Abstract
Gastric cancer is the fourth cause of cancer death globally, and gastric adenocarcinoma is its most common type. Efforts for the treatment of gastric cancer have increased its median survival rate by only seven months. Due to the relatively low response of gastric cancer to surgery and adjuvant therapy, as well as the complex role of risk factors in its incidences, such as protein-pomp inhibitors (PPIs) and viral and bacterial infections, we aimed to study the pathological pathways involved in gastric cancer development and investigate possible medications by systems biology and bioinformatics tools. In this study, the protein–protein interaction network was analyzed based on microarray data, and possible effective compounds were discovered. Non-coding RNA versus coding RNA interaction network and gene-disease network were also reconstructed to better understand the underlying mechanisms. It was found that compounds such as amiloride, imatinib, omeprazole, troglitazone, pantoprazole, and fostamatinib might be effective in gastric cancer treatment. In a gene-disease network, it was indicated that diseases such as liver carcinoma, breast carcinoma, liver fibrosis, prostate cancer, ovarian carcinoma, and lung cancer were correlated with gastric adenocarcinoma through specific genes, including hgf, mt2a, mmp2, fbn1, col1a1, and col1a2. It was shown that signaling pathways such as cell cycle, cell division, and extracellular matrix organization were overexpressed, while digestion and ion transport pathways were underexpressed. Based on a multilevel systems biology analysis, hub genes in gastric adenocarcinoma showed participation in the pathways such as focal adhesion, platelet activation, gastric acid secretion, HPV infection, and cell cycle. PPIs are hypothesized to have a therapeutic effect on patients with gastric cancer. Fostamatinib seems a potential therapeutic drug in gastric cancer due to its inhibitory effect on two survival genes. However, these findings should be confirmed through experimental investigations.
Subject terms: Computational biology and bioinformatics, Gastroenterology
Introduction
Gastric cancer was responsible for nearly one million new cancer cases in 2020, ranking the fifth among all cancers in regard to incidence. It was the fourth cause of cancer death, with approximately 769,000 deaths in 20201. Gastric adenocarcinoma, the most common type of gastric cancer, originates from stomach mucosal epithelium and accounts for nearly 90% of all gastric cancer cases2. Gastric cancer incidence varies in different parts of the world. The highest incidence rate of gastric cancer is found in Central and East Asia as well as Latin America, with the first rank belonging to South Korea with an average incidence rate of 60 per 100,000 for men and 25 per 100,000 for women. North and East Africa have the lowest incidence rate3.
There are different risk factors for gastric cancer, including Helicobacter pylori, cigarette smoking, alcohol, dietary salt, food preservation, and genetic syndromes4, among them H. pylori is the most salient one. According to recent studies, the risk of gastric cancer decreased in patients undergoing H. pylori eradication therapy (pooled incidence rate ratio of 0.53, 95% CI 0.44–0.64)5. Beyond that, genetic factors have a profound impact on gastric cancer; it seems that E-cadherin (cdh1) mutation leads to a specific type of gastric cancer called hereditary diffuse gastric cancer6. Some viruses such as Epstein-Barr virus (EBV) and human papilloma virus (HPV) may also induce gastric cancer. EBV has been found in nearly 9% of patients with gastric cancer7, but HPV prevalence is widely different. While the HPV DNA detection in the tumor cells of gastric cancers was not confirmed in Central Europe8, in two studies performed in China, the HPV DNA was detected in 29% and 47.5% of cases9,10. Gastric cancer survival rate also varies in different parts of the world; in some countries such as the UK, New Zealand, Australia, Canada, Denmark, and Norway, the survival rate is lower than 40%, while it is higher in the high-incidence countries including South Korea and Japan. The probable reason is that the two latter countries have an appropriate plan for screening patients by using radiography and endoscopy, which may cause the survival rate to increase up to 70%11.
A multidisciplinary approach is essential for the treatment of gastric cancer, which should be decided by a team including at least one surgeon, a pathologist, a gastroenterologist, as well as medical and radiation oncologists12. In case of surgical intervention, which is the gold standard treatment for gastric cancer13, complete surgical resection with suitable lymphadenectomy and endoscopic mucosal resection (EMR) can be appropriate to treat early gastric cancer14. Furthermore, adjuvant and neoadjuvant therapy, including chemotherapy and radiotherapy, are very effective in treating gastric cancer. This treatment strategy, which is widely used in some areas of the world, such as Asia and the United States, can reduce tumor size or its invasive behaviors. It may also improve the efficacy of surgical resection as the main way of gastric cancer treatment and could even reduce the side effects after surgery15. In this regard, pre-operative chemotherapy with epirubicin, cisplatin, and infused fluorouracil (ECF) regimen could significantly decrease tumor size and increase the patient overall survival rate16.
Although there is still doubt about the effectiveness of gastric cancer drugs, several molecules such as bemarituzumab, which targets proto-oncogene c-SRC (src), tyrosine-protein kinase (ptk), and Mastinib, which targets fibroblast growth factor 2 (fgf2), may be the potential drugs under investigation in clinical trials17. Several pathways have a substantial role in gastric cancer development, such as the vascular endothelial growth factor (VEGF) pathway, phosphatidylinositol 3-kinase (PI3K)/ AKT/ mammalian target of rapamycin (mTOR) signaling pathway, hepatocyte growth factor (HGF)/ tyrosine-protein kinase Met (MET) signaling pathway, Janus kinase (JAK)/ signal transducer and activator of transcription proteins (STAT) signaling pathway, and Wnt signaling pathway18. Thus, studying these pathways could help in finding appropriate targets and discovering suitable drugs to improve patients’ survival19.
Proton-pomp inhibitor (PPI) drugs reduce acid secretion by blocking the gastric H+, K+-ATPase. They can be administered in some conditions such as gastro-esophageal reflux19. It is noted that PPIs could have dual roles in gastric cancer; some researchers believe that the long-term use of PPIs can increase the risk of gastric cancer, while others claim that they can support chemotherapy efficacy in gastric cancer treatment20. This dual role can be the subject of discussion and further study, which can be done through a systemic approach.
Systems biology is a popular way to understand the complexity of systems. It employs computational modeling to better understand the biological processes and analyze them21. There are some studies using this approach for finding biomarkers, differentially expressed genes, and important pathways in gastric cancer. For instance, Echizen et al. find out the crucial role of NOX1/ROS signaling pathway in gastric cancer tumorigenesis22. In another study by Vizeacoumar and colleagues, candidates for potential biomarkers were discovered including CST1, INHBA, STMN123. He et al. also found potential immune-related prognostic biomarkers of gastric cancer including INHBA, ANGPTL1, ACKR1, GHR24. These studies can show the wide perspective of this approach for a better understanding of human diseases.
In this study, systems biology approaches are used to find hub (the most important nodes in the networks) genes in gastric adenocarcinoma, study the related important pathways, and finally suggest potential drugs for treating gastric adenocarcinoma.
Methods
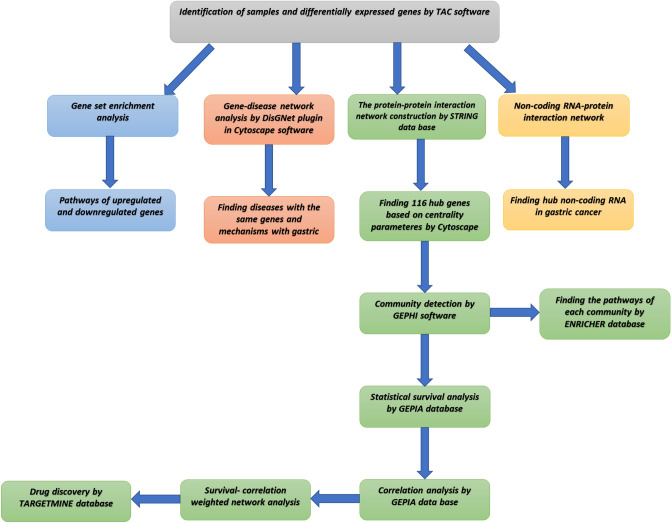
The approach used in this study is as follows in brief: gastric cancer was modeled by network analysis tools based on gene expression data, and potential compounds that can affect patients’ survival were detected. Besides, non-coding RNA interactions, viral causes, and pathways were analyzed to better understand the pathological mechanisms of gastric cancer development. A summary of the method used is shown in Fig. 1.
Figure 1.
Method diagram showing the summary of applied steps in this study.
Identification of samples and differentially expressed genes in gastric cancer
The gene expression omnibus (GEO) database (https://www.ncbi.nlm.nih.gov/geo) was used to download a microarray dataset related to gastric cancer. This database provides a great number of microarrays, RNA seq, and other data freely, so it can be a very useful database for working in the genetics and bioinformatics field. In the advanced search section, all microarray profiles with “gastric cancer” and “u133” keywords in their title or abstract were searched. Forty-five studies about Homo sapiens were found. Among them, GSE79973 consists of 10 pairs of gastric cancer tissue and adjacent non-tumor samples with HG-U133_Plus_2 platforms (a single array representing 14,500 well-characterized human genes, which can be used to explore human biology and disease processes), and was selected for further analyses25. The samples were analyzed by using transcriptome analysis console (TAC) software with the help of affymetrics U133-plus2 library to identify differentially expressed genes (DEGS). RMA algorithm was applied for data normalization. Then, PCA was used to reduce the dimensions of the dataset, so it could be evaluated if normal versus tumor was separated well. Comparison analysis (t-test) was also done on the dataset to identify differentially expressed genes with a significant p-value (p-value ≤ 0.05). Finally, gene set enrichment analysis helped to discover the possible related pathways associated with each group of genes. Two outlier samples were excluded. After that in PCA, normal and tumor samples were well separated. Finally, for further analysis, DEGS were detected based on their fold changes (FC ≥ 2.3 or FC ≤ -2.3) at a significance level of (p-value ≤ 0.05) (Table S1).
