Abstract
The diagnostic utility of neuroradiologic signs associated with idiopathic intracranial hypertension (IIH) for the evaluation of patients presenting with papilloedema remains yet to be elucidated. This multicentre retrospective cohort study assessed consecutive patients presenting with suspected papilloedema to Auckland District Health Board (NZ) and Stanford University Medical Centre (US), between 2005 to 2019, undergoing magnetic resonance imaging and venography (MRI/MRV) or computed tomography and venography (CT/CTV) prior to lumbar puncture assessment for diagnostic suspicion of IIH. Data were collected regarding demographic, clinical, radiologic, and lumbar puncture parameters, and the diagnosis of IIH was determined according to the Friedman criteria for primary pseudotumor cerebri syndrome. A total of 204 participants (174 females; mean±SD age 29.9±12.2 years) were included, and 156 (76.5%) participants fulfilled the diagnostic criteria for IIH. The presence of any IIH-associated radiologic sign on MRI/MRV demonstrated a sensitivity (95% CI) of 74.8% (65.8%−82.0%) and specificity (95% CI) of 94.7% (82.7%−98.5%), while radiologic signs on CT/CTV exhibited a sensitivity (95% CI) of 61.0% (49.9%−71.2%) and specificity (95% CI) of 100.0% (83.2%−100.0%). In summary, the modest sensitivities of radiologic signs of IIH would support the routine use of lumbar puncture assessment following neuroimaging to secure the diagnosis. However, the high specificities might lend limited support for the judicious deferment of lumbar puncture assessment among typical IIH demographic patients who consent to the inherent small risk of missed pathology, which has been proposed by some clinicians.
Keywords: Intracranial hypertension, pseudotumor cerebri, diagnosis, radiology, imaging, computed tomography, magnetic resonance imaging
INTRODUCTION
Idiopathic intracranial hypertension (IIH) is a complex neuro-ophthalmic condition, characterised by increased intracranial pressure in the absence of an identifiable causative factor, such as an intracranial tumour or other central nervous system disease.[1] The primary symptoms of IIH include headache, nausea and vomiting, pulsatile tinnitus, vision loss, and diplopia,[2, 3] which are recognised to have profound impacts on quality of life and visual function.[4–6] The condition demonstrates a striking predilection towards obese women of reproductive age,[7, 8] and the population incidence has been projected to rise with the growing prevalence of obesity globally.[8, 9]
According to the updated Friedman (2013) criteria for primary pseudotumor cerebri syndrome, the diagnosis of IIH requires the presence of papilloedema; the exclusion of hydrocephalus, intracranial mass, structural and vascular lesions on neuroimaging; normal cerebrospinal fluid (CSF) composition and elevated opening pressure on lumbar puncture; and the exclusion of other treatable etiological causes.[1] Gadolinium-enhanced magnetic resonance imaging and venography (MRI/MRV) is typically recommended as the neuroimaging modality of choice to minimise radiation exposure, although contrast-enhanced computed tomography and venography (CT/CTV) can also be used as an alternative when MRI/MRV is unavailable.[1]
In recent decades, there has been growing recognition of a constellation of neuroradiologic signs associated with IIH, including empty sella, posterior scleral flattening, optic nerve sheath distension, transverse venous sinus stenosis, and cerebellar tonsillar herniation.[10–14] A number of case-control studies comparing patients diagnosed with IIH and healthy control subjects have demonstrated potential diagnostic utility of neuroradiologic signs.[10, 11, 14–27] Recent diagnostic criteria have included neuroradiologic signs to support the diagnosis of IIH in patients with lumbar puncture opening pressures below the cut-off.[1, 28] However, the discriminative performance reported in earlier case-control studies might be overestimated secondary to selection bias of control subjects, and the studies were often limited to single centre settings.[10, 11] Furthermore, the diagnostic utility of neuroradiologic signs for the evaluation of patients presenting with bilateral papilloedema remains yet to be elucidated, and is a common clinical situation encountered by ophthalmologists, neurologists, and emergency physicians. The purpose of this multicentre retrospective cohort study was therefore to assess the diagnostic performance of neuroradiologic signs among patients with suspected IIH presenting with papilloedema.
