Abstract
Chronic pain, pain catastrophizing, and mental health disorders such as anxiety or depression frequently occur together and are challenging to treat. To help understand the relationship between these conditions, we sought to identify distinct phenotypes associated with worse pain and function. In a cohort of people with chronic pain on opioids seeking medical cannabis in New York, we conducted latent class analysis to identify clusters of participants based on pain catastrophizing and mental health symptoms of depression, anxiety, post-traumatic stress disorder (PTSD) and attention deficit/hyperactivity disorder (ADHD). We then compared clusters with respect to sociodemographic and clinical characteristics using descriptive statistics. Among 185 participants, we identified four discrete groups: low pain catastrophizing and low mental health symptoms (49% of participants), low pain catastrophizing and ADHD-predominant mental health symptoms (11%), high pain catastrophizing and anxiety-predominant mental health symptoms (11%), and high pain catastrophizing and high mental health symptoms (30%). The group with high pain catastrophizing and high mental health symptoms had the worst pain intensity and interference, disability, insomnia, and quality of life, compared to the two groups with lower pain catastrophizing, though not all differences were statistically significant. Our findings highlight the importance of identifying and addressing pain catastrophizing in patients with comorbid chronic pain and mental health symptoms.
Keywords: chronic pain, pain catastrophizing, latent class analysis, refractory pain
INTRODUCTION
Chronic pain, defined as pain experienced on most or all days for at least three months (Treede et al., 2015), is a growing public health challenge that is inextricable from mental health (Goldberg and McGee, 2011). Globally, 1.5 billion people are estimated to have chronic pain and the prevalence is expected to rise as the population ages (James, 2018). Up to half of adults with chronic pain have comorbid serious mental health conditions including depression, anxiety, post-traumatic stress disorder (PTSD), and attention deficit/hyperactivity disorder (ADHD) (Bair et al., 2003; Goesling et al., 2018; Kroenke et al., 2011; Lépine and Briley, 2004; Miller and Cano, 2009). Chronic pain and mental health conditions are mutually contributive. Among adults with chronic pain, those with comorbid mental health conditions experience greater pain intensity, disability, and poorer quality of life, compared with those without mental health conditions (Dionne et al., 1997; Lamb et al., 2000; Wells et al., 1989). Additionally, chronic pain can worsen mental health symptoms, including low energy, sleep disruption, decreased concentration and attention, and anhedonia (Betrus et al., 1995; Burton et al., 1995; Fuller-Thomson et al., 2016; Moore et al., 2012; Scott et al., 2009; Von Korff et al., 2005). Most published research on chronic pain and mental health conditions centers on the relationship between chronic pain and depression, anxiety and PTSD. Recently, there has been an increasing body of research linked ADHD symptoms and diagnosis and chronic pain.(Asztély et al., 2019; Fuller-Thomson et al., 2016; Reyero et al., 2011; Stray et al., 2013) Patients with both chronic pain and comorbid mental health conditions tend to have complex treatment courses and less robust responses to traditional treatments for both mental health conditions and pain, compared to patients with only chronic pain or mental health conditions (Gureje, 2008; Karp et al., 2005).
An important psychologic factor that impacts patient outcomes in chronic pain is pain catastrophizing, which occurs in up to 30% of patients with chronic pain (Dong et al., 2020; Quartana et al., 2009). Pain catastrophizing is a set of negative emotions and cognitions experienced in response to actual or anticipated pain (Quartana et al., 2009; Sullivan et al., 2001). It is characterized by feeling desperate or fatalistic about one’s pain, for example, feeling that one’s pain will never improve. Pain catastrophizing is thought to be distinct from pain intensity because it varies among individuals even in response to the same painful stimuli (Leung, 2012). Pain catastrophizing is highly intertwined with psychological distress, and sometimes thought to be indistinguishable from depression and anxiety (Quartana et al., 2009). Among individuals with chronic pain, those with pain catastrophizing have higher comorbid depression, anxiety, and suicidal ideation compared to those without pain catastrophizing (Betrus et al., 1995; Burton et al., 1995; Dionne et al., 1997; Wells et al., 1989). Beyond depression and anxiety, pain catastrophizing has also been associated with impairment in attention, emotional processing, and PTSD symptoms (Davis et al., 2000; Gracely et al., 2004; Schrooten et al., 2013; Sullivan et al., 2001).
In individuals with mental health conditions, pain catastrophizing is associated with failure of traditional therapeutic interventions for both mental health conditions and chronic pain (Osborne et al., 2007; Suso-Ribera et al., 2017). For example, several studies have found that traditional cognitive behavioral therapy (CBT) approaches to address anxiety or depression are less effective in patients with catastrophic thinking about their pain in several studies (Morley et al., 2008; Sullivan et al., 2006). Among patients with chronic pain, those with high pain catastrophizing have poor response to pain treatments including pharmacotherapy and physical therapy (Domenech et al., 2013; Schiphorst Preuper et al., 2014). Among individuals with chronic pain and high pain catastrophizing, fear that activity will worsen pain can result in avoiding activity, and subsequent pain exacerbation (Birch et al., 2019; Edwards et al., 2011). This can, in turn, impact mental health symptoms (Betrus et al., 1995; Burton et al., 1995; Fuller-Thomson et al., 2016; Moore et al., 2012; Scott et al., 2009; Von Korff et al., 2005). Research is lacking to guide clinicians in identifying patients who would benefit from an approach that targets pain catastrophizing in addition to their mental health conditions and chronic pain in a holistic manner.
To optimize treatment for people with refractory chronic pain and comorbid mental health symptoms, it is necessary to identify which patients — such as those with pain catastrophizing — might benefit from additional or alternative treatment modalities. To address this, among participants of the Medical Marijuana and Opioids (MEMO) Study, we explored how pain catastrophizing and mental health symptoms clustered together, and how these clusters were related to pain intensity and functional characteristics (pain intensity and interference, disability, insomnia, and quality of life) (Cunningham et al., 2020). The MEMO Study is a longitudinal cohort study of individuals taking opioids for chronic pain who are newly certified for medical cannabis in New York State.
