Abstract
Most studies regarding the beneficial effect of sulforaphane (SFN) on non-alcoholic fatty liver disease (NAFLD) have focused on nuclear factor E2-related factor 2 (Nrf2). But the molecular mechanisms underlying the beneficial effect of SFN in the treatment of NAFLD remain controversial. Fibroblast growth factor (FGF) 21 is a member of the FGF family expressed mainly in liver but also in adipose tissue, muscle and pancreas, which functions as an endocrine factor and has been considered as a promising therapeutic candidate for the treatment of NAFLD. In the present study we investigated whether FGF21 was involved in the therapeutic effect of SFN against NAFLD. C57BL/6J mice were fed a high-fat diet (HFD) for 12 weeks to generate NAFLD and continued on the HFD for additional 6 weeks with or without SFN treatment. We showed that administration of SFN (0.56 g/kg) significantly ameliorated hepatic steatosis and inflammation in NAFLD mice, along with the improved glucose tolerance and insulin sensitivity, through suppressing the expression of proteins responsible for hepatic lipogenesis, while enhancing proteins for hepatic lipolysis and fatty acids oxidation. SFN administration significantly increased hepatic expression of FGFR1 and fibroblast growth factor 21 (FGF21) in NAFLD mice, along with decreased phosphorylation of p38 MAPK (the downstream of FGF21). HepG2 cells were treated in vitro with FFAs (palmitic acid and oleic acid) followed by different concentrations of SFN. We showed that the effects of SFN on FGF21 and FGFR1 protein expression were replicated in FFAs-treated HepG2 cells. Moreover, the increased FGFR1 protein occurred earlier than increased FGF21 protein. Interestingly, the rapid effect of SFN on FGFR1 protein was not regulated by the FGFR1 gene transcription. Knockdown of FGFR1 and p38 genes weakened SFN-reduced lipid deposition in FFAs-treated HepG2 cells. SFN administration in combination with rmFGF21 (1.5 mg/kg, i.p., every other day) for 3 weeks further suppressed hepatic steatosis in NAFLD mice. In conclusion, SFN ameliorates lipid metabolism disorders in NAFLD mice by upregulating FGF21/FGFR1 pathway. Our results verify that SFN may become a promising intervention to treat or relieve NAFLD.
Keywords: non-alcoholic fatty liver disease, sulforaphane, fibroblast growth factor 21, fibroblast growth factor receptor-1, p38MAPK, insulin sensitivity
Introduction
Non-alcoholic fatty liver disease (NAFLD), arising from the input/output imbalance of hepatic free fatty acid metabolism, encompasses a broad-spectrum ranging from the simple steatosis to steatohepatitis, cirrhosis and primary liver cancer [1, 2]. NAFLD pathogenesis is widely described as a “two-hit” theory. Obesity and insulin resistance as a “first hit” causes the accumulation of fat in the liver, leading to simple fatty liver. The “second hit” is characterized as inflammation, endoplasmic reticulum stress (ER stress), oxidative stress, mitochondrial dysfunction and other factors, leading to steatohepatitis and fibrosis [3, 4]. However, clinical studies have demonstrated a high degree of heterogeneity in the pathogenesis and clinical manifestations of the disease [5]. Currently, there are no pharmacological approaches for treating this disease [6, 7], while the only remedy is the intensive lifestyle change, including calorie restriction and exercise [8].
Broccoli sprout powder has been found to improve liver function in non-obese patients with fatty liver disease [9]. Sulforaphane (SFN) is a naturally occurring isothiocyanate derived from cruciferous vegetables such as broccoli, cabbage and kale. Bioactive SFN is demonstrated for a promising chemo-preventive compound with anti-oxidant, anti-cholesterol, and anti-cancer properties [10]. SFN was recently reported to decrease weight gain and visceral adiposity in high-fat diet-fed (HFD) mice and alcohol-induced steatosis [11, 12]. Other studies have provided strong evidence for the efficacy of SFN on patients with type 2 diabetes [13, 14]. However, the mechanism underlying SFN-mediated NAFLD remains elusive.
As SFN is a natural nuclear factor E2-related factor 2 (Nrf2) activator, many studies have elucidated the role of SFN in obesity by using either Nrf2 knockout (KO) and Kelch-like ECH-associated protein 1 (Keap1) knockdown (KD) in mice or Nrf2 activators [15, 16]. Despite extensive researches, the results of Nrf2 are inconsistent in hepatic steatosis and insulin resistance. In this sense, fibroblast growth factor (FGF) 21 is a promising therapeutic candidate for the treatment of NAFLD due to its beneficial effects on lipid homeostasis. This growth factor is a notable member of the FGF family that functions as an endocrine agent, which is expressed predominantly in liver [17] but also in adipose tissue, muscle, and pancreas [18–21]. Most studies indicate that the administration or overexpression of FGF21 in obese animals has positive metabolic effects [22, 23] while FGF21 knockout mice have an increased hepatic lipid content when challenged with a HFD [24]. However, paradoxically elevated protein expression of FGF21 is evident in liver and white adipose tissue (WAT) of both diet-induced obese (DIO) mice and genetically obese ob/ob or db/db mice [25–27]. Furthermore, increased level of FGF21 correlates not only with liver fat content in NAFLD but also with body mass index (BMI) in human subjects [28, 29], which has raised the concept of FGF21 resistance [30]. FGF21 actions are mediated through a heterodimeric receptor complex comprising FGF receptor 1 (FGFR1) and βKlotho [31]. Diet‐induced obese (DIO) mice have diminished expression of these receptor components in liver and WAT, attenuated FGF21 signaling response and impaired induction of FGF21 target genes. Thus, is there any possibility to restore the endogenous FGF21 response in obese subjects improving their metabolic parameters and the risk of developing obesity‐associated pathologies?
To confirm our hypothesis, we investigated the effects of SFN on hepatic steatosis in mice induced by a HFD and in HepG2 cells induced by free fatty acids (FFAs), with a goal of understanding the potential signaling pathway between SFN and FGF21 in NAFLD.
Materials and methods
Ethical statement
All experimental procedures involving mice were carried out according to protocols approved by the Institutional Animal Ethics Committee of Jiangnan University (JN. No20160303-20161125[14] and 20170509-20170930[58]). All efforts were made to minimize suffering of experimental mice in this research. Animal studies are reported in compliance with the ARRIVE guidelines [32] and adhere to the National Institutes of Health (NIH) standards [33].
