Summary
Background
The NHS Diabetes Prevention Programme (DPP) is the first nationwide type 2 diabetes prevention programme targeting people with prediabetes. It was rolled out across England from 2016 in three waves. We evaluate the population level impact of the NHS DPP on incidence rates of type 2 diabetes.
Methods
We use data from the National Diabetes Audit, which records all individuals across England who have been diagnosed with type 2 diabetes by 2019. We use difference-in-differences regression models to estimate the impact of the phased introduction of the DPP on type 2 diabetes incidence. We compare patients registered with the 3,282 general practices enrolled from 2016 (wave 1) and the 1,610 practices enrolled from 2017 (wave 2) to those registered with the 1,584 practices enrolled from 2018 (final wave).
Findings
Incidence rates of type 2 diabetes in wave 1 practices in 2018 and 2019 were significantly lower than would have been expected in the absence of the DPP (difference-in-differences Incident Rate Ratio (IRR) = 0·938 (95% CI 0·905 to 0·972)). Incidence rates were also significantly lower than expected for wave 2 practices in 2019 (difference-in-differences IRR = 0·927 (95% CI 0·885 to 0·972)). These results remained consistent across several robustness checks.
Interpretation
Introduction of the NHS DPP reduced population incidence of type 2 diabetes. Longer follow-up is required to explore whether these effects are maintained or if diabetes onset is delayed.
Funding
This research was funded by the National Institute for Health and Care Research (Health Services and Delivery Research, 16/48/07 – Evaluating the NHS Diabetes Prevention Programme (NHS DPP): the DIPLOMA research programme (Diabetes Prevention – Long Term Multimethod Assessment)). The views and opinions expressed in this manuscript are those of the authors and do not necessarily reflect those of the NHS, the National Institute for Health and Care Research or the Department of Health and Social Care.
Keywords: Diabetes prevention, Prediabetes, Public health, Health policy, Type 2 Diabetes
Research in context.
Evidence before this study
A large literature exists evaluating the use of lifestyle interventions to prevent or delay the onset of type 2 diabetes amongst individuals with prediabetes, as well as several systematic reviews and meta-analyses of these studies. One such review, by Barry et al. published in the BMJ in 2017, identified 50 trials evaluating interventions aiming to prevent diabetes amongst a prediabetic population. A meta-analysis of these studies showed that lifestyle interventions were able to reduce the relative risk of developing diabetes by 31% (95% CI 15% to 44%), on the condition that the intervention lasted between 6 months and 2 years. This review cited the need for future research to focus on pragmatic evaluations of prediabetes interventions, beyond analyses conducted alongside clinical trials.
Another review by Schellenberg et al. published in 2013 in the Annals of Internal Medicine noted that most of the trials evaluating diabetes prevention assessed secondary outcomes (such as weight change, blood pressure, and lipids) instead of focusing on diabetes conversion.
Added value of this study
The national implementation of the NHS Diabetes Prevention Programme meant that individuals not participating in the programme may have nevertheless been affected, for example through increased identification and diagnosis of prediabetes. This is the first study to evaluate the impact of the programme for the entire population, not just the programme participants, within a real world setting. Up until now, studies have focused on the individual level effect of prevention programmes, focusing on highly selected individuals often within trial settings.
Our findings will be of use to other countries who have yet to introduce such prevention programmes or those looking to recommission such programmes.
Implications of all the available evidence
Our study suggests that the NHS Diabetes Prevention Programme has reduced rates of type 2 diabetes incidence at the population level. This shows that diabetes prevention delivered on a national scale can produce results, aligning with existing evidence from trials regarding the effectiveness of the underpinning behaviour change interventions.
Future research is needed to identify whether this impact is maintained and thus whether type 2 diabetes has been prevented completely or delayed.
Alt-text: Unlabelled box
Introduction
Diabetes prevalence is increasing internationally, and the most recent Global Burden of Disease Study estimates that conditions associated with high fasting plasma glucose (including diabetes) are now the sixth leading cause of disability worldwide.1
Before developing type 2 diabetes, many individuals first develop non-diabetic hyperglycaemia or ‘prediabetes’, characterised by elevated blood glucose levels that are below the threshold of type 2 diabetes, but above normal ranges. It is possible for these individuals to progress to type 2 diabetes or return to normal blood glucose levels. It is estimated that 11% of individuals with obesity and non-diabetic hyperglycaemia will progress to type 2 diabetes annually,2 and a study amongst adults in the United Kingdom has estimated conversion rates of 7% within the first year.3
There are genetic risk factors associated with developing type 2 diabetes, such as a family history of the disease and being from South-Asian, African-Caribbean or Black African descent. Several sociodemographic factors have been associated with higher rates of conversion; socio-economic disadvantage, economic inactivity or limiting long-standing illness.4 There is therefore considerable interest in attempting to prevent this progression.5,6
Prevalence of type 2 diabetes has more than doubled in the United Kingdom between 2000 and 2013, from 2·4 to 5·3% of the general population.7 In an effort to combat this rising trend, the NHS Diabetes Prevention Programme (DPP) was introduced across England.8 The DPP is a group-based lifestyle change programme focusing on nutrition, physical activity and weight loss (see next section for a detailed description of the programme). There is evidence from randomised trials,9,10 implementation studies11 and systematic reviews12,13 that diabetes prevention strategies similar to those used in the NHS DPP are effective at an individual level for those who participate. Evidence from the NHS DPP itself has shown that completion of the programme is associated with an average weight loss of 3.6kg (95% CI -3·6 to -3·514 and a reduction in glycated haemoglobin (HbA1c) of 2·04 mmol/mol (95% CI -2·12 to -1.96).15
However, it is not yet known whether mass roll-out of these prevention programmes has had an impact on population trends of type 2 diabetes incidence. For example, it is unclear whether the results observed at the ‘individual’ level can be scaled. The effects found in previous research studies (such as trials) focus on select individuals.16 As such, the effectiveness may be overestimated compared to what is achievable in a real-world setting, where individuals are ‘unselected’ and treatments are given outside a controlled context. Secondly, to have an impact on population trends, not only does the programme need to be effective but it also needs to have considerable reach, with access to a significant proportion of those at high-risk of developing diabetes.
