Abstract
Background
Since 2015, the European pulmonary hypertension guidelines recommend the use of combination therapy in most patients with pulmonary arterial hypertension (PAH). However, it is unclear to what extent this treatment strategy is adopted in clinical practice and if it is associated with improved long-term survival.
Methods
We analysed data from COMPERA, a large European pulmonary hypertension registry, to assess temporal trends in the use of combination therapy and survival of patients with newly diagnosed PAH between 2010 and 2019. For survival analyses, we looked at annualised data and at cumulated data comparing the periods 2010–2014 and 2015–2019.
Results
A total of 2531 patients were included. The use of early combination therapy (within 3 months after diagnosis) increased from 10.0% in patients diagnosed with PAH in 2010 to 25.0% in patients diagnosed with PAH in 2019. The proportion of patients receiving combination therapy 1 year after diagnosis increased from 27.7% to 46.3%. When comparing the 2010–2014 and 2015–2019 periods, 1-year survival estimates were similar (89.0% (95% CI 87.2–90.9%) and 90.8% (95% CI 89.3–92.4%), respectively), whereas there was a slight but nonsignificant improvement in 3-year survival estimates (67.8% (95% CI 65.0–70.8%) and 70.5% (95% CI 67.8–73.4%), respectively).
Conclusions
The use of combination therapy increased from 2010 to 2019, but most patients still received monotherapy. Survival rates at 1 year after diagnosis did not change over time. Future studies need to determine if the observed trend suggesting improved 3-year survival rates can be confirmed.
Short abstract
In this analysis of temporal trends in PAH treatment patterns and survival in 2010–2019, this study found an increase in the use of targeted combination therapies but only a slight, nonsignificant trend towards improved survival 3 years after diagnosis https://bit.ly/3FExlK5
Introduction
The term pulmonary arterial hypertension (PAH) describes a potentially fatal pulmonary vasculopathy characterised by a progressive increase in pulmonary vascular resistance (PVR) that may lead to right-sided heart failure. The most common form of PAH is idiopathic (IPAH), but there are also heritable (HPAH) and drug-associated (DPAH) forms as well as disease manifestation associated with various conditions, such as connective tissue disease (CTD), HIV infection, portal (porto-pulmonary) hypertension and congenital heart disease (CHD) [1].
Before targeted treatments became available, the outlook for patients with PAH was grim. A US registry study published in 1991 found a median survival of patients with IPAH (at that time called primary pulmonary hypertension) of 2.8 years after diagnosis [2]. Over the ensuing 30 years, various treatments have been developed which improve haemodynamics, exercise tolerance and worsening-free survival [3]. Life expectancy of patients with PAH has also increased, with median survival after diagnosis now exceeding 5 years [4–6]. However, for most of the currently approved therapies, no effect on mortality has been demonstrated in randomised clinical trials (except for i.v. epoprostenol, which improved survival in a randomised, open-label study done at a time when no other treatments were available) [7–11].
Over the years, with more PAH drugs becoming available, treatment strategies have evolved. In the 2004 European pulmonary hypertension guidelines, monotherapy was recommended for almost all patients and all disease stages, with combination therapy receiving a “may be considered” recommendation as a last resort [12]. In the 2009 European pulmonary hypertension guidelines, initial monotherapy remained the recommended strategy for most patients, with initial combination therapy to be considered for patients presenting in World Health Organization Functional Class IV and sequential combination therapy in case of an insufficient response to monotherapy [13]. In 2015, the results of the AMBITION (Ambrisentan and Tadalafil in Patients with Pulmonary Arterial Hypertension) study were published showing markedly better treatment outcomes in terms of exercise tolerance and disease progression with initial combination therapy with ambrisentan (an endothelin receptor antagonist (ERA)) and tadalafil (a phosphodiesterase-5 inhibitor (PDE5i)) compared with monotherapy with these compounds [8]. Like other studies in the field, AMBITION was not designed to detect and did not show a survival benefit at the end of the study [8, 11]. Still, the revised European pulmonary hypertension guidelines published in 2015 recommended for the first time to use initial combination therapy in most patients with newly diagnosed PAH [14, 15].
Concurrently with the evolution of PAH therapies, there have been changes in patient phenotypes, particularly in countries with an ageing population. While the disease was originally seen mostly in young, otherwise healthy females, a diagnosis of PAH is nowadays made predominantly in male and female patients of older age. In several recent registries, median age at PAH diagnosis was >60 years and many of the older patients presented with numerous cardiopulmonary comorbidities [6, 16–19]. Such patients were not well represented in the clinical trials that led to the approval of PAH medications. The aforementioned AMBITION study had a small (n=105) subset of older (mean age 62 years) patients with multiple comorbidities that were analysed separately and in whom no clear benefit from combination therapy could be demonstrated [20]. According to registry data, older patients, compared with younger patients, tend to be treated less aggressively, respond less well to PAH medications, have a higher likelihood to discontinue their PAH medications and have a higher mortality risk [5, 6, 17, 21].
It is unclear how the introduction of new treatments and new treatment strategies for PAH and the demographic changes observed in this patient population have affected treatment patterns and survival over time. Here, we present data from COMPERA (Comparative, Prospective Registry of Newly Initiated Therapies for Pulmonary Hypertension). The objective of the present analysis was to describe temporal trends in treatment patterns and survival of patients diagnosed with PAH between 2010 and 2019, i.e. over a 10-year period.
Methods
Database
Details of COMPERA (www.COMPERA.org; ClinicalTrials.gov: NCT01347216) have been reported previously [6, 22]. In brief, COMPERA is an ongoing web-based pulmonary hypertension registry launched in 2007 that prospectively collects baseline, follow-up and outcome data of patients who receive targeted therapies for pulmonary hypertension. Originally, COMPERA was initiated as a registry for patients with PAH who were treated with ERA, but in June 2009, COMPERA evolved into a comprehensive pulmonary hypertension registry enrolling patients with all forms of pulmonary hypertension who receive medical therapy with any drug approved for PAH. Centres must enter their patients within 6 months after the pulmonary hypertension diagnosis to ensure inclusion of newly diagnosed patients. Pulmonary hypertension centres from various European countries participate (Austria, Belgium, Germany, Greece, Hungary, Italy, Latvia, Lithuania, Netherlands, Slovakia, Switzerland and the UK), with ∼80% of the enrolled patients coming from German pulmonary hypertension centres.
COMPERA has been approved by the ethics committees of all participating centres and all patients provide written, informed consent prior to inclusion.
Patients
For the present analysis, patients were selected from the COMPERA database by the following criteria: 1) treatment-naïve patients aged ≥18 years newly diagnosed with any form of PAH between 1 January 2010 and 31 December 2019, 2) at least one follow-up available, and 3) resting mean pulmonary arterial pressure ≥25 mmHg and mean pulmonary arterial wedge pressure ≤15 mmHg at baseline. Patients with suspected or confirmed pulmonary veno-occlusive disease or pulmonary capillary haemangiomatosis were excluded, as were patients with other forms of pulmonary hypertension.
