Abstract
Magnetotactic bacteria (MTB) are a group of phylogenetically diverse and morphologically varied microorganisms with a magnetoresponsive capability called magnetotaxis or microbial magnetoreception. MTB are a distinctive constituent of the microbiome of aquatic ecosystems because they use Earth’s magnetic field to align themselves in a north or south facing direction and efficiently navigate to their favored microenvironments. They have been identified worldwide from diverse aquatic and waterlogged microbiomes, including freshwater, saline, brackish and marine ecosystems, and some extreme environments. MTB play important roles in the biogeochemical cycling of iron, sulphur, phosphorus, carbon and nitrogen in nature and have been recognized from in vitro cultures to sequester heavy metals like selenium, cadmium, and tellurium, which makes them prospective candidate organisms for aquatic pollution bioremediation. The role of MTB in environmental systems is not limited to their lifespan; after death, fossil magnetosomal magnetic nanoparticles (known as magnetofossils) are a promising proxy for recording paleoenvironmental change and geomagnetic field history. Here, we summarize the ecology, evolution, and environmental function of MTB and the paleoenvironmental implications of magnetofossils in light of recent discoveries.
Subject terms: Bacteria, Environmental microbiology
Introduction
The terms biomineralization and magnetoreception are invariably associated with magnetotactic bacteria (MTB). Magnetoreception involves the use of Earth’s magnetic field for motility and navigation and biomineralization is a capability by which organisms produce minerals1,2. MTB are so far the only known group of prokaryotes with the ability to perform both biomineralization and magnetoreception3. MTB have been proposed to represent some of the most ancient organisms capable of biomineralization4. This group of bacteria was discovered in 1963 by Salvatore Bellini5, and was rediscovered independently in 1974 by Richard Blakemore6. The latter study also identified magnetosomal magnetic particles for the first time, which were referred to as “iron-rich particles”. MTB form magnetite (Fe3O4) and/or greigite (Fe3S4) crystals, generally in bead-like chains7 (Fig. 1). Magnetotaxis is one of their primary modes of motility7. Many organisms can use the geomagnetic field for navigation. Birds, fish, and certain migratory animals among others top this list, while desert ants, newts, spiny lobsters, snails, Bogong moths, and certain other invertebrates have been recently found to possess a magnetic sense of some sort8–11. Some mammals, too, can respond to Earth’s magnetic field. Laboratory experiments indicate that wood mice and mole rats make use of magnetic field lines while siting nests; certain cattle and deer use the field for body orientation while grazing; and dogs appear to orient toward north or south when they excrete12–14. Magnetic orientation is associated with many organisms, and understanding its origin is a subject of extensive research.
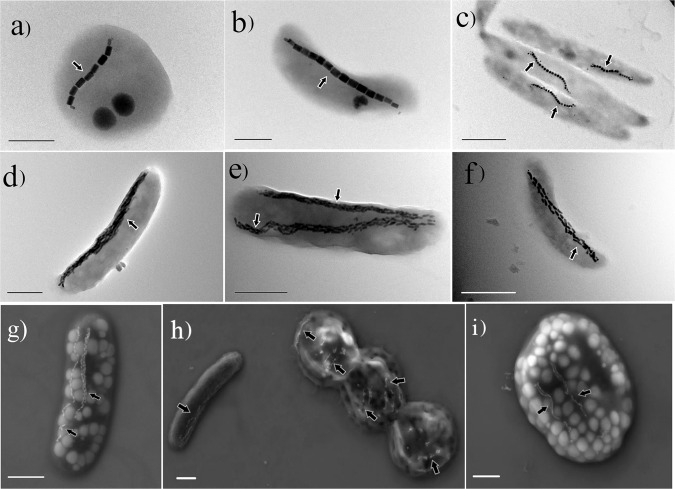
Fig. 1. Electron microscope images of MTB.
Transmission electron microscope (a–f) and scanning electron microscope (g–i) images of various MTB. Black arrows indicate magnetosome chains. Scale bars: a, b = 0.5 μm, c–i = 1 μm.
Magnetoreception represents a spectrum of capabilities of which magnetotaxis is a subset. While magnetoreception is mostly associated with higher organisms that use a magnetic sense for mobility, magnetotaxis is associated with microorganisms. It is related to previously discovered methods of taxis such as chemotaxis and aerotaxis, which are common modes of transportation and translocation by bacteria and archaea15,16. However, unlike chemotaxis or aerotaxis, which are multidirectional, magnetotaxis mostly involves upward/downward movement in search of optimal microenvironments near chemical gradients in water/sediment, aligning passively along Earth’s magnetic field17. It is thought that magnetotaxis along with chemotaxis/aerotaxis provides an additional benefit to MTB by permitting a one-dimensional search along the oxic–anoxic interface (OAI) in aquatic environments to enable MTB to find optimal oxygen concentrations to carry out necessary physiological functions18. MTB ecology, diversity, and evolution have been reviewed previously19–21; however, recent developments of omics, cultivation and magnetic measurements have expanded our understanding of MTB and magnetofossils. Multiple studies have pointed out that magnetotaxis is monophyletic in origin; that is, it originated from a single common ancestor22–24. This would make it a primordial physiological phenomenon and (probably) the earliest case of magnetoreception and systematic biomineralization on Earth4,25. Following this discovery, diverse multidisciplinary studies have sought to answer several significant questions. Are MTB widespread across the domain Bacteria? How did magnetotaxis originate and evolve? Do MTB have a significant role in biogeochemical element cycling? These questions are of multidisciplinary interest to microbiologists, geologists, physicists, and chemists. In this paper, we review progress to date in addressing these questions. We discuss the phylogenetic diversity of MTB and their potential role in the biogeochemical cycling of elements, and compare the metabolic pathways of all sixteen phylum-level MTB lineages identified so far. Moreover, we discuss expanding oxygen minimum zones (OMZs) in the oceans; diverse MTB likely live in OMZs and their environmental role in such settings has been understudied.
MTB diversity and ecology
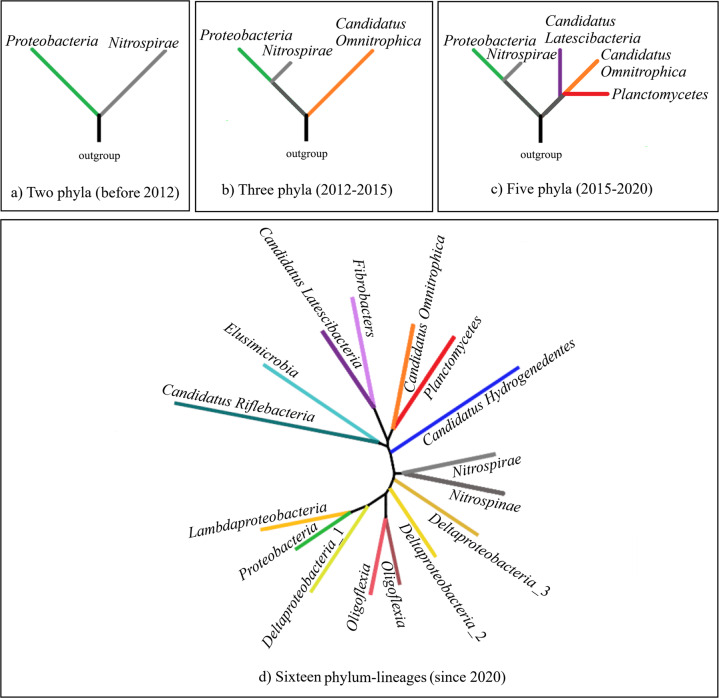
MTB occur in multiple lineages of the bacterial tree of life, although with a patchy distribution. They include cocci, vibrio, rod, spirilla, and multicellular morphotypes19,20,26,27. Until 2012, both cultured and uncultured MTB could be grouped mostly into the Proteobacteria phylum (including the Alphaproteobacteria, Gammaproteobacteria and Deltaproteobacteria classes) and a few in the Nitrospirae phylum27 (Fig. 2a). In 2012, a novel uncultivated ovoid MTB (designated SKK-01) from Lake Chiemsee, Germany, was discovered and characterized to belong to the candidate phylum Omnitrophica (also known as candidate division OP3)28 (Fig. 2b). In 2015 and 2017, two additional bacterial phyla, the candidate phylum Latescibacteria29 and the phylum Planctomycetes21, respectively, were identified to contain MTB based on the presence of magnetosome gene clusters (MGCs, a group of genes responsible for magnetosome biomineralization and magnetotaxis) in their genomes (Fig. 2c). More recently, advances in metagenomic approaches, large-scale field studies and public database mining studies have expanded the total number of MTB-containing phylum-level lineages from five to sixteen24,30,31, which greatly increases the taxonomic and genomic diversity of MTB across the domain Bacteria (Fig. 2d). These findings highlight a much higher diversity of MTB lineages in the domain Bacteria than previously anticipated. On the basis of MGC types in MTB genomes, putative Fe3O4-producing MTB have been identified in all MTB lineages so far except for the Latescibacteria phylum, while putative Fe3S4-producing MTB exist in at least five bacterial phyla31.
Fig. 2. Schematic representation of the gradual expansion of the phylogenetic tree of MTB in the Bacteria domain.
a Before 2012, only two phyla (Proteobacteria and Nitrospirae) were identified to contain MTB. b Between 2012 and 2015, an additional branch for the Candidatus Omnitrophica phylum was added28. c Subsequently, two bacterial phyla (Candidatus Latescibacteria29 and Planctomycetes21) were added to the MTB tree of life based on the presence of magnetosome gene clusters in their genomes. d The latest addition to the MTB tree has expanded its branches to a total of sixteen bacterial phylum-level lineages30,31. All taxonomic groupings are based on the NCBI taxonomy (https://www.ncbi.nlm.nih.gov/taxonomy).
MTB affiliated with the Nitrospirae phylum are biogeochemically important because many members of this group produce several hundred magnetosomal magnetite crystals per cell rather than the normal 10–50 crystals in most MTB26,32. Characteristic examples are the Candidatus Magnetobacterium bavaricum, which was discovered by Vali et al.33 and was subsequently phylogenetically defined by Spring et al.26 as magnetotactic rods that form elongated, bullet-shaped and kinked crystals arranged in a parallel chain-like fashion that appear as rope-like strands or bundles. Hanzlik et al.34,35 studied the chain architecture of Ca. Magnetobacterium bavaricum in detail. Apart from producing magnetite within magnetosomes, cells of Ca. Magnetobacterium bavaricum also harbor sulphur globules, solely comprising cyclo-octasulphur (S8), which gives them a probable role in both iron and sulphur cycling in nature36. The second example is Ca. Magnetobacterium casensis, which is smaller than Ca. Magnetobacterium bavaricum and produces around 200–500 magnetosomal Fe3O4 particles per cell37–39. Recent 16S rRNA-based and metagenomic surveys suggest that magnetotactic Nitrospirae are more diverse and more widely distributed than previously thought31,40, which emphasizes their environmental importance in various ecosystems.
Apart from single-celled bacteria, multicellular magnetic prokaryotes (MMPs) are flagella-aided motile organisms that also produce magnetic nanocrystals, as first reported by Farina et al.41. Despite their multicellular organization, MMPs have coherent magnetosome-chain polarity across their cells41. Many studies have now been carried out involving especially the spherical MMP morphotype (sMMP)42–48. A second morphotype, ellipsoidal MMP (eMMP), has been found in the Mediterranean Sea and Pacific Ocean49–57. MMPs are affiliated within the Deltaproteobacteria and MTB from this group produce Fe3O4 and/or Fe3S4 nanoparticles23,31. Studies of MMPs also provide insights into the origin and evolution of bacterial multicellularity, which is one of the most prevalent evolutionary innovations in the bacterial world58.
MTB have been identified from diverse biomes with highly variable, including extreme environments. For instance, MTB flourish in shallow hemipelagic sediments (at ~600 m depths) at 8 °C, in Santa Barbara Basin, Eastern Pacific Ocean59. Petermann and Bleil60 discovered live MTB from pelagic and hemipelagic sediments in the eastern South Atlantic Ocean. They observed assorted cocci, spirilla, vibrioids, and even rod-shaped MTB morphologies in pelagic environments on the African continental margin and on Walvis Ridge at ~3000 m and ~1000 m water depths, respectively. More recently, MTB have been observed from tropical marine environments in Singapore, which adds to equatorial MTB biodiversity61. A recent phylogenetic analysis of 16S rRNA gene sequences of microbial communities from a seamount in the Mariana volcanic arc (238–2023 m) near Challenger Deep, tropical western Pacific Ocean, revealed 16 novel MTB populations62. Abundant MTB have also been observed in seafloor sediments of the Yellow Sea with Nitrospirae being the dominant group63. In more extreme environments, Lefèvre et al.64 isolated three MTB strains of Desulfonatronum thiodismutans from hyper-alkaliphilic habitats in California, USA, that are obligate alkaliphiles (with optimal growth at pH 9.0–9.5) and sulphate reducers. Essential genes (i.e., dissimilatory sulphite reductase (dsrAB) and adenosine-5′-phosphate reductase (aprA)) were identified that verify that the novel strains are sulphate reducers. Psychrophilic MTB have been characterized from water and sediment samples from Admiralty Bay, King George Island, Antarctica, where average water temperatures during sampling ranged between 0.1 and 0.8 °C65. Nash et al.66 characterized a thermophilic MTB variety belonging to Nitrospirae from Little Hot Creek Hot Springs, California, and another Gammaproteobacteria MTB from the hyper-alkaline and hypersaline Mono Lake. Lefèvre et al.67 discovered a thermophilic MTB that they named Candidatus Thermomagnetovibrio paiutensis from mud and water samples in hot springs of the Great Boiling Springs geothermal field in Gerlach, northern Nevada. Two rod-shaped Gammaproteobacteria MTB strains, BW-2 and SS-5, from the hypersaline Salton Sea were the first isolated MTB from the Gammaproteobacteria class that biomineralize magnetite68. Further Gammaproteobacteria MTB strains FZSR-1 and FZSR-2 were identified recently from a salt evaporation pool in Fuzhou saltern, Bohai Bay, China69. Lin et al.31 also discovered living MTB cells in a relatively acidic peatland soil with draft genomes dominantly comprising magnetotactic Nitrospirae, Omnitrophica and Deltaproteobacteria.
