Abstract
Calcitonin gene-related peptide (CGRP) is probably the most potent vasodilator in cerebral circulation. Forty years after its discovery, the new CGRP-targeted therapy monoclonal antibodies, and the small molecule gepants, are now available for clinical practice. While randomized controlled trials and real-world experience consistently demonstrated the high efficacy and tolerability of monoclonal antibodies, limited evidence is available to characterize gepants fully. Depending on pharmacokinetics, these CGRP receptor antagonists can be used for acute (ubrogepant, rimegepant, and the not yet approved zavegepant) or preventive (atogepant and rimegepant) migraine treatment. Randomized placebo-controlled trials demonstrated gepants efficacy in treating acute attacks to obtain 2 h pain freedom in about 20% of patients and pain relief in about 60%, while up to 60% of treated patients with episodic migraine may experience a 50% reduction in monthly migraine days. The most common treatment-related emergent adverse events were gastrointestinal (nausea, constipation) for the acute or preventive use. No vascular or hepatic concerns have emerged so far. More studies are ongoing to investigate gepant tolerability and safety also if associated with monoclonal antibodies targeting CGRP and other therapeutic classes. Gepants are also under investigation to treat other painful and non-painful conditions. Real-life studies are necessary to confirm the trials’ findings and investigate more practical clinical aspects.
Keywords: Calcitonin gene-related peptide, Gepants, Migraine treatment, Real world
Introduction
Migraine is among the most disabling neurological conditions, affecting around 1.04 billion people, mainly in their productive age, impairing work performances and social and familial contexts [1]. Moreover, about 8% of migraine patients encounter a progressive increase in attacks’ frequency to the point where migraine becomes chronic [2]. Until 2018, preventive migraine management relied on drugs [3] not specifically developed for migraine treatment and was burdened by poor long-term adherence because of adverse events and often inadequate effectiveness [4]. Not least, migraine often comes associated with different comorbidities, which may further restrict the range of therapeutic options [5, 6]. In this scenario, calcitonin gene-related peptide (CGRP)-targeted therapies came as a real revolution in migraine management [7].
The history of CGRP-targeted therapy development
It is now 40 years since the CGRP discovery when Amara and colleagues identified alternative processing of the calcitonin gene [8]. They observed that the CGRP-specific mRNA predominates in the hypothalamus and hypothesized CGRP as a hypothalamic peptide with a hormonal effect. Soon after, the same group observed that the distribution of CGRP-producing cells and pathways in the brain included the olfactory and gustatory system (hypoglossal, facial and vagal nuclei, the hypothalamus, and the limbic regions), the nociceptive and thermal sensory pathways (trigeminal and spinal sensory ganglion cells), and the visceral motor functions mediated by the vagus nerve (rostral parts of the nucleus ambiguous). CGRP distribution in brain structures and other tissues suggested that the peptide played multiple functions such as nociception, feeding behavior, and modulation of the autonomic and endocrine systems [9]. A few years later, CGRP was also shown to be a potent vasodilator [10]. Its vasodilatory potency is now known to be tenfold higher than prostaglandins and up to 100 times more than other vasodilators such as acetylcholine, thus making CGRP the most potent peripheral and cerebral vasodilator discovered so far [11, 12]. Altogether, these observations inspired the first hypothesis of the involvement of CGRP in migraine [13]. The subsequent report that the activated trigeminovascular system releases CGRP in the extra-cerebral circulation provided new insights into the putative role of vasoactive peptides in the pathophysiology of migraine [14]. The definite unquestionable demonstration of the primary involvement of CGRP in migraine came from the occurrence of CGRP release (but not of other peptides) during spontaneous migraine attacks [15]. Later, CGRP infusion showed also to trigger attacks in migraine patients [16].
We can define these studies as the first building blocks of specific migraine therapies. In the early 1990s, triptans were described to inhibit CGRP release induced by trigeminal activation and normalize its levels during attacks, in association with pain relief [17].
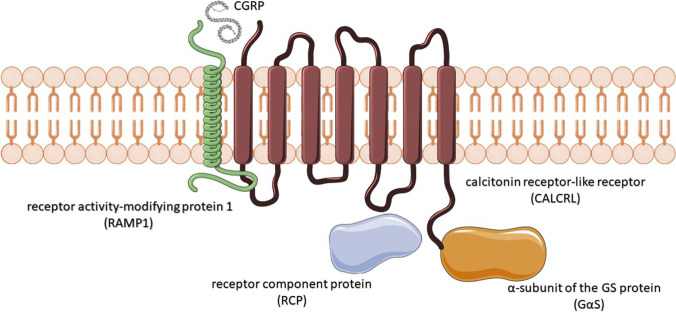
The characterization of the CGRP receptor (CGRPr) represented a further important step. The CGRPr is constituted of three subunits: the calcitonin receptor-like receptor (CALCRL), the receptor activity-modifying protein 1 (RAMP1), and the receptor component protein (RCP) [18]. The CALCRL is a G-protein-coupled receptor essential in binding CGRP and adrenomedullin, but it is not sufficient to bind CGRP effectively unless it forms a heterodimer with RAMP1. RAMPs are single transmembrane-spanning proteins that modify the functions of G-protein-coupled receptors, including pharmacological properties and cell trafficking. The CGRP binds the CGRPr ligand cleft in the interface between CALCRL and RAMP1 (Fig. 1). Once CGRPr is activated, RCP facilitates the coupling of the Gαs subunit of the G protein, which in turn initiates intracellular adenylyl cyclase and cyclic adenosine monophosphate (cAMP)-dependent signaling and, in the cerebral vessel smooth muscle, ultimately produces an increase in c-AMP resulting in vasorelaxation.
Fig. 1.
Schematic representation of the CGRP receptor complex
Once characterized the CGRPr, different studies evaluated the effects of blocking CGRP in different conditions to explore the therapeutic potential, especially in pain control [19, 20]. At the beginning of the new millennium, BIBN4096BS, the first selective small-molecule CGRP antagonist, was administered to marmoset monkeys to investigate its capability to counteract the effects on facial blood flow of CGRP released by the stimulation of the trigeminal ganglion [21]. The positive results obtained allowed the authors to conclude that BIBN4096BS was a potent and selective CGRP antagonist. A few years later, this encouraging result has brought to the first phase I study [22] and then to a clinical trial on BIBN4096BS administered intravenously as acute attack medication in 126 migraine patients [23]. The 2.5-mg dose had a response rate (RR) of 66% (vs. 27% for placebo, p = 0.001). Moreover, the active drug showed superiority over placebo also on the pain-free rate at 2 h (44% vs. 2%), the rate of sustained response over 24 h, the rate of recurrence of headache, and on the improvement of bothersome symptoms such as nausea, photophobia, phonophobia, and functional capacity. The effect was also rapid as it became apparent after 30 min and increased over the next few hours. The overall rate of adverse events (AE) was 25% (vs. 12% for placebo). Paresthesia was the most frequently reported side effect, while no serious adverse events were reported. The safety of this molecule was also preliminarily confirmed on cerebral or systemic hemodynamics in a very small group of healthy volunteers [24].