Cell culture and transfection
The human hepatoma HepG2 cell line was purchased from Cell Bank of the Shanghai Institute of Cells, Chinese Academy of Science (Shanghai, China). Cells were maintained in DMEM media (C11965500BT, Gibco, New York, NY, USA) with 10% FBS (10099-141, Gibco) and 100 μg/mL penicillin/streptomycin in a humidified atmosphere containing 5% CO2 at 37 °C. Palmitic acid and oleic acid (57-10-3, 112-80-1, Nu-Chek Prep, Elysian, MN, USA) were mixed thorough in media, then SFN (S6317, Sigma, Shanghai, China) was added into media. For small RNA interference, HepG2 cells were transfected with FGFR1-specific siRNA, p38MAPK-specific siRNA and scramble siRNA (GenePharma, Shanghai, China) for 24 h by using Jet-Prime Transfection Reagent (114-15, Polyplus, New York, NY, USA) according to the manufacturer’s instructions. The transfected cells were cultured containing either vehicle or FFAs for 6 h, and then stimulated by SFN for another 6 h.
Establishment of NAFLD animal model and dosage information
Male C57BL/6J mice (7 weeks old, weighing 20 ± 2 g) were purchased from SLAC (C57BL/6Slac, Shanghai, China). All mice studied were maintained on a 12 h light/dark cycle at 24 ± 2 °C with free access to food and water. Normal diets (ND; 10% energy from fat) were purchased from Xietong Organism (AIN93, Nanjing, China) and high-fat diets (HFD; 60% energy from fat) were purchased from HFK Bioscience Co; Ltd (H10010, Beijing, China). Sulforaphane (90% purity) was obtained from Pioneer Herb Industrial Co; Ltd (SF-010P, Ganzhou, China) and added to HFD diet (0.56 g/kg) (China). Recombinant mouse FGF21 was prepared by College of Life Science, Henan Normal University (Xinxiang, China).
After a week acclimation, mice were fed a ND or HFD diet for 12 weeks. Then the mice in HFD group (average 40 g) were randomly divided into HFD and HFD + SFN group, followed by additional 6 weeks of feeding with the respective diet (Supplementary Fig. S1a).
To set-up recombinant FGF21 mouse groups (rmFGF21), HFD-induced obese mice, with either vehicle (HFD) or HFD + SFN (0.56 g/kg) for 3 weeks, were subjected to intraperitoneal injection of rmFGF21 (1.5 mg/kg, i.p., every other day) for another 3 weeks (Supplementary Fig. S1b).
Hematoxylin and eosin and oil red O staining
For hematoxylin and eosin (H&E) staining, tissues were fixed in 4% paraformaldehyde overnight and embedded in paraffin. Sections (5-μm) were stained with H&E dyes and evaluated using a digital slice scanner (Pannoramic MIDI, 3DHistech, Budapest, Hungary). For oil red O staining, fresh liver tissues were embedded in optimum cutting temperature (OCT) compound and cryo-sectioned. The sections were stained with 0.5% oil red O according to standard procedures.
Plasma lipid profile
Plasma triglyceride (TG), cholesterol (TC), low density lipoprotein (LDL), high density lipoprotein (HDL), alanine transaminase (ALT) and aspartate transaminase (AST) levels were measured by Roche Modular P800 Automatic Analyzer (Roche, Rotkreuz, Switzerland).
Enzyme-linked immunosorbent assays (ELISA)
Hepatic interleukin 1β (IL-1β), tumor necrosis factor alpha (TNF-α) and monocyte chemoattractant protein-1 (MCP-1) levels were measured with ELISA kits from R&D Systems (DY401-05, DY410-05, DY479-05, Minneapolis, MN, USA) according to the protocols of the manufacturer. Absorbance was measured at 450 nm with a microplate reader Multiclan GO (Thermo Fisher Scientific Inc, Waltham, MA, USA). Hepatic tissue samples were homogenized in a saline solution (1:19, w/v) using a homogenizer (Polytron, Ningbo, China) at 55 Hz for 1 min. Samples were centrifuged at 4 °C, 10,000 × g for 10 min. Protein concentrations were determined by BCA Protein Assay Kit (P0010S, Beyotime, Shanghai, China). Insulin levels in plasma were measured by the Mercodia Mouse Insulin ELISA kit (10-1247-01, Uppsala, Sweden) according to the standard procedure.
Hepatic TG, TC contents and citrate synthase activity
Hepatic TG (K622-100), TC (K603-100) levels and citrate synthase activity (K318) were quantified in liver homogenates according to Biovision kit (Milpitas, CA, USA). Briefly, frozen livers were weighed, homogenized and centrifuged and the supernatant was collected for measurement.
Glucose and insulin tolerance tests
Glucose tolerance tests (GTTs) and insulin tolerance tests (ITTs) were performed as in previous reports [17]. For the GTTs, mice were fasted overnight for 12 h, and glucose (2 g/kg) was injected intraperitoneally. For the ITTs, insulin (0.75 U/kg) was injected intraperitoneally.
Immunoblotting
Cells and tissues samples were lysed by RIPA buffer (containing protease inhibitors and phosphatase inhibitors). Protein concentration was quantified by a BCA Protein Assay Kit (Beyotime). Equal amounts of proteins were electrophoretically separated in SDS–PAGE gels and transferred onto PVDF membranes (Millipore, Billerica, MA, USA). Primary antibodies were incubated overnight at 4 °C and then probed with secondary horseradish peroxidase-labeled antibody. Antibodies for P-HSL (Ser660, #4126), HSL (#4107), p-p38 MAPK (Thr180/Tyr182, #4511), p38 MAPK (#9212), p-p44/42 MAPK (ERK1/2) (Thr202/Tyr204, #9101), p44/42 MAPK (ERK1/2) (#9102), p-SAPK/JNK (Thr183/Tyr185, #4668), SAPK/JNK (#9258), FAS (C20G5, #3180), CHOP (L63F7, #2895) and FGF Receptor 1 (D8E4, #9740) were purchased from Cell Signaling Technology (Danvers, MA, USA). Antibodies for FGF21 were purchased from Abcam (#ab171941, Cambridge, UK). Antibodies for βKlotho were purchased from R&D Systems (#AF2619). Antibodies for PPARα (H-98, #sc-9000), SREBP1 (F-10, #sc-365514), PPARγ (H-100, #sc-7196), GRP78 (A-10, #sc-376768), ATF6 (F-7, #sc-166659) and β-actin (C4, #sc-47778) were purchased from Santa Cruz Biotechnology (Dallas, TX, USA). The signals were visualized by Plus-enhanced chemiluminiscence using FluorChem FC3 (ProteinSimple, San Jose, CA, USA). The densitometric analyses of protein expression were performed by AlphaView Software (ProteinSimple).
RNA isolation and real-time quantitative PCR (RT-qPCR)
Total RNA was extracted from cells and tissues using TRIzol according to the manufacturer’s protocol and reversely transcribed to cDNA using PrimeScriptTM RT Master Mix (RR036A, Takara, Kyoto, Japan). Primers (Supplementary Table S1) were used to perform RT-qPCR with Absolute Q-PCR SYBR Green Supermix (172-5124, Bio-Rad, Irvine, CA, USA) with CFX ConnectTM Real-Time System (Bio-Rad).
