Abstract
Non-melanoma carcinoma has high incidence rates and has two most common subtypes: basal cell carcinoma and squamous cell carcinoma. This type of carcinoma is usually not fatal; however, it can destroy sensory organs such as the nose, ears, and lips. The treatment of these injuries using non-invasive methods is thus strongly recommended. Some treatments for non-melanoma carcinoma are already well defined, such as surgery, cryosurgery, curettage and electrode section, and radiotherapy; however, these conventional treatments cause inflammation and scarring. In the non-surgical treatment of non-melanoma carcinoma, the topical administration of chemotherapeutic drugs contributes for an effective treatment with reduced side effects. However, the penetration of anticancer drugs in the deeper layers of the skin is required. Lipid delivery systems (liposomes, solid lipid nanoparticles, nanostructured lipid carriers) have been developed to overcome epidermal barrier of the skin and to allow the drugs to reach tumor cells. These lipid nanoparticles contribute to control the release profile of the loaded chemotherapeutic drugs, maintaining their stability and increasing death of tumor cells. In this review, the characteristics of non-melanoma carcinoma will be discussed, describing the main existing treatments, together with the contribution of lipid delivery systems as an innovative approach to increase the effectiveness of topical therapies for non-melanoma carcinomas.
Keywords: basal cell carcinoma, squamous cell carcinoma, liposomes, solid lipid nanoparticles, nanostructured lipid carriers
Introduction
Non-melanoma skin cancer is a current public health problem worldwide. Based on the most recent statistic report, there are annually recognized about 5.4 million cases of basal cell carcinoma and squamous cell, while in the United States among 3.3 million patients are diagnosed [1, 2]. Although most non-melanoma skin cancers are reported to be treatable and are not deadly, they can destroy facial sensory organs, such as the nose, ears, lips, and eyelids, as late diagnosis and treatment may result in the growth of tumour cells and their expansion, requiring greater excisions, resulting in a greater cosmetic impact and loss of motor functions [3, 4]. Therefore, prevention, early diagnosis, as well as the development of non-invasive treatments are in demand.
Certain characteristics, such as age, light skin and light-coloured eyes, freckles, and family history of skin cancer, are risk factors for the development of non-melanoma skin cancer [5]. Among these, excessive exposure to ultraviolet (UV) radiation is regarded as the main risk factor for the development of this type of cancer [6, 7], mainly due to the decrease in the stratospheric ozone layer, which consequently increases human exposure to unfiltered ultraviolet radiation in the ozone layer [8], [9], [10].
The intake of certain nutrients can also contribute to the development and progression of skin cancer, but may also interfere with its prevention and treatment [11], [12], [13], [14]. This is the case of excessive intake of fats, which can increase the carcinogenic effect of UV radiation, as lipid peroxidation gives rise to unsaturated aldehydes α and β, which are mutagenic and carcinogenic [15, 16]. Another example is folate, one of the B vitamins, which at high levels may increase the risk of non-melanoma skin cancer [17]. On the other hand, vitamin A is essential for the proliferation, differentiation, and maintenance of epithelial cells and for reducing the amount of ultraviolet radiation that reaches the strata below the epidermis, by increasing its thickness [18]. Retinoids thus contribute to normal cell differentiation and inhibit the growth of malignant cells [19]. Carotenoids may play a role in inhibiting tumours, converting the human body to retinol, or acting as an antioxidant and reducing deoxyribonucleic acid (DNA) modifications caused by sun exposure [20, 21]. Vitamin D shows a protective effect against non-melanoma skin cancer, by inhibiting strong carcinogens such as 7,12-dimethylbenzanthracene, exercising a photoprotective function against exposure to UV radiation [22]. The polyphenols existing in black tea may also contribute to the inhibition of tumours derived from UV radiation, through chemoprotective mechanisms, such as the elimination of reactive oxygen species (ROS) or the increase in apoptosis of cancer cells [23, 24]. However, no nutrient has conclusively been shown to prevent or reduce the risk of non-melanoma skin cancer. The reduction of fats and the use of retinol in a diet may contribute to small health benefits for patients with non-melanoma skin cancer [25, 26].
Non-melanoma skin cancer has 82 types of tumours, with different diagnoses, many of which are difficult to detect and diagnose [27, 28]. This review addresses the most common types of non-melanoma skin cancers, focusing on the main characteristics of each type, as well as their molecular mechanisms. Thus, current treatments for non-melanoma carcinoma, and the role played by lipid delivery systems (namely, liposomes, solid lipid nanoparticles and nanostructured lipid carriers) to overcome some limitations of existing treatments, will be highlighted.
Non-melanoma carcinoma
Non-melanoma skin cancer has two most common types, basal cell or basaloid carcinoma and squamous cell or squamous cell carcinoma, with the former having a higher incidence than the latter [29, 30]. Other less common types of non-melanoma skin cancer are Merkel's carcinoma, Kaposi's sarcoma, and T-cell lymphoma [31, 32].
Basal cell carcinoma
Basal cell carcinoma is the most common malignant skin tumour, the lesions of which occur more frequently in areas subject to greater sun exposure, particularly on the head and neck [33, 34]. Its designation is related to the fact that the carcinoma is similar to the basal layer of the epidermis, its origin is associated with the germ cells of the hair follicle [35, 36]. This type of carcinoma has certain common characteristics, such as the presence of small and round basaloid cells, an oval or elongated nucleus with condensed chromatin, and little cytoplasm [37]. At the tumour boundary, cells tend to line up perpendicularly to the peripheral layer [3].
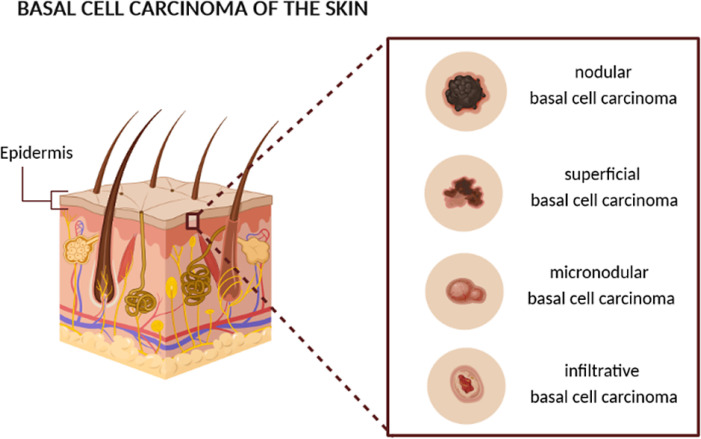
The histology of basal cell carcinoma, as well as the risk of recurrence and metastasis [38], provides the basis for its categorization into subtypes [39, 40], shown in Figure 1, allowing a more detailed and effective diagnosis and treatment [41].
Figure 1.
Schematic representation of the different subtypes of basal cell carcinoma [authors’ own drawing].
Nodular basal cell carcinoma is the most common subtype and is histologically well defined [42]. The peripheral nucleus cells are palisaded, and this subtype of basal cell carcinoma is characterized by the formation of cracks that result from the contraction of mucin during tissue fixation and staining [41, 43]. Nodular basal cell carcinoma has a low risk of recurrence because it is well known clinically and consequently the tumour boundary for further treatment is well delineated [44].
Superficial basal cell carcinoma is a subtype that affects the trunk and extremities of the body more, compared to the other subtypes [45]. This subtype of basal cell carcinoma is characterized by a smooth or red stain and irregular proliferation of tumour cells in the epidermis with little or no penetration into the dermis [41]. Recurrence is common, as the lesions of this subtype of basal cell carcinoma are difficult to define clinically [46].
The infiltrative and micronodular basal cell carcinoma are two subtypes of basal cell carcinoma with a high risk of local recurrence [46]. The infiltrative basal cell carcinoma is an aggressive subtype, the definition of which refers to the fact that it expands peripherally as well as deeply, penetrating the dermis [40]. The micronodular basal cell carcinoma is similar to the infiltrative one, however, these neoplasms are constituted by aggregates of small and round basaloid cells, unlike the other subtypes that present large aggregates [47, 48].
Squamous cell carcinoma
Squamous cell carcinoma, the second most common type of skin cancer, is more invasive than basal cell carcinoma and has a greater predisposition to develop in certain cervicofacial regions compared to basal cell carcinoma, such as in the ears and lower lip [49, 50]. This type of carcinoma is more common in the older population; however, young people are not immune to this disease. As with basal cell carcinoma, the main cause of squamous cell carcinoma is excessive exposure to ultraviolet radiation [6]. However, other factors such as chemical carcinogenesis and human papillomavirus can lead to the development of squamous cell carcinoma, in this case in regions other than those caused by sun exposure, such as the endometrium [51, 52].
Squamous cell carcinoma corresponds to actinic keratoses, which involve only a part of the epidermis [53]. However, in addition to actinic keratosis, it is possible to differentiate squamous cell carcinoma in situ, which occupies the entire thickness of the epidermis, and invasive squamous cell carcinoma, which penetrates the basal layer of the epidermis [54]. All of these lesions present a disorderly maturation of the cells as they progress from the basal to the superficial layer, as well as variation in the size, shape, and colour of the nucleus and an abnormally high mitotic activity.
The pathological characteristics related to the aggressiveness of squamous cell carcinoma include the size, depth, location, and differentiation of the lesion, that is, tumours larger than 2 cm in diameter are more likely to recur and metastasize, as well as tumours more than 4 mm deep have a high probability of metastasis [55]. Regarding differentiation, the well-defined squamous cell carcinoma presents a differentiated cytological appearance, irregular infiltration of the dermis by neoplastic keratinocytes, and different degrees of inflammation and fibrosis under the lesion. Moderately defined squamous cell carcinoma has a deeper invasion and greater mitotic activity, and blood vessel invasion is also possible. Squamous cell carcinoma with little differentiation usually infiltrates the hypodermis and has little keratinization [56].
The existence of a neoplasm with intermediate histology between basal cell carcinoma and squamous cell carcinoma defines basal squamous cell carcinoma. Despite presenting an ambiguous definition, the term “basal squamous cell carcinoma” refers to basal cell carcinoma with areas made up of squamous cell carcinoma cells [57, 58]. This type of carcinoma is more common in the head and neck than in the trunk and it is argued that it is related to the collision of primary lesions of the basal cell carcinoma and squamous cell carcinoma that are nearby [59, 60]. On the other hand, it is argued that basal squamous cell carcinoma corresponds to basal cell carcinoma capable of forming keratoses. The histological definition that brings together greater consensus argues that basal squamous cell carcinoma corresponds to the infiltrative growth of basaloid cells larger than cells of the common basal cell carcinoma, presenting little cytoplasm and large, uniform, and pale nuclei [61]. These cells form a cluster, in which aggregates of squamous cell carcinoma cells can form, either in the centre or spread over the lesion [62] .
Other Non-Melanoma Carcinomas
T-cell lymphoma, Merkel's carcinoma as well as Kaposi's sarcoma refer to different types of skin cancers of the rarer non-melanoma type [31, 63]. The predominant cells of the adaptive immune system are lymphocytes, which divide into B cells and T cells [64]. With advancing age, several changes occur in the immune system, which is responsible for the state of immunosuppression, the most significant change being related to T cells, in that there is a marked decrease in T lymphocytes and inability to respond to some signs of activation [65]. This decrease in T lymphocyte response is caused by the permanent loss of CD28 protein expression, an important receptor, the absence of which makes T lymphocytes unable to undergo clonal expansion [66]. The decrease in CD28 protein on the surface of T cells and the increase in CD95 expression contribute to a greater tendency for cells to undergo apoptosis. These changes increase the occurrence of a certain type of non-melanoma skin cancer called T-cell lymphoma, which belongs to a rare group of lymphomas, being quite biologically heterogeneous [67]. T-cell lymphoma represents the second stage of aggressive non-Hodgkin lymphoma, having been categorized according to the immunophenotyping of T cells and their pathological characteristics, which allowed the definition of a wide spectrum of the different types of T cell neoplasms [68, 69].
Merkel's carcinoma is a rare but very aggressive neuroendocrine carcinoma, for which excessive sun exposure appears once again as the main risk factor for its development [58]. The histological features of Merkel's carcinoma include neuroendocrine granules and intercellular junctions, presenting fast-growing painless nodules. Merkel's carcinoma also has a high incidence in individuals with immunosuppression of T lymphocytes, such as patients with human immunodeficiency syndrome (AIDS), leukaemia, or recipients of an organ transplant [58, 70]. These factors indicate the existence of an infectious agent, and a polyomavirus integrated into the genome of Merkel's carcinoma cells has recently been discovered. The then-called Merkel cell polyomavirus was detected in several Merkel carcinomas, suggesting that this polyomavirus may be the origin of this type of non-melanoma carcinoma in immunosuppressed patients [71, 72]. Kaposi's sarcoma is an antiproliferative disorder that is characterized by neoangiogenesis, inflammation, and oedema and can be classified in four main ways: classic, endemic (Africa), associated with immunosuppression, and associated with aids. Classic Kaposi's sarcoma typically affects elderly men of European origin and presents with paranodular lesions on the skin [73]. Endemic Kaposi's sarcoma, as well as aids-associated Kaposi's sarcoma, have a higher incidence in Africa, among young men and children, the latter presenting in a more aggressive form, with skin lesions and viscera [74]. Kaposi's sarcoma-associated with immunosuppression develops due to immunosuppressive therapies in transplant patients and the use of immunosuppressive pharmacologically active substances (PAS) [74, 75]. Kaposi's carcinomas result from the transmission of human herpesvirus type 8, saliva being the main source of transmission. in the case of transplanted patients, Kaposi's sarcoma may occur due to the reactivation of human herpesvirus type 8 [76, 77]. Kaposi's sarcoma is classified as an intermediate neoplasm, as it does not have the conventional characteristics of malignant disease [78]. Since basal cell carcinoma and squamous cell carcinoma are the most common, this chapter will focus on the topics covered in these two types of non-melanoma carcinoma.
Molecular mechanisms of non-melanoma carcinoma
Sun exposure is, as already mentioned, the main factor for the development of non-melanoma carcinoma [79], [80], [81]. Mutations caused by ultraviolet radiation cause the development of neoplasms, by altering the genes responsible for regulating cell growth and development [79]. These mutations are common in tumour cells of basal cell carcinoma as well as squamous cell carcinoma, although some changes are specific to each type of carcinoma (Table 1).