METHODS
Patients
This multicentre retrospective cohort study followed the tenets of the Declaration of Helsinki and was approved by the two institutional review boards. A waiver of informed consent was granted due to the retrospective non-interventional study design. Consecutive patients presenting with bilateral optic nerve head appearances concerning for papilloedema, between June 2005 to March 2017 at Greenlane Clinical Centre, Auckland District Health Board, Auckland, New Zealand, and between March 2010 to July 2019 at Stanford University Medical Centre, Palo Alto, California, United States, were screened for inclusion from electronic and written medical records. Eligibility criteria required patients to have undergone a complete dilated ophthalmic assessment by a general or neuro-ophthalmologist. Patients were also required have undergone gadolinium-enhanced MRI/MRV or contrast-enhanced CT/CTV prior to lumbar puncture CSF analysis and opening pressure assessment for diagnostic suspicion of IIH. Exclusion criteria included previous established diagnosis of IIH or raised intracranial pressure; previous optic nerve sheath fenestration, CSF diversion, or venous sinus stenting procedures; previous history of head trauma, congenital cranial malformation, or subarachnoid haemorrhage; previous established diagnosis of underlying optic nerve or retinal disease; and previous history of malignancy. Patients who had symptoms of meningitis, including fever and nuchal rigidity, were also excluded. None of the subjects underwent any clinical interventions between neuroimaging and lumbar puncture.
Measurements
Clinical records were reviewed to extract data on demographic and clinical characteristics including age, sex, body mass index, and lumbar puncture opening pressure, as well as the presence of neuroradiologic signs associated with IIH, including optic nerve sheath distension, posterior scleral flattening, transverse venous sinus stenosis, empty or partially empty sella, increased optic nerve sheath tortuosity, caudal descent of cerebellar tonsils, and protrusion of the optic nerve head.[10–14] Gadolinium-enhanced MRI/MRV were completed with 1.5T (1.5T SIGNA, Chicago, US) or 3T MRI scanners (3T Siemens Skyra, Munich, Germany) with standard head coils and a routine protocol including sagittal T1, axial T2, FLAIR, and susceptibility weighted sequences. Contrast-enhanced CT/CTV were obtained using 128-slice CT scanners (Siemens Definition FLASH, Munich, Germany or Phillips Brilliance iCT, Best, Netherlands). All MRI/MRV and CT/CTV imaging were reviewed by experienced consultant radiologists that were not blinded to the clinical indication for neuroimaging to generate clinical radiology reports from which the presence of the signs was abstracted.
The diagnosis of IIH was determined according to the updated Friedman (2013) criteria for primary pseudotumor cerebri syndrome,[1] which requires the presence of papilloedema; normal neurological examination except for cranial nerve abnormalities; normal brain parenchyma on neuroimaging without hydrocephalus, mass, structural lesions or meningeal enhancement; normal CSF composition; an elevated lumbar puncture opening pressure of ≥25 cm CSF in adults, and ≥28 cm CSF in children; and the exclusion of other treatable etiological causes.
Statistics
Statistical analysis was performed using R version 4.0.2 (R Foundation, Vienna, Austria). Inter-group comparisons of continuous variables were performed using the independent samples t-test following confirmation with normality testing (Shapiro-Wilk p>0.05), and categorical data analysed using Fisher’s exact test. Diagnostic accuracy values for neuroradiologic signs in classifying IIH were evaluated, and confidence intervals about proportions were calculated using the Wilson score method. All tests were two-tailed, and p<0.05 was considered significant.
RESULTS
Participant flow is illustrated in Figure 1, and demographic and clinical characteristics summarised in Table 1. A total of 204 participants (30 males and 174 females), with a mean ± SD age of 29.9 ± 12.2 years, met the eligibility criteria for study inclusion. 156 (76.5%) participants fulfilled the diagnostic criteria for IIH, and 48 (23.5%) participants did not. Participants with IIH were younger (27.8 ± 9.6 versus 36.5 ± 16.5 years, p<0.001) and had higher body mass index than those that did not (36.1 ± 8.3 versus 29.7 ± 7.5 kg/m2, p<0.001). The diagnoses of participants without IIH are summarised in Figure 1, and included 27 patients with pseudopapilloedema, 9 patients with non-idiopathic causes for intracranial hypertension, 7 patients with migraine headache, and 5 patients with other causes of optic nerve oedema.