Origin and evolution of MTB
Ancient atmospheric molecular O2 in the Archean, 2.4 billion years ago (Ga), was less than 0.001% of the atmosphere today70 and Fe(II) concentrations probably ranged between 0.03 and 0.5 mM71,72 (modern oceanic iron concentrations range between 0.05 and 2.0 nM73). Archean ocean sulphate concentrations were also less than 1/100 of present levels, with concentrations lower than 200 µM74,75 and N2 was a bulk gas with similar levels to today76. Oxygen concentrations in the atmosphere and surface ocean first rose at ~2.4 Ga in the Great Oxygenation Event (GOE)77 with a second increase in the Neoproterozoic Oxygenation Event78 (NOE), which established a more modern ocean redox profile. The GOE is generally thought to have facilitated the emergence of eukaryotes79 while Betts et al.80 argue that the NOE was associated with emergence of large and complex multicellular organisms. Thus, the GOE and NOE were fundamental pacemakers for evolution of life on Earth, although the beginning of microbial life must have been much earlier than the GOE. There is evidence of microbial life originating sometime during the early Archean at ~3.5–3.8 Ga79,81. The presence of biogenic carbon has been uncovered in detrital zircon grains from Jack Hills, Western Australia82; if verified by further studies, the beginning of microbial life can be traced to ~4.1 Ga in the late Hadean Eon. Further evidence is required to corroborate this early age, which potentially transforms the way scientists view paleo-environments on Earth.
Fossilized magnetosomal magnetic particles (referred to as magnetofossils) can be preserved in sediments or rocks. Magnetofossil records trace an evolutionary history of MTB to the Cretaceous and with less certainty to the Paleoproterozoic at around ∼1.9 Ga83–85, which suggests that MTB should have an early origin. Furthermore, phylogenetic and molecular clock analyses suggest an Archean origin for genetic functionality of magnetosome biomineralization (which is needed to perform magnetotaxis); specifically, the emergence of MGCs is estimated to date to the Archean Eon (∼3.38–3.21 Ga)25 or even earlier24,31. It is important to remember that atmospheric oxygen was most likely sparse in the Archean so that an anoxic environment prevailed86–89. In addition to magnetotaxis, magnetosomal particles have been proposed to act as iron storage and sequestration organelles, or as gravity detection units or “geobatteries” that provided energy from elemental oxidation-reduction cycling90. It has recently been proposed that the initial role of magnetosomal magnetic particles was to mitigate intracellular reactive oxygen species (ROS) toxicity and that, eventually, they were co-opted for magnetotaxis in early Earth environments4. A photoferrotrophy-driven origin of magnetotaxis has also been proposed; that is, magnetosome formation was a by-product of Archean iron cycling and magnetotaxis evolved as a result of environmental pressure of co-evolved cyanobacteria and metabolically accumulated magnetite91. Magnetosomes have also been proposed to provide a protective shield in a metal-stressed environment92. To ascertain the evolutionary history of MTB, integration of microbiology, evolutionary biology, geobiology, biogeochemistry, and geochronology is required.
The recent development of metagenomics and single-cell genomics allows direct reconstruction of MTB genomes from environmental samples without cultivation. Comparison of these novel genomes with those from cultivated MTB strains provides insights into the evolution of magnetotaxis. Magnetosome protein phylogeny largely mirrors that of organisms at or above the class or phylum level, which suggests that vertical inheritance followed by multiple independent MGC losses mainly drove bacterial evolution of magnetotaxis at higher taxonomic levels23,25,31. Subsequent evolutionary trajectories of magnetotaxis at lower taxonomic ranks appear to be much more complicated and multiple evolutionary processes including horizontal gene transfers, gene duplications and/or gene losses may have been involved93–95. Metagenomic sequences with similarity to known magnetosome genes have been found in the microbiomes of some animals and even humans, which might suggest that MTB sensed by their hosts may produce symbiotic magnetoreception in these organisms96–98. It has also been suggested recently that the ability to biologically control magnetite precipitation might have been a fundamental feature of eukaryotic biology that was likely present in the last common ancestor of some archaea and extant eukaryotes99.
Magnetic characterization and quantification of MTB and magnetofossils
Magnetofossils are distributed widely in freshwater and marine sediments83. Recently, their presence has also been reported in ferromanganese (Fe–Mn) crusts and abyssal manganese nodules100–103. Several methods exist for identifying magnetofossils. The most direct way is to observe them under a transmission electron microscope (TEM). Kirschvink and Chang104 first observed magnetofossils under a TEM from deep-sea sediments of the Southwest Atlantic, Equatorial Pacific, and South Atlantic Oceans. Petersen et al.105 found additional magnetofossil morphotypes from South Atlantic marine sediments, with bullet-, prismatic- and octahedral shapes that they considered to be the predominant natural remanent magnetization (NRM) carrier in sediments with ages from Quaternary to Eocene. Magnetosomal magnetite crystals usually have a [111] elongation direction, while Li et al.106 found that bullet-shaped biogenic magnetite grows in a two-stage process with the second stage involving elongation in the [100] direction. Kopp and Kirschvink83 proposed a series of criteria to identify magnetofossils. For instance, biogenic magnetosomal magnetite crystals have a narrow size distribution and distinctive morphologies with blunt crystal edges, high chemical purity, crystallographic perfection and chain arrangement.
Magnetic techniques provide complementary approaches that are commonly used to initially identify magnetofossils before direct TEM observation. These methods include low-temperature remanence measurements, first-order reversal curve (FORC) diagrams, and ferromagnetic resonance (FMR) spectroscopy. Moskowitz et al.107 suggested that low-temperature isothermal remanent magnetization (IRM) measurements can be used to distinguish magnetite magnetosome chains. They defined δ as δ = (IRM80K-IRM150K)/IRM80K and the δ ratio = δFC/δZFC, where δFC and δZFC are the difference between field cooled (FC) and zero-field cooled (ZFC) IRM curves at 150 K and 80 K, respectively. Moskowitz et al.107 concluded that biogenic magnetite chains are present when the δ ratio >2. However, when magnetosome chains are oxidized, the δ ratio becomes less diagnostic. The test suggested by Chang et al.108, based on low-temperature cycling of a saturation IRM imparted at room temperature, is more robust for oxidized magnetosomes. Chang et al.109 reported that a double Verwey transition temperature signal can also be used to identify magnetofossils and suggested that ~100 K and ~120 K are the Verwey transition temperatures of biogenic and detrital magnetite, respectively, although it has also been suggested that the two Verwey transitions could result from other factors110.
FORC diagrams are normally interpreted in terms of the magnetic coercivity distribution and magnetostatic interactions among single-domain magnetic particles111–113. When interactions among uniaxial single-domain particles are negligible or weak, FORC diagrams will have a central ridge along the horizontal Bc axis, with a small vertical distribution along the vertical Bu axis. Although magnetic particles in magnetosome chains have strong interactions among them, the entire chain will act as a single needle with uniaxial magnetization that does not interact with other chains114. Therefore, MTB samples produce a central ridge in FORC diagrams115–119. However, when magnetofossil chains are broken, magnetic particles clump together so that interactions become strong; a central ridge usually persists and FORC distributions spread vertically in the Bu direction120. Inorganic magnetite with weak interactions also produce a central ridge FORC signature. Thus, if a FORC central ridge is observed, further TEM observations are needed to demonstrate the presence of magnetofossils.
FMR spectroscopy is sensitive to the magnetic anisotropy of the chain configuration121. The magnetic anisotropy of MTB arises mainly from dipolar interactions among adjacent magnetic particles in a chain, which behaves like an elongated single-domain particle114; therefore, FMR is used to indicate the presence of magnetosome chains122,123. The main FMR parameters are as follows: the effective g-factor (geff), asymmetry ratio (A), and empirical parameter (α), where geff = hv/βBeff, h is Planck’s constant, v is the microwave frequency, β is the Bohr magneton and Beff is the maximum absorption field, A = ΔBhigh/ΔBlow, where ΔBhigh = Bhigh − Beff, and ΔBlow = Beff − Blow. The full width at half maximum is defined as ΔBFWHM = Bhigh + Blow (Fig. 3e). Weiss et al.122 proposed that a magnetosome chain will have A < 1 and geff < 2.12. Kopp et al.123 proposed an empirical parameter, defined as α = 0.17 A + 9.8 × 10−4 ΔBFWHM/mT, and concluded that A < 1 and geff < 2.12 are insufficient to ensure the presence of magnetofossils. However, all of their measured MTB had α < 0.25, and magnetofossil-bearing samples have α = 0.25–0.30. When α is larger, magnetofossil contents are lower. Kodama et al.124 suggested that detrital and extracellularly produced authigenic magnetite (D + Ex) dominate sediments with α > 0.40, while magnetofossil-rich sediments have α < 0.35 and A < 1. However, inorganic elongated single-domain particles in a volcanic tuff also have 0.3 < α < 0.35 and A < 1125. Kind et al.126 investigated magnetic components in Holocene Lake sediments by combining anhysteretic remanent magnetization (ARM), IRM, FORC diagrams and FMR spectra and suggested a combination of FORC and FMR measurements to detect magnetofossils in natural environments. Blattmann et al.127 applied quantitative FMR to analyze magnetofossil variations in Lake Constance, which records sediment–water interface redox changes. FMR spectroscopy is also used widely to detect magnetofossils in pre-Quaternary marine sediments116,128.
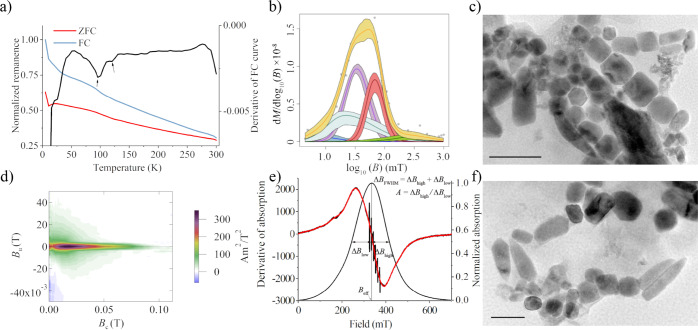
Fig. 3. Methods for identifying magnetofossils in samples from core MD01-2444 at 26.74 m depth.
a Low-temperature magnetic measurements (blue and red curves: normalized zero-field cooled (ZFC) and field cooled (FC) curves, respectively; black curve: normalized first derivative of the FC curve; peaks with arrows (~100 K and ~115 K) are indicative of biogenic and detrital magnetite, respectively). b Coercivity distribution from IRM acquisition curve unmixing (gray dots: IRM acquisition data; orange curve: spline fit based on measured data; purple and red curves: biogenic soft and biogenic hard components, respectively; shaded areas for each curve are 95% confidence intervals). c, f TEM images of magnetic extracts from the same sample. Scale bars: c = 200 nm, f = 100 nm. The main magnetofossil morphotypes are prisms, octahedra and bullets. d FORC diagram with a central ridge signature that is indicative of non-interacting single-domain magnetic particles. e Ferromagnetic resonance (FMR) and FMR absorption spectra (black curve: measured FMR spectrum; red curve: fitted FMR spectrum; black humped curve: normalized FMR absorption spectrum). Definition of commonly used magnetofossil-indicative parameters are shown in (e)123.
To better understand the magnetic properties of magnetofossils, micromagnetic modeling has been applied to build a link between magnetofossil size distributions and magnetic properties. Muxworthy and Williams129 found that the largest reported interacting magnetosomal magnetite particles still have single-domain behavior based on micromagnetic simulations. Chang et al.130 used micromagnetic models to build a link between magnetic properties and magnetofossil size distributions from Ocean Drilling Program (ODP) Site 1263 during the Paleocene–Eocene thermal maximum (PETM). Moreover, micromagnetic modeling demonstrates that magnetosome-chain structures play a more important role in controlling magnetic particle coercivity than morphology130. Different MTB species have different chain structures and magnetic particle numbers. Therefore, magnetofossil diversity probably affects the magnetic properties of bulk samples, especially when magnetofossils are the main NRM carrier. Berndt et al.131 modeled magnetosome chain morphologies (including crystal size, elongation, chain length, and intra-chain spacing) and found that intra-chain spacing can control the magnetism of magnetosomal magnetic particles such as coercivity, coercivity of remanence, and saturation magnetization.
Heslop et al.132 calculated the vector difference sum (VDS) of components in NRM demagnetization curves to quantify magnetofossil contents from sediments offshore of northwestern Australia. In addition, IRM unmixing and principal component analyses applied to FORCs (FORC-PCA) are used to quantify magnetic components in sediments. Two categories of methods are used to unmix IRM acquisition curves. One is single sample IRM unmixing, which includes fitting of log-normal, skewed generalized Gaussian, and Burr-XII distribution models133–135. Egli136 concluded that the dispersion parameter of biogenic magnetite is <0.2, while values for detrital magnetite particles are between 0.3 and 0.4. Chang et al.130 analyzed magnetic components from PETM sediments using a cumulative log-Gaussian function and decomposed IRM acquisition curves into three components, including biogenic soft and hard magnetite, and a soft detrital component. They suggested that magnetofossil components contribute 76% of the total remanent magnetization during the PETM onset. A further IRM unmixing approach is endmember-based IRM unmixing, with non-negative matrix factoring137. Usui et al.138 reconstructed the biogenic proportion of particles using this approach and obtained a two biogenic magnetite endmember solution by combining χARM/IRM ratios (a magnetic mineral grain size proxy) and FORC diagrams. They concluded that the two biogenic magnetite endmembers represent isotropic and bullet-shaped magnetofossils.