Statistical analysis
Statistical analysis was undertaken only when each group size has a minimum of n = 5 independent samples/individuals, and in a blinded manner. Normal distribution was confirmed using the Kolmogorov-Smirnov test. Statistical analysis between two groups was performed by independent t-test, or when multiple comparisons were made, by one-way analysis of variance (ANOVA) followed by Tukey’s post-hoc test using GraphPad Prism 8.0.2 (GraphPad Software Inc., San Diego, CA, USA). For all one-way ANOVAs, post-hoc tests were run only if F achieved P < 0.05 and there was no significant variance in homogeneity. For Western blot and RT-qPCR analysis, the relative protein or mRNA expression values were expressed as “fold difference” by comparing to the corresponding control value, and the control value was normalized to 1.0. Potential outliers were tested using Grubbs’ test. *P < 0.05 were considered as a statistically significant difference. Data were presented as mean with standard deviations (SD).
Results
SFN alleviated hepatic damage in mice fed a HFD along with improvement of hepatic steatosis and inflammation
To investigate the role of SFN in non-alcoholic fatty liver, NAFLD mice were generated through 12 weeks HFD feeding and then followed with SFN supplement for another 6 weeks (Supplementary Fig. S1a). The pathological changes of the liver demonstrated a significant increase in the size (Fig. 1a) as well as the weight of liver and the indices of liver/body weight (Fig. 1b). Severe hepatic steatosis and inflammation assessed by H&E staining (Fig. 1c), while hepatic fat droplets were assessed by oil red O staining (Fig. 1d). Notably, SFN supplement markedly reduced the size of fat droplets in liver in conjunction with a reduction in hepatic content of TG and TC (Fig. 1e). Despite of unaltered circulating TG, plasma levels of TC, LDL and HDL were significantly reduced in HFD + SFN group compared to HFD group, so were the levels of ALT and AST (Fig. 1f, g). The gene and protein expression levels of pro-inflammatory cytokines IL-1β, TNF-ɑ and MCP-1 were increased in the HFD group, but decreased following SFN treatment (Fig. 1h, i). Together, these results demonstrated that SFN supplement significantly improved hepatic steatosis and inflammation.
Fig. 1. Effects of SFN on HFD-induced hepatic steatosis and inflammation.
a Representative livers from the mice fed a ND, a HFD, or HFD + SFN. Scale bar, 10 mm. b Liver weight (left panel) and liver-to-body weight indices (%) (right panel). Representative images of lipid droplets by (c) H&E staining (original magnification, ×200; scale bars, 100 μm) and by (d) oil red O (original magnification, ×100; scale bars, 200 μm). e Content of liver TG (left panel) and TC (right panel). f Levels of plasma ALT (left panel) and AST (right panel). g Levels of plasma TG, TC, LDL and HDL. h Hepatic ELISA levels of IL-1β, TNF-α and MCP-1; i Quantification of the hepatic genes involved in inflammation. n = 8. Data are mean ± SD. *P < 0.05, **P < 0.01, ***P < 0.001.
SFN increases fatty acids metabolism in mice fed a HFD
As hepatic steatosis was dramatically reduced in mice with SFN supplement, we then investigated if SFN could play a role in mediating fatty acids metabolism. Firstly, we detected the expression of molecules involved in lipogenesis and lipolysis. The protein levels of hepatic sterol regulatory element binding protein-1 (SREBP1), peroxisome proliferator-activated receptor gamma (PPARγ) and fatty acid synthase (FAS) were significantly decreased by SFN supplement in mice fed a HFD (Fig. 2a, b), indicating the reduction of lipogenesis. Consistently, the mRNA levels of aforementioned molecules showed a similar change compared to protein levels (Fig. 2c). In contrast, hepatic peroxisome proliferator-activated receptor α (PPARα) protein level (Fig. 2a, b) and mRNA levels of Ppara (Fig. 2d) were elevated by SFN supplement, accompanied by increased mRNA levels of Cpt1a, Acox1, Acadm, Acadvl (Fig. 2d) as well as an increase in hepatic citrate synthase activity (Fig. 2e), suggesting an enhanced fatty acids oxidation in liver. Additionally, the protein level of hormone-sensitive lipase (HSL) and its mRNA level Lipe were unchanged among three groups, but SFN significantly increased the phosphorylation level of HSL (Fig. 2f–h) and mRNA of Pnpla2, indicative of enhanced lipolysis. Thus, reduced hepatic steatosis by SFN supplementation is associated with upregulating hepatic lipolysis and fatty acids oxidation while down-regulating lipogenesis.
Fig. 2. Regulation of hepatic lipogenesis, lipolysis and fatty acid oxidation by SFN.
Representative blots for (a) hepatic lipogenesis proteins and (b) their densitometric analysis. Quantification of the hepatic genes involved in (c) fatty acid synthesis and in (d) fatty acid oxidation. e Level of liver citrate synthase activity (n = 8). f Quantification of the hepatic genes involved in fatty acid lipolysis. Representative blots for (g) p-HSL and HSL proteins and (h) their densitometric analysis. n = 6. Data are mean ± SD. *P < 0.05, **P < 0.01, ***P < 0.001.
SFN improves HFD-induced insulin resistance
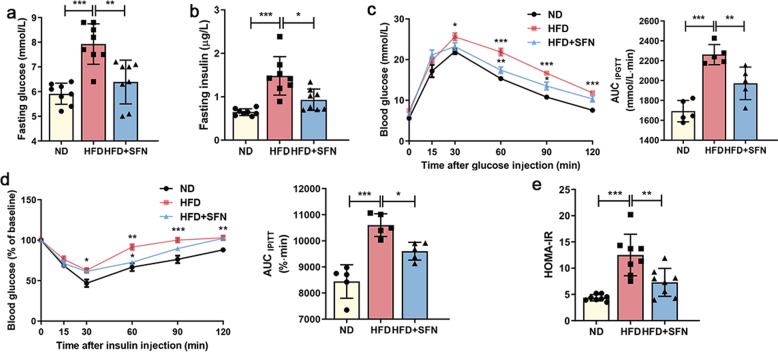
Insulin resistance has been recognized as the key risk factor for NAFLD. To understand if SFN plays a role in altering insulin resistance, GTTs and ITTs were performed to assess the glucose homeostasis and insulin sensitivity in mice with or without SFN supplement. At the baseline, both fasting glucose and insulin levels were higher in mice fed a HFD compared to mice fed a ND. Of which, the lower levels were observed in HFD-mice with SFN supplement (Fig. 3a, b). In addition, a decreased response to glucose load in the HFD-mice was reversed post SFN supplement (Fig. 3c), indicative of an effect of SFN on improving glucose tolerance. Consistently, a rapid response of glucose to insulin load was evident in HFD-mice with SFN supplement (Fig. 3d), along with a decreased insulin resistant index (HOMA-IR) score (Fig. 3e). Thus, SFN supplement in mice significantly improves obesity-related insulin sensitivity and glucose tolerance.