Table 1.
Target molecules of non-melanoma type carcinoma based on the most recent scientific reports.
| Protein | Type of non-melanoma carcinoma | Reference |
|---|---|---|
| p53 | Squamous cell carcinoma, Basal cell carcinoma | [82], [83], [84], [85], [86] |
| Ras | Squamous cell carcinoma, Basal cell carcinoma | [86], [87], [88], [89] |
| E-cadherin | Squamous cell carcinoma | [90, 91] |
| Sonic hedgehog (Shh) | Basal cell carcinoma | [92], [93], [94] |
Ultraviolet A radiation (320-400 nm) is 10,000 times less mutagenic than ultraviolet B radiation (290-320 nm), the latter having a wavelength that is strongly absorbed by the nitrogenous bases of DNA and a spectrum of mutations distinct from those caused by other types of mutagenesis [95], [96], [97]. Mutations caused by ultraviolet B radiation tend to occur in two or more consecutive pyrimidines, consisting of the replacement of nitrogenous bases, most often in the transition Cytosine (C) → Thymine (T) and the formation of thymine dimers (CC → TT) [96, 98, 99].
DNA changes caused by solar radiation only progress to skin cancer if they are consecutive and repeated, moving on to a phase of replication [100, 101]. Mutations can occur in two alleles of a tumour suppressor, in two different proto-oncogenes, or a proto-oncogene and a tumor suppressor gene. Proto-oncogenes are normal genes that, when mutated, become active oncogenes or encode proteins with new functions. Tumour suppressor genes, when mutated or eliminated, lose their negative influence on controlling tumour growth [100].
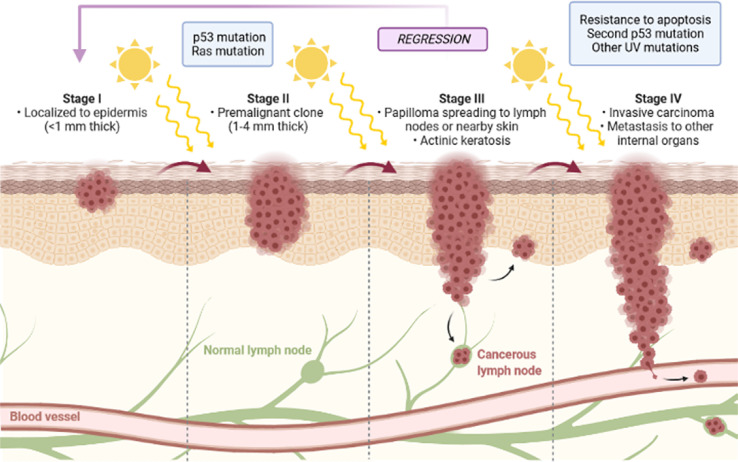
The evolution of squamous cell carcinoma is associated with different stages in which cellular and molecular changes occur, which, in turn, are related to the transition from keratinocytes to papilloma and consequently to carcinoma [102, 103], as shown in Figure 2.
Figure 2.
Schematic representation of the different stages of evolution of squamous cell carcinoma [authors’ own drawing].
In an initial phase, a mutation in a proto-oncogene occurs, with clonal expansion into pre-malignant cells. The progress of the neoplasia is characterized by an increase in cell proliferation resulting in a benign squamous cell papilloma [104]. The evolution to malignant squamous cell carcinoma corresponds to the last stage and occurs in a spontaneous process associated with aneuploidy and chromosomal aberrations [105, 106]. Regression means the partial or complete disappearance of a malignant tumour confirmed by microscopic examination in the absence of treatment, however, this phenomenon occurs infrequently. Among the most common neoplasms to regress are melanoma, renal cell carcinoma, and neuroblastoma [107].
Most cancers have mutations in the p53 tumour suppressor gene [108], with non-melanoma carcinoma being one of them, with this type of mutation being observed in basal cell carcinoma and a large percentage in squamous cell carcinoma. This protein plays a fundamental role in the cellular response to DNA damage, rapidly accumulating in the nucleus of damaged cells and causing a delay in the G1 phase of the cell cycle, allocating more time for DNA repair or elimination of the cell by apoptosis [84, 109]. The p53 protein is essential for inducing apoptosis of epidermal cells, whose DNA has been altered by ultraviolet radiation [82, 86]. Since ultraviolet radiation is a promoter of non-melanoma carcinomas and the fact that the death of your cells is dependent on the p53 protein [84], it then works as a mechanism to prevent the development of the carcinoma [110]. Mutations of the p53 protein in squamous cell carcinoma are predominantly transitions of type C → T and CC → TT, proving the origin of mutagenesis in ultraviolet B radiation [82]. Basal cell carcinoma also presents a mutation of the p53 protein in about 50% of the cases examined [111]. While squamous cell carcinoma loses one p53 allele and isolated mutations in another, basal cell carcinoma tends to mutate in two p53 protein alleles [112].
Ras protein mutations are also associated with the early development of neoplasms in humans [113]. The Ras protein is responsible for regulating proliferation, angiogenesis, apoptosis, and cell morphology, encoding the G protein that is responsible for the hydrolysis of guanosine triphosphate (GTP). Ras oncogene encodes the Ras protein, which prevents the hydrolysis of GTP from occurring [114]. The Ras oncogene acts together with other oncogenes, such as the Fos oncogene, and when introduced into normal keratinocytes, they cause their transformation [86]. The Ras oncogene plays a crucial role in the evolution of non-melanoma carcinomas, with mutations provoked in the Ras gene by ultraviolet B radiation, observed in basal cell carcinoma, as well as squamous cell carcinoma [89].
E-cadherin is a membrane protein involved in cell adhesion, which is expressed in epithelial tissues and with the important function of maintaining epithelial stability [115]. The inactivation of this protein is associated with the invasion of tumour cells and the metastasis of several cancers, including squamous cell carcinoma [116]. The inactivation of E-cadherin occurs through its mutation and by hypermethylation of the E-cadherin gene promoter, a mechanism related to gene silencing [90, 117]. The increase in E-cadherin hypermethylation is directly related to the greater invasive character of the tumour [117].
The expression of the Sonic hedgehog (SHH) glycoprotein in human skin contributes to the acquisition of characteristics of basal cell carcinoma [30, 118]. Activation and changes in the SHH signalling pathway and loss of function of the receptor of the gene encoding the SHH protein (PTCH1) are relevant to the development of basal cell carcinoma, resulting in abnormal gene expression [30].
Treatments of non-melanoma carcinomas
Several surgical and non-surgical treatments have been developed over the last few years for non-melanoma carcinoma [28], with the main objective of obtaining the highest possible percentage of carcinoma elimination, with minimal tissue destruction and with acceptable cosmetic results [119]. Treatment must be individualized, based on the characteristics of the tumour, the patient, and the dermatologist's experience with the various existing techniques. The traditional treatment of non-melanoma carcinoma includes surgery, cryosurgery, curettage and electrode section, and radiotherapy [119, 120]. However, alternatives to the traditional treatment of non-melanoma carcinoma have been developed, with different levels of effectiveness, such as lasers, topical pharmacotherapy, and photodynamic therapy.
Surgery emerges as the most effective treatment for non-melanoma carcinoma, as it allows a histological assessment of the tumour margins, both in its extension and depth, and has a low percentage of recurrence. The surgery consists of excising the tumour with a safety margin, with the application of local anaesthesia in most cases, even though the treatment is individualized, it may require surgery with general anaesthesia [121].
Other treatments, such as cryosurgery and curettage, and electrode section, also have a high cure rate for the disease. In cryosurgery, biological changes in the skin are caused by freezing and thawing, causing tissue necrosis. Generally, this technique involves the use of an aerosol or a cryoprobe, and its main advantages are being safe, fast, effective, low cost, and with satisfactory cosmetic results [120]. Curettage and electrodissection are recommended for the treatment of well-defined, low-risk carcinomas [122]. This technique consists of curettage followed by electrodissection of the site to be treated, with repetition of the procedure as many times as necessary. It is a simple technique, of low cost and with satisfactory cosmetic results. However, these techniques have some disadvantages, as they do not allow the histological evaluation of the tumour margins, increase the risk of pigmentary changes and despite satisfactory cosmetic results, the use of these techniques can lead to the formation of hypertrophic or atrophic scars [123].
When the patient is not a candidate for surgery and when the tumour is more than 15 mm in diameter in high-risk areas or more than 20 mm in diameter in medium-risk areas, radiotherapy appears with the technique used for the treatment of carcinoma basal cell and squamous cell carcinoma [124]. The typical patient for this type of treatment is an elderly individual with an advanced stage of the disease. Radiotherapy has a high cure rate, as well as a low recurrence rate of basal cell carcinoma, however, the results are not as satisfactory and effective as those obtained by surgery [125]. Squamous cell carcinoma does not have as high success rates with radiotherapy treatment as basal cell carcinoma, and it can reoccur quickly after radiotherapy, being a technique that also does not allow the histological evaluation of the tumour margins [50, 126]. These factors together with advances in surgical techniques have contributed to the use of radiotherapy only in very specific situations, as previously mentioned [127].
Despite the surgical procedure emerging as the standard treatment for non-melanoma carcinomas, presenting low rates of recurrence and allowing histological studies of the tumour, in certain circumstances alternatives to surgery and less invasive options, are needed for the treatment of non-melanoma carcinoma [128]. In the last decade, new techniques have been developed and improved, emerging as more recent non-surgical treatments, laser therapy, topical pharmacotherapy (5-fluorouracil and imiquimod), and photodynamic therapy [28, 129].
Laser therapy appears as an alternative treatment for low-risk skin tumours and consists of applying a light source to eliminate the tumour [130], [131], [132]. The improvement of laser technology has resulted in small and portable lasers suitable for the removal of skin lesions, with reduction of thermal diffusion and unwanted thermal necrosis, contributing to the minimization of tissue damage, ease of recovery, and less formation of scars [133]. The carbon dioxide laser and the pulsed contrast laser are the most used in the treatment of non-melanoma carcinomas [134], more specifically for basal cell carcinoma, revealing very high cure rates and satisfactory cosmetic results, with the disadvantage of not allowing to perform histological assessments [135, 136].
The treatment of non-melanoma carcinoma by topical pharmacotherapy has been reported to use semi-solid formulations of 5-fluorouracil and imiquimod [137]. 5-fluorouracil is a chemotherapeutic agent used in the treatment of basal cell carcinoma and squamous cell carcinoma topically, with a concentration of 5% [138], [139], [140]. This anti-cancer is structurally similar to thiamine and inhibits the enzyme thymidylate synthase, blocking DNA synthesis in proliferative cells. The main side effect of 5-fluorouracil is the development of serious inflammatory reactions. Another disadvantage is related to the fact that it is recommended only for the treatment of superficial carcinomas and when patients cannot undergo surgery, it is not effective for the treatment of lesions in deeper layers of the skin [141]. This limitation makes it necessary to use alternative methods to improve the permeation of this anti-cancer drug into the skin.
Imiquimod acts as an immune response modifier, stimulating the innate and adaptive immune response [142]. Imiquimod 5% cream was initially approved for the treatment of genital and perianal warts and later for the treatment of superficial basal cell carcinomas and actinic keratoses of patients who would not be candidates for surgery. Treatment with imiquimod reveals similar disadvantages to treatment with 5-fluorouracil, exhibiting, in some cases, local inflammation [143].
Photodynamic therapy is a relatively recent therapeutic method [144], also approved for the treatment of non-melanoma carcinoma and results in the administration of a drug or pro-drug, which will contribute to the formation of a photosensitive compound, which will accumulate in tumour tissues, followed by irradiation with visible light, which causes a set of photochemical reactions that cause the elimination of target cells [130, 145]. Photosensitization obtained for photodynamic therapy of superficial carcinomas is usually obtained by topical application of 5-aminolevulinic acid or its methyl ester, which will increase the formation of protoporphyrin IX in the skin, since 5-aminolevulinic acid is the precursor of protoporphyrin IX [146]. Subsequently, the tumour will be irradiated with light with a wavelength corresponding to the maximum absorption of protoporphyrin IX, initiating a complex of chemical, biological and physiological reactions, in which very reactive oxygen singlets are produced, which cause necrosis and/or apoptosis of cells [147]. Thus, for the treatment to be specific for the death of tumour cells, the photosensitizer must accumulate in high concentrations in the target cells, that is, 5-aminolevulinic acid must selectively accumulate in the tumour tissue so that consequently there is an increase in the concentration of protoporphyrin in that location [148]. This fact appears as a limitation for this technique, in the treatment of carcinomas in deeper and non-superficial skin strata, as there is a greater difficulty for 5-aminolevulinic acid to penetrate the skin and reach these deeper strata [149]. Several methods have been developed to overcome this barrier, such as the development of lipid vectors, which will be addressed later. Photodynamic therapy is a non-invasive, effective method with very satisfactory cosmetic results, the formation of scars being an exception and not a consequence of treatment, as in the case of surgery. The fact that it is a very selective treatment, as it only acts on the area in the tumour cells, means that the risk of loss of function is very low. Photodynamic therapy may also appear as a supplement to surgery, with a reduction in the size of the tumour to simplify its surgical removal [150].
These recent topical treatments are fundamentally approved for the treatment of superficial non-melanoma carcinomas and are only used in the treatment of more invasive carcinomas when the patient cannot undergo surgery, as chemotherapeutic drugs may not reach invasive carcinoma throughout its depth [141]. It is essential to emphasize once again the need to develop new methods that increase the penetration of these drugs into the skin for the treatment of different types of non-melanoma carcinoma [53, 151]. Several studies have been reported to overcome the limitations of existing treatments, such as the use of lipid delivery systems [152], for the delivery of chemotherapeutic drugs aiming at reducing their adverse side effects [153, 154].
Stimuli responsive lipid vectors for the treatment of non-melanoma carcinoma
A remarkable characteristic of cancerous cells is their low cytosol pH when compared to other cells. Increased hyperthermia sensitivity is also another almost exclusive feature of cancer cells [155]. Specific systems can be engineered to deliver their content only when exposed to proper environmental stimulus like light, pH gradients, sound, magnetic fields, or epitopes in the organism, leading to a controlled release both in time and space of drugs which can be very useful to target cytotoxic treatments [156, 157].