METHODS
Overview
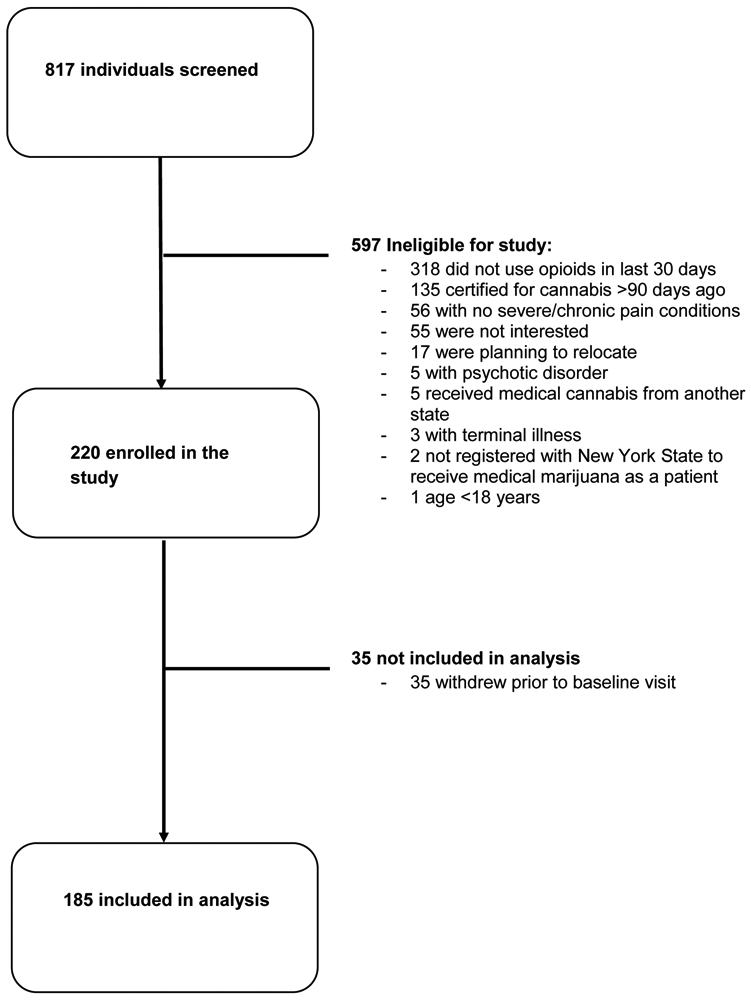
We analyzed baseline data from the first 185 patients of the ongoing MEMO longitudinal cohort study enrolled in the study from September 2018 to August 2021. This study was carried out in accordance with the latest version of the Declaration of Helsinki. The MEMO Study was approved by the Montefiore Medical Center/Albert Einstein College of Medicine institutional review board (IRB protocol number: 2017-7857) and a detailed description of the study design has been described previously (Cunningham et al., 2020).
Setting
Recruitment and study visits for the MEMO Study took place at Montefiore Medical Center and four medical cannabis dispensaries in the New York City metropolitan area. Montefiore is the largest healthcare system in the Bronx, with primary, specialty, surgical, and acute care at four hospitals, four emergency rooms, and over 20 outpatient practices. The four medical cannabis dispensaries were operated by Vireo Health and Columbia Care and at the time of the study provided services to over 30,000 patients.
Study participants
Inclusion criteria for MEMO Study participants were: 1) ≥18 years old, 2) fluency in English or Spanish, 3) new certification for medical cannabis in New York within 90 days, 4) no medical cannabis use in the six months prior to certification, 5) medical cannabis qualifying condition of “chronic or severe pain” or “pain that degrades health and functional capability as an alternative to opioid use,” or qualifying complication of “severe or chronic pain resulting in substantial limitation of function,” and 6) use of prescribed or illicit opioids in the past 30 days. Exclusion criteria were: 1) inability to provide informed consent, 2) inability to complete study visits over 18 months, 3) qualifying conditions for medical cannabis in New York that are likely to cause unique pain syndromes (cancer, epilepsy, multiple sclerosis, spinal cord injury, amyotrophic lateral sclerosis, Parkinson’s disease, inflammatory bowel disease, Huntington’s disease), 4) terminal illness, and 5) current or prior psychotic disorder based on self-report and medical record review.
Participants completed an oral consent prior to screening for eligibility, and informed consent after their eligibility was determined and after the nature of the procedures of the MEMO Study were fully explained (Cunningham et al., 2020).
Data Collection
During quarterly research visits, data were collected from MEMO participants via quarterly questionnaires. Questionnaires were administered using Audio Computer-Assisted Self-Interview (ACASI) technology, which displays questions on a computer screen while playing an audio-recording of the questions. Participants entered responses directly into the computer over 45-60 minutes. Due to the COVID-19 pandemic, no in-person visits occurred after April 2020; during this time study staff administered the ACASI questionnaire over the phone; this applies to 114 ACASI interviews. Web-based questionnaires were completed via personalized links sent by text or email from TelASK Technologies, Inc. (Nepean, ON, Canada).
Measures
We examined participants’ baseline symptoms in five domains: 1) pain catastrophizing, 2) depression, 3) anxiety, 4) PTSD, and 5) ADHD. These domains were chosen based on previously established relationships between each domain and pain outcomes (Bair et al., 2003; Goesling et al., 2018; Kroenke et al., 2011; Lépine and Briley, 2004; Miller and Cano, 2009; Schrooten et al., 2013). We chose to dichotomize each symptom scale to maximize generalizability and external validity. Dichotomized scales are easy to interpret clinically and extrapolate to real world situations. Each symptom scale was dichotomized into “high” vs. “low” based on interpretation guides and prior studies, consistent with moderate to severe symptoms. Pain catastrophizing was measured using the Pain Catastrophizing Scale (PCS), a 13-item scale that assesses negative perceptions related to pain. Participants indicate the degree to which they experience rumination, magnification, and helplessness when experiencing pain. Higher scores indicated worse catastrophizing (range=0-52, high≥30) (Sullivan et al., 2001; Sullivan et al., 1995). The PCS is well-validated including in patients with chronic pain and comorbid mental health conditions and is the most commonly used instrument for measuring pain catastrophizing (Leung, 2012). Symptoms of depression were measured using the 9-item Patient Health Questionnaire-9 (PHQ-9). Higher scores indicated worse severity of depressive symptoms (range=0-27, high≥10) (Kroenke et al., 2001). Symptoms of anxiety were measured using the Generalized Anxiety Disorder 7-Item Scale (GAD-7). Higher scores indicated worse severity of anxiety symptoms (range=0-21, high≥10) (Spitzer et al., 2006). Symptoms of PTSD were measured using the 6-item Abbreviated Post-Traumatic Stress Disorder Checklist (PCL-C). Higher scores indicated worse severity of PTSD symptoms (range=6-30, high ≥14) (Lang and Stein, 2005; Lang et al., 2012). Symptoms of ADHD were measured using the 6-item Adult ADHD Self-Report Screening Scale for DSM-5 (ASRS-5). Higher scores indicated worse severity of ADHD symptoms (range=0-25, high ≥14) (Ustun et al., 2017).
We also examined pain intensity and functional characteristics (pain intensity and interference, disability, insomnia, and quality of life). Pain intensity and interference were measured using the Pain, Enjoyment of Life and General Activity Scale (PEG-3) (Krebs et al., 2009). Higher scores indicate worse pain intensity and interference and a score of 5 or higher indicates at least moderate to severe pain (range=0-10) (Von Korff et al., 2016). Participants self-reported the location(s) of their pain, categorized as back or neck, limb (including knee, hip, elbow, shoulder, arm or leg), abdomen or chest, head, or pain in the whole body. Disability was measured using the 11-item Roland Morris Disability Questionnaire (RMDQ); higher scores indicate worse disability (range=0-11) (Stroud et al., 2004). Insomnia was measured using the Insomnia Severity Index (ISI) (range=0-28); higher scores indicate worse insomnia and a score equal to or greater than 15 is considered moderate to severe insomnia (Morin et al., 2011). Quality of life was measured using the EuroQol 5-Dimension Scale (EQ-5D-5L); a score of 1 represents full health and a score of 0 represents death (range=0-1) (EuroQol Research Foundation, 2019).
Other self-reported descriptive variables included sociodemographic characteristics (gender, age, education, race, ethnicity, employment) and substance use in the prior 30 days (Addiction Severity Index [ASI]),(McLellan et al., 1992) including non-medical cannabis, cocaine, and heroin use, tobacco use (National Cancer Institute, 2019) and hazardous alcohol use (Alcohol Use Disorders Identification Test score >7 [AUDIT-7]) (Saunders et al., 1993).