Lascu et al.139 and Harrison et al.140 demonstrated that FORC-PCA can help to determine biogenic and detrital magnetite abundances. FORC diagrams enable the characterization of magnetic minerals with different domain states and interaction fields. Channell et al.141 applied this method to trace variations of three magnetic endmembers (EMs) in the Rockall Trough (NE Atlantic), including a magnetofossil EM. Roberts et al.142 used FORC-PCA to detect magnetic property variations during early diagenetic reduction, including loss of a magnetofossil component due to dissolution and to estimate the proportions of different minerals in different diagenetic systems. Yamazaki et al.143 applied FORC-PCA to two cores from the western North Pacific Ocean and the South Pacific Ocean and identified two non-interacting single-domain EMs, which represent biogenic soft and biogenic hard components, respectively144. Qian et al.145 used FORC-PCA analysis of eastern Mediterranean sediments to demonstrate that elevated magnetofossil abundances occur at oxidation fronts above organic-rich intervals. FORC-PCA is becoming increasingly common for quantifying sedimentary magnetofossil contents in sediments146–148. Combining magnetic measurements and TEM or scanning electron microscope (SEM) observations can provide information to identify, characterize and quantify magnetofossils in natural samples (Fig. 3).
Paleoenvironmental and paleomagnetic implications of magnetofossils
Varying proportions of different magnetofossil morphotypes can reflect the paleoredox level of sediments100,101,103,149–151. Abundant bullet-shaped magnetofossils have been detected in reducing environments with higher total organic carbon (TOC), while octahedral magnetofossils appear to dominate relatively oxic environments. However, Lean and McCave152 found that more elongated magnetofossils occur in low-TOC horizons. Therefore, the paleoenvironmental implications of magnetofossil morphologies need further investigation, especially the relationship between magnetofossil abundance and sediment nutrient content. Moreover, if magnetofossils undergo diagenetic modification, the relationship between their abundance and paleoenvironment will change153. For example, moderate TOC availability promotes mild diagenetic iron reduction that reduces sedimentary iron so that it becomes bioavailable to MTB116. However, high TOC produces sulphidic diagenetic environments in which magnetite magnetofossils dissolve154. Equant magnetofossils dissolve more easily than bullet-shaped forms155, and bullet-shaped magnetofossils are more easily dissolved than hexagonal prisms and octahedral forms153. Magnetofossils are ideal ancient magnetic field recorders. In environments where magnetofossils are preserved, Ouyang et al.156 and Chen et al.157 demonstrated that that they have a higher magnetic recording efficiency than detrital magnetite, while Li et al.158 found that biogenic magnetite can be less efficiently magnetized than detrital magnetite. The debate also remains about whether magnetofossils record a biogeochemical remanent magnetization (BGRM). Tarduno et al.159 suggested that magnetofossils that survive burial below the Fe-redox boundary can produce delayed BGRM acquisition. Roberts et al.116 noted that paleomagnetic signals carried by magnetofossils must be acquired at shallow depths based on comparison between two nearby records. Tests for offsets between paleomagnetic signals carried by detrital and biogenic magnetite indicate no depth lag, so the evidence is still lacking for the BGRM mechanism156,157. Yamazaki et al.160 found that elongated magnetofossils inhabit lower parts of a redox zonation, while isotropic forms occupy shallower levels.
Although there are few reports of greigite magnetofossils, it has been suggested that they can be reliable paleomagnetic recorders161. Pósfai et al.162 proposed that greigite in Miocene sedimentary rocks is similar to biogenic crystals produced by MMP and Chang et al.163 concluded that greigite magnetofossils are potentially widespread in ancient sediments. The favored MTB habitat depth relates to their ability to contribute to a BGRM; future detailed studies of the vertical distribution of MTB populations within the water column or sediments are required to constrain the habitat range of MTB.
Ecosystem functions of MTB
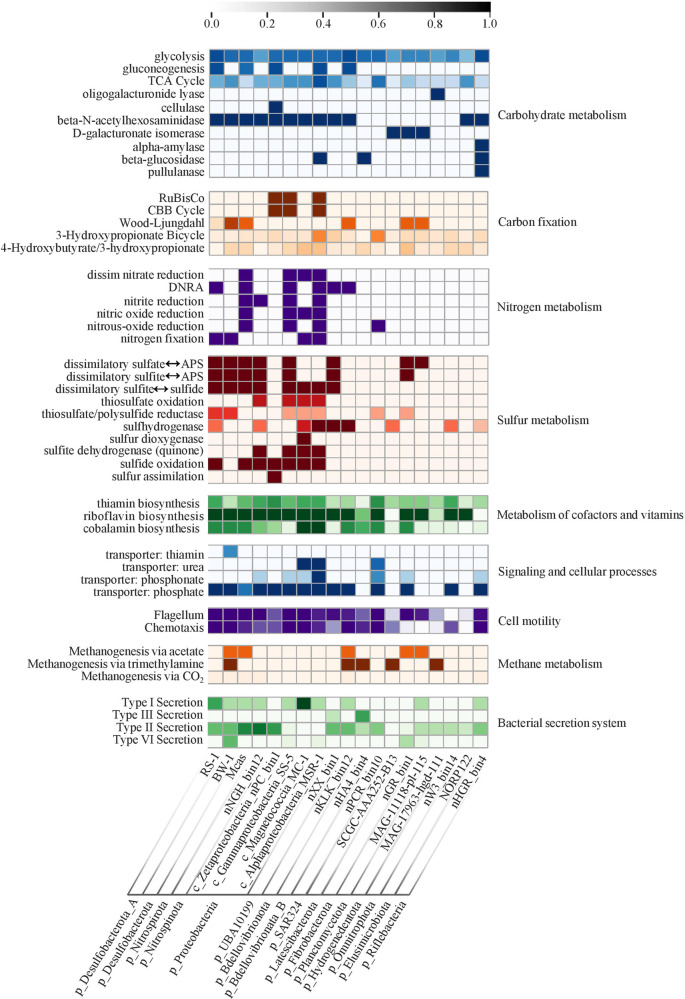
MTB are distributed globally in aquatic ecosystems21. They can comprise up to ~30% of the microbiome in some habitats26 and their biomineralizing capability is assumed to be important for biogeochemical cycling of iron in sediments90. The recent discovery of an Archean origin for MTB further suggests that they may have contributed to iron cycling throughout Earth history. With reports of the ability of MTB to fix nitrogen164,165, sequester carbon166, and to incorporate elements like sulphur, phosphorus, selenium, calcium, etc., into biominerals (Fig. 4)28,32,39,57,64,95,166–171, evidence is growing to indicate that MTB play important roles in global biogeochemical cycling. Here, we compared the major KEGG pathways of representative MTB genomes across all known 16 bacterial phylum-level lineages, which suggest that MTB have phylogenetically diverse metabolic features (Fig. 5). What is not yet clear is the extent to which they impact the total cycling budget of each element.
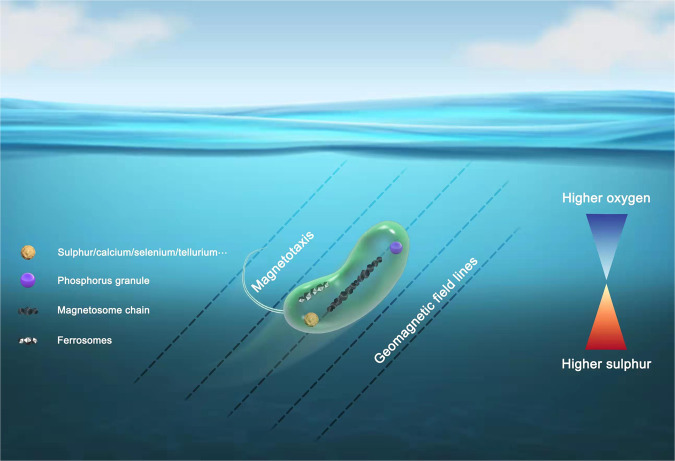
Fig. 4. Magnetotaxis, combined with chemotaxis and aerotaxis, allows MTB to efficiently locate and maintain an optimal position for survival and growth in habitats with vertical redox concentration gradients in water columns and sediments around an oxic–anoxic interface (OAI).
MTB are widely distributed in aquatic environments from marine to freshwater ecosystems and are thought to play important roles in the cycling of various elements (e.g., Fe, C, N, S, P, and some heavy metals, such as tellurium and selenium).
Fig. 5. Metabolic pathways of representative MTB genomes across 16 bacterial phylum-level lineages.
The color gradient represents the completeness of major metabolic pathways inferred from the presence or absence of genes. Dark represents a complete or nearly complete pathway, and white represents a pathway that is absent or mainly incomplete. Note that most available MTB genomes are draft or incomplete, so we cannot rule out that the absence or incompleteness of metabolic pathways maybe due to the fragmented nature of draft genomes. All taxonomic groupings are based on the GTDB taxonomy (Release 89, https://gtdb.ecogenomic.org).
To better understand iron biomineralization, the processes and products of biomineralization, the relevant genetics, and magnetotaxis need to be analyzed. The ability to navigate by magnetotaxis depends on whether magnetosomal magnetite particles are fully developed19. Magnetotaxis is best achieved with fully developed crystals (~30–150 nm)7,59,172 rather than with immature crystals (~30 nm or smaller). Cornejo et al.172 studied the biomineralization mechanism for mature octahedral crystal formation inside magnetosome membranes in Magnetospirillum magneticum strain AMB-1 (AMB-1), whereas Li et al.173 studied the mechanism for mature bullet-shaped crystal formation in Deltaproteobacterial strain WYHR-1. These and further studies could help to better understand crystal biomineralization in various MTB strains. Magnetic particle growth is important because assemblages of mature magnetosomal magnetite particles in chain-like structures produce an intracellular magnetic dipole that interacts with Earth’s magnetic field to assist MTB cells in magnetic navigation114,174–176. Therefore, research on the growth stage of magnetosomal crystals in different MTB populations would help to better understand underlying magnetotaxis mechanisms.
Bioavailable iron is scarce in many environments today, which may limit MTB population growth113. In particular, iron is scarce in high-nitrate low-chlorophyll (HNLC) oceans. Addition of iron from, for example, eolian dust or volcanic ash inputs, can fertilize surface ocean primary productivity, which increases the chlorophyll content in iron-limited biomes177–179. Solubilization of iron from such inputs plays a key role in carbon and nitrogen fixation into biomass, which makes iron bioavailability responsible for primary production in these ecosystems180–183. Export of carbon from the surface ocean to the seafloor can also stimulate mild diagenetic release of iron in the uppermost sediment to release a limitation on MTB productivity in pelagic environments and allows MTB populations to grow116. Considering the global MTB distribution in aquatic ecosystems, it has been suggested that MTB communities play significant roles in present-day global iron cycling20,184.
Apart from MTB, magnetic inclusions have been reported within the purple-sulphur bacterial genera, Rhodopseudomonas and Ectothiorhodospira185. These inclusions have no prismatic crystal structure and they lack a lipid-membrane to enclose magnetosomes. Unlike the microaerophilic model strains, AMB-1 and Magnetospirillum gryphiswaldense strain MSR-1 (MSR-1), recent analysis of an anaerobic sulphate-reducer, Desulfuvibrio magneticus strain RS-1 (RS-1), is gaining popularity as a model MTB organism186,187. This species constructs sub-cellular electron-dense Fe-rich granules (sometimes including phosphorus and oxygen) that are encased by a lipid-like membrane. These “ferrosome” (iron body) granules are independent and separate entities from magnetosomes and are proposed to play a vital role in storing iron under anaerobic respiration during extreme iron starvation conditions188. This aligns with the findings of Amor et al. that magnetite in AMB-1 only represents about ~25–30% of the total iron mass in a bacterial cell189. Broader iron storage remains to be explored in relation to other MTB species to explore their probably crucial role in global iron cycling.
Apart from the well-studied Fe3O4 and Fe3S4 biomineralization products within MTB, diverse other inclusions contain elemental sulphur, polyphosphate, calcium, etc. Sulphur-rich inclusions have been identified in Magnetovibrio strains MV-1 and MV-2 of the Alphaproteobacteria class and in many Nitrospirae MTB7,28,32,57,190,191. It was initially proposed that some MTB, like Nitrospirae MTB, are capable of adapting to chemical gradients near the OAI by adjusting their metabolic strategies—either by moving downward to accumulate reduced sulphur species or upward to oxidize stored sulphur with oxygen192. This redox-controlled response is supported by analysis of the vertical distribution of Ca. Magnetobacterium bavaricum in sediments32 and by genomic characterization of Ca. Magnetobacterium casensis38. Ca. Magnetobacterium casensis and Ca. Magnetobacterium bavaricum are proposed to conduct sulphur oxidation with nitrate and/or oxygen as electron acceptors in the upper micro-oxic layer and to perform sulphate reduction in the anoxic lower layer, suggesting a complex metabolic strategy of Nitrospirae MTB and electron shuttling depending on redox conditions38,39,193.
Bazylinski and Blakemore164 revealed that Magnetospirillum magnetotacticum strain MS-1 (MS-1) fixes nitrogen at rates equivalent to those of Azospirillum lipoferum when cultured microaerobically under nitrogen limiting conditions, where Azospirillum species were among the earliest known potential nitrogen fixers for cereal plants194. Apart from MS-1, two Magnetospirillum strains, including MSR-1 and AMB-1, when proliferated in nitrogen limiting, semi-solid media can also fix nitrogen via nitrogenase164. MSR-1 contains indispensable nitrogenase structural genes required for nitrogen fixation in the presence of the DRAT-DRAG nitrogenase post-translational regulatory system165 (Fig. 5).
Carbon sequestration is a key consideration when analyzing pollution mitigation potency of a plant or microorganism, both in terrestrial and aquatic habitats. Microorganisms and other autotrophs drive the carbon cycle because they are at the forefront of carbon cycle feedback mechanisms and their primary production is critical in carbon cycling195. Marine phytoplankton have a well-known role in CO2 sequestration196; chemolithoautotrophs do the same under dark conditions in deeper, less-oxic/anoxic parts of ocean water or sediment redox gradients197. If not obligate, most MTB are facultative chemolithoautotrophs, which might suggest an underlying role in CO2 fixation19. Most terrestrial plants and microorganisms contain genes that encode a key metabolic pathway known as the Calvin–Benson–Bassham pathway (CBB) that allows the plant/microbe to sequester bioavailable carbon from the environment. Ribulose-1,5-bisphosphate carboxylase/oxygenase (RuBisCO) is an abundant protein that catalyzes the CO2 fixation reaction198. The cbbM gene that encodes form II RuBisCO was identified in magnetotactic chemolithoautotrophs Magnetovibrio strains MV-1 and MV-2 and Magnetospirillum strain MS-1; these species are proposed to be important in carbon cycling and primary productivity166. Gammaproteobacteria MTB strains BW-2 and SS-5 from the hypersaline Salton Sea have a partial RuBisCO gene sequence and potentially use the CBB pathway for autotrophy68. MTB from the Etaproteobacteria (Magnetococcus marinus strain MC-1) use the reverse tricarboxylic acid (rTCA) cycle for autotrophy199, while magnetotactic Nitrospirae are proposed to fix CO2 via the reductive acetyl-CoA (Wood-Ljungdahl or WL) and/or reductive tricarboxylic acid (rTCA) pathways38,193,200 (Fig. 5).
