Abstract
Objective:
To determine the effect of prophylactic dextrose gel on the infant gut microbiome.
Design:
Observational cohort study nested in a randomised trial.
Setting:
Three maternity hospitals in New Zealand.
Patients:
Infants at risk of neonatal hypoglycaemia whose parents consented to participation in the hPOD (hypoglycaemia Prevention in newborns with Oral Dextrose) trial. Infants were randomised to receive prophylactic dextrose gel or placebo gel, or were not randomised and received no gel (controls). Stool samples were collected on days 1, 7 and 28.
Main outcome measures:
The primary outcome was microbiome beta-diversity at 4 weeks. Secondary outcomes were beta-diversity, alpha-diversity, bacterial DNA concentration, microbial community stability and relative abundance of individual bacterial taxa at each time point.
Results:
We analysed 434 stool samples from 165 infants using 16S rRNA gene amplicon sequencing. There were no differences between groups in beta-diversity at 4 weeks (p=0.49). There were also no differences between groups in any other microbiome measures including beta-diversity (p=0.53 at day 7), alpha-diversity (p=0.46 for day 7 and week 4), bacterial DNA concentration (p=0.91), microbial community stability (p=0.52) and microbial relative abundance at genus level. There was no evidence that exposure to any dextrose gel (prophylaxis or treatment) had any effect on the microbiome. Mode of birth, type of milk fed, hospital of birth and ethnicity were all associated with differences in the neonatal microbiome.
Conclusions:
Clinicians and consumers can be reassured that dextrose gel used for prophylaxis or treatment of neonatal hypoglycaemia does not alter the neonatal gut microbiome.
INTRODUCTION
Neonatal hypoglycaemia is common and may be one of the most readily preventable causes of neurodevelopmental impairment.(1) Dextrose gel is widely used as a first-line treatment due to its cost-effectiveness and ease of administration.(2, 3) Two recent studies (4), (5) have also established that prophylactic dextrose gel can reduce the incidence of hypoglycaemia in infants at risk.(6)
The establishment of the neonatal gut microbiome is a highly dynamic process, particularly in the first 6 weeks after birth,(7) and can be influenced by many external influences including mode of delivery,(8) feeding methods,(9, 10) antibiotics,(11) and probiotic use.(12) In the long term, altered microbial composition can result in dysbiosis, which has been associated with many diseases including inflammatory bowel conditions,(13, 14) metabolic disorders,(15, 16) and cardiovascular disease.(17) However, there is not yet evidence to suggest that the external influences on the microbiome around the time of birth persist to contribute to these later disease outcomes.
Clinicians and parents have expressed concerns about the possibility that dextrose gel administration may affect the developing neonatal gut microbiome and hence potentially influence future health.
Therefore, we sought to determine whether early dextrose gel administration altered the neonatal gut microbiome.
METHODS
Study design & participants
We undertook this nested observational study within the hPOD (hypoglycaemia Prevention in newborns with Oral Dextrose) multicentre randomised trial (ACTRN 12614001263684), which compared 40% dextrose with placebo gel prophylaxis for neonatal hypoglycaemia in at-risk neonates.(5)
In brief, eligible infants were at risk of hypoglycaemia (infant of diabetic mother, preterm (< 37 weeks), small (< 2.5kg or <10th centile) or large (> 4.5kg or > 90th centile) birth-weight), ≥ 35 weeks gestation, birth weight > 2.2kg, < 1 hour old, no apparent indication for neonatal nursery (NICU) admission at time of randomisation, unlikely to require NICU admission for other reasons, and mother intended to breastfeed. Eligible infants were randomised to 40% dextrose gel or 2% hydroxymethylcellulose placebo gel, 0.5 ml/kg, massaged into the buccal mucosa at one hour after birth. Infants who developed hypoglycaemia were treated according to usual hospital practices which could include additional feeding, formula, treatment 40% dextrose gel and intravenous dextrose.
Infants in this sub-study were born at Auckland City, North Shore or Waikato hospitals, New Zealand, between October 2018 and September 2019, were eligible to participate in the hPOD trial, and parental consent had been obtained. Infants who were not randomised due to insufficient staff within the first hour after birth, so did not receive either hPOD trial gel, were included as controls.
Stool sample collection and analysis
Stool samples were collected in DNA/RNA shield™ fecal collection tubes (Zymo Research, California) on day 1, (meconium wherever possible), at 7 ± 3 days and at 4 weeks ± 7 days. DNA was extracted using the ZymoBIOMICS™ 96 MagBead DNA Kit (Zymo Research, California) and 16S rRNA amplicon sequencing targeting V3 and V4 regions was conducted using Illumina MiSeq instrument. Sequencing data were processed using DADA2 algorithm (18) and statistical analyses (PERMANOVA tests for beta-diversities, Student’s t-test, Kruskal-Wallis test or linear mixed models for univariate tests) were carried out using R version 4.0.3. Additional details of sample collection, randomisation, bioinformatics, and statistics are provided in Supplemental Material 1.
