Abstract
Background/Aims
Circadian rhythms in behavior and physiology are programmed by the suprachiasmatic nucleus (SCN) of the hypothalamus. A subset of SCN neurons produce the neuropeptide arginine vasopressin (AVP), but it remains unclear whether AVP signaling influences the SCN clock directly.
Methods
Here we test that AVP signaling acting through V1A and V1B receptors influences molecular rhythms in SCN neurons. V1 receptor agonists were applied ex vivo to PERIOD2∷LUCIFERASE SCN slices, allowing for real-time monitoring of changes in molecular clock function.
Results
V1A/B agonists reset the phase of the SCN molecular clock in a time-dependent manner, with larger magnitude responses by the female SCN. Further, we find evidence that both Gαq and Gαs signaling pathways interact with V1A/B-induced SCN resetting, and that this response requires vasoactive intestinal polypeptide (VIP) signaling.
Conclusions
Collectively, this work indicates that AVP signaling resets SCN molecular rhythms in conjunction with VIP signaling and in a manner influenced by sex. This highlights the utility of studying clock function in both sexes and suggests that signal integration in central clock circuits regulates emergent properties important for the control of daily rhythms in behavior and physiology.
Keywords: Vasopressin signaling, suprachiasmatic nucleus, circadian, AVP, VIP, signal integration, sex differences
Introduction
Daily rhythms are pervasive, regulating everything from cell division to sleep, hormone release, and cognition. Designed to maintain temporal homeostasis, daily rhythms allow organisms to anticipate environmental changes important for survival. Circadian rhythms are generated endogenously at the cellular level, driven by a molecular clock that controls the daily timing of gene expression [1]. Nearly every type of cell in the body displays daily rhythms in clock gene expression, and these mammalian clock cells form a system of tissues orchestrated by a central clock in the suprachiasmatic nucleus (SCN) of the hypothalamus. As the central clock, the SCN coordinates tissue clocks throughout the body [2] so that tissues in the system are adjusted to one another and the solar day [3, 4]. In the SCN itself, clock neurons of different types communicate with one another and send outputs to downstream tissues [5]. Network communication is critical for SCN function, and these clock neurons appear to use multiple signals to influence one another, (i.e., GABA, neuropeptides [6–10]) in ways that remain poorly defined.
The neuropeptide arginine vasopressin (AVP) is released by a large subset of SCN neurons [11, 12], but the role of this neuropeptide in regulating network function is not clear. Long recognized as an important SCN output signal [13], recent work indicates that altering the molecular clock of SCN AVP neurons will translate into changes in locomotor rhythms [14, 15] and deficits in SCN network function [16]. This complements ex vivo studies suggesting that AVP signaling regulates SCN function itself [9, 17–19]. Indeed, the SCN expresses both V1A and V1B receptors [19], which are G-protein coupled receptors that activate Gαq signaling cascades [20]. Consistent with this receptor profile, V1 signaling elevates intracellular calcium and depolarizes SCN neurons [21–24]. Further, Gαq signaling is implicated in SCN resetting [25–27], which is important for entrainment of the circadian system. Lastly, daily rhythms in locomotor behavior are altered by the loss of AVP, V1A and/or V1B [28–30], with some effects localized to the SCN itself [29] and with sex differences in the resulting phenotype [30]. Despite this work, it remains unclear whether V1 signaling directly influences the SCN molecular clock, how it might do so, and whether this response differs by sex.
Here we test the hypothesis that AVP influences the SCN molecular clock. Using PERIOD2∷LUCIFERASE (PER2∷LUC) mice, we measured changes in SCN molecular rhythms in response to ex vivo application of a V1A/B receptor agonist cocktail. V1 signaling reset the SCN molecular clock in a dose- and time-dependent manner, with the female SCN showing greater magnitude responses relative to the male SCN. Further, we find that V1A/B-induced resetting involves both Gαq and Gαs signaling, Lastly, V1A/B-induced SCN resetting was occluded by genetic or pharmacological abrogation of vasoactive intestinal polypeptide (VIP) signaling. These results provide novel insights into the role of V1 signaling in the SCN network, demonstrating that resetting by this pathway is sexually dimorphic and that circuit- or cellular-level integration of multiple peptide-signaling pathways modulates circadian timekeeping in central clock neurons.
Results
V1A/B agonists phase delay SCN PER2∷LUC rhythms in a sex-biased manner
To test whether AVP signaling affects the SCN molecular clock, a V1A/B agonist cocktail or vehicle was applied ex vivo to PER2∷LUC SCN slices. Three consecutive SCN slices were collected through the anteroposterior axis of the network (Figure 1), and data were analyzed with a Marginal Multilevel Model to control for repeated observations collected from a single mouse (c.f., Statistical analyses in Methods). Following baseline recording, V1A/B agonists were applied on the fourth cycle in vitro at the predicted time of peak V1A expression in the SCN. Specifically, V1a mRNA is rhythmically expressed, with highest expression during late subjective night [31, 19], which occurs 8–10 h after peak PER2∷LUC expression and the start of locomotor activity in vivo (i.e., Circadian Time 12, CT12). Therefore, we applied V1A/B agonists to SCN slices 10 h after peak PER2∷LUC expression (CT22) to also account for the predicted 2–4 h lag required for translation [32]. Timing of V1A/B agonist or vehicle administration was predicted based on the phase and period of PER2∷LUC rhythms for each SCN slice over the first three cycles in vitro (Figure 1, Figure 2A).
Figure 1. PER2∷LUC rhythms in vehicle- and 10μM V1A/B-treated slices collected from the anterior, middle, and posterior SCN of adult male and female mice.
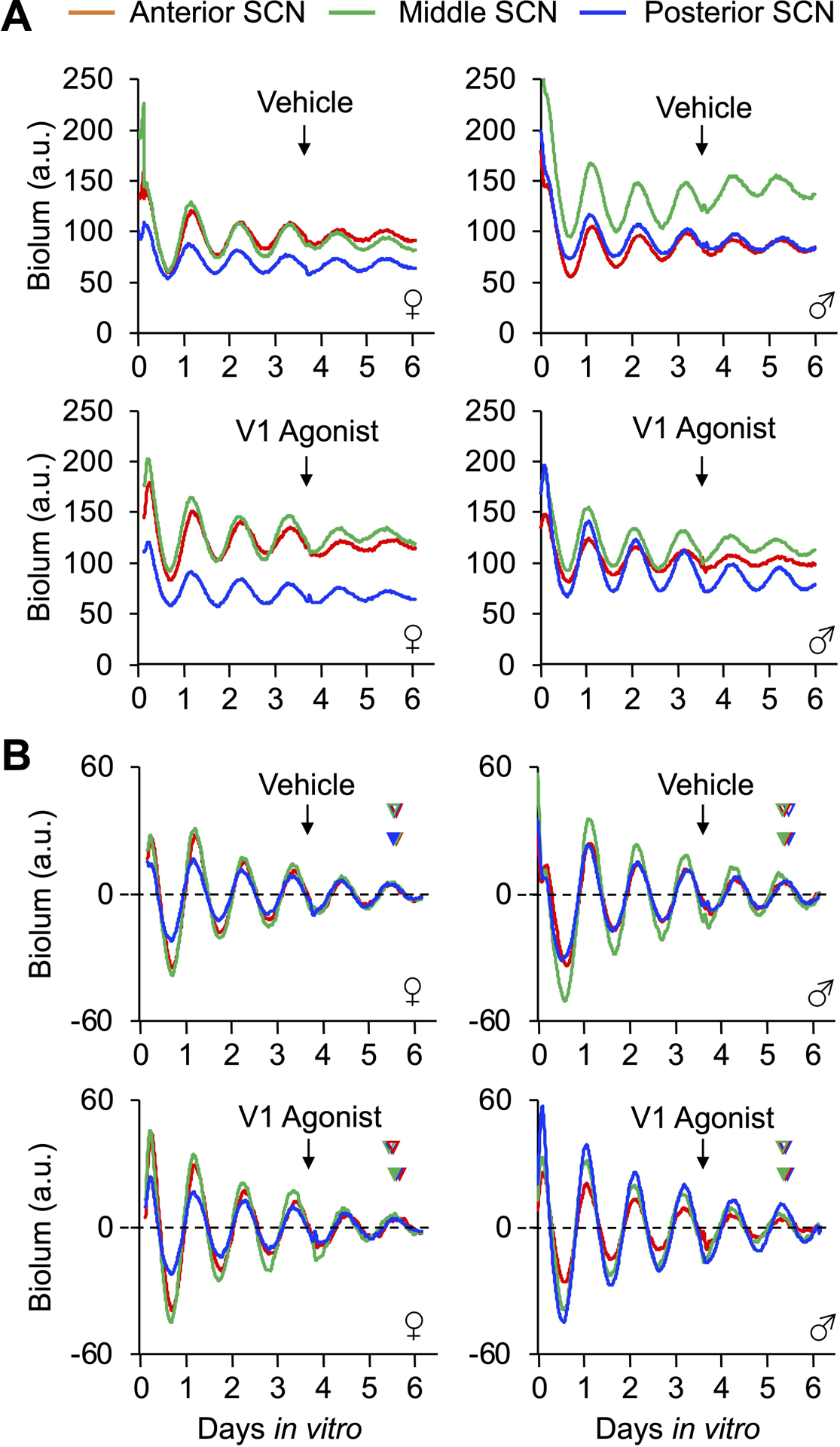
A. Absolute PER2∷LUC levels in three slices collected from a mouse in each group. B Baseline-subtracted PER2∷LUC rhythms in the same three slices in A). Arrow represents time of treatment in vitro for each panel. In B, predicted and actual post-treatment peak times are indicated by open and filled arrowheads, respectively (color coded by slice).
Figure 2. V1A/B agonists reset SCN PER2∷LUC rhythms in a dose- and sex-dependent manner.
