Functional constipation (FC) is one of the most frequently encountered gastrointestinal conditions in practice.1 Practice guidelines universally recommend that patients with typical constipation symptoms and no alarm features be treated empirically with dietary/lifestyle interventions and laxative therapy.2,3 Unfortunately, by the time a patient reaches a gastroenterologist, these treatments frequently have already been tried. Anorectal function testing (anorectal manometry [ARM] and balloon expulsion test [BET]) is the next best step in management guidelines in this all-too-common scenario, because treatment can then be targeted toward pelvic floor dysfunction or colon transit abnormalities. Unfortunately, more than 95% of patients continue to take only over-the-counter laxatives and receive empirical dietary advice, whereas fewer than 2% undergo physiologic evaluation to ascertain the cause of their symptoms.4 Indeed, more than 90% of patients desire more effective treatment options. These observations call into question the wisdom of a management strategy that fails to recognize the intrinsic diversity of the constipation universe and reinforces the misguided “one size fits all” empirical treatment strategy.
In defense of health care providers managing patients with chronic constipation, the small number of tertiary centers that perform ARM and BET and the high cost of testing make it impossible to offer anorectal function testing to all in whom it is indicated.5,6 BET is a conceptually simple test in which a balloon is inserted into the rectum and then inflated with water or air to a certain volume using a separate syringe.7 In practice, this disrupts workflow. The multistep process, which requires separate pieces of equipment, is inconvenient and disruptive to workflow, an issue that makes BET a nonstarter in busy clinical practices, which demand simplicity and time efficiency.
To address these gaps in standard practice between specialty centers and community practice, we simplified the concept of balloon expulsion to develop an office-based, point-of-care device called rectal expulsion device (RED) that streamlines and simplifies testing so that it can be implemented in an office-based setting.8 In this manuscript, we report the results of a proof-of-concept study comparing the performance characteristics of RED with a gold standard, commercially available BET.
In this proof-of-concept study, we enrolled 20 patients with FC (Rome IV criteria) referred to the Michigan Medicine GI Physiology Laboratory who had failed to respond to over-the-counter and/or prescription treatments and 5 healthy volunteers without gastrointestinal complaints between September 2019 and February 2020. This study was approved by Michigan Medicine IRBMED. A 500-mL tap water enema was administered followed by a digital rectal examination. All patients underwent ARM, BET, and RED. ARM was performed on all patients (Diversatek Healthcare, Highlands Ranch, CO). Following ARM, subjects were randomized to undergo BET (Part# SR1B, Mui Scientific, Mississauga, Ontario, Canada) followed by RED or vice versa. To perform BET, the lubricated device was inserted rectally and the balloon was inflated with 50 mL of water using a separate syringe followed by closing a stopcock.7 RED was engineered to mimic the consistency of Bristol IV stool (as opposed to water or air that is typically used in BET) (Figure 1A). RED was performed by inserting the lubricated business end of the device (Figure 1B) into the rectum. Once inserted, the intrarectal portion of RED was inflated to the predetermined dimensions, simply by removing a cap on the end of the extracorporeal tubing (Figure 1C). Following placement of BET or RED, patients transferred to a seated position on a private toilet and the time-to-expel was measured before removing the device. An abnormal RED or BET was defined by retaining either device for ≥60 seconds. Tolerability was assessed on perceived smoothness, shape, size, overall comfort, and procedural duration on post-procedure self-report questionnaires. Statistical analysis on continuous variables was performed using a Student t test and intertest reliability was compared using a Cohen kappa (k) statistic.
Figure 1.
