Abstract
Background
We aim to evaluate the impact of prepregnancy overweight on treatment modalities of Gestational Diabetes Mellitus (GDM). We assessed the association of increased pregravid Body Mass Index (BMI) with dosing of basal and rapid acting insulin as well as pregnancy outcome.
Methods
We included 509 gestational diabetic women (normal weight: 200, overweight: 157, obese: 152), attending the pregnancy outpatient clinic at the Department of Obstetrics and Gynecology, Medical University of Vienna, in this retrospective study. We used a prospectively compiled database to assess patient characteristics, treatment approaches – particularly maximum doses of basal and rapid acting insulin or metformin – and pregnancy outcome.
Results
Increased BMI was associated with the need of glucose lowering medication (odds ratio (OR): 1.08 for the increase of 1 kg/m² BMI, 95%CI 1.05–1.11, p<0.001). Mothers with pregestational obesity received the highest amount of insulin. Metformin was more often used in patients with obesity who also required higher daily doses. Maternal BMI was associated with increased risk of cesarean section (OR 1.04, 95%CI 1.01–1.07, p<0.001) and delivering large for gestational age offspring (OR 1.09, 95%CI 1.04–1.13, p<0.001). Birthweight percentiles were highest in patients with obesity who required glucose lowering therapy.
Conclusions
Treatment modalities and outcome in GDM pregnancies are closely related to the extent of maternal BMI. Patients with obesity required glucose lowering medication more often and were at higher risk of adverse pregnancy outcomes. It is crucial to further explore the underlying pathophysiologic mechanisms to optimize clinical management and individual treatment approaches.
Keywords: gestational diabetes (GDM), obesity, pharmacotherapy, pregnancy outcome, birth weight
Introduction
Growing evidence suggests that GDM comprises a broad spectrum of metabolic entities. It is crucial to identify factors which contribute to the observed heterogeneity in treatment modalities and outcomes of the disease (1), so that the clinical management can be tailored to individual patients.
Increased maternal BMI has a high prevalence in women developing GDM [approximately 65–75% are either overweight or obese (2, 3)] and was shown to be independently associated with pregnancy complications (such as fetal growth excess and adiposity) in the HAPO (Hyperglycaemia and Adverse Pregnancy Outcomes) study (4). Moreover, maternal obesity is associated with a higher level of insulin resistance, which is closely related to adverse pregnancy outcomes (like the development of large for gestational age offspring) (5, 6). In one of our previous studies we found that impaired insulin action at early pregnancy predicts the later requirement of glucose lowering medications, and therefore, an increased level of insulin resistance in mothers with overweight or obesity has possible impact on treatment modalities in those patients who develop GDM (7). While prepregnancy weight loss interventions are often not successful and are not recommended during pregnancy (8), a better understanding of the pathophysiological mechanisms linking pregestational BMI, the effectiveness of GDM treatment and adverse pregnancy outcomes could be used to further tailor the clinical pregnancy management of patients with obesity to their individual risk profile.
However, despite of major clinical importance, data on differences in treatment regimen and pregnancy outcomes in relation to the degree of maternal BMI in patients with GDM is sparsely available.
This study aims to evaluate differences in treatment modalities including use of insulin and metformin in relation to different degrees of obesity in pregnant women affected by GDM, taking into special consideration dosing regimens of basal insulin and rapid acting formulations. An analysis of pregnancy outcome and its association with maternal obesity is a further objective of this study.