Fig. 3. Effects of SFN on glucose tolerance and insulin sensitivity.
Levels of (a) blood glucose and (b) plasma insulin in mice after fasting for 12 h (n = 8). c Glucose tolerance tests (GTTs, 2 g/kg) and the calculation of corresponding area under the curve (AUC) (n = 5). d Insulin tolerance tests (ITTs, 0.75 U/kg), presented as % of baseline glucose to control for differences in baseline glucose, and the AUC calculations (n = 5). e HOMA-IR was calculated based on (a) fasting glucose and (b) insulin levels (n = 8). Data are mean ± SD. *P < 0.05, **P < 0.01, ***P < 0.001.
SFN induces hepatic FGF21 signaling and inhibits p38MAPK
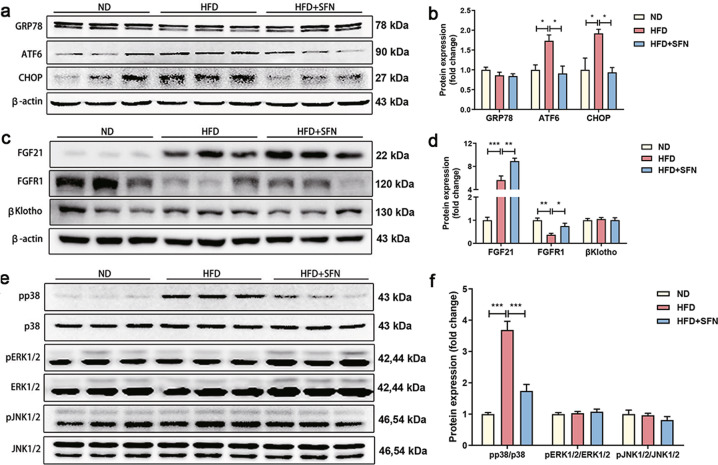
Previous studies have shown that ER stress is involved in the regulation of hepatic steatosis. To determine whether ER stress was associated with SFN-ameliorated NAFLD, glucose regulated protein 78 (GRP78), activating transcription factor 6 (ATF6) and C/EBP homologous protein (CHOP) protein levels were examined by immunoblotting. Although GRP78 protein level was unaltered, enhanced ATF6 and CHOP protein levels were observed in mice fed a HFD compared to mice fed a chow diet, suggesting obesity-induced hepatic ER stress. Of interest, elevated protein expression of hepatic ATF6 and CHOP were significantly reduced by SFN supplement (Fig. 4a, b), indicating an inhibitory role of SFN in ER stress.
Fig. 4. Regulation of hepatic ER stress, FGF21-related and MAPK protein expression levels by SFN.
a, b Representative blots of ER stress-related proteins and the densitometric analysis. c, d Representative blots of FGF21-related proteins and the densitometric analysis. e, f Representative blots of MAPK-related proteins and the densitometric analysis. n = 6. Data are mean ± SD. *P < 0.05, **P < 0.01, ***P < 0.001.
Considerable literatures have shown that FGF21 is not only an energy metabolic regulator but also a stress hormone to maintain metabolic homeostasis [34–36]. To understand if FGF21 was involved in the above effects of SFN, we detected the protein expressions of FGF21, FGF receptor 1 (FGFR1) and obligatory co-receptor βKlotho in liver, epididymal white adipose tissue (eWAT) and brown adipose tissue (BAT). Hepatic FGF21 protein level was increased in HFD-fed mice and further aggravated by SFN (Fig. 4c, d). However, no change in FGF21 protein expression was observed in eWAT and BAT between HFD and HFD + SFN group (Supplementary Fig. S2). The protein expression of hepatic FGFR1 was reduced by HFD, which was prevented upon SFN supplement (Fig. 4c, d). However, the alterations of hepatic FGFR1 did not occur in eWAT and BAT (Supplementary Fig. S2). In addition, the expression of βKlotho was affected neither in liver nor in eWAT and BAT between HFD and HFD + SFN group (Supplementary Fig. S2). Next, we examined the signaling of MAPK, including p38, ERK1/2 and JNK1/2 as the downstream of FGF21. SFN supplement significantly suppressed phosphorylation of p38MAPK level without affecting ERK and JNK in the liver (Fig. 4e, f). Thus, SFN increased hepatic FGF21 and FGFR1 expressions and reduced p38MAPK phosphorylation in mice with NAFLD.
Beneficial effect of SFN on NAFLD is diminished with the knockdown of FGFR1 in vitro
To further understand the mechanism of SFN in NAFLD, we treated the HepG2 cells with FFAs for 24 h to establish an in vitro model, then followed by incubating with SFN at different doses for additional 24 h. SFN (≤20 μM) has been proven not to affect the viability of HepG2 cells at 24 h and 48 h (Supplementary Fig. S3a). We found that 20 μM SFN increased FGF21 protein level in the absence of FFAs (Fig. 5a). Of interest, FFAs treatment also increased FGF21 protein expression (Fig. 5a), which were further enhanced by SFN in a dose-dependent manner (Fig. 5a). 20 µM SFN showed the best effect on down-regulating FAS, PPARγ, whereas downregulation of phosphorylated p38MAPK was evident even with 10 µM SFN (Fig. 5a, b). In consistent with results in cellular FGF21, secreted FGF21 was also increased post SFN treatment (Supplementary Fig. S3b). Unlike FGF21, the protein expression of FGFR1 and βKlotho was reduced in the presence of FFAs, of which, the reduction was prevented by SFN treatment (Fig. 5c, d). Importantly, the recovery of FGFR1 protein occurred earlier at 1 h than βKlotho at 2 h and FGF21 at 4 h post SFN (Fig. 5c, d), implicating a functional reaction of SFN on FGFR1 molecules before the sequential reactions on βKlotho and FGF21 molecules. In consistent, a decrease in phosphorylated p38MAPK was evident at 1 h post SFN (Fig. 5c, d). Treatments with SFN alone also increased FGF21, FGFR1 and βKlotho protein levels at different time points (Supplementary Fig. S3c). To further understand the relationship of SFN with FGFR1 and FGF21, we checked if SFN-associated alterations on their protein levels were due to any effect on gene transcription. The mRNA levels of FGF21 and FGFR1 were unaffected at 1 h, 2 h and 4 h post SFN treatment (Fig. 5e), which means SFN affected protein of FGFR1 not mRNA.
