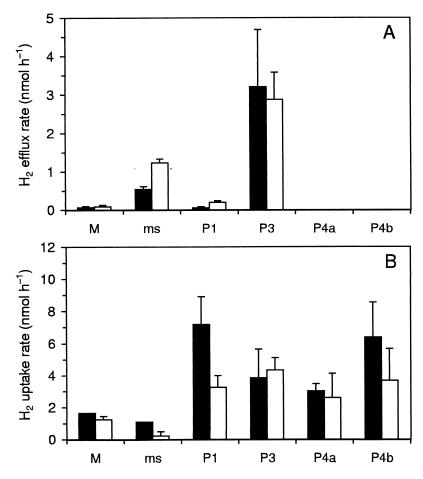
Hydrogen fluxes were estimated from the slopes of the gradients directly above each gut segment. The highest H2 emission rates were obtained with P3, which was followed by the mixed segment, whereas those of the midgut and P1 were much lower (Fig. 4A). When incubated under anoxic conditions, the H2 emission rates of the anterior segments increased more than twofold; only those of the P3 segment were not significantly affected. When exogenous H2 was added, all major hindgut compartments consumed H2 with roughly similar rates when compared on a per segment basis (Fig. 4B). Under oxic conditions, the sum of potential H2 uptake rates of all hindgut compartments was more than an order of magnitude higher than the efflux rates of endogenously produced H2 from the anterior region.
FIG. 4.
Hydrogen emission rates (A) and potential hydrogen uptake rates (B) of different gut regions of Cubitermes orthognathus, calculated from the slopes of the H2 gradients directly above each gut segment (see Fig. 3 for examples) and by approximating each segment as an endless cylinder. The gas headspace consisted of air (closed bars) or N2 (open bars); for H2 uptake rates, H2 (20 kPa) was added to the headspace gas. The values are means (± standard deviations) of three to five different profiles.
Methane and hydrogen emission by living termites.
The specific rates of CH4 emission of all termite species included in this study were roughly in the same range as their H2 emission rates; the emission rates for both gases increased only slightly when the termites were incubated under anoxic conditions (Table 1). The average of the methane emission rates of the soil-feeding species included in this study (0.180 ± 0.048 μmol g−1 h−1; air headspace) is only slightly lower than that of the rates reported by Nunes et al. (25) for a larger set of “field-sampled” species (0.238 ± 0.059 μmol g−1 h−1), whereas both data sets fall significantly behind the average of the methane emission rates found by Brauman et al. (7) for a comparable set of species sampled “directly from the nest” (0.730 ± 0.270 μmol g−1 h−1).
TABLE 1.
Hydrogen and methane emission rates of living termites under different headspace gases
| Termite speciesa | Fresh wt (mg) | H2 emission rate (nmol g [fresh wt]−1 h−1) for n samplesb
|
CH4 emission rate (nmol g [fresh wt]−1 h−1) for n samplesb
|
|||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Air | N2 | n | Air | N2 | n | Air + H2c | N2 + H2c | n | ||
| Nasutitermes arborum (w) | 2.8 | 122 ± 22 | 180 ± 16 | 4 | 143 | 149 | 1 | 784 ± 28 | 838 ± 31 | 2 |
| Reticulitermes flavipes (w) | 4.0 | 165 ± 68 | 244 ± 128 | 8 | 116 ± 22 | 131 ± 8 | 4 | 587 ± 180 | 695 ± 133 | 4 |
| Anoplotermes pacificus (s) | 1.1 | 195 ± 64 | 399 ± 146 | 5 | 260 ± 69 | 369 ± 72 | 2 | 1,674 ± 110 | 1,831 ± 266 | 2 |
| Cubitermes orthognathus (s) | 6.8 | NDd | ND | 158 ± 28 | 160 ± 29 | 3 | 1,123 ± 88 | 1,176 ± 254 | 3 | |
| Noditermes sp. (s) | 3.7 | 285 ± 110 | 449 ± 219 | 3 | 134 | 196 | 1 | 1,338 ± 352 | 1,311 ± 20 | 3 |
| Procubitermes sp. (s) | 11.5 | 127 ± 48 | 196 ± 54 | 3 | 181 | 200 | 1 | 1,015 ± 165 | 1,462 ± 169 | 3 |
| Thoracotermes macrothorax (s) | 11.8 | 122 ± 95 | 225 ± 56 | 3 | 168 ± 35 | 238 | 3 | 1,461 ± 338 | 1,372 ± 316 | 3 |
Wood-feeding (w) or soil-feeding (s) characteristic is indicated in parentheses.
