Abstract
Background
It is currently unknown whether early B cell reconstitution (EBR) in MS patients under rituximab is associated with a risk of relapse or progression.
Objectives
Analyzing EBR in rituximab-treated patients and its putative association with clinical findings.
Methods
Prospective lymphocytes immunophenotyping was performed in a monocentric cohort of MS patients treated by rituximab for 2 years. EBR was defined when B cells concentration was > 5 cells/mm3. B cell subsets were retrospectively associated with clinical data. Clinical and radiological monitoring included relapses, EDSS (Expanded Disability Status Scale), SDMT (Symbol Digit Modalities Test), and MRI.
Results
182 patients were analyzed (61 remitting-relapsing and 121 progressive-active). 38.5% experienced EBR at least once, but very few (7/182) showed systematic reconstitution. Most patients remained stable upon treatment, regardless of the occurrence of EBR. Dynamics of B cell reconstitution featured increased naïve/transitional B cells, and decreased memory subsets. Homeostasis of the B cell compartment differed at baseline between patients experiencing or not EBR upon treatment. In patients with EBR, reciprocal dynamics of transitional and pro-inflammatory double-negative B cell subsets was associated with better response to rituximab treatment.
Conclusion
EBR is common in rituximab-treated MS patients and is not associated with clinical disease activity. EBR in the peripheral blood may reflect regulatory immunological phenomena in subgroup of patients.
Keywords: Multiple sclerosis, Rituximab, B cells, Immunophenotyping, Monitoring-B cell reconstitution
Introduction
Multiple sclerosis (MS) is the most frequent chronic, inflammatory, and demyelinating disease of the central nervous system (CNS). This pathology is believed to be of autoimmune origin, and was considered for a long time as mainly, maybe exclusively, T cell mediated [1]. However, since the 1990s, many published data have underlined the importance of B cells in MS pathophysiology [2].
Those works led to the use of B cell-depleting treatments in MS [3]. Anti-CD20 biotherapies such as rituximab and, more recently, ocrelizumab and ofatumumab, were successfully tested in MS. Rituximab is a chimeric monoclonal antibody targeting the CD20 molecule expressed by B cells from the pre-B to the plasmablast stage [4]. It induces B cell death by apoptosis induction, antibody-dependent cell cytotoxicity (ADCC), and/or complement-dependent cytotoxicity (CDC) [5]. Anti-CD20 therapies showed efficacy in relapsing–remitting MS (RRMS) and progressive MS when there is signs of inflammatory activity (progressive active MS–PAMS) [3, 6, 7].
The depleting effect of rituximab, leading to a total loss of circulating B cells, is sustained for at least 6 to 8 months in rheumatoid arthritis (RA), but can last up to 26 months in ANCA-associated vasculitis [8, 9]. At the end of rituximab treatment, B cell reconstitution progresses from immature, transitional B cells, followed by mature naïve B cells, but memory (CD27+) subsets remain at low levels for extended period of times [10, 11]. Interestingly, early reappearance of memory B cells is associated with an increased risk of relapse in RA, ANCA-associated vasculitis or myasthenia gravis [9, 12, 13]. In inflammatory diseases of the CNS, such as MS or neuromyelitis optical spectrum disorders (NMO-SD), rituximab is used as a long-term maintenance regimen, meaning that B cell depletion is sustained without possibility for reconstitution of this cellular compartment. However, early B cell repopulation (EBR) may be observed in several patients. In NMO-SD, EBR, and more specifically early memory B cells reconstitution, is associated with an increased risk of relapse [14, 15].
The occurrence of EBR has not been deeply studied in the context of rituximab treatment for MS. Thus, we undertook this work to study this biological phenomenon in MS and its putative impact on the clinical course.
Materials and methods
Patients, clinical and radiological data
A retrospective study was performed at the University Hospital of Toulouse. All MS patients having initiated rituximab and having received at least two cycles of infusion between January 1st, 2016, and June 30th, 2019, were included. All of them fulfilled McDonald 2010 MS diagnostic criterion [16] in effect before 2018, and McDonald 2017 MS diagnostic criterion since 2018 [17]. To avoid confounding factors, patients with other autoimmune disease(s) associated with MS were excluded.
During the routine follow-up, we prospectively collected clinical scores referring to global disability (EDSS–Expanded Disability Status Scale [18]), and cognitive processing speed (SDMT–Symbol Digit Modalities Test or CSCT–Computerized Speed Cognitive Test [19]). MRIs were performed according to the OFSEP’s (Observatoire Français de la Sclérose En Plaques–French MS Office) protocol [20]. Radiological and clinical evaluation were performed at 6 months (re-baseline) and then annually; in some patients, evaluations were performed at earlier timepoints, according to the treating neurologist’s expertise.
This research protocol complies with the Declaration of Helsinki on the Protection of Persons.
Treatment protocol
Treatment induction consisted of two 1 g rituximab infusions at Day 1 and Day 15. Before 2017, maintenance infusions included the same regimen (i.e., 1 g rituximab at Day 1 and Day 15), repeated every six months. Since 2017, only one semestrial infusion of 1 g of rituximab was performed after the first infusion cycle. Indication for rituximab therapy was validated in therapeutic concertation meeting for each patient.
Clinical and radiological evaluation
A new T2-weighted lesion on brain MRI or a relapse were considered significant only beyond 6 months and re-baseline MRI. For the EDSS test, was considered significant a 6-months confirmed increase of 1 point between 0 and 5 points and a 6-months confirmed increase of 0.5 points between 5 and 10 points [21]. For the SMDT/CSCT tests, a modification over 0.5 DS between two tests and confirmed on a third one was considered significant [22].
A patient was classified as “improved” if EDSS and/or SDMT/CSCT was improved while the other scores remained stable. Reciprocally, a patient was classified as “worsened” if at least one test was worsened and the others stable.
Immunophenotyping
Blood lymphocytes enumeration and immunophenotyping were performed prospectively for each patient at initiation and immediately before each cycle of infusion. Fresh blood was collected on EDTA-coated tubes by venipuncture, and rapidly transferred to the clinical lab for analysis.