Fig. 5. FGF21 signaling was the target of SFN and the beneficial role of SFN was influenced by the knockdown of FGFR1 in vitro.
a, b HepG2 cells were pre-treated with FFAs for 24 h and continued stimulated with three concentrations of SFN for 24 h. Representative blots of FGF21, FAS, PPARγ, pp38 and densitometric analysis. c, d Effects of SFN on FGF21 pathway and pp38 expression in a time-dependent manner and densitometric analysis. e Quantification of the genes of FGF21 and FGFR1 in HepG2 cells. f, g WB of HepG2 cells transfected with “scrambled” siRNA (Control siRNA) or FGFR1 siRNA and densitometric analysis. h, i pp38 and FGF21 were detected by immunoblotting in HepG2 cells with FGFR1 knockdown and densitometric analysis. j, k WB of HepG2 cells transfected with “scrambled” siRNA (Control siRNA) or p38 siRNA and densitometric analysis. l, m PPARγ and FAS protein levels were detected in HepG2 cells with p38 knockdown and densitometric analysis. Data are mean ± SD of three independent experiments. *P < 0.05, **P < 0.01, ***P < 0.001.
In addition, we also investigated the effect of SFN on the downstream of FGF21/FGFR1 axis by knockdown of FGFR1 via RNA interference. The higher silencing efficiency siRNA Oligos were used in subsequent study after Western blot tests (Fig. 5f, g, j, k). SFN-mediated a rapid up-regulation of FGF21 and downregulation of phosphorylated p38MAPK were weakened with RNA interference of FGFR1 (Fig. 5h, i). Furthermore, knockdown of p38 blunted SFN-mediated downregulation of FAS and PPARγ protein levels (Fig. 5l, m). Consistently, the reduced lipid deposition in SFN treated HepG2 cells was weakened after FGFR1 and p38 knockdown (Supplementary Fig. S3d, e). Taken together, SFN acts on FGFR1 rather than FGF21 to rescue mice from FGF21-resistant status.
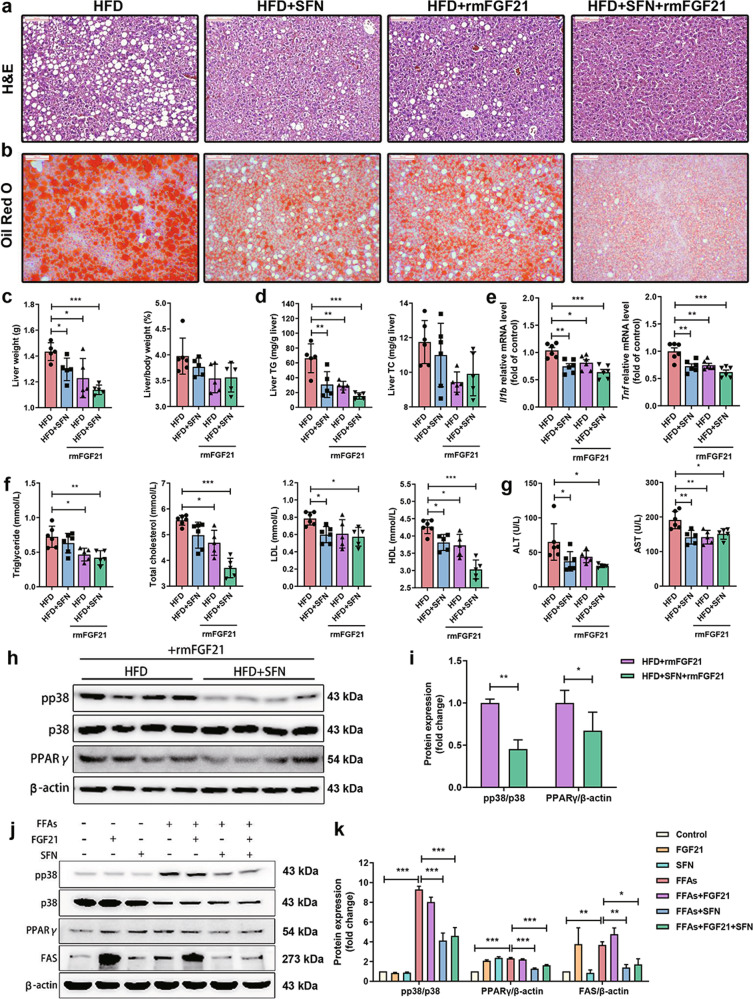
SFN combines with exogenous FGF21 to further improve hepatic steatosis in HFD-fed mice
To further clarify the association between FGF21 and SFN in mediating hepatic steatosis and inflammation, mice were administered rmFGF21 (1.5 mg/kg, i.p., every other day) for 3 weeks with or without SFN supplement (Supplementary Fig. S1b). Formation of fat droplets was reduced in the livers of HFD-mice with single SFN or rmFGF21 treatment. Of which, a further reduction was evident in mice with combination of SFN and rmFGF21 (Fig. 6a, b). Consistently, lowest levels of liver weight and hepatic TG were observed in mice with combination of SFN and rmFGF21 relative to single SFN or rmFGF21 treatment (Fig. 6c, d). However, neither the indices of liver to body weight nor level of hepatic TC was altered in mice following treatment with single SFN, rmFGF21 and a combination of SFN and rmFGF21 (Fig. 6c, d). The gene expression levels of pro-inflammatory cytokines Il1b and Tnf were decreased in the single SFN and rmFGF21 group and further decreased following combined treatment (Fig. 6e). Plasma lipid results showed that circulating TG, TC and HDL levels were significantly reduced in SFN + rmFGF21 group compared to single SFN or rmFGF21 treatment, while LDL, ALT and AST levels had no change among three groups (Fig. 6f, g). Furthermore, hepatic p38MAPK phosphorylation and PPARγ levels were dramatically reduced in mice with combined treatment compared to single rmFGF21 treatment (Fig. 6h, i and Supplementary Fig. S4a–c). Interestingly, FAS protein levels were increased dramatically under exogenous FGF21 treatment with or without FFAs, but returned to normal level when treatment with combination of SFN and FGF21 in the HepG2 cells. Similar results were observed in PPARγ protein expression (Fig. 6j, k), indicating an inhibitory effect of SFN on FAS and PPARγ expression. Thus, FGF21 is indispensable in SFN-ameliorated hepatic steatosis and SFN might concert with exogenous FGF21 to protect NAFLD.
Fig. 6. SFN combines with exogenous FGF21 to further improve hepatic steatosis in vivo and in vitro.
a, b Representative images of lipid droplets by H&E staining (original magnification, ×200; scale bars, 100 μm) and by oil red O (original magnification, ×100; scale bars, 200 μm). c Liver weight (left panel) and liver-to-body weight indices (%) (right panel). d Content of liver TG (left panel) and TC (right panel). e Quantification of the hepatic genes involved in inflammation. f Levels of plasma TG, TC, LDL and HDL. g Levels of plasma ALT (left panel) and AST (right panel). h, i Representative blots of pp38 and PPARγ in liver and densitometric analysis. j, k pp38, PPARγ and FAS protein levels were determined by immunoblotting with or without exogenous treatment with FGF21 under SFN treatment and densitometric analysis (n are of three independent experiments). n = 8. Data are mean ± SD. *P < 0.05, **P < 0.01, ***P < 0.001.
