Dear Editor,
Multiple endocrine neoplasia 1 (MEN1) syndrome, a tumor predisposition disease caused by MEN1 germline mutation, is characterized by combined occurrence of neuroendocrine tumors (NETs) in multiple organs.1 Pancreatic neuroendocrine tumors (PanNETs) are the major cause of the increased mortality in MEN1 patients due to the most malignant potential.1,2 Treatment of NETs with MEN1 is challenging due to concomitant development of multiple tumors and the limited treatment efficiency, where first-line therapy of somatostatin analogues (SSA) shows no more than 10% objective response rate.2 Improved treatments for MEN1 NETs are urgently required.2
In this study, we performed a CRISPR-Cas9-based synthetic lethal screening and identified an interaction between MEN1 mutation and dihydroorotate dehydrogenase (DHODH) inhibition. Leflunomide, an inhibitor of DHODH, efficiently targeted MEN1-mutated tumors in cancer cell lines, xenograft models, as well as patients in a clinical trial. More importantly, leflunomide eliminated almost all the spontaneous development of neuroendocrine tumors in mice with a germline mutation in Men1, providing new insights into the chemoprevention of MEN1 syndrome.
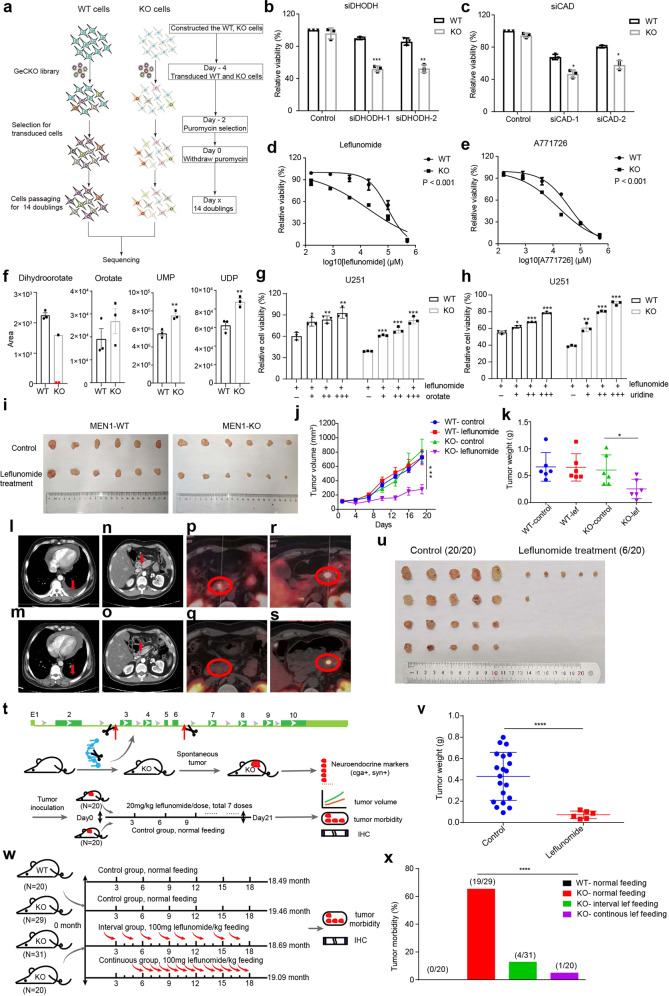
We constructed a MEN1 knockout cell line in U251 and performed a genome-wide CRISPR-Cas9 based knockout screening in isogenic cell lines U251-MEN1+/+ (MEN1-WT) and U251-MEN1–/– (MEN1-KO) (Fig. 1a; Supplementary information, Fig. S1). Of the 19,114 genes tested, we identified 26 genes that were essential for the viability of MEN1-KO cells (Supplementary information, Table S1, Fig. S2). Among them, CAD and DHODH, the pivotal rate-limiting enzymes for de novo pyrimidine synthesis, exhibited the most significant synthetic lethal effect in MEN1-KO cells. Most MEN1-KO cells were dead after transfection with siRNA to knock down DHODH or CAD, while viability of MEN1-WT cells was only slightly reduced (Fig. 1b, c; Supplementary information, Fig. S3a–d). Similar phenomenon was observed in hTERT RPE-1 cells (Supplementary information, Fig. S3e–j).
