Abstract
Background
Fibroblast growth factor 21 (FGF-21), alpha-amylase, and alpha-glucosidase are key proteins implicated in metabolic dysregulations. Bisphenol A (BPA) is an environmental toxicant known to cause endocrine dysregulations. Hesperidin from citrus is an emerging flavonoid for metabolic diseases management. Through computational approach, we investigated the potentials of hesperidin in abrogating BPA interference in metabolism. The 3D crystal structure of the proteins (FGF-21, α-amylase, and α-glucosidase) and the ligands (BPA and hesperidin) were retrieved from the PDB and PubChem database respectively. Using Autodock plugin Pyrx, molecular docking of the ligands and individual proteins were performed to ascertain their binding affinities and their potentials to compete for the same binding site. Validation of the docking study was considered as the ability of the ligands to bind at the same site of each proteins. The docking poses were visualized using UCSF Chimera and Discovery Studio 2020, respectively to reveal each of the protein-ligands interactions within the binding pockets. Using SwissAdme and AdmeSar servers, we further investigated hesperidin’s ADMET profile. Hesperidin used was purchased commercially.
Results
Hesperidin and BPA competitively bound to the same site on each protein. Interestingly, hesperidin had greater binding affinities (Kcal/mol) − 5.80, − 9.60, and − 9.60 than BPA (Kcal/mol) − 4.40, − 7.20, − 7.10 for FGF-21, α-amylase, and α-glucosidase respectively. Visualizations of the binding poses showed that hesperidin interacted with stronger bonds than BPA within the proteins’ pockets. Although hesperidin violated Lipinski rule of five, this however can be optimized through structural modifications.
Conclusions
Hesperidin may be an emerging natural product with promising therapeutic potentials against metabolic and endocrine derangement.
Keywords: Fibroblast growth factor-21, α-amylase, α-glucosidase, Bisphenol A, Hesperidin, Endocrine disruption chemicals
Background
The prevalence of metabolic disorders is on the increase globally due to several factors including environmental toxicants such as heavy metals and several industrial products that could modulate and disrupt insulin secretion and signalling leading to dysregulations in glucose metabolism [1–3]. Previous studies have shown that multiple complex interplays between insulin secretions, signalling, and actions are responsible for efficient glucose metabolism in the body [4–6]. Hence, dysregulation of these pathways especially by endocrine-disrupting chemicals (EDC) at the genomic or proteomic levels may lead to dysfunction in the signaling molecules of glucose metabolism and consequently result in several metabolic syndromes such as obesity and diabetes [7–9]. Diabetes, for instance, typically results from a combination of peripheral insulin resistance and defects in pancreatic β-cell functions, which could be modulated by the deleterious activities of these EDC on entrance into the physiological system. Being an emerging area of research in biomedical science, the mechanisms of metabolic disruptions by various identified EDCs are poorly known; hence developing therapeutic agents to modulate their interference remains a huge task for researchers in this area.
Bisphenol A (BPA), 2,2-bis(4-Hydroxyphenyl) propane has been used in packaging materials by the food and beverage industries since the 1960s. It is one of the major structural components found in polycarbonate beverage bottles, which are commonly used by food industries. It is also used for the manufacture of epoxy and polycarbonate resins used in food containers, such as child feeding bottles and plastic bottles, or as epoxy resins coating food and beverage-containing metallic cans [10, 11]. BPA is a component in the metal can coatings, which protect food substances from directly contacting metal surfaces of the food container. Recent studies have revealed that small amounts of BPA are transferred to stored food and water from the polymer containers or from the epoxy resins lining the metallic cans, especially when exposed to high temperatures (as during sterilization cycles) [11]. Additionally, human exposure to BPA occurs through the consumption of contaminated food and drinking water, which leads to several adverse effects in the body [12, 13]. BPA has since become one of the most controversial endocrine disruptor chemicals that interferes directly with the regulation of glucose metabolism both at the genomic and proteomic levels respectively [14–16]. It has also been implicated by several studies in the pathogenesis of diabetes and other metabolic diseases [17–19]. Moreover, how BPA interferes with glucose metabolism leading to endocrine disruption in the human body remains elusive and requires more insight and mechanistic understanding.
Fibroblast growth factor 21 (FGF-21) has recently gained evident attention as one of the key endocrine hormones involved in glucose metabolism [20]. In obese diabetic rodents [21, 22] and rhesus monkeys [23, 24], studies have shown that injection of FGF-21 can result in a drastic decrease in fasting glucose, insulin, glucagon, and triglycerides. Endogenous FGF-21 functions in various physiological conditions as a stress-responsive hormone protecting the cell against distinct metabolic or environmental stress [25]. Pancreatic α-amylase and α- glucosidase are key digestive enzymes found in the epithelial mucosa of the small intestine and are involved in carbohydrate metabolism in the physiological milieu. Hence, inhibition of these enzymes can significantly reduce the post-prandial increase in blood glucose level making their therapeutic modulation essential in diabetes management [26, 27]. Hesperidin is a plant flavonoid from citrus fruits with an emerging therapeutic potential against several disease conditions such as diabetes and other metabolic syndromes [28–31]. We demonstrated most recently in our lab that hesperidin could markedly increase insulin level in cadmium-induced pancreatitis in rats [32], thus opening a new horizon for the use of hesperidin in therapeutically modulating metabolic dysregulations caused by several endocrine disruptor chemicals (EDC) that find their way into the body system. In this present study, using in silico molecular docking techniques, we investigated whether one of the molecular mechanisms of BPA endocrine disruption is through its binding with FGF-21, α-amylase, and α-glucosidase and if hesperidin could interfere with this binding, thus ameliorating metabolic dysregulations elicited by BPA.
Methods
Retrieval of the 3D crystal structure of target proteins
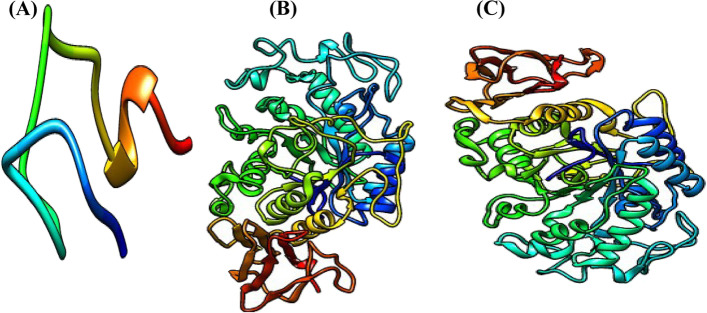
The 3D crystal structures of the proteins FGF-21 (PDB: 5VAQ), pancreatic α-amylase (PDB ID: 2QMK) pancreatic α-glucosidase (PDB ID: 1U33) were fetched by IDs from the Protein Data Bank (http://www.rcsb.org) into UCSF Chimera changed to publication quality and saved as an image (Fig. 1).
Fig. 1.