Low intracellular pH values enable the delivery of pH-sensitive nanoparticles through rupture of acidic-sensitive bonds with the consequent dismantling of the lattice covering the drug content, promoting drug release [158, 159]. Some examples in pH-sensitive systems are those possessing hydrazone bonds (sensitive to acidic environments) as in gemcitabine-loaded micelles with a stearic acid skeleton, GemC18 [160]. When GemC18 and micelles with the same drug but pH-insensitive are compared, GemC18 demonstrated higher cytotoxicity than the prior ones. The low-pH hydrolysis reaction is also a fundamental example of such phenomena, as in doxorubicin-loaded methacrylamide micelles (DOX-MA) [160, 161]. When applied to the in vivo cancer mouse model B16-F10, DOX-MA resulted in preeminent cytotoxic and pro-death effects than doxorubicin molecules alone [162].
The energy available as heat can be generated using the transformation of electromagnetic energy through electromagnetic coils (generating an electromagnetic field) or using radio shortwaves. Two mechanisms are usually considered when disserting about killing cancer cells - death by the direct incidence of heat or by targeting these cells with scaffolds made of heat-sensitive polymers allowing specific delivery of the drugs. An example of a system applied to clinical purposes is an inhibitor of heat-shock protein 90 (Hsp 90) – an anti-apoptotic protein which decreases the sensitivity of cancer cells to hyperthermia and reduces the expression of Akt kinase protein - geldanamycin (GA), which combined with ferromagnetic thermosensitive nanoparticles (FTN) and generates a GA-FTN formulation sensitive to magnetic fields [163, 164]. Compared to the prior formulations with GA molecules alone is distinguishable a prominent cytotoxic effect of the formulation.
To synthesize light-sensitive delivery systems some materials with unique properties (light responsiveness) shall be used during the process of formulation. They bring several advantages as the capacity to trigger a reaction only when exposed to specific wavelengths, during a minimum period, and with a minimum required intensity. The light used to trigger the therapeutic action of these systems may range the ultraviolet, visible, and infrared spectra [165]. A study using quantum dots composed of cadmium telluride wrapped in silica allowed, after laser incidence, to prevent the growth of melanoma with significant differences when compared to the tissues unexposed to the laser beam [165, 166].
Photodynamic therapy (PDT) uses three critical elements: oxygen, light with a specific wavelength, and a photosensitizer (PS) [167, 168]. This last element is an innocuous molecule but, when exposed to light in a certain wavelength (specific for each PS) the molecule becomes “active”, generating a flow of electrons that are transposed to molecular oxygen generating both reactive oxygen species (ROS) via type I reaction, or singlet oxygen (a very reactive form of molecular oxygen) via type II reaction [169]. The presence of these products leads to cell impairment and death. The PS is also preferably taken by cancer cells than by normal ones. This therapeutic option carries with it the advantages of being small invasive and presenting no adverse effects in the long-term which makes it one of the most capable weapons to deal with cancer [170]. Despite such achievements, PDT is restricted on what concerns PS dependent on visible light as it cannot penetrate deeply into the skin. For this purpose, other PS molecules may be used such as those sensitive to infrared (IR) radiation to treat wider and deeper tumours [171]. Several studies point to the efficacy of this remarkable therapy such as chitosan-derived nanoparticles constituted by aminolaevulinic acid to penetrate the skin [172]. Another study demonstrated the efficacy of nanoparticles of mesoporous silica entrapping two PS molecules that are sensitive to IR radiation and yielded significant stoppage of cancer development [168].
Ultrasounds are applied to deliver a sort of products including drugs, genes, and antigens. A brief example of this technology being employed is the liposomes, filled with perfluorpropane gas and specific melanoma cell antigens, that are placed in contact with dendritic cells. When the ultrasound stimulus is applied these liposomes are internalized and posteriorly activated generating crosstalk between dendritic cells and T-lymphocytes which lead to melanoma cell death [173].
In the last decades, several nanometric systems have been proposed for the diagnosis and treatment of different types of cancers [174, 175]. The development of therapeutic products at the nanoscale appears with the main objective of obtaining a controlled release of drugs to increase their effectiveness by the specific targeting to the site of action (tumour cells), consequently reducing the side effects, since healthy cells are not affected.
In the treatment of non-melanoma carcinoma, the stratum corneum of the epidermis appears as the greatest barrier for the penetration of drugs. The transport of drugs occurs mainly in the lipid region of the stratum corneum, which consists of ceramides, cholesterol, free fatty acids, and devoid of phospholipids [151]. Thus, in topical administration of drugs intended to reach deeper layers of the skin is essential to overcome this barrier so that sufficient amounts of drug reach the target cells. Lipid delivery systems have structural characteristics that allow the encapsulation of substances with different solubilities and increase the ability of chemotherapeutic drugs to reach tumour cells, making them more stable and reducing skin irritation, since they prevent direct contact of drug with the skin surface [176, 177].
Liposomes
Liposomes are the most studied lipid delivery systems for the treatment of cancer and are accepted in several strategies for systemic administration of drugs [178]. These lipid vectors consist of spherical vesicles with biocompatible and biodegradable phospholipid bilayers, with amphiphilic characteristics that allow the encapsulation of hydrophilic and lipophilic compounds, in the polar zone or within the lipid bilayer, respectively [173]. The main lipid material used in the production of liposomes is phosphatidyl choline [179]. The selection of the phospholipid bilayer composition is instrumental to determine the rigidity of the liposomal vesicles. Unsaturated phosphatidyl choline obtained from natural sources (e.g. soybean, egg) offers higher permeability but less stable bilayers, whereas saturated phospholipids with long acyl chains (e.g. dipalmitoylphos phatidylcholine) increase the rigidity and impermeability of liposomes [180]. The higher the rigidity of the vesicles, the higher the controlled release of the loaded drugs.
The lipid bilayers have a structural affinity with the stratum corneum of the epidermis, allowing an optimized release of the drug in the different strata of the skin. Many of the studies involving liposomes in the treatment of non-melanoma carcinoma are related to photodynamic therapy [177]. The 5-aminolevulinic acid used in this technique is a hydrophilic molecule and has zwitterionic properties at physiological pH, which results in the main limitations of this compound in the ability to cross the stratum corneum of the skin. Thus, the encapsulation of 5-aminolevulinic acid in liposomes has been investigated to overcome the limitations of photodynamic therapy [181]. Pierre et al. reported on a controlled release system of 5-aminolevulinic acid-based on liposomes made up of lipids similar to those of the stratum corneum of the epidermis [182]. These studies revealed that the liposomes with incorporated 5-aminolevulinic acid present adequate retention of the compound in the epidermis without stratum corneum and in the dermis, and low retention in the stratum corneum [181]. This behaviour indicates that the developed liposomes allow the controlled release of 5-aminolevulinic acid in tumour cells in deeper layers of the skin, for treatment by topical photodynamic therapy [150]. Studies carried out on the influence of different vectors on the formation of protoporphyrin IX by topical application of 5-aminolevulinic acid show the accumulation of the latter in tumour tissues when encapsulated in liposomes, as well as their elimination from the blood circulation after 24 hours of administration, maintaining them if, after this time, only in the tumour cells [150, 181, 183]. The development of ultra-deformable liposomes with different charges on the surface has recently been reported, with greater efficiency in cationic liposomes, which revealed greater stability and permeability, and a release of 5-aminolevulinic acid in deeper layers of the skin by topical application, contributing to the optimization of photodynamic therapy [150, 184]. The encapsulation of polyphenols present in green tea in liposomes has also been studied, to increase their antioxidant efficacy [185] and anti-cancer activity in the treatment of basal cell carcinoma [23]. As in previous studies, these lipid vectors promoted the accumulation of polyphenols in the tumour and induced cell death from basal cell carcinoma more effectively than the polyphenol hydroalcoholic solution [186]. These results are because liposomes can keep polyphenols stable, and it is possible to conclude that these lipid vectors are quite effective for the controlled release of drug, ensuring their accumulation in the target cells as well as their anticancer activity [187].
Fang et al. [188] carried out a comparative study between liposomes and ethosomes to verify the efficacy of the ethosomes with encapsulated 5-aminolevulinic acid to promote the production of a greater amount of protoporphyrin IX in the target cells, due to a greater accumulation of 5- aminolevulinic. The ethosomes correspond to a "new generation" of liposomes being a modified form of them and consist of the mixture of phosphatidylcholine with ethanol showing greater efficiency in the release of drugs by topical administration in terms of quantity and depth concerning the liposomes. The study carried out by Fang et al. [188] allowed us to confirm this fact, having obtained an average size of the ethosomes smaller than the liposomes and a greater capacity of penetration in the strata of the skin of the former about the latter. Ethanol emerges as the main responsible for the ethosomes having a smaller size than the liposomes, producing compact and deformable vesicles, since ethanol has a steric stabilization due to the modification of the total ethosomes charge, which is fundamental for the reduction of the size of the vesicles [189]. The greater capacity of penetration into the skin strata of ethosomes with liposomes is also related to ethanol, but also the surfactant used. Ethanol interacts with the skin and with the lipids of the stratum corneum, making them more fluid and creating channels that allow the increased targeting and delivery of drugs [189].
Pandey et al. proposed to encapsulate the anti-cancer paclitaxel, in ethosomes to promote a controlled release of the drug in the target cells for the treatment of squamous cell carcinoma. Paclitaxel is an extremely hydrophobic molecule, which easily accumulates in the stratum corneum, not penetrating deeper layers of the skin. In this way, the ethosomes allowed to increase the proliferative activity of paclitaxel, since they improved the permeation of drugs in the stratum corneum of the epidermis [190]. The percutaneous permeation profile of paclitaxel encapsulated in ethosomes was approximately 23.2 times higher than that observed for paclitaxel in a hydroalcoholic suspension, suggesting a much greater capacity of the encapsulated drug to reach deeper strata and exercise its function for the elimination of squamous cell carcinoma [190], [191], [192].
Other liposomes-based vesicles have also been developed to improve cutaneous permeation of 5-fluorouracil. This anti-cancer is hydrophilic and shows low skin permeation capacity, which consequently reduces its activity when topically administered. The encapsulation of 5-fluorouracil in niosomes allows the penetration of drug through the stratum corneum, allowing to increase its cytotoxic effect in the intended tissues. Niosomes, like ethosomes, are vesicles similar to liposomes, however, they are nonionic. The ability to increase the skin permeability of niosomes is related to the flexibility and deformability that these vesicles present and the fact that they allow them to pass through the skin layers [178].
Lipid nanoparticles
Lipid nanoparticles also play an important role in improving the efficacy and safety of chemotherapy in non-melanoma skin cancers [193, 194]. Solid lipid nanoparticles (SLN) consist of a solid lipid matrix (solid at room temperature) that allows the release of poorly soluble anti-cancers into target cells.
Nanostructured lipid carriers (NLC) are made up of a mixture of solid lipid and liquid lipid (in a combination that is also solid at room temperature) and have also proven the ability to deliver drugs to deeper layers of the skin, maintaining the stability of drugs and ensuring their accumulation in the skin, tumor cells and their anti-cancer function [178, 195]. The solid lipids commonly used in the production SLN and NLC include fatty acids (e.g., stearic acid, palmitic acid, myristic acid), triglycerides (e.g., tribehenate, tristearate, tripalmitate, trimyristate), cholesterol, waxes (e.g., bee wax, cetyl palmitate), and less pure mixtures (e.g., Imwitor®, Softisan®, Witepsol®). The liquid lipids that are in the composition of NLC are usually natural oils that can have anti-cancer properties (e.g., essential oils, monoterpenes [176, [196], [197], [198]]) or medium chain triglycerides [199].
Yu et al. [200] reported a higher concentration of paclitaxel in cancer cells, demonstrating an increase in the capacity of penetration, a controlled release, and an increase in the anti-cancer activity of drug in the target cells when delivered by SLN.
Venturini et al. [201] proposed co-encapsulation of imiquimod and copaiba oil into lipid nanoparticles to show a synergistic effect against basal cell carcinoma. The chemical imiquimod approved for the treatment of this type of cancer and copaiba oil is known for its anti-proliferative properties. From the in vitro biocompatibility testing in healthy keratinocytes cell cells (HaCaT), no morphological changes were observed, whereas the skin permeation testing in pig skin the authors reported an increased drug retention in the skin layers when using the lipid nanoparticles for the delivery of chemotherapeutic drug.
Ali-von Laue et al. [202] produced SLN to load a guanosine-analogue phosphonate (OxBu) for the treatment of actinic keratosis, a precursor of cutaneous squamous cell carcinoma. The tumor response to the formulation was confirmed by increases in caspase-cleaved fragment of keratin-18, caspase-7 activation and reduced of matrix metallopeptidase-2 and Ki-67 expression. The drug efficacy was also found superior to the equimolar 5-fluorouracil solution.
Doxorubicin-loaded cationic lipid nanoparticles were successfully combined with iontophoresis to improve the bioavailability of the drug to skin tumour [203]. The loading of doxorubicin significantly changed partition coefficient through the diffusion cells and increased permeation of stratum corneum to the drug. The association with anodic iontophoresis in squamous cell carcinoma induced in nude BALB/c mice increased 50-fold the doxorubicin penetration in vivo. This combined approach effectively inhibited tumor cell survival and tumor growth followed by increased keratinization and cell death.
Topotecan loaded solid lipid nanoparticles were proposed for the treatment of cervical cancer [204]. The cytotoxicity of loaded particles was assessed against cervical squamous cell carcinoma cell line (HeLa) and human squamous cell carcinoma cell line (SiHa), confirming the biocompatibility of the developed systems.