Uncultured MTB cells from the Seine River, France, contain vesicles rich in barium and calcium oxide171 and MTB from Lake Pavin, France, biomineralize CaCO3 in addition to magnetosomal magnetite201. Instances of phosphorous sequestration by Magnetococcaceae alongside magnetite chains have also been demonstrated in ferruginous Lake Pavin168 and in the anoxic Black Sea169. Schulz-Vogt et al.169 suggested that magnetotactic cocci act as a shuttle (using magnetotaxis) to transport phosphorous from the upper to the lower stratum of the suboxic zone, which prevents eutrophication resulting from excess phosphorus accumulation in the upper stratum.
Elements can also deposit on MTB cell surfaces, which is important in analyzing the role of MTB in heavy-metal bioremediation and magnetic separation of metal-loaded MTB cells from aquatic bodies. For example, Arakaki and colleagues202 observed electron-dense Cd2+ deposits enveloping RS-1 cell surfaces under TEM when cells were cultured in growth media containing 1.3 ppm Cd2+. Mono-dispersed crystalline inclusions have 20–40 nm sizes and were easily distinguished from magnetic particles in TEM images202. A novel Alphaproteobacterium MTB species grown in a cobalt supplemented medium has efficient biosorption competence with 89% cobalt removed by magnetic separation of the biomass203. Tellurite (O3Te2−) uptake by MTB followed by biomineralization of discrete tellurium crystals alongside and separate from magnetite crystals was observed by Tanaka et al.204. These authors then demonstrated that when a growth medium is supplemented with selenite (SeO32−), elemental selenium nanoparticles with no definitive form gradually built up and some precipitated within cells, in conjunction with and autonomous from magnetite particles170. Quantitative analysis revealed that MTB accumulate selenium at a higher rate per cell than other non-iron element accumulations. For example, Se accumulated by MTB is higher than O3Te2− uptake and Cd adsorbed by factors of 2.4 and 174, respectively170. Elemental adsorption on cell surfaces is not specific to MTB, but they can contribute to metal pollutant removal via magnetic separation. Heavy-metal recovery from diodes and resistors of waste printed circuit boards using MTB is a latest discovery in terms of MTB applications in bioremediation205. Although such high metal concentrations are usually not encountered in natural environments, analysis of samples from polluted water bodies will help to identify the usefulness of MTB for bioremediation, e.g., MTB morphotypes have been observed in hydrocarbon-contaminated microcosms from the Gulf of Fos, France, and hypothesized to be involved in degrading hydrocarbons or other aromatic compounds206. It remains to be shown if MTB extracted from such microcosms can be grown in defined induction experiments using aromatic substrates under anaerobic conditions. MTB could, therefore, be potent model organisms for bioremediating contaminated water bodies and surface sediment. As new MTB genomes are obtained by metagenomics and single-cell genomics of environmental samples, identification and characterization of key proteins involved in these metabolic pathways will provide an important way to understand the ecological functions of MTB.
MTB as a potential important constituent of OMZ microbiomes
Oxygen minimum zones (OMZs) are oxygen delimiting/nutrient-rich sections of an oceanic gradient207. Waters above and below the OMZ have higher oxygen concentrations compared to the OMZ interval. OMZs are global sinks for reactive nitrogen because they have the highest microbial activity that conserves available oxygen and produces N2 and N2O as respiration by-products208 and have been termed “microbial reactors” with global significance209. OMZs have expanded gradually over the past 50 years due to ocean warming, which reduces oxygen solubility210–212. Microbiological analyses of oceanic OMZs reveal that the most abundant phyla are Proteobacteria, Bacteroidetes, Actinobacteria, Planctomycetes, and Marinimicrobia (previously known as Marine Group A)213. A characteristic feature of the OMZ microbiome is its predominant role in the biogeochemical cycling of marine nitrogen214,215, sulphur216–219, and carbon215. Rhoads et al.220 showed that MTB can occur within an OMZ at oxygen levels as low as <4 mg L−1. The range of dissolved oxygen contents in an OMZ appears to be optimal for MTB to thrive (0.1–0.5 mg L−1). It has been hypothesized that marine dysaerobic zones (with 0.1–0.5 mL L−1 dissolved oxygen concentrations) have a biogenic magnetite inventory based on TEM results in which fine-grained (80–100 nm) subrounded cubic magnetite predominates220. High-nitrate concentrations in OMZs suggest that these oxygen-limiting microenvironments should harbor greater denitrifying MTB populations220. Symbiotic occurrences have been proven by studying the “cryptic” sulphur cycle in OMZs around the world221. OMZ biogeochemistry is important in the greater oceanic system with implications even outside the oceans. For example, consortia of ectosymbiotic, sulfate-reducing, chemolithoautotrophic Deltaproteobacteria, and Excavata protists have been reported222. Sulphur-reducing bacteria with magnetosomes have also been observed in microbial mats on carbonate concretions from the Black Sea alongside other archaea that aid methane oxidation223. Sulphur-reducing gammaproteobacterial MTB even occur as extracellular symbionts on marine bivalves224. OAIs and OMZs are ecological niches in which MTB thrive within chemical gradients; tweaking the chemistry of such environments will likely affect much of the microbial community. The increasing importance of OMZs in the modern ocean could imply that global MTB populations will increase over time with accelerating ocean warming and that they could help to limit deterioration of ocean ecosystem health.
Outlook
MTB are distributed in aquatic ecosystems globally. Understanding of the phylogenetic taxonomy and metabolic flexibility of MTB has expanded greatly over the past decade, which suggests an unexpected natural diversity of these microorganisms. Continued discovery of novel MTB lineages from different environments highlights our limited knowledge of their diversity and ecology. Continuing cultivation-dependent and -independent MTB analyses will be crucial to understand their diversity and taxonomy more fully. We anticipate great progress in defining the phylogenetic diversity and evolutionary origin of MTB in the coming years. MTB incorporate elements from their surroundings to produce several biomineral types, with magnetic iron-bearing minerals being the primary biomineralization product. In addition to iron biomineralization, MTB can fix nitrogen, oxidize/reduce sulphur and sequester carbon and phosphorus from the environment. These findings suggest that MTB play important roles in biogeochemical elemental cycling through time, although their contributions are yet to be evaluated quantitatively. Moreover, MTB in marine OMZs/OAIs may play a role in ocean biogeochemical cycling and, in turn, in trimming eutrophication. If this is demonstrated by further work, MTB could be a natural eutrophication repressor that could become important with ongoing global warming. In addition, MTB can be used in pollution bioremediation by accumulating heavy metals on their surfaces by adsorption (e.g., Cd) and intracellularly (e.g., O3Te2− and Se). The magnetism of MTB ensures that metal-loaded MTB cells can be separated magnetically from contaminated waters. Magnetofossils are important in paleoenvironmental, paleoclimatic and paleomagnetic interpretations of sediments and sedimentary rocks. Further research is needed to understand the environmental adaptability of MTB and limiting factors that affect magnetofossil preservation to develop robust magnetofossil proxies for past environmental changes.
Acknowledgements
We thank Runjia Ji and Jia Liu for preparing Fig. 1 and Runjia Ji for her help with Figs. 4 and 5. This work was supported by the CAS-TWAS President’s Fellowship (Series No 2019-054), National Natural Science Foundation of China (NSFC) Grants 41822704 and 41621004, the Key Research Program of the Chinese Academy of Sciences (ZDBS-SSW-TLC001), the Key Research Program of the Institute of Geology and Geophysics, Chinese Academy of Sciences (IGGCAS-202102), the Youth Innovation Promotion Association of the Chinese Academy of Sciences and Australian Research Council grant DP140104544.
Author contributions
W.L., A.P.R., and P.G. conceived the review. All authors contributed to the writing and editing.
Data availability
All NCBI accession numbers of MTB genomes used in Fig. 5 are as follows-HG794546.1: Magnetospirillum gryphiswaldense MSR-1; CP000471.1: Magnetococcus marinus MC-1; CP032508.2: Gamma proteobacterium SS-5; JADFZC010000123.1: Oligoflexales bacterium isolate nHA4_bin4; JADFZW010000010.1: Oligoflexia bacterium isolate nKLK_bin12; AP010904.1: Desulfovibrio magneticus RS-1; FWEV01000001.1: Candidatus Desulfamplus magnetomortis BW-1 PRJEB14757; NVTF01000001.1: Elusimicrobia bacterium isolate NORP122; JADFYW010000100.1: Fibrobacteria bacterium isolate nGR_bin1; DUZM01000001.1: Candidatus Hydrogenedentes bacterium isolate MAG_17963_hgd_111; ASWY01000001.1: Latescibacteria bacterium SCGC AAA252-B13; JADGAO010000100.1: Nitrospinae bacterium isolate nNGH_bin12; JMFO00000000: Candidatus Magnetobacterium casensis; JADGBR010000010.1: Candidatus Omnitrophica bacterium isolate nW3_bin14; DUZJ01000001.1: Planctomycetes bacterium isolate MAG_11118_pl_115; JADGAS010000010.1: Zetaproteobacteria bacterium isolate nPC_bin1; JADFZL010000010.1: Candidatus Riflebacteria bacterium isolate nHGR_bin4; JADGAT010000100.1: SAR324 cluster bacterium isolate nPCR_bin10; JADGCS010000100.1: Deltaproteobacteria bacterium isolate nXX_bin1. The original data for Fig. 3 can be found at 10.6084/m9.figshare.17122277.v1.
Code availability
MagCluster (https://github.com/RunJiaJi/magcluster), code: magcluster prokka 19mtb_genomes; KofamKOALA server (https://www.genome.jp/tools/kofamkoala/) 10.1093/bioinformatics/btz859KEGG-Decoder10.1038/s41396-018-0091-3, code: KEGG-decoder -input 19mtb.txt -output out.list -vizoption static -m myorder.txt. Fig. 3b was produced using by Max UnMix and Fig. 3d was drawn using FORCinel v3.06 (https://wserv4.esc.cam.ac.uk/nanopaleomag/?page_id=51).
Competing interests
The authors declare no competing interests.
Footnotes
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Andrew P. Roberts, Email: andrew.roberts@anu.edu.au
Wei Lin, Email: weilin@mail.iggcas.ac.cn.
References
- 1.Kirschvink, J. L., Jones, D. S. & MacFadden, B. J. Magnetite biomineralization and magnetoreception in organisms (Springer US, 1985).
- 2.Frankel, R. B. & Blakemore, R. P. Iron Biominerals (Springer US, 1991).
- 3.Uebe R, Schüler D. Magnetosome biogenesis in magnetotactic bacteria. Nat. Rev. Microbiol. 2016;14:621–637. doi: 10.1038/nrmicro.2016.99. [DOI] [PubMed] [Google Scholar]
- 4.Lin W, Kirschvink JL, Paterson GA, Bazylinski DA, Pan Y. On the origin of microbial magnetoreception. Natl Sci. Rev. 2020;7:472–479. doi: 10.1093/nsr/nwz065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bellini S. On a unique behavior of freshwater bacteria. Chin. J. Oceanol. Limnol. 2009;27:3–5. doi: 10.1007/s00343-009-0003-5. [DOI] [Google Scholar]
- 6.Blakemore RP. Magnetotactic bacteria. Science. 1975;190:377–379. doi: 10.1126/science.170679. [DOI] [PubMed] [Google Scholar]
- 7.Bazylinski DA, Frankel RB. Magnetosome formation in prokaryotes. Nat. Rev. Microbiol. 2004;2:217–230. doi: 10.1038/nrmicro842. [DOI] [PubMed] [Google Scholar]
- 8.Boles LC, Lohmann KJ. True navigation and magnetic maps in spiny lobsters. Nature. 2003;421:60–63. doi: 10.1038/nature01226. [DOI] [PubMed] [Google Scholar]
- 9.Nordmann GC, Hochstoeger T, Keays DA. Unsolved mysteries: Magnetoreception—a sense without a receptor. PLoS Biol. 2017;15:e2003234. doi: 10.1371/journal.pbio.2003234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Wajnberg E, et al. Magnetoreception in eusocial insects: an update. J. R. Soc. Interface. 2010;7:207–225. doi: 10.1098/rsif.2009.0526.focus. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Dreyer D, et al. The Earth’s magnetic field and visual landmarks steer migratory flight behavior in the nocturnal Australian Bogong moth. Curr. Biol. 2018;28:2160–2166. doi: 10.1016/j.cub.2018.05.030. [DOI] [PubMed] [Google Scholar]
- 12.Malkemper EP, et al. Magnetoreception in the wood mouse (Apodemus sylvaticus): influence of weak frequency-modulated radio frequency fields. Sci. Rep. 2015;5:9917. doi: 10.1038/srep09917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Begall S, Malkemper EP, Cervený J, Němec P, Burda H. Magnetic alignment in mammals and other animals. Mamm. Biol. 2013;78:10–20. doi: 10.1016/j.mambio.2012.05.005. [DOI] [Google Scholar]
- 14.Hart V, et al. Dogs are sensitive to small variations of the Earth’s magnetic field. Front. Zool. 2013;10:80. doi: 10.1186/1742-9994-10-80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Engelmann TW. Neue methode zur untersuchung der sauerstoffausscheidung pflanzlicher und thierischer organismen. Pflüger Arch. Gesammt. Physiol. Menschen Thiere. 1881;25:285–292. doi: 10.1007/BF01661982. [DOI] [Google Scholar]
- 16.Adler J. Chemotaxis in bacteria. Science. 1966;153:708–716. doi: 10.1126/science.153.3737.708. [DOI] [PubMed] [Google Scholar]
- 17.Vali, H. & Kirschvink, J. L. Observations of magnetosome organization, surface structure, and iron biomineralization of undescribed magnetic bacteria: evolutionary speculations. In Iron Biominerals (eds. Frankel, R. B. & Blakemore, R. P.) 97–115 (Springer US, 1991).