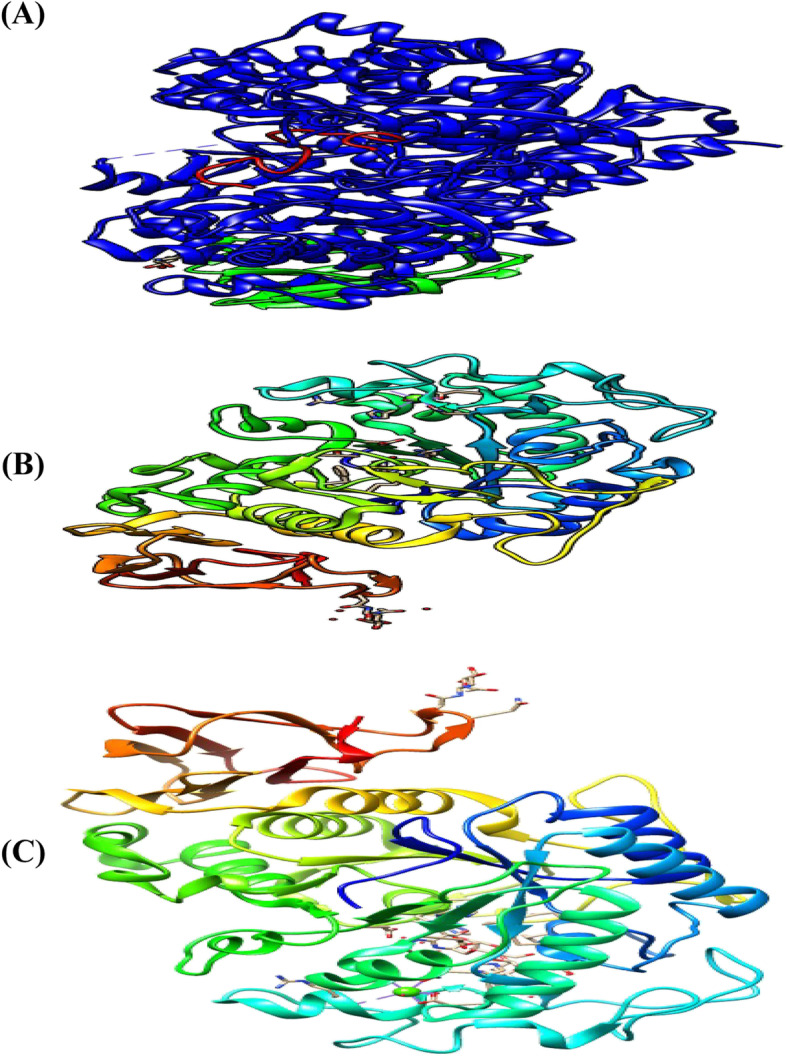
The 3D crystal structure of target proteins displayed UCSF Chimera. (A). FGF-21. (B). Pancreatic α-amylase. (C). Pancreatic α-glucosidase
Retrieval of the 3D structure of the ligands
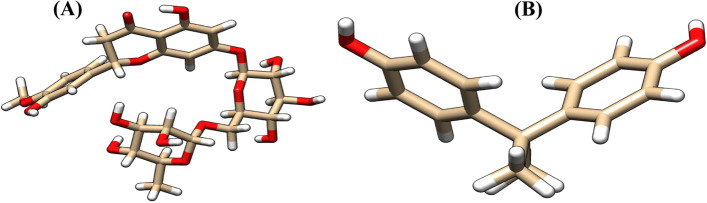
The ligands used for the study are bisphenol A (PubChem CID: 6623) and hesperidin (PubChem CID: 10621), respectively. Their 3D structures were fetched by IDs from PubChem (www.pubchem.org) into UCSF Chimera and saved in a structure data file (SDF) and image (see Fig. 2) format, respectively.
Fig. 2.
Three-dimensional structures of (A) hesperidin and (B) bisphenol A
Preparation of the target proteins
The target proteins were prepared and saved separately. The 3D crystal structure of each of the proteins was fetched into UCSF Chimera by their PDB ID vis-à-vis 5VAQ, 2QMK, and 1U33 respectively. The FGF-21 was in a complex with Beta-klotho and complex chains (Fig. 3A, B) were selected and deleted so that only a single-chain (Fig. 3C) which is the FGF-21 was left displayed while pancreatic α-amylase and pancreatic α-glucosidase were single chains (see Fig. 3). For each protein, structure editing was carried out by minimizing the structure under the default specifications of the steepest descent slope 100; size 0.02 Ả; gradient steps:10; conjugate 0.02; update intervals 10. After preparations, the proteins were saved in PDB format for use in molecular docking.
Fig. 3.
Three-dimensional crystal structure of prepared proteins. (A). FGF-21. (B). Pancreatic α-amylase. (C). Pancreatic α-glucosidase
Molecular docking
The molecular docking studies were carried out using Autodock Vina plugged in Pyrx. The 3D structure of the ligands (hesperidin and bisphenol A) previously retrieved from PubChem (www.pubchem.org) were individually imported into the Pyx in Chemical Table-SDF format. The ligands were minimized in the default with the addition of hydrogen and charge Gastiger, then, converted to PDBQT format. In turn, the prepared proteins as shown in Fig. 3 were loaded into the Pyrx. Using the Autodock, the proteins were made macromolecules in PDBQT format. For each turn, the program was run using a searching grid extended over target proteins with box dimension 24.26 × 31.65 × 48.11 and x, y, z coordinates 47.76, 32.05, and 40.10 (5VAQ); dimension 72.39 × 84.39 × 67.64 and x, y, z coordinates 8.02, − 29.05, 18.99 (2QMK), and 70.69 × 82.91 × 57.87 and x, y, z coordinates 10.09, 24.12, 48.53 (1U33) respectively [33] with exhaustiveness 8 while other parameters were set at default. The ligands were docked into the crystal structure of the proteins in turns so that the highest-scoring pose was selected for each of the ligands and the best docking poses are predicted to be the most stable conformation of each ligand for binding to proteins [34]. Selection of best pose is particularly at the binding site where the majority (or all) of the bisphenol A binds in the eighth models and if hesperidin binds with the highest docking scores in that particular site. Further, the best poses were visualized using the Discovery Studio Visualizer 2020 [35] to ascertain the amino acids in the binding sites and predict the types of bonds with which the ligands bind to the target proteins.
ADMET predictions of hesperidin
ADMET (absorption, distribution, metabolism, excretion, and toxicity) analysis, which constitutes the pharmacokinetics of a drug-like molecule [36] was carried out on hesperidin. Here, web servers use structure-activity relationship (similarity search) to compare and predict the ADMET properties of drug candidates or environmental chemicals with properties of known compounds in their database. In this work, prediction and significant descriptors of drug-likeness such as mutagenicity, toxicological dosage level, and pharmacologically relevant properties of the hesperidin were predicted using Swissadme (http://www.swissadme.ch) and admetSAR (lmmd.ecust.edu.cn:8000) webservers.
Results
Docking scores and binding energy analysis
The best poses of the ligands with the proteins
Ligands’ pose with FGF-21
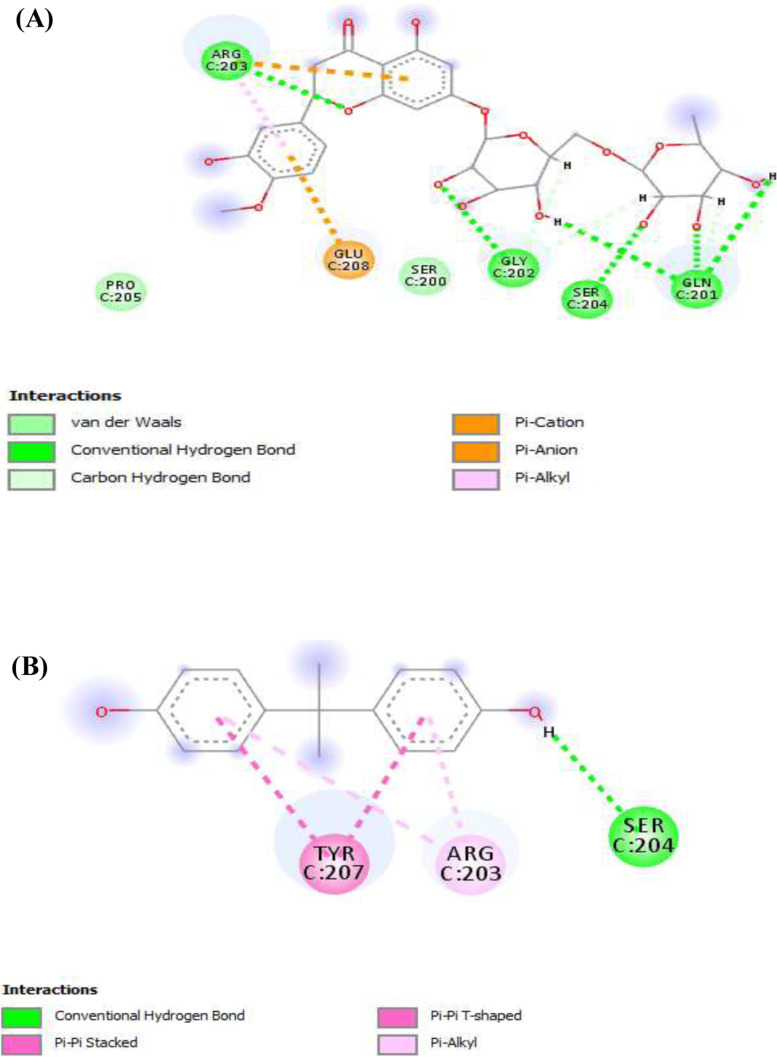
Figure 4 shows the two best conformations (Fig. 4A, B) of the binding poses and interactions between FGF-21, hesperidin, and BPA. Figure 4A represents the best model of hesperidin in complex with all the different models of bisphenol A while Fig. 4B is the binding pose of the best conformation/models of both ligands and the protein. All the binding conformations consistently revealed that both ligands bind at the same binding pocket on the protein with hesperidin having a stronger binding affinity than all the models of BPA at the same site, which enhanced its potential of dislodging BPA from its binding pocket on the protein molecule. Hesperidin, therefore, has the potential to favourably compete for the same binding site as BPA and perhaps may inhibit the binding of BPA on its binding pocket on the protein three-dimensional conformations.