Rates are given as means ± standard deviations of n independent assays.
H2 partial pressure was 20 kPa.
ND, not determined.
This may be related to our observation that both the CH4 and H2 emission rates of soil-feeding termites decreased progressively after the time of collection. Four months after collection, H2 emission rates of Thoracotermes macrothorax and Noditermes sp. had decreased to 13 to 14% of the values determined 2 weeks after collection. Also the H2 partial pressures in the anterior hindgut were much lower than in the first weeks after collection, probably caused by an insufficient supply of fermentable substrates in laboratory colonies which are presumably feeding mainly on mound material (see above). The hydrogen limitation of methanogenesis was completely relieved when exogenous H2 was provided in the headspace gas. In all termite species tested, including well-nourished wood-feeding laboratory cultures taken directly from their substrate, CH4 emission rates increased 5- to 10-fold and were almost unaffected by the presence of oxygen (Table 1).
Methane formation by gut sections.
In order to localize the methanogenic activities within the gut, we determined the CH4 production rates for individual gut sections of C. orthognathus incubated under oxic or anoxic conditions and in the presence or in the absence of externally supplied H2 (Table 2). The highest CH4 emission rates of all sections were observed with the P3/4a compartment, whereas only low rates were observed with the P1 and P4b compartments. In case of P4b, however, high potential methanogenic activities became apparent when external H2 or formate was added. Also the CH4 emission rates of the P3/4a section was strongly stimulated by external H2 or formate. Generally, H2-dependent methanogenesis was slightly higher in the absence of O2, and stimulation of methanogenesis by formate was higher than that by H2.
TABLE 2.
Methane emission rates of individual gut sections of Cubitermes orthognatus compared to those of living termitesa
| Termite type or section | CH4 emission rate (nmol termite−1 h−1)b
|
||||
|---|---|---|---|---|---|
| Air | N2 | Air + H2 | N2 + H2 | N2 + formate | |
| Live | 1.09 ± 0.11 | 1.12 ± 0.13 | 8.02 ± 0.09 | 8.09 ± 1.16 | NAc |
| M | —d | — | — | — | NDe |
| P1 | 0.09 ± 0.01 | 0.09 ± 0.01 | 0.11 ± 0.01 | 0.11 ± 0.02 | 0.15 |
| P3/4a | 0.21 ± 0.04 | 0.57 ± 0.13 | 1.51 ± 0.09 | 2.43 ± 0.72 | 4.73 |
| P4b | 0.02 ± 0.02 | 0.09 ± 0.02 | 0.13 ± 0.09 | 0.47 ± 0.11 | 0.51 |
| P5 | — | — | — | 0.05 ± 0.01 | ND |
Gut sections were incubated for 1.5 to 2 h and suspended in a shallow layer of BSS. The gas headspace consisted of air or N2, with or without the addition of H2 (20 kPa); formate (5 mM) was added to BSS.
Values are means ± mean deviations of two independent assays.
NA, not applicable.
—, below the detection limit (0.02 nmol termite−1 h−1).
ND, not determined.
Methane emission rates of living termites in the presence of external H2 were two- to threefold higher than the sum of the CH4 emission rates of separated gut segments under the same conditions, indicating that in the case of separated gut segments, the CH4 production rates have been underestimated. This is not astonishing considering that the shallow but unstirred layer of BSS covering the gut sections represents a considerably larger diffusion barrier for hydrogen than the tracheal system of the termites, in which virtually all diffusive transport occurs in the gas phase.