Outcomes
The primary outcome was stool microbiota beta-diversity (weighted UniFrac distance) at 4 weeks of age. Secondary outcomes were stool microbiota beta-diversity at day 7, microbial community stability and microbial alpha-diversity (Shannon diversity index) at day 7 and week 4, total microbial load at all time points, and individual microbial taxa differences. These measures of beta- and alpha-diversity were chosen as some of the most widely used diversity metrics in this field.
This study was approved by the Health and Disability Ethics Committees of New Zealand (13/NTA/8). Written informed consent was obtained from parents for the hPOD study, and additionally for this sub-study.
RESULTS
165 infants provided at least one stool sample; 45 in the dextrose gel, 49 in the placebo gel and 72 in the control group. Groups were well matched for baseline maternal and neonatal characteristics, although more control infants were born at Auckland City and North Shore hospitals (Table 1). Few infants were exposed to probiotics (n = 11 at week 4) or antibiotics (n = 7 at week 4), so these covariates were not analysed further.
Table 1.
Baseline Characteristics of the Study Groups
| Dextrose Gel | Placebo Gel | Control | p | |
|---|---|---|---|---|
| Mothers (n = 161)* | N = 44 | N = 49 | N = 69 | |
| Maternal Age (years) | 31.9 (4.5) | 32.0 (4.6) | 32.6 (5.2) | 0.69 |
| Prioritised Ethnicity | ||||
| Māori | 7 (15.9) | 9 (18.4) | 4 (5.8) | 0.10 |
| Pacific | 1 (2.3) | 3 (6.1) | 4 (5.8) | |
| Asian | 10 (22.7) | 11 (22.4) | 21 (30.4) | |
| Indian | 7 (15.9) | 11 (22.4) | 9 (13.0) | |
| Other | 2 (4.5) | 2 (4.1) | 12 (17.4) | |
| European | 17 (38.6) | 13 (26.5) | 19 (27.5) | |
| Diabetes | ||||
| Type 1 Diabetes | 1 (2.3) | 1 (2.0) | 0 (0.0) | 0.60 |
| Type 2 Diabetes | 2 (4.6) | 5 (10.2) | 4 (5.8) | |
| Gestational Diabetes | 25 (56.8) | 26 (53.1) | 46 (66.7) | |
| Diabetes Management | ||||
| Diet | 9 (33) | 9 (29) | 15 (34) | 0.89 |
| Metformin | 7 (26) | 7 (23) | 13 (30) | 0.80 |
| Insulin | 13 (48) | 21 (68) | 22 (50) | 0.22 |
| Antenatal Corticosteroids | 2 (4.5) | 3 (6.1) | 1 (1.4) | 0.43 |
| Prelabour prolonged rupture of membranes | 4 (9.1) | 4 (8.2) | 3 (4.3) | 0.62 |
| Chorioamnionitis | 0 (0.0) | 0 (0.0) | 1 (1.4) | 0.48 |
| Mode of Delivery | ||||
| Normal vaginal | 19 (43.2) | 19 (38.8) | 33 (48.0) | 0.12 |
| Instrumental vaginal | 7 (15.9) | 6 (12.2) | 8 (11.6) | |
| Elective caesarean | 9 (20.5) | 11 (22.4) | 5 (7.2) | |
| Emergency caesarean | 9 (20.5) | 13 (26.5) | 23 (33.3) | |
| Babies (n = 165) | N = 44 | N = 49 | N = 72 | |
| Singleton | 42 (96) | 48 (98) | 60 (90 | 0.16 |
| Girls | 21 (47.7) | 21 (42.9) | 33 (45.8) | 0.89 |
| Gestational age (weeks) | 38.6 (1.1) | 38.6 (1.1) | 38.4 (1.4) | 0.99 |
| Birthweight (g) | 3377.5 (805.0) | 3317.3 (601.6) | 3138.7 (620.3) | 0.15 |
| Length (cm) | 49.8 (3.1) | 50.2 (2.7) | 49.8 (3.3) | 0.83 |
| Head Circumference (cm) | 34.6 (2.2) | 34.6 (1.8) | 34.3 (2.9) | 0.65 |
| Birthweight z -score | 0.26 (1.54) | 0.15 (1.05) | −0.19 (1.01) | 0.11 |