Fig. 4.
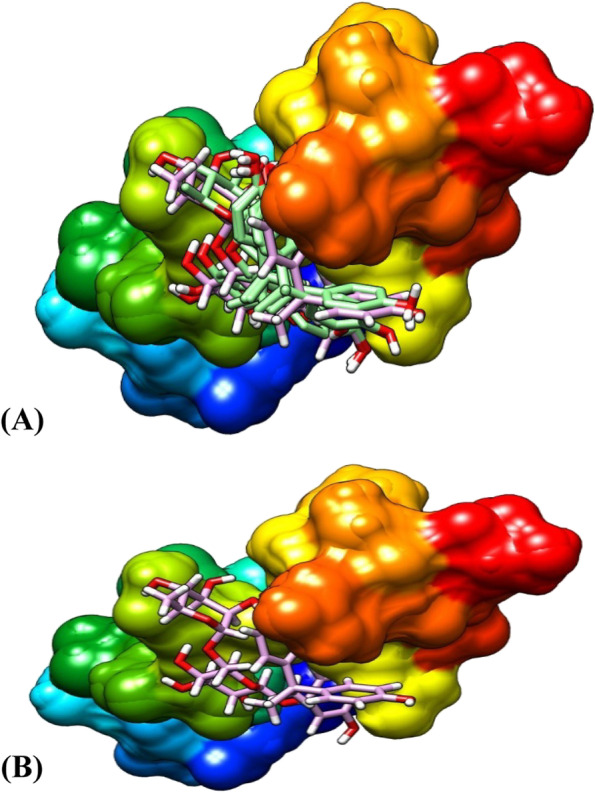
The binding pose of the ligands at the same binding site of FGF-21. (A). The best model of hesperidin in complex with 8 models of bisphenol A. (B) The binding pose of the best conformation of the Ligands and the protein
Ligands’ pose with α-amylase
Figure 5 shows two best conformations (Fig. 5A, B) of the binding poses and interactions between α-amylase, hesperidin, and BPA. Figure 4A represents the best model of hesperidin in complex with seven different models of bisphenol A while Fig. 4B is the binding pose of the best conformation/models of both ligands and the protein. All the binding conformations consistently revealed that both ligands bind at the same binding pocket on the enzyme with hesperidin having a stronger binding affinity than all the models of BPA at the same site, which enhanced its potential of dislodging BPA from its binding pocket on the enzyme. Hesperidin, therefore, has the potential to compete for the same binding site as BPA and perhaps may prevent the binding of BPA on its binding pocket on the α-amylase three-dimensional enzyme conformations.
Fig. 5.
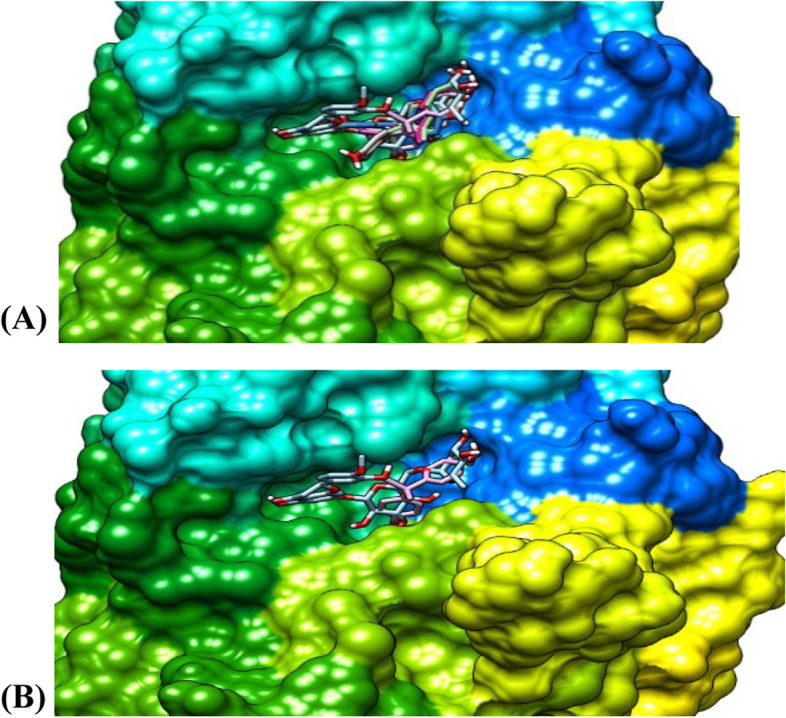
The binding pose of the ligands at the same binding site of α-amylase. (A). The best model of hesperidin in complex with 7 models of bisphenol A. (B) The binding pose of the best conformation/models of the ligands and the protein
Ligands’ pose with α-glucosidase
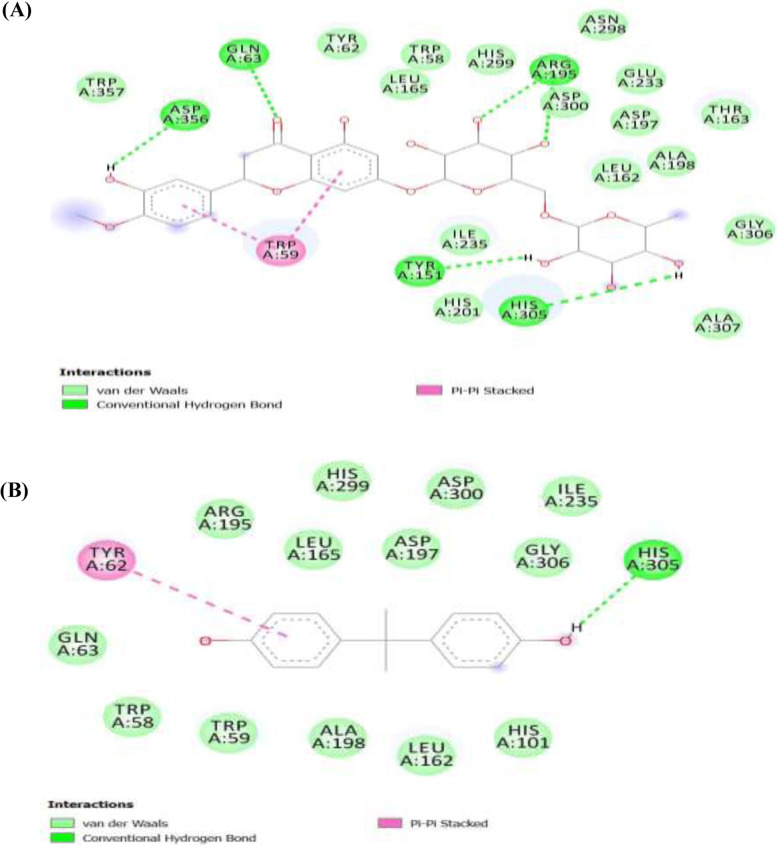
Figure 6 shows the two best conformations (Fig. 6A, B) of the binding poses and interactions between α-glucosidase, hesperidin, and BPA. Figure 6A represents the best model of hesperidin in complex with six different models of bisphenol A while Fig. 6B is the binding pose of the best conformation/models of both ligands and the protein. All the binding conformations consistently revealed that both ligands bind at the same binding pocket on the enzyme with hesperidin having a stronger binding affinity than BPA at the same site, which enhanced its potential of dislodging BPA from its binding pocket on the enzyme. Hesperidin, therefore, has the potential to compete for the same binding site as BPA and may inhibit the binding of BPA on its binding pocket on the α-glucosidase three-dimensional enzyme conformations.