Figure 1:
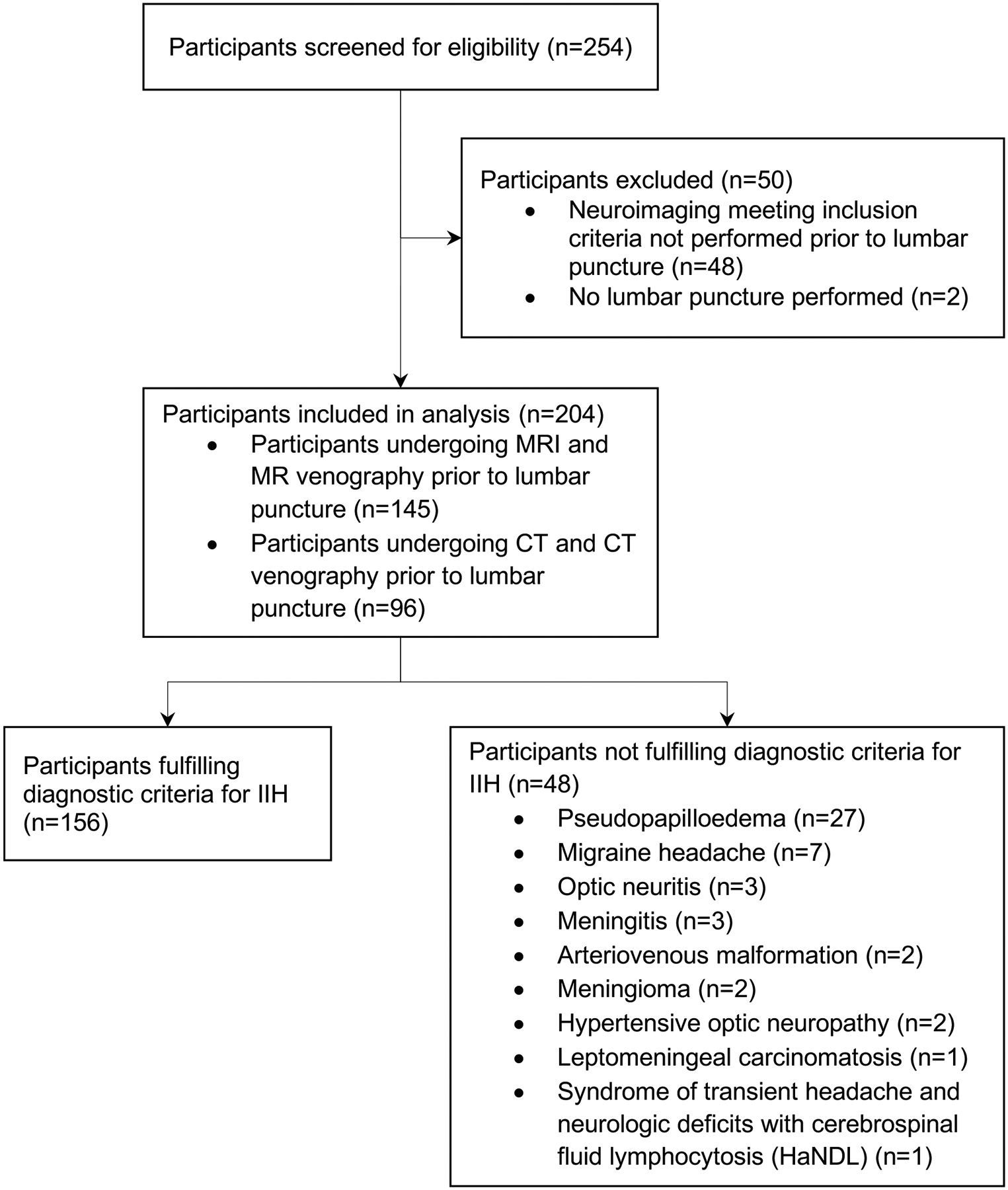
Study participant flow.
Table 1:
Demographic and clinical characteristics of patients.
| Characteristic | Total (n=204) |
Idiopathic intracranial hypertension | ||
|---|---|---|---|---|
| Present (n=156) |
Absent (n=48) |
p-value | ||
| Age (years) | 29.9 ± 12.2 | 27.8 ± 9.6 | 36.5 ± 16.5 | <0.001 |
| Male sex | 30 (14.7%) | 19 (12.2%) | 11 (22.9%) | 0.10 |
| Body mass index (kg/m2) | 34.5 ± 8.6 | 36.1 ± 8.3 | 29.7 ± 7.5 | <0.001 |
| MRI and MR venography performed | 145 (71.1%) | 107 (68.6%) | 38 (79.2%) | 0.20 |
| CT and CT venography performed | 96 (47.1%) | 77 (49.4%) | 19 (39.6%) | 0.25 |
| Lumbar puncture opening pressure (cmCSF) | 34.2 ± 10.5 | 37.6 ± 8.1 | 23.5 ± 10.0 | <0.001 |
Diagnostic accuracy values of neuroradiologic signs for detecting IIH are presented in Table 2. Overall, the presence of any radiologic sign on MRI/MRV demonstrated a sensitivity (95% CI) of 74.8% (65.8%−82.0%) and specificity (95% CI) of 94.7% (82.7%−98.5%), while the presence of any radiologic sign on CT/CTV demonstrated a sensitivity (95% CI) of 61.0% (49.9%−71.2%) and specificity (95% CI) of 100.0% (49.9%−71.2%). Individual radiologic signs of IIH on MRI/MRV and CT/CTV had sensitivities ranging from 0.9–74.8% and specificities ranging from 94.7% to 100.0%. Transverse venous sinus stenosis was the single radiologic feature demonstrating the highest sensitivity value for both neuroimaging modalities, with a sensitivity (95% CI) of 53.3% (43.9%−62.4%) for MRI/MRV, and a sensitivity (95% CI) of 45.5% (34.8%−56.5%) for CT/CTV.