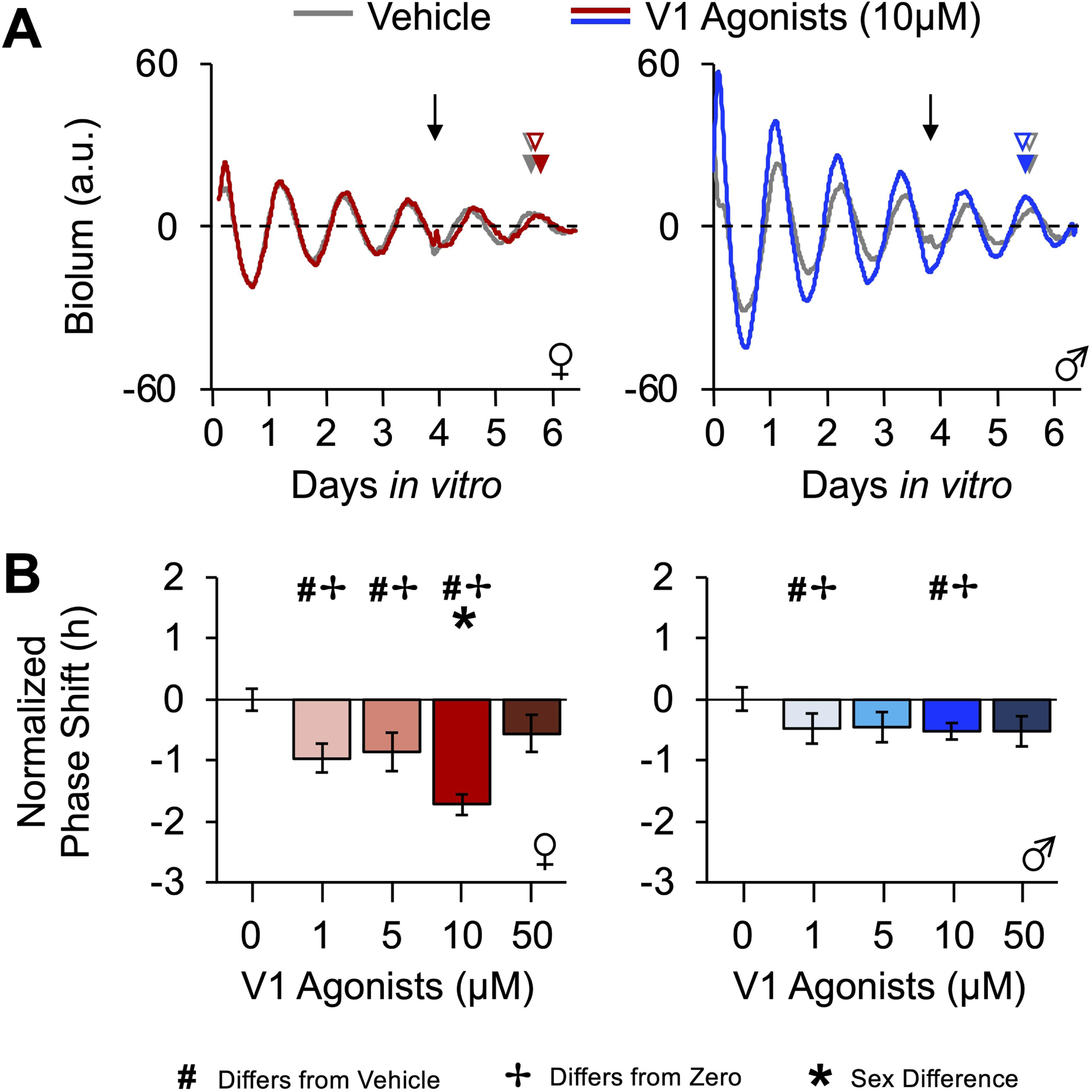
A. Representative, baseline-subtracted time series illustrating PER2∷LUC rhythms from posterior SCN slices treated with vehicle or 10μM V1A/B agonists. Arrow represents time of treatment for each panel. Predicted and actual post-treatment peak times are indicated by open and filled arrowheads, respectively (color coded by sample). B. SCN PER2∷LUC rhythms are reset by V1A/B agonists in a dose-dependent manner influenced by sex. # Different from 0μM, Estimated Marginal Means Contrasts: p < 0.05. * Different by sex, Estimated Marginal Means Contrasts: p < 0.05. ✢ Different from zero, Single sample t-test: p < 0.05. For raw resetting data c.f., Figure S1.
Select properties of SCN PER2∷LUC rhythms differed by sex prior to drug administration. Male and female SCN did not differ in the peak time of PER2∷LUC on the first cycle in vitro or damping rate over the first three cycles (Table S1). However, the period of PER2∷LUC rhythms was longer in the female SCN before treatment (t(54) = −2.5, p < 0.05, d = 0.67), which was observed across multiple experiments (Table S1). Despite the sex difference in period, the error rate for a “mock” prediction of PER2∷LUC phase for peak time on the third cycle did not differ by sex (Table S1), indicating that phase can be predicted accurately in both male and female slices. Lastly, vehicle administration tended to shift the female SCN earlier than in the male SCN (Figure S2A, Marginal Multilevel Model, Sex: F (1,9) = 4.68, p = 0.06, Slice: F (2,18) = 0.18, p > 0.8, Sex*Slice: F (2,18) = 0.28, p > 0.7; Estimated Marginal Means Contrasts: p = 0.06, d = 0.67), and vehicle responses were more variable for the female SCN (Figure S1B, Levene’s test, F(1,31) = 6.5, p = 0.02). As a result, V1A/B-induced responses were normalized by subtraction of sex-specific vehicle data (Figure 2B) to facilitate comparison of drug effects and control for sex differences in non-specific procedural responses. Raw phase resetting data are provided in supplemental materials, and analyses of non-normalized responses produced similar results.
V1A/B agonists shifted the phase of SCN PER2∷LUC rhythms later (i.e., phases delayed) in a dose-dependent manner that varied by sex (Figure 2B, Marginal Multilevel Model, Concentration: F (4,32) = 12.1, p < 0.0001, Sex: F (1,32) = 15.7, p < 0.0001, Concentration*Sex: F (4,32) = 5.3, p = 0.002). Specifically, V1A/B agonists elicited 1–2 h phase delays in female SCN at 1, 5, and 10 μM doses (Figure 2B, Estimated Marginal Means Contrasts: p < 0.05, d = 1.14–2.28), whereas this treatment elicited < 1 h phase delays in the male SCN at the 1μM and 10μM doses (Figure 2B, Estimated Marginal Means Contrasts: p < 0.05, d = 1.17–1.60). At 10μM, V1A/B agonists phase delayed the female SCN more than the male SCN (Figure 2B, Estimated Marginal Means Contrasts: p < 0.05, d = 1.57). Similar dose response curves were displayed by the three anteroposterior SCN slices (Figure S1B, Marginal Multilevel Model, Slice*Concentration: F (8,33) = 0.8, p = 0.57). However, the magnitude of V1A/B-induced SCN resetting varied across this axis in a manner that interacted with sex (Figure 3A, Marginal Multilevel Model, Slice: F (2,32) = 6.2, p = 0.005, Sex*Slice: F (2,32) = 4.8, p = 0.01, Sex*Slice*Concentration: F (8,32) = 0.8, p = 0.59). Relative to vehicle controls, 10μM V1A/B agonists reset all three female SCN slices (Figure 3A, Estimated Marginal Means Contrasts: p < 0.05, d = 1.14–2.72), but male responses were restricted to the posterior SCN (Figure 3A, Estimated Marginal Means Contrasts: p < 0.05, d = 0.88).
Figure 3. SCN responses to 10μM V1A/B agonists varies by region and is blocked by co-culture with 10μM V1A/B antagonists.
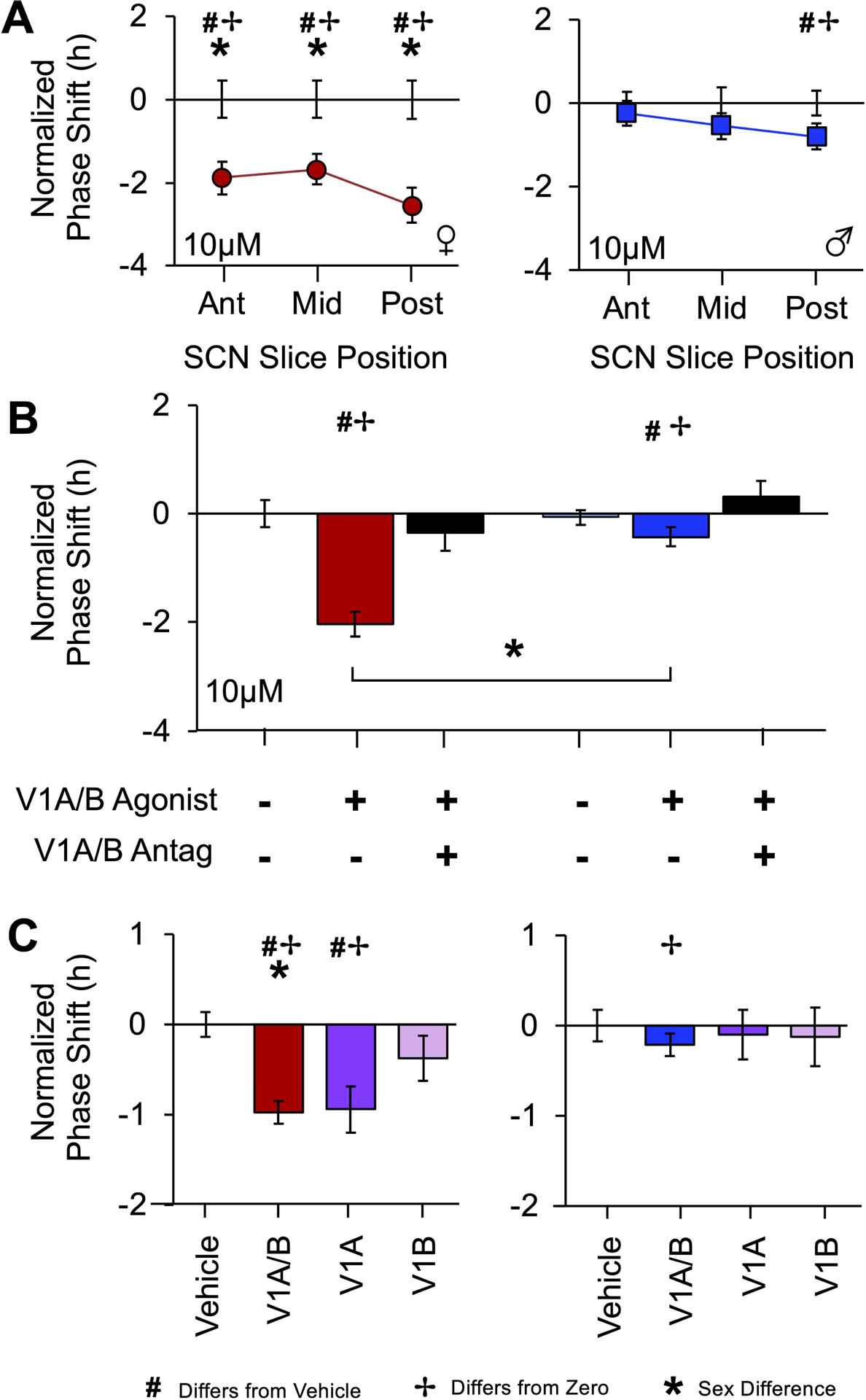
A. Resetting after 10μM V1A/B agonist administration was consistently larger in female SCN compared to males, with significant resetting responses across the anteroposterior axis in female SCN and only posterior responses in male SCN. Error bars plotted on the abscissa represent variability for vehicle controls. B. Female SCN displayed larger resetting responses to 10μM V1A/B agonists, and this response was blocked by V1A/B antagonist co-administration. C. Responses to V1A/B and single receptor agonists differ by sex. Note 0 and 10μM V1A/B data in A-B are re-plotted from Figure2. # Different from 0μM, Estimated Marginal Means Contrasts: p < 0.05. * Different by sex, Estimated Marginal Means Contrasts: p < 0.05. ✢ Different from zero, Single sample t-test: p < 0.05. For raw resetting data c.f., Figures S1–2.
