Graphical abstract
Keywords: Microwave, Quinoa, Textual properties, Starch, Gelatinization
Highlights
-
•
Microwave dispersed quinoa starch aggregates into independent granules.
-
•
Dispersed starch granules were hydrated and gelatinized to form network structure.
-
•
Microwave maintained the crystal form while changed the crystallinity.
-
•
Excessive microwave makes the starch partially gelatinize and formed blocks.
-
•
Moderate microwave treatment can improve the hardness and stickiness of quinoa.
Abstract
Texture characteristics of quinoa under microwave (MW) irradiation were studied from the perspective of starch gelatinization. MW reduced the light transmittance and increased the hardness and stickiness of quinoa. Microstructure showed that MW dispersed the vesicular structure of starch aggregates into separate starch particles, resulting in the full hydration of starch and water molecules to form denser network structures. The value of peak viscosity and setback decreased in RVA after MW treatment, but the gelatinization temperature remained stable. DSC further proved that moderate MW treatment could reduce the gelatinization enthalpy of starch and made quinoa accessible to gelatinize. However, XRD showed that the crystal structure of starch was preserved, but the crystallinity increased. Finally, low field NMR showed that moderate MW stimulated the full hydration of starch to form denser network structures, while excessive MW treatment made starch partially gelatinize and form rigid structure, resulting in negative texture properties.
1. Introduction
Quinoa is native to the Andean mountain region on the west coast of the American continent and has a long history of cultivation (Li & Zhu, 2018). Quinoa seeds are rich in six nutrients and bioactive substances needed by the human body and have great potential for development, such as starch, fat, protein, soluble sugar, cellulose and phenols (Le et al., 2021). Quinoa, recommended by the Food and Agriculture Organization of the United Nations as the only perfect food, has high stress resistance and rich nutritional characteristics. Quinoa is superior to most grains in cultivation and food nutrition, which can meet all the basic material needs of the human body. Quinoa has stronger antioxidant activity with excellent health care effects to human body, just like preventing diabetes, hypertension, hyperlipidemia, heart disease and cancer (Sekhavatizadeh, Hosseinzadeh, & Mohebbi, 2021). Quinoa also can promote endocrine stability, improving immunity, and maintain a good acid-base balance in the human body (Tafadzwa, Zvamaziva, Charles, Amiel, Pepukai, & Shepherd, 2021). The average lipid content of quinoa grain is 5–7.2%, which is higher than that of corn (4.7%) and other general grains. Quinoa can be used as natural antioxidants to prevent the rapid oxidation of quinoa oil (Mazaheri, Torbati, Azadmard-Damirchi, & Savage, 2019). Starch is the main carbohydrate in quinoa, the content varies according to species and region, but basically the content is in between 38% and 61%. The amylopectin content of natural quinoa accounted for a larger proportion of total starch (Li, Hemar, & Zhu, 2021). Quinoa starch has good anti-retrogradation ability and can be widely used in food industry.
Quinoa can be used as staple food to replace the rice of daily life. The saponin content of quinoa is relatively high. In industry, quinoa saponins are usually removed by water grinding and then dried. Traditional high temperature drying method can inhibit the activity of key enzymes in quinoa storage characteristics. However, due to high temperature and long time, high temperature drying often faces the problem of negative nutritional quality and poor volatile substances of quinoa. Therefore, there is an urgent need for a convenient and efficient heating method for drying treatment. As a short-term and efficient treatment method, MW has been widely used in food processing industry and home cooking. MW can generate hot spots in local materials and make the temperature rise rapidly. The alternating electric field of MW can cause mechanical damage to the molecular structure of enzyme, so that the enzymes can inactivated quickly, with little damage to the nutritional activity quality of food, which can just meet the needs of quinoa processing. Moderate processing of grain is a popular way to improve the development and application of grain. In order to improve the storage stability of quinoa, microwave treatment is often used to dry the water in quinoa and inhibit the activity of its endogenous enzymes in the actual production and application.
Therefore, it is particularly significant to study the effects of MW on the quality characteristics of quinoa. MW heating is produced by the interaction between microwave, charged ions and polar molecules. Therefore, during MW treatment, the MW environment and the internal tissue of food are heated rapidly at the same time. Studies have found that MW can change the structure of the composition and the quality of food, such as oil degradation (Ries, Srinivasan, Abida, Caufield, Liao, & Lo, 2021), protein structure (Wang, Dong, Zhu, Shen, Wu, & Zhang, 2021) and starch crystallinity (Harasym & Oledzki, 2018). In the process of cooking, quinoa is faced with such phenomena as granule rupture, starch dissolution, starch gelatinization and retrogradation. Studies have shown that MW heating can change the gelatinization characteristics of starch and the order of its molecular structure. At high power (for example, 10 W/g), MW will fully destroy the hydrogen bond and interaction between glucose chains in starch molecules, resulting in the improvement of rice texture (Li et al., 2019). At the same time, since starch accounts for a large proportion in quinoa, it is very necessary to study the quality characteristics of quinoa grains from the perspective of quinoa starch. What’s more, studies have also shown that the properties and content of quinoa starch have a significant correlation with the texture characteristics of quinoa after cooking (Wu, Morris, & Murphy, 2017). At the same time, we are also eager to explore whether MW can improve the quality of quinoa, and whether these changes are related to the effect of MW on the properties of quinoa starch. Therefore, it is necessary to study the effect of MW pretreatment on the textural properties of cooked quinoa from the perspective of starch gelatinization properties in quinoa.
In order to study the effect mechanism of MW on the texture change of quinoa, this research started with the largest quantity of starch in quinoa and analyzed the internal causes of texture change from the perspective of starch gelatinization. Therefore, this paper aims to study the effect of MW on the texture characteristics and quality change principle from the perspective of starch gelatinization, in order to provide theoretical basis for the utilization of MW pretreatment technology in quinoa food processing.
2. Materials and methods
2.1. Materials
White quinoa (the moisture is 11.75%) was purchased from Ganzhou Kangrui Agriculture Products CO. LTD. (Ganzhou Jiangxi Province, China). Potassium iodide (KI), iodine (I2) and hydrogen chloride (HCL) were purchased from Sinopharm Chemical Reagent Co., Ltd (Shanghai, China). All chemicals, reagents, and solvents were purchased and were of analytical grade unless stated otherwise.
2.2. Microwave treatment of quinoa
According to the actual MW output power obtained by previous methods (Benlloch-Tinoco, Igual, Rodrigo, & Martínez-Navarrete, 2013). Deionized water (1L) was heated at a certain microwave power for a certain period of time t (the Water temperature difference should be more than 20 °C), then stirred rapidly and determine temperature difference (△T). The actual output power of microwave is calculated as following Equation (1–2). Quinoa was treated by MW Synthesis Platform (XH-200A, Xianghu, Beijing, China) with 9 W/g power density for different time (0 s, 10 s, 20 s, 30 s, 40 s, 60 s), and the corresponding temperature is (24 °C, 41.5 °C, 52.5 °C, 57.4 °C, 74.5 °C, 81.9 °C) respectively (Fig. s1). All samples are sealed and stored in a 4 °C refrigerator for subsequent experiments.
| (1) |
Then the power level of certain weight of surimi (m) was calculated as.
| (2) |
2.3. Determination of moisture
Determination of moisture content in quinoa treated with 9 W/g MW power density for different time by direct drying method (Covarrubias et al., 2020). Quinoa was placed in a dry weighing dish and dried to constant weight at 105 °C.