Table 2:
Diagnostic accuracy values of radiologic signs in detecting the presence of idiopathic intracranial hypertension.
| Radiologic sign | MRI and MR venography (n=145) IIH present (n=107); absent (n=38) |
CT and CT venography (n=96) IIH present (n=77); absent (n=19) |
||||||
|---|---|---|---|---|---|---|---|---|
| Sensitivity (95% CI) | Specificity (95% CI) | Positive likelihood ratio (95% CI) | Negative likelihood ratio (95% CI) | Sensitivity (95% CI) | Specificity (95% CI) | Positive likelihood ratio (95% CI) | Negative likelihood ratio (95% CI) | |
| Any radiologic feature of IIH | 74.8% (65.8%−82.0%) |
94.7% (82.7%−98.5%) |
14.21 (3.67–54.99) |
0.27 (0.19–0.37) |
61.0% (49.9%−71.2%) |
100.0% (83.2%−100.0%) |
- | 0.39 (0.29–0.52) |
| Optic nerve sheath distension | 31.8% (23.7%−41.1%) |
94.7% (82.7%−98.5%) |
6.04 (1.52–23.93) |
0.72 (0.62–0.84) |
16.9% (10.1%−26.8%) |
100.0% (83.2%−100.0%) |
- | 0.83 (0.75–0.92) |
| Posterior scleral flattening | 24.3% (17.2%−33.3%) |
100.0% (90.8%−100.0%) |
- | 0.76 (0.68–0.84) |
6.5% (2.8%−14.3%) |
100.0% (83.2%−100.0%) |
- | 0.94 (0.88–0.99) |
| Transverse venous sinus stenosis | 53.3% (43.9%−62.4%) |
97.4% (86.5%−99.5%) |
20.24 (2.90–141.17) |
0.48 (0.39–0.59) |
45.5% (34.8%−56.5%) |
100.0% (83.2%−100.0%) |
- | 0.55 (0.44–0.67) |
| Empty or partially empty sella | 29.9% (22.1%−39.2%) |
97.4% (86.5%−99.5%) |
11.36 (1.61–80.33) |
0.72 (0.63–0.82) |
40.3% (30.0%−51.4%) |
100.0% (83.2%−100.0%) |
- | 0.60 (0.50–0.72) |
| Increased tortuosity of optic nerve sheath | 5.6% (2.6%−11.7%) |
100.0% (90.8%−100.0%) |
- | 0.94 (0.90–0.99) |
2.6% (0.7%−9.0%) |
100.0% (83.2%−100.0%) |
- | 0.97 (0.94–1.01) |
| Caudal descent of cerebellar tonsils | 3.7% (1.5%−9.2%) |
100.0% (90.8%−100.0%) |
- | 0.96 (0.93–1.00) |
3.9% (0.8%−11.0%) |
100.0% (83.2%−100.0%) |
- | 0.96 (0.92–1.01) |
| Protrusion of optic nerve head | 3.7% (1.5%−9.2%) |
97.4% (86.5%−99.5%) |
1.42 (0.16–12.32) |
0.99 (0.93–1.05) |
2.6% (0.7%−9.0%) |
100.0% (83.2%−100.0%) |
- | 0.97 (0.94–1.01) |
False positive neuroimaging signs were detected in 2 patients (both female, aged 36 and 44 years, Table 3), including 1 patient diagnosed with meningitis, and 1 patient with leptomeningeal carcinomatosis. Both patients had elevated lumbar puncture opening pressure, but had no previous history of malignancy, and exhibited no symptoms of meningitis, such as fever or nuchal rigidity.