- 18.Mao X, Egli R, Petersen N, Hanzlik M, Liu X. Magneto-chemotaxis in sediment: first insights. PLoS ONE. 2014;9:e102810. doi: 10.1371/journal.pone.0102810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lefèvre CT, Bazylinski DA. Ecology, diversity, and evolution of magnetotactic bacteria. Microbiol. Mol. Biol. Rev. 2013;77:497–526. doi: 10.1128/MMBR.00021-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lin W, Bazylinski DA, Xiao T, Wu LF, Pan Y. Life with compass: diversity and biogeography of magnetotactic bacteria. Environ. Microbiol. 2014;16:2646–2658. doi: 10.1111/1462-2920.12313. [DOI] [PubMed] [Google Scholar]
- 21.Lin W, Pan Y, Bazylinski DA. Diversity and ecology of and biomineralization by magnetotactic bacteria. Environ. Microbiol. Rep. 2017;9:345–356. doi: 10.1111/1758-2229.12550. [DOI] [PubMed] [Google Scholar]
- 22.Abreu F, et al. Common ancestry of iron oxide- and iron-sulfide-based biomineralization in magnetotactic bacteria. ISME J. 2011;5:1634–1640. doi: 10.1038/ismej.2011.35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lefèvre CT, et al. Monophyletic origin of magnetotaxis and the first magnetosomes. Environ. Microbiol. 2013;15:2267–2274. doi: 10.1111/1462-2920.12097. [DOI] [PubMed] [Google Scholar]
- 24.Lin W, et al. Genomic expansion of magnetotactic bacteria reveals an early common origin of magnetotaxis with lineage-specific evolution. ISME J. 2018;12:1508–1519. doi: 10.1038/s41396-018-0098-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lin W, et al. Origin of microbial biomineralization and magnetotaxis during the Archean. Proc. Natl Acad. Sci. USA. 2017;114:2171–2176. doi: 10.1073/pnas.1614654114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Spring S, et al. Dominating role of an unusual magnetotactic bacterium in the microaerobic zone of a freshwater sediment. Appl. Environ. Microbiol. 1993;59:2397–2403. doi: 10.1128/aem.59.8.2397-2403.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Amann, R., Peplies, J. & Schüler, D. Diversity and taxonomy of magnetotactic bacteria. In Magnetoreception and Magnetosomes in Bacteria (ed. Schüler, D.) 25–36 (Springer, 2006).
- 28.Kolinko S, et al. Single-cell analysis reveals a novel uncultivated magnetotactic bacterium within the candidate division OP3. Environ. Microbiol. 2012;14:1709–1721. doi: 10.1111/j.1462-2920.2011.02609.x. [DOI] [PubMed] [Google Scholar]
- 29.Lin W, Pan Y. A putative greigite-type magnetosome gene cluster from the candidate phylum. Latescibacteria. Environ. Microbiol. Rep. 2015;7:237–242. doi: 10.1111/1758-2229.12234. [DOI] [PubMed] [Google Scholar]
- 30.Uzun M, Alekseeva L, Krutkina M, Koziaeva V, Grouzdev D. Unravelling the diversity of magnetotactic bacteria through analysis of open genomic databases. Sci. Data. 2020;7:252. doi: 10.1038/s41597-020-00593-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Lin W, et al. Expanding magnetic organelle biogenesis in the domain Bacteria. Microbiome. 2020;8:1–28. doi: 10.1186/s40168-020-00931-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Jogler C, et al. Cultivation-independent characterization of ‘Candidatus Magnetobacterium bavaricum’ via ultrastructural, geochemical, ecological and metagenomic methods. Environ. Microbiol. 2010;12:2466–2478. doi: 10.1111/j.1462-2920.2010.02220.x. [DOI] [PubMed] [Google Scholar]
- 33.Vali H, Forster O, Amarantidis G, Petersen N. Magnetotactic bacteria and their magnetofossils in sediments. Earth Planet. Sci. Lett. 1987;86:389–400. doi: 10.1016/0012-821X(87)90235-4. [DOI] [Google Scholar]
- 34.Hanzlik M, Winklhofer M, Petersen N. Spatial arrangement of chains of magnetosomes in magnetotactic bacteria. Earth Planet. Sci. Lett. 1996;145:125–134. doi: 10.1016/S0012-821X(96)00191-4. [DOI] [Google Scholar]
- 35.Hanzlik M, Winklhofer M, Petersen N. Pulsed-field-remanence measurements on individual magnetotactic bacteria. J. Magn. Magn. Mater. 2002;248:258–267. doi: 10.1016/S0304-8853(02)00353-0. [DOI] [Google Scholar]
- 36.Eder SHK, Gigler AM, Hanzlik M, Winklhofer M. Sub-micrometer-scale mapping of magnetite crystals and sulfur globules in magnetotactic bacteria using confocal Raman micro-spectrometry. PLoS ONE. 2014;9:1–12. doi: 10.1371/journal.pone.0107356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Lin W, Li J, Schüler D, Jogler C, Pan Y. Diversity analysis of magnetotactic bacteria in Lake Miyun, northern China, by restriction fragment length polymorphism. Syst. Appl. Microbiol. 2009;32:342–350. doi: 10.1016/j.syapm.2008.10.005. [DOI] [PubMed] [Google Scholar]
- 38.Lin W, et al. Genomic insights into the uncultured genus ‘Candidatus Magnetobacterium’ in the phylum Nitrospirae. ISME J. 2014;8:2463–2477. doi: 10.1038/ismej.2014.94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Li J, Liu P, Wang J, Roberts AP, Pan Y. Magnetotaxis as an adaptation to enable bacterial shuttling of microbial sulfur and sulfur cycling across aquatic oxic‐anoxic interfaces. J. Geophys. Res. Biogeosci. 2020;125:e2020JG006012. [Google Scholar]
- 40.Qian X, et al. Identification of novel species of marine magnetotactic bacteria affiliated with Nitrospirae phylum. Environ. Microbiol. Rep. 2019;11:330–337. doi: 10.1111/1758-2229.12755. [DOI] [PubMed] [Google Scholar]
- 41.Farina M, Lins de Barros HGP, Esquivel DMS, Danon J. Ultrastructure of a magnetotactic microorganism. Biol. Cell. 1983;48:85–88. [Google Scholar]
- 42.Winklhofer M, Abraçado LG, Davila AF, Keim CN, Lins De Barros HGP. Magnetic optimization in a multicellular magnetotactic organism. Biophys. J. 2007;92:661–670. doi: 10.1529/biophysj.106.093823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Keim CN, et al. Multicellular life cycle of magnetotactic prokaryotes. FEMS Microbiol. Lett. 2004;240:203–208. doi: 10.1016/j.femsle.2004.09.035. [DOI] [PubMed] [Google Scholar]
- 44.Abreu F, et al. ‘Candidatus Magnetoglobus multicellularis’, a multicellular, magnetotactic prokaryote from a hypersaline environment. Int. J. Syst. Evol. Microbiol. 2007;57:1318–1322. doi: 10.1099/ijs.0.64857-0. [DOI] [PubMed] [Google Scholar]
- 45.Lins U, Keim C, Evans F, Farina M, Buseck P. Magnetite (Fe3O4) and greigite (Fe3S4) crystals in multicellular magnetotactic prokaryotes. Geomicrobiol. J. 2007;24:43–50. doi: 10.1080/01490450601134317. [DOI] [Google Scholar]
- 46.Wenter R, Wanner G, Schüler D, Overmann J. Ultrastructure, tactic behaviour and potential for sulfate reduction of a novel multicellular magnetotactic prokaryote from North Sea sediments. Environ. Microbiol. 2009;11:1493–1505. doi: 10.1111/j.1462-2920.2009.01877.x. [DOI] [PubMed] [Google Scholar]
- 47.Zhou K, et al. Adaptation of spherical multicellular magnetotactic prokaryotes to the geochemically variable habitat of an intertidal zone. Environ. Microbiol. 2013;15:1595–1605. doi: 10.1111/1462-2920.12057. [DOI] [PubMed] [Google Scholar]
- 48.Zhang R, et al. Characterization and phylogenetic identification of a species of spherical multicellular magnetotactic prokaryotes that produces both magnetite and greigite crystals. Res. Microbiol. 2014;165:481–489. doi: 10.1016/j.resmic.2014.07.012. [DOI] [PubMed] [Google Scholar]
- 49.Lefèvre C, et al. Characterization of Mediterranean magnetotactic bacteria. J. Ocean Univ. China. 2007;6:355–359. doi: 10.1007/s11802-007-0355-4. [DOI] [Google Scholar]
- 50.Zhou K, et al. A novel genus of multicellular magnetotactic prokaryotes from the Yellow Sea. Environ. Microbiol. 2012;14:405–413. doi: 10.1111/j.1462-2920.2011.02590.x. [DOI] [PubMed] [Google Scholar]
- 51.Du H, et al. Temporal distributions and environmental adaptations of two types of multicellular magnetotactic prokaryote in the sediments of Lake Yuehu, China. Environ. Microbiol. Rep. 2015;7:538–546. doi: 10.1111/1758-2229.12284. [DOI] [PubMed] [Google Scholar]
- 52.Chen YR, et al. A novel species of ellipsoidal multicellular magnetotactic prokaryotes from Lake Yuehu in China. Environ. Microbiol. 2015;17:637–647. doi: 10.1111/1462-2920.12480. [DOI] [PubMed] [Google Scholar]
- 53.Chen YR, et al. Novel species and expanded distribution of ellipsoidal multicellular magnetotactic prokaryotes. Environ. Microbiol. Rep. 2016;8:218–226. doi: 10.1111/1758-2229.12371. [DOI] [PubMed] [Google Scholar]
- 54.Dong Y, et al. The detection of magnetotactic bacteria in deep sea sediments from the East Pacific Manganese Nodule Province. Environ. Microbiol. Rep. 2016;8:239–249. doi: 10.1111/1758-2229.12374. [DOI] [PubMed] [Google Scholar]
- 55.Leao P, et al. Ultrastructure of ellipsoidal magnetotactic multicellular prokaryotes depicts their complex assemblage and cellular polarity in the context of magnetotaxis. Environ. Microbiol. 2017;19:2151–2163. doi: 10.1111/1462-2920.13677. [DOI] [PubMed] [Google Scholar]
- 56.Teng Z, et al. Diversity and characterization of multicellular magnetotactic prokaryotes from coral reef habitats of the Paracel Islands, South China Sea. Front. Microbiol. 2018;9:2135. doi: 10.3389/fmicb.2018.02135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Qian XX, et al. Juxtaposed membranes underpin cellular adhesion and display unilateral cell division of multicellular magnetotactic prokaryotes. Environ. Microbiol. 2020;22:1481–1494. doi: 10.1111/1462-2920.14710. [DOI] [PubMed] [Google Scholar]
- 58.Lyons NA, Kolter R. On the evolution of bacterial multicellularity. Curr. Opin. Microbiol. 2015;24:21–28. doi: 10.1016/j.mib.2014.12.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Stolz JF, Chang SBR, Kirschvink JL. Magnetotactic bacteria and single-domain magnetite in hemipelagic sediments. Nature. 1986;321:849–851. doi: 10.1038/321849a0. [DOI] [Google Scholar]
- 60.Petermann H, Bleil U. Detection of live magnetotactic bacteria in South Atlantic deep-sea sediments. Earth Planet. Sci. Lett. 1993;117:223–228. doi: 10.1016/0012-821X(93)90128-V. [DOI] [Google Scholar]
- 61.Tan SM, Ismail MH, Cao B. Biodiversity of magnetotactic bacteria in the tropical marine environment of Singapore revealed by metagenomic analysis. Environ. Res. 2021;194:110714. doi: 10.1016/j.envres.2021.110714. [DOI] [PubMed] [Google Scholar]
- 62.Liu J, et al. Bacterial community structure and novel species of magnetotactic bacteria in sediments from a seamount in the Mariana volcanic arc. Sci. Rep. 2017;7:1–11. doi: 10.1038/s41598-016-0028-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Xu C, Zhang W, Pan H, Du H, Xiao T. Distribution and diversity of magnetotactic bacteria in sediments of the Yellow Sea continental shelf. J. Soils Sediment. 2018;18:2634–2646. doi: 10.1007/s11368-018-1912-8. [DOI] [Google Scholar]
- 64.Lefèvre CT, Frankel RB, Posfai M, Prozorov T, Bazylinski DA. Isolation of obligately alkaliphilic magnetotactic bacteria from extremely alkaline environments. Environ. Microbiol. 2011;13:2342–2350. doi: 10.1111/j.1462-2920.2011.02505.x. [DOI] [PubMed] [Google Scholar]
- 65.Abreu, F., Carolina, A., Araujo, V., Leão, P., Silva, K.T., and Carvalho, F.M.D. Culture-independent characterization of novel psychrophilic magnetotactic cocci from Antarctic marine sediments. Environ Microbiol18, 4426–4441 (2016). [DOI] [PubMed]
- 66.Nash, C. Z. Mechanisms and Evolution of Magnetotactic Bacteria (California Institute of Technology, 2008).
- 67.Lefèvre CT, et al. Moderately thermophilic magnetotactic bacteria from hot springs in Nevada. Appl. Environ. Microbiol. 2010;76:3740–3743. doi: 10.1128/AEM.03018-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Lefèvre CT, et al. Novel magnetite-producing magnetotactic bacteria belonging to the Gammaproteobacteria. ISME J. 2012;6:440–450. doi: 10.1038/ismej.2011.97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Liu P, et al. Identification and characterization of magnetotactic Gammaproteobacteria from a salt evaporation pool, Bohai Bay, China. Environ. Microbiol. 2021 doi: 10.1111/1462-2920.15516. [DOI] [PubMed] [Google Scholar]
- 70.Lyons TW, Reinhard CT, Planavsky NJ. The rise of oxygen in Earth’s early ocean and atmosphere. Nature. 2014;506:307–315. doi: 10.1038/nature13068. [DOI] [PubMed] [Google Scholar]
- 71.Holland HD. The oceans: a possible source of iron in iron-formations. Econ. Geol. 1973;68:1169–1172. doi: 10.2113/gsecongeo.68.7.1169. [DOI] [Google Scholar]
- 72.Konhauser KO, et al. Iron formations: a global record of Neoarchaean to Palaeoproterozoic environmental history. Earth-Sci. Rev. 2017;172:140–177. doi: 10.1016/j.earscirev.2017.06.012. [DOI] [Google Scholar]
- 73.de Baar, H. J. W. & de Jong, J. T. M. Distributions, sources and sinks of iron in seawater. in The Biogeochemistry of Iron in Seawater (eds. Turner, D. R. & Hunter, K. A.) 123–253 (John Wiley & Sons Ltd., 2001).