In 2008, the randomized clinical trials (RCTs) on the first oral CGRPr antagonist, MK-0974 (telcagepant), demonstrated a 2-h pain-free RR of 24.3% for the 400-mg dose and 32.1% for the 600-mg dose (vs. 33.4% for the sumatriptan 10 mg) [25]. Similar efficacy was observed compared to zolmitriptan 5 mg in a cohort of 687 patients receiving telcagepant [26]. Since then, several trials have explored the efficacy and tolerability of telcagepant for the acute treatment of migraine without safety concerns. The half-life of telcagepant of 5–8 h provided the basis for its use as a preventive treatment. However, the NCT00797667 trial (telcagepant 140 mg or 280 mg or placebo twice daily) was prematurely stopped by the safety monitoring board due to hepatotoxicity concerns. Thirteen patients in the telcagepant groups had an alanine aminotransferase (ALT) elevation ≥ 3 times above the normative ranges. Of these, two patients had very high symptomatic transaminase elevations within 2–6 weeks since treatment initiation and resolved after treatment discontinuation [27]. To note, no hepatotoxic effects were observed during the intermittent use as an abortive migraine drug over an 18-month period.
The term Hy’s law was coined to define drug-induced jaundice caused by hepatocellular injury. It indicates hepatocellular injury without a significant obstructive component, which is associated with death or liver transplantation in up to 50% of cases. The FDA (Food and Drug Administration) Hy’s law definition includes 4 components: alanine aminotransferase (ALT) or aspartate aminotransferase (AST) elevation of > 3 times the normal upper limit, total bilirubin elevation of > 2 × , no findings of cholestasis before treatment, and no other explanation for the combined increase in transaminase and total bilirubin [28].
However, this unhappy end did not discourage the scientific efforts to produce more efficient and safe molecules targeting CGRPr. Other small molecules were tested. Some are not available in clinical practice because of observed (MK-3207 [29]) or feared hepatotoxicity (BI 44,370 TA [30]). The FDA has finally approved others (e.g., BMS-927711-rimegepant; MK-1602 — ubrogepant, MK-8031 — atogepant), which are currently commercialized in the USA.
Mechanism of action
Gepants display their anti-migraine action by binding CGRPr and inhibiting its activation. A unique binding interaction in the amino terminus of CLR is consistent with the observation that these compounds also interact with the extracellular region of RAMP1 and could suggest the formation of a binding pocket between the two proteins [31].
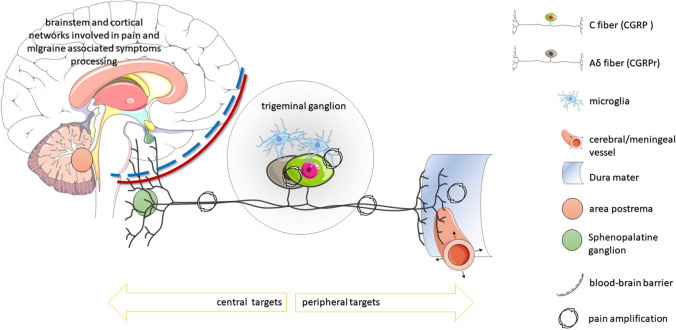
The physiopathology of migraine (syndrome) and attacks is multifactorial, where most probably vasodilation is only an epiphenomenon of neural activation mediated by CGRP release. The activated CGRPr indeed serves several functions during migraine attacks. A primary site where CGRP and its receptor induce (or perpetuate) migraine pain is the trigeminal ganglion (TG) [32].
The neurons populating the TG are mainly primary afferent of the pseudo-unipolar type and glial cells. Around 50% of neurons in the TG express CGRP; these are primarily C-type sensory pain fibers [7] which usually respond to stimuli with stronger intensities and account for the slow, lasting, and spread out of pain. Some TG CGRP neurons also synthesize other neurotransmitters such as pituitary adenylate-cyclase-activating polypeptide (PACAP) or substance P (SP) and express vanilloid-sensitive transient receptor potential (TRPV1) cation channels. Not least, most CGRP neurons in the human TG also express 5-HT1B and 1D receptors.
Separately from CGRP neurons, one-third of TG neurons exhibit CGRPr and appear as Aδ fibers, devoted to receiving and transmitting nociception related to acute, sharp pain. Moreover, CGRPr can also be seen in the glial cells surrounding neuronal cell bodies, particularly CGRP neurons. These observations support the existence of a functional link between TG C fiber neurons with surrounding glia and TG Aδ fiber neurons, mediated by CGRP. This circuitry is likely involved in the perpetuation of migraine attacks: the activated TG C fiber CGRP neurons by descending pathways would release CGRP, which bind CGRPr on the TG “sharp pain” Aδ fiber neurons and the glial cells that, in turn, further activate CGRP neurons by unleashing oxidative inflammatory mediators (e.g., nitric oxide and cytokines) [7]. This vicious spiral circle might be responsible for pain amplification in the TG.
A similar pattern is present in the trigeminal nerve, where the expression of CGRP and CGRPr is segregated for C and Aδ fibers, respectively, and the presence of CGRPr in the Schwann cells indicates a reinforcement of the perpetuating pain transmission also at this site [33].
Peripherally, trigeminal CGRP nerves innervate cerebral blood vessels producing, as described above, vasorelaxation. This neurogenic influence on cerebral hemodynamics is part of a very sophisticated orchestral action with myogenic (i.e., autoregulation), endothelial (i.e., endothelial reactivity), and metabolic responses (i.e., vasomotor reactivity). The astrocyte production of prostaglandins and nitric oxide (NO) responds to the neuronal firing (i.e., neurovascular coupling), mediating smaller intraparenchymal arterioles’ dilatation. The neurogenic control of medium and small size arteries, on the other hand, occurs through the activation of sympathetic, parasympathetic, and sensory neurons. The last ones act by secreting CGRP, NO, serotonin, and PACAP [34, 35]. However, as discussed above, vasodilation is not essential to provoke migraine attacks. Moreover, the effect of CGRP on vessel reactivity is mainly mediated by the innervation of smooth muscles. In contrast, the intraluminal effects (i.e., cerebral circulation) are less prominent as the endothelium does not display CGRP receptors and limits its diffusion to the outliers of the vessel wall (with CGRPr) [36]. In summary, although the CGRP-induced vasodilatation is a prominent clinical feature of migraine attacks, it probably has a minor role in determining headache.
Another peripheral target of trigeminal CGRP nerves is the dura mater (both in pial vessels and nonvascular regions). Also at this site, CGRP release and CGRPr are differently present in C and Aδ fibers. The dural CGRP neurons are also peripherally activated by noxious stimuli. This produces the peripheral release of CGRP (with local vasodilatation and subsequent increase in blood flow) and the input transmission of pain. Substance P is also released, producing, in association with CGRP, plasma extravasation secondary to capillary leakage, edema, and mast cell degranulation (neurogenic inflammation). These phenomena are primarily involved in maintaining migraine pain (peripheral sensitization). It is possible that the neurons and immune cells dialogue in driving such a sterile neuroinflammatory state in migraine pathophysiology [37]. This reflex serves as a further peripheral amplifier of migraine pain. However, it is less likely that this neurogenic inflammation is a migraine trigger [7].
In the central ascending pathways, CGRP neurons and fibers expressing CGRPr are abundant in the trigeminal nucleus and the cervical C1 and 2 spinal cord. The colocalization with glutamate receptors at this site suggests that CGRP can enhance glutamatergic synaptic transmission favoring central sensitization [38].
CGRP pathways were also implied in light adversion acting at multiple sites in the visual network and structures involved in nausea and vomiting and autonomic symptoms such as area postrema and the shenopalatine ganglion [7].
Finally, there is a complex intersection between CGRP and cortical spreading depression (CSD). CGRP release seems not to trigger CSD, while the opposite is true [39]. On the other hand, CGRP can modulate CSD propagation[39] via the cerebral blood flow contra-regulation of neural activity (the so-called vascular-neural coupling) [40]. In this view, the physiological advantage of CGRP release would be to support the rapid wave of depolarization, increasing blood flow to meet the amplified metabolic demands.