It is also possible that individual level studies may fail to capture the wider effects of the programme. For example, the programme may prompt increased identification of individuals with prediabetes. Indeed, receipt of a prediabetes diagnosis may itself trigger behaviour change in individuals,17 and primary care professionals may subsequently work with these patients to reduce their risks in other ways if they do not wish to participate in the DPP. It is therefore important to assess the effectiveness of the programme amongst all individuals at-risk of developing type 2 diabetes, not just those who participate in the programme.
Therefore, evaluating the effects of a nationwide programme requires a real-world evaluation, beyond the randomised controlled studies previously conducted. The large scale implementation across England, combined with a detailed understanding of how the programme was introduced in practice and the availability of national datasets, provides a unique opportunity to measure the impact of the DPP on population health trends. We use difference-in-differences techniques to estimate the impact of the DPP on incidence rates of type 2 diabetes, exploiting the gradual rollout of the programme to compare to a control group in a period before they were affected by the DPP.
Methods
NHS Diabetes Prevention Programme
The DPP is a nationwide programme, targeting adults across England at high-risk of developing type 2 diabetes with the aim of preventing progression to diabetes. The programme is jointly led by NHS England, Public Health England and Diabetes UK, but is delivered by private providers selected via a national, competitive procurement process.
Prior to delivery, NHS England conducted a DPP impact assessment, which estimated that in a cohort of 390,000 DPP participants, a potential 14,000 to 21,000 cases of diabetes could be prevented or delayed within the first 5 years of the programme.18
The DPP was rolled out across England from 1st June 2016 in three waves, with approximately 50% of English general practices enrolled in the first wave. A further 25% enrolled in the second wave starting from 1st April 2017, and from 1st April 2018 the DPP was available nationwide. The DPP has since undergone changes through new ‘frameworks’, including additional commercial providers and the provision of a digital option. Our analysis examines the first framework, before such changes were introduced (June 2016 to March 2020).
For participating individuals, the DPP involves attending at least 13 group-based behaviour change sessions which incorporate structured education on nutrition, physical activity and weight loss.19 The programme usually takes 9–12 months for an individual to complete.
Access to the programme follows a set pathway. Adults 18 years and over are eligible if a recent blood test shows a concentration of HbA1c within the range of 42–47 mmol/mol (6·0–6·4%) or fasting plasma glucose (FPG) level of 5·5–6·9 mmol/L.20 Individuals are usually identified in general practice during an NHS Health Check, opportunistically during a consultation, or via searches of general practice records where letters may be sent to patients inviting them to self-refer.21 For practitioners to make a referral they need to gain patient consent to pass their details onto the local DPP provider, who then arranges an initial assessment to confirm eligibility. Individuals then start the programme, dependent on course availability and other individual factors such as the proximity of the course location and time of day that the course runs.
By April 2020, DPP providers had received 513,312 referrals, of which 271,208 (52·8% of total referrals) had attended an initial assessment and 101,175 (19·7% of total referrals) had attended at least 60% of the programme's sessions.
Data
We used data from multiple sources to create a practice level dataset containing counts of the number of new type 2 diabetes cases identified per year (our outcome of interest), in addition to practice and population characteristics which we control for in our analyses.
National Diabetes Audit
We used pseudo-anonymised data from the core diabetes module of the National Diabetes Audit.22
This data was obtained via an application to NHS Digital, which required approval by their Independent Group Advising on the Release of Data (IGARD). We also received ethical approval from the North West - Greater Manchester East Research Ethics Committee (REC reference 17/NW/0426).
Our data extract from the core diabetes module only covered individuals diagnosed with type 2 diabetes. Individuals diagnosed with other types of diabetes, such as type 1 and gestational diabetes, were not included in the data we examine. Diagnosis was determined by the presence of Read codes within an individual's primary care record.22 Individuals are included in the audit extract if a Read code indicating a diagnosis of type 2 diabetes is present in their healthcare records by the final date of the audit period.
We used three data extracts from the core diabetes module covering: 1st April 2017 – 31st March 2018, 1st January 2018 – 31st March 2019, and 1st January 2019 – 31st March 2020. These extracts contain information from individuals’ healthcare records. This includes information recorded during the audit period such as weight and HbA1c readings, as well as historic data such as the calendar year an individual was first diagnosed with type 2 diabetes (which may have occurred prior to the audit extract dates). This allowed us to calculate annual rates of type 2 diabetes incidence retrospectively in addition to incidence rates observed during the audit periods.
We compiled a list of the unique general practices with which individuals within the National Diabetes Audit were registered. For each practice, we then produced practice level counts of the number of new diagnoses of type 2 diabetes for each full calendar year from 2010 to 2019. We excluded from these counts individuals for whom the year of diagnosis or general practice with which they were registered was missing (1·8% of total records).
The National Diabetes Audit covered 99·3% of practices in England in 2019/20.23
Characteristics of the practice population
We obtained data on the number of people registered with each practice on the 1st April of each year from 2010 to 2019, from data published by NHS Digital. Using this information and the National Diabetes Audit data on the number of people diagnosed with type 2 diabetes each year, we calculated the number of people eligible to benefit from the DPP, that is: the number of patients over 15 years of age registered with each practice who were not already diagnosed with type 2 diabetes.
We also obtained the following practice population characteristics: age distribution, ethnicity and socio-economic deprivation. Details of how these were derived and the sources of data are provided in Supplementary Material.
General practice characteristics
NHS England provided data on when practices first began to participate in the DPP. We assigned all practices to one of three groups: those enrolled in wave 1 (which started on 1st June 2016); wave 2 (which started 1st April 2017); or the final wave (which started from 1st April 2018).