Discussion
In the present study, we provide several novel findings. Firstly, SFN alleviated hepatic steatosis and inflammation in association with reducing lipogenesis, increasing lipolysis and fatty acid oxidation. Secondly, HFD caused a compensatory increase in hepatic FGF21 and decreased FGFR1 expression along with an elevated phosphorylation of p38MAPK in mouse liver. However, SFN prevented HFD-mediated reduction of FGFR1 along with reducing the phosphorylation of p38MAPK. In addition, the effect of SFN on FGF21 and FGFR1 protein was replicated in the HepG2 cells in the presence of FFAs. More importantly, SFN-mediated recovery of FGFR1 occurred at the earlier time point before enhancing FGF21, in conjunction with a decrease in the phosphorylation of p38MAPK. Furthermore, the effect of SFN on preserving FGFR1 protein was on the translational rather than transcriptional level as mRNA levels of FGFR1 was unaffected by SFN. Lastly, gene silencing of FGFR1 abolished the effect of SNF on upregulating FGF21 and down-regulating phosphorylated p38MAPK, and gene silencing of p38 blunted SFN-mediated downregulation of FAS and PPARγ. Meanwhile, SFN with exogenous FGF21 improved hepatic steatosis in HFD-fed mice. Thus, SFN may serve as a stabilizer of FGFR1 and βKlotho, thereby enhancing the expression of FGF21. Through which, it prevents the phosphorylation of p38MAPK, thereby alleviating lipid metabolism disorders in vivo and in vitro.
It is well known that effective signal transduction of FGF21 is promoted after its binding to FGFR1 and βKlotho [37, 38]. Paradoxically, obesity increases circulating FGF21 in both mice [25] and humans [39], most likely as a result of increased fatty liver, which suggests that obesity leads to an FGF21-resistant state [40]. Although the physiology of FGF21 is complicated because it is synthesized in multiple organs and can act on multiple target tissues, SFN enhances FGF21 expression only in mouse liver. This suggests a liver specificity of SFN/FGF21 signaling that can modulate NAFLD. As FGF21 binds to FGF receptors with extremely low affinity, although FGFR1 has the highest affinity for FGF21 [37], one would propose that the increased level of FGF21 in obesity lacks of sufficient amount of FGFR1 to form a functional complex. However, SFN rapidly enhances the protein levels of FGFR1 along with a decrease in the phosphorylation of p38MAPK, which strongly indicates that SFN promotes FGF21 signaling in vivo that starts from modulating FGFR1. It is currently unknown whether SFN prevented degradation of FGFR1 or enhanced translation of FGFR1. It would be interesting to investigate this with the follow-up studies. This notion is further supported by the result that SFN does not have a rapid gene effect on FGFR1 and FGF21. Meanwhile, the maximum reduction in the phosphorylation of p38MAPK is evident only with additional elevated FGF21 protein by SFN, demonstrating that SFN enables an effective signal transduction of FGF21 through promoting interaction of FGF21 with FGFR1 and βKlotho.
Treatment with SFN does not affect HFD-induced weight gain (Supplementary Fig. S5a, b), but reduced hepatic TG levels. It is noteworthy that the combination of SFN and FGF21 displays a better effect on improving hepatic steatosis than single SFN or FGF21 supplement. This suggests that, apart from stabilizing FGFR1, SFN may also promote effective signaling transduction by employing efficient amount of FGF21 to enhance the formation of FGF21 with FGFR1. Combining the RT-qPCR results of lipid metabolism genes, the role of SFN administration in inhibiting excessive fat deposition was through altering lipid metabolism, via a pathway that may be associated with p38MAPK mediated pathway [41–43]. This notion is further supported by our results that HFD or FFAs-mediated increase in p38MAPK phosphorylation and FAS expression is restored by SFN supplement. In addition, SFN-mediated decrease in FAS and PPARγ protein expression in HepG2 cells is weakened by knocking down gene of p38MAPK, indicative of the involvement of p38MAPK in SFN-mediated fatty acid metabolism. However, the question as to how p38MAPK contributes to lipogenesis needs further studies to clarify. In addition, obesity-related hepatic ER stress wase suppressed after SFN supplement, mainly through reducing ATF6 and CHOP protein expression rather than GRP78, suggesting that ER stress might not be the main potential mechanism in SFN-ameliorated NAFLD. In our study, HFD increased the protein level of Nrf2 in whole liver extracts (Supplementary Fig. S6a, b), which is consistent with the data that obese patients with hepatic steatosis have higher Nrf2 protein level in liver [44]. However, SFN supplement could not further increase the protein level of Nrf2, which enlighten us to explore more potential molecular mechanisms involved in the pathogenesis of NAFLD.
In conclusion, SFN can alleviate hepatic triglyceride accumulation, inflammation and improve insulin sensitivity in HFD-mice. On the molecular level, SFN enables effective signaling transduction of FGF21 through enhancing the levels of FGFR1 protein thereby rescuing mice from hepatic FGF21-resistant state. On the other hand, SFN can also combine with exogenous FGF21 to improve hepatic steatosis in HFD-fed mice (Fig. 7). Our studies verified that SFN may become a promising drug to treat or relieve NAFLD. Further investigation is required for elucidation of efficacy and safety of combination of SFN and FGF21 on improvement of NAFLD patients.
Fig. 7. Schematic diagram showing the effect of SFN on NAFLD.
SFN decreased the severity of experimental NAFLD via mechanisms likely to involve the up-regulation of FGF21/FGFR1. SFN functions on FGFR1 first then trigger FGF21/FGFR1 signaling, thereby the signaling can pass on p-p38MAPK. Thus, SFN alleviated steatosis and inflammation induced by fatty acids.
Supplementary Information
Acknowledgements
This research was supported by the National Natural Science Foundation of China (Grant No. 31871773 to QXZ, 81600664 to XLY and 31471321 to ZH).
Author contributions
YKW performed experiments and analyzed data. ZNR and SLZ assisted the experiments. GW, HZ and WC critically reviewed the manuscript. YZW and XLY provided the FGF21 growth factor. YKW and QXZ designed and interpreted experiments. YKW, ZH and QXZ wrote the paper.
Data availability
The authors declare that the main data supporting the findings of this study are available within the article and its Supplementary Information files. Extra data are available from the corresponding author upon request.
Competing interests
The authors declare no competing interests.
Contributor Information
Xian-long Ye, Email: yexianlong1988@163.com.
Qi-xiao Zhai, Email: zhaiqixiao@sina.com.
Supplementary information
The online version contains supplementary material available at 10.1038/s41401-021-00786-2.