Although the gepants’ molecular weight is around 600 KDa and thus ~ 250 smaller than monoclonal antibodies (mAbs) targeting CGRP or its receptor, they do not consistently cross the blood–brain barrier (BBB). The CSF/plasma ratio for telcagepant in primates is around 1.4%, suggesting that a small amount of the circulating molecule penetrates across BBB [41]. Consequently, in physiological conditions (i.e., the integrity of BBB), gepants exert their anti-migraine effects outside the BBB.
Gepants (and mAbs targeting the CGRP pathway) seem to block the above-described pain amplification and perpetuation processes taking place at the TG, trigeminal nerve fibers, and the dura. More specifically, they interrupt the resonance of CGRP-mediated pain signals occurring via neuroglial, neuroneuronal, and neurovascular signaling (Fig. 2) [7]. Moreover, they may hinder other bothersome symptoms, such as nausea and autonomic activation, acting at the area postrema and the shenopalatine ganglion outside BBB and receiving trigeminal nerve projections.
Fig. 2.
Schematic representation of CGRP pathways within and emerging from the trigeminal ganglion to the central (on the left) and peripheral (on the right) targets of the CGRP neuron projections. To note, neurons releasing CGRP and those carrying CGRP receptors interact at different sites producing pain amplification
Clinical trials
Initially synthesized for migraine attacks, depending on the half-life, gepants are now approved as acute and preventive treatments. All currently available gepants are eliminated primarily via hepatic metabolism, mainly mediated by the cytochrome P3A4 (CYP3A4 = (go.drugbank.com). For this reason, co-administration with a potent CYP3A4 inhibitor should be avoided, while dose adjustment is usually recommended with concomitant use of moderate CYP3A4 inhibitors.
Acute migraine treatment
For about 20 years, triptans were the benchmark for acute migraine therapy. Activating the serotonin 5-HT 1D and 1B receptors, triptans were initially assumed to relieve migraine through direct vasoconstriction and the regulation of trigeminal inflammatory transmitter release. The observation that sumatriptan reduces trigeminal sensory nerve activation, inhibiting the release of vasoactive peptides, including SP and CGRP, clarified their key therapeutic mechanism, suggesting that they indirectly share a common pathway with CGRP-targeted therapy. Interestingly, a good response to triptans is a predictive factor for a positive response to mAb vs. CGRP [42]
Ubrogepant
Ubrogepant (MK-1602) was the first oral CGRPr antagonist approved for the acute treatment of migraine by the FDA on December 23, 2019. The recommended dose of ubrogepant to treat acute attack is 50 mg or 100 mg taken orally with or without food.
A second dose may be taken at least 2 h after the initial dose if needed. The maximum daily dose is 200 mg. A dose adjustment is required in patients with severe renal or hepatic failure, with an initial dose of 50 mg and the second dose of 50 mg if needed. In end-stage renal disease (CLcr < 15 mL/min), ubrogepant should be avoided. Peak plasma concentration (Tmax) occurs between 0.7 and 1.5 h. When administered with a high-fat meal, Tmax is delayed by approximately 2 h. The half-life is 5–7 h [43].
The safety of treating with ubrogepant more than 8 monthly migraine attacks has not been yet established.
Different phases 1, 2, and 3 [42, 43] trials have demonstrated its efficacy and safety in treating migraine attacks, and further phase 3 and real-life randomized studies are ongoing. FDA approval came after the favorable results of the phase 3 trial ACHIEVE-I NCT02828020 [44] on 1672 participants randomized to receive placebo, ubrogepant 50 mg or 100 mg for a single migraine attack. Participants on placebo were pain-free at 2 h in 11.8% of cases, in 19.2% those on ubrogepant 50 mg [95% CI OR 1.83 (1.25–2.66)], and 21.2% in the 100-mg ubrogepant group [95% CI OR 2.04 (1.41–2.95)], (p < 0.001). The most commonly reported adverse events (AEs) were nausea, somnolence, and dry mouth (reported in 0.4 to 4.1%), more frequent in the 100-mg ubrogepant group (reported in 2.1 to 4.1%). No serious AEs occurred within 48 h after the dose intake, while within 30 days, appendicitis, spontaneous abortion, pericardial effusion, and seizure were reported in the ubrogepant groups.
The ACHIEVE-2 trial (NCT02867709) [45] compared placebo, ubrogepant 25 mg and 50 mg in 1686 participants. Pain freedom at 2 h was reported by 21.8% patients in the ubrogepant 50 mg (95% CI, 2.6–12.5%; p = 0.01), 20.7% in the 25-mg arm (95% CI, 1.5–11.5%; p = 0.03), and 14.3% in the placebo receivers.
A post hoc analysis of the ACHIEVE 1 and 2 trials considering the common dosage of 50 mg reported pain relief at 2 h in 62% of participants on ubrogepant (49% for placebo). Moreover, at 2 h, 39% of patients on ubrogepant reported the absence of the most bothersome symptom (MBS). The most common adverse events in the first 48 h were nausea (1.9% vs.1.8% for placebo) and dizziness (1.2% vs.1.1%) [46].
The 52-week extension trial (NCT02873221) on 1230 participants compared the safety and tolerability of ubrogepant 50 mg or 100 mg and the standard of care. After upper respiratory tract infection, nausea was the most frequently reported AE (4.6–4.7%). The authors concluded that the long-term intermittent acute use of ubrogepant 50 and 100 mg given as 1 or 2 doses per attack is safe and well-tolerated, as indicated by a low incidence of treatment-related adverse events. Twenty cases of ALT/AST levels of ≥ 3 times the upper limit of normal were reported. There were no cases of Hy’s law. The efficacy of ubrogepant seems not to be influenced by previous exposure or response to triptans [47].
Another post hoc analysis of the ACHIEVE 1 and 2 trials explored the safety and efficacy of ubrogepant in patients with major cardiovascular risk factors. The trial participants were classified with a cardiovascular risk assessment algorithm based on the National Cholesterol Education Program (NCEP; National Institutes of Health, 2001) and Framingham risk factors along with the presence of CV heart disease or other forms of vascular disease as well as diabetes. According to the classification, 11% of participants were categorized as having a moderate-high risk. No evidence of increased treatment-emergent adverse events (TEAEs) or cardiac adverse events and no safety concerns were identified in this population [48].
Rimegepant
Rimegepant (BMS-927711) is an oral antagonist of the CGRPr approved by the FDA on February 27, 2020, for the acute treatment of migraine headaches. However, since the elimination half-life in healthy subjects is approximately 11 h, it was investigated with positive results also for the preventive treatment of migraine in adults (see below). It is available as disintegrating tablets at a dose of 75 mg, which is the maximum daily dose. The maximum peak concentration is reached at 1.5 h after intake, but it is delayed to 2.5 h if administered after a high-fat meal. It should be avoided in patients with severe hepatic impairment or end-stage renal disease [49].
Two phase 3 trials (NCT03461757 [50], NCT03237845 [51]) described the efficacy and safety of the rimegepant 75 mg to treat a single migraine attack. The coprimary endpoints were freedom from pain and freedom from the MBS at 2-h postdose. Among patients treated with rimegepant, the pain freedom at 2 h was achieved in 19.6 and 21%, respectively, of cases (12–11% for placebo), MBS in 35 and 37.6% (27–25.2% for placebo), and pain relief in 58.1 and 59.3% (42.8–43.3% for placebo). The most common adverse events were nausea (rimegepant [1.8–2%], placebo [1 − < 1%]) and urinary tract infection (rimegepant [1.5–1%]; [1.1–1%]). Serum non-serious transaminase increase was similarly observed in the two treatment arms. Treated participants reported no serious adverse events (SAE).