DISCUSSION
This is the first report on hydrogen partial pressures in the hindgut of soil-feeding termites. The results of a previous study, which so far represented the only study of this subject in insect guts, had shown that H2 accumulates to high concentrations in the hindgut paunch of the lower termite Reticulitermes flavipes (17) and had helped considerably in resolving the enigmatic predominance of reductive acetogens in wood-feeding termites (11, 32). Our current study demonstrates that the situation in the soil-feeding Termitinae subfamily is much more complex. In this group of termites, the hindgut is differentiated into several consecutive compartments, two of which are extremely alkaline (P1 and P3). Despite the anoxic conditions at the center of all major hindgut dilations, only those anterior to the P4a segment accumulated H2 to significant partial pressures, whereas H2 was generally below the detection limit in all hindgut compartments posterior to the P3 segment.
The posterior gut sections (P3/4a and P4b) also contained virtually all of the methanogenic activities in the hindgut. The efficient sinks for exogenous H2 in all hindgut compartments, the large CH4 emission rates of the isolated P3/4a section in the absence of exogenous electron donors, and the stimulation of CH4 emission by externally added H2 are strong indications that the low H2 partial pressures in the P4a segment are due not to the absence of H2-forming activities but rather to the efficient removal of endogenously formed H2, analogous to the situation in anoxic sediments (14). In contrast, endogenous sources of reducing equivalents in the P4b section seem to be negligible, since its methanogenic capacities became evident only when exogenous H2 or formate was added to the isolated gut sections.
Methane emission rates of all termite species tested in the present study increased considerably when living termites were incubated in the presence of exogenous H2, which is in agreement with two previous reports for Reticulitermes flavipes (17) and Zootermopsis angusticollis (23). It appears that both in lower and higher termites, irrespective of their feeding guild, methanogenic activities are strongly hydrogen limited. Nevertheless, all termites tested also emitted H2 at rates which were in the same range as those of CH4 emission (Table 1).
Hydrogen emission by the wood-feeding Reticulitermes flavipes has been attributed to the considerable accumulation of H2 in the lumen (17) and a certain degree of patchiness in the epithelial colonization by methanogenic archaea (21), which represent a major hydrogen sink in the gut periphery (17) and probably control H2 efflux. In soil-feeding members of Termitinae, however, there is an axial separation of H2-producing and H2-consuming activities, and the hydrogen profiles do not signify the presence of efficient H2 sinks at the epithelium of the anterior hindgut compartments (Fig. 3). This is in agreement with microscopic observations indicating the absence of any gut wall colonization in the P1 and P3 segments of Procubitermes aburiensis and Cubitermes umbratus (5, 34) and with the large amounts of endogenous H2 escaping from isolated guts (Fig. 4). At first glance, it is therefore quite astonishing that the H2 emission rates of soil-feeding termites did not significantly exceed the values observed with the wood-feeding Reticulitermes flavipes (Table 1) or other wood- and litter-feeding species (1, 25, 26, 36).
Most probably, the explanation lies in the proximity of the different hindgut regions within the abdomen of the insect (Fig. 5), which allows part of the H2 emitted from the anterior region (midgut, mixed segment, and P1 and P3 segments) to diffuse across the epithelia into the adjacent posterior segments (P4a or P4b) containing efficient hydrogen sinks. Also, formate, which was found in a high concentration (>2 mM) in the hemolymph of Cubitermes orthognathus (34) and which strongly stimulates the CH4 emission of isolated P3/4a and P4b sections (Table 2), has to be considered as a possible candidate for the transfer of reducing equivalents between the compartments. This is supported by results of Brauman et al. (8, 29), who found hydrogen- and formate-utilizing methanogens to occur in similar numbers in gut homogenates of Cubitermes speciosus and other soil-feeding members of the subfamily Termitinae.
FIG. 5.
In situ orientation of the different gut segments within the abdomen of a Cubitermes sp. worker termite. Both dorsal (A) and ventral (B) aspects are shown; for segment nomenclature, see the legend to Fig. 1.
