Abstract
Methanogenesis and homoacetogenesis occur simultaneously in the hindguts of almost all termites, but the reasons for the apparent predominance of methanogenesis over homoacetogenesis in the hindgut of the humivorous species is not known. We found that in gut homogenates of soil-feeding Cubitermes spp., methanogens outcompete homoacetogens for endogenous reductant. The rates of methanogenesis were always significantly higher than those of reductive acetogenesis, whereas the stimulation of acetogenesis by the addition of exogenous H2 or formate was more pronounced than that of methanogenesis. In a companion paper, we reported that the anterior gut regions of Cubitermes spp. accumulated hydrogen to high partial pressures, whereas H2 was always below the detection limit (<100 Pa) in the posterior hindgut, and that all hindgut compartments turned into efficient H2 sinks when external H2 was provided (D. Schmitt-Wagner and A. Brune, Appl. Environ. Microbiol. 65:4490–4496, 1999). Using a microinjection technique, we found that only the posterior gut sections P3/4a and P4b, which harbored methanogenic activities, formed labeled acetate from H14CO3−. Enumeration of methanogenic and homoacetogenic populations in the different gut sections confirmed the coexistence of both metabolic groups in the same compartments. However, the in situ rates of acetogenesis were strongly hydrogen limited; in the P4b section, no activity was detected unless external H2 was added. Endogenous rates of reductive acetogenesis in isolated guts were about 10-fold lower than the in vivo rates of methanogenesis, but were almost equal when exogenous H2 was supplied. We conclude that the homoacetogenic populations in the posterior hindgut are supported by either substrates other than H2 or by a cross-epithelial H2 transfer from the anterior gut regions, which may create microniches favorable for H2-dependent acetogenesis.
In the absence of other electron acceptors, CO2 is the terminal electron sink in anoxic environments. Formate and molecular hydrogen are typical products formed in the fermentative degradation of organic compounds. Their reducing equivalents are used either by methanogenic archaea for the direct reduction of CO2 to CH4 or by homoacetogenic bacteria for the reduction of CO2 to acetate, which is subsequently converted to CO2 and CH4 by aceticlastic methanogens (32, 34).
In most environmental situations, the free-energy change (per carbon) of CO2 reduction to CH4 is larger than that of CO2 reduction to acetate (32). It has been shown that the minimum H2 partial pressures in pure cultures of methanogens are roughly 1 order of magnitude lower (3 to 10 Pa) than those achieved by homoacetogens (50 to 100 Pa) under similar conditions (12, 34). This probably explains why methanogenesis usually predominates over homoacetogenesis as the terminal electron sink reaction (32). Only at lower temperatures does the thermodynamic advantage change in favor of homoacetogenesis, and also in slightly acidic environments, methanogens may not be as competitive as homoacetogens as a hydrogen sink (for reviews, see references 31, 32, and 34).
The intestinal tracts of animals are generally characterized by the coexistence of homoacetogenic and methanogenic microorganisms (5, 39). In principle, homoacetogenesis is considered advantageous for the host organism, which is able to use acetate as a carbon and/or energy source (7). However, the numbers of H2-oxidizing methanogenic archaea cultivated from rumen or cecum contents of different mammals are often orders of magnitude higher than those of homoacetogenic bacteria (23). Especially in ruminants, the energy loss via methane production is considerable, and many efforts have been made to shift the ruminal fermentation towards acetogenesis in order to reduce methanogenesis and thereby increase feed exploitation by sheep and cattle (22, 25).
Although in situ rates of H2-dependent homoacetogenesis are generally low in ruminal samples and pig hindgut, they increase when methanogenesis is inhibited by bromoethanesulfonate (BES) or when the H2 partial pressure is increased (13, 25). Inhibition of methanogens by BES also allowed the demonstration of significant numbers of H2-oxidizing homoacetogens in rumen and hindgut contents of animals or in human feces (14). The competition for H2 seems to be a decisive factor: in rumen samples incubated under an H2 headspace, reductive acetogenesis was significantly stimulated by the addition of the homoacetogenic Acetitomaculum ruminis or Peptostreptococcus productus only when the methanogens were simultaneously inhibited with BES (22, 25).
Unfortunately, not much is known about the hydrogen partial pressures in the intestinal tracts of animals in which homoacetogenesis has been identified as the dominating process (7). Considering the metabolic versatility of homoacetogens, which are capable of utilizing other substrates in the absence of H2 or mixotrophic with H2 (5), and the apparently genetic determination in different animal lineages of the ability to host methanogens in their intestinal tracts (17), the actual reasons governing the coexistence of both metabolic groups are complex and may differ in each case.
Termite guts are an excellent example of this situation (6). Breznak and coworkers showed that CO2 reduction to acetate in gut homogenates of most wood-feeding termites exceeds methane emission rates by an order of magnitude (3, 8). In Reticulitermes flavipes, the explanation for the predominance of homoacetogens was found in the high hydrogen partial pressures in the hindgut, which may exceed the typical threshold values of homoacetogenic bacteria by more than 2 orders of magnitude (16). Since the methanogenic population in this termite is restricted almost exclusively to the microoxic gut periphery (16, 20), it has been postulated that in this case, the predominance of homoacetogenesis is founded on a spatial separation of the methanogenic and homoacetogenic populations (11, 16).
In the much more abundant and globally very important group of soil-feeding termites (40), which exhibit a strong hindgut compartmentalization (1, 24) and an extreme alkalinity (>pH 12) in the anterior hindgut (2, 10), the digestive physiology is still largely obscure (11). In an earlier study, Brauman et al. (3) had shown that in contrast to wood-feeding termites, the soil-feeding species emit relatively large amounts of CH4, while the rates of homoacetogenesis in their gut homogenates are an order of magnitude lower. At that time, the authors had already cautioned that homogenization and dilution of the gut contents inevitably disrupt the physical interaction of hydrogen-producing and hydrogen-consuming microorganisms within the hindgut, which might lead to a serious underestimation of homoacetogenesis in situ (3).
Our current study of soil-feeding Cubitermes spp. tries to resolve this issue by addressing the spatial distribution of homoacetogenic and methanogenic microorganisms in the different gut compartments and their respective activities under in situ conditions. In a companion paper, we reported that (i) H2 accumulates only in the anterior gut regions, while H2 partial pressures in the posterior hindgut are always below the detection limit (<100 Pa); (ii) only the anterior gut regions represent hydrogen sources, whereas all hindgut regions turn into H2 sinks when external H2 is provided; and (iii) methanogenic activities are localized only in the posterior gut regions (33).
In the present paper, we used radiotracer techniques to estimate the distribution and numerical abundance of H2-oxidizing methanogenic and homoacetogenic populations in the hindgut. By microinjection of minute amounts of radiotracers, we determined the localization and the in situ activities of reductive acetogenesis in the individual hindgut compartments. Finally, we discuss the results together with those of the microsensor studies described in the companion paper (33).
MATERIALS AND METHODS
Termites.
Cubitermes orthognathus Emerson and Cubitermes umbratus Williams (Termitidae: Termitinae) were collected near Busia (Kenya) and in the Shimba Hills Natural Reserve (Kenya), respectively. Nest fragments with termites were brought to the laboratory in polypropylene containers together with soil from the collection site; measurements were generally performed within 1 to 2 months of collection. Worker caste termites were used for all experiments.
