Abstract
Growing concern for public health has increased the need to change the paradigm towards a healthcare system that advocates holistic practices while reducing adverse effects. Herbal therapy is becoming an integral part of the therapeutic arsenal, and several successful plant-derived compounds/molecules are being introduced into the market. The medicinal plants belonging to the genus Thymus are among the most important species within the Lamiaceae family. One of them is Thymus algeriensis which is mainly distributed in the Mediterranean region. For a long time, this species has been used in traditional medicine to treat several disorders and diseases including inflammation, diabetes, rheumatism, digestive, and respiratory affections. This review describes the traditional uses, phytochemical composition, and biological and pharmacological activities of T. algeriensis extracts. Data were obtained using electronic databases such as SciFindern, ScienceDirect, Scopus, and Web of Science. Several plant-based extracts and a broad spectrum of identified secondary metabolites were highlighted and discussed with respective activities and modes of action. T. algeriensis represents a promising natural resource for the pharmaceutical industry mainly for antioxidant, anti-inflammatory, antimicrobial, and anticancer activities. Considering these findings, more research is needed to transmute the conventional uses of T. algeriensis into scientifically sound information. Moreover, extensive preclinical, clinical, toxicological, and pharmacokinetic trials on this species and its derivatives compounds are required to underpin the mechanisms of action and ensure its biosafety and efficiency. This comprehensive review provides a scientific basis for future investigations on the use of T. algeriensis and derived compounds in health maintenance and promotion and disease prevention.
1. Introduction
According to the World Health Organization (WHO), about 80% of the earth's population relies on folk medicine. Most of ethnopharmacological practices involve the use of plant-based extracts and their bioactive constituents as natural healing remedies [1]. Plants have been used for therapeutic purposes worldwide for thousands of years and still provide the largest drugs to humankind. Therefore, scientists have dedicated a lot of effort to drug discovery willing to identify natural molecules/compounds from plants [2]. Until now, the international market of medicinal and aromatic plants has reached over 60 billion dollars per year, and it is still increasing gradually [3]. In addition, the pharmaceutical industry values medicinal plants for their bioactive constituents such as flavonoids, polyphenols, alkaloids, tannins, and glycosides, which are used as agents in drugs' synthesis [4]. Nowadays, plant-derived molecules are continuously enriching our drug arsenal (e.g., galantamine, vinblastine, vincristine, and artemisinin) [5].
Thymus is a large plant genus comprising up to 400 species of aromatic and medicinal herbaceous, perennials, and shrubs. They are widely distributed in the Mediterranean region and Asia. Thymus species are used traditionally as herbal teas, culinary spices, and condiments [6]. Additionally, their essential oils are listed as one of the world's top ten essential oils and known for their broad spectrum of biological activities including antioxidant, antibacterial, and age-delaying properties [7].
One of the most renowned North African Thymus species is T. algeriensis. The previous investigations carried out on this plant species have been mainly oriented towards its biological activities and clinical attributes. According to the Scopus database, more than 43.4% of the works relate to biological, pharmacological, biochemical, microbiological, and immunological aspects. The largest number of these studies was carried out by North African researchers and institutions, particularly Algerian, Moroccan, and Tunisian. In the Scopus database, the TITLE-ABS-KEY (thymus AND algeriensis) research resulted in 951 documents gathering articles (95.5%), reviews (2.2%), book chapters, and data papers (2.2%) with an increasing trend of document numbers over the years. For instance, it went from 4 to 15 documents per year between 2013 and 2021 indicating the importance that this species arouses among researchers. Therefore, T. algeriensis is a promising endemic resource for drug discovery and healthcare systems. Since this Thymus species has been the subject of a multitude of studies using both in vitro and in vivo approaches, and the data related to its phytochemistry and biological properties are distributed in several documents, we thought, here, to comprehensively summarize and review the phytochemical composition of T. algeriensis tissues with reference to the biological and pharmacological activities of its various extracts to have a holistic and synoptic view of its benefits and curative potentialities and track down research gaps and future prospects.
2. Botanical Description and Distribution
T. algeriensis is an endemic species of North Africa (Morocco, Algeria, Tunisia, and Libya) (Figure 1) [8]. The Thymus genus is represented by numerous aromatic plant species, including T. algeriensis which is a short lived, diploid (2n = 2x = 30) and gynodioecious shrub [9, 10] belonging to the Hyphodromi section and the Subbracteati subsection [11]. It grows wildly in diverse bioclimatic areas extending from the subhumid to the lower arid and on poor fertile calcareous soils [12]. In Morocco, it is found in the Middle, the High, and the Western Anti-Atlas, the Rif, and the Oriental (Figure 1).
Figure 1.
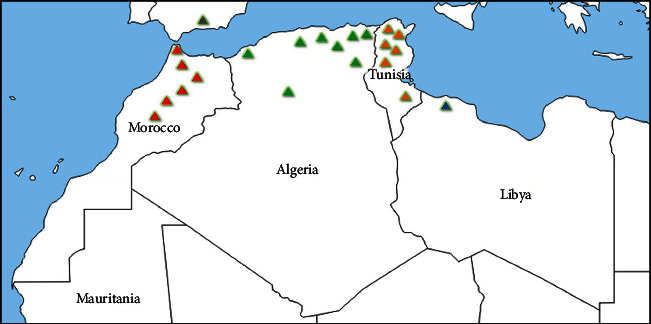
Map of distribution of T. algeriensis Boiss. & Reut. across North Africa.
T. algeriensis is a perennial plant with 4 to 7 mm long internodes emerging as a tuft from the short woody stump [13] (Figure 2(a)). The leaves are opposite with linear-lanceolate (6–12 mm) and have both green surfaces. The flowers are small (5 –7 mm) and have a white purplish or pinkish purple corolla color, with the upper lip cleft and the lower one divided into three lopes (Figure 2(b)). Flowering and fruiting time takes place from late April to June [14, 15].
Figure 2.

(a) Thymus algeriensis Boiss. & Reut. plant; (b) aerial part of Thymus algeriensis Boiss. & Reut. (Source: https://www.biodiversidadvirtual.org/).
Due to the anthropic pressures (overcollection, overgrazing, clearing, etc.), Thymus populations and cultivars from natural areas are severely affected and tend to occur in scattered metapopulations, often characterized by a low size [9]. Many factors influence the level of differentiation and the genetic drift of T. algeriensis populations, mainly habitat fragmentation, special isolation, ecological conditions, and gene flow limitation reducing their adaptation to ecological changes [16]. Several chemotypes were described in T. algeriensis according to their phytochemical composition (essential oils and main compounds) [15]. The genetic diversity among populations are also reported to be influenced by the level of site destruction, the number of initial founders in populations, and their dispersal and reproductive potentials [16].
3. Sources, Search Strategy, and Eligibility Criteria
The chemical composition and the biological and pharmacological activities of T. algeriensis were obtained using the electronic databases SciFinderⁿ®, Web of Science, Google Scholar, and Scopus. The search term used was Thymus algeriensis Boiss. & Reut. (1210 records). When crosslinked with specified terms, mainly “chemical compounds” (1010 records) and “activities” (1050 records), the number of documents decreased to 989. The cumulative results were then crosslinked with biological activities, in vitro, in vivo, or pharmacology which resulted in 155 records. In addition, titles and abstracts were screened and subjected to inclusion criteria that were as follows: phytochemical constituents of Thymus algeriensis extracts and their biological and pharmacological activities both in vitro and in vivo. Exclusion criteria were also considered and included other applications of the plant such as agriculture, non-English documents, duplicated papers, and the inability to locate full text. This selection resulted in relevant literature of 87 records that was used to retrieve data represented in this review. Other than the aforementioned records, many other references were sourced from citations of eligible studies.
4. Phytochemical Composition of T. algeriensis
The phytochemical analysis of different parts of T. algeriensis has shown the presence of diverse phytochemicals like polyphenols, flavonoids, terpenoids, sterols, and volatile compounds. This might be attributed to different factors such as geographic location, temperature, and harvesting time. Furthermore, phytochemical content is reported to vary with the extraction method employed and compounds identified in various parts of T. algeriensis. The major active constituents are flavonoids (Figure 3). The plant is also known by the volatile compounds characterizing the essential oil. Tables 1 and 2 demonstrate the reported compounds from T. algeriensis of different parts including aerial parts, leaves, flowers, and stem bark. The presence of various phytochemicals in T. algeriensis suggests its pluripharmacological properties, and a comprehensive assessment of the various activities of different phytochemicals is included in the sections below.
Figure 3.
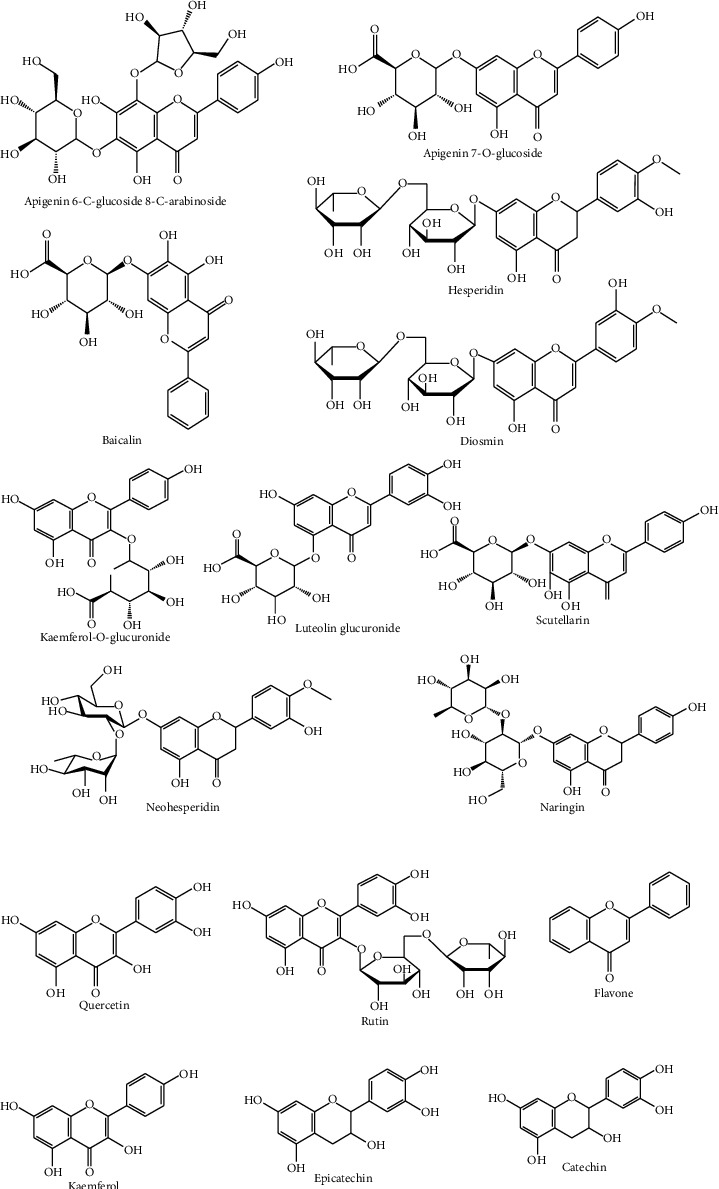
Flavonoids identified from T. algeriensis.
Table 1.
Chemical constituents of T. algeriensis extracts.
| Compound name | Extract type | Ref. |
|---|---|---|
| Algeria | ||
| Apigenin-6,8-C-dihexosideb | H2O & EtOH–H2O | [7] |
| Apigenin-7-O-glucuronideb | H2O & EtOH–H2O | [7] |
| Aringina | EtOH | [23] |
| Baicalinb | n–BuOH | [17] |
| Benzoic acidb | H2O | [18] |
| Caffeoyl rosmarinic acida | MeOH–H2O | [24] |
| Chlorogenic acidb | H2O | [18] |
| Clovane-2,9-diola | MeOH | [25] |
| Diosminb | n–BuOH | [17] |
| Ellagic acida | EtOH | [23] |
| Fumaric acidb | n–BuOH | [17] |
| Gentisic acidb | n–BuOH | [17] |
| Hesperidinb | n–BuOH | [17] |
| Isovanillinb | EtOH–H2O & H2O | [18] |
| Kaempferol-O-glucuronideb | H2O & EtOH–H2O | [7] |
| Lithospermic acidb | H2O & EtOH–H2O | [7] |
| Luteolin glucuronidea | MeOH | [22] |
| Methyl ursolatea | MeOH | [25] |
| Naringinb | EtOH–H2O, H2O, & n–BuOH | [17, 18] |
| Neohesperidinb | n–BuOH | [17] |
| O-Coumaric acidb | H2O | [18] |
| Oleanolic acida | MeOH | [25] |
| p-Coumaric acidb | EtOH–H2O, H2O | [18] |
| Epicatechina | EtOH | [23] |
| Rosmarinic acid glucosidea | MeOH | [22] |
| Salvianolic acid Kab | MeOH, H2O, & EtOH–H2O | [7, 22] |
| Scutellarinb | n–BuOH | [17] |
| Sinapinic acidb | EtOH–H2O, H2O | [18] |
| t-Ferulic acidb | H2O | [18] |
| Ursolic acida | MeOH | [25] |
| β-Sitosterola | MeOH | [25] |
| 2,5-Dihydroxybenzoic acida | EtOH | [23] |
| 2,3-Dimethoxybenzoic acidb | EtOH–H2O, H2O | [18] |
| 3-Hydroxybenzoic acidb | EtOH–H2O, H2O | [18] |
| 4-Hydroxybenzoic acidb | H2O, EtOH–H2O, & n–BuOH | [18] |
| Algeria and Tunisia | ||
| Catechinb | H2O | [14, 18] |
| Gallic acidb | H2O & MeOH | [18–20] |
| Kaempferolab | MeOH–H2O, H2O, & MeOH | [14, 19, 24] |
| Quercetinb | H2O & MeOH | [14, 18, 19, 22] |
| Rosmarinic acidab | MeOH–H2O & MeOH | [7, 21, 24] |
| Rutinab | EtOH–H2O, H2O, & MeOH | [14, 18–20, 23] |
| Syringic acidb | H2O & MeOH | [18, 20] |
| Vanillic acidab | EtOH, MeOH, & H2O | [18–20, 23] |
| Epicatechinb | EtOH–H2O & H2O | [14, 18, 19] |
| Tunisia | ||
| Gallic acidb | H2O | [14] |
| Naringeninb | [14, 19] | |
| Coumaric acidb | [14, 19] | |
| Caffeic acidb | MeOH | [20] |
| Ferulic acidb | [20] | |
| Flavoneb | [20] | |
| Hydroxyphenylic acidb | [20] | |
| Methyl gallateb | [20] | |
| (+)-Catechin hydrateb | [20] | |
| Carvacrola | [21] | |
| Kaempferol-O-hexosidea | [21] | |
| Kaempferol-O-hexuronidea | [21] | |
| Apigeninb | H2O, MeOH | [14, 19] |
aLeaves, baerial parts.
Table 2.
Chemical constituents of T. algeriensis essential oils (EO).
| Compound name | Plant part | Quantity (%) | Country | Ref. |
|---|---|---|---|---|
| 1,8-Cineole | Aerial parts | 17.70% | Tunisia | [9] |
| 7.55-22.07% ∗ | [15] | |||
| 20.98% | [12] | |||
| 19.96% | [20] | |||
| Leaves | 11.60% | [28] | ||
| 12.05% | [69] | |||
| Flowers | 9.12% | [69] | ||
| Leaves and flowers | 5.54% | [29] | ||
| 5.16-11.21%∗∗ | Algeria | [42] | ||
| 7.69% | [34] | |||
| 6.00% | [136] | |||
| 5.94% | [30] | |||
| 4-Terpineol | Aerial parts | 1.55-11.86%∗ | Tunisia | [15] |
| Leaves and flowers | 7.36% | [29] | ||
| Borneol | Aerial parts | 11.16-22.2%∗∗ | Algeria | [42] |
| 5.74% | [136] | |||
| Stem bark | 11.16% | [30] | ||
| Aerial parts | 28% | Morocco | [137] | |
| 18.30% | [53] | |||
| 23.48% | [66] | |||
| 59% | [93] | |||
| Camphene | Aerial parts | 7.53-12.86%∗∗ | Algeria | [42] |
| Stem bark | 12.78% | [30] | ||
| Aerial parts | 20.90% | Morocco | [137] | |
| 11.80% | [53] | |||
| Camphor | Aerial parts | 17.45-32.56%∗∗ | Algeria | [42] |
| 13.62% | [136] | |||
| 14.22% | [46] | |||
| 17.68% | [67] | |||
| Stem bark | 22.60% | [30] | ||
| Aerial parts | 15.70% | Morocco | [137] | |
| 10.00% | [53] | |||
| 27.70% | [50] | |||
| 27.70% | [79] | |||
| 27.70% | [26] | |||
| 19.20% | Tunisia | [20] | ||
| 6.8-19.93%∗ | [15] | |||
| 7.46% | [12] | |||
| 13.82% | [49] | |||
| 8.20% | [9] | |||
| Leaves | 10.40% | [28] | ||
| Leaves and flowers | 7.82% | [29] | ||
| Carvacrol | Aerial parts | 48.40% | Algeria | [78] |
| 28.10% | [138] | |||
| Leaves | 64.6-65.9%¥ | [126] | ||
| 4% | [139] | |||
| Aerial parts | 80.90% | Libya | [6] | |
| 14% | [73] | |||
| 4.59% | [43] | |||
| 36.78% | [124] | |||
| Aerial parts | 85% | Morocco | [93] | |
| Caryophyllene oxide | Stems | 17.80% | Tunisia | [28] |
| Roots | 21.10% | [28] | ||
| cis-Sabinene hydrate | Aerial parts | 0.10-12.95%∗ | Tunisia | [15] |
| Leaves and flowers | 5.29% | [29] | ||
| Elemol | Aerial parts | 18.38% | Algeria | [46] |
| Leaves | 3.98% | Tunisia | [69] | |
| Flowers | 11.30% | [69] | ||
| Stems | 10.20% | [28] | ||
| Geraniol | Aerial parts | 19.60% | Algeria | [35] |
| Leaves | 7.30% | Morocco | [140] | |
| Linalool | Aerial parts | 3.93% | Algeria | [136] |
| 30.40% | [35] | |||
| 47.30% | [141] | |||
| 78.80% | [142] | |||
| 22.15% | Tunisia | [12] | ||
| 17.62% | [49] | |||
| Leaves | 3.20% | [69] | ||
| p-Cymene | Aerial parts | 8.00% | Algeria | [138] |
| 20.04% | [67] | |||
| 14.70% | [78] | |||
| 6.80% | [141] | |||
| Leaves | 6.2-6.9%¥ | [126] | ||
| 3% | [139] | |||
| Aerial parts | 7.70% | Libya | [6] | |
| 8.91% | [43] | |||
| 23% | Morocco | [93] | ||
| 27.18% | Tunisia | [77] | ||
| Thymol | Aerial parts | 20.83% | Algeria | [67] |
| 5.60% | [78] | |||
| 20.20% | [35] | |||
| 29.20% | [141] | |||
| 62.70% | [124] | |||
| Leaves | 71% | [139] | ||
| Aerial parts | 56.00% | Libya | [73] | |
| 38.50% | [43] | |||
| 12.45% | [124] | |||
| Aerial parts | 42% | Morocco | [93] | |
| Aerial parts | 36.94% | Tunisia | [77] | |
| Viridiflorol | Aerial parts | 4.00% | Algeria | [136] |
| 0-11.49%∗ | Tunisia | [15] | ||
| Roots | 17.20% | [28] | ||
| α-Pinene | Aerial parts | 6.80% | Algeria | [23] |
| 27.14% | [34] | |||
| Stem bark | 5.01% | [30] | ||
| Aerial parts | 20.50% | Morocco | [50] | |
| 20.50% | [79] | |||
| 20.50% | [26] | |||
| 7.41-13.94%∗ | Tunisia | [15] | ||
| 21.31% | [12] | |||
| 11.49% | [12] | |||
| 15.50% | [9] | |||
| Leaves | 19.50% | [28] | ||
| 2.97% | [69] | |||
| Leaves and flowers | 6.75% | [29] | ||
| α-Terpinene | Aerial parts | 10.66% | Libya | [124] |
| 3.24% | Tunisia | [77] | ||
| 6.41% | [49] | |||
| β-Caryophyllene | Aerial parts | 11.00% | Algeria | [143] |
| Leaves | 3.0-3.4%¥ | [126] | ||
| γ-Terpinene | Aerial parts | 14.90% | Algeria | [78] |
| Leaves | 5.9-6.7%¥ | [126] | ||
| 0.50% | [139] | |||
| Aerial parts | 7.19% | Libya | [43] | |
| 9.90% | Tunisia | [77] | ||
| δ-Cadinene | Aerial parts | 4.00% | Algeria | [143] |
| 3.39% | [34] | |||
| α -Caryophyllene | Aerial parts | 9.68% | Algeria | [46] |
| α-Terpinyl acetate | 47.40% | [23] | ||
| β-Eudesmol | 11.50% | [46] | ||
| Bornyl acetate | 3.86-7.92%∗ | [140] | ||
| γ-Terpinene | 25.70% | [67] | ||
| Germacrene D | 29.60% | [143] | ||
| Neryl acetate | 9.60% | [23] | ||
| Eucalyptol | 10.04% | [67] | ||
| Bicyclogermacrene | 4.40% | [143] | ||
| -β-Farnesene | 7.80% | [143] | ||
| 2,3-Dehydro-1,4-cineol | 36% | [144] | ||
| Linalyl acetate | 6.39% | Tunisia | [12] | |
| α-Humulene | 5.72% | [12] | ||
| α-Terpenyl acetate | 6.27% | [49] | ||
| β-Linalool | 3.15% | [77] | ||
| Methyl eugenol | 6.78% | [12] | ||
| Terpinen-4-ol | 6.80% | [49] | ||
| Terpenyl acetate | 0-14.92%∗∗ | [15] | ||
| β-Myrcene | 20.22% | Libya | [124] | |
| Myrcene | 8.60% | Morocco | [53] | |
| trans-Caryophyllene | Leaves | 2.40% | Morocco | [140] |
| Geranyl acetate | 80.80% | [140] | ||
| Acorenone | Stem bark | 5.84% | Algeria | [30] |
∗Collected during the vegetative and flowering stages and from eight different geographic regions; ∗∗collected before, during, and after flowering stage; ¥effect of different gamma irradiation doses.