2.4. Determination of cooking time
MW treated quinoa (3 g) were weighed and placed in plate with a diameter of 5 cm. According to the rice-water ratio of 1:2, distilled water (6 g) was added and steamed at 100 °C. The best cooking time of quinoa was the time when the white core disappeared (Thirumdas, Deshmukh, & Annapure, 2015).
2.5. Water absorption index
MW treated quinoa (2 g, m0) was accurately weighed and placed in a glass dish in water of 100 °C for the best cooking time. The weight of 50 mL centrifuge tube was recorded as m1, and the steamed quinoa was dried with filter paper. After standing and cooling, it was poured into the centrifuge tube to weigh the total mass of the centrifuge tube and cooked quinoa (CQ) rice (recorded as m2) (Thirumdas, Deshmukh, & Annapure, 2015).
| (3) |
2.6. Cooking loss
Weigh exactly 2 g of MW treated quinoa (marked as m0) and cook it in the same way described above. Rinse the quinoa and contents with hot water and pour them into a 50 mL centrifuge tube, centrifuging at 4000 g for 6 min. Then pour the supernatant from the centrifuge tube into a weighing dish (mass m3) and bake at 105 °C for 15 h until constant weight, and weigh the total mass as m4 (Sirisoontaralak, Nakornpanom, Koakietdumrongkul, & Panumaswiwath, 2015).
| (4) |
N: 10000, unit conversion factor.
2.8. Transmittance and iodine blue value
MW treated quinoa flour sample (5 g) was accurately weighed, then 25 mL distilled water was added to vibrate and extract at 40 °C for 1 h, and then the volume was fixed to 50 mL. Centrifuged at 3000g for 15 min. Record absorbance at 620 nm (Ma, Wang, Cheng, Zhang, & Lyu, 2020).
| (5) |
A: the absorbance of samples at 620 nm.
Then taken 5 mL of supernatant, 0.5 mL KI-I2 solution and 0.5 mL 0.1 mol/L HCL were added, water was added to make total volume is 50 mL, then colorimetric determination at wavelength 620 nm after standed for 15 min, absorbance was expressed as iodine blue value. The blank solution was 0.5 mL iodine solution with 0.5 mL 0.1 mol/L HCl solution and distilled water constant volume to 50 mL.
2.9. Texture properties
MW treated quinoa was weighed and cooked for 3 g. After cooking, take them out and cool for 20 min. Randomly selected 15 CQ, measured their texture properties by texture analyzer (Stable Micro System, TA. XT-Plus, Surrey, UK) (Wu et al., 2014, Wu et al., 2017). The parameters for texture determination: The speed before test was 2.0 mm/s, the test speed was 1.0 mm/s, the speed after test was 10.0 mm/s, the compression distance was 10 mm, and the probe model was SMS P36R.
2.11. Scanning electron microscopy (SEM)
The MW treated quinoa and MW treated CQ were sliced, then the samples (9 W/0s, 9 W/10 s, 9 W/20 s, 9 W/30 s, 9 W/40 s, 9 W/60 s) were pasted on the black tape, placed the samples on the sample table for gold spraying treatment, and observed under scanning electron microscope (Tescan 3 Brno, Chech) with an accelerating voltage of 30 kV, and take clear photos under appropriate magnification(Contreras-Jiménez et al., 2019, Li and Zhu, 2018).
2.12. Pasting properties
Accurately weighed 3.0 g powder of samples (dry basis) into RVA (RVA4500, Perten Co., Australia) tank, distilled water was added to obtain a total weight of 28.0 g, and adjusted the moisture content to 14%. Program selection: The quinoa flour sample was equilibrated at 50 °C for 1 min and heated to 95 °C at a heating rate of 12 °C/min. Keep it at 95 °C for 2.5 min, cool it to 50 °C at 12 °C/min, and keep it at 50 °C for 2 min. The paddle was stirred at 960 rpm for the first ten seconds and 160 rpm for other times. Record the peak viscosity, valley viscosity, breakdown, final viscosity, setback, peak time and gelatinization temperature (Li, Luo, Zhang, & Yu, 2020).
2.13. Differential scanning calorimetry (DSC)
Accurately weigh 3 mg of sample into DSC aluminum plate (DSC 3500 Sirius, Germany), press the tablet after adding 6 μL water and balance it at room temperature for 24 h. The temperature was raised from 30 °C to 100 °C in the procedure of 5 °C/min. Record the start temperature (T0), peak temperature (TP), end temperature (TC) and gelatinization enthalpy (△H) (Bai et al., 2021).
2.14. X-ray diffraction (XRD)
Crystal structure of quinoa flour was determined by XRD (D2 PHASER, Bruker, Germany). Before the experiment, samples were compacted. The experimental instrument adopts Cu Target and graphite monochromator, and the diffraction scanning area is 5° to 60° (2θ). The target voltage is 40 kV, the current is 30 mA, scanning speed is 2°/min, step width is 0.02°. The X-ray diffraction spectrum of quinoa flour is measured, and the spectrum is automatically completed by the instrument itself, and the peak position and relevant parameters are determined (Pereira et al., 2017). The relative crystallinity of quinoa flour refers to the methods of Tian (Tian et al., 2018).
| (6) |
Ac:area of crystalline phase;
Aa:area of amorphous phase.
2.15. Determination of water distribution by low-field NMR
Moisture distribution of MW treated CQ was determined by low-field nuclear magnetic resonance spectrometer (NMI 20-025V–I, Suzhou Niumag Analytical Instrument Co., Ltd., Suzhou, China). The parameters were as follows: TD, 400200; TR, 500 ms; NS, 64; NECH, 1000. The T2 curves was inversed using SIRT model (Rolandelli, Farroni, & Buera, 2022).
2.16. Statistics analysis
All the experiments were repeated at least 3 times, the statistics analysis and drawing were used by Origin 2018 software (Origin Lab Corporation, USA), SPSS statistics 25 software (IBM Corporation, New York, USA). Analysis of variance (ANOVA) was used to determine whether there were significant differences in characteristics between samples.