Interestingly, a post hoc analysis from the open-label safety study conducted between 2017 and 2019 on 3019 episodic patients with at least 6 monthly migraine days (MMDs) treating acute attacks with rimegepant 75 mg reported a decrease also in MMDs without an increase in monthly tablet intake and improved health-related quality of life [52].
More observations of rimegepant in the acute treatment of migraine are about to come. A real-world head-to-head comparison randomized phase 4 study is ongoing for the use of rimegepant 75 mg vs. diclofenac 50 mg for the acute treatment of migraine (NCT05211154) having 2 h of pain freedom as the primary endpoint.
A phase 3, multicenter, open-label study is also ongoing to assess the long-term safety and tolerability of rimegepant (50 or 75 mg) for the acute treatment of migraine (with or without aura) in children and adolescents ≥ 6 to < 18 years of age (NCT04743141).
Although no indications can be provided on pregnancy and breastfeeding, the relative infant dose was determined after administering one tablet of rimegepant 75 mg in 12 healthy lactating women. This small preliminary study demonstrated that on a weight-adjusted basis, the mean relative infant dose of rimegepant was < 1% of the maternal dose [53]. Two observational studies (patient registry) are also ongoing to evaluate the risk of pregnancy and infant outcomes among women with migraine exposed to rimegepant during pregnancy (NCT05198245, NCT05046613). The expected completion years are respectively 2028 and 2034.
Zavegepant
Zavegepant (BHV-3500/BMS-742413, formerly known as “vazegepant,” is now referred to as “zavegepant”) is the first intranasally administered CGRPr antagonist [54]. Although some registration trials have been completed, at the time we have written this review, no results were available [54].
The first phase 2/3, double-blind, randomized, placebo-controlled, dose-ranging trial (NCT03872453) evaluated the safety and efficacy of three different intranasal dose levels of BHV-3500 relative to placebo in the acute treatment of moderate to severe migraine in 2154 patients. The study planned 4 arms: active treatment at 5 mg, 10 mg, 20 mg, and placebo. It was completed in November 2019.
A phase 3 trial (NCT04571060) assessing the safety and efficacy of BHV-3500 versus placebo in the acute treatment of moderate or severe migraine in 1405 patients ended on October 2021, but its results have not yet been published. The primary outcome of this double-blind, placebo-controlled RCT was pain freedom at 2 h measured on a 4-point Likert scale (0 = none, 1 = mild, 2 = moderate, 3 = severe) and the absence of MBS (nausea, phonophobia, or photophobia).
The long-term safety was investigated by a phase 2/3 open-label trial (NCT04408794). It enrolled 608 participants to test 10 mg intranasal (IN) up to 8 times per month for 1 year. It ended in December 2021.
The phase 2/3 trial NCT04804033 is recruiting to investigate the efficacy and safety of oral zavegepant in migraine prevention. It has been reported that CGRP potently constricts airway smooth muscle in humans and has a significant role in eosinophilia in allergic inflammation. CGRP was also found to activate receptors enriched on endothelial cells, leading to reduced cellular junction gene expression, increased endothelium permeability, excess lung fluid, and hypoxemia. In this line, it was found to increase in human lung diseases with excess fluid, such as acute respiratory distress syndrome (ARDS)[55]. Based on these observations, since April 2020, the phase 2 clinical trial NCT04346615 is recruiting to evaluate the safety and efficacy trial of intranasal zavegepant intranasal for hospitalized patients with COVID-19 requiring supplemental oxygen began to investigate the use of intranasally administered zavegepant to combat the acute respiratory distress syndrome (ARDS) sometimes seen in patients with COVID-19. Moreover, the phase 1 NCT04987944 trial is ongoing to assess the safety and efficacy of oral zavegepant (150 mg bid) in subjects with mild allergic asthma.
Migraine prevention
Atogepant
Atogepant (AGN-241689/MK-8031) was approved by FDA (September 2021) for episodic migraine prevention. The recommended dosage of atogepant is 10 mg, 30 mg, or 60 mg taken orally once daily with or without food. Following oral administration, the time to peak plasma concentration is approximately 2–3 h, while the half-life is around 11 h. While no dose adjustments are required for patients with mild or moderate hepatic impairment, atogepant should be avoided in patients with severe hepatic impairment. Similarly, no dose adjustments are required for patients with mild or moderate renal impairment, but patients with severe renal impairment or concomitant use of potent CYP3A4 inhibitors should be limited to a maximum daily dose of 10 mg[56]. Seven clinical trials on the use of atogepant for migraine prevention have now been concluded. Of these, three studies have available results.
The first dose-finding phase 2/3 trial (NCT02848326) on the use of atogepant started in September 2016 and ended in April 2018 after the enrolment of 834 participants with episodic migraine (from 4 to 14 monthly migraine days, MMDs) [57]. The trial included 6 arms: placebo, 10 mg or 30 mg or 60 mg once a day, 30 mg or 60 mg twice a day for 12 weeks. The arms on investigational drug presented a mean decrease in MMDs of respectively − 4.0, − 3.8, − 3.6, − 4.2, and − 4.1, while the MMDs reduction observed in the placebo was − 2.9. The 50% response rate (RR, i.e., the reduction of at least half of MMDs) in the six arms was as follows: 40% for placebo, 58% for atogepant 10 mg QD, 53% for the 30 mg dose QD, 52% for 60 mg QD, 58% for 30 mg BID, and 62% for 60 mg BID.
Overall, atogepant was well tolerated. Treatment-related AEs frequency ranged from 18% for 10 mg once daily to 26% for 60 mg twice daily, versus 16% for placebo. Seven participants reported a total of eight serious TEAEs which were unrelated to treatment. The more common treatment-related TEAEs reported were nausea, constipation, and fatigue, whose frequency seemed to be dose-related. Nausea, the most common, occurred in 3–6% of once-daily dose groups and 6–9% of twice-daily dose groups. Importantly, no evidence of liver toxicity was observed.
From December 2018 to June 2020, the phase 3 ADVANCE NCT03777059 trial evaluated the safety and tolerability of atogepant 10 mg, 30 mg, and 60 mg once a day to prevent episodic migraine[58]. The changes from baseline across 12 weeks of treatment were − 3.7 days with atogepant 10 mg, − 3.9 days with atogepant 30 mg, − 4.2 days with atogepant 60 mg, and − 2.5 days with placebo. The 50% RR was observed in 55.6% of patients on atogepant 10 mg, in 58.7% for the 30 mg, 60.8% for the 60 mg, and 29.0% for placebo.
This trial also aimed at measuring the changes from baseline in mean monthly performance of daily activities domain score of the activity impairment (AIM-D) and change from baseline in mean monthly physical impairment domain score of the AIM-D in migraine diaries. These scores favored atogepant over placebo except for the 10-mg dose. The most common adverse events were constipation (6.9 to 7.7% across atogepant doses) and nausea (4.4 to 6.1% across atogepant doses).
Finally, a phase 3 open-label randomized trial compared atogepant 60 mg with the standard of care (SOC) for episodic migraine prevention over 1 year in 744 participants (NCT03700320) with a primary safety endpoint. Of the entire population, 546 patients were randomized to receive atogepant. Constipation was the only TEAE more frequently reported in 7.18% of patients (3.06% in the SOC group), while other adverse events were more frequently reported in the SOC than in the atogepant group, particularly fatigue (6.12% vs. 2.58%), weight increase (5.61% vs. 1.29%), and dizziness (11.22% vs. 3.13%).
To further test atogepant safety, supratherapeutic doses were administered in two clinical trials to investigate cardiac repolarization [59] and alanine aminotransferase elevations [60] in healthy adults.