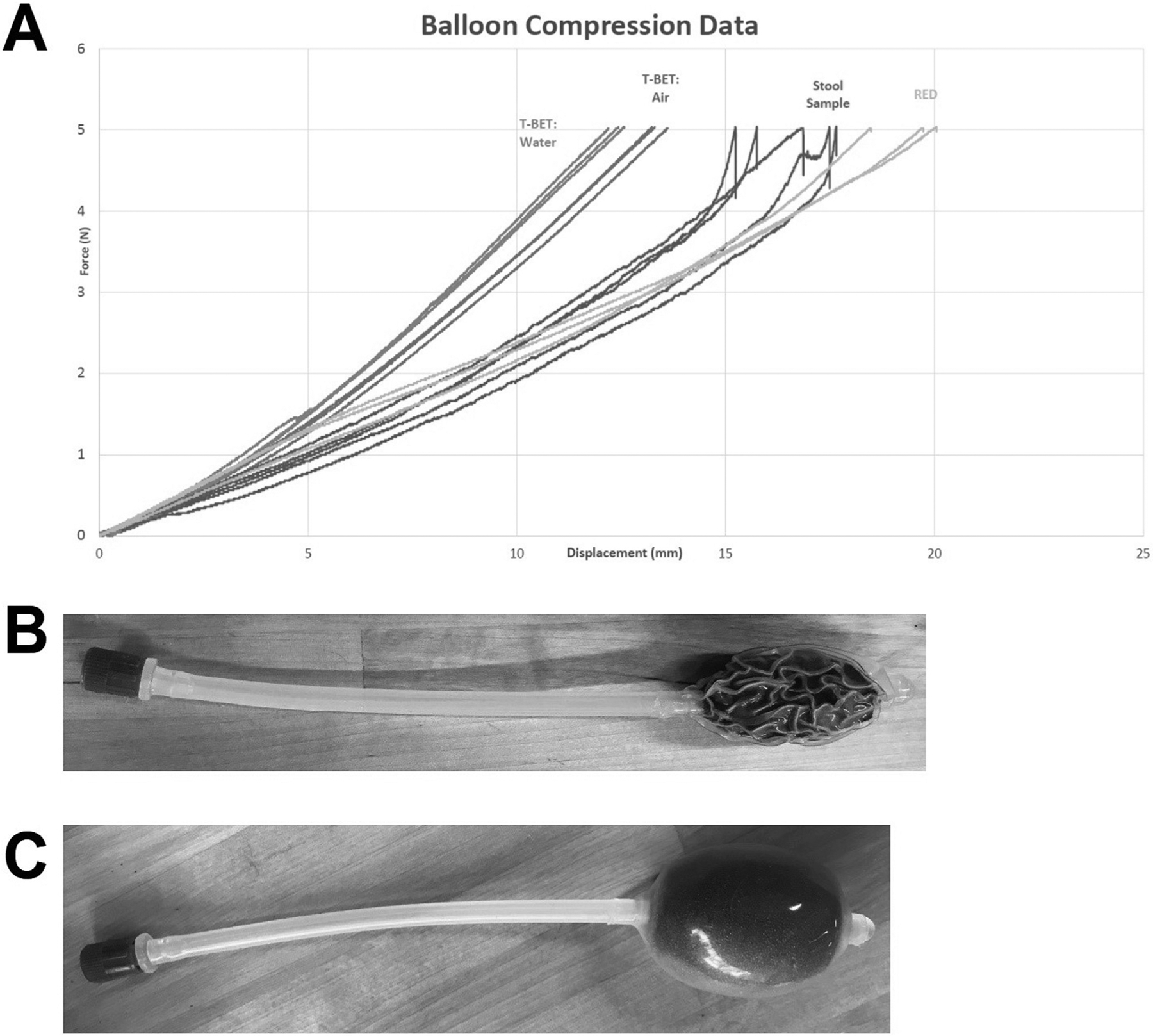
As part of the design process, RED was designed to mimic the consistency of stool rather than water or air. (A) Force-displacement curve that demonstrates our preliminary testing in identifying the foam compound used in RED to mimic stool and also demonstrates that the force-displacement of stool differs from that of water or air that is used in traditional balloon expulsion (T-BET). RED is shown in its initial compressed state (B) and subsequent inflated state following rectal insertion simply by removing the cap on the end of the device (C).
The demographics of subjects with FC (mean age, 50.0 years [range, 22–76]; body mass index, 28.0 kg/m2 [range, 21.3–39.7]; 94.7% women) and healthy subjects (mean age, 47.5 years [range, 28–69]; body mass index, 28.6 kg/m2 [range, 18.2–38.2]; 83.3% women) were similar. The mean anal relaxation was 66.7% (standard deviation [SD], 16.3%) compared with baseline during simulated defecation on ARM for subjects with FC and 34.1% (SD, 36.0%) for healthy subjects. In the pooled cohort, all 5 healthy subjects expelled BET and RED within 60 seconds. In subjects with FC, 8/20 (40.0%) expelled BET within 60 seconds compared with 9/20(45.0%) for RED (k = 0.90).