Statistical Analysis
We conducted a two-part analysis. First, we determined if phenotypes exist varying in pain catastrophizing and mental health symptoms. We used latent class analysis (LCA) to divide the sample into mutually exclusive latent classes (phenotypes) based on high vs low self-reported symptoms in the five domains of pain catastrophizing, depression, anxiety, PTSD, and ADHD symptoms, using the cut-offs described above and treating these variables as categorical variables in the models. There were no missing data for these five variables. We conducted LCA using Mplus software, Version 7.4 (Los Angeles, CA, USA). We applied maximum likelihood estimation via the EM algorithm (Dempster et al., 1977). To enhance generalizability by avoiding locally optimal solutions, we estimated the LCA model with automatic random starting values and set the number of iterations to be 1000. We fit three and four-latent class models. The appropriate number of classes was determined using both statistical criteria and the clinical experience of the investigators, who have extensive experience with managing chronic pain and psychiatric diagnoses as internal medicine physicians, psychiatrists, and psychologists. Statistical tests included: the value of the Bayesian Information Criterion (BIC), where lower values indicate better fit; the significance of the Lo-Mendell-Rubin Adjusted Likelihood Ratio Test (LMR-LRT), with significant values indicating a better fit of the X model compared to X - 1; and model entropy, where values closer to 1.0 indicate a higher accuracy of classifying individuals into latent classes. We did not fit a five-class model because some investigators have cautioned against over-extraction of latent classes due to the small sample size and the presence of non-normal data (Bauer & Curran, 2003). After deciding the number of the latent classes, we assigned each participant to the class with the largest Bayesian posterior probability (BPP). A major advantage of LCA is that it assigns individuals into classes on a probabilistic basis, which allows us to compare the presence of high pain catastrophizing and mental health symptoms across classes. We also conducted sensitivity analyses using continuous measures in the five domains treating them as censored normal variables in the models. The LMR-LRT statistic indicates that the four-class solution is not significantly different from the three-class solution.
Second, we described the sociodemographic characteristics of each phenotype, and determined whether pain severity and interference, disability, insomnia, and quality of life differed between the phenotypes using chi-square tests, t-tests and F-tests when appropriate. To address cells with n<5, we conducted Fisher’s exact test rather than chi-squared test. These analyses were conducted using SAS, Version 9.4 (Cary, NC, USA).
RESULTS
Of 185 study participants, 102 (55%) identified as female and the mean age was 53.9 years (Table 1). Thirty-seven percent identified as white and non-Hispanic, 26% as Hispanic or Latinx, 30% as Black, and 7% as other race/ethnicity. Most reported pain in their limbs (85%) and back or neck (82%). A third (37%) reported current tobacco use and 30% reported non-medical cannabis use. Cocaine and heroin use were uncommon (2% and 1%, respectively) as was hazardous alcohol use (5%). Thirty-two (17%) participants were living with HIV (Table 1). Overall, the sample had moderate to severe pain, disability, insomnia, and quality of life. The mean PEG score was 7.2 (SD= 1.9); the mean disability score (RMDQ) was 8.8 (SD= 2.3); the mean insomnia score (ISI) was 12.3 (SD= 7.0); and the mean quality of life score (EQ-5D-5L) was 0.56 (SD= 0.18). Overall, 43% had high pain catastrophizing (PCS), 46% had moderate to severe depression symptoms (PHQ-9), 35% had moderate to severe anxiety symptoms (GAD-7), 39% had high PTSD symptoms (PCL-C), and 41% had high ADHD symptoms (ASRS-5); 73% of participants had high symptoms in at least one of these measures.
Table 1:
Participant characteristics
| All (N=185) N (%) |
Low PC/ Low MHS (n=90) N (%) |
Low PC/ ADHD- Predominant (n=20) N (%) |
High PC/ Anxiety- Predominant (n=20) N (%) |
High PC/High MHS (n=55) N (%) |
P-value | |
|---|---|---|---|---|---|---|
| Gender | 0.35C | |||||
| Female | 102 (55.1) | 52 (57.8) | 9 (45) | 8 (40) | 33 (60) | |
| Age, mean (SD) | 53.9 (13.1) | 56.5 (13.1) | 53.3 (12.2) | 51.5 (13) | 50.8 (12.9) | 0.06F |
| Education | 0.42E | |||||
| 12 years or more | 159 (86) | 78(86.7) | 18 (90) | 19 (95) | 44 (80) | |
| Race/ethnicity | 0.11E | |||||
| White, non-Hispanic | 68 (37) | 29 (32.6) | 14 (70) | 8 (40) | 17 (30.9) | |
| Black or African American, non-Hispanic | 56 (30.4) | 32 (36) | 4 (20) | 6 (30) | 14 (25.5) | |
| Hispanic or Latinx | 47 (25.5) | 21 (23.6) | 2 (10) | 4 (20) | 20 (36.4) | |
| Other, non-Hispanic | 13 (7.1) | 7 (7.8) | 0 (0) | 2 (10) | 4 (7.3) | |
| Employment Status | 0.59E | |||||
| Not employed | 144 (77.8) | 68 (75.6) | 17 (85) | 14 (70) | 45 (81.2) | |
| HIV status | 0.51 | |||||
| Seropositive | 32 (17.3) | 17 (18.9) | 1 (5) | 3 (15) | 11 (20) | |
| Substance Use, past 30 days | ||||||
| Tobacco | 68 (36.8) | 29 (32.2) | 4 (20) | 9 (45) | 26 (47.3) | 0.09E |
| Non-medical cannabis | 55 (29.7) | 32 (35.6) | 4 (20) | 4 (20) | 15 (27.2) | 0.36E |
| Cocaine | 4 (2.2) | 3 (3.3) | 0 (0) | 0 (0) | 1 (1.8) | 1.00E |