Methods
Study Design and Participants
In this study, we included a total of 509 digitally registered patients with GDM, attending the pregnancy outpatient clinic at the Department of Obstetrics and Gynecology, Medical University of Vienna, between May 2015 and December 2017. Of a total of 617 originally registered patients during this period, 68 women were excluded because of preexisting diabetes, 10 patients were excluded because of pregestational diabetes, which was unrecognized before pregnancy, 25 women were excluded because of unclear GDM diagnosis (e.g. reported as GDM but negative OGTT results) and 5 patients were excluded because of missing pregestational BMI. Study participants self-reported their pregestational BMI (BMIPG) at their first visit (9, 10) and were categorized into women with normal weight (BMIPG <25 kg/m2), overweight (BMIPG ≥25 and <30 kg/m2) or obesity (BMIPG ≥30 kg/m2). GDM was diagnosed by use of a 75g 2h-OGTT according to the International Diabetes in Pregnancy Study Groups (IADPSG) recommendation (11). Glucose lowering medication (insulin and/or metformin) was started if glycemic targets were not achieved by lifestyle modification (i.e. if fasting or postprandial glucose levels exceeded 95 mg/dl or 140 mg/dl, according to international guidelines) (12). Data was assessed retrospectively; the following variables were extracted from a prospectively compiled database: age, height, weight before pregnancy, singleton or multiple pregnancy, OGTT glucose values (fasting, 60’ and 120’ after oral glucose load). Additionally, we assessed data on insulin treatment strategies: use and dose of metformin or insulin as well as details about insulin formulations (basal insulin such as intermediate or long-acting insulin analogues or rapid-acting insulin). All patients were treated with fixed insulin doses and the maximum doses per day were assessed. Moreover, neonatal outcome (birth weight, length and head circumference, gestational age at delivery (GAD), transfer to neonatal intensive care unit) and mode of delivery were assessed by referring to the medical history. Calculations of age and sex adjusted percentiles of the Austrian population were based on an analysis of the local growth standard curves. Small (SGA) and large for gestational age (LGA) were defined as bodyweight below the 10th and above the 90th percentile, respectively.
This study was approved by the local ethics committee and performed in accordance with the Declaration of Helsinki.
Statistical Analysis
Continuous variables were summarized by mean ± standard deviation or as median and interquartile ranges (IQR) in case of skewed distribution and compared by analysis of variance or rank based inference, respectively. Dichotomous variables were summarized by counts and percentages, and compared by binomial logistic regression. Odds ratios (OR) and 95% confidence intervals (95%CI) were additionally calculated for binary outcomes. Fisher protected Least Significant Difference (LSD) tests were used for multiple comparisons (k=3 groups) to achieve a 95% coverage probability. Statistical analysis was performed with R (version 4.0.2) and contributing packages (13). A two-sided p-value of ≤0.05 was considered statistically significant.
Results
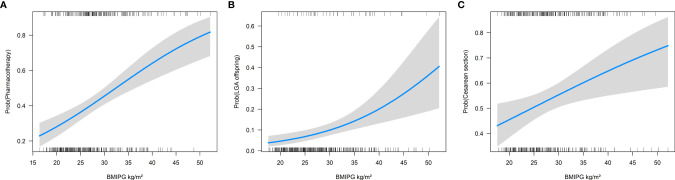
In Table 1 main characteristics of the study sample are provided; categorized according to their pregestational BMI into GDM patients with normal weight (GDM-NW: n=200), overweight (GDM-OW: n=157) and obesity (GDM-OB: n=152). As compared to patients with normal weight, patients with overweight (mean difference, MD: 4.4 mg/dl, 95%CI 1.9 to 6.8, p<0.001) or obesity (MD: 6.7 mg/dl, 95%CI 4.3 to 9.2, p<0.001) had significantly higher fasting glucose concentrations, whereas OGTT glucose levels at 60’ and 120’ were comparable. Of note, obese women were diagnosed at an earlier gestational age. Higher pregestational BMI was significantly associated with the need of glucose lowering medications (OR: 1.08 for the increase of 1 kg/m² BMI, 95%CI 1.05 to 1.11, p<0.001), as visualized in Figure 1A. Insulin (basal or rapid acting) was more often prescribed for patients with overweight or obesity, whereby the latter showed the highest requirement of total insulin units per day (Table 1). There was no difference in the daily dose of basal insulin formulations between groups. However, GDM patients with obesity required a higher amount of rapid acting insulin as compared to normal weight patients. Metformin was more often used in patients with obesity who also required higher daily doses as compared to normal weight women. Moreover, a combination therapy with insulin and metformin was more often observed in gestational diabetic mothers with obesity. Glycemic control was evaluated in a subgroup of 220 patients at 38 (IQR: 37-39) weeks of gestation. Adequate glycemic control was protocolled in 74 (83%) normal weight, 49 (67%) overweight and 42 (72%) obese patients. Although there was a tendency that overweight and obese patients reached glycemic targets less often, the observed difference didn’t reach significance (p=0.052). Moreover, weight gain was assessed in 340 women around delivery (median 1.0, IQR: 0.0-2.0 weeks before delivery). It was found that normal weight patients had a weight gain of 13.2 ± 6.7 kg vs. 12.2 ± 6.1 kg in overweight patients (p=0.504). Obese patients gained 10.7 ± 7.6 kg which was significantly lower as compared to overweight (p=0.04) or obese women (p=0.006).