14CO2 reduction by gut homogenates.
Termites were dissected and guts were homogenized (10 guts ml−1) in anoxic buffered salt solution (BSS [37]), reduced with 1 mM dithiothreitol (DTT), by using a glass tissue homogenizer. Aliquots of the suspension (0.75 ml) were dispensed into 5-ml glass serum vials and sealed with butyl rubber stoppers. The whole procedure was performed in an anoxic glove box under N2 (2 to 5% H2); all equipment was preincubated in the glove box for 48 h.
In the experiments with C. umbratus, the vials were gassed with H2 or N2 for 2 min, and 25 μl of an anoxic aqueous solution of NaH14CO3 (0.71 μmol; 0.15 MBq) was immediately injected. Considering the internal CO2 pool of C. umbratus hindguts (78 nmol termite−1 [36]), the final amount of CO2 in the assay was 1.3 μmol. In the case of C. orthognathus, bicarbonate buffer (30 mM) was added to the BSS, and the vials were gassed with H2-CO2 or N2-CO2 gas mixtures (both 80/20 [vol/vol]), increasing the total amount of CO2 to 57.1 μmol. Fifty microliters of an anoxic aqueous solution of Na214CO3 (1.44 μmol; 2.8 MBq) or [14C]Na-formate (4.1 μmol; 7.8 MBq) was injected. The final pH of the assays was between 7.2 and 7.4.
Vials were incubated at 30°C on a rotary shaker (200 rpm), and samples were taken every hour by using syringes equipped with gas-tight valves and flushed with N2. Headspace samples (50 μl) were analyzed for 14CH4 and 14CO2 by gas chromatography as described below. Liquid-phase samples (50 μl) were combined with 50 μl of a solution containing nonradioactive standards (acetate, formate, lactate, propionate, butyrate, isobutyrate, and ethanol at 5 mM each) in NaOH (0.4 M). After centrifugation (5 min at 14,000 × g), the supernatant was analyzed for radioactivity and label distribution by liquid scintillation counting (LSC) and by high-performance liquid chromatography (HPLC) (see below). The pellet was washed and analyzed by LSC after heat treatment (15 min at 80°C), and a complete radioactivity balance was performed.
Microinjection of 14C-labeled compounds.
For microinjection of radiolabeled compounds, we used a hydraulic system consisting of a 25-μl Hamilton syringe actuated by means of a micrometer drive (10-μm minimum step increment) and connected to a 50-μl glass micropipette via PEEK high-pressure capillaries and fittings. The system was filled with silicone oil, and great care was taken to purge out all air bubbles. The micropipette was drawn to a fine tip with a pipette puller, broken back to a tip diameter of 7 to 12 μm, and heat polished. The inner surface was coated with paraffin by filling the tip with a mixture of toluene and paraffin oil (95%/5% [vol/vol]) and drying the micropipette for 24 h at 100°C. Micropipettes were positioned by means of a manual micromanipulator, and the position of the tip was controlled visually with a stereomicroscope; the whole setup was essentially the same as that used for the microsensor studies (33).
The micropipette was filled with 1 to 2 μl of an aqueous solution of the respective radioactive metabolite kept under silicone oil to avoid evaporation. Immediately before injection, termites were dissected and hindguts were embedded flat and fully extended (see Fig. 1 in the companion paper [33]) in a glass microchamber by using agarose made up with insect Ringer’s solution (33) and preincubated under a controlled headspace of air or H2; the setup was as described by Ebert and Brune (16). The micropipette was inserted into individual gut sections, with the tip positioned at the center of the respective compartment, and minute volumes of the label (40 to 80 nl) were injected via hydraulic pressure. Micropipettes were calibrated before and after each experiment by delivering aliquots of the respective tracer solution directly into LSC vials filled with scintillation cocktail. Over all pipettes tested, the deviation from the average of the injected label was 3.8% ± 1.7% (n = 111).
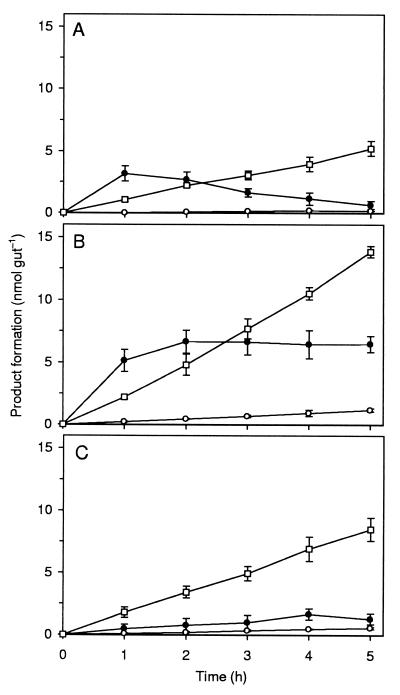
FIG. 1.
Formation of acetate (○), formate (●), and CH4 (□) from H14CO3− in gut homogenates of C. orthognathus under N2-CO2 atmosphere (A), under H2-CO2 atmosphere (B), or under N2-CO2 atmosphere in the presence of 5 mM unlabeled formate (C). Values are means of two replicate assays. In the case of acetate, it was assumed that both C atoms stemmed from CO2. Similar results were obtained for C. umbratus (Table 1).
At different time intervals after injection, the guts were removed from the agarose and immediately disrupted by sonication in 0.1 ml of NaOH (0.2 M) containing nonlabeled standards (see above), by using an ultrasonic probe with a microtip (60 W; Ultraschallprozessor 50 H; Dr. Hielscher GmbH, Teltow, Germany). After centrifugation (14,000 × g for 5 min), aliquots of the supernatant were analyzed for radioactivity and label distribution by LSC and HPLC. The pellet was analyzed as described above. The agarose block (∼0.5 cm3) which had surrounded the guts was transferred into 0.5 ml of 0.2 M NaOH containing nonlabeled standards (see above) and was cut into pieces. After 3 days of equilibration at 4°C, aliquots of the liquid were analyzed by LSC and HPLC. For a complete radioactivity balance of the injected label, the remaining mixture was added quantitatively to scintillation vials, and the recovery was determined by comparing the sum of the radioactivities in the pellet, supernatant, and agarose.
Enumeration of bacteria.
Three-tube most-probable-number (MPN) determinations were performed essentially as described before (37), with anoxic, bicarbonate-buffered mineral medium AM 4 (37) containing bovine rumen fluid (2% [vol/vol]), acetate (1 mM), and resazurin (10 mg liter−1) as a redox indicator and a headspace of H2-CO2 (80/20 [vol/vol]). All tubes contained a palladium catalyst (5% palladium on activated carbon; Aldrich, Steinheim, Germany; final catalyst concentration, 50 mg liter−1) to ensure reducing conditions; hydrogen-free controls were reduced with DTT (1 mM). When indicated, a mixture of additional unlabeled substrates (d-glucose, 5 mM; methanol, 10 mM; formate, 10 mM) or BES (3 mM) was added. All tubes received Na214CO3 (10.3 kBq ml−1, 0.22 GBq mmol−1), and were incubated for 10 weeks at 30°C on a rotary shaker. Tubes were scored as positive for H2-dependent acetogenesis, methanogenesis, or formate production when the amount of labeled product was above that of H2-free controls. Background growth was followed photometrically by measuring the turbidity of the cultures at 600 nm. MPNs were computed with the universal equation for MPN calculation (19).