The randomized, double-blind, phase 1 crossover study compared the cardiac repolarization effect measured as change from baseline in Fridericia-corrected QT intervals of a single atogepant supratherapeutic 300-mg dose vs. placebo in healthy adults. Moxifloxacin 400 mg was the open-label active control. The trial did not report serious adverse events or elevated liver enzymes, nor the impact on cardiac repolarization in healthy participants [59].
Similarly, the administration of atogepant 170 mg for 28 consecutive days did not produce an ALT elevation above 1.5 × the upper limit of normal in 18 healthy subjects. The change from baseline in serum ALT levels was not different compared with 10 participants receiving a placebo [60].
Moreover, a long-term open-label 40-week extension phase 3 trial (NCT03939312) evaluates the safety and tolerability of atogepant 60 mg once a day to prevent high frequency (i.e., 8–14 MMDs) episodic migraine ended patients’ enrolment on March 2021:t the results are due.
Rimegepant
Rimegepant pharmacokinetics have been described above. It received FDA approval for episodic migraine prevention in May 2021 after completing a phase 2/3 randomized, double-blind, placebo-controlled trial (NCT03732638 [61]).
The trial compared the 12-week administration of rimegepant 75 mg (n = 373 subjects) or placebo (n = 374) every other day (EOD) with the primary endpoint to assess change from baseline in the mean MMDs in the last 4 weeks of the double-blind phase.
To note, this trial enrolled patients with 4 to 18 MMDs and allowed as add-on 1 medication with possible migraine-prophylactic effects. The MMDs change from the observation period to 9–12 weeks was − 4.3 days with rimegepant and − 3.5 with placebo (p = 0.0099). The 50% RR in the same period was 49% [95% CI 44 to 54] for the active arm and 41% [95% CI 36 to 47] for the placebo. Nausea was the most common TEAE, reported in 3% of patients (1% in the placebo group).
A phase 3, randomized, double-blind, placebo-controlled study has begun at the end of February 2022 to evaluate the efficacy and safety of rimegepant in migraine prevention in children and adolescents ≥ 6 to < 18 years of age, and it is expected to end in September 2026.
Interestingly, a phase 2 double-blind, placebo-controlled, crossover trial on rimegepant 75 mg for the treatment of refractory trigeminal neuralgia (NCT03941834) is ongoing, and an open-label pilot study on rimegepant (150 mg oral disintegrating tablet EOD) as a preventive treatment for cluster headache (NCT05264714) is about to start.
Rimegepant is also under investigation for other non-neurological disorder as moderate plaque-type psoriasis (NCT04629950) and acute treatment of chronic rhinosinusitis (NCT05248997) and temporomandibular disorders (NCT05262517).
Future directions
Safety
The availability in clinical practice of the new CGRP-targeted therapy raised some concerns about the potential risk of blocking such a potent vasodilator, at least in patients with vascular hemodynamic impairment [62]. Indeed, CGRPr antagonists worsen cerebral ischemic outcomes in mice [63]. As described before, CGRP is one of the neuropeptides (together with PACAP and 5-HT) having a role in the sensory innervation of vessels to provide the neurogenic control of hemodynamics as part of a more complex system of blood flow control that includes autoregulation, vasomotor reactivity, and endothelial activation, suggesting that under physiological conditions, other mechanisms effectively counterbalance CGRP pathway inhibition. To confirm this hypothesis, erenumab does not impair vasodilatory or contractile responses to other vasoactive agents in human isolated cranial arteries [64], nor impairs cerebral and systemic hemodynamics under physiological conditions in migraineurs without aura [65]. In this line, RCTs and the broad real-world experience with mAbs targeting the CGRP pathway did not raise any vascular alert [42, 66, 67]. Gepants cross the intact BBB only in a marginal amount, making unlikely a detrimental effect on cerebral hemodynamics in normal conditions. On the other side, the frequent use of triptans can disrupt the hemodynamic balance toward vasoconstriction [68].
With these premises, it is wise to avoid the use or consider with caution the use of CGRP-targeted therapy in patients with a high risk of vascular accident or already symptomatic of vascular impairment [69].
As the mAbs targeting the CGRP pathway becomes increasingly prescribed for migraine prevention, the CGRPr antagonist availability for the acute treatment raised the question of whether the concomitant use of gepants and mAbs may induce any pharmacokinetics and safety concerns. A randomized phase 1b drug-drug interaction study was conducted in 40 patients (20 per arm) to investigate this issue by comparing the concomitant use of ubrogepant with erenumab or galcanezumab. The pharmacokinetic profile of ubrogepant was not significantly changed, nor were safety concerns identified. The reported TEAEs were similar to those with each treatment alone. Besides, no serious TEAEs, or TEAEs leading to discontinuation, or clinically relevant changes in laboratory parameters or vital signs were detected [70]. A similar smaller open-label study was performed for the use of rimegepant in 13 patients on mAb [71].
Similarly, the concomitant use of gepants as acute and preventive treatment should be assessed. A phase 1b, open-label, fixed-sequence, safety, tolerability, and drug-drug interaction study between atogepant and ubrogepant in 26 participants with a migraine history was concluded in June 2021 (NCT04818515). Its results are not still available yet. Further clinical information will come from the phase 4, open-label study evaluating the safety, tolerability, and efficacy of the concomitant use of ubrogepant for the acute treatment of migraine in subjects taking atogepant for episodic migraine prevention (NCT05264129). The trial is expected to be completed on September 2023 after the enrollment of 235 patients.
Finally, nowadays, migraine patients often remain socially and professionally engaged in older ages, with lesser relief from the migraine symptoms than observed with aging in the past [72]. This issues a new challenge in managing polytherapy and multiple health problems, physiological aging changes (i.e., slowing of gastric emptying, reduced hepatic and renal drug clearance efficiency), and the concomitant use of pain-killers for other pain conditions [5]. Unfortunately, for most migraine drugs, both for acute and preventive treatment (also for mAbs anti-CGRP), efficacy studies are lacking for patients ≥ 65 years. Interestingly, most trials involving gepants did not pose higher age limits, or the upper limit was not younger than 75 years old. This will provide a pool of data on a large sample of elderly patients.
Meanwhile, careful clinical vigilance should be kept to unveil important indications for gepants’ use in clinical practice.
Chronic migraine
Patients with chronic migraine (CM) experience pain as part of a constellation of symptoms, including non-cephalalgic pain, emotional distress, sleep, gastrointestinal, and other somatic conditions [1, 5]. The management of chronic migraine is complex, often requiring a multidisciplinary approach. CGRP-targeted mAbs had a significant favorable impact on CM management [73]. However, having more therapeutic chances would represent an important step forward. Preventive trials on gepants have focused so far on patients with episodic migraine, but the results of trials designed for chronic patients are expected.
A phase 3, multicenter, randomized, double-blind, placebo-controlled, parallel-group study investigated the efficacy, safety, and tolerability of atogepant (30 mg BID or 60 mg QD) to prevent chronic migraine (NCT03855137) was concluded in January 2022. In the same month, an open-label study on the use of atogepant in China was completed (NCT04829747).
Moreover, a phase 3, multicenter, open-label 104-week extension study is ongoing to evaluate the long-term safety and tolerability of oral atogepant 60-mg QD to prevent chronic or episodic migraine (NCT04686136).
Interestingly, the NCT05216263 study of oral atogepant tablets in add-on to onabotulinumtoxinA (botox) is ongoing to assess adverse events and changes in disease activity in chronic migraine.