Table 1.
Characteristics of the study sample.
| GDM-NW | GDM-OW | GDM-OB | p-value | |
|---|---|---|---|---|
| (n=200) | (n=157) | (n=152) | ||
| Age (years) | 31.6 ± 4.8 | 32.6 ± 4.9 | 32.0 ± 4.6 | 0.192 |
| Height (cm) | 164 ± 6.8 | 163 ± 6.8 | 164 ± 6.2 | 0.280 |
| Weight, before pregnancy (kg) | 59.0 ± 7.0 | 72.8 ± 6.7* | 95.5 ± 15.3*† | <0.001 |
| BMI, before pregnancy (kg/m2) | 21.9 ± 2.0 | 27.4 ± 1.3* | 35.6 ± 4.8*† | <0.001 |
| Multiple pregnancy (%) | 23 (11.4) | 15 (9.6) | 10 (6.6) | 0.295 |
| OGTT Glucose 0’ (mg/dl) | 88.4 ± 12.2 | 92.8 ± 9.9* | 95.2 ± 9.0* | <0.001 |
| OGTT Glucose 60’ (mg/dl) | 174.6 ± 33.2 | 179.1 ± 34.4 | 180.3 ± 33.4 | 0.336 |
| OGTT Glucose 120’ (mg/dl) | 135.2 ± 30.9 | 139.8 ± 31.4 | 138.8 ± 35.1 | 0.482 |
| Gestational age at GDM diagnosis (weeks) | 28.0 (26.0-29.5) | 27.0 (25.0-30.0) | 26.5 (23.0-28.0)*† | <0.001 |
| Pharmacotherapy (Metformin and/or Insulin) | 52 (26.0) | 75 (47.8)* | 84 (55.3)* | <0.001 |
| Metformin only (%) | 12 (6.0) | 15 (9.6) | 15 (9.9) | 0.317 |
| Metformin total (%) | 16 (8.0) | 19 (12.1) | 27 (17.9)* | 0.022 |
| Metformin total (g/d) | 1.50 (1.25-1.85) | 1.50 (1.00-2.50) | 2.50 (1.50-2.50)* | 0.032 |
| Insulin and Metformin (%) | 4 (2.0) | 4 (2.5) | 12 (7.9)*† | 0.016 |
| Insulin BI or RAI total (%) | 40 (20.0) | 60 (38.2)* | 69 (45.3)* | <0.001 |
| Insulin BI or RAI total (IU/d) | 24.0 (15.5-31.5) | 30.0 (18.0-40.0) | 40.0 (27.0-52.0)*† | 0.019 |
| Insulin BI total (%) | 31 (15.5) | 55 (35.0)* | 63 (41.4)* | <0.001 |
| Insulin BI total (IU/d) | 12 (9-24) | 12 (10-21) | 16 (12-27) | 0.122 |
| Insulin RAI total (%) | 24 (12.1) | 31 (20.0)* | 34 (22.4)* | 0.026 |
| Insulin RAI total (IU/d) | 10 (4.5-15) | 10 (5-19) | 14 (10-26)* | 0.029 |
Data are mean ± SD or median (IQR) and count (%) for patients with GDM and normal weight (GDM-NW) vs. overweight (GDM-OW) and obese patients (GDM-OB). BMI, body mass index; OGTT, oral glucose tolerance test; Insulin BI, basal insulin; insulin RAI, rapid acting insulin.
*p<0.05 vs. GDM-NW.
†p<0.05 vs. GDM-OW.
Figure 1.
Association of pregestational BMI (BMIPG) with the probability of receiving pharmacotherapy (A), for delivering large for gestational age (LGA) offspring (B) and having delivery by cesarean section (C).