4.1. Aerial Parts
Most of the studies have been focused on the aerial parts of T. algeriensis. Different studies reported that the hydroalcoholic extracts were shown to contain kaempferol-O-glucuronide, apigenin-6,8-C-dihexoside and apigenin-7-O-glucuronide, and naringenin, identified from the Algerian plants [7, 17, 18], while apigenin was annotated in the polar extracts of the Tunisian plants [14, 19] (Figure 3). In the Tunisian plants, catechin, epicatechin, rutin, flavone, and (+)-catechin hydrate have been identified in the hydroalcoholic and methanolic extracts (Figure 3) [14, 18, 19].
Phenolic acids such as O-coumaric, p-coumaric, salvianolic, and t-ferulic were documented from the Algerian flora [7, 18], while gallic, rosmarinic, syringic, and vanillic acids were detected in the extracts from the Tunisian and Egyptian plants (Figure 4). In contrast, caffeic and ferulic acids characterized the methanolic extract of the plant harvested from Tunisia [20]. In the same line, 2,3-dimethoxybenzoic acid, 3-hydroxybenzoic acid, and 4-hydroxybenzoic acid were identified in the ethanol, hydroalcoholic, and butanolic extracts of the Algerian plants (Figure 5) [18].
Figure 4.
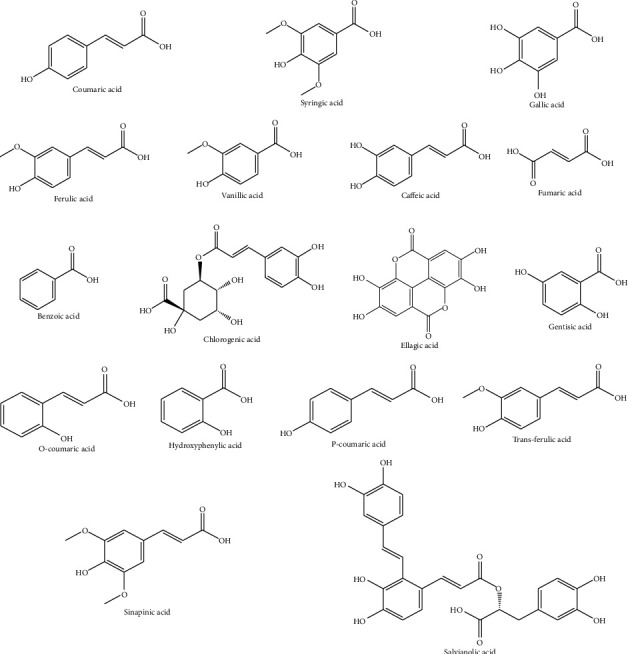
Phenolic and carboxylic acids identified from T. algeriensis.
Figure 5.
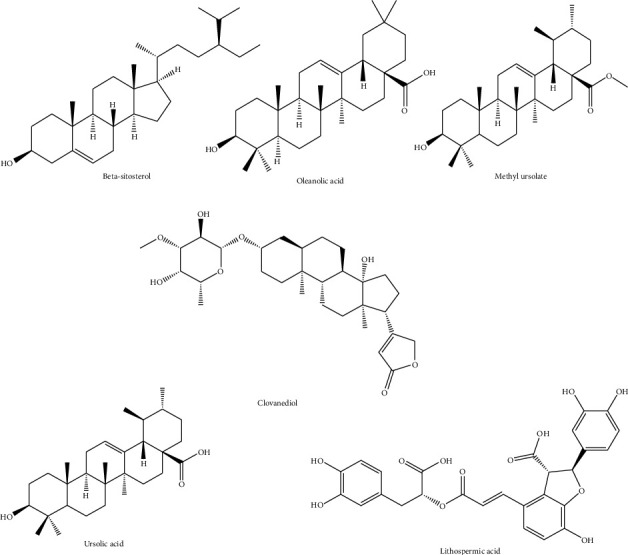
Other compounds identified from T. algeriensis.
4.2. Leaves
T. algeriensis has a high antioxidant activity mainly due to its high content in flavonoids (Figure 3). Most of the flavonoids present in the leaves are in the flavanol and glycoside forms. Kaempferol and rutin were detected in the polar leaves' extract of the plants from Algeria and Tunisia. Kaempferol-O-hexoside and kaempferol-O-hexuronide have been identified in the methanolic extract of the leaves collected from Tunisia [21], while luteolin glucuronide was identified in the Algerian plants (Figure 3) [22]. From all phenolic acids (Figure 4), the leaves of T. algeriensis are rich in vanillic and rosmarinic acids, well-known phenolic acids with previously confirmed biological and pharmacological activities. These two compounds were characterized in the Algerian and Tunisian plants [7, 18–21, 23, 24]. The plant also contained phenolic acid derivatives such as rosmarinic acid glucoside, characterized in the methanolic extract of the Algerian plants [22]. Ursolic acid, a triterpenoid, was identified in the methanolic extract of the leaves. In addition, sterols such as β-sitosterol have been identified in the methanolic extract of the leaves. In addition, oleanolic acid, a triterpenoid, was identified in the leaves [25] (Figure 5).
4.3. Essential Oils of T. algeriensis
Essential oils are naturally defined as volatile secondary metabolites of plants and characterized by a strong aromatic nature and a complex chemical composition. The aroma of T. algeriensis is strong and contains large quantities of volatile compounds [26, 27]. More than 40 volatile compounds have been identified in T. algeriensis (Table 2 and Figure 6).
Figure 6.
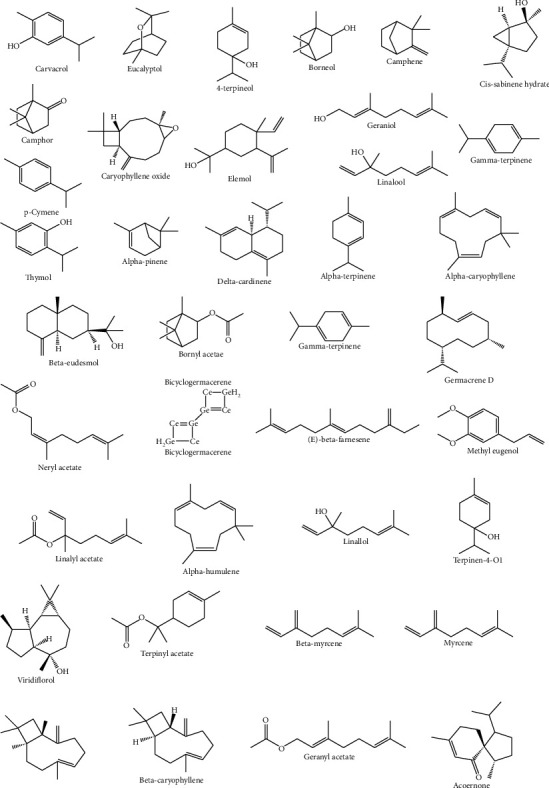
Selected volatile compounds identified from T. algeriensis.
Several investigations reported the phytochemical composition of the essential oils of different specimen of T. algeriensis from different regions including Algeria, Libya, Tunisia, and Morocco (Table 2). The monoterpenoids and sesquiterpenoids dominated the oil. For instance, the main components of the essential oils of the aerial parts from the Tunisian flora were 1,8-cineole and 4-terpineol [9, 15], while borneol, camphene, camphor, and carvacrol dominated the Algerian and Moroccan plants. p-Cymene and thymol were detected in all flora. In the Tunisian flora, caryophyllene oxide and cis-sabinene hydrate were identified from different parts of the plant with different percentages [15, 28, 29]. Limited studies focused on the isolation and identification of bioactive compounds from the flowers of T. algeriensis. Like the leaves, the flowers were shown to contain 1,8-cineole identified in the Algerian plants. Elemol was identified from the stem bark from the Tunisian plants while acorenone and α-pinene were characterized in the stem bark of the Algerian plants [23, 30].
The variations in the phytochemical composition of the essential oils could be attributed to the harvesting time and stage and the drying methods as well as extraction methods. Some factors like environmental conditions, genetic variations, physiological condition of plants, regions, and evolution also influence the phytochemical variability of T. algeriensis [31]. Overall, T. algeriensis alongside other Lamiaceae species contains high contents of polyphenols, flavonoids, terpenoids, sterols, and volatile compounds.
5. Traditional Uses and Ethnomedicinal Properties
Thymus species have been used by the populations of the Mediterranean and Asian countries for thousands of years [32, 33]. Traditional health uses of Thymus species show high applicability as flu controller, anti-inflammatory, sedative, antirheumatic, analgesic, antiseptic, astringent, and diuretic agents. Generally, Thymus flowers and leaves are mainly used as herbal teas and aromatic and flavoring preparations to treat common cold, cough, sore throat, and indigestion symptoms [33]. Thymus leaves are also used as astringent, expectorant, antiseptic, antirheumatic, diuretic, analgesic, and cicatrizing agents [18]. T. algeriensis is also used either fresh or dried for its antispasmodic, antiabortive, and antifungal properties [34, 35]. In Morocco, its vernacular name is either Zîtra, Tazouknit, or Mantha [36]. Infusion and decoction of its stems and/or leaves are traditionally used to treat diabetes and digestive and respiratory infections [37, 38]. To sum up, T. algeriensis preparations are traditionally known for their multiple benefits and uses in gastronomy, digestive and cold problems, analgesia, microbial infections, and perfume preparations.
6. In Vitro Pharmacological Properties
Many studies have shown that T. algeriensis extracts have several in vitro biological properties mainly antioxidant, anti-inflammatory, antimicrobial, and anticancer activities.
6.1. In Vitro Antioxidant Activity
The oxidation process causes cellular damage by interacting with biological materials within the cell leading to several disorders and chronic diseases such as cardiovascular diseases and cancer. In addition, oxidation forms secondary reaction products in food and alters its nutritional quality and safety [4].
Antioxidant activity was defined as a delay or inhibition of the oxidation of cell molecules mainly proteins, lipids, DNA, and sugars by limiting the oxidative chain reactions. Due to their phytochemicals, plants are generally known as the best source of active antioxidants [39]. This is mainly attributed to their capacity to prevent the oxidation of a substrate by neutralizing reactive oxygen species (ROS) such as superoxide radical and hydroxyl radical. Common mechanisms by which plant-based extracts/molecules block ROS formation include mainly free radical scavenging (e.g., lipoxygenase inhibition, transition-metal-chelating activity, and singlet-oxygen-quenching capacity) and lipid peroxidation inhibition [40, 41]. Hence, there is a growing interest in the antioxidant activities of plant-based compounds and their role in promoting health and preventing diseases.
The in vitro antioxidant activities of T. algeriensis extracts have been extensively explored (Table 3). They have been determined by various methods mainly 2,2-diphenyl-1-picrylhydrazil (DPPH) radical scavenging, ferric-reducing antioxidant power (FRAP), β–carotene bleaching, oxygen radical absorbance capacity (ORAC), thiobarbituric acid reactive substances (TBARS), reducing power (RP), phosphomolybdenum, lipid peroxidation inhibition, total antioxidant capacity (TAC), hydroxyl radical scavenging (HRS), metal ion chelation, and superoxide anion scavenging assays (Table 3).
Table 3.
In vitro antioxidant activities of T. algeriensis extracts.
| Extract | Used method | Effects | Ref. |
|---|---|---|---|
| Algeria | |||
| Aerial parts | |||
| PE, CHCl3, & n–BuOH | DPPH | IC50 (mg/mL) = 69.50 ± 0.68 (PE), 79.92 ± 0.30 (CHCl3), and 5.05 ± 0.12 (n–BuOH) | [44] |
| CUPRAC | A0.50 (μg/mL) = 22.28 ± 0.24 (PE), 27.81 ± 3.06 (CHCl3), and 0.94 ± 0.06 (n–BuOH) | ||
| RP | A0.50 (μg/mL) = 25.25 ± 0.08 (PE), 24.5 ± 0.52 (CHCl3), and 4.98 ± 0.48 (n–BuOH) | ||
| TAC | TAC (μg EAA/mg (dw)) = 15.69 ± 0.001 (PE), 16.21 ± 0.02 (CHCl3), and 20.79 ± 0.19 (n–BuOH) | ||
| FTC | %of inhibition = 27.80 ± 0.37 (PE), 24.25 ± 0.45 (CHCl3), and 47.43 ± 0.58 (n–BuOH) | ||
| EtOH & H2O | DPPH | IC50 (mg/mL) = 0.052 ± 0.004 (EtOH), not active (H2O) | [145] |
| ABTS | IC50 (μg/mL) = 42 ± 0.99 (EtOH), 52 ± 31 (H2O) | ||
| MeOH–H2O | DPPH | IC50 (μg/mL) = 7.4 ± 0.3 | [24] |
| Iron chelating | EC50 (μg/mL) = 512 ± 0 | ||
| β–Carotene bleaching | %of inhibition = 90 ± 2 | ||
| TAC | TAC (μg AAE/mg) = 268 ± 4 | ||
| FRAP | FRAP (mM FeSO4/mg) = 5.3 ± 0.0 | ||
| H2O & EtOH–H2O | DPPH | EC50 (μg/mL) = 64.8 ± 0.7 (H2O), 131 ± 3 (EtOH–H2O) | [7] |
| RP | EC50 (μg/mL) = 54.0 ± 0.5 (H2O), 100.2 ± 0.5 (EtOH–H2O) | ||
| β–Carotene bleaching | EC50 (μg/mL) = 149 ± 3 (H2O), 85 ± 3 (EtOH–H2O) | ||
| TBARS | EC50 (μg/mL) = 26.3 ± 0.2 (H2O), 40.3 ± 0.3 (EtOH–H2O) | ||
| EA & n–BuOH | DPPH | EC50 (mg/mL) = 0.290 (EA) EC50 (mg/mL) = 1.45 (n–BuOH) |
[46] |
| EO | DPPH | IC50 (mg/mL) = 10.2 ± 0.9–>45.0 | [67] |
| Phosphomolybdenum assay | AEAC (mg/mL) = 0.148 ± 0.003–0.220 ± 0.022 | ||
| MeOH–H2O, acetone–H2O, MeOH, acetone–H2O | DPPH, ABTS, phosphomolybdenum | All extracts possess potential antioxidant activities compared to standards | [105] |
| EO | TBARS | Not active | [62] |
| ABTS | IC50 (mg/mL) = 0.150 ± 0.002 | ||
| DPPH | IC50 (mg/mL) = 0.235 ± 0.018 | ||
| ORAC | ORAC (μmol Trolox equivalent/g) = 38.47 ± 39.71 | ||
| RP | IC50 (mg/mL) = 0.025 ± 0.006 | ||
| Chelating metal ions | Not active | ||
| HRS | Not active | ||
| Superoxide anion scavenging assay (nonenzymatic method) | Not active | ||
| EO | HRS | IC50 (μg/mL) = 2.2 ± 0.03–8.5 ± 0.1 (ALG1–ALG3) | [35] |
| DPPH | %of inhibition = 1.6 ± 0.0–53.4 ± 0.2 (chemotype and dose-dependent effect) | ||
| TBARS | IC50 (μg/mL) = 106.7 ± 8.4–911.6 ± 7.4 (ALG1–ALG3) | ||
| Leaves | |||
| MeOH | DPPH | EC50 (μg/mL) = 1.60 ± 0.13 | [45] |
| β–Carotene bleaching test | %of inhibition = 64.31 ± 1.9 | ||
| Lipophilic extract using olive oil (OO) | RPlip | Significantly higher (RPlip = 50 mg BHT eq/g (dw)) than that of OO only (40 mg BHT eq/g OO) | [146] |
| EtOH & EO | DPPH | IC50 (mg/mL) = 1.560 ± 0.010 (EtOH), 1.437 ± 4.51 (EO) | [23] |
| ABTS | IC50 (mg/mL) = 1.743 ± 0.195 (EtOH), 0.8960 ± 0.203 (EO) | ||
| RP | AEAC–FRAP assay (μg/mL) = 0.897 ± 0.064 (EtOH), 1.387 ± 0.265 (EO) | ||
| Phosphomolybdenum | AEAC (mg/mL) = 0.007 ± 0.0006 (EtOH), 0.432 ± 0.001 (EO) | ||
| MeOH | DCFDA | No significant modification in ROS levels in HaCaT cells | [47] |
| Western blot analyses | Significant increase in nuclear levels of Nrf–2 (nuclear factor erythroid 2) by up to 180% after incubation of the HaCaT cells for 15 min | ||
| H2O & EO | DPPH | IC50 (mg/L) = 404.08 ± 5.87 (EO), 22.26 ± 0.07 (H2O) | [126] |
| ABTS | IC50 (mg/L) = 10.48 ± 0.49 (EO), 25.29 ± 0.21 (H2O) | ||
| TBARS | IC50 (mg/L) = 23.54 ± 0.37 (EO), not active (H2O) | ||
| RP | IC50 (mg/L) = 347.84 ± 3.02 (EO), 59.53 ± 0.70 (H2O) | ||
| EO | DPPH | IC50 (mg/mL) = 41.09 | [42] |
| ABTS | IC50 (mg/mL) = 10.84 | ||
| TAC | TAC (U/L) = 39.27 ± 3.47 | ||
| Stem bark | |||
| EO | DPPH | IC50 (mg/mL) = 83.8 | [30] |
| Tunisia | |||
| Aerial parts | |||
| EO | DPPH | IC50 (mg/mL) = 0.8 | [29] |
| β–Carotene bleaching | IC50 (mg/mL) = 0.5 | ||
| EO | DDPH | IC50 (μg/mL) = 3155 ± 27.56 | [49] |
| EO aqueous extract | DPPH FRAP |
IC50 (μg/mL) = 0.06 | [127] |
| IC50 (μg/mL) = 0.04 | |||
| H2O & hexane | DPPH | IC50 (μg/mL) = 6.7 (H2O) | [14] |
| FRAP | Samples at vegetative and flowering stages (200, 300, 400, and 500 μg/mL) reduced the Fe3+ to Fe2+ with lower potency than BHT | ||
| EO | DPPH | %of inhibition = 52–91.96% | [118] |
| EtOH & H2O | DPPH | IC50 (μg/mL) = 43.5 ± 1.36 | [147] |
| FRAP | IC50 (μg/mL) = 378.5 ± 5.24 | ||
| β–Carotene bleaching | IC50 (μg/mL) = 1430 ± 10.79 | ||
| EO & MeOH | DPPH | %of inhibition = 81 ± 0.26–93 ± 0.06 (MeOH), 82 ± 0.52–85 ± 0.57 (EO) | [20] |
| ABTS | %of inhibition = 22 ± 0.9–75 ± 0.72 (MeOH), 8 ± 0.7–19 ± 0.33 (EO) | ||
| β–Carotene bleaching | %of inhibition = 25 ± 0.08–50 ± 0.12 (MeOH), 4 ± 0.44–10 ± 0.52 (EO) | ||
| Leaves | |||
| MeOH | DPPH | IC50 (μg/mL) = 8.9 ± 0.1–68.8 ± 1.0 | [21] |
| FRAP | EC50 (mmol Fe2+/L) = 1.0 ± 0.0–20.6 ± 0.2 | ||
| β–Carotene bleaching | EC50 (μg/mL) = 0.03 ± 0.0–1.81 ± 0.0 | ||
| EO | DPPH | IC50 (mg/mL) = 4.31 ± 0.7–9.23 ± 1.8 | [28] |
| ABTS | ABTS (μg Trolox equivalent/mg DW) = 11.69 ± 0.64–18.13 ± 0.92 | ||
| Leaves & flowers | |||
| EO | DPPH | IC50 (μg/mL) = 0.347 ± 0 (L), 0.349 ± 0 (F) | [69] |
| MeOH & H2O | IC50 (μg/mL) = 3.46 ± 0.010–3.88 ± 0.015 (H2O), 3.13 ± 0.011–4.27 ± 0.010 (MeOH) | [19] | |
| Morocco | |||
| Aerial parts | |||
| EA & MeOH | DPPH | IC50 (μg/mL) = 14.8 | [148] |
| β–Carotene bleaching | IC50 (μg/mL) = 59.85 ± 1.98 | ||
| EO | DPPH | IC50 (μg/mL) = 745.6 | [50] |
| IC50 (μg/mL) = 1800 | [53] | ||
| EO | DPPH | IC50 (μg/mL) = 67.85 | [131] |
| Leaves | |||
| H2O | DPPH | IC50 (μg/mL) = 32.40 | [140] |
| EO | DPPH | IC50 (μg/mL) = 6.88 | [149] |
| ABTS | IC50 (μg/mL) = 6.96 | ||
| Libya | |||
| Aerial parts | |||
| EO | DPPH | EC50 (mg/mL) = 1.64 ± 0.05 | [73] |
| RP | EC50 (mg/mL) = 0.68 ± 0.01 | ||
| β–Carotene bleaching | EC50 (mg/mL) = 1.56 ± 0.12 | ||
| TBARS | EC50 (mg/mL) = 0.31 ± 0.01 | ||
| DPPH | EC50 (mg/mL) = 0.299 | [43] | |
| DPPH | EC50 (mg/mL) = 0.132 | [6] | |
ABTS: 2,2′-azino-bis 3-ethylbenzothiazoline-6-sulfonic acid; AEAC: ascorbic acid equivalent antioxidant capacity; BHT: butyl-hydroxytoluene; CUPRAC: cupric reducing antioxidant capacity; DCFDA: dichlorodihydrofluorescein diacetate; DPPH: 2,2-diphenyl-1-picryl-hydrazyl-hydrate; EA: ethyl acetate; EAA: equivalents of ascorbic acid; FRAP: ferric-reducing antioxidant power; FTC: ferric thiocyanate; HRS: hydroxyl radical scavenging; ORAC: oxygen radical absorbance capacity; PE: petroleum ether; RP: reducing power; TAC: total antioxidant capacity; TBARS: thiobarbituric acid reactive substances.