Statistical analyses
This was a post hoc analysis of prospectively collected variables. Continuous data are presented as mean with standard deviation or as median (interquartile range). Categorical data are presented as number (percentage). The dataset as of 1 September 2021 was analysed. Annualised data on the use of monotherapy and combination therapy within 3 months after diagnosis, also termed early combination therapy in this article, and after 1 year (±6 months) were shown based on the year of diagnosis. Ascertainment of vital status was done by on-site visits or phone calls to the patients or their caregivers. Patients who underwent lung transplantation and patients who were lost to follow-up were censored at the date of the last contact. Survival was evaluated using Kaplan–Meier analysis and the log-rank test. Estimated survival probability at 1 and 3 years was shown with 95% confidence intervals for each year of diagnosis. All trends were visualised in figures without formal statistical testing. The 1-year survival analyses were performed for all patients diagnosed between 2010 and 2019, while the 3-year survival analysis was done only for patients diagnosed between 2010 and 2018. All analyses were done for the entire cohort, for subgroups of patients divided by age (<65 versus ≥65 years), for subgroups of patients with I/H/D-PAH and CTD-PAH, and for a subgroup of patients with I/H/D-PAH who had no comorbidities and a diffusing capacity of the lung for carbon monoxide (DLCO) >45% predicted [23, 24]. In addition, we compared the cumulative survival rates of patients diagnosed between 2010 and 2014 with those of patients diagnosed between 2015 and 2019.
All statistical analyses were performed using R version 3.5.2 (www.r-project.org).
Results
Patient characteristics, treatment and survival of the entire cohort
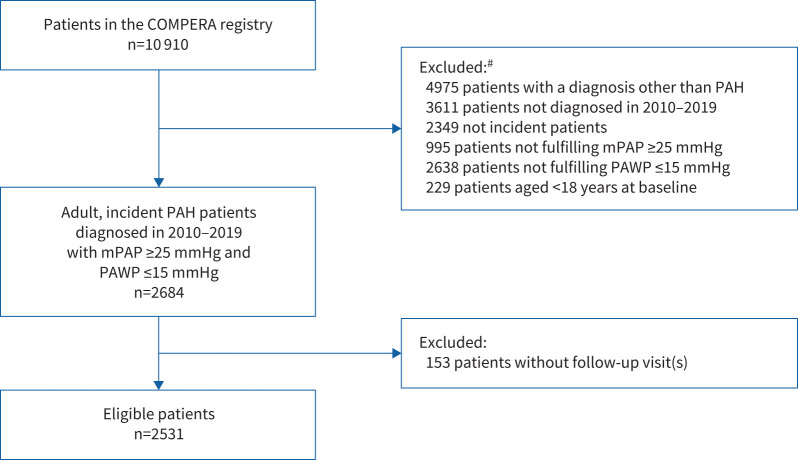
A total of 2531 patients were included in the present analysis (figure 1). The patients’ baseline characteristics, including those of the subgroups of patients with I/H/D-PAH and CTD-PAH, are shown in table 1. The baseline characteristics of the patient cohorts <65 years (n=1031 (40.7%)), ≥65 years (n=1500 (59.3%)), and I/H/D-PAH without comorbidities and DLCO >45% predicted (n=128 (5.1%)) are shown in supplementary table S1a–c. The annualised baseline characteristics did not suggest temporal trends in patient demographics or disease severity over time (supplementary table S2 and supplementary figure S1).
FIGURE 1.
STROBE (Strengthening the Reporting of Observational Studies in Epidemiology) diagram showing eligibility for analysis. PAH: pulmonary arterial hypertension; mPAP: mean pulmonary arterial pressure; PAWP: pulmonary arterial wedge pressure. #: more than one reason for exclusion could apply.
TABLE 1.
Baseline characteristics of patients newly diagnosed with pulmonary arterial hypertension (PAH) between 2010 and 2019
| All PAH | I/H/D-PAH | CTD-PAH | CHD-PAH | HIV-PAH | POPH | |
| Patients | 2531 (100) | 1698 (67.1) | 536 (21.2) | 128 (5.1) | 24 (0.9) | 145 (5.7) |
| Age, years | 64.6±15.8 | 65.9±15.9 | 66.3±13.1 | 50.6±18.1 | 45.8±12.1 | 57.8±11.7 |
| Female | 1609 (63.6) | 1016 (59.8) | 434 (81.0) | 84 (65.6) | 11 (45.8) | 64 (44.1) |
| BMI, kg·m−2 | 28.0±6.1 | 28.4±6.1 | 26.7±5.4 | 26.3±6.5 | 27.6±8.2 | 29.6±6.2 |
| WHO FC | ||||||
| I | 15 (0.6) | 7 (0.4) | 2 (0.4) | 4 (3.5) | 1 (4.2) | 1 (0.7) |
| II | 373 (15.7) | 244 (15.3) | 78 (15.4) | 26 (22.8) | 6 (25.0) | 19 (13.8) |
| III | 1675 (70.4) | 1117 (69.9) | 369 (73.1) | 73 (64.0) | 14 (58.3) | 102 (73.9) |
| IV | 315 (13.2) | 229 (14.3) | 56 (11.1) | 11 (9.6) | 3 (12.5) | 16 (11.6) |
| 6MWD, m | 297.1±127.1 | 297.4±127.3 | 278.4±124.2 | 344.6±112.9 | 366.0±146.7 | 310.7±129.1 |
| Pulmonary function | ||||||
| TLC, % pred | 89.6±17.4 | 91.7±16.4 | 84.0±18.8 | 84.0±15.2 | 88.7±11.5 | 94.7±16.9 |
| FVC, % pred | 80.1±20.3 | 80.4±20.0 | 80.0±21.5 | 70.2±18.5 | 85.5±10.6 | 82.5±19.1 |
| FEV1, % pred | 76.4±19.8 | 76.5±20.0 | 76.9±19.6 | 66.0±16.9 | 85.0±15.3 | 78.8±19.6 |
| DLCO, % pred | 50.5±21.7 | 52.1±22.6 | 42.6±17.0 | 65.8±18.9 | 47.0±17.5 | 57.9±17.8 |
| PaO2, mmHg | 65.0±12.3 | 64.5±12.6 | 65.2±12.0 | 66.0±10.1 | 69.8±19.3 | 68.9±10.8 |
| PaCO2, mmHg | 35.1±6.5 | 35.6±6.4 | 34.3±6.8 | 35.9±6.0 | 35.2±1.3 | 33.0±5.9 |
| Smoking history | ||||||
| Current | 96 (6.9) | 76 (6.6) | 5 (3.3) | 3 (10.0) | 0 (0.0) | 12 (25.5) |
| Former | 594 (43.0) | 490 (42.6) | 66 (43.7) | 13 (43.3) | 2 (50.0) | 23 (48.9) |
| Never | 693 (50.1) | 585 (50.8) | 80 (53.0) | 14 (46.7) | 2 (50.0) | 12 (25.5) |
| Pack-years# | 30.0 (15.0–40.0) | 30.0 (15.0–45.0) | 20.0 (10.0–30.0) | 12.5 (10.0–28.8) | NA (NA–NA) | 20.0 (20.0–37.5) |
| Comorbid conditions | ||||||
| Obesity¶ | 810 (32.7) | 589 (35.1) | 120 (23.7) | 34 (26.6) | 6 (28.6) | 61 (42.4) |
| Hypertension | 1329 (60.7) | 1007 (65.6) | 225 (56.0) | 36 (32.4) | 7 (46.7) | 54 (43.2) |