Gene set enrichment analysis
DEGs were imported in STRING v11.0 (https://string-db.org/)26 and ranked based on their fold change (proteins with value/rank section). The STRING database can help to find the relation between different proteins in different species, including homo sapiens. Enrichment analysis was done using the gene ontology (GO) database (http://geneontology.org/), which has a good potential for finding different pathways wherein a group of genes has a role. Pathways were filtered based on the false discovery rate (FDR ≤ 0.05).
The protein–protein interaction network construction
The STRING v11.0 database was employed to construct the protein–protein interaction network of DEGs, and Cytoscape v3.7.2 software was used for visualization and network parameter calculation27. Centrality parameters including degree, betweenness, and closeness were calculated for the identification of hub genes in the network using Gephi. Gephi is a powerful software for the visualization and analysis of networks28. It was employed for the identification of smaller communities that a group of hub genes form, which are called modules, among 116 selected hub genes. Gephi uses Louvain method for modularity analysis, which is a popular method in community identification29. As the last step, two databases of KEGG and gene ontology (GO) at https://maayanlab.cloud/Enrichr/ were used to show the related biological pathways of each module30–32. These two databases are good online tools for the detection of related pathways to a group of genes. The upregulated and downregulated hub genes were imported separately to ENRICHR to find the upregulated and downregulated pathways. ENRICHR is a professional online database, which can link a group of genes to other databases, showing the related pathways, diseases, drugs, and other data33.
Non-coding RNA–protein interaction network construction
In this study, non-coding RNA network construction was considered to get a better view on the pathogenesis of gastric cancer. Non-coding RNA expression data were separated from DEGs at the significance level of (p-value ≤ 0.05, FC ≥ 2.3 or FC ≤ -2.3). The non-coding versus coding RNA network was constructed using the RNA Interactome Database (https://www.rna-society.org/rnainter/)34. A python script was also employed. The network was constructed between 116 and 37 coding hub and non-coding genes, respectively. STRING v11.0 protein–protein interaction network was also added to obtain a complete interaction network.
Gene-disease network
The 116 hub genes were exported to ENRICHR, and their correlated diseases were extracted from the DisGNet plugin to identify the best matched diseases with these hub genes, based on the p-value. The DisGNet plugin in Cytoscape was then used to find the interaction of our 116 hub genes with diseases and their involvement in several diseases35. The collected data can suggest the genes that might have role in the metastasis of gastric cancer or other related cancers based on the networks.
Statistical survival analysis
The GEPIA database (http://gepia.cancer-pku.cn/) was employed for survival analysis36. GEPIA is an online tool that can be used for survival, correlation analysis, and gene expression analysis in different types of cancer and normal tissues. We hypothesized survival analysis can discover more reliable drug targets among our hub genes, because it can show the patient outcome in association with the underexpression or overexpression of genes, so a gene with both high centrality parameters in graph and significant impact on the patient survival at the same time might have stronger and more valid evidence for tumor growth progression or inhibition.
The GEPIA uses TCGA and GTEx project data to perform overall survival or disease-free survival analysis using log-rank t-test with adjustable thresholds. It can also calculate the cox proportional hazard ratio and shows 95% confidence interval on survival plots. Log-rank < 0.05 was assumed as the significance level, and the threshold was set on 50 percent.
All the survival plots of 116 hub genes were checked, and their cox proportional hazard ratio was calculated to find the hub genes that had a significant effect on the survival rate. The survival gene expression between tumor and normal data in GEPIA were also checked, and the survival genes without significance differential expression based on GEPIA analyses were excluded.
Correlation analysis
GEPIA was also used to identify the linear correlation between the discovered survival genes and the rest of hubs. Pearson method was used, and the significance level was set on “R coefficient ≥ 0.5, p-value ≤ 0.05” to discover the linear correlation. Again, the genes without significant expression in the tumor and normal samples were excluded based on the GEPIA analysis. The pathways in which a survival gene and its correlations were involved, were found using KEGG and GO in the ENRICHR database. It allowed to identify all possible drug targets with a high effect on tumor growth in order to use them in the experiments for validation.
Survival-correlation weighted network analysis
To have a better analysis that can be attributed to all gastric cancer populations, the box plot of the GEPIA database was used. In our study, an interaction network was constructed between survivals and their correlations to understand the hypothetical mechanisms that result in poor prognosis in gastric cancer. The weight of edges in this network was the correlation coefficient retrieved from GEPIA.
Drug discovery
The Targetmine database (https://targetmine.mizuguchilab.org/) was used to find drugs and compounds that have interactions with genes/proteins37. Targetmine can search multiple genes in Drug bank and other databases at the same time and show the results. Based on the aforementioned database, the survival genes whose overexpression resulted in better patient survival rates could be targeted with cognate agonists. In this study, to discover any possible synergistic effects of drugs, the genes that had a positive linear correlation with survivals needing agonists were detected. Antagonists were selected against the genes whose underexpression either was related with better survival rates or had a positive linear correlation with this type of survival genes.
Ethical considerations
The human gene data obtained in the study was derived from a publicly available repository. All experiments were conducted in accordance with relevant guidelines and regulations in the main manuscript.
Results
Differentially expressed genes (DEGs) analysis
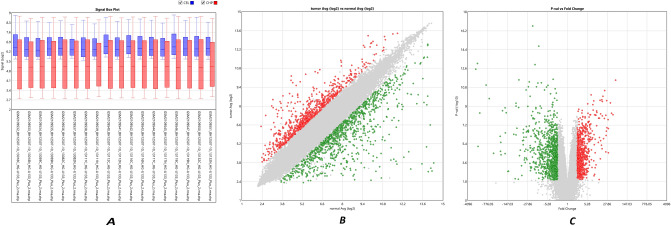
The normalized data of gastric adenocarcinoma samples versus paired normal tissue and the box plot gene expression distribution in each patient are shown in Fig. 2A. The microarray gene expression distribution before normalization is shown in blue boxes (CEL). A CEL file is a data file created by Affymetrix DNA microarray image analysis software. It contains the data extracted from "probes" on an Affymetrix GeneChip and can store thousands of data points, which may make the file size large. CEL files can be processed by software algorithms and visualized on a 2D grid as part of an overall genome experiment.
Figure 2.
Normalization and gene filtering of the microarray data. (A) Box plot gene expression distribution in each patient and data normalization of microarray samples. For each microarray sample, blue box plot (CEL) shows microarray gene expression distribution before normalization, and red box plot (CHP) represents gene expression distibution after normalization. (B) The scatter plot of DEGs (differentially expressed genes). It shows varience of the average gene expression in normal versus tumor patients. (C) Volcano plot of DEGs (differentially expressed genes) between gastric adenocarcinoma samples versus paired normal tissue. It shows signficant gene expression based on ther p-value and fold change. Note: In (B) and (C), red and green dots show upregulated and downregulated genes, respectively. Besides, gray nodes are the DEGS whose fold change is below the determined limit in the study.
The gene expression distribution after normalization is represented in red boxes (CHP). CHP is a file format generated by Transcriptome analysis console (TAC) after normalization of the expression data by RMA, MSA5 or DABG algorithms. It saves algorithm parameters and summary statistics.
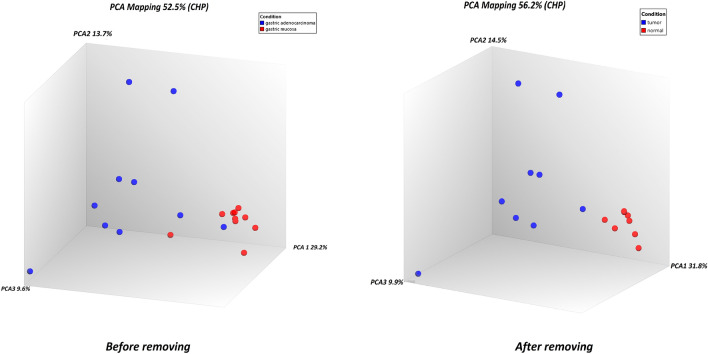
According to the PCA results, two samples, including GSM2109548_BH12507-10_13130C_HG-U133_Plus_2.CEL and GSM2109549_BH12507-10_13130N_HG-U133_Plus_2.CEL, were excluded, and others entered further analysis (Fig. 3). DEGs were identified at a significance level of p-value ≤ 0.05, FC ≤ − 2.3 or FC ≥ 2.3 (Fig. 2B, C). There were 1768 DEGs, among which 775 genes were overexpressed, and 993 genes were downregulated (Supplementary Table S1).
Figure 3.
The PCA graph of gastric adenocarcinoma samples versus paired normal tissues before and after removing two outlier samples. Normal patients and tumor patintes are well separated on principal component 1 (PCA1). Note: Red dots are normal samples, and blue dots are tumor tissue samples.