Many studies have been carried out on the T. algeriensis aerial parts using mainly EO. However, other extracts such as methanolic, aqueous, and ethanolic were also tested (Table 3). Recently, Ouakouak et al. [42] showed that the EO from the leaves of Algerian T. algeriensis are endowed with a moderate antioxidant activity using DPPH (IC50 (mg/mL) = 41.09), ABTS (IC50 (mg/m) = 10.84), and TAC (TAC (U/L) = 39.27 ± 3.47) assays. This activity was corroborated in other studies that showed that the EO extracted from the aerial parts of T. algeriensis grown in Libya possessed a strong antioxidant activity (IC50 = 0.299 mg/mL) better than thymol [43] and inhibited the deoxyribose degradation better than mannitol [35]. The only study on the antioxidant potential of EO from T. algeriensis stem bark was conducted in Algeria and showed that the plant exhibited moderate DPPH scavenging activity (IC50 = 83.8 mg/mL) [30]. Furthermore, Mokhtari et al. [44] tested the antioxidant activities of other different extracts and showed that, using the DPPH assay, chloroform, petroleum ether, and n–BuOH extracts demonstrated IC50 values of 79.92 ± 0.30, 69.50 ± 0.68, and 5.05 ± 0.12 μg/mL, respectively. Noteworthy, the antioxidant potential of all these extracts was dependent on the method used. For instance, using the CUPRAC assay, the chloroform extract was the most effective while the n–BuOH extract was the most active using the FTC assay. In another work, the aqueous extract of leaves of Algerian T. algeriensis was active only using ABTS (IC50 = 52 ± 31 μg/mL) while the ethanolic extract was active using both the DPPH and ABTS assays with IC50 (μg/mL) of 52 ± 4 and 42 ± 0.99, respectively [45]. In contrast, Ziani et al. showed that both aqueous and hydroethanolic extracts exhibit antioxidant activities using DPPH, RP, β–carotene bleaching, and TBARS assays, with a high efficiency of the hydroethanolic fraction except when using β–carotene bleaching in which the aqueous extract demonstrated the highest activity [7]. It was also shown that the ethyl acetate extract is also endowed with an antioxidant capacity (IC50 = 0.290 mg/mL) higher than the n–BuOH extract (IC50 = 1.45 mg/mL) and ascorbic acid [46]. Interestingly, Rezq et al. [47] demonstrated that methanolic extracts of T. algeriensis leaves induced significant increase in the nuclear levels of Nrf–2 (nuclear factor erythroid 2) transcription factor (Table 3). The Nrf–2 upregulates the antioxidant response element-mediated expression of antioxidant enzymes and cytoprotective proteins and protects against oxidative pulmonary injury, abnormal inflammatory and immune responses, and apoptosis [48]. The various antioxidant activities of T. algeriensis extracts were largely studied and correlated with their phytochemical composition. In this regard, it has been shown that the Algerian T. algeriensis EO with poor content of thymol and carvacrol exhibited significantly lower antioxidant effect [35]. Similarly, Guesmi et al. [20] concluded that the higher the polarity of the Tunisian T. algeriensis extract, the stronger is the antioxidant effect. In addition, the antioxidant effect was chemotype dependent [20]. This was also reported by Jaouadi et al. [21] who showed that T. algeriensis plants growing in upper arid climatic zones of Tunisia were endowed with the best reducing, antiradical, and β–carotene bleaching inhibition potential. Likewise, the antioxidant effect of EO from leaves was reported to vary according to localities [28]. Furthermore, the antioxidant capacity was also reported to be influenced by the development stage of the plant. For instance, the aqueous extract extracted during the flowering stages of Tunisian T. algeriensis had the highest scavenging potential, while the extracts of both vegetative and flowering periods were able to reduce Fe3+ to Fe2+ [14].
Several studies have compared Thymus species to other plants in terms of their antioxidant activities. For instance, Ahmed et al. observed that EO of T. algeriensis from Tunisia presented a moderate DPPH scavenging effects compared to EO from other plants such as Eucalyptus globulus, Pinus halepensis, Pituranthos tortuosus, Rosmarinus officinalis, and Tetraclinis articulata [49]. Similarly, the EO of Moroccan T. algeriensis had a lower antioxidant activity comparatively to other species mainly T. capitatus, T. ciliatus, and T. bleicherianus [50]. However, because of the bioassay used, it is often biased and difficult to compare the antioxidant activities reported by many different studies [51].
The antioxidant effect of T. algeriensis extracts and EO is attributed to their free radical scavenging and metal chelating properties having a chemoprotective activity against oxidative stress [35]. This should be due to the richness of T. algeriensis in phytochemical active compounds dominated by certain phenolic constituents as well as the synergetic effect between its compounds even at small concentrations such as δ-cadinene and germacrene D [52]. Next to T. algeriensis, other Thymus species such asT. vulgaris, T. serpyllum, T. bleicherianus, and T. hyemalis have been widely explored for their antioxidant potential [50, 53–56]. As a result, the antioxidant potential has diverse downstream biological effects, including anti-inflammatory, anticarcinogenic, and antiatherosclerosis activities [57]. Same as other Lamiaceae species as well as most medicinal plants, T. algeriensis can serve as a good source for antioxidant compounds/molecules with potential applications in pharmaceutical, nutraceutical, and cosmeceutical industries.
6.2. Anti-Inflammatory Activity
Inflammation is a healing process induced by inflammation-inducing factors such as pathogens, toxic substances, and irradiation. These stimuli trigger the immune system and induce inflammatory responses in the host's organs which may lead to cell damage and/or diseases [58]. Many diseases are associated with inflammatory processes including type 2 diabetes, asthma, rheumatoid arthritis, neurodegenerative diseases, chronic inflammatory bowel diseases, and cancer [59]. Prominent anti-inflammatory molecules have been isolated from plants and clinically tested in humans. They mainly include curcumin, resveratrol, colchicine, quercetin, capsaicin, and epigallocatechin-3-gallate [5]. The in vitro anti-inflammatory effect of T. algeriensis has been reported by many studies. It was evaluated using egg albumin denaturation, cyclooxygenase (COX–1 and COX–2), and lipoxygenase inhibition assays (Table 4).
Table 4.
In vitro activities of T. algeriensis extracts.
| Extract | Activity | Used method | Country | Effects | Ref |
|---|---|---|---|---|---|
| Aerial part | |||||
| PE, CHCl3, and n–BuOH | Antihemolytic | Erythrocyte osmotic fragility | Algeria | IC50 (μg/mL) = 19.51 ± 0.17 (PE), 443.25 ± 0.52 (CHCl3), 322.85 ± 0.87 (n–BuOH) | [44] |
| Anti-inflammatory | Egg albumin denaturation | %inhibition = 30.26 (PE), 45.27 (CHCl3), 26.03 (n–BuOH) | |||
| EA & MeOH | Anticorrosive | Gravimetric and electrochemical | Morocco | %inhibition = 87% (MeOH) IC50 (μg/mL) = 59.85 ± 1.98 |
[148] |
| EO | Antitumor and cytotoxic | Sulforhodamine B | Libya | GI50 (μg/mL) = 62.12–64.79 | [73] |
| Hepatotoxicity evaluation | None of the EO showed toxicity at tested concentrations (>400 g/mL) for porcine liver primary cell culture | ||||
| EO | Anti-inflammatory | 5–Lipoxygenase | Algeria | IC50 (μg/mL) = 0.083 ± 0.005 | [62] |
| EO | Leishmanicidal | MTT assay | Tunisia | L.infantum IC50 (μg/mL) = 0.25 | [49] |
| L.major IC50 (μg/mL) = 0.43 | |||||
| Cytotoxic | IC80 (μg/mL) = 0.67 (murine macrophages RAW264.7) | ||||
| EO | ACE inhibition | Spectrophotometry | Tunisia | IC50 (μg/mL) = 150 | [29] |
| EO | Anticorrosive | Weight loss measurement | Morocco | Inhibited the corrosion rate (CR) of mild steel at all concentrations (CR = 0.012 (EO) versus 1.23 mg/cm2 h (blank) at 313 K) | [137] |
| Potentiodynamic polarization | Acted as mixed-type inhibitor | ||||
| Electrochemical impedance spectroscopy | Inhibition efficiency (ERT (%)) = 46–93 | ||||
| Leaves | |||||
| EO | Phytotoxic | In vitro seed germination inhibition (allelopathic effect) | Tunisia | 100% inhibition of M. sativa at 1 mg/mL (dose-dependent effect) | [28] |
| Insecticidal | Fumigant bioassay against Spodoptera littoralis Boisd. | LC50 (μL/L air) (LLD–ULD) = 41.75–131 LC90 (μL/L air) (LLD–ULD) = 53.25–189.25 |
|||
| EO | Cytotoxic | Mitochondrial-dependent reduction of yellow | Algeria | LC50 (μg/mL) = 39.8, LC90 (μg/mL) = 59.6 (cytotoxic on HCT116 cell line) | [42] |
| LC50 and LC90 (μg/mL) > 100 (limited activity against the HePG2 cell line) | |||||
| MeOH | COX inhibition | EIA | Algeria | IC50 (μM) = 12.4 ± 0.49 (COX–1), 0.05 ± 0.01 (COX–2) | [22] |
| LOX inhibition | Lipoxygenase inhibitor screening | IC50 (μM) = 2.70 ± 0.23 | |||
| MeOH | Cytotoxic | MTT | Algeria | Biocompatible on both the immortalized tested cell lines HaCaT and BALB/c-3T3 and slightly toxic on A431 and SVT2 cancer cells at high concentrations (100 μg/mL) | [47] |
| MeOH | Silver nanoparticle biosynthesis | Dropwise addition of the plant extract to the silver nitrate solution | Algeria | The extract acts as a reducing as well as a stabilizing agent Extract at 10% induced a consistent increase in the intensity of the surface plasmon peak absorbance for AgNPs |
[71] |
| EtOH and EO | Anticancer | MTT | Algeria | LC50 (μg/mL) ≥ 10,000 (EtOH), 300 ± 13–1067 ± 96 (EO) | [23] |
| MeOH | Acetylcholinesterase inhibition | Spectrophotometry | Tunisia | %inhibition = 94.5% | [21] |
| Flowers & leaves | |||||
| MeOH & H2O | Cytotoxic | MTT | Tunisia | CC50 (μg/mL) = 508 ± 45.32–516.81 ± 47.42 (H2O), 520.12 ± 32.56–528.05 ± 31.37 (MeOH) | [19] |
| EO | Cytotoxic | MTT | Tunisia | CC50 (μg/mL) = 725.92 ± 195.25 (L), 733.53 ± 141.96 (F) | [69] |
| EO | Anticancer | MTT | Morocco | Ta1 is more cytotoxic (100% lysis) than Tb2 (60% lysis) against P815 tumor cell line | [93] |
| PBMC | Increased viability by 200% | ||||
ACE: angiotensin I-converting enzyme; CC: half maximal cytotoxic concentration; COX: cyclooxygenase; EIA: enzyme immunoassay; IC50: half maximal inhibitory concentration; IC80: concentration resulting in 80% inhibition; LC50: half maximal lethal concentration; LC90: concentration resulting in 90% lethality; LOX: lipoxygenase; MTT: methyl tetrazolium test; PBMC: peripheral blood mononuclear cells.
Recently, Mokhtari et al. [44] showed that petroleum ether, chloroform, and n–BuOH extracts from the aerial parts of Algerian T. algeriensis inhibited egg albumin denaturation in a concentration-dependent effect. The highest inhibitory effect was observed using the chloroform extract with 45.27% inhibition, followed by petroleum ether (30.26%) and then n–BuOH (26.03%) extracts [44]. This activity could be useful in inhibiting protein denaturation during inflammatory conditions characterized by the production of autoantigens during disorder conditions such as rheumatic arthritis, cancer, and diabetes [60]. As previously suggested, the in vitro antidenaturation of protein activity of these extracts could be due to the interactions of the phytocompounds and/or molecules such as polyphenols, phenylpropanoids, and the alpha-lipoic acid with the aliphatic regions surrounding the lysine residue of the albumin protein [61].
Additionally, it was revealed that the methanolic extract of T. algeriensis leaves showed higher selectivity against cyclooxygenase (COX–2) (IC50 = 0.05 ± 0.01 μM) than both diclofenac and indomethacin used as standard references. Moreover, T. algeriensis extract induced similar potency like zileuton and diclofenac to inhibit 5–lipoxygenase (5–LOX) in vitro (IC50 = 2.70 ± 0.23 μM) [22]. Similarly, the EO of the aerial parts from T. algeriensis grown in Algeria were the most potent in inhibiting the lipoxygenase comparatively to those extracted from Mentha spicata and Ocimum basilicum [62]. This could be associated with the volatile compounds in EO such as 1,8-cineole, borneol, and camphor which were reported to be determinant for the anti-inflammatory activity [63]. For instance, carnosol and rosemary oil inhibited the adhesion of TNF-α-induced monocytes to endothelial cells during inflammation and suppressed the expression of intercellular adhesion molecule at the transcriptional scale [64]. Using molecular docking of some T. algeriensis compounds characterized in the methanolic extract of leaves, Ref. [22] demonstrated that salvianolic acid A and apigenin 6,8-di-C-hexosides interact with amino acid residues of COX–1 and 5–LOX, while rosmarinic acid glucoside interacts with amino acid residues of COX–2. In addition, salvianolic acid A and quercetin pentoside target the amino acid residues of the 5–LOX activating protein (FLAP). This in silico study showed that identified compounds especially rosmarinic acid glucoside, salvianolic acid A, quercetin pentoside, and apigenin 6,8-di-C-hexosides could be developed as safer anti-inflammatory agents. Noteworthy, the anti-inflammatory potential of T. algeriensis species growing in other North African countries, namely, Morocco, Tunisia, and Libya, has not been evaluated yet (Table 4). In conclusion, T. algeriensis is endowed with an exceptional anti-inflammatory power thanks to its compounds and active molecules that target key stages and mediators of the inflammatory process of different pathologies.
6.3. Antimicrobial Activities
The overuse of antibiotics has become one of the greatest challenges in human health. In addition, the rapid spread of antibiotic resistant pathogens is alarming. Therefore, research and development of a new generation of antimicrobials has become imperative. Antimicrobial agents are a group of materials that selectively destroy pathogens by interfering with their growth or survival. With the emergence of resistance phenomenon to current antibiotics, new alternative compounds such as plant-derived compounds are being explored. Due to the advantages of their inherent biochemical and biophysical properties including biocompatibility, biodegradability, and low cytotoxicity, plant biomolecules have huge potential for the antimicrobial application and have been extensively studied in recent years.
Several methods are used to evaluate the antimicrobial activities of plant extracts mainly agar disk diffusion, agar well diffusion, and macrodilution or microdilution methods [65]. These methods determine the inhibition zone diameter and the minimal inhibitory (MIC) and bactericidal concentrations (MBC). The antimicrobial activities of the T. algeriensis extracts and EO against a broad set of pathogenic bacteria, fungi, and yeasts were exhaustively studied (Tables 5 and 6).
Table 5.
Antibacterial activities of T. algeriensis extracts.
| Extract | Tested strains | Key results | Ref. |
|---|---|---|---|
| Aerial part | |||
| MeOH–H2O | S. aureus ATCC 29213 | Resistant to all the extracts | [44] |
| E. faecalis ATCC 29212 | MIC (μg/mL) = 6.25 (n–BuOH) MIC (μg/mL) = 12.5 (PE & CHCl3) |
||
| E. coli ATCC 25922 | MIC (μg/mL) = 25 (PE, CHCl3 & n–BuOH) | ||
| P. aeruginosa DMS 1117 | Resistant to all the extracts | ||
| EO | E. coli SB3 | MBC (mg/mL) = 25, MIC (mg/mL) = 12.5 | [150] |
| K. pneumoniae SB4 | MBC (mg/mL) = 25, MIC (mg/mL) = 12.5 | ||
| K. pneumoniae SB5 | MBC (mg/mL) = 25, MIC (mg/mL) = 3.12 | ||
| K. pneumoniae SB6 | MBC (mg/mL) = 25, MIC (mg/mL) = 1.56 | ||
| EO | M. luteus ATCC 9314 | IZ (mm) = 18.0 ± 0.6 | [42] |
| S. aureus ATCC 43,300 | IZ (mm) = 18.0 ± 0.7 | ||
| E. coli | IZ (mm) = 13.0 ± 0.9 | ||
| EO | S. aureus | MIC (μg/mL) ≤ 0.5 | [75] |
| L. monocytogenes (EGD-e) | MIC (μg/mL) ≤ 0.5 | ||
| L. monocytogenes (4b) | MIC (μg/mL) ≤ 0.5 | ||
| E. faecalis | MIC (μg/mL) ≤ 0.5 | ||
| S. Enteritidis | MIC (μg/mL) = 1.0 | ||
| E. coli O157:H7 | MIC (μg/mL) = 1.0 | ||
| P. aeruginosa | MIC (μg/mL) = 1.0 | ||
| EO | L. monocytogenes (ATCC 19118) | MIC (%) = 0.025, MBC (%) = 0.05 | [68] |
| S. aureus (ATCC 25923) | MIC (%) = 0.020, MBC (%) = 0.05 | ||
| E. coli (ATCC 25922) | MIC (%) = 0.025, MBC (%) = 0.05 | ||
| P. aeruginosa (ATCC 27853) | MIC (%) = 0.025, MBC (%) = 0.05 | ||
| S. typhimurium (ATCC 1402) | MIC (%) = 0.025, MBC (%) = 0.05 | ||
| MeOH–H2O | B. cereus (ATCC10876) | MIC (mg/mL) = 2.34 | [24] |
| M. luteus (NRLL B-4375) | MIC (mg/mL) = 7.03 | ||
| P. mirabilis (ATCC35659) | MIC (mg/mL) = 4.68 | ||
| E. coli (ATCC25922) | MIC (mg/mL) = 9.37 | ||
| S. typhimurium (ATCC13311) | MIC (mg/mL) = 7.06 | ||