| Length z-score | 0.26 (1.25) | 0.34 (1.01) | 0.19 (1.23) | 0.83 |
| Head Circumference z-score | 0.40 (1.42) | 0.36 (1.09) | 0.14 (1.88) | 0.63 |
| Apgar score < 7 at 5 minutes | 2 (4.6) | 0 (0.0) | 1 (1.4) | 0.56 |
| Primary Reason for Risk of Hypoglycaemia | ||||
| Infant of mother with diabetes | 28 | 32 | 51 | 0.31 |
| Preterm (< 37 weeks’ gestation) | 4 | 7 | 6 | |
| Small (< 2.5kg or < 10th centile) | 6 | 5 | 13 | |
| Large (> 4.5kg or > 90th centile) | 6 | 5 | 2 | |
| One Risk Factor | 37 (84.1) | 44 (89.8) | 66 (91.7) # | 0.06 |
| Two Risk Factors | 7 (15.9) | 5 (10.2) | 4 (5.6) | |
| Three Risk Factors | 0 (0.0) | 0 (0.0) | 2 (2.8) | |
| Hospital site | ||||
| Auckland City Hospital | 10 (22.7) | 13 (26.5) | 32 (44.4) | <0.0001 ~ |
| North Shore Hospital | 14 (31.8) | 19 (38.8) | 37 (51.4) | |
| Waikato Hospital | 20 (29.5) | 17 (34.7) | 3 (4.2) | |
| Admission to NICU | 0 (0.0) | 3 (6.1) | 6 (9.0) | 0.13 |
| Duration of hospital stay (hours) | 61.7 (45.7 - 95.7) | 72.0 (45.6 - 92.7) | 78.7 (45.2 - 106.6) | 0.49 |
| Received treatment dextrose gel | 10 (22.7) | 18 (36.7) | 21 (29.2) | 0.34 |
| Antibiotic exposure ^ | ||||
| Day 1 | 0/44 (0.0) | 2/49 (4.1) | 3/71 (4.2) | 0.39 |
| Day 7 | 1/38 (2.6) | 0/45 (0.0) | 3/59 (5.0) | 0.30 |
| Week 4 | 1/34 (2.9) | 1/35 (2.9) | 5/60 (8.3) | 0.40 |
| Probiotic exposure @ | ||||
| Day 1 | 1/44 (2.3) | 0/49 (0.0) | 1/71 (1.4) | 0.60 |
| Day 7 | 1/38 (2.6) | 1/44 (2.3) | 0/59 (0.0) | 0.48 |
| Week 4 | 5/34 (14.7) | 2/35 (5.7) | 4/60 (3.4) | 0.32 |
| Milk Type | ||||
| Day 1 | ||||
| Breastmilk only | 36 (81.8) | 39 (81.3) | 48 (68.6) | 0.42 |
| Formula only | 0 (0.0) | 1 (2.0) | 2 (2.9) | |
| Breastmilk and formula | 8 (18.1) | 9 (18.8) | 20 (28.6) | |
| Day 7 | ||||
| Breastmilk only | 29 (76.3) | 28 (62.2) | 41 (69.5) | 0.39 |
| Formula only | 0 (0.0) | 1 (2.2) | 32 (5.1) | |
| Breastmilk and formula | 9 (23.7) | 16 (36.6) | 15 (25.4) | |
| Week 4 | ||||
| Breastmilk only | 23 (67.7) | 21 (60.0) | 40 (66.7) | 0.88 |
| Formula only | 2 (5.9) | 2 (5.7) | 5 (8.3) | |
| Breastmilk and formula | 9 (26.5) | 12 (34.3) | 15 (25.4) |
Data are n (%), mean (SD) or median (interquartile range). P values are for comparison across the three groups.
One mother had a twin in the dextrose gel group and another in the placebo gel group and is thus included in both groups.
Control significantly different from both glucose and placebo groups on post hoc analysis.
Number of risk factors unknown for 5 babies in the control group.
Maternal antibiotic exposure during pregnancy or infant antibiotic exposure since birth.
Infant probiotic exposure since birth.
Of 446 stool samples received, three were misplaced, four were duplicates, and three were taken outside the required timeframes, leaving 436 samples for sequencing: 162 day 1, 145 day 7 and 129 week 4 samples (Figure 1). One day 7 and one week 4 sample contained <3,000 sequencing reads post DADA2 sequence processing and were excluded, leaving 434 samples from 165 babies for analysis.