We collated data on practice characteristics based on our previous analysis of practice referral activity.24 Characteristics included: the practice's contract type; whether the practice could dispense as well as prescribe; whether the practice was located in an urban or rural area; total payments received per registered patient weighted for need by the Carr-Hill capitation formula;25 whether the practice received additional income than was recommended by the capitation formula through the Minimum Practice Income Guarantee (MPIG); and the first quality rating that the practice received from the national regulator, the Care Quality Commission.
We also obtained data on practices’ diabetes management from the Quality and Outcomes Framework (QOF). We include scores for: percentage of QOF points awarded for diabetes management; and percentage of registered patients with diabetes who were ‘exception reported’.
The data sources for these practice characteristics are described in Table S1 in the Supplementary Material.
Analysis sample
From the set of general practices included in the National Diabetes Audit (n = 7,413), we excluded practices that did not maintain a registered population size of over 1,000 patients during the audit periods (n = 534, 7·2%). We also excluded any practices that did not contribute to the National Diabetes Audit in all of the three audit periods (n = 351, 4·7%). In order to retain sample size, we used the missing indicator method and created a ‘missing’ category for each categorical variable.26 However, we omitted practices from our sample that were missing data on the continuous covariates (n = 52, 0·7%).
Analysis
We first examined the annual rates of type 2 diabetes incidence by DPP wave descriptively. We calculated weighted-mean incidence rates in each year for all practices in each of the three waves, using the size of the at-risk population in each practice as weights, and presented these graphically. We used Poisson regression models to estimate cross-sectional, unadjusted Incidence Rate Ratios (IRRs) for the average differences between the waves in each year. These simple descriptive statistics show how the raw differences in type 2 diabetes incidence rates between the groups changed over time. We then used a difference-in-differences approach27 to estimate the impact of introducing the DPP on incidence rates of type 2 diabetes across England.
Difference-in-differences is a commonly used method for evaluating the impact of health policies in the absence of randomisation.28 This approach compares outcome trends in a treated group to those in an unaffected comparison group, using the comparison group to provide a counterfactual trend that would have been observed in the absence of treatment. It relies on the assumption that, in the absence of treatment, the outcome trends would have been the same in the treated group as in the comparison group. This is referred to as the parallel trends assumption, and supporting evidence for its validity is sought by examining whether the trends are parallel in the pre-intervention period.
The trend in outcomes of the comparator group is then used to account for common trends and changes experienced by both groups during the intervention period (for example, the general trend over time in type 2 diabetes incidence and any other national changes to diagnosis and care activity). The difference in outcomes observed for the treatment group after the intervention was introduced, over and above the difference in outcomes for the comparator group over the same period can then be attributed to the intervention. Difference-in-differences is a pre-post evaluation design, with the addition of a control group to account for background changes in outcomes that occur over time.
We exploit the gradual rollout of the DPP to implement a difference-in-differences design with staggered adoption.29 We examine the impact of the DPP on type 2 diabetes incidence rates amongst practices belonging to waves 1 and 2 of the DPP, using practices belonging to the final wave as the comparator group. We compare to the final wave practices during the period before we would expect that the DPP could have had an impact on type 2 diabetes incidence in final wave practice populations. Due to the differential timing of the DPP for each of the waves, we carry out separate analyses for wave 1 and wave 2 practices, comparing each to final wave practices in separate models.
We define three time periods for our analyses: pre-DPP, DPP implementation, and post-DPP introduction. We treat 2010–2015 as the pre-period, capturing the trends in type 2 diabetes incidence prior to the introduction of the DPP in any part of the country. We test for plausible evidence of whether parallel trends assumption holds by carrying out t-tests of the significance of a wave and time trend interaction in the pre-period.
We then define an implementation period which allows for the fact that a practice enrolling in the DPP will not see an immediate effect on their population's type 2 diabetes incidence rates. This is because individuals must first be identified as having prediabetes before they can be offered a referral to the DPP. If an individual accepts this referral, programme completion takes a minimum of 9 months from first session attendance. We allow for a minimum of 19 months after the introduction of the DPP to practices in a wave before measuring the incidence of type 2 diabetes.
More specifically, we treat 2016 and 2017 as the implementation period for practices belonging to wave 1 of the DPP and 2016 to 2018 as the implementation period for practices belonging to wave 2. We omit this implementation period from our assessment of outcomes, and examine the impact of the DPP on incidence of type 2 diabetes during the post-DPP introduction period. In our main analyses this post period is 2018-2019 for the comparison of wave 1 practices to final wave practices, and 2019 for the comparison of wave 2 practices to the final wave practices.
We estimate the difference-in-differences models using Poisson regressions with random effects at the practice level, and present the results in terms of IRRs. Poisson regression models were used because the dependent variables of interest (rates of new type 2 diabetes) was a non-negative count.
These regressions also included the following practice covariates, as detailed in the data section: contract type, dispensing permissions, rural location, total payments received per registered weighted patient, MPIG additional income receipt, Care Quality Commission rating, QOF diabetes management scores. We also accounted for the following characteristics of the practice population: age distribution, ethnicity, and socio-economic deprivation. Further details of the model specification can be found in the Supplementary Material.
Sensitivity analyses
We conducted a number of additional analyses to investigate the sensitivity of our results to the assumptions regarding the timing of the DPP's effects and the DPP implementation period.
First, we explored the impact of retaining additional years within the pre-DPP period rather than treating them as implementation years which are omitted from our assessment of outcomes. For wave 1 practices we examined the inclusion of 2016 in the pre-DPP period, and then 2016 to 2017. For wave 2 practices we examined the inclusion of 2016 in the pre-DPP period, then 2016 to 2017, and finally 2016 to 2018.