Table 3:
Summary of cases with false positive neuroimaging findings.
| Age range | Sex | Body mass index (kg/m2) | Neuroimaging features | Lumbar puncture opening pressure (cm CSF) | Diagnosis |
|---|---|---|---|---|---|
| 30s | Female | 27.5 | Transverse sinus narrowing, optic nerve sheath distension, empty sella, protrusion of optic nerve head on MRI and MR venography | 34 | Meningitis |
| 40s | Female | 46.3 | Optic nerve sheath distension on MRI and MR venography. | 30 | Leptomeningeal carcinomatosis |
False negative neuroimaging signs were detected in 27 patients undergoing MRI/MRV, and 30 patients undergoing CT/CTV (Table 4). For both neuroimaging modalities, there were no significant differences in age, sex, body mass index, and lumbar puncture opening pressure between false negative and true positive cases (all p>0.30).
Table 4:
Comparison of cases with false negative and true positive neuroimaging findings.
| MRI and MR venography | CT and CT venography | |||||
|---|---|---|---|---|---|---|
| True positive cases (n=80) |
False negative cases (n=27) |
p-value | True positive cases (n=47) |
False negative cases (n=30) |
p-value | |
| Age (years) | 28.6 ± 9.7 | 26.4 ± 11.3 | 0.33 | 28.2 ± 10.0 | 28.1 ± 10.2 | 0.97 |
| Male sex | 11 (13.8%) | 6 (22.2%) | 0.36 | 3 (6.4%) | 2 (6.7%) | >0.99 |
| Body mass index (kg/m2) | 35.7 ± 8.9 | 35.2 ± 6.6 | 0.79 | 36.5 ± 9.3 | 36.4 ± 8.0 | 0.96 |
| Lumbar puncture opening pressure (cmCSF) | 37.4 ± 8.1 | 36.9 ± 7.7 | 0.76 | 38.8 ± 8.8 | 38.2 ±7.8 | 0.76 |
DISCUSSION
The findings of this multicentre retrospective cohort study clarified the diagnostic potential of neuroradiologic signs in IIH suspects presenting with papilloedema, which is the patient population most commonly referred to the neuro-ophthalmic clinic. Neuroradiologic signs of IIH detected on MRI/MRV and CT/CTV exhibited high diagnostic specificity values of 95% or higher, but only modest sensitivities of 75% or lower. Furthermore, there were no significant differences in the overall discriminative performance between the two neuroimaging modalities, MRI/MRV and CT/CTV.
The high specificity values of neuroimaging signs in both modalities in the current study are consistent with the trends highlighted in previous reports,[10, 11, 14–27] and reinforce that neuroimaging signs support a diagnosis of IIH in a patient with papilloedema. In the current cohort, the presence of any radiologic signs on MRI/MRV demonstrated a diagnostic specificity of 94.7%, while the detection of any radiologic features on CT/CTV exhibited a specificity of 100.0%. Specificity values of individual radiologic signs on MRI/MRV and CT/CTV for diagnosing IIH also ranged between 94.7 to 100.0%. However, it is noted that the two false positive cases had elevated lumbar puncture opening pressure, and thus the specificities for the detection of raised intracranial pressure would be even higher. The two patients had no previous history of malignancy, and exhibited no symptoms of meningitis, such as fever or nuchal rigidity. In both cases, a provisional diagnosis of IIH based on neuroimaging features and lumbar puncture opening pressure assessment, prior to obtaining the results of CSF analysis, caused some degree of delay in initiating medically appropriate treatment.