Figure 1 –
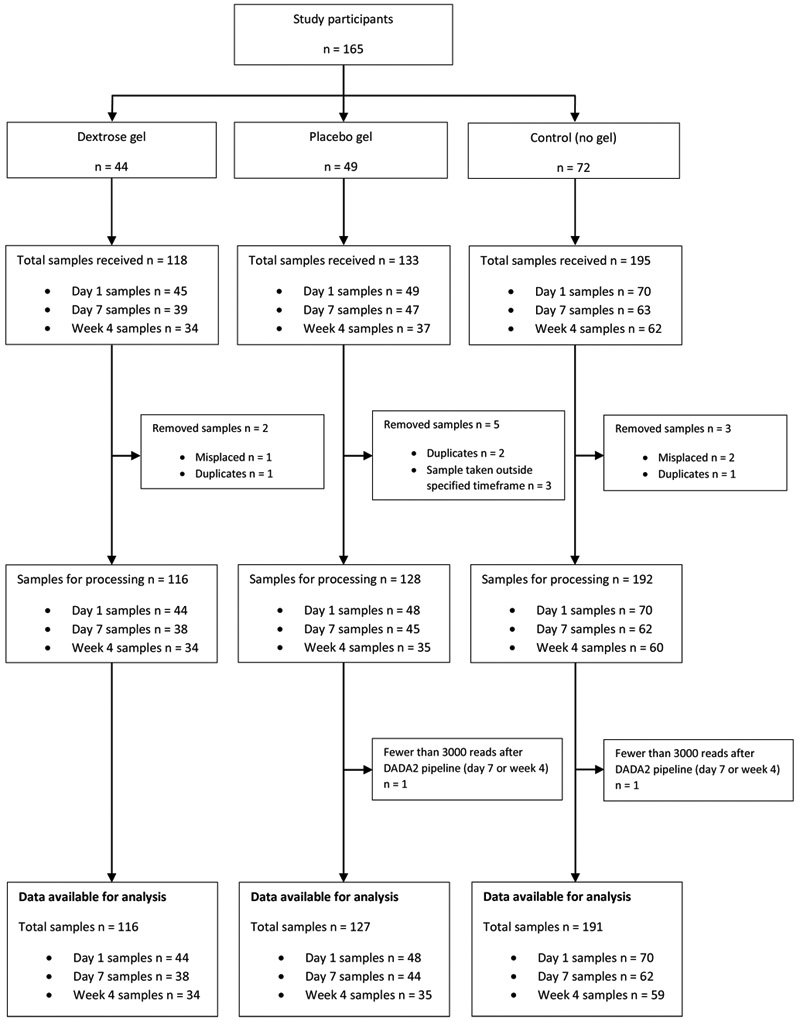
Flow of participants and samples.
Taxonomic assignment identified 8,604 amplicon sequence variants (ASVs). The frequency-based method identified 110 and the prevalence-based method identified 39 contaminant ASVs, all of which were removed, leaving 8,459 ASVs from 434 stool samples and 21 controls for analysis (Supplemental Material 2).
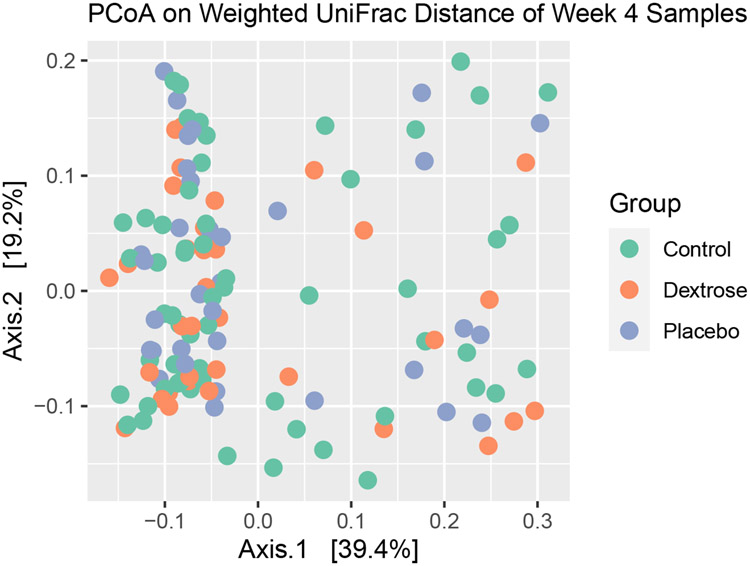
There was no significant difference in beta-diversity between groups in the 128 week 4 samples in the unadjusted analysis (PERMANOVA, p = 0.89, Figure 2) or after adjustment (p = 0.49). There was also no significant difference in beta-diversity between groups in the day 7 samples in the unadjusted analysis (p = 0.51) or after adjustment (p = 0.53).
Figure 2 -. Principle Coordinates Analysis Plot for stool samples collected in week 4 from babies who received dextrose gel, placebo gel or no prophylactic gel (control).
The PCoA visualises the weighted UniFrac dissimilarity for 128 week 4 stool samples. Each dot indicates one of these samples. This plot displays the PERMANOVA result, and shows no clustering related to study group. However other clustering is apparent, indicating the potential influence of another factor.
On sensitivity analysis, findings for week 4 samples were unchanged when excluding infants exposed to antibiotics (n = 7, p = 0.51), probiotics (n = 9, p = 0.48), admitted to NICU (n = 8, p = 0.40) or treated with dextrose gel (n = 43, p = 0.27).
There were no significant differences between groups in stool DNA concentration, reflecting total microbial load (ANOVA, p = 0.91, Supplemental Material 3). As expected, day 1 samples exhibited low DNA concentrations resulting in noisy amplicon sequence profiles. Day 1 samples were therefore excluded from further analyses.