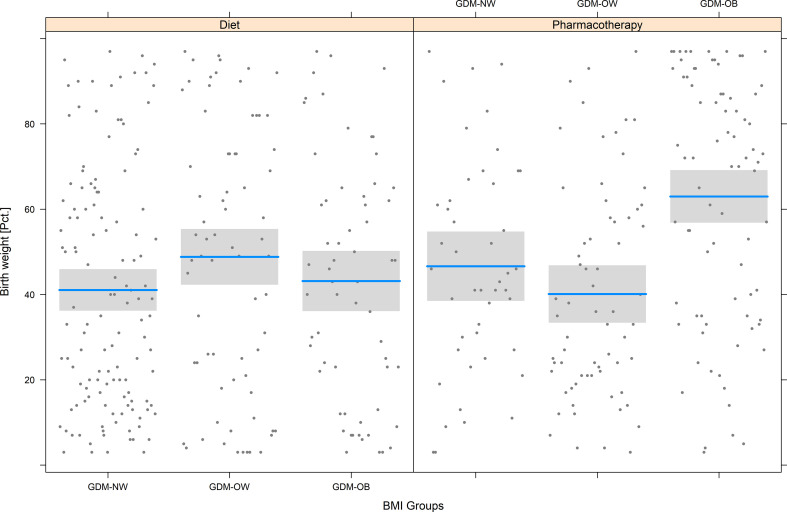
Assessment of pregnancy outcome data showed that pregestational BMI was associated with an increased risk of delivering LGA offspring (OR 1.09 for the increase of 1 kg/m² BMI, 95%CI 1.04 to 1.13, p<0.001, Figure 1B) as well as with an increased cesarean section rate (OR 1.04 for the increase of 1 kg/m² BMI, 95%CI 1.01 to 1.07, p<0.001, Figure 1C). Consequently, offspring of mothers with obesity showed higher birth weight percentiles as compared to normal weight and overweight patients as provided in Table 2. However, analysis of interaction indicated that the association of obesity status and birth weight percentiles of newborns was modified if pharmacotherapy was necessary in these patients (p<0.001 for the interaction between overweight or obesity status and requirement of pharmacotherapy for GDM). As compared to not medicated mothers with obesity (which showed mean birth weight of 43.2 ± 28.2 Pct.), birthweight percentiles were increased in patients with obesity who received glucose lowering medication (63.0 ± 28.8 Pct.), leading to a significant interaction (Figure 2). However, weight gain during pregnancy showed no significant association with LGA development in our study (p=0.591).
Table 2.
Fetal biometry and pregnancy outcomes (multiple pregnancies and cases with missing pregnancy outcome data are excluded).
| GDM-NW | GDM-OW | GDM-OB | p-value | |
|---|---|---|---|---|
| (n=177) | (n=142) | (n=142) | ||
| Cesarean Section | 79 (44.6) | 76 (53.5) | 90 (63.4)* | 0.004 |
| Neonatal Intensive Care | 13 (7.3) | 10 (7.2) | 11 (7.9) | 0.976 |
| GAD (weeks) | 40 (39-41) | 39 (39-41) | 39 (39-40)* | 0.028 |
| Preterm Delivery (<37 GW) | 17 (9.6) | 11 (7.7) | 9 (6.3) | 0.558 |
| Birth Weight (Percentile) | 42.5 ± 27.3 | 44.6 ± 28.6 | 54.4 ± 30.1*† | <0.001 |
| Birth Length (Percentile) | 46.9 ± 26.3 | 48.2 ± 26.9 | 49.2 ± 28.7 | 0.758 |
| Birth Head Circumference (Percentile) | 42.2 ± 27.1 | 44.1 ± 28.9 | 53.3 ± 29.4*† | 0.002 |
| SGA (<10. Pct) | 20 (11.5) | 18 (13.0) | 15 (10.6) | 0.819 |
| LGA (>90. Pct) | 10 (5.7) | 10 (7.2) | 23 (16.3)*† | 0.005 |
Data are mean ± SD or median (IQR) and count (%) for patients with GDM and normal weight (GDM-NW) vs. overweight (GDM-OW) and obese patients (GDM-OB). GAD, gestational age at delivery; SGA, small for gestational age offspring; LGA, large for gestational age offspring.
*p<0.05 vs. GDM-NW.
†p<0.05 vs. GDM-OW.
Figure 2.
Birth weight percentiles in normal weight (GDM-NW), overweight (GDM-OW) or obese patients with GDM (GDM-OB), categorized further into patients who received glucose lowering pharmacotherapy (PT) or diet only. The grey box represents the interquartile range. The median is indicated by a blue line.