| n–BuOH | E. coli (ATCC25922) | IZ (mm) = 7 | [17] |
| P. aeruginosa (ATCC27853) | IZ (mm) = 6.5 ± 0.7 | ||
| S. aureus (ATCC25923) | IZ (mm) = 8 | ||
| E. faecalis (ATCC29212) | IZ (mm) = 7 | ||
| EtOH & EO | S. epidermidis ATCC12228 | MIC (μg/mL) = 128 (EtOH), 32 (EO) | [23] |
| S. aureus ATCC25923 | MIC (μg/mL) = 128 (EtOH), 32 (EO) | ||
| B. subtilis ATCC11562 | MIC (μg/mL) = 64 (EtOH), 32 (EO) | ||
| E. coli ATCC29425 | MIC (μg/mL) = 256 (EtOH), 64 (EO) | ||
| P. aeruginosa ATCC15442 | MIC (μg/mL) = 512 (EtOH), 512 (EO) | ||
| K. pneumoniae ATCC43816 | MIC (μg/mL) = 256 (EtOH), 256 (EO) | ||
| H2O & EO | P. aeruginosa | IZ (mm) = 19–55 (H2O) | [14] |
| E. coli | IZ (mm) = 35–44 (EO) | ||
| S. aureus | IZ (mm) = 44–55 (EO) | ||
| E. aerogenes | IZ (mm) = 19–34 (EO) | ||
| MeOH & EtOH | E. coli ATCC 25922 | IZ (mm) = 13 (MeOH), 10 (EtOH), MIC (μg/mL) = 220 (MeOH), 270 (EtOH) | [70] |
| K. pneumonia ATCC 4352 | IZ (mm) = 0 (MeOH), 0 (EtOH), MIC (μg/mL) = 0 (MeOH), 0 (EtOH) | ||
| P. aeruginosa ATCC 27853 | IZ (mm) = 16.5 (MeOH), 14 (EtOH), MIC (μg/mL) = 185 (MeOH), 150 (EtOH) | ||
| S. typhimurium ATCC 13311 | IZ (mm) = 9 (MeOH), 12 (EtOH), MIC (μg/mL) = 110 (MeOH), 130 (EtOH) | ||
| E. cloacae ATCC 49452 | IZ (mm) = 7 (M), 0 (EtOH), MIC (μg/mL) = 160 (MeOH), 0 (EtOH) | ||
| E. faecalis ATCC 49452 | IZ (mm) = 12.5 (MeOH), 17 (EtOH), MIC (μg/mL) = 80 (M), 105 (EtOH) | ||
| S. aureus ATCC 25923 | IZ (mm) = 19 (MeOH), 15.5 (EtOH), MIC (μg/mL) = 40 (MeOH), 65 (EtOH) | ||
| EO, EtOH, & H2O | E. coli | IZ (mm) = 11.53 ± 0.43 (EO), 10.91 ± 0.05 (EtOH) | [72] |
| S. aureus | IZ (mm) = 11.52 ± 0.41 (EO) | ||
| P. aeruginosa | IZ (mm) = 0 (EO) | ||
| S. enterica | IZ (mm) = 12.51 ± 0.19 (EO) | ||
| EtOH–H2O & H2O | M. morganii | MIC (mg/mL) = 10 (H2O)–5 (EtOH–H2O) | [7] |
| P. aeruginosa | MIC (mg/mL) = 20 (H2O)–20 (EtOH–H2O) | ||
| E. coli | MIC (mg/mL) = 5 (H2O)–5 (EtOH–H2O) | ||
| E. coli extended producer of β–lactamases (ESBL) | MIC (mg/mL) = 5 (H2O)–5 (EtOH–H2O) | ||
| K. pneumoniae | MIC (mg/mL) = 10 (H2O)–5 (EtOH–H2O) | ||
| K. pneumoniae extended producer of β–lactamases (ESBL) | MIC (mg/mL) = 10 (H2O)–5 (EtOH–H2O) | ||
| E. faecalis | MIC (mg/mL) = 10 (H2O)–10 (EtOH–H2O) | ||
| L. monocytogenes | MIC (mg/mL) = 10 (H2O)–10 (EtOH–H2O) | ||
| S. aureus (MSSA) | MIC (mg/mL) = 5 (H2O)–2.5 (H2O & H2O) | ||
| S. aureus (MRSA) | MIC (mg/mL) = 5 (H2O)–2.5 (H2O & H2O) | ||
| EO | E. coli ATCC 25922 | IZ (mm) = 28 ± 1.5 | [151] |
| S. typhimurium ATCC 1402 | IZ (mm) = 20 ± 1.73 | ||
| S. aureus ATCC 25923 | IZ (mm) = 12 ± 1.33 | ||
| P. aeruginosa ATCC 27853 | IZ (mm) = 13 ± 1 | ||
| EO | E. coli | IZ (mm) = 10–13 | [118] |
| S. aureus | IZ (mm) = 8–36 | ||
| B. subtilis | IZ (mm) = 10–13 | ||
| K. pneumoniae | IZ (mm) = 0 | ||
| H2O & MeOH | S. typhimurium | MIC (mg/mL) = 0.25–0.5 (H2O), 0.12–0.25 (MeOH) | [19] |
| E. coli | MIC (mg/mL) = 0.12–0.5 (H2O), 0.12–0.25 (MeOH) | ||
| S. aureus | MIC (mg/mL) = 0.5–1 (H2O), 1 (MeOH) | ||
| S. epidermis | MIC (mg/mL) = 0.12–0.5 (H2O), 0.5–1 (MeOH) | ||
| EO | S. aureus (ATCC 6538) | MIC (mg/mL) = 0.08 ± 0.03, MBC (mg/mL) = 0.15 ± 0.05 | [6] |
| S. typhimurium (ATCC 13311) | MIC (mg/mL) = 0.09 ± 0.04, MBC (mg/mL) = 0.18 ± 0.07 | ||
| E. cloacae (human isolate) | MIC (mg/mL) = 0.05 ± 0.04, MBC (mg/mL) = 0.11 ± 0.07 | ||
| E. coli (ATCC 35210) | MIC (mg/mL) = 0.08 ± 0.03, MBC (mg/mL) = 0.11 ± 0.07 | ||
| P. aeruginosa (ATCC 27853) | MIC (mg/mL) = 0.05 ± 0.00, MBC (mg/mL) = 0.11 ± 0.01 | ||
| L. monocytogenes (NCTC 7973) | MIC (mg/mL) = 0.04 ± 0.00, MBC (mg/mL) = 0.09 ± 0.02 | ||
| M. flavus (ATCC 10240) | MIC (mg/mL) = 0.03 ± 0.00, MBC (mg/mL) = 0.05 ± 0.00 | ||
| B. cereus (clinical isolate) | MIC (mg/mL) = 0.04 ± 0.01, MBC (mg/mL) = 0.08 ± 0.02 | ||
| EO | E. coli ATCC 25922 | MIC (mg/mL) = 2.5 mg/mL | [136] |
| P. aeruginosa ATCC 27853 | MIC (mg/mL) = 1.66 mg/mL | ||
| S. aureus ATCC 25923 | MIC (mg/mL) = 0.20 mg/mL | ||
| EO | K. pneumoniae | MIC (mg/mL) = 2.030–2.114, MBC (mg/mL) ≥ 4.227 | [67] |
| P. aeruginosa | MIC (mg/mL) ≥ 4.227, MBC (mg/mL) ≥ 4.227 | ||
| S. Typhi | MIC (mg/mL) = 2.114–3.004, MBC (mg/mL) = 4.059–3.044 | ||
| E. coli | MIC (mg/mL) = 3.004–3.044, MBC (mg/mL) ≥ 4.059 | ||
| B. cereus | MIC (mg/mL) = 0.264–1.015, MBC (mg/mL) = 0.528–1.015 | ||
| S. aureus | MIC (mg/mL) = 1.015–1.057, MBC (mg/mL) = 1.015–1.057 | ||
| S. aureus (MRSA) | MIC (mg/mL) = 0.528–1.015, MBC (mg/mL) = 2.030–3.044 | ||
| E. faecalis | MIC (mg/mL) = 0.507–0.528, MBC (mg/mL) = 1.015–1.057 | ||
| EO | E. coli GM 109 | MIC (mg/mL) = 1.80–4.20 | [20] |
| P. aeruginosa | MIC (mg/mL) = 0.90–0.90 | ||
| S. enteritidis ATCC 502 | MIC (mg/mL) = 1.50–22.00 | ||
| S. aureus ATCC 25923 | MIC (mg/mL) = 1.70–4.50 | ||
| B. subtilis 166 | MIC (mg/mL) = 4.00–5.50 | ||
| L. monocytogenes | MIC (mg/mL) = 2.00–7.50 | ||
| EO | S. mutans (IBR S001) | MIC (μg/mL) = 40 ± 1.15, MBC (μg/mL) = 80 ± 2.25 | [73] |
| S. aureus (ATCC 25923) | MIC (μg/mL) = 80 ± 2.25, MBC (μg/mL) = 160 ± 4.50 | ||
| S. salivarius (IBR S006) | MIC (μg/mL) = 40 ± 3.00, MBC (μg/mL) = 80 ± 5.95 | ||
| S. sanguinis (IBR S002) | MIC (μg/mL) = 40 ± 0.00, MBC (μg/mL) = 80 ± 0.00 | ||
| S. pyogenes (IBR S004) | MIC (μg/mL) = 40 ± 0.00, MBC (μg/mL) = 80 ± 0.00 | ||
| E. feacalis (IBR E001) | MIC (μg/mL) = 20 ± 3.40, MBC (μg/mL) = 40 ± 6.75 | ||
| P. aeruginosa (IBR P001) | MIC (μg/mL) = 80 ± 2.25, MBC (μg/mL) = 160 ± 4.50 | ||
| L. acidophilus (IBR L001) | MIC (μg/mL) = 40 ± 0.00, MBC (μg/mL) = 80 ± 0.00 | ||
| EO | E. coli (ATCC 35210) | MIC (mg/mL) = 0.002, MBC (mg/mL) = 0.004 | [48] |
| P. aeruginosa (ATCC 27853) | MIC (mg/mL) = 0.003, MBC (mg/mL) = 0.05 | ||
| S. typhimurium (ATCC 13311) | MIC (mg/mL) = 0.05, MBC (mg/mL) = 0.05 | ||
| P. mirabilis (human isolate) | MIC (mg/mL) = 0.003, MBC (mg/mL) = 0.05 | ||
| L. monocytogenes (NCTC 7973) | MIC (mg/mL) = 0.001, MBC (mg/mL) = 0.05 | ||
| B. cereus (clinical isolate) | MIC (mg/mL) = 0.001, MBC (mg/mL) = 0.0025 | ||
| M. flavus (ATCC 10240) | MIC (mg/mL) = 0.001, MBC (mg/mL) = 0.0025 | ||
| S. aureus (ATCC 6538) | MIC (mg/mL) = 0.002, MBC (mg/mL) = 0.003 | ||
| EO | E. coli O157:H7 VTEC (phage type 34) | Inactivation of 5 log10 cycles of E. coli O157:H7 at both pH and of L. monocytogenes EGD-e at pH 4. | [35] |
| L. monocytogenes EGD-e | |||
| EO | S. enteritidis (CECT 4155) | IZ (mm) = 15.6 ± 2.4 | [96] |
| E. coli O157:H7 (CECT 4267) | IZ (mm) = 17.8 ± 1.7 | ||
| P. aeruginosa (CECT 110) | IZ (mm) = 15.2 ± 1.0 | ||
| S. aureus (CECT 239) | IZ (mm) = 51.0 ± 3.4 | ||
| E. aecalis (CECT 410) | IZ (mm) = 14.7 ± 1.2 | ||
| L. monocytogenes 4b (CECT 935) | IZ (mm) = 26.7 ± 2.3 | ||
| L. monocytogenes (EGD-e) | IZ (mm) = 33.7 ± 0.4 | ||
| EO | E. coli ATCC 25922 | IZ (mm) = 14 ± 1 mm, MIC (μL/mL) = 6 | [28] |
| P. aeruginosa ATCC 27853 | IZ (mm) = 14.5 ± 0.5 mm, MIC (μL/mL) = 6 | ||
| K. pneumoniae ATCC 13883 | IZ (mm) = 13.5 ± 0.5 mm, MIC (μL/mL) = 6 | ||
| S. typhimurium NRRLB 4420 | IZ (mm) = 15 ± 0.5 mm, MIC (μL/mL) = 6 | ||
| B. cereus ATCC 11778 | IZ (mm) = 30 ± 2 mm, MIC (μL/mL) = 1 | ||
| E. faecalis ATCC 29212 | IZ (mm) = 18.5 ± 0.5 mm, MIC (μL/mL) = 3 | ||
| EO | B. subtilis | MIC (v/v) = 1/250 | [57] |
| E. coli | MIC (v/v) = 1/500 | ||
| M. luteus | MIC (v/v) = 1/500 | ||
| S. aureus | MIC (v/v) = 1/500 | ||
| EO | S. aureus CFSA2 | IZ (mm) = 9.33 mm | [50] |
| L. monocytogenes EGD | IZ (mm) = 11.66 mm | ||
| B. cereus C1060 | IZ (mm) = 17.00 mm | ||
| Salmonella sp. | IZ (mm) = 8.33 mm | ||
| H. pylori strains J99 and 26695 | IZ (mm) = 13–30 mm | ||
| EO | B. subtilis ATCC 6633 | IZ (mm) = 42 mm, MIC (μL/mL) = 0.5 | [52] |
| S. aureus CIP 7625 | IZ (mm) = 0 mm, MIC (μL/mL) = 2 | ||
| E. coli CIP 54.8 | IZ (mm) = 0 mm, MIC (μL/mL) = 5 | ||
| P. aeruginosa CIP A22 | IZ (mm) = 0 mm, MIC (μL/mL) = 2 | ||
| Leaves | |||
| H2O | S. aureus ATCC 25923 | Not active towards any of the microorganisms | [89] |
| P. aeruginosa ATCC 27853 | |||
| E. coli ATCC 25922 | |||
| B. cereus ATCC 10876 | |||
| MeOH | S. aureus | MIC (mg/mL) = 1.4 | [25] |
| S. faecalis | MIC (mg/mL) = 1.4 | ||
| B. cereus | MIC (mg/mL) = 1.4 | ||
| S. epidermis | MIC (mg/mL) = 1.4 | ||
| P. aeruginosa | MIC (mg/mL) = 1.4 | ||
| E. coli | MIC (mg/mL) = 1.4 mg/mL, MBC (mg/mL) = 1.4 | ||
| K. pneumonia | MIC (mg/mL) = 1.4 | ||
| EO | E. coli ATCC 25.922 | MIC (μL/mL) = 3.25–5 | [26] |
| P. aeruginosa ATCC 9027 | MIC (μL/mL) = 3.5–5 | ||
| S. aureus ATTCC 25.923 | MIC (μL/mL) = 1.25–2.5 | ||
| L. monocytogenes ATCC 7644 | MIC (μL/mL) = 1.75–4.5 | ||
| B. cereus ATCC 11.778 | MIC (μL/mL) = 1–2.5 | ||
| EO | K. pneumoniae | IZ (mm) = 25 | [46] |
| E. coli | IZ (mm) = 46 | ||
| P. aeruginosa | IZ (mm) = 75 | ||
| M. luteus | IZ (mm) = 15 | ||
| S. aureus | IZ (mm) = 60 | ||
| S. epidermidis | IZ (mm) = 28 | ||
| B. bronchiseptica | IZ (mm) = 25 | ||
| E. faecalis | IZ (mm) = 49 | ||
| Leaves & flowers | |||
| EO | S. typhimurium | MIC (mg/mL) = 0.5 | [27] |
| E. coli | MIC (mg/mL) = 0.5 | ||
| S. aureus | MIC (mg/mL) = 0.5 | ||
| S. epidermis | MIC (mg/mL) = 0.5 | ||
| Aerial parts & leaves | |||
| EO | E. coli O157:H7 VTEC (phage type 34) | Inactivation of the initial cell populations by 4–5 log10 cycles in combination with high hydrostatic pressure | [101] |
| L. monocytogenes EGD-e | |||
IC50: half-maximal inhibitory concentration; IZ: inhibition zone; MBC: minimum bactericidal concentration; MIC: minimum inhibitory concentration; MRSA: meticillin-resistant Staphylococcus aureus; MSSA: meticillin-sensitive Staphylococcus aureus.
Table 6.
Antifungal activities of T. algeriensis extracts.
| Tested strains | Key results | Ref. |
|---|---|---|
| Aerial parts | ||
| EO | ||
| C. tropicalis | IZ (mm) = 2.04 ± 0.8 | [42] |
| C. albicans IPA200 | IZ (mm) = 13.0 ± 0.4 | |
| C. glabrata | IZ (mm) = 18.0 ± 0.6 | |
| C. glabrata | MIC (μL/mL) ≤ 0.5 | [75] |
| A. fumigates (human isolate) | MIC (mg/mL) = 0.01 ± 0.00, MFC (mg/mL) = 0.03 ± 0.00 | [6] |
| A. versicolor (ATCC 11730) | MIC (mg/mL) = 0.04 ± 0.01, MFC (mg/mL) = 0.04 ± 0.03 | |
| A. ochraceus (ATCC 12066) | MIC (mg/mL) = 0.01 ± 0.00, MFC (mg/mL) = 0.03 ± 0.00 | |
| A. niger (ATCC 6275) | MIC (mg/mL) = 0.01 ± 0.00, MFC (mg/mL) = 0.01 ± 0.00 | |
| T. viride (IAM 5061) | MIC (mg/mL) = 0.01 ± 0.00, MFC (mg/mL) = 0.01 ± 0.00 | |
| P. funiculosum (ATCC 36839) | MIC (mg/mL) = 0.01 ± 0.01, MFC (mg/mL) = 0.03 ± 0.02 | |
| P. ochrochloron (ATCC 9112) | MIC (mg/mL) = 0.01 ± 0.02, MFC (mg/mL) = 0.03 ± 0.02 | |
| P. aurantiogriseum (food isolate) | MIC (mg/mL) = 0.02 ± 0.01, MFC (mg/mL) = 0.04 ± 0.01 | |
| C. albicans | MIC (mg/mL) = 4.510–4.697, MFC (mg/mL) ≥ 4.697 | [67] |
| V. destructor | EO at 0.5% decreased the rate of infestation and caused a mortality rate of 32.6% | [78] |
| P. infestans | IC50 (μL/L) = nondetermined value | [77] |
| P. ultimum | IC50 (μL/L) = nondetermined value | |
| B. cinerea | IC50 (μL/L) ≥ 300 | |
| R. solani | IC50 (μL/L) = nondetermined value | |
| F. oxysporum f. sp. radicis-lycopersici | IC50 (μL/L) ≥ 200 | |
| C. albicans ATCC 10231 | MIC (μg/mL) = 10 ± 0.2, MFC (μg/mL) = 20 ± 0.6 | [73] |
| C. tropicalis ATCC 750 | MIC (μg/mL) = 5 ± 0.0, MFC (μg/mL) = 10 ± 0.0 | |
| A. flavus (ATCC 9643) | MIC (mg/mL) = 0.002, MFC (mg/mL) = 0.004 | [43] |
| A. fumigatus (human isolate) | MIC (mg/mL) = 0.002, MFC (mg/mL) = 0.003 | |
| A. niger (ATCC 6275) | MIC (mg/mL) = 0.001, MFC (mg/mL) = 0.003 | |
| A. ochraceus (ATCC 12066) | MIC (mg/mL) = 0.001, MFC (mg/mL) = 0.0025 | |
| P. funiculosum (ATCC 36839) | MIC (mg/mL) = 0.001, MFC (mg/mL) = 0.002 | |
| P. ochrochloron (ATCC 9112) | MIC (mg/mL) = 0.001, MFC (mg/mL) = 0.0025 | |
| T. viride (IAM 5061) | MIC (mg/mL) = 0.0005, MFC (mg/mL) = 0.001 | |
| C. albicans (human isolate) | MIC (mg/mL) = 0.025, MFC (mg/mL) = 0.05 | |
| F. solani | IZ (mm) = 31 ± 1.5, MIC (μL/mL) = 1 | [29] |
| A. niger | IZ (mm) = 64 ± 3, MIC (μL/mL) = 2 | |
| A. niger | MIC (v/v) = 1/500 | [27] |
| P. expansum | MIC (v/v) = 1/500 | |
| P. digitatum | MIC (v/v) = 1/500 | |
| G. trabeum | MIC (v/v) = 1/500 | [26] |
| P. placenta | MIC (v/v) = 1/250 | |
| C. puteana | MIC (v/v) = 1/500 | |
| C. versicolor | MIC (v/v) = 1/250 | |
| C. albicans | IZ (mm) = 9.66 | [35] |
| S. aureus CFSA2 | IZ (mm) = 9.33 | |
| C. albicans | IZ (mm) = 32, MIC (μL/mL) = 1 | [141] |
| S. cerevisiae | IZ (mm) = 46, MIC (μL/mL) = 1 | |
| M. ramanniamus NRRL 6606 | IZ (mm) = 28, MIC (μL/mL) = 0.5 | |
| F. oxysporum f. sp. albedinis | IZ (mm) = 34, MIC (μL/mL) = 1 | |
| EtOH & EO | ||
| C. glabrata ATCC22553 | MIC (μg/mL) = 128 (EtOH)–32 (EO) | [23] |
| C. albicans ATCC1023 | MIC (μg/mL) = 128 (EtOH)–64 (EO) | |
| H2O & MeOH | ||
| A. flavus | %inhibition = 5.33 ± 1.15–8 ± 2 (H2O), 0–11.33 ± 1.15 (MeOH) | [19] |
| A. niger | %inhibition = 46.03 ± 2.74–63.83 ± 6.88 (H2O), 42.53 ± 0.54–75.04 ± 4.12 (MeOH) | |
| Leaves | ||
| H2O | ||
| C. albicans ATCC 10231 | Not active | [71] |
| EO | ||
| C. albicans | IZ (mm) = 23 | [139] |
| S. cerevisiae | IZ (mm) = 47 | |
| H2O & EtOH | ||
| P. megakarya | EC50 = 32.35 ± 2.02 (H2O), EC90 = 112.55 ± 16.57 (H2O) | [76] |
| EC50 = 1.32 ± 0.4 (EtOH), EC90 = 24.97 ± 4.9 (EtOH) | ||
| Leaves & Flowers | ||
| EO | ||
| A. niger | Not active | [69] |
| A. flavus | 42.86% inhibition |
EC50: half maximal effective concentration; EC90: 90% maximal effective concentration; IC50: half maximal inhibitory concentration; IZ: inhibition zone; MBC: minimum bactericidal concentration; MFC: minimum fungicidal concentration; MIC: minimum inhibitory concentration.
According to literature, EO were the most explored for the antimicrobial potential. For instance, Ait-Ouazzou et al. tested the effect of the EO from the aerial parts of T. algeriensis growing in Morocco against seven bacteria and one fungus and showed their very high effectiveness (MIC ≤ 0.5 μL/mL) towards S. aureus, E. faecalis, L. monocytogenes, and C. glabrata and a moderate activity (MIC = 1.0 μL/mL) against P. aeruginosa, S. enteritidis, and E. coli O157:H7 [66]. These bacteria especially P. aeruginosa were sometimes shown to exhibit a remarkable resistance against OE of T. algeriensis from other countries such as Tunisia and Algeria [20, 28, 67]. Interestingly, Hazzit et al. [35] noticed that Helicobacter pylori strains J99 and 26695 were more sensitive (IZ = up to 30 mm) to EO from Algerian T. algeriensis aerial parts than all the other five tested strains (IZ = 9.33–17 mm) including Salmonella sp. and S. aureus CFSA2. Similarly, it was found that Bacillus cereus (ATCC10876), Micrococcus luteus (NRLL B-4375), and Proteus mirabilis (ATCC35659) were inhibited at 2.34, 7.03, and 4.68 mg/mL, respectively, of the EO extracted from the aerial parts of T. algeriensis growing in the north of Tunisia [68]. In addition, Zaïri et al. revealed that the best antibacterial activity was obtained using the EO from T. algeriensis leaves (MIC = 0.5 mg/mL) which also inhibited the fungus Aspergillus flavus by up to 42.86% [69]. Moreover, Rezzoug et al. [23] and Guesmi et al. [14] observed that the EO from the aerial parts of Algerian and Tunisian T. algeriensis demonstrated better antimicrobial activity that the ethanolic and aqueous extracts, respectively. For instance, S. epidermidis ATCC12228 and S. aureus ATCC25923 were inhibited at 128 μg/mL using the methanolic extract and at only 32 μg/mL using EO. More importantly, the antimicrobial efficiency of T. algeriensis EO from Libya was shown to be stronger than reference antibiotics such as ampicillin and streptomycin [6]. Additionally, Messaoudi et al. reported that the aerial part's ethanolic extract of T. algeriensis collected in the Algerian southwest has no effect on the growth of E. cloacae ATCC 49452 while it inhibited widely the growth of both E. faecalis ATCC 49452 and S. aureus ATCC 25923 [70]. However, aqueous extracts from leaves and aerial parts of Algerian T. algeriensis showed no inhibitory effect (no halo zone) against five human pathogens. Using methanol as the solvent, the leaves of T. algeriensis from the upper arid area of Tunisia showed a bacteriostatic effect against seven human pathogenic bacterial strains (MIC = 1.4 mg/mL) while the bactericidal activity was restricted to E. coli [71, 72].
Extract characterization revealed that samples containing carvacrol elicited a remarkable antibacterial activity [21]. Elsewhere, Nikolić et al. found a positive correlation between the antimicrobial activity of selected EO of T. serpyllum, T. algeriensis, and T. vulgaris and their chemical composition, which indicated that the activity may be ascribed to the presence of thymol because it occurs in high proportions [73].