The mixed segment and the third proctodeal segment (P3) are the major sources of H2 in the hindgut of the three soil-feeding Termitinae species tested in this study. Luminal H2 partial pressures were in the same range as those in the hindgut paunch of Reticulitermes flavipes (17). However, the anterior hindgut of soil-feeding Termitinae is characterized by an extremely high luminal pH, extending from the mixed segment to the P3, with an alkalinity maximum (pH 12) in the P1 compartment (12), which also accumulates less H2 than the mixed segment or P3. The P1 compartment of Procubitermes aburiensis and Cubitermes umbratus contains a significantly lower density of microorganisms than all other hindgut compartments (5, 34), and O2 consumption in the alkaline gut regions has been attributed in part to the autoxidation of phenolic residues (20). It is not yet clear which organisms or processes are responsible for the H2 production.
The large O2-consuming activities of all gut regions, the peripheral penetration of O2 into the hindgut lumen, and the anoxic status of the gut center are in agreement with previous results obtained for several other termites (13, 17, 20) and corroborate that also in soil feeders, a close juxtaposition of oxidative and fermentative processes within each hindgut dilation has to be expected. The increased H2 uptake rates of several hindgut compartments under oxic conditions may represent aerobic H2-oxidizing activities, but it is also possible that O2 affects the intestinal H2 production directly by its influence on the product pattern of the fermentative gut microbiota, as discussed by Ebert and Brune (17). In that case, decreased H2 uptake rates under anoxic conditions would merely reflect a partial saturation of the H2-oxidizing processes by the increased internal H2 production.
It is likely that the methanogenic activities in the P3/4a compartment are restricted to the P4a segment, which contains microorganisms with green F420-like autofluorescence (34). In contrast to the P4a segment, the P3 segment accumulated endogenous H2 to high concentrations (Fig. 3), but in view of the high alkalinity (pH ∼10) of the P3 segment in all soil-feeding species of Termitinae so far investigated (12), it can be speculated that H2-dependent acetogenesis also will be restricted to the posterior, methanogenic gut region. In those compartments, H2-dependent acetogens would have to compete with methanogens for H2. Even if methanogens prevent a luminal accumulation of H2 above the threshold values of homoacetogenic bacteria (15) in the hindgut region posterior to the P3, cross-epithelial H2 transfer may still create potential microniches for homoacetogens situated within the hydrogen gradient (e.g., directly below the gut epithelium).
In that context, it seems important to recall that the hypothetical predominance of methanogenesis over reductive acetogenesis in soil-feeding termites is so far based only on the comparison of CH4 emission rates of living termites with the potential rates of H2-dependent acetogenesis in gut homogenates (7). The in situ rates of reductive acetogenesis and the axial distribution of homoacetogenic bacteria remain to be determined. We have addressed these issues in a companion paper (33).
ACKNOWLEDGMENTS
This study was supported by a grant of the Deutsche Forschungsgemeinschaft (DFG) within the program “Structural and Functional Analysis of Natural Microbial Communities.”
We thank Erika Banchio, Andrea Ebert, and Thorsten Lemke for help with measurements and Bernhard Schink for continuing support. We are indebted to several colleagues who helped collect the termite species used in this study.