3. Results and discussion
3.1. Effect of MW on basic quality index of MW treated quinoa
Moisture content has great influence on texture characteristics of quinoa. Water absorption is mainly achieved by starch leaching from the cell gap of quinoa. The cell gap of the abdomen is different from the back of quinoa; the abdominal cell gap is larger. When the moisture of quinoa is low (14%), the abdomen will absorb water rapidly, which produced a moisture content difference with the back. At the same time, in the process of quinoa cooking, quinoa granules cracked on its surface due to the existence of moisture difference, resulting in quinoa starch gushing from the crack place, making quinoa viscosity increased. The moisture content of raw quinoa was 11.75%. After 9 W/g MW treatment, the moisture of quinoa decreased significantly, which was due to the combination of thermal and non-thermal effects of MW electromagnetic fields. In the MW process, for the existence of electromagnetic waves, the internal material began to heat up, and the water migrated from the internal material to the surface. Water continues to heat up and escaped from the intercellular space, leading to a decrease of moisture content. When treated with 9 W/g for 60 s, the moisture content of quinoa declined from 11.75% to 8.77% (Table 1). MW slightly reduced the cooking water absorption of quinoa, but there were no significant effects. Excessive MW pretreatment has a negative impact on the cooking quality of quinoa, because the cooking loss increased at 60 s (Table 1). The iodine blue value indicates the concentration of starch dissolved in the quinoa soup during cooking. Iodine blue value was positively correlated with edible quality. The object of this study is quinoa grains. However, MW’ alternating electric field can only destroy the internal molecules and some chemical bonds of quinoa, and it has lightly destroyed the surface structure of quinoa. This phenomenon led to that there are no huge significant differences in some indexes, but the more important is the change of their light transmittance. Transmittance is an indicator of short-term retrogradation of starch. MW treatment promoted the retrogradation of starch and reduced the light transmittance of quinoa (Dalbhagat & Mishra, 2019). Dry noodles, bean vermicelli, and bean jelly are taken the advantage of starch retrogradation (Zhang et al., 2022). One reason might be that MW treatment leads to higher starch dissolution and gelatinization degree. Another explanation was that MW heating leads to the loss of water in quinoa and the decrease of transmittance.
Table 1.
Effects of microwave treatment on basic quality index of quinoa.
| Microwave group | Moisture content (%) |
Water absorption index | Cooking loos (mg/g) |
Iodine blue value | Transmittancy |
|---|---|---|---|---|---|
| 9 W/0s | 11.75 ± 0.08a | 1.62 ± 0.08a | 0.27 ± 0.01a | 0.10 ± 0.00a | 46.57 ± 1.21a |
| 9 W/10 s | 11.61 ± 0.02a | 1.56 ± 0.01a | 0.31 ± 0.01bc | 0.10 ± 0.00a | 28.65 ± 0.84b |
| 9 W/20 s | 10.59 ± 0.03b | 1.52 ± 0.09a | 0.29 ± 0.02ab | 0.11 ± 0.01ab | 23.02 ± 0.60c |
| 9 W/30 s | 9.95 ± 0.06bc | 1.54 ± 0.10a | 0.30 ± 0.01b | 0.11 ± 0.00b | 20.80 ± 0.41c |
| 9 W/40 s | 9.56 ± 0.49 cd | 1.56 ± 0.06a | 0.28 ± 0.01ab | 0.11 ± 0.00b | 21.33 ± 0.49c |
| 9 W/60 s | 8.77 ± 0.21d | 1.56 ± 0.06a | 0.33 ± 0.00c | 0.12 ± 0.00c | 20.51 ± 0.00c |
Note: Values are mean ± SD based on three times measurements. Values with different letters in the same column are significantly different (P < 0.05). 9 W/0s–9 W/60 s represents quinoa at different microwave times at the microwave power density of 9 W/g.
3.2. Textural properties
The texture of quinoa is closely related to the quality of quinoa, the most important parameters of texture are hardness and stickiness. A high hardness value means a harder and more chewable quality. Quinoa with stickiness less than 0.3 gf*s belongs to weak viscosity. Grains with too small stickiness are often accompanied by dry characters. Especially when the stickiness is less than 0.2 gf*s, the shape of CQ is scattered and has poor palatability. The optimum stickiness of quinoa is about 0.6 gf*s, at which quinoa has moderate stickiness and good quality. It was showed in Table 2 that the hardness of CQ increased after MW treatment. When treated for 20 s, the hardness of quinoa reached to 577.21 gf which may be related to the loss of water and the change of internal microstructure of quinoa during MW treatment (Gu et al., 2021). Yu et al. (2017) claimed that low water absorption ratio would lead to higher hardness of cooked rice, which was consistent with water absorption index result (Table 1). The exudation of amylose molecules during cooking formed a rigid structure on the surface of quinoa during cooling and retrogradation, which increased the hardness of quinoa. It is obviously that there were no significant differences between the elasticity of CQ and control group (Table 2). The range of elasticity is 0.58 to 0.62, which has little changed. Because the part produces elasticity was the endosperm part of quinoa, the endosperm of quinoa was preserved after MW treatment, so there was no difference in elasticity. The stickiness of cooked grain is an important index to evaluate the taste quality and consumer acceptance (Bai et al., 2021). As shown in Table 2, with the increase of MW treated time, the stickiness of quinoa increased. After MW treated for 20 s, the stickiness of CQ tended to be stable, and the difference became insignificant. Higher stickiness is not only related to the starch, but also to the lipids and proteins inside quinoa. During MW and cooking heat treatment, proteins and lipids easily form complexes with starch exuded from quinoa, which increased the stickiness and affected quality characteristics. In addition, dry matter leaching during cooking also affected the stickiness of CQ (Wu, Morris, & Murphy, 2017), and the increase of stickiness of MW treated quinoa (Table 2) was consisted with the previous results of cooking loss (Table 1). Cohesiveness refers to the contraction force inside the food. There is a positive correlation between stickiness and food chewing resistance. The cohesiveness and elasticity of CQ did not change significantly after MW treatment, indicating that MW could not destroy the original quality of quinoa, but it was beneficial to maintain the original quality of quinoa while improving its hardness and stickiness. Therefore, MW treated for 20 s has the best effect on improving the texture properties of quinoa.
Table 2.
Effects of microwave treatment on textural properties of quinoa.
| Microwave group | Hardness (gf) |
Stickiness(gf * s) | Chewiness (gf) |
Gumminess (gf) |
Elasticity | Cohesiveness |
|---|---|---|---|---|---|---|
| 9 W/0s | 443.26 ± 33.27a | 0.30 ± 0.08a | 174.09 ± 23.84ab | 279.17 ± 33.21ab | 0.62 ± 0.03a | 0.63 ± 0.04b |
| 9 W/10 s | 456.71 ± 47.53a | 0.80 ± 0.3b | 158.74 ± 26.75a | 257.69 ± 41.38a | 0.62 ± 0.05a | 0.56 ± 0.04a |
| 9 W/20 s | 512.85 ± 38.08b | 0.66 ± 0.22b | 174.71 ± 25.63ab | 300.52 ± 38.20b | 0.58 ± 0.03a | 0.58 ± 0.05a |
| 9 W/30 s | 577.21 ± 48.02c | 0.38 ± 0.12a | 222.21 ± 25.86c | 360.60 ± 24.65c | 0.62 ± 0.04a | 0.63 ± 0.03b |
| 9 W/40 s | 535.03 ± 47.83bc | 0.42 ± 0.09a | 198.32 ± 31.37bc | 311.43 ± 33.57b | 0.62 ± 0.05a | 0.60 ± 0.03ab |
| 9 W/60 s | 538. 01 ± 58.85bc | 0.46 ± 0.06a | 186.28 ± 31.73ab | 310.90 ± 42.91b | 0.60 ± 0.04a | 0.58 ± 0.04a |
Note: Values are mean ± SD based on three times measurements. Values with different letters in the same column are significantly different (P < 0.05). 9 W/0s–9 W/60 s represents quinoa at different microwave times at the microwave power density of 9 W/g.