The synergistic combination effect of T. algeriensis EO from Morocco and heat or pulsed electric fields against E. coli and L. monocytogenes was shown to inactivate the bacterial growth by of 5 log10 cycles [66]. Moreover, T. algeriensis EO were capable of inactivating 4–5 log10 cycles of the initial cell populations (E. coli O157:H7 VTEC (phage type 34) and L. monocytogenes (EGD-e)) in combination with high hydrostatic pressure [74]. Comparatively to other plants such as E. globulus and R. officinalis, EO from T. algeriensis were the most potent in inhibiting seven human pathogenic bacteria [75].
Plant pathogens such as Phytophthora megakarya (black pod of cocoa) were dose-dependently inhibited using both aqueous and ethanolic extracts of T. algeriensis leaves bought at a local market in Cameroon and completely inhibited at 60 and 125 mg/mL, respectively [76]. Similarly, Botrytis cinerea was inhibited by the EO from wild Tunisian T. algeriensis at >300 μL/L [77]. Another investigation showed that 0.5% EO of T. algeriensis collected in northern Algeria decreased the rate of infestation of Varroa destructor, causing a mortality rate of 32.6% [78] (Table 6). Taken together, the EO of T. algeriensis are more potent than the different other extracts against human and plant pathogenic strains as well as other microbial species. Antibacterial activity of other Moroccan Thymus species was also reported in the literature such as T. bleicherianus [79], T. serpyllum [56], T. munbyanus [80], T. hyemalis, T. vulgaris [54], and T. zygis [81].
The mechanisms by which plant extracts trigger microbe survival and growth involve the integrity and the permeability of the membranes as well as the efflux pump systems of resistant Gram-negative bacteria such as E. coli, E. aerogenes, K. pneumoniae, P. aeruginosa, and S. enterica Typhimurium [82–84]. These effects are often explained by the hydrophobic nature of plant extracts allowing them to accumulate in bacterial cell membranes and disrupt their structure. In addition, the ability of some compounds to chelate transition metals such as copper and iron reduces their bioavailability to microorganisms [85]. Some researchers have observed that the antibacterial activity is correlated with the high contents of monoterpenes, phenols, aldehydes, and ketones in plant extracts that affect the cell membrane of microorganisms [86]. Others have been reported to induce a deficiency in microbial enzyme systems due to leakage of intracellular constituents leading to apoptosis [87]. Plant extracts can also trigger the phospholipids of the bacterial cell membrane, coagulate the cytoplasm, and attack lipids and proteins [88]. For instance, a study reported that Salix tetrasperma stem bark extract impaired P. aeruginosa virulence by inhibiting swimming and swarming mobilities as well as by reducing its hemolytic and proteolytic activities [89]. The antibacterial activities of plant extracts have been associated with several bioactive compounds such as polyphenols and molecules, namely, furocoumarins and furanocoumarins [85]. In general, the nature of a phytochemical may influence its mechanism of action and therefore its antibacterial activity. Finally, most antimicrobial activities of T. algeriensis extracts have been tested in vitro. However, it is interesting to evaluate their potential in vivo using animal models.
The antimicrobial effects of T. algeriensis extracts against human pathogenic bacteria and fungi make it an important plant to be explored using fractionation and testing procedures in order to isolate potentially active molecules that can be tested in vivo and through clinical trials.
6.4. Anticancer and Cytotoxic Activity
Nowadays, cancer is the most lethal disease [90]. Although several chemotherapeutic molecules are being used to manage cancer, development of drug resistance, problems of selective toxicity, and severe side effects are still challenging. Therefore, discovering new anticancer drugs is becoming more and more imperative [91]. In this regard, plant-derived compounds are a promising source of chemotherapeutic drugs. In the market today, many of the currently used chemotherapeutic medicines such as taxanes (docetaxel and paclitaxel), vinca alkaloids (vincristine, vinblastine, and semisynthetic drugs vindesine and vinorelbine), camptothecin derivatives (camptothecin and irinotecan), and epipodophyllotoxins (teniposide and etoposide) were initially isolated from medicinal plants [92].
The cytotoxic activity of T. algeriensis against cancer cells has been extensively studied using the methyl tetrazolium test (MTT), sulforhodamine B assay, and histopathological analysis (Table 4). Cytotoxicity was mostly assessed using EO. According to Ouakouak et al. [42], leaves' EO from the Algerian T. algeriensis showed high toxicity against the HCT116 tumor cell line (LC90 = 59.6 μL/mL) while limited effect was seen towards the HePG2 hepatocellular carcinoma cell line (LC90 > 100 μL/mL). Similarly, it was reported that the EO of T. algeriensis leaves and flowers exhibited cytotoxic activities at concentrations higher than 400 μg/mL with CC50 = 725.92 ± 195.25 μg/mL and 733.53 ± 141.96 μg/mL, respectively [69]. Toxicities against other tumor cells such as NCI-H460 (non-small cell lung cancer), MCF-7 (breast), HCT-15 (colon), and AGS (gastric) were also reported using Libya's T. algeriensis EO with CC50 ranging from 62.12 to 64.79 μg/mL. However, no toxicity was noticed on the porcine liver primary cell lines at concentrations > 400 g/mL [73]. Noteworthy, Jaafari et al. observed that the EO from the chemotype-rich Moroccan T. algeriensis which is carvacrol were more toxic than the other chemotypes' EO against the p815 mastocytoma tumor model cell line (up to 100% lysis) and had an important proliferative effect on the peripheral blood mononuclear cells (PBMC) [93]. Besides EO, other T. algeriensis extracts were also shown to be potent against cancer cell lines. For instance, the leaf methanolic extract of Algerian T. algeriensis was slightly toxic on A431 and SVT2 cancer cells at 100 μg/mL. However, biocompatibility on both the immortalized tested cell lines HaCaT and BALB/c-3T3 was observed [47]. The anticancer potential of Tunisian T. algeriensis extracts of leaves and flowers on the HCT116 cell line revealed that both the aqueous and methanolic extracts are toxic with CC50 (μg/mL) of 516.81 ± 47.42 and 528.05 ± 31.37, respectively. However, using T. capitatus and R. officinalis extracts, the aqueous extracts showed very low toxicities compared with the methanolic extracts [19].
The mechanisms underpinning the cytotoxic activity against cancer cells were linked to the nature of secondary metabolites in the plant extracts. For instance, berberine alkaloids are known to activate the apoptosis-inducing enzymes (caspase 3/9) of the human leukemia HL-60 cells by downregulating telomerase activity and nucleophosmin/B23 [94]. Additionally, some plant molecules such as curcumin have the potential to downregulate miR-21 expression in MCF-7 cells through the upregulation of the PTEN/Akt signaling pathway [95]. The anticancer potential of phytochemicals and their regulatory aspects were exhaustively described by Khan et al. [94]. In 2021, a study showed that even at low concentrations, T. algeriensis (from Tunisia) oily fraction inhibited HCT116 cell growth. Cleavage of poly(ADP-ribose) polymerase (PARP) and activation of the initiator and effector caspases show that T. algeriensis causes apoptotic cell death (caspases 3, 8, and 9). It also increased the expression of death receptors (DRs) while decreasing the expression of TNF-α-related apoptosis-inducing ligand (TRAIL) decoy receptors, according to the findings (DcRs). This study also showed that T. algeriensis increased MAPK pathway signaling molecules (p38 kinase, ERK, and JNK), downregulated c-FLIP, and overexpressed SP1 and CHOP. The in vivo model of cancer showed that intragastric injection of T. algeriensis extract (12.5 and 50 mg/mL) inhibited colorectal carcinogenesis in an animal model by preventing multiple phases in the carcinoma [96]. To sum up, the anticancer potential of T. algeriensis extracts is mainly attributed to their capacity to enhance the apoptosis-stimulating enzymes and receptors in tumor cells, to downregulate the oncogenic miRNA and genes, and to inhibit the antiapoptotic proteins. The anticancer and cytotoxic activity of T. algeriensis extracts is evident as has been widely proven. It turns out that this plant hides an enormous potential in the induction of apoptosis of cancer cells; that said, it will be extremely interesting to go further through in vivo and clinical experiments and to identify, like the compounds mentioned above, potential anticancer molecules with promising medical applications.
6.5. Miscellaneous Activities
T. algeriensis appears to be endowed with other biological activities such as antihemolytic, antiacetylcholinesterase, antilipase, insecticidal, leishmanicidal, and angiotensin converting enzyme (ACE) inhibitory activities (Table 4). Using erythrocyte osmotic fragility test, petroleum ether, chloroform, and n–BuOH extracts of the Algerian T. algeriensis (aerial part) induced resistance of erythrocytes to hemolysis. The highest antihemolytic effect was noted using the n–BuOH extract (IC50 = 322.85 ± 0.87 μg/mL), followed by the chloroform extract (IC50 = 443.25 ± 0.52 μg/mL). The petroleum ether extract displayed the lowest activity with 19.51% inhibition at 800 μg/mL [44]. The antihemolytic effect of T. algeriensis extracts could be linked to their chemical composition, particularly flavonoids and polyphenols. In fact, previous research has shown that the presence of polyphenols and flavonoids in the crude extracts enhanced the stability of the erythrocyte membrane and inhibited hemolysis [97]. However, the exact mechanism of action of the membrane stabilization by the extract is yet to be elucidated [98]. Studies have also related the antihemolytic effect to the occurrence of molecules having antioxidant activities due to their ability to scavenge free radicals [99].
Previous studies showed that the methanolic extract from the T. algeriensis leaves (Tunisia) showed various abilities to inhibit acetylcholinesterase (AChE) with significant variations among T. algeriensis populations. It was observed that populations growing in the arid zone were the most potent (EC50 = 0.1–0.2 mg/mL) [21]. The modulation of the activity of AChE has been linked to the phenolic compounds [100, 101]. These latter effects have been reported to protect against neurodegenerative diseases [102]. For instance, rosmarinic acid was reported as an Aβ aggregation inhibitor [103, 104]. Besides, Benguechoua et al. showed, for the first time, that an acetone/water extraction mixture with ethyl acetate and butanol fractions from Algerian T. algeriensis inhibited Candida rugosa lipase [105].
Leaves' EO from Tunisian T. algeriensis used as a fumigant exhibited insecticidal activity against Spodoptera littoralis Boisd. with LC50 ranging from 41.75 to 131 μL/L air [28]. The insecticidal effect of T. algeriensis EO is likely due to their major constituents such as α-pinene, 1,8-cineole, caryophyllene oxide, camphor, linalool, camphene, and p-eugenol, which were found to be toxic in many previous studies [106, 107]. This level of larvae mortality is higher than those observed using other medicinal plants such as Thymus mastichina, Origanum majorana, and Salvia sclarea [108]. According to Al-Nagar et al., terpene compounds constitute a major group of insect feeding deterrents and display a very strong inhibitory activity against larvae of S. littoralis [109]. Tunisian OE from the T. algeriensis aerial parts were also endowed with leishmanicidal activity against two Leishmania species: L. infantum and L. major with IC50 of 0.25 and 0.43 μg/mL, respectively [49]. Some investigations indicated that the plant extracts including EO can target the metabolic pathways involved in Leishmania species survival such as MAP kinases, glyoxalase, metacaspases, and sterol pathways [110, 111]. These plant-based compounds include flavones, azoles, and staurosporine [111, 112]. Similarly, Melo et al. demonstrated that oleanolic acid, which possessed a good activity against L. amazonensis, L. braziliensis, and L. infantum (IC50<70 μM in promastigotes and amastigotes), did not provoke FN-γ–assisted macrophage activation [113]. These findings indicated that this type of triterpene most likely acts on targets located in Leishmania parasites rather than in the host's macrophages.
Finally, Zouari et al. showed that T. algeriensis EO could be used as angiotensin I-converting enzyme (ACE) inhibitor for hypertension treatment (IC50 = 150 μg/mL) [29]. To elucidate the mechanisms underlying ACE inhibition, it was suggested that the flavonoids such as vitexin and isovitexin isolated from various plants that inhibited ACE activity at 0.33 mg/mL by 20% and 45%, respectively, act by competing with the substrate for the ACE active site [114]. In addition, flavonoids are reported to form chelate complexes with the zinc atom within the active site of zinc-dependent metallopeptidases to which ACE belongs [115, 116].
It can be concluded that T. algeriensis extracts act as an antihemolytic by protecting the integrity of the erythrocyte's membrane, as antiacetylcholinesterase agents by inhibiting the Aβ aggregation, as an insecticidal and leishmanicidal by targeting the metabolic pathways for insects' survival, and as angiotensin converting enzyme (ACE) inhibitors by competing with the ACE active site. In the light of these various potentialities, some of which are being used traditionally, it would be original to investigate T. algeriensis extracts more deeply for these activities to spotlight the underlying mechanisms both in vitro and in vivo.
7. In Vivo Pharmacological Activities
In the biological systems, there is a growing need for in vivo experiments as most studies focus on in vitro and cell culture-based assays. However, these studies require verification using animal experiments to avoid misleading data and toxicity issues [117]. Indeed, the in vivo studies carried out on T. algeriensis extracts were comparatively limited and were only performed on species growing in Algeria and Tunisia using aerial part and leaf hydromethanolic extracts and EO. Reported studies are summarized in Table 7.
Table 7.
In vivo activities of T. algeriensis extracts.
| Extract | Doses | Model | Activity | Effects | Ref. |
|---|---|---|---|---|---|
| Algeria | |||||
| Leaves | |||||
| 80% MeOH | 200, 400, and 600 mg/kg | Male rats | Anti-inflammatory activity | Significant mild reduction in edema thickness using 400 mg/kg by up to 30% | [22] |
| Leukocyte's recruitment | A dose-dependent reduction in the total leukocyte number at three doses tested by up to 62% | ||||
| 200, 400, and 600 mg/kg | Swiss albino mice | Acetic acid-induced vascular permeability | Attenuation of vasodilation and decreased vascular permeability in mice at 400 or 600 mg/kg by 63 and 58%, respectively | ||
| 200 and 400 mg/kg | Antinociceptive activity by hot plate test | A dose-dependent increase in response latency using 200 and 400 mg/kg by up to 200% | |||
| 200 and 400 mg/kg | Antinociceptive activity by acetic acid-induced abdominal writhing | 94% reduction of the writhing response using 400 mg/kg | |||
| 200 and 400 mg/kg | Male Wistar rats | Heat hyperalgesia | Restoration of heat response latency when measured at day 14 post chronic constriction injury (CCI) by about 160 and 200% using 200 and 400 mg/kg, respectively | [47] | |
| Mechanical hyperalgesia (pinprick test) | Increased the withdrawal time of injured hind paw by 8.4- and 6-folds after 7 and 14 days, respectively | ||||
| Acetone drop test (paw cold allodynia) | Decreased cold allodynia score by about 16- and 10-folds after 7 and 14 days using 200 and 400 mg/kg, respectively | ||||
| Paint-brush test (mechanical dynamic allodynia) | Attenuated the dynamic allodynia score when assessed at day 7 by up to 1.75-folds Normalization of dynamic response score by 400 mg/kg dose level when measured at day 14 post surgery |
||||
| Aqueous extract | 2000 and 5000 mg/kg | Albino Wistar rats | Acute toxicity study for 14 days | No mortality or signs of toxicity and no significant differences in body weight, food consumption, and absolute organ weights between controls and treated animals | [119] |
|
| |||||
| Aerial part | |||||
| MeOH–H2O | 200, 400, and 800 mg/kg | Albino male mice | Safety evaluation | No influence on the levels of AST and ALT A significant reduction in phosphatase alkaline levels by up to 35% using 400 mg/kg No effect on the levels of urea and creatinine |
[24] |
| Antioxidant activity | Increased the plasma antioxidant levels by 3-folds (22% of inhibition) using 800 mg/kg | ||||
| Increased the iron reducing ability (908 μM FeSO4 eq/mL) using 800 mg/kg by 2-folds compared to the nontreated group (405 μM FeSO4 eq/mL) Improved CAT activity by 24 to 86% using 200 to 800 mg/kg Increased GSH levels in mice treated with 400 and 800 mg/kg (34 to 45 nmol/mL) compared to those of the nontreated group (30 nmol/mL) Decreased the MDA levels in the plasma of treated groups at 200 and 400 mg/kg by 50 and 63%, respectively | |||||
| Tunisia | |||||
| Aerial part | |||||
| EO | 180 mg/kg per day dissolved in normal saline | Sprague Dawley rats | Body weight gain, toxicity, and mortality | Body weight gain, no mortality, and no sign of toxicity after 15 days of experiment | [118] |
| Assessment of lipid profile | Decreased the MDA levels (743.57 ± 41.12 nmol MDA/mg protein) compared to the control group (3648.47 ± 33.22 nmol MDA/mg protein) | ||||
| Assessment of antioxidant defense enzymes | Prevented the toxicity effect of H2O2 on the nonenzymatic antioxidant GSH level and the activities of SOD, CAT, GPx, and GST | ||||
| Histopathological examination | No pathological change, normal histoarchitecture, and significant reduction in neuronal damage induced by H2O2 | ||||
| AChE inhibition | Decreased the AChE activity by up to 31.5% | ||||
| 150 mg/kg | Adult male Wistar rats | Reproductive organs weights | Weight gain by up to 92% | [121] | |
| Sperm morphology | No effect on sperm's morphology, counts, and mobility | ||||
| Sperm count and motility | |||||
| Sperm viability | Increased the sperm's viability by up to 46% | ||||
| Histopathological studies | Improvement in morphological abnormalities (amorphous head, hookless head, doublet heads, compact head tail with a cytoplasmic droplet, irregular tail, and coiled tail) of sperms | ||||
| DNA fragmentation analysis using gel electrophoresis | Protection against H2O2-induced DNA fragmentation in testis | ||||
| Lipid peroxidation | Reduction of the levels of MDA in testicular cells induced by H2O2 at 1 mmol/L | ||||
| Assessment of nonenzymatic antioxidants | Prevention of the H2O2-induced alterations in GSH level | ||||
| Protein estimation | Increase in total protein | ||||
| 180 mg/kg per day | Sprague Dawley rats | Hepatic and renal functional marker enzymes | Attenuation of the increase in AST and reduction in urea and creatinine levels in H2O2-treated group | [122] | |
| Enzymatic antioxidants and lipid profile | Recovered the levels of CAT (up to 150% increase), SOD (up to 233% increase), GST (up to 15.7% increase) and GPx (up to 71.4% increase) activities, and GSH (up to 98% increase) | ||||
| Histopathological examination | No histopathological changes in the liver and kidney Alleviation of the injuries in the glomeruli and proximal tubules (77.7% reduction in damage score) |
||||
| Body and organ weights | Prevention of H2O2-induced liver, kidney, and weight loss | ||||
| 54, 117, and 180 mL/kg | Adult male and female Wistar rats | Histology of gastric lesions | Lesions inhibition mainly at doses of 180 mg/kg for male rats (88%) and between 117 and 180 mg/kg for female rats (96.25 and 98.85%) | [20] | |
| Assessment of enzymatic and nonenzymatic antioxidants | Increase in SOD, CAT, GPx, and GST activities and GSH content | ||||
| Measurement of mucus production | Increase in the mucus production of gastric mucosa compared to control group | ||||
| Acute toxicity study in rodents | No signs of toxicity and absence of abnormal organic damage to the rats' organs | ||||
AChE: acetylcholine; ALP: alanine transaminase; AST: aspartate transaminase; CAT: catalase; CCI: chronic constriction injury; CDNB: 2,4-dinitrochlorobenzene; GPx: glutathione peroxidase; GSH: glutathione; GST: glutathione S-transferases; MDA: malondialdehyde; SOD: superoxide dismutase. All experiments were done orally.
7.1. Safety Studies
As the interactions of plant constituents with biological components such as tissues, cells, proteins, and DNA could disrupt host's immune response and metabolism, assessing their in vivo behavior would provide a basis for beneficial as well as toxic responses and, more importantly, could lead to predictive models to assess their pharmacokinetics and intended effects [117].
Here, the in vivo toxicological evaluation of T. algeriensis extracts was investigated using animal models mainly Albino male mice and Sprague Dawley rats. Toxicity parameters were mostly related to hepatic and renal functions, mortality, body and organ weights, and histopathological evaluation of tissues (Table 7). Righi et al. showed that the hydromethanolic extract of T. algeriensis aerial parts (Algeria) administrated orally to Albino male mice at 200–800 mg/kg did not influence the hepatic (AST and ALT) and renal functional markers (urea and creatine) [24]. Similarly, Guesmi et al. observed no sign of toxicity or mortality after 15 days of oral administration of EO from Tunisian T. algeriensis at 180 mg/kg per day dissolved in normal saline to Sprague Dawley rats with no pathological change and normal histoarchitecture [118]. In another study, they showed that T. algeriensis EO exhibited no signs of acute toxicity with the absence of abnormal organic damage to the rats' organs and prevented H2O2-induced liver, kidney, and organ weight loss [20].
The leaf aqueous extract of Algerian T. algeriensis administered by oral route to Albino Wistar male and female mice at the doses of 2000 mg/kg and 5000 mg/kg induced no significant differences in body weight, no mortality, and/or no signs of toxicity. In addition, treated mice showed no change in the biochemical parameters and no histological abnormalities in their organs compared to control animals [119].
The major challenge encountering plant-derived agents is the prediction of their trajectory and location after in vivo administration. Hence, the relationship between the properties of a molecule and its distribution and behavior with the biological system should be studied as it is developed by pharmaceutical approaches for drugs. This is known as structure–function/toxicity relationship models [120]. Such data could be investigated before evaluating the systematic effect [117].
According to these studies, it seems that T. algeriensis is far from being toxic at least in tested models and at administered doses. However, more in-depth studies using pharmacokinetics and downstream targets of hepatic metabolites resulting from its compounds will have to be explored to ensure its biosafety for humans.