| Coronary heart disease | 554 (26.1) | 435 (29.0) | 89 (23.2) | 10 (9.3) | 1 (6.7) | 19 (15.8) |
| Diabetes mellitus | 590 (27.2) | 489 (32.1) | 61 (15.5) | 18 (16.2) | 0 (0.0) | 22 (17.5) |
| Haemodynamics | ||||||
| RAP, mmHg | 8.3±4.8 | 8.3±4.7 | 7.8±4.9 | 8.6±5.5 | 8.3±4.1 | 9.8±5.3 |
| mPAP, mmHg | 44.2±12.9 | 44.3±12.7 | 41.3±11.5 | 50.8±19.1 | 47.0±11.4 | 47.4±10.8 |
| PAWP, mmHg | 9.5±3.4 | 9.5±3.3 | 9.3±3.4 | 9.6±3.6 | 7.9±3.0 | 9.7±3.5 |
| CI, L·min−1·m−2 | 2.3±0.8 | 2.2±0.8 | 2.4±0.8 | 2.8±1.2 | 2.3±1.0 | 2.7±1.1 |
| PVR, dyn·s·cm−5 | 742.7±402.4 | 764.8±412.5 | 675.5±353.8 | 789.4±511.0 | 871.2±348.3 | 669.7±321.6 |
| PVR, WU | 9.3±5.0 | 9.6±5.2 | 8.4±4.4 | 9.9±6.4 | 10.9±4.4 | 8.4±4.0 |
| SvO2, % | 62.8±8.6 | 62.3±8.5 | 63.5±8.7 | 65.4±10.1 | 57.2±7.7 | 64.5±7.5 |
| Laboratory findings | ||||||
| Creatinine, µmol·L−1 | 101.7±55.3 | 106.1±60.2 | 95.3±41.9 | 86.5±50.9 | 83.0±24.6 | 89.1±33.9 |
| Uric acid, µmol·L−1 | 436.6±151.6 | 443.1±146.5 | 430.2±154.1 | 393.6±188.6 | 383.3±123.1 | 424.8±161.6 |
| Bilirubin, µmol·L−1 | 15.6±14.2 | 14.7±11.8 | 12.9±12.2 | 16.5±21.6 | 32.0±34.6 | 29.5±20.0 |
| NT-proBNP, pg·mL−1 | 1454 (480–3341) | 1574 (566–3498) | 1504 (432–3430) | 513 (262–1163) | 1212 (209–2542) | 848 (233–2128) |
| BNP, pg·mL−1 | 206 (94–497) | 200 (102–462) | 231 (78–636) | 154 (62–315) | 175 (150–201) | 240 (143–454) |
| Risk | ||||||
| Low | 246 (9.8) | 153 (9.1) | 49 (9.2) | 22 (17.6) | 6 (25.0) | 16 (11.0) |
| Intermediate | 1698 (67.6) | 1144 (67.8) | 349 (65.8) | 91 (72.8) | 15 (62.5) | 99 (68.3) |
| High | 568 (22.6) | 391 (23.2) | 132 (24.9) | 12 (9.6) | 3 (12.5) | 30 (20.7) |
Data are presented as n (%), mean±sd or median (interquartile range). I/D/H: idiopathic/drug-associated/heritable; CTD: connective tissue disease; CHD: congenital heart disease; POPH: porto-pulmonary hypertension; BMI: body mass index; WHO FC: World Health Organization Functional Class; 6MWD: 6-min walk distance; TLC: total lung capacity; FVC: forced vital capacity; FEV1: forced expiratory volume in 1 s; DLCO: diffusing capacity of the lung for carbon monoxide; PaO2: arterial oxygen tension; PaCO2: arterial carbon dioxide tension; RAP: right atrial pressure; mPAP: mean pulmonary arterial pressure; PAWP: pulmonary arterial wedge pressure; CI: cardiac index; PVR: pulmonary vascular resistance; SvO2: mixed venous oxygen saturation; NT-proBNP: N-terminal fragment of pro-brain natriuretic peptide; BNP: brain natriuretic peptide. #: if current or former smoker; ¶: BMI ≥30 kg·m−2.
The use of PAH medications, cumulated over the entire enrolment period, is shown in table 2 for the entire group and in supplementary table S3a–e for the subgroups. Overall, 19.6% of the patients received combination therapy within 3 months after diagnosis, 42.9% after 1 year and 49.8% after 3 years. In patients who received combination therapy, ERA and PDE5i were used in about two-thirds of the cases. Other combination therapies were used less frequently, and triple combination therapy including i.v. or s.c. prostacyclin analogues (PCAs) was used in 0.9% of the patients at entry and in 3.5% of the patients during follow-up. The median dosages of PAH medications at 3 months and 1 year are shown in supplementary table S4.
TABLE 2.
Use of drugs to treat pulmonary arterial hypertension (PAH) within 3 months and 1, 2 and 3 years (±6 months) after diagnosis
| 3 months (n=2375) | 1 year (n=2136) | 2 years (n=1727) | 3 years (n=1299) | |
| No therapy | 8 (0.3) | 73 (3.4) | 61 (3.5) | 48 (3.7) |
| Monotherapy | 1901 (80.0) | 1146 (53.7) | 841 (48.7) | 604 (46.5) |
| ERA | 401 (21.1) | 223 (19.5) | 145 (17.2) | 99 (16.4) |
| PDE5i | 1375 (72.3) | 851 (74.3) | 642 (76.3) | 467 (77.3) |
| sGC | 63 (3.3) | 40 (3.5) | 31 (3.7) | 23 (3.8) |
| PCA | 6 (0.3) | 3 (0.3) | 1 (0.1) | 1 (0.2) |
| PCA oral or inhaled# | 1 (16.7) | 2 (66.7) | 1 (100.0) | 0 (0.0) |
| PCA i.v. or s.c.¶ | 5 (83.3) | 1 (33.3) | 0 (0.0) | 1 (100.0) |
| CCB | 51 (2.7) | 29 (2.5) | 22 (2.6) | 14 (2.3) |
| Other PAH treatment | 5 (0.3) | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| Combination therapy | 466 (19.6) | 917 (42.9) | 825 (47.8) | 647 (49.8) |
| ERA+PDE5i | 320 (68.7) | 619 (67.5) | 539 (65.3) | 400 (61.8) |
| Other than ERA+PDE5i | 146 (31.3) | 298 (32.5) | 286 (34.7) | 247 (38.2) |
| ERA+PDE5i+PCA | 33 (22.6) | 106 (35.6) | 110 (38.5) | 104 (42.1) |
| Triple combination therapy including i.v. or s.c. PCA+ | 21 (14.4) | 44 (14.8) | 47 (16.4) | 45 (18.2) |
Data are presented as n (%); percentages refer to subgroups only, if applicable. ERA: endothelin receptor antagonist; PDE5i: phosphodiesterase-5 inhibitor; sGC: soluble guanylate cyclase stimulator; PCA: prostacyclin analogue; CCB: calcium channel blocker. #: selexipag, iloprost inhaled, treprostinil inhaled, beraprost; ¶: epoprostenol, iloprost i.v., treprostinil i.v./s.c.; +: all triple combination therapies that include i.v. or s.c. PCA therapy.