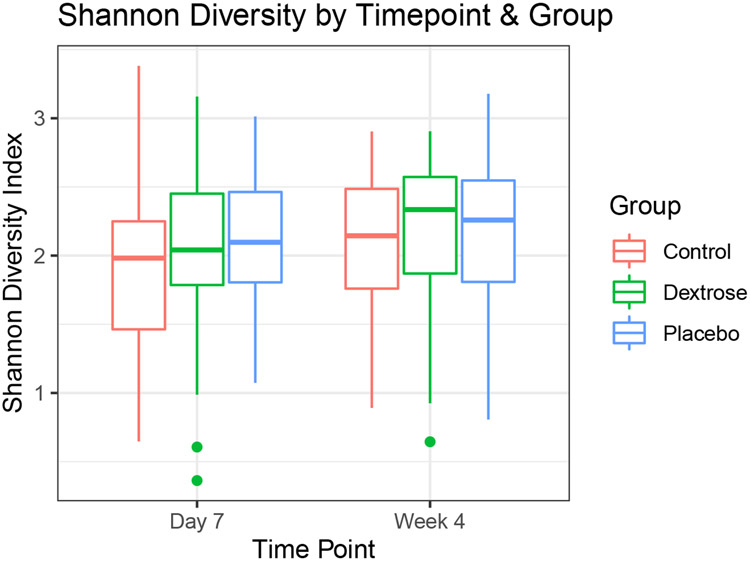
There were no significant differences between groups for microbial community stability (Kruskal-Wallis, p = 0.52). There were also no significant differences in relative abundance amongst the 367 identified genera. Mean dissimilarity score within subjects (0.195, σ2 = 0.013) was lower than between subjects (0.288, σ2 = 0.015, p = 4.69e-15). There were no significant differences in Shannon diversity index between day 7 and week 4 samples (n = 271, ANOVA, p = 0.46, Figure 3). Hierarchical clustering of the samples did not correspond to the study groups (Supplemental Material 5).
Figure 3 -. Alpha diversity in samples from babies who received dextrose gel, placebo gel or no prophylactic gel (control) collected on day 7 and week 4.
The boxes represent interquartile range (IQR), the horizontal line indicates the median, whiskers indicate range and the dots represent outlier values (further than 1.5 times IQR from the box). There were no statistically significant differences between the groups or between day 7 and week 4.
Exploratory analyses
When we compared prophylactic dextrose gel and placebo groups, there were no significant differences in beta-diversity at any time point, alpha-diversity (ANOVA, p = 0.75), DNA concentration (ANOVA, p = 0.70) or relative abundance of individual genera.
Beta diversity was significantly different between infants born vaginally and those born by caesarean section on day 1 (PERMANOVA, p = 0.005), day 7 (p = 1e-4) and week 4 (p = 1e-4) but there were no significant differences in alpha diversity at day 7 and week 4 (ANOVA, p = 0.73), or in DNA concentration (p = 0.32). There were 14 genera with statistically significant differences in relative abundance between vaginal and caesarean births in day 7 and week 4 samples (q < 0.05, Supplemental Material 4).
Beta-diversity was significantly different between infants who received breastmilk and those who received formula with or without breastmilk on day 1 (PERMANOVA, p = 0.027) and week 4 (p = 0.012), but not on day 7 (p = 0.091). Formula fed infants exhibited greater alpha diversity than those who were solely breastfed (ANOVA, p = 0.0002). There were no significant differences in DNA concentration between feeding groups (ANOVA, p = 0.89). MaAsLin2 showed differences in the relative abundance of the genera Staphylococcus, Veillonella, Gamella and Haemophilus between feeding groups (Supplemental Material 4).
Beta diversity was significantly different between babies born at the three recruitment sites on day 1 (PERMANOVA, p = 2e-4) and day 7 (p = 0.020), but not week 4 (p = 0.15). There were also apparent but not statistically significant differences in alpha diversity (ANOVA, p = 0.093), no significant differences in DNA concentration (p = 0.94) or individual genera (q > 0.05).
There were no significant differences between prioritised ethnic groups in beta-diversity, alpha-diversity or DNA concentration. Enterococcus (FDR-corrected p = 8.038e-4) and Veillonella (p = 3.849e-3) were both more abundant in Indians compared to other ethnic groups (Supplemental Material 4).
There were no significant differences in beta-diversity at any time point between infants with different primary reasons for risk of hypoglycaemia. Large birth-weight infants and infants of diabetic mothers had higher Shannon diversity index (ANOVA, p = 0.015). There were no significant differences between risk groups in DNA concentration (p = 0.88) or relative abundance of individual genera (q > 0.05).
There were no significant differences between infants who had received any dextrose gel (as prophylaxis or treatment) and those who had not in beta-diversity at any time point, alpha-diversity (ANOVA, p = 0.85), DNA concentration (p = 0.85) or relative abundance of individual genera (q > 0.05).