7.2. Antioxidant Activity
As mentioned above, T. algeriensis extracts are reported to be endowed with a high antioxidant activity in vitro. This was corroborated using in vivo experiments especially by Guesmi et al. [20, 118, 121, 122]. Various methods were used to investigate the antioxidant effect in vivo, mainly the assessment of the antioxidant machinery (enzymatic and nonenzymatic antioxidants), lipid peroxidation, and ferric reducing ability of plasma (Table 7). For instance, in 2016, these researchers demonstrated that Tunisian EO administrated orally to Sprague Dawley rats (150 mg/kg per day) subjected to low- and high-dose H2O2 treatment recovered the catalase (CAT), superoxide dismutase (SOD), and glutathione peroxidase (GPx) activities and glutathione (GSH) levels in liver and renal tissues of treated animals. In another study, they showed that T. algeriensis EO administered to adult male Wistar rats preserved the sperms' viability, improved the morphological abnormalities, and prevented induced DNA fragmentation in their testis. An increase in total protein in the H2O2-treated group was also noticed [121]. These researchers' investigations corroborated the same finding [20, 118]. Moreover, it was reported that the aerial part hydromethanolic extract of Algerian T. algeriensis increased the plasma antioxidant levels by 3-folds following treatment of Albino male mice with the dose of 800 mg/kg. At the same concentration, the iron reducing ability (908 μM FeSO4eq/mL) was twofolds higher than that of the untreated group (405 μM FeSO4/mL). In addition, a significant improvement in CAT activity and GSH levels was seen in mice treated with 400 and 800 mg/kg compared to the nontreated group accompanied with a significant decrease in the MDA levels in the plasma of treated groups by 200 and 400 mg/kg [24]. To sum up, T. algeriensis extracts displayed substantial antioxidant activities in several experimental models (in vitro: DPPH, FRAP, β–carotene bleaching, ORAC, TBARS, RP, phosphomolybdenum, lipid peroxidation inhibition, TAC, HRS, metal ions chelation, and superoxide anion scavenging assays, and in vivo: rats and mice) via diverse molecular targets/mechanisms by increasing the activities of antioxidant enzyme activities such as SOD, GST, and CAT and nonenzymatic antioxidants like GSH, reducing the MDA levels, and preventing the DNA damage. All these protective effects of T. algeriensis extracts against oxidative stress sustain the in vitro findings and highlight its benefits in the prevention and protection against oxidative stress and its radicals in related disorders and diseases.
7.3. Anti-Inflammatory Activity
The in vivo effectiveness of T. algeriensis as a source of anti-inflammatory agents has been investigated by Sobeh et al. using the leaf extracts of Algerian species [22] (Table 7). The study showed that the oral administration of the 80% MeOH extract to male rats injected with carrageenan induced a significant mild reduction in edema thickness using the doses of 200 and 400 mg/kg compared to the control rats. In addition, the increased vascular permeability induced by acetic acid injection in mice was reduced by 63 and 58% when animals were pretreated with T. algeriensis extracts at 400 or 600 mg/kg, respectively. These effects on the first phase of inflammation were attributed to the capacity of T. algeriensis extracts to inhibit the vasodilatation and the release of inflammatory mediators. In conclusion, T. algeriensis extracts exhibited prominent anti-inflammatory activities both in vitro and in vivo by inhibiting the inflammatory mediators such as COX, LOX, PGE2, LT, IL-1β, TNF-α, and IL-6 and by increasing the levels of Nrf–2 which controls the intracellular redox environment and redox homeostasis genes. The in vivo effects corroborate the anti-inflammatory activities reported in vitro and open promising prospects in using T. algeriensis-based compounds and derivatives in treating inflammatory related disorders in humans.
7.4. Leukocyte Recruitment
The effect of T. algeriensis leaf extracts on carrageenan-induced leukocyte migration into the peritoneal cavity in mice was also evaluated by Sobeh et al. [22] (Table 7). Following intraperitoneal injection of carrageenan, mice pretreated with T. algeriensis extract doses (200, 400, and 600 mg/kg) showed a dose-dependent decrease in the number of total leukocytes with up to 62%. This effect was more important that those observed using diclofenac and dexamethasone, which induced 39 and 30% decrease in leucocyte number, respectively. The observed reduction in leucocyte number has been explained by the suppression of inflammatory mediators such as prostaglandins and proinflammatory cytokines. It was also shown that the inhibition of leukocyte migration could be the result of the suppression of adhesion molecule and/or inhibition of the chemotactic of substances' expression [123]. This investigation confirms the in vitro anti-inflammatory activity of T. algeriensis extracts and highlights its positive effect on leukocyte migration and inflammation mediation during the immune response process.
7.5. Antinociceptive Activity
The central and peripheral analgesic activity of T. algeriensis leaf extract was studied in mice using the hot plate test and acetic acid-induced abdominal writhing assay, respectively [22] (Table 7). The study showed that oral pretreatment of mice with T. algeriensis extracts at 200 and 400 mg/kg prior to acetic acid injection induced a significant peripheral analgesic activity with a dose-dependent reduction in induced writhes. The effect observed by the dose 400 mg/kg (94% reduction) was higher than that achieved by dexamethasone (2 mg/kg) or diclofenac (20 mg/kg). Moreover, central antinociceptive activity was also shown using the extract's doses represented by a longer response latency. In addition, the extract at 400 mg/kg showed similar activity to the reference narcotic analgesic (nalbuphine). It was presumed that the leaf hydroalcoholic extract of T. algeriensis could act on the opioid system and/or other central nervous system (CNS) receptors involved in the antinociception. To sum up, T. algeriensis extracts exerted an antinociceptive effect in vivo by targeting the opioid system receptors (i.e., mu, delta, and kappa) and CNS nociceptors. This prominent analgesic effect of T. algeriensis extracts arouses a lot of interest in the search for metabolites endowed with antinociceptive activity which will certainly lead to good research and application perspectives of plant-based painkillers from T. algeriensis.
7.6. Gastroprotective and Antiulcerogenic Activity
The effect of T. algeriensis EO was investigated on Tunisian species using induced gastric lesions in adult male and female Wistar rats by 0.3 M HCl/60% ethanol [20] (Table 7). The results showed that oral treatment of rats by the EO (54–180 mg/kg, p.o.) induced a dose-dependent decrease in gastric lesions and reduced the ulcer index and percentage of inhibition mainly at doses of 180 mg/kg for male rats (88%) and between 117 and 180 mg/kg for female rats (96.25 and 98.85%). In addition, the gastric acidity of female rats pretreated with EO was significantly reduced comparatively to the ulcer control rats. The mucosal damage induced by HCl/ethanol in the control group was alleviated using the T. algeriensis EO. In fact, mucus production increased significantly in both male and female rats treated with 180 mg/kg. As compared to the acidified group in which visible change in the gross appearance of the gastric mucosa was seen, stomachs of male and female rats treated with the dose of 180 mg/kg showed a normal appearance. The authors suggested that the antiulceration effect of EO from T. algeriensis is attributed to their antioxidant effect and inhibition of neutrophil infiltration into ulcerated tissues. Nevertheless, the molecules and their structural aspects determining the observed effect are not yet established. The gastroprotective and antiulcerogenic activities of T. algeriensis EO seen in animal models explain its uses in traditional medicine for this purpose and open a great potential of using this plant's volatile constituents in the treatment of peptic ulcers and gastric troubles.
7.7. Neuroprotective Activity
The effects of T. algeriensis extracts on chronic neuropathic pain and the underpinning mechanisms were investigated by Rezq et al. [47] (Table 7). Using the chronic constriction injury (CCI) model in male Wistar rats, 80% MeOH leaf extracts of Algerian T. algeriensis (200 mg/kg and 400 mg/kg) prominently attenuated hyperalgesia and allodynia at day 14 of surgery. Noteworthy, the extracts showed a higher effect than the standard drug (pregabalin) towards both stimuli. In addition, they restored the normal appearance of the nerve fascicle which regained its regular form. Finally, the degeneration of most neurons caused by CCI was restored by the T. algeriensis extract at 400 mg/kg with few pyknotic neurons and low and deeply stained nuclei. The extracts also mitigated the increase of caspase 3 and synaptophysin. The underlying mechanisms of the observed neuroprotective activity were (i) inhibition of NOX–1, iNOS; (ii) increase of catalase activity; and (iii) inhibition of inflammatory mediators such as TNF–α, NF–κB, COX–2, and PGE2. These effects suggest the substantial potential of T. algeriensis extracts in improving painful peripheral neuropathy. Furthermore, another earlier study on hydrophobic fractions of T. algeriensis (HFTS) growing in Tunisia showed that HFTS mitigates neuroinflammation via AChE inhibition and attenuates H2O2-induced brain toxicity [118]. In conclusion, T. algeriensis attenuated neuropathic pains and degeneration of neurons via antioxidant and anti-inflammatory properties. This opens new avenues for research of molecules exhibiting neuronal activities. Hence, bio-guided assays on T. algeriensis extracts can be envisaged with the aim of isolating molecules with neuroprotector power, unraveling their functional features, identifying their targets, and understanding their mode of actions.
7.8. Hot Topic Analysis of T. algeriensis
In this study, the bibliometric data used for the mapping tool is from the Web of Science database. The cooccurrence analysis of more than 50 high frequency keywords has been made to construct a knowledge map of the main strong domain of research related to T. algeriensis. In the visualization results, each keyword is represented by a circle. From the analysis of Figure 7, four topic groups are identified, which are “the different chemotypes of the plant,” “the chemical composition of the essential oil,” “the biological activities,” and the” in vivo activities.” Among them, the field of biological activities is the link between the field of chemical composition and the chemotype of the plant as there is a close relationship with these fields. After further domain structure detection as shown in Figure 8, the main topics in the field of biological activities of T. algeriensis were the antibacterial and antioxidant activities, directly related to the richness of the essential oil of the aerial parts of the plant in thymol, carvacrol, and p-cymene. The occurrences of these compounds, their absence, or their presence determine the chemotype of the plant.
Figure 7.
Coword map network visualization of T. algeriensis.
Figure 8.
Knowledge map of T. algeriensis.
8. Discussion
To the best of our knowledge, this is the first review to summarize and critically evaluate the chemical composition, biological and pharmacological activities, efficacy, and safety of T. algeriensis extracts and essential oils. T. algeriensis is one of the most well-known Thymus species in northern Africa. Most of the prior research on this plant species has focused on its biological activities and pharmacological and preclinical characteristics. Many chemical constituents have been identified from T. algeriensis extracts such as flavonoids, mainly kaempferol and rutin, besides luteolin, apigenin, hesperidin, and neohesperidin among others. They also contain phenolic acids such as rosmarinic acid and vanillic acid. Other constituents include sterols (e.g., β-sitosterol) and triterpenoid (e.g., oleanolic acid) [22, 23].
Current evidence revealed that the chemical composition of T. algeriensis essential oils (EO) differs according to its source. Its EO contains 40 volatile compounds represented mainly by monoterpenes and sesquiterpenoids. EO from T. algeriensis growing in Tunisia contains mainly 1,8-cineole and 4-terpineol [9, 15], while borneol, camphene, camphor, and carvacrol were listed from the Algerian and Moroccan plants. p-Cymene and thymol were detected in all flora. β-Myrcene (20.22%) has only been identified in the aerial parts of the Libyan plants [124], while myrcene characterized the EO of the Moroccan plants [53].
Free radicals are unstable and reactive because they have an unpaired electron. Superoxide, nitric oxide, and hydroxyl radicals, the most reactive and poisonous ROS, are among them. Hydrogen peroxide, singlet oxygen, and ozone are nonradical oxidants that produce free radicals in tissues through a variety of chemical processes. ROS are produced normally in mitochondria during aerobic respiration, by macrophages to fight infection, during induction of cytochrome p450, and by peroxisomes in the cells. Many exogenous factors can stimulate ROS production which damages cells and causes cellular injury and disease development such as tobacco smoke, ionizing radiation, UV light, industrial toxins such as carbon-tetrachloride, drugs, and charcoal-broiled foods [125]. Natural products with antioxidant properties such as T. algeriensis are beneficial in disease prevention and treatment. Antioxidant effects of T. algeriensis were investigated using different methods including DPPH, ABTS, FRAP, TBRS, TAC, HRS, and β-carotene bleaching assays [42, 126–128]. These studies showed that T. algeriensis extracts and EO had antioxidant properties. Furthermore, in vivo studies confirmed these in vitro tests. Although the in vitro studies investigated the antioxidant effects of this species, more comparative studies between extracts and EO of T. algeriensis obtained from various geographical areas are required. Moreover, in vivo antioxidant effects of T. algeriensis extracts and EO are not sufficient, and more studies are required.
Inflammation is now recognized as a major factor in cellular and subcellular disorders. Few cells are engaged in this process, which boosts the production of proinflammatory chemical mediators (IL, TNF-α, NO, and PGs). Stimuli and tissue injuries influence mediator overproduction [129]. It was reported that phenolic compounds are similar to nonsteroidal anti-inflammatory drugs (NSAIDs) in their ability to decrease the chemical mediators of inflammation [130]. As mentioned previously in this review, T. algeriensis extracts and EO are rich in different phenolic compounds. In vitro studies showed their ability to inhibit inflammatory enzymes such as COX–1, COX–2, and 5-LOX [22, 44]. Besides, in vivo studies showed that the extracts suppressed the production of proinflammatory cytokines such as NF-κB, TNF-α, lipoxygenase, COX–2 enzymes, and PGE2 [47, 131]. Additionally, the administration of T. algeriensis extracts to animal models enhanced their enzymatic and nonenzymatic antioxidant machinery (CAT, SOD, GPx, and GSH) in response to stress induction (Figure 9). Nevertheless, more studies on the different extracts and EO from T. algeriensis on inflammatory cell lines and in vivo models are still needed.
Figure 9.
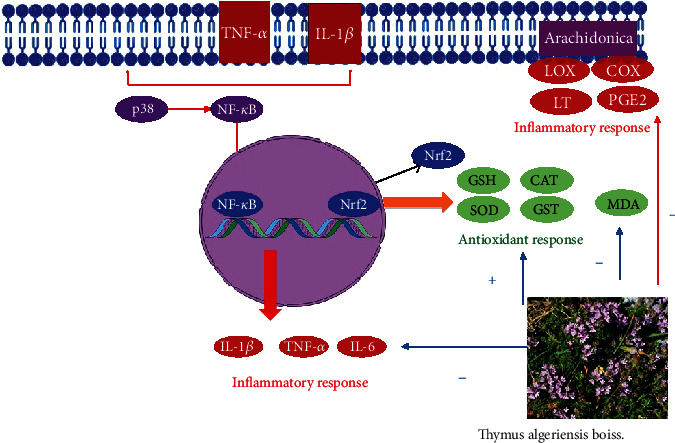
Mechanisms of antioxidant and anti-inflammatory effects of T. algeriensis extracts. Oxidative stress is associated with increased lipid peroxidation product (MDA) and decreased antioxidant enzyme activities such as SOD, GST, and CAT and nonenzymatic antioxidants such as GSH. Increased oxidative stress is a trigger for release of inflammatory cytokines such as TNF-α and IL-1β which increase p38 and subsequent phosphorylation and translocation of NF-κB to the nucleus and subsequent transcription of genes responsible for formation of inflammatory cytokines creating a vicious cycle of inflammation. Furthermore, oxidative stress activates inflammatory enzymes such as LOX and COX with subsequent increase in both leukotriene and PG syntheses, respectively. T. algeriensis through its potent antioxidant effects can suppress all these inflammatory pathways and thus protect susceptible tissues against oxidative and inflammatory reactions. Abbreviations: LOX: lipoxygenase; COX: cyclooxygenase; LT: leukotrienes; PGE2: prostaglandin E2; GSH: reduced glutathione; GST: glutathione transferase; CAT: catalase; SOD: superoxide dismutase; MDA: malondialdehyde; IL-1β: interleukin-1β; IL-6: interleukin-6; NF-κB: nuclear factor kappa B; Nrf–2: nuclear factor erythroid 2; p38: mitogen-activated protein kinase; TNF-α: tumor necrosis factor.
Moreover, in vitro studies showed that T. algeriensis extracts and EO had anticholinesterase activities [132], while in vivo studies on their use in treatment of Myasthenia gravis, Alzheimer diseases, or other diseases treated with anticholinesterases are lacking. In the same context, in vitro studies on ACE inhibition are promising [133], but no in vivo studies to our knowledge studied the antihypertensive effects or compared the extract effects with known ACE inhibitors (ACEIs). Similarly, α-glucosidase inhibitory effects of T. algeriensis extracts were explored, but no in vivo study on their antidiabetic effects was carried out [134].
Although gastroprotective, antinociceptive, anti-inflammatory, antioxidant, neuroprotective, and anticancer properties have all been documented for T. algeriensis extracts and EO, several studies are required on other disease models.
9. Conclusions and Future Perspectives
This review profiles the chemical composition of T. algeriensis and synthesizes the biological and pharmacological activities of its extracts and EO. Based on this comprehensive overview, the use of this plant's extracts in biotechnology seems to be promising because of their wealth in bioactive molecules mainly for antioxidant, antimicrobial, anticancer, anti-inflammatory, antinociceptive, gastro- and neuroprotective, and antiacetylcholinesterase purposes. However, as most of the biological activities have been evaluated in vitro, it would be interesting to extend their in vivo activities and uncover the underpinning mechanisms of action. In addition, the activities of T. algeriensis extracts vary depending on the solvents utilized. Moreover, environmental factors, geographical regions of growth, seasonal variations, phenological stages, degree of ripeness, plant part used, and harvesting time as well as postharvest treatment and processing could greatly influence the chemical composition of the plant and thus the related activities. This led to result inconsistency which represents a real issue for the development of bioactive preparations and their reliability. Thus, it could be interesting to study the effect of T. algeriensis domestication on its chemical composition and biological activities as it is a solution to conserve this plant species.
Since T. algeriensis was shown to improve sperm viability and mobility, further investigation could shed light on the potential estrogenic activity of this plant and its effect on reproductive hormones as Lamiaceae species including Thymus genus are traditionally used in Morocco to treat infertility disorders [135]. Additionally, as the revolution for patient safety has gained momentum importance, the safety of T. algeriensis cannot be definitively established from reported studies. More attention should still be given to the toxicity aspect in a more in-depth manner especially when using EO that could be harmful in some individuals. Although the preclinical investigations (in vitro, cell-based, and animal studies) on this plant species are available in a large quantity, they should be translated into evidence-based clinical progress using human trials which are completely missing. Having said that, there are many opportunities for the healthcare industry to explore the benefits of T. algeriensis because of the potential growth market of medicinal plants. Therefore, future prospective studies should screen fractions or individual constituents that exhibit health-protecting and curative activities from T. algeriensis.
Conflicts of Interest
The authors declare no competing interest.
Authors' Contributions
I.M., W.B.B., and M.F. reviewed the literature and wrote the manuscript. G.T.M.B. and H.A. revised the manuscript. M.S. revised the manuscript and designed and conceived the work. All authors approved the final version.
Funding
The APC was funded by UM6P.
