Abstract
This case report describes the findings of septic pulmonary embolism (SPE) in a young adult male with a history of intravenous drug use who initially presented with signs and symptoms of acute sepsis. The patient underwent evaluation by computed tomography (CT) imaging as well as blood cultures and echocardiography, which confirmed the diagnosis of SPE secondary to Staphylococcus aureus positive bacterial endocarditis. In this case report, we discuss the presentation and characteristic CT imaging findings of SPE as well as highlight the value of this imaging modality in the timely diagnosis and management of this urgent condition.
Keywords: Septic embolism, Computed Tomography, Endocarditis
Introduction
Septic pulmonary embolism (SPE) is a relatively rare form of pulmonary embolism that presents following the embolization of pathogen-containing emboli to the lungs from the pulmonary arteries [1]. The most common predisposing conditions that can precipitate SPE are bacterial endocarditis of the right heart (often in intravenous drug users), infected indwelling catheters or devices, periodontal disease, skin and soft tissue infections, alcoholism, and immunodeficiency, with Staphylococcus aureus being the most seen culprit [2], [3], [4]. In 1978, a case series study analyzing 60 SPE cases found that 78% of these patients were active intravenous drug users [5]. In addition to thorough clinical evaluation and laboratory testing as indicated, radiographic imaging also plays a critical role in the diagnosis and management of pulmonary embolism (PE). SPE is associated with unique imaging findings on computed tomography (CT), which can yield valuable information for reaching a timely and accurate diagnosis [1,[4], [5], [6], [7], [8], [9], [10], [11], [12].
Case report
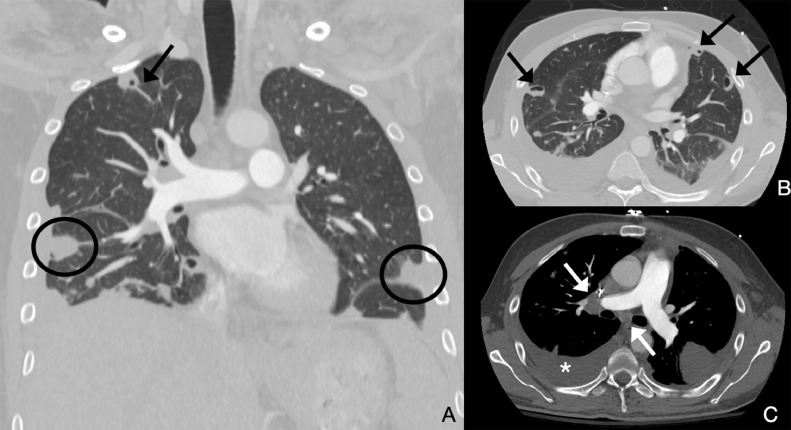
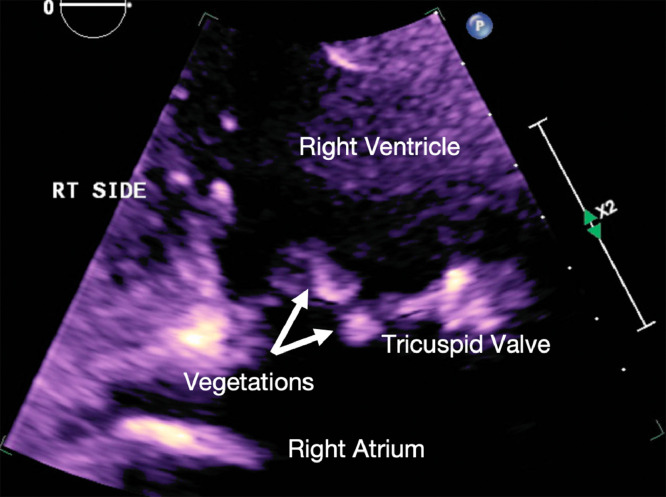
A 28-year-old male with a history of intravenous drug use presented with fever, chills, hypotension, and tachypnea. Blood cultures drawn at this time returned positive for methicillin-susceptible S aureus (MSSA). Transthoracic echocardiogram demonstrated right heart failure with moderate to severe tricuspid regurgitation and 2 vegetations attached to the anterior leaflet of the tricuspid valve measuring 1.6 × 1 cm and 0.8 × 0.6 cm (Fig. 1), indicating bacterial endocarditis. Computed tomography of the chest (Fig. 2) revealed multiple bilateral peripheral wedge-shaped opacities; multiple peripheral pulmonary nodules with central cavitation, some showing a “feeding vessel sign”; reactive lymphadenopathy; and a small right-sided pleural effusion. Antibiotic therapy was initiated with intravenous daptomycin and oral doxycycline for a duration of 3 weeks. Cardiothoracic surgery evaluated the patient and determined that he did not meet the criteria for surgical intervention at this time. The patient's hospital course was complicated by a transient ischemic attack secondary to septic emboli and endocarditis-related glomerulonephritis. Following completion of antibiotic therapy with supportive care and normalization of creatinine, the patient was discharged and directed to follow up with cardiology, nephrology, and infectious disease.