- 74.Walker JCG, Brimblecombe P. Iron and sulfur in the pre-biologic ocean. Precambrian Res. 1985;28:205–222. doi: 10.1016/0301-9268(85)90031-2. [DOI] [PubMed] [Google Scholar]
- 75.Habicht KS, Gade M, Thamdrup B, Berg P, Canfield DE. Calibration of sulfate levels in the Archean ocean. Science. 2002;298:2372–2374. doi: 10.1126/science.1078265. [DOI] [PubMed] [Google Scholar]
- 76.Catling DC, Zahnle KJ. The Archean atmosphere. Sci. Adv. 2020;6:eaax1420. doi: 10.1126/sciadv.aax1420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Holland HD. The chemical evolution of the atmosphere and oceans. Geol. Mag. 1985;122:404–405. doi: 10.1017/S0016756800031873. [DOI] [Google Scholar]
- 78.Canfield DE, Poulton SW, Narbonne GM. Late-Neoproterozoic deep-ocean oxygenation and the rise of animal life. Science. 2007;315:92–95. doi: 10.1126/science.1135013. [DOI] [PubMed] [Google Scholar]
- 79.Knoll AH, Nowak MA. The timetable of evolution. Sci. Adv. 2017;3:e1603076. doi: 10.1126/sciadv.1603076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Betts HC, et al. Integrated genomic and fossil evidence illuminates life’s early evolution and eukaryote origin. Nat. Ecol. Evol. 2018;2:1556–1562. doi: 10.1038/s41559-018-0644-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Nutman AP, Bennett VC, Friend CRL, van Kranendonk MJ, Chivas AR. Rapid emergence of life shown by discovery of 3,700-million-year-old microbial structures. Nature. 2016;537:535–538. doi: 10.1038/nature19355. [DOI] [PubMed] [Google Scholar]
- 82.Bell EA, Boehnke P, Harrison TM, Mao WL. Potentially biogenic carbon preserved in a 4.1 billion-year-old zircon. Proc. Natl Acad. Sci. USA. 2015;112:14518–14521. doi: 10.1073/pnas.1517557112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Kopp RE, Kirschvink JL. The identification and biogeochemical interpretation of fossil magnetotactic bacteria. Earth-Sci. Rev. 2008;86:42–61. doi: 10.1016/j.earscirev.2007.08.001. [DOI] [Google Scholar]
- 84.Hounslow MW, Maher BA. Quantitative extraction and analysis of carriers of magnetization in sediments. Geophys. J. Int. 1996;124:57–74. doi: 10.1111/j.1365-246X.1996.tb06352.x. [DOI] [Google Scholar]
- 85.Montgomery P, Hailwood EA, Gale AS, Burnett JA. The magnetostratigraphy of Coniacian late Campanian chalk sequences in southern England. Earth Planet. Sci. Lett. 1998;156:209–224. doi: 10.1016/S0012-821X(98)00008-9. [DOI] [Google Scholar]
- 86.Blankenship RE. Early evolution of photosynthesis. Plant Physiol. 2010;154:434–438. doi: 10.1104/pp.110.161687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Williams BP, Aubry S, Hibberd JM. Molecular evolution of genes recruited into C 4 photosynthesis. Trends Plant Sci. 2012;17:213–220. doi: 10.1016/j.tplants.2012.01.008. [DOI] [PubMed] [Google Scholar]
- 88.Ilbert M, Bonnefoy V. Insight into the evolution of the iron oxidation pathways. Biochim. Biophys. Acta Bioenerg. 2013;1827:161–175. doi: 10.1016/j.bbabio.2012.10.001. [DOI] [PubMed] [Google Scholar]
- 89.Camacho A, Walter XA, Picazo A, Zopfi J. Photoferrotrophy: remains of an ancient photosynthesis in modern environments. Front. Microbiol. 2017;8:323. doi: 10.3389/fmicb.2017.00323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Simmons, S. L. & Edwards, K. J. Geobiology of magnetotactic bacteria. In Magnetoreception and Magnetosomes in Bacteria (ed. Schüler, D.) 77–102 (Springer, 2006).
- 91.Strbak O, Dobrota D. Archean iron-based metabolism analysis and the photoferrotrophy-driven hypothesis of microbial magnetotaxis origin. Geomicrobiol. J. 2019;36:278–290. doi: 10.1080/01490451.2018.1554013. [DOI] [Google Scholar]
- 92.Muñoz D, et al. Magnetosomes could be protective shields against metal stress in magnetotactic bacteria. Sci. Rep. 2020;10:11430. doi: 10.1038/s41598-020-68183-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Monteil CL, et al. Genomic study of a novel magnetotactic Alphaproteobacteria uncovers the multiple ancestry of magnetotaxis. Environ. Microbiol. 2018;20:4415–4430. doi: 10.1111/1462-2920.14364. [DOI] [PubMed] [Google Scholar]
- 94.Du H, et al. Magnetosome gene duplication as an important driver in the evolution of magnetotaxis in the Alphaproteobacteria. mSystems. 2019;4:e00315–e00319. doi: 10.1128/mSystems.00315-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Monteil CL, et al. Repeated horizontal gene transfers triggered parallel evolution of magnetotaxis in two evolutionary divergent lineages of magnetotactic bacteria. ISME J. 2020;14:1783–1794. doi: 10.1038/s41396-020-0647-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Natan, E., Fitak, R. R., Werber, Y. & Vortman, Y. Symbiotic magnetic sensing: raising evidence and beyond. Philos. Trans. R. Soc. Lond. B375, 20190595 (2020). [DOI] [PMC free article] [PubMed]
- 97.Natan E, Vortman Y. The symbiotic magnetic-sensing hypothesis: do magnetotactic bacteria underlie the magnetic sensing capability of animals? Mov. Ecol. 2017;5:22. doi: 10.1186/s40462-017-0113-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Simon RA, et al. Magnetotactic bacteria from the human gut microbiome associated with orientation and navigation regions of the brain. J. Oceanol. Limnol. 2021;39:2044–2052. doi: 10.1007/s00343-021-0495-1. [DOI] [Google Scholar]
- 99.Bellinger, M. R. et al. Conservation of magnetite biomineralization genes in all domains of life and implications for magnetic sensing. Proc. Natl. Acad. Sci. USA119, e2108655119 (2022). [DOI] [PMC free article] [PubMed]
- 100.Yuan W, Zhou H, Yang Z, Hein JR, Yang Q. Magnetite magnetofossils record biogeochemical remanent magnetization in hydrogenetic ferromanganese crusts. Geology. 2020;48:298–302. doi: 10.1130/G46881.1. [DOI] [Google Scholar]
- 101.Jiang XD, et al. Characterization and quantification of magnetofossils within abyssal manganese nodules from the Western Pacific Ocean and implications for nodule formation. Geochem. Geophys. Geosyst. 2020;21:e2019GC008811. [Google Scholar]
- 102.Hassan Mbin, et al. Presence of biogenic magnetite in ferromanganese nodules. Environ. Microbiol. Rep. 2020;12:288–295. doi: 10.1111/1758-2229.12831. [DOI] [PubMed] [Google Scholar]
- 103.Oda H, Nakasato Y, Usui A. Characterization of marine ferromanganese crust from the Pacific using residues of selective chemical leaching: Identification of fossil magnetotactic bacteria with FE-SEM and rock magnetic methods. Earth, Planets Space. 2018;70:1–10. doi: 10.1186/s40623-018-0924-3. [DOI] [Google Scholar]
- 104.Kirschvink JL, Chang SBR. Ultrafine-grained magnetite in deep-sea sediments—possible bacterial magnetofossils. Geology. 1984;12:559–562. doi: 10.1130/0091-7613(1984)12<559:UMIDSP>2.0.CO;2. [DOI] [Google Scholar]
- 105.Petersen N, von Dobeneck T, Vali H. Fossil bacterial magnetite in deep-sea sediments from the South-Atlantic Ocean. Nature. 1986;320:611–615. doi: 10.1038/320611a0. [DOI] [Google Scholar]
- 106.Li J, et al. Biomineralization, crystallography and magnetic properties of bullet-shaped magnetite magnetosomes in giant rod magnetotactic bacteria. Earth Planet. Sci. Lett. 2010;293:368–376. doi: 10.1016/j.epsl.2010.03.007. [DOI] [Google Scholar]
- 107.Moskowitz BM, Frankel RB, Bazylinski DA. Rock magnetic criteria for the detection of biogenic magnetite. Earth Planet. Sci. Lett. 1993;120:283–300. doi: 10.1016/0012-821X(93)90245-5. [DOI] [Google Scholar]
- 108.Chang L, et al. Low-temperature magnetic properties of pelagic carbonates: oxidation of biogenic magnetite and identification of magnetosome chains. J. Geophys. Res. Solid Earth. 2013;118:6049–6065. doi: 10.1002/2013JB010381. [DOI] [Google Scholar]
- 109.Chang L, Heslop D, Roberts AP, Rey D, Mohamed KJ. Discrimination of biogenic and detrital magnetite through a double Verwey transition temperature. J. Geophys. Res. Solid Earth. 2016;121:3–14. doi: 10.1002/2015JB012485. [DOI] [Google Scholar]
- 110.Jackson MJ, Moskowitz B. On the distribution of Verwey transition temperatures in natural magnetites. Geophys. J. Int. 2021;224:1314–1325. doi: 10.1093/gji/ggaa516. [DOI] [Google Scholar]
- 111.Pike CR, Roberts AP, Verosub KL. Characterizing interactions in fine magnetic particle systems using first order reversal curves. J. Appl. Phys. 1999;85:6660–6667. doi: 10.1063/1.370176. [DOI] [Google Scholar]
- 112.Roberts AP, Pike CR, Verosub KL. First-order reversal curve diagrams: a new tool for characterizing the magnetic properties of natural samples. J. Geophys. Res. Solid Earth. 2000;105:28461–28475. doi: 10.1029/2000JB900326. [DOI] [Google Scholar]
- 113.Roberts AP, Heslop D, Zhao X, Pike CR. Understanding fine magnetic particle systems through use of first-order reversal curve diagrams. Rev. Geophys. 2014;52:557–602. doi: 10.1002/2014RG000462. [DOI] [Google Scholar]
- 114.Dunin-Borkowski RE, et al. Magnetic microstructure of magnetotactic bacteria by electron holography. Science. 1998;282:1868–1870. doi: 10.1126/science.282.5395.1868. [DOI] [PubMed] [Google Scholar]
- 115.Pan YX, et al. Rock magnetic properties of uncultured magnetotactic bacteria. Earth Planet. Sci. Lett. 2005;237:311–325. doi: 10.1016/j.epsl.2005.06.029. [DOI] [Google Scholar]
- 116.Roberts AP, et al. Magnetotactic bacterial abundance in pelagic marine environments is limited by organic carbon flux and availability of dissolved iron. Earth Planet. Sci. Lett. 2011;310:441–452. doi: 10.1016/j.epsl.2011.08.011. [DOI] [Google Scholar]
- 117.Chen AP, Egli R, Moskowitz BM. First-order reversal curve (FORC) diagrams of natural and cultured biogenic magnetic particles. J. Geophys. Res. Solid Earth. 2007;112:B08S90. [Google Scholar]
- 118.Egli R, Chen AP, Winklhofer M, Kodama KP, Horng C-S. Detection of noninteracting single domain particles using first-order reversal curve diagrams. Geochem. Geophys. Geosyst. 2010;11:Q01Z11. doi: 10.1029/2009GC002916. [DOI] [Google Scholar]
- 119.Li J, Wu W, Liu Q, Pan Y. Magnetic anisotropy, magnetostatic interactions and identification of magnetofossils. Geochem. Geophys. Geosyst. 2012;13:Q10Z51. doi: 10.1029/2012GC004384. [DOI] [Google Scholar]
- 120.Amor M, et al. Key signatures of magnetofossils elucidated by mutant magnetotactic bacteria and micromagnetic calculations. J. Geophys. Res. Solid Earth. 2022;127:e2021JB023239. doi: 10.1029/2021JB023239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Charilaou M, Winklhofer M, Gehring AU. Simulation of ferromagnetic resonance spectra of linear chains of magnetite nanocrystals. J. Appl. Phys. 2011;109:093903. doi: 10.1063/1.3581103. [DOI] [Google Scholar]
- 122.Weiss BP, et al. Ferromagnetic resonance and low-temperature magnetic tests for biogenic magnetite. Earth Planet. Sci. Lett. 2004;224:73–89. doi: 10.1016/j.epsl.2004.04.024. [DOI] [Google Scholar]
- 123.Kopp RE, et al. Ferromagnetic resonance spectroscopy for assessment of magnetic anisotropy and magnetostatic interactions: a case study of mutant magnetotactic bacteria. J. Geophys. Res. Solid Earth. 2006;111:B12S25. doi: 10.1029/2006JB004529. [DOI] [Google Scholar]
- 124.Kodama KP, Moeller RE, Bazylinski DA, Kopp RE, Chen AP. The mineral magnetic record of magnetofossils in recent lake sediments of Lake Ely, PA. Glob. Planet. Change. 2013;110:350–363. doi: 10.1016/j.gloplacha.2013.03.012. [DOI] [Google Scholar]
- 125.Chang L, et al. Magnetic detection and characterization of biogenic magnetic minerals: a comparison of ferromagnetic resonance and first‐order reversal curve diagrams. J. Geophys. Res. Solid Earth. 2014;119:6136–6158. doi: 10.1002/2014JB011213. [DOI] [Google Scholar]
- 126.Kind J, Gehring AU, Winklhofer M, Hirt AM. Combined use of magnetometry and spectroscopy for identifying magnetofossils in sediments. Geochem. Geophys. Geosyst. 2011;12:Q08008. doi: 10.1029/2011GC003633. [DOI] [Google Scholar]
- 127.Blattmann TM, et al. Ferromagnetic resonance of magnetite biominerals traces redox changes. Earth Planet. Sci. Lett. 2020;545:116400. doi: 10.1016/j.epsl.2020.116400. [DOI] [Google Scholar]