References
- 1.Gilani A. H., Rahman A. Trends in ethnopharmacology. Journal of Ethnopharmacology . 2005;100(1-2):43–49. doi: 10.1016/j.jep.2005.06.001. [DOI] [PubMed] [Google Scholar]
- 2.Krishnaiah D., Sarbatly R., Nithyanandam R. A review of the antioxidant potential of medicinal plant species. Food and Bioproducts Processing . 2011;89(3):217–233. doi: 10.1016/j.fbp.2010.04.008. [DOI] [Google Scholar]
- 3.Gunjan M., Naing T. W., Saini R. S., Ahmad A., Naidu J. R., Kumar I. Marketing trends & future prospects of herbal medicine in the treatment of various disease. World Journal of Pharmaceutical Research . 2015;4(9):132–155. [Google Scholar]
- 4.Škrovánková S., Mišurcová L., Machů L. Chapter three - antioxidant activity and protecting health effects of common medicinal plants. In: Henry J., editor. Advances in Food and Nutrition Research . Academic Press; 2012. pp. 75–139. [DOI] [PubMed] [Google Scholar]
- 5.Fürst R., Zündorf I. Plant-derived anti-inflammatory compounds: hopes and disappointments regarding the translation of preclinical knowledge into clinical progress. Mediators of Inflammation . 2014;2014:9. doi: 10.1155/2014/146832.146832 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bukvicki D., Giweli A., Stojkovic D., et al. Short communication: cheese supplemented with _Thymus algeriensis_ oil, a potential natural food preservative. Journal of Dairy Science . 2018;101(5):3859–3865. doi: 10.3168/jds.2017-13714. [DOI] [PubMed] [Google Scholar]
- 7.Ziani B. E. C., Heleno S. A., Bachari K., et al. Phenolic compounds characterization by LC-DAD-ESI/MSn and bioactive properties of Thymus algeriensis Boiss. & Reut. and Ephedra alata Decne. Food Research International . 2019;116:312–319. doi: 10.1016/j.foodres.2018.08.041. [DOI] [PubMed] [Google Scholar]
- 8.Le Floc'h E., Boulos L. Flore de Tunisie . Montpellier, France: Catalogue synonymique commenté; 2008. [Google Scholar]
- 9.Ali E. H., Ben I., Zaouali Y., Bejaoui A., Boussaid M. Variation of the chemical composition of essential oils in Tunisian populations of Thymus algeriensis Boiss. Et Reut. (Lamiaceae) and implication for conservation. Chemistry & Biodiversity . 2010;7(5):1276–1289. doi: 10.1002/cbdv.200900248. [DOI] [PubMed] [Google Scholar]
- 10.Morales R. Studies on the genus . Thymus; 1996. [Google Scholar]
- 11.Stahl-Biskup E., Sáez F. Thyme: The Genus Thymus . CRC Press; 2002. [DOI] [Google Scholar]
- 12.Ali B. E. H., Imen A. G., Boussaid M. Chemical and genetic variability of Thymus algeriensis Boiss. et Reut. (Lamiaceae), a North African endemic species. Industrial Crops and Products . 2012;40:277–284. doi: 10.1016/j.indcrop.2012.03.021. [DOI] [Google Scholar]
- 13.Benabid A. Flore et Écosystèmes Du Maroc: Evaluation et Préservation De La Biodiversité . Paris (France): Ibis Press; 2000. [Google Scholar]
- 14.Guesmi F., Saidi I., Bouzenna H., Hfaiedh N., Landoulsi A. Phytocompound variability, antioxidant and antibacterial activities, anatomical features of glandular and aglandular hairs of Thymus hirtus Willd. Ssp. Algeriensis Boiss. And Reut. over developmental stages. South African Journal of Botany . 2019;127:234–243. doi: 10.1016/j.sajb.2019.09.004. [DOI] [Google Scholar]
- 15.Zouari N., Ayadi I., Fakhfakh N., Rebai A., Zouari S. Variation of chemical composition of essential oils in wild populations of Thymus algeriensis Boiss. Et Reut., a north African endemic species. Lipids in Health and Disease . 2012;11(1):p. 28. doi: 10.1186/1476-511X-11-28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ali B. E. H., Imen A. G., Boussaid M. Effect of habitat fragmentation on the genetic structure of the gynodioecious Thymus algeriensis Boiss. et Reut. (Lamiaceae) in Tunisia. Plant Biosystems-An International Journal Dealing with all Aspects of Plant Biology . 2014;148(2):217–226. doi: 10.1080/11263504.2012.761290. [DOI] [Google Scholar]
- 17.Kaki F. A., Benkiniouar R., Demirtas I., Merzoug A., Touil A. Phytochemical study of two Algerian plants Origanum vulgare L. Sbsp. glandulosum (Desf) Ietswaart and Thymus algeriensis (Boiss. And Reut) Asian Journal of Chemistry . 2019;31(5):1105–1109. doi: 10.14233/ajchem.2019.21805. [DOI] [Google Scholar]
- 18.Boutaoui N., Zaiter L., Benayache F., et al. Qualitative and quantitative phytochemical analysis of different extracts from Thymus algeriensis aerial parts. Molecules . 2018;23(2):p. 463. doi: 10.3390/molecules23020463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Zairi A., Nouir S., M'hamdi N., et al. Antioxidant, antimicrobial and the phenolic content of infusion, decoction and methanolic extracts of Thyme and Rosmarinus species. Current Pharmaceutical Biotechnology . 2018;19(7):590–599. doi: 10.2174/1389201019666180817141512. [DOI] [PubMed] [Google Scholar]
- 20.Guesmi F., Ali M. B., Barkaoui T., et al. Effects of Thymus hirtus Sp. Algeriensis Boiss. Et Reut. (Lamiaceae) essential oil on healing gastric ulcers according to sex. Lipids in Health and Disease . 2014;13(1):p. 138. doi: 10.1186/1476-511X-13-138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Jaouadi R., Silva A. M. S., Boussaid M., Yahia I. B. H., Cardoso S. M., Zaouali Y. Differentiation of phenolic composition among Tunisian Thymus algeriensis Boiss. et Reut. (Lamiaceae) populations: correlation to bioactive activities. Antioxidants . 2019;8(11):p. 515. doi: 10.3390/antiox8110515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Sobeh M., Rezq S., Cheurfa M., et al. Thymus algeriensis and Thymus fontanesii: chemical composition, in vivo antiinflammatory, pain killing and antipyretic activities: a comprehensive comparison. Biomolecules . 2020;10(4):p. 599. doi: 10.3390/biom10040599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Rezzoug M., Bakchiche B., Gherib A., et al. Chemical composition and bioactivity of essential oils and ethanolic extracts of Ocimum basilicum L. and Thymus algeriensis Boiss. & Reut. from the Algerian Saharan atlas. BMC Complementary and Alternative Medicine . 2019;19(1):p. 146. doi: 10.1186/s12906-019-2556-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Righi N., Boumerfeg S., Fernandes P. A. R., et al. Thymus algeriensis Bioss & Reut: relationship of phenolic compounds composition with _in vitro/in vivo_ antioxidant and antibacterial activity. Food Research International . 2020;136, article 109500 doi: 10.1016/j.foodres.2020.109500. [DOI] [PubMed] [Google Scholar]
- 25.Gedara S. R. Terpenoid content of the leaves of Thymus algeriensis Boiss. Mansoura Journal of Pharmaceutical Sciences . 2008;24:133–143. [Google Scholar]
- 26.Ajjouri E., Mustapha M. G., Satrani B., et al. Composition chimique et activité antifongique des huiles essentielles de Thymus algeriensis Boiss. & Reut. et Thymus ciliatus (Desf.) Benth. contre les champignons de pourriture du bois. Acta botanica gallica . 2010;157(2):285–294. doi: 10.1080/12538078.2010.10516206. [DOI] [Google Scholar]
- 27.Amarti F., Satrani B., Ghanmi M., et al. Chemical composition and antimicrobial activity of the Thymus algeriensis Boiss. & Reut, and Thymus ciliatus (Desf.) Benth. essential oils of Morocco. Biotechnologie, Agronomie, Société et Environnement . 2010;14(1):141–148. [Google Scholar]
- 28.Ali B. E. H., Imen M. C., Bahri R., Chaieb I., Boussaïd M., Harzallah-Skhiri F. Chemical composition and antioxidant, antibacterial, allelopathic and insecticidal activities of essential oil of Thymus algeriensis Boiss. et Reut. Industrial Crops and Products . 2015;77:631–639. doi: 10.1016/j.indcrop.2015.09.046. [DOI] [Google Scholar]
- 29.Zouari N., Fakhfakh N., Zouari S., et al. Chemical composition, angiotensin I-converting enzyme inhibitory, antioxidant and antimicrobial activities of essential oil of Tunisian Thymus algeriensis Boiss. et Reut. (Lamiaceae) Food and Bioproducts Processing . 2011;89(4):257–265. doi: 10.1016/j.fbp.2010.11.006. [DOI] [Google Scholar]
- 30.Hamza O., Fahima A., Hassani A. Chemical composition, antioxidant activity of the essential oil of Thymus algeriensis Boiss, North Algeria. International Letters of Chemistry, Physics and Astronomy . 2015;59:72–80. doi: 10.18052/www.scipress.com/ILCPA.59.72. [DOI] [Google Scholar]
- 31.Zhang W., Li H.-J., Chen L., et al. Fructan from Polygonatum cyrtonema Hua as an eco-friendly corrosion inhibitor for mild steel in HCl media. Carbohydrate Polymers . 2020;238, article 116216 doi: 10.1016/j.carbpol.2020.116216. [DOI] [PubMed] [Google Scholar]
- 32.Russo R., Corasaniti M. T., Bagetta G., Morrone L. A. Exploitation of cytotoxicity of some essential oils for translation in cancer therapy. Evidence-based Complementary and Alternative Medicine . 2015;2015:9. doi: 10.1155/2015/397821.397821 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Salehi B., Abu-Darwish M. S., Tarawneh A. H., et al. Thymus spp. plants - Food applications and phytopharmacy properties. Trends in Food Science & Technology . 2019;85:287–306. doi: 10.1016/j.tifs.2019.01.020. [DOI] [Google Scholar]
- 34.Giordani R., Hadef Y., Kaloustian J. Compositions and antifungal activities of essential oils of some Algerian aromatic plants. Fitoterapia . 2008;79(3):199–203. doi: 10.1016/j.fitote.2007.11.004. [DOI] [PubMed] [Google Scholar]
- 35.Hazzit M., Baaliouamer A., Veríssimo A. R., Faleiro M. L., Miguel M. G. Chemical composition and biological activities of Algerian Thymus oils. Food Chemistry . 2009;116(3):714–721. doi: 10.1016/j.foodchem.2009.03.018. [DOI] [Google Scholar]
- 36.Mariam R., Allal D., Zidane L. Étude ethnobotanique des plantes médicinales dans le Parc National de Talassemtane (Rif occidental du Maroc) Journal of Applied Biosciences . 2016;97 doi: 10.4314/jab.v97i1.5. [DOI] [Google Scholar]
- 37.Hachi M., Ouafae B., Hachi T., et al. Contribution to the ethnobotanical study of antidiabetic medicinal plants of the Central Middle Atlas region (Morocco) Lazaroa . 2016;37 doi: 10.5209/LAZAROA.51854. [DOI] [Google Scholar]
- 38.Fakchich J., Elachouri M. An overview on ethnobotanico-pharmacological studies carried out in Morocco, from 1991 to 2015: systematic review (part 1) Journal of Ethnopharmacology . 2021;267, article 113200 doi: 10.1016/j.jep.2020.113200. [DOI] [PubMed] [Google Scholar]
- 39.Guclu G., Kelebek H., Selli S. Chapter 26 - antioxidant activity in olive oils. In: Preedy V. R., Watson R. R., editors. Olives and Olive Oil in Health and Disease Prevention . Second Edition. San Diego: Academic Press; 2021. pp. 313–325. [DOI] [Google Scholar]
- 40.Santos-Sánchez N. F., Salas-Coronado R., Villanueva-Cañongo C., Hernández-Carlos B., editors. Antioxidant Compounds and Their Antioxidant Mechanism . London, UK: IntechOpen; 2019. [DOI] [Google Scholar]
- 41.El-Beltagi H. S., Badawi M. H. Comparison of antioxidant and antimicrobial properties for Ginkgo biloba and Rosemary (Rosmarinus officinalis L.) from Egypt. Notulae Botanicae Horti Agrobotanici Cluj-Napoca . 2013;41(1):126–135. doi: 10.15835/nbha4118928. [DOI] [Google Scholar]
- 42.Ouakouak H., Benarfa A., Messaoudi M., et al. Biological properties of essential oils from Thymus algeriensis Boiss. Plants . 2021;10(4):p. 786. doi: 10.3390/plants10040786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Giweli A. A., Džamić A. M., Soković M. D., Ristić M. S., Marin P. D. Chemical composition, antioxidant and antimicrobial activities of essential oil of Thymus algeriensis wild-growing in Libya. Central European Journal of Biology . 2013;8(5):504–511. doi: 10.2478/s11535-013-0150-0. [DOI] [Google Scholar]
- 44.Mokhtari M., Chabani S., Mouffouk S., et al. Phytochemicals, antihemolytic, anti-inflammatory, antioxidant, and antibacterial activities from Thymus algeriensis. Journal of Herbs, Spices & Medicinal Plants . 2021;27(3):253–266. doi: 10.1080/10496475.2021.1891174. [DOI] [Google Scholar]
- 45.Bouguerra A., Djebili S., Zouaoui N., Barkat M. Evaluation of phenolic contents and antioxidant activities of some medicinal plants growing in Algerian Aurès mountains. Acta Scientifica Naturalis . 2020;7(2):15–30. doi: 10.2478/asn-2020-0017. [DOI] [Google Scholar]
- 46.Benabdallah F. Z., Zellagui A., Demirtas I. Chemical composition of essential oils and antioxidant activities of extracts of two endemic plants from Algeria. International Journal of Pharmaceutical Sciences and Research . 2017;8(1):p. 244. [Google Scholar]
- 47.Rezq S., Alsemeh A. E., D’Elia L., et al. Thymus algeriensis and Thymus fontanesii exert neuroprotective effect against chronic constriction injury-induced neuropathic pain in rats. Scientific Reports . 2020;10(1, article 20559) doi: 10.1038/s41598-020-77424-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Zhao H., Eguchi S., Alam A., Ma D. The role of nuclear factor-erythroid 2 related factor 2 (Nrf-2) in the protection against lung injury. American Journal of Physiology. Lung Cellular and Molecular Physiology . 2017;312(2):L155–L162. doi: 10.1152/ajplung.00449.2016. [DOI] [PubMed] [Google Scholar]
- 49.Ahmed S. B. H., Sghaier R. M., Guesmi F., et al. Evaluation of antileishmanial, cytotoxic and antioxidant activities of essential oils extracted from plants issued from the leishmaniasis-endemic region of Sned (Tunisia) Natural Product Research . 2011;25(12):1195–1201. doi: 10.1080/14786419.2010.534097. [DOI] [PubMed] [Google Scholar]
- 50.Amarti F., Satrani B., Ghanmi M., et al. Activité antioxydante et composition chimique des huiles essentielles de quatre espèces de thym du Maroc. Acta botanica gallica . 2011;158(4):513–523. doi: 10.1080/12538078.2011.10516292. [DOI] [Google Scholar]
- 51.Konishi T. Chapter 4 - antioxidant property is the basic feature of Kampo medicine. In: Arumugam S., Watanabe K., editors. Japanese Kampo Medicines for the Treatment of Common Diseases: Focus on Inflammation . Academic Press; 2017. pp. 33–40. [DOI] [Google Scholar]
- 52.Čančarević A., Bugarski B., Šavikin K., Zdunić G. Biological activity and ethnomedicinal use of Thymus vulgaris and Thymus serpyllum. Lekovite sirovine . 2013;33:3–17. [Google Scholar]
- 53.Hamdani I., Bouyanzer A., Hammouti B., et al. Chemical composition and antioxidant activity of essential oils of _Thymus broussonetii_ Boiss. and _Thymus algeriensis_ Boiss. from Morocco. Asian Pacific Journal of Tropical Disease . 2014;4(4):281–286. doi: 10.1016/S2222-1808(14)60573-9. [DOI] [Google Scholar]
- 54.Zeroual A., Eloutassi N., Sakar E. H., et al. Antimicrobial and antioxidant activities of crude extracts and essential oils from two Thyme species: Thymus vulgaris and Thymus hyemalis from northern Morocco. International Journal of Biosciences . 2018;12:391–399. [Google Scholar]
- 55.Jennan S., Farah A., Bennani B., Fouad R., Mahjoubi F. Antibacterial and antioxidant activities of the essential oils of Thymus hyemalis and Thymus bleicherianus from Morocco. International Journal of Pharmacognosy and Phytochemistry . 2013;28(2):1173–1178. [Google Scholar]
- 56.Alaoui Jamali C., Kasrati A., Fadli M., Hassani L., Leach D., Abbad A. Synergistic effects of three Moroccan Thyme essential oils with antibiotic cefixime. Phytothérapie . 2017 doi: 10.1007/s10298-017-1107-2. [DOI] [Google Scholar]
- 57.Kasote D. M., Katyare S. S., Hegde M. V., Bae H. Significance of antioxidant potential of plants and its relevance to therapeutic applications. International Journal of Biological Sciences . 2015;11(8):982–991. doi: 10.7150/ijbs.12096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Chen L., Deng H., Cui H., et al. Inflammatory responses and inflammation-associated diseases in organs. Oncotarget . 2018;9(6):7204–7218. doi: 10.18632/oncotarget.23208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Scrivo R., Vasile M., Bartosiewicz I., Valesini G. Inflammation as "common soil" of the multifactorial diseases. Autoimmunity Reviews . 2011;10(7):369–374. doi: 10.1016/j.autrev.2010.12.006. [DOI] [PubMed] [Google Scholar]
- 60.Sangeetha G., Vidhya R. In vitro anti-inflammatory activity of different parts of Pedalium murex (L.) Inflammation . 2016;4(3):31–36. [Google Scholar]
- 61.Williams L. A. D., O'connar A., Latore L., et al. The in vitro anti-denaturation effects induced by natural products and non-steroidal compounds in heat treated (immunogenic) bovine serum albumin is proposed as a screening assay for the detection of anti-inflammatory compounds, without the use of animals, in the early stages of the drug discovery process. West Indian Medical Journal . 2008;57(4) [PubMed] [Google Scholar]
- 62.Boulanouar B., Abdelaziz G., Aazza S., Gago C., Graça Miguel M. Antioxidant activities of eight Algerian plant extracts and two essential oils. Industrial Crops and Products . 2013;46:85–96. doi: 10.1016/j.indcrop.2013.01.020. [DOI] [Google Scholar]
- 63.Ehrnhöfer-Ressler M. M., Fricke K., Pignitter M., et al. Identification of 1,8-cineole, borneol, camphor, and thujone as anti-inflammatory compounds in a Salvia officinalis L. infusion using human gingival fibroblasts. Journal of Agricultural and Food Chemistry . 2013;61(14):3451–3459. doi: 10.1021/jf305472t. [DOI] [PubMed] [Google Scholar]
- 64.Lian K.-C., Chuang J.-J., Hsieh C.-W., et al. Dual mechanisms of Nf-Κb inhibition in carnosol-treated endothelial cells. Toxicology and Applied Pharmacology . 2010;245(1):21–35. doi: 10.1016/j.taap.2010.01.003. [DOI] [PubMed] [Google Scholar]
- 65.Balouiri M., Sadiki M., Ibnsouda S. K. Methods for in vitro evaluating antimicrobial activity: a review. Analysis . 2016;6(2):71–79. doi: 10.1016/j.jpha.2015.11.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Ait-Ouazzou A., Espina L., Cherrat L., et al. Synergistic combination of essential oils from Morocco and physical treatments for microbial inactivation. Innovative Food Science & Emerging Technologies . 2012;16:283–290. doi: 10.1016/j.ifset.2012.07.002. [DOI] [Google Scholar]
- 67.Benabed H., Khadidja N. G., Ouinten M., Bombarda I., Yousfi M. Chemical composition, antioxidant and antimicrobial activities of the essential oils of three Algerian Lamiaceae species. Current Nutrition & Food Science . 2017;13(2):97–109. doi: 10.2174/1573401313666170104105521. [DOI] [Google Scholar]
- 68.Jayari A., Jouini A., Boukhris H., et al. Essential oils from Thymus capitatus and Thymus algeriensis as antimicrobial agents to control pathogenic and spoilage bacteria in ground meat. Journal of Food Quality . 2021;2021:9. doi: 10.1155/2021/5599374.5599374 [DOI] [Google Scholar]
- 69.Zaïri A., Nouir S., Khalifa M. A., et al. Phytochemical analysis and assessment of biological properties of essential oils obtained from Thyme and Rosmarinus species. Current Pharmaceutical Biotechnology . 2020;21(5):414–424. doi: 10.2174/1389201020666191019124630. [DOI] [PubMed] [Google Scholar]
- 70.Messaoudi M., Benreguieg M., Merah M., Messaoudi Z. A. Antibacterial effects of Thymus algeriensis extracts on some pathogenic bacteria. Acta Scientiarum. Biological Sciences . 2019;41:p. e48548. doi: 10.4025/actascibiolsci.v41i1.48548. [DOI] [Google Scholar]
- 71.Beldjilali M., Mekhissi K., Khane Y., Chaibi W., Belarbi L., Bousalem S. Antibacterial and antifungal efficacy of silver nanoparticles biosynthesized using leaf extract of Thymus algeriensis. Journal of Inorganic and Organometallic Polymers and Materials . 2020;30(6):2126–2133. doi: 10.1007/s10904-019-01361-3. [DOI] [Google Scholar]
- 72.Bouatrous Y. Antibacterial activity of an essential oil and various extracts of the medicinal plant Thymus hirtus Sp. algeriensis Boiss. & Reut. Annals of Phytomedicine: An International Journal . 2019;8(2) doi: 10.21276/ap.2019.8.2.13. [DOI] [Google Scholar]
- 73.Nikolić M., Glamočlija J., Ferreira I. C. F. R., et al. Chemical composition, antimicrobial, antioxidant and antitumor activity of Thymus serpyllum L., Thymus algeriensis Boiss. and Reut and Thymus vulgaris L. essential oils. Industrial Crops and Products . 2014;52:183–190. doi: 10.1016/j.indcrop.2013.10.006. [DOI] [Google Scholar]
- 74.Espina L., García-Gonzalo D., Laglaoui A., Mackey B. M., Pagán R. Synergistic combinations of high hydrostatic pressure and essential oils or their constituents and their use in preservation of fruit juices. International Journal of Food Microbiology . 2013;161(1):23–30. doi: 10.1016/j.ijfoodmicro.2012.11.015. [DOI] [PubMed] [Google Scholar]
- 75.Ait-Ouazzou A., Lorán S., Bakkali M., et al. Chemical composition and antimicrobial activity of essential oils of Thymus algeriensis, Eucalyptus globulus and Rosmarinus officinalis from Morocco. Journal of the Science of Food and Agriculture . 2011;91(14):2643–2651. doi: 10.1002/jsfa.4505. [DOI] [PubMed] [Google Scholar]
- 76.Fovo J. D., Ntongo V. M., Kouam E. B., Nsouga F. R. A., Ntsomboh-Ntsefong G. Pod resistance of cocoa clones to black pod disease and antifungal properties of phytoextracts against Phytophthora megakarya. International Journal of Biosciences . 2018;13(3):94–104. doi: 10.12692/ijb/13.3.94-104. [DOI] [Google Scholar]