DISCUSSION
We sought to determine whether prophylactic dextrose gel altered the gut microbiome of infants at risk of neonatal hypoglycaemia. We randomised babies to dextrose or placebo gel and included a control group to account for the possibility that placebo gel may also alter the gut microbiome. The groups were well matched for baseline and clinical characteristics, and repeated samples were available for most babies allowing study of potential longitudinal as well as cross-sectional changes in the gut microbiome. We found no evidence that dextrose gel alters the gut microbiome up to 4 weeks of age. Specifically, there were no differences between dextrose gel, placebo gel and control groups in beta-diversity, alpha-diversity, total microbial load, microbial community stability or bacterial relative abundance at the genus level. These findings should be reassuring for both clinicians and families that prophylactic dextrose gel is very unlikely to affect early gut microbiome development or impact future health through altered gut microbiome.
Beta-diversity reflects differences in microbiome structure between microbial communities, while alpha-diversity indicates the diversity within a microbial community. If dextrose gel affected microbiome composition, we would expect to see differences in beta-diversity, alpha-diversity, or both. Total microbial load was measured to determine if there were any differences in stool bacterial concentrations. Microbial community stability was also measured to detect significant fluctuations in the establishment of the neonatal microbiome during the early weeks. Bacterial relative abundance at the genus level was measured to determine whether the gel contributed to altered levels of specific genera in the infants’ gut. However, none of these measures differed between groups
Our finding that dissimilarity scores within subjects were significantly lower than between subjects is consistent with the idea of personalised microbiomes, whereby the gut microbiomes are more similar over time within a subject than between subjects.(19) Our findings were consistent with previous reports that the developing neonatal microbiome is influenced by a number of early exposures, including mode of birth, feeding, hospital of birth and ethnicity.(8, 9, 20)
We found that Bacteroides was more abundant in microbiomes of vaginally-born infants than in those born by caesarean section, supporting previous reports that children born by caesarean section lack this particular bacterial genus.(21) Vaginally-born infants are thought to acquire bacteria from multiple maternal body sites.(22) The impact of reduced Bacteroides for long-term health remains unclear, although caesarean section has been associated with increased risk of a number of long-term health problems, potentially linked to gut microbiome shifts, including asthma and childhood obesity.(23)
We also found that type of feeding had no effect on total microbial load but significantly altered microbial composition, consistent with the results of other studies.(9) Infants exclusively receiving breast-milk had a higher abundance of the Staphylococcus genus, which is consistent with its predominance in breastmilk.(24) Infants who received formula had a higher alpha-diversity than those exclusively receiving breastmilk, indicating a more diverse bacterial flora. Beta-diversity was also significantly different. The simple sugars in formula can be utilised by a range of gut bacteria, potentially contributing to higher diversity in infants receiving formula, whereas human milk oligosaccharides can only be utilised by a limited number of specialised bacteria.(25) The reduced diversity in infants receiving breastmilk has also been attributed to lactic acid-producing bacteria in the breastmilk which inhibit pathogenic bacterial growth.(26)
Amongst infants born at the three recruiting hospitals we found significant differences in beta-diversity on days 1 and 7, but not at week 4. Similar results were also observed in another New Zealand-based trial undertaken over similar time periods and the same hospitals.(27) Since on average babies spent only 3 days in hospital, this altered beta-diversity could reflect differences between hospital-specific environmental microbiomes that had a persistent effect on the neonatal gut microbiome for at least a few days but had dissipated by week 4. It is also possible that bacterial contamination from hospitals occurred in the day 1 samples, although this would not explain differences in day 7 samples which were collected after discharge.
Ethnicity has also been reported to be associated with differences in gut microbiota composition, potentially linked to genetic, dietary, socioeconomic or lifestyle factors.(20) Ethnic differences in the neonatal microbiome have been reported, particularly in Pacific infants.(27) However, we found only minor differences between ethnic groups, with Indians having a greater abundance of the genera Enterococcus and Veillonella. Our study was not powered to detect small differences between ethnic groups and small numbers of Pacific infants were combined with Other ethnicities for subgroup analysis.
The primary reason for risk of hypoglycaemia was associated with few differences in the gut microbiome. Samples from large birth-weight infants and those of diabetic mothers had a higher Shannon Diversity index, indicating a more diverse gut bacterial composition. Differences in diet may contribute to these findings, since breastmilk of mothers with gestational diabetes has altered composition, with reduced amino acids and organic acids, as well as decreased fat and energy content compared to breastmilk of mothers without diabetes.(28, 29)
To determine effects of dextrose gel on the neonatal microbiome, we compared infants randomised to prophylactic dextrose or placebo gel. Interpretation was potentially confounded by some infants also receiving dextrose gel as standard clinical treatment for hypoglycaemia. However, in exploratory analyses comparing infants who received any dextrose gel with those who received none there were still no differences in any of the microbiome measures. Since the dose of dextrose gel and the way it is administered are the same, whether used as prophylaxis or as treatment for neonatal hypoglycaemia, these findings suggest that use of dextrose gel for either purpose does not alter neonatal microbial composition.