Fig. 6.
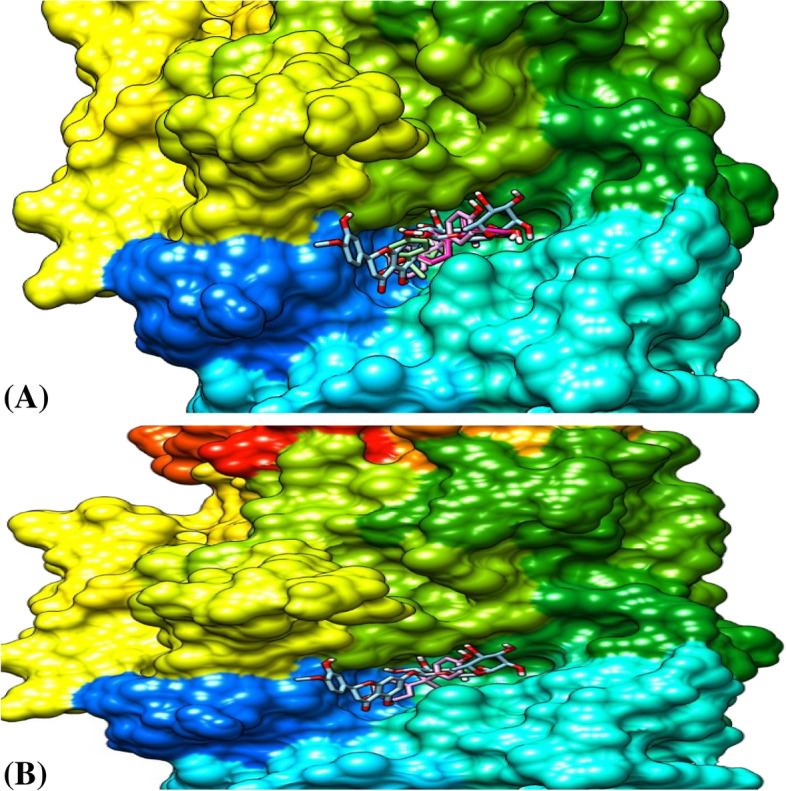
The binding pose of the ligands at the same binding site of α-glucosidase. (A). The best model of hesperidin and 6 models of bisphenol A. (B). The binding pose of the best conformation of the ligands and the protein
Protein-ligands interactions
FGF-21-ligands interactions
Figure 7 shows the bidirectional binding interactions of the ligands (Fig. 7A) hesperidin and (Fig. 7B) BPA and the amino acids of the FGF-21 at the binding pocket of the protein. The result revealed that hydrogen and alkyl bonds were the prominent bonds involved in the interactions for both of the ligands, although the alkyl bond was more prominent in the interactions of BPA with the protein. Moreover, hydrogen bond was more prominent for hesperidin-FGF-21 interactions adding to our finding that hesperidin binds stronger than BPA on the protein and perhaps may be an effective inhibitor of BPA. Further molecular inquiry revealed that BPA and hesperidin interacted with the same binding site on the FGF-21 structure through the three main amino acids (Tyr207, Arg203, and Ser204) that made up the binding pocket. Specifically, the ligands interacted with traditional hydrogen bonds in FGF-21, especially using Ser204, which is a strong bond. Besides, along with Ser204, hesperidin has three other amino acids (Gly202, Gln201, and Arg203) with which it also interacted by traditional hydrogen bonding with the binding site.
Fig. 7.
FGF-21-Ligands interaction. (A). Hesperidin. (B). Bisphenol A. Dark green: conventional hydrogen bond; light green: van der Waals; Pink-violet: π-T shape/alkyl; brown: π-cation/anion. Red: unfavorable donor-donor; grey: carbon-hydrogen bond
α-amylase ligands interactions
Figure 8 shows the bidirectional binding interactions of the ligands (Fig. 8A) hesperidin and (Fig. 8B) BPA and the amino acids of the pancreatic enzyme α-amylase at the binding pocket of the enzyme. The image revealed that hydrogen bond and van der Waals forces were the prominent bonds involved in the interactions for both of the ligands, although van der Waals forces were more prominent in the interactions of BPA with the enzyme. Moreover, hydrogen bond was more prominent for hesperidin-α-amylase interactions adding to our finding that hesperidin binds stronger than BPA on the enzyme and thus would be an effective inhibitor of BPA. The interaction with α-amylase ligands showed that both bisphenol A and hesperidin interacted with traditional hydrogen of various amino acids in the binding pockets of the enzyme as revealed in Fig. 8 (bisphenol A: Asp300, Arg195, hesperidin: Asp300, Lys200, Gln63, and Trp59). Our investigation further revealed that each of the ligands interacted with Asp300.
Fig. 8.
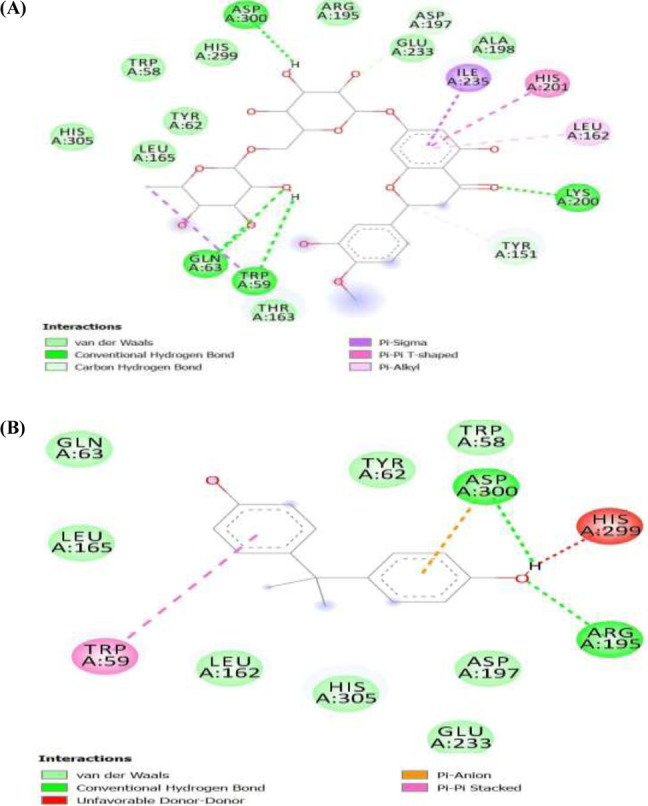
α-amylase-ligands interaction. (A). Hesperidin. (B). Bisphenol A. Deeper green: conventional hydrogen bond; light green: van der Waals; Pink-violet: π-T shape/alkyl/sigma; brown: π-cation/anion. Red: unfavourable donor-donor; grey: carbon-hydrogen bond
α-glucosidase ligands interactions
Figure 9 shows the separate binding interactions of the ligands (Fig. 9A) hesperidin and (Fig. 9B) BPA and the amino acids of the pancreatic enzyme α-glucosidase at the binding pocket of the enzyme. The image revealed that hydrogen bond and van der Waals forces were the major bonds involved in the interactions for both of the ligands, albeit van der Waals forces were more prominent in all. Moreover, hydrogen bonds in hesperidin-α-glucosidase interactions were greater than the hydrogen bonds in BPA-α-glucosidase interactions adding to our finding that hesperidin binds stronger than BPA on the enzyme and thus would be an effective inhibitor of BPA. For the α-glucosidase enzyme, the ligands interacted with the standard hydrogen bond of the enzyme using His305 as shown in Fig. 9. Gln53, Asp356, Arg195, and Try159 also interacted with hesperidin. Although both bisphenol A and hesperidin interacted with the target protein using traditional hydrogen bonds and at least with the main amino acid, hesperidin interacted with more amino acids than BPA indicating the possibility of preventing the binding of BPA and cleaving off bisphenol A from its most potent protein binding sites.