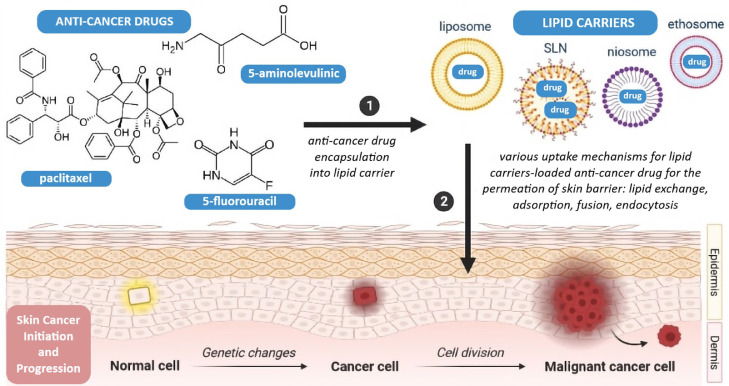
Teaima et al. [205] described the development of a transdermal emulgel containing pomegranate extract-loaded SLN for the treatment of Ehrlich ascites carcinoma. The ex vivo permeation assay showed a non-Fickian diffusion transport through mouse skin. On a mouse model of skin bearing a solid form of Ehrlich ascites carcinoma, the authors confirmed statistically significant anticancer effects of the SLN-containing emulgel. Figure 3 illustrates a variety of lipid carriers that can be used in non-melanoma skin cancer, and Table 2 summarizes examples of anticancer-drugs formulated into lipid carriers, their lipid composition, associated treatment, and in vitro/in vivo results.
Figure 3.
Schematic illustration of non-melanoma skin cancer treatment using lipid delivery systems for chemotherapeutic drugs [authors’ own drawing].
Table 2.
Examples of lipid delivery systems recommended for the treatment of non-melanoma skin cancers, their lipid composition, loaded anti-cancer drug, associated treatment, and in vitro/in vivo results.
| Lipid Delivery System | Lipid composition | Anti-cancer Drug | Associated Treatment | In vitro and/or in vivo results | Reference |
|---|---|---|---|---|---|
| Liposomes | Hydrogenated soy phosphatidylcholine | Curcumin | Photodynamic therapy | Decreased cytotoxicity of phototoxic agents loaded in liposomes toward normal skin cells | [206] |
| AS1411-aptamer conjugated liposomes | Egg Yolk Phosphatidylcholine and cholesterol | 5-Fluorouracil (5-FU) | - | Semi-solid hydrogel based on sodium alginate and hyaluronic acid containing 5-FU-loaded AS1411-aptamer conjugated liposomes increased drug permeability in comparison to free liposomes, with higher anti-tumor efficiency and lower irritation potential on the skin | [207] |
| PEGylated solid lipid nanoparticles | Tefose 1500 | Curcumin | Photodynamic therapy | Skin accumulation of drug in vivo was twice when delivered by SLN in comparison to free suspension; cytotoxicity in A431 cells increased with the loading and with irradiation | [208] |
| Lipid nanoparticles dispersed in sodium carboxy methylcellulose hydrogel | Stearic acid and lecithin | 5-Fluorouracil (5-FU) | - | Mice-bearing Ehrlich's ascites carcinoma treated with 5-FU-loaded SLN exhibited reduced inflammatory reactions, reduced keratosis, and reduced symptoms of angiogenesis when compared to mice with non-loaded 5-FU. | [209] |
| Lipid nanoparticles dispersed in a cream | Glycery monostearate or cetyl alcohol, and lecithin | Sesamol | - | In vitro antiproliferative and DNA fragmentation assays pm HL 60 cell lines confirmed sesamol-induced apoptosis. Significant retention of sesamol in the skin with minimal flux across skin when administered in cream-based SLN both in vitro and in vivo. In vivo anticancer studies on TPA†-induced and benzopyrene-initiated tumour production (ROS mediated) in mouse epidermis showed the histological normalization of skin cancers post their induction. | [210] |
| Cationic lipid nanoparticles | Stearic acid and monoolein | Doxorubicin (DOX) | Iontophoresis | Loading DOX increased drug in the lipid matrix of the stratum corneum. The association with iontophoresis created DOX reservoirs in the follicles of the skin. Nude BALB/c mice-bearing squamous cell carcinoma treated with DOX-SLN and iontophoresis was effective in inhibiting tumor cell survival and tumor growth, with increased keratinization and cell death. | [211] |
| Nanostructured lipid carriers | Sorbitan monooleate; cupuaçu butter | Imiquimod and copaiba oil | - | Imiquimod-loaded NLC did not show any changes in healthy skin cells (keratinocytes, HaCaT); in vitro skin permeation/penetration using pig skin resulted in increased drug retention in the skin layers. | [201] |
TPA, 12-O-tetradecanoylphorbol-13-acetate.
Conclusions
The treatment of non-melanoma carcinoma has been intensively studied and several currently available techniques allow dermatologists to choose the best solution for individualized and more effective treatment. However, existing topical therapies have some limitations and lipid delivery systems appear as very promising approach to reduce serious adverse side effects of local chemotherapy. The studies carried out show the advantage of encapsulating anti-cancer drugs in liposomes, ethosomes and in lipid nanoparticles, to increase the drug stability, penetration into the deeper skin strata, the accumulation in the target cells by controlled release thereof, and consequently increased cytotoxicity of drug in carcinogenic cells. The ultimate aim is to increase anticancer bioavailability in the site of action, sparing healthy cells from the contact with the drug thereby reducing the serious cytotoxic effects of chemotherapy.
Authors Contribution
Conceptualization: ELIANA B. SOUTO, ALEKSANDRA ZIELINSKA, JOÃO DIAS-FERREIRA
writing—original draft preparation: ELIANA B. SOUTO, VÂNIA VIEIRA, J.F.F, JOÃO DIAS-FERREIRA, AMANDA CANO and ALEKSANDRA ZIELINSKA
writing—review and editing ELIANA B. SOUTO, RAFAŁ STASZEWSKI, ALEKSANDRA ZIELINSKA and JACEK KARCZEWSKI
visualization: ALEKSANDRA ZIELINSKA
supervision: ELIANA B. SOUTO and JACEK KARCZEWSKI
funding acquisition: RAFAŁ STASZEWSKI All authors have read and agreed to the published version of the manuscript.
Acknowledgments
Authors wish to acknowledge the National Science Centre within the MINIATURA 4 for a single research activity carried out by Dr. Aleksandra Zielińska (grant no: 2020/04/X/ST5/00789), the Institute of Human Genetics, Polish Academy of Sciences by the internal grant for the implementation of a single scientific activity, and the Poznan University of Medical Sciences. Authors also wish to thank the Portuguese Science and Technology Foundation (FCT) from the Ministry of Science and Technology (MCTES) for sponsoring the project UIDB/04033/2020 (CITAB), co-funded by European Funds (PRODER/COMPETE) and FEDER, under the Partnership Agreement PT2020.
Contributor Information
Eliana B. Souto, Email: ebsouto@ff.up.pt.
Jacek Karczewski, Email: jkarczewski@ump.edu.pl.
References
- 1.Thomas SM, Lefevre JG, Baxter G, Hamilton NA. Interpretable deep learning systems for multi-class segmentation and classification of non-melanoma skin cancer. Medical Image Analysis. 2021;68 doi: 10.1016/j.media.2020.101915. [DOI] [PubMed] [Google Scholar]
- 2.(ASCO) ASoCO (2021). Skin Cancer (Non-Melanoma): StatisticsCancer Facts & Figures
- 3.Cives M, Mannavola F, Lospalluti L, Sergi MC, Cazzato G, Filoni E, Cavallo F, Giudice G, Stucci LS, Porta C. Non-Melanoma Skin Cancers: Biological and Clinical Features. International Journal of Molecular Sciences. 2020;21:5394. doi: 10.3390/ijms21155394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Marson JW, Maner BS, Harding TP, VII JM, Solomon JA, Leavitt M, Levin NJ, Dellavalle R, Brooks I, Rigel DS. The magnitude of COVID-19′s effect on the timely management of melanoma and nonmelanoma skin cancers. Journal of the American Academy of Dermatology. 2021;84:1100–1103. doi: 10.1016/j.jaad.2020.12.065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Apalla Z, Lallas A, Sotiriou E, Lazaridou E, Ioannides D. Epidemiological trends in skin cancer. Dermatol Pract Concept. 2017;7:1–6. doi: 10.5826/dpc.0702a01. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Watson M, Holman DM, Maguire-Eisen M. Ultraviolet Radiation Exposure and Its Impact on Skin Cancer Risk. Semin Oncol Nurs. 2016;32:241–254. doi: 10.1016/j.soncn.2016.05.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Miligi L. Ultraviolet radiation exposure: Some observations and considerations, focusing on some Italian experiences, on cancer risk, and primary prevention. Environments. 2020;7:10. [Google Scholar]
- 8.Apalla Z, Nashan D, Weller RB, Castellsagué X. Skin cancer: epidemiology, disease burden, pathophysiology, diagnosis, and therapeutic approaches. Dermatology and therapy. 2017;7:5–19. doi: 10.1007/s13555-016-0165-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bais AF, Lucas RM, Bornman JF, Williamson CE, Sulzberger B, Austin AT, Wilson SR, Andrady AL, Bernhard G, McKenzie RL, et al. Environmental effects of ozone depletion, UV radiation and interactions with climate change: UNEP Environmental Effects Assessment Panel, update 2017. Photochem Photobiol Sci. 2018;17:127–179. doi: 10.1039/c7pp90043k. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Neale RE, Barnes PW, Robson TM, Neale PJ, Williamson CE, Zepp RG, Wilson SR, Madronich S, Andrady AL, Heikkilä AM, et al. Environmental effects of stratospheric ozone depletion, UV radiation, and interactions with climate change: UNEP Environmental Effects Assessment Panel, Update 2020. Photochemical & Photobiological Sciences. 2021;20:1–67. doi: 10.1007/s43630-020-00001-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Pullar JM, Carr AC, Vissers MCM. The Roles of Vitamin C in Skin Health. Nutrients. 2017;9:866. doi: 10.3390/nu9080866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Georgescu S-R, Sârbu M-I, Matei C, Ilie MA, Caruntu C, Constantin C, Neagu M. Capsaicin: friend or foe in skin cancer and other related malignancies? Nutrients. 2017;9:1365. doi: 10.3390/nu9121365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.de Castro LL, Aguiar JRS, de Araújo CGB, Visacri MB, Tuan BT, de Carvalho Pincinato E, Moriel P. Antioxidant capacity total in non-melanoma skin cancer and its relationship with food consumption of antioxidant nutrients. Nutricion hospitalaria. 2015;31:1682–1688. doi: 10.3305/nh.2015.31.4.8331. [DOI] [PubMed] [Google Scholar]
- 14.Gracia-Cazaña T, González S, Parrado C, Juarranz Á, Gilaberte Y (2020). Influence of the exposome on skin cancerActas Dermo-Sifiliográficas (English Edition). [DOI] [PubMed]
- 15.Schuch AP, Moreno NC, Schuch NJ, Menck CFM, Garcia CCM. Sunlight damage to cellular DNA: Focus on oxidatively generated lesions. Free Radical Biology and Medicine. 2017;107:110–124. doi: 10.1016/j.freeradbiomed.2017.01.029. [DOI] [PubMed] [Google Scholar]
- 16.Papuc C, Goran GV, Predescu CN, Nicorescu V. Mechanisms of oxidative processes in meat and toxicity induced by postprandial degradation products: A review. Comprehensive Reviews in Food Science and Food Safety. 2017;16:96–123. doi: 10.1111/1541-4337.12241. [DOI] [PubMed] [Google Scholar]
- 17.Dhana A, Yen H, Li T, Holmes MD, Qureshi AA, Cho E. Intake of folate and other nutrients related to one-carbon metabolism and risk of cutaneous melanoma among US women and men. Cancer Epidemiol. 2018;55:176–183. doi: 10.1016/j.canep.2018.06.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kim J, Park MK, Li W-Q, Qureshi AA, Cho E. Association of vitamin A intake with cutaneous squamous cell carcinoma risk in the United States. JAMA dermatology. 2019;155:1260–1268. doi: 10.1001/jamadermatol.2019.1937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Zasada M, Budzisz E. Retinoids: active molecules influencing skin structure formation in cosmetic and dermatological treatments. Postepy Dermatol Alergol. 2019;36:392–397. doi: 10.5114/ada.2019.87443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Koklesova L, Liskova A, Samec M, Buhrmann C, Samuel SM, Varghese E, Ashrafizadeh M, Najafi M, Shakibaei M, Büsselberg D. Carotenoids in Cancer Apoptosis—The Road from Bench to Bedside and Back. Cancers. 2020;12:2425. doi: 10.3390/cancers12092425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Chinembiri TN, Du Plessis LH, Gerber M, Hamman JH, Du Plessis J. Review of natural compounds for potential skin cancer treatment. Molecules. 2014;19:11679–11721. doi: 10.3390/molecules190811679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ombra MN, Paliogiannis P, Doneddu V, Sini MC, Colombino M, Rozzo C, Stanganelli I, Tanda F, Cossu A, Palmieri G. Vitamin D status and risk for malignant cutaneous melanoma: recent advances. European Journal of Cancer Prevention. 2017;26:532. doi: 10.1097/CEJ.0000000000000334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Sharma P, Montes de Oca MK, Alkeswani AR, McClees SF, Das T, Elmets CA, Afaq F. Tea polyphenols for the prevention of UVB-induced skin cancer. Photodermatol Photoimmunol Photomed. 2018;34:50–59. doi: 10.1111/phpp.12356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Sharma P, Montes de Oca MK, Alkeswani AR, McClees SF, Das T, Elmets CA, Afaq F. Tea polyphenols for the prevention of UVB-induced skin cancer. Photodermatol Photoimmunol Photomed. 2018;34:50–59. doi: 10.1111/phpp.12356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Russo I, Caroppo F, Alaibac M. Vitamins and Melanoma. Cancers. 2015;7:1371–1387. doi: 10.3390/cancers7030841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Katta R, Brown DN. Diet and Skin Cancer: The Potential Role of Dietary Antioxidants in Nonmelanoma Skin Cancer Prevention. Journal of Skin Cancer. 2015;2015 doi: 10.1155/2015/893149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Farasat S, Yu S.S., Neel V.A., Nehal K.S., Lardaro T., Mihm M.C., Byrd D.R., Balch C.M., Califano J.A., Chuang A.Y., Sharfman W.H., Shah J.P., Nghiem P., Otley C.C., Tufaro A.P., Johnson T.M., Sober A.J., Liegeois N.J. A New American Joint Committee On Cancer Staging System For Cutaneous Squamous Cell Carcinoma: Creation And Rationale For Inclusion Of Tumor (T) Characteristics. J Am Acad Dermatol. 2011;64:1051–1059. doi: 10.1016/j.jaad.2010.08.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Fahradyan A, Howell AC, Wolfswinkel EM, Tsuha M, Sheth P, Wong AK. Updates on the Management of Non-Melanoma Skin Cancer (NMSC) Healthcare (Basel. 2017;5:82. doi: 10.3390/healthcare5040082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Cohen PR, Torres-Quiñones M, Uebelhoer NS. Red Dot Basal Cell Carcinoma: Literature Review of a Unique Clinical Subtype of Basal Cell Carcinoma. Dermatology and Therapy. 2021;11:401–413. doi: 10.1007/s13555-021-00496-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Campione E, Di Prete M, Lozzi F, Lanna C, Spallone G, Mazzeo M, Cosio T, Rapanotti C, Dika E, Gaziano R, et al. High-Risk Recurrence Basal Cell Carcinoma: Focus on Hedgehog Pathway Inhibitors and Review of the Literature. Chemotherapy. 2020;65:2–10. doi: 10.1159/000509156. [DOI] [PubMed] [Google Scholar]