Analytical methods.
To analyze the total amount of radioactivity in liquid samples, 10-μl aliquots were added to 3.5 ml of Pico Aqua liquid scintillation cocktail (Canberra Packard, Frankfurt, Germany) and were analyzed with a liquid scintillation counter (LS 1801; Beckman Instruments, München, Germany). Duplicate assays were performed; all values were quench corrected by using n-[14C]hexadecane as internal standard.
Liquid samples (50 μl) were analyzed by HPLC with a system equipped with an ion-exclusion column and a refractive-index detector (37); radioactivity was measured with an on-line flow scintillation analyzer (Ramona 2000; Raytest, Straubenhardt, Germany) with a cell volume of 1.2 ml. The scintillation cocktail (Quicksafe Flow 2; Zinsser Analytic, Eschborn, Germany) was used at a buffer/cocktail ratio of 1:3. Radioactive gases (14CH4 and 14CO2) were detected with a gas chromatographic system equipped with a molecular sieve column (29), a methanizer (for the catalytic reduction of CO2 to CH4), a flame-ionization detector, and a radioactivity monitor (RAGA 2026/2028; Raytest). The detection limit was between 2 and 5 Bq per peak for soluble organic compounds and was 25 Bq for radioactive gases. Radiolabeled products were identified by cochromatography with labeled and unlabeled standards.
Chemicals.
Radiochemicals were purchased from Sigma, Deisenhofen, Germany, with the following radiochemical purities and specific activities, respectively: [U-14C]Na-acetate, 98.7% and 2.3 GBq mmol−1; [14C]Na-formate, 97.4% and 2.1 GBq mmol−1; [14C]polyethylene glycol 4000, 99.7% and 1.9 GBq mmol−1; and NaH14CO3, 210 MBq mmol−1. Na214CO3 (1.9 GBq mmol−1) was from Moravek Biochemicals (Brea, Calif.). All other chemicals were of the highest available purity. Gases were supplied by SWF, Friedrichshafen, Germany, and were 99.999% pure.
RESULTS
14CO2 reduction by gut homogenates.
Gut homogenates of both Cubitermes spp. reduced 14CO2 to methane, acetate, and formate. Methane and acetate accumulated linearly with time, whereas formate accumulated only transiently under an N2 atmosphere, but reached a steady-state concentration when hydrogen was present. The time courses of product formation were basically identical for both termite species; therefore, the time course is shown in detail only for C. orthognathus (Fig. 1).
Methane formation rates were always much higher than those of acetate formation, but homoacetogenesis was stimulated much more than methanogenesis when external H2 was provided (Table 1). Considering the differences in fresh weight (10.6 mg per termite for C. umbratus and 6.8 mg per termite for C. orthognathus), there were only small differences in the potential rates of H2-dependent methanogenesis (0.303 and 0.391 μmol g−1 h−1) and acetogenesis (0.025 and 0.034 μmol g−1 h−1) between the two species.
TABLE 1.
Formation of labeled acetate and CH4 from 14CO2 in gut homogenates of Cubitermes spp. in the presence and absence of external electron donors.
| Termite species | Electron donor | Formation rate (nmol gut−1 h−1)a
|
|
|---|---|---|---|
| Acetateb | CH4 | ||
| C. umbratus | Nonec | 0.016 | 1.60 |
| H2d | 0.27 | 3.21 | |
| C. orthognathus | Nonec | 0.037 | 1.03 |
| H2d | 0.23 | 2.66 | |
| Formatec | 0.12 | 1.70 | |
Rates were calculated by linear regression by using the data points of two replicate assays (r2 ≥ 0.95).
Calculated assuming that both C atoms of acetate stem from CO2.
Incubation atmosphere: N2-CO2 (80/20 [vol/vol]).
Incubation atmosphere: H2-CO2 (80/20 [vol/vol]).
In contrast to CH4 and acetate, formate did not accumulate with a linear rate. The initial rates of formate formation under an N2-CO2 or H2-CO2 atmosphere were quite high (4.2 to 5.4 and 3.2 to 5.1 nmol gut−1 h−1 for C. umbratus and C. orthognathus, respectively), but declined already within the first hour of incubation (Fig. 1A and B). 14CO2 reduction to acetate and methane was stimulated as well by the addition of unlabeled formate as an electron donor (Fig. 1C [tested only with gut homogenates of C. orthognathus] and Table 1). When labeled formate was injected, however, labeled methane was formed only at very low rates (<0.01 nmol gut−1 h−1), in both the presence and absence of external H2, and labeled acetate was never detected.
No labeled products other than CH4, acetate, and formate were detected. The recovery of added radioactive label was close to unity in all assays (95.5 to 99.7%) and did not differ significantly between the two incubation atmospheres. Less than 0.5% of the radioactivity was recovered in the particulate fraction. All rates were proportional to the amount of gut homogenate added, and product formation could be abolished by boiling the homogenates for 10 min (not shown).
Microinjection of radiotracers.
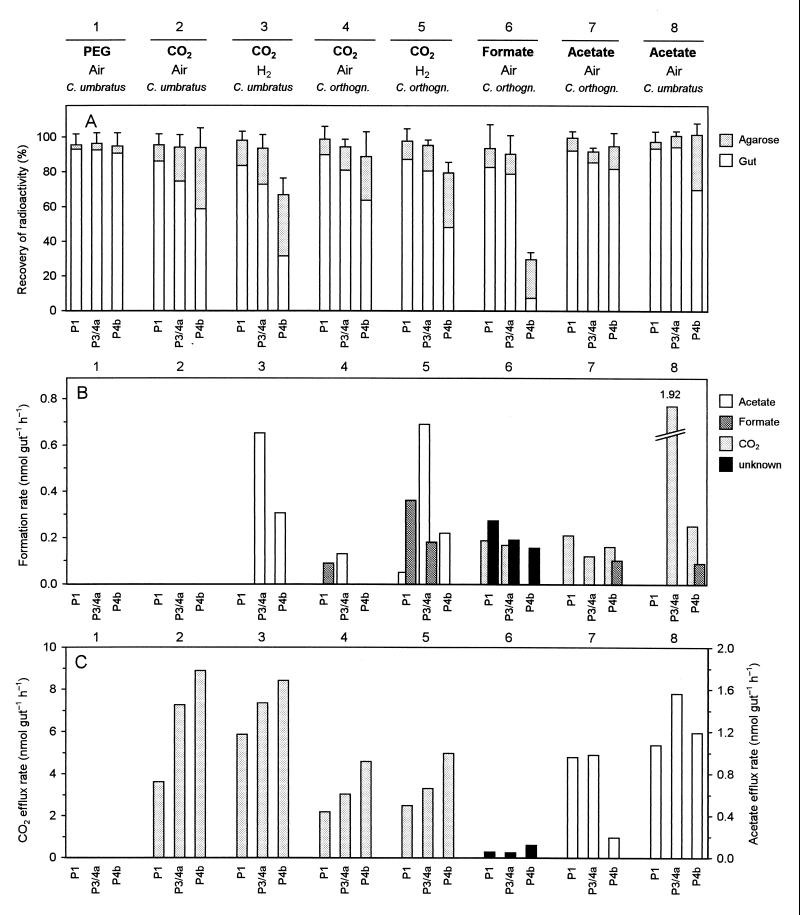
In order to evaluate the in situ rates and the localization of homoacetogenic activities, we injected minute amounts of radiolabeled metabolites into individual sections of agarose-embedded guts of C. umbratus and C. orthognathus. Figure 2A gives an overview of the different experimental series and the specific incubation conditions and illustrates the distribution of injected radioactivity between gut and agarose at the end of the incubation period.