In the same line, trials on the acute treatment with gepant posed an upper limit of intake to 8 treating days per month for ubrogepant and 18 for rimegepant. Unfortunately, the clinical experience shows that patients with high frequency and chronic migraine, regardless of clinician indications, are often forced to consume analgesics in search of pain relief, resulting in medication overuse, which worsens patients’ quality of life and represents a risk factor for migraine chronification [74]. Observational real-world studies are necessary to assess the safety of a more frequent intake and the related risk of developing medication overuse.
Conclusion
After 40 years since discovering CGRP, the gepants, antagonists of the CGRPr, are finally available for clinical use. RCTs demonstrated gepants’ efficacy in treating acute attacks to obtain 2 h pain freedom in about 20% of patients and pain relief in about 60% of them, with a more favorable safety profile than triptans [75]. Gepants were also the first oral agents specifically designed to prevent migraine. Up to 60% of treated patients may experience a 50% reduction in migraine frequency. The most common treatment-related emergent adverse events were gastrointestinal (nausea, constipation) for the acute or preventive use. No vascular or hepatic concerns have emerged so far for the FDA-approved molecules. More studies are ongoing to investigate gepant tolerability and safety also in association with monoclonal antibodies and other therapeutic classes for acute or preventive treatment. Interestingly, gepants are also under investigation to treat other painful (e.g., cluster headache and trigeminal neuralgia) and non-painful (e.g., psoriasis or COVID-19) conditions. Real-life studies are necessary to confirm the RCTs findings and investigate more practical clinical aspects. We had walked a long way to cure, but there is still a long way to go.
Funding
This research received no specific grant from any funding agency in the public, commercial, or not-for-profit sectors.
Declarations
Ethical approval and informed consent
This review did not involve human participants and/or animals, so no informed consent was necessary.
Conflict of interest
Claudia Altamura received travel grants and honoraria from Novartis, Eli Lilly, Lusofarmaco, Laborest, Allergan, and Almirall; Fabrizio Vernieri received travel grants, honoraria for advisory boards, speaker panels, or clinical investigation studies from Allergan-Abbvie, Amgen, Angelini, Eli-Lilly, Lundbeck, Novartis, and Teva; and Luisa Fofi received travel grants and honoraria from Teva, Eli-Lilly, and Novartis. Nicoletta Brunelli and Marilena Marcosano declare no competing interests.
Footnotes
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Ashina M, Katsarava Z, Do TP, et al. Migraine: epidemiology and systems of care. Lancet. 2021;397:1485–1495. doi: 10.1016/S0140-6736(20)32160-7. [DOI] [PubMed] [Google Scholar]
- 2.Buse DC, Fanning KM, Reed ML, et al. Life with migraine: effects on relationships, career, and finances from the chronic migraine epidemiology and outcomes (CaMEO) study. Headache. 2019;59:1286–1299. doi: 10.1111/head.13613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Evers S, Áfra J, Frese A, et al. EFNS guideline on the drug treatment of migraine - revised report of an EFNS task force. Eur J Neurol. 2009;16:968–981. doi: 10.1111/j.1468-1331.2009.02748.x. [DOI] [PubMed] [Google Scholar]
- 4.Hepp Z, Dodick DW, Varon SF, et al. Persistence and switching patterns of oral migraine prophylactic medications among patients with chronic migraine: a retrospective claims analysis. Cephalalgia. 2017;37:470–485. doi: 10.1177/0333102416678382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Altamura C, Corbelli I, de Tommaso M, et al. Pathophysiological bases of comorbidity in migraine. Front Hum Neurosci. 2021;15:640574. doi: 10.3389/fnhum.2021.640574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Whyte CA, Tepper SJ. Adverse effects of medications commonly used in the treatment of migraine. Expert Rev Neurother. 2009;9:1379–1391. doi: 10.1586/ern.09.47. [DOI] [PubMed] [Google Scholar]
- 7.Edvinsson L, Haanes KA, Warfvinge K, DiN K. CGRP as the target of new migraine therapies — successful translation from bench to clinic. Nat Rev Neurol. 2018;14:338–350. doi: 10.1038/s41582-018-0003-1. [DOI] [PubMed] [Google Scholar]
- 8.Amara SG, Jonas V, Rosenfeld MG, et al. (1982) Alternative RNA processing in calcitonin gene expression generates mRNAs encoding different polypeptide products. Nat. 1982;2985871(298):240–244. doi: 10.1038/298240a0. [DOI] [PubMed] [Google Scholar]
- 9.Rosenfeld MG, Mermod JJ, Amara SG, et al. Production of a novel neuropeptide encoded by the calcitonin gene via tissue-specific RNA processing. Nature. 1983;304:129–135. doi: 10.1038/304129A0. [DOI] [PubMed] [Google Scholar]
- 10.Brain SD, Williams TJ, Tippins JR, et al. Calcitonin gene-related peptide is a potent vasodilator. Nature. 1985;313:54–56. doi: 10.1038/313054A0. [DOI] [PubMed] [Google Scholar]
- 11.Russell FA, King R, Smillie S-J, et al. Calcitonin gene-related peptide: physiology and pathophysiology. Physiol Rev. 2014;94:1099–1142. doi: 10.1152/physrev.00034.2013.-Calcitonin. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Argunhan F, Brain SD (2022) The vascular-dependent and -independent actions of calcitonin gene-related peptide in cardiovascular disease. Front Physiol 1310.3389/fphys.2022.833645 [DOI] [PMC free article] [PubMed]
- 13.Edvinsson L. Functional role of perivascular peptides in the control of cerebral circulation. Trends Neurosci. 1985;8:126–131. doi: 10.1016/0166-2236(85)90050-5. [DOI] [Google Scholar]
- 14.Goadsby PJ, Edvinsson L, Ekman R. Release of vasoactive peptides in the extracerebral circulation of humans and the cat during activation of the trigeminovascular system. Ann Neurol. 1988;23:193–196. doi: 10.1002/ana.410230214. [DOI] [PubMed] [Google Scholar]
- 15.Goadsby PJ, Edvinsson L, Ekman R. Vasoactive peptide release in the extracerebral circulation of humans during migraine headache. Ann Neurol. 1990;28:183–187. doi: 10.1002/ANA.410280213. [DOI] [PubMed] [Google Scholar]
- 16.Lassen LH, Haderslev PA, Jacobsen VB, et al. CGRP may play a causative role in migraine. Cephalalgia. 2002;22:54–61. doi: 10.1046/J.1468-2982.2002.00310.X. [DOI] [PubMed] [Google Scholar]
- 17.Goadsby P, Edvinsson L. Peripheral and central trigeminovascular activation in cat is blocked by the serotonin (5HT)-I D receptor agonist 311C90. Headache J Head Face Pain. 1994;34:394–399. doi: 10.1111/J.1526-4610.1994.HED3407394.X. [DOI] [PubMed] [Google Scholar]
- 18.Poyner DR, Sexton PM, Marshall I, et al. International Union of Pharmacology. XXXII. The mammalian calcitonin gene-related peptides, adrenomedullin, amylin, and calcitonin receptors. Pharmacol Rev. 2002;54:233–246. doi: 10.1124/pr.54.2.233. [DOI] [PubMed] [Google Scholar]