REFERENCES
- 1.Anklin-Mühlemann R, Bignell D E, Veivers P C, Leuthold R H, Slaytor M. Morphological, microbiological and biochemical studies of the gut flora in the fungus-growing termite Macrotermes subhyalinus. J Insect Physiol. 1995;41:929–940. [Google Scholar]
- 2.Bignell D E. Soil-feeding and gut morphology in higher termites. In: Hunt J H, Nalepa C A, editors. Nourishment and evolution in insect societies. Boulder, Colo: Westview Press; 1994. pp. 131–158. [Google Scholar]
- 3.Bignell D E, Anderson J M. Determination of pH and oxygen status in the guts of lower and higher termites. J Insect Physiol. 1980;26:183–188. [Google Scholar]
- 4.Bignell D E, Eggleton P. On the elevated intestinal pH of higher termites (Isoptera: Termitidae) Insectes Soc. 1995;42:57–69. [Google Scholar]
- 5.Bignell D E, Oskarsson H, Anderson J M. Distribution and abundance of bacteria in the gut of a soil-feeding termite Procubitermes aburiensis (Termitidae, Termitinae) J Gen Microbiol. 1980;117:393–403. doi: 10.1099/00221287-117-2-393. [DOI] [PubMed] [Google Scholar]
- 6.Bignell D E, Eggleton P, Nunes L, Thomas K L. Termites as mediators of carbon fluxes in tropical forests: budgets for carbon dioxide and methane emissions. In: Watt A B, Stork N E, Hunter M D, editors. Forests and insects. London, United Kingdom: Chapman and Hall; 1997. pp. 109–134. [Google Scholar]
- 7.Brauman A, Kane M D, Labat M, Breznak J A. Genesis of acetate and methane by gut bacteria of nutritionally diverse termites. Science. 1992;257:1384–1387. doi: 10.1126/science.257.5075.1384. [DOI] [PubMed] [Google Scholar]
- 8.Brauman A, Labat M, Garcia J L. Preliminary studies on the gut microbiota of the soil-feeding termite: Cubitermes speciosus. In: Lésel R, editor. Microbiology in Poecilotherms. Amsterdam, The Netherlands: Elsevier; 1990. pp. 73–77. [Google Scholar]
- 9.Breznak J A. Acetogenesis from carbon dioxide in termite guts. In: Drake H L, editor. Acetogenesis. New York, N.Y: Chapman and Hall; 1994. pp. 303–330. [Google Scholar]
- 10.Breznak J A, Brune A. Role of microorganisms in the digestion of lignocellulose by termites. Annu Rev Entomol. 1994;39:453–487. [Google Scholar]
- 11.Brune A. Termite guts: the world’s smallest bioreactors. Trends Biotechnol. 1998;16:16–21. [Google Scholar]
- 12.Brune A, Kühl M. pH profiles of the extremely alkaline hindguts of soil-feeding termites (Isoptera: Termitidae) determined with microelectrodes. J Insect Physiol. 1996;42:1121–1127. [Google Scholar]
- 13.Brune A, Emerson D, Breznak J A. The termite gut microflora as an oxygen sink: microelectrode determination of oxygen and pH gradients in guts of lower and higher termites. Appl Environ Microbiol. 1995;61:2681–2687. doi: 10.1128/aem.61.7.2681-2687.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Conrad R. Soil microbial processes involved in production and consumption of atmospheric trace gases. Adv Microb Ecol. 1995;14:207–250. [Google Scholar]
- 15.Cord-Ruwisch R, Seitz H-J, Conrad R. The capacity of hydrogenotrophic anaerobic bacteria to compete for traces of hydrogen depends on the redox potential of the electron acceptor. Arch Microbiol. 1988;149:350–357. [Google Scholar]
- 16.Crank J. The mathematics of diffusion. 2nd ed. Oxford, United Kingdom: Clarendon Press; 1975. [Google Scholar]
- 17.Ebert A, Brune A. Hydrogen concentration profiles at the oxicanoxic interface: a microsensor study of the hindgut of the wood-feeding lower termite Reticulitermes flavipes (Kollar) Appl Environ Microbiol. 1997;63:4039–4046. doi: 10.1128/aem.63.10.4039-4046.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Friedrich M, Schink B. Hydrogen formation from glycolate driven by reversed electron transport in membrane vesicles of a syntrophic glycolate-oxidizing bacterium. Eur J Biochem. 1993;217:233–240. doi: 10.1111/j.1432-1033.1993.tb18238.x. [DOI] [PubMed] [Google Scholar]
- 19.Kaiser P. Anoplotermes pacificus, eine mit Pflanzenwurzeln vergesellschaftet lebende Termite. Zool Staatsinst Zool Museum (Hamburg) Mitt. 1953;52:77–92. [Google Scholar]
- 20.Kappler, A., and A. Brune. Influence of gut alkalinity and oxygen status on mobilization and size-class distribution of humic acids in the hindgut of soil-feeding termites. Appl. Soil Ecol., in press.