3.3. SeM
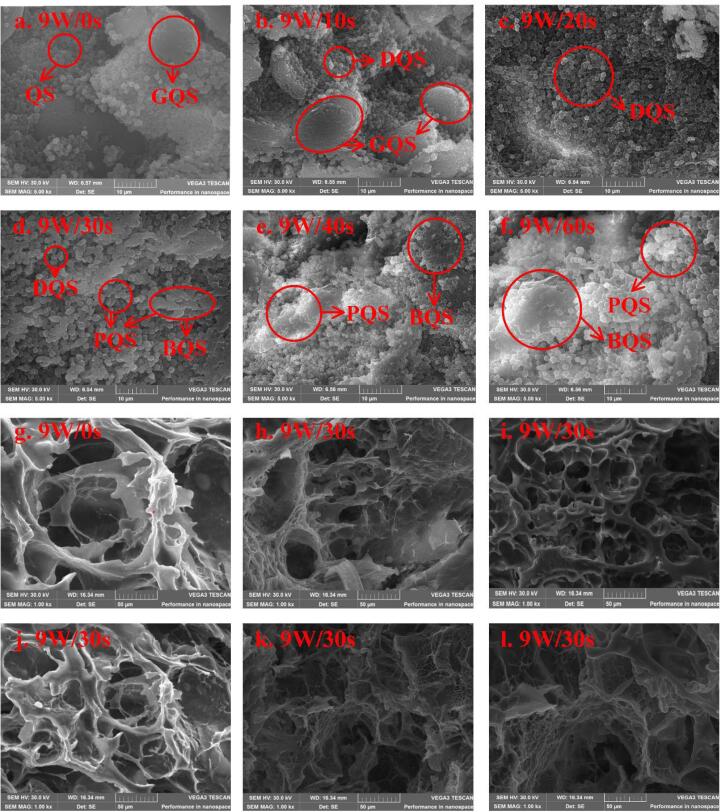
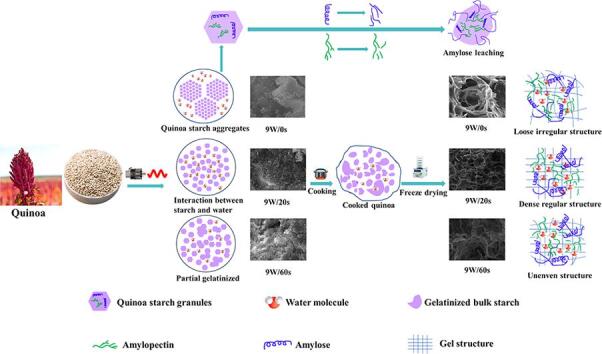
The starch particles in natural quinoa were polygonal, irregular and angular, and the shape were small (Fig. 1a). The starch of natural quinoa gathered into vesicles to form large starch aggregates. Compared with natural starch, under the action of MW alternating electric field, the molecular interaction and chemical bonds such as van der waals force and hydrogen bond of quinoa starch molecules were destroyed. Starch aggregates began to disintegrate gradually, the internal starch molecules dispersed with the destruction of the shape of starch aggregates. But the general shape of starch still can be seen (Fig. 1b). After MW treatment for 20 s, most of the starch molecules were dispersed and large starch particles began to rupture. Due to the increase of MW time, continuous thermal effects of MW caused the loss of moisture inside quinoa. At the same time, the alternating electric and thermal effect of MW acted together on quinoa, resulting in the destruction of the chemical bonds among starch molecules, and between starch and water molecules. The double helix ordered structure of starch disappeared, resulting in the destruction of starch particles morphology. Many starches were no longer regular polygons, but irregular slightly gelatinized forms (Fig. 1c-e). However, as the MW treated time increased continuously, starch molecules began to paste with each other into a frit (Fig. 1f), which might be related to the starch melting transition during the glassy transition phase cause by MW thermal effect and non-thermal effect of MW electromagnetic filed (Zou, Xu, Tang, Wen, & Yang, 2020).
Fig. 1.
Scanning electron microscopy images of raw and cooked quinoa treated with 9 W/g microwave at different time points. QS: quinoa starch; GQS: quinoa starch aggregates; DQS: dispersed quinoa starch; PQS: pasting quinoa starch; BQS: bulk quinoa starch. Fig. a-f is microwave treated quinoa; Fig. g-l is microwave treated cooked quinoa.
Microstructure of CQ was shown in Fig. 1 (g-i). Raw quinoa has a loose internal structure after cooking. Large holes were caused by the infiltration of water into quinoa during cooking, water filled entire quinoa and expanded quinoa tissues. As the cooking continues, further evaporation of the water led to larger holes left behind. It was obviously that MW heating can make the structure and holes inside quinoa denser and more uniform, which promoted the formation of starch hydration gel network. The dense and tidy structure of quinoa may also be contributing to the increase of hardness. However, as MW time continues to 60 s, MW was heated directly inside the material by electromagnetic waves immediately. Temperature inside quinoa rose rapidly when excessive MW treating was carried, which caused a small part of the internal starch to gelatinize, thus forming a rigid shell near the surface of the quinoa. This made it difficult for quinoa to absorb water during cooking and absorbing water, the infiltrate of moisture was not excessively. Therefore, moderate MW treatment could make the interior of quinoa forms a structure which was beneficial to the gelatinization of starch and the quality of quinoa. However, excessive MW treatment caused the formation of frit, which affected the gelatinization and quality characteristics of quinoa, at which point the gelatinization of quinoa was more thorough.
At the same time, we also treated quinoa by hot air heating with different temperature gradients. It can be clearly seen that when the hot air heating temperature is 35 °C, the morphology of quinoa starch vesicular aggregates hardly changed, and the starch particles were closely connected (Fig. s2A). Therefore, when there is no effect of microwave alternating electric field, the chemical bond between starch molecules cannot be destroyed only by conventional hot air heating. When heated to 45 °C (Fig. s2B), the structure of quinoa starch aggregates were persevered, there were still many starch vesicular aggregates, and the shape of individual quinoa starch particles showed a natural hexagonal state. On the contrary, when treated with 9 W/g microwave for 10 s (which temperature is 41.5 °C), the vesicular of starch aggregates were cracked (Fig. 1b). However, when conventionally hot air treatment heated to 55 °C (Fig. s2C), individual starch aggregates were partially destroyed and disintegrated into separate starch particles. When microwave treatment reaches a similar temperature (9 W/20 s, 52.5 °C), quinoa starch has been completely dispersed into separate scattered starch particles, which undoubtedly shows that the effect of microwave alternating electric field has a significant impact on the starch properties gelatinization properties of quinoa starch (Figs. s1, s2).