Next, we examined whether SCN V1A/B-induced phase resetting would be blocked by V1A/B antagonists and mimicked by V1A and/or V1B specific agonists. For V1A/B occlusion, we selected a cocktail of 10μM V1A/B antagonists, a dose shown previously to not alter SCN phase, period, or damping when applied alone [19]. Co-administration with V1A/B antagonists blocked 10μM V1A/B-induced SCN resetting (Figure 3B, Marginal Multilevel Model, Drug Group: F (2,25) = 17.4, p < 0.0001, Sex: F (1,25) = 12.8, p = 0.001, Drug Group*Sex: F (2,25) = 6.0, p = 0.007, Slice: F (2,25) = 0.3, p > 0.7, Slice*Sex: F (2,25) = 1.3, p > 0.2, Drug Group*Slice: F (4,25) = 1.7, p > 0.1, Drug Group*Slice*Sex: F (4,25) = 0.6, p > 0.6, Estimated Marginal Means Contrasts: p < 0.05, d = 0.84–0.85). When effects of the two V1 receptors were dissected in an independent experiment, SCN resetting responses to the V1A/B cocktail and V1A agonist differed by sex (Figure S3C, Figure S2, Marginal Multilevel Model, Sex: F (1,67) = 7.7, p < 0.01, V1 Agonist: F (3,67) = 6.6, p = 0.001, Sex* V1 Agonist: F (3,67) = 2.0, p > 0.1, Sex*Slice F (2,67) = 2.4 p = 0.1, V1 Agonist*Slice: F (6,67) = 0.4, p > 0.8, Sex*V1 Agonist*Slice: F (6, 67) = 1.2, p > 0.3). Once again vehicle responses tended to differ by sex (Figure S2, Estimated Marginal Means Contrasts: p = 0.08, d = 0.44), and female SCN displayed larger shifts to V1A/B treatment relative to male SCN (Figure S3C, Figure S2, Estimated Marginal Means Contrasts: p < 0.05, d = 0.42). Relative to vehicle, V1A/B agonists reset the female SCN (Figure S3C, Figure S2, Estimated Marginal Means Contrasts: p < 0.05, d = 1.09), but not the male SCN (Figure S3C, Figure S2, Estimated Marginal Means Contrasts: p > 0.3, d = 0.24). SCN responses to the V1A agonist were also sexually dimorphic. Female SCN phase shifted in response to the V1A agonist (Figure S3C, Figure S2, Estimated Marginal Means Contrasts: p < 0.05, d = 1.04), but not the V1B agonist (Figure S3C, Figure S2, Estimated Marginal Means Contrasts: p > 0.1, d = 0.42). Male SCN did not respond to either V1A or V1B alone (Figure S3C, Figure S2, Estimated Marginal Means Contrasts: p > 0.7, d = 0.11–0.14).
To further test the basis of V1A/B-induced phase shifts, we examined whether other SCN clock properties were affected by V1A/B agonist treatment. SCN PER2∷LUC expression was not influenced acutely by V1A/B agonist administration (Figure 1, Figure 2A), nor was the damping of PER2∷LUC rhythms increased by V1A/B agonists (Table S2). Administration of 50μM V1A/B agonists decreased PER2∷LUC period in female SCN (Table S2). However, 10μM V1A/B agonists did not alter SCN period in either sex (Table S2), indicating that the phase shift in response to this dose does not reflect a change in period. Collectively, these data indicate that the 10μM V1A/B agonist resets the molecular clock by shifting its phase, with larger responses in the female SCN. Further, the sex difference in 10μM V1A/B-induced phase resetting does not appear to be driven by effects on other clock properties. Based on this pattern of results, we selected the 10μM dose to test how V1A/B signaling resets the SCN molecular clock.
V1A/B-induced resetting varies across the circadian cycle in a sexually dimorphic manner
Given that SCN responses to other stimuli vary across the circadian cycle (e.g., light, VIP, melatonin), we speculated that sex-biased responses at CT22 could reflect sex differences in the daily rhythm of V1A/B-induced resetting rather than the absolute magnitude of phase shifts. To test for sex differences in the rhythm of V1A/B-induced resetting, we constructed a phase response curve (PRC) by applying V1A/B agonists to SCN slices at one of four timepoints spanning the circadian cycle. Response to vehicle alone varied by time of day, consistent with previous work [33]. Notably, time-dependent effect of vehicle interacted with sex, but not slice position (Figure S3B, Marginal Multilevel Model, CT: F (3,22) = 8.9, p < 0.0001, Sex: F (1,22) = 7.7, p = 0.01, Slice: F (2,22) = 0.0, p > 0.9, CT*Sex: F (3,22) = 1.0, p = 0.4, Slice*Sex: F (2,22) = 0.1, p > 0.9, Slice*CT: F (6,22) = 2.1, p = 0.09, Slice*CT*Sex: F (6,22) = 0.4, p > 0.8). Thus, data for each timepoint was normalized to sex- and phase-specific vehicle controls to evaluate effects of drug application relative to non-specific factors (Figure 4A, Figure S3).
Figure 4. V1A/B agonists reset SCN PER2∷LUC rhythms in a time-, sex-, and region-specific manner.
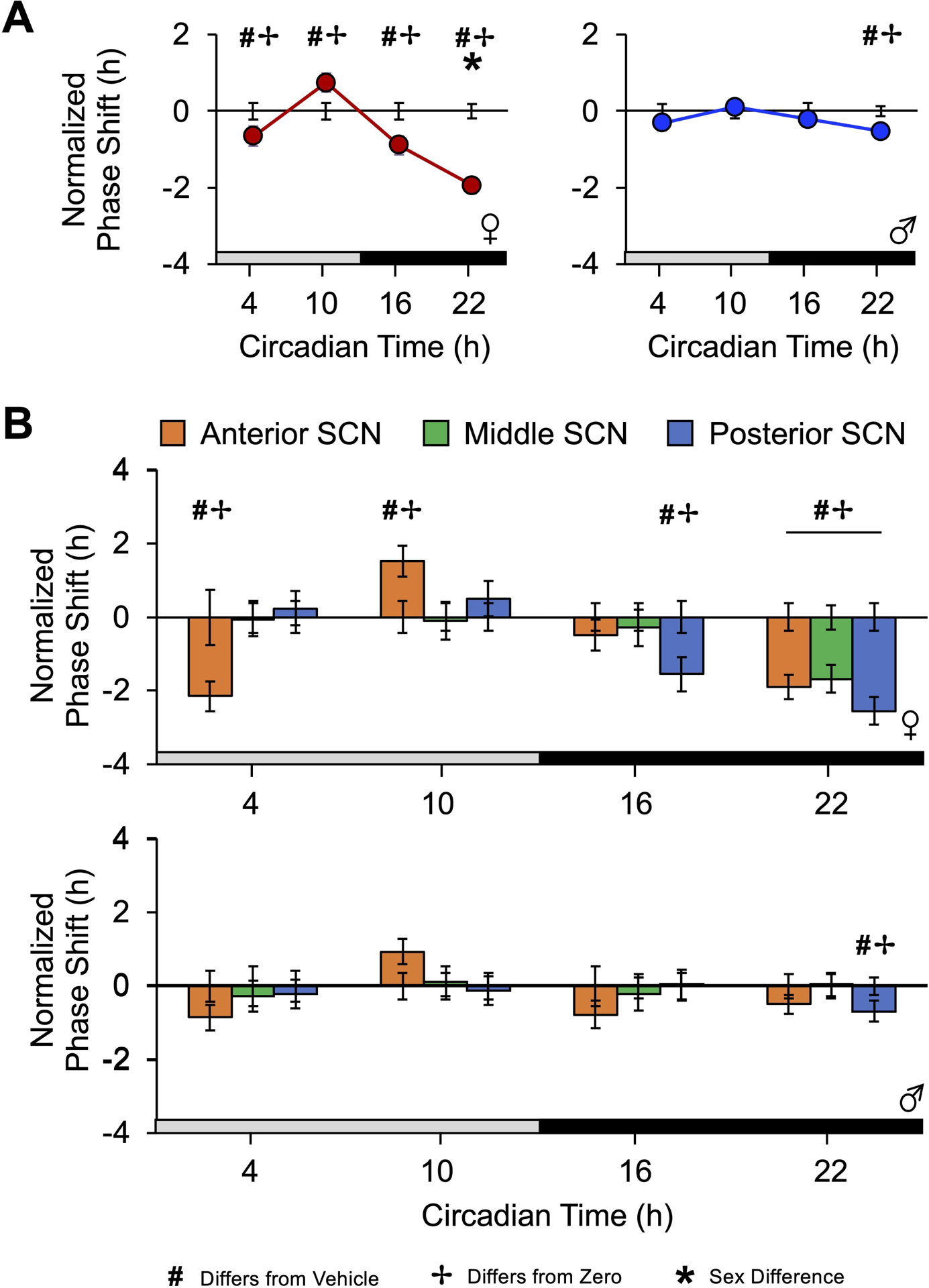
A. The V1A/B agonists resetting response across four times spanning the circadian cycle divided by sex. B. V1A/B-induced resetting varies by SCN region in each sex. Error bars plotted on the abscissa represent variability for vehicle controls. Note CT22 0 and 10μM V1A/B data are re-plotted from Figure2. # Different from vehicle, Estimated Marginal Means Contrasts: p < 0.05. * Different by sex, Estimated Marginal Means Contrasts: p < 0.05. ✢ Different from zero, Single sample t-test: p < 0.05. For raw resetting data c.f., Figure S3.
V1A/B agonists induced time-dependent resetting responses, and the overall magnitude of the V1A/B-induced resetting rhythm was larger in female SCN (Figure 4A, Figure S3A, Marginal Multilevel Model, CT: F (3,39) = 20.9, p < 0.001, Sex: F (1,39) = 9.1, p < 0.05, CT*Sex: F (3,39) = 3.45, p < 0.05, V1 Agonist: F (1,39) = 14.8, p < 0.001, CT*V1 Agonist: F (3,39) = 8.8, p < 0.05, Sex*V1 Agonist: F (1,39) = 4.52, p < 0.05). In addition to CT22, the female SCN responded to V1A/B agonists with phase delays at CT16 and CT04 (Figure 4A, Figure S3A, Estimated Marginal Means Contrasts: p < 0.05, d = 0.82–1.12), but V1A/B treatment at CT10 induced phase advances when compared to vehicle controls (Figure 4A, Estimated Marginal Means Contrasts: p < 0.05, d = 0.94). In contrast, V1A/B-induced responses in male SCN were only detected at CT22 (Figure 4A–B, Estimated Marginal Means Contrasts: p < 0.05, d = 0.66). When collapsed by sex, the daily rhythm in V1A/B agonist resetting resembled that for the female SCN (Figure S3B).
The daily rhythm in V1A/B-induced resetting also varied by SCN slice position (Figure 4B, Marginal Multilevel Model, Slice*CT: F (6,39) = 6.3, p < 0.001, Sex*Slice*CT: F (6,39) = 2.0, p = 0.08, Slice: F (2,39) = 1.2, p > 0.3, Slice*V1 Agonist: F (2,39) = 0.42, p > 0.5, Slice*Sex: F (2,39) = 0.1, p > 0.9, Slice*CT*Sex*V1 Agonist: F (6,39) = 1.44, p > 0.1). At CT16, V1A/B-induced responses were restricted to the posterior SCN in females (Figure 4B, Estimated Marginal Means Contrasts: p < 0.05, d = 1.5). In contrast, phase delays at CT04 and phase advances at CT10 were displayed by the anterior SCN (Figure 4B, Estimated Marginal Means Contrasts: p < 0.05, d = 1.58–1.92). A smaller, non-significant phase advance was also observed in the anterior SCN of males at CT10 (Figure 4B, Estimated Marginal Means Contrasts: p > 0.1, d = 0.47). Collectively, these data indicate that the magnitude of V1A/B-induced resetting is influenced by sex, and the rhythm in V1A/B-induced resetting varies in a SCN region-specific manner.