Fig. 1. Genome-wide CRISPR-Cas9 screening identifies synthetic lethal partners of MEN1 deficiency.
a Screening strategy for identification of synthetic lethal partners of MEN1 deficiency. b Relative cell viability of WT (MEN1-WT) and KO (MEN1-KO) cells with DHODH siRNA knockdown assessed with the CCK8 assay at OD 450. c Relative cell viability of WT and KO cells with CAD siRNA knockdown assessed with the CCK8 assay at OD 450. d, e Cell viability assessed with the CCK8 in WT and KO cells in the absence or presence of leflunomide or A771726 at multiple concentrations. f Relative metabolite concentrations of dihydroorotate, orotate, UMP and UDP in MEN1-WT and MEN1-KO cells. DHODH catalyzes the conversion of dihydroorotate to orotate. UMP, Uridine 5’-monophosphate; UDP, Uridine 5’-diphosphate. The red dot represents the undetectable value. g, h The relative cell viability of WT and KO cells treated with 100 µM leflunomide in combination with different concentrations of orotate (g) and uridine (h) for 48 h assessed with CCK8 assay. i Representative images of MEN1-WT and MEN1-KO U251 xenograft tumors from nude mice treated with leflunomide or vehicle control after 7 doses. j Quantification of xenograft tumor volume at different days after administration (n = 6, for both vehicle-treated and leflunomide-treated groups). k Weight of tumors harvested from animals after treatment with leflunomide or vehicle control. l Chest CT before taking the leflunomide. m Chest CT after 2 months of medication. n Abdominal CT before taking the leflunomide. o Abdominal CT after 2 months of medication. p Tumor in the head of pancreas with high SSTR2 expression (SUVmax 10.4). q Susceptive tumor in the head of pancreas with almost no SSTR2 expression (SUVmax 5.6) by GA68-PETCT at third visit. r Tumor in the tail of pancreas with high SSTR2 expression (SUVmax 27.8). s Tumor in the tail of pancreas with decreased SSTR2 expression (SUVmax 18.7). t Schematic of the construction of Men1 knockout mice with CRISPR-Cas9 technology and the subsequent drug delivery over 21 days. u Images of spontaneous tumors in Men1+/− mice under leflunomide or vehicle treatment for 7 doses. v Quantification of spontaneous tumor weight after treatment with leflunomide or control. w Schematic of the time line and leflunomide treatment for prevention of tumor development in Men1+/− mice. x Tumor morbidity for the 4 different groups of mice based on feeding with or without leflunomide: WT-normal feeding, KO-normal feeding, KO-interval treatment group and KO-continuous treatment group.
Leflunomide is a potent DHODH inhibitor that has been used to treat rheumatoid arthritis for 30 years and shows antitumor potency in recent studies.3–6 The IC50 of leflunomide in the MEN1-KO cell line was 32 μM, which was 4-fold lower compared with that in MEN1-WT cells (Fig. 1d). When MEN1 expression was reconstituted in MEN1-KO cells, the sensitivity to leflunomide was comparable to that in MEN1-WT cells (Fig. S4a). The IC50 of leflunomide was also significantly lower in MEN1-mutated cell lines compared with MEN1-WT cell lines generated from other tumor types, including breast (ZR-751 vs MCF7) and prostate cancer (22RV1 vs LNCaP) (Supplementary information, Fig. S4b, c). Furthermore, we treated MEN1-WT and MEN1-KO cells with A771726, the active metabolite of leflunomide, and found that the MEN1-KO cells were more sensitive to A771726 than MEN1-WT cells (Fig. 1e). Similar phenomenon was observed in the MEN1-mutated cancer cell lines (Supplementary information, Fig. S4d). These data suggest that DHODH is a specific synthetic lethal target of MEN1 deficiency.