Immunophenotyping was performed with the Cytostat Tetrachrome reagents (Beckman Coulter, Brea, CA, USA) for identification of CD3+ (CD4+ and CD8+) T cells, CD19+ B cells, and CD3−CD56+CD16+ NK cells. Enumeration was performed with Flow count fluorospheres (Beckman Coulter). Data acquisition was carried out on a Navios® flow cytometer (Beckman Coulter).
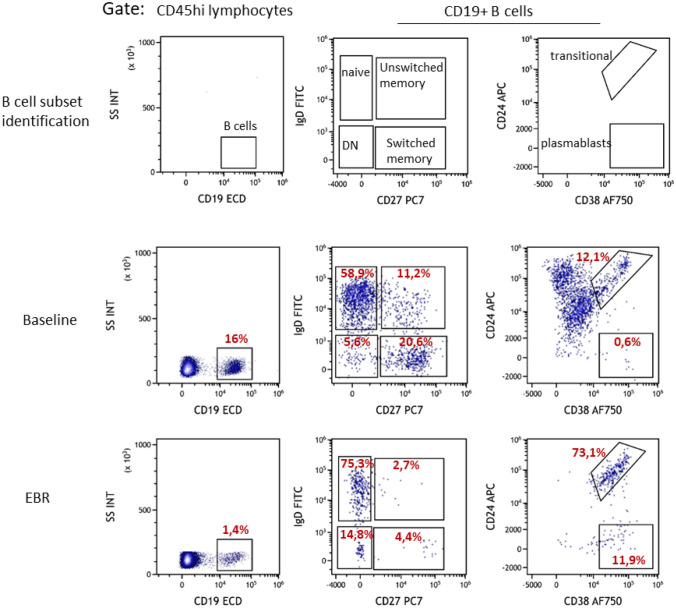
Early B cell repopulation (EBR) was considered when B cells concentration was > 5 cells/mm3. B cells subsets phenotyping was performed when B cell concentration reached > 10 cells/mm3, using the Duraclone IM B cells kit (Beckman Coulter). Data were analyzed with the Kaluza® software (Beckman Coulter). The identification of the indicated subsets was based on the following phenotypes: naïve (CD27−), memory (CD27+), transitional (CD24hiCD38hi), plasmablasts (CD27+CD24loCD38hi), switched memory (CD27+ IgD−IgM−), unswitched memory (CD27+IgD+IgM+) and double negative (CD27− IgD−) subsets (Fig. 1).
Fig. 1.
Flow cytometry analysis of B cell subsets. Flow cytometry analyses are performed directly from whole blood. Samples are stained using the Duraclone IM B cells analysis kit (Beckman Coulter), followed by erythrocyte lysis and data acquisition. Upper panels: identification of major B cells subsets, based on CD19, IgD, CD27, CD24, and CD38 expression. Middle panel: example of B cells phenotyping in one rituximab-treated patient, at baseline (before the first infusion). Lower panel: example of B cells phenotyping in the same patient, at the first EBR (before next infusion). DN: double negative CD27- IgD- B cells
Statistical analysis
Data were analyzed using the Student’s t test for parametric quantitative variables, and Mann–Whitney’s test for nonparametric quantitative variables if not paired, Wilcoxon’s test otherwise. Parametric categorial variables were analyzed by Chi square test and nonparametric categorial variables by the Fisher’s exact test. Normality was tested by the Shapiro–Wilk test. Finally, inter-group analyses were performed by mixed effect. Statistical analyses were performed with Prims® version 8.0 (GraphPad Software, San Diego, CA, USA).
Results
Demographical characteristics
One hundred and eighty-seven patients started rituximab for MS during the study period. Five were excluded because of another autoimmune disease associated with MS (2 rheumatoid arthritis, 1 myasthenia gravis, 1 anti-NMDAR autoimmune encephalitis and 1 undetermined systemic disease).
RRMS forms represented 28.57% (N = 61) of the patients (Table 1). PAMS included transitional forms switching from RRMS to secondary progressive MS (SPMS, N = 92), and primary progressive-relapsing MS (PRMS, N = 29). Females represented 58% of the cohort. The mean age was 47.7 years (range 19–76). Mean disease duration was 15.1 years (range 0–48). Almost one-third of the patients (29.7%) was naïve of previous DMT. The mean EDSS at baseline was moderate (4.5) but with significant inter-individual variability (range 0–8.0). Mean SDMT/CSCT score (− 1.1 standard deviation) was under the expected score for the general MS population but remained over the pathological cut-off with significant interindividual variability (range − 3.0 to + 3 SD). Gadolinium enhancement on the baseline brain MRIs was rare (7.7%). Similarly, few patients were treated with fampridine (12.6%).
Table 1.