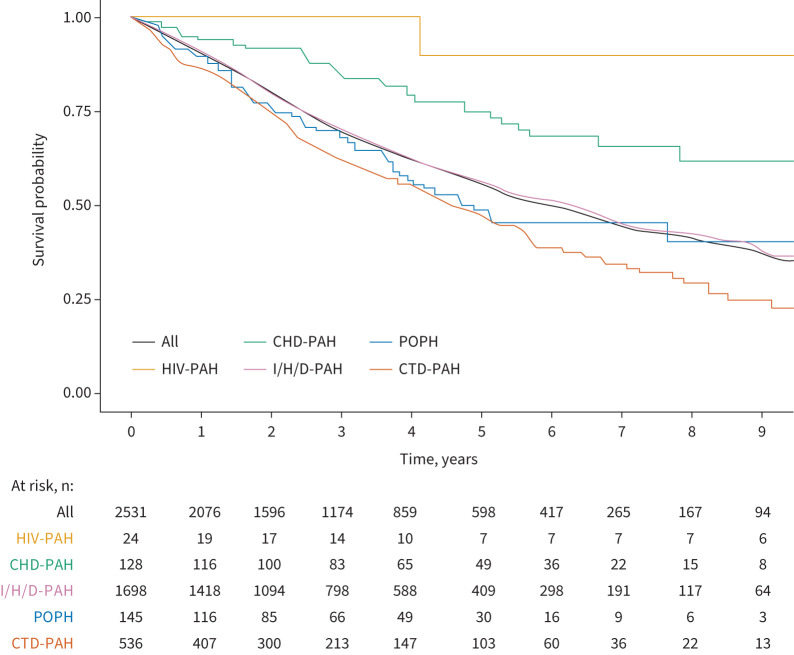
The median follow-up was 2.8 years for the entire cohort, and 3.3 and 2.5 years, respectively, for the subgroups of patients aged <65 and ≥65 years. A total of 978 (38.6%) patients died during the observation period, 29 (1.1%) underwent lung transplantation and 160 (6.3%) were lost to follow-up. The Kaplan–Meier estimated survival curves are shown in figure 2. The estimated survival rates of the entire cohort were 90.0% after 1 year, 69.2% after 3 years and 55.3% after 5 years. Patients with HIV-PAH and CHD-PAH had a better survival than patients with other forms of PAH, whereas patients with CTD-PAH had the worst outcome. The survival estimates for the cohorts of patients in the subgroups are shown in supplementary figure S2a–c.
FIGURE 2.
Kaplan–Meier survival estimates overall and by pulmonary arterial hypertension (PAH) subtype. CHD: congenital heart disease; POPH: porto-pulmonary hypertension; I/H/D: idiopathic/heritable/drug-associated; CTD: connective tissue disease.
Temporal trends in PAH therapy
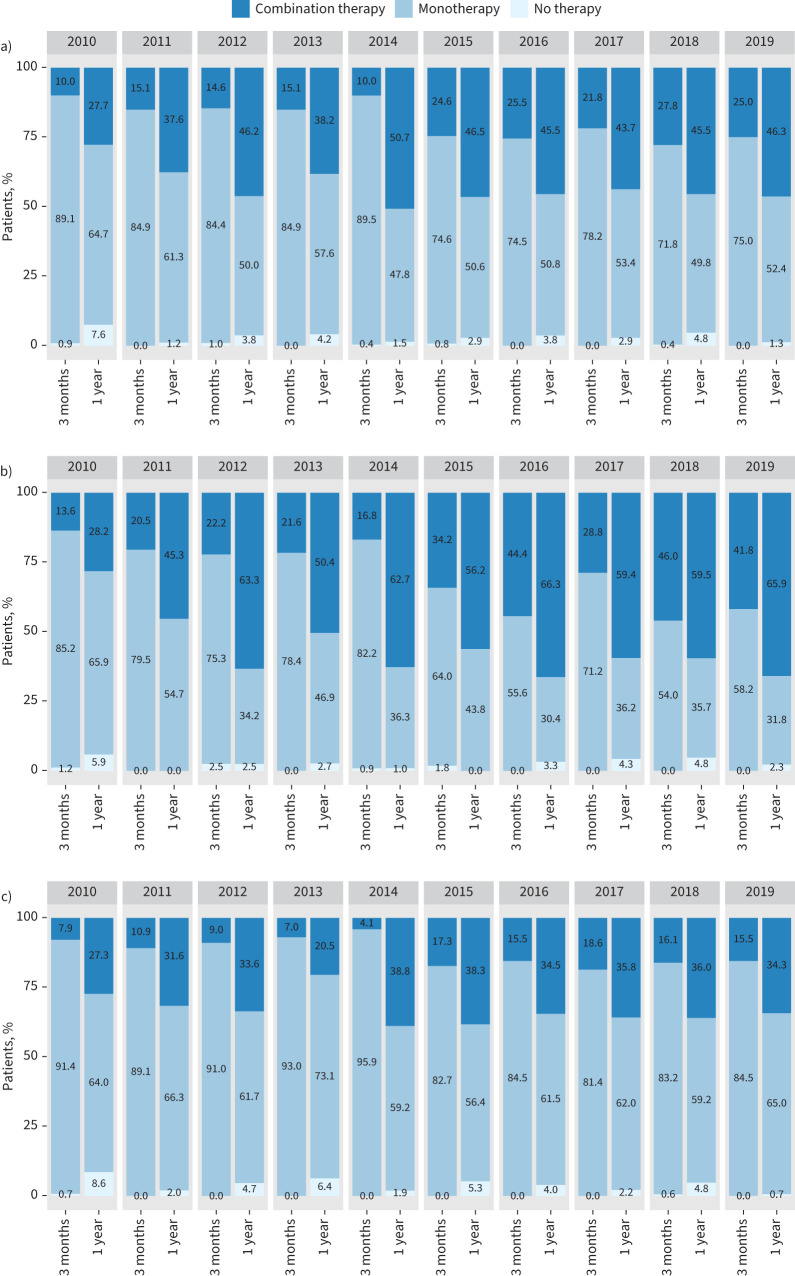
The annualised trends for the use of combination therapy within 3 months after diagnosis and after 1 year are shown in figure 3. In the entire cohort, the use of early combination therapy increased from 10.0% in patients diagnosed with PAH in 2010 to 25.0% in patients diagnosed with PAH in 2019. The steepest increase in the use of early combination therapy was seen between 2014 and 2015. The proportion of patients who received combination therapy 1 year after diagnosis increased from 27.7% to 46.3% (figure 3a).
FIGURE 3.
Temporal trends in the use of initial combination therapy and combination therapy 1 year after pulmonary arterial hypertension diagnosis in a) the entire cohort, and in the subgroups of patients aged b) <65 years and c) ≥65 years.
In the subgroup of patients with PAH who were aged <65 years at the time of diagnosis, the use of early combination therapy increased from 13.6% to 41.8% and the use of combination therapy 1 year after diagnosis increased from 28.2% to 65.9% during the observation period (figure 3b).
In the subgroup of patients with PAH who were aged ≥65 years at the time of diagnosis, combination therapy was given less frequently than in the younger cohort. The use of combination therapy increased from 7.9% to 15.5% after 3 months and from 27.3% to 34.3% after 1 year (figure 3c).
Temporal trends in the use of combination therapy in the subgroups of patients with I/H/D-PAH, CTD-PAH, and I/H/D-PAH without comorbidities and DLCO >45% predicted were in line with the changes in the whole group (supplementary figure S3a–c).