The specificity values reported in the current study were comparable with a recent meta-analysis of 21 case-control studies incorporating 724 patients, which reported that individual radiologic signs on MRI/MRV demonstrated pooled specificity values ranging from 84.0 to 97.1%.[10] Another pooled analysis of 14 case-control studies also reported that individual radiologic signs on MRI/MRV or CT/CTV demonstrated pooled specificity values ranging from 83 to 95%.[11] It is possible that the slightly higher specificity values observed in the current cohort might have been partially attributed to the study inclusion criteria, which required the presence of papilloedema with suspicion for IIH, and would make the study conclusions particularly relevant to the clinical management of patients typically referred to the neuro-ophthalmic clinic for diagnostic workup for IIH. These findings are similar to those reported in earlier case-control studies which recruited healthy controls as a comparison group to patients diagnosed with IIH,[10, 11] and complementary to a recent study of patients undergoing MRI for any reason which did not support diagnostic utility of MRI signs of raised intracranial pressure in this broader population.[29]
In agreement with the results reported in previous case-control studies,[10, 11, 14–27] the sensitivity values of radiologic signs in both neuroimaging modalities were relatively modest in the current cohort, and reinforce that the absence of neuroimaging signs does not exclude a diagnosis of IIH. The presence of any radiologic signs on MRI/MRV demonstrated a sensitivity of 74.8%, while the detection of any radiologic features on CT/CTV exhibited a sensitivity of 61.0%. Sensitivity values of individual radiologic signs of IIH on MRI/MRV and CT/CTV, also ranged between 0.9 to 53.3%. These trends are comparable with those highlighted in two earlier meta-analyses, which report that individual radiologic features on MRI/MRV or CT/CTV demonstrated pooled sensitivity values ranging from 6.1 to 84.4%.[10, 11] It is acknowledged that the lower sensitivity values of some of the individual neuroimaging signs may potentially be related to underreporting by radiologists in diagnostic study reports, particularly in time-pressured clinical contexts. In the current cohort, transverse venous sinus stenosis was the single radiologic feature demonstrating the highest sensitivities for both neuroimaging modalities, which is also consistent with the findings reported in previous case-control studies.[10, 11, 16, 19]
Overall, the modest sensitivity values of 75% or less observed in the current study would reaffirm that the absence of radiologic features on neuroimaging is insufficient to exclude out a diagnosis of IIH. This supports the routine requirement for lumbar puncture opening pressure assessment and CSF analysis in patients with absent neuroradiologic signs, consistent with the updated Friedman (2013) criteria for primary pseudotumor cerebri syndrome and other IIH diagnostic criteria.[1] Though the specificities of neuroimaging signs were high, there were 2 (1.0%) cases of false positive neuroradiologic signs, both of which had alternative diagnoses necessitating different treatment that were identified on the basis of abnormal CSF analysis following lumbar puncture. This supports the routine requirement for lumbar puncture opening pressure assessment and CSF analysis in patients with radiographic signs of IIH. However, the high specificities of 95% or more might lend limited support for the judicious deferment of lumbar puncture assessment among typical IIH demographic patients who consent to the inherent small risk of missed pathology, in settings where the condition is responsive to medical treatment, as proposed by a subset of clinicians.[30–32]
The results of this study also showed that the overall discriminative performance of radiologic signs detected on MRI/MRV and CT/CTV were similar. These findings would support the recommendations of the updated Friedman (2013) criteria, which suggest the use of CT/CTV as an alternative neuroimaging modality when MRI/MRV are unavailable.[1] However, it is noted that the conduct of non-invasive venography did not differ significantly between the two neuroimaging modalities in the current study. The most sensitive radiologic sign on CT/CTV was transverse sinus stenosis, which highlights the importance of ensuring the incorporation of non-invasive venography when selecting computed tomography as a substitute modality for magnetic resonance imaging.[1]
This study is not without limitations. It is acknowledged that the retrospective design and the inclusion criteria requiring the presence of papilloedema with diagnostic suspicion for IIH may introduce selection bias. However, the inclusion criteria of the current study were intended to facilitate investigation of the diagnostic utility of neuroimaging among patients with suspected IIH presenting with papilloedema, in contrast to previous case-control studies that compared patients diagnosed with IIH and healthy control subjects.[10, 11] In addition, it is acknowledged that the findings of the current study might not be generalisable to atypical presentations of IIH, including cases of abducens nerve palsy in the absence of papilloedema. The assessing radiologists were not blinded to the clinical indication for neuroimaging, and the potential for this to lead to overcalling of certain radiologic signs cannot be excluded. It is also possible that not all neuroimaging signs were comprehensively evaluated, secondary to the time constraints associated with radiology reporting in the clinical setting. Nevertheless, the study findings are clinically relevant from the standpoint of the diagnosing clinician making management decisions on the basis of radiology reports alone.
In conclusion, the modest sensitivities of radiologic signs of IIH would support the routine requirement for lumbar puncture assessment following neuroimaging. However, the high specificities might lend limited support to the proposals from a subset of clinicians for judicious deferment of lumbar puncture assessment among typical IIH demographic patients who consent to the inherent small risk of missed pathology, in settings where the condition is responsive to medical treatment.
HIGHLIGHTS.
Multicentre retrospective analysis of 204 patients with suspected papilloedema.
156 participants fulfilled the Friedman criteria for primary pseudotumor cerebri.
MRI/MRV signs exhibited a sensitivity of 74.8% and specificity of 94.7% for IIH.
CT/CTV signs exhibited a sensitivity of 61.0% and specificity of 100.0% for IIH.