- 128.Larrasoaña JC, et al. Magnetotactic bacterial response to Antarctic dust supply during the Palaeocene-Eocene thermal maximum. Earth Planet. Sci. Lett. 2012;333:122–133. doi: 10.1016/j.epsl.2012.04.003. [DOI] [Google Scholar]
- 129.Muxworthy AR, Williams W. Critical single-domain/multidomain grain sizes in noninteracting and interacting elongated magnetite particles: implications for magnetosomes. J. Geophys. Res. Solid Earth. 2006;111:B12S12. [Google Scholar]
- 130.Chang L, et al. Coupled microbial bloom and oxygenation decline recorded by magnetofossils during the Palaeocene–Eocene thermal maximum. Nat. Commun. 2018;9:4007. doi: 10.1038/s41467-018-06472-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Berndt TA, Chang L, Pei Z. Mind the gap: towards a biogenic magnetite palaeoenvironmental proxy through an extensive finite-element micromagnetic simulation. Earth Planet. Sci. Lett. 2020;532:116010. doi: 10.1016/j.epsl.2019.116010. [DOI] [Google Scholar]
- 132.Heslop D, et al. Quantifying magnetite magnetofossil contributions to sedimentary magnetizations. Earth Planet. Sci. Lett. 2013;382:58–65. doi: 10.1016/j.epsl.2013.09.011. [DOI] [Google Scholar]
- 133.Kruiver PP, Dekkers MJ, Heslop D. Quantification of magnetic coercivity components by the analysis of acquisition curves of isothermal remanent magnetisation. Earth Planet. Sci. Lett. 2001;189:269–276. doi: 10.1016/S0012-821X(01)00367-3. [DOI] [Google Scholar]
- 134.Egli R. Analysis of the field dependence of remanent magnetization curves. J. Geophys. Res. Solid Earth. 2003;108:2081. doi: 10.1029/2002JB002023. [DOI] [Google Scholar]
- 135.Zhao X, Fujii M, Suganuma Y, Zhao X, Jiang Z. Applying the Burr type XII distribution to decompose remanent magnetization curves. J. Geophys. Res. Solid Earth. 2018;123:8298–8311. doi: 10.1029/2018JB016082. [DOI] [Google Scholar]
- 136.Egli R. Characterization of individual rock magnetic components by analysis of remanence curves. 2. Fundamental properties of coercivity distributions. Phys. Chem. Earth. 2004;29:851–867. [Google Scholar]
- 137.Heslop D, Dillon M. Unmixing magnetic remanence curves without a priori knowledge. Geophys. J. Int. 2007;170:556–566. doi: 10.1111/j.1365-246X.2007.03432.x. [DOI] [Google Scholar]
- 138.Usui Y, Yamazaki T, Saitoh M. Changing abundance of magnetofossil morphologies in pelagic red clay around Minamitorishima, Western North Pacific. Geochem. Geophys. Geosyst. 2017;18:4558–4572. doi: 10.1002/2017GC007127. [DOI] [Google Scholar]
- 139.Lascu I, et al. Magnetic unmixing of first-order reversal curve diagrams using principal component analysis. Geochem. Geophys. Geosyst. 2015;16:2900–2915. doi: 10.1002/2015GC005909. [DOI] [Google Scholar]
- 140.Harrison RJ, et al. An improved algorithm for unmixing first‐order reversal curve diagrams using principal component analysis. Geochem. Geophys. Geosyst. 2018;19:1595–1610. doi: 10.1029/2018GC007511. [DOI] [Google Scholar]
- 141.Channell JET, et al. Magnetic record of deglaciation using FORC-PCA, sortable-silt grain size, and magnetic excursion at 26 ka, from the Rockall Trough (NE Atlantic) Geochem. Geophys. Geosyst. 2016;17:1823–1841. doi: 10.1002/2016GC006300. [DOI] [Google Scholar]
- 142.Roberts AP, et al. Signatures of reductive magnetic mineral diagenesis from unmixing of first-order reversal curves. J. Geophys. Res. Solid Earth. 2018;123:4500–4522. doi: 10.1029/2018JB015706. [DOI] [Google Scholar]
- 143.Yamazaki T, Fu W, Shimono T, Usui Y. Unmixing biogenic and terrigenous magnetic mineral components in red clay of the Pacific Ocean using principal component analyses of first-order reversal curve diagrams and paleoenvironmental implications. Earth, Planets Space. 2020;72:120. doi: 10.1186/s40623-020-01248-5. [DOI] [Google Scholar]
- 144.Egli R. Characterization of individual rock magnetic components by analysis of remanence curves, 1. Unmixing natural sediments. Stud. Geophys. Geod. 2004;48:391–446. doi: 10.1023/B:SGEG.0000020839.45304.6d. [DOI] [Google Scholar]
- 145.Qian Y, et al. Assessment and integration of bulk and component-specific methods for identifying mineral magnetic assemblages in environmental magnetism. J. Geophys. Res. Solid Earth. 2020;125:1–19. doi: 10.1029/2019JB019024. [DOI] [Google Scholar]
- 146.Inoue K, Yamazaki T, Usui Y. Influence of magnetofossils on paleointensity estimations inferred from principal component analyses of first‐order reversal curve diagrams for sediments from the Western Equatorial Pacific. Geochem. Geophys. Geosyst. 2021;22:1–15. doi: 10.1029/2021GC010081. [DOI] [Google Scholar]
- 147.Wagner CL, et al. Diversification of iron-biomineralizing organisms during the Paleocene-Eocene thermal maximum: evidence from quantitative unmixing of magnetic signatures of conventional and giant magnetofossils. Paleoceanogr. Paleoclimatol. 2021;36:1–25. doi: 10.1029/2021PA004225. [DOI] [Google Scholar]
- 148.Zhang Q, et al. Magnetotactic bacterial activity in the North Pacific Ocean and its relationship to Asian dust inputs and primary productivity since 8.0 Ma. Geophys. Res. Lett. 2021;48:1–11. [Google Scholar]
- 149.Hesse PP. Evidence for bacterial palaeoecological origin of mineral magnetic cycles in oxic and sub-oxic Tasman Sea sediments. Mar. Geol. 1994;117:1–17. doi: 10.1016/0025-3227(94)90003-5. [DOI] [Google Scholar]
- 150.Yamazaki T, Kawahata H. Organic carbon flux controls the morphology of magnetofossils in marine sediments. Geology. 1998;26:1064–1066. doi: 10.1130/0091-7613(1998)026<1064:OCFCTM>2.3.CO;2. [DOI] [Google Scholar]
- 151.He K, Pan Y. Magnetofossil abundance and diversity as paleoenvironmental proxies: a case study from Southwest Iberian margin sediments. Geophys. Res. Lett. 2020;47:e2020GL087165. [Google Scholar]
- 152.Lean CMB, McCave IN. Glacial to interglacial mineral magnetic and palaeoceanographic changes at Chatham Rise, SW Pacific Ocean. Earth Planet. Sci. Lett. 1998;163:247–260. doi: 10.1016/S0012-821X(98)00191-5. [DOI] [Google Scholar]
- 153.Yamazaki T. Reductive dissolution of biogenic magnetite. Earth, Planets Space. 2020;72:150. doi: 10.1186/s40623-020-01290-3. [DOI] [Google Scholar]
- 154.Mathias GL, et al. A multi-proxy approach to unravel late Pleistocene sediment flux and bottom water conditions in the western South Atlantic Ocean. Paleoceanogr. Paleoclimatol. 2021;36:e2020PA004058. doi: 10.1029/2020PA004058. [DOI] [Google Scholar]
- 155.Rodelli D, et al. Diagenetic fate of biogenic soft and hard magnetite in chemically stratified sedimentary environments of Mamangua Ria, Brazil. J. Geophys. Res. Solid Earth. 2019;124:2313–2330. doi: 10.1029/2018JB016576. [DOI] [Google Scholar]
- 156.Ouyang T, et al. Variable remanence acquisition efficiency in sediments containing biogenic and detrital magnetites: implications for relative paleointensity signal recording. Geochem. Geophys. Geosyst. 2014;15:2780–2796. doi: 10.1002/2014GC005301. [DOI] [Google Scholar]
- 157.Chen L, et al. Remanence acquisition efficiency in biogenic and detrital magnetite and recording of geomagnetic paleointensity. Geochem. Geophys. Geosyst. 2017;18:1435–1450. doi: 10.1002/2016GC006753. [DOI] [Google Scholar]
- 158.Li J, et al. Paleomagnetism of a sediment core taken from the Ontong-Java Plateau: for better understanding of the role of biogenic magnetite in geomagnetic paleointensity recording. Preprint. 2021 doi: 10.1002/essoar.10507999.1. [DOI] [Google Scholar]
- 159.Tarduno JA, Tian WL, Wilkison S. Biogeochemical remanent magnetization in pelagic sediments of the western equatorial Pacific Ocean. Geophys. Res. Lett. 1998;25:3987–3990. doi: 10.1029/1998GL900079. [DOI] [Google Scholar]
- 160.Yamazaki T, Suzuki Y, Kouduka M, Kawamura N. Dependence of bacterial magnetosome morphology on chemical conditions in deep-sea sediments. Earth Planet. Sci. Lett. 2019;513:135–143. doi: 10.1016/j.epsl.2019.02.015. [DOI] [Google Scholar]
- 161.Vasiliev I, et al. Putative greigite magnetofossils from the Pliocene epoch. Nat. Geosci. 2008;1:782–786. doi: 10.1038/ngeo335. [DOI] [Google Scholar]
- 162.Pósfai M, et al. Crystal-size distributions and possible biogenic origin of Fe sulfides. Eur. J. Mineral. 2001;13:691–703. doi: 10.1127/0935-1221/2001/0013-0691. [DOI] [Google Scholar]
- 163.Chang L, et al. Identification and environmental interpretation of diagenetic and biogenic greigite in sediments: a lesson from the Messinian Black Sea. Geochem. Geophys. Geosyst. 2014;15:3612–3627. doi: 10.1002/2014GC005411. [DOI] [Google Scholar]
- 164.Bazylinski DA, Blakemore RP. Nitrogen fixation (acetylene reduction) in Aquaspirillum magnetotacticum. Curr. Microbiol. 1983;9:305–308. doi: 10.1007/BF01588824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Jiang W, et al. Submerged culture of Magnetospirillum gryphiswaldense under N2-fixing condition and regulation of activity of nitrogen fixation. Chin. Sci. Bull. 2002;47:2095–2099. doi: 10.1360/02tb9455. [DOI] [Google Scholar]
- 166.Bazylinski DA, et al. Chemolithoautotrophy in the marine, magnetotactic bacterial strains MV-1 and MV-2. Arch. Microbiol. 2004;182:373–387. doi: 10.1007/s00203-004-0716-y. [DOI] [PubMed] [Google Scholar]
- 167.Schultheiss D, Handrick R, Jendrossek D, Hanzlik M, Schüler D. The presumptive magnetosome protein Mms16 is a poly(3-hydroxybutyrate) granule-bound protein (phasin) in Magnetospirillum gryphiswaldense. J. Bacteriol. 2005;187:2416–2425. doi: 10.1128/JB.187.7.2416-2425.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Rivas-Lamelo S, et al. Magnetotactic bacteria as a new model for P sequestration in the ferruginous Lake Pavin. Geochem. Perspect. Lett. 2017;5:35–41. doi: 10.7185/geochemlet.1743. [DOI] [Google Scholar]
- 169.Schulz-Vogt HN, et al. Effect of large magnetotactic bacteria with polyphosphate inclusions on the phosphate profile of the suboxic zone in the Black Sea. ISME J. 2019;13:1198–1208. doi: 10.1038/s41396-018-0315-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Tanaka M, et al. Biomagnetic recovery and bioaccumulation of selenium granules in magnetotactic bacteria. Appl. Environ. Microbiol. 2016;82:3886–3891. doi: 10.1128/AEM.00508-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Isambert A, Menguy N, Larquet E, Guyot F, Valet J-P. Transmission electron microscopy study of magnetites in a freshwater population of magnetotactic bacteria. Am. Mineral. 2007;92:621–630. doi: 10.2138/am.2007.2278. [DOI] [Google Scholar]
- 172.Cornejo E, Subramanian P, Li Z, Jensen GJ, Komeili A. Dynamic remodeling of the magnetosome membrane is triggered by the initiation of biomineralization. MBio. 2016;7:e01898–15. doi: 10.1128/mBio.01898-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Li J, et al. Bullet-shaped magnetite biomineralization within a magnetotactic Deltaproteobacterium: implications for magnetofossil identification. J. Geophys. Res. Biogeosci. 2020;125:e2020JG005680. [Google Scholar]
- 174.Bazylinski DA. Structure and function of the bacterial magnetosome. ASM N. 1995;61:337–343. [Google Scholar]
- 175.Simmons SL, Bazylinski DA, Edwards KJ. South-seeking magnetotactic bacteria in the Northern Hemisphere. Science. 2006;311:371–374. doi: 10.1126/science.1122843. [DOI] [PubMed] [Google Scholar]
- 176.Faivre D, Fischer A, Garcia-Rubio I, Mastrogiacomo G, Gehring AU. Development of cellular magnetic dipoles in magnetotactic bacteria. Biophys. J. 2010;99:1268–1273. doi: 10.1016/j.bpj.2010.05.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177.Coale KH, et al. Southern ocean iron enrichment experiment: carbon cycling in high- and low-Si waters. Science. 2004;304:408–414. doi: 10.1126/science.1089778. [DOI] [PubMed] [Google Scholar]