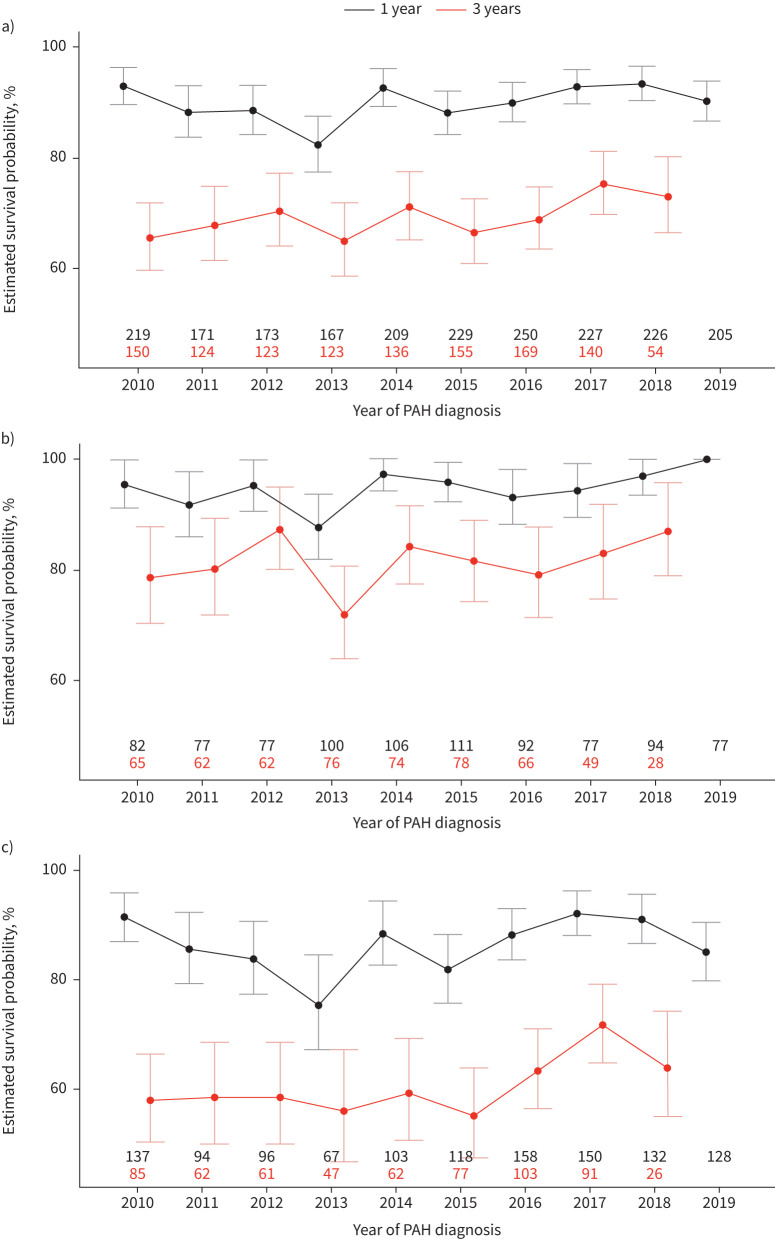
Survival over time
Temporal trends in 1- and 3-year survival are shown in figure 4. In the entire cohort, the 1-year survival rates varied between 82.3% (95% CI 77.4–87.5%) and 93.3% (95% CI 90.3–96.4%), with no clear improvement over time (figure 4a). The annualised 3-year survival rates of the entire cohort ranged between 65.0% (95% CI 58.8–71.8%) and 75.3% (95% CI 69.9–81.2%); here, a slight trend towards improvement cannot be excluded (figure 4a).
FIGURE 4.
Annualised survival rates (95% CI) at 1 and 3 years after pulmonary arterial hypertension (PAH) diagnosis in a) the entire cohort, and in the subgroups of patients aged b) <65 years and c) ≥65 years. The black and red numbers indicate the numbers of patients available for the annualised 1- and 3-year survival rates, respectively.
In the patients who were aged <65 years at the time of diagnosis, the annualised 1-year survival estimates varied between 87.7% (95% CI 82.1–93.8%) and 100.0% (95% CI 100.0–100.0%), and the 3-year survival estimates ranged between 72.0% (95% CI 64.2–80.8%) and 87.3% (95% CI 80.3–95.0%) (figure 4b), also suggesting a possible improvement over time.
In the patients who were aged ≥65 years at the time of diagnosis, the annualised 1-year survival estimates varied between 75.3% (95% CI 67.3–84.5%) and 92.1% (95% CI 88.1–96.1%), and the 3-year survival estimates ranged between 55.1% (95% CI 47.6–63.9%) and 71.7% (95% CI 64.9–79.3%) (figure 4c). In both cohorts, there were fluctuations over time and no clear trend in the 1-year survival estimates, whereas there was a possible increase in the 3-year survival estimates. Similar trends were observed in the subgroups of patients with I/H/D-PAH and CTD-PAH (supplementary figure S4a and b).
The cumulative 1- and 3-year survival rates of patients diagnosed with PAH between 2010 and 2014 and between 2015 and 2019 are shown in table 3. In this analysis, the 1-year survival of the entire cohort did not differ between the two time periods, whereas the 3-year survival did improve slightly (albeit with overlapping 95% confidence intervals). A similar pattern was seen in all subgroups.
TABLE 3.
Estimated survival probability at 1 and 3 years in patients diagnosed with pulmonary arterial hypertension (PAH) between 2010 and 2014 and between 2015 and 2019
| 2010–2014 | 2015–2019 | |||
| 1-year survival | 3-year survival | 1-year survival | 3-year survival | |
| Entire cohort | 89.0 (87.2–90.9) | 67.8 (65.0–70.8) | 90.8 (89.3–92.4) | 70.5 (67.8–73.4) |
| Patients aged <65 years | 93.3 (91.1–95.5) | 80.0 (76.4–83.8) | 96.0 (94.3–97.8) | 83.4 (79.7–87.2) |
| Patients aged ≥65 years | 85.5 (82.8–88.4) | 58.1 (54.2–62.3) | 87.7 (85.5–90.0) | 63.1 (59.5–66.9) |
| I/H/D-PAH | 90.2 (88.0–92.5) | 69.4 (66.0–73.0) | 90.8 (89.0–92.7) | 70.4 (67.1–73.8) |
| CTD-PAH | 85.3 (81.0–89.9) | 57.4 (51.3–64.3) | 88.2 (84.3–92.2) | 67.1 (60.9–74.0) |
| I/H/D-PAH with no comorbidities and DLCO >45% predicted | 90.7 (82.4–99.8) | 86.0 (76.2–97.0) | 97.5 (94.2–100.0) | 90.1 (82.6–98.2) |
Data are presented as mean % (95% CI). I/H/D: idiopathic/heritable/drug-associated; CTD: connective tissue disease; DLCO: diffusing capacity of the lung for carbon monoxide.
Discussion
In the present study, we assessed the COMPERA database to describe temporal trends in treatment strategies and survival of patients newly diagnosed with PAH between 2010 and 2019, i.e. over a 10-year period. During this time, we observed an increase in the use of oral combination therapies, especially in younger patients. While survival at 1 year after diagnosis did not improve over time, a slight improvement in 3-year survival rates is possible, but unproven.
Although the use of combination therapy increased over time, it remained unexpectedly low. The steepest increase in the use of early combination therapy was seen between 2014 and 2015, presumably resulting from the publication of the AMBITION study, which showed that patients with PAH who received initial combination therapy with an ERA and a PDE5i had more pronounced clinical improvement and fewer clinical worsening events than patients receiving initial monotherapy [8]. Still, even at the end of the observation period, early combination therapy was used in only 25% of the patients in the entire cohort and in <50% of the patients who were aged <65 years at the time of diagnosis. In the subgroup of patients who were aged ≥65 years at the time of diagnosis, combination therapy was used much less frequently than in younger patients, both initially and 1 year after diagnosis.
The reasons for the relatively infrequent use of combination therapies are unclear. Safety concerns may play a role. However, in the AMBITION study, initial combination therapy was well tolerated and there were no more premature study drug discontinuations with initial combination therapy than with monotherapy [8]. In addition, several other large-scale studies have demonstrated the safety and efficacy of various sequential combination therapies [9, 10]. However, neither these studies nor meta-analyses have shown a survival advantage with the use of combination therapies over monotherapies [8–11, 25], which may discourage physicians from a broader utilisation of combination therapies.