Discussion
We aimed to assess characteristics of treatment modalities, neonatal outcome and delivery mode in women with GDM, categorized according to their pregestational BMI into patients with normal-weight, overweight or obesity. We found that pregestational BMI was significantly associated with an earlier diagnosis of GDM and increased requirement of glucose lowering medication, with mothers with obesity needing the highest amount of insulin units per day. This observation is in line with previous publications: Langer and co-workers found that mothers with overweight and obesity required a significantly higher total daily insulin dose (88 and 121 IU/d) as compared to normal weight GDM patients (69 IU/d) (14). Of note, the number of patients who received pharmacotherapy (69% vs. 41%) as well as the amount of required insulin was markedly higher as compared to our study. This might be explained by the use of different diagnosis criteria. There is some data suggesting that the IADPSG approach (which was used in our study) identifies more, but also less severe, cases of GDM as compared to other approaches [such as the Carpenter-Coustan criteria, which were used by Langer et al. (14)] (15). More recently, Machado et al. evaluated the impact of pregestational obesity on pregnancy outcomes in GDM pregnancies and observed that the need of pharmacological intervention was higher in patients with obesity (54%) as compared to patients with overweight (42%) or normal weight (29%) (16). This is comparable to our findings; however, the amount of insulin or metformin doses per day were not reported. In line with the above mentioned findings, another retrospective cohort study concluded that maternal BMI was the only predictor for the need of treatment with glucose lowering agents in patients with GDM (17).
The higher need of pharmacotherapy in patients with obesity may be explained by impaired insulin action, which is often prevalent already before conception. With advancing pregnancy insulin sensitivity decreases, leading to manifest hyperglycemia in patients who do not adequately compensate impaired insulin action by increased insulin release from the pancreatic β-cells (18–20). In this context, we observed increased fasting plasma glucose levels in patients with overweight and obesity at the time of GDM diagnosis, whereas glucose levels after oral glucose load were comparable between groups. Higher fasting glucose concentrations are pointing towards a primary defect in hepatic insulin sensitivity [as fasting glucose is mostly regulated by the liver (21)] in GDM patients with overweight or obesity and may be an underlying reason why basal insulin [which suppresses hepatic gluconeogenesis (22)] was more often used in those patients in our study. The higher requirement of rapid acting insulin may be due to additional defects in β-cell function.
Interestingly, GDM patients with obesity were more often treated with metformin [which also suppresses hepatic gluconeogenesis (23)] and required a higher dosage of this medication. We also observed that obesity resulted in an increased complementary treatment with insulin. This may possibly indicate an increased risk of secondary treatment failure of metformin in pregnant women with obesity. In this context, results from the MIG (Metformin in Gestational Diabetes) trial and other studies suggest that its use in pregnancy is save and has some advantages over insulin (especially being weight neutral and without risk of hypoglycemia) (24, 25). However, a significant treatment failure rate of metformin was already reported in the MIG trial, where 46% of the patients received supplemental insulin therapy (24). Other studies (26, 27) reported lower rates of metformin treatment failure. Previous research, aiming to assess the contributors to the observed failure rate of metformin monotherapy in pregnant women, concluded that the chance for responding to metformin was lower in patients with increased BMI (17, 28, 29) and higher fasting glucose levels (26, 28, 29). It is also noteworthy that large intervention studies failed to identify a protective effect of metformin on the risk of GDM, further suggesting that metformin is less effective in mothers with obesity (30, 31).
A number of studies indicated an increased risk of cesarean section delivery in mothers with obesity, potentially related to fetal adiposity (18). As shown in a secondary analysis of the HAPO study, the impact of increased maternal obesity on the risk of fetal growth excess is aggravated by hyperglycemia (32). This is in line with our results; however, our data further indicates that the well-known association between maternal and fetal adiposity is markedly increased in GDM patients with obesity who required glucose lowering therapy. There are two possible explanations for this observation: First; mothers with obesity have poor glycemic control despite receiving glucose lowering medication. Second; there is an underlying pathophysiologic factor (e.g. impaired insulin sensitivity) responsible for both, exceeded fetal growth as well as treatment failure of life-style modification, leading to the requirement of pharmacologic intervention in some patients. The latter theory is supported by observations suggesting that impaired insulin action in early pregnancy is per se contributing to fetal adiposity (due to hyperinsulinism and placental changes leading to increased nutrient supply to the offspring) but is also acting as a risk factor for the need of glucose lowering medication as described above (7, 18). We are not able to further elaborate those theories due to lack of detailed data about insulin sensitivity status in this retrospective study which is a limitation of this work. Impaired insulin sensitivity can possibly lead to increased maternal lipids, which can possibly explain the increased rates of LGA offspring in obese women (33, 34). We suggest further investigation of the possible interaction between the extent of insulin resistance (as well as other glucometabolic parameters in early pregnancy) and fetal development in patients with obesity. Also, the role of maternal body composition (especially the amount of visceral fat), lipid profiles and inflammatory markers should be further investigated to further disentangle the interaction between BMI, poor glycemic control and detrimental neonatal and maternal pregnancy outcomes in women with obesity.