3.4. Gelatinization characteristics
Gelatinization characteristic is an important index reflecting the quality of quinoa, and it is also an important parameter affecting the cooking quality of quinoa. As can be seen from Table s1, there is little fluctuation in the final viscosity and pasting temperature. Peak viscosity refers to the equilibrium viscosity value between the increase of starch gelatinization viscosity and the decrease of starch particle fracture viscosity under the action of mechanical shear force. According to Table s1, the peak viscosity of quinoa in the control group was 1433.50 ± 65.76 m Pa/s, which was decreased by MW, especially to 1313.40 ± 55.86 m Pa/s after MW for 40 s. The slightly decrease of peak viscosity was related to the formation of porous morphology. The formation of porous morphology contributed the starch particles easily hydrolyzed and loosened their resistance to swelling (Gutierrez-Osnaya et al., 2020). Studies had shown that the reduction of peak viscosity improved cooking resistance and quality of grain. Wang et al. (Wang, Wu, Wang, Yang, & Zhou, 2019) found that after MW treatment of rice, due to the heating effect of MW, the complex reaction between starch-lipid complex and nitrogen-containing compounds inside the grain, which could improve the stability of starch clusters inside the grain in the cooking process. Therefore, the decrease of peak viscosity indicating that MW treatment for 20 s has the potential to improve the cooking properties of quinoa. However, when the MW time reached 60 s, the peak viscosity increases significantly again, which might be related to the dual effects of electromagnetic effect and thermal effect of MW. As this time, the temperature of MW treated quinoa achieved 81.9 °C (Fig. s1), while quinoa’s gelatinization temperature is around 61℃. Polar water molecules and glycosidic bonds of starch in quinoa have electromagnetic introduction to MW field, and some water molecules are very close to starch, even combined with starch. The starch inside quinoa partially gelatinized after MW, possibly accompanied by protein denaturation, caused a sharp decline in the cooking quality of quinoa. Furthermore, this is also related to the production of starch lipid complex promoted by MW (Fig. s3).
The value of breakdown is the most sensitive index of gelatinization properties, which represents the degree of breakage of starch particles. High breakdown value is often accompanied by more starch rupture during heating, and the internal starch molecules will be released. Devraj et al.’s (2020) study showed that the breakdown of rice during gelatinization was significantly related to the amylopectin content and microstructure in rice. As the MW time increased, the decrease of breakdown may be due to the evaporation and immigration of free water in quinoa under MW field. However, the hydroxyl groups in the starch combined more closely with the weak water and strengthened the double helix structure of the starch. Thus, the microcrystalline structure of amylopectin was strengthened which decreased the outflow of starch during cooking and aggrandize the hardness of quinoa. When MW treatment lasted for 60 s, the breakdown increased significantly. For the MW field caused the high frequency vibration of the polar groups such as hydroxyl on starch molecules during the cooking process of quinoa, which generated heat energy and destructed the hydrogen bonds. The microcrystalline structure of the starch was destroyed; a rigid structure was formed within the starch. This structure was manifested as breakdown and hardness increase.
MW had no significant effect on the final viscosity of quinoa, which represents the gelatinization degree during cooling, and is also related to the formation of sticky paste and gelatinization function of starch after cooking and cooling (Thuengtung & Ogawa, 2020). Pasting temperature refers to the irreversible expansion temperature of starch particles in the process of quinoa cooking (Huang & Lai, 2014). MW treatment has no obvious effect on the pasting temperature of quinoa, which was 61.88 °C. The setback indicated the stability and aging trend of starch paste. A high setback value is interrelated to the retrogradation and negative quality of quinoa. When MW treatment time was 20 s, the setback reached the minimum value (238.50 ± 31.82 m Pa/s), which was 91.5 m Pa/s lower than that of the control group. Suggesting that 9 W/20 s MW reduced the retrogradation ability of quinoa and improved its quality. Because quinoa is a mixed system, MW can enhance the disulfide bond bridging force between the proteins in the starch, leading to the decrease of protein-starch crosslinking and inhibit the aging of starch (Wang, Wu, Wang, Yang, & Zhou, 2019).
3.5. Thermal analysis
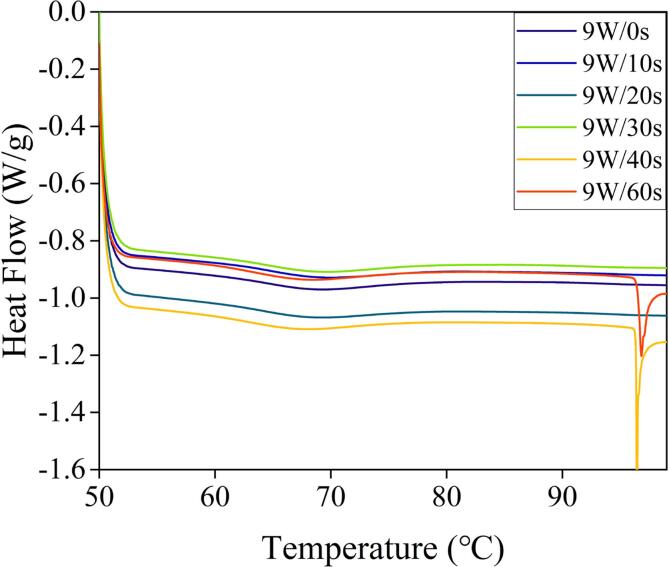
Fig. 1 and Table s2 shows the thermodynamic parameters of natural and MW quinoa. The starting temperature (To), peak temperature (Tp), final temperature (Tf) and temperature range (Tf - Ton, △T) reflects the gelatinization characteristics of starch. Those with small parameter values are more prone to gelatinize (Xing et al., 2021). Gelatinization enthalpy (△H) is related to the crystal structure of crystalline region and amylose double helix structure of amorphous region and it represents the energy of dissociation of double helix structure. After MW treatment of quinoa, the △H decreased first and then increased. The decrease of △H illustrated that the content of double helix structure was reduced, and molecular arrangement of starch particles became disorderly, which was caused by the partial gelatinization of starch particles (Huang, Zhou, Jin, Xu, & Chen, 2016). It also signified that quinoa starch tended to gelatinize after MW. The increase of △H was due to the increase of MW time, which led to recrystallization between starch molecules, so that the structure of starch molecules were re-stabilized, more energy was needed to destroy crystallization zone of quinoa.
MW had little effect on the thermodynamic parameters of quinoa (To, Tp, Tf), To was around 61 °C, but the temperature transition range (△T) was lower than natural quinoa. The transition temperature (To、Tp、Tf) represents the ordered double helix structure of starch, To and Tf represents the melting temperature of the weakest and strongest crystal structure. The To of MW treated quinoa was little higher than that of the natural quinoa though there is no significant difference, which manifested that the order of the weakest crystal structure was protected and the thermal stability of the sample was enhanced in the MW process. Whereas, excessive MW treatment contributed to the destruction of thermal stability. The endothermic peak of the starch-lipid complex occurred at 100 °C (Zhou et al., 2021). The level of starch-lipid complex in MW treated quinoa less than 30 s was very low, and no endothermic process occurred (Fig. 2). Conversly, an endothermic peak was observed at 100 °C when MW was treated for 40 s to 60 s. This implied that excessive MW treatment increased the strength and content of starch-lipid complex in quinoa, which was consistent with XRD’s results.