- 178.Jacq V, Ridame C, L’Helguen S, Kaczmar F, Saliot A. Response of the unicellular diazotrophic cyanobacterium Crocosphaera watsonii to iron limitation. PLoS ONE. 2014;9:e86749. doi: 10.1371/journal.pone.0086749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.Martínez-García A, Winckler G. Iron fertilization in the glacial ocean. PAGES Mag. 2014;22:82–83. doi: 10.22498/pages.22.2.82. [DOI] [Google Scholar]
- 180.Street JH, Paytan A. Iron, phytoplankton growth, and the carbon cycle. Met. Ions Biol. Syst. 2005;43:153–193. doi: 10.1201/9780824751999.ch7. [DOI] [PubMed] [Google Scholar]
- 181.Aumont O, Bopp L. Globalizing results from ocean in situ iron fertilization studies. Glob. Biogeochem. Cycles. 2006;20:GB2017. doi: 10.1029/2005GB002591. [DOI] [Google Scholar]
- 182.Wambeke Fvan, et al. Nutrient limitation of primary productivity in the Southeast Pacific. Biogeosciences. 2008;5:215–225. doi: 10.5194/bg-5-215-2008. [DOI] [Google Scholar]
- 183.Schoffman H, Lis H, Shaked Y, Keren N. Iron-nutrient interactions within phytoplankton. Front. Plant Sci. 2016;7:1223. doi: 10.3389/fpls.2016.01223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184.Amor M, Tharaud M, Gélabert A, Komeili A. Single-cell determination of iron content in magnetotactic bacteria: implications for the iron biogeochemical cycle. Environ. Microbiol. 2020;22:823–831. doi: 10.1111/1462-2920.14708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185.Vainshtein M, Suzina N, Sorokin V. A new type of magnet-sensitive inclusions in cells of photosynthetic purple bacteria. Syst. Appl. Microbiol. 1997;20:182–186. doi: 10.1016/S0723-2020(97)80064-1. [DOI] [Google Scholar]
- 186.Sakaguchi T, Arakaki A, Matsunaga T. Desulfovibrio magneticus sp. nov., a novel sulfate-reducing bacterium that produces intracellular single-domain-sized magnetite particles. Int. J. Syst. Evol. Microbiol. 2002;52:215–221. doi: 10.1099/00207713-52-1-215. [DOI] [PubMed] [Google Scholar]
- 187.Rahn-Lee L, et al. A genetic strategy for probing the functional diversity of magnetosome formation. PLoS Genet. 2015;11:e1004811. doi: 10.1371/journal.pgen.1004811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 188.Grant, C. R. et al. Distinct gene clusters drive formation of ferrosome organelles in bacteria. Nature (2022) 10.1038/s41586-022-04741-x. [DOI] [PMC free article] [PubMed]
- 189.Amor M, et al. Magnetotactic bacteria accumulate a large pool of iron distinct from their magnetite crystals. Appl. Environ. Microbiol. 2020;86:1–20. doi: 10.1128/AEM.01278-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 190.Lefèvre CT, Frankel RB, Abreu F, Lins U, Bazylinski DA. Culture‐independent characterization of a novel, uncultivated magnetotactic member of the Nitrospirae phylum. Environ. Microbiol. 2011;13:538–549. doi: 10.1111/j.1462-2920.2010.02361.x. [DOI] [PubMed] [Google Scholar]
- 191.Lin W, Li J, Pan Y. Newly isolated but uncultivated magnetotactic bacterium of the phylum Nitrospirae from Beijing, China. Appl. Environ. Microbiol. 2012;78:668–675. doi: 10.1128/AEM.06764-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 192.Spring S, Bazylinski DA. Magnetotactic bacteria. Prokaryotes. 2006;2:842–862. doi: 10.1007/0-387-30742-7_26. [DOI] [Google Scholar]
- 193.Kolinko S, Richter M, Gloeckner F-O, Brachmann A, Schüler D. Single-cell genomics of uncultivated deep-branching magnetotactic bacteria reveals a conserved set of magnetosome genes. Environ. Microbiol. 2016;18:21–37. doi: 10.1111/1462-2920.12907. [DOI] [PubMed] [Google Scholar]
- 194.Sen J. The role of associated nitrogen-fixing bacteria on nitrogen nutrition of cereal crops. Agric. J. India. 1929;24:967–980. [Google Scholar]
- 195.Rubin-Blum M, Dubilier N, Kleiner M. Genetic evidence for two carbon fixation pathways (the Calvin-Benson-Bassham Cycle and the Reverse Tricarboxylic Acid Cycle) in symbiotic and free-living bacteria. Msphere. 2019;4:e00394–18. doi: 10.1128/mSphere.00394-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 196.Hoppenrath, M., Elbrächter, M. & Drebes, G. Marine Phytoplankton: Selected Microphytoplankton Species from the North Sea Around Helgoland and Sylt (E. Schweizerbart’sche Verlagsbuchhandlung, 2009).
- 197.Matin A. Organic nutrition of chemolithotrophic bacteria. Annu. Rev. Microbiol. 1978;32:433–468. doi: 10.1146/annurev.mi.32.100178.002245. [DOI] [PubMed] [Google Scholar]
- 198.Paoli GC, Morgan NS, Tabita FR, Shively JM. Expression of the cbbLcbbS and cbbM genes and distinct organization of the cbb Calvin cycle structural genes of Rhodobacter capsulatus. Arch. Microbiol. 1995;164:396–405. [PubMed] [Google Scholar]
- 199.Williams TJ, Zhang CL, Scott JH, Bazylinski DA. Evidence for autotrophy via the reverse tricarboxylic acid cycle in the marine magnetotactic coccus strain MC-1. Appl. Environ. Microbiol. 2006;72:1322–1329. doi: 10.1128/AEM.72.2.1322-1329.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 200.Zhang W, Wang Y, Liu L, Pan Y, Lin W. Identification and genomic characterization of two previously unknown magnetotactic Nitrospirae. Front. Microbiol. 2021;12:690052. doi: 10.3389/fmicb.2021.690052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 201.Monteil CL, et al. Intracellular amorphous Ca-carbonate and magnetite biomineralization by a magnetotactic bacterium affiliated to the Alphaproteobacteria. ISME J. 2021;15:1–18. doi: 10.1038/s41396-020-00747-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 202.Arakaki A, Takeyama H, Tanaka T, Matsunaga T. Cadmium recovery by a sulfate-reducing magnetotactic bacterium, Desulfovibrio magneticus RS-1, using magnetic separation. Appl. Biochem. Biotech. 2002;98:833–840. doi: 10.1385/ABAB:98-100:1-9:833. [DOI] [PubMed] [Google Scholar]
- 203.Tajer-Mohammad-Ghazvini P, et al. Cobalt separation by Alphaproteobacterium MTB-KTN90: Magnetotactic bacteria in bioremediation. Bioprocess Biosyst. Eng. 2016;39:1899–1911. doi: 10.1007/s00449-016-1664-z. [DOI] [PubMed] [Google Scholar]
- 204.Tanaka M, Arakaki A, Staniland SS, Matsunaga T. Simultaneously discrete biomineralization of magnetite and tellurium nanocrystals in magnetotactic bacteria. Appl. Environ. Microbiol. 2010;76:5526–5532. doi: 10.1128/AEM.00589-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 205.Sannigrahi S, Suthindhiran K. Metal recovery from printed circuit boards by magnetotactic bacteria. Hydrometallurgy. 2019;187:113–124. doi: 10.1016/j.hydromet.2019.05.007. [DOI] [Google Scholar]
- 206.Postec A, et al. Magnetotactic bacteria in microcosms originating from the French Mediterranean coast subjected to oil industry activities. Microb. Ecol. 2012;63:1–11. doi: 10.1007/s00248-011-9910-z. [DOI] [PubMed] [Google Scholar]
- 207.Sewell RBS, Fage L. Minimum oxygen layer in the ocean. Nature. 1948;162:949–951. doi: 10.1038/162949a0. [DOI] [Google Scholar]
- 208.Cavicchioli R, et al. Scientists’ warning to humanity: microorganisms and climate change. Nat. Rev. Microbiol. 2019;17:569–586. doi: 10.1038/s41579-019-0222-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 209.Ulloa O, Canfield DE, DeLong EF, Letelier RM, Stewart FJ. Microbial oceanography of anoxic oxygen minimum zones. Proc. Natl Acad. Sci. USA. 2012;109:15996–16003. doi: 10.1073/pnas.1205009109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 210.Schmidtko S, Stramma L, Visbeck M. Decline in global oceanic oxygen content during the past five decades. Nature. 2017;542:335–339. doi: 10.1038/nature21399. [DOI] [PubMed] [Google Scholar]
- 211.Breitburg, D. et al. Declining oxygen in the global ocean and coastal waters. Science359, eaam7240 (2018). [DOI] [PubMed]
- 212.Bertagnolli AD, Stewart FJ. Microbial niches in marine oxygen minimum zones. Nat. Rev. Microbiol. 2018;16:723–729. doi: 10.1038/s41579-018-0087-z. [DOI] [PubMed] [Google Scholar]
- 213.Wright JJ, Konwar KM, Hallam SJ. Microbial ecology of expanding oxygen minimum zones. Nat. Rev. Microbiol. 2012;10:381–394. doi: 10.1038/nrmicro2778. [DOI] [PubMed] [Google Scholar]
- 214.Paulmier A, Ruiz-Pino D. Oxygen minimum zones (OMZs) in the modern ocean. Progr. Oceanogr. 2009;80:113–128. doi: 10.1016/j.pocean.2008.08.001. [DOI] [Google Scholar]
- 215.Ulloa O, Pantoja S. The oxygen minimum zone of the eastern South Pacific. Deep-Sea Res. II. 2009;56:987–991. doi: 10.1016/j.dsr2.2008.12.004. [DOI] [Google Scholar]
- 216.Stevens H, Ulloa O. Bacterial diversity in the oxygen minimum zone of the eastern tropical South Pacific. Environ. Microbiol. 2008;10:1244–1259. doi: 10.1111/j.1462-2920.2007.01539.x. [DOI] [PubMed] [Google Scholar]
- 217.Lavik G, et al. Detoxification of sulphidic African shelf waters by blooming chemolithotrophs. Nature. 2009;457:581–584. doi: 10.1038/nature07588. [DOI] [PubMed] [Google Scholar]
- 218.Walsh DA, et al. Metagenome of a versatile chemolithoautotroph from expanding oceanic dead zones. Science. 2009;326:578–582. doi: 10.1126/science.1175309. [DOI] [PubMed] [Google Scholar]
- 219.Canfield DE, et al. A cryptic sulfur cycle in oxygen-minimum-zone waters off the Chilean coast. Science. 2010;330:1375–1378. doi: 10.1126/science.1196889. [DOI] [PubMed] [Google Scholar]
- 220.Rhoads DC, Mulsow SG, Gutschick R, Baldwin CT, Stolz JF. The dysaerobic zone revisited: a magnetic facies? Geol. Soc. Lond. Spec. Publ. 1991;58:187–199. doi: 10.1144/GSL.SP.1991.058.01.13. [DOI] [Google Scholar]
- 221.Brune A, Frenzel P, Cypionka H. Life at the oxic-anoxic interface: microbial activities and adaptations. FEMS Microbiol. Rev. 2000;24:691–710. doi: 10.1016/S0168-6445(00)00054-1. [DOI] [PubMed] [Google Scholar]
- 222.Monteil CL, et al. Ectosymbiotic bacteria at the origin of magnetoreception in a marine protist. Nat. Microbiol. 2019;4:1088–1095. doi: 10.1038/s41564-019-0432-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 223.Reitner J, et al. Concretionary methane-seep carbonates and associated microbial communities in Black Sea sediments. Palaeogeogr. Palaeoclimatol. Palaeoecol. 2005;227:18–30. doi: 10.1016/j.palaeo.2005.04.033. [DOI] [Google Scholar]
- 224.Dufour SC, et al. Magnetosome-containing bacteria living as symbionts of bivalves. ISME J. 2014;8:2453–2462. doi: 10.1038/ismej.2014.93. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
All NCBI accession numbers of MTB genomes used in Fig. 5 are as follows-HG794546.1: Magnetospirillum gryphiswaldense MSR-1; CP000471.1: Magnetococcus marinus MC-1; CP032508.2: Gamma proteobacterium SS-5; JADFZC010000123.1: Oligoflexales bacterium isolate nHA4_bin4; JADFZW010000010.1: Oligoflexia bacterium isolate nKLK_bin12; AP010904.1: Desulfovibrio magneticus RS-1; FWEV01000001.1: Candidatus Desulfamplus magnetomortis BW-1 PRJEB14757; NVTF01000001.1: Elusimicrobia bacterium isolate NORP122; JADFYW010000100.1: Fibrobacteria bacterium isolate nGR_bin1; DUZM01000001.1: Candidatus Hydrogenedentes bacterium isolate MAG_17963_hgd_111; ASWY01000001.1: Latescibacteria bacterium SCGC AAA252-B13; JADGAO010000100.1: Nitrospinae bacterium isolate nNGH_bin12; JMFO00000000: Candidatus Magnetobacterium casensis; JADGBR010000010.1: Candidatus Omnitrophica bacterium isolate nW3_bin14; DUZJ01000001.1: Planctomycetes bacterium isolate MAG_11118_pl_115; JADGAS010000010.1: Zetaproteobacteria bacterium isolate nPC_bin1; JADFZL010000010.1: Candidatus Riflebacteria bacterium isolate nHGR_bin4; JADGAT010000100.1: SAR324 cluster bacterium isolate nPCR_bin10; JADGCS010000100.1: Deltaproteobacteria bacterium isolate nXX_bin1. The original data for Fig. 3 can be found at 10.6084/m9.figshare.17122277.v1.
MagCluster (https://github.com/RunJiaJi/magcluster), code: magcluster prokka 19mtb_genomes; KofamKOALA server (https://www.genome.jp/tools/kofamkoala/) 10.1093/bioinformatics/btz859KEGG-Decoder10.1038/s41396-018-0091-3, code: KEGG-decoder -input 19mtb.txt -output out.list -vizoption static -m myorder.txt. Fig. 3b was produced using by Max UnMix and Fig. 3d was drawn using FORCinel v3.06 (https://wserv4.esc.cam.ac.uk/nanopaleomag/?page_id=51).