Fig. 9.
α-glucosidase ligands interaction. (A). Hesperidin. (B). Bisphenol A. Dark green: conventional hydrogen bond; light green: van der Waals; Pink: π-π stacked
ADME/T prediction profile of hesperidin
Discussion
The human physiological system is frequently exposed to several endocrine disruptor chemicals (EDC) either through environmental toxicants such as heavy metals and/or industrial products such as BPA [37, 38]. These EDCs upon entrance into the body interfere with normal metabolic regulations of the body leading to disease conditions. Bisphenol A is a well-known EDC whose molecular mechanisms of action has remained opaque over the years [39]. Clearer understanding of the mechanistic approach of BPA in causing endocrine disruption and other metabolic dysregulations would be helpful in developing possible new therapeutics against this environmental toxicant. Our study for the first time used in silico molecular techniques to investigate possible novel mechanistic approaches of BPA in inducing endocrine disruption and other metabolic dysregulations. In silico molecular methods is a modern veritable tool in drug discovery and development. Although, modern experimental approaches are able to elucidate ADMET properties with great degree of accuracy, however, they are time-consuming with high cost implications. In silico molecular method therefore remains an efficient key approach in drug discovery and development [40, 41].
In molecular docking, binding free energies or docking scores describe the affinity of a ligand for protein molecules. This is usually denoted with negative values and a higher negative value of docking score results in a higher binding affinity vice versa. Interestingly, our docking score results shown in Table 1, revealed high negative values for all the binding poses between BPA and the three proteins as well as hesperidin and the proteins respectively. Protein-ligand binding interactions is a reversible non-covalent interaction that occur through various molecular mechanics involving conformational changes leading to high affinity and low affinity states. These molecular interactions are essential to all life processes and thus are strongly considered in drug discovery and development of new therapeutics [42]. Our results revealed that BPA can bind with FGF-21, α-amylase, and α-glucosidase respectively, suggesting a novel mechanistic approach of BPA interference with glucose metabolism through interactions with these metabolic regulators. FGF-21, α-amylase, and α-glucosidase are key proteins involved in the metabolism of glucose in the body [43–45]. Although inhibition of α-amylase and α-glucosidase may be seen to be a potential action of BPA in regulating glucose level, it is not clear how this may manifest in the physiological system in vivo studies. Inhibiting α-amylase and α-glucosidase in addition to other metabolic targets of BPA may rather exacerbate glucotoxicity and metabolic dysfunctions in the body. FGF-21 is an indicator of metabolic syndrome and endocrine dysfunction [46] and BPA binding affinity to FGF-21 may be a novel mechanism of action of BPA in disrupting metabolic regulations leading to metabolic derangement in the body.
Table 1.
Molecular docking scores for the best pose of the ligands
| Protein | Ligand | Binding energy (Kcal/mol) |
|---|---|---|
| FGF21 | Hesperidin | − 5.80 |
| Bisphenol A | − 4.40 | |
| α-amylase | Hesperidin | − 9.60 |
| Bisphenol A | − 7.20 | |
| α-glucosidase | Hesperidin | − 9.60 |
| Bisphenol A | − 7.10 |
Interestingly, in Figs. 4, 5, and 6, we further demonstrated that hesperidin binding to FGF-21, α-amylase, and α-glucosidase interfered with the binding of BPA to these metabolic regulators. This is further evidenced in the higher binding affinity/negative values of hesperidin in all the binding poses with the proteins investigated. Hesperidin is an emerging natural product recently revealed to have strong therapeutic potentials for the treatment of metabolic diseases such as diabetes and obesity [30]. Our results revealed that although bisphenol A bound with all 8, 6, or 7 of its conformational models to specific sites on the proteins; hesperidin bound at the same site with BPA with stronger affinity demonstrating that hesperidin outcompeted with BPA on its binding pockets on FGF-21, alpha-amylase, and α-glucosidase 3-D structure. Proteins usually recruit its residues during ligands binding, which subsequently enhance its interactions with the ligands especially at the binding pockets. The greater the residues involved in the binding, the greater the affinity of the protein to the ligand [47, 48]. In all the three proteins investigated, the binding of hesperidin led to the recruitment of more residues of the proteins than when BPA bound. Thus further indicating that hesperidin has greater binding affinity than BPA. Hesperidin may therefore be a promising strong inhibitor of BPA binding especially against these proteins investigated. The interactions of the ligands (BPA and hesperidin) with the proteins as competitive inhibitors are shown in Figs. 7, 8, and 9.
To further position hesperidin as a strong lead compound in drug development, we conducted ADME/T studies to enhance our understanding of its druggability. In decision-making to boost the success rates in the early drug development process, knowledge relating to the drug-like appearance of compounds is imperative [34]. Interestingly, many methods and instruments can be used in testing a molecule’s physicochemical properties that can influence its pharmacokinetic and pharmacodynamic properties in vivo [49]. ADME properties of hesperidin were evaluated and the selected properties correlated with metabolism, cell permeation, and bioavailability. Hesperidin proved to be a strong inhibitor of bisphenol A binding, however violated Lipinski’s five-molecular weight rule of 610.56, H-bond Acceptor 15, and H-bond Donor 8 (Table 2). Moreover, investigation of human intestinal absorption (HIA) usually reveals human intestinal permeability as shown in Table 3 and better absorption through the intestine is reflected by a probability value closer to 1 [34]. Interestingly, hesperidin displayed a strong absorption value of 0.8161 and displayed near values for the in silico simulation (Caco-2) in the human cell line used. As shown from the Ames test, hesperidin would be neither mutagenic nor carcinogenic in vivo. In addition, the LD50 of hesperidin was high, which indicates that hesperidin would not be toxic in vivo even at a high dosage.
Table 2.
Lipinski rule of five from SwissAdme server
| Physical property | Value |
|---|---|
| Molecular weight | 610.56* |
| LogP | 2.6 |
| H-bond acceptor | 15* |
| H-bond donor | 8* |
| Rotatable bonds | 7 |
*Lipinski rule of five violations
Table 3.
In silico absorption and toxicity profile of the compounds as obtained from the AdmetSar server.
| Absorption/toxicity profile | Value | Probability |
|---|---|---|
| Human blood-brain barrier (BBB) | − | 0.9570 |
| Intestinal absorption (HIA) | + | 0.8161 |
| Caco-2 | − | 0.8816 |
| Plasma protein binding | 1.096436381 | 1.00 |
| AMES Test | − | 0.6600 |
| Carcinogenicity | − | 0.9714 |
| UGT catalysed | + | 0.6000 |
| Acute oral toxicity (LD50; kg/mol) | 2.257 | |
| Hepatotoxicity | + | 0.6500 |
In silico distribution profile showed that hesperidin is a non-substrate and non-inhibitor of P-glycoprotein (P-GP) (Table 4). P-glycoprotein is one of the major drug transporters that is involved in the uptake and efflux of drugs and xenobiotics in and out of the cell. This process significantly affects plasma and tissue concentrations of such drug in addition to their therapeutic profile [50]. P-glycoprotein is strongly implicated in drug-drug interactions in the physiological system and understanding its interactions with drugs are necessary in new drug development [51]. It has been shown that drugs that induce P-glycoprotein, has the ability to reduce the bioavailability of some other drugs. Additionally, inhibitors of P-glycoprotein, could increase the bioavailability of susceptible drugs transported by P-glycoprotein [52]. Interestingly, our investigation revealed that hesperidin is a non-substrate and non-inhibitor of P-glycoprotein, thus a strong pharmacological advantage to hesperidin as there may not be significant alterations in its therapeutic effects due to the activity of P-glycoprotein when they are administered into the body.