Fig. 2.
Thermodynamic curves of microwave-treated quinoa.
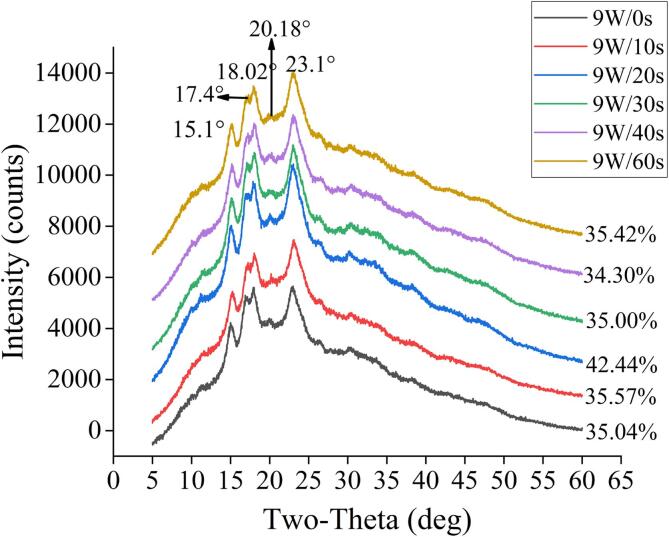
3.6. Crystal type and crystallinity
X-ray diffraction results of MW treated quinoa were shown in Fig. 3. There were no new peaks and no significant shifts in peak location. After 9 W/g MW treatment, the intensity of each absorption peak of quinoa increased, the absorption peak intensity of quinoa treated by MW for 20 s was the highest, and then gradually declined to the same intensity as the control group with the increase of MW time. Apparently, experimental group and the control group have absorption peak in 15.10°, 17.30°, 18.02 °and 23.00°, these peaks represented the A crystal type which is no difference from natural quinoa. Meanwhile, a small absorption peak could be seen at 2θ = 20.04° (V-type), which was caused by the presence of starch-lipid complex. The crystal of A-type and V-type were typical cereal starches (Xing et al., 2021). No change of crystal shape and peak position testified that the change of properties of quinoa starch was mainly owing to the change of amorphous region (Zhou et al., 2021).
Fig. 3.
XRD diffraction patterns of quinoa at 9 W/g microwave treatment.
The crystallinity of quinoa was between 21.5%–43.03% (Li & Zhu, 2018). In this experiment, the crystallinity of quinoa treated with 9 W/g for 0 s, 10 s, 20 s, 30 s, 40 s, 60 s were 35.04%, 35.57%, 42.44%, 35.00%, 34.30% and 35.42%, respectively. After MW treatment for 20 s, the crystallinity of quinoa reached maximum. MW increased the crystallinity of quinoa starch, indicating that the moderate MW would make the crystal structure of quinoa starch more complete, the double helix structure of moderately damaged starch rearranged into a more orderly crystal structure. The amylose content was negatively correlated with crystallinity (Li & Zhu, 2018). What’s more, the chain length distribution of amylopectin also affects its crystallinity. Excessive MW treatment had degraded and destroyed the crystal structure of starch that has been rearranged, leading to a decrease of crystallinity (Oh, Bae, & Lee, 2018). Because quinoa is high in amylopectin, amylopectin has a higher molecular weight and steric resistance, so the starch molecules would not be destroyed by MW quickly. However, with the increase of MW time, the crush effects of MW electromagnetic on amylopectin were enhanced. Microwave vibration destroyed the chemical bond between starch molecules, promotes the hydration of starch during heating, and generated the gelatinization of quinoa. Starch molecules cannot recover into a new unstable crystal quickly, resulting in a decrease in crystallization, but also accompanied by a decrease in water absorption capacity (Table 1).
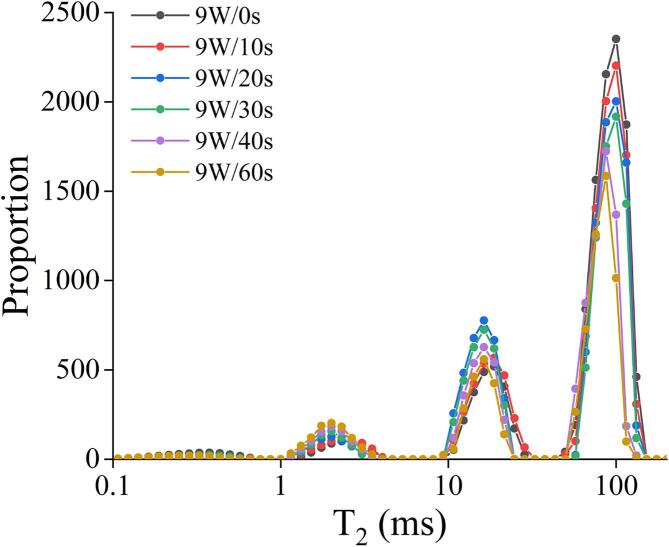
3.7. Moisture distribution of microwave-treated quinoa
T21a (0.01–0.75 ms) and T21b (1–4 ms) in low-field NMR refers to the relaxation time of strongly bound water, which is the strongest combination of quinoa ingredients and the weakest mobility of water. T22 (10–30 ms) stands for immobilized water and refers to the relaxation time of water whose fluidity can be detected between bound water and free water (Chen et al., 2019). T23 (50–140 ms) represents free water (Fig. 4). After MW treatment, the peak position of T21a (0.3 ms) did not change, but the peak area decreased slightly, a small amount of moisture migrated to the relaxation time of T21b, indicating that the tightness of the smallest structure inside the material with water had not destroyed, but this part of moisture content was lower than that of natural quinoa under microscopic conditions. Peak position of T21b (2.3 ms) moved towards the direction of weakening fluidity after MW treatment, but it was still tightly bound water, and the content of this part of bound water tended to increase. This might because the appropriate expansion of the double helix structure of starch molecules in quinoa caused by MW alternating electric field, shortening the distance between starches and water. Starch molecular network embeds some weak flowing water into starch microstructure, which enhanced the ability of starch microstructure to bind water (Tang et al., 2018). The relaxation time of T22 water moved left when MW was less than 20 s, and then moved to right when MW was longer than 20 s. The corresponding peak area had similar trends. This meant that the fluidity of the less mobile water in quinoa decreased and then increased with the increasing of MW time. Network structure of quinoa shown in the electron microscope images also reflected such a phenomenon. Proper MW treatment (<20 s) made the pores between the tissues of quinoa became denser and more regular. Therefore, it was easier for water to enter the interstitium of quinoa cells and form a three-dimensional gel during cooking and heating. However, with the increase of MW time, the electromagnetic effect and heating effect of MW pretreatment would lead to excessive gelatinization of quinoa, forming a rigid structure inside quinoa. The rigid structure would weaken the ability of binding water in the interstitium of quinoa during subsequent cooking. By observing the relaxation time of free water T23, it can be found that appropriate MW treatment had no effect on the binding capacity of free water, but the peak position moves towards the direction of weakened mobility when treated for 40 s, which may also relate to the forms of rigid structures. With the increase of MW time, free water content showed a decreasing trend, which was closely related to the gelatinization and texture properties of quinoa starch such as the increasing of hardness (Yan & Lu, 2021).