| Heroin | 1 (0.5) | 0 (0) | 0 (0) | 1 (5) | 0 (0) | 0.22E |
| Hazardous alcohol use | 9 (4.9) | 4 (4.4) | 2 (10) | 2 (10) | 1 (1.8) | 0.21E |
| Location of pain | ||||||
| Back or neck | 151 (81.6) | 69 (76.7) | 17 (85) | 16 (80) | 49 (89.1) | 0.30C |
| Limb | 157 (84.9) | 75 (83.3) | 16 (80) | 16 (80) | 50 (90.9) | 0.48C |
| Abdomen or chest | 41 (22.2) | 11 (12.2) | 6 (30) | 4 (20) | 20 (36.4) | 0.005E |
| Head | 50 (27) | 21 (23.3) | 4 (20) | 5 (25) | 20 (36.4) | 0.35E |
| Whole bodv | 30 (16.2) | 10 (11.1) | 6 (30) | 2 (10) | 12 (21.8) | 0.10E |
PC= pain catastrophizing; MHS= mental health symptoms; SD= standard deviation
=Chi-squared test
=Fisher’s exact test
=F-test
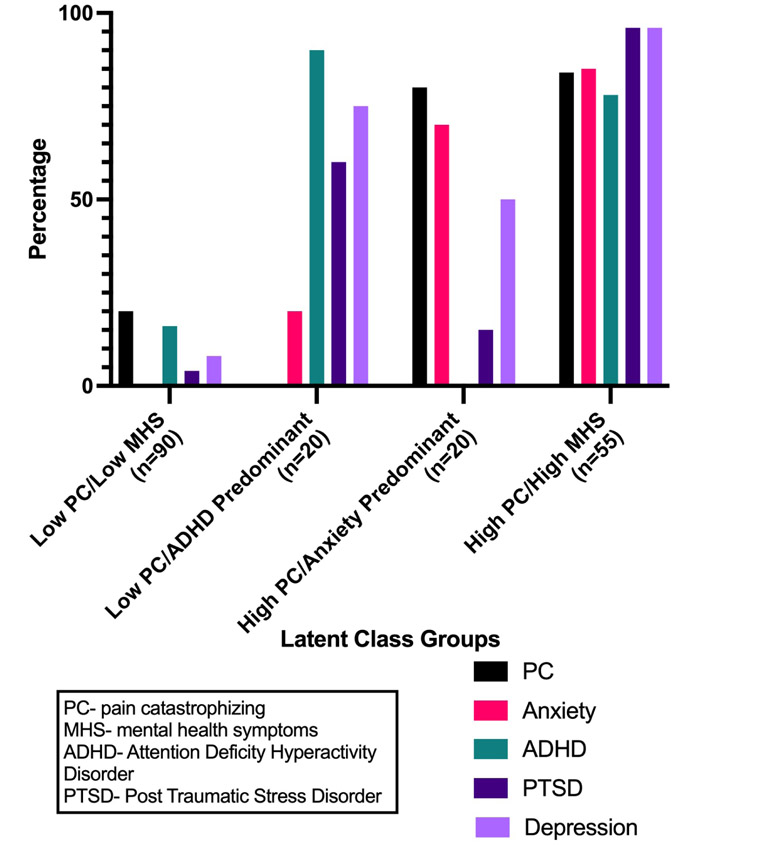
The latent class analysis identified four discrete groups, which differed by the presence and absence of high or moderate to severe symptoms in the five domains (pain catastrophizing, depression, anxiety, PTSD, and ADHD), presented in Figure 1. Compared to the three-class solution, the four-class solution had a slightly higher BIC (1124.1 vs 1104), similar entropy (0.78 vs 0.74) and was significantly different using the LMR-LRT statistic (p=0.04, one-tailed). In discussing the classes, the investigative team concluded that despite a similar fit in the statistical models, the four-class solution had greater clinical significance. For example, the four-class solution identified a group with low pain catastrophizing, in which many participants have high ADHD symptoms. This is a clinically significant group we see in practice which was not identified in the three-group solution. We thus reached a consensus that the four-class solution was preferred. The range of average BPP for cluster membership is 82% - 91%, which indicates that the classes do not significantly overlap. The first group, which we refer to as the “low pain catastrophizing and low mental health symptoms” (low PC/low MHS) group included 90 (49%) of the participants. In this group, a minority of participants had high pain catastrophizing (20%), ADHD (16%) and PTSD (4%) symptoms; a minority had moderate to severe anxiety symptoms (0%) and depression (8%) symptoms. The next group, which we refer to as the low PC/ADHD-predominant group, included 20 (11%) of the participants. Within this group, no participants had high pain catastrophizing and 20% had moderate to severe anxiety symptoms, while most had high ADHD (90%), moderate to severe depression symptoms (75%), and high PTSD (60%) symptoms. The third group, which we refer to as the high PC/anxiety-predominant group, included 20 (11%) of the participants. Within this group, most had high symptoms of pain catastrophizing (80%) and moderate to severe anxiety symptoms (70%), 50% had moderate to severe depression symptoms, a minority of the participants had high PTSD symptoms (15%), and none of the participants had ADHD symptoms. The fourth group, which we refer to as the high PC/high MHS group, included 55 (30%) of the participants. In this group, most participants reported high symptoms of pain catastrophizing (84%), moderate to severe depression symptoms (96%), high PTSD symptoms (96%), moderate to severe anxiety symptoms (85%), and high ADHD symptoms (78%). Table 2 presents the mean (standard deviation [SD]) of the original continuous scores on the five domains by the four groups.
Figure 1.
Study Flowchart
Table 2.
Mean continuous scores in each domain by each latent class group
| All (N=185) Mean (SD) |
Low PC/ Low MHS (n=90) Mean (SD) |
Low PC/ ADHD Predominant (n=20) Mean (SD) |
High PC/ Anxiety Predominant (n=20) Mean (SD) |
High PC/High MHS (n=55) Mean (SD) |
P-value (F-test) |
|
|---|---|---|---|---|---|---|
| Pain catastrophizing symptoms | 26.4 (13.4) | 18.9 (11.7) | 19 (6.4) | 36.1 (7.6) | 37.7 (8.6) | <0.001 |
| Anxiety symptoms | 8.1 (5.7) | 4 (2.7) | 8 (4.7) | 10.4 (4.2) | 14 (4.3) | <0.001 |
| Depression symptoms | 9.6 (6.4) | 5.1 (3.5) | 11.5 (5) | 8.9 (3.2) | 16.4 (5.1) | <0.001 |
| PTSD symptoms | 12.7 (5.5) | 9.2 (2.9) | 14.3 (4) | 10.8 (3.7) | 18.6 (4.5) | <0.001 |
| ADHD symptoms | 11.4 (5.6) | 8.2 (4.7) | 15.5 (2.4) | 8.9 (3.1) | 16.1 (4.2) | <0.001 |
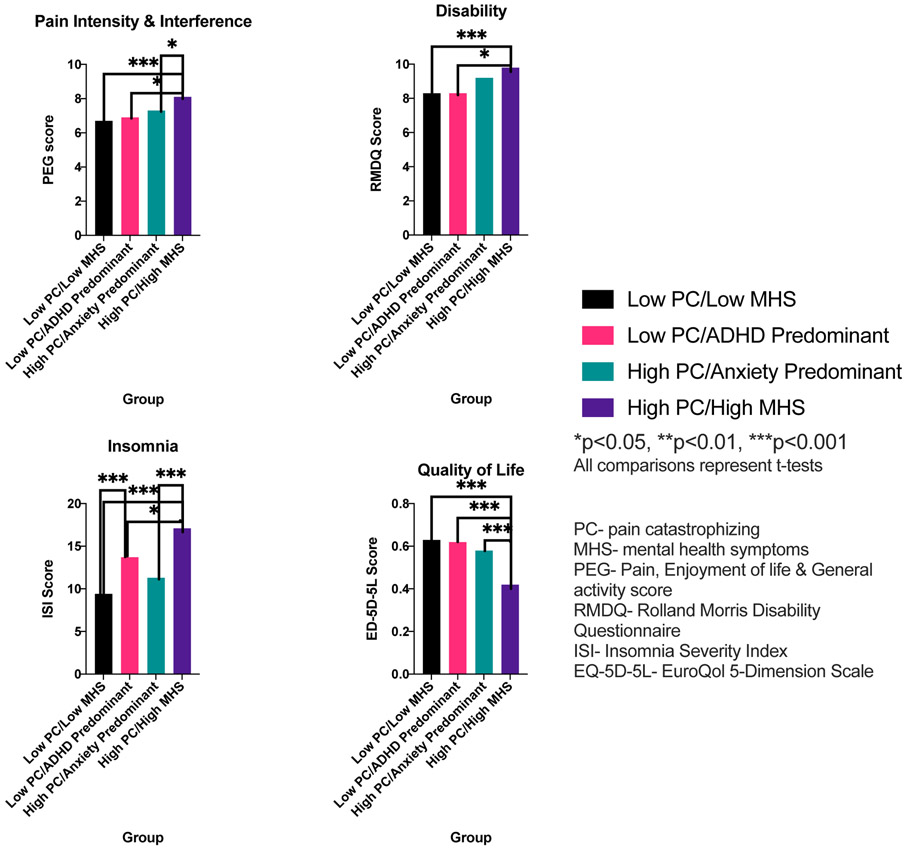
The four groups differed in pain and functional characteristics (Figure 2). The high PC/high MHS group had the poorest outcomes in all measures among the groups. Compared with low PC/low MHS, the high PC/high MHS group had higher pain intensity and interference (mean PEG score of 8.1 vs. 6.7, p<0.001), higher disability (mean RMDQ score of 9.8 vs. 8.3 p<0.001), higher insomnia (mean ISI score of 17.1 vs. 9.4, p<0.001), and lower quality of life (mean EQ-5D-5L score of 0.42 vs. 0.63, p<0.001). The high PC/high MHS group also had higher pain intensity and interference (8.1 vs. 6.9, p=0.03), higher disability (9.8 vs. 8.3, p=0.04), higher insomnia (17.1 vs. 13.7, p=0.04) and lower quality of life (0.42 vs. 0.62, p<0.001) when compared with the low PC/ADHD-predominant group. When compared with the high PC/anxiety-predominant group, the high PC/high MHS group had higher pain intensity and interference (8.1 vs. 7.3, p=0.04), higher insomnia (17.1 vs. 11.3, p<0.001), and lower quality of life (0.42 vs. 0.58, p<0.001). The low PC/ADHD-predominant group in turn had worse insomnia (13.7 vs. 9.4, p<0.01) when compared with the low PC/low MHS group.