In the present series, older patients with PAH had more comorbidities, were less likely to receive combination therapy and had a higher mortality risk than younger patients, similar to what has been observed in other PAH registries [16–18]. With older patients having a higher mortality risk, one might expect that combination therapy would be used more frequently than in younger patients, whereas the opposite is being observed. We have reported previously that the less frequent use of combination therapies in elderly patients results not only from a lower initiation rate but also from a higher likelihood of drug discontinuations [6, 21]. Physicians seem to be less confident with the use of combination therapy in older patients with PAH and multiple comorbidities. This may be related, at least in part, to the fact that such patients were under-represented in the pivotal trials that have been conducted in this area. Clinical inertia, i.e. recognition of a problem, but failure to act, a phenomenon well known in other disease areas [26], may also play a role. Prospective studies are underway to further explore the reasons for withholding combination therapy in patients with PAH.
Although we cannot exclude a slight increase in the 3-year survival rates over time, we were surprised to find no clearer signal, not even in younger patients, which we had expected to see with the increasing use of combination therapies. Several reasons may apply. First, although the use of combination therapy increased over time, most patients received monotherapy, so that any effect of combination therapy on survival might have been diluted. Second, the drugs mainly used to treat PAH in the present series were already available in 2010, i.e. when this analysis was started. The only drugs that were newly introduced in Europe during the observation period were macitentan, an ERA [9], selexipag, a prostacyclin receptor agonist [10], and riociguat, a stimulator of soluble guanylate cyclase [27]. For all three compounds, improvement in event-free survival, but not overall survival, has been demonstrated [9, 10, 27]. The concept of a goal-oriented use of combination therapy was already introduced in 2005 [28] and had probably been adopted by many pulmonary hypertension centres in 2010, i.e. the onset of the observation period of the present study. This and the observation that clinical studies using combination therapies have consistently shown an improvement in event-free survival, but not in overall survival [8–10], may suggest that treatment escalation at the time of a clinical worsening event might delay further clinical worsening and death in some patients. If this is the case, early combination therapy would be expected to affect overall survival to a lesser extent than time to clinical worsening.
Our findings corroborate those of Boucly et al. [5] who recently analysed initial treatment strategies and long-term outcomes from the French Pulmonary Hypertension Registry and found no differences in survival of patients who received initial monotherapy or initial oral combination therapy (except for a small but statistically significant survival difference in patients at intermediate risk). Of note, in the French series, the only treatment strategy that was associated with a long-term survival benefit was initial triple combination therapy including ERA, PDE5i and i.v. or s.c. PCAs [5]. This treatment strategy was used in ∼5% of the patients in the French series but in <1% of the patients in our present series, indicating that initial triple combination therapy including i.v. or s.c. prostacyclin cannot yet be considered standard of care. However, as two large registry studies based on 1611 patients [5] and 2531 patients (our study) now independently suggest that initial or early oral combination therapy may not necessarily be associated with a long-term survival benefit over oral monotherapy, it becomes increasingly important to further explore the effects of initial triple combination therapy including i.v. or s.c. PCAs on long-term outcome. In addition, future studies exploring treatment strategies in PAH should be designed to show potential survival differences.
It is important to note that our observations should not discourage physicians from using initial or early oral combination therapy in patients with PAH. This treatment strategy is recommended by current guidelines based on prospective randomised controlled trials showing meaningful improvements in several clinically important end-points [3, 8, 15]. Given the post hoc nature of our study as well as the study by Boucly et al. [5], our findings should be considered hypothesis generating and we need prospective studies to compare the effects of various initial treatment strategies on long-term survival. Registry-based pragmatic trials that have been used successfully in other disease areas [29] may be considered to approach this fundamentally important question.
Our study has strengths and limitations. Strengths include the large sample size and the relatively constant number of newly enrolled patients throughout the study period. The main limitations are those inherent to registries, including the post hoc analysis and the potential of missing or wrongly entered data. To minimise such risks, COMPERA uses several preventive strategies including automated plausibility checks, regular queries of implausible entries and independent on-site monitoring with source data verification. Moreover, dividing the age groups at 65 years for the subgroup analyses was arbitrary, although this threshold is often used to define old age. In the setting of PAH, this threshold may be of limited value as previous studies have shown differences in treatment patterns, treatment response and survival in patients with PAH aged 18–45 years and those aged 46–64 years [16].
In summary, the present analysis of the COMPERA database showed that there was an increase in the use of oral combination therapy over the 10-year period between 2010 and 2019, but about half of the patients were still receiving monotherapy 1 year after diagnosis. While 1-year survival rates did not change over time, there was a slight improvement in 3-year survival rates which was, however, not statistically significant. It will be an important future task to find out if a more proactive use of combination therapy including i.v. or s.c. PCAs translates into better long-term survival in patients with PAH.
Supplementary material
Please note: supplementary material is not edited by the Editorial Office, and is uploaded as it has been supplied by the author.
Supplementary material ERJ-02024-2021.Supplement (236.3KB, pdf)
Shareable PDF
Acknowledgements
The authors are indebted to the COMPERA investigators and their co-workers.
Footnotes
This article has an editorial commentary: https://doi.org/10.1183/13993003.00390-2022
Conflicts of interest: M.M. Hoeper has received fees for lectures and/or consultations from Acceleron, Actelion, Bayer, GlaxoSmithKline, Janssen, MSD and Pfizer.
Conflicts of interest: C. Pausch has no disclosures.
Conflicts of interest: E. Grünig has received fees for lectures and/or consultations from Actelion, Bayer, GlaxoSmithKline, Janssen, MSD, Pfizer and United Therapeutics.
Conflicts of interest: G. Staehler has received honoraria for lectures and/or consultancy for Actelion, Bayer, GlaxoSmithKline, Novartis and Pfizer.
Conflicts of interest: D. Huscher has received consulting fees from Actelion.
Conflicts of interest: D. Pittrow has received fees for consultations from Actelion, Amgen, Aspen, Bayer, Biogen, Boehringer Ingelheim, Daiichi Sankyo, Sanofi, Takeda and Viatris.
Conflicts of interest: K.M. Olsson has received fees for lectures and/or consultations from Acceleron, Actelion, Bayer, GlaxoSmithKline, Janssen, MSD, Pfizer and United Therapeutics.
Conflicts of interest: C.D. Vizza has received fees for lectures and/or consultations from Acceleron, Actelion, Bayer, GlaxoSmithKline, Janssen, MSD, Pfizer and United Therapeutics.
Conflicts of interest: H. Gall reports personal fees from Actelion, AstraZeneca, Bayer, BMS, GlaxoSmithKline, Janssen-Cilag, Lilly, MSD, Novartis, OMT, Pfizer and United Therapeutics.
Conflicts of interest: O. Distler has/had consultancy relationship and/or has received research funding from 4D Science, Actelion, Active Biotec, Bayer, Biogen Idec, Boehringer Ingelheim Pharma, BMS, ChemoAb, EpiPharm, Ergonex, EspeRare Foundation, GlaxoSmithKline, Genentech/Roche, Inventiva, Janssen, Lilly, medac, MedImmune, Mitsubishi Tanabe, Pharmacyclics, Pfizer, Sanofi, Serodapharm and Sinoxa, in the area of potential treatments of scleroderma and its complications including PAH; in addition, the author has a patent “mir-29 for the treatment of systemic sclerosis” licensed.