Table 4.
In silico distribution profile of hesperidin as obtained from the AdmetSar server
| Distribution substrate/inhibitor | Profile |
|---|---|
| p-gp substrate/inhibitor probability | Non-substrate/non-inhibitor |
| CYP-2C9 substrate/inhibitor | Non-substrate/non-inhibitor |
| CYP-2D6 substrate/inhibitor | Non-substrate/non-inhibitor |
| CYP-3A4 substrate/inhibitor | Substrate/non-inhibitor |
| CYP-1A2 inhibitor | Non-inhibitor |
| CYP-2C19 inhibitor | Non-inhibitor |
| CYP inhibitory promiscuity | 0.6670 |
Cytochrome P450 is a family of microsomal enzymes involved in the metabolism of xenobiotics including drugs and many drug-drug interactions are as a result of alterations in the metabolism of CYP450 [36, 53]. They however can be inhibited or induced by drugs and their metabolites, leading to drug-drug interactions with significant clinical relevance [54]. Such drug-drug interactions could lead to adverse effects or therapeutic failures of clinically administered drugs [55]. They are therefore strongly considered in the development of new drug candidates especially as it relates to drug-drug interactions [56, 57]. We therefore assessed the cytochrome P450 inhibition profile for hesperidin for the five major isoforms (2C9, 2D6, 3A4, 1A2, and 2C19), which are involved in the metabolism of many drugs. Our result revealed that hesperidin is neither a substrate nor an inhibitor of these isoforms (2C9 and 2D6) of cytochrome P450. It was also specifically found not to be an inhibitor of 3A4, 1A2, and 2C19 isoforms although a substrate 3A4 cytochrome P450 isoform. Inhibitors of CYP450 enzymes usually block the metabolic activity of one or more CYP450 enzymes [55]. Moreover, the extent to which an inhibitor affects the CYP450 metabolism of a specific drug depends on many factors such as drug dosage and the inhibitor’s ability to effectively bind to the enzyme. Interestingly, our result revealed that hesperidin do no inhibit any of the major isoforms of CYP450, which is a strong feature of good drug candidate.
More so, several drugs interact with the CYP450 system in many ways. Some drugs may be metabolized by only one CYP450 enzyme whereas others may be metabolized by more than one isoforms of the enzyme. Drugs that are substrates and/or inhibitors of any of the cytochrome P450 isoforms are known to interfere with the metabolism of others drugs that depend on the isoform leading to drug-drug interactions [58]. However, the more CYP450 enzymes they are substrate to, the more they are able to cause drug-drug interactions [59]. Interestingly, our study revealed that hesperidin is a substrate of only CYP3A4 isoform of CYP450 enzyme system, thus making it to have lower risk of triggering drug-drug interactions when it is administered into the body for therapeutic purposes (Table 4).
In general, hesperidin is an active inhibitor of bisphenol A binding to FGF-21, alpha-amylase, and alpha-glucosidase, which are known regulators of metabolism. Conversely, despite the revealed potentials of hesperidin to inhibiting these target proteins, its high molecular weight could lead to decreased solubility and bioavailability within the physiological system when administered for therapeutic purposes. However, this can be improved through pharmaceutical modifications and optimizations of the hesperidin molecular structure to produce derivatives with greater solubility and bioavailability in vivo, thus enhancing it prospects of meeting up with Lipinski’s five-molecular weight rule.
Conclusions
In this present study, we showed perhaps a novel mechanistic approach of BPA endocrine disruption through inhibition of FGF-21, α-amylase, and α-glucosidase, which are key endocrine regulators of glucose metabolism. Interestingly, hesperidin a natural product strongly disrupted the binding of BPA to these metabolic regulators by interfering with its binding at the same binding pocket of the regulators with stronger affinity with α-amylase, and α-glucosidase producing the highest binding affinity with hesperidin. Hesperidin further showed a promising ADME/T profile hence enhancing its potential for further drug development. Hesperidin may be a promising drug candidate for the development of therapeutics against metabolic-related diseases.
Acknowledgements
We acknowledge Jaris Computational Biology Centre Jos for materials and software used in the studies.
Abbreviations
- FGF-21
Fibroblast Growth Factor-21
- BPA
Bisphenol A
- ADME/T
Administration, Distribution, Metabolism, Excretion, and Toxicity
- EDC
Endocrine disrupting chemical
- PDB
Protein Data Bank
- CYP
Cytochrome
Authors’ contributions
APM conceived and designed the study, and performed experimental aspect (software). AJN designed the study, wrote and edited the manuscript. APC performed the experimental aspect of the study (software) and wrote the manuscript. AA and AB performed experimental aspect (software). EE performed the experimental aspect of the study (software) and wrote the manuscript. II, OO, AO, and IU contributed to the experimental design and proofreading of the manuscript. AB performed the experimental aspect of the study (software). All authors have read and approved the manuscript.
Funding
The researchers received no funding for this study.
Availability of data and materials
We have included all the data.
Declarations
Ethics approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Footnotes
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Fu Z, Xi S. The effects of heavy metals on human metabolism. Toxicol Mech Methods. 2020;30:167–176. doi: 10.1080/15376516.2019.1701594. [DOI] [PubMed] [Google Scholar]
- 2.Li X, Brejnrod AD, Ernst M, Rykær M, Herschend J, Olsen NMC, Dorrestein PC, Rensing C, Sørensen SJ. Heavy metal exposure causes changes in the metabolic health-associated gut microbiome and metabolites. Environ Int. 2019;126:454–467. doi: 10.1016/j.envint.2019.02.048. [DOI] [PubMed] [Google Scholar]
- 3.Żwierełło W, Maruszewska A, Skórka-Majewicz M, Goschorska M, Baranowska-Bosiacka I, Dec K, Styburski D, Nowakowska A, Gutowska I. The influence of polyphenols on metabolic disorders caused by compounds released from plastics – Review. Chemosphere. 2020;240:124901. doi: 10.1016/j.chemosphere.2019.124901. [DOI] [PubMed] [Google Scholar]
- 4.Zamora M, Villena JA. Contribution of impaired insulin signaling to the pathogenesis of diabetic cardiomyopathy. Int J Mol Sci. 2019;20:2833. doi: 10.3390/ijms20112833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Friesen M, Cowan CA. Adipocyte metabolism and insulin signaling perturbations: insights from genetics. Trends Endocrinol Metabol. 2019;30:396–406. doi: 10.1016/j.tem.2019.03.002. [DOI] [PubMed] [Google Scholar]
- 6.Fernandez-Mejıa C. Oxidative stress and chronic degenerative diseases role for antioxidants. Mexico City: InTech; 2013. [Google Scholar]
- 7.Olga P, Eleni KA, George P, Evanthia D-K. Endocrine disrupting chemicals: an occult mediator of metabolic disease. Front Endocrinol. 2019;10:112. doi: 10.3389/fendo.2019.00112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Sargis RM, Simmons RA. Environmental neglect: endocrine disruptors as underappreciated but potentially modifiable diabetes risk factors. Diabetologia. 2019;62:1811–1822. doi: 10.1007/s00125-019-4940-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Dominic JW, Morris W. Perspective: the insulin signaling system—a common link in the pathogenesis of type 2 diabetes. Endocrinology. 2020;141:3–5. doi: 10.1210/endo.141.6.7584. [DOI] [PubMed] [Google Scholar]
- 10.Menale C, Mita DG, Diano N, Diano S. Adverse effects of bisphenol A exposure on glucose metabolism regulation. Open Biotechnol J. 2016;10:122–130. doi: 10.2174/1874070701610010122. [DOI] [Google Scholar]
- 11.US Food and Drug Administration (FDA, 2021) Bisphenol A (BPA): Use in food contact application update on bisphenol A (BPA) for use in food contact applications. January 2010; March 30, 2012; Updated March 2013; July 2014; Retrieved November 2021, 10.00am WAT. https://www.fda.gov/food/food-additives-petitions/bisphenol-bpa-use-food-contact-application.