Figure 2.
Percent of participants with high pain catastrophizing and mental health symptoms by latent class group
Discussion
In this cohort of adults with chronic pain on opioids who were initiating medical cannabis, latent class analysis identified four phenotypes, distinguishable by differences in pain catastrophizing and mental health symptoms. The most symptomatic group, those with high pain catastrophizing and high mental health symptoms, comprised just under one-third of the study cohort. This group had worse pain, higher disability, worse insomnia, and lower quality of life, compared with groups not reporting high pain catastrophizing, although not all of these differences reached statistical significance. Our findings highlight that pain catastrophizing and mental health symptoms are deeply interrelated in patients with refractory chronic pain seeking alternative treatments such as medical cannabis, and they advance our understanding of how they are interrelated. These findings call attention to the need for mental health and pain providers to identify their patients who have comorbid high pain catastrophizing and mental health symptoms and to address these conditions concurrently.
Our finding of high mental health comorbidity in this cohort of patients with chronic pain corroborates and extends the prior literature (Bair et al., 2004; Bair et al., 2003; Goesling et al., 2018). Given that mental health comorbidity is associated with poor pain treatment outcomes including high pain intensity, high disability, and worse responsiveness to conventional treatments for pain (Betrus et al., 1995; Burton et al., 1995; Dionne et al., 1997; Lamb et al., 2000; Scott et al., 2009; Von Korff et al., 2005; Wells et al., 1989), it is not surprising that a cohort of people with refractory chronic pain would have particularly high prevalence of depression, anxiety, PTSD, and ADHD.
The relationship between ADHD symptoms and functional outcomes are particularly intriguing. In this cohort of patients with refractory chronic pain, 41% had symptoms of ADHD. This is comparable to other cohorts of patients with chronic pain (Asztély et al., 2019; van Rensburg et al., 2018). Chronic pain is known to lead to interruption of attention, particularly complex attention, such as attention span, attentional switch, and divided attention (Moore et al., 2012). In a relatively small study using quantitative sensory testing, people with ADHD were found to have lower pain thresholds and pain tolerance than healthy controls; this was reversed when patients were treated with methylphenidate, suggesting that pain in patients with ADHD may be reversible if ADHD is treated (Treister et al., 2015). People with ADHD have a high prevalence of non-medical cannabis use (Lee et al., 2011; Molina et al., 2013). It is possible that patients with comorbid chronic pain and ADHD symptoms may be more likely to seek out medical cannabis to manage not only pain symptoms but also ADHD symptoms.
It has been reported that several cohorts of patients with chronic pain had a high proportion of individuals with ADHD (Asztély et al., 2019; Reyero et al., 2011; van Rensburg et al., 2018), and that patients with ADHD have higher pain intensity compared to those without ADHD (Stray et al., 2013). However, prior studies had not examined the relationship of ADHD with pain catastrophizing. Our findings suggest that in people with refractory chronic pain, the relationship between pain catastrophizing and ADHD is different than the relationship between pain catastrophizing and other mental health symptoms. Patients in the low MHS/ADHD-predominant group had functional outcomes that were not significantly different from patients in the low PC/low MHS group, indicating that ADHD symptoms may not have the same negative impact on pain and function that depression or anxiety do; or alternatively, poor pain and function do not contribute to ADHD symptoms in the way they contribute to other mental health symptoms. The only exception to this was insomnia, which was significantly worse in the low PC/ADHD-predominant group compared with the low PC/low MHS group. Insomnia is a known complication of ADHD, though the etiology is not well understood (Wynchank et al., 2017). One explanation is that insomnia is a side effect of stimulants that patients are prescribed for management of their ADHD (Bijlenga et al., 2019). While this is certainly possible, our sample’s ADHD symptoms were identified not through clinical diagnosis, but rather through identifying symptoms of ADHD with the ASRS-5, making it much less likely that patients were taking stimulants for ADHD. Insomnia was even worse in the high PC/high MHS group when compared to the low PC/ADHD-predominant group. This is consistent with the finding that among people with refractory chronic pain and ADHD symptoms, comorbidity with other mental health symptoms, like anxiety, depression, and PTSD lead to worse insomnia (Wynchank et al., 2017).
We found that, compared with the other groups, the high PC/high MHS group had worse pain intensity and interference, disability, insomnia, and quality of life. Differences were statistically significant for all of these outcomes compared with the low PC/low MHS group. The high PC/high MHS also had significantly worse pain intensity and interference than the low PC/ADHD predominant group and the high PC/anxiety predominant group, worse disability compared with the low PC/ADHD predominant group, and significantly worse insomnia compared to all other groups. These results suggest that pain catastrophizing and mental health symptoms may interact to affect pain and function. These findings support previous research that pain catastrophizing is associated with poor physical function (Dance et al., 2017; Jensen et al., 1991; Roth et al., 2012; Vienneau et al., 1999) and that depression, anxiety, PTSD, and ADHD are associated with poor pain and physical function outcomes (Taylor et al., 2013; Watrous et al., 2020; Wylie et al., 2016).
While assessing mental health symptoms is routine in mental health care and gaining traction in chronic pain care, assessing pain catastrophizing is extremely limited in both settings. For example, major guidelines for treatment of chronic pain and for treatment of depression do not mention pain catastrophizing (Dowell et al., 2016; McQuaid et al., 2019). Treatment interventions targeting pain catastrophizing, including cognitive behavioral therapy (CBT) approaches, have been successful in improving pain (Morley et al., 2008; Sullivan et al., 2006). Thus, integrated treatment of pain catastrophizing into treatment for mental health conditions such as depression or PTSD is feasible and has potential to improve outcomes including quality of life. Further, repeated measures of pain catastrophizing over a treatment course could be an important measure to consider in patients with refractory chronic pain and comorbid mental health symptoms (Darnall et al., 2017). A more active role by mental health providers in assessing chronic pain may maximize collaborative care with primary care providers. Increased collaborative care between mental health and primary care providers has shown to enhance patient satisfaction, improve treatment outcomes, and decrease medical utilization and healthcare costs (Balestrieri et al., 1988; Katon et al., 1996).