In summary, we found that treatment modalities and outcomes in GDM pregnancies are strongly associated with the extent of maternal BMI and obesity status. Patients with obesity showed higher requirement of glucose lowering medication and were at higher risk of adverse pregnancy outcomes, such as cesarean section or LGA offspring. Our finding of markedly exceeded birth weight in infants of mothers with obesity who required glucose lowering medication is interesting and points towards an underlying role of early pregnancy insulin resistance, which interferes either with glycemic control or the development of fetal adiposity. It is crucial to further explore the underlying pathophysiologic mechanisms to optimize individual treatment approaches in these patients with particular risk for poor obstetric outcomes.
Data Availability Statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Ethics Statement
This study was reviewed and approved by the Ethics Committee of the Medical University of Vienna, Borschkegasse 8b/E06, 1090 Vienna, Austria. The patients/participants provided their written informed consent to participate in this study.
Author Contributions
CG conceived the study. Data assessment was performed by CM, AE, IR, DE, TL and CG. Calculations and data interpretation were performed by CG. Statistical analysis was performed by CG. CSG prepared tables and figures. The manuscript was written by CG and TL. CM, IR, DE, FG, KR, IH, EH critically revised the manuscript. All authors contributed to the article and approved the submitted version.
Funding
The study was funded by a research grant from Sanofi to CSG.
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s Note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
- 1. Wexler DJ, Powe CE, Barbour LA, Buchanan T, Coustan DR, Corcoy R, et al. Research Gaps in Gestational Diabetes Mellitus: Executive Summary of a National Institute of Diabetes and Digestive and Kidney Diseases Workshop. Obstetrics Gynecol (2018) 132:496–505. doi: 10.1097/AOG.0000000000002726 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Black MH, Sacks DA, Xiang AH, Lawrence JM. Clinical Outcomes of Pregnancies Complicated by Mild Gestational Diabetes Mellitus Differ by Combinations of Abnormal Oral Glucose Tolerance Test Values. Diabetes Care (2010) 33:2524–30. doi: 10.2337/dc10-1445 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Ouzounian JG, Hernandez GD, Korst LM, Montoro MM, Battista LR, Walden CL, et al. Pre-Pregnancy Weight and Excess Weight Gain Are Risk Factors for Macrosomia in Women With Gestational Diabetes. J Perinatol (2011) 31:717–21. doi: 10.1038/jp.2011.15 [DOI] [PubMed] [Google Scholar]
- 4. Study Cooperative Research Group. Hyperglycaemia HAPO. And Adverse Pregnancy Outcome (HAPO) Study: Associations With Maternal Body Mass Index. BJOG (2010) 117:575–84. doi: 10.1111/j.1471-0528.2009.02486.x [DOI] [PubMed] [Google Scholar]
- 5. Powe CE, Allard C, Battista M-C, Doyon M, Bouchard L, Ecker JL, et al. Heterogeneous Contribution of Insulin Sensitivity and Secretion Defects to Gestational Diabetes Mellitus. Dia Care (2016) 39:1052–5. doi: 10.2337/dc15-2672 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Benhalima K, Van Crombrugge P, Moyson C, Verhaeghe J, Vandeginste S, Verlaenen H, et al. Characteristics and Pregnancy Outcomes Across Gestational Diabetes Mellitus Subtypes Based on Insulin Resistance. Diabetologia (2019) 62:2118–28. doi: 10.1007/s00125-019-4961-7 [DOI] [PubMed] [Google Scholar]