DHODH plays a key role in the conversion of dihydroorotate to orotate and links mitochondria to the biosynthesis of pyrimidine nucleotides.7,8 When we profiled the metabolites in this pathway, liquid chromatography tandem mass spectrometry (LC-MS/MS) results revealed that MEN1 deficiency dramatically decreased the level of dihydroorotate, while the levels of the downstream products including UMP and UDP increased significantly. Orotate was not significantly increased, possibly because the orotate accumulation through increased production from dihydroorotate was compromised by increased transfer to UMP and UDP (Fig. 1f; Supplementary information, Table S2). This is consistent with previous reports that the de novo pyrimidine synthesis is regulated by two rate limiting steps catalyzed by CAD and DHODH.4 This phenomenon inspired us to investigate the expression of DHODH after MEN1 knockout. The protein expression level of DHODH was upregulated in MEN1-KO cells while decreased in MEN1-reconstituted cells (Supplementary information, Fig. S5a). Moreover, we performed Assay for Transposase-Accessible Chromatin using sequencing (ATAC-seq) and RNA-sequencing (RNA-seq) on MEN1-WT and MEN1-KO cells. The results showed that the enhancements from MEN1-KO group over WT group were 1.6-fold and 1.5-fold in promoter accessibility and transcription level, respectively (Supplementary information, Fig. S5b). Thus, we speculated that MEN1-KO cells were addicted to the increased level of pyrimidine metabolites through upregulating the expression of DHODH. Next, we treated cells with orotate or uridine and the growth inhibitory effect caused by leflunomide was rescued with the increasing concentrations of orotate or uridine (Fig. 1g, h). Similarly, we performed the rescue experiment on MEN1-mutated cells including ZR-751 and 22RV1, and the cells showed a higher viability under leflunomide treatment when orotate was added (Supplementary information, Fig. S5c, d). These results indicate that leflunomide selectively kills MEN1 deficient cells due to the insufficient metabolites of pyrimidine biosynthesis.
Next, we constructed a xenograft model in nude mice to explore the antitumor properties of leflunomide in vivo. MEN1-WT and MEN1-KO cells were implanted in mice, and 20 mg/kg leflunomide was administered by gavage, once every 3 days. After 7 doses, MEN1-KO xenografts under leflunomide treatment exhibited 2.5-fold reduced volume and weight relative to MEN1-WT tumors (Fig. 1i–k).
We further set up a clinical trial to test the efficacy of leflunomide for patients with MEN1-mutated tumors. Three MEN1 syndrome patients with PanNETs were enrolled and the efficacy was evaluated every 2 months with CT or GA68-PET-CT. Patient A, a 43 y/o male, went to hospital due to abdominal pain and was diagnosed to have PanNETs with multiple metastasis. His disease progressed in two months with enlarged metastases and bone metastases. After leflunomide treatment, he showed stable disease (SD) at all the four visits in 11 months. Furthermore, the left pleural effusion almost disappeared after 2 months of treatment (Fig. 1l–o). Patient B, a 39 y/o male, underwent mediastinal neuroendocrine tumor (G2) resection with postoperative radiotherapy and chemotherapy since July 2019. The patient developed PanNET (G2) in May 2020, and underwent surgical resection with postoperative first-line standard treatment (Octreotide Acetate Microspheres) for 3 periods. The PanNET recurred in October 2020. After receiving leflunomide treatment, the patient showed SD in five visits in 11 months. Although the size of the tumor was not decreased, there was a decrease in the signal value of the tumor in the dynamic CT scan in the evaluations compared to baseline. Furthermore, the tumor in the head of pancreas almost disappeared and the maximum standardized uptake value (SUVmax) of the pancreatic tail tumor has significantly decreased (from 27.8 to 18.7) by GA68-PET-CT which was profiled at the third visit (Fig. 1p–s). Patient C, a 40 y/o male, underwent mediastinal neuroendocrine tumor resection with postoperative radiotherapy since August 2016. The patient developed PanNETs (G1) with local thoracic vertebrae metastasis in May 2020. He received radiotherapy for 8 times and first-line standard treatment (Octreotide Acetate Microspheres) combined with everolimus for 4 months. The disease progressed with multiple thoracic vertebrae metastasis and liver metastasis in November 2020. After receiving leflunomide treatment from February 2021, Patient C has shown SD in four visits for 8 months. No severe adverse reactions were observed in all the patients.