Fig. 4.
Low field NMR moisture distribution of 9 W/g microwave-treated quinoa.
4. Conclusion
With the extension of MW treatment time, the texture characteristics of quinoa first increased and then decreased. The alternating electromagnetic field of MW destroyed the original vesicular aggregation structure of starch particles, so that the starch dispersed into separate particles from the aggregation state. However, high intensity MW treatment for a long time was prone to cause partial gelatinization of quinoa starch and form a rigid structure, these changes have impacts on the gelatinization and thermodynamic properties of quinoa starch, but there was no difference in gelatinization temperature. MW could affect the rotational state of double helix structure of starch, resulting in the increase and decrease of starch crystallinity. MW alternating electric field could also change some chemical bonds on amylopectin branches, making starch tends to combine with water to form an ordered gel network structure, which is conducive to the enhancement of quality of quinoa.
CRediT authorship contribution statement
Hongwei Cao: Conceptualization, Investigation. Rulian Sun: Writing – original draft. Yu Liu: Formal analysis. Xiaoxue Wang: Software. Xiao Guan: Funding acquisition, Supervision. Kai Huang: Visualization. Yu Zhang: Writing – review & editing.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgment
This work was supported by Shanghai Agriculture Applied Technology Development Program (2021-02-08-00-12-F00780), National Natural Science Foundation of China (32102140); Shanghai Sailing Program (20YF1433400) and the Domestic Science and Technology Cooperation Projects of Shanghai (21015801100).
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.fochx.2022.100347.
Appendix A. Supplementary data
The following are the Supplementary data to this article:
References
- Bai T.-G., Zhang L., Qian J.-Y., Jiang W., Wu M., Rao S.-Q.…Wu C. Pulsed electric field pretreatment modifying digestion, texture, structure and flavor of rice. Lwt. 2021;138:110650. [Google Scholar]
- Benlloch-Tinoco M., Igual M., Rodrigo D., Martínez-Navarrete N. Comparison of microwaves and conventional thermal treatment on enzymes activity and antioxidant capacity of kiwifruit puree. Innovative Food Science & Emerging Technologies. 2013;19:166–172. [Google Scholar]
- Chen C., Fu W., Chang Q., Zheng B., Zhang Y.i., Zeng H. Moisture distribution model describes the effect of water content on the structural properties of lotus seed resistant starch. Food Chemistry. 2019;286:449–458. doi: 10.1016/j.foodchem.2019.01.214. [DOI] [PubMed] [Google Scholar]
- Contreras-Jiménez B., Torres-Vargas O.L., Rodríguez-García M.E. Physicochemical characterization of quinoa (Chenopodium quinoa) flour and isolated starch. Food Chemistry. 2019;298:124982. doi: 10.1016/j.foodchem.2019.124982. [DOI] [PubMed] [Google Scholar]
- Covarrubias N., Sandoval S., Vera J., Nunez C., Alfaro C., Lutz M. Moisture, protein and mineral content of ten varieties of Chilean quinoa grown in different geographic zones. Revista Chilena De Nutricion. 2020;47(5):730–737. [Google Scholar]
- Dalbhagat C.G., Mishra H.N. Effects of extrusion process conditions on system parameters; physicochemical properties and cooking characteristics of extruded fortified rice kernels. Journal of Cereal Science. 2019;89:102782. [Google Scholar]
- Devraj L., Natarajan V., Vadakkeppulpara Ramachandran S., Manicakam L., Sarvanan S. Influence of microwave heating as accelerated aging on physicochemical, texture, pasting properties, and microstructure in brown rice of selected Indian rice varieties. Journal of Texture Studies. 2020;51(4):663–679. doi: 10.1111/jtxs.12522. [DOI] [PubMed] [Google Scholar]
- Gu R., Chang X., Bai G., Li X., Di Y., Liu X.…Wang Y. Effects of household cooking methods on changes of tissue structure, phenolic antioxidant capacity and active component bioaccessibility of quinoa. Food Chemistry. 2021;350 doi: 10.1016/j.foodchem.2021.129138. [DOI] [PubMed] [Google Scholar]
- Gutiérrez-Osnaya L.J., Hernández-Uribe J.P., Castro-Rosas J., Román-Gutiérrez A.D., Camacho-Díaz B.H., Palma-Rodríguez H.M.…Guzmán-Ortiz F.A. Influence of germination time on the morphological, morphometric, structural, and physicochemical characteristics of Esmeralda and Perla barley starch. International Journal of Biological Macromolecules. 2020;149:262–270. doi: 10.1016/j.ijbiomac.2020.01.245. [DOI] [PubMed] [Google Scholar]
- Harasym J., Olędzki R. The mutual correlation of glucose, starch, and beta-glucan release during microwave heating and antioxidant activity of oat water extracts. Food and Bioprocess Technology. 2018;11(4):874–884. [Google Scholar]
- Huang T.T., Zhou D.N., Jin Z.Y., Xu X.M., Chen H.Q. Effect of repeated heat-moisture treatments on digestibility, physicochemical and structural properties of sweet potato starch. Food Hydrocolloids. 2016;54:202–210. [Google Scholar]
- Huang Y.C., Lai H.M. Characteristics of the starch fine structure and pasting properties of waxy rice during storage. Food Chemistry. 2014;152:432–439. doi: 10.1016/j.foodchem.2013.11.144. [DOI] [PubMed] [Google Scholar]
- Le L., Gong X., An Q.i., Xiang D., Zou L., Peng L.…Wan Y. Quinoa sprouts as potential vegetable source: Nutrient composition and functional contents of different quinoa sprout varieties. Food Chemistry. 2021;357:129752. doi: 10.1016/j.foodchem.2021.129752. [DOI] [PubMed] [Google Scholar]
- Li C., Luo J.-X., Zhang C.-Q., Yu W.-W. Causal relations among starch chain-length distributions, short-term retrogradation and cooked rice texture. Food Hydrocolloids. 2020;108:106064. [Google Scholar]
- Li G., Hemar Y., Zhu F. Relationships between supramolecular organization and amylopectin fine structure of quinoa starch. Food Hydrocolloids. 2021;117 [Google Scholar]
- Li G., Zhu F. Quinoa starch: Structure, properties, and applications. Carbohydrate Polymers. 2018;181:851–861. doi: 10.1016/j.carbpol.2017.11.067. [DOI] [PubMed] [Google Scholar]
- Li N., Cai Z., Guo Y., Xu T., Qiao D., Zhang B.…Lin Q. Hierarchical structure and slowly digestible features of rice starch following microwave cooking with storage. Food Chemistry. 2019;295:475–483. doi: 10.1016/j.foodchem.2019.05.151. [DOI] [PubMed] [Google Scholar]