V1A/B-induced resetting requires both Gαq and Gαs signaling
Having found V1A/B agonists reset the molecular clock in a time-dependent manner, next we decided to interrogate the required signaling cascades. V1A and V1B are both Gαq coupled receptors, which are blocked by the Phospholipase C (PLC) inhibitor U-73122 (10μM, as in [26]). Given precedent that Gαs coupled pathways are involved in SCN resetting [26], we also used the adenylate cyclase (AC) inhibitor MDL-12,330A (2μM, as in [26]). PLC and AC inhibition without V1A/B agonists affected SCN phase (Figure S4A), but not period or damping of SCN rhythms (Table S2). Thus, we visualized responses specific to V1A/B agonists by normalizing to inhibitor-specific controls for each sex (Figure 5A).
Figure 5. V1A/B agonists do not reset SCN PER2∷LUC rhythms in the absence of Gα signaling or VIP.
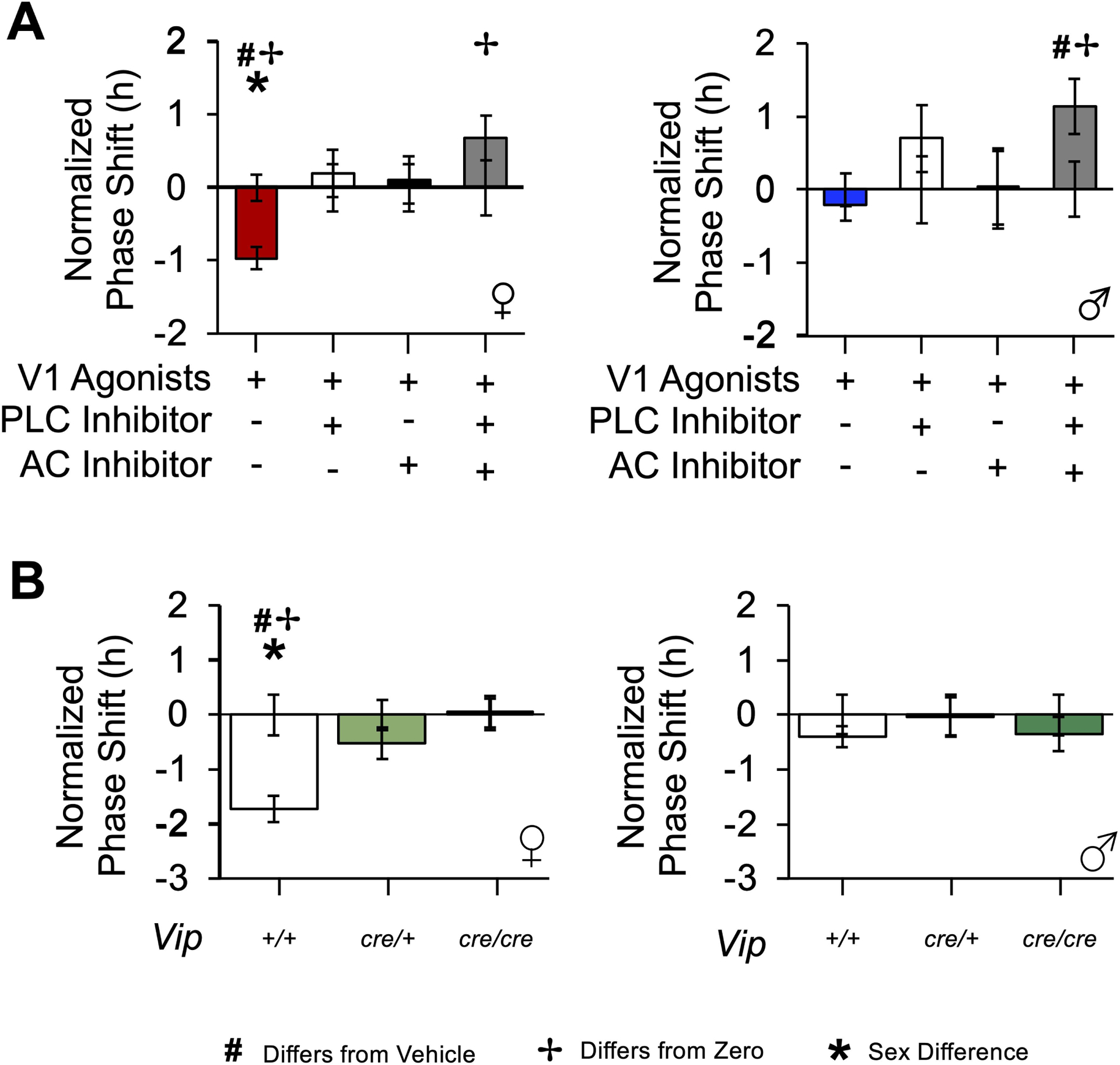
A. V1A/B agonists do not phase delay PER2∷LUC rhythms at CT22 when PLC or AC signaling is blocked. B. V1A/B-induced resetting is eliminated in the VIP-Cre SCN at CT22. Error bars plotted on the abscissa represent variability for vehicle controls. * Different by sex, Estimated Marginal Means Contrasts: p < 0.05. ✢ Different from zero, Single sample t-test: p < 0.05. For raw resetting data c.f., Figures S4–5.
PLC and AC inhibitors blocked V1A/B-induced phase delays at CT22 when administered alone and in combination (Figure 5A, Marginal Multilevel Model, Inhibitor: F (3,52) = 15.9, p < 0.0001, Inhibitor*V1 Agonist: F (2,52) = 6.7, p < 0.05, V1 Agonist: F (1,52) = 1.1, p > 0.2, Sex: F (1,52) = 1.2, p = 0.27, Inhibitor*Sex: F (3,52) = 1.5, p = 0.24, Slice: F (2,52) = 1.3, p = 0.28, Slice*V1 Agonist: F (2,52) = 0.3, p > 0.7, Slice*Sex: F (2,52) = 0.2, p > 0.8, Slice*Inhibitor: F (6,52) = 0.9, p = 0.52, Slice*Inhibitor*Sex: F (6,52) = 0.7, p > 0.6, Sex*V1 Agonist: F (1,52) = 0.5, p > 0.4). Relative to V1A/B agonists alone, the PLC inhibitor suppressed V1A/B-induced phase delays in the female SCN (Figure 5A, Estimated Marginal Means Contrasts: p < 0.05, d = 1.03), which was also observed with the AC inhibitor (Figure 5A, Estimated Marginal Means Contrasts: p < 0.05, d = 0.95). Interestingly, applying both PLC and AC inhibitors together with the V1A/B agonists caused the SCN to shift in the opposite direction in both sexes (Figure 5A, Estimated Marginal Means Contrasts: p < 0.05, d = 1.17–1.46). Overall, these data suggest that Gαq and Gαs pathways play an ongoing role in the unstimulated SCN and that V1A/B-induced SCN resetting involves both signaling cascades. Given that V1A and V1B are both coupled to Gαq signaling pathways, we speculated that the effectiveness of the AC inhibitor in blocking V1A/B-induced resetting may reflect a contribution of another SCN receptor mechanism.
VIP signaling is required for V1A/B-induced resetting
Because there is precedent for AVP and VIP to interact in the SCN [34, 9, 19, 35], we next tested whether VIP was necessary for V1A/B-induced resetting using both a pharmacological and genetic approach [36, 10, 37, 35]. The latter, genetic approach involved collecting SCN slices from VIP-IRES-Cre mice, which are VIP-deficient in a copy-dependent manner [37, 35]. As expected, there were genotype differences in SCN properties prior to treatment, which interacted with sex (Table S1). Prior to V1 agonist treatment, SCN period was shorter in female mice with at least one copy of a VIP-IRES-Cre transgene (Table S1), but the male Vipcre/cre SCN had a longer period (Table S1). Further, the peak time on the first cycle in vitro was earlier in Vipcre/+ and Vipcre/cre female SCN (Table S1). Importantly, the ability to predict the phase of SCN PER2∷LUC rhythms did not differ by genotype (Table S1), and there were no genotype or sex differences in vehicle responses (Figure S5).
Using both approaches, we found evidence that VIP signaling is required for V1A/B-induced SCN resetting. First, V1A/B-induced resetting was blocked by VIP deficiency in the VIP-IRES-Cre model (Figure 5B, Marginal Multilevel Model, Genotype: F (2,45) = 13, p < 0.0001, Sex: F (1,45) = 7.8, p < 0.01, Genotype*Sex: F (2,45) = 8.6, p = 0.001, V1 Agonist: F (1,45) = 9.4, p < 0.005, Slice: F (2,45) = 6.7, p = 0.005, Slice*V1 Agonist: F (2,45) = 3.07, p = 0.06, Sex*Slice: F (2,45) = 0.2, p > 0.8, Slice*Genotype: F (4,45) = 1.2, p = 0.35, Sex*V1 Agonist: F (1,45) = 2.8, p = 0.1, Sex*Slice*Genotype: F (4,45) = 0.5, p > 0.7). Interestingly, V1A/B-induced phase shifts were eliminated in both Vipcre/cre and Vipcre/+ female mice (Figure 5B, Estimated Marginal Means Contrasts: p < 0.05, d = 0.96–1.40). Importantly, the occlusion of V1A/B-induced resetting was not attributable to genotype differences in the period response to drug application (Table S2). Complementary results were obtained with a pharmacological approach, and co-administration of the 10μM V1A/B agonist cocktail with the 20μM VPAC2 antagonist blocked SCN resetting (Figure S6, Marginal Multilevel Model, V1 Agonist*VPAC2 Antagonist: F (1,61) = 4.9, p < 0.05, V1 Agonist: F (1,61) = 2.8, p = 0.1, VPAC2 Antagonist: F (1,61) = 7.9, p = 0.008, Sex: F (1,61) = 5.2, p < 0.05, Sex* V1 Agonist: F (1,61) = 1.2, p > 0.2, Sex*VPAC2 Antagonist: F (2,61) = 3.4, p = 0.07, Slice: F (2,61) = 0.6, p = 0.54, Slice*V1 Agonist: F (2,61) = 0.3, p > 0.7, Slice*Sex: F (2,61) = 0.9, p > 0.4, Slice*VPAC2 Antagonist: F (2,61) = 1.1, p = 0.35, Sex*Slice*VPAC2 Antagonist: F (2,67) = 0.8, p > 0.4). As expected, the VPAC2 antagonist shortened SCN period regardless of V1 agonist administration (Table S2). Overall, these data indicate that V1 and VPAC2 pathways interact to reset the SCN molecular clock.
Discussion
Here we find that V1 agonists can reset the phase of the SCN molecular clock, with a larger magnitude response in the female SCN. Interestingly, the magnitude of the sex difference in V1A/B-induced resetting was influenced by SCN slice position, which may reflect sex differences in regional V1A and/or V1B expression. Both V1A and V1B appear to contribute to SCN resetting, with an effect of V1A alone in females but not males. At the intracellular level, both Gαq and Gαs appear to contribute to SCN V1A/B-induced resetting, consistent with previous work indicating SCN resetting involves both these signaling pathways [26]. Lastly, we find evidence that V1A/B-induced resetting in the SCN requires VIP signaling, which suggests that AVP and VIP signaling pathways interact at the cellular and/or network level (Figure 6). Overall, this work provides insight into how AVP signaling modulates local SCN clock function, reveals that response to V1A/B signaling is influenced by biological sex, and uncovers a novel way in which neuropeptide integration modulates central clock function.