Funding statement:
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors. WX was supported by a summer student scholarship from the University of Auckland. HM received support from NIH P30 026877 and an unrestricted grant from Research to Prevent Blindness. The funding sources had no role in study design; the collection, analysis and interpretation of data; the writing of the report; or the decision to submit the article for publication.
Footnotes
Declarations of interest: None.
REFERENCES
- [1].Friedman DI, Liu GT, Digre KB. Revised diagnostic criteria for the pseudotumor cerebri syndrome in adults and children. Neurology. 2013;81:1159–65. [DOI] [PubMed] [Google Scholar]
- [2].Wall M, Kupersmith MJ, Kieburtz KD, Corbett JJ, Feldon SE, Friedman DI, et al. The idiopathic intracranial hypertension treatment trial: clinical profile at baseline. JAMA Neurol. 2014;71:693–701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].D DA, Curone M, Ciasca P, Cammarata G, Melzi L, Bussone G, et al. Headache prevalence and clinical features in patients with idiopathic intracranial hypertension (IIH). Neurol Sci. 2013;34 Suppl 1:S147–9. [DOI] [PubMed] [Google Scholar]
- [4].Digre KB, Bruce BB, McDermott MP, Galetta KM, Balcer LJ, Wall M. Quality of life in idiopathic intracranial hypertension at diagnosis: IIH Treatment Trial results. Neurology. 2015;84:2449–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Mulla Y, Markey KA, Woolley RL, Patel S, Mollan SP, Sinclair AJ. Headache determines quality of life in idiopathic intracranial hypertension. J Headache Pain. 2015;16:521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Bruce BB, Preechawat P, Newman NJ, Lynn MJ, Biousse V. Racial differences in idiopathic intracranial hypertension. Neurology. 2008;70:861–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Adderley NJ, Subramanian A, Nirantharakumar K, Yiangou A, Gokhale KM, Mollan SP, et al. Association Between Idiopathic Intracranial Hypertension and Risk of Cardiovascular Diseases in Women in the United Kingdom. JAMA Neurol. 2019;76:1088–98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Mollan SP, Aguiar M, Evison F, Frew E, Sinclair AJ. The expanding burden of idiopathic intracranial hypertension. Eye (Lond). 2019;33:478–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Kilgore KP, Lee MS, Leavitt JA, Mokri B, Hodge DO, Frank RD, et al. Re-evaluating the Incidence of Idiopathic Intracranial Hypertension in an Era of Increasing Obesity. Ophthalmology. 2017;124:697–700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Kwee RM, Kwee TC. Systematic review and meta-analysis of MRI signs for diagnosis of idiopathic intracranial hypertension. Eur J Radiol. 2019;116:106–15. [DOI] [PubMed] [Google Scholar]
- [11].Bidot S, Saindane AM, Peragallo JH, Bruce BB, Newman NJ, Biousse V. Brain Imaging in Idiopathic Intracranial Hypertension. J Neuroophthalmol. 2015;35:400–11. [DOI] [PubMed] [Google Scholar]
- [12].Lublinsky S, Kesler A, Friedman A, Horev A, Shelef I. Quantifying response to intracranial pressure normalization in idiopathic intracranial hypertension via dynamic neuroimaging. J Magn Reson Imaging. 2018;47:913–27. [DOI] [PubMed] [Google Scholar]
- [13].Hoffmann J, Huppertz HJ, Schmidt C, Kunte H, Harms L, Klingebiel R, et al. Morphometric and volumetric MRI changes in idiopathic intracranial hypertension. Cephalalgia. 2013;33:1075–84. [DOI] [PubMed] [Google Scholar]
- [14].Brodsky MC, Vaphiades M. Magnetic resonance imaging in pseudotumor cerebri. Ophthalmology. 1998;105:1686–93. [DOI] [PubMed] [Google Scholar]
- [15].Delen F, Peker E, Onay M, Altay ÇM, Tekeli O, Togay Işıkay C. The Significance and Reliability of Imaging Findings in Pseudotumor Cerebri. Neuroophthalmology. 2019;43:81–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Morris PP, Black DF, Port J, Campeau N. Transverse Sinus Stenosis Is the Most Sensitive MR Imaging Correlate of Idiopathic Intracranial Hypertension. AJNR Am J Neuroradiol. 2017;38:471–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Pellerin A, Aguilar Garcia J, David A, Meyer J, Guyomarch Delasalle B, De Gaalon S, et al. A quantitative and semi-automatic measurement of transverse sinus stenosis improves idiopathic intracranial hypertension diagnostic accuracy. J Neuroradiol. 2018;45:329–32. [DOI] [PubMed] [Google Scholar]