FIG. 2.
Microinjection of various 14C-labeled metabolites into individual sections of agarose-embedded guts of Cubitermes spp. (C. umbratus and C. orthognathus) incubated under air or H2 atmosphere. (A) Distribution of radioactivity between guts and agarose at the end of the incubation period. Incubation times were 45 min, except for series 6 (20 min) and 7 and 8 (15 min). Each bar represents the mean of three to four injections into separate guts, except for PEG (six to eight injections per section). Error bars indicate standard deviations of the total recovery. (B) Product formation rates from labeled substrates. (C) Efflux rates of labeled metabolites into the surrounding agarose. In the case of the unidentified product (series 6), the rates were calculated for a C1 compound. Bar definition in panel C is the same as in panel B. For definitions of the sections, see Fig. 1 in the companion paper (33).
When polyethylene glycol (PEG) was injected, the radiolabel was always retained within the gut. The amount of radiolabel recovered from the surrounding agarose directly after microinjection (2.9% ± 1.0%; n = 20) was not significantly different from that after 45 min of incubation (3.4% ± 1.0%; n = 20). There was also no significant difference between the individual gut sections (Fig. 2A; series 1). Since PEG does not diffuse through epithelia, this series represented an important control which ensured that (i) the injected label was indeed located within the gut, (ii) the initially injected label could be completely recovered, and (iii) radioactivity could escape into the surrounding agarose only if there was a selective permeability of the gut epithelium for the respective compound. Since there was no reason to assume a different situation in C. orthognathus, these controls were performed only with C. umbratus.
Also after microinjection of radiolabeled HCO3−, acetate, or formate, recovery of radioactivity at the end of the incubation period was complete for most sections. Only in the case of the P4b segment were considerable amounts of radiolabel missing when HCO3− was injected and the guts were incubated under H2 (Fig. 2A, series 3 and 5), or when formate was injected and the guts were incubated under air (Fig. 2A, series 6). Since the P4b segment of C. orthognathus has been shown to form substantial amounts of CH4 when incubated in the presence of H2 or formate (33), this gap in the recovery is most likely attributable to the formation of methane from 14CO2. Unfortunately, the microinjection assay procedure did not allow the detection of 14CH4. In all series, substantial amounts of the injected label were recovered from the agarose at the end of the incubation period, indicating that the respective gut sections were permeable for the injected compounds or their metabolites (see below).
Product formation rates.
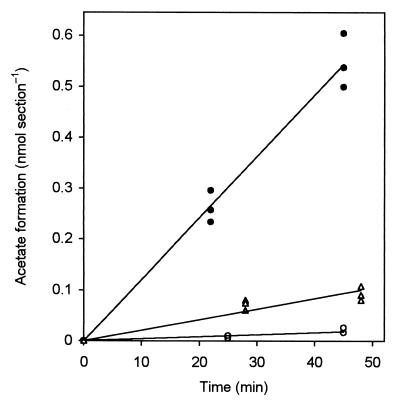
In order to calculate the product formation rates (Fig. 2B) and, when applicable, the efflux rates of the injected substances or their metabolites into the agarose (Fig. 2C), the radioactivity values were transformed into absolute amounts of metabolites by using the specific radioactivity of the injected substrate and correcting for the pool of unlabeled substrate already present in the respective gut section (Table 2). Due to the large internal pools of CO2 and acetate in all gut sections, pool sizes were not significantly affected by the injection. The amount of labeled products increased linearly with incubation time, as exemplified by the results obtained for series 5 (Fig. 3), and product formation rates (Fig. 2B) were calculated by linear regression. Only in the case of formate were the internal pools so small that the pool concentrations were significantly increased by the injected label (Table 2). Nevertheless, injected formate was turned over already within the first incubation interval of 20 min, and only the minimal rates could be reported.
TABLE 2.
Pool sizes of selected metabolites in the different gut sections of Cubitermes spp. and the injected amounts of the corresponding radiolabeled tracersa
| Metabolite | Pool size (nmol section−1)b
|
Injected amt
|
||||||
|---|---|---|---|---|---|---|---|---|
|
C. umbratus
|
C. orthognathus
|
nmol | Bq | |||||
| P1 (1.55 ± 0.23 μl) | P3/4a (0.97 ± 0.16 μl) | P4b (0.32 ± 0.16 μl) | P1 (0.76 ± 0.19 μl) | P3/4a (0.54 ± 0.15 μl) | P4b (0.12 ± 0.02 μl) | |||
| Acetate | 6.80 ± 0.39 | 5.86 ± 0.68 | 0.73 ± 0.06 | 3.30 ± 0.07 | 3.83 ± 0.06 | 0.35 ± 0.12 | 0.21 | 61 |
| CO2c | 26.0 ± 1.5 | 22.4 ± 0.3 | 13.2 ± 2.1 | 13.1 ± 0.9 | 11.0 ± 0.2 | 6.9 ± 1.1 | 1.50 | 315 |
| Formate | 0.03 ± 0.03 | <0.01 | 0.06 ± 0.05 | <0.01 | 0.09 ± 0.08 | <0.01 | 0.17 | 340 |
The average volume of the sections (given in parentheses above) was determined by geometric approximation. A complete data set including all metabolites will be published elsewhere (36). For definitions of the sections, see Fig. 1 in the companion paper (33).
Values are means (± standard deviations) of three independent values, determined as described elsewhere (35).
Total dissolved inorganic C (CO2 plus HCO3− plus CO32−).
FIG. 3.
Formation of labeled acetate after microinjection of H14CO3− into the P1 (○), P3/4a (●), and P4b (C) sections of C. orthognathus hindguts incubated under H2 (Fig. 2B, series 5). Each data point represents an injection into a separate gut. For definitions of the sections, see Fig. 1 in the companion paper (33).
When HCO3− was injected into C. umbratus and C. orthognathus hindguts incubated under air, no labeled products could be detected (results with C. umbratus are shown in Fig. 2B, series 2). After we had realized that intestinal H2 production in Cubitermes spp. decreased progressively during the first weeks after collection, but could be restored by feeding fresh soil (33), we repeated the experiment with a batch of C. orthognathus fed with topsoil from the collection site 24 h before the experiment. The hindguts now formed labeled formate in the P1 section and acetate in the P3/4a section, albeit at low rates (Fig. 2B, series 4).