Limitations of the study include that the sample size was slightly smaller than intended because of closure of the hPOD trial, resulting in limited power to detect small differences between groups, particularly in the subgroup analyses. There was also some loss to follow-up with fewer samples provided at week 4 than on day 1. However, this was similar in all groups, so was unlikely to have affected our findings. Nevertheless, using the original power calculations, we had 76% power to detect the expected 20% differences in the frequency of the five most common taxa between groups, allowing for 10% diversity within groups if there were only 34 samples per group, and 86% power if there were the actual average of 42 per group. In addition, our finding of the expected differences in gut microbiome between babies with different modes of birth, feeding and birth hospital provides reassurance that the methods used were appropriate and sensitive to the changes that might be clinically relevant in this context.
In summary, we found no significant differences in gut microbiome composition between babies exposed to prophylactic dextrose gel, placebo gel or no gel. Clinicians and consumers should be reassured that there is no evidence that use of dextrose gel for prophylaxis or treatment of neonatal hypoglycaemia alters the neonatal microbiome.
Supplementary Material
What is already known on this topic:
Oral dextrose gel is effective for treatment and prophylaxis of neonatal hypoglycaemia
Establishment of the neonatal gut microbiome is readily perturbed by early environmental exposures and may be important for lifelong health
Concerns have been expressed that administration of dextrose gel may alter the neonatal microbiome
What this study adds:
No differences in neonatal gut microbial composition were detected up to 4 weeks of age in infants given prophylactic dextrose gel, placebo or no gel.
The expected differences in neonatal gut microbial composition were detected between infants with different modes of birth, feeding, birth hospital and ethnicity.
Use of dextrose gel for prophylaxis or treatment of neonatal hypoglycaemia is extremely unlikely to alter the neonatal microbiome.
ACKNOWLEDGEMENTS
The authors acknowledge the generosity of all families who participated in the study: the hPOD Study Steering Group; Jane Harding (chair), Jane Alsweiler, Caroline Crowther, Richard Edlin, Gregory Gamble, and Joanne Hegarty: study coordinators Kelly Fredell and Coila Bevan: and the study staff at the recruiting sites; Auckland City Hospital, Auckland: Jane Alsweiler, Celia Grigg, Jodi Guthrie-Mart, Joanne Hegarty, Sabine Hulh, Andrew Meisner, Carla Saunders, and Robyn Wilkinson, North Shore Hospital, Auckland: Dianne Allan, Susan Law, Maree Young, Jutta van den Boom, and Stephanie Williams, and Waikato Hospital, Hamilton: Alana Cumberpatch, Deborah Harris, and Rachel Ladd.
The authors thank Victoria Peters for conducting DNA extractions on the stool samples.
FUNDING
The hPOD trial was funded by a grant from the Health Research Council of New Zealand (13/131). The microbiome analyses were funded by the Liggins Institute New Staff Research Fund (3717847).
SLSC was funded by a clinical research internship from the Aotearoa Foundation (9909494).
JEH was funded in part by a grant from the Eunice Kennedy Shriver National Institute of Child Health & Human Development of the National Institutes of Health (R01HD091075). The content is solely the responsibility of the authors and does not necessarily represent the official views of the Eunice Kennedy Shriver National Institute of Child Health and Human Development or the National Institutes of Health.
Footnotes
COMPETING INTERESTS
Authors declare no competing interests.
References
- 1.Kaiser JR, Bai S, Gibson N, et al. Association Between Transient Newborn Hypoglycemia and Fourth-Grade Achievement Test Proficiency: A Population-Based Study. JAMA Pediatr 2015;169(10):913–21. [DOI] [PubMed] [Google Scholar]
- 2.Harris DL, Weston PJ, Signal M, et al. Dextrose gel for neonatal hypoglycaemia (the Sugar Babies Study): a randomised, double-blind, placebo-controlled trial. Lancet 2013;382(9910):2077–83. [DOI] [PubMed] [Google Scholar]
- 3.Alsweiler JM, Woodall SM, Crowther CA, et al. Oral dextrose gel to treat neonatal hypoglycaemia: Clinician survey. J Paediatr Child Health 2019. Jul;55(7):844–50. [DOI] [PubMed] [Google Scholar]
- 4.Hegarty JE, Harding JE, Gamble GD, et al. Prophylactic Oral Dextrose Gel for Newborn Babies at Risk of Neonatal Hypoglycaemia: A Randomised Controlled Dose-Finding Trial (the Pre-hPOD Study). PLoS Med 2016. Oct 25;13(10):e1002155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Harding JE, Hegarty JE, Crowther CA, et al. Evaluation of oral dextrose gel for prevention of neonatal hypoglycemia (hPOD): A multicenter, double-blind randomized controlled trial. PLoS Med 2021. Jan 28;18(1). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Harris DL, Weston PJ, Harding JE. Incidence of neonatal hypoglycemia in babies identified as at risk. J Pediatr 2012. Nov;161(5):787–91. [DOI] [PubMed] [Google Scholar]