Patients’ characteristics at baseline
| Whole cohort | RRMS | PAMS | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Without EBR | EBR | Without EBR | EBR | |||||||||
| N | %/Range | N | %/Range | N | %/Range | p | N | %/Range | N | %/Range | p | |
| N | 182 | 32 | 52% | 29 | 48% | ns | 80 | 66% | 41 | 34% | ns | |
| Mean age (years) | 47.7 | [19–76 | 41.3 | [23–62] | 36.3 | [19–57] | ns | 52.9 | [31–76] | 50.2 | [22–69] | ns |
| Male | 77 | 42% | 10 | 31% | 12 | 41% | ns | 31 | 39% | 21 | 51% | ns |
| Disease duration (mean, years) | 15.1 | [0–48] | 11.5 | [0–37] | 10.0 | [0–24] | ns | 17.5 | [1–48] | 16.6 | [1–40] | ns |
| BMI (mean) | 23.9 | [14.5–40.4] | 23.2 | [16.8–38.4] | 24.4 | [17.9–36.1] | ns | 23.9 | [14.5–40.4] | 24.3 | [18.5–32.8] | ns |
| Without previous DMT | 54 | 30% | 1 | 3% | 2 | 7% | ns | 32 | 40% | 19 | 46% | ns |
| Number of previous DMT (median) | 2 | [0–5] | 2 | [0–4] | 2 | [0–3] | ns | 1 | [0–5] | 1 | [0–5] | ns |
| Initial EDSS (mean) | 4.4 | [0–8] | 2.4 | [0–6] | 2.9 | [0–6.5] | ns | 5,5 | [2–8] | 5.3 | [2–7] | ns |
| Initial CSCT/SDMT (mean) | − 1.1 SD | [−3;3] | − 0.8 SD | [−3; 2] | −0.6 SD | [−3; 3] | ns | −1.3 SD | [−3; 1] | −1.3 SD | [−3; 1.5] | ns |
| Gadolinium enhancement | 14 | 8% | 0 | 0% | 7 | 24% | 0.0040 | 3 | 4% | 4 | 10% | ns |
| Fampridine | 23 | 13% | 1 | 3% | 1 | 3.5% | ns | 17 | 21% | 4 | 10% | ns |
Statistical analysis: Student’s t test, Fisher’s exact test, and Mann–Whitney’s test
EBR early B repopulation, RRMS relapsing–remitting multiple sclerosis, PAMS progressive active multiple sclerosis, DMT disease modifying treatment, BMI body mass index
Because some patients were routinely followed outside the University Hospital, there was no strict homogeneity in their follow-up, notably for SDMT/CSCT. Thus, a large majority of patients had available annual brain MRI (90%) and clinical evaluation including EDSS (82%); some patients had no MRI or EDSS available because the study ended before they reached a full year of follow-up. Finally, 43% of patients had at least two available SDMT/CSCT tests.
Early B cells repopulation is common during rituximab treatment for MS
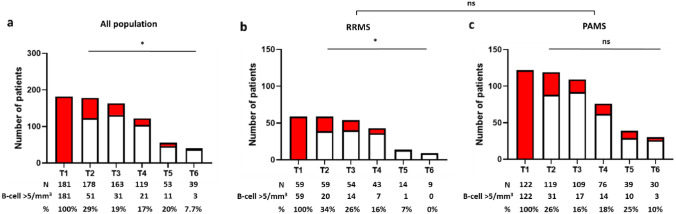
Seventy patients (38.5%) showed detectable B cells at least once (Fig. 2a), 23% (16/70, including 15 RRMS forms) of which as a single episode (not shown). Only 8% (6/70) showed systematic EBR before each next infusion. EBR was slightly more frequent in RRMS (48% of EBR versus 34% in PAMS), in younger patients (36.3 vs 41.3 y.o.), and was significantly associated with gadolinium enhancement (Table 1). The distribution of patients experiencing a single EBR episode did not differ between RRMS and PAMS (52% versus 42%, p = 0.4789, Fisher’s exact test). The frequency of EBR decreased overtime in RRMS, suggesting a cumulative effect of rituximab on B cell repopulation (Fig. 2b, c).
Fig. 2.
Early B cell repopulation over time. Histograms show the number of patients displaying EBR (red) versus no EBR (white) at each cycle of rituximab infusion. T1 represents baseline numbers, and analyses from T2 to T6 are performed hours before each administration. The number of patients with or without EBR are indicated, as well as the percentage of EBR+ patients at each cycle. a Overall population, b remitting-relapsing multiple sclerosis (RRMS), c progressive active multiple sclerosis (PAMS). Statistical analysis: Chi square test and Fisher’s exact test (* ≤ 0.05, ns: non-significant)
Mean inter-cure delay was 188.3 days for patients without EBR (minimum 173 days; maximum 234 days) and 184.5 days for patients of the EBR group (minimum 160 days; maximum 230 days), suggesting that EBR was not associated with an increased interval between cycles. Further, only 7 EBR cases occurred when the maintenance infusion was performed over 190 days (maximum 234 days), for medical (mainly infectious) or logistical reasons. Interestingly, 6 out of those 7 patients also showed EBR while the interval between two infusions was shorter than 190 days.
The impact of previous use of DMT was considered. However, neither of these treatments, nor the number of previous DMT, showed any impact on the occurrence of EBR. Likewise, this phenomenon was independent of disease duration (Table 1 and Fig. 3).
Fig. 3.
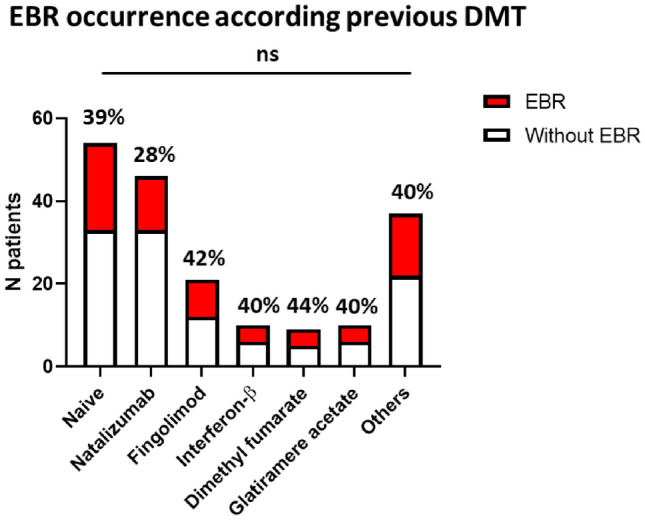
Impact of previous disease-modifying treatments (DMT) on EBR occurrence. The figure shows the number of patients with (red) or without (white) EBR, according to their use, or absence thereof (Naïve), of previous DMTs. The frequency of EBR+ patients in each group is indicated. Patients are classified as EBR+ when they experience at least one episode of EBR. The most frequently used DMTs are shown individually; the “Others” group included cyclophosphamide (N = 9), corticosteroids (N = 9), teriflunomide (N = 7), methotrexate (N = 1), mycophenolate mofetil (N = 1), alemtuzumab (N = 1), and azathioprine (N = 1). Chi-square test is used for statistical analysis
Altogether, these data show that the phenomenon of EBR is common during MS treatment with rituximab, and probably independent of an acquired resistance to B cell depletion.