Fig. 1.
Multiple images from the CT of the chest performed as pulmonary embolism protocol. Multiple peripheral wedge-shaped nodules are seen in both lungs (black circles in A), some showing cavitation (black arrows in A and B). A right-sided pleural effusion is present (asterisk in D) along with right hilar and subcarinal lymphadenopathy.
Fig. 2.
Two-dimensional echocardiogram image demonstrates two echogenic nodular foci (white arrows) at the tricuspid valve, compatible with vegetations from infective endocarditis.
Discussion
Septic pulmonary embolism (SPE) is an uncommon yet crucial diagnosis to consider when evaluating patients with signs of septicemia [1]. In a Japanese study reviewing 11,367 PE cases identified on postmortem examination, the incidence of SPE was 247 (2.2%) [2]. Furthermore, a case series study in 1978, analyzing 60 SPE cases found that 78% of these patients were active intravenous drug users [5]. However, in a 2013 study of 137 SPE cases, only 26% were intravenous drug users [3]. This decrease in proportion is likely attributed to the increased usage of indwelling catheters and pacemakers in recent decades [3].
To achieve optimal outcomes and avoid potentially catastrophic complications, early identification and treatment are of the utmost importance in the management of SPE. Although typically linked to bacteria such as S aureus, fungal and parasitic infections have also been observed to cause SPE. Patients with suspected SPE commonly present with symptoms of fever, dyspnea, tachypnea, cough, and pleuritic chest pain. In more severe cases or in patients with delayed treatment, septic shock, and multisystem organ failure may also be seen [4]. Other rare complications include empyema, bronchopleural fistula, and pneumothorax [8]. Given the difficulty of reaching a definitive diagnosis based on initial clinical examination alone and the fact that blood cultures are often negative during the early course of the disease process, computed tomography (CT) serves as a valuable tool and the preferred imaging modality in the early detection of acute SPE [1].
SPE presents specific findings on CT that can facilitate diagnosis. Characteristic CT findings include bilateral nodules with varying degrees of cavitation predominantly in the peripheral and lower lung fields, wedge-shaped lesions with or without necrosis because of septic infarcts, air bronchograms within nodules, and airspace opacities [4,7,[9], [10], [11], [12]. Of these, the most noted features on spiral CT are numerous peripheral nodules and wedge-shaped peripheral opacities [12]. In some cases, “ground-glass halo sign”—or ground-glass opacity surrounding a nodule—can also be seen, which could indicate infarction or hemorrhage [1,4]. Oftentimes a “feeding vessel sign” can also be appreciated, which is seen as a vessel directly communicating with the nodule [6,7].
Certain CT findings may also be more so associated with 1 bacterial group over another, which could assist in identifying the causative pathogen and guiding antibiotic therapy while blood culture results are pending. In the retrospective review by Kwon et al, cavitary parenchymal nodules and air bronchograms were more likely to be observed in patients with gram-positive septicemia than in those with gram-negative infections [4]. Moreover, the nodules in the gram-positive group were noted to be larger in size than those in the gram-negative group. Conversely, the gram-negative group was more likely to exhibit the CT findings of “halo sign,” “feeding vessel sign,” and well-demarcated nodules [4].
On imaging, the differential diagnosis of SPE should also include granulomatosis with polyangiitis, cavitary pulmonary metastases, infarcts associated with pulmonary thromboembolism, and rheumatoid lung nodules. SPE can be differentiated from these mimicking conditions by confirmation of infection on 2 sets of blood cultures and visualization of right-sided heart failure and vegetation on an echocardiogram [4].
Given the high mortality associated with SPE, prompt management is necessary. The mainstay of treatment remains source control and parenteral antibiotic therapy. The choice of antimicrobial agent should be directed against the causative pathogen, as identified by blood cultures [7,13]. Vascular or cardiothoracic surgery may be indicated in some cases for the mechanical removal of infected thrombi or valve replacement in advanced endocarditis. In patients who develop pleural effusion with or without infection, drainage via thoracostomy tube should be considered [7,13]. Likewise, thoracoscopic surgery may be required for drainage and decortication in cases complicated by empyema [7].
Conclusion
SPE is a rare condition that can result from a variety of causative factors, the most common of which being S aureus-mediated bacterial endocarditis of the right heart secondary to intravenous drug use. In order to achieve the best possible prognosis and prevent fatal complications, early diagnosis and treatment are required. In addition to the initial clinical assessment, patients with suspected SPE should be promptly evaluated by blood cultures, CT imaging, and echocardiography. Characteristic CT findings of SPE include multiple peripheral nodules with or without cavitation, peripheral wedge-shaped opacities, air bronchograms within nodules, infiltrates, “ground-glass halo sign,” and “feeding vessel sign.” Although all cases of SPE can present with these findings, gram-positive and gram-negative bacteria may exhibit differing tendencies to present with certain characteristic findings. Treatment of SPE should include prompt initiation of targeted parenteral antibiotic therapy. Surgical intervention or drainage via thoracostomy tube may also be indicated in select cases.