Second, we varied the assumptions regarding the period over which the DPP could have had an effect on type 2 diabetes incidence. This involved retaining additional years within the post-DPP introduction period rather than treating them as implementation years which are omitted from our assessment of outcomes. For wave 1 practices we examined the inclusion of 2017 in the post-DPP period. For wave 2 practices we examined the inclusion of 2018 in the post-DPP period. In doing so we reduce the time period between the DPP first becoming available and the point at which we start to examine any potential impacts of the programme on type 2 diabetes incidence from the 19 month interval (used in our main analysis) to 7 months.
Finally, for wave 1 practices we estimate the effect of the DPP for the years of 2018 and 2019 separately, to examine whether the magnitude of the impacts varies over time.
All analyses were conducted in Stata 14.
Role of the funding source
Neither the study sponsor nor the funder had any input into the study design; in the collection, analysis, and interpretation of data; in the writing of the report; or in the decision to submit the paper for publication.
Results
Descriptive statistics
Our sample consisted of 6,476 practices (94·1% of all practices in England) covering over 47 million registered patients that were over 15 years of age. Of these practices, 3,282 (50·7%) were wave 1, 1,610 (24·9%) wave 2 and 1,584 (24·5%) final wave practices (Table 1). A detailed description of the number of practices excluded and the related reasons is provided in the Supplementary Material.
Table 1.
Patient population and practice characteristics by wave of DPP enrolment, measured as of 1st April 2018.
| Wave of practice enrolment: | Wave 1 |
Wave 2 |
Final Wave |
|||
|---|---|---|---|---|---|---|
| Practice Characteristics | Mean | SD | Mean | SD | Mean | SD |
| Registered population | 8,911·8 | 5,723·8 | 9,008·8 | 5,396·0 | 8,431·0 | 5,425·8 |
| Receiving Minimum Practice Income Guarantee, % | 38·2 | 36·1 | 33·7 | |||
| First Care Quality Commission Rating, % | ||||||
| Inadequate | 3·1 | 2·3 | 3·2 | |||
| Requires Improvement | 14·1 | 10·3 | 11·4 | |||
| Good | 78·2 | 82·9 | 79·8 | |||
| Outstanding | 4·3 | 4·0 | 4·9 | |||
| Missing | 0·2 | 0·4 | 0·6 | |||
| Contract Type, % | ||||||
| Alternative Provider Medical Services | 2·3 | 2·0 | 1·1 | |||
| Personal Medical Services | 24·5 | 29·8 | 27·2 | |||
| General Medical Services | 73·2 | 68·2 | 71·7 | |||
| Dispensing Practice, % | 15·1 | 12·9 | 18·6 | |||
| Rural practice, % | 15·5 | 13·7 | 20·6 | |||
| Payment per weighted patient, ln(£s) | 5·0 | 0·2 | 5·0 | 0·2 | 5·0 | 0·2 |
| Quality and Outcomes Framework, % | ||||||
| Clinical points awarded for Diabetes management | 92·2 | 9·6 | 92·8 | 9·4 | 92·1 | 10·4 |
| Exception reporting rate | 11·4 | 5·0 | 12·2 | 5·2 | 11·9 | 5·2 |
| Patient Characteristics | ||||||
| Male, % | 50·2 | 2·5 | 50·1 | 2·0 | 50·0 | 2·0 |
| Ethnicity, % | ||||||
| White | 80·3 | 22·9 | 84·5 | 20·3 | 87·0 | 19·8 |
| Mixed | 1·6 | 1·5 | 1·1 | 1·1 | 1·0 | 1·1 |
| Asian | 10·2 | 15·6 | 9·3 | 15·2 | 7·5 | 13·6 |
| Black | 4·5 | 7·1 | 2·6 | 4·7 | 2·3 | 4·7 |
| Other | 3·5 | 4·5 | 2·6 | 3·5 | 2·3 | 3·7 |
| Index of Multiple Deprivation, % | ||||||
| 1st decile (Most deprived) | 10·3 | 18·9 | 15·6 | 20·8 | 12·5 | 20·6 |
| 2nd decile | 11·4 | 13·3 | 12·3 | 13·4 | 9·5 | 12·0 |
| 3rd decile | 11·8 | 12·8 | 9·6 | 9·2 | 10·2 | 10·9 |
| 4th decile | 10·8 | 10·7 | 8·8 | 9·2 | 10·9 | 12·0 |
| 5th decile | 10·4 | 10·2 | 8·3 | 8·8 | 11·6 | 12·0 |
| 6th decile | 9·9 | 10·3 | 8·5 | 9·3 | 11·0 | 11·8 |
| 7th decile | 9·2 | 9·8 | 9·3 | 9·8 | 9·8 | 10·7 |
| 8th decile | 8·8 | 10·4 | 9·7 | 10·5 | 9·2 | 10·1 |
| 9th decile | 8·8 | 11·4 | 9·1 | 11·5 | 8·7 | 11·5 |
| 10th decile (Least deprived) | 8·6 | 15·2 | 8·9 | 14·8 | 6·7 | 12·9 |
| Age, % | ||||||
| Below 40 years | 51·5 | 10·1 | 50·8 | 9·8 | 48·3 | 9·3 |
| 40 to 49 years | 13·7 | 2·0 | 13·2 | 1·6 | 12·9 | 1·6 |
| 50 to 59 years | 13·3 | 2·6 | 13·4 | 2·6 | 13·9 | 2·2 |
| 60 to 69 years | 9·8 | 3·1 | 10·2 | 2·8 | 11·2 | 3·0 |
| 70 to 79 years | 7·4 | 3·3 | 7·7 | 3·0 | 8·6 | 3·3 |
| 80 years and over | 4·4 | 2·1 | 4·5 | 2·4 | 5·1 | 2·1 |
| Number of General Practices | 3,282 (50·7%) | 1,610 (24·9%) | 1,584 (24·5%) | |||
Wave 1 practices had on average 8,911 registered patients, wave 2 practices had 9,008 and final wave practices 8,431 (Table 1). Wave 1 practices had a lower percentage of White patients (80·3% compared to 84·5% in wave 2 and 87·0% in final wave practices). Otherwise, the characteristics that may predict differences in incidence rates appeared broadly similar across waves.