This study has limitations. The sample size was relatively small and limited to the New York City metropolitan area. Symptoms of pain and mental health symptoms were self-reported and clinical diagnostic interviews were not conducted. This was a cross-sectional study at the time of enrollment in the parent study. Further, in our latent class analysis, we decided upon a four-group solution based on clinical relevance, rather than choosing the solution that was best based on statistics. While the four-group solution was not better than the three-group solution statistically, it is important to accurately represent what is seen clinically. Future studies are needed to examine the persistence and clinical significance of the four phenotypes longitudinally, in larger sample sizes, and in other settings. Ongoing longitudinal data from this cohort will allow us to examine this as well as the impact of pain catastrophizing and mental health symptoms on health outcomes over time, and the impact of medical cannabis on pain catastrophizing, mental health symptoms, and chronic pain.
This study has implications for assessment and treatment for both mental health providers and pain providers. Cross collaboration between mental health providers, primary care providers, and pain management providers, can help identify patients with pain who have comorbid mental health symptoms and pain catastrophizing. Identifying which group a patient belongs to may help clinicians identify the patients with most severe impact on pain and other factors important to patients and providers like disability, insomnia, and quality of life, and those patients most in need of enhanced treatment. By addressing all of these conditions collaboratively, it is possible that patients will have improved health, mental health and pain outcomes. Studies are needed to test and disseminate interventions that address pain catastrophizing in people seeking mental health care. Further research should examine how static or dynamic the groups are, whether interventions could result in individuals moving between groups (i.e., reducing pain catastrophizing and reducing mental health symptoms) and whether doing so could in turn improve pain, quality of life, and other clinical outcomes. Overall, the findings are a call for mental health providers and pain providers to collaborate on the care of patients with refractory chronic pain and mental health symptoms.
Figure 3.
Pain and functional characteristics by latent class group membership
Conflict of interest
This work was supported by the National Institutes of Health’s National Institute on Drug Abuse (R01DA044171, K24DA036955, K24DA046309); by the National Institute of Mental Health (K23 MH114752); by the National Center for Advancing Translational Sciences (UL1TR001073); and by the Einstein-Rockefeller-CUNY Center for AIDS Research (P30-AI124414), which is supported by the following NIH Co-Funding and Participating Institutes and Centers: NIAID, NCI, NICHD, NHBL, NIDA, NIMH, NIA, FIC, and OAR. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health. No others have conflicts of interest to report.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
CRediT author statement
Deepika E. Slawek- Writing-Original Draft, Conceptualization, Visualization; Madiha Syed- Writing- Review & Editing; Chinazo O. Cunningham- Funding acquisition, Project administration, Writing- Review & Editing, Conceptualization; Chenshu Zhang- Methodology, Formal analysis, Data curation; Jonathan Ross- Writing- Review & Editing; Merrill Herman- Writing- Review & Editing; Nancy Sohler- Writing- Review & Editing; Haruka Minami- Writing- Review & Editing; Frances R. Levin- Writing- Review & Editing, Julia H. Arnsten- Writing- Review & Editing, Supervision, Joanna L. Starrels- Writing- Review & Editing, Supervision, Conceptualization
References
- Asztély K, Kopp S, Gillberg C, Waern M, Bergman S, 2019. Chronic Pain And Health-Related Quality Of Life In Women With Autism And/Or ADHD: A Prospective Longitudinal Study. J Pain Res 12, 2925–2932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bair MJ, Robinson RL, Eckert GJ, Stang PE, Croghan TW, Kroenke K, 2004. Impact of pain on depression treatment response in primary care. Psychosom Med 66(1), 17–22. [DOI] [PubMed] [Google Scholar]
- Bair MJ, Robinson RL, Katon W, Kroenke K, 2003. Depression and pain comorbidity: a literature review. Arch Intern Med 163(20), 2433–2445. [DOI] [PubMed] [Google Scholar]
- Balestrieri M, Williams P, Wilkinson G, 1988. Specialist mental health treatment in general practice: A meta-analysis. Psychological Medicine 18(3), 711–717. [DOI] [PubMed] [Google Scholar]
- Betrus PA, Elmore SK, Hamilton PA, 1995. Women and somatization: unrecognized depression. Health Care Women Int 16(4), 287–297. [DOI] [PubMed] [Google Scholar]
- Bijlenga D, Vollebregt MA, Kooij JJS, Arns M, 2019. The role of the circadian system in the etiology and pathophysiology of ADHD: time to redefine ADHD? Atten Defic Hyperact Disord 11(1), 5–19. [DOI] [PubMed] [Google Scholar]
- Birch S, Stilling M, Mechlenburg I, Hansen TB, 2019. The association between pain catastrophizing, physical function and pain in a cohort of patients undergoing knee arthroplasty. BMC Musculoskelet Disord 20(1), 421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burton AK, Tillotson KM, Main CJ, Hollis S, 1995. Psychosocial predictors of outcome in acute and subchronic low back trouble. Spine (Phila Pa 1976) 20(6), 722–728. [DOI] [PubMed] [Google Scholar]
- Cunningham CO, Starrels JL, Zhang C, Bacchuber MA, Sohler N, Levin FR, Minami H, Slawek D, Arnsten J, 2020. The Medical Marijuana and Opioids (MEMO) Study: A longitudinal cohort study to examine if medical cannabis reduces opioid use among adults with chronic pain. British Medical Journal Open. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dance C, DeBerard MS, Gundy Cuneo J, 2017. Pain acceptance potentially mediates the relationship between pain catastrophizing and post-surgery outcomes among compensated lumbar fusion patients. J Pain Res 10, 65–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Darnall BD, Sturgeon JA, Cook KF, Taub CJ, Roy A, Burns JW, Sullivan M, Mackey SC, 2017. Development and Validation of a Daily Pain Catastrophizing Scale. J Pain 18(9), 1139–1149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davis KD, Hutchison WD, Lozano AM, Tasker RR, Dostrovsky JO, 2000. Human anterior cingulate cortex neurons modulated by attention-demanding tasks. J Neurophysiol 83(6), 3575–3577. [DOI] [PubMed] [Google Scholar]
- Dempster AP, Laird NM, Rubin DB, 1977. Maximum likelihood from incomplete data via the EM algorithm. Journal of the Royal Statistical Society: Series B (Methodological) 39(1), 1–22. [Google Scholar]