- 31.Csoboz B, Rasheed K, Sveinbjørnsson B, Moens U. Merkel cell polyomavirus and non-Merkel cell carcinomas: guilty or circumstantial evidence? Apmis. 2020;128:104–120. doi: 10.1111/apm.13019. [DOI] [PubMed] [Google Scholar]
- 32.Choi FD, Kraus CN, Elsensohn AN, Carley SK, Lehmer LM, Nguyen RT, Linden KG, Shiu J. Programmed cell death 1 protein and programmed death-ligand 1 inhibitors in the treatment of nonmelanoma skin cancer: A systematic review. Journal of the American Academy of Dermatology. 2020;82:440–459. doi: 10.1016/j.jaad.2019.05.077. [DOI] [PubMed] [Google Scholar]
- 33.Kawaguchi M, Kato H, Tomita H, Hara A, Suzui N, Miyazaki T, Matsuyama K, Seishima M, Matsuo M. Magnetic resonance imaging findings differentiating cutaneous basal cell carcinoma from squamous cell carcinoma in the head and neck region. Korean journal of radiology. 2020;21:325. doi: 10.3348/kjr.2019.0508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Yan F, Knochelmann HM, Morgan PF, Kaczmar JM, Neskey DM, Graboyes EM, Nguyen SA, Ogretmen B, Sharma AK, Day TA. The evolution of care of cancers of the head and neck region: State of the science in 2020. Cancers. 2020;12:1543. doi: 10.3390/cancers12061543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Castanheira A, Boaventura P, Clemente MP, Soares P, Mota A, Lopes JM. Head and neck cutaneous basal cell carcinoma: what should the otorhinolaryngology head and neck surgeon care about? Acta Otorhinolaryngologica Italica. 2020;40:5. doi: 10.14639/0392-100X-2245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Peterson SC, Eberl M, Vagnozzi AN, Belkadi A, Veniaminova NA, Verhaegen ME, Bichakjian CK, Ward NL, Dlugosz AA, Wong SY. Basal cell carcinoma preferentially arises from stem cells within hair follicle and mechanosensory niches. Cell stem cell. 2015;16:400–412. doi: 10.1016/j.stem.2015.02.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Goyal S, Rathore R, Sharma S, Arora VK, Das GK, Singal A. Cutaneous basal cell carcinoma with mixed histology: Cytomorphological features of two unusual cases. J Cytol. 2017;34:115–118. doi: 10.4103/0970-9371.203566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Puig S, Berrocal A. Management of high-risk and advanced basal cell carcinoma. Clinical and Translational Oncology. 2015;17:497–503. doi: 10.1007/s12094-014-1272-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Peris K, Fargnoli MC, Garbe C, Kaufmann R, Bastholt L, Seguin NB, Bataille V, Vd Marmol, Dummer R, Harwood CA, et al. Diagnosis and treatment of basal cell carcinoma: European consensus–based interdisciplinary guidelines. European Journal of Cancer. 2019;118:10–34. doi: 10.1016/j.ejca.2019.06.003. [DOI] [PubMed] [Google Scholar]
- 40.Villani R, Murigneux V, Alexis J, Sim S-L, Wagels M, Saunders N, Soyer HP, Parmentier L, Nikolaev S, Fink JL (2021). Subtype-Specific Analyses Reveal Infiltrative Basal Cell Carcinomas Are Highly Interactive with their EnvironmentJournal of Investigative Dermatology. [DOI] [PubMed]
- 41.Fania L, Didona D, Morese R, Campana I, Coco V, Di Pietro FR, Ricci F, Pallotta S, Candi E, Abeni D, et al. Basal Cell Carcinoma: From Pathophysiology to Novel Therapeutic Approaches. Biomedicines. 2020;8:449. doi: 10.3390/biomedicines8110449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Attia ABE, Chuah SY, Razansky D, Ho CJH, Malempati P, Dinish U, Bi R, Fu CY, Ford SJ, Lee JS-S. Noninvasive real-time characterization of non-melanoma skin cancers with handheld optoacoustic probes. Photoacoustics. 2017;7:20–26. doi: 10.1016/j.pacs.2017.05.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Suppa M, Fontaine M, Dejonckheere G, Cinotti E, Yélamos O, Diet G, Tognetti L, Miyamoto M, Orte Cano C, Perez-Anker J. Line-field confocal optical coherence tomography of basal cell carcinoma: a descriptive study. Journal of the European Academy of Dermatology and Venereology. 2021;35:1099–1110. doi: 10.1111/jdv.17078. [DOI] [PubMed] [Google Scholar]
- 44.Altun E, Schwartzman G, Cartron AM, Khachemoune A. Beyond Mohs surgery and excisions: A focused review of treatment options for subtypes of basal cell carcinoma. Dermatologic Therapy. 2021;34:e14476. doi: 10.1111/dth.14476. [DOI] [PubMed] [Google Scholar]
- 45.Pyne JH, Myint E, Barr EM, Clark SP, David M, Na R, Hou R. Superficial basal cell carcinoma: A comparison of superficial only subtype with superficial combined with other subtypes by age, sex and anatomic site in 3150 cases. Journal of cutaneous pathology. 2017;44:677–683. doi: 10.1111/cup.12959. [DOI] [PubMed] [Google Scholar]
- 46.Cameron MC, Lee E, Hibler BP, Barker CA, Mori S, Cordova M, Nehal KS, Rossi AM. Basal cell carcinoma: Epidemiology; pathophysiology; clinical and histological subtypes; and disease associations. Journal of the American Academy of Dermatology. 2019;80:303–317. doi: 10.1016/j.jaad.2018.03.060. [DOI] [PubMed] [Google Scholar]
- 47.Carr RA, Taibjee S.M., Sanders D.S.A. Basaloid Skin Tumours: Basal Cell Carcinoma. Current Diagnostic Pathology. 2007;13:252–272. [Google Scholar]
- 48.Mcguire JF, Ge N.N., Dyson S. Nonmelanoma Skin Cancer Of The Head And Neck I: Histopathology And Clinical Behavior. Am J Otolaryngol. 2009;30:121–133. doi: 10.1016/j.amjoto.2008.03.002. [DOI] [PubMed] [Google Scholar]
- 49.Stratigos AJ, Garbe C, Dessinioti C, Lebbe C, Bataille V, Bastholt L, Dreno B, Fargnoli MC, Forsea AM, Frenard C. European interdisciplinary guideline on invasive squamous cell carcinoma of the skin: Part 1. epidemiology, diagnostics and prevention. European journal of cancer. 2020;128:60–82. doi: 10.1016/j.ejca.2020.01.007. [DOI] [PubMed] [Google Scholar]
- 50.Howell JY, Ramsey ML (2019). Cancer, squamous cell of the skinStatPearls [Internet].
- 51.Becerril S, Corchado-Cobos R, García-Sancha N, Revelles L, Revilla D, Ugalde T, Román-Curto C, Pérez-Losada J, Cañueto J. Viruses and Skin Cancer. International Journal of Molecular Sciences. 2021;22:5399. doi: 10.3390/ijms22105399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Smola S. Human Papilloma Viruses and Skin Cancer. Sunlight, Vitamin D and Skin Cancer. 2014:192–207. [Google Scholar]
- 53.Reinehr CPH, Bakos RM. Actinic keratoses: review of clinical, dermoscopic, and therapeutic aspects. An Bras Dermatol. 2019;94:637–657. doi: 10.1016/j.abd.2019.10.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Casari A, Chester J, Pellacani G. Actinic Keratosis and Non-Invasive Diagnostic Techniques: An Update. Biomedicines. 2018;6:8. doi: 10.3390/biomedicines6010008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Alam M, Armstrong A, Baum C, Bordeaux JS, Brown M, Busam KJ, Eisen DB, Iyengar V, Lober C, Margolis DJ. Guidelines of care for the management of cutaneous squamous cell carcinoma. Journal of the American Academy of Dermatology. 2018;78:560–578. doi: 10.1016/j.jaad.2017.10.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Stratigos A, Garbe C, Lebbe C, Malvehy J, del Marmol V, Pehamberger H, Peris K, Becker JC, Zalaudek I, Saiag P, et al. Diagnosis and treatment of invasive squamous cell carcinoma of the skin: European consensus-based interdisciplinary guideline. European Journal of Cancer. 2015;51:1989–2007. doi: 10.1016/j.ejca.2015.06.110. [DOI] [PubMed] [Google Scholar]
- 57.Korhonen N, Ylitalo L, Luukkaala T, Itkonen J, Häihälä H, Jernman J, Snellman E, Palve J. Premalignant lesions, basal cell carcinoma and melanoma in patients with cutaneous squamous cell carcinoma. Archives of Dermatological Research. 2020:1–6. doi: 10.1007/s00403-020-02114-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Zelin E, Zalaudek I, Agozzino M, Dianzani C, Dri A, Di Meo N, Giuffrida R, Marangi GF, Neagu N, Persichetti P. Neoadjuvant Therapy for Non-melanoma Skin Cancer: Updated Therapeutic Approaches for Basal, Squamous, and Merkel Cell Carcinoma. Current treatment options in oncology. 2021;22:1–24. doi: 10.1007/s11864-021-00826-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Mangkorntongsakul V, Tng ETV, Scurry J, Gourlay R. Symbiotic collision tumour of the scalp: squamous cell carcinoma and malignant melanoma. Australasian Journal of Dermatology. 2020;61:e229–e230. doi: 10.1111/ajd.13218. [DOI] [PubMed] [Google Scholar]
- 60.Li R, Lee G, Huang M, El-Sherief A. Rare basal cell metastasis of a basal-squamous skin collision tumour to the lung and axillary lymph node. BMJ Case Reports CP. 2019;12 doi: 10.1136/bcr-2019-231487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Fania L, Didona D, Morese R, Campana I, Coco V, Di Pietro FR, Ricci F, Pallotta S, Candi E, Abeni D. Basal Cell Carcinoma: From Pathophysiology to Novel Therapeutic Approaches. Biomedicines. 2020;8:449. doi: 10.3390/biomedicines8110449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Pasquali P, Segurado-Miravalles G, Castillo M, Fortuño Á, Puig S, González S. Use of Cytology in the Diagnosis of Basal Cell Carcinoma Subtypes. J Clin Med. 2020;9:612. doi: 10.3390/jcm9030612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Kallini JR, Hamed N, Khachemoune A. Squamous cell carcinoma of the skin: epidemiology, classification, management, and novel trends. International journal of dermatology. 2015;54:130–140. doi: 10.1111/ijd.12553. [DOI] [PubMed] [Google Scholar]
- 64.Raverdeau M, Cunningham SP, Harmon C, Lynch L. γδ T cells in cancer: a small population of lymphocytes with big implications. Clin Transl Immunology. 2019;8:e01080. doi: 10.1002/cti2.1080. -e01080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Schrom KP, Kim I, Baron ED. Springer; 2020. The Immune System and Pathogenesis of Melanoma and Non-melanoma Skin Cancer; pp. 211–226. [DOI] [PubMed] [Google Scholar]
- 66.Nowak E, Sulicka-Grodzicka J, Strach M, Bukowska-Strakova K, Siedlar M, Korkosz M, Grodzicki T. Decreased number of regulatory T lymphocytes is related to inflammation and number of CD8+ T cells expressing programmed cell death protein-1 in common variable immunodeficiency. Folia Medica Cracoviensia. 2020 doi: 10.24425/fmc.2020.135791. 5-16-15-16. [DOI] [PubMed] [Google Scholar]
- 67.Jung JW, Overgaard NH, Burke MT, Isbel N, Frazer IH, Simpson F, Wells JW. Does the nature of residual immune function explain the differential risk of non-melanoma skin cancer development in immunosuppressed organ transplant recipients? International journal of cancer. 2016;138:281–292. doi: 10.1002/ijc.29450. [DOI] [PubMed] [Google Scholar]
- 68.Perrotta RE, Giordano M, Malaguarnera M. Non-melanoma skin cancers in elderly patients. Critical reviews in oncology/hematology. 2011;80:474–480. doi: 10.1016/j.critrevonc.2011.04.011. [DOI] [PubMed] [Google Scholar]