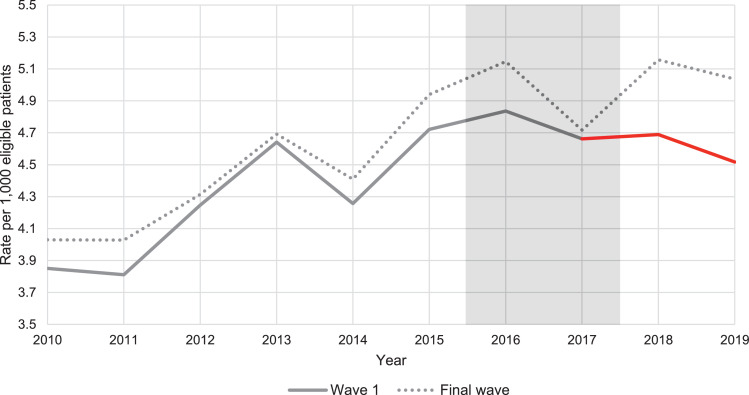
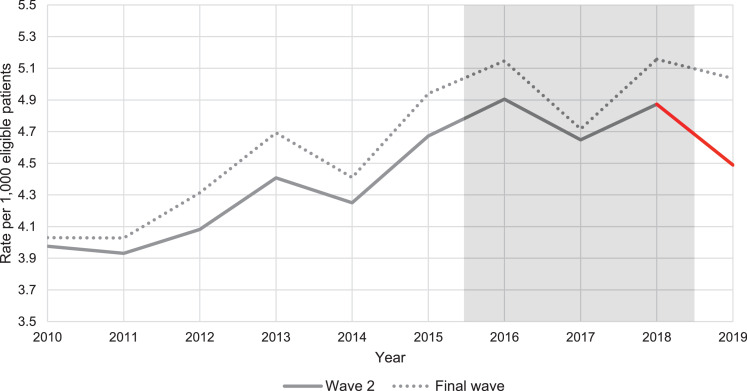
Rates of new type 2 diabetes per 1,000 eligible patients were higher amongst the final wave practices throughout the period, these are shown graphically in Figure 1, Figure 2 and descriptively in Table S2 of the Supplementary Material, along with the individual year, unadjusted Poisson regressions.
Figure 1.
Weighted average practice rates of type 2 diabetes incidence per 1,000 eligible patients from 2010 to 2019, for wave 1 compared to final wave practices [Note: Axis does not start from 0, weighting by number of eligible individuals for each practice, by year].
Figure 2.
Weighted average practice rates of type 2 diabetes incidence per 1,000 eligible patients from 2010 to 2019, for wave 2 compared to final wave practices [Note: Axis does not start from 0, weighting by number of eligible individuals for each practice, by year].
Parallel trends assumption
The annual increase in incidence rates was on average 0·182 cases per 1,000 in final wave practices between 2010 and 2015. During the same period, the trends in incidence rate were very similar in wave 1 practices (IRR for interaction of wave 1 with time trend: 1·001, 95% CI 0·995 to 1·007) and slightly lower in wave 2 practices (IRR for interaction of wave 2 with time trend: 0·991, 95% CI 0·984 to 0·997).
Regression results
The main regression results are shown in Table 2. The estimated coefficients on the covariates are shown in the Supplementary Material Tables S4 and S5, and the sensitivity analyses results are presented in Supplementary Material Table S6.
Table 2.
Difference-in-differences results on type 2 diabetes incidence.
| Type 2 diabetes incidence Years: 2018 to 2019 |
||
|---|---|---|
| Difference-in-differences | IRR | 95% Confidence Interval |
| Wave 1 vs Final Wave | 0·938⁎⁎⁎ | (0·905 to 0·972) |
| Observations=38,647 | ||
| Type 2 diabetes incidence Years: 2019 | ||
| Wave 2 vs Final Wave | 0·927* | (0·885 to 0·972) |
| Observations=22,142 | ||
Notes:
p < 0·05.
**p < 0·01.
p < 0·001. Incident rate ratios from random effects Poisson regression models. Models also include the following covariates measured at April 2018: Payment received per weighted patient; whether the practice received additional income than was recommended by the capitation formula through the Minimum Practice Income Guarantee (MPIG); contract type; dispensing status; rural location; QOF achievement for diabetes and exception reporting; and quality rating from the Care Quality Commission; percentages of registered population in each deprivation decile, and the ethnicity, gender and age composition of the practice's registered patients.
The incidence rates in wave 1 practices were significantly lower in 2018 and 2019 than would have been expected in the absence of the DPP (difference-in-differences IRR = 0·938 (95% CI 0·905 to 0·972)) (Table 2). When we estimated the effects for each of these years separately, we found a statistically significant difference-in-differences IRR of 0·944 (95% CI 0·909 to 0·981) in 2018, and an IRR of 0·932 (95% CI 0·896 to 0·969) for 2019. This suggests that the change in incidence rates amongst wave 1 practices relative to final wave practices was slightly larger in 2019 than in 2018.
The incidence rate in wave 2 practices was also significantly lower than expected in 2019 (difference-in-differences IRR = 0·927 (95% CI 0·885 to 0·972)).
We tested the sensitivity of our results to assumptions regarding the timing of effects and the implementation period. First, we explored the impact of retaining additional years within the pre-period rather than treating them as implementation years.
When we retained 2016 as a pre-DPP year, the wave 1 IRR increased to 0·943 (95% CI 0·910 to 0·976) and the wave 2 effect remained at 0·927 (95% CI 0·884 to 0·972). When we also retained 2017 as a pre-DPP year, the estimated effect size of wave 1 increased, IRR 0·939 (0·909 to 0·970) and for wave 2 the IRR reduced to 0·922 (95% CI 0·881 to 0·965). The difference-in-differences IRR reduced to 0·925 (95% CI 0·889 to 0·962) when we retained 2018 as a pre-DPP year within the wave 2 analysis.