Conflicts of interest: C. Opitz has received speaker fees and honoraria for consultations from Actelion, Bayer, GlaxoSmithKline, Lilly, Novartis and Pfizer.
Conflicts of interest: J.S.R. Gibbs has received fees for lectures and/or consultations from Acceleron, Actelion, Aerovate, Bayer, Complexia, Janssen, MSD, Pfizer and United Therapeutics.
Conflicts of interest: M. Delcroix reports research grants from Actelion/J&J, speaker and consultant fees from Bayer, MSD, Acceleron, AOP and Daiichi Sankyo, outside the submitted work; and is holder of the Janssen Chair for Pulmonary Hypertension at the KU Leuven.
Conflicts of interest: H.A. Ghofrani has received honoraria for consultations and/or speaking at conferences from Bayer HealthCare AG, Actelion, Encysive, Pfizer, Ergonex, Lilly and Novartis; is member of advisory boards for Acceleron, Bayer HealthCare AG, Pfizer, GlaxoSmithKline, Actelion, Lilly, Merck, Encysive and Ergonex; and has also received governmental grants from the German Research Foundation (DFG), Excellence Cluster Cardiopulmonary Research (ECCPS), State Government of Hessen (LOEWE) and the German Ministry for Education and Research (BMBF).
Conflicts of interest: S. Rosenkranz has received fees for lectures and/or consultations from Abbott, Acceleron, Actelion, Bayer, BMS, Gilead, GlaxoSmithKline, Janssen, MSD, Novartis, Pfizer, United Therapeutics and Vifor; research grants to institution from AstraZeneca, Actelion, Bayer Janssen and Novartis.
Conflicts of interest: D-H. Park has nothing to disclose.
Conflicts of interest: R. Ewert has received speaker fees and honoraria for consultations from Actelion, Bayer, GlaxoSmithKline, Janssen, Lilly, MSD, Novartis, Pfizer and United Therapeutics.
Conflicts of interest: H. Kaemmerer has received honoraria for lectures and/or consultancy from Actelion, BMS and Janssen.
Conflicts of interest: T.J. Lange has received speaker fees and honoraria for consultation from Acceleron, Actelion, Bayer, GlaxoSmithKline, Janssen-Cilag, MSD, Pfizer and United Therapeutics.
Conflicts of interest: H-J. Kabitz has received fees from Löwenstein Medical, Weinmann, Philips Respironics, ResMed, Vivisol, Sapio Life and Sanofi-Genzyme.
Conflicts of interest: D. Skowasch received fees for lectures and/or consulting and/or research support to institution from Actelion, Bayer, GlaxoSmithKline, Janssen, MSD and Pfizer.
Conflicts of interest: A. Skride reports no conflicts of interest.
Conflicts of interest: M. Claussen reports honoraria for lectures from Boehringer Ingelheim Pharma GmbH and Roche Pharma, and for serving on advisory boards from Boehringer Ingelheim.
Conflicts of interest: J. Behr received grants from Actelion, Bayer, Biogen, Boehringer Ingelheim, Galapagos, Novartis, Roche and Sanofi/Genzyme.
Conflicts of interest: K. Milger has received fees from Actelion, AstraZeneca, GlaxoSmithKline, Janssen, MSD, Novartis and Sanofi-Aventis.
Conflicts of interest: M. Halank has received speaker fees and honoraria for consultations from Acceleron, Actelion, AstraZeneca, Bayer, BerlinChemie, GlaxoSmithKline, Janssen and Novartis.
Conflicts of interest: H. Wilkens received personal fees from Actelion, Bayer, Biotest, Boehringer, GlaxoSmithKline, Janssen, Pfizer and Roche.
Conflicts of interest: H-J. Seyfarth has received speaker fees and honoraria for consultations from Actelion, Bayer, GlaxoSmithKline, Janssen and MSD.
Conflicts of interest: M. Held has received speaker fees and honoraria for consultations from Actelion, Bayer, Boehringer Ingelheim Pharma, GlaxoSmithKline, Janssen, MSD, Novartis, Pfizer, Nycomed, Roche and Servier.
Conflicts of interest: D. Dumitrescu declares honoraria for lectures and/or consultancy from Actelion, AstraZeneca, Bayer, GlaxoSmithKline, Janssen, MSD, Novartis, Pfizer and Servier.
Conflicts of interest: I. Tsangaris has received fees from Actelion, Bayer, ELPEN, GlaxoSmithKline, Janssen, MSD, Pfizer and United Therapeutics.
Conflicts of interest: A. Vonk-Noordegraaf reports receiving fees for lectures and/or consultations from Actelion, Bayer, GlaxoSmithKline, Janssen, MSD and Pfizer.
Conflicts of interest: S. Ulrich reports personal fees from Actelion, Janssen and MSD outside the submitted work.
Conflicts of interest: H. Klose has received speaker fees and honoraria for consultations from Actelion, Bayer, GlaxoSmithKline, Janssen, MSD, Novartis, Pfizer and United Therapeutics.
Support statement: This work was supported by the German Center for Lung Research (DZL). COMPERA is funded by unrestricted grants from Acceleron, Bayer, GlaxoSmithKline, Janssen and OMT. These companies were not involved in data analysis or the writing of the manuscript.
References
- 1.Simonneau G, Montani D, Celermajer DS, et al. . Haemodynamic definitions and updated clinical classification of pulmonary hypertension. Eur Respir J 2019; 53: 1801913. doi: 10.1183/13993003.01913-2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.D'Alonzo GE, Barst RJ, Ayres SM, et al. . Survival in patients with primary pulmonary hypertension. Results from a national prospective registry. Ann Intern Med 1991; 115: 343–349. doi: 10.7326/0003-4819-115-5-343 [DOI] [PubMed] [Google Scholar]
- 3.Galiè N, Channick RN, Frantz RP, et al. . Risk stratification and medical therapy of pulmonary arterial hypertension. Eur Respir J 2019; 53: 1801889. doi: 10.1183/13993003.01889-2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Benza RL, Kanwar MK, Raina A, et al. . Development and validation of an abridged version of the REVEAL 2.0 risk score calculator, REVEAL Lite 2, for use in patients with pulmonary arterial hypertension. Chest 2021; 159: 337–346. doi: 10.1016/j.chest.2020.08.2069 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Boucly A, Savale L, Jaïs X, et al. . Association between initial treatment strategy and long-term survival in pulmonary arterial hypertension. Am J Respir Crit Care Med 2021; 204: 842–854. doi: 10.1164/rccm.202009-3698OC [DOI] [PubMed] [Google Scholar]
- 6.Hoeper MM, Pausch C, Grunig E, et al. . Idiopathic pulmonary arterial hypertension phenotypes determined by cluster analysis from the COMPERA registry. J Heart Lung Transplant 2020; 39: 1435–1444. doi: 10.1016/j.healun.2020.09.011 [DOI] [PubMed] [Google Scholar]
- 7.Barst RJ, Rubin LJ, Long WA, et al. . A comparison of continuous intravenous epoprostenol (prostacyclin) with conventional therapy for primary pulmonary hypertension. N Engl J Med 1996; 334: 296–301. doi: 10.1056/NEJM199602013340504 [DOI] [PubMed] [Google Scholar]