- 19.Ménard DP, Van Rossum D, Kar S, et al. A calcitonin gene-related peptide receptor antagonist prevents the development of tolerance to spinal morphine analgesia. J Neurosci. 1996;16:2342–2351. doi: 10.1523/jneurosci.16-07-02342.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Feuerstein G, Willette R, Aiyar N. Clinical perspectives of calcitonin gene related peptide pharmacology. Can J Physiol Pharmacol. 1995;73:1070–1074. doi: 10.1139/Y95-152. [DOI] [PubMed] [Google Scholar]
- 21.Doods H, Hallermayer G, Wu D, et al. Pharmacological profile of BIBN4096BS, the first selective small molecule CGRP antagonist. Br J Pharmacol. 2000;129:420–423. doi: 10.1038/sj.bjp.0703110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Iovino M, Feifel U, Yong CL, et al. Safety, tolerability and pharmacokinetics of BIBN 4096 BS, the first selective small molecule calcitonin gene-related peptide receptor antagonist, following single intravenous administration in healthy volunteers. Cephalalgia. 2004;24:645–656. doi: 10.1111/J.1468-2982.2004.00726.X. [DOI] [PubMed] [Google Scholar]
- 23.Olesen J, Diener H-C, Husstedt IW, et al (2004) Calcitonin gene-related peptide receptor antagonist BIBN 4096 BS for the acute treatment of migraine [DOI] [PubMed]
- 24.Petersen KA, Birk S, Lassen LH, et al. The CGRP-antagonist, BIBN4096BS does not affect cerebral or systemic haemodynamics in healthy volunteers. Cephalalgia. 2005;25:139–147. doi: 10.1111/j.1468-2982.2004.00830.x. [DOI] [PubMed] [Google Scholar]
- 25.Ho TW, Mannix LK, Fan X, et al. Randomized controlled trial of an oral CGRP receptor antagonist, MK-0974, in acute treatment of migraine. Neurology. 2008;70:1304–1312. doi: 10.1212/01.WNL.0000286940.29755.61. [DOI] [PubMed] [Google Scholar]
- 26.Ho TW, Ferrari MD, Dodick DW, et al. Efficacy and tolerability of MK-0974 (telcagepant), a new oral antagonist of calcitonin gene-related peptide receptor, compared with zolmitriptan for acute migraine: a randomised, placebo-controlled, parallel-treatment trial. Lancet (London, England) 2008;372:2115–2123. doi: 10.1016/S0140-6736(08)61626-8. [DOI] [PubMed] [Google Scholar]
- 27.Ho TW, Connor KM, Zhang Y, et al. Randomized controlled trial of the CGRP receptor antagonist telcagepant for migraine prevention. Neurology. 2014;83:958–966. doi: 10.1212/WNL.0000000000000771. [DOI] [PubMed] [Google Scholar]
- 28.Temple R. Hy’s law: predicting serious hepatotoxicity. Pharmacoepidemiol Drug Saf. 2006;15:241–243. doi: 10.1002/PDS.1211. [DOI] [PubMed] [Google Scholar]
- 29.Hewitt DJ, Aurora SK, Dodick DW, et al. Randomized controlled trial of the CGRP receptor antagonist MK-3207 in the acute treatment of migraine. Cephalalgia. 2011;31:712–722. doi: 10.1177/0333102411398399. [DOI] [PubMed] [Google Scholar]
- 30.Diener HC, Barbanti P, Dahlöf C, et al. BI 44370 TA, an oral CGRP antagonist for the treatment of acute migraine attacks: results from a phase II study. Cephalalgia. 2011;31:573–584. doi: 10.1177/0333102410388435. [DOI] [PubMed] [Google Scholar]
- 31.Salvatore CA, Mallee JJ, Bell IM, et al. Identification and pharmacological characterization of domains involved in binding of CGRP receptor antagonists to the calcitonin-like receptor. Biochemistry. 2006;45:1881–1887. doi: 10.1021/BI052044W. [DOI] [PubMed] [Google Scholar]
- 32.Messlinger K, Russo AF. Current understanding of trigeminal ganglion structure and function in headache. Cephalalgia. 2019;39:1661–1674. doi: 10.1177/0333102418786261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Eftekhari S, Salvatore CA, Johansson S, et al. Localization of CGRP, CGRP receptor, PACAP and glutamate in trigeminal ganglion. Relation to the blood-brain barrier. Brain Res. 2015;1600:93–109. doi: 10.1016/J.BRAINRES.2014.11.031. [DOI] [PubMed] [Google Scholar]
- 34.Frederiksen SD, Haanes KA, Warfvinge K, Edvinsson L. Perivascular neurotransmitters: regulation of cerebral blood flow and role in primary headaches. J Cereb Blood Flow Metab. 2019;39:610–632. doi: 10.1177/0271678X17747188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Altamura C, Vernieri F. Commentary: enhanced hemodynamic and clinical response to αCGRP in migraine patients—a TCD study. Front Neurol. 2021;12:663818. doi: 10.3389/fneur.2021.663818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Petersen KA, Nilsson E, Olesen J, Edvinsson L. Presence and function of the calcitonin gene-related peptide receptor on rat pial arteries investigated in vitro and in vivo. Cephalalgia. 2005;25:424–432. doi: 10.1111/J.1468-2982.2005.00869.X. [DOI] [PubMed] [Google Scholar]
- 37.Ramachandran R. Neurogenic inflammation and its role in migraine. Semin Immunopathol. 2018;40:301–314. doi: 10.1007/S00281-018-0676-Y. [DOI] [PubMed] [Google Scholar]
- 38.Russo AF. Calcitonin gene-related peptide (CGRP): a new target for migraine. Annu Rev Pharmacol Toxicol. 2015;55:533–552. doi: 10.1146/annurev-pharmtox-010814-124701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Melo-Carrillo A, Schain AJ, Stratton J, et al. Fremanezumab and its isotype slow propagation rate and shorten cortical recovery period but do not prevent occurrence of cortical spreading depression in rats with compromised blood-brain barrier. Pain. 2020;161:1037–1043. doi: 10.1097/j.pain.0000000000001791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Kim KJ, Diaz JR, Iddings JA, Filosa JA. Vasculo-neuronal coupling: retrograde vascular communication to brain neurons. J Neurosci. 2016;36:12624–12639. doi: 10.1523/JNEUROSCI.1300-16.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Edvinsson L, Lars Edvinsson P (2015) CGRP receptor antagonists and antibodies against CGRP and its receptor in migraine treatment10.1111/bcp.12618 [DOI] [PMC free article] [PubMed]
- 42.Vernieri F, Altamura C, Brunelli N, et al. Galcanezumab for the prevention of high frequency episodic and chronic migraine in real life in Italy: a multicenter prospective cohort study (the GARLIT study) J Headache Pain. 2021;22:35. doi: 10.1186/s10194-021-01247-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.FDA approved drug products: Ubrelvy (ubrogepant) oral tablets
- 44.Dodick DW, Lipton RB, Ailani J, et al. Ubrogepant for the treatment of migraine. N Engl J Med. 2019;381:2230–2241. doi: 10.1056/nejmoa1813049. [DOI] [PubMed] [Google Scholar]
- 45.Lipton RB, Dodick DW, Ailani J, et al. Effect of ubrogepant vs placebo on pain and the most bothersome associated symptom in the acute treatment of migraine: the ACHIEVE II randomized clinical trial. JAMA. 2019;322:1887–1898. doi: 10.1001/JAMA.2019.16711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Goadsby PJ, Blumenfeld AM, Lipton RB, et al Time course of efficacy of ubrogepant for the acute treatment of migraine: clinical implications. 10.1177/0333102420970523 [DOI] [PMC free article] [PubMed]
- 47.Blumenfeld AM, Goadsby PJ, Dodick DW, et al. Efficacy of ubrogepant based on prior exposure and response to triptans: a post hoc analysis. Headache. 2021;61:422–429. doi: 10.1111/HEAD.14089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Hutchinson S, Silberstein SD, Blumenfeld AM, et al (2021) Safety and efficacy of ubrogepant in participants with major cardiovascular risk factors in two single-attack phase 3 randomized trials: ACHIEVE I and II. Cephalalgia 4110.1177/03331024211000311 [DOI] [PubMed]