- 69.Narsale A, Moya R, Ma J, Anderson LJ, Wu D, Garcia JM, Davies JD. Cancer-driven changes link T cell frequency to muscle strength in people with cancer: a pilot study. Journal of cachexia, sarcopenia and muscle. 2019;10:827–843. doi: 10.1002/jcsm.12424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Wessely A, Steeb T, Leiter U, Garbe C, Berking C, Heppt MV. Immune Checkpoint Blockade in Advanced Cutaneous Squamous Cell Carcinoma: What Do We Currently Know in 2020? International Journal of Molecular Sciences. 2020;21:9300. doi: 10.3390/ijms21239300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Mertz KD, Pfaltz M., Junt T., Schmid M., Fernandez Figueras M.T., Pfaltz K., Barghorn A., Kempf W. Merkel Cell Polyomavirus Is Present In Common Warts And Carcinoma In Situ Of The Skin. Human Pathology. 2010;41:1369–1379. doi: 10.1016/j.humpath.2010.01.023. [DOI] [PubMed] [Google Scholar]
- 72.Kassem A, Schopflin A., Diaz C., Weyers W., Stickeler E., Werner M., Zur Hausen A. Frequent Detection Of Merkel Cell Polyomavirus In Human Merkel Cell Carcinomas And Identification Of A Unique Deletion In The Vp1 Gene. Cancer Res. 2008;68:5009–5013. doi: 10.1158/0008-5472.CAN-08-0949. [DOI] [PubMed] [Google Scholar]
- 73.Cesarman E, Damania B, Krown SE, Martin J, Bower M, Whitby D. Kaposi sarcoma. Nat Rev Dis Primers. 2019;5:9. doi: 10.1038/s41572-019-0060-9. -9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Oluoch PO, Oduor CI, Forconi CS, Ong'echa JM, Münz C, Dittmer DP, Bailey JA, Moormann AM. Kaposi Sarcoma-Associated Herpesvirus Infection and Endemic Burkitt Lymphoma. The Journal of infectious diseases. 2020;222:111–120. doi: 10.1093/infdis/jiaa060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Fröhlich J, Grundhoff A. Vol. 42. Springer; City: 2020. pp. 143–157. (Epigenetic control in Kaposi sarcoma-associated herpesvirus infection and associated disease). Editor (ed)^(eds) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Chen L, Feng Z, Yuan G, Emerson CC, Stewart PL, Ye F, Jin G. HIV-associated exosomes promote infection of Kaposi sarcoma-associated herpesvirus via epidermal growth factor receptor. Journal of Virology. 2020 doi: 10.1128/JVI.01782-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Dupin N. Update on oncogenesis and therapy for Kaposi sarcoma. Current opinion in oncology. 2020;32:122–128. doi: 10.1097/CCO.0000000000000601. [DOI] [PubMed] [Google Scholar]
- 78.Krown SE, Moser CB, MacPhail P, Matining RM, Godfrey C, Caruso SR, Hosseinipour MC, Samaneka W, Nyirenda M, Busakhala NW. Treatment of advanced AIDS-associated Kaposi sarcoma in resource-limited settings: a three-arm, open-label, randomised, non-inferiority trial. The Lancet. 2020;395:1195–1207. doi: 10.1016/S0140-6736(19)33222-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Calzavara-Pinton P, Ortel B, Venturini M. Non-melanoma skin cancer, sun exposure and sun protection Giornale italiano di dermatologia e venereologia: organo ufficiale. Societa italiana di dermatologia e sifilografia. 2015;150:369–378. [PubMed] [Google Scholar]
- 80.Wei L, Christensen SR, Fitzgerald ME, Graham J, Hutson ND, Zhang C, Huang Z, Hu Q, Zhan F, Xie J. Ultradeep sequencing differentiates patterns of skin clonal mutations associated with sun-exposure status and skin cancer burden. Science advances. 2021;7:eabd7703. doi: 10.1126/sciadv.abd7703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Loney T, Paulo M, Modenese A, Gobba F, Tenkate T, Whiteman D, Green AC, John S. Global evidence on occupational sun exposure and keratinocyte cancers: a systematic review. British Journal of Dermatology. 2021;184:208–218. doi: 10.1111/bjd.19152. [DOI] [PubMed] [Google Scholar]
- 82.Quinlan S, May S, Weeks R, Yuan H, Luff J. Canine Papillomavirus 2 E6 Does Not Interfere With UVB-Induced Upregulation of p53 and p53-Regulated Genes. Frontiers in veterinary science. 2021;8:135. doi: 10.3389/fvets.2021.570982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Bikle DD. Vitamin D regulation of and by long non coding RNAs. Molecular and Cellular Endocrinology. 2021 doi: 10.1016/j.mce.2021.111317. [DOI] [PubMed] [Google Scholar]
- 84.Loureiro J, Abrantes M, Oliveira P, Saraiva L. P53 in skin cancer: From a master player to a privileged target for prevention and therapy. Biochimica et Biophysica Acta (BBA)-Reviews on Cancer. 2020 doi: 10.1016/j.bbcan.2020.188438. [DOI] [PubMed] [Google Scholar]
- 85.Reichrath J, Reichrath S, Vogt T, Römer K. Springer; 2020. Crosstalk Between Vitamin D and p53 Signaling in Cancer: An Update; pp. 307–318. [DOI] [PubMed] [Google Scholar]
- 86.Wang Y, Bensen S, Wen X, Hao D, Du D, He G, Jiang X. The roles of lncRNA in cutaneous squamous cell carcinoma. Frontiers in oncology. 2020;10:158. doi: 10.3389/fonc.2020.00158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Nikolouzakis TK, Falzone L, Lasithiotakis K, Krüger-Krasagakis S, Kalogeraki A, Sifaki M, Spandidos DA, Chrysos E, Tsatsakis A, Tsiaoussis J. Current and future trends in molecular biomarkers for diagnostic, prognostic, and predictive purposes in non-melanoma skin cancer. J Clin Med. 2020;9:2868. doi: 10.3390/jcm9092868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Mendez T, Saffari S, Cowan JM, Laver NM, Baleja JD, Alt-Holland A. Delineating cell behavior and metabolism of non-melanoma skin cancer in vitro. In Vitro Cellular & Developmental Biology-Animal. 2020;56:165–180. doi: 10.1007/s11626-019-00416-6. [DOI] [PubMed] [Google Scholar]
- 89.Liu-Smith F, Jia J, Zheng Y. UV-induced molecular signaling differences in melanoma and non-melanoma skin cancer. Ultraviolet Light in Human Health, Diseases and Environment. 2017:27–40. doi: 10.1007/978-3-319-56017-5_3. [DOI] [PubMed] [Google Scholar]
- 90.Ji Y, Feng G, Hou Y, Yu Y, Wang R, Yuan H. Long noncoding RNA MEG3 decreases the growth of head and neck squamous cell carcinoma by regulating the expression of miR-421 and E-cadherin. Cancer medicine. 2020;9:3954–3963. doi: 10.1002/cam4.3002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Kang B-H, Shu C-W, Chao J-K, Lee C-H, Fu T-Y, Liou H-H, Ger L-P, Liu P-F. HSPD1 repressed E-cadherin expression to promote cell invasion and migration for poor prognosis in oral squamous cell carcinoma. Scientific reports. 2019;9:1–12. doi: 10.1038/s41598-019-45489-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Charazac A, Fayyad N, Beal D, Bourgoin-Voillard S, Seve M, Sauvaigo S, Lamartine J, Soularue P, Moratille S, Martin MT. Impairment of Base Excision Repair in Dermal Fibroblasts Isolated From Nevoid Basal Cell Carcinoma Patients. Frontiers in oncology. 2020;10:1551. doi: 10.3389/fonc.2020.01551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Bellei B, Caputo S, Carbone A, Silipo V, Papaccio F, Picardo M, Eibenschutz L. The Role of Dermal Fibroblasts in Nevoid Basal Cell Carcinoma Syndrome Patients: An Overview. International journal of molecular sciences. 2020;21:720. doi: 10.3390/ijms21030720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Villani A, Fabbrocini G, Costa C, Scalvenzi M. Sonidegib: safety and efficacy in treatment of advanced basal cell carcinoma. Dermatology and therapy. 2020;10:401–412. doi: 10.1007/s13555-020-00378-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Fontanillas P, Alipanahi B, Furlotte NA, Johnson M, Wilson CH, Pitts SJ, Gentleman R, Auton A. Disease risk scores for skin cancers. Nature communications. 2021;12:1–13. doi: 10.1038/s41467-020-20246-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Lohakul J, Chaiprasongsuk A, Jeayeng S, Saelim M, Muanjumpon P, Thanachaiphiwat S, Tripatara P, Soontrapa K, Lumlerdkij N, Akarasereenont P. The protective effect of polyherbal formulation, Harak formula, on UVA-induced photoaging of human dermal fibroblasts and mouse skin via promoting Nrf2-regulated antioxidant defense. Frontiers in pharmacology. 2021;12 doi: 10.3389/fphar.2021.649820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Baseggio Conrado A, Fanelli S, McGuire VA, Ibbotson SH. Role of Hypotaurine in Protection against UVA-Induced Damage in Keratinocytes. Photochemistry and Photobiology. 2021;97:353–359. doi: 10.1111/php.13334. [DOI] [PubMed] [Google Scholar]
- 98.Ibuki Y, Komaki Y, Yang G, Toyooka T. Long-wavelength UVA enhances UVB-induced cell death in cultured keratinocytes: DSB formation and suppressed survival pathway. Photochemical & Photobiological Sciences. 2021:1–14. doi: 10.1007/s43630-021-00050-w. [DOI] [PubMed] [Google Scholar]
- 99.Besaratinia A, Yoon J-I, Schroeder C, Bradforth SE, Cockburn M, Pfeifer GP. Wavelength dependence of ultraviolet radiation-induced DNA damage as determined by laser irradiation suggests that cyclobutane pyrimidine dimers are the principal DNA lesions produced by terrestrial sunlight. FASEB J. 2011;25:3079–3091. doi: 10.1096/fj.11-187336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Jackson M, Marks L, May GHW, Wilson JB. The genetic basis of disease. Essays Biochem. 2018;62:643–723. doi: 10.1042/EBC20170053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Cadet J, Douki T. Formation of UV-induced DNA damage contributing to skin cancer development. Photochemical & Photobiological Sciences. 2018;17:1816–1841. doi: 10.1039/c7pp00395a. [DOI] [PubMed] [Google Scholar]
- 102.Dotto GP, Rustgi AK. Squamous Cell Cancers: A Unified Perspective on Biology and Genetics. Cancer Cell. 2016;29:622–637. doi: 10.1016/j.ccell.2016.04.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Sánchez-Danés A, Blanpain C. Deciphering the cells of origin of squamous cell carcinomas. Nat Rev Cancer. 2018;18:549–561. doi: 10.1038/s41568-018-0024-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Thaiwong T, Sledge DG, Wise AG, Olstad K, Maes RK, Kiupel M. Malignant transformation of canine oral papillomavirus (CPV1)-associated papillomas in dogs: An emerging concern? Papillomavirus Res. 2018;6:83–89. doi: 10.1016/j.pvr.2018.10.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Vargas-Rondón N, Villegas VE, Rondón-Lagos M. The Role of Chromosomal Instability in Cancer and Therapeutic Responses. Cancers. 2018;10 doi: 10.3390/cancers10010004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Grade M, Difilippantonio MJ, Camps J. Patterns of Chromosomal Aberrations in Solid Tumors. Recent Results Cancer Res. 2015;200:115–142. doi: 10.1007/978-3-319-20291-4_6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Divakar P, Khan MJ, Polacco M, Kerr DA, Paydarfar JA. Spontaneous regression of squamous cell carcinoma in the setting of dental infection and needle biopsy. Clinical Case Reports. 2020;8:1919–1923. doi: 10.1002/ccr3.3024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Rivlin N, Brosh R, Oren M, Rotter V. Mutations in the p53 Tumor Suppressor Gene: Important Milestones at the Various Steps of Tumorigenesis. Genes Cancer. 2011;2:466–474. doi: 10.1177/1947601911408889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Williams AB, Schumacher B. p53 in the DNA-Damage-Repair Process. Cold Spring Harb Perspect Med. 2016;6 doi: 10.1101/cshperspect.a026070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Chen J. The Cell-Cycle Arrest and Apoptotic Functions of p53 in Tumor Initiation and Progression. Cold Spring Harb Perspect Med. 2016;6 doi: 10.1101/cshperspect.a026104. -a026104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Montagna E, Lopes OS. Molecular basis of basal cell carcinoma. An Bras Dermatol. 2017;92:517–520. doi: 10.1590/abd1806-4841.20176544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Pellegrini C, Maturo MG, Di Nardo L, Ciciarelli V, Gutiérrez García-Rodrigo C, Fargnoli MC. Understanding the Molecular Genetics of Basal Cell Carcinoma. International journal of molecular sciences. 2017;18:2485. doi: 10.3390/ijms18112485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Prior IA, Hood FE, Hartley JL. The frequency of Ras mutations in cancer. Cancer research. 2020;80:2969–2974. doi: 10.1158/0008-5472.CAN-19-3682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Simanshu DK, Nissley DV, McCormick F. RAS Proteins and Their Regulators in Human Disease. Cell. 2017;170:17–33. doi: 10.1016/j.cell.2017.06.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Trillsch F, Kuerti S, Eulenburg C, Burandt E, Woelber L, Prieske K, Eylmann K, Oliveira-Ferrer L, Milde-Langosch K, Mahner S. E-Cadherin fragments as potential mediators for peritoneal metastasis in advanced epithelial ovarian cancer. British journal of cancer. 2016;114:213–220. doi: 10.1038/bjc.2015.436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Kang B-H, Shu C-W, Chao J-K, Lee C-H, Fu T-Y, Liou H-H, Ger L-P, Liu P-F. HSPD1 repressed E-cadherin expression to promote cell invasion and migration for poor prognosis in oral squamous cell carcinoma. Scientific Reports. 2019;9:8932. doi: 10.1038/s41598-019-45489-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Kaszak I, Witkowska-Piłaszewicz O, Niewiadomska Z, Dworecka-Kaszak B, Ngosa Toka F, Jurka P. Role of Cadherins in Cancer-A Review. International journal of molecular sciences. 2020;21:7624. doi: 10.3390/ijms21207624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Carpenter RL, Ray H. Safety and Tolerability of Sonic Hedgehog Pathway Inhibitors in Cancer. Drug Saf. 2019;42:263–279. doi: 10.1007/s40264-018-0777-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Ariza S, Espinosa S, Naranjo M. Nonsurgical therapies for basal cell carcinoma: a review. Actas Dermo-Sifiliográficas (English Edition) 2017;108:809–817. doi: 10.1016/j.ad.2017.01.018. [DOI] [PubMed] [Google Scholar]