Immunological profiling of T and B cells in patients with EBR
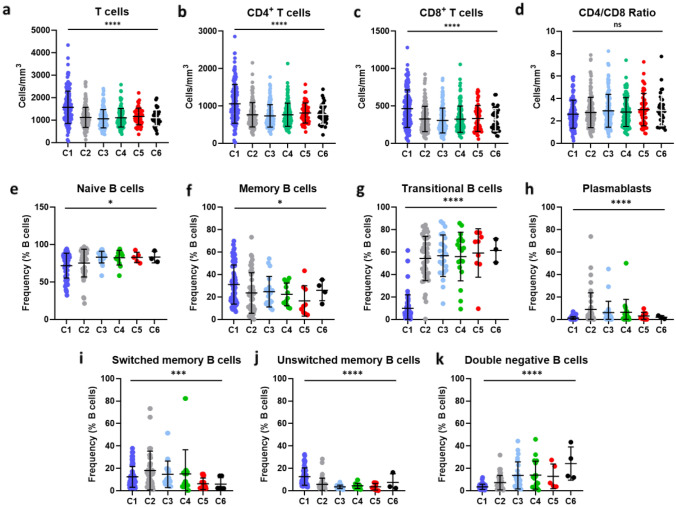
Rituximab induced a sustained T cell depletion (Fig. 4a). This depletion was observed in both CD4 + and CD8+ T (Fig. 4b, c), with a stable CD4/CD8 ratio (Fig. 4d). T cell depletion was similar in RRMS and PAMS, as well as in patients with or without EBR (Table 2).
Fig. 4.
Dynamic of T cell and B cells subsets over each cycle of treatment. a–c CD3+ T cells (a), CD4+ (b) and CD8+ (c) concentrations at baseline (C1) and before each new rituximab infusion (C2 to C6). Each dot represents a patient. Bars show mean concentrations with standard deviation. e–k Major B cell subsets were quantified by flow cytometry, as in the method section, before each rituximab infusion, in EBR+ patients with blood CD19+ B cells > 10 cells/mm3. Each dot corresponds to one patient. Bars represent mean frequencies with standard deviation. Statistical analyses were performed with Kruskal-Walli’s test (* ≤ 0.05, ** ≤ 0.01, *** ≤ 0.001, **** ≤ 0.0001)
Table 2.
Cellular population changes between T0 and the last EBR episode
| RRMS | PAMS | |||||
|---|---|---|---|---|---|---|
| Baseline | Last EBR | p | Baseline | Last EBR | p | |
| Total lymphocytes (median cells/mm3) | 2111 | 1305 | 0.0023 | 1811 | 1256 | 0.0022 |
| T cells (median cells/mm3) | 1592 | 1179 | 0.0337 | 1380 | 1057 | 0.0435 |
| CD4 + T cells (median cells/mm3) | 961 | 732 | 0.0367 | 954 | 700 | 0.0087 |
| CD8 + T cells (median cells/mm3) | 477 | 356 | 0.0322 | 391 | 270 | 0.0086 |
| CD4/CD8 ratio (median) | 2.0 | 2.1 | ns | 2.4 | 2.8 | ns |
| NK cells (median cells/mm3) | 156 | 163 | 0.0339 | 140 | 182 | 0.0222 |
| B cells (median cells/mm3) | 366 | 14 | < 0.0001 | 315 | 21 | < 0.0001 |
| Naïve B cells (median % B cells) | 68.8 | 80.7 | 0.0110 | 80.9 | 82.8 | ns |
| Memory B cells (median % B cells) | 36.4 | 26.6 | 0.0411 | 20.4 | 23.3 | ns |
| Switched memory B cells (median % B cells) | 20.3 | 12.2 | 0.0248 | 8.9 | 9.5 | ns |
| Unswitched memory B cells (median % B cells) | 13.2 | 3.9 | < 0.0001 | 9.0 | 3.4 | < 0.0001 |
| Double negative B cells (median % B cells) | 4 | 6.8 | 0.0063 | 2.3 | 7.4 | < 0.0001 |
| Transitional B cells (median % B cells) | 4.9 | 59 | < 0.0001 | 5.8 | 56.5 | < 0.0001 |
| Plasmablasts (median % B cells) | 0.6 | 3.7 | 0.0191 | 0.9 | 1.1 | 0.0340 |
Statistical analysis: Mann–Whitney’s test
EBR early B cell repopulation, RRMS relapsing–remitting multiple sclerosis, PAMS progressive active multiple sclerosis
After each cycle of rituximab infusion, repopulating B cell subsets were dominated by high levels of transitional and naïve B cells, whereas memory B cells frequencies were low (Fig. 4E–G). This skewed distribution was amplified after each cycle, suggesting a cumulative effect of rituximab on B cell reconstitution. Interestingly, we observed an increasing frequency of the IgD− CD27− double negative (DN) B pro-inflammatory B cell subset with the number of cycles (Fig. 4I–K). This pattern of B cell reconstitution was grossly similar in RRMS compared with PAMS patients (Table 2). However, the frequencies of naïve, total memory and switched memory B cells were not significantly different between EBR and baseline in PAMS patients. This was mostly due to different proportions of these B cell subsets between PAMS and RRMS at baseline, rather than different reconstitution dynamics.