Smoothness (score = 8.2 [SD, 1.9] on 11-point Likert scale), shape (8.5 [SD, 1.7]), size (8.2 [SD, 2.1]), overall comfort (8.4 [SD, 1.8]), and procedural duration(9.2 [SD, 1.5]) of RED were similar to the smoothness(8.4 [SD, 1.7]), shape (8.2 [SD, 2.0]), size (8.00 [SD,2.22]), overall comfort (8.15 [SD, 2.03]), and procedural duration (8.20 [SD, 2.40]) of BET (P > .50 for all tolerability variables).
RED is a novel, point-of-care, investigational, single-use disposable device intended to identify constipated patients with an evacuation disorder. In this proof-of-concept study, RED yielded similar results and tolerability to BET, the current gold standard in clinical practice. From a clinical standpoint, BET has been found to predict treatment outcomes with biofeedback therapy in randomized controlled trials in patients with chronic constipation.9,10 Unlike BET, which requires water insufflation, has multiple components, and is typically performed in motility laboratories, RED has been engineered to overcome these obstacles by providing an all-inclusive device that provides a streamlined patient and provider experience.
RED is performed simply by inserting the device, removing a cap to inflate the device, and asking the patient to attempt defecation. During this brief time, a provider could leave the room to chart for 2 minutes. If the patient is unable to expel RED, the provider can positively diagnose an evacuation disorder and the device can be easily removed at bedside simply by pulling the device from the rectum without requiring additional staff or additional steps. Alternatively, a nurse or medical assistant could perform RED.
In summary, RED delivers a strong value proposition to potentially disrupt the paradigm in evaluating/managing chronic constipation as an easy-to-use, point-of-care device to rapidly screen for an evacuation disorder and immediately triage the 98% of patients with laxative-refractory chronic constipation who never undergo anorectal function testing to appropriate therapy in community practice.4
Acknowledgment
The authors would like to thank the University of Michigan Fast Forward Medical Innovation GI Innovation Fund and the AGA Research Foundation for supporting the conduct of this work.
Conflicts of interest
These authors disclose the following: William D. Chey consulted for AbbVie, Alnylamm, Allakos, Arena, Biomerica, Gemelli, Ironwood, Isothrive, Nestle, Progenity, Salix, Takeda, Uorvant, and Vibrant; has research grants from Biomerica, Commonwealth Diagnostics International, QOL Medical, and Salix; and has stock options in GastroGirl and Modify Health. Jason R. Baker consulted for Diversatek Healthcare, Medtronic, GI Supply, and Coloplast. Eric D. Shah consulted for GI Supply/Laborie and Bausch Health. William D. Chey, Eric D. Shah, Jason R. Baker, Adrienne Harris, and the Regents of the University of Michigan are holders of patent PCT/US2019/050155. The remaining authors disclose no conflicts.
Funding
This study was supported by the University of Michigan Fast Forward Medical Innovation GI Innovation Fund, which was not involved in the conduct of this study. Eric D. Shah is supported by the AGA Research Foundation’s 2019 American Gastroenterological Association-Shire Research Scholar Award in Functional GI and Motility Disorders.
Contributor Information
ADRIENNE HARRIS, In2Being LLC, Saline, Michigan.
ERIC D. SHAH, Division of Gastroenterology and Hepatology, Michigan Medicine, Ann Arbor, Michigan; Section of Gastroenterology and Hepatology, Dartmouth-Hitchcock Medical Center, Lebanon, New Hampshire.
References
- 1.Barberio B, et al. Lancet Gastroenterol Hepatol 2021;6:638–648. [DOI] [PubMed] [Google Scholar]
- 2.Paquette IM, et al. Dis Colon Rectum 2016;59:479–492. [DOI] [PubMed] [Google Scholar]
- 3.Bharucha AE, et al. Gastroenterology 2013;144:218–238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Oh SJ, et al. Am J Gastroenterol 2020;115:895–905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Carrington EV, et al. Neurogastroenterol Motil 2020;32:e13679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ortengren AR, et al. Neurogastroenterol Motil 2021. Available at: https://onlinelibrary.wiley.com/doi/10.1111/nmo.14137. Accessed May 21, 2021. [DOI] [PMC free article] [PubMed]
- 7.Shah ED, et al. Am J Gastroenterol 2018;113:1613–1620. [DOI] [PubMed] [Google Scholar]
- 8.Servoss J, et al. J Clin Trans Sci 2017;1:271–277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Chiarioni G, et al. Gastroenterology 2005;129:86–97. [DOI] [PubMed] [Google Scholar]
- 10.Rao SSC, et al. Clin Gastroenterol Hepatol 2007;5:331–338. [DOI] [PubMed] [Google Scholar]