- 120.Akarsu S, Kamberoglu I. BoD–Books on Demand; 2018. Cryotherapy for Common Premalignant and Malignant Skin Disorders; pp. 27–46. [Google Scholar]
- 121.Quazi SJ, Aslam N, Saleem H, Rahman J, Khan S. Surgical Margin of Excision in Basal Cell Carcinoma: A Systematic Review of Literature. Cureus. 2020;12:e9211. doi: 10.7759/cureus.9211. -e9211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Bailey A, Vasicek B, Tao J, Janeczek M, Mitri A, Tung R. Management of keratinocyte carcinoma - Special considerations in the elderly. Int J Womens Dermatol. 2019;5:235–245. doi: 10.1016/j.ijwd.2019.05.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Fournier S, Laroche A, Leblanc M, Bourgeault E, Ulrich Singbo MN, Turcotte S, Blouin M-M, Alain J. Prospective Clinical Trial Comparing Curettage and Cryosurgery to Curettage and Electrodesiccation in the Management of Minimally Invasive Basal and Squamous Cell Carcinomas. Journal of Cutaneous Medicine and Surgery. 2020;24:596–600. doi: 10.1177/1203475420943258. [DOI] [PubMed] [Google Scholar]
- 124.Puig S, Berrocal A. Management of high-risk and advanced basal cell carcinoma. Clin Transl Oncol. 2015;17:497–503. doi: 10.1007/s12094-014-1272-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Sreekantaswamy S, Endo J, Chen A, Butler D, Morrison L, Linos E. Aging and the treatment of basal cell carcinoma. Clin Dermatol. 2019;37:373–378. doi: 10.1016/j.clindermatol.2019.06.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Cameron MC, Lee E, Hibler BP, Giordano CN, Barker CA, Mori S, Cordova M, Nehal KS, Rossi AM. Basal cell carcinoma: Contemporary approaches to diagnosis, treatment, and prevention. Journal of the American Academy of Dermatology. 2019;80:321–339. doi: 10.1016/j.jaad.2018.02.083. [DOI] [PubMed] [Google Scholar]
- 127.Potenza C, Bernardini N, Balduzzi V, Losco L, Mambrin A, Marchesiello A, Tolino E, Zuber S, Skroza N, Proietti I. A Review of the Literature of Surgical and Nonsurgical Treatments of Invasive Squamous Cells Carcinoma. BioMed Research International. 2018;2018 doi: 10.1155/2018/9489163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Ferry AM, Sarrami SM, Hollier PC, Gerich CF, Thornton JF. Treatment of Non-melanoma Skin Cancers in the Absence of Mohs Micrographic Surgery. Plast Reconstr Surg Glob Open. 2020;8:e3300. doi: 10.1097/GOX.0000000000003300. -e3300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Dasari S, Yedjou CG, Brodell RT, Cruse AR, Tchounwou PB. Therapeutic strategies and potential implications of silver nanoparticles in the management of skin cancer. Nanotechnology Reviews. 2020;9:1500–1521. doi: 10.1515/ntrev-2020-0117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Cohen DK, Lee PK. Photodynamic Therapy for Non-Melanoma Skin Cancers. Cancers. 2016;8:90. doi: 10.3390/cancers8100090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Kianmehr Z, Khorsandi K, Mohammadi M, Hosseinzadeh R. Low-level laser irradiation potentiates anticancer activity of p-coumaric acid against human malignant melanoma cells. Melanoma research. 2020;30:136–146. doi: 10.1097/CMR.0000000000000603. [DOI] [PubMed] [Google Scholar]
- 132.Sima LE, Chiritoiu G, Negut I, Grumezescu V, Orobeti S, Munteanu CV, Sima F, Axente E. Functionalized graphene oxide thin films for anti-tumor drug delivery to melanoma cells. Frontiers in chemistry. 2020;8:184. doi: 10.3389/fchem.2020.00184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Peinemann F, Behrouz-Pirnia A. Quality issues identified in systematic reviews on early laser intervention to reduce scar formation in wound healing. Journal of Plastic, Reconstructive & Aesthetic Surgery. 2020;73:1357–1404. doi: 10.1016/j.bjps.2020.03.003. [DOI] [PubMed] [Google Scholar]
- 134.Ebrahimi A, Rezaei M, Kavoussi R, Eidizadeh M, Madani SH, Kavoussi H. Superpulsed CO2 Laser with Intraoperative Pathologic Assessment for Treatment of Periorbital Basal Cell Carcinoma Involving Eyelash Line. Dermatology Research and Practice. 2014;2014 doi: 10.1155/2014/931657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.He Y, Wang L, Shi J, Yao J, Li L, Zhang R, Huang C-H, Zou J, Wang LV. In vivo label-free photoacoustic flow cytography and on-the-spot laser killing of single circulating melanoma cells. Scientific reports. 2016;6:1–8. doi: 10.1038/srep39616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Soleymani T, Abrouk M, Kelly KM. An Analysis of Laser Therapy for the Treatment of Nonmelanoma Skin Cancer. Dermatol Surg. 2017;43:615–624. doi: 10.1097/DSS.0000000000001048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Ermertcan AT, Hellings PW, Cingi C. Nonmelanoma skin cancer of the head and neck: nonsurgical treatment. Facial Plastic Surgery Clinics. 2012;20:445–454. doi: 10.1016/j.fsc.2012.08.004. [DOI] [PubMed] [Google Scholar]
- 138.Micali G, Lacarrubba F, Nasca MR, Ferraro S, Schwartz RA. Topical pharmacotherapy for skin cancer: part II. Clinical applications. Journal of the American Academy of Dermatology. 2014;70(979):e971. doi: 10.1016/j.jaad.2013.12.037. -979. e912. [DOI] [PubMed] [Google Scholar]
- 139.Josiah AJ, Twilley D, Pillai SK, Ray SS, Lall N. Pathogenesis of Keratinocyte Carcinomas and the Therapeutic Potential of Medicinal Plants and Phytochemicals. Molecules. 2021;26:1979. doi: 10.3390/molecules26071979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Moscarella E, Di Brizzi EV, Casari A, De Giorgi V, Di Meo N, Fargnoli MC, Lacarrubba F, Micali G, Pellacani G, Peris K. Italian expert consensus paper on the management of patients with actinic keratoses. Dermatologic Therapy. 2020;33:e13992. doi: 10.1111/dth.13992. [DOI] [PubMed] [Google Scholar]
- 141.Voiculescu VM, Lisievici CV, Lupu M, Vajaitu C, Draghici CC, Popa AV, Solomon I, Sebe TI, Constantin MM, Caruntu C. Mediators of Inflammation in Topical Therapy of Skin Cancers. Mediators Inflamm. 2019;2019 doi: 10.1155/2019/8369690. -8369690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Hanna E, Abadi R, Abbas O. Imiquimod in dermatology: an overview. International journal of dermatology. 2016;55:831–844. doi: 10.1111/ijd.13235. [DOI] [PubMed] [Google Scholar]
- 143.Bubna AK. Imiquimod - Its role in the treatment of cutaneous malignancies. Indian J Pharmacol. 2015;47:354–359. doi: 10.4103/0253-7613.161249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Au - Austin E, Au - Jagdeo J. An In Vitro Approach to Photodynamic Therapy. JoVE. 2018:e58190. doi: 10.3791/58190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Cantisani C, Paolino G, Faina V, Frascani F, Cantoresi F, Bianchini D, Fazia G, Calvieri S. Overview on Topical 5-ALA Photodynamic Therapy Use for Non Melanoma Skin Cancers. International Journal of Photoenergy. 2014;2014 [Google Scholar]
- 146.Tewari KM, Eggleston IM. Chemical approaches for the enhancement of 5-aminolevulinic acid-based photodynamic therapy and photodiagnosis. Photochemical & Photobiological Sciences. 2018;17:1553–1572. doi: 10.1039/c8pp00362a. [DOI] [PubMed] [Google Scholar]
- 147.van Straten D, Mashayekhi V, de Bruijn HS, Oliveira S, Robinson DJ. Oncologic Photodynamic Therapy: Basic Principles, Current Clinical Status and Future Directions. Cancers. 2017;9:19. doi: 10.3390/cancers9020019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Kou J, Dou D, Yang L. Porphyrin photosensitizers in photodynamic therapy and its applications. Oncotarget. 2017;8:81591–81603. doi: 10.18632/oncotarget.20189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Collier NJ, Rhodes LE. Photodynamic Therapy for Basal Cell Carcinoma: The Clinical Context for Future Research Priorities. Molecules (Basel, Switzerland) 2020;25:5398. doi: 10.3390/molecules25225398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Champeau M, Vignoud S, Mortier L, Mordon S. Photodynamic therapy for skin cancer: How to enhance drug penetration? Journal of photochemistry and photobiology B, Biology. 2019;197 doi: 10.1016/j.jphotobiol.2019.111544. [DOI] [PubMed] [Google Scholar]
- 151.Laikova KV, Oberemok VV, Krasnodubets AM, Gal'chinsky NV, Useinov RZ, Novikov IA, Temirova ZZ, Gorlov MV, Shved NA, Kumeiko VV, et al. Advances in the Understanding of Skin Cancer: Ultraviolet Radiation, Mutations, and Antisense Oligonucleotides as Anticancer Drugs. Molecules (Basel, Switzerland) 2019;24:1516. doi: 10.3390/molecules24081516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Padya BS, Pandey A, Pisay M, Koteshwara K, Biswas S, Hariharapura RC, Bhat KU, Mutalik S. Stimuli-responsive and cellular targeted nanoplatforms for multimodal therapy of skin cancer. European Journal of Pharmacology. 2020 doi: 10.1016/j.ejphar.2020.173633. [DOI] [PubMed] [Google Scholar]
- 153.Paolino G, Donati M, Didona D, Mercuri SR, Cantisani C. Histology of non-melanoma skin cancers: an update. Biomedicines. 2017;5:71. doi: 10.3390/biomedicines5040071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Maner BS, Dupuis L, Su A, Jueng JJ, Harding TP, Meisenheimer J, Siddiqui FS, Hardack MR, Aneja S, Solomon JA. Overview of genetic signaling pathway interactions within cutaneous malignancies. Journal of Cancer Metastasis and Treatment. 2020;6 [Google Scholar]
- 155.Liu X, Zhang Y, Wang Y, Zhu W, Li G, Ma X, Zhang Y, Chen S, Tiwari S, Shi K, et al. Comprehensive understanding of magnetic hyperthermia for improving antitumor therapeutic efficacy. Theranostics. 2020;10:3793–3815. doi: 10.7150/thno.40805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Li L, Yang WW, Xu DG. Stimuli-responsive nanoscale drug delivery systems for cancer therapy. J Drug Target. 2019;27:423–433. doi: 10.1080/1061186X.2018.1519029. [DOI] [PubMed] [Google Scholar]
- 157.Kato Y, Ozawa S, Miyamoto C, Maehata Y, Suzuki A, Maeda T, Baba Y. Acidic extracellular microenvironment and cancer. Cancer Cell Int. 2013;13:89. doi: 10.1186/1475-2867-13-89. -89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Zhuo S, Zhang F, Yu J, Zhang X, Yang G, Liu X. pH-Sensitive Biomaterials for Drug Delivery. Molecules. 2020;25:5649. doi: 10.3390/molecules25235649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Wu W, Luo L, Wang Y, Wu Q, Dai H-B, Li J-S, Durkan C, Wang N, Wang G-X. Endogenous pH-responsive nanoparticles with programmable size changes for targeted tumor therapy and imaging applications. Theranostics. 2018;8:3038–3058. doi: 10.7150/thno.23459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Song N, Zhou L, Li J, Pan Z, He X, Tan H, Wan X, Li J, Ran R, Fu Q. Inspired by nonenveloped viruses escaping from endo-lysosomes: a pH-sensitive polyurethane micelle for effective intracellular trafficking. Nanoscale. 2016;8:7711–7722. doi: 10.1039/c6nr00859c. [DOI] [PubMed] [Google Scholar]
- 161.Jiang Y, Zhou Y, Zhang CY, Fang T. Co-delivery of paclitaxel and doxorubicin by pH-responsive prodrug micelles for cancer therapy. International Journal of Nanomedicine. 2020;15:3319. doi: 10.2147/IJN.S249144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Licarete E, Rauca VF, Luput L, Drotar D, Stejerean I, Patras L, Dume B, Toma VA, Porfire A, Gherman C. Overcoming Intrinsic Doxorubicin Resistance in Melanoma by Anti-Angiogenic and Anti-Metastatic Effects of Liposomal Prednisolone Phosphate on Tumor Microenvironment. International journal of molecular sciences. 2020;21:2968. doi: 10.3390/ijms21082968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Den RB, Lu B. Heat shock protein 90 inhibition: rationale and clinical potential. Ther Adv Med Oncol. 2012;4:211–218. doi: 10.1177/1758834012445574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Mielczarek-Lewandowska A, Hartman ML, Czyz M. Inhibitors of HSP90 in melanoma. Apoptosis. 2020;25:12–28. doi: 10.1007/s10495-019-01577-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Das SS, Bharadwaj P, Bilal M, Barani M, Rahdar A, Taboada P, Bungau S, Kyzas GZ. Stimuli-responsive polymeric nanocarriers for drug delivery, imaging, and theragnosis. Polymers. 2020;12:1397. doi: 10.3390/polym12061397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Chu M, Pan X, Zhang D, Wu Q, Peng J, Hai W. The therapeutic efficacy of CdTe and CdSe quantum dots for photothermal cancer therapy. Biomaterials. 2012;33:7071–7083. doi: 10.1016/j.biomaterials.2012.06.062. [DOI] [PubMed] [Google Scholar]