The immunological profile at baseline is associated with the occurrence of EBR
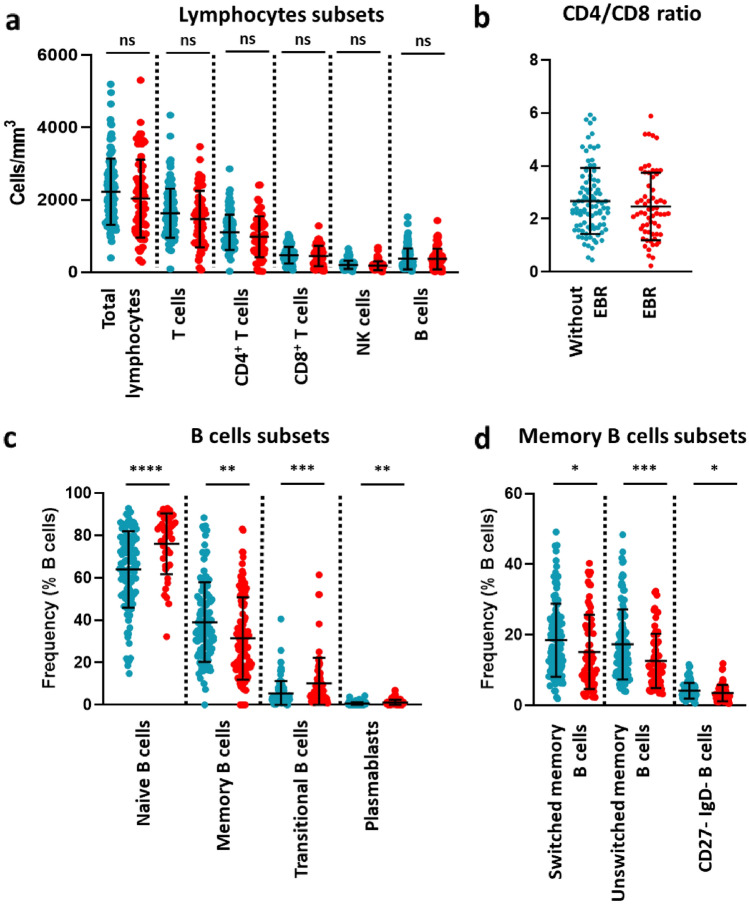
We next compared the immunological profile of patients with or without EBR at baseline. There was no significant differences in total lymphocytes, B cells, T cells, CD4+ T cells, CD8+ T cells and NK cells levels (Fig. 5a), and in CD4/CD8 ratio (Fig. 5b) at baseline, either in the RRMS or PAMS group (Table 3).
Fig. 5.
Lymphocyte subsets numbers and B cell subsets frequencies at baseline in patients with MS treated by rituximab. a Total lymphocyte, and major subsets thereof, concentrations. b CD4/CD8 ratio. c B cell subsets frequencies. d Memory B cell subsets frequencies. EBR+ patients are indicated in red, EBR-negative in blue. Each dot corresponds to one patient. Bars represent mean frequencies with standard deviation. Statistical analyses were performed with Mann-Whitney’s test (* = p ≤ 0.05, ** = p ≤ 0.01, *** = p ≤ 0.001, **** = p < 0.0001, ns non-significant)
Table 3.
Cell populations at baseline in patients with or without early B cell repopulation under rituximab
| RRMS | PAMS | |||||
|---|---|---|---|---|---|---|
| Without EBR | EBR | p | Without EBR | EBR | p | |
| Total lymphocytes (median cells/mm3) | 2540 | 2111 | ns | 1971 | 1811 | ns |
| T cells (median cells/mm3) | 1660 | 1592 | ns | 1466 | 1380 | ns |
| CD4 + T cells (median cells/mm3) | 1115 | 941 | ns | 950 | 931 | ns |
| CD8 + T cells (median cells/mm3) | 613 | 409 | ns | 384 | 361 | ns |
| CD4/CD8 ratio (median) | 2.2 | 2.1 | ns | 2.7 | 2.4 | ns |
| NK cells (median cells/mm3) | 235 | 156 | ns | 179 | 140 | ns |
| B cells (median cells/mm3) | 583 | 366 | ns | 222 | 315 | ns |
| Naïve B cells (median % B cells) | 59.8 | 68.8 | ns | 68.1 | 80.9 | 0.0005 |
| Memory B cells (median % B cells) | 43.6 | 36.4 | ns | 34.0 | 20.4 | 0.0002 |
| Switched memory B cells (median % B cells) | 21.9 | 20.4 | ns | 15.0 | 8.9 | 0.0014 |
| Unswitched memory B cells (median % B cells) | 16.7 | 13.6 | 0.0349 | 14.0 | 9.0 | 0.0006 |
| Double negative B cells (median % B cells) | 3.9 | 4 | ns | 3.3 | 2.3 | 0.0050 |
| Transitional B cells (median % B cells) | 3.0 | 4.9 | 0.0267 | 4.0 | 5.8 | 0.0014 |
| Plasmablasts (median % B cells) | 0.3 | 0.6 | 0.0182 | 0.5 | 0.9 | 0.0461 |
Statistical analysis: Mann–Whitney’s test
EBR early B cell repopulation RRMS relapsing–remitting multiple sclerosis, PAMS progressive active multiple sclerosis
In contrast, B cells subsets frequencies differed at baseline between patients with and without EBR. Indeed, when considering the whole MS cohort, higher proportions of naïve, transitional B cells and plasmablasts were noticed in the EBR group, contrasting with a lower proportion of memory B cells, including the switched, unswitched, and DN B cell subsets (Fig. 5c, d). Within the two subgroups of MS patients, these differences were mostly associated with PAMS; however, the increased frequencies of transitional B cells and plasmablasts were also significant in RRMS (Table 3). Thus, pre-treatment immunological profile is a predictor of the probability of EBR occurrence.
Immunological profile association with clinical evolution
A large majority of the patients remained stable in both groups. As expected, there was no relapse during the follow-up and few patients exhibited new lesion on MRI during the follow-up (n = 2); both with enhanced lesion after gadolinium injection (Table 4). There was no significant difference between patients with and without EBR regarding clinical outcome, either in the sense of improvement or worsening. It was noted, however, a trend towards more improved, and less worsened patients in the group with EBR than without EBR, especially in patients with RRMS. For instance, in the EBR + group, 16% showed EDSS improvement and 8% worsening, contrasting with 4% improved and 20% worsened in the EBR-negative group.
Table 4.