Gene set enrichment analysis
The DEGs were ranked based on their fold change and were imported to STRING v11.0 for gene set enrichment analysis based on the GO biological pathways. Filtering of pathways based on false discovery rate (FDR ≤ 0.05) showed the involvement of the overexpressed genes in the extracellular matrix organization, while the downregulated genes were found to play a role in ion transportation and digestion (Supplementary Table S2).
Protein–protein interaction network analysis
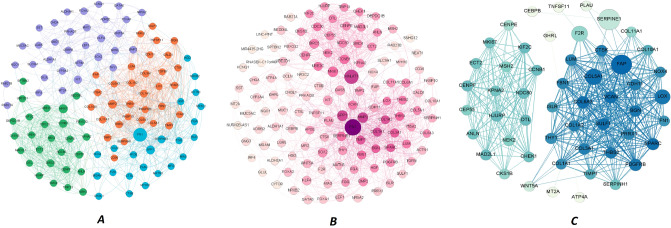
Fibronectin 1 (fn1), collagen type I alpha 1 chain (col1a1), matrix metallopeptidase 2 (mmp2), collagen type I alpha 2 chain (col1a2), secreted protein acidic and cysteine-rich (sparc), biglycan (bgn) were the first five hub genes based on eigenvector. These genes possibly can control most of the feedback loops involved in tumor cell survival, and interventions against their function may result in dramatic changes in the gastric cancer tumor cells’ growth. The first 116 hub genes had four modules, and module enrichment analysis based on the KEGG pathways showed the involvement of these modules in pathways such as cell cycle, focal adhesion, platelet activation, and gastric acid secretion. It also suggested the HPV infection pathway, which was nearly correlated with extracellular matrix organization. All these pathways were upregulated except gastric acid secretion. Targeting these genes may result in numerous side effects, so we decided to target the hub genes that specifically affected gastric cancer patients’ survival (Supplementary Table S3, Fig. 4A).
Figure 4.
(A) 116 hub genes (the most important nodes in the network) and their interaction in the network. Node size represents betweenness, and node color shows modularity. Purple nodes: module 0; Green nodes: module1; Orange nodes: module 2; Blue nodes: module 3. (B) Hub genes and their correlating microRNA networks in gastric cancer. It shows the effect of microRNA in the hub genes network. The node size demonstrates the degree, while the node color represents betweenness. (C) Weighted network analysis between the survival genes and their correlations, which shows separation between agonist and antagonist requiring targets. Node size represents degree, and node color shows betweenness. Edge thickness shows the correlation of nodes.
Survival analysis
The survival analysis performed on the overexpressed and underexpressed hub genes represented the significant role of hub genes in the survival of patients suffering from gastric cancer. The overexpression of some hub genes such as fn1, platelet-derived growth factor receptor beta (pdgfrb), col1a1, versican (vcan), plasminogen activator inhibitor-1 (serpine1), immunoglobulin superfamily containing leucine rich repeat (islr), cluster of differentiation 36 (cd36), fibrinogen gamma chain (fgg), ghrelin and obestatin prepropeptide (ghrl), and coagulation factor II thrombin receptor (f2r) showed a negative impact on the survival of patients suffering from gastric cancer. In this group, all of the genes except cd36, ghrl, and kit proto-oncogene (kit) displayed overexpression in gastric cancer, which might suggest their oncogenic effect. On the other hand, the overexpression of some hub genes such as centrosomal protein 55 (cep55), centrosome-associated protein E (cenpe), and epithelial cell transforming 2 (ect2) indicated a positive effect on the survival of gastric cancer cases. Moreover, these genes were overexpressed in the tumor tissue, which might show their role in tumor growth inhibition. Checking the hub genes in GEPIA revealed that they all had significant roles in the prognosis of gastric cancer.
Correlation analysis
It is speculated that the linear correlated expression between the hub genes might have a synergistic effect on tumor progression, especially if the correlation between non-survival and survival hub genes is of a casual type. Although most of the genes in the network with high centrality parameters were not survival-affecting genes, they had a linear correlation with the survival genes. Thus, they might have a causal effect on the expression of the survival-affecting hub genes. However, it must be investigated in an experimental approach. Our results showed that some genes namely col3a6, pdgfrb, col1a2, fn1, thbs2, sulf1, and vcan had a positive linear correlation with col1a1 gene. Genes col1a2, col6a3, thbs2, fibroblast activation protein (fap), sulf1, and bgn showed a positive linear correlation with vcan gene, while genes col1a2, wnt5a, col6a3, thbs1, thy1, vcan, sparc, and bgn indicated a positive linear correlation with pdgfrb gene. Genes mki67, anln, ccnb1, cenpf, chek1, dtl, and ndc80 displayed a positive linear correlation with cep55 gene.
It was also found that the genes with a significant role in survival were correlated with each other. Several paired genes including (f2r, islr), (fn1, col1a1), (islr, col1a1), (islr, f2r), (pdgfrb, col1a1), (pdgfrb, f2r), and (vcan, col1a1) showed a positive correlation with each other, so that using antagonists against both of them could have a synergistic effect on the gastric cancer survival. Moreover, as mentioned above, the overexpression of two other paired genes, i.e. (cep55, cenpe) and (cep55, ect2), revealed a good impact on survival, so that using their agonist may have a synergistic effect on gastric cancer survival. Interestingly, in this analysis, cep55 overexpression was found to relate to a positive prognosis in gastric cancer patients.
Non-coding RNA–protein interaction network construction
Using the complete constructed non-coding RNA protein interaction network, network centrality parameters were calculated for both hub coding and non-coding genes using Gephi. Fn1, growth arrest-specific transcript 5 (gas5), and metastasis-associated lung adenocarcinoma transcript 1 (malat1) were detected as hubs based on degree and betweenness in the network. It showed the central role of fn1 and malat1 in the gastric cancer pathogenesis (Fig. 4B).
Gene-disease network
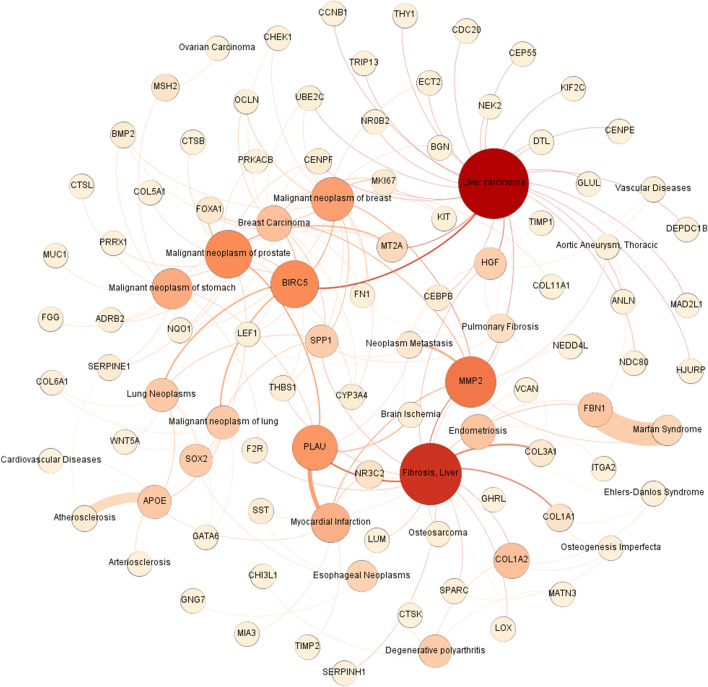
It was found that 116 hub genes were also involved in liver carcinoma, breast carcinoma, liver fibrosis, prostate cancer, ovarian carcinoma, lung cancer, brain ischemia, atherosclerosis, arteriosclerosis, aortic aneurysm, cardiovascular diseases, pulmonary fibrosis, Ehler-danlos syndrome, Marfan syndrome, osteogenesis imperfecta, esophageal neoplasm, osteosarcoma, and endometriosis. Beyond that, this network showed the hub genes with higher betweenness, such as hgf, metallothionein 2a (mt2a), mmp2, fibrillin-1 (fbn1), col1a1, and col1a2 could not only play a role in several diseases but also cause metastasis38–40. Moreover, it showed that survival hub genes, such as fn1 and serpine1, could be involved in several diseases. The genes acting in the components of the extracellular matrix (ECM) pathway and HPV infection were also identified in this network (Fig. 5).
Figure 5.
Gene-disease network. It shows not only the diseases and syndromes that have common genes and pathways with gastric adenocarcinoma, but also the common genes participating in different diseases and syndromes. Node size represents the degree (the bigger size shows the higher degree). The node color exhibits betweenness (the darker color indicates the higher betweenness).
Survival-correlation weighted network analysis
Interestingly, the agonist target network and the antagonist target network were separated, meaning that targeting one of the genes in each network did not have a significant impact on other networks. This can potentially reduce the chance of drug side effects (Fig. 4C). Based on this network, the antagonist targets mostly showed involvement in the ECM-receptor interaction and focal adhesion, while the agonist targets represented participation in the cell cycle pathway.
Drug discovery
The genes with a negative correlation with better survival rates were searched in the Targetmine database to identify their antagonists. Some of the genes included fn1, K+/H+ ATPase transporting subunit alpha (atp4a), serpine1, ctsk, pdgfrb, and kit. There were also some genes that needed to be overexpressed, probably through using an agonist. Some of these genes were cep55, cenpe, ect2, anillin (anln), and checkpoint kinase 1 (chek1). Moreover, some candidate drugs for repositioning were found. Some of them were sorafenib, fostamatinib, troglitazone, quercetin (a natural flavonol found in vegetables preventing cancer and inflammation), and pantoprazole (Table 1).