- 167.Abrahamse H, Hamblin MR. New photosensitizers for photodynamic therapy. The Biochemical journal. 2016;473:347–364. doi: 10.1042/BJ20150942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Niculescu A-G, Grumezescu AM. Photodynamic Therapy—An Up-to-Date Review. Applied Sciences. 2021;11:3626. [Google Scholar]
- 169.Paszko E, Ehrhardt C, Senge MO, Kelleher DP, Reynolds JV. Nanodrug applications in photodynamic therapy. Photodiagnosis and photodynamic therapy. 2011;8:14–29. doi: 10.1016/j.pdpdt.2010.12.001. [DOI] [PubMed] [Google Scholar]
- 170.Senapati S, Mahanta AK, Kumar S, Maiti P. Controlled drug delivery vehicles for cancer treatment and their performance. Signal Transduct Target Ther. 2018;3:7. doi: 10.1038/s41392-017-0004-3. -7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Dai Z. Vol. 6. Springer; 2015. (Advances in nanotheranostics I: design and fabrication of theranosic nanoparticles). [Google Scholar]
- 172.Patra JK, Das G, Fraceto LF, Campos EVR, Rodriguez-Torres MDP, Acosta-Torres LS, Diaz-Torres LA, Grillo R, Swamy MK, Sharma S, et al. Nano based drug delivery systems: recent developments and future prospects. J Nanobiotechnology. 2018;16:71. doi: 10.1186/s12951-018-0392-8. -71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Bozzuto G, Molinari A. Liposomes as nanomedical devices. International journal of nanomedicine. 2015;10:975–999. doi: 10.2147/IJN.S68861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Wang X, Li H, Chen G. Elsevier; 2018. Core-shell nanoparticles for cancer imaging and therapy; pp. 143–175. [Google Scholar]
- 175.Zielińska A, Szalata M, Gorczyński A, Karczewski J, Eder P, Severino P, Cabeda JM, Souto EB, Słomski R. Cancer nanopharmaceuticals: physicochemical characterization and in vitro/in vivo applications. Cancers. 2021;13:1896. doi: 10.3390/cancers13081896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Zielińska A, Martins-Gomes C, Ferreira NR, Silva AM, Nowak I, Souto EB. Anti-inflammatory and anti-cancer activity of citral: Optimization of citral-loaded solid lipid nanoparticles (SLN) using experimental factorial design and LUMiSizer®. International journal of pharmaceutics. 2018;553:428–440. doi: 10.1016/j.ijpharm.2018.10.065. [DOI] [PubMed] [Google Scholar]
- 177.Lenders V, Koutsoumpou X, Sargsian A, Manshian BB. Biomedical nanomaterials for immunological applications: ongoing research and clinical trials. Nanoscale Advances. 2020;2:5046–5089. doi: 10.1039/d0na00478b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.Rigon RB, Oyafuso MH, Fujimura AT, Gonçalez ML, AHd Prado, Gremião MPD, Chorilli M. Nanotechnology-Based Drug Delivery Systems for Melanoma Antitumoral Therapy: A Review. BioMed Research International. 2015;2015 doi: 10.1155/2015/841817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.Teixeira MC, Carbone C, Souto EB. Beyond liposomes: Recent advances on lipid based nanostructures for poorly soluble/poorly permeable drug delivery. Prog Lipid Res. 2017;68:1–11. doi: 10.1016/j.plipres.2017.07.001. [DOI] [PubMed] [Google Scholar]
- 180.Akbarzadeh A, Rezaei-Sadabady R, Davaran S, Joo SW, Zarghami N, Hanifehpour Y, Samiei M, Kouhi M, Nejati-Koshki K. Liposome: classification, preparation, and applications. Nanoscale Res Lett. 2013;8:102. doi: 10.1186/1556-276X-8-102. -102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181.Lin M-W, Huang Y-B, Chen C-L, Wu P-C, Chou C-Y, Wu P-C, Hung S-Y. A formulation study of 5-aminolevulinic encapsulated in DPPC liposomes in melanoma treatment. International journal of medical sciences. 2016;13:483. doi: 10.7150/ijms.15411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182.Pierre MB, Tedesco A.C., Marchetti J.M., Bentley M.V. Stratum Corneum Lipids Liposomes For The Topical Delivery Of 5-Aminolevulinic Acid In Photodynamic Therapy Of Skin Cancer: Preparation And In Vitro Permeation Study. Bmc Dermatol. 2001;1:5. doi: 10.1186/1471-5945-1-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183.Casas A, Fukuda H., Di Venosa G., Batlle A.M. The Influence Of The Vehicle On The Synthesis Of Porphyrins After Topical Application Of 5-Aminolaevulinic Acid. Implications In Cutaneous Photodynamic Sensitization. Br J Dermatol. 2000;143:564–572. doi: 10.1111/j.1365-2133.2000.03711.x. [DOI] [PubMed] [Google Scholar]
- 184.Oh EK, Jin S.E., Kim J.K., Park J.S., Park Y., Kim C.K. Retained Topical Delivery Of 5-Aminolevulinic Acid Using Cationic Ultradeformable Liposomes For Photodynamic Therapy. Eur J Pharm Sci. 2011;44:149–157. doi: 10.1016/j.ejps.2011.07.003. [DOI] [PubMed] [Google Scholar]
- 185.Souto EB, da Ana R, Souto SB, Zielińska A, Marques C, Andrade LN, Horbańczuk OK, Atanasov AG, Lucarini M, Durazzo A. In Vitro Characterization, Modelling, and Antioxidant Properties of Polyphenon-60 from Green Tea in Eudragit S100-2 Chitosan Microspheres. Nutrients. 2020;12:967. doi: 10.3390/nu12040967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186.Katiyar SK. Green tea prevents non-melanoma skin cancer by enhancing DNA repair. Arch Biochem Biophys. 2011;508:152–158. doi: 10.1016/j.abb.2010.11.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 187.Pimentel-Moral S, Teixeira M, Fernandes A, Arráez-Román D, Martinez-Ferez A, Segura-Carretero A, Souto E. Lipid nanocarriers for the loading of polyphenols–A comprehensive review. Advances in colloid and interface science. 2018;260:85–94. doi: 10.1016/j.cis.2018.08.007. [DOI] [PubMed] [Google Scholar]
- 188.Fang YP, Tsai Y.H., Wu P.C., Huang Y.B. Comparison Of 5-Aminolevulinic Acid-Encapsulated Liposome Versus Ethosome For Skin Delivery For Photodynamic Therapy. International Journal Of Pharmaceutics. 2008;356:144–152. doi: 10.1016/j.ijpharm.2008.01.020. [DOI] [PubMed] [Google Scholar]
- 189.Abdulbaqi IM, Darwis Y, Khan NAK, Assi RA, Khan AA. Ethosomal nanocarriers: the impact of constituents and formulation techniques on ethosomal properties, in vivo studies, and clinical trials. International journal of nanomedicine. 2016;11:2279–2304. doi: 10.2147/IJN.S105016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 190.Pandey V, Golhani D, Shukla R. Ethosomes: versatile vesicular carriers for efficient transdermal delivery of therapeutic agents. Drug Delivery. 2015;22:988–1002. doi: 10.3109/10717544.2014.889777. [DOI] [PubMed] [Google Scholar]
- 191.Paolino D, Celia C, Trapasso E, Cilurzo F, Fresta M. Paclitaxel-Loaded Ethosomes®: Potential Treatment Of Squamous Cell Carcinoma, A Malignant Transformation Of Actinic Keratoses. European Journal Of Pharmaceutics And Biopharmaceutics. 2012;81:102–112. doi: 10.1016/j.ejpb.2012.02.008. [DOI] [PubMed] [Google Scholar]
- 192.Farrukh MA, Koprowski R. Nanomedicines. 2019 Vol. [Google Scholar]
- 193.Barua S, Mitragotri S. Challenges associated with Penetration of Nanoparticles across Cell and Tissue Barriers: A Review of Current Status and Future Prospects. Nano Today. 2014;9:223–243. doi: 10.1016/j.nantod.2014.04.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 194.Ghasemiyeh P, Mohammadi-Samani S. Potential of Nanoparticles as Permeation Enhancers and Targeted Delivery Options for. Skin: Advantages and Disadvantages. 2020;14:3271–3289. doi: 10.2147/DDDT.S264648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 195.Shah P, Desai P., Channer D., Singh M. Enhanced Skin Permeation Using Polyarginine Modified Nanostructured Lipid Carriers. Journal Of Controlled Release. 2012;161:735–745. doi: 10.1016/j.jconrel.2012.05.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 196.Carbone C, Martins-Gomes C, Caddeo C, Silva AM, Musumeci T, Pignatello R, Puglisi G, Souto EB. Mediterranean essential oils as precious matrix components and active ingredients of lipid nanoparticles. Int J Pharm. 2018;548:217–226. doi: 10.1016/j.ijpharm.2018.06.064. [DOI] [PubMed] [Google Scholar]
- 197.Zielińska A, Ferreira NR, Feliczak-Guzik A, Nowak I, Souto EB. Loading, release profile and accelerated stability assessment of monoterpenes-loaded solid lipid nanoparticles (SLN) Pharmaceutical development and technology. 2020;25:832–844. doi: 10.1080/10837450.2020.1744008. [DOI] [PubMed] [Google Scholar]
- 198.Zielinska A, Ferreira NR, Durazzo A, Lucarini M, Cicero N, Mamouni SE, Silva AM, Nowak I, Santini A, Souto EB. Development and Optimization of Alpha-Pinene-Loaded Solid Lipid Nanoparticles (SLN) Using Experimental Factorial Design and Dispersion Analysis. Molecules. 2019;24 doi: 10.3390/molecules24152683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 199.Doktorovova S, Shegokar R, Souto EB (2017). Chapter 30 - Role of Excipients in formulation development and biocompatibility of lipid nanoparticles (SLNs/NLCs). Ficai D and Grumezescu AM (eds). Elsevier, pp. 811-843.
- 200.Yu YH, Kim E., Park D.E., Shim G., Lee S., Kim Y.B., Kim C.W., Oh Y.K. Cationic Solid Lipid Nanoparticles For Co-Delivery Of Paclitaxel And Sirna. European Journal Of Pharmaceutics And Biopharmaceutics. 2012;80:268–273. doi: 10.1016/j.ejpb.2011.11.002. [DOI] [PubMed] [Google Scholar]
- 201.Venturini CG, Bruinsmann FA, Contri RV, Fonseca FN, Frank LA, D'Amore CM, Raffin RP, Buffon A, Pohlmann AR, Guterres SS. Co-encapsulation of imiquimod and copaiba oil in novel nanostructured systems: promising formulations against skin carcinoma. European Journal of Pharmaceutical Sciences. 2015;79:36–43. doi: 10.1016/j.ejps.2015.08.016. [DOI] [PubMed] [Google Scholar]
- 202.Ali-von Laue C, Zoschke C, Do N, Lehnen D, Kuchler S, Mehnert W, Blaschke T, Kramer KD, Plendl J, Weindl G, et al. Improving topical non-melanoma skin cancer treatment: In vitro efficacy of a novel guanosine-analog phosphonate. Skin Pharmacol Physiol. 2014;27:173. doi: 10.1159/000354118. [DOI] [PubMed] [Google Scholar]
- 203.Huber LA, Pereira TA, Ramos DN, Rezende LC, Emery FS, Sobral LM, Leopoldino AM, Lopez RF. Topical Skin Cancer Therapy Using Doxorubicin-Loaded Cationic Lipid Nanoparticles and lontophoresis. J Biomed Nanotechnol. 2015;11:1975–1988. doi: 10.1166/jbn.2015.2139. [DOI] [PubMed] [Google Scholar]
- 204.Chen ZJ, Zhang Z, Xie BB, Zhang HY. Development and evaluation of topotecan loaded solid lipid nanoparticles: A study in cervical cancer cell lines. Journal of photochemistry and photobiology B, Biology. 2016;165:182–188. doi: 10.1016/j.jphotobiol.2016.10.019. [DOI] [PubMed] [Google Scholar]
- 205.Teaima MH, Badawi NM, Attia DA, El-Nabarawi MA, Elmazar MM, Mousa SA. Efficacy of pomegranate extract loaded solid lipid nanoparticles transdermal emulgel against Ehrlich ascites carcinoma. Nanomedicine. 2022;39 doi: 10.1016/j.nano.2021.102466. [DOI] [PubMed] [Google Scholar]
- 206.Woźniak M, Nowak M, Lazebna A, Więcek K, Jabłońska I, Szpadel K, Grzeszczak A, Gubernator J, Ziółkowski P. The Comparison of In Vitro Photosensitizing Efficacy of Curcumin-Loaded Liposomes Following Photodynamic Therapy on Melanoma MUG-Mel2, Squamous Cell Carcinoma SCC-25, and Normal Keratinocyte HaCaT Cells. Pharmaceuticals (Basel) 2021;14:374. doi: 10.3390/ph14040374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 207.Cadinoiu AN, Rata DM, Atanase LI, Mihai CT, Bacaita SE, Popa M. Formulations Based on Drug Loaded Aptamer-Conjugated Liposomes as a Viable Strategy for the Topical Treatment of Basal Cell Carcinoma-In Vitro Tests. Pharmaceutics. 2021;13:866. doi: 10.3390/pharmaceutics13060866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 208.Abdel Fadeel DA, Kamel R, Fadel M. PEGylated lipid nanocarrier for enhancing photodynamic therapy of skin carcinoma using curcumin: in-vitro/in-vivo studies and histopathological examination. Scientific Reports. 2020;10:10435. doi: 10.1038/s41598-020-67349-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 209.Khallaf RA, Salem HF, Abdelbary A. 5-Fluorouracil shell-enriched solid lipid nanoparticles (SLN) for effective skin carcinoma treatment. Drug Deliv. 2016;23:3452–3460. doi: 10.1080/10717544.2016.1194498. [DOI] [PubMed] [Google Scholar]
- 210.Geetha T, Kapila M, Prakash O, Deol PK, Kakkar V, Kaur IP. Sesamol-loaded solid lipid nanoparticles for treatment of skin cancer. J Drug Target. 2015;23:159–169. doi: 10.3109/1061186X.2014.965717. [DOI] [PubMed] [Google Scholar]
- 211.Huber LA, Pereira TA, Ramos DN, Rezende LCD, Emery FS, Sobral LM, Leopoldino AM, Lopez RFV. Topical Skin Cancer Therapy Using Doxorubicin-Loaded Cationic Lipid Nanoparticles and Iontophoresis. Journal of Biomedical Nanotechnology. 2015;11:1975–1988. doi: 10.1166/jbn.2015.2139. [DOI] [PubMed] [Google Scholar]