Patient consent
A written informed consent was obtained from the patient for the publication of this case report.
Footnotes
Competing Interests: None. The authors declare that they have no conflicting interests and have not been supported or funded by any drug company or authority.
Acknowledgment: This project did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Authorship: The authors declare that this is their original work and they all approve of the content of this manuscript. They confirm that this manuscript has not been published previously, in any language, in whole or in part, and is not currently under consideration elsewhere.
Ethical Clearance: This project did not involve any research and no ethical clearance was required.
References
- 1.Kwon WJ, Jeong YJ, Kim KI, Lee IS, Jeon UB, Lee SH, et al. Computed tomographic features of pulmonary septic emboli: comparison of causative microorganisms. J Comput Assist Tomogr. 2007;31(3):390–394. doi: 10.1097/01.rct.0000243455.23308.a9. [DOI] [PubMed] [Google Scholar]
- 2.Sakuma M, Sugimura K, Nakamura M, Takahashi T, Kitamukai O, Yazu T, et al. Unusual pulmonary embolism: septic pulmonary embolism and amniotic fluid embolism. Circ J. 2007;71(5):772–775. doi: 10.1253/circj.71.772. [DOI] [PubMed] [Google Scholar]
- 3.Ye R, Zhao L, Wang C, Wu X, Yan H. Clinical characteristics of septic pulmonary embolism in adults: a systematic review. Respir Med. 2014;108(1):1–8. doi: 10.1016/j.rmed.2013.10.012. [DOI] [PubMed] [Google Scholar]
- 4.Ufuk F, Kaya F, Sagtas E, Kupeli A. Non-thrombotic pulmonary embolism in emergency CT. Emerg Radiol. 2020;27(3):343–350. doi: 10.1007/s10140-020-01755-8. [DOI] [PubMed] [Google Scholar]
- 5.MacMillan JC, Milstein SH, Samson PC. Clinical spectrum of septic pulmonary embolism and infarction. J Thorac Cardiovasc Surg. 1978;75(5):670–679. [PubMed] [Google Scholar]
- 6.Han D, Lee KS, Franquet T, Muller NL, Kim TS, Kim H, et al. Thrombotic and nonthrombotic pulmonary arterial embolism: spectrum of imaging findings. Radiographics. 2003;23(6):1521–1539. doi: 10.1148/rg.1103035043. [DOI] [PubMed] [Google Scholar]
- 7.Khashper A, Discepola F, Kosiuk J, Qanadli SD, Mesurolle B. Nonthrombotic pulmonary embolism. AJR Am J Roentgenol. 2012;198(2):W152–W159. doi: 10.2214/AJR.11.6407. [DOI] [PubMed] [Google Scholar]
- 8.Jaffe RB, Koschmann EB. Septic pulmonary emboli. Radiology. 1970;96(3):527–532. doi: 10.1148/96.3.527. [DOI] [PubMed] [Google Scholar]
- 9.Huang RM, Naidich DP, Lubat E, Schinella R, Garay SM, McCauley DI. Septic pulmonary emboli: CT-radiographic correlation. AJR Am J Roentgenol. 1989;153(1):41–45. doi: 10.2214/ajr.153.1.41. [DOI] [PubMed] [Google Scholar]
- 10.Kuhlman JE, Fishman EK, Teigen C. Pulmonary septic emboli: diagnosis with CT. Radiology. 1990;174(1):211–213. doi: 10.1148/radiology.174.1.2294550. [DOI] [PubMed] [Google Scholar]
- 11.Iwasaki Y, Nagata K, Nakanishi M, Natuhara A, Harada H, Kubota Y, et al. Spiral CT findings in septic pulmonary emboli. Eur J Radiol. 2001;37(3):190–194. doi: 10.1016/s0720-048x(00)00254-0. [DOI] [PubMed] [Google Scholar]
- 12.Cook RJ, Ashton RW, Aughenbaugh GL, Ryu JH. Septic pulmonary embolism: presenting features and clinical course of 14 patients. Chest. 2005;128(1):162–166. doi: 10.1378/chest.128.1.162. [DOI] [PubMed] [Google Scholar]
- 13.Goswami U, Brenes JA, Punjabi GV, LeClaire MM, Williams DN. Associations and outcomes of septic pulmonary embolism. Open Respir Med J. 2014;8:28–33. doi: 10.2174/1874306401408010028. [DOI] [PMC free article] [PubMed] [Google Scholar]