- 21.Leadbetter J R, Breznak J A. Physiological ecology of Methanobrevibacter cuticularis sp. nov. and Methanobrevibacter curvatus sp. nov., isolated from the hindgut of the termite Reticulitermes flavipes. Appl Environ Microbiol. 1996;62:3620–3631. doi: 10.1128/aem.62.10.3620-3631.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Leadbetter J R, Schmidt T M, Graber J R, Breznak J A. Acetogenesis from H2 plus CO2 by spirochetes from termite guts. Science. 1999;283:686–689. doi: 10.1126/science.283.5402.686. [DOI] [PubMed] [Google Scholar]
- 23.Messer A C, Lee M J. Effect of chemical treatments on methane emission by the hindgut microbiota in the termite Zootermopsis angusticollis. Microb Ecol. 1989;18:275–284. doi: 10.1007/BF02075814. [DOI] [PubMed] [Google Scholar]
- 24.Noirot C. From wood- to humus-feeding: an important trend in termite evolution. In: Billen J, editor. Biology and evolution of social insects. Leuven, Belgium: Leuven University Press; 1992. pp. 107–119. [Google Scholar]
- 25.Nunes L, Bignell D E, Lo N, Eggleton P. On the respiratory quotient (RQ) of termites (Insecta: Isoptera) J Insect Physiol. 1997;43:749–758. doi: 10.1016/s0022-1910(97)00036-x. [DOI] [PubMed] [Google Scholar]
- 26.Odelson D A, Breznak J A. Volatile fatty acid production by the hindgut microbiota of xylophagous termites. Appl Environ Microbiol. 1983;45:1602–1613. doi: 10.1128/aem.45.5.1602-1613.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Platen H, Schink B. Methanogenic degradation of acetone by an enrichment culture. Arch Microbiol. 1987;149:136–141. doi: 10.1007/BF00425079. [DOI] [PubMed] [Google Scholar]
- 28.Revsbech N P. An oxygen microelectrode with a guard cathode. Limnol Oceanogr. 1989;34:472–476. [Google Scholar]
- 29.Rouland C, Brauman A, Labat M, Lepage M. Nutritional factors affecting methane emission from termites. Chemosphere. 1993;26:617–622. [Google Scholar]
- 30.Sanderson M G. Biomass of termites and their emissions of methane and carbon dioxide: a global database. Global Biogeochem Cycles. 1996;10:543–557. [Google Scholar]
- 31.Schink B. Energetics of syntrophic cooperation in methanogenic degradation. Microbiol Mol Biol Rev. 1997;61:262–280. doi: 10.1128/mmbr.61.2.262-280.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Tholen, A., and A. Brune. Unpublished data.
- 33.Tholen A, Brune A. Localization and in situ activities of homoacetogenic bacteria in the highly compartmentalized hindgut of soil-feeding higher termites (Cubitermes spp.) Appl Environ Microbiol. 1999;65:4497–4505. doi: 10.1128/aem.65.10.4497-4505.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Tholen, A., and A. Brune. Impact of oxygen on metabolic fluxes and in situ rates of reductive acetogenesis in the hindgut of the wood-feeding termite. Reticulitermes flavipes, determined by microinjection of radiotracers. Submitted for publication. [DOI] [PubMed]
- 35.Tholen A, Schink B, Brune A. The gut microflora of Reticulitermes flavipes, its relation to oxygen, and evidence for oxygen-dependent acetogenesis by the most abundant Enterococcus sp. FEMS Microbiol Ecol. 1997;24:137–149. [Google Scholar]
- 36.Williams C M, Veivers P C, Slaytor M, Cleland S V. Atmospheric carbon dioxide and acetogenesis in the termite Nasutitermes walkeri (Hill) Comp Biochem Physiol. 1994;107A:113–118. [Google Scholar]
- 37.Witty J F. Microelectrode measurements of hydrogen concentrations and gradients in legume nodules. J Exp Bot. 1991;42:765–771. [Google Scholar]
- 38.Wood T G, Johnson R A. The biology, physiology and ecology of termites. In: Vinson S B, editor. Economic impact and control of social insects. New York, N.Y: Praeger; 1986. pp. 1–68. [Google Scholar]