As the clinical trial was limited to advanced-stage and fast-progressing cases, we tried to test the efficacy of leflunomide on early-stage tumors with animal model (Supplementary information, Fig. S6a). We constructed a mouse model harboring Men1 germline mutation and fed the Men1+/− mice with normal food until one mouse developed a tumor. Western blot analysis confirmed the loss of menin expression (Men1−/−) in the tumor (Supplementary information, Fig. S6b). This tumor tissue was expanded through two successive passages, and an equal volume of small tissue pieces was implanted subcutaneously into healthy wild type Men1+/+ mice. On the third day after implantation, 20 mg/kg leflunomide was administered by gavage once every 3 days in a treatment group of 20 mice, while corn oil was administered as vehicle control in the control group (Fig. 1t). After 7 cycles of therapy, tumor incidence was 100% (20/20) in the control group and 30% (6/20) in the treatment group, and the tumor weight in the 6 mice of the treatment group was significantly lower than that in the control group (Fig. 1u, v). During treatment, there was no significant difference in body weight between the two groups of mice (Supplementary information, Fig. S6c).
Next, we explored whether leflunomide could prevent the spontaneous development of neuroendocrine tumors in Men1+/− mouse model of MEN1 syndrome (Supplementary information, Fig. S7a). The mice were fed with continuous, interval or no supply of leflunomide (Fig. 1w). All mice were sacrificed at the age of 19 months and the pancreas were collected for analysis, as PanNET is the most frequent tumor type appearing in Men1+/− mice and the leading cause of death in MEN1 syndrome patients1,9,10. PanNETs developed only in Men1+/− mice (0%, 0/20 in Men1+/+ mice feeding group). The incidence of spontaneous tumors was markedly reduced in Men1+/− mice under the treatment of leflunomide, and continuous treatment showed greater efficacy than interval treatment (5%, 1/20 in continuous treatment; 12.9%, 4/31 in interval treatment; and 65.5%, 19/29 in no treatment group of Men1+/− mice) (Fig. 1x; Supplementary information, Fig. S7b). The strong preventive performance could be due to the early start of treatment and the specificity in targeting Men1−/− cells, enabling tumor inhibition since the initial step of tumorigenesis, when a Men1+/− normal cell loses the wild type allele to be a single tumor cell of Men1−/−.
In summary, we identified leflunomide as a potential treatment targeting MEN1-mutated tumors. In the clinical trial, leflunomide showed SD, instead of partial response (PR) or complete response (CR), to the advanced-stage tumors which were progressing after first-line treatments. The drug could have better efficacy when applied as first-line treatment or to early-stage tumors. More importantly, leflunomide could show much stronger performance when applied to prevent spontaneous development of tumors in the high-risk individuals with MEN1 syndrome, or to prevent recurrence by targeting minimal residual cancer cells after a radical resection. Compared with advanced-stage tumors, leflunomide showed much stronger efficacy when targeting tumors in small size or in the stage of single tumor cells. Meanwhile, as an anti-rheumatoid drug approved and used for > 30 years,3 leflunomide is relatively safe for long term application and the cost is less than 5% of octreotide, making it feasible for chemoprevention.