- 49.FDA approved drug products: Nurtec ODT (rimegepant) orally disintegrating tablets
- 50.Croop R, Goadsby PJ, Stock DA, et al. Efficacy, safety, and tolerability of rimegepant orally disintegrating tablet for the acute treatment of migraine: a randomised, phase 3, double-blind, placebo-controlled trial. Lancet. 2019;394:737–745. doi: 10.1016/S0140-6736(19)31606-X. [DOI] [PubMed] [Google Scholar]
- 51.Lipton RB, Croop R, Stock EG, et al. Rimegepant, an oral calcitonin gene-related peptide receptor antagonist, for migraine. N Engl J Med. 2019;381:142–149. doi: 10.1056/NEJMOA1811090. [DOI] [PubMed] [Google Scholar]
- 52.Johnston K, Harris L, Powell L, et al (2022) Monthly migraine days, tablet utilization, and quality of life associated with rimegepant - post hoc results from an open label safety study (BHV3000-201). J Headache Pain 2310.1186/S10194-021-01378-5 [DOI] [PMC free article] [PubMed]
- 53.Baker TE, Croop R, Kamen L, et al. Human milk and plasma pharmacokinetics of single-dose rimegepant 75 mg in healthy lactating women. Breastfeed Med. 2022;17:277–282. doi: 10.1089/BFM.2021.0250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.clinicaltrials.gov. https://clinicaltrials.gov
- 55.Xu J, Xu L, Sui P, et al. Excess neuropeptides in lung signal through endothelial cells to impair gas exchange. Dev Cell. 2022 doi: 10.1016/J.DEVCEL.2022.02.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.FDA approved drug products: Qulipta (atogepant) tablets for oral use
- 57.Goadsby PJ, Dodick DW, Ailani J, et al. Safety, tolerability, and efficacy of orally administered atogepant for the prevention of episodic migraine in adults: a double-blind, randomised phase 2b/3 trial. Lancet Neurol. 2020;19:727–737. doi: 10.1016/S1474-4422(20)30234-9. [DOI] [PubMed] [Google Scholar]
- 58.Ailani J, Lipton RB, Goadsby PJ, et al. Atogepant for the preventive treatment of migraine. N Engl J Med. 2021;385:695–706. doi: 10.1056/NEJMOA2035908. [DOI] [PubMed] [Google Scholar]
- 59.Boinpally R, McNamee B, Yao L, et al. A single supratherapeutic dose of atogepant does not affect cardiac repolarization in healthy adults: results from a randomized, single-dose, phase 1 crossover trial. Clin Pharmacol drug Dev. 2021;10:1099–1107. doi: 10.1002/CPDD.940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Min KC, Kraft WK, Bondiskey P, et al. Atogepant is not associated with clinically meaningful alanine aminotransferase elevations in healthy adults. Clin Transl Sci. 2021;14:599–605. doi: 10.1111/CTS.12917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Croop R, Lipton RB, Kudrow D, et al. Oral rimegepant for preventive treatment of migraine: a phase 2/3, randomised, double-blind, placebo-controlled trial. Lancet. 2021;397:51–60. doi: 10.1016/S0140-6736(20)32544-7. [DOI] [PubMed] [Google Scholar]
- 62.Mathew PG, Klein BC. Getting to the heart of the matter: migraine, triptans, dhe, ditans, cgrp antibodies, first/second-generation gepants, and cardiovascular risk. Headache. 2019;59:1421–1426. doi: 10.1111/head.13601. [DOI] [PubMed] [Google Scholar]
- 63.Mulder IA, Li M, de Vries T, et al. Anti-migraine calcitonin gene–related peptide receptor antagonists worsen cerebral ischemic outcome in mice. Ann Neurol. 2020;88:771–784. doi: 10.1002/ana.25831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Ohlsson L, Haanes KA, Kronvall E, et al. Erenumab (AMG 334), a monoclonal antagonist antibody against the canonical CGRP receptor, does not impair vasodilatory or contractile responses to other vasoactive agents in human isolated cranial arteries. Cephalalgia. 2019;39:1745–1752. doi: 10.1177/0333102419867282. [DOI] [PubMed] [Google Scholar]
- 65.Altamura C, Viticchi G, Fallacara A, et al. Erenumab does not alter cerebral hemodynamics and endothelial function in migraine without aura. Cephalalgia. 2021;41:90–98. doi: 10.1177/0333102420956692. [DOI] [PubMed] [Google Scholar]
- 66.Kudrow D, Pascual J, Winner PK, et al. Vascular safety of erenumab for migraine prevention. Neurology. 2020;94:E497–E510. doi: 10.1212/WNL.0000000000008743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Barbanti P, Aurilia C, Cevoli S, et al. Long-term (48 weeks) effectiveness, safety, and tolerability of erenumab in the prevention of high-frequency episodic and chronic migraine in a real world: results of the EARLY 2 study. Headache J Head Face Pain. 2021;61:1351–1363. doi: 10.1111/head.14194. [DOI] [PubMed] [Google Scholar]
- 68.Roberto G, Piccinni C, D’Alessandro R, Poluzzi E. Triptans and serious adverse vascular events: data mining of the FDA Adverse Event Reporting System database. Cephalalgia. 2014;34:5–13. doi: 10.1177/0333102413499649. [DOI] [PubMed] [Google Scholar]
- 69.Breen ID, Brumfiel CM, Patel MH, et al. Evaluation of the safety of calcitonin gene-related peptide antagonists for migraine treatment among adults with Raynaud phenomenon. JAMA Netw Open. 2021;4:217934. doi: 10.1001/jamanetworkopen.2021.7934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Jakate A, Blumenfeld AM, Boinpally R, et al. Pharmacokinetics and safety of ubrogepant when coadministered with calcitonin gene-related peptide-targeted monoclonal antibody migraine preventives in participants with migraine: a randomized phase 1b drug-drug interaction study. Headache. 2021;61:642–652. doi: 10.1111/HEAD.14095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Berman G, Croop R, Kudrow D, et al. Safety of rimegepant, an oral CGRP receptor antagonist, plus CGRP monoclonal antibodies for migraine. Headache. 2020;60:1734–1742. doi: 10.1111/HEAD.13930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Feigin VL, Krishnamurthi RV, Theadom AM, et al. Global, regional, and national burden of neurological disorders during 1990–2015: a systematic analysis for the Global Burden of Disease Study 2015. Lancet Neurol. 2017;16:877–897. doi: 10.1016/S1474-4422(17)30299-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Soni P, Chawla E. Efficacy and safety of anti-calcitonin gene-related peptide monoclonal antibodies for treatment of chronic migraine: a systematic review and network meta-analysis. Clin Neurol Neurosurg. 2021;209:106893. doi: 10.1016/j.clineuro.2021.106893. [DOI] [PubMed] [Google Scholar]
- 74.Schwedt TJ, Hentz JG, Sahai-Srivastava S, et al. Headache characteristics and burden from chronic migraine with medication overuse headache: cross-sectional observations from the Medication Overuse Treatment Strategy trial. Headache J Head Face Pain. 2021;61:351–362. doi: 10.1111/head.14056. [DOI] [PubMed] [Google Scholar]
- 75.Yang CP, Liang, Chih-Sung, Chang CM, et al. Comparison of new pharmacologic agents with triptans for treatment of migraine a systematic review and meta-analysis + supplemental content. JAMA Netw Open. 2021;4:2128544. doi: 10.1001/jamanetworkopen.2021.28544. [DOI] [PMC free article] [PubMed] [Google Scholar]