- 12.Kang JH, Kondo F, Katayama Y. Human exposure to bisphenol A. Toxicology. 2006;226:79–89. doi: 10.1016/j.tox.2006.06.009. [DOI] [PubMed] [Google Scholar]
- 13.Errico S, Bianco M, Mita L, Migliaccio M, Rossi S, Nicolucci C, Menale C, Portaccio M, Gallo P, Mita DG, Diano N. Migration of bisphenol A into canned tomatoes produced in Italy: dependence on temperature and storage conditions. Food Chem. 2014;160:157–164. doi: 10.1016/j.foodchem.2014.03.085. [DOI] [PubMed] [Google Scholar]
- 14.Song S, Zhang L, Zhang H, Wei W, Jia L. Perinatal BPA exposure induces hyperglycemia, oxidative stress, and decreased adiponectin production in later life of male rat offspring. Int J Environ Res Public Health. 2014;11:3728–3742. doi: 10.3390/ijerph110403728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Fenichel P, Chevalier N, Brucker-Davis F. Bisphenol A: an endocrine and metabolic disruptor. Annales d'Endocrinologie. 2013;74:211–220. doi: 10.1016/j.ando.2013.04.002. [DOI] [PubMed] [Google Scholar]
- 16.Li L, Wang Q, Zhang Y, Niu Y, Yao X, Liu H. The molecular mechanism of bisphenol A (BPA) as an endocrine disruptor by interacting with nuclear receptors: insights from molecular dynamics (MD) simulations. PLoS ONE. 2015;10:e0120330. doi: 10.1371/journal.pone.0120330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Murphy L, Mérida-Ortega Á, Cebrián ME. Exposure to bisphenol A and diabetes risk in Mexican women. Environ Sci Pollut Res. 2019;26:26332–26338. doi: 10.1007/s11356-019-05731-9. [DOI] [PubMed] [Google Scholar]
- 18.Magliano DJ, Lyons JG. Bisphenol A, and diabetes, insulin resistance, cardiovascular disease and obesity: controversy in a (Plastic) Cup? J Clin Endocrinol Metabol. 2013;98:502–504. doi: 10.1210/jc.2012-3058. [DOI] [PubMed] [Google Scholar]
- 19.LaKind JS, Goodman M, Mattison DR. Bisphenol A and indicators of obesity, glucose metabolism/type 2 diabetes and cardiovascular disease: a systematic review of epidemiologic research. Crit Rev Toxicol. 2014;44:121–150. doi: 10.3109/10408444.2013.860075. [DOI] [PubMed] [Google Scholar]
- 20.Fisher FM, Maratos-Flier E. Understanding the physiology of FGF21. Ann Rev Physiol. 2016;78:223–241. doi: 10.1146/annurev-physiol-021115-105339. [DOI] [PubMed] [Google Scholar]
- 21.Coskun T, Bina HA, Schneider MA, Dunbar JD, Hu CC, Chen Y, Moller DE, Kharitonenkov A. Fibroblast growth factor 21 corrects obesity in mice. Endocrinology. 2008;149:6018–6027. doi: 10.1210/en.2008-0816. [DOI] [PubMed] [Google Scholar]
- 22.Xu J, Lloyd DJ, Hale C, Stanislaus S, Chen M, Sivits G, Vonderfecht S, Hecht R, Li Y-S, Lindberg RA, Chen J-L, Jung DY, Zhang Z, Ko HJ, Kim JK, Véniant MM. Fibroblast growth factor 21 reverses hepatic steatosis, increases energy expenditure and improves insulin sensitivity in diet-induced obese mice. Diabetes. 2009;58:250–259. doi: 10.2337/db08-0392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kharitonenkov A, Wroblewski VJ, Koester A, Chen Y-F, Clutinger CK, Tigno XT, Hansen BC, Shanafelt AB, Etgen GJ. The metabolic state of diabetic monkeys is regulated by fibroblast growth factor-21. Endocrinology. 2007;148:774–781. doi: 10.1210/en.2006-1168. [DOI] [PubMed] [Google Scholar]
- 24.Foltz IN, Hu S, King C, Wu X, Yang C, Wang W, Weiszmann J, Stevens J, Chen JS, Nuanmanee N, Gupte J, Komorowski R, Sekirov L, Hager T, Arora T, Ge H, Baribault H. Treating diabetes and obesity with an FGF21-mimetic antibody activating the betaKlotho/FGFR1c receptor complex. Sci Translat Med. 2012;4:162ra153. doi: 10.1126/scitranslmed.3004690. [DOI] [PubMed] [Google Scholar]
- 25.Kim KH, Lee MS. FGF21 as a stress hormone: the roles of FGF21 in stress adaptation and the treatment of metabolic diseases. Diabetes Metabol J. 2014;38:245–251. doi: 10.4093/dmj.2014.38.4.245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Shobana S, Sreerama SR, Soheila M, Ahmad H, Mahmood RM. Kinetics of α-glucosidase inhibition by different fractions of three species of Labiatae extracts a new diabetes treatment model. Pharm Biol. 2017;55:1483–1488. doi: 10.1080/13880209.2017.1306569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Shai L-J, Masoko P, Mokgotho M-P, Magano S-R, Mogale A, Boaduo N, Eloff J-N. Yeast alpha-glucosidase inhibitory and antioxidant activities of six medicinal plants collected in Phalaborwa, South Africa. South Afr J Botany. 2010;76:465–470. doi: 10.1016/j.sajb.2010.03.002. [DOI] [Google Scholar]
- 28.Campanero MA, Escolar M, Perez G, Garcia-Quetglas E, Sadaba B, Azanza JR. Simultaneous determination of diosmin and diosmetin in human plasma by ion trap liquid chromatography–atmospheric pressure chemical ionization tandem mass spectrometry: Application to a clinical pharmacokinetic study. J Pharmacol Biomed Anal. 2010;51:875–881. doi: 10.1016/j.jpba.2009.09.012. [DOI] [PubMed] [Google Scholar]
- 29.Rehman K, Munawar SM, Akash MSH, Buabeid MA, Chohan TA, Tariq M, Komal JK, Arafa EA. Hesperidin improves insulin resistance via downregulation of inflammatory responses: Biochemical analysis and in silico validation. PLoS ONE. 2020;15:e0227637. doi: 10.1371/journal.pone.0227637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Akiyama S, Katsumata S, Suzuki K, Ishimi Y, Wu J, Uehara M. Dietary hesperidin exerts hypoglycemic and hypolipidemic effects in streptozotocin-induced marginal type 1 diabetic rats. J Clin Biochem Nutr. 2010;46:87–92. doi: 10.3164/jcbn.09-82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Agrawal YO, Sharma PK, Shrivastava B, Ojha S, Upadhya HM, Arya DS, Goyal SN. Hesperidin produces cardioprotective activity via the PPAR-gamma pathway in ischemic heart disease model in diabetic rats. PloS one. 2014;9:e111212. doi: 10.1371/journal.pone.0111212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Aja PM, Izekwe FI, Famurewa AC, Ekpono EU, Nwite FE, Igwenyi IO, Awoke JN, Ani OG, Aloke C, Obasi NA, Udeh KU, Ale BA. Hesperidin protects against cadmium-induced pancreatitis by modulating insulin secretion, redox imbalance and iNOS/NF-ĸB signaling in rats. Life Sci. 2020;259:118268. doi: 10.1016/j.lfs.2020.118268. [DOI] [PubMed] [Google Scholar]
- 33.Elekofehinti OO, Omotuyi IO, Kamdem JP, Ejelonu OC, Alves GV, Adanlawo IO, Rocha JBT. Saponin as a regulator of biofuel: implication for ethnobotanical management of diabetes. J Physiol Biochem. 2014;70:555–567. doi: 10.1007/s13105-014-0325-4. [DOI] [PubMed] [Google Scholar]