Supplementary information
Acknowledgements
We thank Drs. D. Guo, H. Yan, and Y. He for helpful discussions, and Dr. M. Li for data analysis. We would also thank Dr. Xiaohui Liu at the Metabolomics Center at Tsinghua University-National Protein Science Facility (Beijing) for the technical assistance. The project is supported by the National Key R&D Program of China (2021YFC2501004, 2021YFC2500900), the National Natural Science Foundation Fund (81772490, 82172988, 81972311, 82141127), the Chinese Academy of Medical Sciences (CAMS) Innovation Fund for Medical Sciences (CIFMS) (2021-1-I2M-018, 2021-I2M-1-067, 2021-1-I2M-012 and 2021-I2M-1-066), the Non-profit Central Research Institution Fund of Chinese Academy of Medical Sciences (2019PT310026) and Sanming Project of Medicine in Shenzhen (SZSM202011010).
Author contributions
Y.J., H.Z., and Y.-X.Z. were the principal investigators who conceived and designed the study; Y.M. and Q.Z. performed the experiments and wrote the manuscript; X.W. and M.L. coordinated the experimental work with cell lines and animal models; Q.C. and H.Z. supervised the design and implementation of the clinical trial; L.J., and Y.C. contributed to the enrollment of patients; Y.J. and Y.-X.Z. revised the manuscript. All authors critically reviewed the article and approved the final manuscript.
Competing interests
The authors declare no competing interests.
Footnotes
These authors contributed equally: Yarui Ma, Qing Zhu, Xiaobing Wang.
Contributor Information
Yi-Xin Zeng, Email: zengyx@sysucc.org.cn.
Hong Zhao, Email: zhaohong@cicams.ac.cn.
Yuchen Jiao, Email: Jiaoyuchen@cicams.ac.cn.
Supplementary information
The online version contains supplementary material available at 10.1038/s41422-022-00613-1.
References
- 1.Al-Salameh A, Cadiot G, Calender A, Goudet P, Chanson P. Nat. Rev. Endocrinol. 2021;17:207–224. doi: 10.1038/s41574-021-00468-3. [DOI] [PubMed] [Google Scholar]
- 2.Frost M, Lines KE, Thakker RV. Nat. Rev. Endocrinol. 2018;14:216–227. doi: 10.1038/nrendo.2018.3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Goldenberg MM. Clin. Ther. 1999;21:1837–1852. doi: 10.1016/S0149-2918(00)86732-6. [DOI] [PubMed] [Google Scholar]
- 4.Mathur D, et al. Cancer Discov. 2017;7:380–390. doi: 10.1158/2159-8290.CD-16-0612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Brown KK, Spinelli JB, Asara JM, Toker A. Cancer Discov. 2017;7:391–399. doi: 10.1158/2159-8290.CD-16-0611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bajzikova M, et al. Cell Metab. 2019;29:399–416. doi: 10.1016/j.cmet.2018.10.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Loffler M, Jockel J, Schuster G, Becker C. Mol. Cell Biochem. 1997;174:125–129. doi: 10.1023/A:1006859115450. [DOI] [PubMed] [Google Scholar]
- 8.Reis RAG, Calil FA, Feliciano PR, Pinheiro MP, Nonato MC. Arch. Biochem. Biophys. 2017;632:175–191. doi: 10.1016/j.abb.2017.06.019. [DOI] [PubMed] [Google Scholar]
- 9.Mohr H, Pellegata NS. Endocr. Relat. Cancer. 2017;24:T161–T177. doi: 10.1530/ERC-17-0249. [DOI] [PubMed] [Google Scholar]
- 10.Crabtree JS, et al. Proc. Natl. Acad. Sci. USA. 2001;98:1118–1123. doi: 10.1073/pnas.98.3.1118. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.