References
- 1.Rinella ME. Nonalcoholic fatty liver disease: a systematic review. JAMA. 2015;313:2263–73. doi: 10.1001/jama.2015.5370. [DOI] [PubMed] [Google Scholar]
- 2.Yang G, Lee HE, Lee JY. A pharmacological inhibitor of NLRP3 inflammasome prevents non-alcoholic fatty liver disease in a mouse model induced by high fat diet. Sci Rep. 2016;6:24399. doi: 10.1038/srep24399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Yao HR, Liu J, Plumeri D, Cao YB, He T, Lin L, et al. Lipotoxicity in HepG2 cells triggered by free fatty acids. Am J Transl Res. 2011;3:284–91. [PMC free article] [PubMed] [Google Scholar]
- 4.Tilg H, Moschen AR. Evolution of inflammation in nonalcoholic fatty liver disease: the multiple parallel hits hypothesis. Hepatology. 2010;52:1836–46.. doi: 10.1002/hep.24001. [DOI] [PubMed] [Google Scholar]
- 5.Alonso C, Fernandez-Ramos D, Varela-Rey M, Martinez-Arranz I, Navasa N, Van Liempd SM, et al. Metabolomic identification of subtypes of nonalcoholic steatohepatitis. Gastroenterology. 2017;152:1449–61 e7. doi: 10.1053/j.gastro.2017.01.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Dietrich MO, Horvath TL. Limitations in anti-obesity drug development: the critical role of hunger-promoting neurons. Nat Rev Drug Discov. 2012;11:675–91. doi: 10.1038/nrd3739. [DOI] [PubMed] [Google Scholar]
- 7.Sanyal AJ, Chalasani N, Kowdley KV, McCullough A, Diehl AM, Bass NM, et al. Pioglitazone, vitamin E, or placebo for nonalcoholic steatohepatitis. N Engl J Med. 2010;362:1675–85. doi: 10.1056/NEJMoa0907929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Dowman JK, Armstrong MJ, Tomlinson JW, Newsome PN. Current therapeutic strategies in non-alcoholic fatty liver disease. Diabetes Obes Metab. 2011;13:692–702. doi: 10.1111/j.1463-1326.2011.01403.x. [DOI] [PubMed] [Google Scholar]
- 9.Kikuchi M, Ushida Y, Shiozawa H, Umeda R, Tsuruya K, Aoki Y, et al. Sulforaphane-rich broccoli sprout extract improves hepatic abnormalities in male subjects. World J Gastroenterol. 2015;21:12457–67. doi: 10.3748/wjg.v21.i43.12457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Su X, Jiang X, Meng L, Dong X, Shen Y, Xin Y. Anticancer activity of sulforaphane: the epigenetic mechanisms and the Nrf2 signaling pathway. Oxid Med Cell Longev. 2018;2018:5438179. doi: 10.1155/2018/5438179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Choi KM, Lee YS, Kim W, Kim SJ, Shin KO, Yu JY, et al. Sulforaphane attenuates obesity by inhibiting adipogenesis and activating the AMPK pathway in obese mice. J Nutr Biochem. 2014;25:201–7. doi: 10.1016/j.jnutbio.2013.10.007. [DOI] [PubMed] [Google Scholar]
- 12.Zhou R, Lin J, Wu D. Sulforaphane induces Nrf2 and protects against CYP2E1-dependent binge alcohol-induced liver steatosis. Biochimica et biophysica acta. 2014;1840:209–18. doi: 10.1016/j.bbagen.2013.09.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Axelsson AS, Tubbs E, Mecham B, Chacko S, Nenonen HA, Tang Y, et al. Sulforaphane reduces hepatic glucose production and improves glucose control in patients with type 2 diabetes. Sci Transl Med. 2017;9:eaah4477. doi: 10.1126/scitranslmed.aah4477. [DOI] [PubMed] [Google Scholar]
- 14.Bahadoran Z, Tohidi M, Nazeri P, Mehran M, Azizi F, Mirmiran P. Effect of broccoli sprouts on insulin resistance in type 2 diabetic patients: a randomized double-blind clinical trial. Int J food Sci Nutr. 2012;63:767–71. doi: 10.3109/09637486.2012.665043. [DOI] [PubMed] [Google Scholar]
- 15.Chartoumpekis DV, Ziros PG, Psyrogiannis AI, Papavassiliou AG, Kyriazopoulou VE, Sykiotis GP, et al. Nrf2 represses FGF21 during long-term high-fat diet-induced obesity in mice. Diabetes. 2011;60:2465–73. doi: 10.2337/db11-0112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Xu J, Kulkarni SR, Donepudi AC, More VR, Slitt AL. Enhanced Nrf2 activity worsens insulin resistance, impairs lipid accumulation in adipose tissue, and increases hepatic steatosis in leptin-deficient mice. Diabetes. 2012;61:3208–18. doi: 10.2337/db11-1716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Markan KR, Naber MC, Ameka MK, Anderegg MD, Mangelsdorf DJ, Kliewer SA, et al. Circulating FGF21 is liver derived and enhances glucose uptake during refeeding and overfeeding. Diabetes. 2014;63:4057–63. doi: 10.2337/db14-0595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hondares E, Iglesias R, Giralt A, Gonzalez FJ, Giralt M, Mampel T, et al. Thermogenic activation induces FGF21 expression and release in brown adipose tissue. J Biol Chem. 2011;286:12983–90. doi: 10.1074/jbc.M110.215889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Fisher FM, Kleiner S, Douris N, Fox EC, Mepani RJ, Verdeguer F, et al. FGF21 regulates PGC-1alpha and browning of white adipose tissues in adaptive thermogenesis. Genes Dev. 2012;26:271–81. doi: 10.1101/gad.177857.111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Adams AC, Coskun T, Cheng CC, O Farrell LS, Dubois SL, Kharitonenkov A. Fibroblast growth factor 21 is not required for the antidiabetic actions of the thiazoladinediones. Mol Metab. 2013;2:205–14. doi: 10.1016/j.molmet.2013.05.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ribas F, Villarroya J, Hondares E, Giralt M, Villarroya F. FGF21 expression and release in muscle cells: involvement of MyoD and regulation by mitochondria-driven signalling. Biochem J. 2014;463:191–9. doi: 10.1042/BJ20140403. [DOI] [PubMed] [Google Scholar]