- 7. Falcone V, Kotzaeridi G, Breil MH, Rosicky I, Stopp T, Yerlikaya-Schatten G, et al. Early Assessment of the Risk for Gestational Diabetes Mellitus: Can Fasting Parameters of Glucose Metabolism Contribute to Risk Prediction? Diabetes Metab J (2019) 43:785–93. doi: 10.4093/dmj.2018.0218 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Institute of Medicine (US) and National Research Council (US) Committee to Reexamine IOM Pregnancy Weight Guidelines . Weight Gain During Pregnancy: Reexamining the Guidelines (2009). Washington (DC: National Academies Press (US. Available at: http://www.ncbi.nlm.nih.gov/books/NBK32813/ (Accessed August 31, 2021). [PubMed] [Google Scholar]
- 9. Natamba BK, Sanchez SE, Gelaye B, Williams MA. Concordance Between Self-Reported Pre-Pregnancy Body Mass Index (BMI) and BMI Measured at the First Prenatal Study Contact. BMC Pregnancy Childbirth (2016) 16:187. doi: 10.1186/s12884-016-0983-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Holland E, Moore Simas TA, Doyle Curiale DK, Liao X, Waring ME. Self-Reported Pre-Pregnancy Weight Versus Weight Measured at First Prenatal Visit: Effects on Categorization of Pre-Pregnancy Body Mass Index. Matern Child Health J (2013) 17:1872–8. doi: 10.1007/s10995-012-1210-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. International Association of Diabetes and Pregnancy Study Groups Consensus Panel . International Association of Diabetes and Pregnancy Study Groups Recommendations on the Diagnosis and Classification of Hyperglycemia in Pregnancy. Diabetes Care (2010) 33:676–82. doi: 10.2337/dc09-1848 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. American Diabetes Association . Management of Diabetes in Pregnancy: Standards of Medical Care in Diabetes—2020 . Dia Care (2020) 43:S183–92. doi: 10.2337/dc20-S014 [DOI] [PubMed] [Google Scholar]
- 13. R: The R Project for Statistical Computing . Available at: https://www.r-project.org/ (Accessed May 21, 2018).
- 14. Langer O, Yogev Y, Xenakis EMJ, Brustman L. Overweight and Obese in Gestational Diabetes: The Impact on Pregnancy Outcome. Am J Obstetrics Gynecol (2005) 192:1768–76. doi: 10.1016/j.ajog.2004.12.049 [DOI] [PubMed] [Google Scholar]
- 15. Huhn EA, Rossi SW, Hoesli I, Göbl CS. Controversies in Screening and Diagnostic Criteria for Gestational Diabetes in Early and Late Pregnancy. Front Endocrinol (Lausanne) (2018) 9:696. doi: 10.3389/fendo.2018.00696 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Machado C, Monteiro S, Oliveira MJ, Grupo de Estudo de Diabetes e Gravidez da Sociedade Portuguesa de Diabetologia . Impact of Overweight and Obesity on Pregnancy Outcomes in Women With Gestational Diabetes - Results From a Retrospective Multicenter Study. Arch Endocrinol Metab (2020) 64:45–51. doi: 10.20945/2359-3997000000178 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Ali A, Shastry S, Nithiyananthan R, Ali A, Ganapathy R. Gestational Diabetes–Predictors of Response to Treatment and Obstetric Outcome. Eur J Obstetrics Gynecol Reprod Biol (2018) 220:57–60. doi: 10.1016/j.ejogrb.2017.11.014 [DOI] [PubMed] [Google Scholar]
- 18. Catalano PM, Shankar K. Obesity and Pregnancy: Mechanisms of Short Term and Long Term Adverse Consequences for Mother and Child. BMJ (2017) 356:j1. doi: 10.1136/bmj.j1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Stopp T, Feichtinger M, Rosicky I, Yerlikaya-Schatten G, Ott J, Egarter HC, et al. Novel Indices of Glucose Homeostasis Derived From Principal Component Analysis: Application for Metabolic Assessment in Pregnancy. J Diabetes Res (2020) 2020:4950584. doi: 10.1155/2020/4950584 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Catalano PM. Trying to Understand Gestational Diabetes. Diabetes Med (2014) 31:273–81. doi: 10.1111/dme.12381 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Abdul-Ghani MA, Tripathy D, DeFronzo RA. Contributions of Beta-Cell Dysfunction and Insulin Resistance to the Pathogenesis of Impaired Glucose Tolerance and Impaired Fasting Glucose. Diabetes Care (2006) 29:1130–9. doi: 10.2337/diacare.2951130 [DOI] [PubMed] [Google Scholar]