- Ma Z.H., Wang Y.B., Cheng H.T., Zhang G.C., Lyu W.Y. Biochemical composition distribution in different grain layers is associated with the edible quality of rice cultivars. Food Chemistry. 2020;311 doi: 10.1016/j.foodchem.2019.125896. [DOI] [PubMed] [Google Scholar]
- Mazaheri Y., Torbati M., Azadmard-Damirchi S., Savage G.P. Effect of roasting and microwave pre-treatments of Nigella sativa L. seeds on lipase activity and the quality of the oil. Food Chemistry. 2019;274:480–486. doi: 10.1016/j.foodchem.2018.09.001. [DOI] [PubMed] [Google Scholar]
- Oh I.K., Bae I.Y., Lee H.G. Effect of dry heat treatment on physical property and in vitro starch digestibility of high amylose rice starch. International Journal of Biological Macromolecules. 2018;108:568–575. doi: 10.1016/j.ijbiomac.2017.11.180. [DOI] [PubMed] [Google Scholar]
- Pereira J.M., Evangelho J.A., Moura F.A., Gutkoski L.C., Zavareze E.R., Dias A.R.G. Crystallinity, thermal and gel properties of oat starch oxidized using hydrogen peroxide. International Food Research Journal. 2017;24(4):1545–1552. [Google Scholar]
- Ries S., Srinivasan A., Abida O., Caufield K., Liao P.H., Lo K.V. Microwave enhanced advanced oxidation treatment of fat, oil and grease (FOG) with organic co-substrates. Environmental Technology. 2021;42(28):4500–4510. doi: 10.1080/09593330.2020.1767700. [DOI] [PubMed] [Google Scholar]
- Rolandelli G., Farroni A.E., Buera M.D.P. Analysis of molecular mobility in corn and quinoa flours through (1)H NMR and its relationship with water distribution, glass transition and enthalpy relaxation. Food Chemistry. 2022;373:131422. doi: 10.1016/j.foodchem.2021.131422. [DOI] [PubMed] [Google Scholar]
- Sekhavatizadeh S.S., Hosseinzadeh S., Mohebbi G. Nutritional, antioxidant and polyphenol content of quinoa (Chenopodium quinoa Wind.) cultivated in Iran. Future of Food-Journal on Food Agriculture and Society. 2021;9(2):68–79. [Google Scholar]
- Sirisoontaralak P., Nakornpanom N.N., Koakietdumrongkul K., Panumaswiwath C. Development of quick cooking germinated brown rice with convenient preparation and containing health benefits. Lwt-Food Science and Technology. 2015;61(1):138–144. [Google Scholar]
- Tafadzwa M.J., Zvamaziva J.T., Charles M., Amiel M., Pepukai M., Shepherd M. Proximate, physico-chemical, functional and sensory properties OF quinoa and amaranth flour AS potential binders in beef sausages. Food Chemistry. 2021;365 doi: 10.1016/j.foodchem.2021.130619. [DOI] [PubMed] [Google Scholar]
- Tang X.J., Liu N., Huang W.N., Cheng X.Y., Wang F., Zhang B.L.…Li Z.B. Syneresis rate, water distribution, and microstructure of wheat starch gel during freeze-thaw process: Role of a high molecular weight dextran produced by Weissella confusa QS813 from traditional sourdough. Cereal Chemistry. 2018;95(1):117–129. [Google Scholar]
- Thirumdas R., Deshmukh R.R., Annapure U.S. Effect of low temperature plasma processing on physicochemical properties and cooking quality of basmati rice. Innovative Food Science & Emerging Technologies. 2015;31:83–90. [Google Scholar]
- Thuengtung S., Ogawa Y. Comparative study of conventional steam cooking and microwave cooking on cooked pigmented rice texture and their phenolic antioxidant. Food Science & Nutrition. 2020;8(2):965–972. doi: 10.1002/fsn3.1377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tian J., Cai Y., Qin W., Matsushita Y., Ye X., Ogawa Y. Parboiling reduced the crystallinity and in vitro digestibility of non-waxy short grain rice. Food Chemistry. 2018;257:23–28. doi: 10.1016/j.foodchem.2018.03.005. [DOI] [PubMed] [Google Scholar]
- Wang H., Wu Y., Wang N., Yang L., Zhou Y. Effect of water content of high-amylose corn starch and glutinous rice starch combined with lipids on formation of starch-lipid complexes during deep-fat frying. Food Chemistry. 2019;278:515–522. doi: 10.1016/j.foodchem.2018.11.092. [DOI] [PubMed] [Google Scholar]
- Wang L., Dong J.L., Zhu Y.Y., Shen R.L., Wu L.G., Zhang K.Y. Effects of microwave heating, steaming, boiling and baking on the structure and functional properties of quinoa (Chenopodium quinoaWilld.) protein isolates. International Journal of Food Science and Technology. 2021;56(2):709–720. [Google Scholar]
- Wu G., Morris C.F., Murphy K.M. Evaluation of texture differences among varieties of cooked quinoa. Journal of Food Science. 2014;79(11):S2337–S2345. doi: 10.1111/1750-3841.12672. [DOI] [PubMed] [Google Scholar]
- Wu G., Morris C.F., Murphy K.M. Quinoa starch characteristics and their correlations with the texture profile analysis (TPA) of cooked quinoa. Journal of Food Science. 2017;82(10):2387–2395. doi: 10.1111/1750-3841.13848. [DOI] [PubMed] [Google Scholar]
- Xing B., Teng C., Sun M., Zhang Q., Zhou B., Cui H.…Qin P. Effect of germination treatment on the structural and physicochemical properties of quinoa starch. Food Hydrocolloids. 2021;115:106604. [Google Scholar]
- Yan, H. L., & Lu, Q. Y. (2021). Physicochemical properties of starch-wheat germ oil complex and its effects on water distribution and hardness of noodles. Lwt-Food Science and Technology, 135.
- Yu Y., Pan F., Ramaswamy H.S., Zhu S., Yu L., Zhang Q. Effect of soaking and single/two cycle high pressure treatment on water absorption, color, morphology and cooked texture of brown rice. Journal of Food Science and Technology-Mysore. 2017;54(6):1655–1664. doi: 10.1007/s13197-017-2598-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang M., Chen G., Li M., Niu H., Chen Y.e., Jiang P., Li S. Effects of microwave on microscopic, hydration, and gelatinization properties of oat and its application on noodle processing. Journal of Food Processing and. 2022 [Google Scholar]
- Zhou Y.-l., Cui L.-H., You X.-Y., Jiang Z.-H., Qu W.-H., Liu P.-D.…Cui Y.-Y. Effects of repeated and continuous dry heat treatments on the physicochemical and structural properties of quinoa starch. Food Hydrocolloids. 2021;113:106532. [Google Scholar]
- Zou J., Xu M., Tang W., Wen L., Yang B. Modification of structural, physicochemical and digestive properties of normal maize starch by thermal treatment. Food Chemistry. 2020;309:125733. doi: 10.1016/j.foodchem.2019.125733. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.