- [18].Carvalho GB, Matas SL, Idagawa MH, Tibana LA, de Carvalho RS, Silva ML, et al. A new index for the assessment of transverse sinus stenosis for diagnosing idiopathic intracranial hypertension. J Neurointerv Surg. 2017;9:173–7. [DOI] [PubMed] [Google Scholar]
- [19].Maralani PJ, Hassanlou M, Torres C, Chakraborty S, Kingstone M, Patel V, et al. Accuracy of brain imaging in the diagnosis of idiopathic intracranial hypertension. Clin Radiol. 2012;67:656–63. [DOI] [PubMed] [Google Scholar]
- [20].Ibrahim YA, Mironov O, Deif A, Mangla R, Almast J. Idiopathic Intracranial Hypertension: Diagnostic Accuracy of the Transverse Dural Venous Sinus Attenuation on CT Scans. Neuroradiol J. 2014;27:665–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Agid R, Farb RI, Willinsky RA, Mikulis DJ, Tomlinson G. Idiopathic intracranial hypertension: the validity of cross-sectional neuroimaging signs. Neuroradiology. 2006;48:521–7. [DOI] [PubMed] [Google Scholar]
- [22].Butros SR, Goncalves LF, Thompson D, Agarwal A, Lee HK. Imaging features of idiopathic intracranial hypertension, including a new finding: widening of the foramen ovale. Acta Radiol. 2012;53:682–8. [DOI] [PubMed] [Google Scholar]
- [23].Lim MJ, Pushparajah K, Jan W, Calver D, Lin JP. Magnetic resonance imaging changes in idiopathic intracranial hypertension in children. J Child Neurol. 2010;25:294–9. [DOI] [PubMed] [Google Scholar]
- [24].Görkem SB, Doğanay S, Canpolat M, Koc G, Dogan MS, Per H, et al. MR imaging findings in children with pseudotumor cerebri and comparison with healthy controls. Childs Nerv Syst. 2015;31:373–80. [DOI] [PubMed] [Google Scholar]
- [25].Ranganathan S, Lee SH, Checkver A, Sklar E, Lam BL, Danton GH, et al. Magnetic resonance imaging finding of empty sella in obesity related idiopathic intracranial hypertension is associated with enlarged sella turcica. Neuroradiology. 2013;55:955–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Aiken AH, Hoots JA, Saindane AM, Hudgins PA. Incidence of cerebellar tonsillar ectopia in idiopathic intracranial hypertension: a mimic of the Chiari I malformation. AJNR Am J Neuroradiol. 2012;33:1901–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Farb RI, Vanek I, Scott JN, Mikulis DJ, Willinsky RA, Tomlinson G, et al. Idiopathic intracranial hypertension: the prevalence and morphology of sinovenous stenosis. Neurology. 2003;60:1418–24. [DOI] [PubMed] [Google Scholar]
- [28].Mollan SP, Davies B, Silver NC, Shaw S, Mallucci CL, Wakerley BR, et al. Idiopathic intracranial hypertension: consensus guidelines on management. J Neurol Neurosurg Psychiatry. 2018;89:1088–100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].Chen BS, Meyer BI, Saindane AM, Bruce BB, Newman NJ, Biousse V. Prevalence of Incidentally Detected Signs of Intracranial Hypertension on Magnetic Resonance Imaging and Their Association With Papilledema. JAMA Neurol. 2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Vosoughi AR, Margolin E, Micieli JA. Can Lumbar Puncture Be Safely Deferred in Patients With Mild Presumed Idiopathic Intracranial Hypertension? Poster presented at: North American Neuro-Ophthalmology Society Annual Meeting. February 20–23, 2021. [DOI] [PubMed] [Google Scholar]
- [31].Margolis MS, DeBusk AA, Moster ML, Falardeau JM, Eggenberger ER, Sergott RC, et al. Lumbar Puncture for Diagnosis of Idiopathic Intracranial Hypertension in Typical Patients. J Neuroophthalmol. 2021;41:375–8. [DOI] [PubMed] [Google Scholar]
- [32].Moss HE, Margolin EA, Lee AG, Van Stavern GP. Should Lumbar Puncture Be Required to Diagnose Every Patient With Idiopathic Intracranial Hypertension? J Neuroophthalmol. 2021;41:379–84. [DOI] [PMC free article] [PubMed] [Google Scholar]