The apparent H2 limitation of reductive acetogenesis was confirmed when the guts were incubated under an H2 atmosphere, which increased the rate of acetate production from 14CO2 considerably. Acetate was the major product in the P3/4a and P4b sections of both termites (Fig. 2B, series 3 and 5); the combined in situ rates of all gut sections (Table 3) were roughly 3 to 4 times higher than the rates observed in gut homogenates under external H2 (Table 1). The specific rates of homoacetogenesis under an H2 atmosphere were slightly higher in C. orthognathus than in C. umbratus (0.141 and 0.091 μmol g−1 h−1, respectively).
TABLE 3.
In situ rates of product formation after microinjection of H14CO3− into the major gut sections of agarose-embedded hindguts of Cubitermes spp. incubated under air or an H2 atmospherea
| Termite species | Incubation atmosphere | Product | Formation rate (nmol gut−1 h−1)
|
||
|---|---|---|---|---|---|
| P1 | P3/4a | P4b | |||
| C. umbratus | H2 | Acetate | —b | 0.65 | 0.31 |
| Formate | — | — | — | ||
| C. orthognathus | Air | Acetate | — | 0.12 | — |
| Formate | 0.09 | — | — | ||
| H2 | Acetate | 0.05 | 0.69 | 0.22 | |
| Formate | 0.36 | 0.18 | — | ||
C. orthognathus was fed with fresh soil 24 h before the measurements. C. umbratus guts did not form any products when incubated under air (see text).
—, below the detection limit (0.02 nmol section−1 h−1).
Also formate formation from 14CO2 in C. orthognathus increased when the guts were incubated under H2, and formate was formed in both the P1 and the P3/4a compartments (Fig. 2B, series 5). These gut sections (incubated under air) rapidly oxidized injected formate to CO2 (Fig. 2B, series 6). A consistent discrepancy between the total radioactivity recovered in the supernatant and that detected by HPLC analysis indicated the production of further, so far unidentified product(s) in all gut sections when formate was injected. It should be considered that microinjection of formate increased the intestinal pool concentrations considerably (Table 2), which might cause overestimation of in situ rates, whereas the complete turnover of injected formate already within the first incubation interval (see above) led to underestimated values. Therefore, both the rates of formate oxidation and the formation of the unidentified product(s) should be regarded with caution.
Hindguts of both termites (incubated under air) oxidized injected acetate to CO2 (Fig. 2B, series 7 and 8), although the rates were lower than those for acetate formation from CO2 in the respective gut sections. The only exception was the P3/4a section of C. umbratus, which showed an exceptionally high rate of acetate oxidation, while acetate oxidation in the P1 section of this termite was at the detection limit. In the P4b compartment of both termites, injected acetate was also converted to formate at a low rate.
Efflux of metabolites from the gut.
After microinjection of CO2 or acetate into any of the hindgut compartments, the labeled substrates were always recovered as well from the agarose, and efflux rates (Fig. 2C) were determined by the calculation procedure described above. However, in the cases in which acetate or CO2 was formed as a labeled product within the gut (Fig. 2B), they were never detected in the agarose, probably due to the trapping of the respective product label in the large internal pools of acetate and CO2 (Table 2). Apparently, this does not apply to the unidentified product(s) formed from formate, since slight but significant discrepancies between total and HPLC-detectable radioactivity were also found in the agarose samples (Fig. 2C, series 6).
In general, CO2 efflux rates were significantly higher than those for acetate. When compared between the gut sections, the efflux rates were similar, except for the low rate of acetate efflux from the P4b section of C. orthognathus (series 7). Injected formate was not recovered from the agarose, presumably due to its rapid turnover. The apparent discrepancy between the relative amounts of label recovered from the agarose (Fig. 2A) and the rates of acetate efflux from the different sections (Fig. 2C) is explained by the different pool sizes of the respective metabolites in each compartment (Table 2).
Enumeration of CO2-reducing bacteria.
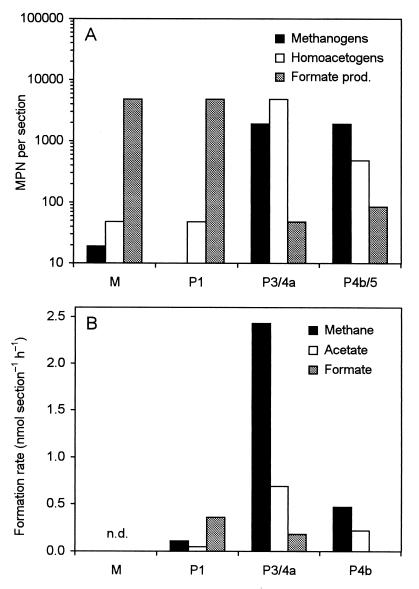
The MPNs of microorganisms capable of H2-dependent CO2 reduction in the different gut sections of C. umbratus were determined by serial dilution. We used 14CO2 to increase sensitivity and to reliably distinguish reductive acetogenesis from acetate formation by fermentative processes, especially when additional substrates were included. The highest MPNs of homoacetogenic and methanogenic bacteria were obtained in the posterior hindgut, i.e., in the P3/4a and the P4b/5 sections (Fig. 4A). When the MPNs are converted to population densities, using the average volume of the respective sections (Table 2), the concentrations of homoacetogenic and methanogenic bacteria in the two sections are similar (4.9 × 106 and 2.0 × 106 cells ml−1 in the P3/4a section and 1.1 × 106 and 4.4 × 106 cells ml−1 in the P4b/5 section).
FIG. 4.
Comparison of the MPNs of CO2-reducing methanogenic, homoacetogenic, and formate-producing microorganisms in the different hindgut sections of C. umbratus (A) with the potential rates of H2-dependent methanogenesis, acetogenesis, and formicogenesis from CO2 in the respective hindgut sections of C. orthognathus (B). The rates for methanogenesis are from the companion paper (33). n.d., not determined. For definitions of the sections, see Fig. 1 in the companion paper (33).
In the anterior gut sections, the MPNs of homoacetogens and methanogens were negligible (Fig. 4A). Instead, both the M and the P1 sections harbored large numbers of bacteria that reduced CO2 to formate, which corresponded to cell densities of 1.3 × 107 and 3.1 × 106 cells ml−1, respectively. Such bacteria may be responsible for the high rates of H2-dependent 14CO2 reduction to formate observed in the P1 section of C. orthognathus (Fig. 4B).
The MPNs did not increase when glucose, methanol, and formate were included as additional substrates. H2-dependent acetogenesis and formicogenesis from CO2 were not detected when BES, an inhibitor of methanogenesis, was omitted or when the tubes were incubated under an N2-CO2 atmosphere. In the latter case, no methanogenesis was detected.
DISCUSSION
This paper represents the first report on the localization and in situ activities of homoacetogenic bacteria in the highly compartmentalized hindgut of soil-feeding termites and, together with the studies of the wood-feeding termites Nasutitermes walkeri (38) and Reticulitermes flavipes (35), is one of the few studies addressing the metabolic rates of the gut microbiota within the intact intestinal tract of insects.