- 77.Maissa B. J., Walid H. Antifungal activity of chemically different essential oils from wild Tunisian Thymus spp. Natural Product Research . 2015;29(9):869–873. doi: 10.1080/14786419.2014.984182. [DOI] [PubMed] [Google Scholar]
- 78.Kouache B., Brada M., Saadi A., Fauconnier M. L., Lognay G., Heuskin S. Chemical composition and acaricidal activity of Thymus algeriensis essential oil against Varroa destructor. Natural Product Communications . 2017;12(1):p. 1934578X1701200. doi: 10.1177/1934578X1701200138. [DOI] [PubMed] [Google Scholar]
- 79.Amarti F., Satrani B., Aafi A., et al. Composition chimique et activité antimicrobienne des huiles essentielles de Thymus capitatus et de Thymus bleicherianus du Maroc. Phytothérapie . 2008;6(6):342–347. doi: 10.1007/s10298-008-0346-7. [DOI] [Google Scholar]
- 80.Moukhles A., Mansour A., Ellaghdach A., Abrini J. Chemical composition and in vitro antibacterial activity of the pure essential oils and essential oils extracted from their corresponding hydrolats from different wild varieties of Moroccan thyme. Journal of Materials and Environmental Science . 2018;9:235–244. doi: 10.26872/jmes.2018.9.3.87. [DOI] [Google Scholar]
- 81.Smahane B., Mounyr B., Faisl B., Stephane M., Sghir M., Dalila B. Antimicrobial activities of essential oil of five plant species from Morocco against some microbial strains. International Journal of Pharmacognosy and Phytochemical Research . 2016;8(11):1901–1906. [Google Scholar]
- 82.Fadli M., Chevalier J., Bolla J.-M., Mezrioui N.-E., Hassani L., Pages J.-M. Thymus maroccanus essential oil, a membranotropic compound active on Gram-negative bacteria and resistant isolates. Journal of Applied Microbiology . 2012;113(5):1120–1129. doi: 10.1111/j.1365-2672.2012.05401.x. [DOI] [PubMed] [Google Scholar]
- 83.Fadli M., Chevalier J., Saad A., Mezrioui N.-E., Hassani L., Pages J.-M. Essential oils from Moroccan plants as potential chemosensitisers restoring antibiotic activity in resistant Gram-negative bacteria. International Journal of Antimicrobial Agents . 2011;38(4):325–330. doi: 10.1016/j.ijantimicag.2011.05.005. [DOI] [PubMed] [Google Scholar]
- 84.Raccach M. The antimicrobial activity of phenolic antioxidants in foods: a REVIEW. Journal of Food Safety . 1984;6(3):141–170. doi: 10.1111/j.1745-4565.1984.tb00479.x. [DOI] [Google Scholar]
- 85.Vasconcelos N. G., Croda J., Simionatto S. Antibacterial mechanisms of cinnamon and its constituents: a review. Microbial Pathogenesis . 2018;120:198–203. doi: 10.1016/j.micpath.2018.04.036. [DOI] [PubMed] [Google Scholar]
- 86.Chaillot J., Tebbji F., Remmal A., et al. The monoterpene carvacrol generates endoplasmic reticulum stress in the pathogenic Fungus candida albicans. Antimicrobial Agents and Chemotherapy . 2015;59(8):4584–4592. doi: 10.1128/AAC.00551-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Lv F., Liang H., Yuan Q., Li C. In vitro antimicrobial effects and mechanism of action of selected plant essential oil combinations against four food-related microorganisms. Food Research International . 2011;44(9):3057–3064. doi: 10.1016/j.foodres.2011.07.030. [DOI] [Google Scholar]
- 88.Viuda-Martos M., Mohamady M. A., Fernández-López J., et al. In vitro antioxidant and antibacterial activities of essentials oils obtained from Egyptian aromatic plants. Food Control . 2011;22(11):1715–1722. doi: 10.1016/j.foodcont.2011.04.003. [DOI] [Google Scholar]
- 89.Mostafa I., Abbas H. A., Ashour M. L., et al. Polyphenols from Salix tetrasperma impair virulence and inhibit quorum sensing of Pseudomonas aeruginosa. Molecules . 2020;25(6):p. 1341. doi: 10.3390/molecules25061341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Siegel R., Naishadham D., Jemal A. Cancer statistics, 2012. CA: a Cancer Journal for Clinicians . 2012;62(1):10–29. doi: 10.3322/caac.20138. [DOI] [PubMed] [Google Scholar]
- 91.Desai A. G., Qazi G. N., Ganju R. K., et al. Medicinal plants and cancer chemoprevention. Current Drug Metabolism . 2008;9(7):581–591. doi: 10.2174/138920008785821657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Uğur D., Güneş H., Güneş F., Mammadov R. Cytotoxic activities of certain medicinal plants on different cancer cell lines. Turkish journal of pharmaceutical sciences . 2017;14(3):222–230. doi: 10.4274/tjps.80299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Jaafari A., Mouse H. A., Rakib E. M., et al. Chemical composition and antitumor activity of different wild varieties of Moroccan thyme. Revista Brasileira de Farmacognosia . 2007;17(4) doi: 10.1590/S0102-695X2007000400002. [DOI] [Google Scholar]
- 94.Khan T., Ali M., Khan A., Nisar P. Anticancer plants: A review of the active phytochemicals, applications in animal models, and regulatory aspects. Biomolecules . 2019;10(1):p. 47. doi: 10.3390/biom10010047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Wang X., Hang Y., Liu J., Hou Y., Wang N., Wang M. Anticancer effect of curcumin inhibits cell growth through Mir-21/Pten/Akt pathway in breast cancer cell. Oncology Letters . 2017;13(6):4825–4831. doi: 10.3892/ol.2017.6053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Guesmi F., Prasad S., Ali M. B., Ismail I. A., Landoulsi A. Thymus hirtus Sp. algeriensis Boiss. and Reut. volatile oil enhances Trail/Apo2l induced apoptosis and inhibits colon carcinogenesis through upregulation of death receptor pathway. Aging (Albany NY) . 2021;13(18):21975–21990. doi: 10.18632/aging.203552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Awe E. O., Makinde J. M., Adeloye O. A., Banjoko S. O. Membrane stabilizing activity of Russelia equisetiformis, Schlecht & Chan. Journal of Natural Products (India) . 2009;2:3–9. [Google Scholar]
- 98.Dima J., Raghda L., Jalil G. A. Evaluation of hemolytic and anti-hemolytic activity of the aerial parts of Sonchus oleraceus extracts. International Journal of Pharmaceutical Sciences and Nanotechnology . 2017;10(3):3745–3751. doi: 10.37285/ijpsn.2017.10.3.7. [DOI] [Google Scholar]
- 99.Simon M., Horovská Ľ., Greksak M., Dušinský R., Nakano M. Antihemolytic effect of Rooibos tea (Aspalathus linearis) on red blood cells of Japanese quails. General Physiology and Biophysics . 2000;19(4):365–371. [PubMed] [Google Scholar]
- 100.Williams P., Sorribas A., Howes M.-J. R. Natural products as a source of Alzheimer's drug leads. Natural Product Reports . 2011;28(1):48–77. doi: 10.1039/C0NP00027B. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Costa P., Gonçalves S., Valentão P., Andrade P. B., Romano A. Accumulation of phenolic compounds in in vitro cultures and wild plants of Lavandula viridis L’hér and their antioxidant and anti-cholinesterase potential. Food and Chemical Toxicology . 2013;57:69–74. doi: 10.1016/j.fct.2013.03.006. [DOI] [PubMed] [Google Scholar]
- 102.Ramassamy C. Emerging role of polyphenolic compounds in the treatment of neurodegenerative diseases: a review of their intracellular targets. European Journal of Pharmacology . 2006;545(1):51–64. doi: 10.1016/j.ejphar.2006.06.025. [DOI] [PubMed] [Google Scholar]
- 103.Hamaguchi T., Ono K., Murase A., Yamada M. Phenolic compounds prevent Alzheimer's pathology through different effects on the amyloid-β aggregation pathway. The American Journal of Pathology . 2009;175(6):2557–2565. doi: 10.2353/ajpath.2009.090417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Iuvone T., De Filippis D., Esposito G., D'Amico A., Izzo A. A. The spice sage and its active ingredient rosmarinic acid protect PC12 cells from amyloid-β peptide-induced neurotoxicity. Journal of Pharmacology and Experimental Therapeutics . 2006;317(3):1143–1149. doi: 10.1124/jpet.105.099317. [DOI] [PubMed] [Google Scholar]
- 105.Benguechoua M., Nia S., Benarous K., Khachba I., Yousfi M. Inhibition of Candida rugosa lipase by different extracts of five Algerian plants and their antioxidant activities. Current Enzyme Inhibition . 2015;10(2):121–128. doi: 10.2174/1573408010666140812233628. [DOI] [Google Scholar]
- 106.Liu Y. Q., Xue M., Zhang Q. C., Zhou F. Y., Wei J. Q. Toxicity of Β-caryophyllene from Vitex negundo (Lamiales: Verbenaceae) to Aphis gossypii Glover (Homoptera: Aphididae) and its action mechanism. Acta Entomologica Sinica . 2010;53(4):396–404. [Google Scholar]
- 107.Wang J. L., Li Y., Lei C. L. Evaluation of monoterpenes for the control of Tribolium castaneum (Herbst) and Sitophilus zeamaise Motschulsky. Natural Product Research . 2009;23(12):1080–1088. doi: 10.1080/14786410802267759. [DOI] [PubMed] [Google Scholar]
- 108.Pavela R. Insecticidal activity of some essential oils against larvae of Spodoptera littoralis. Fitoterapia . 2005;76(7-8):691–696. doi: 10.1016/j.fitote.2005.06.001. [DOI] [PubMed] [Google Scholar]
- 109.Al-Nagar N. M. A., Abou-Taleb H. K., Shawir M. S., Abdelgaleil S. A. M. Comparative toxicity, growth inhibitory and biochemical effects of terpenes and phenylpropenes on Spodoptera littoralis (Boisd.) Journal of Asia-Pacific Entomology . 2020;23(1):67–75. doi: 10.1016/j.aspen.2019.09.005. [DOI] [Google Scholar]
- 110.Singh N., Mishra B. B., Bajpai S., Singh R. K., Tiwari V. K. Natural product based leads to fight against Leishmaniasis. Bioorganic & Medicinal Chemistry . 2014;22(1):18–45. doi: 10.1016/j.bmc.2013.11.048. [DOI] [PubMed] [Google Scholar]
- 111.Tiwari N., Gedda M. R., Tiwari V. K., Singh S. P., Singh R. K. Limitations of current therapeutic options, possible drug targets and scope of natural products in control of Leishmaniasis. Mini Reviews in Medicinal Chemistry . 2018;18(1):26–41. doi: 10.2174/1389557517666170425105129. [DOI] [PubMed] [Google Scholar]
- 112.McCall L.-I., El Aroussi A., Choi J. Y., et al. Targeting ergosterol biosynthesis in Leishmania donovani: essentiality of sterol 14 alpha-demethylase. PLoS Neglected Tropical Diseases . 2015;9(3):p. e0003588. doi: 10.1371/journal.pntd.0003588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Melo T. S., Gattass C. R., Soares D. C., et al. Oleanolic acid (OA) as an antileishmanial agent: biological evaluation and in silico mechanistic insights. Parasitology International . 2016;65(3):227–237. doi: 10.1016/j.parint.2016.01.001. [DOI] [PubMed] [Google Scholar]
- 114.Lacaille-Dubois M. A., Franck U., Wagner H. Search for potential angiotensin converting enzyme (ace)-inhibitors from plants. Phytomedicine . 2001;8(1):47–52. doi: 10.1078/0944-7113-00003. [DOI] [PubMed] [Google Scholar]
- 115.Spyroulias G. A., Galanis A. S., Pairas G., Manessi-Zoupa E., Cordopatis P. Structural features of angiotensin-I converting enzyme catalytic sites: conformational studies in solution, homology models and comparison with other zinc metallopeptidases. Current Topics in Medicinal Chemistry . 2004;4(4):403–429. doi: 10.2174/1568026043451294. [DOI] [PubMed] [Google Scholar]
- 116.Boeing T., Cechinel-Filho V., Niero R., Mota da Silva L., de Souza P., Faloni de Andrade S. 1,3,5,6-Tetrahydroxyxanthone, a natural xanthone, induces diuresis and saluresis in normotensive and hypertensive rats. Chemico-Biological Interactions . 2019;311, article 108778 doi: 10.1016/j.cbi.2019.108778. [DOI] [PubMed] [Google Scholar]
- 117.Fischer H. C., Chan W. C. W. Nanotoxicity: the growing need for in vivo study. Current Opinion in Biotechnology . 2007;18(6):565–571. doi: 10.1016/j.copbio.2007.11.008. [DOI] [PubMed] [Google Scholar]
- 118.Guesmi F., Bellamine H., Landoulsi A. Hydrogen peroxide-induced oxidative stress, acetylcholinesterase inhibition, and mediated brain injury attenuated by Thymus algeriensis. Applied Physiology, Nutrition, and Metabolism . 2018;43(12):1275–1281. doi: 10.1139/apnm-2018-0107. [DOI] [PubMed] [Google Scholar]
- 119.Merghem, Mounira, Hani M., Dahamna S. Acute toxicity study of aqueous extract of leaves of Thymus algeriensis in Swiss albino mice. Plant Cell Biotechnology And Molecular Biology . 2021:79–86. [Google Scholar]
- 120.Kar S., Roy K. Qsar of phytochemicals for the design of better drugs. Expert Opinion on Drug Discovery . 2012;7(10):877–902. doi: 10.1517/17460441.2012.716420. [DOI] [PubMed] [Google Scholar]
- 121.Guesmi F., Beghalem H., Tyagi A. K., et al. Prevention of H2O2 induced oxidative damages of rat testis by Thymus algeriensis. Biomedical and Environmental Sciences . 2016;29(4):275–285. doi: 10.3967/bes2016.035. [DOI] [PubMed] [Google Scholar]
- 122.Guesmi F., Tyagi A. K., Bellamine H., Landoulsi A. Antioxidant machinery related to decreased Mda generation by Thymus algeriensis essential oil-induced liver and kidney regeneration. Biomedical and Environmental Sciences . 2016;29(9):639–649. doi: 10.3967/bes2016.086. [DOI] [PubMed] [Google Scholar]
- 123.Sherwood E. R., Toliver-Kinsky T. Mechanisms of the inflammatory response. Best Practice & Research. Clinical Anaesthesiology . 2004;18(3):385–405. doi: 10.1016/j.bpa.2003.12.002. [DOI] [PubMed] [Google Scholar]
- 124.Aboutabl E. A., El-Dahmy S. I. Chemical composition and antimicrobial activity of essential oil of Thymus algeriensis Boiss. Bulletin of Faculty of Pharmacy, Cairo University . 1995;33:87–90. [Google Scholar]
- 125.Brieger K., Schiavone S., Miller F. J., Krause K.-H. Reactive oxygen species: from health to disease. Swiss Medical Weekly . 2012;142, article w13659 doi: 10.4414/smw.2012.13659. [DOI] [PubMed] [Google Scholar]
- 126.Douar-Latreche S., Benchabane O., Sahraoui N., Hazzit M., Mouhouche F., Baaliouamer A. Effect of gamma irradiation on the chemical composition and antioxidant activity of Thymus algeriensis extracts. Journal of Essential Oil-Bearing Plants . 2018;21(2):449–461. doi: 10.1080/0972060X.2017.1421869. [DOI] [Google Scholar]
- 127.Ghorbel A., Fakhfakh J., Brieudes V., et al. Chemical composition, antibacterial activity using micro-broth dilution method and antioxidant activity of essential oil and water extract from aerial part of TunisianThymus algeriensisBoiss. & Reut. Journal of Essential Oil-Bearing Plants . 2021;24(6):1349–1364. doi: 10.1080/0972060X.2021.2021112. [DOI] [Google Scholar]
- 128.Bouhrim M., Mechchate H., Al-zahrani M., et al. Phytochemical analysis, antimicrobial and antioxidant properties of Thymus zygis L. And Thymus willdenowii Boiss. essential oils. Plants . 2021;11(1):p. 15. doi: 10.3390/plants11010015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Rubió L., Motilva M.-J., Romero M.-P. Recent advances in biologically active compounds in herbs and spices: a review of the most effective antioxidant and anti-inflammatory active principles. Critical Reviews in Food Science and Nutrition . 2013;53(9):943–953. doi: 10.1080/10408398.2011.574802. [DOI] [PubMed] [Google Scholar]
- 130.Ambriz-Pérez D. L., Leyva-López N., Gutierrez-Grijalva E. P., Heredia J. B. Phenolic compounds: natural alternative in inflammation treatment. A review. Cogent Food & Agriculture . 2016;2(1, article 1131412) doi: 10.1080/23311932.2015.1131412. [DOI] [Google Scholar]
- 131.El Ouahdani K., Es-Safi I., Mechchate H., et al. Thymus algeriensis and Artemisia herba-alba essential oils: chemical analysis, antioxidant potential and in vivo anti-inflammatory, analgesic activities, and acute toxicity. Molecules . 2021;26(22):p. 6780. doi: 10.3390/molecules26226780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Bendjabeur S., Benchabane O., Bensouici C., Hazzit M., Baaliouamer A., Bitam A. Antioxidant and anticholinesterase activity of essential oils and ethanol extracts of Thymus algeriensis and Teucrium polium from Algeria. Journal of Food Measurement and Characterization . 2018;12(4):2278–2288. doi: 10.1007/s11694-018-9845-x. [DOI] [Google Scholar]
- 133.Radović J., Suručić R., Niketić M., Kundakovic-Vasovic T. Angiotensin I-converting Enzyme (ACE) Inhibitory Activity and Chemical Composition of Alchemilla Viridiflora Rothm . University of Belgrade Faculty of Pharmacy; 2021. [Google Scholar]
- 134.Zohra B. F., Amar Z., Chawki B. Chemical analysis, antioxidant, anti-Alzheimer and anti-diabetic effect of two endemic plants from Algeria: Lavandula antineae and Thymus algeriensis. Jordan Journal of Biological Sciences. All rights reserved-Volume . 2021;14(3):551–558. doi: 10.54319/jjbs/140322. [DOI] [Google Scholar]
- 135.Slighoua M., Mahdi I., Ez-zahra Amrati F., et al. Ethnopharmacological survey of medicinal plants used in the traditional treatment of female infertility in Fez region, Morocco. Phytothérapie . 2019 doi: 10.3166/phyto-2020-0194. [DOI] [Google Scholar]
- 136.Mehalaine S., Belfadel O., Menasria T., Messaili A. Chemical composition and antibacterial activity of essential oils of three medicinal plants from Algerian semi-arid climatic zone. Phytothérapie . 2017;16(S1):S155–S163. doi: 10.3166/phyto-2019-0150. [DOI] [Google Scholar]
- 137.Hamdani I., El-Ouariachi E., Mokhtari O., et al. Chemical constituents and corrosion inhibition of mild steel by the essential oil of Thymus algeriensis in 1.0 M hydrochloric acid solution. Der Pharma Chemica . 2015;7(8):252–264. [Google Scholar]
- 138.Hazzit M., Baaliouamer A. Essential oil composition of Thymus algeriensis Boiss. et Reut. and Thymus numidicus Poiret from Algeria. Rivista Italiana EPPOS . 2007;17(43):p. 11. [Google Scholar]
- 139.Chemat S., Cherfouh R., Meklati B. Y., Belanteur K. Composition and microbial activity of thyme (Thymus algeriensis genuinus) essential oil. Journal of Essential Oil Research . 2012;24(1):5–11. doi: 10.1080/10412905.2012.645303. [DOI] [Google Scholar]
- 140.Salhi A., Bouyanzer A., El Mounsi I., et al. Chemical composition, antioxidant and anticorrosive activities of Thymus algeriensis. Journal of Materials and Environmental Science . 2016;7(11):3949–3960. [Google Scholar]
- 141.Dob T., Dahmane D., Benabdelkader T., Chelghoum C. Studies on the essential oil composition and antimicrobial activity of Thymus algeriensis Boiss. et Reut. International Journal of Aromatherapy . 2006;16(2):95–100. doi: 10.1016/j.ijat.2006.04.003. [DOI] [Google Scholar]
- 142.Houmani Z., Azzoudj S., Naxakis G., Skoula M. The essential oil composition of Algerian Zaatar: Origanum spp. and Thymus spp. Journal of Herbs, Spices & Medicinal Plants . 2002;9(4):275–280. doi: 10.1300/J044v09n04_03. [DOI] [Google Scholar]
- 143.Kebbi S., Fadel H., Chalchat J.-c., et al. Chemical composition of Algerian Thymus algeriensis Boiss. & Reut. and Marrubium vulgare L. (Lamiaceae) essential oils from the Aures region. Acta Scientifica Naturalis . 2020;7(2):1–14. doi: 10.2478/asn-2020-0016. [DOI] [Google Scholar]
- 144.Khemkham A., Belhadj S., Meddour R., et al. Hs-Spme-Gc/Ms analysis of 3 Lamiaceae plants: Ajuga Iva (L.) Schreb., Salvia Verbenaca L. and Thymus Algeriensis Boiss. & Reut. Journal of Fundamental and Applied Sciences . 2020;12(2) doi: 10.4314/jfas.v12i2.12. [DOI] [Google Scholar]
- 145.Rezzoug M., Bakchiche B., Gherib A., Elasri O. Phytochemical screening and antioxidant activities of different organic extracts of three Algerian plants. Journal of Drug Delivery and Therapeutics . 2020;10(2-s):75–79. doi: 10.22270/jddt.v10i2-s.4038. [DOI] [Google Scholar]
- 146.Allane T., Benamara S. Determination of reducing power of 56 Algerian plant products using olive (Olea europaea) oil as extraction solvent. Chemical Engineering Communications . 2019;206(1):12–21. doi: 10.1080/00986445.2018.1469016. [DOI] [Google Scholar]
- 147.Neffati N., Aloui Z., Karoui H., Guizani I., Boussaid M., Zaouali Y. Phytochemical composition and antioxidant activity of medicinal plants collected from the Tunisian flora. Natural Product Research . 2017;31(13):1583–1588. doi: 10.1080/14786419.2017.1280490. [DOI] [PubMed] [Google Scholar]
- 148.Salhi A., Hamdani I., Bouyanzer A., et al. Phytochemical analysis, antioxidant and anticorrosive activities of Thymus algeriensis extracts. Analytical and Bioanalytical Electrochemistry . 2018;10:1587–1610. [Google Scholar]
- 149.Labiad M. H., Belmaghraoui W., Ghanimi A., et al. Biological properties and chemical profiling of essential oils of Thymus(vulgaris, algeriensis and broussonettii) grown in Morocco. Collections . 2022;37, article 100797 doi: 10.1016/j.cdc.2021.100797. [DOI] [Google Scholar]
- 150.Sara M., Yamina B., Ramazan E., Mesut G., Selma A. Dietary risk of blaESBL producing multidrug resistant Enterobacteriaceae and their inhibition by Artemisia herba-alba and Thymus algeriensis essential oils. Journal of Essential Oil-Bearing Plants . 2021;24(3):658–670. doi: 10.1080/0972060X.2021.1937334. [DOI] [Google Scholar]
- 151.Jayari A., El Abed N., Jouini A. Antibacterial activity of Thymus capitatus and Thymus algeriensis essential oils against four food-borne pathogens inoculated in minced beef meat. Journal of Food Safety . 2018;38(1, article e12409) doi: 10.1111/jfs.12409. [DOI] [Google Scholar]