- 22.Xu J, Lloyd DJ, Hale C, Stanislaus S, Chen M, Sivits G, et al. Fibroblast growth factor 21 reverses hepatic steatosis, increases energy expenditure, and improves insulin sensitivity in diet-induced obese mice. Diabetes. 2009;58:250–9. doi: 10.2337/db08-0392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kharitonenkov A, Shiyanova TL, Koester A, Ford AM, Micanovic R, Galbreath EJ, et al. FGF-21 as a novel metabolic regulator. J Clin Invest. 2005;115:1627–35. doi: 10.1172/JCI23606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Badman MK, Koester A, Flier JS, Kharitonenkov A, Maratos-Flier E. Fibroblast growth factor 21-deficient mice demonstrate impaired adaptation to ketosis. Endocrinology. 2009;150:4931–40.. doi: 10.1210/en.2009-0532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Muise ES, Azzolina B, Kuo DW, El-Sherbeini M, Tan Y, Yuan X, et al. Adipose fibroblast growth factor 21 is up-regulated by peroxisome proliferator-activated receptor gamma and altered metabolic states. Mol Pharmacol. 2008;74:403–12. doi: 10.1124/mol.108.044826. [DOI] [PubMed] [Google Scholar]
- 26.Zhang X, Yeung DC, Karpisek M, Stejskal D, Zhou ZG, Liu F, et al. Serum FGF21 levels are increased in obesity and are independently associated with the metabolic syndrome in humans. Diabetes. 2008;57:1246–53. doi: 10.2337/db07-1476. [DOI] [PubMed] [Google Scholar]
- 27.Badman MK, Kennedy AR, Adams AC, Pissios P, Maratos-Flier E. A very low carbohydrate ketogenic diet improves glucose tolerance in ob/ob mice independently of weight loss. Am J Physiol Endocrinol Metab. 2009;297:E1197–204. doi: 10.1152/ajpendo.00357.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Dostalova I, Kavalkova P, Haluzikova D, Lacinova Z, Mraz M, Papezova H, et al. Plasma concentrations of fibroblast growth factors 19 and 21 in patients with anorexia nervosa. J Clin Endocrinol Metab. 2008;93:3627–32. doi: 10.1210/jc.2008-0746. [DOI] [PubMed] [Google Scholar]
- 29.Yan H, Xia M, Chang X, Xu Q, Bian H, Zeng M, et al. Circulating fibroblast growth factor 21 levels are closely associated with hepatic fat content: a cross-sectional study. PLoS One. 2011;6:e24895. doi: 10.1371/journal.pone.0024895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kralisch S, Fasshauer M. Fibroblast growth factor 21: effects on carbohydrate and lipid metabolism in health and disease. Curr Opin Clin Nutr Metab Care. 2011;14:354–9. doi: 10.1097/MCO.0b013e328346a326. [DOI] [PubMed] [Google Scholar]
- 31.Geng L, Lam KSL, Xu A. The therapeutic potential of FGF21 in metabolic diseases: from bench to clinic. Nat Rev Endocrinol. 2020;16:654–67.. doi: 10.1038/s41574-020-0386-0. [DOI] [PubMed] [Google Scholar]
- 32.Kilkenny C, Browne W, Cuthill IC, Emerson M, Altman DG, Group NCRRGW. Animal research: reporting in vivo experiments: the ARRIVE guidelines. Br J Pharmacol. 2010;160:1577–9. doi: 10.1111/j.1476-5381.2010.00872.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Landis SC, Amara SG, Asadullah K, Austin CP, Blumenstein R, Bradley EW, et al. A call for transparent reporting to optimize the predictive value of preclinical research. Nature. 2012;490:187–91. doi: 10.1038/nature11556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Schaap FG, Kremer AE, Lamers WH, Jansen PL, Gaemers IC. Fibroblast growth factor 21 is induced by endoplasmic reticulum stress. Biochimie. 2013;95:692–9. doi: 10.1016/j.biochi.2012.10.019. [DOI] [PubMed] [Google Scholar]
- 35.Kim KH, Lee MS. FGF21 as a mediator of adaptive responses to stress and metabolic benefits of anti-diabetic drugs. J Endocrinol. 2015;226:R1–16. doi: 10.1530/JOE-15-0160. [DOI] [PubMed] [Google Scholar]
- 36.Kim SH, Kim KH, Kim HK, Kim MJ, Back SH, Konishi M, et al. Fibroblast growth factor 21 participates in adaptation to endoplasmic reticulum stress and attenuates obesity-induced hepatic metabolic stress. Diabetologia. 2015;58:809–18. doi: 10.1007/s00125-014-3475-6. [DOI] [PubMed] [Google Scholar]
- 37.Yie J, Wang W, Deng L, Tam LT, Stevens J, Chen MM, et al. Understanding the physical interactions in the FGF21/FGFR/beta-Klotho complex: structural requirements and implications in FGF21 signaling. Chem Biol drug Des. 2012;79:398–410. doi: 10.1111/j.1747-0285.2012.01325.x. [DOI] [PubMed] [Google Scholar]
- 38.Micanovic R, Raches DW, Dunbar JD, Driver DA, Bina HA, Dickinson CD, et al. Different roles of N- and C- termini in the functional activity of FGF21. J Cell Physiol. 2009;219:227–34.. doi: 10.1002/jcp.21675. [DOI] [PubMed] [Google Scholar]
- 39.Dushay J, Chui PC, Gopalakrishnan GS, Varela-Rey M, Crawley M, Fisher FM, et al. Increased fibroblast growth factor 21 in obesity and nonalcoholic fatty liver disease. Gastroenterology. 2010;139:456–63. doi: 10.1053/j.gastro.2010.04.054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Fisher FM, Chui PC, Antonellis PJ, Bina HA, Kharitonenkov A, Flier JS, et al. Obesity is a fibroblast growth factor 21 (FGF21)-resistant state. Diabetes. 2010;59:2781–9. doi: 10.2337/db10-0193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Hirosumi J, Tuncman G, Chang L, Gorgun CZ, Uysal KT, Maeda K, et al. A central role for JNK in obesity and insulin resistance. Nature. 2002;420:333–6. doi: 10.1038/nature01137. [DOI] [PubMed] [Google Scholar]
- 42.Jager J, Corcelle V, Gremeaux T, Laurent K, Waget A, Pages G, et al. Deficiency in the extracellular signal-regulated kinase 1 (ERK1) protects leptin-deficient mice from insulin resistance without affecting obesity. Diabetologia. 2011;54:180–9. doi: 10.1007/s00125-010-1944-0. [DOI] [PubMed] [Google Scholar]
- 43.Cao W, Collins QF, Becker TC, Robidoux J, Lupo EG, Jr., Xiong Y, et al. p38 Mitogen-activated protein kinase plays a stimulatory role in hepatic gluconeogenesis. J Biol Chem. 2005;280:42731–7. doi: 10.1074/jbc.M506223200. [DOI] [PubMed] [Google Scholar]
- 44.Bricambert J, Alves-Guerra MC, Esteves P, Prip-Buus C, Bertrand-Michel J, Guillou H, et al. The histone demethylase Phf2 acts as a molecular checkpoint to prevent NAFLD progression during obesity. Nat Commun. 2018;9:2092. doi: 10.1038/s41467-018-04361-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The authors declare that the main data supporting the findings of this study are available within the article and its Supplementary Information files. Extra data are available from the corresponding author upon request.