Table 1.
Drugs and compounds targeting survival genes and their correlated targets derived from Targetmine.
| DrugBank interaction protein name | Action Type | DrugBank interaction compound identifier | DrugBank interaction compound name |
|---|---|---|---|
| Tumor necrosis factor ligand superfamily member 11 | Inhibitor | Drug Bank: DB00480 | Lenalidomide |
| Urokinase-type plasminogen activator | Inhibitor | DrugBank: DB00594 | Amiloride |
| Fibrinogen alpha chain | Antagonist | DrugBank: DB00364 | Sucralfate |
| Fibrinogen alpha chain | Antagonist | KEGG DRUG: D00446 | Sucralfate |
| Fibronectin | Cleavage | DrugBank: DB08888 | Ocriplasmin |
| Plasminogen activator inhibitor 1 | Antagonist | DrugBank: DB00197 | Troglitazone |
| Platelet-derived growth factor receptor beta | Antagonist | DrugBank: DB00619 | Imatinib |
| Platelet-derived growth factor receptor beta | Inhibitor | DrugBank: DB12010 | Fostamatinib |
| Mast/stem cell growth factor receptor Kit | Antagonist | DrugBank: DB00619 | Imatinib |
| Mast/stem cell growth factor receptor Kit | Inhibitor | DrugBank: DB12010 | Fostamatinib |
| CCAAT/enhancer-binding protein beta | Inhibitor | DrugBank: DB04216 | Quercetin |
| Potassium-transporting ATPase alpha chain 1 | Inhibitor | DrugBank: DB00213 | Pantoprazole |
| Potassium-transporting ATPase alpha chain 1 | Inhibitor | DrugBank: DB00338 | Omeprazole |
| Potassium-transporting ATPase alpha chain 1 | Inhibitor | DrugBank: DB00448 | Lansoprazole |
| Potassium-transporting ATPase alpha chain 1 | Inhibitor | DrugBank: DB00736 | Esomeprazole |
| Potassium-transporting ATPase alpha chain 1 | Inhibitor | DrugBank: DB01129 | Rabeprazole |
| Potassium-transporting ATPase alpha chain 1 | Inhibitor | DrugBank: DB05351 | Dexlansoprazole |
| Proteinase-activated receptor 1 | Antagonist | DrugBank: DB09030 | Vorapaxar |
| Cathepsin K | Inhibitor | DrugBank: DB06670 | Odanacatib |
Discussion
Recent studies showed that not only genetic factors but also some non-genetic factors contribute to gastric cancer41. Some pathways, including cell cycle, WNT/β-catenin signaling, focal adhesion, and nucleotide excision repair, are among the most important pathways in gastric cancer42. Currently, the main way of treating gastric cancer is still surgery, which might be followed by adjuvant therapy. However, surgery and adjuvant chemotherapy, as the gold standard therapy in gastric cancer, can increase median survival by only seven months43. Hence, understanding tumor biological pathways can be useful in finding new drugs and therapeutic methods for gastric cancer. In this study, using different bioinformatics tools and databases, the gene expression data in gastric cancer was analyzed and compared to a normal condition to identify the genes that are upregulated or downregulated in gastric cancer. The focus was given to survival genes, and the potential compounds that can affect patients’ survival were detected by performed analyses. To further understand pathological mechanisms of gastric cancer development, non-coding RNA interactions, viral causes, and pathways were also analyzed.
Cell cycle, which regulates cell proliferation and tumor growth, is one of the key pathways in all cancers including gastric cancer. According to our analysis, several genes including cdc20, ccnb1, chek1, and mad2l1 participate in the cell cycle pathway. This pathway interacts with most pathways in gastric cancer, as discussed in the following.
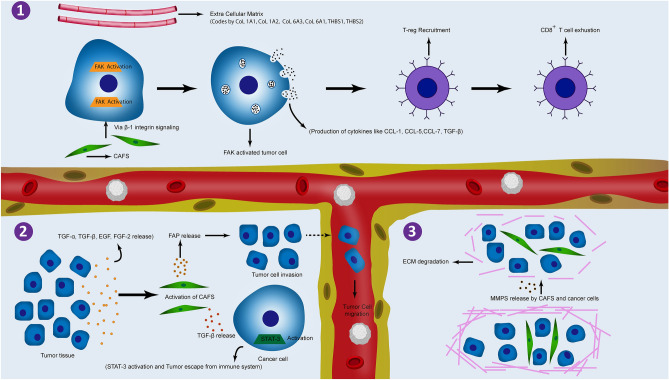
Based on our analysis, focal adhesion is a promising pathway, which was found to be upregulated in our analysis. It participates in multiple activities in tumor microenvironment (TME), immunosuppression, and metastasis. According to our analysis, col1a1, col1a2, col6a3, col6a1, thbs1, and thbs2 are the main hub genes of this pathway, which mostly produce ECM and have an initial role in the activation of focal adhesion pathway30–32. Focal adhesion kinase (FAK) is an important part of the focal adhesion pathway, whose activation by these ECM compounds leads to an increase in cancer cell migration and survival, angiogenesis, cytokine production, and abnormal ECM accumulation44. Some of these cytokines and chemokines, namely CCL1, CCL5, CCL7, CXCL10, and TGF-β2. are responsible for T-reg cell recruitment, leading to CD8+ (cytotoxic T cell) exhaustion, which results in the reduction of tumoricidal cell function and helps cancer cells to escape from the immune system45. On the other hand, FAK activation can be induced by cancer-associated fibroblasts (CAFs) via β1 integrin, which can lead to proliferation, migration, and invasion potential in gastric cancer cells46. CAFs are crucial cells in tumor formation process. They can promote tumor cell growth via the promotion of cancer stemness or prevention of cancer cell recognition by T-cells. They are activated by pro-inflammatory cytokines such as TGF-α, TGF-β, FGF-2, and EGF, which are mainly secreted from tumor cells. Additionally, CAFs can release TGF-β leading to activation of STAT3 signaling pathway in tumor cells. CAFs and tumor cells can also secrete proteases, including matrix metalloproteinase, resulting in the breakdown of the cell’s basement membrane, which has a key role in cancer metastasis47. CAFs can also activate fap transcription, which was found in our analysis as a hub gene in the antagonist network (Fig. 4C). Since CAFs are overexpressed in the gastric cancer tissue and have a crucial role in tumor cell migration and invasion48, it can be concluded that focal adhesion and CAFs can be activated via different pathways, and they play key roles in cancer in different ways (Fig. 6).
Figure 6.
Focal adhesion signaling. (1) ECM and CAFs activating focal FAK in cancer cells leading to cytokine production. These cytokines can finally inhibit CD8+ T-cell activation. (2) Tumor cells release different cytokines resulting in CAF activation. This can finally cause tumor metastasis and escaping from immune cells induced by activated CAFs. (3) Activated CAFs and tumor cells can release MMPs leading to ECM degradation, which promotes tumor metastasis. *ECM: extracellular matrix; CAFs: cancer associated fibroblasts; FAK: focal adhesion kinase.
The current study found that platelet activation is an important pathway in gastric cancer. Col1a1, fga, fgg, f2r, col3a1, and col1a2 are the important genes of this pathway, which are upregulated in gastric cancer. According to KEGG, collagen fibers (col1a1, col3a1, col1a2) can activate GPVI, which leads to the initiation of platelet activation30–32.
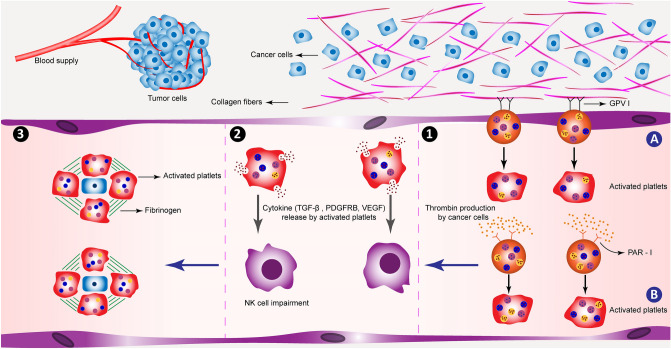
Thrombin is another molecule with multiple functions, which can not only attach to its receptor (PAR 1, coded by f2r gene) and activate platelets but also support angiogenesis by activating VEGF. Likewise, it could activate fibrinogen, which may cause cell proliferation and tumor growth in collaboration with PAR 149. Platelet activation can help cancer development via different mechanisms. It may result in the release of transforming growth factor beta (TGF-β), VEGF, and platelet-derived growth factor (PDGF), which can cause tumor cell growth, angiogenesis, and neovascularization. Platelet and fibrinogen could coat tumor cells in the vascular and lymphatic systems, thereby supporting the escape of tumor cells from natural killer (NK) cells49,50. Platelets release TGF-β leads to the impairment of NK cell function50. This may imply that these mechanisms can hide tumor cells from the immune system and help them to metastasize into other organs and tissues (Fig. 7).
Figure 7.