Main characteristics during follow-up for RRMS (relapsing–remitting multiple sclerosis) and PAMS (progressive active MS) patients
| RRMS | PAMS | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Without EBR | EBR | p | Without EBR | EBR | p | |||||
| N | % | N | % | N | % | N | % | |||
| N | 31 | 29 | 81 | 41 | ||||||
| Relapse | 0 | 0 | 0 | 0 | ns | 0 | 0 | 0 | 0 | ns |
| MRI | 29 | 28 | 66 | 39 | ||||||
| New MRI lesion | 0 | 0 | 1 | 3 | ns | 1 | 1 | 0 | 0 | ns |
| EDSS | 25 | 25 | 60 | 37 | ||||||
| EDSS improvement | 1 | 4 | 4 | 16 | ns | 6 | 10 | 1 | 3 | ns |
| EDSS worsening | 5 | 20 | 1 | 8 | ns | 9 | 15 | 5 | 14 | ns |
| SDMT/CSCT | 15 | 14 | 27 | 22 | ||||||
| SDMT/CSCT improvement | 3 | 20 | 5 | 36 | ns | 5 | 19 | 6 | 27 | ns |
| SDMT/CSCT worsening | 1 | 7 | 1 | 7 | ns | 3 | 11 | 3 | 14 | ns |
Statistical analysis: Fisher’s exact test and Mann–Whitney test
Finally, when considering only the subgroup of patients with EBR, we observed that patients with improvement (EDSS and/or SDMT/CSCT) displayed upon reconstitution less pro-inflammatory DN B cells (7.7% vs 18.6%, p = 0.0254), and a higher transitional/DN ratio (25.12 vs 5.667, p = 0.0350) (Table 5). Thus, the occurrence of EBR in patients treated with rituximab was not associated with relapse or progression. Further, the dynamics of B cell reconstitution may be associated with clinical evolution in these patients.
Table 5.
Main lymphocyte subsets changes in case of EBR, according to clinical outcome
| Improved | Worsened | p | |
|---|---|---|---|
| Baseline | |||
| N | 15 | 10 | |
| Double negative (DN) B cells (median % B cells) | 3.243 | 3.120 | ns |
| Transitional B cells (median % B cells) | 7.647 | 9.140 | ns |
| Transitional/DN ratio (median) | 3.267 | 4.961 | ns |
| Last available | |||
| DN B cells (median % B cells) | 7.727 | 18.61 | 0.0254 |
| Transitional B cells (median % B cells) | 63.34 | 47.81 | ns |
| Transitional/DN ratio (median) | 25.12 | 5.667 | 0.0350 |
Statistical analysis: Mann–Whitney’s test
DN double negative (CD27− IgD−) B cells
Discussion
We show here that EBR upon iterative rituximab treatment for MS is not associated with a risk of relapse of clinical worsening. Further, in some patients, EBR seems to correlate with an increased clinical benefit.
The absence of association between the occurrence of EBR and a less favorable evolution may seem in contradiction with what is described in other neurological autoimmune diseases, in which EBR is associated with a higher risk of relapse [15]. However, the decoupling between B cell repopulation and MS course has been suggested in several studies. Indeed, recent works showed that delaying rituximab infusions over 6 months because of SARS-CoV-2 pandemic a situation susceptible to increase cases of B cells repopulation before maintenance infusion was not associated with adverse outcomes [23–26].
B cell repopulation pattern, including more frequent naïve and transitional subsets, and less frequent memory B cells, is consistent with recently reported results in MS [27] and is also similar to observations made in other pathologies such as rheumatoid arthritis and systemic lupus erythematosus (SLE) [4, 9, 12]. In contrast with the situation in NMOSD, we found no association between the frequency of memory B cells at the time of reconstitution and the occurrence of relapses [15, 28, 29]. There are different hypotheses that could explain these data; one of them involves the pathophysiology of MS and the role of B cells in the disease. Whereas B cells in NMSD primarily mediate disease through the production of autoantibodies, other functions may be implicated in MS, such as antigen presentation or inflammatory cytokine secretion [2, 30]. The fact that patients are very rapidly re-treated after EBR could also be important since the dynamics of B cell reconstitution on a longer time frame could be different and also informative.
Indeed, patients with clinical worsening despite rituximab administration showed higher proportion of DN B cells and a higher DN-to-transitional B cells ratio at the time of B cell reconstitution. Interestingly, transitional B cells are known to include a significant part of regulatory B cells (Bregs) [31], whereas DN B cells are expanded in MS and display a proinflammatory profile [32]. Thus, we speculate that the EBR, at least in a subset of patients, skews B cells subsets towards a higher ratio of regulatory to inflammatory B cells. The trend towards clinical improvement and, particularly, cognitive tests improvement, could be due to a better control of CNS inflammation, allowing a better expression of neuronal plasticity. On the other hand, in some patients, rituximab may preferentially induce a Bregs depletion, leading to a pro-inflammatory profile of myeloid cells, as it was recently reported [27]. This phenomenon could explain, in addition with higher DN B cells, clinical worsening. Additional studies, involving the assessment of B cell functions, could be performed to confirm these hypotheses.
Rituximab treatment induced partial T cell depletion, as already observed in other autoimmune diseases [8–10], regardless of the presence of an EBR. T cells are involved in brain tissues destruction in MS [33, 34]. Especially, some CD8+ autoreactive T cells targeting myelin antigens exhibit CD20 marker on their surface and were recently described to be particularly affected by anti-CD20 therapies [35]. A recent article reported a decreased CD8 frequency in ocrelizumab-treated patients with low lymphocyte counts [36]. Whether or not T cell depletion is predictive of long-term rituximab efficacy remains to be studied.
Subgroup analysis showed grossly similar clinical results for RRMS and PMS than for the whole MS population. There were, however, some apparent differences between these two subgroups, such as the frequency of EBR (more frequent in RRMS) and the B cell subsets distribution at baseline according to the presence of EBR (with more differences in PAMS than in RRMS). The reason for these discrepancies is not clear; they may involve statistical power (as there was twice as many patients with PAMS was than with RRMS), but also specific pathophysiological processes, including the more inflammatory nature of RRMS compared to PAMS. More studies, and a longer follow-up, are required to confirm these differences and unravel putative consequences. Still, the trends were the same in the two groups, orienting to a similar biological effect of anti-CD20 in RRMS and PAMS.