Figure 6. Putative intracellular and intercellular mechanisms by which AVP and VIP signaling interact to reset the SCN molecular clock.
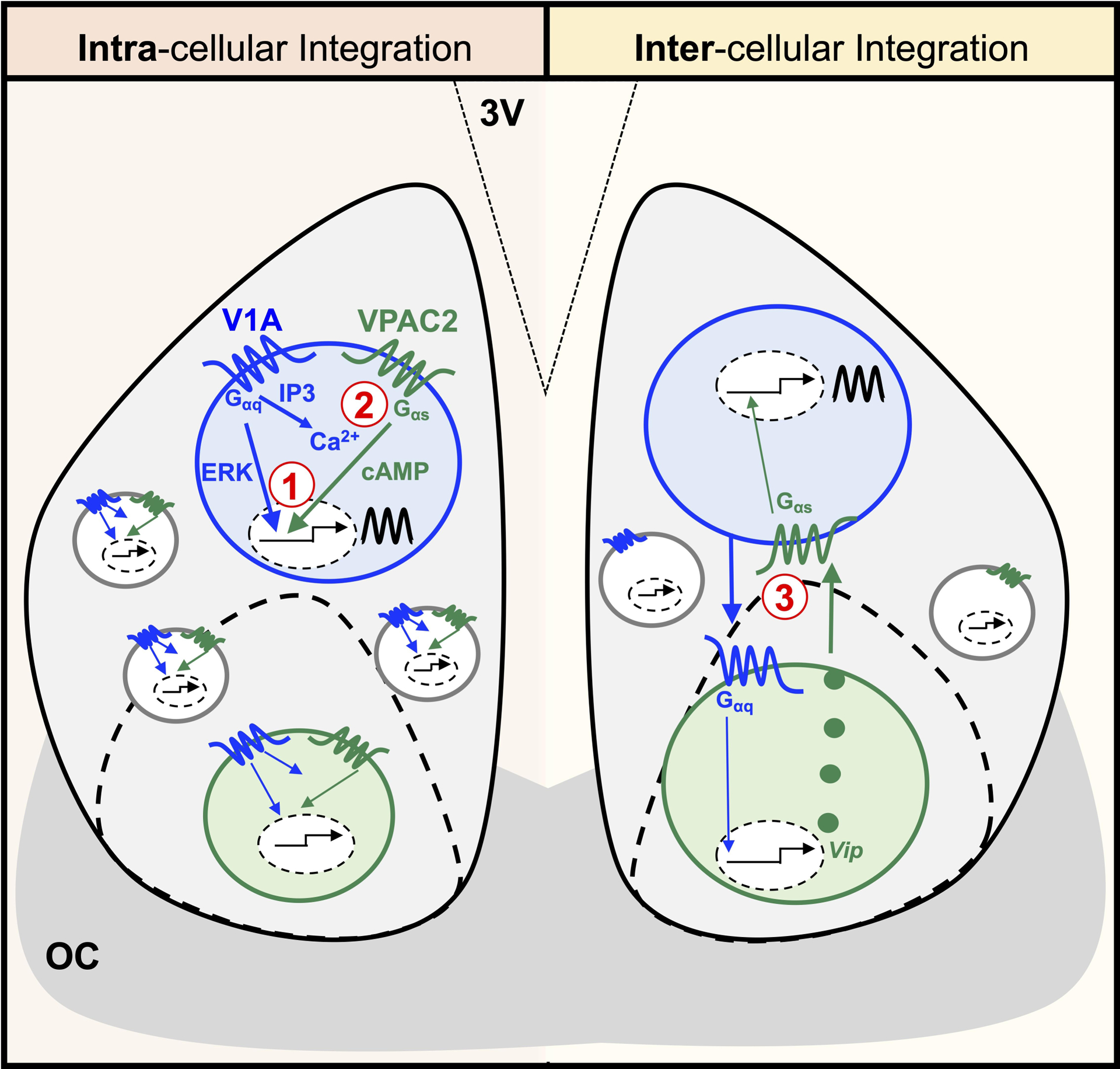
Left: At the single cell level, temporal and spatial co-expression may lead to interaction between V1- and VPAC2-induced signaling cascades. 1) Transcriptional integration: V1-activated extracellular signal-regulated kinase (ERK)/mitogen activated protein kinase (MAPK) and VPAC2-activated cAMP-response element binding protein (CREB) signaling may synergistically regulate gene expression to shift the SCN clock. 2) Cytosolic integration: V1-activated diacylglycerol (DAG)/inositol triphosphate (IP3) could increase intracellular calcium to potentiate VPAC2-activated cAMP regulation of gene expression to shift the SCN clock. Right: At the network level, AVP and VIP signaling may interact through poly-synaptic circuits involving V1-induced up-regulation of Vip to shift the SCN clock. Note: Interactions between AVP and VIP neurons could occur either directly as shown or indirectly via connections with other SCN neurons. Further, other intracellular and intercellular signaling pathways may also be involved (not shown). 3V = third ventricle. OC = optic chiasm.
V1A/B agonists can reset the phase of the SCN molecular clock
We demonstrate that V1A/B agonists reset the phase of SCN PER2∷LUC rhythms, with more modest effects on other properties. At the 10μM dose selected, V1A/B agonists did not decrease the quality of PER2∷LUC rhythms or alter SCN period. This may not discount potential effects of V1A/B signaling on period since repeated or long-term drug application is often required for modulation of this parameter [26, 39]. V1A/B agonists did not reset SCN PER2∷LUC rhythms when co-applied with 20μM V1A/B antagonists, which is a dose previously shown to not alter SCN function when applied alone [19]. This indicates V1A/B receptor signaling is required for this response, which is consistent with the high specificity and efficacy of agonists selected here [40, 41]. An important consideration is that V1A and V1B agonists can each stimulate V1A, V1B and oxytocin receptors when applied at high doses [41], oxytocin receptors have been detected in the rat SCN [42]. The interface slice preparation used here (i.e., slice suspended above medium on membrane) is expected to reduce the agonist dose within the tissue itself. In addition to V1A and V1B. Previous work indicates that SCN responses to nonapeptide ligands are largely driven by V1-induced signaling [22], but it remains difficult to dismiss potential contributions of other receptor types to the SCN response to V1A/B agonist administration. Additional limitations of this approach include damping of SCN PER2∷LUC rhythms that occurs acutely after dissection, high variability in vehicle control responses, and effects of inhibitors when applied alone. Despite its limitations, continued optimization and use of this technique may be used to delineate mechanisms by which V1 signaling modulates molecular clock function.
SCN resetting induced by V1A/B agonists is influenced by biological sex
One of the most interesting results is that V1A/B-induced resetting was larger in the female SCN than in the male SCN. In fact, the female SCN responded to the V1A/B agonist cocktail more consistently at lower doses, across experiments, and across circadian time than seen in the male SCN. Similar to the lack of large male SCN responses to V1A/B agonists, male hamsters do not display behavioral resetting when AVP is injected into the SCN region in vivo [43]. A sex difference in the role of AVP in circadian timekeeping is consistent with previous work using an AVP-deficient mouse [37, 30]. In this genetic model, AVP deficiency decreased the precision of free-running locomotor rhythms and increased the duration of the active phase in both sexes, but only female AVP-deficient mice displayed longer period under free-running conditions [30]. Combined with the present results, these data indicate that AVP regulates multiple properties of circadian timekeeping and that these effects can differ by sex. In non-SCN tissues, AVP neurons play sexually dimorphic roles in the regulation of social behaviors, which are thought to reflect sex differences in AVP synthesis and V1A binding [44]. It remains to be determined what mechanisms may drive sex differences in V1A/B-induced resetting of the SCN, but this represents an exciting area for future work.
Although the mechanistic basis of sex differences in V1A/B-induced SCN resetting remains to be determined, previous work indicates that daily rhythms in Avp and V1a transcription in the SCN do not differ by sex [45, 46, 44]. Nevertheless, it remains possible that there are sex differences in the expression or function of SCN nonapeptide receptors. Sex differences in the ex vivo SCN response to V1A/B agonist administration may reflect hormonal and/or non-hormonal mechanisms acting in vivo prior to tissue collection [47]. For example, the SCN expresses both testosterone and estrogen receptors, and sex steroid signaling modulates photic phase resetting in vivo [47]. Further, hormonal and non-hormonal mechanisms may sculpt SCN circuits in other ways. In the present work, we observed a modest, but consistent sex difference in SCN period, consistent with a trend present in the one other study that has systematically investigated sex differences in PER2∷LUC rhythms [48]. It is perhaps surprising that sex differences in SCN function can be detected in the ex vivo preparation used here since this approach does not fully recapitulate the in vivo condition (e.g., lack of gonadal sex hormones), but this suggests that mechanisms driving these sex differences produce long-lasting changes in the central clock network. Given that larger V1A/B-induced resetting in females facilitated examination of cellular mechanisms in the present work, these results highlight the benefit of routine inclusion of females in preclinical research [49]. Further work leveraging sex as a biological variable may be useful in delineating SCN mechanisms and consequences of V1 signaling on cellular clock function. Overall, the present results indicate that SCN circuitry is influenced by sex, and a deeper understanding of SCN sexual dimorphism will benefit from more studies that include both sexes [48, 50].
V1A and V1B contributions to V1A/B-induced SCN resetting
Our data indicate that both V1A and V1B are required for SCN resetting in males, but V1A agonist alone can reset the female SCN. In both male and female SCN, the largest response to V1A/B agonists was observed at CT22, which is predicted to be the phase of highest V1A expression in the SCN (based on male data; [19]). Further, similar dose response curves were observed for all three SCN slices collected here, consistent with V1a expression spanning the anteroposterior axis of the SCN network [19]. In the male SCN, significant phase shifts at CT22 were largest in the posterior SCN, which is the region with the densest V1a expression [19]. Previous work suggests that V1a is expressed by SCN neurons that produce Avp-Nms, Vip-Nms, and Cck-Clql3 in male mice [51], but SCN AVP receptor expression has not been systematically studied in both sexes. Further, it remains unclear which subpopulations of SCN neurons respond to V1A/B agonists in the current study because luminometry is a population-level approach that may largely reflect rhythms from the SCN shell (e.g., AVP neurons [52]). Future work using real-time imaging techniques may be used to visualize the spatiotemporal kinetics of V1A/B-induced SCN resetting and clarify the identity of V1A/B-responsive SCN neurons in each sex [39, 16].