Platelet activation signaling pathway. (1) Platelets can be activated via two ways: (A) The GPVI receptor on platelets’ surface binds to collagen fibers in the ECM of tumor tissue; (B) Platelets can also be activated through binding to thrombin, which is produced by cancer cells via PAR-1 receptors on the platelets’ surface. Activated platelets can promote tumor metastasis via different ways. (2) They can also release different cytokines that impair NK cell’s function. These cytokines also can help tumor cell growth and vascularization. (3) They can cover up metastatic cells in collaboration with fibrinogen fibers helping metastatic cells to escape from the immune system. *NK: natural killer; GPVI: glycoprotein VI.
Another finding is that platelet activation by tumor tissue can cause a hypercoagulable state; consequently, patients have a higher risk of thrombotic conditions, namely deep vein thrombosis (DVT)51. Therefore, aspirin, as an anti-thrombotic agent, could be used in targeting platelet activation via acetylation of cyclooxygenase (COX)52. COX enzyme has two isoforms (COX1 and COX2), which are both inhibited by aspirin. COX1 inhibition leads to the anti-thrombotic effect of aspirin, while aspirin inhibition on COX2 enzyme results in anti-inflammatory effects. Its inhibitory effect on COX2 is 200 folds less potent than COX152. The results of this study suggest that aspirin could act as a potential therapeutic agent in cancer. According to recent studies, the long-term use of aspirin was associated with a reduced risk of having gastric cancer, especially non-cardiac type gastric cancer53,54. In addition, aspirin could inhibit the growth of gastric cancer cell lines via suppressing the survivin protein and induction of apoptosis55,56. Thus, although aspirin is associated with stomach mucosal damage57, it might prevent gastric cancer occurrence via inhibition of platelet activation and survivin, as well as apoptosis induction.
It was also found that upregulation of the HPV infection pathway plays an important role in gastric adenocarcinoma. HPV is one of the most important carcinogenic viruses in humans. Different HPV serotypes have been discovered, among which HPV-16 and HPV-18 are the most prevalent mucosal high-risk serotypes, associated with nearly 90% of human cervical cancers and 20% of oral cancers58–60. The E6 and E7 genes of HPV play a causative role in cancer progression; the former promotes degradation of p53 through its interaction with E6AP, an E3 ubiquitin ligase, while the latter binds to the retinoblastoma protein (prb) and disrupts its complex formation with E2F transcription factors so that p53 is suppressed and rb is promoted causing a higher rate of cell division61. According to our study, pdgfrb, which is a mitogen for the cells with a mesenchymal origin, is an important survival gene whose underexpression is associated with better outcomes in gastric cancer patients. On the other side, according to VIRHOSTNET 3.0, available at https://virhostnet.prabi.fr/, the E6 protein of HPV can directly induce pdgfrb gene expression62.
The result of our study is consistent with a meta-analysis of 30 studies, including 1,917 gastric cancer patients and 576 controls. It was revealed that HPV prevalence was 28% higher in patients with gastric cancer, which was significant compared to the control group. It was found that HPV-positive patients with gastric cancer were higher in the Chinese than non-Chinese population, which may be the reason for the higher prevalence of gastric cancer in China63. Interestingly, the microarray database used in this study was prepared from people of Hangzhou in China25. Therefore, it is hypothesized that HPV may be a risk factor for gastric adenocarcinoma due to its direct effect on survival genes, as it is associated with a higher risk of cervical, colorectal, esophageal, and oropharyngeal cancers64. Nevertheless, there are other studies against our findings, which did not detect HPV in the gastric cancer samples65. Hence, more studies in various countries are needed to verify this hypothesis.
Survival and drug analysis
The results indicated that gastric acid secretion, which is a crucial pathway to digest food in normal stomach physiology, plays a key role in gastric adenocarcinoma progression. Atp4a had a strong positive linear correlation with ghrl (among survival genes), and both of them were underexpressed in gastric cancer according to GEPIA boxplot, which resulted in gastric acid secretion downregulation. Therefore, ghrl and possibly atp4a have a protective role in gastric cancer.
In our study, PPI drugs were shown to target the atp4a gene, so they might help patients to have a better prognosis. However, some studies showed PPIs as a risk factor in developing gastric cancer66. According to these contrary results, we decided to study the mechanism of gastric acid secretion to gain a better insight into PPIs’ effect on atp4a and ghrelin interaction. Since a few studies have shown the relationship between atp4a and ghrelin, we were not certain if atp4a expression was the result of ghrelin expression. On the other hand, gastrin can stimulate atp4a, which can code for potassium hydrogen ATPase channels and increase acid secretion, as an important risk factor for gastric cancer67.
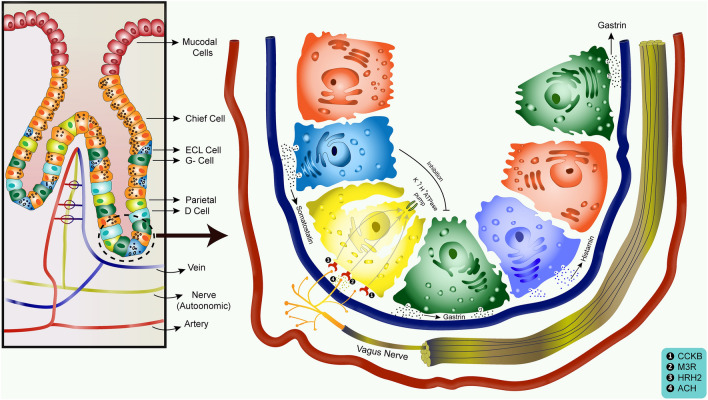
Gastrin hormone induces tumor cell growth, migration, autophagy, and survival68, and its secretion by G-cells is inhibited by somatostatin (SST) release through D cells69. Gastrin also can stimulate cholecystokinin β receptors (CCKβR), leading to the growth of gastric cancer tumor cells70. Thus, it is hypothesized that a lower level of SST leads to a higher level of gastrin, which increases gastric cancer occurrence. Downregulation of SST was also found in the DEGs of our study. Based on the aforementioned findings, SST decrease and gastrin increase have a synergistic effect on gastric cancer development, while ATP4A and GHRL decrease act oppositely so that their net effect on gastric cancer progression depends on the influences of each axis of gastric cancer development. Based on the results of our study, gastrin upregulation can also increase histamine release, and the downregulation of SST has the same effect. The result may imply that the underexpression of ghrl gene possibly affects enterochromafin cells (ECL) through decreasing histamine release; the effect of ghrl on histamine is contrary to gastrin and somatostatin71. Histamine can act through histamine receptor h2 (hrh2), which plays a stimulatory effect on acid secretion72. Based on GEPIA, hrh2, the same as ghrl, is a survival gene in gastric cancer whose underexpression is associated with better survival in patients (downregulated based on our DEGs) (Fig. 8). These data suggest the same mechanisms of gastric cell protective response against cancer development.
Figure 8.
Gastric acid secretion signaling pathway. Gastrin is mainly produced by G- cells that induce CCKβR function. It can result in gastric cancer progression and prompts acid formation in parietal cells. Acetylcholine (ACH) released from the vagus nerve ending binds to Muscarinic M3 receptors (M3R). Histamine produced by ECL cells binds to HRH2. All these together result in the activation of atp4a gene, which codes K+/H+ ATPase pumps and increases acid secretion. D-cells release somatostatin, which has an inhibitory effect on G-cells finally resulting in reducing the acid secretion. *HRH2: histamine receptor H2; ECL: enterochromafin cells.
Based on these findings, PPIs may have therapeutic effects on gastric cancer. According to Cheung and Leung, the long-term use of PPIs could increase the risk of gastric cancer by nearly twofold66, while in other studies investigating PPI association with gastric cancer cell lines, rabeprazole, a second-generation PPI drug, could reduce gastric cancer cell survival in vitro73. The probable interpretation could be that although the long-term use of PPIs can lead to higher rates of gastric cancer in the normal population, PPI drugs could reduce cancer progression in patients diagnosed with gastric adenocarcinoma.
According to our analysis, pdgfrb is mostly involved in pathways such as focal adhesion, PI3K-AKT signaling pathway, the pathways in cancer, and HPV infection. Meanwhile, kit mostly participates in PI3K-AKT signaling pathway and the pathways in cancer, and both pdgfrb and kit are survival genes whose overexpression leads to poor survival for patients. Fostamatinib, which is used for rheumatoid arthritis and immune thrombocytopenic purpura (ITP) treatment74,75, can also be used as a potential inhibitor of pdgfrb and kit76. Thus, fostamatinib may be a probable small molecule for treating gastric cancer to reduce cell proliferation, metastasis, and cancer progression. A recent clinical trial for fostamatinib performed on multiple malignancies (advanced colorectal, non-small cell lung, head and neck, thyroid, and renal cell carcinomas, as well as pheochromocytoma) showed its anti-tumor activity in several patients77. However, its effect on gastric adenocarcinoma has not been studied yet.