- 7.Chu DM, Ma J, Prince AL, et al. Maturation of the infant microbiome community structure and function across multiple body sites and in relation to mode of delivery. Nat Med 2017. Mar;23(3):314–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Biasucci G, Rubini M, Riboni S, et al. Mode of delivery affects the bacterial community in the newborn gut. Early Hum Dev 2010. Jul;86(Suppl 1):13–5. [DOI] [PubMed] [Google Scholar]
- 9.Vatanen T, Franzosa EA, Schwager R, et al. The human gut microbiome in early-onset type 1 diabetes from the TEDDY study. Nature 2018. Oct;562(7728):589–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bäckhed F, Roswall J, Peng Y, et al. Dynamics and Stabilization of the Human Gut Microbiome during the First Year of Life. Cell Host Microbe 2015. May 13;17(5):690–703. [DOI] [PubMed] [Google Scholar]
- 11.Persaud R, Azad MB, Konya T, et al. Impact of perinatal antibiotic exposure on the infant gut microbiota at one year of age. Allergy Asthma Clin Immunol 2014. Mar 03;10(S1):A31. [Google Scholar]
- 12.Quin C, Estaki M, Vollman DM, et al. Probiotic supplementation and associated infant gut microbiome and health: a cautionary retrospective clinical comparison. Sci Rep 2018. May 29;8(1):8283–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Morgan XC, Tickle TL, Sokol H, et al. Dysfunction of the intestinal microbiome in inflammatory bowel disease and treatment. Genome Biol 2012. Apr 16;13(9):R79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Takahashi K, Nishida A, Fujimoto T, et al. Reduced Abundance of Butyrate-Producing Bacteria Species in the Fecal Microbial Community in Crohn's Disease. Digestion 2016;93(1):59–65. [DOI] [PubMed] [Google Scholar]
- 15.Qin J, Li Y, Peng Y, et al. A metagenome-wide association study of gut microbiota in type 2 diabetes. Nature 2012;490(7418):55–60. [DOI] [PubMed] [Google Scholar]
- 16.Turnbaugh PJ, Hamady M, Egholm M, et al. A core gut microbiome in obese and lean twins. Nature 2009;457(7228):480–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Wang Z, Klipfell E, Wu Y, et al. Gut flora metabolism of phosphatidylcholine promotes cardiovascular disease. Nature 2011;472(7341):57–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Callahan BJ, McMurdie PJ, Rosen MJ, et al. DADA2: high-resolution sample inference from Illumina amplicon data. Nat Methods 2016. Jul;13(7):581–3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Franzosa Eric A., Huang Katherine, Meadow James F., et al. Identifying personal microbiomes using metagenomic codes. Proc Natl Acad Sci U S A 2015. Jun 2;112(22):E2930–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Deschasaux M, Bouter KE, Prodan A, et al. Depicting the composition of gut microbiota in a population with varied ethnic origins but shared geography. Nat Med 2018. Oct;24(10):1526–31. [DOI] [PubMed] [Google Scholar]
- 21.Mitchell CM, Mazzoni C, Hogstrom L, et al. Delivery Mode Affects Stability of Early Infant Gut Microbiota. Cell Rep Med 2020. Dec 22;1(9):100156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ferretti P, Pasolli E, Tett A, et al. Mother-to-Infant Microbial Transmission from Different Body Sites Shapes the Developing Infant Gut Microbiome. Cell Host Microbe 2018;24(1):133–145.e5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Sandall J, Tribe RM, Avery L, et al. Short-term and long-term effects of caesarean section on the health of women and children. Lancet 2018. Oct 13;392(10155):1349–57. [DOI] [PubMed] [Google Scholar]
- 24.Cabrera-Rubio R, Collado MC, Laitinen K, et al. The human milk microbiome changes over lactation and is shaped by maternal weight and mode of delivery. Am J Clin Nutr 2012. Sep;96(3):544–51. [DOI] [PubMed] [Google Scholar]
- 25.Lawson MAE, O'Neill IJ, Kujawska M, et al. Breast milk-derived human milk oligosaccharides promote Bifidobacterium interactions within a single ecosystem. ISME J 2020. Feb;14(2):635–48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Zimmermann P, Curtis N. Breast milk microbiota: A review of the factors that influence composition. J Infect 2020;81(1):17–47. [DOI] [PubMed] [Google Scholar]
- 27.Chong CYL, Vatanen T, Alexander T, et al. Factors Associated With the Microbiome in Moderate–Late Preterm Babies: A Cohort Study From the DIAMOND Randomized Controlled Trial. Front Cell Infect Microbiol 2021. Mar 1;11:595323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Shapira D, Mandel D, Mimouni FB, et al. The effect of gestational diabetes mellitus on human milk macronutrients content. J Perinatol 2019. Jun;39(6):820–3. [DOI] [PubMed] [Google Scholar]
- 29.Bardanzellu F, Puddu M, Fanos V. The Human Breast Milk Metabolome in Preeclampsia, Gestational Diabetes, and Intrauterine Growth Restriction: Implications for Child Growth and Development. J Pediatr 2020;221S:S20–8. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.