SCN response to V1A/B signaling most resembles that induced by non-photic stimuli
In addition, we investigated how V1A/B-induced resetting varies over the circadian cycle to test how this signal is interpreted by the SCN. Time-dependent responses is a classic feature of circadian stimuli, with the magnitude and direction of phase resetting varying over the circadian cycle. This type of daily rhythm is driven by cellular signaling mechanisms and is most often illustrated using a phase response curve [53]. There are two main types of stimuli that have been classified in the field: photic and non-photic (e.g., arousal, food, temperature). Both photic and non-photic resetting is characterized by time-dependent responses, although the nature of these rhythms differs based on stimulus class [53–55]. Whereas responses to photic stimuli are typically restricted to subjective night [53, 56], non-photic stimuli often induce responses during both subjective day and night [53, 54, 56, 55]. We find here that the rhythm in V1A/B-induced SCN resetting resembles that for a non-photic stimulus, with phase delays elicited during subjective night and phase advances elicited during subjective day. In contrast, photic stimuli induce phase delays during early subjective night and phase advances during late subjective night [53, 54, 56, 55]. With this in mind, it is also of interest that application of V1A/B agonists in the current study did not induce acute PER2∷LUC expression. Photic stimuli up-regulate PER and other immediate early genes to shift the molecular clock [57–60], whereas non-photic stimuli down-regulate immediate early genes or have no immediate effect [61–64]. Overall, the SCN response to AVP appears to be distinct from that induced by other SCN peptides. For example, VIP and GRP are most often associated with light-like resetting responses [65, 66, 26, 67] and induction of immediate gene expression [68, 69, 39]. Given the general similar resetting response to AVP and other signaling molecules that provide feedback to the SCN (e.g., melatonin, NPY, 5HT), this suggests the interesting possibility that AVP regulates SCN function by acting like a local feedback signal.
V1A/B-induced SCN resetting involves both Gαq and Gαs signaling
We also find that V1A/B agonists administered at CT22 failed to delay the SCN when PLC and/or AC was inhibited. These results suggest that multiple signaling cascades are required for V1A/B-induced resetting. Previous work indicates that PLC and AC inhibition blocks VIP-induced SCN resetting [26], and PLC is required for Per1/2 induction [69]. PLC/AC inhibitors affected SCN rhythms without administration of V1A/B agonists and co-administration of V1A/B agonists with PLC+AC inhibitors reversed the direction of SCN V1A/B-induced phase resetting. The response to PLC/AC inhibitors alone makes it difficult to cleanly parse the role of these pathways in V1A/B-mediated responses, but these results suggest that Gαq and Gαs signaling influence SCN phase under basal conditions in ways that are independent of AVP. Consistent with an ongoing role of these intracellular signaling pathways, the SCN displays daily rhythms in the phosphorylation of PLC and CREB that are important for SCN clock function [70–72]. Previous work using these same compounds found that these inhibitors at these doses did not phase shift PER2∷LUC rhythms when applied at CT12 [26], further suggesting that the ongoing activity of these pathways may be rhythmic. The identity of downstream effectors through which Gαq and Gαs pathways may intersect with one another to reset SCN molecular clock warrants further investigation, as does the potential role of additional signaling pathways not studied here [39, 73].
V1A/B-induced SCN resetting requires VIP signaling
Using two complementary approaches, we find that VIP signaling is necessary for V1A/B-induced SCN resetting. Specifically, V1A/B-induced resetting was blocked by both co-administration of VPAC2 antagonists and genetic VIP deficiency. In the latter study, neither Vipcre/cre or Vipcre/+ SCN responded to V1A/B agonists, suggesting that even partial loss of VIP [35] is sufficient to block V1A/B effects. Although Vipcre/+ mice do not display a phenotype in basic circadian phenotyping assays, Vip and VIP expression is reduced in Vipcre /+ mice [37, 35]. Unlike Vip−/− mice [19], Vipcre/cre and Vipcre/+ mice do not show pronounced deficits in AVP signaling or SCN function [37, 35], making this an excellent model for use in the present studies. Collectively, these results indicate AVP and VIP signaling interact, which is consistent with their overlapping time of neuropeptide and receptor expression. Both AVP and VIP are produced during late subjective day, with peak receptor expression for each occurring during mid-to-late subjective night [31, 45, 74, 46, 51]. Similarly, both AVP and VIP when applied separately cause the SCN to phase delay during early subjective night [75, 76]. However, the idea that these two signals interact in a cooperative manner is difficult to reconcile with previous work indicating that AVP and VIP influence SCN neurophysiology in distinct ways. For instance, VIP decreases firing rate in SCN neurons [77], whereas AVP increases firing rate [22]. Likewise, AVP and VIP elicit opposite calcium responses, with VIP reducing intracellular calcium during subjective day and AVP increasing intracellular calcium during subjective day and night [24]. Further, AVP and VIP produce distinct effects on the SCN molecular clock, with VIP producing light-like resetting, lengthened period, and acute PER induction [39]. Although opposite SCN responses to AVP and VIP signaling would be predicted to abrogate one another, there is precedent for different classes of stimuli producing antagonist or synergistic effects depending on the timing of stimuli administration [78–82].
Although little is known about how AVP and VIP may interact to reset the SCN molecular clock, the underlying cellular mechanism could involve integration at the intracellular and/or intercellular levels (Figure 6). At the intracellular level, V1 and VPAC2 signaling could interact via transcriptional (1) or cytosolic synergy (2) to phase shift the SCN molecular clock (Figure 6). At the intercellular level, AVP and VIP signaling may interact through network-level circuits (Figure 6). In this second model, V1 signaling would cause the release of VIP to shift the SCN molecular clock, and the nature of this response would be predicted to resemble that elicited by VIP alone. Thus, the observation that V1A/B-induced SCN resetting is distinct from that elicited by VIP [26, 67] may provide evidence against a model involving only network-level integration. Further, SCN electrical responses to AVP are not abolished in Vipr2 knockout mice [83], suggesting that V1A/B-induced signaling is able to influence SCN properties in the absence of VIP signaling. Thus, intracellular integration of AVP and VIP signaling seems the more parsimonious model to account for the present results. Although it is not known if V1a and Vipr2 are co-expressed in single SCN neurons, both V1a and Vipr2 are expressed by Avp-Nms and Vip-Nms SCN neurons [51]. Thus, future work could test the validity of the intracellular integration model for AVP and VIP signaling by examining whether V1a and Vipr2 are co-expressed in time and space at the single cell level in both sexes.
Methods
Mice and husbandry conditions
Homozygous PERIOD2∷LUCIFERASE (PER2∷LUC) mice [84] were bred and raised under a 24-hour light-dark cycle with 12 hours of light and 12 hours of darkness (LD12:12; lights off: 1800 CST, defined as Zeitgeber Time 12, i.e., ZT12). Throughout life, ambient temperature was maintained at 22°C ± 2°C, and mice had ad libitum access to water and food (Teklad Rodent Diet 8604). Founder homozygous VIP-IRES-Cre mice were obtained from Jackson Laboratory (Vip<tm1(cre)Zjh>/J; Stock No: 010908) and bred to PER2∷LUC mice for at least 4 generations. At weaning, mice were group housed in cages without running wheels.
PER2∷LUC recording
Coronal SCN slices (150 μm) were collected from PER2∷LUC mice (10–18 weeks old) and cultured as described previously [19]. Briefly, male and female mice were anesthetized with isoflurane and sacrificed using cervical dislocation 4–6 h before lights off to minimize dissection-induced resetting [33]. Three consecutive SCN slices were collected using a vibratome (Leica VT1200S) and trimmed by hand under a dissecting microscope. After collection, SCN slices were placed on a membrane insert in a dish containing 1.2mL of luciferin-treated recording medium: air-buffered Dulbecco’s modified explant medium (DMEM, Sigma D2902) supplemented with 0.1mM beetle luciferin, 0.02% B27 (Gibco 17504), 0.01% HEPES (Gibco 15630), 0.005% NaCHO3 (Gibco 25080), 0.004% Dextrose (Sigma G7021), and 0.01% penicillin/streptomycin (Gibco 15140). SCN were cultured at 37°C for at least 6 days with a luminometer (Actimetrics, LumiCycle 32).
Pharmacology
Drugs were added to the media after three cycles in vitro and not washed out. To assess how AVP signaling resets the molecular clock, responses were compared between samples receiving vehicle control (<0.001% DMSO) and a drug cocktail of AVP agonists targeting both V1A and V1B receptors (V1A-[Phe2, Ile3,Orn8]-Vasopressin, Phoenix Pharmaceuticals, Inc., Cat#065–30; V1B-d[Leu4, Lys8]-VP, Tocris, Cat#3127). To test the necessity of V1 signaling, a subset of samples were co-treated with a drug cocktail of V1A and V1B antagonists at the time of V1 agonist administration (V1A-OPC21268, 10μM, Tocris, Cat# 3924; V1B-SSR149415, 10μM, Axon Medchem Cat# 1116, as in [19]). In addition, we tested involvement of different intracellular signaling cascades [26] using inhibitors of phospholipase C (U-73122, 10μM, Sigma, Cat#U6756) and adenylate cyclase (MDL-12,330A, 2μM, Sigma, Cat#M182). Lastly, the role of VIP signaling was tested using the VPAC2 antagonist [4Cl-D-Phe6,Leu17] VIP (20μM, Tocris, Cat# 3054, as in [35]). SCN slices treated with 0 and 10uM V1A/B agonists were collected at CT22 in two independent replicates each spanning a two-year period to serve as negative and positive controls for experiments conducted in parallel.
PER2∷LUC analyses
PER2∷LUC rhythms were analyzed with Lumicycle- and Excel-based analyses, as in previous work [38, 10]. The PER2∷LUC rhythm for each sample was detrended and fit with a damped sine wave on the three cycles before and after drug administration, and the time of peak PER2∷LUC expression were determined to quantify SCN resetting. The circadian time (CT) of drug administration was projected using the average cycle-to-cycle period length and the peak time on the last cycle prior to drug application. The shift in the PER2∷LUC rhythm was measured as the discrepancy between the projected peak time versus actual peak time after treatment. In addition, period, goodness of fit, and damping rate (i.e., number of days for rhythm amplitude to decrease ca. 37%) was recorded from sine wave fit to cycles before and after drug administration. Drug-induced changes in period were quantified by the difference between post-treatment period and pre-treatment period (positive values indicate period was lengthened).
Statistical analyses
Data are represented in figures and tables as mean ± SEM. Statistical analyses were performed with JMP software (SAS Institute, Cary, NC) and SPSS software (IBM Corporation, Armonk, NY). To examine group differences in pre-treatment PER2∷LUC rhythms, data from three slices/mouse were averaged, then assessed with Student’s t test, one-way ANOVA or Full Factorial ANOVA as appropriate (Table S1). To examine post-treatment responses, resetting data from all three slices were included in a Marginal Multilevel Model, which is a Full Factorial Linear Mixed Model well suited to analyze datasets that lack independence, sphericity, and/or compound symmetry due to collection of multiple observations from individuals (i.e., Slice Position, Anterior, Middle, and Posterior SCN). For each experimental dataset, the Marginal Multilevel Model included all relevant factors: 1) Biological Sex, 2) V1A/B Dose, 3) Antagonist, 4) Circadian Time, 5) VIP Genotype and/or 6) Gαq/Gαs Intracellular Inhibitor. Post hoc tests were performed with Estimated Marginal Means Contrasts and one-sample t tests. Effect sizes of pairwise comparisons were estimated with Cohen’s d. To facilitate comparison of drug effects across sex, data from each vehicle control was subtracted in a sex-specific manner for main figures. Raw phase shifting data is provided supplemental materials. Marginal Multilevel analyses of both raw and normalization data yielded similar conclusions. Post-treatment resetting, period modulation, and damping data (averaged across slice position) are provided in Table S2 for all experiments. Statistical significance was set at p < 0.05.
Supplementary Material
Acknowledgements
We would like to thank Harshida Pancholi, Alec Huber, Austin Fritsch, Alecia Bjerke, Erika Johnson, Audrey Konieczny, and Berri Foreman for providing care to the animals used for this research.