Fibrinolytic system can also be a potential target for cancer therapy. Plasmin is the key compound of this system, which is derived from plasminogen. Plasminogen is an inactive enzyme, which is converted to plasmin by tissue- or urokinase-type plasminogen activators (tPA or uPA). uPA is coded by plau gene. Fibrinolytic system takes part in cancer in different ways. Plasmin can activate MMPs, which have a crucial role in ECM degradation, cancer cell migration, and metastasis. The uPA system can induce cell proliferation via increasing growth factors such as VEGF, EGF, FGF2, and TGF-β. It also causes a reduction in apoptosis, leading to cancer cell immortality78,79. Hence, using uPA inhibitors could be useful in cancer treatment. Amiloride is a selective uPA inhibitor commonly used for inhibiting sodium reabsorption through sodium channels in the renal epithelial cells80. It can reduce gastric cancer peritoneal metastasis, which could be useful at the end stages of gastric cancer81. The serpine1 gene codes plasminogen activator inhibitor 1 (PAI-1). Thus, PAI-1 overexpression, as the inhibitor of uPA system, must have a positive effect on patients’ survival by the inhibition of cell proliferation, metastasis, and apoptosis induction79. However, in our analysis, we found that plau has a positive linear correlation with serpine1, so inhibiting both of them may have a synergistic effect in gastric cancer treatment. The PAI-1 level is higher in gastric cancer tissue than in normal tissue, and its overexpression leads to poor survival in gastric cancer patients via increasing tumorigenicity and inhibition of cancer cells apoptosis82–84, as was confirmed by GEPIA. Therefore, using a PAI-1antagonist as well as uPA antagonists, can be useful in gastric cancer treatment. Troglitazone, a PAI-1inhibitor, was once used as an anti-hyperglycemic agent, but it was withdrawn in the year 2000 because of its hepatotoxicity. Thus, finding a way to reduce its toxicity can candidate it as a potential drug for gastric cancer80.
MicroRNA analysis
MicroRNAs are small non-coding RNAs with important effects on gene regulation. Their regulation is being interrupted in a variety of cancers. Many different mechanisms are involved in microRNA dysregulation, such as amplification or deletion of microRNA genes, epigenetic changes and defects in the microRNA biogenesis machinery, and abnormal transcriptional control of microRNAs. They also have a considerable role in cancers by regulating cell proliferation signaling pathways, cell resistance to apoptosis, invasion, metastasis, and inactivating of tumor suppressor mechanisms, which help tumor cells to grow and build a tumor mass. They also affect the clinical features and therapeutic outcomes of tumors, which could be sensitive and specific biomarkers for diagnostic processes and therapeutic goals85. MicroRNAs also have a key role in gastric cancer. According to our studies, MALAT1 is a hub microRNA among others in gastric cancer (Fig. 4B). According to recent studies, MALAT1 function varies in different tumors, which may act as a tumor suppressor gene or have an oncogenic effect on different cancers. MALAT1 directly binds to SOX2 mRNA, which enhances its stability so that it can have a positive effect on the regulation of stemness of gastric cancer cells86. Likewise, MALAT1 can be a powerful candidate for prognostic goals in gastric cancer. Some studies reveal that MALAT1 level is higher in patients with a distant gastric cancer metastasis than those without distant metastasis in the control group87. As discussed above, the role of microRNAs in different cancers is proved88, but still more investigations are needed to reveal other dimensions of their effect on cancers. They can be very good biomarkers to solve challenges in finding specific and sensitive biomarkers for different types of cancers.
Gene disease network analysis
Gastric cancer is a complicated disease, and different pathways take part in its pathogenesis. Gastric cancer can lead to secondary metastatic cancers in other tissues. According to our analysis, different diseases and syndromes are associated with gastric cancer, such as liver carcinoma, malignant neoplasia of the prostate, atherosclerosis, liver fibrosis, myocardial infarction (MI), and lung carcinoma. Among these diseases, liver carcinoma, gastric cancer, and lung carcinoma are correlated via the baculoviral inhibitor of apoptosis repeat-containing 5 (birc5) gene. Birc5 is a member of the inhibitor of apoptosis (IAP) gene family, which codes proteins that can prevent cell apoptosis89. According to GEPIA, birc5 is overexpressed in these three cancers36, so that it can lead to the activation of the anti-apoptotic pathway, which is a crucial pathway in cancer progression. Gastric cancer and atherosclerosis are connected to each other via the serpine1 gene. On the other hand, gastric cancer and MI are similar in plau gene. As discussed above, these two genes participate in the complement coagulation pathway. In fact, the complement coagulation pathway is an interplay between the complement system as a part of innate immunity and the coagulation pathway. Inflammation is one of the key factors that can activate the complement coagulation pathway90. In both MI and atherosclerosis, inflammation is an important part of the pathogenesis, so that this pathway can interconnect MI and atherosclerosis with gastric cancer. The malignant neoplasias of prostate and gastric cancers also share many genes, one of the most important of which is mt2a. The mt2a gene is a member of the metallothionein family of genes, which code proteins that have a substantial role in the hemostatic control of metals in cells and detoxification of heavy metals influences apoptotic and autophagy pathways89. According to GEPIA, mt2a is underexpressed in both of these cancers36, which leads to less detoxification of heavy metals and increased risk of these cancers in the same way.
These data show an interesting correlation between gastric cancer and other diseases, which can help in predicting other diseases induced by gastric cancer tumor or other diseases with a predisposing role in gastric cancer.
Limitations and perspectives for future studies
1- Although the microarray dataset was paired data, and its PCA graph was well separated, it was not mentioned which part of the stomach was used for collecting the tumor samples.
2- For a better analysis of each type of cancer, it is better to have samples that are collected from the same disease stage. However, it was not mentioned in the source of microarray data.
3- In this study, all the linear relationships that existed between gene expression were used. Further inclusion of nonlinear relationships in future studies might generate more accurate data on the relationship between proteins, and probably more drugs could be suggested.
4- Using RNA-Seq technology to measure gene expression might lead to more reliable results. However, due to the unavailability of paired RNA-Seq data in this study, paired microarray data was used, which matched better and could produce more valid results. Besides, to increase accuracy in this study, key genes with significant differential expression in both microarray dataset and TCGA data (analyzed by GEPIA) were employed for drug target discovery.
Conclusion
Totally based on a multilevel systems biology analysis, the hub genes in gastric adenocarcinoma showed participation in the pathways such as focal adhesion, platelet activation, gastric acid secretion, HPV infection, and cell cycle. In the survival and drug analysis, fostamatinib and troglitazone were found as potential drugs targeting survival and hub genes in gastric adenocarcinoma. The fibrinolytic system and gastric acid secretion are two important pathways for drug analysis. PPIs are hypothesized to have a therapeutic effect on patients with gastric cancer, but their long-term administration can induce cancer in the normal population. Although microRNA analysis showed the potential role of MALAT1 in gastric cancer pathogenesis, there are presently few studies available on this subject. More future studies may help in finding novel therapies or new biomarkers.
Moreover, gene-disease network showed many mechanisms and genes being shared in gastric cancer and other diseases. Therefore, a drug or therapeutic approach might be useful for more than one of these diseases due to their similar pathways. Through these analyses, a new window is opened to achieve a better understanding of gastric cancer as one of the most complicated tumors. Further research in this field can deepen our knowledge and drive advancement in developing novel therapeutic approaches for gastric cancer as well as some other related diseases.
Supplementary Information
Author contributions
S.R.S., M.K., and M.J.T. designed the research, provided technical support, and supervised the analyses. S.R.S. and M.K. performed the experiments. S.R.S. and M.M. collected the data. S.R.S. and M.K. analyzed the data. S.R.S., M.J.T., M.K., and M.M. drafted the manuscript. M.N. supervised the project, edited, and revised the manuscript. All authors contributed to the article and approved the submitted version.
Data availability
The dataset used in this study was obtained from a publicly available repository, gene expression omnibus (GEO) at https://www.ncbi.nlm.nih.gov/geo/. All primary expression files are deposited in the CEL files.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
These authors contributed equally: Seyed Reza Salarikia and Mohammad Kashkooli.
Supplementary Information
The online version contains supplementary material available at 10.1038/s41598-022-13052-0.