- 22. Hedrington MS, Pulliam L, Davis SN. Basal Insulin Treatment in Type 2 Diabetes. Diabetes Technol Ther (2011) 13:S–33-S-42. doi: 10.1089/dia.2011.0062 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Ferrannini E. The Target of Metformin in Type 2 Diabetes. N Engl J Med (2014) 371:1547–8. doi: 10.1056/NEJMcibr1409796 [DOI] [PubMed] [Google Scholar]
- 24. Rowan JA, Hague WM, Gao W, Battin MR, Moore MP. Metformin Versus Insulin for the Treatment of Gestational Diabetes. N Engl J Med (2008) 358:2003–15. doi: 10.1056/NEJMoa0707193 [DOI] [PubMed] [Google Scholar]
- 25. Butalia S, Gutierrez L, Lodha A, Aitken E, Zakariasen A, Donovan L. Short- and Long-Term Outcomes of Metformin Compared With Insulin Alone in Pregnancy: A Systematic Review and Meta-Analysis. Diabetes Med (2017) 34:27–36. doi: 10.1111/dme.13150 [DOI] [PubMed] [Google Scholar]
- 26. Ashoush S, El-Said M, Fathi H, Abdelnaby M. Identification of Metformin Poor Responders, Requiring Supplemental Insulin, During Randomization of Metformin Versus Insulin for the Control of Gestational Diabetes Mellitus: Metformin in GDM. J Obstet Gynaecol Res (2016) 42:640–7. doi: 10.1111/jog.12950 [DOI] [PubMed] [Google Scholar]
- 27. Silva JC, Fachin DRRN, Coral ML, Bertini AM. Perinatal Impact of the Use of Metformin and Glyburide for the Treatment of Gestational Diabetes Mellitus. J Perinat Med (2012) 40:225–8. doi: 10.1515/jpm-2011-0175 [DOI] [PubMed] [Google Scholar]
- 28. de Souza MLR, Silva R, Silva T, de Oliveira LC, Dienstmann G, do Nascimento IB, et al. Factors Associated With the Need for Insulin as a Complementary Treatment to Metformin in Gestational Diabetes Mellitus. Rev Bras Ginecol Obstet (2019) 41:697–702. doi: 10.1055/s-0039-1700796 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Gante I, Melo L, Dores J, Ruas L, Almeida M do C. Metformin in Gestational Diabetes Mellitus: Predictors of Poor Response. Eur J Endocrinol (2018) 178:129–35. doi: 10.1530/EJE-17-0486 [DOI] [PubMed] [Google Scholar]
- 30. Syngelaki A, Nicolaides KH, Balani J, Hyer S, Akolekar R, Kotecha R, et al. Metformin Versus Placebo in Obese Pregnant Women Without Diabetes Mellitus. N Engl J Med (2016) 374:434–43. doi: 10.1056/NEJMoa1509819 [DOI] [PubMed] [Google Scholar]
- 31. Chiswick C, Reynolds RM, Denison F, Drake AJ, Forbes S, Newby DE, et al. Effect of Metformin on Maternal and Fetal Outcomes in Obese Pregnant Women (EMPOWaR): A Randomised, Double-Blind, Placebo-Controlled Trial. Lancet Diabetes Endocrinol (2015) 3:778–86. doi: 10.1016/S2213-8587(15)00219-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Catalano PM, McIntyre HD, Cruickshank JK, McCance DR, Dyer AR, Metzger BE, et al. The Hyperglycemia and Adverse Pregnancy Outcome Study: Associations of GDM and Obesity With Pregnancy Outcomes. Diabetes Care (2012) 35:780–6. doi: 10.2337/dc11-1790 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Bozkurt L, Göbl CS, Hörmayer AT, Lugert A, Pacini G, Kautzky-Willer A, et al. The impact of Preconceptional Obesity on Trajectories of Maternal Lipids During Gestation. Sci Rep (2016) 6:29971. doi: 10.1038/srep29971 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Göbl CS, Handisurya A, Klein K, Bozkurt L, Luger A, Bancher-Todesca D, et al. Changes in Serum Lipid Levels During Pregnancy in Type 1 and Type 2 Diabetic Subjects. Diabetes Care (2010) 33:2071–3. doi: 10.2337/dc10-0484 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.