Funding Statement
The National Institutes of Health (R01091234), the Whitehall Foundation (2014-12-65), and the Charles E Kubly Mental Health Research Center supported this work but had no role in the study design; data collection, analysis, or interpretation.
Footnotes
Statement of Ethics
All procedures were conducted according to the National Institutes of Health Guide for the Care and Use of Animals and were approved by the Institutional Animal Care and Use Committee at Marquette University (Protocol# AR-282).
Conflict of Interest Statement
The authors have no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Data Availability Statement
All data generated during this study are included in this article or its supplementary material files. Data can be obtained from the corresponding author upon request.
References
- 1.Partch CL, Green CB, Takahashi JS. Molecular architecture of the mammalian circadian clock. Trends in cell biology. 2014. Feb;24(2):90–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Mohawk JA, Green CB, Takahashi JS. Central and peripheral circadian clocks in mammals. Annu Rev Neurosci. 2012. Apr 5;35:445–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Rea MA. Photic entrainment of circadian rhythms in rodents. Chronobiol Int. 1998. Sep;15(5):395–423. [DOI] [PubMed] [Google Scholar]
- 4.Aranda ML, Schmidt TM. Diversity of intrinsically photosensitive retinal ganglion cells: circuits and functions. Cellular and molecular life sciences : CMLS. 2020. Sep 23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Evans JA. Collective timekeeping among cells of the master circadian clock. J Endocrinol. 2016. May 6;230(1):R27–49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Harmar AJ, Marston HM, Shen S, Spratt C, West KM, Sheward WJ, et al. The VPAC(2) receptor is essential for circadian function in the mouse suprachiasmatic nuclei. Cell. 2002. May 17;109(4):497–508. [DOI] [PubMed] [Google Scholar]
- 7.Aton SJ, Colwell CS, Harmar AJ, Waschek J, Herzog ED. Vasoactive intestinal polypeptide mediates circadian rhythmicity and synchrony in mammalian clock neurons. Nat Neurosci. 2005. Apr;8(4):476–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Aton SJ, Huettner JE, Straume M, Herzog ED. GABA and Gi/o differentially control circadian rhythms and synchrony in clock neurons. Proc Natl Acad Sci U S A. 2006. Dec 12;103(50):19188–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Maywood ES, Chesham JE, O’Brien JA, Hastings MH. A diversity of paracrine signals sustains molecular circadian cycling in suprachiasmatic nucleus circuits. Proc Natl Acad Sci U S A. 2011. Jul 25;108(34):14306–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Evans JA, Leise TL, Castanon-Cervantes O, Davidson AJ. Dynamic interactions mediated by nonredundant signaling mechanisms couple circadian clock neurons. Neuron. 2013. Nov 20;80(4):973–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Schwartz WJ, Reppert SM. Neural regulation of the circadian vasopressin rhythm in cerebrospinal fluid: a pre-eminent role for the suprachiasmatic nuclei. J Neurosci. 1985. Oct;5(10):2771–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Taub A, Carbajal Y, Rimu K, Holt R, Yao Y, Hernandez AL, et al. Arginine Vasopressin-Containing Neurons of the Suprachiasmatic Nucleus Project to CSF. eNeuro. 2021. Mar-Apr;8(2). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kalsbeek A, Fliers E, Hofman MA, Swaab DF, Buijs RM. Vasopressin and the output of the hypothalamic biological clock. J Neuroendocrinol. 2010. May;22(5):362–72. [DOI] [PubMed] [Google Scholar]
- 14.Mieda M, Ono D, Hasegawa E, Okamoto H, Honma K, Honma S, et al. Cellular clocks in AVP neurons of the SCN are critical for interneuronal coupling regulating circadian behavior rhythm. Neuron. 2015. Mar 4;85(5):1103–16. [DOI] [PubMed] [Google Scholar]
- 15.Mieda M, Okamoto H, Sakurai T. Manipulating the cellular circadian period of arginine vasopressin neurons alters the behavioral circadian period. Curr Biol. 2016. Sep 26;26(18):2535–42. [DOI] [PubMed] [Google Scholar]
- 16.Shan Y, Abel JH, Li Y, Izumo M, Cox KH, Jeong B, et al. Dual-Color Single-Cell Imaging of the Suprachiasmatic Nucleus Reveals a Circadian Role in Network Synchrony. Neuron. 2020. Oct 14;108(1):164–79 e7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Edwards MD, Brancaccio M, Chesham JE, Maywood ES, Hastings MH. Rhythmic expression of cryptochrome induces the circadian clock of arrhythmic suprachiasmatic nuclei through arginine vasopressin signaling. Proc Natl Acad Sci U S A. 2016. Mar 8;113(10):2732–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ono D, Honma S, Honma K. Differential roles of AVP and VIP signaling in the postnatal changes of neural networks for coherent circadian rhythms in the SCN. Science advances. 2016. Sep;2(9):e1600960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Bedont JL, Rohr KE, Bathini A, Hattar S, Blackshaw S, Sehgal A, et al. Asymmetric vasopressin signaling spatially organizes the master circadian clock. J Comp Neurol. 2018. Sep 1;526(13):2048–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Koshimizu TA, Nakamura K, Egashira N, Hiroyama M, Nonoguchi H, Tanoue A. Vasopressin V1a and V1b receptors: from molecules to physiological systems. Physiol Rev. 2012. Oct;92(4):1813–64. [DOI] [PubMed] [Google Scholar]
- 21.Liou SY, Albers HE. Single unit response of suprachiasmatic neurons to arginine vasopressin (AVP) is mediated by a V1-like receptor in the hamster. Brain Res. 1989. Jan 16;477(1–2):336–43. [DOI] [PubMed] [Google Scholar]
- 22.Mihai R, Coculescu M, Wakerley JB, Ingram CD. The effects of [Arg8]vasopressin and [Arg8]vasotocin on the firing rate of suprachiasmatic neurons in vitro. Neuroscience. 1994. Oct;62(3):783–92. [DOI] [PubMed] [Google Scholar]
- 23.Mihai R, Juss TS, Ingram CD. Suppression of suprachiasmatic nucleus neurone activity with a vasopressin receptor antagonist: possible role for endogenous vasopressin in circadian activity cycles in vitro. Neurosci Lett. 1994. Sep 26;179(1–2):95–9. [DOI] [PubMed] [Google Scholar]
- 24.Irwin RP, Allen CN. Neuropeptide-mediated calcium signaling in the suprachiasmatic nucleus network. Eur J Neurosci. 2010. Nov;32(9):1497–506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Meyer-Spasche A, Piggins HD. Vasoactive intestinal polypeptide phase-advances the rat suprachiasmatic nuclei circadian pacemaker in vitro via protein kinase A and mitogen-activated protein kinase. Neurosci Lett. 2004. Mar 25;358(2):91–4. [DOI] [PubMed] [Google Scholar]
- 26.An S, Irwin RP, Allen CN, Tsai CA, Herzog ED. Vasoactive intestinal polypeptide requires parallel changes in adenylate cyclase and phospholipase C to entrain circadian rhythms to a predictable phase. J Neurophysiol. 2011. Mar 9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Brancaccio M, Maywood ES, Chesham JE, Loudon AS, Hastings MH. A Gq-Ca2+ axis controls circuit-level encoding of circadian time in the suprachiasmatic nucleus. Neuron. 2013. May 22;78(4):714–28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Li JD, Burton KJ, Zhang C, Hu SB, Zhou QY. Vasopressin receptor V1a regulates circadian rhythms of locomotor activity and expression of clock-controlled genes in the suprachiasmatic nuclei. Am J Physiol Regul Integr Comp Physiol. 2009. Mar;296(3):R824–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Yamaguchi Y, Suzuki T, Mizoro Y, Kori H, Okada K, Chen Y, et al. Mice genetically deficient in vasopressin V1a and V1b receptors are resistant to jet lag. Science. 2013. Oct 4;342(6154):85–90. [DOI] [PubMed] [Google Scholar]
- 30.Rohr KE, Telega A, Savaglio A, Evans JA. Vasopressin regulates daily rhythms and circadian clock circuits in a manner influenced by sex. Horm Behav. 2020. Dec 14;127:104888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Young WS 3rd, Kovacs K, Lolait SJ. The diurnal rhythm in vasopressin V1a receptor expression in the suprachiasmatic nucleus is not dependent on vasopressin. Endocrinology. 1993. Aug;133(2):585–90. [DOI] [PubMed] [Google Scholar]
- 32.An S, Tsai C, Ronecker J, Bayly A, Herzog ED. Spatiotemporal distribution of vasoactive intestinal polypeptide receptor 2 in mouse suprachiasmatic nucleus. J Comp Neurol. 2012. Aug 15;520(12):2730–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Davidson AJ, Castanon-Cervantes O, Leise TL, Molyneux PC, Harrington ME. Visualizing jet lag in the mouse suprachiasmatic nucleus and peripheral circadian timing system. Eur J Neurosci. 2009. Jan;29(1):171–80. [DOI] [PubMed] [Google Scholar]
- 34.Harmar AJ. An essential role for peptidergic signalling in the control of circadian rhythms in the suprachiasmatic nuclei. J Neuroendocrinol. 2003. Apr;15(4):335–8. [DOI] [PubMed] [Google Scholar]
- 35.Joye DAM, Rohr KE, Keller D, Inda T, Telega A, Pancholi H, et al. Reduced VIP Expression Affects Circadian Clock Function in VIP-IRES-CRE Mice (JAX 010908). J Biol Rhythms. 2020. Aug;35(4):340–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Atkins N Jr., Mitchell JW, Romanova EV, Morgan DJ, Cominski TP, Ecker JL, et al. Circadian integration of glutamatergic signals by little SAAS in novel suprachiasmatic circuits. PLoS One. 2010;5(9):e12612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Cheng AH, Fung SW, Cheng HM. Limitations of the Avp-IRES2-Cre (JAX #023530) and Vip-IRES-Cre (JAX #010908) Models for Chronobiological Investigations. J Biol Rhythms. 2019. Dec;34(6):634–44. [DOI] [PubMed] [Google Scholar]
- 38.Evans JA, Leise TL, Castanon-Cervantes O, Davidson AJ. Intrinsic regulation of spatiotemporal organization within the suprachiasmatic nucleus. PLoS One. 2011;6(1):e15869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Hamnett R, Crosby P, Chesham JE, Hastings MH. Vasoactive intestinal peptide controls the suprachiasmatic circadian clock network via ERK1/2 and DUSP4 signalling. Nat Commun. 2019. Feb 1;10(1):542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Pena A, Murat B, Trueba M, Ventura MA, Bertrand G, Cheng LL, et al. Pharmacological and physiological characterization of d[Leu4, Lys8]vasopressin, the first V1b-selective agonist for rat vasopressin/oxytocin receptors. Endocrinology. 2007. Sep;148(9):4136–46. [DOI] [PubMed] [Google Scholar]