Second, we varied the assumptions regarding the period over which the DPP could have had an effect on diabetes incidence. When we included 2017 in the post-DPP introduction period over which we estimated impact in the wave 1 analysis, we find a difference-in-differences IRR of 0·970 (95% CI 0·942 to 0·9997). The impact of including 2018 in the effectiveness estimates for the wave 2 analysis resulted in a difference-in-differences IRR of 0·951 (95% CI 0·917 to 0·986).
Discussion
Statement of principal findings
We evaluated the impact of the DPP on new cases of type 2 diabetes within England, by exploiting its gradual roll-out and using real world programme evaluation methods. Our difference-in-differences analyses suggest that introduction of the DPP was associated with a decrease in type 2 diabetes incidence. This conclusion remains consistent across several robustness checks, with IRR estimates marginally changing but not being significantly different from one another, in that their confidence intervals overlap with one another.
Using the main analysis estimates and the observed number of new cases of diabetes from the National Diabetes Audit data, it is possible to calculate the number of cases prevented. During 2018 to 2019 there were 208,420 new cases of type 2 diabetes amongst wave 1 practices. Using the difference-in-differences IRR of 0·938 (95% CI 0·905 to 0·972), it is possible to calculate that in the absence of the DPP we would have expected 222,196 cases, which is 13,776 cases (95% CI 6,004 to 21,878) prevented. Performing similar calculations for the wave 2 analysis, with an IRR of 0·927 (95% CI 0·885 to 0·972) we estimate that 4,032 cases were prevented (95% CI 1,475 to 6,652) for the year 2019.
This is the first study to examine the national impact of the DPP in terms of population rates of new type 2 diabetes. Whilst other studies have demonstrated the effectiveness of prevention programmes, they focused only on those who participated in the programme, and were often delivered at much reduced scale in more controlled ‘research’ environments using more selected groups of patients.
When scaling-up programmes of this nature, it is possible that the programmes are not delivered as intended leading to ‘program drift’, or that the effectiveness is diluted, referred to as a ‘voltage drop’ whereby the effect of an intervention decreases as testing moves from efficacy to effectiveness to implementation.30 To ensure that programmes such as the DPP are impactful public health interventions, they need to both retain their potency in unselected patient populations and have sufficient reach amongst the at-risk population. The RE-AIM framework suggests five elements for determining the public health impact of programmes.31 Previous research has reported on the reach,32 adoption, and implementation33 of the NHS DPP. In this paper we examine the remaining two elements, effectiveness and maintenance (at the individual level). Our results suggest that the DPP was effective at reducing the incidence of type 2 diabetes compared to population health trends over an extended period.
Other studies
Our finding that the DPP reduced diabetes incidence is consistent with previous studies of similar prevention initiatives, but our effectiveness estimates are evaluated at the population level.
The United States Diabetes Prevention Programme (US DPP) enrolled 3,234 highly selected participants to either: a lifestyle change programme, metformin, or placebo. During the study period incidence was 4·8 cases (95% CI 4·1–5·7) per 100 person years in the intensive lifestyle intervention and 11·0 cases (95% CI 9·8–12·3) in the placebo group.34 At 10 years post randomisation, type 2 diabetes incidence rates were reduced by 34% in the lifestyle group compared to placebo.35 A meta-analysis of studies evaluating lifestyle interventions found that they reduced the relative risk of developing diabetes by 31% (95% CI 15% to 44%), on the condition that the intervention lasted between 6 months and 2 years.12
Strengths and limitations
A key strength of this study is that it considers DPP effectiveness in terms of type 2 diabetes incidence rather than a proxy outcome measure linked to type 2 diabetes risk, such as weight loss. This is relatively rare in the published literature, as evidenced by one meta-analysis evaluating real-world translational studies from the US DPP, finding zero out of the 28 studies identified to have assessed changes in diabetes incidence.36
This is also the first study to assess the population level effect of the NHS DPP. In evaluating the programme at the national level we explored the effect not just on those participating in the programme, but also potential wider effects such as those resulting from increased identification and diagnosis of patients with prediabetes.
We use routinely collected data from the National Diabetes Audit which covers over 99% of practices across England, not just those who volunteered to submit data. The data were routinely collected independent of this study, although this meant that we did not have control over which variables were collected and how frequently.
The observational nature of this study does, however, means that it is subject to residual confounding. To mitigate against this, we included a rich set of covariates measuring characteristics of practices as well as their registered patient populations. In addition, the historic rates of type 2 diabetes were calculated retrospectively from the National Diabetes Audit data, which only includes individuals that are still alive as of the extraction dates. The incidence rates examined are therefore likely to be an underestimate. It is also important to note that we are only able to observe diagnoses of type 2 diabetes recorded in primary care, meaning that we cannot examine the impact of the NHS DPP on cases of undiagnosed diabetes.
The date of diagnosis available in our National Diabetes Audit dataset was limited to calendar year. We were therefore unable to produce these annual counts according to financial year, which would have better aligned with the timing of the wave rollouts. In our analysis, when calculating the eligible population for each practice we considered individuals without a diagnosis of diabetes over 15 years of age. This was to align with the data available in the National Diabetes Audit (all individuals over 15 years of age) but did not directly align with the minimum age of participating in the DPP, which was 18 years and over. It is, however, still possible that these individuals may have benefited from a general practice being active in the DPP.
Implications for future research
It is unclear whether the effect of the NHS DPP is to completely prevent type 2 diabetes or instead delay the onset of the condition. Whilst we use data over the longest time period currently available, the longer-term effects are unknown. Future research should address this question when longer-term follow-up data becomes available.