- 34.Elekofehintia OO, Ejelonub OC, Kamdemc JP, Akinlosotub OB, Famutib A, Adebowaleb DD, Iwaloyeb O, Bulud YI, Kadee IJ, Rochaf JBT. Discovery of potential visfatin activators using in silico docking and ADME predictions as therapy for type 2 diabetes. Beni Suef Univ J Basic Appl Sci. 2018;7:241–249. [Google Scholar]
- 35.Herowati R, Widodo GP (2017) Molecular docking analysis: Interaction studies of natural compounds to anti-inflammatory targets. Quantitative Structure-activity Relationship 63. 10.5772/IntechOpen.68666
- 36.Nisha CM, Kumar A, Nair P, Gupta N, Silakari C, Tripathi T, Kumar A. Molecular Docking and In Silico ADMET Study Reveals Acylguanidine 7a as a Potential Inhibitor of β-Secretase. Adv Bioinformat. 2016;2016:6. doi: 10.1155/2016/9258578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Orji OU, Awoke JN, Harbor C, Igwenyi IO, Obasi OD, Ezeani NN, Aloke C. Ethanol leaf extract of Psychotria microphylla rich in quercetin restores heavy metal-induced redox imbalance in rats. Heliyon. 2020;6:e04999. doi: 10.1016/j.heliyon.2020.e04999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Awoke JN, Orji OU, Aja PM, Ezeani NN, Aloke C, Obasi OD. Ethanol leaf extract of ruspolia hypocrateriformis abrogated hepatic redox imbalance and oxidative damage induced by heavy metal toxicity in rats. Arab J Chem. 2020;13(11):8133–8145. doi: 10.1016/j.arabjc.2020.09.045. [DOI] [Google Scholar]
- 39.Vom Saal FS, Nagel SC, Coe BL, Angle BM, Taylor JA. The estrogenic endocrine-disrupting chemical bisphenol A (BPA) and obesity. Mol Cell Endocrinol. 2012;354:74–84. doi: 10.1016/j.mce.2012.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Kirchmair J, Göller AH, Lang D, Kunze J, Testa B, Wilson ID, Schneider G. Predicting drug metabolism: Experiment and/or computation? Nat Rev Drug Discov. 2015;14:387–404. doi: 10.1038/nrd4581. [DOI] [PubMed] [Google Scholar]
- 41.Tyzack JD, Kirchmair J. Computational methods and tools to predict cytochrome P450 metabolism for drug discovery. Chem Biol Drug Des. 2019;93:377–386. doi: 10.1111/cbdd.13445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Li L, Koh C, Reker D. Predicting protein-ligand interactions based on bow-pharmacological space and Bayesian additive regression trees. Sci Rep. 2019;9:7703. doi: 10.1038/s41598-019-43125-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Li H, Wu G, Fang Q, Zhang M, Hui X, Sheng B, Wu L, Bao Y, Li P, Xu A, Jia W. Fibroblast growth factor 21 increases insulin sensitivity through a specific expansion of subcutaneous fat. Nat Commun. 2018;9:272. doi: 10.1038/s41467-017-02677-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Fisher FM, Kleiner S, Douris N, Fox EC, Mepani JR, Verdeguer F, Wu J, Kharitonenkov A, Flier JS, Maratos-Flier E, Spiegelman BM. FGF21 regulates PGC-1alpha and browning of white adipose tissues in adaptive thermogenesis. Gene Dev. 2012;26:271–281. doi: 10.1101/gad.177857.111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Shobana S, Sreerama Y, Malleshi N. Composition and enzyme inhibitory properties of finger millet (Eleusine coracana L.) seed coat phenolics: Mode of inhibition of a-glucosidase and pancreatic amylase. Food Chem. 2017;115:1268–1273. doi: 10.1016/j.foodchem.2009.01.042. [DOI] [Google Scholar]
- 46.Mraz M, Bartlova M, Lacinova Z, Michalsky D, Kasalicky M, Haluzikova D, Matoulek M, Dostalova I, Humenanska V, Haluzik M. Serum concentrations and tissue expression of a novel endocrine regulator fibroblast growth factor-21 in patients with type 2 diabetes and obesity. Clin Endocrinol (Oxford) 2009;71:369–375. doi: 10.1111/j.1365-2265.2008.03502.x. [DOI] [PubMed] [Google Scholar]
- 47.Dunn MF (2001) Protein–Ligand Interactions: General Description. In eLS, (Ed.). 10.1002/9780470015902.a0001340.pub2
- 48.Xia C-Q, Pan X, Shen H-B. Protein–ligand binding residue prediction enhancement through hybrid deep heterogeneous learning of sequence and structure data. Bioinformatics. 2020;36:3018–3027. doi: 10.1093/bioinformatics/btaa110. [DOI] [PubMed] [Google Scholar]
- 49.Matias I, Diniz LP, Buosi A, Neves G, Stipursky J, Gomes FCA. Flavonoid hesperidin induces synapse formation and improves memory performance through the astrocytic TGF-beta1. Front Aging Neurosci. 2017;9:184. doi: 10.3389/fnagi.2017.00184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Elmeliegy M, Vourvahis M, Guo C. Effect of P-glycoprotein (P-gp) inducers on exposure of P-gp substrates: review of clinical drug–drug interaction studies. Clin Pharmacokinet. 2020;59:699–714. doi: 10.1007/s40262-020-00867-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Lund M, Petersen TS, Dalhoff KP. Clinical implications of P-glycoprotein modulation in drug–drug interactions. Drugs. 2017;77:859–883. doi: 10.1007/s40265-017-0729-x. [DOI] [PubMed] [Google Scholar]
- 52.Konig J, Muller F. Transporters and drug-drug interactions: Important determinants of drug disposition and effects. Pharmacol Rev. 2013;65:944–966. doi: 10.1124/pr.113.007518. [DOI] [PubMed] [Google Scholar]
- 53.Luthra A, Denisov IG, Sligar SG. Spectroscopic features of cytochrome P450 reaction intermediates. Arch Biochem Biophys. 2011;507:26–35. doi: 10.1016/j.abb.2010.12.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Elfaki I, Mir R, Almutairi FM, Duhier F. Cytochrome P450: polymorphisms and roles in cancer, diabetes and atherosclerosis. APJCP. 2018;19:2057–2070. doi: 10.22034/APJCP.2018.19.8.2057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Srinivas M, Thirumaleswara G, Pratima S. Cytochrome p450 enzymes, drug transporters and their role in pharmacokinetic drug-drug interactions of xenobiotics: a comprehensive review. Peertechz J Med Chem Res. 2017;3:001–011. doi: 10.17352/ojc.000006. [DOI] [Google Scholar]
- 56.Guengerich FP. Intersection of the roles of cytochrome P450 enzymes with xenobiotic and endogenous substrates, relevance to toxicity and drug interactions. Chem Res Toxicol. 2017;30:2–12. doi: 10.1021/acs.chemrestox.6b00226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Navarro-Mabarak C, Camacho-Carranza R, Espinosa-Aguirre JJ. Cytochrome P450 in the central nervous system as a therapeutic target in neurodegenerative diseases. Drug Metabol Rev. 2018;50:95–108. doi: 10.1080/03602532.2018.1439502. [DOI] [PubMed] [Google Scholar]
- 58.Lynch T, Price A. The effect of cytochrome P450 metabolism on drug response, interactions, and adverse effects. Am Fam Phys. 2007;76:390–396. [PubMed] [Google Scholar]
- 59.Barnaba C, Sahoo BR, Ravula T, Medina-Meza IG, Im SC, Anantharamaiah GM, Waskell L, Ramamoorthy A. Chem Int Ed. 2018;57:3391. doi: 10.1002/anie.201713167. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
We have included all the data.