- 41.Manning M, Misicka A, Olma A, Bankowski K, Stoev S, Chini B, et al. Oxytocin and vasopressin agonists and antagonists as research tools and potential therapeutics. J Neuroendocrinol. 2012. Apr;24(4):609–28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Vaccari C, Lolait SJ, Ostrowski NL. Comparative distribution of vasopressin V1b and oxytocin receptor messenger ribonucleic acids in brain. Endocrinology. 1998. Dec;139(12):5015–33. [DOI] [PubMed] [Google Scholar]
- 43.Albers HE, Ferris CF, Leeman SE, Goldman BD. Avian pancreatic polypeptide phase shifts hamster circadian rhythms when microinjected into the suprachiasmatic region. Science. 1984. Feb 24;223(4638):833–5. [DOI] [PubMed] [Google Scholar]
- 44.Dumais KM, Veenema AH. Vasopressin and oxytocin receptor systems in the brain: Sex differences and sex-specific regulation of social behavior. Frontiers in neuroendocrinology. 2016. Jan;40:1–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Krajnak K, Kashon ML, Rosewell KL, Wise PM. Sex differences in the daily rhythm of vasoactive intestinal polypeptide but not arginine vasopressin messenger ribonucleic acid in the suprachiasmatic nuclei. Endocrinology. 1998. Oct;139(10):4189–96. [DOI] [PubMed] [Google Scholar]
- 46.Mahoney MM, Ramanathan C, Hagenauer MH, Thompson RC, Smale L, Lee T. Daily rhythms and sex differences in vasoactive intestinal polypeptide, VIPR2 receptor and arginine vasopressin mRNA in the suprachiasmatic nucleus of a diurnal rodent, Arvicanthis niloticus. Eur J Neurosci. 2009. Oct;30(8):1537–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Joye DAM, Evans JA. Sex differences in daily timekeeping and circadian clock circuits. Semin Cell Dev Biol. 2021. May 13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Kuljis DA, Loh DH, Truong D, Vosko AM, Ong ML, McClusky R, et al. Gonadal- and sex-chromosome-dependent sex differences in the circadian system. Endocrinology. 2013. Apr;154(4):1501–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Shansky RM, Murphy AZ. Considering sex as a biological variable will require a global shift in science culture. Nat Neurosci. 2021. Apr;24(4):457–64. [DOI] [PubMed] [Google Scholar]
- 50.Lee R, Tapia A, Kaladchibachi S, Grandner MA, Fernandez FX. Meta-analysis of light and circadian timekeeping in rodents. Neurosci Biobehav Rev. 2021. Apr;123:215–29. [DOI] [PubMed] [Google Scholar]
- 51.Wen S, Ma D, Zhao M, Xie L, Wu Q, Gou L, et al. Spatiotemporal single-cell analysis of gene expression in the mouse suprachiasmatic nucleus. Nat Neurosci. 2020. Mar;23(3):456–67. [DOI] [PubMed] [Google Scholar]
- 52.Azzi A, Evans JA, Leise T, Myung J, Takumi T, Davidson AJ, et al. Network dynamics mediate circadian clock plasticity. Neuron. 2017. Jan 18;93(2):441–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Johnson CH. Forty years of PRCs--what have we learned? Chronobiol Int. 1999;16(6):711–43. [DOI] [PubMed] [Google Scholar]
- 54.Rosenwasser AD SM. Circadian phase shifting: Relationships between photic and nonphotic phase-response curves. Physiology and Behavior. 2001;73:175–83. [DOI] [PubMed] [Google Scholar]
- 55.Challet E Minireview: Entrainment of the suprachiasmatic clockwork in diurnal and nocturnal mammals. Endocrinology. 2007. Dec;148(12):5648–55. [DOI] [PubMed] [Google Scholar]
- 56.Johnson CH, Elliott JE, Foster R. Entrainment of circadian programs. Chronobiology International. 2003;20:741–73. [DOI] [PubMed] [Google Scholar]
- 57.Earnest DJ, Olschowka JA. Circadian regulation of c-fos expression in the suprachiasmatic pacemaker by light. J Biol Rhythms. 1993;8 Suppl:S65–71. [PubMed] [Google Scholar]
- 58.Shigeyoshi Y, Taguchi K, Yamamoto S, Takekida S, Yan L, Tei H, et al. Light-induced resetting of a mammalian circadian clock is associated with rapid induction of the mPer1 transcript. Cell. 1997. Dec 26;91(7):1043–53. [DOI] [PubMed] [Google Scholar]
- 59.Yan L, Silver R. Resetting the brain clock: Time course and localization of mPER1 and mPER2 protein expression in suprachiasmatic nuclei during phase shifts. Eur J Neurosci. 2004. Feb;19(4):1105–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Schwartz WJ, Tavakoli-Nezhad M, Lambert CM, Weaver DR, de la Iglesia HO. Distinct patterns of Period gene expression in the suprachiasmatic nucleus underlie circadian clock photoentrainment by advances or delays. Proc Natl Acad Sci U S A. 2011. Oct 11;108(41):17219–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Mikkelsen JD, Vrang N, Mrosovsky N. Expression of Fos in the circadian system following nonphotic stimulation. Brain Res Bull. 1998;47(4):367–76. [DOI] [PubMed] [Google Scholar]
- 62.Maywood ES, Mrosovsky N, Field MD, Hastings MH. Rapid down-regulation of mammalian period genes during behavioral resetting of the circadian clock. Proc Natl Acad Sci U S A. 1999. Dec 21;96(26):15211–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Maywood ES, Okamura H, Hastings MH. Opposing actions of neuropeptide Y and light on the expression of circadian clock genes in the mouse suprachiasmatic nuclei. Eur J Neurosci. 2002. Jan;15(1):216–20. [DOI] [PubMed] [Google Scholar]
- 64.Mendoza JY, Dardente H, Escobar C, Pevet P, Challet E. Dark pulse resetting of the suprachiasmatic clock in Syrian hamsters: behavioral phase-shifts and clock gene expression. Neuroscience. 2004;127(2):529–37. [DOI] [PubMed] [Google Scholar]
- 65.Piggins HD, Antle MC, Rusak B. Neuropeptides phase shift the mammalian circadian pacemaker. J Neurosci. 1995. Aug;15(8):5612–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Gamble KL, Allen GC, Zhou T, McMahon DG. Gastrin-releasing peptide mediates light-like resetting of the suprachiasmatic nucleus circadian pacemaker through cAMP response element-binding protein and Per1 activation. J Neurosci. 2007. Oct 31;27(44):12078–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Mazuski C, Abel JH, Chen SP, Hermanstyne TO, Jones JR, Simon T, et al. Entrainment of Circadian Rhythms Depends on Firing Rates and Neuropeptide Release of VIP SCN Neurons. Neuron. 2018. Aug 8;99(3):555–63 e5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Aida R, Moriya T, Araki M, Akiyama M, Wada K, Wada E, et al. Gastrin-releasing peptide mediates photic entrainable signals to dorsal subsets of suprachiasmatic nucleus via induction of Period gene in mice. Mol Pharmacol. 2002. Jan;61(1):26–34. [DOI] [PubMed] [Google Scholar]
- 69.Nielsen HS, Hannibal J, Fahrenkrug J. Vasoactive intestinal polypeptide induces per1 and per2 gene expression in the rat suprachiasmatic nucleus late at night. Eur J Neurosci. 2002. Feb;15(3):570–4. [DOI] [PubMed] [Google Scholar]
- 70.Obrietan K, Impey S, Smith D, Athos J, Storm DR. Circadian regulation of cAMP response element-mediated gene expression in the suprachiasmatic nuclei. J Biol Chem. 1999. Jun 18;274(25):17748–56. [DOI] [PubMed] [Google Scholar]
- 71.Jenkins TC, Andrews JB, Meyer-Bernstein EL. Daily oscillation of phospholipase C beta4 in the mouse suprachiasmatic nucleus. Brain Res. 2007. Oct 31;1178:83–91. [DOI] [PubMed] [Google Scholar]
- 72.O’Neill JS, Reddy AB. The essential role of cAMP/Ca2+ signalling in mammalian circadian timekeeping. Biochem Soc Trans. 2012. Feb 1;40(1):44–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Hegazi S, Lowden C, Rios Garcia J, Cheng AH, Obrietan K, Levine JD, et al. A Symphony of Signals: Intercellular and Intracellular Signaling Mechanisms Underlying Circadian Timekeeping in Mice and Flies. International journal of molecular sciences. 2019. May 13;20(9). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Shinohara K, Funabashi T, Kimura F. Temporal profiles of vasoactive intestinal polypeptide precursor mRNA and its receptor mRNA in the rat suprachiasmatic nucleus. Brain Res Mol Brain Res. 1999. Jan 8;63(2):262–7. [DOI] [PubMed] [Google Scholar]
- 75.Ingram CD, Ciobanu R, Coculescu IL, Tanasescu R, Coculescu M, Mihai R. Vasopressin neurotransmission and the control of circadian rhythms in the suprachiasmatic nucleus. Prog Brain Res. 1998;119:351–64. [DOI] [PubMed] [Google Scholar]
- 76.Pakhotin P, Harmar AJ, Verkhratsky A, Piggins H. VIP receptors control excitability of suprachiasmatic nuclei neurones. Pflugers Archiv : European journal of physiology. 2006. Apr;452(1):7–15. [DOI] [PubMed] [Google Scholar]
- 77.Reed HE, Cutler DJ, Brown TM, Brown J, Coen CW, Piggins HD. Effects of vasoactive intestinal polypeptide on neurones of the rat suprachiasmatic nuclei in vitro. J Neuroendocrinol. 2002. Aug;14(8):639–46. [DOI] [PubMed] [Google Scholar]
- 78.Joy JE, Turek FW. Combined effects on the circadian clock of agents with different phase response curves: phase-shifting effects of triazolam and light. J Biol Rhythms. 1992;7(1):51–63. [DOI] [PubMed] [Google Scholar]
- 79.Ralph MR, Mrosovsky N. Behavioral inhibition of circadian responses to light. J Biol Rhythms. 1992;7(4):353–9. [DOI] [PubMed] [Google Scholar]
- 80.Abraham U, Gwinner E, Van’t Hof TJ. Exogenous melatonin reduces the resynchronization time after phase shifts of a nonphotic zeitgeber in the house sparrow (Passer domesticus). J Biol Rhythms. 2000. Feb;15(1):48–56. [DOI] [PubMed] [Google Scholar]
- 81.Christian CA, Harrington ME. Three days of novel wheel access diminishes light-induced phase delays in vivo with no effect on per1 induction by light. Chronobiol Int. 2002. Jul;19(4):671–82. [DOI] [PubMed] [Google Scholar]
- 82.Edelstein K, de la Iglesia HO, Schwartz WJ, Mrosovsky N. Behavioral arousal blocks light-induced phase advances in locomotor rhythmicity but not light-induced Per1 and Fos expression in the hamster suprachiasmatic nucleus. Neuroscience. 2003;118(1):253–61. [DOI] [PubMed] [Google Scholar]
- 83.Cutler DJ, Haraura M, Reed HE, Shen S, Sheward WJ, Morrison CF, et al. The mouse VPAC2 receptor confers suprachiasmatic nuclei cellular rhythmicity and responsiveness to vasoactive intestinal polypeptide in vitro. Eur J Neurosci. 2003. Jan;17(2):197–204. [DOI] [PubMed] [Google Scholar]
- 84.Yoo SH, Yamazaki S, Lowrey PL, Shimomura K, Ko CH, Buhr ED, et al. PERIOD2∷LUCIFERASE real-time reporting of circadian dynamics reveals persistent circadian oscillations in mouse peripheral tissues. Proc Natl Acad Sci U S A. 2004. Apr 13;101(15):5339–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
All data generated during this study are included in this article or its supplementary material files. Data can be obtained from the corresponding author upon request.