It has been suggested that methanogenesis outprocesses homoacetogenesis in soil-feeding termites (3). To date, the activities of homoacetogens had been measured only in gut homogenates and were found to vary strongly, depending on the hydrogen supply, and information on methanogenesis in homogenates was scarce (3). Our results demonstrate that methanogens outcomplete homoacetogens in gut homogenates of Cubitermes spp., but addition of H2 or formate stimulated homoacetogenesis much more than methanogenesis (Table 1). In view of the considerable axial and radial differences in H2 partial pressure in the hindgut of these termites (33), homogenization is likely to cause considerable artifacts, and the possibility of a strong homogenization bias underlines the necessity for measuring the rates of reductive acetogenesis under more realistic conditions.
In situ activities and their localization.
Using a microinjection assay originally developed to study metabolic fluxes in wood-feeding termites (35), we found that the potential rates of H2-dependent acetogenesis in intact hindguts of Cubitermes umbratus and C. orthognathus were roughly three times higher than those obtained in homogenates. A similar discrepancy exists between the potential rates of methanogenesis in homogenates (Table 1) and in living termites (33), indicating that homogenization has a negative effect on both processes.
Also the endogenous rates of reductive acetogenesis were as severely H2 limited in intact hindguts as in homogenates, which is apparent from the stimulation with exogenously supplied H2, but is also indicated by the differences observed between starved and freshly fed termites. Most interestingly, the homoacetogenic activities in Cubitermes spp. are not located in gut regions with high H2 partial pressures, as previously shown for the wood-feeding R. flavipes (16, 35). Instead, they are restricted to the posterior hindgut, a region which does not accumulate H2 from endogenous sources (except for the P3 compartment) and which also harbors all methanogenic activities (33).
The coexistence of methanogens and homoacetogens in the posterior hindgut compartments is unexpected, but is supported by the results of the differential enumeration of homoacetogenic and methanogenic microorganisms, which demonstrated high numbers for both metabolic groups in these gut sections, although the absolute numbers have to be regarded with caution and are by no means realistic estimates of the total populations. In the P4b/5 section, the MPN of methanogens is fourfold higher than that of the homoacetogens, which is in good agreement with the potential rates of methanogenesis and homoacetogenesis in the P4b compartment (Fig. 4). In the P3/4a section, the MPN of homoacetogens exceeds that of methanogens, but the potential rate of methanogenesis is more than threefold higher than that of homoacetogenesis in this compartment. This may merely reflect a cultivation bias, i.e., an underestimation of the methanogenic population, but might also indicate that due to differences in the spatial distribution of the two populations within this gut section, homoacetogens are still limited for H2 even when H2 is supplied in the headspace.
Notably, there are distinct differences in the in situ rates of methanogenesis and homoacetogenesis between the P3/4a and P4b compartments in the absence of external electron donors. While the P3/4a compartment forms methane already from endogenous sources, methanogenesis is virtually absent in the P4b compartment unless exogenous H2 is provided (33). The same is true for H2-dependent acetogenesis, although the endogenous rates in the P3/4a compartment were above the detection limit only when the termites were supplied with fresh soil 24 h before the experiment (Table 3). It has already been suggested that the close contact of anterior and posterior hindgut within the abdomen would allow a cross-epithelial H2 transfer which would drive methanogenesis and homoacetogenesis in the posterior compartments, especially in the P4b compartment, which seems to have small or no endogenous sources of reductants (33).
Another exogenous electron donor for methanogenesis and homoacetogenesis could be formate. Formate is present in the hemolymph of C. orthognathus in appreciable concentrations (2.6 mM [36]) and is most probably a product of microbial fermentations in the anterior gut compartments (see below). The stimulation of methane emission of isolated P3/4a and P4b sections by formate is even stronger than that by exogenous H2 (33), and H2-oxidizing and formate-oxidizing methanogens have been found to occur in similar numbers in Cubitermes speciosus and other soil-feeding members of the subfamily Termitinae (4, 30). It is not clear whether formate also functions as an electron donor of homoacetogenesis in situ. Labeled formate was not converted to acetate when injected into C. orthognathus hindguts, and in gut homogenates of this termite, it was only oxidized to CO2 and never reduced to methane or acetate. However, when formate was added as an electron donor, it stimulated both homoacetogenesis and methanogenesis from 14CO2 almost as strongly as the addition of H2 (Table 1). It is possible that formate serves only as electron donor for CO2 reduction, but is itself not reduced in the C1 pathway.
Hindgut homogenates of both C. orthognathus and C. umbratus also formed formate from 14CO2 at high rates when H2 was present (Fig. 1B). The same reaction had been previously observed in gut homogenates of the closely related C. speciosus (4) and of the wood-feeding termites R. flavipes, Zootermopsis angusticollis, Prorhinotermes simplex, Nasutitermes costalis, and N. nigriceps (8). However, formate did not accumulate in intact guts of R. flavipes (35), Nasutitermes walkeri (38), and C. umbratus (this study). Only in C. orthognathus hindguts did we find low rates of formate formation after microinjection of 14CO2 into the alkaline P1 compartment and, in the presence of exogenous H2, also into the P3/4a compartment, whereas injected formate was rapidly oxidized to CO2 in both compartments (Fig. 2A). It is possible that formate accumulation is an artifact caused by homogenization or by high H2 partial pressures. Also the large microbial population(s) catalyzing the H2-dependent reduction of CO2 to formate in the anterior gut region (Fig. 4B) may catalyze the observed reactions only under high H2 partial pressures and may actually represent fermenting bacteria involved in the formation of H2 and formate in situ. They are most likely also responsible for the high consumption rates of externally added H2 in the anterior gut regions observed with hydrogen microsensors (33).
Importance of reductive acetogenesis in vivo.
The metabolic rates of soil-feeding termites are much lower than those of wood-feeding species (26, 28, 30). If one bases the in vivo rates of methanogenesis and the in situ rates of homoacetogenesis on the overall electron flow during oxidation of organic matter, it becomes apparent that methanogenesis is of similar importance as a terminal electron sink in hindgut metabolism in the soil-feeding C. orthognathus as homoacetogenesis is in the wood-feeding R. flavipes; both contribute almost 10% to the overall electron flow (Table 4). Conversely, the contributions of methanogenesis in R. flavipes and of homoacetogenesis (from endogenous substrates) in C. orthognathus are hardly significant.
TABLE 4.
Comparison of the relative contributions of methanogenesis and homoacetogenesis to the overall electron flow in a soil-feeding termite and a wood-feeding termite
| Parameter | Result fora:
|
|||
|---|---|---|---|---|
|
Cubitermes orthognathus
|
Reticulitermes flavipes
|
|||
| Rate (μmol g−1 h−1) | Electron flow (%)b | Rate (μmol g−1 h−1) | Electron flow (%) | |
| Respiration rate (CO2 formation); In vivo | 3.68 (36) | 15.90 (27) | ||
| Methanogenesis; In vivo | 0.158 (33) | 8.6 | 0.116 (16) | 1.5 |
| Homoacetogenesis | ||||
| In situ | 0.018c | 1.0 | 0.833 (35) | 10.5 |
| In situ + exogenous H2 | 0.141c | 7.7 | 0.855 (35) | 10.8 |
References are given in parentheses.
Calculated from respiratory CO2 formation, assuming organic substrates with an average oxidation state of zero.
Calculated from the combined rates of the individual compartments (Table 3).