- 8.Galiè N, Barbera JA, Frost AE, et al. . Initial use of ambrisentan plus tadalafil in pulmonary arterial hypertension. N Engl J Med 2015; 373: 834–844. doi: 10.1056/NEJMoa1413687 [DOI] [PubMed] [Google Scholar]
- 9.Pulido T, Adzerikho I, Channick RN, et al. . Macitentan and morbidity and mortality in pulmonary arterial hypertension. N Engl J Med 2013; 369: 809–818. doi: 10.1056/NEJMoa1213917 [DOI] [PubMed] [Google Scholar]
- 10.Sitbon O, Channick R, Chin KM, et al. . Selexipag for the treatment of pulmonary arterial hypertension. N Engl J Med 2015; 373: 2522–2533. doi: 10.1056/NEJMoa1503184 [DOI] [PubMed] [Google Scholar]
- 11.Hoeper MM, McLaughlin VV, Barbera JA, et al. . Initial combination therapy with ambrisentan and tadalafil and mortality in patients with pulmonary arterial hypertension: a secondary analysis of the results from the randomised, controlled AMBITION study. Lancet Respir Med 2016; 4: 894–901. doi: 10.1016/S2213-2600(16)30307-1 [DOI] [PubMed] [Google Scholar]
- 12.Galiè N, Torbicki A, Barst R, et al. . Guidelines on diagnosis and treatment of pulmonary arterial hypertension. The Task Force on Diagnosis and Treatment of Pulmonary Arterial Hypertension of the European Society of Cardiology. Eur Heart J 2004; 25: 2243–2278. doi: 10.1016/j.ehj.2004.09.014 [DOI] [PubMed] [Google Scholar]
- 13.Galiè N, Hoeper MM, Humbert M, et al. . Guidelines for the diagnosis and treatment of pulmonary hypertension: the Task Force for the Diagnosis and Treatment of Pulmonary Hypertension of the European Society of Cardiology (ESC) and the European Respiratory Society (ERS), endorsed by the International Society of Heart and Lung Transplantation (ISHLT). Eur Heart J 2009; 30: 2493–2537. doi: 10.1093/eurheartj/ehp297 [DOI] [PubMed] [Google Scholar]
- 14.Galiè N, Humbert M, Vachiery JL, et al. . 2015 ESC/ERS Guidelines for the diagnosis and treatment of pulmonary hypertension: The Joint Task Force for the Diagnosis and Treatment of Pulmonary Hypertension of the European Society of Cardiology (ESC) and the European Respiratory Society (ERS). Eur Respir J 2015; 46: 903–975. doi: 10.1183/13993003.01032-2015 [DOI] [PubMed] [Google Scholar]
- 15.Galiè N, Humbert M, Vachiery JL, et al. . 2015 ESC/ERS Guidelines for the diagnosis and treatment of pulmonary hypertension: The Joint Task Force for the Diagnosis and Treatment of Pulmonary Hypertension of the European Society of Cardiology (ESC) and the European Respiratory Society (ERS). Eur Heart J 2016; 37: 67–119. doi: 10.1093/eurheartj/ehv317 [DOI] [PubMed] [Google Scholar]
- 16.Hjalmarsson C, Radegran G, Kylhammar D, et al. . Impact of age and comorbidity on risk stratification in idiopathic pulmonary arterial hypertension. Eur Respir J 2018; 51: 1702310. doi: 10.1183/13993003.02310-2017 [DOI] [PubMed] [Google Scholar]
- 17.Ling Y, Johnson MK, Kiely DG, et al. . Changing demographics, epidemiology, and survival of incident pulmonary arterial hypertension: results from the pulmonary hypertension registry of the United Kingdom and Ireland. Am J Respir Crit Care Med 2012; 186: 790–796. doi: 10.1164/rccm.201203-0383OC [DOI] [PubMed] [Google Scholar]
- 18.Frost AE, Badesch DB, Barst RJ, et al. . The changing picture of patients with pulmonary arterial hypertension in the United States: how REVEAL differs from historic and non-US contemporary registries. Chest 2011; 139: 128–137. doi: 10.1378/chest.10-0075 [DOI] [PubMed] [Google Scholar]
- 19.Kylhammar D, Kjellstrom B, Hjalmarsson C, et al. . A comprehensive risk stratification at early follow-up determines prognosis in pulmonary arterial hypertension. Eur Heart J 2018; 39: 4175–4181. doi: 10.1093/eurheartj/ehx257 [DOI] [PubMed] [Google Scholar]
- 20.McLaughlin VV, Vachiery JL, Oudiz RJ, et al. . Patients with pulmonary arterial hypertension with and without cardiovascular risk factors: results from the AMBITION trial. J Heart Lung Transplant 2019; 38: 1286–1295. doi: 10.1016/j.healun.2019.09.010 [DOI] [PubMed] [Google Scholar]
- 21.Opitz CF, Hoeper MM, Gibbs JS, et al. . Pre-capillary, combined, and post-capillary pulmonary hypertension: a pathophysiological continuum. J Am Coll Cardiol 2016; 68: 368–378. doi: 10.1016/j.jacc.2016.05.047 [DOI] [PubMed] [Google Scholar]
- 22.Vizza CD, Hoeper MM, Huscher D, et al. . Pulmonary hypertension in patients with COPD: results from the Comparative, Prospective Registry of Newly Initiated Therapies for Pulmonary Hypertension (COMPERA). Chest 2021; 160: 678–689. doi: 10.1016/j.chest.2021.02.012 [DOI] [PubMed] [Google Scholar]
- 23.Trip P, Nossent EJ, de Man FS, et al. . Severely reduced diffusion capacity in idiopathic pulmonary arterial hypertension: patient characteristics and treatment responses. Eur Respir J 2013; 42: 1575–1585. doi: 10.1183/09031936.00184412 [DOI] [PubMed] [Google Scholar]
- 24.Hoeper MM, Meyer K, Rademacher J, et al. . Diffusion capacity and mortality in patients with pulmonary hypertension due to heart failure with preserved ejection fraction. JACC Heart Fail 2016; 4: 441–449. doi: 10.1016/j.jchf.2015.12.016 [DOI] [PubMed] [Google Scholar]
- 25.Lajoie AC, Lauziere G, Lega JC, et al. . Combination therapy versus monotherapy for pulmonary arterial hypertension: a meta-analysis. Lancet Respir Med 2016; 4: 291–305. doi: 10.1016/S2213-2600(16)00027-8 [DOI] [PubMed] [Google Scholar]
- 26.Phillips LS, Branch WT, Cook CB, et al. . Clinical inertia. Ann Intern Med 2001; 135: 825–834. doi: 10.7326/0003-4819-135-9-200111060-00012 [DOI] [PubMed] [Google Scholar]
- 27.Ghofrani HA, Galiè N, Grimminger F, et al. . Riociguat for the treatment of pulmonary arterial hypertension. N Engl J Med 2013; 369: 330–340. doi: 10.1056/NEJMoa1209655 [DOI] [PubMed] [Google Scholar]
- 28.Hoeper MM, Markevych I, Spiekerkoetter E, et al. . Goal-oriented treatment and combination therapy for pulmonary arterial hypertension. Eur Respir J 2005; 26: 858–863. doi: 10.1183/09031936.05.00075305 [DOI] [PubMed] [Google Scholar]
- 29.Ford I, Norrie J. Pragmatic trials. N Engl J Med 2016; 375: 454–463. doi: 10.1056/NEJMra1510059 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Please note: supplementary material is not edited by the Editorial Office, and is uploaded as it has been supplied by the author.
Supplementary material ERJ-02024-2021.Supplement (236.3KB, pdf)
This one-page PDF can be shared freely online.
Shareable PDF ERJ-02024-2021.Shareable (642KB, pdf)