- Dionne CE, Koepsell TD, Von Korff M, Deyo RA, Barlow WE, Checkoway H, 1997. Predicting long-term functional limitations among back pain patients in primary care settings. J Clin Epidemiol 50(1), 31–43. [DOI] [PubMed] [Google Scholar]
- Domenech J, Sanchis-Alfonso V, López L, Espejo B, 2013. Influence of kinesiophobia and catastrophizing on pain and disability in anterior knee pain patients. Knee Surgery, Sports Traumatology, Arthroscopy 21(7), 1562–1568. [DOI] [PubMed] [Google Scholar]
- Dong HJ, Gerdle B, Bernfort L, Levin L, Dragioti E, 2020. Pain Catastrophizing in Older Adults with Chronic Pain: The Mediator Effect of Mood Using a Path Analysis Approach. J Clin Med 9(7). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dowell D, Haegerich TM, Chou R, 2016. CDC Guideline for Prescribing Opioids for Chronic Pain—United States, 2016. JAMA 315(15), 1624–1645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edwards RR, Cahalan C, Mensing G, Smith M, Haythornthwaite JA, 2011. Pain, catastrophizing, and depression in the rheumatic diseases. Nat Rev Rheumatol 7(4), 216–224. [DOI] [PubMed] [Google Scholar]
- EuroQol Research Foundation, 2019. EQ-5D-5L User Guide. [Google Scholar]
- Fuller-Thomson E, Lewis DA, Agbeyaka SK, 2016. Attention-deficit/hyperactivity disorder casts a long shadow: findings from a population-based study of adult women with self-reported ADHD. Child Care Health Dev 42(6), 918–927. [DOI] [PubMed] [Google Scholar]
- Goesling J, Lin LA, Clauw DJ, 2018. Psychiatry and Pain Management: at the Intersection of Chronic Pain and Mental Health. Curr Psychiatry Rep 20(2), 12. [DOI] [PubMed] [Google Scholar]
- Goldberg DS, McGee SJ, 2011. Pain as a global public health priority. BMC Public Health 11, 770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gracely RH, Geisser ME, Giesecke T, Grant MA, Petzke F, Williams DA, Clauw DJ, 2004. Pain catastrophizing and neural responses to pain among persons with fibromyalgia. Brain 127(Pt 4), 835–843. [DOI] [PubMed] [Google Scholar]
- Gureje O, 2008. Treating chronic pain in the context of comorbid depression. Pain 134(1-2), 3–4. [DOI] [PubMed] [Google Scholar]
- James, S.e.a., 2018. Global, regional, and national incidence, prevalence, and years lived with disability for 354 diseases and injuries for 195 countries and territories, 1990-2017: a systematic analysis for the Global Burden of Disease Study 2017. Lancet 392(10159), 1789–1858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jensen MP, Turner JA, Romano JM, Karoly P, 1991. Coping with chronic pain: a critical review of the literature. Pain 47(3), 249–283. [DOI] [PubMed] [Google Scholar]
- Karp JF, Scott J, Houck P, Reynolds CF 3rd, Kupfer DJ, Frank E, 2005. Pain predicts longer time to remission during treatment of recurrent depression. J Clin Psychiatry 66(5), 591–597. [DOI] [PubMed] [Google Scholar]
- Katon W, Robinson P, Von Korff M, Lin E, Bush T, Ludman E, Simon G, Walker E, 1996. A multifaceted intervention to improve treatment of depression in primary care. Arch Gen Psychiatry 53(10), 924–932. [DOI] [PubMed] [Google Scholar]
- Krebs EE, Lorenz KA, Bair MJ, Damush TM, Wu J, Sutherland JM, Asch SM, Kroenke K, 2009. Development and initial validation of the PEG, a three-item scale assessing pain intensity and interference. J Gen Intern Med 24(6), 733–738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kroenke K, Spitzer RL, Williams JB, 2001. The PHQ-9: validity of a brief depression severity measure. J Gen Intern Med 16(9), 606–613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kroenke K, Wu J, Bair MJ, Krebs EE, Damush TM, Tu W, 2011. Reciprocal relationship between pain and depression: a 12-month longitudinal analysis in primary care. J Pain 12(9), 964–973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lamb SE, Guralnik JM, Buchner DM, Ferrucci LM, Hochberg MC, Simonsick EM, Fried LP, 2000. Factors that modify the association between knee pain and mobility limitation in older women: the Women's Health and Aging Study. Ann Rheum Dis 59(5), 331–337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lang AJ, Stein MB, 2005. An abbreviated PTSD checklist for use as a screening instrument in primary care. Behav Res Ther 43(5), 585–594. [DOI] [PubMed] [Google Scholar]
- Lang AJ, Wilkins K, Roy-Byrne PP, Golinelli D, Chavira D, Sherbourne C, Rose RD, Bystritsky A, Sullivan G, Craske MG, Stein MB, 2012. Abbreviated PTSD Checklist (PCL) as a guide to clinical response. Gen Hosp Psychiatry 34(4), 332–338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee SS, Humphreys KL, Flory K, Liu R, Glass K, 2011. Prospective association of childhood attention-deficit/hyperactivity disorder (ADHD) and substance use and abuse/dependence: a meta-analytic review. Clin Psychol Rev 31(3), 328–341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lépine JP, Briley M, 2004. The epidemiology of pain in depression. Hum Psychopharmacol 19 Suppl 1, S3–7. [DOI] [PubMed] [Google Scholar]
- Leung L, 2012. Pain catastrophizing: an updated review. Indian J Psychol Med 34(3), 204–217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McLellan AT, Kushner H, Metzger D, Peters R, Smith I, Grissom G, Pettinati H, Argeriou M, 1992. The Fifth Edition of the Addiction Severity Index. J Subst Abuse Treat 9(3), 199–213. [DOI] [PubMed] [Google Scholar]
- McQuaid JR, Lin EH, Barber JP, Cuijpers P, Greenberg LS, Jones VY, Kessler M, Nezu AM, Reynolds CF 3rd, Scogin F, 2019. Clinical Practice Guideline for the Treatment of Depression Across Three Age Cohorts. https://www.apa.org/depression-guideline/guideline.pdf. (Accessed May 26, 2021 2021). [Google Scholar]
- Miller LR, Cano A, 2009. Comorbid chronic pain and depression: who is at risk? J Pain 10(6), 619–627. [DOI] [PubMed] [Google Scholar]
- Molina BS, Hinshaw SP, Eugene Arnold L, Swanson JM, Pelham WE, Hechtman L, Hoza B, Epstein JN, Wigal T, Abikoff HB, Greenhill LL, Jensen PS, Wells KC, Vitiello B, Gibbons RD, Howard A, Houck PR, Hur K, Lu B, Marcus S, 2013. Adolescent substance use in the multimodal treatment study of attention-deficit/hyperactivity disorder (ADHD) (MTA) as a function of childhood ADHD, random assignment to childhood treatments, and subsequent medication. J Am Acad Child Adolesc Psychiatry 52(3), 250–263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moore DJ, Keogh E, Eccleston C, 2012. The interruptive effect of pain on attention. Q J Exp Psychol (Hove) 65(3), 565–586. [DOI] [PubMed] [Google Scholar]
- Morin CM, Belleville G, Belanger L, Ivers H, 2011. The Insomnia Severity Index: psychometric indicators to detect insomnia cases and evaluate treatment response. Sleep 34(5), 601–608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morley S, Williams A, Hussain S, 2008. Estimating the clinical effectiveness of cognitive behavioural therapy in the clinic: evaluation of a CBT informed pain management programme. Pain 137(3), 670–680. [DOI] [PubMed] [Google Scholar]