Our results show that the DPP had a demonstrable impact on type 2 diabetes incidence at the population level. However some patient groups may have benefited more than others. Future research investigating whether the impact of the DPP on developing type 2 diabetes differs across patient groups could inform future refinements of the programme to target those likely to experience the greatest benefit, and provide evidence on the equity implications of initial implementation. An assessment of the cost-effectiveness of the DPP is also vital to determine whether the benefits achieved by the programme represent an efficient use of NHS resources.
Conclusion
This is the first study to evaluate the population impact of the NHS DPP on type 2 diabetes incidence in England. Comparing practices enrolled in earlier waves of the programme to those enrolled in the final wave, we find that the introduction of the DPP was associated with a decrease in diabetes incidence. Further research is needed to explore whether these effects are maintained in the long term and whether the DPP represents value for money.
Contributors
MS, RM, BP and EM conceived the idea for the paper.
EM and MS undertook the analysis. EM took the lead in writing the manuscript. All authors were involved in editing the manuscript.
EM, BP and MS have had full access to and verify the underlying study data. They accept responsibility to submit for publication.
Declaration of interests
All authors are funded by the National Institute for Health Research (Health Services and Delivery Research, 16/48/07 – Evaluating the NHS Diabetes Prevention Programme (NHS DPP): the DIPLOMA research Programme (Diabetes Prevention – Long Term Multimethod Assessment)). They have no other financial or personal relationships to declare.
Acknowledgments
Data sharing statement
The National Diabetes Audit data used in this study was obtained upon application to NHS Digital (reference: DARS-NIC-196221-K4K3Y). The authors are not permitted to share this data. The use of this data is subject to various output rules, including rounding count values to the nearest 5. All other data used in this study is publicly available and referenced within the manuscript and supplementary material. The wider project protocol is available from: https://fundingawards.nihr.ac.uk/award/16/48/07 and ethical approval was received from the North West - Greater Manchester East Research Ethics Committee (REC reference 17/NW/0426).
Footnotes
Supplementary material associated with this article can be found in the online version at doi:10.1016/j.lanepe.2022.100420.
Appendix. Supplementary materials
References
- 1.Murray C.J., Aravkin A.Y., Zheng P., et al. Global burden of 87 risk factors in 204 countries and territories, 1990–2019: a systematic analysis for the Global Burden of Disease Study 2019. Lancet N Am Ed. 2020;396(10258):1223–1249. doi: 10.1016/S0140-6736(20)30752-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Tabák A.G., Herder C., Rathmann W., Brunner E.J., Kivimäki M. Prediabetes: a high-risk state for diabetes development. Lancet N Am Ed. 2012;379(9833):2279–2290. doi: 10.1016/S0140-6736(12)60283-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ravindrarajah R., Reeves D., Howarth E., et al. The epidemiology and determinants of non-diabetic hyperglycaemia and its conversion to type 2 diabetes mellitus, 2000-2015: cohort population study using UK electronic health records. BMJ Open. 2020 doi: 10.1136/bmjopen-2020-040201. (In press) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Chatzi G., Mason T., Chandola T., et al. Sociodemographic disparities in non-diabetic hyperglycaemia and the transition to type 2 diabetes: evidence from the English Longitudinal Study of Ageing. Diabet Med. 2020;37(9):1536–1544. doi: 10.1111/dme.14343. [DOI] [PubMed] [Google Scholar]
- 5.Bergman M., Buysschaert M., Schwarz P.E., Albright A., Narayan K.V., Yach D. Diabetes prevention: global health policy and perspectives from the ground. Diabetes Manag. 2012;2(4):309. doi: 10.2217/dmt.12.34. London, England. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Saeedi P., Petersohn I., Salpea P., et al. Global and regional diabetes prevalence estimates for 2019 and projections for 2030 and 2045: results from the International diabetes federation diabetes atlas. Diabetes Res Clin Pract. 2019;157 doi: 10.1016/j.diabres.2019.107843. [DOI] [PubMed] [Google Scholar]
- 7.Sharma M., Nazareth I., Petersen I. Trends in incidence, prevalence and prescribing in type 2 diabetes mellitus between 2000 and 2013 in primary care: a retrospective cohort study. BMJ Open. 2016;6(e010210) doi: 10.1136/bmjopen-2015-010210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.NHS England . 2019. NHS Diabetes Prevention Programme (NHS DPP)https://www.england.nhs.uk/diabetes/diabetes-prevention/ Accessed 27th November 2019. [Google Scholar]
- 9.Lindström J., Eriksson J.G., Valle T.T., et al. Prevention of diabetes mellitus in subjects with impaired glucose tolerance in the Finnish diabetes prevention study: results from a randomized clinical trial. J Am Soc Nephrol. 2003;14(suppl 2):S108–SS13. doi: 10.1097/01.asn.0000070157.96264.13. [DOI] [PubMed] [Google Scholar]
- 10.Rubin R.R., Fujimoto W.Y., Marrero D.G., et al. The diabetes prevention program: recruitment methods and results. Control Clin Trials. 2002;23(2):157–171. doi: 10.1016/s0197-2456(01)00184-2. [DOI] [PubMed] [Google Scholar]
- 11.Tabak R.G., Sinclair K.A., Baumann A.A., et al. A review of diabetes prevention program translations: use of cultural adaptation and implementation research. Trans Behav Med. 2015;5(4):401–414. doi: 10.1007/s13142-015-0341-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Barry E., Roberts S., Oke J., Vijayaraghavan S., Normansell R., Greenhalgh T. Efficacy and effectiveness of screen and treat policies in prevention of type 2 diabetes: systematic review and meta-analysis of screening tests and interventions. BMJ. 2017:356. doi: 10.1136/bmj.i6538. [DOI] [PubMed] [Google Scholar]