References
- 1.Sung H, et al. Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA A Cancer J. Clin. 2021;71:209–249. doi: 10.3322/caac.21660. [DOI] [PubMed] [Google Scholar]
- 2.Kumar V, Abbas AK, Aster JC. Robbins Basic Pathology E-book. Elsevier; 2017. [Google Scholar]
- 3.Rawla P, Barsouk A. Epidemiology of gastric cancer: Global trends, risk factors and prevention. Przeglad Gastroenterol. 2019;14:26. doi: 10.5114/pg.2018.80001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Thrumurthy SG, Chaudry MA, Hochhauser D, Mughal M. The diagnosis and management of gastric cancer. BMJ. 2013;347:1125. doi: 10.1136/bmj.f6367. [DOI] [PubMed] [Google Scholar]
- 5.Lee Y-C, et al. Association between Helicobacter pylori eradication and gastric cancer incidence: A systematic review and meta-analysis. Gastroenterology. 2016;150:1113–1124. doi: 10.1053/j.gastro.2016.01.028. [DOI] [PubMed] [Google Scholar]
- 6.Guilford P, Blair V, More H, Humar B. A short guide to hereditary diffuse gastric cancer. Hered. Cancer Clin. Pract. 2007;5:1–12. doi: 10.1186/1897-4287-5-4-183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Murphy G, Pfeiffer R, Camargo MC, Rabkin CS. Meta-analysis shows that prevalence of Epstein-Barr virus-positive gastric cancer differs based on sex and anatomic location. Gastroenterology. 2009;137:824–833. doi: 10.1053/j.gastro.2009.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Snietura M, et al. Potential role of human papilloma virus in the pathogenesis of gastric cancer. World J. Gastroenterol. WJG. 2014;20:6632. doi: 10.3748/wjg.v20.i21.6632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ding G-C, et al. Human papillomavirus DNA and P16INK4A expression in concurrent esophageal and gastric cardia cancers. World J. Gastroenterol. WJG. 2010;16:5901. doi: 10.3748/wjg.v16.i46.5901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Xu W, et al. Detection of human papillomavirus type 16 E6 mRNA in carcinomas of upper digestive tract. Zhonghua Yi Xue Za Zhi. 2003;83:1910–1914. [PubMed] [Google Scholar]
- 11.Hamashima C. The burden of gastric cancer. Ann. Transl. Med. 2020;8:1102. doi: 10.21037/atm-2020-gc-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Sitarz R, Kocemba K, Maciejewski R, Polkowski W. Effective cancer treatment by multidisciplinary teams. Polish J. Surg. 2012;84:371–376. doi: 10.2478/v10035-012-0063-7. [DOI] [PubMed] [Google Scholar]
- 13.Choi YY, Noh SH, Cheong J-H. Evolution of gastric cancer treatment: From the golden age of surgery to an Era of precision medicine. Yonsei Med. J. 2015;56:1177–1185. doi: 10.3349/ymj.2015.56.5.1177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Sitarz R, et al. Gastric cancer: Epidemiology, prevention, classification, and treatment. Cancer Manag. Res. 2018;10:239. doi: 10.2147/CMAR.S149619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Newton AD, Datta J, Loaiza-Bonilla A, Karakousis GC, Roses RE. Neoadjuvant therapy for gastric cancer: Current evidence and future directions. J. Gastrointest. Oncol. 2015;6:534–543. doi: 10.3978/j.issn.2078-6891.2015.047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Cunningham D, et al. Perioperative chemotherapy versus surgery alone for resectable gastroesophageal cancer. N. Engl. J. Med. 2006;355:11–20. doi: 10.1056/NEJMoa055531. [DOI] [PubMed] [Google Scholar]
- 17.Wang Y, et al. Therapeutic target database 2020: Enriched resource for facilitating research and early development of targeted therapeutics. Nucleic Acids Res. 2020;48:D1031–D1041. doi: 10.1093/nar/gkz981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Riquelme I, et al. Molecular classification of gastric cancer: Towards a pathway-driven targeted therapy. Oncotarget. 2015;6:24750. doi: 10.18632/oncotarget.4990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Shin JM, Sachs G. Pharmacology of proton pump inhibitors. Curr. Gastroenterol. Rep. 2008;10:528–534. doi: 10.1007/s11894-008-0098-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Joo MK, Park J-J, Chun HJ. Proton pump inhibitor: The dual role in gastric cancer. World J. Gastroenterol. 2019;25:2058. doi: 10.3748/wjg.v25.i17.2058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Breitling R. What is systems biology? Front. Physiol. 2010;1:159. doi: 10.3389/fphys.2010.00009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Echizen K, et al. NF-κB-induced NOX1 activation promotes gastric tumorigenesis through the expansion of SOX2-positive epithelial cells. Oncogene. 2019;38:4250–4263. doi: 10.1038/s41388-019-0702-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Vizeacoumar FS, et al. Mining the plasma-proteome associated genes in patients with gastro-esophageal cancers for biomarker discovery. Sci. Rep. 2021;11:1–13. doi: 10.1038/s41598-021-87037-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.He Y, Song H, Jiang Y, Ren W. Identification of immune-related prognostic markers in gastric cancer. J. Healthcare Eng. 2022;2022:1125. doi: 10.1155/2022/7897274. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 25.He J, et al. Downregulation of ALDOB is associated with poor prognosis of patients with gastric cancer. Onco. Targets. Ther. 2016;9:6099. doi: 10.2147/OTT.S110203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Szklarczyk D, Gable A, Lyon D. STRING v11: Protein-protein association networks with increased All rights reserved. No reuse allowed without permission. Coverage, supporting functional discovery in genome-wide experimental datasets. Nucleic Acids Res. 2019;47:D607–D613. doi: 10.1093/nar/gky1131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Shannon P, et al. Cytoscape: A software environment for integrated models of biomolecular interaction networks. Genome Res. 2003;13:2498–2504. doi: 10.1101/gr.1239303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Bastian, M., Heymann, S. & Jacomy, M. in Proceedings of the International AAAI Conference on Web and Social Media. 361–362.
- 29.Blondel VD, Guillaume J-L, Lambiotte R, Lefebvre E. Fast unfolding of communities in large networks. J. Stat. Mech. Theory Exp. 2008;2008:P10008. doi: 10.1088/1742-5468/2008/10/P10008. [DOI] [Google Scholar]
- 30.Kanehisa M, Goto S. KEGG: Kyoto encyclopedia of genes and genomes. Nucleic Acids Res. 2000;28:27–30. doi: 10.1093/nar/28.1.27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kanehisa M. Toward understanding the origin and evolution of cellular organisms. Protein Sci. Publ. Protein Soc. 2019;28:1947–1951. doi: 10.1002/pro.3715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kanehisa M, Furumichi M, Sato Y, Ishiguro-Watanabe M, Tanabe M. KEGG: Integrating viruses and cellular organisms. Nucleic Acids Res. 2021;49:D545–d551. doi: 10.1093/nar/gkaa970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Chen EY, et al. Enrichr: Interactive and collaborative HTML5 gene list enrichment analysis tool. BMC Bioinf. 2013;14:128. doi: 10.1186/1471-2105-14-128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Lin Y, et al. RNAInter in 2020: RNA interactome repository with increased coverage and annotation. Nucleic Acids Res. 2020;48:D189–D197. doi: 10.1093/nar/gkz804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Bauer-Mehren A, et al. Gene-disease network analysis reveals functional modules in mendelian, complex and environmental diseases. PLoS ONE. 2011;6:e20284. doi: 10.1371/journal.pone.0020284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Tang Z, et al. GEPIA: A web server for cancer and normal gene expression profiling and interactive analyses. Nucleic Acids Res. 2017;45:W98–W102. doi: 10.1093/nar/gkx247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Chen Y-A, et al. The TargetMine data warehouse: Enhancement and updates. Front. Genet. 2019;934:1226. doi: 10.3389/fgene.2019.00934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Li J, Ding Y, Li A. Identification of COL1A1 and COL1A2 as candidate prognostic factors in gastric cancer. World J. Surg. Oncol. 2016;14:1–5. doi: 10.1186/s12957-016-1056-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Koh SA, Lee KH. Function of hepatocyte growth factor in gastric cancer proliferation and invasion. Yeungnam Univ. J. Med. 2020;37:73. doi: 10.12701/yujm.2019.00437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Bibak F, Ahmadi S, Khateri Z, Ahmadi A, Yari K. The role of matrix metalloproteinase-2 expression in gastric cancer susceptibility: A Systematic Review. Int. J. Cancer Manag. 2019;12:94185. doi: 10.5812/ijcm.94185. [DOI] [Google Scholar]
- 41.Anvar MS, Minuchehr Z, Shahlaei M, Kheitan S. Gastric cancer biomarkers; A systems biology approach. Biochem. Biophys. Rep. 2018;13:141–146. doi: 10.1016/j.bbrep.2018.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Molaei F, Forghanifard MM, Fahim Y, Abbaszadegan MR. Molecular signaling in tumorigenesis of gastric cancer, Iran. Biomed. J. 2018;22:217. doi: 10.22034/ibj.22.4.217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Jameson JL, et al. Harrison's Principles of Internal Medicine. McGraw-hill education; 2018. [Google Scholar]
- 44.Murphy JM, Rodriguez YA, Jeong K, Ahn E-YE, Lim S-TS. Targeting focal adhesion kinase in cancer cells and the tumor microenvironment. Exp. Mol. Med. 2020;52:877–886. doi: 10.1038/s12276-020-0447-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Borst J, Ahrends T, Bąbała N, Melief CJ, Kastenmüller W. CD4+ T cell help in cancer immunology and immunotherapy. Nat. Rev. Immunol. 2018;18:635–647. doi: 10.1038/s41577-018-0044-0. [DOI] [PubMed] [Google Scholar]
- 46.Wang X, et al. Cancer-associated fibroblast-derived Lumican promotes gastric cancer progression via the integrin β1-FAK signaling pathway. Int. J. Cancer. 2017;141:998–1010. doi: 10.1002/ijc.30801. [DOI] [PubMed] [Google Scholar]
- 47.Winkler J, Abisoye-Ogunniyan A, Metcalf KJ, Werb Z. Concepts of extracellular matrix remodelling in tumour progression and metastasis. Nat. Commun. 2020;11:1–19. doi: 10.1038/s41467-020-18794-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Wang R-F, et al. Effects of the fibroblast activation protein on the invasion and migration of gastric cancer. Exp. Mol. Pathol. 2013;95:350–356. doi: 10.1016/j.yexmp.2013.10.008. [DOI] [PubMed] [Google Scholar]
- 49.Schlesinger M. Role of platelets and platelet receptors in cancer metastasis. J. Hematol. Oncol. 2018;11:1–15. doi: 10.1186/s13045-018-0669-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Palacios-Acedo AL, et al. Platelets, thrombo-inflammation, and cancer: Collaborating with the enemy. Front. Immunol. 2019;2:1805. doi: 10.3389/fimmu.2019.01805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Lee EC, Cameron SJ. Cancer and thrombotic risk: The platelet paradigm. Front. Cardiovasc. Med. 2017;4:67. doi: 10.3389/fcvm.2017.00067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Schrör, K. in Seminars in Thrombosis and Hemostasis. 349–356 (Copyright© 1997 by Thieme Medical Publishers, Inc.).