Our study has several limitations, most of which are inherent to its monocentric, retrospective design and limited follow-up, mainly under 2 years. First, there is no strict consensus about the positioning of anti-CD20 treatment for MS; hence, the use of rituximab varies from one center to another, leading to a possible bias in the patients’ characteristics. Furthermore, this real-life study was associated with modifications in the protocol over time, notably regarding infusion and evaluation timepoints, and rituximab posology. However, there was no clinical nor biological difference between patients receiving 1 g or 2 g of rituximab in maintenance infusions (data not shown). Finally, the demography of our cohort differs significantly from the patients treated with new-generation anti-CD20, such as ocrelizumab. Indeed, this study was designed when rituximab was used off-label as third-line treatment for patients with RRMS, but more often in active PMS patients. Thus, the patients studied here show a high mean EDSS, a large age range, and in their majority have progressive MS. The preliminary observations of putative differences between RRMS and PMS patients in our study underscore the importance of confirming our results with new cohorts of patients treated with ocrelizumab.
In conclusion, rituximab is associated with at least one episode of EBR during the two first years of treatment in 38% of MS patients. This EBR is not predictive of relapse or clinical worsening; by contrast, it could be more predictive of increased benefit in some patients. This observation, in agreement with the recent notion than an increased inter-cure period does not negatively affect treatment efficacy, could lead to personalized strategies of anti-CD20 usage in MS patients, provided it is confirmed by future large-scale studies. Particularly, if confirmed, the interest of the transitional/double negative B cells ratio may allow some patients to be spared treatment to mitigate side-effects.
Funding
This research received no specific fundings.
Declarations
Conflicts of interest
The authors report no disclosures relevant to the manuscript.
Ethical standard
This study was performed in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki Declaration.
Research ethic and patient consent
This work is based on retrospective data collection, obtained from the routine monitoring of patients’ treatments and their clinical evaluation in the hospital setting. As such, and according to French regulations, it does not require specific approval from the ethics committee.
Footnotes
Guillaume Dorcet and Hugo Migné have contributed equally.
References
- 1.Lassmann H. Multiple sclerosis pathology. Cold Spring Harb Perspect Med. 2018 doi: 10.1101/cshperspect.a028936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Li R, Patterson KR, Bar-Or A. Reassessing B cell contributions in multiple sclerosis. Nat Immunol. 2018;19:696–707. doi: 10.1038/s41590-018-0135-x. [DOI] [PubMed] [Google Scholar]
- 3.Hauser SL, Waubant E, Arnold DL, et al. B-cell depletion with rituximab in relapsing-remitting multiple sclerosis. N Engl J Med. 2008;358:676–688. doi: 10.1056/NEJMoa0706383. [DOI] [PubMed] [Google Scholar]
- 4.Theunissen PMJ, van den Branden A, Van Der Sluijs-Gelling A, et al. Understanding the reconstitution of the B-cell compartment in bone marrow and blood after treatment for B-cell precursor acute lymphoblastic leukaemia. Br J Haematol. 2017;178:267–278. doi: 10.1111/bjh.14685. [DOI] [PubMed] [Google Scholar]
- 5.Weiner GJ. Rituximab: mechanism of action. Semin Hematol. 2010;47:115–123. doi: 10.1053/j.seminhematol.2010.01.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Montalban X, Hauser SL, Kappos L, et al. Ocrelizumab versus placebo in primary progressive multiple sclerosis. N Engl J Med. 2017;376:209–220. doi: 10.1056/NEJMoa1606468. [DOI] [PubMed] [Google Scholar]
- 7.Naegelin Y, Naegelin P, von Felten S, et al. Association of rituximab treatment with disability progression among patients with secondary progressive multiple sclerosis. JAMA Neurol. 2019;76:274–281. doi: 10.1001/jamaneurol.2018.4239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Leandro MJ, Cambridge G, Ehrenstein MR, Edwards JCW. Reconstitution of peripheral blood B cells after depletion with rituximab in patients with rheumatoid arthritis. Arthritis Rheum. 2006;54:613–620. doi: 10.1002/art.21617. [DOI] [PubMed] [Google Scholar]
- 9.Thiel J, Rizzi M, Engesser M, et al. B cell repopulation kinetics after rituximab treatment in ANCA-associated vasculitides compared to rheumatoid arthritis, and connective tissue diseases: a longitudinal observational study on 120 patients. Arthritis Res Ther. 2017;19:101. doi: 10.1186/s13075-017-1306-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Roll P, Palanichamy A, Kneitz C, et al. Regeneration of B cell subsets after transient B cell depletion using anti-CD20 antibodies in rheumatoid arthritis. Arthritis Rheum. 2006;54:2377–2386. doi: 10.1002/art.22019. [DOI] [PubMed] [Google Scholar]
- 11.Colucci M, Carsetti R, Cascioli S, et al. B Cell Reconstitution after rituximab treatment in idiopathic nephrotic syndrome. J Am Soc Nephrol JASN. 2016;27:1811–1822. doi: 10.1681/ASN.2015050523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Pers J-O, Daridon C, Bendaoud B, et al. B-cell depletion and repopulation in autoimmune diseases. Clin Rev Allergy Immunol. 2008;34:50–55. doi: 10.1007/s12016-007-8015-4. [DOI] [PubMed] [Google Scholar]