- 13.Schellenberg E.S., Dryden D.M., Vandermeer B., Ha C., Korownyk C. Lifestyle interventions for patients with and at risk for type 2 diabetes: a systematic review and meta-analysis. Ann Intern Med. 2013;159(8):543–551. doi: 10.7326/0003-4819-159-8-201310150-00007. [DOI] [PubMed] [Google Scholar]
- 14.Marsden A.M., Bower P., Howarth E., Soiland-Reyes C., Sutton M., Cotterill S. ‘Finishing the race’–a cohort study of weight and blood glucose change among the first 36,000 patients in a large-scale Diabetes Prevention Programme. Int J Behav Nutr Phys Act. 2022;19(1):1–10. doi: 10.1186/s12966-022-01249-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Valabhji J., Barron E., Bradley D., et al. Early outcomes from the English National health service Diabetes Prevention Programme. Diabetes Care. 2020;43(1):152–160. doi: 10.2337/dc19-1425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Wareham N.J. Mind the gap: efficacy versus effectiveness of lifestyle interventions to prevent diabetes. lancet Diabetes Endocrinol. 2015;3(3):160–161. doi: 10.1016/S2213-8587(15)70015-X. [DOI] [PubMed] [Google Scholar]
- 17.French D.P., Sutton S. Methods: does measuring people change them? Psychologist. 2011;24(4):272–274. [Google Scholar]
- 18.NHS England . NHS England; 2016. NHS England Impact Analysis of implementing NHS Diabetes Prevention Programme, 2016 to 2021.https://www.england.nhs.uk/wp-content/uploads/2016/08/impact-assessment-ndpp.pdf Accessed 11th October 2019. [Google Scholar]
- 19.Hawkes R.E., Cameron E., Cotterill S., Bower P., French D.P. The NHS Diabetes Prevention Programme: an observational study of service delivery and patient experience. BMC Health Serv. Res. 2020;20(1):1–12. doi: 10.1186/s12913-020-05951-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Bansal N. Prediabetes diagnosis and treatment: a review. World J Diabetes. 2015;6(2):296. doi: 10.4239/wjd.v6.i2.296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.NHS England . NHS England; 2016. Diabetes Prevention Programme Information Governance and Data Flows.https://www.england.nhs.uk/wp-content/uploads/2019/09/diabetes-prevention-programme-information-governance-and-data-flows-framework.pdf Accessed 26th April 2021. [Google Scholar]
- 22.NHS Digital, Read Codes, 2020, NHS Digital. https://digital.nhs.uk/services/terminology-and-classifications/read-codes. Accessed 6th October 2021.
- 23.NHS Digital . NHS Digital; 2021. National Diabetes Audit, 2019-20, Report 1: Care Processes and Treatment Targets.https://files.digital.nhs.uk/42/B253B1/National%20Diabetes%20Audit%202019-20%20Full%20Report%201.pdf Accessed 13th November 2021. [Google Scholar]
- 24.McManus E., Elliott J., Meacock R., Wilson P., Gellatly J., Sutton M. The effects of structure, process and outcome incentives on primary care referrals to a national prevention programme. Health Econ. 2021;30(6):1393–1416. doi: 10.1002/hec.4262. [DOI] [PubMed] [Google Scholar]
- 25.British Medical Association Global sum allocation formula. British Medical Association. 20202020 https://www.bma.org.uk/advice-and-support/gp-practices/funding-and-contracts/global-sum-allocation-formula [Google Scholar]
- 26.Groenwold R.H., White I.R., Donders A.R.T., Carpenter J.R., Altman D.G., Moons K.G. Missing covariate data in clinical research: when and when not to use the missing-indicator method for analysis. CMAJ. 2012;184(11):1265–1269. doi: 10.1503/cmaj.110977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Dimick J.B., Ryan A.M. Methods for evaluating changes in health care policy: the difference-in-differences approach. JAMA. 2014;312(22):2401–2402. doi: 10.1001/jama.2014.16153. [DOI] [PubMed] [Google Scholar]
- 28.Wing C., Simon K., Bello-Gomez R.A. Designing difference in difference studies: best practices for public health policy research. Annu Rev Public Health. 2018;39:453–469. doi: 10.1146/annurev-publhealth-040617-013507. [DOI] [PubMed] [Google Scholar]
- 29.Callaway B., Sant'Anna P.H. Difference-in-differences with multiple time periods. J Econometrics. 2021;225(2):200–230. [Google Scholar]
- 30.Chambers D.A., Glasgow R.E., Stange K.C. The dynamic sustainability framework: addressing the paradox of sustainment amid ongoing change. Implement Sci. 2013;8(1):1–11. doi: 10.1186/1748-5908-8-117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Glasgow R.E., Vogt T.M., Boles S.M. Evaluating the public health impact of health promotion interventions: the RE-AIM framework. Am J Public Health. 1999;89(9):1322–1327. doi: 10.2105/ajph.89.9.1322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Barron E., Clark R., Hewings R., Smith J., Valabhji J. Progress of the healthier you: NHS Diabetes Prevention Programme: referrals, uptake and participant characteristics. Diabet Med. 2018;35(4):513–518. doi: 10.1111/dme.13562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.French D.P., Hawkes R.E., Bower P., Cameron E. Is the NHS Diabetes Prevention Programme intervention delivered as planned? An observational study of fidelity of intervention delivery. Ann Behav Med. 2021;55(11):1104–1115. doi: 10.1093/abm/kaaa108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Diabetes Prevention Program Research Group Reduction in the incidence of type 2 diabetes with lifestyle intervention or metformin. N Engl J Med. 2002;346(6):393–403. doi: 10.1056/NEJMoa012512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Diabetes Prevention Program Research Group 10-year follow-up of diabetes incidence and weight loss in the diabetes prevention program outcomes study. Lancet N Am Ed. 2009;374(9702):1677–1686. doi: 10.1016/S0140-6736(09)61457-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Ali M.K., Echouffo-Tcheugui J.B., Williamson D,F. How effective were lifestyle interventions in real-world settings that were modeled on the diabetes prevention program? Health Aff. 2012;31(1):67–75. doi: 10.1377/hlthaff.2011.1009. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.