- 53.Kim Y-I, et al. Long-term low-dose aspirin use reduces gastric cancer incidence: A nationwide cohort study. Cancer Res. Treat. Off. J. Korean Cancer Assoc. 2016;48:798. doi: 10.4143/crt.2015.117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Niikura R, et al. Effect of aspirin use on gastric cancer incidence and survival: A systematic review and meta-analysis. JGH Open. 2020;4:117–125. doi: 10.1002/jgh3.12226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Yang L, et al. Aspirin suppresses growth of human gastric carcinoma cell by inhibiting survivin expression. J. Biomed. Res. 2011;25:246–253. doi: 10.1016/S1674-8301(11)60033-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Wong B, Zhu G, Lam S. Aspirin induced apoptosis in gastric cancer cells. Biomed. Pharmacother. 1999;53:315–318. doi: 10.1016/S0753-3322(00)88503-0. [DOI] [PubMed] [Google Scholar]
- 57.Cryer B, Mahaffey KW. Gastrointestinal ulcers, role of aspirin, and clinical outcomes: Pathobiology, diagnosis, and treatment. J. Multidiscip. Healthc. 2014;7:137. doi: 10.2147/JMDH.S54324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Münger K, et al. Mechanisms of human papillomavirus-induced oncogenesis. J. Virol. 2004;78:11451–11460. doi: 10.1128/JVI.78.21.11451-11460.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Negahdaripour M, et al. A novel HPV prophylactic peptide vaccine, designed by immunoinformatics and structural vaccinology approaches. Infect. Genet. Evol. 2017;54:402–416. doi: 10.1016/j.meegid.2017.08.002. [DOI] [PubMed] [Google Scholar]
- 60.Negahdaripour M, et al. Production and preliminary in vivo evaluations of a novel in silico-designed L2-based potential HPV vaccine. Curr. Pharm. Biotechnol. 2020;21:316–324. doi: 10.2174/1389201020666191114104850. [DOI] [PubMed] [Google Scholar]
- 61.Narisawa-Saito M, Kiyono T. Basic mechanisms of high-risk human papillomavirus-induced carcinogenesis: Roles of E6 and E7 proteins. Cancer Sci. 2007;98:1505–1511. doi: 10.1111/j.1349-7006.2007.00546.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Guirimand T, Delmotte S, Navratil V. VirHostNet 2.0: Surfing on the web of virus/host molecular interactions data. Nucleic Acids Res. 2015;43:D583–D587. doi: 10.1093/nar/gku1121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Zeng Z-M, et al. Human papillomavirus as a potential risk factor for gastric cancer: A meta-analysis of 1,917 cases. Oncol. Targets. Ther. 2016;9:7105. doi: 10.2147/OTT.S115053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Bucchi D, Stracci F, Buonora N, Masanotti G. Human papillomavirus and gastrointestinal cancer: A review. World J. Gastroenterol. 2016;22:7415. doi: 10.3748/wjg.v22.i33.7415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Kirkegård J, et al. Conization as a marker of persistent cervical human papillomavirus (HPV) infection and risk of gastrointestinal cancer: A Danish 34-year nationwide cohort study. Cancer Causes Control. 2014;25:1677–1682. doi: 10.1007/s10552-014-0473-4. [DOI] [PubMed] [Google Scholar]
- 66.Cheung KS, Leung WK. Long-term use of proton-pump inhibitors and risk of gastric cancer: A review of the current evidence. Ther. Adv. Gastroenterol. 2019;12:1756284819834511. doi: 10.1177/1756284819834511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Prinz C, et al. Acid secretion and the H, K ATPase of stomach. Yale J. Biol. Med. 1992;65:577. [PMC free article] [PubMed] [Google Scholar]
- 68.Rao SV, et al. Gastrin activates autophagy and increases migration and survival of gastric adenocarcinoma cells. BMC Cancer. 2017;17:1–13. doi: 10.1186/s12885-017-3055-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Li Y-Y. Mechanisms for regulation of gastrin and somatostatin release from isolated rat stomach during gastric distention. World J. Gastroenterol. 2003;9:129. doi: 10.3748/wjg.v9.i1.129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Smith, J., Nadella, S. & Osborne, N.
- 71.Fauci AS. Harrison's Principles of Internal Medicine. McGraw-Hill Education; 2015. [Google Scholar]
- 72.Ca GC. Histamine and gastric acid secretion. Acta Gastroenterol. Latinoam. 1980;10:77–84. [PubMed] [Google Scholar]
- 73.Gu M, et al. Rabeprazole exhibits antiproliferative effects on human gastric cancer cell lines. Oncol. Lett. 2014;8:1739–1744. doi: 10.3892/ol.2014.2354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Weinblatt ME, et al. Effects of fostamatinib, an oral spleen tyrosine kinase inhibitor, in rheumatoid arthritis patients with an inadequate response to methotrexate: Results from a phase III, multicenter, randomized, double-blind, placebo-controlled, parallel-group study. Arthritis Rheumatol. 2014;66:3255–3264. doi: 10.1002/art.38851. [DOI] [PubMed] [Google Scholar]
- 75.Bussel J, et al. Fostamatinib for the treatment of adult persistent and chronic immune thrombocytopenia: Results of two phase 3, randomized, placebo-controlled trials. Am. J. Hematol. 2018;93:921–930. doi: 10.1002/ajh.25125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Rolf MG, et al. In vitro pharmacological profiling of R406 identifies molecular targets underlying the clinical effects of fostamatinib. Pharmacol. Res. Perspect. 2015;3:e00175. doi: 10.1002/prp2.175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Park SR, et al. A multi-histology trial of fostamatinib in patients with advanced colorectal, non-small cell lung, head and neck, thyroid, and renal cell carcinomas, and pheochromocytomas. Cancer Chemother. Pharmacol. 2013;71:981–990. doi: 10.1007/s00280-013-2091-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Lin H, et al. Therapeutics targeting the fibrinolytic system. Exp. Mol. Med. 2020;52:367–379. doi: 10.1038/s12276-020-0397-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Mahmood N, Mihalcioiu C, Rabbani SA. Multifaceted role of the urokinase-type plasminogen activator (uPA) and its receptor (uPAR): Diagnostic, prognostic, and therapeutic applications. Front. Oncol. 2018;8:24. doi: 10.3389/fonc.2018.00024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Wishart DS, et al. DrugBank 5.0: A major update to the DrugBank database for 2018. Nucleic Acids Res. 2018;46:D1074–D1082. doi: 10.1093/nar/gkx1037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Ding Y, et al. u-PA inhibitor amiloride suppresses peritoneal metastasis in gastric cancer. World J. Surg. Oncol. 2012;10:1–7. doi: 10.1186/1477-7819-10-270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Brungs D, et al. The urokinase plasminogen activation system in gastroesophageal cancer: A systematic review and meta-analysis. Oncotarget. 2017;8:23099. doi: 10.18632/oncotarget.15485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Plebani M, et al. Urokinase-type plasminogen activator receptor in gastric cancer: Tissue expression and prognostic role. Clin. Exp. Metas. 1997;15:418–426. doi: 10.1023/A:1018454305889. [DOI] [PubMed] [Google Scholar]
- 84.Zhu E-D, et al. miR-30b, down-regulated in gastric cancer, promotes apoptosis and suppresses tumor growth by targeting plasminogen activator inhibitor-1. PLoS ONE. 2014;9:e106049. doi: 10.1371/journal.pone.0106049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Peng Y, Croce CM. The role of MicroRNAs in human cancer. Signal Transduct. Target. Ther. 2016;1:1–9. doi: 10.1038/sigtrans.2015.4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Xiao Y, Pan J, Geng Q, Wang G. Lnc RNA MALAT 1 increases the stemness of gastric cancer cells via enhancing SOX 2 mRNA stability. FEBS Open Biol. 2019;9:1212–1222. doi: 10.1002/2211-5463.12649. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 87.Xia H, et al. The lncRNA MALAT1 is a novel biomarker for gastric cancer metastasis. Oncotarget. 2016;7:56209. doi: 10.18632/oncotarget.10941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Negahdaripour M, et al. Small extracellular vesicles (sEVs): Discovery, functions, applications, detection methods and various engineered forms. Expert Opin. Biol. Ther. 2021;21:371–394. doi: 10.1080/14712598.2021.1825677. [DOI] [PubMed] [Google Scholar]
- 89.Tatusova T, et al. NCBI prokaryotic genome annotation pipeline. Nucleic Acids Res. 2016;44:6614–6624. doi: 10.1093/nar/gkw569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Oikonomopoulou, K., Ricklin, D., Ward, P. A. & Lambris, J. D. in Seminars in Immunopathology. 151–165 (Springer). [DOI] [PMC free article] [PubMed]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The dataset used in this study was obtained from a publicly available repository, gene expression omnibus (GEO) at https://www.ncbi.nlm.nih.gov/geo/. All primary expression files are deposited in the CEL files.