- National Cancer Institute, 2019. Tobacco Use Supplement to the Current Population Survey Harmonized Data, 1992–2015. [Google Scholar]
- Osborne TL, Jensen MP, Ehde DM, Hanley MA, Kraft G, 2007. Psychosocial factors associated with pain intensity, pain-related interference, and psychological functioning in persons with multiple sclerosis and pain. Pain 127(1-2), 52–62. [DOI] [PubMed] [Google Scholar]
- Quartana PJ, Campbell CM, Edwards RR, 2009. Pain catastrophizing: a critical review. Expert Rev Neurother 9(5), 745–758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reyero F, Ponce G, Rodriguez-Jimenez R, Fernandez-Dapica P, Taboada D, Martin V, Navio M, Jimenez-Arriero MA, Hoenicka J, Palomo T, 2011. High frequency of childhood ADHD history in women with fibromyalgia. European Psychiatry 26(8), 482–483. [DOI] [PubMed] [Google Scholar]
- Roth RS, Geisser ME, Williams DA, 2012. Interventional pain medicine: retreat from the biopsychosocial model of pain. Transl Behav Med 2(1), 106–116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saunders JB, Aasland OG, Babor TF, de la Fuente JR, Grant M, 1993. Development of the Alcohol Use Disorders Identification Test (AUDIT): WHO Collaborative Project on Early Detection of Persons with Harmful Alcohol Consumption--II. Addiction 88(6), 791–804. [DOI] [PubMed] [Google Scholar]
- Schiphorst Preuper HR, Geertzen JH, van Wijhe M, Boonstra AM, Molmans BH, Dijkstra PU, Reneman MF, 2014. Do analgesics improve functioning in patients with chronic low back pain? An explorative triple-blinded RCT. Eur Spine J 23(4), 800–806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schrooten MG, Karsdorp PA, Vlaeyen JW, 2013. Pain catastrophizing moderates the effects of pain-contingent task interruptions. Eur J Pain 17(7), 1082–1092. [DOI] [PubMed] [Google Scholar]
- Scott KM, Von Korff M, Alonso J, Angermeyer MC, Bromet E, Fayyad J, de Girolamo G, Demyttenaere K, Gasquet I, Gureje O, Haro JM, He Y, Kessler RC, Levinson D, Medina Mora ME, Oakley Browne M, Ormel J, Posada-Villa J, Watanabe M, Williams D, 2009. Mental-physical co-morbidity and its relationship with disability: results from the World Mental Health Surveys. Psychol Med 39(1), 33–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spitzer RL, Kroenke K, Williams JB, Lowe B, 2006. A brief measure for assessing generalized anxiety disorder: the GAD-7. Arch Intern Med 166(10), 1092–1097. [DOI] [PubMed] [Google Scholar]
- Stray LL, Kristensen Ø, Lomeland M, Skorstad M, Stray T, Tønnessen FE, 2013. Motor regulation problems and pain in adults diagnosed with ADHD. Behav Brain Funct 9, 18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stroud MW, McKnight PE, Jensen MP, 2004. Assessment of self-reported physical activity in patients with chronic pain: development of an abbreviated Roland-Morris disability scale. J Pain 5(5), 257–263. [DOI] [PubMed] [Google Scholar]
- Sullivan MJ, Adams H, Thibault P, Corbière M, Stanish WD, 2006. Initial depression severity and the trajectory of recovery following cognitive-behavioral intervention for work disability. J Occup Rehabil 16(1), 63–74. [DOI] [PubMed] [Google Scholar]
- Sullivan MJ, Thorn B, Haythornthwaite JA, Keefe F, Martin M, Bradley LA, Lefebvre JC, 2001. Theoretical perspectives on the relation between catastrophizing and pain. Clin J Pain 17(1), 52–64. [DOI] [PubMed] [Google Scholar]
- Sullivan MJL, Bishop SR, Pivik J, 1995. The Pain Catastrophizing Scale: Development and validation. Psychological Assessment 7(4), 524–532. [Google Scholar]
- Suso-Ribera C, García-Palacios A, Botella C, Ribera-Canudas MV, 2017. Pain Catastrophizing and Its Relationship with Health Outcomes: Does Pain Intensity Matter? Pain Res Manag 2017, 9762864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor B, Carswell K, Williams AC, 2013. The interaction of persistent pain and post-traumatic re-experiencing: a qualitative study in torture survivors. J Pain Symptom Manage 46(4), 546–555. [DOI] [PubMed] [Google Scholar]
- Treede RD, Rief W, Barke A, Aziz Q, Bennett MI, Benoliel R, Cohen M, Evers S, Finnerup NB, First MB, Giamberardino MA, Kaasa S, Kosek E, Lavand'homme P, Nicholas M, Perrot S, Scholz J, Schug S, Smith BH, Svensson P, Vlaeyen JWS, Wang SJ, 2015. A classification of chronic pain for ICD-11. Pain 156(6), 1003–1007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Treister R, Eisenberg E, Demeter N, Pud D, 2015. Alterations in pain response are partially reversed by methylphenidate (Ritalin) in adults with attention deficit hyperactivity disorder (ADHD). Pain Pract 15(1), 4–11. [DOI] [PubMed] [Google Scholar]
- Ustun B, Adler LA, Rudin C, Faraone SV, Spencer TJ, Berglund P, Gruber MJ, Kessler RC, 2017. The World Health Organization Adult Attention-Deficit/Hyperactivity Disorder Self-Report Screening Scale for DSM-5. JAMA Psychiatry 74(5), 520–527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Rensburg R, Meyer HP, Hitchcock SA, Schuler CE, 2018. Screening for Adult ADHD in Patients with Fibromyalgia Syndrome. Pain Med 19(9), 1825–1831. [DOI] [PubMed] [Google Scholar]
- Vienneau TL, Clark AJ, Lynch ME, Sullivan MJL, 1999. Catastrophizing, Functional Disability and Pain Reports in Adults with Chronic Low Back Pain. Pain Research and Management 4, 201231. [Google Scholar]
- Von Korff M, Crane P, Lane M, Miglioretti DL, Simon G, Saunders K, Stang P, Brandenburg N, Kessler R, 2005. Chronic spinal pain and physical-mental comorbidity in the United States: results from the national comorbidity survey replication. Pain 113(3), 331–339. [DOI] [PubMed] [Google Scholar]
- Von Korff M, Scher AI, Helmick C, Carter-Pokras O, Dodick DW, Goulet J, Hamill-Ruth R, LeResche L, Porter L, Tait R, Terman G, Veasley C, Mackey S, 2016. United States National Pain Strategy for Population Research: Concepts, Definitions, and Pilot Data. J Pain 17(10), 1068–1080. [DOI] [PubMed] [Google Scholar]
- Watrous JR, McCabe CT, Jones G, Farrokhi S, Mazzone B, Clouser MC, Galarneau MR, 2020. Low back pain, mental health symptoms, and quality of life among injured service members. Health Psychol 39(7), 549–557. [DOI] [PubMed] [Google Scholar]
- Wells KB, Golding JM, Burnam MA, 1989. Affective, substance use, and anxiety disorders in persons with arthritis, diabetes, heart disease, high blood pressure, or chronic lung conditions. Gen Hosp Psychiatry 11(5), 320–327. [DOI] [PubMed] [Google Scholar]
- Wylie JD, Suter T, Potter MQ, Granger EK, Tashjian RZ, 2016. Mental Health Has a Stronger Association with Patient-Reported Shoulder Pain and Function Than Tear Size in Patients with Full-Thickness Rotator Cuff Tears. J Bone Joint Surg Am 98(4), 251–256. [DOI] [PubMed] [Google Scholar]
- Wynchank D, Bijlenga D, Beekman AT, Kooij JJS, Penninx BW, 2017. Adult Attention-Deficit/Hyperactivity Disorder (ADHD) and Insomnia: an Update of the Literature. Curr Psychiatry Rep 19(12), 98. [DOI] [PubMed] [Google Scholar]