However, in contrast to R. flavipes, in which reductive acetogenesis is not strongly stimulated by exogenous H2 due to the high intestinal H2 concentration (35), the activities in C. orthognathus will strongly depend on the actual H2 partial pressures in the microniches occupied by the homoacetogens. Considering the strong possibility of a transfer of H2 or other reductants between the compartments (see above), the low endogenous rates of homoacetogenesis obtained with isolated guts probably underestimate the true in vivo rates. The endogenous rates of methanogenesis in isolated gut sections of C. orthognathus (0.047 μmol g−1 h−1) are almost four times lower than the respective in vivo rates, but surpass them when exogenous electron donors are added (33). Therefore, the in vivo rate of homoacetogenesis can also be expected to lie within the range spanned by the in situ rates in the absence and in the presence of exogenous reductants, implying that in living termites, the electron flow towards homoacetogenesis might be as high as that towards methanogenesis (Table 4). In such a scenario, homoacetogenesis would contribute as much to host nutrition in the soil-feeding species as in the wood-feeding species.
Metabolic versatility as a basis for coexistence.
Under starvation conditions, when the P3 compartment does not accumulate H2 to significant concentrations (33), homoacetogenesis in the P3/4a compartment of both Cubitermes spp. was below the detection limit, unless exogenous H2 was added. In freshly fed C. orthognathus, however, the H2 partial pressure in the P3 compartment increased considerably (33), and homoacetogenesis in the P3/4a compartment occurred at significant albeit moderate rates (Table 3). The metabolic versatility of homoacetogens, which are generally capable of utilizing a wide variety of substrates (5, 15), including many of the fermentation products found in the hindgut fluid of soil-feeding species of Termitinae (36) and methoxylated aromatic compounds derived from lignins or humic substances, might help them to maintain an active metabolism during phases of low H2 partial pressure. Also their being capable of mixotrophic growth, i.e., the simultaneous utilization of H2 and organic substrates, as demonstrated for Sporomusa termitida isolated from the wood-feeding Nasutitermes nigriceps (9), would add to their competitiveness in an environment where H2 is only temporarily available.
Unfortunately, there is only little information on the physiology of homoacetogens in soil-feeder hindguts. To date, Clostridium mayombei from the hindgut of C. speciosus (18) remains the only homoacetogen isolated from soil-feeding termites. It has been recently discovered that spirochetes isolated from the wood-feeding Zootermopsis angusticollis are homoacetogenic (21), but the metabolic properties of the spirochetal morphotypes present in the P4b section of C. umbratus (36) remain to be established. More isolates and physiological studies are urgently needed, but it is of equal importance to gain insight into the radial distribution of the homoacetogenic population(s) within the posterior hindgut compartments. In contrast to methanogenic archaea, where information on the spatial distribution can be derived already from the localization of microbial cells with F420-like autofluorescence within the respective compartments (36), the in situ identification of homoacetogens calls for specific molecular probes.
Conclusions and outlook.
Together with the results presented in the companion paper (33), it is now evident that methanogens and homoacetogens coexist within the posterior hindgut of soil-feeding Cubitermes spp. and that the importance of reductive acetogenesis to the overall electron flow might be larger than previously expected. An intercompartment transfer of H2 or formate and fluctuations of H2 partial pressures in the anterior hindgut emerge as important factors sustaining the substantial homoacetogenic populations. Our findings underline the fact that it is necessary to determine microbial activities within their environmental context in order to correctly assess the functional ecology of a microbial population. In situ rates will be affected not only by the metabolic interaction of organisms located within different microniches, but also by temporal fluctuations of metabolite concentrations. Therefore, only an exact knowledge of the spatial distribution of microbial populations and their orientation in the metabolic gradients will allow us to understand diversity and coexistence on the microscale.
ACKNOWLEDGMENTS
This study was supported by a grant of the Deutsche Forschungsgemeinschaft (DFG) within the program “Structural and Functional Analysis of Natural Microbial Communities.”
We thank Hamadi Boga, Lucie Rogo, Nixon Onyimbo, and Patrick Muthama for helping to collect the termites used in this study and Bernhard Schink for continuing support.
REFERENCES
- 1.Bignell D E. Soil-feeding and gut morphology in higher termites. In: Hunt J H, Nalepa C A, editors. Nourishment and evolution in insect societies. Boulder, Colo: Westview Press; 1994. pp. 131–158. [Google Scholar]
- 2.Bignell D E, Eggleton P. On the elevated intestinal pH of higher termites (Isoptera: Termitidae) Insectes Soc. 1995;42:57–69. [Google Scholar]
- 3.Brauman A, Kane M D, Labat M, Breznak J A. Genesis of acetate and methane by gut bacteria of nutritionally diverse termites. Science. 1992;257:1384–1387. doi: 10.1126/science.257.5075.1384. [DOI] [PubMed] [Google Scholar]
- 4.Brauman A, Labat M, Garcia J L. Preliminary studies on the gut microbiota of the soil-feeding termite: Cubitermes speciosus. In: Lésel R, editor. Microbiology in Poecilotherms. Amsterdam, The Netherlands: Elsevier; 1990. pp. 73–77. [Google Scholar]
- 5.Breznak J A. Acetogenesis from carbon dioxide in termite guts. In: Drake H L, editor. Acetogenesis. New York, N.Y: Chapman and Hall; 1994. pp. 303–330. [Google Scholar]
- 6.Breznak J A, Brune A. Role of microorganisms in the digestion of lignocellulose by termites. Annu Rev Entomol. 1994;39:453–487. [Google Scholar]
- 7.Breznak J A, Kane M D. Microbial H2/CO2 acetogenesis in animal guts: nature and nutritional significance. FEMS Microbiol Rev. 1990;87:309–314. doi: 10.1111/j.1574-6968.1990.tb04929.x. [DOI] [PubMed] [Google Scholar]
- 8.Breznak J A, Switzer J M. Acetate synthesis from H2 plus CO2 by termite gut microbes. Appl Environ Microbiol. 1986;52:623–630. doi: 10.1128/aem.52.4.623-630.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Breznak J A, Switzer Blum J. Mixotrophy in the termite gut acetogen, Sporomusa termitida. Arch Microbiol. 1991;156:105–110. [Google Scholar]
- 10.Brune A, Kühl M. pH profiles of the extremely alkaline hindguts of soil-feeding termites (Isoptera: Termitidae) determined with microelectrodes. J Insect Physiol. 1996;42:1121–1127. [Google Scholar]