- 13.Ruetsch-Chelli C, Bresch S, Seitz-Polski B, et al. Memory B Cells predict relapse in rituximab-treated myasthenia gravis. Neurother J Am Soc Exp Neurother. 2021 doi: 10.1007/s13311-021-01006-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Nosadini M, Alper G, Riney CJ, et al. Rituximab monitoring and redosing in pediatric neuromyelitis optica spectrum disorder. Neurol Neuroimmunol Neuroinflamm. 2016;3:e188. doi: 10.1212/NXI.0000000000000188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ellrichmann G, Bolz J, Peschke M, et al. Peripheral CD19+ B-cell counts and infusion intervals as a surrogate for long-term B-cell depleting therapy in multiple sclerosis and neuromyelitis optica/neuromyelitis optica spectrum disorders. J Neurol. 2019;266:57–67. doi: 10.1007/s00415-018-9092-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Polman CH, Reingold SC, Banwell B, et al. Diagnostic criteria for multiple sclerosis: 2010 revisions to the McDonald criteria. Ann Neurol. 2011;69:292–302. doi: 10.1002/ana.22366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Thompson AJ, Banwell BL, Barkhof F, et al. Diagnosis of multiple sclerosis: 2017 revisions of the McDonald criteria. Lancet Neurol. 2018;17:162–173. doi: 10.1016/S1474-4422(17)30470-2. [DOI] [PubMed] [Google Scholar]
- 18.Meyer-Moock S, Feng Y-S, Maeurer M, et al. Systematic literature review and validity evaluation of the expanded disability status scale (EDSS) and the multiple sclerosis functional composite (MSFC) in patients with multiple sclerosis. BMC Neurol. 2014;14:58. doi: 10.1186/1471-2377-14-58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ruet A, Deloire MSA, Charré-Morin J, et al. A new computerised cognitive test for the detection of information processing speed impairment in multiple sclerosis. Mult Scler Houndmills Basingstoke Engl. 2013;19:1665–1672. doi: 10.1177/1352458513480251. [DOI] [PubMed] [Google Scholar]
- 20.Cotton F, Kremer S, Hannoun S, et al. OFSEP, a nationwide cohort of people with multiple sclerosis: consensus minimal MRI protocol. J Neuroradiol J Neuroradiol. 2015;42:133–140. doi: 10.1016/j.neurad.2014.12.001. [DOI] [PubMed] [Google Scholar]
- 21.Calabresi PA, Radue E-W, Goodin D, et al. Safety and efficacy of fingolimod in patients with relapsing-remitting multiple sclerosis (FREEDOMS II): a double-blind, randomised, placebo-controlled, phase 3 trial. Lancet Neurol. 2014;13:545–556. doi: 10.1016/S1474-4422(14)70049-3. [DOI] [PubMed] [Google Scholar]
- 22.Kalb R, Beier M, Benedict RH, et al. Recommendations for cognitive screening and management in multiple sclerosis care. Mult Scler Houndmills Basingstoke Engl. 2018;24:1665–1680. doi: 10.1177/1352458518803785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Boremalm M, Sundström P, Salzer J. Discontinuation and dose reduction of rituximab in relapsing-remitting multiple sclerosis. J Neurol. 2021;268:2161–2168. doi: 10.1007/s00415-021-10399-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Disanto G, Ripellino P, Riccitelli GC, et al. De-escalating rituximab dose results in stability of clinical, radiological, and serum neurofilament levels in multiple sclerosis. Mult Scler Houndmills Basingstoke Engl. 2021;27:1230–1239. doi: 10.1177/1352458520952036. [DOI] [PubMed] [Google Scholar]
- 25.Juto A, Fink K, Al Nimer F, Piehl F. Interrupting rituximab treatment in relapsing-remitting multiple sclerosis; no evidence of rebound disease activity. Mult Scler Relat Disord. 2020;37:101468. doi: 10.1016/j.msard.2019.101468. [DOI] [PubMed] [Google Scholar]
- 26.Maarouf A, Rico A, Boutiere C, et al. Extending rituximab dosing intervals in patients with MS during the COVID-19 pandemic and beyond? Neurol Neuroimmunol Neuroinflammation. 2020;7:e825. doi: 10.1212/NXI.0000000000000825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Nissimov N, Hajiyeva Z, Torke S, et al. B cells reappear less mature and more activated after their anti-CD20-mediated depletion in multiple sclerosis. Proc Natl Acad Sci USA. 2020;117:25690–25699. doi: 10.1073/pnas.2012249117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kim S-H, Huh S-Y, Lee SJ, et al. A 5-year follow-up of rituximab treatment in patients with neuromyelitis optica spectrum disorder. JAMA Neurol. 2013;70:1110–1117. doi: 10.1001/jamaneurol.2013.3071. [DOI] [PubMed] [Google Scholar]
- 29.Cohen M, Romero G, Bas J, et al. Monitoring CD27+ memory B-cells in neuromyelitis optica spectrum disorders patients treated with rituximab: Results from a bicentric study. J Neurol Sci. 2017;373:335–338. doi: 10.1016/j.jns.2017.01.025. [DOI] [PubMed] [Google Scholar]
- 30.Comi G, Bar-Or A, Lassmann H, et al. The role of B cells in multiple sclerosis and related disorders. Ann Neurol. 2021;89:13–23. doi: 10.1002/ana.25927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Mauri C, Menon M. The expanding family of regulatory B cells. Int Immunol. 2015;27:479–486. doi: 10.1093/intimm/dxv038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Claes N, Fraussen J, Vanheusden M, et al. Age-associated B cells with proinflammatory characteristics are expanded in a proportion of multiple sclerosis patients. J Immunol Baltim Md 1950. 2016;197:4576–4583. doi: 10.4049/jimmunol.1502448. [DOI] [PubMed] [Google Scholar]
- 33.Kaskow BJ, Baecher-Allan C. Effector T cells in multiple sclerosis. Cold Spring Harb Perspect Med. 2018;8:a029025. doi: 10.1101/cshperspect.a029025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Spencer CM, Crabtree-Hartman EC, Lehmann-Horn K, et al. Reduction of CD8(+) T lymphocytes in multiple sclerosis patients treated with dimethyl fumarate. Neurol Neuroimmunol Neuroinflamm. 2015;2:e76. doi: 10.1212/NXI.0000000000000076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Sabatino JJ, Wilson MR, Calabresi PA, et al. Anti-CD20 therapy depletes activated myelin-specific CD8+T cells in multiple sclerosis. Proc Natl Acad Sci USA. 2019;116:25800–25807. doi: 10.1073/pnas.1915309116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Abbadessa G, Maida E, Miele G, et al. Lymphopenia in multiple sclerosis patients treated with Ocrelizumab is associated with an effect on CD8 T cells. Mult Scler Relat Disord. 2022;60:103740. doi: 10.1016/j.msard.2022.103740. [DOI] [PubMed] [Google Scholar]