- 11.Brune A. Termite guts: the world’s smallest bioreactors. Trends Biotechnol. 1998;16:16–21. [Google Scholar]
- 12.Cord-Ruwisch R, Seitz H-J, Conrad R. The capacity of hydrogenotrophic anaerobic bacteria to compete for traces of hydrogen depends on the redox potential of the electron acceptor. Arch Microbiol. 1988;149:350–357. [Google Scholar]
- 13.De Graeve K G, Grivet J P, Durand M, Beaumatin P, Cordelet C, Hannequart G, Demeyer D. Competition between reductive acetogenesis and methanogenesis in the pig large-intestinal flora. J Appl Bacteriol. 1994;76:55–61. doi: 10.1111/j.1365-2672.1994.tb04415.x. [DOI] [PubMed] [Google Scholar]
- 14.Dore J, Morvan B, Rieu-Lesme F, Goderel I, Gouet P, Pochart P. Most probable number enumeration of H2-utilizing acetogenic bacteria from the digestive tract of animals and man. FEMS Microbiol Lett. 1995;130:7–12. doi: 10.1016/0378-1097(95)00176-6. [DOI] [PubMed] [Google Scholar]
- 15.Drake H L, Daniel S L, Küsel K, Matthies C, Kuhner C, Braus-Stromeyer S. Acetogenic bacteria: what are the in situ consequences of their diverse metabolic versatilities? Biofactors. 1997;6:13–24. doi: 10.1002/biof.5520060103. [DOI] [PubMed] [Google Scholar]
- 16.Ebert A, Brune A. Hydrogen concentration profiles at the oxic-anoxic interface: a microsensor study of the hindgut of the wood-feeding lower termite Reticulitermes flavipes (Kollar) Appl Environ Microbiol. 1997;63:4039–4046. doi: 10.1128/aem.63.10.4039-4046.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hackstein J H P, van Alen T A. Fecal methanogens and vertebrate evolution. Evolution. 1996;50:559–572. doi: 10.1111/j.1558-5646.1996.tb03868.x. [DOI] [PubMed] [Google Scholar]
- 18.Kane M D, Brauman A, Breznak J A. Clostridium mayombei sp. nov., an H2/CO2 acetogenic bacterium from the gut of the African soil-feeding termite, Cubitermes speciosus. Arch Microbiol. 1991;156:99–104. [Google Scholar]
- 19.Koch A L. Growth measurement. In: Gerhardt P, Murray R G E, Costilow R N, Nester E W, Wood W A, Krieg N R, Phillips G B, editors. Manual of methods for general bacteriology. Washington, D.C.: American Society for Microbiology; 1981. pp. 179–207. [Google Scholar]
- 20.Leadbetter J R, Breznak J A. Physiological ecology of Methanobrevibacter cuticularis sp. nov. and Methanobrevibacter curvatus sp. nov., isolated from the hindgut of the termite Reticulitermes flavipes. Appl Environ Microbiol. 1996;62:3620–3631. doi: 10.1128/aem.62.10.3620-3631.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Leadbetter J R, Schmidt T M, Graber J R, Breznak J A. Acetogenesis from H2 plus CO2 by spirochetes from termite guts. Science. 1999;283:686–689. doi: 10.1126/science.283.5402.686. [DOI] [PubMed] [Google Scholar]
- 22.Le Van T D, Robinson J A, Ralph J, Greening R C, Smolenski W J, Leedle J A Z, Schaefer D M. Assessment of reductive acetogenesis with indigenous ruminal bacterium populations and Acetitomaculum ruminis. Appl Environ Microbiol. 1998;64:3429–3436. doi: 10.1128/aem.64.9.3429-3436.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Morvan B, Bonnemoy F, Fonty G, Gouet P. Quantitative determination of H2-utilizing acetogenic and sulfate-reducing bacteria and methanogenic archaea from digestive tract of different mammals. Curr Microbiol. 1996;32:129–133. doi: 10.1007/s002849900023. [DOI] [PubMed] [Google Scholar]
- 24.Noirot C. From wood- to humus-feeding: an important trend in termite evolution. In: Billen J, editor. Biology and evolution of social insects. Leuven, Belgium: Leuven University Press; 1992. pp. 107–119. [Google Scholar]
- 25.Nollet L, Demeyer D, Verstraete W. Effect of 2-bromoethanesulfonic acid and Peptostreptococcus productus ATCC 35244 addition on stimulation of reductive acetogenesis in the ruminal ecosystem by selective inhibition of methanogenesis. Appl Environ Microbiol. 1997;63:194–200. doi: 10.1128/aem.63.1.194-200.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Nunes L, Bignell D E, Lo N, Eggleton P. On the respiratory quotient (RQ) of termites (Insecta: Isoptera) J Insect Physiol. 1997;43:749–758. doi: 10.1016/s0022-1910(97)00036-x. [DOI] [PubMed] [Google Scholar]
- 27.Odelson D A, Breznak J A. Volatile fatty acid production by the hindgut microbiota of xylophagous termites. Appl Environ Microbiol. 1983;45:1602–1613. doi: 10.1128/aem.45.5.1602-1613.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Peakin G J, Josens G. Respiration and energy flow. In: Brian M V, editor. Production ecology of ants and termites. Cambridge, United Kingdom: Cambridge University Press; 1978. pp. 111–163. [Google Scholar]
- 29.Platen H, Schink B. Methanogenic degradation of acetone by an enrichment culture. Arch Microbiol. 1987;149:136–141. doi: 10.1007/BF00425079. [DOI] [PubMed] [Google Scholar]
- 30.Rouland C, Brauman A, Labat M, Lepage M. Nutritional factors affecting methane emission from termites. Chemosphere. 1993;26:617–622. [Google Scholar]
- 31.Schink B. Diversity, ecology, and isolation of acetogenic bacteria. In: Drake H L, editor. Acetogenesis. New York, N.Y: Chapman and Hall; 1994. pp. 197–235. [Google Scholar]
- 32.Schink B. Energetics of syntrophic cooperation in methanogenic degradation. Microbiol Mol Biol Rev. 1997;61:262–280. doi: 10.1128/mmbr.61.2.262-280.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Schmitt-Wagner D, Brune A. Hydrogen profiles and localization of methanogenic activities in the highly compartmentalized hindgut of soil-feeding higher termites (Cubitermes spp.) Appl Environ Microbiol. 1999;65:4490–4496. doi: 10.1128/aem.65.10.4490-4496.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Stams A J M. Metabolic interactions between anaerobic bacteria in methanogenic environments. Antonie Leeuwenhoek. 1994;66:271–294. doi: 10.1007/BF00871644. [DOI] [PubMed] [Google Scholar]
- 35.Tholen, A., and A. Brune. Impact of oxygen on metabolic fluxes and in situ rates of reductive acetogenesis in the hindgut of the wood-feeding termite Reticulitermes flavipes, determined by microinjection of radiotracers. Submitted for publication. [DOI] [PubMed]
- 36.Tholen, A., and A. Brune. Unpublished data.
- 37.Tholen A, Schink B, Brune A. The gut microflora of Reticulitermes flavipes, its relation to oxygen, and evidence for oxygen-dependent acetogenesis by the most abundant Enterococcus sp. FEMS Microbiol Ecol. 1997;24:137–149. [Google Scholar]
- 38.Williams C M, Veivers P C, Slaytor M, Cleland S V. Atmospheric carbon dioxide and acetogenesis in the termite Nasutitermes walkeri (Hill) Comp Biochem Physiol. 1994;107A:113–118. [Google Scholar]
- 39.Wolin M J, Miller T L. Acetogenesis from CO2 in the human colonic ecosystem. In: Drake H L, editor. Acetogenesis. New York, N.Y: Chapman and Hall; 1994. pp. 365–385. [Google Scholar]
- 40.Wood T G, Johnson R A. The biology, physiology and ecology of termites. In: Vinson S B, editor. Economic impact and control of social insects. New York, N.Y: Praeger; 1986. pp. 1–68. [Google Scholar]